Significance
Coculture is a promising strategy in cartilage tissue engineering given its potential to rejuvenate aged mesenchymal stem cells (MSCs) to promote functional chondrogenesis. Identifying the molecular factors mediating this coculture effect, as well as carriers and delivery mechanisms, will forward the development of therapeutics to promote functional cartilage repair by aged autologous MSCs. This will potentially expand the clinical indications of microfracture and other procedures that rely on aged endogenous stem cell populations.
Keywords: extracellular vesicles, intercellular communication, chondrocyte/MSC coculture, cartilage tissue engineering
Abstract
Several recent studies have demonstrated that coculture of chondrocytes (CHs) with bone marrow-derived mesenchymal stem cells (MSCs) improves their chondrogenesis. This implies that intercellular communication dictates fate decisions in recipient cells and/or reprograms their metabolic state to support a differentiated function. While this coculture phenomenon is compelling, the differential chondroinductivity of zonal CHs on MSC cocultures, the nature of the molecular cargo, and their transport mechanisms remains undetermined. Here, we demonstrate that juvenile CHs in coculture with adult MSCs promote functional differentiation and improved matrix production. We further demonstrate that close proximity between the two cell types is a prerequisite for this response and that the outcome of this interaction improves viability, chondrogenesis, matrix formation, and homeostasis in the recipient MSCs. Furthermore, we visualized the transfer of intracellular contents from CHs to nearby MSCs and showed that inhibition of extracellular vesicle (EV) transfer blocks the synergistic effect of coculture, identifying EVs as the primary mode of communication in these cocultures. These findings will forward the development of therapeutic agents and more effective delivery systems to promote cartilage repair.
There are currently no surgical techniques that can treat osteoarthritis and restore the native properties of articular cartilage. As such, tissue-engineering approaches are being developed with the goal of forming functional biologic replacement materials. Toward this, various hydrogels and different cell types have been used to generate cell-laden constructs (1, 2). While chondrocytes (CHs) have been broadly used in cartilage tissue engineering, their clinical application is limited due to a scarcity of healthy tissue. Given these limitations, mesenchymal stem cells (MSCs) have emerged as an alternative cell source (3–5). MSCs are attractive due to their potential autologous sourcing and their capacity to undergo chondrogenesis. However, MSC number and chondrogenic capacity is attenuated with aging (6). This limits clinical therapeutics that rely on endogenous recruitment or application of exogenously expanded autologous MSCs.
To address this deficiency, several studies have introduced coculture (CO) systems of MSCs and CHs and have demonstrated a synergistic improvement in functional outcomes (7–9). While it is generally believed that molecular factors are passed from one cell type to another, findings have varied as to the directionality of this intercellular communication and the underlying mode of communication. In some instances, it is reported that CHs improve MSC chondrogenesis, while other studies indicate the opposite, and some report a mutual beneficial effect (7–15). Moreover, while the zonal origin of CHs (within the depth of the tissue) and the aging of MSCs distinctively define their phenotype and metabolic capacity, the concomitant impact of these factors has not been fully investigated regarding COs. Furthermore, the molecular factors mediating the coculture effect and their delivery mechanisms are not fully understood. Recent studies have suggested that cell-derived extracellular vesicles (EVs) can carry proteins, enzymes, RNA, and DNA (16, 17), and that these vesicular contents reflect the physiology of releasing cells and convey molecular information to recipient cells by receptor-mediated endocytosis (18, 19) or vesicular fusion (20, 21). After internalization, these received molecular factors reprogram the metabolism and activity of recipient cells and so may play an important role in intercellular communication that occurs during coculture (22, 23).
In this study, we demonstrate the chondroinductive impact of CHs from all cartilage zones on cocultured bone marrow-derived MSCs and specifically demonstrate the role of intercellular distance on the success of this interaction. We also identify molecular factors and pathways that are activated as a consequence of coculture. Finally, we track the movement of intracellular contents from releasing to recipient cells and show that secretion and uptake of EVs is essential for this process, explicating the central delivery mode in this intercellular communication. Collectively, these findings improve our understanding of cocultures toward the development of therapeutic agents and more effective delivery systems to promote functional cartilage tissue formation with adult autologous bone marrow-derived MSCs.
Results
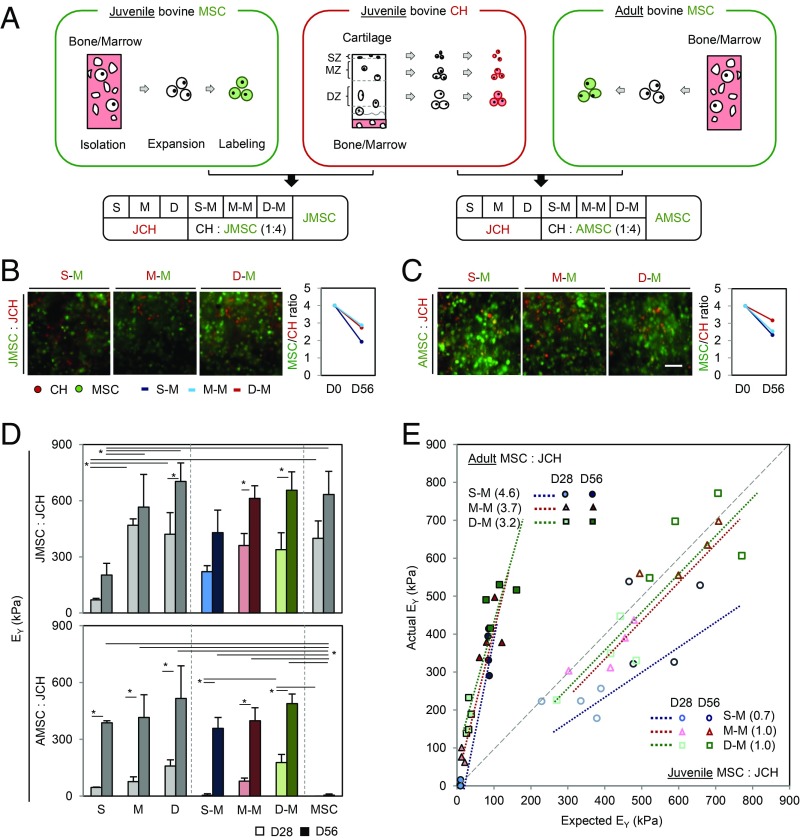
Coculture of Juvenile Chondrocytes with Adult MSCs Derived from Bone Marrow Improves Functional Outcomes.
All cell-laden construct groups grew well over 56 d, except for the adult MSC (AMSC)-laden construct group (SI Appendix, Fig. S1). AMSCs in hydrogels self-aggregated, resulting in a small mass that separated from the hydrogels (SI Appendix, Fig. S1 C and D). The AMSC/juvenile chondrocyte (JCH) construct group contracted slightly early in culture but maintained construct geometry (SI Appendix, Fig. S1 D–F). Both CHs and MSCs in hydrogels retained CellTracker signal after 56 d, and the ratio (MSC/CH) was only slightly lower than on day 0 (Fig. 1 B and C). Mechanical properties and glycosaminoglycan (GAG) content for zonal JCH-laden construct groups [i.e., superficial (S)-, middle (M)-, and deep (D)-zone CHs] increased with time and depended on zonal origin; lower properties were achieved with superficial JCHs (300 kPa and 3.2%WW GAG) and higher properties with deep zone JCHs (610 kPa and 5.7%WW GAG). These passaged JCHs produced robust matrix in a chemically defined media containing TGF-β3 (CM+), and construct properties reached near native levels after 56 d (Figs. 1D and 2A). Juvenile MSCs alone also grew well (633 kPa and 6.1%WW GAG), matching the properties of deep-zone CHs. Construct properties for JMSC/JCH CO groups reflected the zonal origins of the JCHs, but their combination (541 kPa and 5.1%WW GAG) did not enhance functional properties. Conversely, AMSCs cultured alone failed to mature (5 kPa and 1.2%WW GAG). When cocultured with JCHs, however, these constructs achieved markedly higher properties and ECM content (422 kPa and 3.9%WW GAG) (Figs. 1D and 2A). Collagen content was, however, independent of coculture (SI Appendix, Fig. S2A).
Fig. 1.
Impact of zonal CHs cocultured with juvenile MSCs (JMSCs) or AMSCs. (A) Zonal CHs (S, M, and D), and JMSCs and AMSCs were expanded and labeled with CellTracker (MSC, green; CH, red). Zonal CHs were mixed with JMSCs or AMSCs (MSC:CH, 4:1). (B and C) Mixed cell populations were well distributed within the constructs with stable ratios through 56 d of culture (B, JMSC/JCH; C, AMSC/JCH). (D) Equilibrium modulus (EY; in kilopascals). (JMSC/JCH, Top; AMSC/JCH, Bottom; light bar, day 28; dark bar, day 56; n = 4/group, *P < 0.05). (E) Efficacy of coculture (solid, AMSC/JCH; hollow, JMSC/JCH; circle, S–M; triangle, M–M; square, D–M).
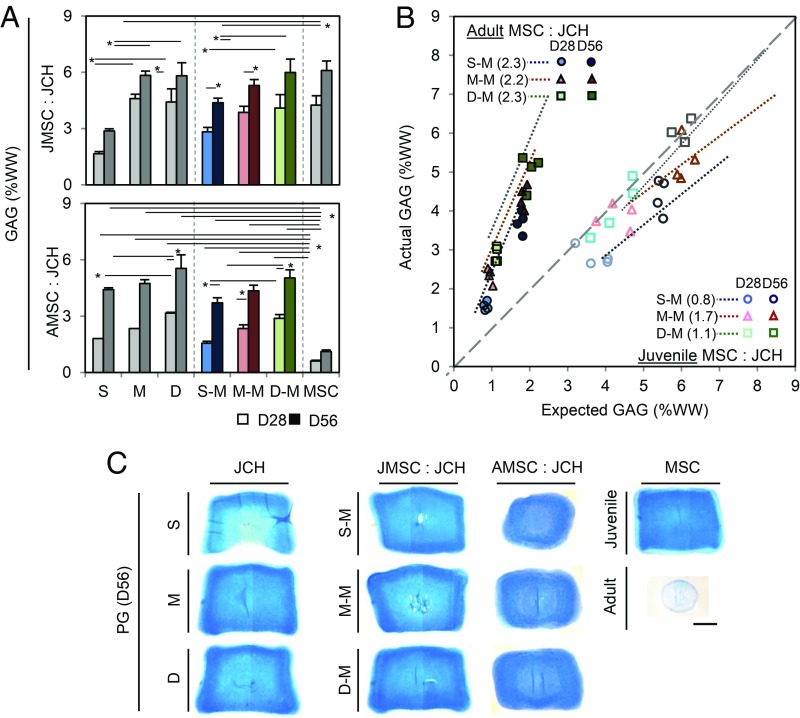
Fig. 2.
PG content of zonal CH/MSC cocultures. (A) GAG content (%WW; JMSC:JCH, Top; AMSC:JCH, Bottom; light bar, day 28; dark bar, day 56; n = 4/group; *P < 0.05); (B) efficacy of coculture (solid, AMSC/JCH; hollow, JMSC/JCH; circle, S–M; triangle, M–M; square, D–M); (C) Alcian blue staining (zonal JCH alone, Left; cocultures, Middle; JMSC or AMSC alone, Right). (Scale bar: 1 mm.)
To evaluate the efficacy of coculture, mechanical properties and biochemical content were plotted on an X–Y plane based on expected vs. actual properties (Figs. 1E and 2B and SI Appendix, Fig. S2B). MSC/CH cocultures made of all juvenile cells (hollow) showed no synergistic effect, with expected contributions from each cell type. Conversely, there was a marked synergistic effect when AMSCs were cocultured with JCHs (AMSC/JCH, solid). Middle- and deep-zone CHs mixed with AMSCs produced greater construct properties in both CO groups. As above, collagen content showed no synergy (SI Appendix, Fig. S2B). Alcian Blue staining for proteoglycan (PG) and immunostaining for chondroitin sulfate (CS) and type II collagen (COL II) were evident for all groups, except for AMSC-laden constructs (Fig. 2C and SI Appendix, Fig. S2 C and E).
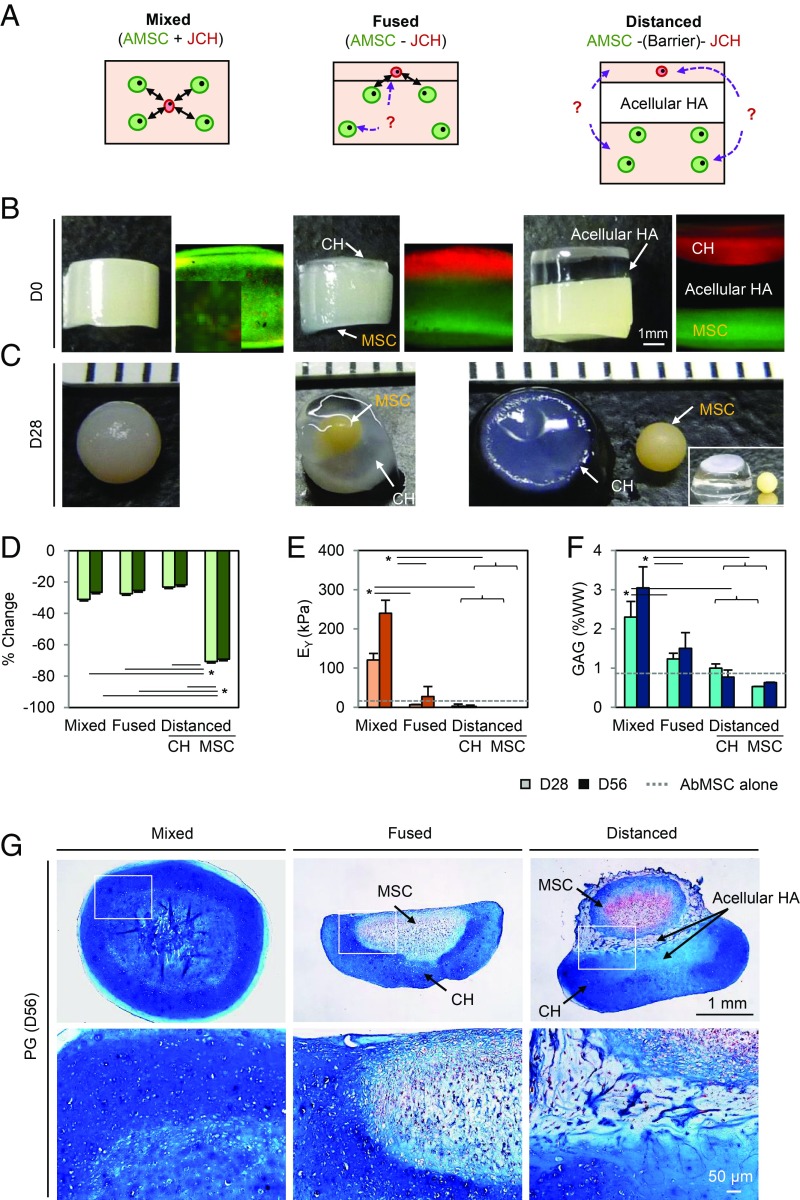
Close Proximity Is Required for a Positive Coculture Response.
To assay the impact of spatial relationships between cell types, AMSCs and JCHs were either well distributed throughout constructs or separated from one another (Fig. 3B). AMSCs in the “fused” and “distanced” groups aggregated together, resulting in the formation of several small masses or a single mass with a 70% decrease in diameter (Fig. 3C and SI Appendix, Fig. S3). Conversely, the “mixed” configuration maintained initial construct geometry with only mild contraction (−25%) (Fig. 3D). Mechanical properties and GAG content for the mixed group increased with time, reaching 240 kPa and 3%WW by day 56, while properties in the fused and distanced groups (combined from both sublayers) were significantly lower (36 kPa and 1.5%WW for the fused group and 2 kPa and 1.4%WW for the distanced group) (Fig. 3 E and F). The equilibrium modulus of the mixed group was 6.7 (P < 0.001) and 120 times (P < 0.001) higher than the fused and distanced groups, respectively. Similarly, GAG content for the mixed group was two times higher than the other groups (P < 0.001). The mixed group showed robust PG deposition throughout the construct at day 56, whereas the fused and distanced groups showed dense matrix deposition only in areas where JCHs were located (Fig. 3G). Interestingly, AMSCs, lacking close contact with JCHs in the fused and distanced groups, produced very little matrix, whereas AMSCs near the border of JCHs produced some matrix in the fused group. These findings support the notion that JCHs and AMSCs must be in close proximity for the coculture response.
Fig. 3.
Close cell proximity is a prerequisite for the coculture effect. (A) AMSCs (green) and JCHs (red) were seeded in 1% MeHA hydrogels (60 million cells per mL; AMSC:JCH, 4:1; the mixed, fused, or distanced groups). (B and C) Gross images at day 0 and 28. (D) Percent change in construct volume. (E) Equilibrium modulus (EY; in kilopascals). (F) GAG content (%WW) (light bar, day 28; dark bar, day 56; dashed line, AMSC alone at day 56; n = 3–5/group; *P < 0.05). (G) Alcian blue staining. (Scale bar: 1 mm.)
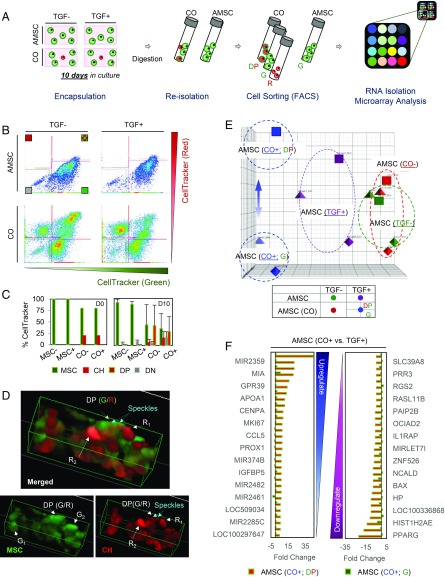
Adult Bone Marrow-Derived MSCs in Coculture Internalize Factors Released from JCHs to Alter Their Transcriptional Profile.
To investigate pathways involved in the coculture effect, we evaluated the molecular profiles of AMSCs that were mixed with JCHs (i.e., CO group) and compared them to AMSCs that were cultured alone (i.e., AMSC group) (Table 1). Cells within the AMSC group were labeled with CellTracker Green (G) and treated with TGF-β3 (TGF+) or were maintained in the absence of TGF-β3 (TGF–). These AMSCs retained their initial green label (G), regardless of TGF-β3 (90% retention) (Fig. 4 B and C). In the CO group, however, some AMSCs (∼50%) retained their original green label, while others became double positive (DP), a 40∼50% shift in the population. To further investigate this phenomenon, we examined constructs at early time points to determine when and how they began to manifest this DP characteristic. Constructs were first observed after 15 h, and no exchange of intracellular contents was observed (SI Appendix, Fig. S4). By day 3 in coculture (CO), however, many AMSCs contained red speckles (indicated as CO±_DP). When these DP cells were reconstructed in 3D, we observed areas of overlap, where red speckles appeared within otherwise green AMSCs (Fig. 4D). This suggests the transfer of intracellular contents from the JCHs to the AMSCs. By day 7, red speckles were more numerous in the green cells (i.e., AMSCs), and some originally red cells (i.e., JCHs) had decreased in staining intensity, suggesting a loss of red dye.
Table 1.
Abbreviations and definition of cell types and experimental groups
| Abbreviation | Definition |
| CH | CH isolated from fetal (FCH), juvenile (JCH), or adult (ACH) cartilage |
| Zonal CH | CH isolated from superficial (S), middle (M), or deep (D) zone cartilage |
| MSC | MSC isolated from fetal (FMSC), juvenile (JMSC), or adult (AMSC) bone marrow |
| AMSC | AMSC cultured alone and with (TGF+) or without (TGF−) TGF-β3 |
| Coculture group (CO) | AMSC in coculture with JCH and with (CO+) or without (CO−) TGF-β3 |
| TGF±_G | AMSC cultured alone that remained green throughout culture and with (TGF+_G) or without (TGF−_G) TGF-β3 |
| CO±_G | AMSC in coculture (CO) that remained green throughout culture and with (CO+_G) or without (CO−_G) TGF-β3 |
| CO±_DP | AMSC in coculture (CO) that took on red speckles in culture (i.e., became DP) and with (CO+_DP) or without (CO−_DP) TGF-β3 |
Fig. 4.
Molecular profiling of coculture induced pathways in AMSCs. (A) AMSC (green) alone or AMSC/JCH (green/red) cocultured populations were seeded in 1% MeHA hydrogels at 20 million cells (AMSC:JCH, 4:1) and cultured in the presence or absence of TGF-β3. After 10 d, cells were reisolated and sorted using FACS. Sorted AMSCs (green or DP) were used for RNA isolation, followed by microarray and pathway analyses. (B and C) FACS results (B) showed DP cells present in the CO groups, regardless of TGF-β3. Nearly one-half of the AMSCs in the coculture became DP (CO_DP) over 10 d (C). (D) Three-dimensional reconstructions from confocal microscopy identified red speckles in AMSCs (green). (E) PCA analysis. (F) List detailing the 30 genes showing the greatest fold change with coculture (positive, Left; negative, Right). AMSCs mixed with JCHs (CO+_DP or CO+_G) were compared with AMSCs cultured alone (TGF+_G) CO+_DP/TGF+ (CO+_G/TGF+).
To explore the impact of transfer of these cellular contents, molecular profiling and principal-component analysis (PCA) were performed. This analysis showed no coculture effect in the absence of TGF-β3 (CO− vs. TGF−) (Fig. 4E). Conversely, in the presence of TGF-β3, the AMSC group (TGF+) shifted in its response (TGF+ vs. TGF−). Likewise, those cells in the CO group supplemented with TGF-β3 (CO+) further shifted in expression compared with the AMSC group (TGF+). Interestingly, within the CO group, AMSCs that were DP (CO+_DP) differed from those that remained green (CO+_G). To further define these differences, we evaluated the fold change in the CO groups (CO+_DP or CO+_G) compared with the AMSC group (CO+_DP/TGF+ or CO+_G/TGF+) and identified the 30 genes with the greatest positive and negative fold changes (a total 60 genes) from the microarray analysis (SI Appendix, Tables S1 and S2). Interestingly, genes showing the highest fold changes were distinct between these groups, based on whether the AMSCs from coculture were DP (i.e., contained red speckles, CO+_DP) or remained green (CO+_G). DP cocultured MSCs (CO+_DP) had higher expression of factors related to cartilage matrix formation and homeostasis (SI Appendix, Tables S3 and S4). Expression in cocultured AMSCs that remained green (CO+_G) were 5- to 44-fold lower. This suggests that the presence of red speckles in AMSCs (making green cells DP) resulted in an increase in chondrogenic activity in these cocultured AMSCs (Fig. 4F).
To further investigate the effect of TGF-β3 and/or molecular factors secreted from JCH on AMSCs, we sorted genes based on biological themes, enriched function-related clusters, and their expression levels, as shown in the Venn diagrams (SI Appendix, Fig. S5 and Tables S5–S8). In the presence of TGF-β3 alone, there was an increase in genes related to cartilage extracellular matrix production (ACAN, CHAD, COMP, and COL12A1), mineralization (AMTN, MATN2, and POSTN), and antiinflammatory/fibrosis (ASPN, CCL20, CXCL3, DEFB1, and MXRA5) (SI Appendix, Fig. S5 A and E). Furthermore, in the CO group, with TGF-β3 and molecular factors secreted from JCHs (i.e., AMSCs that were cocultured with TGF-β3 and were also DP), there were additional increases in expression of matrix proteins (COL2A1, HAPLN1, IGFBP5, and MIA) and a reduction in expression of apoptosis/inflammation (CCL5, IL1RAP, HP, PENK) and hypertrophy (GPR39) markers (SI Appendix, Fig. S5B and Tables S1–S6). Interestingly, AMSCs in coculture that remained green (with no intracellular transfer from cocultured CHs) did not show differences in expression of cartilage matrix proteins compared with AMSCs cultured with TGF-β3 alone (SI Appendix, Fig. S5C and Table S7).
Intercellular Communication in CH/MSC Cocultures Is Mediated by EVs.
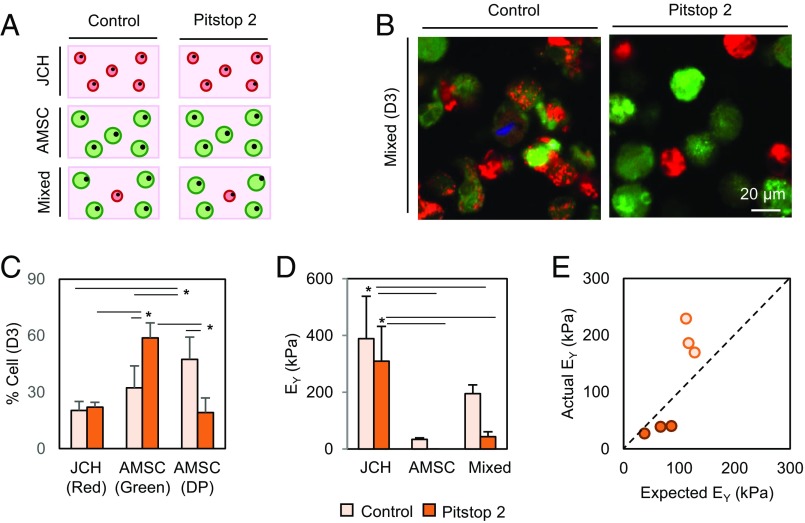
In the earlier study, we noted that AMSCs became DP with culture duration, while JCHs tended to remain red (Fig. 4D). Recent studies have suggested that one mechanism for intercellular communication is through the formation and transfer of EVs. To determine whether the improvement in AMSC chondrogenesis resulted from trafficking of molecular factors through EVs, we used Pitstop2 to block clathrin-mediated endocytic pathways (SI Appendix, Fig. S6). When treated with Pitstop2, the number of AMSCs that had taken on red speckles (i.e., became DP) was notably reduced (2.3 times; 47–19%; P < 0.0001) (Fig. 5 B and C), while the number of AMSCs that remained green increased (32–59%; P < 0.0001). No changes were observed in JCHs regardless of Pitstop2 treatment (20–22%; P = 0.998). Additionally, the equilibrium modulus of JCH- and AMSC-only constructs did not change markedly with Pitstop2 treatment (389–309 kPa, P < 0.615, and 4.3–3.5%WW GAG, P < 0.016 for JCHs; 34–0 kPa and 0.4–0.35%WW GAG, P < 0.09 for AMSCs) (Fig. 5D and SI Appendix, Fig. S6). Conversely, when Pitstop2 (25 µM) was introduced into the CO group, construct properties markedly decreased (195–43 kPa; 4.5-fold decrease; and 2.4–1.9%WW GAG) compared with those in coculture without Pitstop2 inhibition. The synergistic effect of cocultures was thus lost in the presence of Pitstop2 (Fig. 5E), suggesting that clathrin-mediated endocytosis is essential for the positive coculture effect to occur.
Fig. 5.
EVs mediate intercellular communication in cocultures. (A) AMSC alone (green), JCH alone (red), or mixed cocultured populations (AMSC:JCH, 4:1) were seeded in hydrogels and cultured in CM+ with or without Pitstop2 (25 μM). (B and C) Inhibition with Pitstop2 decreased the number of AMSCs that became DP while increasing the number of AMSCs that remained green, as shown visually and quantitatively after 3 d (n = 5–6/group; scale bar, 20 μm; green, red, and DP cells). (D) Equilibrium modulus (EY, in kilopascals; n = 3; *P < 0.05) and (E) efficacy of coculture with addition of Pitstop2 (light bar/dot, control; dark bar/dot, Pitstop2).
Discussion
In this study, we determined the extent to which different zonal CHs mixed with MSCs in 3D culture promoted MSC chondrogenesis, as a function of MSC age. JCHs seeded in HA hydrogels and cultured with TGF-β3 retained their native production levels and zonal characteristics, even after extensive culture expansion. We also found that juvenile MSCs in HA hydrogel grew well on their own, achieving functional properties equivalent to those of middle- or deep-zone CH-laden constructs. Given this already robust growth, no significant increases were observed in juvenile CH/MSC cocultures, with measured (actual) properties mostly matching expected properties based on a simple mixture analysis. This result showed that coculturing with JCHs did not improve juvenile MSC chondrogenesis, indicating that young MSCs do not require additional chondrogenic induction via coculture.
Unlike juvenile MSCs, AMSCs failed to mature in chemically defined media containing TGF-β3, and instead aggregated together in the hydrogel. When these AMSCs were placed in coculture with JCHs (AMSC/JCH) in the presence of TGF-β3, however, a synergistic enhancement was observed. These results are consistent with studies using human MSCs from aged patients (7–9), where the chondrogenic capacity of aged MSCs was lower than young MSCs. AMSCs thus appear to be more sensitive to molecular factors secreted from JCHs in the coculture system. Taken together, these findings show that a small population of JCHs cultured with AMSCs can rejuvenate the chondrogenic capacity of AMSC population. This is consistent with recent single-stage cell-based cartilage regeneration studies using chondron/MSC cocultures [i.e., instant MSC product accompanying autologous chondron transplantation (IMPACT)]. In that study, Bekkers et al. (15) optimized the mixture ratio of chondrons and MSCs and showed that, when the mixture ratio of chondrons reached 50%, GAG content markedly increased. From this, it appears that the optimal fraction is between 10 and 50%, which is consistent with our findings and other reports in the literature.
To better understand the operative conditions of coculture, we next queried how the distance between the two cell populations, which may be critical for this intercellular communication, mediated the coculture effect. We tested conditions in which molecular factors would travel through the HA network itself (direct) or through the tissue culture media (indirect). Based on our data and experimental framework, if molecular factors from CHs could travel over a long distance, then all configurations tested (i.e., mixed, fused, and distanced) should have had similar results in terms of rejuvenating AMSCs and increasing matrix production. We found that constructs with mixed cell populations maintained their initial geometry, had improved mechanical properties, and had better histological appearance, whereas AMSC layers in the fused and distanced groups aggregated, with layers that gradually separated from one another. This suggests that the molecular factors operative in JCHs and AMSC coculture cannot traverse a great distance. Diffusion properties vary depending on the material and also changes as cells produce matrix with time in culture (24). Our HA hydrogels begin with a very low polymer density (1%) and so are permissive of the diffusion of large molecules. In other studies, it has been shown that factors derived by injected MSCs can influence the whole joint, across a greater distance. It may be, in that circumstance, that MSCs release factors with longer half-lives to act on a number of joint tissues (including CHs) (13). In the context of coculture under chondrogenic conditions, our results support the idea that proximity is essential for intercellular communication (25–27).
To determine the molecular factors and pathways mediating the coculture effect, we carried out genome-wide analyses of fluorescently labeled AMSC populations (Table 1). AMSCs cultured alone mostly retained their initial green label regardless of the presence or absence of TGF-β3. However, in the CO groups, 50% of AMSCs became DP, while the remainder stayed green (G). Confocal microscopy confirmed that red speckles from JCHs appeared in green AMSCs, but not vice versa, suggesting directional transport of intracellular contents. Microarray analysis showed a shift in expression patterns in AMSCs in the presence of TGF-β3 compared with the absence of TGF-β3. AMSCs cocultured with JCHs (CO+) shifted in their expression compared with AMSCs that were chondrogenically induced with TGF-β3 alone. Interestingly, those AMSCs that were cocultured but remained green (no intracellular transfer) were distinct from those that had become DP (with evidence of intracellular transfer).
Analysis of expression profiles from DP AMSCs in coculture compared with AMSCs cultured alone identified altered regulation of a number of genes related to cartilage matrix formation, homeostasis, and anti-apoptosis/inflammation. We also noted that expression of PPARγ was lower in DP AMSCs in coculture compared with in AMSCs alone. Moreover, increased expression of GPR39 and RGS2 in the DP group may have contributed to AMSC chondrogenesis by blocking hedgehog signaling that causes heterotopic ossification (28, 29) or suppressing hypertrophic differentiation (30) (SI Appendix, Tables S3 and S4). These data suggest that, for AMSCs, the combined provision of TGF-β3 and factors secreted/transferred from JCHs supports a more robust and stable chondrogenesis than does TGF-β3 alone.
Unlike AMSCs, juvenile MSCs have a very robust chondrogenic capacity in the presence of TGF-β3, and tissue-engineered constructs formed from these young cells achieve functional properties that are nearly equivalent to those obtained from JCHs (Figs. 1 and 2). This suggests that TGF can activate pathways in juvenile MSCs that are reactivated in AMSCs only with coculture. To directly assess this, we compared the results of adult, TGF-β3–treated, cocultured, DP MSCs with data previously generated using juvenile MSCs cultured in the presence of TGF-β3 for a similar time period (31). Based on this, we identified 30 genes with the greatest positive and negative fold changes using the Database for Annotation, Visualization and Integrated Discovery (DAVID) gene ontology software (SI Appendix, Fig. S7 and Tables S9–S12).
Results from this analysis are shown in Venn diagrams (SI Appendix, Figs. S5 A and B, and S6C) and tables (SI Appendix, Tables S5, S6 and S9), grouping gene function and expression levels based on their response to TGF-β3 and/or CH-derived molecular factors in the adult and juvenile MSCs. In the presence of TGF-β3, there were several genes expressed in both adult and juvenile MSCs involved in cartilage matrix production and suppression of inflammation, indicating that many genes important for cartilage formation are stimulated by TGF-β3, regardless of the age-related decline in matrix-forming capacity of the MSCs. Furthermore, there was an increase of genes that promote matrix production and proliferation/mitosis, and also those that suppress inflammation/apoptosis and angiogenesis. Interestingly, however, in the presence of TGF-β3, there was a simultaneous regulation of genes that promote and suppress bone turnover/formation in juvenile MSCs (SI Appendix, Fig. S7C and Table S9).
Likewise, in the presence of TGF-β3, AMSCs showed a mixed expression of genes that promote chondrogenesis/cartilage homeostasis and inhibit calcification, fibrosis, adipogenesis, and apoptosis/inflammation. Simultaneously, TGF-β3 promoted hypertrophy/bone formation, matrix degradation, and angiogenesis in these AMSCs. These findings indicate that AMSCs retain their multipotent capacity and that even AMSCs can be activated by TGF-β3 toward chondrogenesis. However, chondrogenic induction with TGF-β3 seems to excessively activate not only chondrogenic but also osteogenic signals in these adult cells (SI Appendix, Fig. S5A and Table S5).
Conversely, when these AMSCs also took up molecular factors from juvenile CHs in coculture, additional genes were expressed that promote chondrogenesis while attenuating inflammation and ossification (SI Appendix, Fig. S5B and Table S6). This finding suggests that when CH-derived molecular factors are internalized by AMSCs, chondroinductive signals become more predominate, rejuvenating their chondrogenic capacity and maintaining cartilage homeostasis. Whether these CH-derived molecular factors offset a loss in AMSC capacity or trigger AMSCs to regain their more immature function is not clear, but AMSCs under the influence of CH-derived factors remain quite distinct from juvenile MSCs, as shown in the PCA analysis (SI Appendix, Figs. S5E and S7D).
While it is generally believed that there is a synergistic effect in coculture between CHs and MSCs, there have been differing reports with regard to the directionality of this intercellular communication. Several studies (including ours) have shown that a small number of CHs (20%) rejuvenate a larger number of old/infirm MSCs by transferring molecular contents secreted from CHs to MSCs (7–9, 32, 33). Conversely, others have shown that bioactive factors secreted from MSCs help CHs to form functional matrix (10, 11, 14, 15, 34). Early work by Caplan and Dennis (14) suggested a dual role of MSCs. First, MSCs are able to differentiate into several phenotypes and produce the appropriate extracellular matrix (e.g., bone, cartilage, muscle, etc.). Second, bioactive factors secreted from MSCs can influence host cells and/or the tissue environment by suppressing fibrosis and apoptosis and increasing angiogenesis, mitosis, and differentiation. Indeed, studies in the heart suggest that, for some cell types, the directionality is most certainly from the MSC to the recipient cell (14).
Our studies do not rule out the influence of MSCs on recipient CHs but do strongly suggest that CHs support the MSC population, particularly in the aged/infirm scenario. Indeed, when we cocultured juvenile MSCs and JCHs, we saw no synergistic effect, whereas there was a synergistic effect when AMSCs were cocultured with JCHs. We previously reported and show here again that the chondrogenic capacity of MSCs is attenuated with aging in pellet culture and 3D hydrogels (6, 32). This indicates that the directionality of intercellular communication may depend on chondrogenic capacity (where AMSCs have little chondrogenic capacity), rather than implying that MSCs are unable to impact host cells and/or tissues.
To test this concept, we carried out another set of studies using fetal MSCs (FMSCs) cocultured with adult CHs (ACHs) with same mixture ratio (FMSC:ACH, 1: 4). FMSCs possess the highest chondrogenic potential, and ACHs possess the least matrix-producing capacity. As expected, FMSCs alone generated engineered cartilage with greater mechanical properties and GAG content than those formed from ACHs alone. In coculture, however, there was no synergistic effect over 28 d. Furthermore, no vesicular exchange was observed in either direction. These results are similar to that of JCH/JMSC cocultures, in which both grew well on their own, with no synergistic effect (SI Appendix, Fig. S9). This supports that, in our hands, the directionality of transfer is from the CHs to the recipient MSCs, where, in AMSCs, factors from JCHs can rejuvenate function.
Other important molecular factors include miRNAs (22), which have been implicated in intercellular communication. These small noncoding RNAs can be delivered to recipient cells via EVs. Recent studies have shown that EVs specifically target recipient cells to deliver protein, mRNAs, and lipids and also initiate downstream signaling pathways (35, 36). While data from previous studies are somewhat conflicting, it appears that MIR29A is induced by the Wnt signaling pathway and down-regulates expression of fibrillar collagens and proinflammatory factors (37), indicating a potential role in coculture. Interestingly, it has also been reported that MIR29A is down-regulated by TGF-β1 and delays MSC chondrogenesis (38). This is consistent with our microarray data, which showed that AMSCs in coculture with JCHs expressed eightfold greater MIR29A than those cultured with TGF-β3 alone. As described by Guérit et al. (38), the action of MIR29A may be to maintain an undifferentiated and proliferative pool of MSCs. If so, then the effect of coculture (mediated by MIR29A) would be to enhance proliferation of undifferentiated AMSCs concurrently during chondrogenic differentiation. This would ultimately result in more cells in the construct differentiating and producing matrix in the long term. Based on our first set of studies (Figs. 1E and 2B), coculture of JCHs with AMSCs resulted in constructs with higher mechanical properties and biochemical content at 8 wk, and these values consistently increased with time in culture. This may suggest that while TGF-β3 can induce differentiation, doing so in that absence of secreted factors results in commitment of the entire progenitor pool, exhausting their reserve capacity to divide and ultimately produce matrix.
The above findings strongly suggest that EVs are secreted from JCHs and are internalized by the AMSCs to change their phenotype. To validate this hypothesis, we blocked key intercellular communication mechanisms and determined whether such blockade would influence the coculture phenomenon. Results from these studies showed that the equilibrium modulus of cocultured constructs decreased substantially with inhibition of clathrin-mediated endocytosis. Furthermore, confocal microscopy showed a reduction in the number of AMSCs that had become DP.
As a next step, direct isolation and identification of EV contents will be important to fully explicate the mechanism of this coculture response. It will also be important to determine whether other released factors that are not trafficked via EVs play a role in this process. For example, Vonk et al. (13) showed that MSC-conditioned media depleted of EVs could still attenuate proinflammatory signaling and promote matrix deposition, although to a much lesser extent than when EVs were present. It will also be important to assess the role of EVs in an in vivo scenario. Here, we used a chemically defined medium that provides a supportive growth environment, but “conflict” signals in the native joint environment may pose additional challenges (39). Our data support that DP AMSCs have higher expression of antiinflammatory/apoptotic and proliferation/mitosis-related factors (SI Appendix, Tables S1–S11), and therefore EV-mediated factors may help in the transition MSC-based cartilage to the in vivo space.
Caplan et al. first defined the “trophic effect” of MSCs as cells secreting bioactive factors to influence nearby cells and/or tissue (14). This is indisputable, and the data above strongly support that finding. However, our data support a broader definition, where molecular factors secreted from one cell population are transferred to the other, and the directionality of intercellular communication might be determined in a “need-based variable flow.” For example, Strassburg et al. (12) showed the biodirectional exchange of membrane components during the coculture of MSCs and nucleus pulposus cells. Our data, for older/infirm MSCs, support that the transfer resulting in synergistic impact (through exchange of membrane-bound EVs) is in the direction of JCHs to AMSCs. Future studies will explore whether EVs can influence AMSC functionality directly (by providing missing components to the recipient cells) or indirectly (by reactivating debilitated anabolic pathways). Moreover, we will also investigate whether the EV transfer is a selective on-demand secretion and/or reception or rather is the consequence of a random release/intake. Furthermore, it will be interesting to determine whether intercellular communication efficiency and the actual vesicular contents are altered by changes in cell or macromer density of the surrounding hydrogels. Finally, it will be important to determine the specific molecular factors mediating the coculture effect, and which of these are conveyed exclusively by EVs. Delivery of such factors may be used in the development of cell-free therapeutics for cartilage repair.
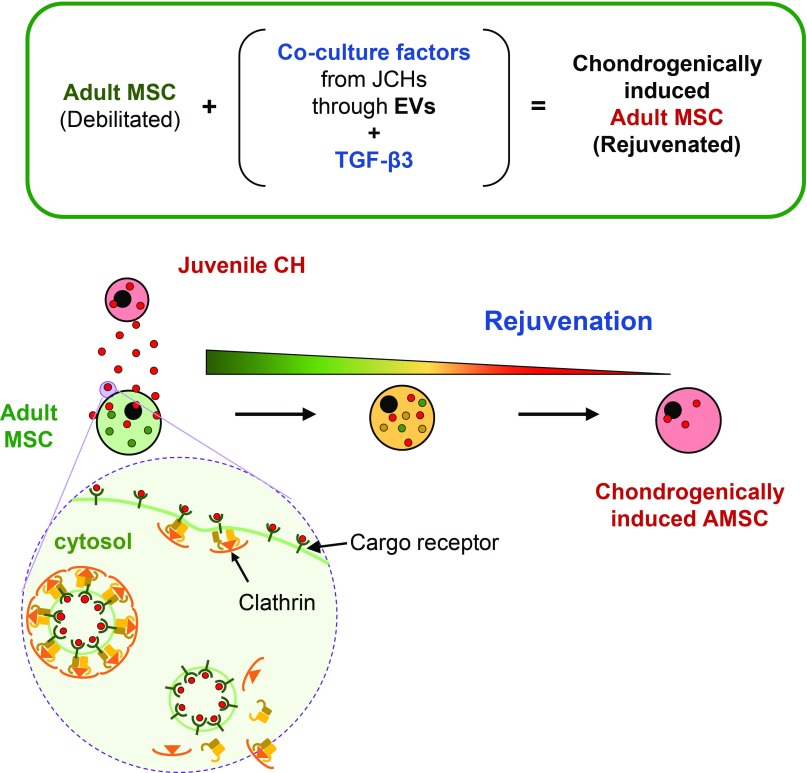
Taken together, this work demonstrated that JCHs retain their chondrogenic capacity (with zonal characteristics) in the presence of TGF-β3 and that rejuvenation of MSCs cocultured with JCHs is age dependent. Additionally, we show that the coculture phenomenon is only operable when cells are close to one another. Studies blocking intercellular communication suggest that EVs released from JCHs shuttle molecular factors to AMSCs using clathrin-mediated endocytosis and that the contents of these EVs activate anabolic signaling pathways while suppressing catabolic activity in recipient cells (Fig. 6). This in turn enables aged MSCs to rejuvenate and perform as though they were younger CH-like cells. Identifying the molecular factors and vesicular pathways that underlie coculture might lead to biologics or other therapeutics to improve functional tissue engineering using aged autologous MSCs, and may ultimately obviate the need for such complicated coculture systems, increasing the likelihood for clinical translation.
Fig. 6.
Schematic illustration of EV-mediated transfer of molecular factors in coculture. EVs secreted from releasing cells (JCHs) travel through the extracellular space to recipient cells (AMSCs) and enter via clathrin-mediated endocytosis. The contents of these internalized vesicles enable AMSCs to take on an improved chondrogenic capacity, enhancing the function of engineered tissues compared with those formed from AMSCs cultured alone.
Materials and Methods
Isolation of Zonal CHs and MSCs.
CHs and MSCs were isolated from bovine stifles [juvenile (J), 1–2 mo; Research 87; adult (A), >1 y; Animal Technologies] (Fig. 1A). Full-thickness juvenile cartilage segments were excised from the femoral condyle and divided into three layers (40, 41). The topmost 100-μm layer at the articular surface was separated and taken as the superficial zone (S). The segment from the bony surface to the tidemark was removed and discarded. The remaining “top” half of the tissue segment was considered as the middle zone (M), and the “bottom” half as the deep zone (D). These separated zonal cartilage tissues were minced and digested with collagenase. MSCs were isolated from juvenile (JMSC) or adult (AMSC) bone marrow as described previously (6). These zonal CHs and MSCs were separately maintained in serum-containing medium (10% FBS) (39). Cells of both types were expanded in culture through passage 3. Cells were trypsinized, washed, and labeled with CellTracker (Molecular Probes; CHs, red; MSCs, green) before encapsulation.
Gel Synthesis and Cell Encapsulation.
Methacrylated hyaluronic acid (MeHA) was synthesized as described previously (42). Briefly, 1% (wt/vol) sodium hyaluronate (65-kDa HA; Lifecore) was reacted with methacrylic anhydride (Sigma) on ice at pH 8.0 for 6 h followed by dialysis (6-kDa Mr cutoff; Spectrum Labs) for 7 d to remove unreacted byproducts. Then, the MeHA solution was lyophilized and stored at −20 °C. The final macromer products were confirmed by 1H NMR to obtain a methacrylation level of ∼30%. To form gels, lyophilized MeHA was dissolved at 1% (wt/vol) in PBS with 0.05% (wt/vol) photoinitiator (Irgacure I2959; CibaGeigy).
Establishment of Zonal CH and MSC Cocultures.
To determine whether zonal CH identity differentially contributed to MSC fate and/or how aging impacted MSC response in coculture, CellTracker-labeled zonal JCHs were mixed with juvenile MSCs or AMSCs (zonal CH:MSC, 1:4). A mixture of 1 wt% MeHA and cells (60 million cells per mL) was added to a gel casting device (Hoefer) and exposed to UV (365 nm) for 10 min. Cylindrical cores (Ø4 mm × 2.25 mm) were removed from the resulting HA gel sheets using a sterile biopsy punch. These constructs were then cultured in chemically defined media (CM) with 10 ng/mL TGF-β3 (CM+; R&D Systems) (39). Media was changed thrice weekly for up to 8 wk.
Mechanical Analysis.
To determine construct mechanical properties, unconfined compression testing was performed as previously described (1). The equilibrium modulus was determined via a stress relaxation test. After stress relaxation, a 1% sinusoidal deformation was applied at 1.0 Hz to obtain the dynamic modulus.
Biochemical Analysis.
After mechanical testing, construct wet weight was measured followed by papain digestion. GAG content was determined using the 1,9-dimethylmethylene blue assay (43). Collagen content was extrapolated from orthohydroxyproline (OHP) content assessed by reaction with chloramine T, using a 1:7.14 (OHP:collagen) ratio (44).
Histological Analysis.
Constructs were fixed in 4% paraformaldehyde and embedded in paraffin. Sections (8 μm thick) were deparaffinized and stained with Alcian Blue. Immunohistochemistry was performed to visualize COL I, COL II, and CS. Primary antibodies for COL I (MAB3391; 10 μg/mL; Millipore) and COL II (II-II6B3; 10 μg/mL; DSHB), and CS (C8035, 1:150; Sigma) were used.
Assessment of Coculture Efficacy.
To determine whether coculture of zonal CHs and MSCs in MeHA hydrogels improved outcomes over CHs or MSCs alone, we determined the efficacy of coculture by computing the ratio of the actual (y axis) to the expected (x axis) outcomes based on the relative contribution of each cell type and their individual performance. Expected values were calculated based on the rule of mixtures, where the expected outcome was the sum of 20% of the performance of zonal CHs on their own and 80% of the performance of MSCs on their own (Expected = 0.2 × ActualCH + 0.8 × ActualMSC). In cases where there was a “synergistic” effect, the actual measured value of the construct was greater than expected (actual > expected), and these samples were located above the diagonal line on the plot. In cases where there was a “negative” effect (actual < expected), samples were located below the line on the plot. Finally, in cases where the two cell types were “independent” of one another (no synergistic effect, actual = expected), the samples fell along the line.
Influence of Intercellular Distance on Coculture Outcomes.
To investigate how far molecular factors might travel from releasing to recipient cells within a 3D environment, JCHs and AMSCs were seeded in constructs at varying distances to provide increasing separation between the two cell populations: these included mixed, fused, or distanced configurations (Fig. 3A). JCHs and AMSCs were isolated, expanded (passage 2), and seeded in 1% (wt/vol) MeHA hydrogels (60 million cells per mL; AMSC:JCH, 4:1) at varying distances. First, mixed populations were seeded as a single mixture in one layer to provide the shortest possible distance. Second, JCH- and AMSC-seeded layers were fused by creating a bilayered construct. Finally, JCH- and AMSC-seeded layers were constructed as a trilayered “barrier” construct, with an acellular MeHA gel segment interposed between the cell-laden layers in the middle of the construct (AMSC–acellular HA gel–JCH). The AMSC-laden layer was four times greater in volume than the JCH-seeded layer for fused and distanced groups (Fig. 3B). These constructs were cultured in CM+ for 56 d. Due to the delamination of sublayers for the distanced group, mechanical and biochemical analyses of sublayers were performed separately, and respective values were combined.
Molecular Profiling of MSCs During Coculture.
To investigate molecular factors and/or pathways that might mediate the coculture effect, we evaluated genome-wide changes in mRNA expression in AMSCs. To enable sorting of distinct cell populations, AMSCs (green) and JCHs (red) were labeled with CellTracker (Molecular Probes) (Fig. 4A). AMSCs alone or mixed cell populations (AMSC:JCH, 4:1) were encapsulated at 20 × 106 cells per mL in 1% MeHA. Constructs (Ø4 × 0.75 mm) were cultured in CM with or without TGF. To ensure a transport of equal amount of nutrient to all cells within the constructs and to facilitate construct digestion and reisolation of cells, construct volume and cell density were reduced. On day 10, constructs were minced and digested with hyaluronidase (42) to reisolate cells from constructs. Reisolated cells underwent FACS.
Sorting resulted in AMSC populations that remained green in coculture (CO_G) or had become DP (CO_DP) as well as those that were cultured alone and remained green (TGF_G) in the presence (+) or absence (−) of TGF-β3 (Table 1). RNA was isolated from these populations (microRNeasy mini kit; Qiagen) and subjected to microarray analysis (Bovine Genome 1.0st; Affymetrix) by the Penn Microarray Facility as described previously (31). The study was repeated three times with mixed donor sets used in each replicate. The Ingenuity software was used to identify pathways associated with coculture. Fold change was compared across four groups (CO+ vs. TGF+, TGF+ vs. TGF−, CO− vs. TGF−, and CO+ vs. TGF−) to assess how the molecular factors secreted from JCHs altered the expression profiles of AMSCs in coculture compared with AMSCs alone with or without TGF-β3. To further investigate the effect of TGF-β3 and/or molecular factors secreted from JCHs on the rejuvenation of AMSCs, we used the list of 30 genes with the greatest positive and negative fold changes (a total 60 genes) from the microarray analysis, and sorted based on biological themes, enriched functional-related gene clusters, and expression levels. For this, we used the DAVID, version 6.8, publicly available bioinformatics resource (https://david.ncifcrf.gov/). Next, using the function-related gene clusters identified in DAVID, we chose three to five subcategorized groups with the greatest number of associated genes and organized these themes using a Venn diagram.
Probing Mechanisms of Intercellular Communication.
To identify whether intercellular communication occurred through the trafficking of EVs, we first inhibited clathrin-mediated vesicular formation using Pitstop2 (Abcam). Cell-laden constructs (JCH, AMSC alone or mixed) were cultured in CM+ with Pitstop2 (0 or 25 μM) for 42 d (Fig. 5A).
Statistical Analysis.
Statistical analysis was performed using the SYSTAT software (version 10.2; SYSTAT Software). Significance was determined by two-way ANOVA with Tukey’s post hoc test (P < 0.05). Data represent the mean ± SD.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01 EB008722 and Department of Veterans Affairs Grant I01 RX000700.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1815447116/-/DCSupplemental.
References
- 1.Mauck RL, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 2.Hunter CJ, Mouw JK, Levenston ME. Dynamic compression of chondrocyte-seeded fibrin gels: Effects on matrix accumulation and mechanical stiffness. Osteoarthritis Cartilage. 2004;12:117–130. doi: 10.1016/j.joca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Yoo JU, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 5.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Erickson IE, van Veen SC, Sengupta S, Kestle SR, Mauck RL. Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent. Clin Orthop Relat Res. 2011;469:2744–2753. doi: 10.1007/s11999-011-1869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 8.Aung A, Gupta G, Majid G, Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 2011;63:148–158. doi: 10.1002/art.30086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian L, Zhai DY, Mauck RL, Burdick JA. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. 2011;17:1137–1145. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meretoja VV, Dahlin RL, Kasper FK, Mikos AG. Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials. 2012;33:6362–6369. doi: 10.1016/j.biomaterials.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012;18:1542–1551. doi: 10.1089/ten.TEA.2011.0715. [DOI] [PubMed] [Google Scholar]
- 12.Strassburg S, Hodson NW, Hill PI, Richardson SM, Hoyland JA. Bi-directional exchange of membrane components occurs during co-culture of mesenchymal stem cells and nucleus pulposus cells. PLoS One. 2012;7:e33739. doi: 10.1371/journal.pone.0033739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vonk LA, et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8:906–920. doi: 10.7150/thno.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 15.Bekkers JE, et al. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: Comparison with microfracture. Am J Sports Med. 2013;41:2158–2166. doi: 10.1177/0363546513494181. [DOI] [PubMed] [Google Scholar]
- 16.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: Mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 19.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 20.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, et al. SNARE proteins synaptobrevin, SNAP-25, and syntaxin are involved in rapid and slow endocytosis at synapses. Cell Rep. 2013;3:1414–1421. doi: 10.1016/j.celrep.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: A new form of intercellular communication. Trends Cell Biol. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Malda J, Boere J, van de Lest CH, van Weeren P, Wauben MH. Extracellular vesicles—new tool for joint repair and regeneration. Nat Rev Rheumatol. 2016;12:243–249. doi: 10.1038/nrrheum.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bian L, et al. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials. 2013;34:413–421. doi: 10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 2013;3:3553. doi: 10.1038/srep03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Windt TS, et al. Direct cell-cell contact with chondrocytes is a key mechanism in multipotent mesenchymal stromal cell-mediated chondrogenesis. Tissue Eng Part A. 2015;21:2536–2547. doi: 10.1089/ten.TEA.2014.0673. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Burdick JA, Mauck RL. Transactions of the 60th Annual Orthopaedic Research Society Meeting, New Orleans, LA. Orthopaedic Research Society; Rosemont, IL: 2014. Mechanism of enhanced functional properties with co-culture of adult MSCs and juvenile chondrocytes in hyaluronic acid hydrogels. [Google Scholar]
- 28.Regard JB, et al. Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med. 2013;19:1505–1512. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassilana F, et al. Target identification for a Hedgehog pathway inhibitor reveals the receptor GPR39. Nat Chem Biol. 2014;10:343–349. doi: 10.1038/nchembio.1481. [DOI] [PubMed] [Google Scholar]
- 30.James CG, Appleton CT, Ulici V, Underhill TM, Beier F. Microarray analyses of gene expression during chondrocyte differentiation identifies novel regulators of hypertrophy. Mol Biol Cell. 2005;16:5316–5333. doi: 10.1091/mbc.E05-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang AH, Stein A, Mauck RL. Evaluation of the complex transcriptional topography of mesenchymal stem cell chondrogenesis for cartilage tissue engineering. Tissue Eng Part A. 2010;16:2699–2708. doi: 10.1089/ten.tea.2010.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M, Burdick JA, Mauck RL. World Congress on Osteoarthritis Research Society International, Philadelphia, PA, 2013. Osteoarthritis Research Society International; Mount Laurel, NJ: 2013. Age-dependent impact on co-cultures with MSCs and zonal chondrocytes in HA hydrogels for cartilage tissue engineering. [Google Scholar]
- 33.Kim M, Burdick JA, Mauck RL. Proceedings of the ASME 2012 Summer Bioengineering Conference, June 20-23, Farjardo, Puerto Rico, USA. American Society of Mechanical Engineers; New York: 2012. Influence of chondrocyte zone on co-cultures with mesenchymal stem cells in HA hydrogels for cartilage tissue engineering. [Google Scholar]
- 34.Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 36.Kosaka N, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldring MB, Marcu KB. Epigenomic and microRNA-mediated regulation in cartilage development, homeostasis, and osteoarthritis. Trends Mol Med. 2012;18:109–118. doi: 10.1016/j.molmed.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guérit D, et al. FOXO3A regulation by miRNA-29a controls chondrogenic differentiation of mesenchymal stem cells and cartilage formation. Stem Cells Dev. 2014;23:1195–1205. doi: 10.1089/scd.2013.0463. [DOI] [PubMed] [Google Scholar]
- 39.Kim M, Garrity ST, Steinberg DR, Dodge GR, Mauck RL. Role of dexamethasone in the long-term functional maturation of MSC-laden hyaluronic acid hydrogels for cartilage tissue engineering. J Orthop Res. 2017;36:1717–1727. doi: 10.1002/jor.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim TK, et al. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11:653–664. doi: 10.1016/s1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 41.Kim M, Farrell MJ, Steinberg DR, Burdick JA, Mauck RL. Enhanced nutrient transport improves the depth-dependent properties of tri-layered engineered cartilage constructs with zonal co-cultures of chondrocytes and MSCs. Acta Biomater. 2017;58:1–11. doi: 10.1016/j.actbio.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 44.Neuman RE, Logan MA. The determination of hydroxyproline. J Biol Chem. 1950;184:299–306. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.