Significance
The study demonstrates that V-domain immunoglobulin suppressor of T cell activation (VISTA) is an immune checkpoint that is preferentially expressed at higher levels in pancreatic cancer. The study gives a detailed analysis of immune infiltration in primary and metastatic pancreatic tumors compared with melanoma, which differs in its tumor/stromal distribution. Our data indicate that human pancreatic tumors express CD68+ macrophages and highlight the inhibitory checkpoint molecule VISTA as a potential immunotherapeutic target in pancreatic cancer.
Keywords: pancreatic cancer, immune monitoring, immune checkpoints, immunopathology, immune infiltrate
Abstract
Immune checkpoint therapy (ICT) has transformed cancer treatment in recent years; however, treatment response is not uniform across tumor types. The tumor immune microenvironment plays a critical role in determining response to ICT; therefore, understanding the differential immune infiltration between ICT-sensitive and ICT-resistant tumor types will help to develop effective treatment strategies. We performed a comprehensive analysis of the immune tumor microenvironment of an ICT-sensitive tumor (melanoma, n = 44) and an ICT-resistant tumor (pancreatic cancer, n = 67). We found that a pancreatic tumor has minimal to moderate infiltration of CD3, CD4, and CD8 T cells; however, the immune infiltrates are predominantly present in the stromal area of the tumor and are excluded from tumoral area compared with melanoma, where the immune infiltrates are primarily present in the tumoral area. Metastatic pancreatic ductal adenocarcinomas (PDACs) had a lower infiltration of total T cells compared with resectable primary PDACs, suggesting that metastatic PDACs have poor immunogenicity. Further, a significantly higher number of CD68+ macrophages and VISTA+ cells (also known as V-domain immunoglobulin suppressor of T cell activation) were found in the pancreatic stromal area compared with melanoma. We identified VISTA as a potent inhibitory checkpoint that is predominantly expressed on CD68+ macrophages on PDACs. These data suggest that VISTA may be a relevant immunotherapy target for effective treatment of patients with pancreatic cancer.
Pancreatic cancer patients have poor prognoses with limited treatment options, having a 5-y survival rate of less than 7% (1–4). Whereas, other immunogenic tumor types, such as melanoma, have demonstrated dramatic clinical responses to immune checkpoint inhibitors, including anti–CTLA-4 and anti–PD-1 antibodies (5–7). In contrast, clinical studies have reported a minimal clinical benefit in response to these antibodies in patients with pancreatic cancer (3, 4, 8). A greater understanding of the tumor immune microenvironment in pancreatic cancer may enable development of effective immunotherapeutic strategies. Immune infiltration into pancreatic tissues has not been well characterized. Some studies have reported that the pancreatic tumor microenvironment (TME) is poorly infiltrated with effector lymphocytes and has multiple immunosuppressive cells, including myeloid-derived suppressor cells and regulatory T cells (9–11). Previous publications largely indicate that pancreatic tumors have minimal infiltration of immune cells (9, 10, 12–14). In contrast, reports from other groups suggest that, apart from a regulatory immune signature, pancreatic tumors also contain an effector immune infiltrate (11, 15–18). A previous genomic study in human pancreatic tumors identified an immunogenic subtype of pancreatic cancer with up-regulated immune networks (19). The expression of specific immune checkpoints in the pancreatic TME, which may represent novel immunotherapy targets, has not been investigated systematically.
Compared with melanomas, pancreatic and other solid tumors have some important architectural histologic differences. In addition, morphological differences exist among different types of melanomas (20–25) and pancreatic cancers (26–28). Cancers do not just comprise malignant cells, but are rather complex matrix-like “organoid formations” defined in the TME (29–32). The two main regions that comprise this complex matrix are parenchymal tumor cells (tumor area) and stromal cells (stromal area). Melanomas are generally described as having melanocytes in an aberrant distribution and grouped nests of melanocytes, which are normally surrounded by or embedded in a thinner matrix of stromal component largely invaded by immune cells. In contrast, pancreatic ductal adenocarcinomas (PDACs) comprise a thicker desmoplastic structure composed of fibrous connective tissue populated with myeloid cells and erratically distributed lymphoid components (see representative microphotographs, SI Appendix, Fig. S1A–C and refs. 20–25).
Melanomas originate in one of the most immunogenically active organs of the body, the skin, which is traversed by a network of lymphatic and blood vessels and contains a large number of immune-competent cells (33), whereas the pancreas has a lymphatic network in which peripheral extensions can be found within the lobules, but intralobular lymphatic extensions are relatively sparse (34). We have no clear understanding of the distribution of immune infiltrates in these architecturally different tumor types, and dissecting these differences may enable development of novel and effective treatment strategies for pancreatic cancer. A detailed analysis of the immune infiltration of pancreatic tumors has not been done, and the extent of infiltration in pancreatic tumors is unclear. The differential distribution of immune cells within the tumor versus the stromal area of the TME of PDACs may have an impact on response to immune checkpoint therapy (ICT). In addition, we do not have a clear understanding of the differences in tumor immune microenvironment of primary (resectable) and metastatic PDACs. In the clinical scenario, treatment with neoadjuvant chemotherapy as standard-of-care may alter or impact the immune TME, which may ultimately impact response to ICT.
To gain more insight into the immune contexture of pancreatic tumors, we compared (i) immune infiltration in pancreatic cancer, which responds poorly to ICT, with that of melanoma, which responds favorably to ICT, and (ii) immune infiltration in primary untreated to primary neoadjuvant-treated PDACs as well as metastatic treated PDACs. We analyzed the microlocalization of the immune infiltration in the TME to determine whether immune cells are localized in close contact with tumor cells or within the tumor (tumor area) or are in the stromal component of the tumor (stromal area). We found that both tumor types have a heterogeneous distribution of immune infiltrates. PDAC was found to have a wide range of CD3, CD4, and CD8 T-cell infiltration, but these subsets were mainly found in the stromal area of the TME. In addition, our data clearly highlight CD68+ macrophages that express the inhibitory immune checkpoint V-domain Ig suppressor of T cell activation (VISTA) as a subset of immune cells that are highly expressed in pancreatic tumors and are frequently present in the stromal area of the tumor.
Results
Expression of T-Cell Coinhibitory Genes Correlates with Overall Survival in Pancreatic Cancer.
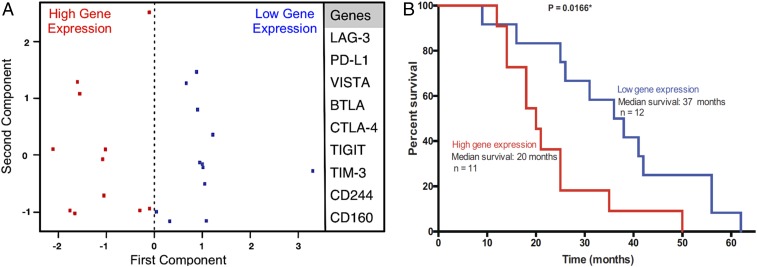
In a cohort of 23 untreated primary PDAC samples, we evaluated the gene expression of nine T-cell coinhibitory genes (LAG-3, PD-L1, VISTA, BTLA, CTLA-4, TIGIT, TIM-3, CD244, and CD160) that were previously published (19) to be associated with the immunogenic subtype of pancreatic cancer. Principal component analysis (PCA) separated patients into two cohorts: those with high T-cell coinhibitory gene expression (n = 11) and those with low T-cell coinhibitory gene expression (n = 12) (Fig. 1A). The expression of the inhibitory immune checkpoint genes was inversely correlated with survival. The median survival of patients whose tumors had low expression of these T-cell coinhibitory genes was significantly higher than patients whose tumors had high expression of these genes (37 mo versus 20 mo; P = 0.016) (Fig. 1B).
Fig. 1.
Expression of T-cell coinhibitory genes correlates with overall survival in pancreatic cancer. (A) PCA showing coinhibitory genes that are associated with the immunogenic subtype of pancreatic cancer (LAG-3, PD-L1, VISTA, BTLA, CTLA-4, TIGIT, TIM-3, CD244, and CD160). Gene expression data from a cohort of 23 untreated pancreatic tumors were analyzed. PCA separated patients into two cohorts: those with high T-cell coinhibitory gene expression (n = 11) and those with low T-cell coinhibitory gene expression (n = 12). (B) Kaplan−Meier survival curves for patients with high (red) or low (blue) T-cell coinhibitory gene expression.
Immune Infiltration in Human Pancreatic Cancer Is Mainly Located in the Stromal Component of the TME.
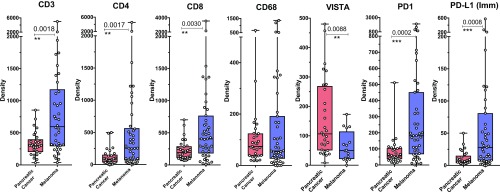
We next compared density of multiple immune subsets in untreated primary PDAC (n = 29) and untreated metastatic melanoma (n = 44). Compared with melanoma, which has a higher overall infiltration, untreated primary PDAC had a broad range of immune cell subset infiltration (Fig. 2). As expected, we observed that melanoma tumors had significantly higher density of T cells represented by CD3, CD4, and CD8 markers (Fig. 2). Melanoma tumors also showed significantly higher density of memory T cells (CD45RO), B cells (CD20), cells expressing the activation markers ICOS and OX40, cytotoxic cells (Gr-B), and regulatory T cells (FoxP3) (SI Appendix, Fig. S3). The density of immune cells expressing PD-1 and PD-L1 was also significantly higher in melanomas compared with PDAC (Fig. 2). The protein expression of PD-L1 on tumor cells was not significantly different between the tumor types (SI Appendix, Fig. S3).
Fig. 2.
Comparison of immune infiltrates in the total TME in pancreatic cancer and melanoma. Bar and dot plots comparing immune subsets (CD3, CD4, CD8, and CD68) and immune cells expressing VISTA, PD-1, and PD-L1 evaluated by IHC at 10× (large square) and 20× (small square) magnification. Each dot represents a patient, and bars with lines indicate median with confidence interval (CI). Only significant P values between groups are indicated. Statistical significance is defined as P < 0.05. **P ≤ 0.01; ***P ≤ 0.001.
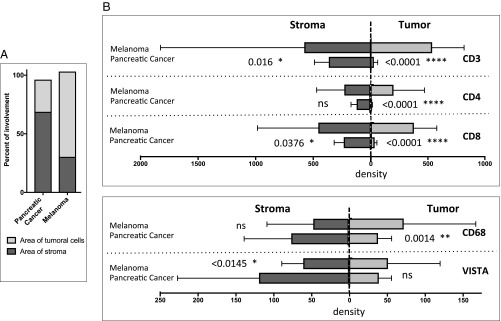
Conventional methods for IHC analyses, such as density (positive cells per square millimeter area of analysis) and percentage (number of positive cells per total number of cells) are appropriate for comparing samples from the same tumor type, since they have similar features, but these measures are inadequate for comparing tumors of different cytomorphologies or that have different tumor/stroma distributions. A description of the analysis of the tumor and stromal areas in the TME is given in SI Appendix, Supplementary Materials and Methods. We observed that melanomas have a higher tumor/stroma ratio, with tumor cells occupying ∼70% of the area, whereas, in PDAC, tumor cells occupy ∼30% of the area (Fig. 3A). To understand whether differences in immune infiltration of melanoma and PDAC are reflected in the differential distribution of various immune cells within the tumor versus stroma, we computed the absolute numbers of cells expressing immunologic markers in the two compartments. CD3, CD4, and CD8 T cells were observed in both tumoral and stromal components for melanoma, but these T cells were predominantly found in the stromal component for PDAC, with minimal infiltration into the tumoral component of PDAC (Fig. 3B). Detailed examination of the other immune subsets (CD45RO, Gr-B, OX40, ICOS, FOXP3, PD-1, PD-L1, and CD20) also demonstrated significantly higher density of these cells in the tumoral component, and, for some subsets, the stromal component, of melanoma compared with PDAC (SI Appendix, Fig. S4).
Fig. 3.
Distribution of immune infiltrates in the tumor and stromal compartments in pancreatic cancer compared with melanoma. (A) Tumor/stroma ratio for each tumor type. (B) Bar plots comparing the median expression of the selected immune subsets (CD3, CD4, CD8, CD68, and VISTA) in each compartment of the total TME. Statistical significance is defined as P < 0.05. *P ≤ 0.05; **P ≤ 0.01; ****P ≤ 0.0001; ns, nonsignificant.
Next, we evaluated macrophage infiltration as defined by CD68 expression and the inhibitory checkpoint VISTA that is predominantly expressed on macrophages (35–37). Total CD68+ macrophages did not significantly differ between melanoma and PDAC (Fig. 2), but we did find higher density of CD68+ macrophages in the tumoral component of melanoma compared with PDAC (Fig. 3B). Despite lower density of CD68+ macrophages, PDAC had significantly higher density of VISTA expression (Figs. 2 and 3B), which highlights VISTA as a potential immunotherapeutic target.
Immune Infiltrates Differ in Primary Untreated, Primary Neoadjuvant-Treated, and Metastatic Treated Pancreatic Tumors.
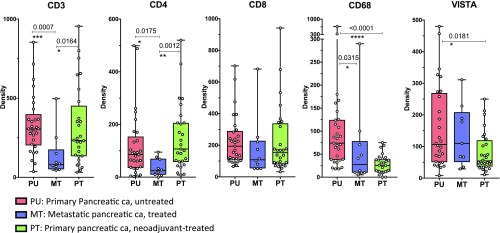
In a complementary analysis, we also compared three cohorts of PDACs: (i) untreated resectable primary PDACs (n = 29); (ii) neoadjuvant-treated primary PDACs (n = 28); and (iii) treated metastatic PDACs (n = 10), which were pancreatic tumor samples obtained from patients with metastatic disease (SI Appendix, Tables S1 and S2 A–C). The density of CD3 T cells in both the neoadjuvant-treated primary tumors (∼250 cells per square millimeter) and untreated primary tumors (∼350 cells per square millimeter) were significantly higher than that in the metastatic tumors (∼100 cells per square millimeter) (Fig. 4). Similarly, the density of CD4 T cells was significantly higher in both the neoadjuvant-treated primary tumors and untreated primary tumors than in the metastatic tumors (Fig. 4). A similar trend for CD8 T cells was also observed (Fig. 4). Although metastatic tumors had lower density of T cells compared with primary tumors, we did observe higher density of ICOS and Gr-B subsets in metastatic tumors (SI Appendix, Fig. S5), which may indicate activated T cells. Overall, immune infiltration was found to be lower in metastatic PDACs compared with primary PDAC (SI Appendix, Fig. S5). Metastatic PDAC also had lower density of CD68+ macrophages compared with primary PDAC, but metastatic PDAC had similar density of VISTA+ cells compared with primary untreated PDAC (Fig. 4).
Fig. 4.
Immune infiltrates in the total TME in primary untreated, neoadjuvant-treated, and metastatic pancreatic tumors. Bar and dot plots compare immune subsets (CD3, CD4, CD8, CD68, and VISTA) evaluated by IHC. Each dot represents one patient; bars with lines indicate medians with confidence intervals. Only significant P values between groups are indicated. Statistical significance is defined as P < 0.05. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. ca, cancer.
VISTA Is Expressed on CD68+ Macrophages, and Blocking VISTA but Not PD-L1 Inhibits Cytokine Production by Tumor-Infiltrating Lymphocytes in Vitro.
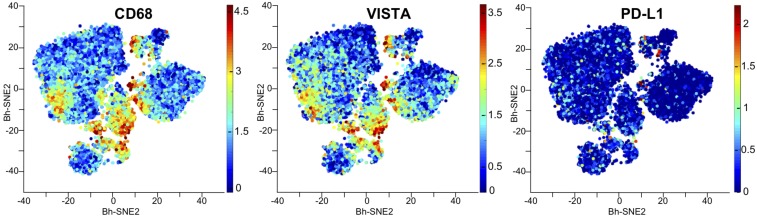
To determine which cells expressed inhibitory checkpoint molecules and whether there is coexpression of VISTA on CD68+ macrophages, we subjected seven pancreatic tumor samples to mass cytometry (also known as cytometry by time-of-flight or CyTOF) analysis. CD45+ immune infiltrates were further analyzed for the expression of immune checkpoints found to be expressed on different immune cell types. The analysis of a representative case is shown in Fig. 5. We observed a greater frequency of VISTA+ cells than PD-L1+ cells (Fig. 5). VISTA expression was predominantly present on CD68+ macrophages (Fig. 5). A pooled analysis of the seven tumors demonstrated the presence of a CD68+ macrophage cluster (cluster 18) that was predominantly VISTA+ (SI Appendix, Fig. S6).
Fig. 5.
CyTOF analysis of CD68, PD-L1, and VISTA expression in human pancreatic tumors. CyTOF data are from a representative primary pancreatic, showing CD68+ macrophages and the inhibitory immune checkpoints PD-L1 and VISTA. Each point on the viSNE maps represents a single cell. Red indicates a high level of marker expression; blue indicates no marker expression.
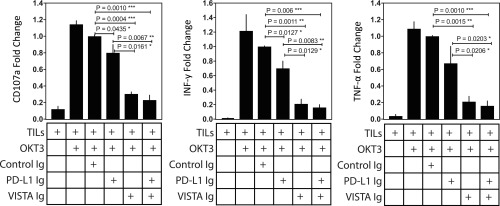
The differential expression of PD-L1 and VISTA on distinct CD68+ subsets suggests that PD-L1 and VISTA represent separate inhibitory pathways that are capable of suppressing antitumor T-cell responses in pancreatic cancer. However, to date, ICT targeting the PD-1–PD-L1 axis has not been successful in pancreatic cancer (3, 4, 8). To dissect the role of each of these inhibitory pathways in pancreatic cancer and to test the idea that both PD-L1 and VISTA can inhibit T-cell responses, we isolated and expanded tumor-infiltrating lymphocytes (TILs) from metastatic pancreatic tumors of three different patients and cultured the CD8+ T cells from each patient’s tumor separately with plate-bound VISTA−Ig and/or or PD-L1–Ig. The proliferation of T cells was not altered upon treatment with control Ig, as shown by the Ki67+ CD8+ T-cell percentage upon stimulation with anti-CD3 (∼80%; SI Appendix, Fig. S7). Degranulation, as measured by CD107a, and cytokine production, as measured by IFN-γ and TNF-α, were significantly inhibited in the presence of PD-L1–Ig (∼1.5× to 2×) or VISTA−Ig (∼3× to 10×); however, the engagement of the VISTA inhibitory pathway resulted in greater decrease in CD8+ T-cell responses than the engagement of PD-L1 pathway (Fig. 6). Furthermore, the presence of PD-L1-Ig and VISTA-Ig did not result in a significant decrease in T cell responses when compared to VISTA-Ig alone (Fig. 6). These data suggest that monotherapy blockade of the PD-1/PD-L1 inhibitory pathway is unlikely to restore effective T-cell responses, because the VISTA inhibitory pathway is also highly expressed and capable of suppressing T-cell responses in human pancreatic cancer.
Fig. 6.
Engagement of VISTA inhibits cytokine production by TILs. TILs from metastatic pancreatic tumors (n = 3) were cultured independently in different experiments with plate-bound VISTA−Ig and/or PD-L1−Ig to evaluate the function by CD107a degranulation assay and intracellular cytokine staining for IFN-γ and TNF-α. Data are expressed as mean with SD for the three independent experiments and represent percentage changes after normalization to control Ig. The differences in degranulation and cytokine release are compared with the control Ig group. Only significant P values between groups are indicated. Statistical significance is defined as P < 0.05. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Discussion
A detailed analysis and understanding of the immune infiltration of pancreatic tumors is essential for immune targeted therapy. A comparison of immune infiltration in the TME of an ICT-responsive tumor like melanoma with an ICT-unresponsive tumor like pancreatic cancer may provide insights for improving ICT responsiveness. In the current study, we observed that pancreatic tumors have a broad range of immune infiltration levels but that main players such as CD3, CD4, and CD8 T cells are preferentially distributed in the stromal area of the TME (Fig. 3B); therefore, these findings suggest that not all pancreatic tumors have low immunogenicity. The gene and protein expression data presented here are in agreement with previously published gene expression data suggesting an immune gene signature-rich subtype of pancreatic cancer (19). Approximately one-third of the human pancreatic tumors in the present study had immune infiltration similar to that of melanoma; however, unlike melanoma, the immune infiltrates were primarily distributed within the stromal area of the TME, with fewer cells in the tumor area (or tumoral parenchyma). Additionally, pancreatic tumors have high numbers of CD68+ macrophages, and, in the present study, we show that these macrophages are preferentially distributed in the stromal area. This may have an important impact on clinical outcomes with ICT therapies. Thus, even for moderately infiltrated pancreatic tumors, we may need approaches which will help mobilize these moderately infiltrated immune cells toward the tumor area for clinical benefit.
A recent study demonstrated that a higher degree of myeloid cell infiltration is correlated with poor clinical responses in patients with pancreatic cancer who received GVAX vaccination, an allogeneic, GM-CSF-secreting cellular immunotherapy (11).Our study highlights VISTA+ macrophages as one of the potential inhibitory cell types, which may affect clinical outcomes in patients with pancreatic cancer. VISTA is predominantly expressed on myeloid cells (35). Preclinical data have established that VISTA can suppress T-cell activation and hinders the development of tumor-specific immunity, but data on the functionality of VISTA+ cells in human cancer are lacking (35, 38). Our group previously reported that anti−CTLA-4 therapy can increase VISTA expression on CD68+ macrophages, which have an M2 phenotype, as a resistance mechanism in prostate tumors and represents an immune checkpoint target (36). Recently, another group reported that pancreatic tumors with high cytolytic activity have increased expression of multiple immune checkpoint genes such as CTLA4, TIGIT, TIM3, and VISTA (39).
Here, we show that pancreatic tumors express significantly higher levels of VISTA than melanoma tumors (Fig. 2), and VISTA expression was found predominantly on CD68+ macrophages (Fig. 5). We additionally show that engagement of VISTA diminishes cytokine production by T cells isolated from metastatic pancreatic tumors (Fig. 6). Therefore, anti-VISTA antibodies may be an effective immunotherapeutic strategy for patients with pancreatic cancer.
Materials and Methods
The details of complete materials and methods are available in SI Appendix. Details of patient materials used and clinicopathological information on pancreatic cases are provided in SI Appendix, Tables S1 and S2 A–C, respectively. The study was approved by MD Anderson Cancer Center’s Institutional Review Board, and all relevant data were retrieved from the computerized database of the MD Anderson Cancer Center Immunotherapy Platform and from the Multidisciplinary Pancreas Database. Nanostring analysis was performed on untreated primary pancreatic tumor samples (n = 23). IHC staining and data analysis on formalin-fixed paraffin-embedded (FFPE) samples was performed on cohorts of pancreatic and melanoma patients as detailed in SI Appendix, Supplementary Materials and Methods. CyTOF analysis was performed on fresh pancreatic tumor samples, and T-cell activation assays were performed on expanded CD8 TILs.
Supplementary Material
Acknowledgments
We thank Ashura Khan, Sheila Duncan, Sumit Subudhi, Jianjun Gao, Nicolle Patterson, and Alexsandra Espejo for technical support. P.S., J.P.A., J.W., and C.Y. are members of the Parker Institute for Cancer Immunotherapy. This research work was supported, in part, by the MD Anderson Cancer Center Immunotherapy Platform, the MD Anderson Pancreatic Cancer Moon Shot program, and National Institutes of Health/National Cancer Institute Grant R01 CA1633793 (to P.S.).
Footnotes
Conflict of interest statement: J.P.A. is an inventor and recipient of royalties from intellectual property licensed to Bristol-Meyer Squibb, Merck, and Jounce. He is a member of the scientific advisory board for Jounce Therapeutics, Neon Therapeutics, Amgen, Apricity, BioAlta, Forty-Seven, Tvardi Therapeutics, TapImmune, ImaginAB, Codiak Biosciences, and Marker Therapeutics. J.P.A. and P.S. own a patent licensed to Jounce Therapeutics. P.S. serves as a consultant for Constellation, Jounce Therapuetics, Kite Pharma, Neon Therapeutics, BioAtla, Pieris Pharmaceuticals, Oncolytics Biotech, Merck, BioMx, Forty-Seven, Polaris, Apricity, Marker Therapeutics, Codiak, ImaginAB, and TapImmune. She also has stock ownership in Jounce, Neon Therapeutics, Constellation, Oncolytics, BioAtlanta, Forty-Seven, Apricity, Polaris, Marker Therapeutics, Codiak, ImaginAB, and TapImmune. A.S. is an employee of Janssen.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811067116/-/DCSupplemental.
References
- 1.Le DT, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2015;381:244–251. doi: 10.1016/j.canlet.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royal RE, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 6.Postow MA, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winograd R, et al. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013;25:200–205. doi: 10.1016/j.coi.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark CE, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 11.Tsujikawa T, et al. Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell Rep. 2017;19:203–217. doi: 10.1016/j.celrep.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melief CJ, Kast WM. T-cell immunotherapy of cancer. Res Immunol. 1991;142:425–429. doi: 10.1016/0923-2494(91)90042-h. [DOI] [PubMed] [Google Scholar]
- 13.Clark CE, Beatty GL, Vonderheide RH. Immunosurveillance of pancreatic adenocarcinoma: Insights from genetically engineered mouse models of cancer. Cancer Lett. 2009;279:1–7. doi: 10.1016/j.canlet.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Helm O, et al. Comparative characterization of stroma cells and ductal epithelium in chronic pancreatitis and pancreatic ductal adenocarcinoma. PLoS One. 2014;9:e94357. doi: 10.1371/journal.pone.0094357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol. 2013;4:210. doi: 10.3389/fphys.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibuya KC, et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS One. 2014;9:e96565. doi: 10.1371/journal.pone.0096565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukunaga A, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Ino Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey P, et al. Australian Pancreatic Cancer Genome Initiative Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 20.Saida T, et al. Histopathological characteristics of malignant melanoma affecting mucous membranes: A unifying concept of histogenesis. Pathology. 2004;36:404–413. doi: 10.1080/00313020412331282753. [DOI] [PubMed] [Google Scholar]
- 21.Mihm MC, Jr, Clark WH, Jr, From L. The clinical diagnosis, classification and histogenetic concepts of the early stages of cutaneous malignant melanomas. N Engl J Med. 1971;284:1078–1082. doi: 10.1056/NEJM197105132841907. [DOI] [PubMed] [Google Scholar]
- 22.Flotte TJ, Mihm MC., Jr Lentigo maligna and malignant melanoma in situ, lentigo maligna type. Hum Pathol. 1999;30:533–536. doi: 10.1016/s0046-8177(99)90197-1. [DOI] [PubMed] [Google Scholar]
- 23.Clark WH, Jr, Elder DE, Van Horn M. The biologic forms of malignant melanoma. Hum Pathol. 1986;17:443–450. doi: 10.1016/s0046-8177(86)80032-6. [DOI] [PubMed] [Google Scholar]
- 24.Clark WH, Jr, et al. A study of tumor progression: The precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984;15:1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- 25.Ackerman AB, David KM. A unifying concept of malignant melanoma: Biologic aspects. Hum Pathol. 1986;17:438–440. doi: 10.1016/s0046-8177(86)80030-2. [DOI] [PubMed] [Google Scholar]
- 26.Scoazec JY, Couvelard A, Reseau T. Réseau TENpath [Classification of pancreatic neuroendocrine tumours: Changes made in the 2017 WHO classification of tumours of endocrine organs and perspectives for the future] Ann Pathol. 2017;37:444–456. doi: 10.1016/j.annpat.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Bosman FT. World Health Organization; International Agency for Research on Cancer . WHO Classification of Tumours of the Digestive System. IARC Press; Lyon, France: 2010. [Google Scholar]
- 28.Bellizzi AM, Frankel WL. Pancreatic pathology: A practical review. Labmedicine. 2009;40:417–426. [Google Scholar]
- 29.Lyssiotis CA, Kimmelman AC. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27:863–875. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ait-Belkacem R, et al. Microenvironment tumor metabolic interactions highlighted by qMSI: Application to the tryptophan-kynurenine pathway in immuno-oncology. SLAS Discovery: Adv Life Sci. 2017;22:1182–1192. doi: 10.1177/2472555217712659. [DOI] [PubMed] [Google Scholar]
- 31.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 33.Salmon JK, Armstrong CA, Ansel JC. The skin as an immune organ. West J Med. 1994;160:146–152. [PMC free article] [PubMed] [Google Scholar]
- 34.O’Morchoe CC. Lymphatic system of the pancreas. Microsc Res Tech. 1997;37:456–477. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<456::AID-JEMT9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23:551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lines JL, Sempere LF, Broughton T, Wang L, Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res. 2014;2:510–517. doi: 10.1158/2326-6066.CIR-14-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Mercier I, et al. VISTA regulates the development of protective antitumor immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune cytolytic activity stratifies molecular subsets of human pancreatic cancer. Clin Cancer Res. 2017;23:3129–3138. doi: 10.1158/1078-0432.CCR-16-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.