Significance
Mitochondria are the primary source of ATP for placental growth, transport, and hormone synthesis. However, to date, little is known about the developmental regulation or functional significance of placental mitochondria during normal or suboptimal intrauterine conditions, such as oxygen deprivation (hypoxia). Here we show that, in the placenta, mitochondria adapt their use of oxygen and nutrients (carbohydrate and fat) to best support both placental growth and function, as well as fetal development, during normal and hypoxic conditions. These data are significant because they improve our mechanistic understanding of human pregnancies compromised by fetal growth restriction at sea level and high altitude.
Keywords: mitochondria, metabolism, fetus, placenta, hypoxia
Abstract
Mitochondria respond to a range of stimuli and function in energy production and redox homeostasis. However, little is known about the developmental and environmental control of mitochondria in the placenta, an organ vital for fetal growth and pregnancy maintenance in eutherian mammals. Using respirometry and molecular analyses, the present study examined mitochondrial function in the distinct transport and endocrine zones of the mouse placenta during normal pregnancy and maternal inhalation hypoxia. The data show that mitochondria of the two zones adopt different strategies in modulating their respiration, substrate use, biogenesis, density, and efficiency to best support the growth and energy demands of fetoplacental tissues during late gestation in both normal and hypoxic conditions. The findings have important implications for environmentally induced adaptations in mitochondrial function in other tissues and for compromised human pregnancy in which hypoxia and alterations in placental mitochondrial function are associated with poor outcomes like fetal growth restriction.
Mitochondria are multifunctional organelles. Their primary role is in ATP generation by oxidative phosphorylation (OXPHOS) using substrates derived from β-oxidation and the tricarboxylic acid cycle. They are also involved in cell signaling via production of reactive oxygen species (ROS) and other molecules, which affect cell homeostasis and survival. ROS are a normal by-product of OXPHOS but, when produced in excess, e.g., during disrupted oxygen (O2) or substrate supply (1), they can cause oxidative stress and damage DNA, lipids, and proteins (2). In endocrine tissues, like the placenta, mitochondria also synthesize steroids, which consumes O2 independently of OXPHOS (3). Consequently, mitochondria vary in number and function between different cell types in relation to metabolic needs (4).
The placenta has a high energy requirement. It synthesizes hormones and other molecules for pregnancy maintenance and actively transports a range of substrates to the fetus for growth and development (4, 5). It also requires energy for its own metabolism, growth, and morphological remodeling (6). In all mammals, placental energy demand is met primarily by OXPHOS (7); thus the placenta has a significant O2 requirement, using 50 to 70% of O2 taken up from the uterine circulation at a mass-specific rate of consumption higher than in adult liver (7). As the fetus grows, the demands on placental energetics increase, yet total mass-specific uteroplacental O2 consumption changes little, if at all, from mid to late gestation in sheep and humans (7, 8). However, there are gestational changes in placental oxidative stress and expression of the mitochondrial-related proteins in several species (9–12), which suggest that function of placental mitochondria changes developmentally to meet the increasing fetal demands for growth toward term.
The placenta is known to adapt its morphology and transport characteristics to optimize fetal growth during suboptimal conditions in several species (4). In humans, hypoxia is the main cause of fetal growth restriction at high altitude and is a common feature of pregnancy complications at sea level (13). In pregnant rodents and guinea pigs, inhalation hypoxia adapts placental morphology and nutrient transport to the fetus dependent upon the degree, timing, and length of O2 restriction (14–19). Changes in placental mitochondrial function are also seen in compromised human pregnancies (3) and in nutritionally induced fetal growth restriction in rodents, in association with changes in mitochondrial function and biogenesis (20, 21). However, the extent to which placental mitochondrial function adapts to environmental cues like hypoxia remains unclear.
Here we comprehensively examine the functional phenotype of placental mitochondria during the last third of mouse pregnancy and in response to maternal inhalation hypoxia in relation to the temporal changes in fetoplacental growth. In rodents, unlike humans, the endocrine and transport functions of the placenta are carried out by structurally distinct regions, the junctional zone (Jz) and labyrinthine zone (Lz), respectively, which differ in morphology, cellular composition, and blood flow (4). Consequently, we investigated mitochondrial function of the two zones separately.
Results
Mitochondrial Respiratory Capacity in the Placenta with Gestation.
C57BL/6J mice were time-mated and the ontogeny of mitochondrial function determined in the placental Jz and Lz on day (D) 14, 16, and 19 of pregnancy (term = ∼D20). This covers the period when mouse fetuses grow most rapidly in absolute terms. We used three respirometry assays to assess the capacity for substrate use and electron transfer system (ETS) function in saponin-permeabilized placental samples, initially in the absence of ADP (O2 consumption without ATP generation; LEAK) and then following the addition of ADP (OXPHOS state). First, Py-supported respiration was measured in the LEAK (PyL) and OXPHOS (PyP) states in the presence of malate. Second, palmitoyl carnitine (Pal)-supported respiration was measured in the LEAK (PalL) and OXPHOS (PalP) states also in the presence of malate. Third, a substrate titration was used to elucidate ETS capacity. Initially, LEAK state respiration in the presence of complex I-linked substrates, glutamate and malate was recorded (GML) before OXPHOS was stimulated (GMP), and, finally, succinate was added (GMSP) to measure OXPHOS capacity when electron entry via complexes I and II of the ETS was saturated. The respiratory medium comprised 0.5 mM EGTA, 3 mM MgCl20.6H2O, 20 mM taurine, 10 mM KH2PO4, 20 mM Hepes, 1 mg/mL of BSA, 60 mM K-lactobionate, 110 mM sucrose, pH 7.1.
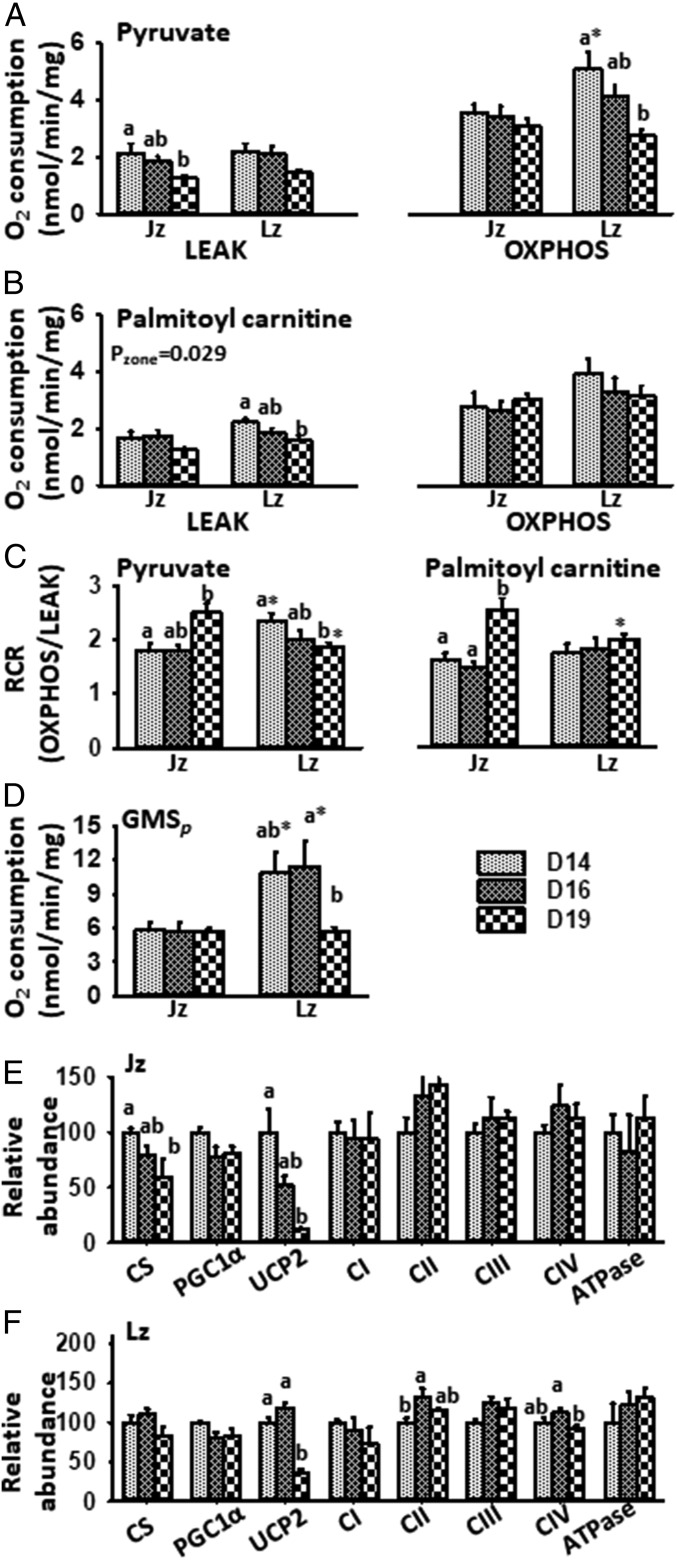
Both Lz and Jz were able to use Py and Pal as respiratory substrates, although respiratory rate varied between D14 and D19. In the Jz, PyL declined between D14 and D19 (Fig. 1A). In contrast, Jz PalL remained stable gestationally (Fig. 1B). The Jz displayed no gestational changes in either PyP or PalP; however, respiratory control ratios (RCRs; OXPHOS/LEAK) for Py and Pal increased from D16 to D19 (Fig. 1 A–C). GML declined between D16 and D19 in the Jz (SI Appendix, Fig. S2). However, GMP and GMSP did not vary between D14 and D19, in line with Jz size (Fig. 1D and SI Appendix, Figs. S1 and S2).
Fig. 1.
Mitochondrial respiration and associated protein abundance in the last third of pregnancy. (A and B) Oxygen consumption in LEAK and OXPHOS states using (A) Py and (B) Pal, (C) RCRs for Py and Pal, and (D) OXPHOS respiration in the presence of malate, glutamate, and succinate (GMSP) in the placental Jz and Lz, as well as (E and F) protein abundance in the (E) Jz and (F) Lz at D14, D16, and D19 of pregnancy. Analyzed by two-way ANOVA (age and zone) with Bonferroni post hoc tests. Different letters represent a significant difference between gestational ages, within a zone (P < 0.05); * denotes a significant difference of Lz to Jz, for a given age (P < 0.05). Outcome of ANOVA is shown if pair-wise comparisons were not significant; n = 6 to 10 per age for A–D, and n = 5 to 6 for E and F per treatment. ATPase, ATP synthase; CI−IV, ETS complexes I to IV.
In the Lz, PyP and PalL declined between D14 and D19 (Fig. 1 A and B). PyP in the Lz was also greatest at D14 and decreased toward term (Fig. 1A). PalP in the Lz instead remained stable between D14 and D19. The RCR for Py was lower in the Lz on D19, relative to D14 (Fig. 1C), due to the greater decline in OXPHOS than LEAK respiration. In contrast, Pal RCRs in the Lz were stable with age (Fig. 1C). GML, GMP, and GMSP in the Lz were greatest on D14 and/or D16, with values decreasing by D19 (Fig. 1D and SI Appendix, Fig. S2). These data suggest more active mitochondrial function at the earlier ages when the Lz is growing most rapidly (SI Appendix, Fig. S1). The lower rates of Lz O2 consumption with age, particularly with Py, also suggest O2 and glucose may be spared by the placenta for fetal transfer during the rapid phase of fetal growth (SI Appendix, Fig. S1).
Mitochondrial Proteins in the Placenta with Gestation.
Next, we investigated whether there were ontogenic changes in the levels of proteins regulating mitochondrial content and function in the two zones. In the Jz, abundance of both citrate synthase (CS), a marker of mitochondrial density, and UCP2 declined with increasing gestational age (Fig. 1E). Similarly, Jz abundance of peroxisome proliferator-activated receptor gamma coactivator-1-alpha (PGC1α), a regulator of mitochondrial biogenesis, showed a trend to decrease gestationally (Fig. 1E, P = 0.054). There was an overall effect of gestational age on protein carbonylation, a marker of oxidative stress, in the Jz; values appeared highest on D16 versus D14 and D19 (relative abundance mean ± SEM: D14, 100 ± 7%; D16, 171 ± 31%; D19: 98 ± 22%; P < 0.05). However, there was no significant change in the Jz abundance of any ETS complex between D14 and D19 (Fig. 1E).
Unlike the Jz, Lz expression of CS and PGC1α were unaffected by gestational age (Fig. 1F). Abundance of Lz UCP2 was similar on D14 and D16, but decreased by D19 (Fig. 1F). Lz abundance of ETS complexes II and IV (Fig. 1F) and protein carbonylation were greater on D16, relative to D14 or D19 (D14, 100 ± 8%; D16, 182 ± 32%; D19: 98 ± 15%; P < 0.05 for D16 versus D14 and D19). Thus, the functionally distinct zones show ontogenic differences in abundance of mitochondrial-regulatory proteins that relates to their respiratory capacity and the pattern of fetoplacental growth during the last third of pregnancy.
Hypoxia and Placental Mitochondrial Respiratory Capacity.
Adaptation in placental mitochondrial function may optimize fetal growth and survival when maternal availability of resources, like inspired O2, are limited. Thus, we sought to address whether placental mitochondrial function is altered by hypoxia in a severity-dependent fashion that relates to the known placental support of fetal growth in these conditions (15). We assessed the effects of both moderate hypoxia (13% inspired O2) between D11 and D16 or D14 and D19 and severe hypoxia (10% inspired O2) from D14 to D19 on placental mitochondrial function on D16 and D19 of pregnancy, relative to normoxic dams (21% O2; N). These levels of hypoxia would be equivalent to altitudes of ∼3,700 m and ∼5,800 m, over which range human and rodent populations decrease from significant to sparse levels (15). As 13% O2 from D11 to D16 and 10% O2 from D14 to D19 were associated with reductions in maternal food intake (15), additional groups of normoxic dams were pair-fed (PF) to match the intakes of the 13% O2 and 10% O2 dams for the same periods of pregnancy, to discriminate between the effects of hypoxia and hypoxia-induced hypophagia. Previously, we have shown that fetal growth is unchanged at D16 and only marginally decreased (by 5%) at D19 in 13% O2, whereas pup weight is reduced by 20% in 10% O2 dams at D19, with intermediate values in the PF dams (15). There was no effect of hypoxia or pair feeding on placental weight at either D16 or D19, although Lz volume was increased by 13% O2 on D16, and Jz volume increased by 10% O2 on D19 (15).
Early Exposure.
Respiratory capacity.
Py-supported Jz respiration was unaffected by 13% O2 on D16. However, Jz PalL in 13% O2 dams was greater than in N dams but similar to PF (Fig. 2B). PalP in the Jz of 13% O2 dams did not differ from N or PF mice; however, values were higher in PF mice, relative to N dams (Fig. 2B). There was no effect of 13% O2 on Py- or Pal-supported RCRs or GML, GMP, and GMSP in the Jz (Fig. 2 C and D and SI Appendix, Fig. S3A).
Fig. 2.
Mitochondrial respiration and associated protein abundance at D16 in response to hypoxia. (A and B) Oxygen consumption in LEAK and OXPHOS states using (A) Py and (B) Pal, (C) RCRs for Py and Pal, and (D) OXPHOS respiration in the presence of malate, glutamate and succinate (GMSP) in the placental Jz and Lz, as well as (E and F) protein abundance in the (E) Jz and (F) Lz following exposure to 13% O2 from D11 to D16 or pair feeding normoxic animals to the food intake of mice in 13% O2 (PF). Analyzed by two-way ANOVA (treatment and zone) with Bonferroni post hoc tests. Different letters represent a significant difference between treatments, within a zone (P < 0.05); * denotes a significant difference of Lz to Jz (P < 0.05). Outcome of ANOVA is shown if pair-wise comparisons were not significant; n = 6 to 10 for A–D and n = 5 for E and F per treatment; txt, treatment.
In the Lz, the majority of differences in placental mitochondrial respiratory function were seen between the 13% O2 and PF dams, with intermediate values in the N group (Fig. 2). Although not different from N dams, Py-supported LEAK and Pal-supported LEAK and OXPHOS respiration rates and RCRs were reduced in the Lz on D16 in 13% O2 relative to PF dams (Fig. 2 A–C). GML, GMP, and GMSP were diminished by 13% O2 compared with either N or PF dams (Fig. 2 A–C and SI Appendix, Fig. S3A).
Mitochondrial proteins.
In the Jz, CS and PGC1α abundance was reduced by 13% O2, relative to N mice on D16 (Fig. 2E). However, Jz CS was greater in 13% O2 mice than in PF, while PGC1α did not differ between 13% O2 and PF. Abundance of UCP2 and ETS complexes in the Jz was not affected by 13% O2 compared with N dams. Jz UCP2 abundance with 13% O2 was also not different from that of PF mice, but values for the PF group were increased relative to N mice (Fig. 2E). Moreover, complexes I and II were greater and ATP synthase was lower in the Jz of 13% O2 mice, relative to PF (Fig. 2E). There was no effect of 13% O2 Jz protein carbonylation (SI Appendix, Fig. S4A).
In the Lz at D16, abundance of PGC1α was greater in both 13% O2 and PF mice, compared with N dams (Fig. 2F). The Lz abundance of ETS complex III and ATP synthase was lower in 13% O2 dams compared with both N and PF dams (Fig. 2F). In the Lz, other ETS complexes, CS, and UCP2 abundance were unaffected by 13% O2. However, Lz complex I was more abundant in PF relative to N dams. There was also no effect of 13% O2 on Lz protein carbonylation (SI Appendix, Fig. S4A). Thus, 13% O2 affected mitochondrial phenotype differentially in the Jz and Lz at D16.
Late Exposure.
Respiratory capacity.
On D19, PyL and PyP respiration and Py-supported RCRs in the Jz or Lz were unaffected by 13% O2 or 10% O2 (Fig. 3 A–C). However, Jz PalP was lower in 13% O2, relative to N. It also tended to be lower than N values in 10% O2 and PF mice, which were similar to each other. However, Jz Pal-supported RCRs, as well as GML, GMP, and GMSP, were unchanged by either 13% O2 or 10% O2 (Fig. 3C and SI Appendix, Fig. S3B).
Fig. 3.
Mitochondrial respiration and associated protein abundance at D19 in response to hypoxia. (A and B) Oxygen consumption in LEAK and OXPHOS states using (A) Py and (B) Pal, (C) RCRs for Py and Pal, and (D) OXPHOS respiration in the presence of malate, glutamate, and succinate (GMSP) in the placental Jz and Lz, as well as (E and F) protein abundance in the (E) Jz and (F) Lz following exposure to 13% O2 or 10% O2 from D14 to D19 or pair feeding normoxic animals to the food intake of mice in 10% O2 (PF). Analyzed by two-way ANOVA (treatment and zone) with Bonferroni post hoc tests; * denotes a significant difference of Lz to Jz (P < 0.05); different letters represent a significant difference between treatments, within a zone (P < 0.05). Outcome of ANOVA is shown if pair-wise comparisons were not significant; and # indicates a significant difference of 13% O2 to N by t test (P < 0.05); n = 9 to 16 for A–D and n = 6 to 9 for E and F per treatment.
In the D19 Lz, both PalL and PalP were lower in both hypoxic groups, relative to N dams (Fig. 3B). Dams PF to the 10% O2 group also exhibited lower Lz PalP, versus N dams (Fig. 3B). In the Lz, Pal-supported RCRs in 13% O2 and 10% O2 dams were similar to N or the respective PF dams (Fig. 3C). GML, GMP, and GMSP was lower in Lz of 13% O2 and 10% O2 dams, relative to N dams (SI Appendix, Fig. S3B and Fig. 3D, by t test for 13% O2 dams). GML, GMP, and GMSP values in the Lz for PF were intermediate between N and 10% O2 dams (Fig. 3D and SI Appendix, Fig. S3B).
Mitochondrial proteins.
On D19, Jz CS, PGC-1α, and UCP2 abundances were increased in 13% and 10% O2 mice, relative to N, but were not different from those of their respective PF group (Fig. 3E, t test for 13% O2 dam). There was no effect of hypoxia on Jz abundance of ETS complexes (Fig. 3E) or protein carbonylation (SI Appendix, Fig. S4B). In the Lz, CS was more abundant in 13% O2, 10% O2 and the respective PF group, compared to N dams (Fig. 3F). Lz expression of PGC-1α tended to increase with hypoxia, with a significant difference between 13% O2 and N dams (Fig. 3F, t test). Overall, there was a significant effect of treatment on Lz abundance of complex II (Fig. 3F). Lz expression of complex IV and ATP synthase was greater in 13% O2 but not 10% O2 relative to N dams (Fig. 3F, by t test). ATP synthase abundance in the Lz was increased by PF compared with N (Fig. 3F). Protein carbonylation was ∼twofold greater in the Lz of 10% O2 mice than in all other experimental groups (SI Appendix, Fig. S4B). Thus, hypoxia, independent of the severity, affects mitochondrial profile differentially in the Jz and Lz at D19.
Discussion
This study in mice shows that placental mitochondria use both fatty acids and carbohydrates as respiratory substrates and adapt their function ontogenically during normal pregnancy and in response to environmental hypoxia. It is comprehensive in demonstrating that there are Lz- and Jz-specific changes in mitochondrial respiration, efficiency, substrate use, biogenesis, density, and ETS complex abundances that depend on gestational age, nutritional intake, and the degree of maternal hypoxia. There were also zonal and age-related differences in placental oxidative stress during normal and hypoxic pregnancy, which probably relate to changes in mitochondrial ROS production with potential consequences for cell damage more widely. These data also emphasize the dynamic nature of mitochondrial phenotype and complexity of the physiological and molecular mechanisms regulating placental mitochondrial function in response to environmental cues.
In both the Lz and Jz, ADP-coupled O2 consumption rates were similar when respiration was supported by either Py or Pal. Previous studies have shown that the placenta expresses fatty acid oxidation enzymes and that trophoblasts oxidize fatty acids in vitro (22, 23). Indeed, the activity of certain fatty acid oxidation enzymes in the human placenta is as high as those in adult liver (23). The present study demonstrates that the placenta can use fatty acid oxidation to support mitochondrial ATP production, in part fulfilling its requirements for growth, transport, and hormone synthesis. Defects in placental fatty acid oxidation in complicated pregnancies may therefore contribute to the poor fetoplacental growth common in these diseases (22, 24).
At the earlier gestational ages studied, the Lz had greater Py and total OXPHOS respiration rates than the Jz. Thus, the energy demands of the transport zone appear greater than those of the endocrine zone at this stage of gestation, consistent with the rapid growth, morphological remodeling, and synthesis of proteins required for Lz nutrient transport between D14 and D16 (25–27). Thereafter, both pyruvate (Py)-supported and maximal Lz respiration rates and RCRs declined toward term, but there were no apparent alterations in Lz mitochondrial biogenesis and density, as indicated by the CS and PGC-1α abundances, that could explain this ontogenic change. The lower rates of Lz mitochondrial O2 consumption, particularly with Py toward term, suggest O2 and glucose may be spared by the placenta for transfer to the fetus during its rapid growth phase. Indeed, OXPHOS rates are lower for the transporting syncytiotrophoblast than the proliferative cytotrophoblast in the term human placenta (28). Since Lz PalP was unaffected by gestational age, while PalL declined, fatty acids may become more important substrates for meeting the energy demands placed on the Lz for transport by the rapidly growing fetus (29). Unlike the Lz, in the Jz, the coupled respiratory rates with Pal and Py and the maximum OXPHOS capacity were stable across the last third of pregnancy, in line with Jz weight and the steady energetic requirements for hormone production (5). The maintained Jz respiratory capacity between D14 and D19 occurred despite decreasing CS abundance. However, the RCRs increased, suggesting that Jz mitochondria become more efficient near term. Indeed, abundance of UCP2 was low in both the Jz and Lz, at D19, which might reduce proton leak and increase coupling of O2 consumption to ATP production.
Since UCP2 attenuates ROS production, the high level of Lz UCP2 abundance at D14 and D16 may help protect against oxidative damage at the time when the Lz is growing most rapidly and placental O2 delivery is still low (27). Indeed, Lz oxidative stress was highest at D16 in the current study. By D19, Lz oxidative stress and UCP2 abundance had decreased, in parallel with the known increase in uteroplacental blood flow toward term in the mouse (29). The ontogenic change in UCP2 abundance in the Lz, therefore, appears to reflect placental O2 availability and the fetoplacental demands for growth. At the earlier ages, the high level of Lz UCP2 appears to act to limit ROS accumulation and the risk of oxidative damage, whereas, at D19, the low UCP2 abundance will increase the efficiency of mitochondrial ATP production by minimizing proton leak when Lz energy requirements are at their highest to support fetal growth.
In this study, placental mitochondria adapted not only developmentally but also in response to environmental hypoxia during late gestation. Exposure to 5 d of 13% O2 led to an overall reduction in Lz mitochondrial oxidative capacity at both D16 and D19. These changes appear to have been a result of hypoxia alone, because dams in normoxia but PF to the 13% O2 intake typically showed increased, rather than decreased rates of Lz respiration. As fatty acids require around 8 to 11% more O2 to generate a given amount of ATP (6), the specific reduction in Lz fatty acid-supported O2 consumption in hypoxic mice at D19 may be a more efficient way of producing ATP in hypoxic conditions near term. There may also be a greater reliance on glycolytic ATP production, as placental accumulation and transplacental transfer of glucose and amino acids are increased, while active transport of amino acids is maintained in 13% O2 dams (15). These reductions in mitochondrial respiration and the change in relative substrate use with 13% O2 would be beneficial in sparing O2 for transfer to the rapidly growing fetus and are consistent with reports of normal fetal oxygen consumption rates in high-altitude human pregnancies (30). In line with this, fetal growth was maintained or only marginally (5%) reduced at D16 and D19, respectively, despite 5 d of hypoxia (15).
In 13% O2 dams at D16, the reduced Lz respiration was associated with decreased abundance of ETS complex III and ATP synthase, whereas complex I abundance was increased. As the major mitochondrial source of ROS in hypoxia is complex III (31), its lower abundance in the Lz of 13% O2 dams may serve to limit the excessive production of hypoxia-induced ROS. Consistent with this, we found no change in the levels of Lz oxidative stress in 13% O2 dams at D16. PGC1α was enhanced in the D16 Lz in 13% O2 dams, suggesting more mitochondrial biogenesis at this stage. However, this was likely to be due to hypoxia-induced hypophagia, as values in PF dams were similar to those in 13% O2 at D16, consistent with the enhanced mitochondrial biogenesis seen previously in the placenta of nutrient-restricted rats (20). At D19, lower respiration rates in hypoxic dams were paradoxically associated with increased abundance of complex II, IV, ATP synthase, PGC1α, and CS. Although reductions in oxidative respiration in hypoxic tissues, including the placenta, are generally associated with decreased mitochondrial biogenesis and ETS complexes (16, 32, 33), hypoxia has also been shown to stimulate mitochondrial biogenesis and lead to greater mitochondrial content in different cell types (34). Moreover, placentas of women evolutionarily adapted to high altitude contain more syncytial mitochondria than those of native sea-level populations (35). Enhanced mitochondrial formation in response to 13% O2 may thus reflect an adaptive attempt to increase Lz bioenergetic capacity. As mitochondria are susceptible to ROS-mediated damage, their enhanced biogenesis in the Lz of 13% O2 dams may be important for replacing damaged mitochondria and preventing excessive ROS production at D19, when there was no evidence of placental oxidative stress.
The effects of more severe hypoxia (10% O2) on Lz mitochondrial respiration and protein abundance in late pregnancy were generally similar to those observed with 13% O2. However, in contrast to 13% O2 dams, placental accumulation and transport of glucose was not up-regulated, while amino acid transport and fetal growth were reduced in 10% O2 dams at D19 (15). These differences were not entirely explained by the hypophagia in the 10% O2 dams; fetuses of PF dams were growth-restricted compared with normoxic animals but significantly larger than their hypoxic counterparts. Placental amino acid transport capacity of PF dams has also been shown to be intermediate between that of the normoxic and 10% O2 groups at D19 (15). Therefore, the more pronounced defects in placental amino acid transport and fetal growth restriction induced by 10% O2 at D19 may be accounted for by the more pronounced Lz oxidative stress in these dams. Certainly, placental oxidative stress is a common feature of fetal growth restriction in human pregnancy and reduces amino acid uptake in human trophoblasts in vitro (36, 37). Collectively, our findings suggest that the changes in Lz mitochondrial and transport phenotype with 10% O2 at D19 are due to complex regulatory interactions between maternal undernutrition, O2 availability, and the degree of ROS production and oxidative stress. This probably involves at least two molecular pathways (e.g., HIF and mTORC1) and energy-sensing molecules such as adenosine monophosphate-activated protein kinase, which is known in increase in abundance specifically in the mouse Lz during late gestation (38).
Compared with the Lz, maternal inhalation hypoxia had little effect on Jz mitochondrial respiration or oxidative stress levels at either gestational age. Since the Jz is devoid of fetal capillaries and does not function in fetal O2 transfer (4), there is little need to down-regulate its respiratory pathways as a mechanism to spare O2 for fetal use during hypoxia at either age. Jz mitochondrial density, biogenesis, and abundance of specific ETS complexes were altered by hypoxia in an age-related manner, with decreased PGC1α, CS, and ATP synthase abundance in 13% O2 dams on D16 but increased protein abundances at D19 irrespective of the degree of hypoxia. However, there were similar alterations in Jz mitochondrial respiration, biogenesis, and density in animals PF to the intakes of the hypoxic groups at both D16 and D19, indicating that many of the changes seen during hypoxia may have been driven largely by maternal undernutrition. Since the Jz is normally less well oxygenated than the Lz (39), it may be more resilient to further reductions in O2 levels than the Lz, although, at D19, abundance of UCP2 in the Jz increased in both hypoxic and the PF groups, which may have protected against excessive ROS production associated with hypoxia and hypophagia. Whatever the mechanisms involved, overall maintenance of Jz respiration rates during hypoxia may help maintain steroidogenesis and other endocrine activities that are essential for pregnancy (5).
While our study has clear strengths, it also has some limitations. Both the Lz and Jz are heterogeneous and vary in cellular composition and mitochondrial ultrastructure during gestation (27, 40). Furthermore, the human placenta (41) and other tissues [e.g., adipose tissue (42)] contain subpopulations of mitochondria with specific functions. Studies using single-cell isolation will be helpful in establishing the contribution of placental cell types and mitochondrial subpopulations to the respiratory profile and function of the Lz and Jz in normoxia and hypoxia. The contribution of O2 consumption by non-OXPHOS–related processes (such as steroid and nitric oxide production) could also be explored in future work using ETS inhibitors (e.g., rotenone and antimycin). Indeed, studies using the mitochondrial-targeted antioxidant MitoQ suggest non-OXPHOS processes may have a significant effect on substrate exchange, placental secretions, and fetal growth in hypoxic rat dams (17, 43).
In summary, our data show that the mouse placental Lz and Jz adopt different strategies at the mitochondrial level to support the growth and other energy-demanding functions of both the placenta and fetus during normal and hypoxic pregnancy (SI Appendix, Table S1). More broadly, our data emphasize that mitochondrial function in the placenta is highly adaptable over the course of normal gestation and in response to environmental cues, which appears to help support normal fetal growth. Our findings are also important clinically, as hypoxia and altered mitochondrial function are reported in the placenta of human pregnancies with poor outcomes such as fetal growth restriction (3). The heterogeneity of pregnancy outcomes for women with gestational hypoxia may be explained, in part, by differences in the adaptive responses of placental mitochondria. Our work, therefore, highlights placental mitochondria as possible mediators and targets for intervention, in hypoxia-induced fetal growth restriction in sea-level and high-altitude human pregnancies.
Materials and Methods
All procedures described were approved by the Ethical Review Committee of the University of Cambridge (Cambridge, United Kingdom) and were carried out in accordance with UK Animals (Scientific Procedures) Act 1986 as previously reported (15). Either on D14, D16, and D19 (ontogeny study) or on D16 or D19 (hypoxia study), dams were killed by cervical dislocation. For the hypoxia study, all dams were anesthetized before death with an i.p. injection of fentanyl-fluanisone and midazolam in sterile water (1:1:2, 10 µg/mL; Janssen Animal Health). The uterus was removed, and each fetus and corresponding placenta were weighed. Two placentas from each litter were separated into Lz and Jz. Zones from one placenta were snap-frozen for quantification of protein abundance. Zones from the other placenta were immediately taken for analysis of mitochondrial respiratory capacity. Data are presented as means ± SEM and were analyzed by one-way or two-way ANOVA with Bonferroni post hoc tests using IBM SPSS statistics or by t test using Excel with statistical significance determined by P < 0.05. For fetal and placental weights, statistics were performed using litter means.
Supplementary Material
Acknowledgments
This work was funded by a PhD studentship (to J.S.H.), an in vivo skills Award BB/F016581/1 (to A.N.S.-P. and A.L.F.) from the British Biotechnology and Biological Sciences Research Council, and a Next Generation Fellowship (to A.N.S.-P.) and PhD studentship (to O.R.V.) from the Centre for Trophoblast Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816056116/-/DCSupplemental.
References
- 1.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 3.Holland O, et al. Review: Placental mitochondrial function and structure in gestational disorders. Placenta. 2017;54:2–9. doi: 10.1016/j.placenta.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol. 2016;7:33. doi: 10.3389/fphys.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front Physiol. 2018;9:1091. doi: 10.3389/fphys.2018.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray AJ. Oxygen delivery and fetal-placental growth: Beyond a question of supply and demand? Placenta. 2012;33:e16–e22. doi: 10.1016/j.placenta.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Carter AM. Placental oxygen consumption. Part I: In vivo studies–A review. Placenta. 2000;21:S31–S37. doi: 10.1053/plac.1999.0513. [DOI] [PubMed] [Google Scholar]
- 8.Fowden AL, Forhead AJ, White KL, Taylor PM. Equine uteroplacental metabolism at mid- and late gestation. Exp Physiol. 2000;85:539–545. [PubMed] [Google Scholar]
- 9.Jones ML, et al. Antioxidant defenses in the rat placenta in late gestation: Increased labyrinthine expression of superoxide dismutases, glutathione peroxidase 3, and uncoupling protein 2. Biol Reprod. 2010;83:254–260. doi: 10.1095/biolreprod.110.083907. [DOI] [PubMed] [Google Scholar]
- 10.Gnanalingham MG, et al. Nutritional manipulation between early to mid-gestation: Effects on uncoupling protein-2, glucocorticoid sensitivity, IGF-I receptor and cell proliferation but not apoptosis in the ovine placenta. Reproduction. 2007;134:615–623. doi: 10.1530/REP-06-0369. [DOI] [PubMed] [Google Scholar]
- 11.Stark MJ, Hodyl NA, Butler M, Clifton VL. Localisation and characterisation of uncoupling protein-2 (UCP2) in the human preterm placenta. Placenta. 2012;33:1020–1025. doi: 10.1016/j.placenta.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Holland OJ, et al. Changes in mitochondrial respiration in the human placenta over gestation. Placenta. 2017;57:102–112. doi: 10.1016/j.placenta.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Giussani DA. The fetal brain sparing response to hypoxia: Physiological mechanisms. J Physiol. 2016;594:1215–1230. doi: 10.1113/JP271099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuffe JS, et al. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592:3127–3141. doi: 10.1113/jphysiol.2014.272856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JS, Vaughan OR, Fernandez de Liger E, Fowden AL, Sferruzzi-Perri AN. Placental phenotype and resource allocation to fetal growth are modified by the timing and degree of hypoxia during mouse pregnancy. J Physiol. 2016;594:1341–1356. doi: 10.1113/JP271057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matheson H, Veerbeek JH, Charnock-Jones DS, Burton GJ, Yung HW. Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy. J Physiol. 2016;594:1371–1388. doi: 10.1113/JP271073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuzzo AM, et al. Placental adaptation to early-onset hypoxic pregnancy and mitochondria-targeted antioxidant therapy in a rodent model. Am J Pathol. 2018;188:2704–2716. doi: 10.1016/j.ajpath.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson LP, Pence L, Pinkas G, Song H, Telugu BP. Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod. 2016;95:128. doi: 10.1095/biolreprod.116.142273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahill LS, et al. Feto- and utero-placental vascular adaptations to chronic maternal hypoxia in the mouse. J Physiol. 2018;596:3285–3297. doi: 10.1113/JP274845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayeur S, et al. Maternal calorie restriction modulates placental mitochondrial biogenesis and bioenergetic efficiency: Putative involvement in fetoplacental growth defects in rats. Am J Physiol Endocrinol Metab. 2013;304:E14–E22. doi: 10.1152/ajpendo.00332.2012. [DOI] [PubMed] [Google Scholar]
- 21.Rebelato HJ, Esquisatto MA, Moraes C, Amaral ME, Catisti R. Gestational protein restriction induces alterations in placental morphology and mitochondrial function in rats during late pregnancy. J Mol Histol. 2013;44:629–637. doi: 10.1007/s10735-013-9522-7. [DOI] [PubMed] [Google Scholar]
- 22.Shekhawat PS, et al. Carnitine content and expression of mitochondrial beta-oxidation enzymes in placentas of wild-type (OCTN2+/+) and OCTN2 null (OCTN2-/-) mice. Pediatr Res. 2004;56:323–328. doi: 10.1203/01.PDR.0000134252.02876.55. [DOI] [PubMed] [Google Scholar]
- 23.Oey NA, et al. High activity of fatty acid oxidation enzymes in human placenta: Implications for fetal-maternal disease. J Inherit Metab Dis. 2003;26:385–392. doi: 10.1023/a:1025163204165. [DOI] [PubMed] [Google Scholar]
- 24.Bartha JL, Visiedo F, Fernández-Deudero A, Bugatto F, Perdomo G. Decreased mitochondrial fatty acid oxidation in placentas from women with preeclampsia. Placenta. 2012;33:132–134. doi: 10.1016/j.placenta.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Bondy CA. Placental glucose transporter gene expression and metabolism in the rat. J Clin Invest. 1993;91:845–852. doi: 10.1172/JCI116305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamson SL, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250:358–373. doi: 10.1016/s0012-1606(02)90773-6. [DOI] [PubMed] [Google Scholar]
- 27.Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 2004;70:1806–1813. doi: 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- 28.Kolahi KS, Valent AM, Thornburg KL. Cytotrophoblast, not syncytiotrophoblast, dominates glycolysis and oxidative phosphorylation in human term placenta. Sci Rep. 2017;7:42941. doi: 10.1038/srep42941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rennie MY, Cahill LS, Adamson SL, Sled JG. Arterio-venous fetoplacental vascular geometry and hemodynamics in the mouse placenta. Placenta. 2017;58:46–51. doi: 10.1016/j.placenta.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illsley NP, Caniggia I, Zamudio S. Placental metabolic reprogramming: Do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol. 2010;54:409–419. doi: 10.1387/ijdb.082798ni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 32.Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta. 2010;1797:1171–1177. doi: 10.1016/j.bbabio.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Colleoni F, et al. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: A role for miRNA-210 and protein synthesis inhibition. PLoS One. 2013;8:e55194. doi: 10.1371/journal.pone.0055194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutsaeva DR, et al. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soma H, et al. Characteristics of histopathological and ultrastructural features of placental villi in pregnant Nepalese women. Med Mol Morphol. 2005;38:92–103. doi: 10.1007/s00795-004-0259-y. [DOI] [PubMed] [Google Scholar]
- 36.Araújo JR, et al. Oxidative stress decreases uptake of neutral amino acids in a human placental cell line (BeWo cells) Reprod Toxicol. 2013;40:76–81. doi: 10.1016/j.reprotox.2013.06.073. [DOI] [PubMed] [Google Scholar]
- 37.Araújo JR, Pereira AC, Correia-Branco A, Keating E, Martel F. Oxidative stress induced by tert-butylhydroperoxide interferes with the placental transport of glucose: In vitro studies with BeWo cells. Eur J Pharmacol. 2013;720:218–226. [PubMed] [Google Scholar]
- 38.Skeffington KL, et al. Hypoxia, AMPK activation and uterine artery vasoreactivity. J Physiol. 2016;594:1357–1369. doi: 10.1113/JP270995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kusinski LC, et al. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am J Physiol Regul Integr Comp Physiol. 2012;303:R86–R93. doi: 10.1152/ajpregu.00600.2011. [DOI] [PubMed] [Google Scholar]
- 40.Coan PM, Ferguson-Smith AC, Burton GJ. Ultrastructural changes in the interhaemal membrane and junctional zone of the murine chorioallantoic placenta across gestation. J Anat. 2005;207:783–796. doi: 10.1111/j.1469-7580.2005.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bustamante J, et al. Oxygen metabolism in human placenta mitochondria. J Bioenerg Biomembr. 2014;46:459–469. doi: 10.1007/s10863-014-9572-x. [DOI] [PubMed] [Google Scholar]
- 42.Benador IY, et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 2018;27:869–885.e6. doi: 10.1016/j.cmet.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips TJ, et al. Treating the placenta to prevent adverse effects of gestational hypoxia on fetal brain development. Sci Rep. 2017;7:9079. doi: 10.1038/s41598-017-06300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.