Abstract
Lignans, neolignans, norlignans and norneolignans constitute a large class of phenolic natural compounds. 9-Norlignans, here defined to contain a β–β’ bond between the two phenylpropanoid units and to lack carbon number 9 from the parent lignan structure, are the most rarely occurring compounds within this class of natural compounds. We present here an overview of the structure, occurrence and biological activity of thirty-five 9-norlignans reported in the literature to date. In addition, we report the semisynthetic preparation of sixteen 9-norlignans using the natural lignan hydroxymatairesinol obtained from spruce knots, as starting material. 9-Norlignans are shown to exist in different species and to have various biological activities, and they may therefore serve as lead compounds for example for the development of anticancer agents. Hydroxymatairesinol is shown to be a readily available starting material for the preparation of norlignans of the imperanene, vitrofolal and noralashinol family.
Keywords: lignans, norlignans, 9-norlignans, semisynthesis, hydroxymatairesinol, bioactivity
1. Introduction
Many secondary metabolites, including lignans, flavonoids, and coumarins are formed from phenylpropanoids originating from the well-known shikimic acid pathway. The parent structures are usually further oxidized and arranged into various structures. Norlignans, a subclass of lignans lacking one or more carbon atoms, seem to be a rather unknown class of natural products. Compared to other phenylpropanoids their occurrence, biosynthetic pathway, and their properties are much less reported in the literature. Especially 9-norlignans (lacking carbon 9 from the parent lignan skeleton) with guaiacyl (3-methoxy-4-hydroxyphenyl) moieties have rarely been reported in the literature, although they would be expected to be common norlignans as guaiacyl lignans are the most abundant class of lignans. To the best of our knowledge, (+)-imperanene, vitrofolal E and F, noralashinol A and C are the only guaiacyl type 9-norlignans which have been reported as plant constituents [1,2,3,4]. The unnatural enantiomer, (−)-R-imperanene, dehydroxyimperanenes and dihydrodehydroxy-imperanenes have also been synthesized [5,6]. Normally, common substitution patterns are found in norlignans, but the more rare 2,4,5-trisubstituted phenyl moieties seem to be present in several 9-norlignans. In this paper we review the occurrence and properties of natural compounds belonging to the class of 9-norlignans, and in addition, we report the semisynthesis of 9-norlignans from the natural lignan hydroxymatairesinol.
1.1. Classification and Nomenclature of Norlignans
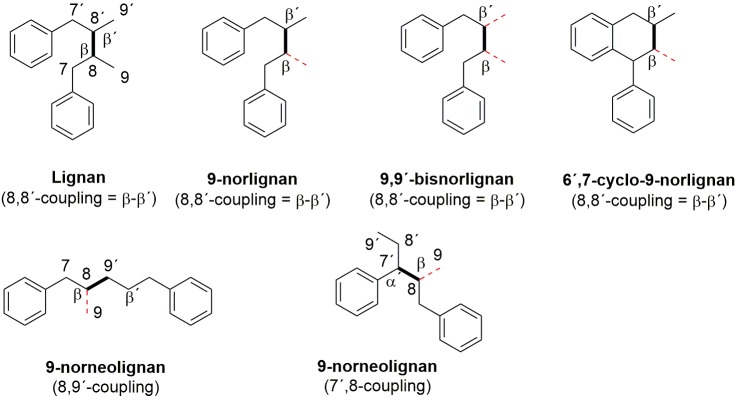
The term lignan is defined as two phenyl propane units coupled together by a β–β’ bond, and if the same structural units are coupled in any other ways the product is called a neolignan. If the coupling between the units contains an ether function (fundamental parent structures) the compound is called an oxyneolignan. According to the present recommendations by the International Union of Pure and Applied Chemistry (IUPAC), the prefix nor (modification of the fundamental parent structure) is used when the lignan, neolignan or oxyneolignan lack one or more carbon atoms [7]. However, in the literature there are today over 60 compounds named as norlignans although the major part of these are lacking the β–β’coupling, which is the definition for the fundamental parent structure named lignan. In our opinion most of these structures should be named as norneolignans or noroxyneolignans (prefix nor + fundamental parent structure). The choice of the numbering of the lost carbon is also somewhat confusing. Numbering of the modified carbon skeleton (in this case nor) should be performed so that the modification is expressed by the lowest locant (number, unprimed). However, the choice of locants for the removal of a carbon atom (nor) is preferred in the order: unprimed, higher number. Consequently, there is a conflict when assigning structures to 9 or 7-norlignans or norneolignans. Although the exact definition of different norlignans seems a bit confusing and vague, we here define a norlignan as a plant-derived compound consisting of two phenylpropane units coupled in the propane moiety with a β–β’ bond and missing one or more carbon atoms.
Moreover, we consider 9-norlignans to be derived from a parental lignan skeleton and thus to have a detectable β–β’ coupling and to lack one (or more, in the case of di(bis)norlignans) of the terminal carbon atoms (carbon 9). In Figure 1, the fundamental structures of different norlignan and norneolignan structures coupled in the propane moiety are displayed (oxyneolignans and others are excluded, dotted lines in red indicate the missing carbon-9, the bold lines indicate the coupling in the propane moiety).
Figure 1.
Fundamental structures for the lignan and different 9-norlignan and 9-norneolignan structures. Dotted (red lines) lines indicate the missing carbon-9 atom.
1.2. 9-Norlignans
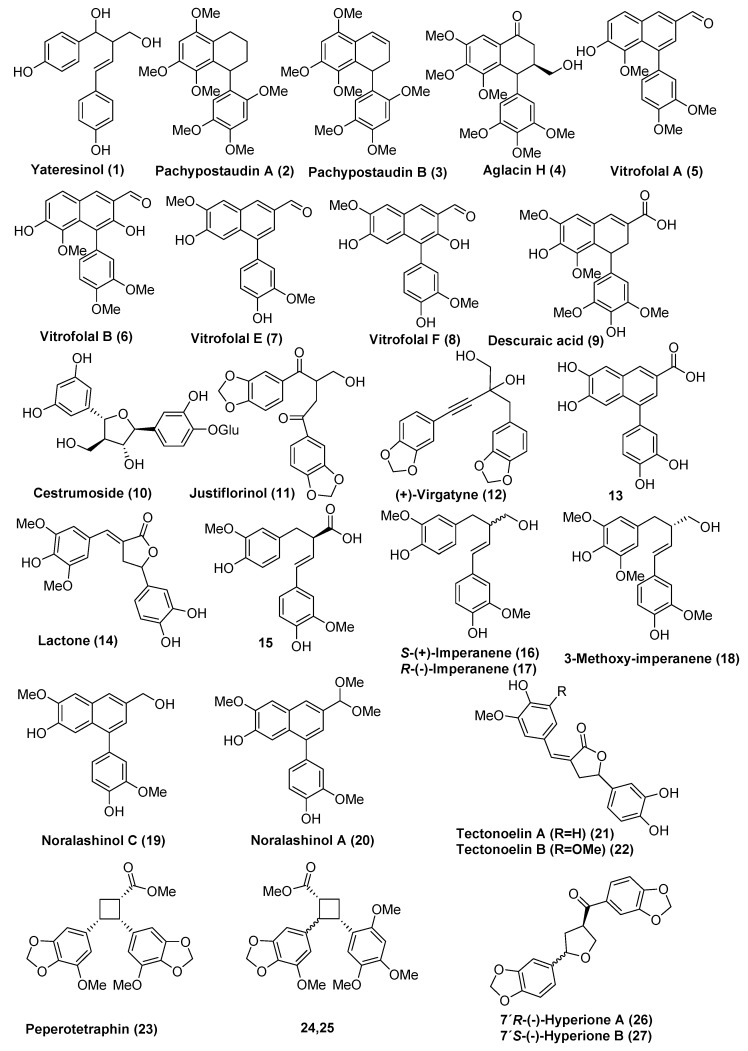
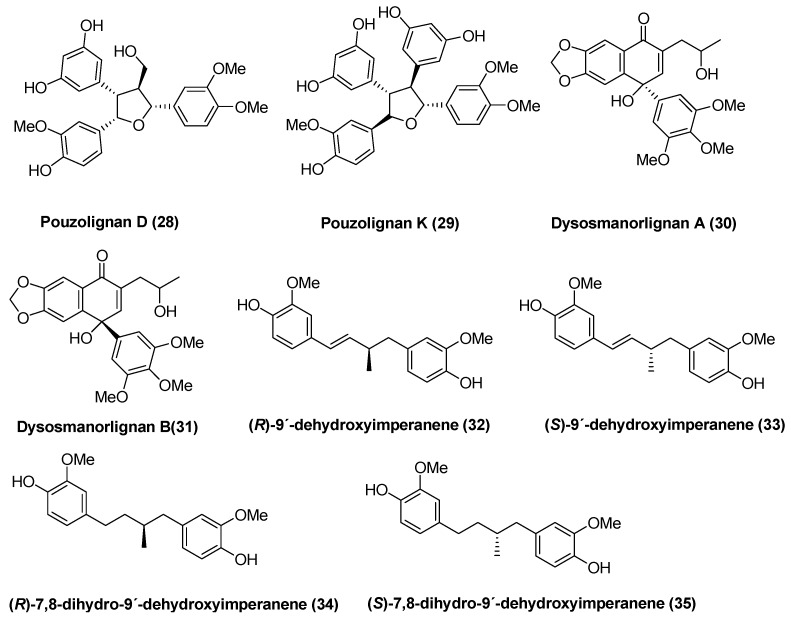
According to the definition above, approximately 25 naturally occurring 9-norlignans can be found in the literature. These are yateresinol (1) [8], pachypostaudin A and B (2,3) [9], aglacin H (4) [10], vitrofolal A,B,E, and F (5,6,7,8) [2,11] descuraic acid (9) [12], cestrumoside (glycoside) (10) [13], (−)-justiflorinol (11) [14], (+)-virgatyne (12) [15], the arylnaphthalene derivative 13 [16] and the lactone 14 [17], compound 15 [18], S-(+)-imperanene (16) [1], 3-methoxy-imperanene (18) [19], noralashinol A and C (19,20) [3,4], tectonoelin A and B (21,22) [20], peperotetraphin (23) [21] the related cyclobutane carboxylates 24 and 25 [22] and hyperione A and B (26,27) [23] (Figure 2). In addition, some related 9-norlignans have been synthesized and some 9-norlignan like compounds have also been reported [6,24,25]. In Figure 3, the structures of compounds 28–35 are shown.
Figure 2.
The structures of previously reported 9-norlignans with natural origin.
Figure 3.
Structures of synthetic 9-norlignans and 9-norlignan related compounds.
1.3. Occurrence and Biological Activity
Yateresinol (1) was first isolated from the heartwood of Libocedrus yateensis in 1979 by Ertman et al. [8]. It has also been found in Cryptomerica japonica D.Don and found to be part of the wood discoloring substances in sapwood [26]. Studies of butt-rot sugi wood suggested that the norlignans may be important for antifungal properties but no clear effect has been shown [27]. Yateresinol has been tested for cytotoxicity towards the human HL60 and Hepa G2 cancer cell lines [28] but was shown to be inactive with the half maximal inhibitory concentration (IC50) values >20 µg/mL. The two 9,9′-bisnorlignans 2 and 3 (pachypostaudin A and B) have been isolated from stem bark of Pachypodanthium staudtii Engl. et. Diels [9]. The same bisnorlignans have also been isolated from bark of the related species Pachypodanthium confine Engl. et Diels [29]. Both species are trees growing in the tropical zone of west and central Africa, and have been used in traditional African medicine. However, no biological activity of the bisnorlignans has been reported. Aglacin H (4) has been isolated from the bark of Aglaia cordata collected in Indonesia [10]. No other sources, and no chemical and biological properties for this norlignan have so far been reported. Norlignans from Vitex species, namely vitrofolal A, B, E, and F (compounds 5–8) are norlignans of the arylnaphthalene type. These compounds were first isolated from Vitex rotundifolia [2,11]. Compounds 5–8 have later also been isolated from the seeds [30] and 7 from the roots of Vitex negundo [31,32]. Compounds 7 and 8 have also been isolated from the fruits of Vitex cannabifolia [33]. Vitrofolal E (7) was also recently reported in the stem and bark of Syringa pinnatifolia [2,34]. Vitrofolals have been shown to have various biological activities. Compound 7 was shown to have antibacterial activity against methicillin-resistant Staphylococcus aureus at concentrations above 64 µg/mL [2]. Compounds 7 and 8 have also been reported to have antioxidant activity by inhibition of lipid peroxidation using the ferric thiocyanate method, and by scavenging of the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH). In both assays 7 and 8 showed comparable or slightly higher antioxidative activity than α-tocopherol, l-cysteine and butylated hydroxyanisole (BHA) [33,35]. Vitrofolal E (7) has also been shown to have moderate activity in the in vitro cholinesterase inhibition assay [31] and to have tyrosinase inhibitory activity in the same µM-range compared to known inhibitors [32]. Vitrofolal F (8) has been shown to have α-chymotrypsin (serine protease) inhibitory effect and has thus been suggested as a specific natural inhibitor of this enzyme [36]. The aryldihydronaphthoic acid derivative, descuraic acid (9), was isolated from seeds of Descurainia Sophia (L.) Webb ex Prantl, an annual/biennial herb [12]. Despite the structural similarities with vitrofolals, no biological properties of this compound have been published to date, however it is used in traditional medicine to alleviate common cold symptoms and to prevent asthma and oedema. The 9-norlignan glycoside cestrumoside (10), was recently identified from methanol extract of Cestrum diurnum L. leaves [13]. No biological effects for this compound were reported, although several species of this plant have been used in traditional Chinese medicine, especially for treatment of burns and swellings. However, recently 10 was claimed to be a protein kinase C inhibitor in an animal feed additive [37]. A study of the cytotoxic effects of the arylnaphthalene lignans from Vietnamese Acanthaceae, Justicia patentiflora revealed the structure of justiflorinol (11). However, the cytotoxic evaluation showed that 11 does not have the same cytotoxic effect as structurally related lignans found in this plant species [14]. Justiflorinol has also been isolated from leaves of Piper sanguineispicum and tested for its antileishmanial activity and for cytotoxicity on MCF7 and Vero cells. No significant biological activity was found [38]. (+)-Virgatyne (12) has been found in whole plant extracts of the Taiwanese annual plant Phyllantus virgatus Frost. F. (Euphorbiaceae) [15]. This plant has traditionally been used to protect the liver and 12 was tested for anti-hepatitis B virus in a MS-G2 cell line, but was shown to be completely inactive [39].
Different liverwort species have been shown to contain dihydronaphthalene and naphthalene lignans and norlignans. The arylnaphthalene derivative 13 was first isolated from Pellia epiphylla and the structure was confirmed by chemical synthesis [16]. The same compound (13) was later found in Jamesoniella autumnalis [40], Bazzania trilobata [41], Lepidozia reptans [42], Lepidozia incurvata, Chiloscyphus polyanthos and Jungermannia exsertifolia ssp. cordifolia. In the last three species conjugates with malic acid, shikimic acid and α-L-rhamnose were also isolated [43], but so far no biological activity has been reported.
The butyrolactone 9-norlignan (14) has been isolated from a methanol-water extract of leaves of Cestrum parqui, originally a South American shrub, but frequently occurring also in the Mediterranean region. Green cestrum (Cestrum parqui) has been shown to have phytotoxic effects and has been studied as a natural herbicide. Compound 14 showed phytotoxic effects on Lactuca sativa (lettuce) and Lycopersicon esculentum (tomato) by reducing the development of the plant [17].
Compound 15 was first identified in alkaline extraction (pulping) of Norway spruce (Picea abies) roots. Later it was also shown to be a degradation product of the abundant guaiacyl type dibenzylbutyrolactone lignan hydroxymatairesinol [18]. The degradation process has been studied in detail and 15 can be obtained by alkaline treatment of hydroxymatairesinol in nearly quantitative yields [5]. The carboxylic acid has not been reported as a constituent in other plants, but the corresponding alcohol ((−)-imperanene (17)), although the other enantiomer (+)-imperanene 16, has been found in the rhizomes of Imperata cylindrica. (+)-Imperanene was shown to have platelet aggregation inhibitory effects and gave a complete inhibition, at 6 × 10−4 M concentration, of rabbit platelet aggregation induced by thrombin [1]. (+)-Imperanene, 3-methoxyimperanene (18) and their glucosides have also been detected in rum distillate wastewater. In the same report, these compounds were shown to be inhibitors of human tyrosinase activity (IC50 = 1.85 mM). (+)-Imperanene showed the highest activity, indicating that the guaiacyl (3-methoxy-4-hydroxyphenyl) substitution pattern is important for the activity [19]. Contrary to many other norlignans, imperanene has been a target for several synthetic works. Noralashinol C (19) and noralashinol A (20), structurally related to the arylnaphthalene norlignans vitrofolals, have been isolated from stem barks of Syringa pinnatifolia. Compound 20 seems to be the acetal of 7 (possibly formed by reaction with methanol). Compound 19 was inactive in a cytotoxicity assay using HepG2 hepatic cancer cells and 20 was inactive in the NO production inhibitory assay [3,4]. Tectonoelin A and B (21,22), and compound 14 have been isolated from the leaves of Tectona grandis and shown to have growth inhibition activity in the etiolated wheat coleoptiles bioassay test [20]. Interestingly, both green cestrum and teak leaves seem to contain these rare norlignans and display herbicidal effects. Cyclobutane-type norlignans peperoteraphin (23) and two related isomers (24,25) have been isolated from Peperomia tetraphylla (whole plant). Compounds 24 and 25 were tested for cytotoxicity in human liver cancer cell lines HepG2, human lung cancer cell lines A549, and human cervical cancer cell lines Hela. Moderate activity with IC50 values around 50 µM for all cell lines, were detected [21,22]. Hyperione A and B (26,27) have been isolated from Hypericum chinense (whole herb). Hypericum species are known for their antibacterial activity but compounds 26 and 27 showed no antibacterial activity [23]. The occurrence and biological activities of 9-norlignans are summarized in Table 1.
Table 1.
9-norlignans, their occurrence and biological activity.
| Compound | Number | Occurrence | Bioactivity | References |
|---|---|---|---|---|
| Yateresinol | 1 | Libocedrus yateensis, Cryptomerica japonica | Antifungal | [8,26] |
| Pachypostaudin A | 2 | Pachypodanthium staudtii, Pachypodanthium confine | [9,29] | |
| Pachypostaudin B | 3 | |||
| Aglacin H | 4 | Aglaia cordata | [10] | |
| Vitrofolal A | 5 | Vitex rotundifolia, Vitex negundo, Vitex cannabifolia, Syringa pinnatifolia | Antibacterial, antioxidant, cholinesterase inhibition, tyrosinase inhibitory effects, chymotrypsin inhibitory effects | [2,11,30,31,32,33,34,36] |
| Vitrofolal B | 6 | |||
| Vitrofolal E | 7 | |||
| Vitrofolal F | 8 | |||
| Descuraic Acid | 9 | Descurainia Sophia | [12] | |
| Cestrumoside | 10 | Cestrum diurnum L. | [13] | |
| (−)-Justiflorinol | 11 | Justicia patentiflora, Piper sanguineispicum | [14,38] | |
| (+)-Virgatyne | 12 | Phyllantus virgatus | [15] | |
| Arylnaphthalene | 13 | Pellia epiphylla, Jamesoniella autumnalis, Bazzania trilobata, Lepidozia reptans, Lepidozia incurvata, Chiloscyphus polyanthos, Jungermannia exsertifolia ssp. cordifolia | [16,40,41,42,43] | |
| Lactone | 14 | Cestrum parqui | Phytotoxic effects | [17] |
| Compound | 15 | Picea abies | [18] | |
| (+)-Imperanene | 16 | Imperata cylindrica, sugarcane rum distillate | Platelet aggregation inhibitory effect, tyrosinase inhibitory effect | [1,19] |
| 3-Methoxy-Imperanene | 18 | Sugarcane rum distillate | Tyrosinase inhibitory effect | [19] |
| Noralashinol C | 19 | Syringa pinnatifolia | [3,4] | |
| Noralashinol A | 20 | |||
| Tectonoelin A | 21 | Tectona grandis | Herbicidal activity | [20] |
| Tectonoelin B | 22 | |||
| Peperoteraphin | 23 | Peperomia tetraphylla | Cytotoxic effect | [21,22] |
| Cyclobutane carboxylate | 24 | |||
| Cyclobutane carboxylate | 25 | |||
| Hyperione A | 26 | Hypericum chinense | [23] | |
| Hyperione B | 27 |
In addition to these compounds, some 9-norlignan-like compounds (although containing additional carbon atoms) have been isolated (Figure 3). Pouzolignan D (28) and K (29) were isolated from the aerial parts of Pouzolzia zeylanica (L.) Benn. var. microphylla (wedd.) W.T. Wang [24] and dysosmanorlignans A (30) and B (31) from the roots of Dysosma versipellis [25]. No biological activity has been reported for these compounds.
Several of these naturally occurring lignans have been the topic for total synthesis, however, the synthetic methods are excluded from this literature review. Some semisynthetic methods as well as the synthesis of interesting derivatives related to the presented norlignans will be discussed briefly. For example, the synthesized dehydroxy- and dihydrodehydroxyimperanene isomers have been evaluated for their plant growth regulatory effects, for larvicidal, antifungal, antibacterial and cytotoxic activity, and compared to dihydroguaiaretic acid isomers. In most studies compounds 32–35 were less or equally active as dihydroguaiaretic acids. In the cytotoxicity study, using HL-60 and HeLa cells, compound 35 showed the highest activity with IC50 values at approximately 6 µM, suggesting that norlignans may serve as lead compounds for anticancer agents [6].
As previously mentioned, imperanene derivatives can also be synthesized from the natural lignan hydroxymatairesinol and these compounds can be further transformed to vitrofolal and noralashinol type norlignans. To expand the substrate scope for investigation of 9-norlignans and to perform structure–activity relationship studies, we have undertaken the semisynthetic preparation of 15 different 9-norlignans with different functionalities using hydroxymatairesinol as starting material. In this strategy we chose to introduce flexibility or rigidity to the basic skeleton. We also varied the functionality at carbon-9 by the preparation of the carboxylic acid, the ester and the alcohol. In addition, the skeleton was also aromatized by oxidation and two different substitution patterns at the aromatic moieties were prepared. The semisynthesis of these compounds from hydroxymatairesinol is presented in detail below.
2. Results
Semisynthesis of Norlignan Derivatives from Hydroxymatairesinol
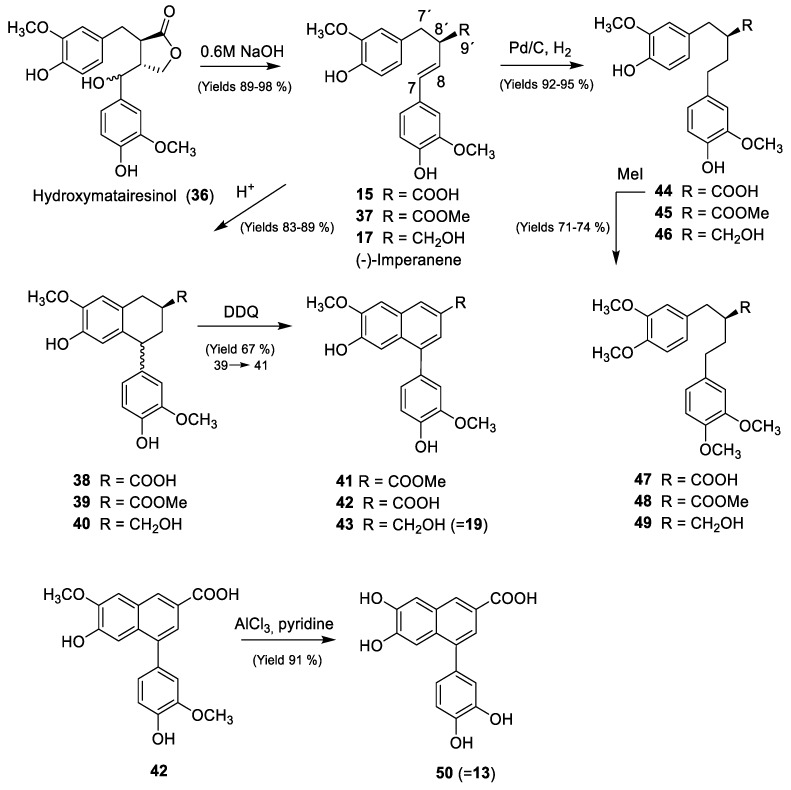
The knots of Norway spruce (Picea abies (L.) H. Karst) have been shown to contain about 10% (w/w) of the dibenzylbutyrolactone hydroxymatairesinol (36, Scheme 1). Methods for the separation of knots and the subsequent isolation of hydroxymatairesinol were developed during the last decade. Today hydroxymatairesinol is isolated on kg-scale, which certainly makes it one of the most readily available lignans in pure form for further studies. In our previous studies, we have shown that hydroxymatairesinol is degraded to norlignan derivative 15 by strong alkali. This retro-aldol-type reaction probably involves a formation of a quinone methide intermediate and the loss of formaldehyde [5].
Scheme 1.
Preparation of various 9-norlignans from hydroxymatairesinol. This generalized scheme shows the fundamental chemical transformations. For more detailed information on the interconversions and transformations including reaction conditions, see the supplementary material.
During degradation, two of the stereocentra of hydroxymatairesinol are destroyed, but the third retains the R-configuration without isomerization, yielding an optically pure product in excellent yields, usually over 95%. Further esterification and reduction of the obtained product (37), by lithium aluminium hydride (LAH) affords the 9-norlignan (−)-imperanene (17), as previously reported. However, the structure of 15 offers many possibilities for modification of the structure, which enabled the preparation of a set of derivatives for structure-activity relationship (SAR) studies of their biological effects (Scheme 1). The double bond was shown to be susceptible for protonation and Friedel–Crafts ring closing to yield two diastereomers of the 6’-7-cyclo-9-norlignan (aryltetralin-type) 38 in a 3:4 ratio. The two diastereomers were not separable by column chromatography. Further modification of the carboxylic acid to the ester 39 and the alcohol 40 was performed equally as in the case of 15 [5]. Oxidation of the cyclonorlignan 39 by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) was utilized to aromatize the aliphatic ring to obtain the corresponding arylnaphthalene vitrofolal-type norlignan 41. This structure was also transformed into the acid 42 and the alcohol 43 by hydrolysis and reduction, respectively. Oxidation of 38 or 40 by DDQ was proven to be unsuccessful. In the case of 38, the structure was easily decarboxylated during the oxidation. The double bond of 15 was also reduced by catalytic hydrogenation in a batch reactor using ethanol, Pd/C and hydrogen at a slightly elevated pressure (2 bar) to give 44. Again, the ester 45 and the alcohol 46 were prepared equally as for the other structures (Scheme 1). The permethylated derivative 48 was obtained by methylation with MeI in dry acetone and K2CO3. The acid 47 and the alcohol 49 were prepared accordingly. With these simple chemical modifications, we obtained a set of compounds (derivatives of imperanenes, vitrofolals and noralashinols) with some structural alterations. In this strategy we chose to introduce both flexibility, by reducing the double bond, and rigidity by introducing the aliphatic ring, at the basic skeleton of imperanene. The nonflexible cyclic structures with a half-boat structure 38–40 adopted a certain non-planar conformation, and were therefore aromatized to yield a planar structure (compounds 41–43) resembling bioactive 9-norlignans of the vitrofolal and noralashinol family. Finally, we chose to prepare the carboxylic acid with an ionizable group, the ester containing the carbonyl function and the alcohol, for comparison of the effects of the functionality at C-9’. Some of the derivatives were also submitted to methylation of the free phenolic groups for comparison of the 3-methoxy-4-hydroxyphenyl and 3,4-dimethoxyphenyl moieties. The liverwort norlignan 13 was obtained from 42 by treatment with AlCl3 in pyridine.
Compounds 15, 44, 48, 37, 38, 40 and 41 have previously been evaluated as inhibitors of multidrug resistance protein 1 (MRP1)-mediated transport using human erythrocytes as model. This was part of a larger study on polyphenolic compounds comprising lignans, norlignans, stilbenes and flavonoids. Compound 15 was shown to be the most active one, with moderate activity at IC50 = 50 µM. The structure-activity relationship study showed that the carbonyl function at C-9’ is crucial for the activity. Furthermore, none of the norlignans showed detrimental effects up to 200 µM, indicating a large therapeutic width. These compounds could therefore be interesting as possible agents reversing multidrug resistance and to potentially sensitize cancer cells to anticancer drugs [44]. A similar set of compounds (15, 38, 40, 41, 44 and 48) were tested for estrogen and antiestrogen activity in the yeast estrogen assay. Compounds 40, 41 and 48 showed antiestrogenic effects, albeit at quite high concentrations (100–500 µM) [45]. Some of these norlignans were also studied for antimicrobial effects in comparison with the effective stilbene trans-pinosylvin (trans-3,5-dihydroxystilbene) and its monomethylated derivative (trans-3-hydroxy-5-methoxystilbene). Although some effects were observed, it was concluded that the norlignans were ineffective against Salmonella infantis, Listeria monocytogenes and Candida tropicalis [46].
3. Discussion and Conclusions
Norlignans of the C-9 type are an uncommon group of polyphenols in nature. Many of these compounds can be found in plants used in traditional Asian medicine, however published scientific data of their bioactivities is in general rather scarce. In most cases, their biological effects have been tested in a few assays mainly for antioxidant, cytotoxic and antimicrobial activities. Clearly, a more broad and systematic screening of bioactivity should be conducted to evaluate these compounds as potential bioactive agents. 9-Norlignans belonging to the vitrofolal-, noralashinol-, and imperanene families have however been shown to be bioactive and some of them could find potential in treatment of cancer. Furthermore, these 9-norlignans have been shown to inhibit specific enzymes, which warrants further investigations of these compounds. In our semisynthetic approach using hydroxymatairesinol as starting material for the preparation of 9-norlignans, we have developed simple chemical transformations to unsaturated, saturated, cyclic and aromatic structures closely resembling those of the vitrofolal, noralashinol and imperanene family. The unsaturated compounds 15, 17 and 37 could also be seen as polyphenolic compounds with both trans-stilbene-like, and lignan-like structures, which could make them interesting as antimicrobial compounds. The cyclic structures were obtained in high yields by treatment with formic acid or trifluoroacetic acid. However, this Friedel–Crafts-type reaction can also be performed in aqueous conditions, using mineral acids. The aromatization was introduced by reaction with DDQ with moderate yields. DDQ was superior for this reaction due to its benzylic hydride abstraction properties, yielding dehydrogenation of the product, rather than oxidative polymerization. Hydrogenation of the double bond was facile, proceeding in high yields. To broaden the substrate scope of the semisynthetic 9-norlignans, the initial carboxylic acid (of compound 15) was esterified and reduced by conventional methods, in relatively high yields. By these interconversions the carboxylic acid, the methyl ester and the alcohol derivatives were obtained for all the basic structures. Some structures were also methylated at the phenolic positions, which gave us 16 norlignans with different structures available for screening of biological activity. This semisynthetic route is not restricted to these compounds and it offers numerous additional chemical modifications and functional group interconversions. The biological testing of some selected semisynthetic 9-norlignans showed that none of these compounds were highly active. However, compound 15 showed moderate activity as an inhibitor of MRP1-mediated transport. The activity was decreased for all other derivatives, which indicated that the double bond and the carboxylic acid function were important for the activity. Due to the limited scope of biotesting for these semisynthetic 9-norlignans, no generalized results of their bioactivity or results on structure-activity relationships can be proposed, without further screening of biological activity.
4. Materials and Methods
All commercially available chemicals were used as supplied by the manufacturers. Hydroxymatairesinol was isolated from Norway spruce (Picea abies (L.) Karst) knots by previously described methods [5]. Knots of Norway spruce were separated, ground and freeze-dried prior to extraction in a Soxhlet apparatus. The raw extract obtained with acetone-water (9:1 v/v), after the removal of lipophilic extractives with hexane was purified by flash chromatography (eluent CH2Cl2:EtOH 98:2 v/v) to yield hydroxymatairesinol.
GC analyses were performed on a standard gas chromatograph (Agilent Technologies, model 6850, Santa Clara, CA, USA) equipped with a HP-5 column and a flame ionization (FI) detector. The samples were silylated using hexamethyldisilazane-chlorotrimethylsilane in pyridine, prior to analyses. Gas chromatography-mass spectrometry (GCMS) analyses were performed essentially the same way (Agilent Technologies, model 7890A+5975C). High-resolution mass spectrometry (HRMS) were recorded using a Micro Q-TOF (Bruker, Billerica, MA, USA) with electrospray ionization (ESI) operated in positive mode or with a ZAB-Spec high-resolution MS-ESI instrument (Fisons Instruments, Ipswich, UK).
1H- and 13C-NMR spectra were recorded on an Avance instrument (Bruker) at 600.13 and 150.90 MHz, respectively. 2D NMR experiments (1H-1H COSY, HSQC, HMBC) were recorded using standard pulse sequences and chemical shifts are reported downfield from tetramethylsilane. Optical rotations were measured with a digital polarimeter model 241 (Perkin Elmer, Waltham, MA, USA) using a 1 dm, 1 mL cell.
The inhibition of multidrug resistance protein 1-mediated transport was studied by measurement of 2′,7′-bis-(carboxypropyl)-5(6)-carboxyfluorescein (BCPCF) efflux in human erythrocytes. Erythrocytes were loaded with 2′,7′-bis-(carboxypropyl)-5-(6)-carboxyfluorescein acetoxymethyl ester (BCPCF-AM) and incubated with or without the norlignans (10–200 mM). The extracellular BCPCF fluorescence intensity was then measured and the IC50 values for BCPCF efflux were determined from dose-response curves. The detailed experimental procedure and results have previously been published [44]. The estrogen and antiestrogen activity was determined using the yeast estrogen assay consisting of a transformed yeast strain (Saccharomyces cerevisiae). Cells were pre-cultured and diluted in a medium containing chlorophenol red-β-d-galactopyranoside. The suspensions with compounds were incubated in 96-well plates for three days before the absorbance at 540 nm was measured. Estrogenic or anti-estrogenic activity was calculated by subtraction of absorbance at 620 nm from that of 540 nm. Dose curves were plotted and the IC50 values were calculated. 17β-Estradiol was used as reference. A more detailed description has been published by Aehle et al. [45].
The antimicrobial activity tests were performed with Listeria monocytogenes L211, Salmonella infantis EELA72 and Candida tropicalis 4068 using an automated incubator and turbidity reader to monitor the microbial growth. The compounds were studied at equimolar concentrations (0.1–1 mM). The growth was calculated by subtraction of the turbidity of the test culture from that of the control and expressed as growth inhibition percentages. The detailed experimental procedure and results have previously been published [46].
Acknowledgments
Annika Smeds is acknowledged for the HRMS analyses and Jan-Erik Lönnqvist for help with the syntheses.
Supplementary Materials
The following are available online. Full experimental procedures for compounds 36–50, including analytical data.
Author Contributions
Conceptualization: P.E., methodology: P.E. and J.-E.R., formal analysis: P.E. and J.-E.R., investigation: P.E. and J.-E.R., resources: P.E., writing—original draft preparation: P.E., writing—review and editing: P.E. and J.-E.R., visualization: P.E., supervision: P.E., project administration: P.E., funding acquisition: P.E.
Funding
This research was part of the BIOSTIMUL (Bioactive and wood-associated stilbenes as multifunctional antimicrobial and health promoting agents) project. Financial support from the Finnish Funding Agency for Technology (Tekes), from the Magnus Ehrnrooth foundation and from the Liv och Hälsa foundation is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Matsunaga K., Shibya M., Ohizumi Y. Imperanene, a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica. J. Nat. Prod. 1995;58:138–139. doi: 10.1021/np50115a022. [DOI] [PubMed] [Google Scholar]
- 2.Kawazoe K., Yutani A., Tamemoto K., Yuasa S., Shibata H., Higuti T., Takaishi Y. Phenylnaphthalene Compounds from the Subterranean Part of Vitex rotundifolia and Their Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus. J. Nat. Prod. 2001;64:588–591. doi: 10.1021/np000307b. [DOI] [PubMed] [Google Scholar]
- 3.Su G., Bai R., Yu X., Cao Y., Yin X., Tu P., Chai X. Noralashinol A, a new norlignan from stem barks of Syringa pinnatifolia. Nat. Prod. Res. 2016;19:2149–2153. doi: 10.1080/14786419.2016.1146886. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R.F., Feng X., Su G.Z., Yin X., Yang X.Y., Zhao Y.F., Li W.F., Tu P.F., Chai X.Y. Noralashinol B, a norlignan with cytotoxicity from stem barks of Syringa pinnatifolia. J. Asian Nat. Prod. Res. 2017;19:416–422. doi: 10.1080/10286020.2017.1307188. [DOI] [PubMed] [Google Scholar]
- 5.Eklund P., Riska A., Sjöholm R. Synthesis of R-(−)-Imperanene from the Natural Lignan Hydroxymatairesinol. J. Org. Chem. 2002;67:7544–7546. doi: 10.1021/jo025985c. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi S., Tanimura R., Nishiwaki H., Nishi K., Sugahara T., Maruyama M., Ano Y., Akiyama K., Kishida T. Enantioselective syntheses of both enantiomers of 9′-dehydroxyimperanene and 7,8-dihydro-9′-dehydroxyimperanene and the comparison of biological activity between 9-norlignans and dihydroguaiaretic acids. Bioorg. Med. Chem. Lett. 2016;26:3019–3023. doi: 10.1016/j.bmcl.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Moss G.P. Nomenclature of Lignans and Neolignans (IUPAC Recommendations 2000) Pure Appl. Chem. 2000;72:1493–1523. doi: 10.1351/pac200072081493. [DOI] [Google Scholar]
- 8.Erdtman H., Harmata J. Phenolic and Terpenoid Heartwood Constituents of Libocedrus Yateensis. Phytochemistry. 1979;18:1495–1500. doi: 10.1016/S0031-9422(00)98482-6. [DOI] [Google Scholar]
- 9.Ngadjui B.T., Lontsi D., Ayafor J.F., Sondengam B.L. Pachypophyllin and pachypostaudins A and B: Three bisnorlignans from Pachypodantum staudtii. Phytochemistry. 1989;28:231–234. doi: 10.1016/0031-9422(89)85044-7. [DOI] [Google Scholar]
- 10.Wang B.-G., Ebel R., Wang C.-Y., Wray V., Proksch P. New methoxylated aryltetrahydronaphthalene lignans and a norlignan from Aglaia cordata. Tetrahedron Lett. 2002;43:5783–5787. doi: 10.1016/S0040-4039(02)01180-2. [DOI] [Google Scholar]
- 11.Kawazoe K., Yutani A., Takaishi Y. Aryl naphthalenes norlignans from Vitex rotundifolia. Phytochemistry. 1999;52:1657–1659. doi: 10.1016/S0031-9422(99)00405-7. [DOI] [Google Scholar]
- 12.Sun K., Li X., Li W., Liu J.-M., Wang J.-H., Yi S. A new nor-lignan from the seeds of Descurainia sophia. Nat. Prod. Res. 2006;20:519–522. doi: 10.1080/14786410500045309. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed K.M., Fouad M.A., Matsunami K., Kamel M.S., Otsuka H. A new norlignan glycoside from Cestrum diurnum L. Arkivoc. 2007;4:63–70. doi: 10.3998/ark.5550190.0008.d09. [DOI] [Google Scholar]
- 14.Susplugas S., Van Hung N., Bignon J., Thoison O., Kruczynski A., Sévenet T., Guéritte F. Cytotoxic Arylnaphthalene Lignans from a Vietnamese Acanthaceae, Justicia patentiflora. J. Nat. Prod. 2005;68:735–738. doi: 10.1021/np050028u. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y.-L., Chen C.-C., Hsu F.-L., Chen C.-F. Tannins, Flavonol Sulfonates, and a Norlignan from Phyllantus virgatus. J. Nat. Prod. 1998;61:1194–1197. doi: 10.1021/np970336v. [DOI] [PubMed] [Google Scholar]
- 16.Rishmann M., Mues R., Geiger H., Laas H.J., Eicher T. Isolation and synthesis of 6,7-dihydroxy-4-(3,4-dihydroxyphenyl)naphthalene-2-carboxylic acid from Pellia epiphylla. Phytochemistry. 1989;28:867–869. doi: 10.1016/0031-9422(89)80132-3. [DOI] [Google Scholar]
- 17.D’Abrosca B., Dellagreca M., Fiorentino A., Golino A., Minaco P., Zarrelli A. Isolation and characterization of new lignans from the leaves of Cestrum parqui. Nat. Prod. Res. 2006;20:293–298. doi: 10.1080/14786410500111234. [DOI] [PubMed] [Google Scholar]
- 18.Ekman R., Sjöholm R.T., Sjöholm R. A Degraded Lignan from Alkaline Hydrolysis of Norway Spruce Root Extractives. Finn. Chem. Lett. 1979;4:126–128. [Google Scholar]
- 19.Takara K., Iwasaki H., Ujihara K., Wada K. Human Tyrosinase Inhibitor in Rum Distillate Wastewater. J. Oleo Sci. 2008;57:191–196. doi: 10.5650/jos.57.191. [DOI] [PubMed] [Google Scholar]
- 20.Lacret R., Varela R.M., Molinillo J.M.G., Nogueiras C., Macías F.A. Tectonoelins, new norlignans from a bioactive extract of Tectona grandis. Phytochem. Lett. 2012;5:382–385. doi: 10.1016/j.phytol.2012.03.008. [DOI] [Google Scholar]
- 21.Li Y.-Z., Huang J., Gong Z., Tian Z.-Q. A Novel Norlignan and a Novel Phenylpropanoid from Peperomia tetraphylla. Helv. Chim. Acta. 2007;90:2222–2226. doi: 10.1002/hlca.200790230. [DOI] [Google Scholar]
- 22.Li Y.-Z., Tong A.P., Huang J. Two New Norlignans and a New Lignanamide from Peperomia tetraphylla. Chem. Biodivers. 2012;9:769–776. doi: 10.1002/cbdv.201100138. [DOI] [PubMed] [Google Scholar]
- 23.Wang W., Zeng Y.H., Osman K., Shinde K., Rahman M., Gibbons S., Mu Q. Norlignans, Acylphloroglucinols, and a Dimeric Xanthone from Hypericum chinense. J. Nat. Prod. 2010;73:1815–1820. doi: 10.1021/np1004483. [DOI] [PubMed] [Google Scholar]
- 24.Zhong C.-Q., Tao S.-H., Yi Z.-B., Guo L.-B., Xie Y.-F., Chen Y.-F. Four New Compounds from Pouzolzia zeylanica (L.) Benn. Var. Microphylla. Heterocycles. 2015;91:1926–1936. doi: 10.3987/COM-15-13300. [DOI] [Google Scholar]
- 25.Zheng Y., Xie Y.-G., Zhang Y., Li T., Li H.-L., Yan S.-K., Jin H.-Z., Zhang W.-D. New norlignans and flavonoids of Dysosma versipellis. Phytochem. Lett. 2016;16:75–81. doi: 10.1016/j.phytol.2016.03.001. [DOI] [Google Scholar]
- 26.Takahashi K., Ogiyama K. Phenols of discolored sugi (Cryptomeria japonica D. Don) sapwood II. Norlignans of discolored sugi sapwood collected in the Kyushu region. Mokuzai Gakkaishi. 1985;31:28–38. [Google Scholar]
- 27.Noguchi T., Ohtani Y., Sameshima K. Static defense components for sugi butt-rot disease. Trans. Mater. Res. Soc. Jpn. 2004;29:2479–2482. [Google Scholar]
- 28.Chen T.-H., Liau B.-C., Wang S.-Y., Jong T.-T. Isolation and cytotoxicity of the lignanoids from Chamaecyparis formosensis. Planta Med. 2008;74:1806–1811. doi: 10.1055/s-0028-1088325. [DOI] [PubMed] [Google Scholar]
- 29.Mathouet H., Elomri A., Lameiras P., Daich A., Vérité P. An alkaloid, two conjugate sesquiterpenes and a phenylpropanoid from Pachypodanthium confine Engl. and Diels. Phytochemistry. 2007;68:1813–1818. doi: 10.1016/j.phytochem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Lou Z.-H., Li H.-M., Gao L.-H., Li R.-T. Antioxidant lignans from the seeds of Vitex negundo var. cannabifolia. J. Asian Nat. Prod. Res. 2014;16:963–969. doi: 10.1080/10286020.2014.929574. [DOI] [PubMed] [Google Scholar]
- 31.Haq A.-U., Malik A., Anis I., Khan S.B., Ahmed E., Ahmed Z., Nawaz S.A., Choudhary M.I. Enzymes Inhibiting Lignans from Vitex negundo. Chem. Pharm. Bull. 2004;52:1269–1272. doi: 10.1248/cpb.52.1269. [DOI] [PubMed] [Google Scholar]
- 32.Haq A.-U., Malik A., Khan M.T.H., Haq A.-U., Khan S.B., Ahmad A., Choudhary M.I. Tyrosinase inhibitory lignans from the methanol extract of the roots of Vitex negundo Linn. And their structure-activity relationship. Phytomedicine. 2006;13:255–260. doi: 10.1016/j.phymed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki T., Kawabata T., Masuoka C., Kinjo J., Ikeda T., Nohara T., Ono M. Two new lignan glucosides from the fruit of Vitex cannabifolia. J. Nat. Med. 2008;62:47–51. doi: 10.1007/s11418-007-0184-1. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q.-H., Huo S.R.N., Bao Y.P., Ao W.L.J. Chemical constituents of Syringa pinnatifolia and its chemotaxonomic study. Chem. Nat. Compd. 2018;54:435–438. doi: 10.1007/s10600-018-2373-4. [DOI] [Google Scholar]
- 35.Ono M., Nishida Y., Masuoka C., Li J.-C., Okawa M., Ikeda T., Nohara T. Lignan Derivatives and a Norditerpene from the seeds of Vitex negundo. J. Nat. Prod. 2004;67:2073–2075. doi: 10.1021/np040102t. [DOI] [PubMed] [Google Scholar]
- 36.Lodhi M.A., Haq A.U., Choudhary M.I., Malik A., Ahmad S. Chymotrypsin inhibition studies on the lignans from Vitex negundo Linn. J. Enzyme Inhib. Med. Chem. 2008;23:400–405. doi: 10.1080/14756360701584653. [DOI] [PubMed] [Google Scholar]
- 37.Neufeld K., Neufeld N. Animal Feed Additive Containing Diurnoside and/or Cestrumoside. WO 2017129732 A1 20170803. PCT Int. Appl. 2017 Aug 3;
- 38.Cabanillas B.J., Le Lamer A.-C., Castillo D., Arevalo J., Rojas R., Odonne G., Bourdy G., Moukarzel B., Sauvain M., Fabre N. Caffeic Acid Esters and Lignans from Piper sanguineispicum. J. Nat. Prod. 2010;73:1884–1890. doi: 10.1021/np1005357. [DOI] [PubMed] [Google Scholar]
- 39.Huang R.L., Huang Y.L., Ou J.C., Chen C.C., Hsu F.L., Chang C. Screening of 25 Compounds isolated from Phyllantus Species for Anti-Human Hepatitis B Virus In Vitro. Phytother. Res. 2003;17:449–453. doi: 10.1002/ptr.1167. [DOI] [PubMed] [Google Scholar]
- 40.Tazaki H., Adam K.-P., Becker H. Five lignan derivatives from in vitro cultures of liverwort Jamesoniella autumnalis. Phytochemistry. 1995;40:1671–1675. doi: 10.1016/0031-9422(95)90024-P. [DOI] [Google Scholar]
- 41.Martini U., Zapp J., Becker H. Lignans from the liverwort Bazzania Trilobata. Phytochemistry. 1998;49:1139–1146. doi: 10.1016/S0031-9422(97)01076-5. [DOI] [Google Scholar]
- 42.Sanderson S. Ph.D. Thesis. Universität des Saarlandes; Saarbrucken, Germany: 1995. Phytochemische Untersuchungen der Ledermoose Jamesoniella rubricaulis (Nees) Grolle und Lepidozia reptans (L.) Dum. [Google Scholar]
- 43.Cullman F., Schmidt A., Schuld F., Trennheuser M.L., Becker H. Lignans from the liverworts Lepidozia incurvata, Chiloscyphus polyanthos and Jungermannia exsertifolia ssp. cordifolia. Phytochemistry. 1999;52:1647–1650. doi: 10.1016/S0031-9422(99)00279-4. [DOI] [Google Scholar]
- 44.Wróbel A., Eklund P., Bobrowska-Hägerstrand M., Hägerstrand H. Lignans and norlignans Inhibit Multidrug Resistance Protein 1(MRP1/ABCC1)-mediated Transport. Anticancer Res. 2010;30:4423–4428. [PubMed] [Google Scholar]
- 45.Aehle E., Müller U., Eklund P.C., Willför S.M., Sippl W., Dräger B. Lignans as food constituents with estrogen and antiestrogen activity. Phytochemistry. 2011;72:2396–2405. doi: 10.1016/j.phytochem.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Plumed-Ferrer C., Väkeväinen K., Komulainen H., Rautiainen M., Smeds A., Raitanen J.-E., Eklund P., Willför S., Alakomi H.-L., Saarela M., et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013;164:99–107. doi: 10.1016/j.ijfoodmicro.2013.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.