Abstract
Human Dental Pulp Stem Cells (hDPSCs) represent a type of adult mesenchymal stem cells that have the ability to differentiate in vitro in several lineages such as odontoblasts, osteoblasts, chondrocytes, adipocytes and neurons. In the current work, we used hDPSCs as the experimental model to study the role of recombinant prion protein 23–231 (recPrPC) in the neuronal differentiation process, and in the signal pathway activation of ERK 1/2 and Akt. We demonstrated that recPrPC was able to activate an intracellular signal pathway mediated by extracellular-signal-regulated kinase 1 and 2 (ERK 1/2) and protein kinase B (Akt). Moreover, in order to understand whether endogenous prion protein (PrPC) was necessary to mediate the signaling induced by recPrPC, we silenced PrPC, demonstrating that the presence of endogenous PrPC was essential for ERK 1/2 and Akt phosphorylation. Since endogenous PrPC is a well-known lipid rafts component, we evaluated the role of these structures in the signal pathway induced by recPrPC. Our results suggest that lipid rafts integrity play a key role in recPrPC activity. In fact, lipid rafts inhibitors, such as fumonisin B1 and MβCD, significantly prevented ERK 1/2 and Akt phosphorylation induced by recPrPC. In addition, we investigated the capacity of recPrPC to induce hDPSCs neuronal differentiation process after long-term stimulation through the evaluation of typical neuronal markers expression such as B3-Tubulin, neurofilament-H (NFH) and growth associated protein 43 (GAP43). Accordingly, when we silenced endogenous PrPC, we observed the inhibition of neuronal differentiation induced by recPrPC. The combined data suggest that recPrPC plays a key role in the neuronal differentiation process and in the activation of specific intracellular signal pathways in hDPSCs.
Keywords: cellular prion protein, shed prion protein, recombinant prion protein, prions, lipid rafts, dental pulp-derived stem cells, mesenchymal stem cells, neural stem cells, adult neurogenesis, neuronal differentiation
1. Introduction
The cellular form of prion protein (PrPC) is a cell surface glycosylphosphatidyl-inositol (GPI)-anchored glycoprotein that was first identified as a molecule that is able to bind Cu2+ in vitro [1]. PrPC is highly conserved in mammalians and it is present in all nucleated cells, although it is mainly expressed in the central and peripheral nervous system [2].
PrPC is located within lipid rafts [3,4], sphingolipid-rich membrane micro domains, and it is present in several kind of cells such as neural and lymphocytic cells [5,6,7]. The membrane-bound isoform can act as a cell surface receptor, or as a co-receptor, recruiting downstream signal transduction pathways [8,9,10,11]. PrPC is involved in a wide range of cellular processes, such as synaptic plasticity, neurite regulation, calcium homeostasis, copper metabolism, apoptosis and cellular resistance to oxidative stress [12,13,14,15,16]. Recent evidence suggests that PrPC plays a possible in the neuronal differentiation processes of stem cells [17,18,19,20,21].
In a previous work, we used human dental pulp stem cell (hDPSCs) as the experimental model to develop an in vitro system used to study proliferation and differentiation process [22]. hDPSCs represent a kind of adult mesenchymal stem cells that show the ability to differentiate in vitro in several multilineages such as odontoblasts, osteoblasts, chondrocytes, adipocytes and neurons [23,24,25,26,27,28,29,30]. The fact that hDPSCs can express basally PrPC [21] and show the ability to differentiate into neuronal-like cells [31] or dopaminergic neuron-like cells [32], prompt it to be considered as a cellular model for the study of neurodegenerative diseases, such as Alzheimer, Parkinson and Huntington diseases [22,33,34].
Several authors showed that PrPC may be subjected to post translational proteolytic processing, including shedding α-β-γ cleavage [35,36,37,38]. These cleavage events have been shown to regulate its physiological functions, produce biologically active fragments such as N1, N2, N3, shed PrP and potentially influence the course of prion diseases [39]. In particular, it has been shown that the shedding of PrPC near the glycosylphosphatidylinositol (GPI) anchor releases almost full-length prion protein from the cell [40] and this phenomenon could be used to regulate the levels of PrPC on the plasma membrane in response to different stimuli [37,39]. Shed PrPC was first identified in the preparation of prion-infected hamster brains where 15% of total PrPSc was found to end with Gly228 [41]. An identical cleavage site, between Gly228 and Arg229, and the responsible protease were later also found [42] and confirmed [38] for murine PrPC using recombinant human ADAM10 [43]. Indeed, shed PrPC has been found in the supernatant of cultured cells [35,40], as well as in human cerebrospinal fluid [44], suggesting a potential physiological role. This phenomenon is not restricted to the nervous system, since lymphoid cells and platelets have also been shown to shed PrPC [6,37,45].
It has been shown that, in cell culture, a small fraction of total PrPC is slowly but constitutively shed into the media and that shed PrPC lacks any parts of the GPI-modification [40]. The cleavage products of PrPC could act as soluble trophic factors in autocrine, paracrine or endocrine ways in different human cell types. Furthermore, as reported by Altmeppen et al. [39], the N1 fragment and shed PrPC could potentially bind β-sheet-rich oligomers, thereby blocking their deleterious effects and directing them to phagocytosis and degradation. In this scenario, increased surface expression and shedding of PrPC might be a mechanism that is able to block out the effects of toxic oligomers. To mimic the effect of shed PrPC, we used recPrPC (23–231) as a molecular model; indeed, as reported by several authors, the C-terminal cleavage close to the membrane, releases nearly full length PrPC from the cell surface [35,40,41,43,44,46].
To date, the information regarding the role of shed PrPC on different cell types is still poor. In this context, our goal is to evaluate the possible effect of recPrPC in signal pathways and in differentiation process using hDPSCs as cellular model system.
Moreover, we will evaluate the possible involvement of endogenous PrPC in recPrPC signal pathways and neuronal differentiation process using siRNA PrP to ablate the expression of prion protein. Since membrane-bound PrPC is a well know raft component, we will also evaluate the possible role of lipid rafts in the signal pathway induced by recPrPC using molecules affecting lipid rafts integrity.
2. Results
2.1. Role of recPrPC in Signal Pathways of hDPSCs
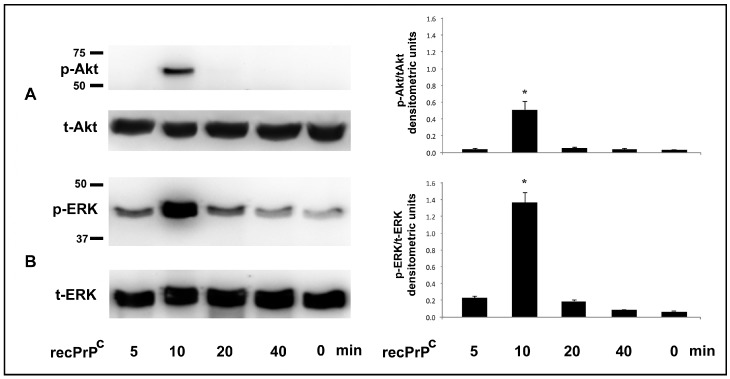
In order to evaluate a possible role of human recPrPC in the activation of intracellular signal pathway, hDPSCs were treated with recPrPC for different times: 5, 10, 20, 40 min at 37 °C. Western blot analysis for pERK1-2 and pAkt expression showed the presence of these active forms upon treatment with recPrPC at 10 min (Figure 1A,B). The upregulation of phosphorylated forms, was also confirmed by densitometric analysis (Figure 1, right panels, bar graphs).
Figure 1.
Effect of recPrPC on Akt and ERK Phosphorylation. hDPSCs, untreated or treated with 0.5 µg/mL of recPrPC for 5, 10, 20, 40 min, were analyzed by Western blot using anti-pAkt, anti-total Akt (A). anti-pERK1/2 and anti-total ERK1/2 (B). Densitometric analysis is shown in the right panel. Results represent the mean ± SD from 3 independent experiments, * p recPrPC treated cells <0.01 vs. untreated cells.
2.2. Role of Endogenous PrPC in the Modulation of Cell Signaling Induced by recPrPC
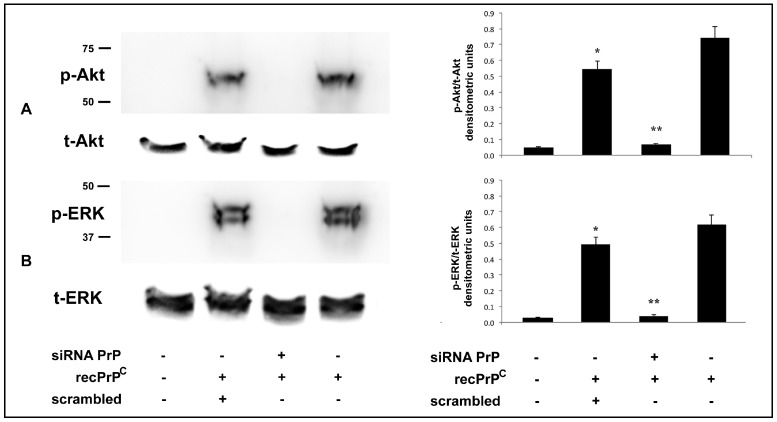
It is well known that the C-terminal cleavage close to the membrane releases nearly full length PrPC from the cell surface. In order to understand whether the signal pathway induced by recPrPC requires full length endogenous PrPC, we used a verified siRNA PrP. With this aim, hDPSCs were pretreated with siRNA PrP for 72 h and, subsequently, were stimulated with recPrPC for 10 min at 37 °C. Western blot analysis demonstrated that pretreatment with siRNA PrP prevented the activation of Akt and ERK 1-2 induced by recPrPC (Figure 2A,B). These results were also confirmed by densitometric analysis (Figure 2, right panels, bar graphs).
Figure 2.
Effect of PrPC silencing on Akt and ERK phosphorylation induced by recPrPC. hDPSCs, untreated or treated with 0.5 µg/mL of recPrPC for 10 min in presence or in absence of pre-treatment with siRNA PrP or scrambled siRNA for 72 h, were analyzed by Western blot using anti-pAkt, anti-total Akt (A). anti-pERK1/2 and anti-total ERK1/2 (B). Densitometric analysis is shown in the right panel. Results represent the mean ± SD from 3 independent experiments, * p recPrPC treated cells <0.01 vs. untreated cells, ** p siRNA PrP + recPrPC treated cells vs. scrambled + recPrPC treated cells. As control, scrambled siRNA was employed in each experiment.
These data indicate that endogenous PrPC is essential for the signal pathway induced by recPrPC.
2.3. Role of Lipid Rafts in the Modulation of Cell Signaling Induced by recPrPC
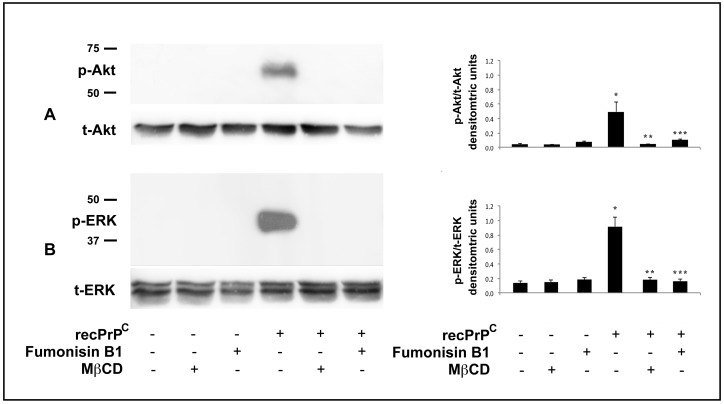
Endogenous PrPC is a well-known raft component, thus we evaluated the role of lipid rafts in the signal pathway induced by recPrPC. To analyze the functional role of lipid rafts in recPrPC signal pathways, cells were preincubated with lipid rafts affecting agents, Fumonisin B1 or methyl-β-cyclodextrin (MβCD) and then stimulated with recPrPC for 10 min at 37 °C. Western blot analysis clearly showed that cell pretreatment with either Fumonisin B1 or methyl-β-cyclodextrin, significantly prevents Akt and ERK 1-2 (Figure 3A,B) phosphorylation induced by recPrPC, indicating that lipid rafts integrity is essential for recPrPC-induced signal pathways of hDPSCs. These results were also confirmed by densitometric analysis (Figure 3, right panels, bar graphs).
Figure 3.
Effect of lipid rafts perturbation on Akt and ERK Phosphorylation induced by recPrPC. hDPSCs, untreated or treated with 0.5 µg/mL of recPrPC for 10 min in the presence or in the absence of Fumonisin B1 or MβCD, were analyzed by Western blot using anti-pAkt and anti-total Akt (A). anti-pERK1/2 and anti-total ERK1/2 (B). Densitometric analysis is shown in the right panel. Results represent the mean ± SD from 3 independent experiments, * p recPrPC treated cells <0.01 vs. untreated cells, ** p recPrPC treated cells + MβCD <0.01 vs. recPrPC treated cells, *** p recPrPC treated cells + Fumonisin B1 <0.01 vs. recPrPC treated cells.
2.4. Role of recPrPC in the Neuronal Differentiation of hDPSCs
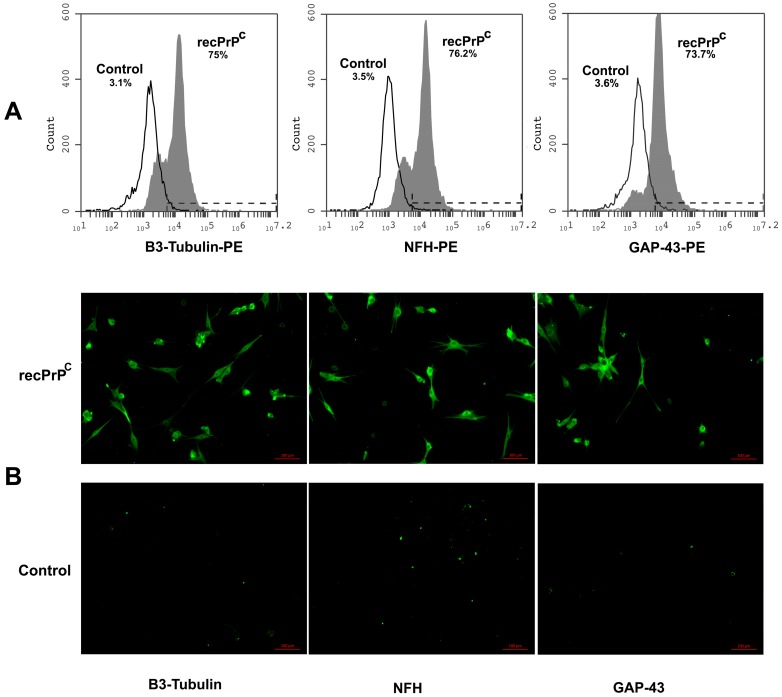
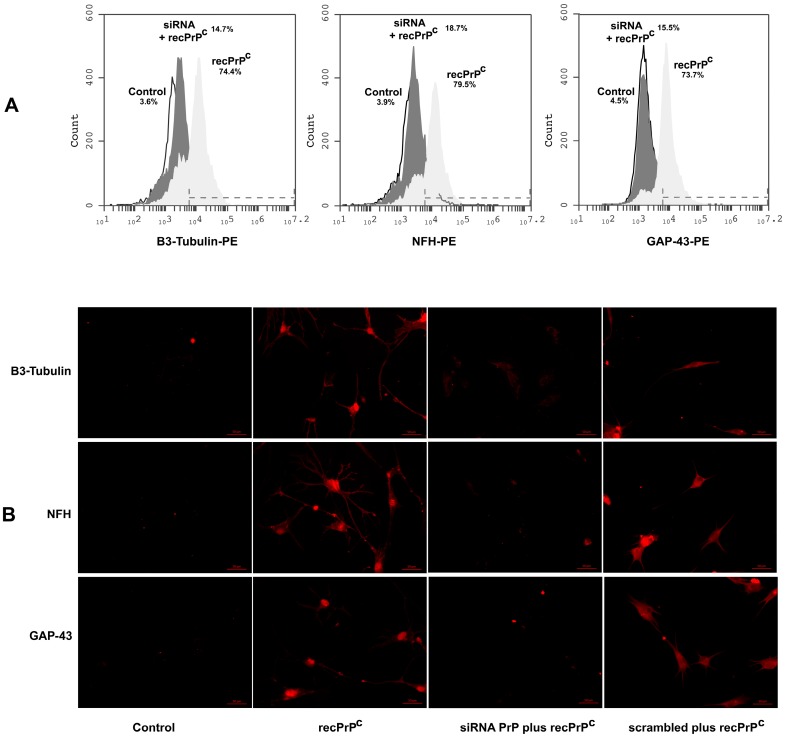
We further analyzed the possible role of recPrPC in the neuronal differentiation of hDPSCs. With this aim, we performed flow cytometry and immunofluorescence analysis of hDPSCs with recPrPC to verify the expression of typical neuronal markers such as B3-Tubulin, NFH and GAP 43. Flow cytometry analysis showed positive values for all the neuronal antigens under testing (>73%) (Figure 4A). Immunofluorescence analysis confirmed the expression of these typical neuronal markers in hDPSCs stimulated with recPrPC for 14 days, but not in control cells (Figure 4B).
Figure 4.
Effect of recPrPC on expression of neuronal markers GAP-43, NFH and B3-Tubulin. hDPSCs, untreated or treated with recPrPC for 14 days, were analyzed by flow cytometry and immunofluorescence analysis using anti-NFH, Anti-B3-Tubulin and anti-GAP-43. (A) Flow Cytometry. Histograms represent log fluorescence vs. cell number, gated on cell population of a side scatter/forward scatter (SS/FS) histogram. Cell number is indicated on the y-axis and fluorescence intensity is represented on the x-axis. Each panel was compared with the corresponding secondary antibody as negative control. A representative experiment among 3 is shown. (B) Immunofluorescence analysis. Alternatively, hDPSCs were analyzed by immunofluorescence analysis using anti-B3-Tubulin, anti-NFH and anti-GAP-43 and observed with a Zeiss Axio Vert. A1 fluorescence microscope (Zeiss, Oberkochen, Germany). Scale bars, 100 µm.
These findings demonstrate that recPrPC is able to induce neuronal differentiation of hDPSCs.
2.5. Role of Endogenous PrPC in the Neuronal Differentiation Process Induced by recPrPC
To analyze whether the effect of recPrPC on neuronal differentiation of hDPSCs requires full length endogenous PrPC, hDPSCs were stimulated with recPrPC in the presence or in the absence of siRNA PrP pre-treatment.
Our results revealed that the expression of the neuronal markers B3-Tubulin, NFH and GAP 43 of hDPSCs induced by recPrPC was significantly inhibited (p < 0.001) by previous silencing of PrP, as revealed by flow cytometry (Figure 5A). These findings were also confirmed by immunofluorescence analysis under the same conditions (Figure 5B).
Figure 5.
Effect of PrPC silencing on expression of neuronal markers B3-Tubulin, NFH and GAP-43. hDPSCs, stimulated with 0.5 µg/mL of recPrPC for 14 days in the presence or in the absence of pre-treatment with siRNA PrP or scrambled siRNA for 72 h, were analyzed by flow cytometry or immunofluorescence analysis using anti-B3-Tubulin, anti-NFH and anti-GAP-43. (A) Flow Cytometry. Histograms represent log fluorescence vs. cell number, gated on cell population of a side scatter/forward scatter (SS/FS) histogram. Cell number is indicated on the y-axis, and fluorescence intensity is represented on the x-axis. Each panel was compared with the corresponding secondary antibody as a negative control. A representative experiment among 3 is shown. (B) Immunofluorescence analysis. Alternatively, hDPSCs were analyzed by immunofluorescence analysis using anti-B3-Tubulin, anti-NFH and anti-GAP-43 and, then, observed with a Zeiss Axio Vert. A1 fluorescence microscope. Scale bars, 50 µm.
This data indicates that the endogenous PrPC is essential for neuronal differentiation induced by recPrPC.
3. Discussion
In this study we have investigated the cellular and molecular mechanisms mediated by recPrPC involved in the neuronal differentiation process of mesenchymal stem cells. Our results confirm and extend previous data which suggested that PrPC is involved in the neuronal differentiation process of adult stem cells [21].
Shed PrPC and the cleavage products N1, N2, N3 are biologically active fragments that may potentially influence the course of prion diseases, or participate with other biological processes. Indeed, proteolytic cleavage events may alter either biological functions of PrPC or produce protein fragments harboring specific intrinsic properties, thus contributing to a higher biological complexity [30]. It is well-known that the cleavage of certain key proteins is a highly relevant mechanism with regard to neurodegenerative diseases [43]. In particular, shed PrPC has been found in supernatants of cultured cells [35,40], as well as in human cerebrospinal fluid [44], which is indicative of its physiological role. There are many observations which highlight that this process is not restricted to the nervous system, since lymphoid cells and platelets have also been shown to shed PrPC [6,37,45]. Moreover, in cell culture, a small fraction of total PrPC is slowly but constitutively shed into the media, and shed PrPC lacks any parts of the GPI-modification [40]. Within this context, it may be interesting to understand the role of shed PrPC and its fragments in physiological or pathological conditions.
Our goal was to understand whether a molecule able to mimic shed PrPC, i.e., recPrPC (23–231 a.a.), was able to activate a signal pathway in our stem cells experimental model, and whether it may influence neuronal differentiation process. hDPSCs represent a type of adult mesenchymal stem cells that have the ability to differentiate in vitro in several multilineages such as odontoblasts, osteoblasts, chondrocytes, adipocytes and neurons [23,24,25,26,27,28,29,30].
Our results demonstrated that recPrPC was able to activate a signal transduction pathway by phosphorylation of ERK 1/2 and Akt-kinase. A particularly interesting matter, is that this activity required an endogenous PrPC to mediate the way of the signal triggered by recPrPC. In fact, when the same experiment of stimulation with recPrP was conducted in cells with a transient silencing of PrP, the phosphorylation of ERK molecules 1/2 and Akt was significantly reduced.
Previous studies [2,47] reported that PrPC was able to form dimeric structures, both in its physiologic and pathologic status. As previously mentioned, shed PrPC and cleavage products, such as PrP-N1, are stimulated as a consequence of intracellular dimerization. Dimerization of membrane-bound PrPC leads to clustering in multimolecular complexes and serves to regulate different aspects of neuronal homeostasis, whereas intracellular dimerization appears to be the most relevant event in neuroprotection, via N1 and C1 prion metabolites [2]. Indeed, the dimerization stimulates α-cleavage and thus the production of the neuroprotective fragments.
Since membrane-bound PrPC is a well know raft component, we also evaluated the possible role of lipid rafts in the signal pathway induced by recPrPC. We used molecules affecting lipid rafts microdomains integrity, such as Fumonisin B1 or MβCD, revealing that cell pretreatment with these molecules significantly prevented ERK 1/2 and Akt phosphorylation induced by recPrPC. These results suggest that lipid rafts integrity is essential for recPrPC-induced signal pathways in hDPSCs, and that both gangliosides and cholesterol play a key role. These data are in agreement with our previous study [48], where we demonstrated that lipid rafts integrity was essential for neuronal differentiation process of hDPSCs induced by epidermal growth factor and basic fibroblast growth factor (EGF/bFGF).
In conclusion, our findings suggest that recPrPC play a key role in the neuronal differentiation process and in the activation of specific intracellular signal pathways. Cellular prion protein and its cleavage products represent enigmatic molecules, since their polymorphic behavior is still not fully understood. We suggest that membrane-bound PrPC and its cleavage products play a role in a type of dynamic equilibrium dependent on cellular conditions, and is able to function as sensors influencing signal pathways through autocrine, paracrine or endocrine mechanisms.
Better knowledge on cellular and molecular pathways playing a role in differentiation mechanisms mediated by endogenous and/or recombinant prion protein could allow to clarify the involvement of stem cells in nerve regeneration processes in order to better direct their use in regenerative medicine.
4. Materials and Methods
4.1. Research Ethics
To obtain hDPSCs, third molars included were excised from patients aged 13–19 years [48,49]. All subjects gave their informed consent for inclusion before their participation in the study, which was conducted in accordance with the Declaration of Helsinki, and the protocol was approved on 26 January 2017 by the Ethics Committee of “Sapienza” University (Project identification code CE:4336).
4.2. Isolation of Stem Cells Derived from Human Dental Pulp
hDPSCs were isolated and cultured as described in previous works [21,48,49] and, subsequently, they were maintained in Dulbecco’s Modified Eagle’s Medium low glucose (DMEM-L), containing 100 units/mL penicillin, 10 mg/mL streptomycin, plus 0.1% amphotericin (Sigma-Aldrich, Milan, Italy), plus fetal bovine serum (FBS) qualified 10% (Life Technologies, Monza, Italy), at 37 °C in humified CO2 atmosphere.
4.3. Treatments
Before treatments, hDPSCs were cultured up to 28 days from the pulp separation. For cell signaling analysis, hDPSCs were stimulated with recPrPC (0.5 μg/mL) (Jena Bioscience, Jena, Germany) several times (5, 10, 20, 40 min) at 37 °C in 5% CO2. To understand the implication of lipid rafts in the signaling of recPrPC, hDPSCs were pretreated with 30 μM Fumonisin B1 (Sigma-Aldrich, Milan, Italy), a compound that blocks the synthesis of sphingolipids, for 24 h at 37 °C or, alternatively, with 5 mM methyl-β-cyclodextrin (MβCD) (Sigma-Aldrich, Milan, Italy), a compound which is known to induce cholesterol efflux from the membrane, for 30 min at 37 °C. Moreover, to assess the role of endogenous PrPC in recPrPC signal pathways and neuronal differentiation induction, hDPSCs were pretreated with siRNA PrP for 72 h as described extensively below. To evaluate the role of recPrPC in neuronal differentiation process, hDPSCs were stimulated with recPrPC (0.5 μg/mL) for a long exposure time (14 days) changing the media every 4 days.
4.4. Knockdown PrPC by siRNA
hDPSCs were seeded (5 × 104 cells/mL) 6-well plates, in DMEM-L containing serum and antibiotics. Twenty-four hours after seeding, cells were transfected with 5 nM siRNA PrP (Flexitube GeneSolution GS5621 for PRNP), using HiPerFect Transfection Reagent (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. As experimental control, cells were also transfected with 5 nM scrambled siRNA (AllStars Negative Control—Qiagen). We verified by TLC analysis that PrP siRNA did not affect both cholesterol and ganglioside content, thus not affecting lipid rafts (data not shown). After 72 h, cells were incubated with recPrPC for 10 min (to evaluate signal pathways) or 14 days (to evaluate neuronal differentiation process) at 37 °C. For long time exposure, (14 days) every 4 days the siRNA PrP was replaced. PrPC expression was verified by flow cytometry analysis using mouse anti-PrP SAF32 mAb (Spi-Bio, Bertin Pharma, France) (Figure S1).
4.5. Western Blot Analysis
hDPSCs were lysed in lysis buffer containing 0.1% Triton X-100, 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4 and 75 U of aprotinin and allowed to stand for 20 min at 4 °C. The cell suspension was mechanically disrupted by Dounce homogenization (10 strokes). The lysate was centrifuged for 5 min at 1300× g to eliminate nuclei and large cellular debris. After protein concentration analysis by Bradford Dye Reagent assay (Bio-Rad, Milano, Italia), the lysate was tested with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, the proteins were electrophoretically transferred to PVDF membranes (Bio-Rad, Milan, Italia) which were blocked with 5% nonfat dried milk (Bio-Rad, Milan, Italia), or Bovine Serum Albumine (BSA) (Sigma-Aldrich, Milan, Italy), in TBS (Bio-Rad, Milan, Italia), containing 0.05% Tween 20 (Bio-Rad, Milan, Italia), and probed with rabbit anti-p-ERK1/2 pAb, rabbit anti-total ERK1/2 pAb, rabbit anti-p-Akt pAb, rabbit anti-total Akt pAb. Antibodies were visualized with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (Cell Signaling Technology Danvers, MA, USA) and immunoreactivity assessed by chemiluminescence reaction, using the ECL detection system (ThermoFisher Scientific, Rockford, IL, USA). Densitometric scanning analysis was accomplished with NIH Image 1.62 software by Mac OS X (Apple Computer International).
4.6. Flow Cytometry Analysis
Flow cytometry was used to quantify neuronal antigen expression on hDPSCs after a long period of stimulation with recPrPC. Briefly, hDPSCs untreated or treated with siRNA PrP or scrambled siRNA for 72 h, were stimulated with recPrPC for 14 days as described below and fixed with 4% paraformaldehyde and permeabilized by 0.1% (v/v) Triton X-100. After washing, cells were incubated with mouse anti-B3-Tubulin mAb, mouse anti-NFH mAb (Cell Signaling Technology Danvers, MA, USA) and mouse anti-GAP43 mAb (Sigma-Aldrich, Milan, Italy) for 1 h at 4 °C, followed by PE-conjugated anti-mouse IgG H&L (Abcam, Cambridge, MA, USA) for additional 30 min. All samples were analyzed with a FACScan cytometer (BD Accuri C6 Flow cytometer) equipped with a blue laser (488 nm) and a red laser (640 nm). At least 20,000 events were acquired.
4.7. Immunofluorescence Analysis
hDPSCs were seeded (2 × 104 cells/mL) 6-well plates, in DMEM-L containing serum and antibiotics. Twenty-four hours after seeding, hDPSCs untreated or treated with siRNA PrP or scrambled for 72 h were stimulated with recPrPC for 14 days and tested for immunofluorescence analysis. Briefly, hDPSCs untreated or treated as above were fixed with 4% paraformaldehyde and permeabilized by 0.1% (v/v) Triton X-100. After washing, cells were incubated with mouse anti-B3-Tubulin mAb, mouse anti-NFH mAb (Cell Signaling Technology Danvers, MA, USA) and mouse anti-GAP43 mAb (Sigma-Aldrich, Milan, Italy) for 1 h at 4 °C, followed by anti-mouse alexa fluor 488 or anti-mouse alexa fluor 594 (Cell Signaling Technology Danvers, MA, USA) for additional 30 min. Finally, cells were observed with a Zeiss Axio Vert. A1 fluorescence microscope (Zeiss, Oberkochen, Germany).
4.8. Statistical Analysis
Western blot images were subjected to densitometric scanning analysis, performed by Mac OS X (Apple Computer International), using NIH Image 1.62 software. The data were analyzed using one-way analysis of variance (ANOVA) after Bartlett’s test for the homogeneity of variances and Kolmogorov-Smirnov’s test for the Gaussian distribution and followed by Newman-Keuls multiple-comparison test or, when appropriate, with Student’s t-test (StatView for Macintosh; SAS Institute, Cary, NC, USA). All data were verified in at least 3 different experiments and reported as mean ± standard deviation (SD). Only p values of <0.01 were considered as statistically significant.
Acknowledgments
This research was supported by Rieti University Hub “Sabina Universitas” and “Fondazione Varrone” to V.M.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/2/345/s1.
Author Contributions
S.M., C.S., V.M. conceptualization. S.M., F.S., L.P., S.D.M. investigation. S.M., F.S., S.D.M. methodology. S.M., V.M. writing—original draft. A.A., R.M., M.S., V.M. writing—review and editing.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Brown D.R., Qin K., Herms J.W., Madlung A., Manson J., Strome R., Fraser P.E., Kruck T., von Bohlen A., Schulz-Schaeffer W., et al. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 2.Mattei V., Martellucci S., Santilli F., Manganelli V., Garofalo T., Candelise N., Caruso A., Sorice M., Scaccianoce S., Misasi R. Morphine Withdrawal Modifies Prion Protein Expression in Rat Hippocampus. PLoS ONE. 2017;12:e0169571. doi: 10.1371/journal.pone.0169571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis V., Hooper N.M. The role of lipid rafts in prion protein biology. Front Biosci. 2011;16:151–168. doi: 10.2741/3681. [DOI] [PubMed] [Google Scholar]
- 4.Sorice M., Mattei V., Tasciotti V., Manganelli V., Garofalo T., Misasi R. Trafficking of PrPC to mitochondrial raft-like microdomains during cell apoptosis. Prion. 2012;6:354–358. doi: 10.4161/pri.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prusiner S.B., Scott M.R., DeArmond S.J., Cohen F.E. Prion protein biology. Cell. 1998;93:337–348. doi: 10.1016/S0092-8674(00)81163-0. [DOI] [PubMed] [Google Scholar]
- 6.Parizek P., Roeckl C., Weber J., Flechsig E., Aguzzi A., Raeber A.J. Similar turnover and shedding of the cellular prion protein in primary lymphoid and neuronal cells. J. Biol. Chem. 2001;276:44627–44632. doi: 10.1074/jbc.M107458200. [DOI] [PubMed] [Google Scholar]
- 7.Mattei V., Garofalo T., Misasi R., Circella A., Manganelli V., Lucania G., Pavan A., Sorice M. Prion protein is a component of the multimolecular signaling complex involved in T cell activation. FEBS Lett. 2004;560:14–18. doi: 10.1016/S0014-5793(04)00029-8. [DOI] [PubMed] [Google Scholar]
- 8.Mouillet-Richard S., Ermonval M., Chebassier C., Laplanche J.L., Lehmann S., Launay J.M., Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–1928. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- 9.Toni M., Spisni E., Griffoni C., Santi S., Riccio M., Lenaz P., Tomasi V. Cellular prion protein and caveolin-1 interaction in a neuronal cell line precedes Fyn/Erk 1/2 signal transduction. J. Biomed. Biotechnol. 2006;2006:69469. doi: 10.1155/JBB/2006/69469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llorens F., Carulla P., Villa A., Torres J.M., Fortes P., Ferrer I., del Río J.A. PrP(C) regulates epidermal growth factor receptor function and cell shape dynamics in Neuro2a cells. J. Neurochem. 2013;127:124–138. doi: 10.1111/jnc.12283. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch T.Z., Martin-Lannerée S., Mouillet-Richard S. Functions of the Prion Protein. Prog. Mol. Biol. Transl. Sci. 2017;150:1–34. doi: 10.1016/bs.pmbts.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Hu W., Kieseier B., Frohman E., Eagar T.N., Rosenberg R.N., Hartung H.P., Stüve O. Prion proteins: Physiological functions and role in neurological disorders. J. Neurol. Sci. 2008;264:1–8. doi: 10.1016/j.jns.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Mattei V., Matarrese P., Garofalo T., Tinari A., Gambardella L., Ciarlo L., Manganelli V., Tasciotti V., Misasi R., Malorni W., et al. Recruitment of cellular prion protein to mitochondrial raft-like microdomains contributes to apoptosis execution. Mol. Biol. Cell. 2011;22:4842–4853. doi: 10.1091/mbc.e11-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garofalo T., Manganelli V., Grasso M., Mattei V., Ferri A., Misasi R., Sorice M. Role of mitochondrial raft-like microdomains in the regulation of cell apoptosis. Apoptosis. 2015;20:621–634. doi: 10.1007/s10495-015-1100-x. [DOI] [PubMed] [Google Scholar]
- 15.Wulf M.A., Senatore A., Aguzzi A. The biological function of the cellular prion protein: An update. BMC Biol. 2017;15:34. doi: 10.1186/s12915-017-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linden R. The Biological Function of the Prion Protein: A Cell Surface Scaffold of Signaling Modules. Front Mol. Neurosci. 2017;10:77. doi: 10.3389/fnmol.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanaani J., Prusiner S.B., Diacovo J., Baekkeskov S., Legname G. Recombinant prion protein induces rapid polarization and development of synapses in embryonic rat hippocampal neurons in vitro. J. Neurochem. 2005;95:1373–1386. doi: 10.1111/j.1471-4159.2005.03469.x. [DOI] [PubMed] [Google Scholar]
- 18.Steele A.D., Emsley J.G., Ozdinler P.H., Lindquist S., Macklis J.D. Prion protein (PrPc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc. Natl. Acad. Sci. USA. 2006;103:3416–3421. doi: 10.1073/pnas.0511290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miranda A., Ramos-Ibeas P., Pericuesta E., Ramirez M.A., Gutierrez-Adan A. The role of prion protein in stem cell regulation. Reproduction. 2013;146:R91–R99. doi: 10.1530/REP-13-0100. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.J., Baskokov I.V. The cellular form of the prion protein guides the differentiation of human embryonic stem cell into neuron-, oligodendrocyte- and astrocyte-committed lineages. Prion. 2014;8:266–275. doi: 10.4161/pri.32079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martellucci S., Manganelli V., Santacroce C., Santilli F., Piccoli L., Sorice M., Mattei V. Role of Prion protein-EGFR multimolecular complex during neuronal differentiation of human dental pulp-derived stem cells. Prion. 2018;12:117–126. doi: 10.1080/19336896.2018.1463797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mediano D.R., Sanz-Rubio D., Ranera R., Bolea I., Martin-Burriel I. The potential of mesenchymal stem cell in prion research. Zoonoses Public Health. 2015;62:165–178. doi: 10.1111/zph.12138. [DOI] [PubMed] [Google Scholar]
- 23.Gronthos S., Brahim J., Li W., Fisher L.W., Cherman N., Boyde A., DenBesten P., Robey P.G., Shi S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 24.Arthur A., Rychkov G., Shi S., Koblar S.A., Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;7:1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 25.Suchanek J., Soukup T., Visek B., Ivancakova R., Kucerova L., Mokry J. Dental pulp stem cells and their characterization. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2009;153:31–35. doi: 10.5507/bp.2009.005. [DOI] [PubMed] [Google Scholar]
- 26.Koyama N., Okubo Y., Nakao K., Bessho K. Evaluation of pluripotency in human dental pulp cells. J. Oral Maxillofac Surg. 2009;67:501–506. doi: 10.1016/j.joms.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Lee S.H., Ryu J.S., Lee J.W., Kwak D.H., Ko K., Choo Y.K. Comparison of ganglioside expression between human adipose- and dental pulp-derived stem cell differentiation into osteoblasts. Arch. Pharm. Res. 2010;33:585–591. doi: 10.1007/s12272-010-0413-0. [DOI] [PubMed] [Google Scholar]
- 28.Bieberich E. It’s a lipid’s world: Bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochem. Res. 2012;37:1208–1229. doi: 10.1007/s11064-011-0698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atari M., Gil-Recio C., Fabregat M., García-Fernández D., Barajas M., Carrasco M.A., Jung H.S., Alfaro F.H., Casals N., Prosper F., et al. Dental pulp of the third molar: A new source of pluripotent-like stem cells. J. Cell Sci. 2012;125:3343–3356. doi: 10.1242/jcs.096537. [DOI] [PubMed] [Google Scholar]
- 30.Young F.I., Telezhkin V., Youde S.J., Langley M.S., Stack M., Kemp P.J., Waddington R.J., Sloan A.J., Song B. Clonal heterogeneity in the neuronal and glial differentiation of dental pulp stem/progenitor cells. Stem Cells Int. 2016;2016 doi: 10.1155/2016/1290561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ullah I., Subbarao R.B., Kim E.J., Bharti D., Jang S.J., Park J.S., Shivakumar S.B., Lee S.L., Kang D., Byun J.H., et al. In vitro comparative analysis of human dental stem cells from a single donor and its neurodifferentiation potential evaluated by electrophysiology. Life Sci. 2016;154:39–51. doi: 10.1016/j.lfs.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Chun S.Y., Soker S., Jang Y.J., Kwon T.G., Yoo E.S. Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. J. Korean Med. Sci. 2016;31:171–177. doi: 10.3346/jkms.2016.31.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S., Kim E., Koh S.E., Maeng S., Lee W.D., Lim J., Shim I., Lee Y.J. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson’s disease model rats and alleviation of asymmetric rotational behaviour. Brain Res. 2012;1466:158–166. doi: 10.1016/j.brainres.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 34.Nesti C., Pardini S., Barachini S., D’Alessandro D., Siciliano G., Murri L., Petrini M., Vaglini F. Human dental pulp stem cells protect mouse dopaminergic neurons against MPP+ orrotenone. Brain Res. 2011;1367:94–102. doi: 10.1016/j.brainres.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 35.Harris D.A., Huber M.T., van Dijken P., Shyng S.L., Chait B.T., Wang R. Processing of a cellular prion protein: Identification of N- and C-terminal cleavage sites. Biochemistry. 1993;32:1009–1016. doi: 10.1021/bi00055a003. [DOI] [PubMed] [Google Scholar]
- 36.Watt N.T., Taylor D.R., Gillott A., Thomas D.A., Perera W.S., Hooper N.M. Reactive oxygen species-mediated beta-cleavage of the prion protein in the cellular response to oxidative stress. J. Biol. Chem. 2005;280:35914–35921. doi: 10.1074/jbc.M507327200. [DOI] [PubMed] [Google Scholar]
- 37.Altmeppen H.C., Prox J., Puig B., Dohler F., Falker C., Krasemann S., Glatzel M. Roles of endoproteolytic α-cleavage and shedding of the prion protein in neurodegeneration. FEBS J. 2013;280:4338–4347. doi: 10.1111/febs.12196. [DOI] [PubMed] [Google Scholar]
- 38.McDonald A.J., Dibble J.P., Evans E.G., Millhauser G.L. A new paradigm for enzymatic control of α-cleavage and β-cleavage of the prion protein. J. Biol. Chem. 2014;289:803–813. doi: 10.1074/jbc.M113.502351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altmeppen H.C., Puig B., Dohler F., Thurm D.K., Falker C., Krasemann S., Glatzel M. Proteolytic processing of the prion protein in health and disease. Am. J. Neurodegener Dis. 2012;1:15–31. [PMC free article] [PubMed] [Google Scholar]
- 40.Borchelt D.R., Rogers M., Stahl N., Telling G., Prusiner S.B. Release of the cellular prion protein from cultured cells after loss of its glycoinositol phospholipid anchor. Glycobiology. 1993;3:319–329. doi: 10.1093/glycob/3.4.319. [DOI] [PubMed] [Google Scholar]
- 41.Stahl N., Borchelt D.R., Prusiner S.B. Differential release of cellular and scrapie prion proteins from cellular membranes by phosphatidylinositol-specific phospholipase C. Biochemistry. 1990;29:5405–5412. doi: 10.1021/bi00474a028. [DOI] [PubMed] [Google Scholar]
- 42.Taylor D.R., Parkin E.T., Cocklin S.L., Ault J.R., Ashcroft A.E., Turner A.J., Hooper N.M. Role of ADAMs in the ectodomain shedding and conformational conversion of the prion protein. J. Biol. Chem. 2009;284:22590–22600. doi: 10.1074/jbc.M109.032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linsenmeier L., Altmeppen H.C., Wetzel S., Mohammadi B., Saftig P., Glatzel M. Diverse functions of the prion protein—Does proteolytic processing hold the key? Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:2128–2137. doi: 10.1016/j.bbamcr.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Tagliavini F., Prelli F., Porro M., Salmona M., Bugiani O., Frangione B. A soluble form of prion protein in human cerebrospinal fluid: Implications for prion-related encephalopathies. Biochem. Biophys. Res. Commun. 1992;184:1398–1404. doi: 10.1016/S0006-291X(05)80038-5. [DOI] [PubMed] [Google Scholar]
- 45.Perini F., Vidal R., Ghetti B., Tagliavini F., Frangione B., Prelli F. PrP27-30 is a normal soluble prion protein fragment released by human platelets. Biochem. Biophys. Res. Commun. 1996;223:572–577. doi: 10.1006/bbrc.1996.0936. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C.C., Steele A.D., Lindquist S., Lodish H.F. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc. Natl. Acad. Sci. USA. 2006;103:2184–2189. doi: 10.1073/pnas.0510577103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rigter A., Langeveld J.P.M., Zijderveld F.G.V., Bossers A. Prion protein self-interactions: A gateway to novel therapeutic strategies? Vaccine. 2010;28:7810–7823. doi: 10.1016/j.vaccine.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Mattei V., Santacroce C., Tasciotti V., Martellucci S., Santilli F., Manganelli V., Piccoli L., Misasi R., Sorice M., Garofalo T. Role of lipid rafts in neuronal differentiation of dental pulp-derived stem cells. Exp. Cell Res. 2015;339:231–240. doi: 10.1016/j.yexcr.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 49.Martellucci S., Santacroce C., Manganelli V., Santilli F., Piccoli L., Cassetta M., Misasi R., Sorice M., Mattei V. Isolation, Propagation and Prion Protein Expression During Neuronal Differentiation Process of Human Dental Pulp Stem Cells. J. Vis. Exp. doi: 10.3791/59282. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.