Abstract
Salmonella is the genus of Gram-negative, facultative intracellular pathogens that have the ability to infect large numbers of animal or human hosts. The S. enterica usg gene is associated with intracellular survival based on ortholog screening and identification. In this study, the λ-Red recombination system was used to construct gene deletion strains and to investigate whether the identified operon was related to intracellular survival. The pdxB-usg-truA-dedA operon enhanced the intracellular survival of S. enterica by resisting the oxidative environment and the usg and truA gene expression was induced by H2O2. Moreover, the genes in this operon (except for dedA) contributed to virulence in mice. These findings indicate that the pdxB-usg-truA-dedA operon functions in resistance to oxidative environments during intracellular survival and is required for in vivo S. enterica virulence. This study provides insight toward a better understand of the characteristics of intracellular pathogens and explores the gene modules involved in their intracellular survival.
Keywords: usg, truA, Salmonella enterica serovar Typhimurium, oxidative stress, intracellular survival
1. Introduction
Salmonella is a genus of Gram-negative and facultative intracellular pathogens that consists of a large group of genetically similar organisms with the ability to infect many animal and human hosts [1]. In 2005, the Salmonella genus was divided into S. bongori and S. enterica species [2]. Salmonella spp. are the second most common causative-agents of gastrointestinal infections in humans, after Campylobacter spp. [3]. Some Salmonella serovars cause large outbreaks of gastroenteritis associated with contaminated meat and produced or processed food [4].
A previous study found that the S. enterica usg gene was associated with intracellular survival based on ortholog screening and identification [5]. Because the complemented STΔusg/p-usg and wild type strains showed significant variation in the infection assay, our lab suggested that the usg gene was located in an operon with other genes. Operon prediction for the S. enterica strain LT2 whole genome sequence [6] found that the pdxB, usg, truA and dedA genes were located in the same operon. The promoter of this operon was located upstream of the pdxB gene (http://www.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb; accessed on 2 May 2018). Based on the genome annotation, the pdxB gene encodes 4-phosphoerythronate dehydrogenase, which is associated with de novo vitamin B6 biosynthesis [7]; the usg gene encodes putative aspartate-semialdehyde dehydrogenase, truA encodes RNA pseudouridine (38–40) synthase, and the dedA gene encodes a hypothetical protein.
In this study, the λ-Red recombination system was used to construct gene deletion strains and investigate whether the identified operon was related to intracellular survival. Furthermore, this study investigated the possible functions of the genes in the operon and assessed their roles in the virulence of S. enterica.
2. Results
2.1. The pdxB-usg-truA-dedA Operon Is Required for Intracellular Survival of S. enterica
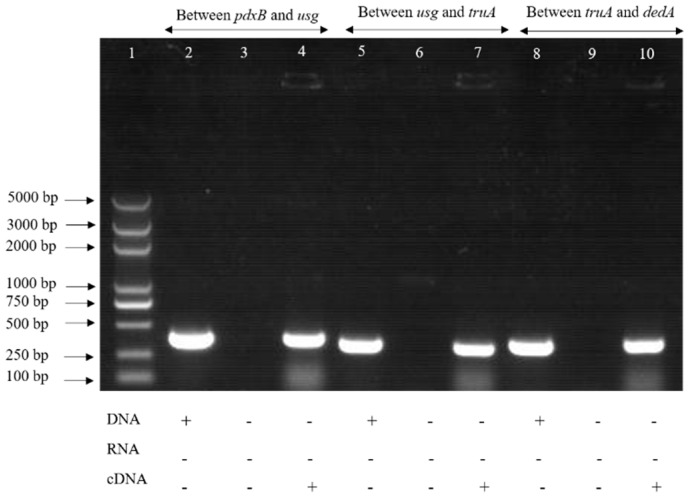
The predicted operon showed that the pdxB, usg, truA and dedA genes were transcribed in the same direction, indicating that these genes may have co-transcription. The primers were used to amplify the cDNA of the upstream and downstream genes and the intergenic regions, and the DNA and RNA were used as controls. The results revealed that the genes in the operon were co-transcribed. The above results indicate that the operon contains four genes: pdxB, usg, truA and dedA (Figure 1). The expression of each gene, using RT-PCR, showed that the genes in the operon had no effect on other genes in the lysogeny broth (LB) medium.
Figure 1.
Analysis of co-transcription detection in the operon. Note: “+” means there was a fragment, “−” means there was no fragment. Lane 1 was a DNA marker, lanes 2, 5 and 8 were amplified using genome DNA; lanes 3, 6 and 9 were amplified using total RNA; lanes 4, 7 and 10 were amplified using cDNA.
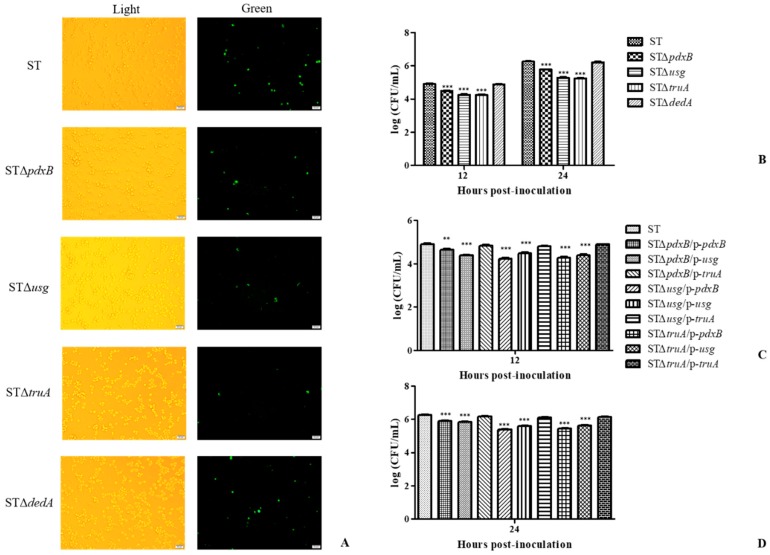
The pdxB, usg, truA and dedA genes were located in the same operon and deletion strains for these genes were constructed using the λ-Red recombination system. The intracellular survival of the gene deletion strains was assessed using J774A.1 macrophage cells (Figure 2). At 12 and 24 h post-infection, the cells infected with the STΔpdxB, STΔusg and STΔtruA strains showed lower bacterial loads than the cells infected with the ST and STΔdedA strains (p < 0.01) (Figure 2A,B). The STΔpdxB, STΔusg and STΔtruA strains had reduced replication abilities inside the J774A.1 macrophages and, therefore, exhibited reduced virulence in vitro. However, the STΔdedA strain did not show reduced replication.
Figure 2.
Intracellular survival of the operon gene deletion mutants and complemented strains in macrophages. (A) The infection process in J774A.1 macrophages by the strains was observed under a microscope at 12 h post-infection (40×). “Light” indicates that the cells were observed under natural light, and “Green” indicates that the cells were under a fluorescence microscope. (B) Bacterial loads of the gene deletion strains at 12 and 24 h post inoculation. (C,D) Bacterial loads of the complemented strains with different plasmids at 12 and 24 h post-inoculation. ** p < 0.01, *** p < 0.001.
Subsequently, the complemented gene plasmids were electroporated into all of the gene deletion mutants. As shown in Figure 2C,D, the STΔpdxB strains electroporated with the recombinant plasmids p-pdxB and p-usg showed significant differences in bacterial loads (p < 0.001), whereas the STΔpdxB strains electroporated with the recombinant plasmid p-truA had almost the same bacterial loads in the macrophages. Similar results were found for the other complemented strains. These results indicated that the truA gene played a key role related to intracellular survival in this operon.
In summary, these results showed that all genes in this operon, except for the dedA gene, confirmed that S. enterica has the ability to survive in macrophages and that the truA gene played a key role in this function.
2.2. The pdxB-usg-truA-dedA Operon Contributes to Virulence in Mice
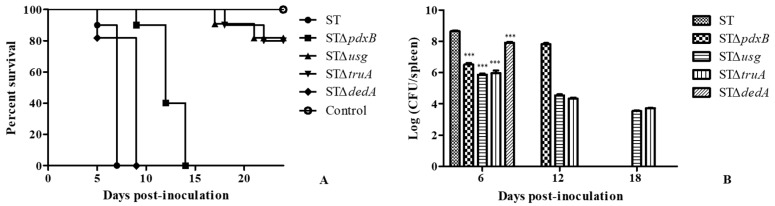
Ten mice were intraperitoneally inoculated with a dose of 105 Colony-Forming Units (CFU) of the STΔpdxB, STΔusg, STΔtruA, STΔdedA and wild type strains. The animals did not survive more than seven days after intraperitoneal inoculation with the wild type strain and not more than nine days post-inoculation with STΔdedA. The mice in the group inoculated with STΔpdxB did not survive more than 15 days. Conversely, the survival rates of the groups inoculated with STΔusg and STΔtruA were both 80% at 24 days post-inoculation (Figure 3A).
Figure 3.
Survival of the gene deletion mutant and wild type strains in mice. (A) Survival curves of the gene deletion and wild type strains. (B) Bacterial loads of the spleens at six, 12, and 18 days post-inoculation with the gene deletion and wild type strains. *** p < 0.001.
Five infected mice from each group were randomly selected at 6, 12, and 18 days post-inoculation. Their spleens were removed to assess the bacterial loads (Figure 3B). At six days post-inoculation, the bacterial loads were significantly lower for the gene deletion strains than for the wild type strain and a large reduction (above 2-log) in the spleen bacterial load was observed in the mice inoculated with STΔusg and STΔtruA compared to the mice infected with the wild type strains. At 12 days post-inoculation, the spleen bacterial load increased in the mice inoculated with STΔpdxB, which was similar to the load measured in the mice inoculated with the wild type strain at six days. The bacterial loads of the mice inoculated with STΔusg and STΔtruA reduced slowly (Figure 3B). The results showed that usg and truA contributed to virulence in mice, which was consistent with the cell infection assay results.
2.3. The usg and truA Expression Levels Were Higher in the Oxidative Environment
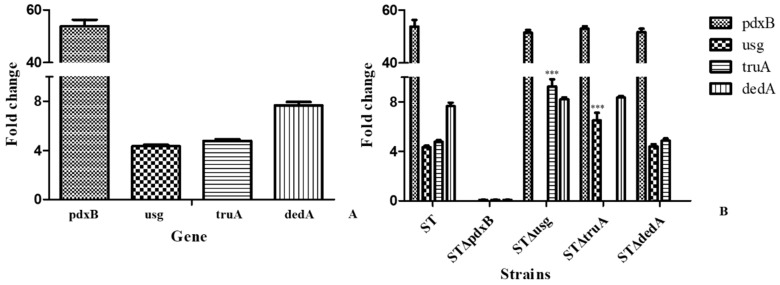
The expression levels of genes in the operon were evaluated in the gene deletion and wild type strains in the routine growth medium and under oxidative conditions (Figure 4). As shown in Figure 3A, pdxB, usg, truA and dedA expression was induced in the wild type strain by H2O2 and was significantly higher in the samples treated with H2O2 than in the untreated controls (Figure 4A).
Figure 4.
Gene expression levels in the bacterial strains. (A) Gene expression levels in the wild type strain under oxidative conditions. (B) Gene expression levels in wild type and gene deletion strains under oxidative conditions. *** p < 0.001.
Under oxidative conditions, the usg, truA and dedA genes were barely expressed when pdxB was deleted compared with the expression levels in the wild type strain. The pdxB gene expression levels were similar in the usg, truA and dedA gene deletion strains. When dedA was deleted, the expression levels of the other genes were not significantly different from the expression levels in the wild type strain. When usg was deleted, truA expression was induced by oxidative conditions and truA expression was significantly higher in these strains compared with the wild type strain. Usg expression also significantly increased when truA was deleted (Figure 4B).
2.4. The pdxB-usg-truA-dedA Operon Contributed to Resistance to Oxidative Conditions
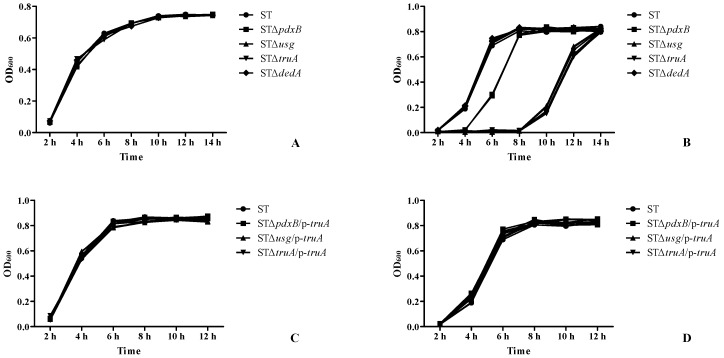
The growth characteristics of the gene deletion and parent strains were determined in an LB medium. No significant variations were observed between the gene deletion and wild type strains (Figure 5A). These results suggested that the genes in the operon did not affect the in vitro growth of Salmonella spp. at normal temperatures. Under oxidative conditions, all of the strains grew slowly for the first 2 h. After 6 h, the growth of the wild type and STΔdedA strains reached the plateau phase (Figure 3B). At this time, the STΔpdxB strain was in the logarithmic phase, and the STΔusg and STΔtruA strains had barely replicated. At 10 h post-infection, the STΔusg and STΔtruA strains began to replicate, whereas the other strains were in the plateau phase (Figure 5B).
Figure 5.
Growth characteristics of the gene deletion and complemented strains in the lysogeny broth (LB) medium and under oxidative conditions. (A) Growth characteristics of the gene deletion strains in the LB medium. (B) Growth characteristics of the gene deletion strains under oxidative conditions. (C) Growth characteristics of the strains complemented with the p-truA plasmid in the LB medium. (D) Growth characteristics of the strains complemented with p-truA plasmid under oxidative conditions.
The growth characteristics and oxidative resistance were also assessed for the STΔpdxB/p-truA, STΔusg/p-truA and STΔtruA/p-truA strains, which were complemented strains with the same recombinant p-truA plasmid (Figure 3C,D). These strains had characteristics similar to the wild type, even under oxidative conditions. All of the results suggested that the operon (except for the dedA gene) contributed to a resistance to oxidative conditions promoted the survival of S. enterica in macrophages.
3. Discussion
S. enterica is a common facultative intracellular pathogen. The main pathway used by macrophages to eliminate invading pathogens are endocytosis and digestion. S. enterica can exploit multiple aspects of host defenses to promote its replication in the host after adaptation to a variety of harsh environments, such as oxidative conditions [8]. A previous study found that the usg gene was related to intracellular survival and that the pdxB, usg, truA and dedA genes were located in the same operon. Another study found that pdxB was related to intracellular survival in S. enterica. This study found that a pdxB mutant strain was sensitive to oxidative conditions and reduced the bacterial load in macrophages. Another study found that pdxB in E. coli and Pseudomonas aeruginosa contained tightly bound NAD+ and/or NADH [7,9,10] and that the nucleotide-binding domains of pdxB were homologous to the corresponding domains of d-3-phosphoglycerate dehydrogenase (PGDHs) from E. coli and Mycobacterium tuberculosis [7]. Because orthologs usually have conserved biological structures and functions [11,12], the results of this study suggest that pdxB in S. enterica has the same function. The usg and truA genes had similar results in the cell assay, similar expression levels under oxidative conditions, and inhibited TNF-α and IL-1β expression in macrophages. One study used random insertions of TnphoA-132 and found that truA was one target of glyoxal [13]. Another study showed that truA was associated with resistance to quinoxaline 1, 4-dioxides (QdNOs) in E. coli, which have been used in animals as antimicrobial agents and growth promoters for decades [14]. The results showed that usg and truA were related to intracellular survival. Although the dedA gene was in the same operon as the other genes, no effect on intracellular survival was observed for this gene.
There were many genes related to intracellular survival via oxidative resistance. The sodA gene deletion strain resulted in a slightly reduced growth rate, low SOD activity, increased susceptibility to reactive oxygen species and chicken serum, and no effect on the motility of the wild type strain [15]. One study found that three catalases (KatE, KatG, and KatN) and two alkyl hydroperoxide reductases (AhpC and TsaA) were related to oxidative resistance using silico genome analysis and gene deletion methods [16]. Large-scale profiling of Salmonella protein expression was performed under H2O2 treatment. The results showed that the abundance of 116 proteins were altered significantly among 1600 quantified proteins and that iron acquisition systems were induced to promote bacterial survival under oxidative stress [17]. Macrophages play important roles in the phagocytosis of pathogens and antigen presentation. Macrophages are immediately activated after phagocytosing pathogens, resulting in a variety of bactericidal mechanisms. These mechanisms include both oxidative and non-oxidative bactericidal mechanisms [8,18]. These poor survival environments lead Salmonella spp. to secrete effectors and generate a replicative compartment known as the Salmonella-containing vacuole (SCV). This study showed that genes in this operon (except for the dedA gene) conferred S. enterica with the ability to survive in macrophages and that the truA gene played a key role. The usg and truA expression levels were increased under oxidative treatment. All of these results suggested that this operon enhanced the intracellular survival of S. enterica by increasing resistance to oxidative environments and that truA played a key role in this function.
Operons are polycistronic clusters of genes transcribed from a promoter at the 5′ end of the cluster [19]. Several operons reportedly related to virulence have been identified in S. enterica [20,21,22,23,24]. Typically, genes in the same operon have similar functions and interact with each other. The amino acid sequences pdxB, usg, truA and dedA were uploaded to the STRING database [25] to predict protein–protein interactions. Usg and truA had the highest combined association score (0.935). Co-expression of usg and truA orthologs was also found in Acinetobacter sp. ADP1 and Pseudomonas aeruginosa. The combined association score of dedA was lower than the association scores for the other genes and no co-expression of dedA orthologs had been found to date. The results of our study are similar to our predictions.
4. Materials and Methods
4.1. Ethics Statement
All animal research was approved by the Beijing Association for Science and Technology. The approval ID is SYXK (Beijing) 2015–0028 (Validity period: 22 September 2015 to 22 September 2020), and the animal research complied with the Beijing Laboratory Animal Welfare and Ethics guidelines of the Beijing Administration Committee of Laboratory Animals.
4.2. Bacterial Strains and Media
All of the bacterial strains and plasmids used in this study are listed in Table 1. The S. typhimurium and E. coli strains, including the parental strain and the derived mutants, were routinely grown or incubated in an LB medium. Antibiotics were added at the following concentrations when required: ampicillin, 100 mg/L and chloramphenicol, 34 mg/L. All bacterial strains were frozen at −80 °C with 15–20% (v/v) glycerol.
Table 1.
Strains and plasmids used in this study.
| Strains or Plasmids | Description/Purpose | Source or Reference |
|---|---|---|
| Strains | ||
| S. typhimurium ATCC14028 (ST) | Wild type (WT) | Guangdong Culture Collection Center |
| STΔpdxB | ΔpdxB mutant of ST by the λ-Red recombination system | This study |
| STΔusg | Δusg mutant of ST by the λ-Red recombination system | Our lab |
| STΔtruA | ΔtruA mutant of ST by the λ-Red recombination system | This study |
| STΔdedA | ΔdedA mutant of ST by the λ-Red recombination system | This study |
| STΔpdxB/p-pdxB | STΔpdxB harboring the pBR322-pdxB plasmid, complement strains | This study |
| STΔpdxB/p-usg | STΔpdxB harboring the pBR322-usg plasmid, complement strains | This study |
| STΔpdxB/p-truA | STΔpdxB harboring the pBR322-truA plasmid, complement strains | This study |
| STΔusg/p-pdxB | STΔusg harboring the pBR322-pdxB plasmid, complement strains | This study |
| STΔusg/p-usg | STΔusg harboring the pBR322-usg plasmid, complement strains | Our lab |
| STΔusg/p-truA | STΔusg harboring the pBR322-truA plasmid, complement strains | This study |
| STΔtruA/p-pdxB | STΔtruA harboring the pBR322-pdxB plasmid, complement strains | This study |
| STΔtruA/p-usg | STΔtruA harboring the pBR322-usg plasmid, complement strains | This study |
| STΔtruA/p-truA | STΔtruA harboring the pBR322-truA plasmid, complement strains | This study |
| DH5α | For cloning | Takara |
| Plasmids | ||
| pKD3, pKD46 and pCP20 | λ-Red recombination system | Datsenko and Wanner, 2000 [26] |
| pBR322 | For constructed complement strains | Virulent laboratory |
| p-pdxB | pdxB of ST product cloned into pBR322 for complementation assay | This study |
| p-usg | usg of ST product cloned into pBR322 for complementation assay | Our lab |
| p-truA | truA of ST product cloned into pBR322 for complementation assay | This study |
4.3. Mice
BALB/c mice (aged 4 to 6 weeks) were purchased from the Weitong Lihua Laboratory Animal Services Center (Beijing, China), and bred in individually ventilated cage rack systems. All experiments involving animals followed the regulations of the Beijing Administration Office for Laboratory Animals.
4.4. Construction of Gene Deletion and Complemented Gene Deletion Mutant Strains
Deletion mutants and their complemented mutants were constructed for all genes in the operon. Gene deletion mutants were constructed using the λ-Red recombination system. After sequencing confirmation, the recombinant plasmids with the coding regions and their promoters were subsequently electroporated into every gene deletion mutant to complement the gene function. The complemented strains were selected from an LB medium containing ampicillin. The primers are shown in Table S1. The gene deletion and complemented gene deletion mutants were confirmed by PCR amplification and sequencing.
Total RNA was extracted from all strains using TRIzol (Invitrogen, Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions and treated with DNase (TaKaRa Bio, Inc., Dalian, China) before reverse transcription to remove DNA contamination. Total RNA was dissolved in diethypyrocarbonate (DEPC)-treated water, and the concentration and purity of the total RNA were estimated by reading the absorbance at 260 and 280 nm, respectively. cDNA was synthesized using the Prime ScriptTM RT Reagent Kit (TaKaRa Bio, Inc., Dalian, China), according to the manufacturer’s instructions. The reverse transcription product was stored at −20 °C. PCR was performed with the primers shown in Table S1 to evaluate gene expression.
To determine if the genes in the operon were co-transcribed, the intergenic regions of genes were amplified by RT-PCR [27]. The RNA of the parental strain was extracted and cDNA was synthesized by reverse transcription using the method descibed before. The gene spacers were amplified using primers (Table S1) while the wild strain genomic DNA and RNA were used as controls. The amplification system and conditions were the same as before.
4.5. Cell Infection Assay
To investigate the intracellular survival of the strains, infection assays were performed using J774A.1 murine macrophages (Key Laboratory of Animal Epidemiology and Zoonosis of the Ministry of Agriculture, Beijing, China). The cells were cultured in 24-well plates and infected with each strain at a multiplicity of infection (MOI) of 10 CFU. Then, the infected plates were centrifuged at 1000 rpm for 5 min at room temperature and incubated at 37 °C in an atmosphere containing 5% (v/v) CO2. After 20 min, the cells were washed three times with phosphate buffered solution (PBS) and incubated in a medium containing gentamycin (50 μg/mL) at 37 °C under 5% CO2 until the end of the infection period. At 12 and 24 h post infection (p.i.), the cells were washed and lysed, and the numbers of bacteria exhibiting intracellular survival were determined through serial dilution and plating on an LB medium.
The pBR322 plasmid encoding the green fluorescent protein (GFP) was transferred into the parent and gene deletion strains using the electroporation method. Recombinant clones were selected from the LB medium containing ampicillin. Then, infection assays were performed as described above. After 12 h of incubation, the macrophages were washed three times with PBS. Infection of the J774A.1 macrophages by the strains was observed under a fluorescence microscope (Olympus, Tokyo, Japan).
4.6. Growth Characteristics and Oxidative Resistance Assay
The in vitro growth analysis of the deletion mutants and complemented strains was described previously. An oxidative resistance assay was performed as follows. One colony of each strain was inoculated into 3 mL of LB or LB with ampicillin medium and cultured overnight at 37 °C with shaking at 200 rpm. Subsequently the cultures were adjusted to the same concentration (OD600 ≈ 1.0) and a 50 μL sample of each strain was inoculated into 5 mL of an LB or LB with ampicillin medium. Then, 30% H2O2 was add to the liquid medium (final concentration 4.4 mM) [28] to provide an oxidative environment. The cultures were incubated at 37 °C with shaking at 200 rpm, and the OD600 value was determined every 2 h using a BioTek microplate reader (Gene Company Limited, Hong Kong, China).
4.7. Gene Expression Levels in an Oxidative Environment
The gene expression levels under oxidative treatment were assessed by real-time PCR (RT-PCR). One colony of the gene deletion and parent strain was inoculated into 3 mL of LB medium and cultured overnight at 37 °C with shaking. The cultures were adjusted to the same concentration (OD600 ≈ 1.0). A 50 μL sample of each strain was inoculated into 5 mL of LB medium and LB medium with H2O2. Total RNA was extracted from all strains, and cDNAs were obtained as described above. The cDNA samples were subjected to quantitative RT-PCR using the SYBR® Premix Ex TaqTM II Kit (TaKaRa Bio, Inc., Dalian, China). Each PCR reaction consisted of 2 μL of cDNA, 0.8 μL of each primer (10 μM), 10 μL of SYBR®Premix Ex TaqTM II, and 20 μL RNase-free water. The cycling conditions were a denaturation step, at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 20 s. The specificity of the RT-PCR products was confirmed using a melting curve analysis. These reactions were repeated in triplicate for every sample as technical replicates. Gene mRNA quantification was performed using the 2−ΔΔCt method to analyze the expression levels. The 16S rRNA expression level in S. enterica was used as a reference to normalize all values. The results presented in this study represent the averages from at least three separate experiments.
4.8. Virulence in BALB/c Mice
There were two experiments. The first experiment concerned the survival of the mice. Ten mice were intraperitoneally inoculated with a dose of 105 CFU of the gene deletion and wild type strains in 100 μL of phosphate-buffered saline (PBS) [29]; the control group included five mice intraperitoneally inoculated with 100 μL of PBS. The survival of the mice was observed over the next 24 days. The second experiment was the virulence of the gene deletion strains. Based on survival time, five mice were intraperitoneally inoculated with the same dose of the gene deletion and wild type strains. Five infected mice from each group were randomly selected at 6, 12, and 18 days post-inoculation. At each time point, the spleens were removed and homogenized individually in an aseptic manner in 1 mL of PBS and then serially diluted to isolate the bacteria. The results are presented as the mean number of CFU per spleen± the standard deviation (SD) in each group.
4.9. Statistical Analysis
The statistical analyses of the data, including the data from the growth curve analysis, cell infection study, oxidative resistance assay and virulence experiments, were performed using IBM SPSS Statistics version 23 (IBM, Armonk, New York, NY, USA, https://www.ibm.com/analytics/data-science/predictive-analytics/spss-statistical-software). A p value < 0.05 obtained through one-way analysis of variance (ANOVA) was considered significant. All graphics were drawn with GraphPad Prism 5 (GraphPad Software, La jolla, CA, USA, https://www.graphpad.com/).
5. Conclusions
In conclusion, this study used the λ-Red recombination system to construct gene deletion strains and determine whether the identified operon was related to intracellular survival. Except for the dedA gene, all of the genes in this operon confirmed the ability of S. enterica to survive in macrophages, and the truA gene played a key role in resistance to oxidative conditions. Moreover, the genes in this operon (except for dedA) contributed to virulence in mice. These findings indicate that the pdxB-usg-truA-dedA operon functions in resistance to oxidative environments and contributes to intracellular survival; moreover, the operon is required for the virulence of S. enterica in vivo. In this study, clues were examined to gain a better understanding of the characteristics of intracellular pathogens and to explore the gene modules involved in the intracellular survival of intracellular pathogens.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/2/380/s1.
Author Contributions
X.Y. conceived of the experiments, interpreted the data, and supervised the research project; X.Y., Z.F., and J.W. performed the experiments and wrote the manuscript; X.Z., X.W., and Q.W. participated in the discussion and revised the manuscript; all authors approved the final draft.
Funding
This work was funded by the National Natural Science Foundation of China (No. 31372446 and No. 31802263), the Research of Key Technology for Prevention of Major Zoonosis in Dairy Cattle (No. 2015NZ0104), and the National Special Foundation for Science and Technology Basic Research (No. 2012FY111000).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Baker S., Dougan G. The genome of Salmonella enterica serovar Typhi. Clin. Infect. Dis. 2007;45(Suppl. 1):S29–S33. doi: 10.1086/518143. [DOI] [PubMed] [Google Scholar]
- 2.Tindall B.J., Grimont P.A., Garrity G.M., Euzeby J.P. Nomenclature and taxonomy of the genus Salmonella. Int. J. Syst.Evol. Microbiol. 2005;55:521–524. doi: 10.1099/ijs.0.63580-0. [DOI] [PubMed] [Google Scholar]
- 3.Lamas A., Miranda J.M., Regal P., Vazquez B., Franco C.M., Cepeda A. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol. Res. 2018;206:60–73. doi: 10.1016/j.micres.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Nyachuba D.G. Foodborne illness: Is it on the rise? Nutr. Rev. 2010;68:257–269. doi: 10.1111/j.1753-4887.2010.00286.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang X., Wang J., Bing G., Bie P., De Y., Lyu Y., Wu Q. Ortholog-based screening and identification of genes related to intracellular survival. Gene. 2018;651:134–142. doi: 10.1016/j.gene.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 6.Taboada B., Ciria R., Martinez-Guerrero C.E., Merino E. ProOpDB: Prokaryotic Operon DataBase. Nucleic Acids Res. 2012;40:D627–D631. doi: 10.1093/nar/gkr1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha J.Y., Lee J.H., Kim K.H., Kim D.J., Lee H.H., Kim H.K., Yoon H.J., Suh S.W. Crystal structure of D-erythronate-4-phosphate dehydrogenase complexed with NAD. J. Mol. Biol. 2007;366:1294–1304. doi: 10.1016/j.jmb.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 8.Behnsen J., Perez-Lopez A., Nuccio S.P., Raffatellu M. Exploiting host immunity: The Salmonella paradigm. Trends Immunol. 2015;36:112–120. doi: 10.1016/j.it.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao G., Pease A.J., Bharani N., Winkler M.E. Biochemical characterization of gapB-encoded erythrose 4-phosphate dehydrogenase of Escherichia coli K-12 and its possible role in pyridoxal 5′-phosphate biosynthesis. J. Bacteriol. 1995;177:2804–2812. doi: 10.1128/jb.177.10.2804-2812.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph J., Kim J., Copley S.D. Multiple turnovers of the nicotino-enzyme PdxB require alpha-keto acids as cosubstrates. Biochemistry. 2010;49:9249–9255. doi: 10.1021/bi101291d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F., Mackey A.J., Vermunt J.K., Roos D.S. Assessing performance of orthology detection strategies applied to eukaryotic genomes. PLoS ONE. 2007;2:e383. doi: 10.1371/journal.pone.0000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Breis J.T., Chiba H., Legaz-Garcia Mdel C., Uchiyama I. The Orthology Ontology: Development and applications. J. Biomed. Semant. 2016;7:34. doi: 10.1186/s13326-016-0077-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee C., Kim J., Kwon M., Lee K., Min H., Kim S.H., Kim D., Lee N., Kim J., Kim D., et al. Screening for Escherichia coli K-12 genes conferring glyoxal resistance or sensitivity by transposon insertions. FEMS Microbiol. Lett. 2016;363 doi: 10.1093/femsle/fnw199. [DOI] [PubMed] [Google Scholar]
- 14.Guo W., Hao H., Dai M., Wang Y., Huang L., Peng D., Wang X., Wang H., Yao M., Sun Y., et al. Development of quinoxaline 1, 4-dioxides resistance in Escherichia coli and molecular change under resistance selection. PLoS ONE. 2012;7:e43322. doi: 10.1371/journal.pone.0043322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Yi L., Zhang J., Sun L., Wen W., Zhang C., Wang S. Functional analysis of superoxide dismutase of Salmonella typhimurium in serum resistance and biofilm formation. J. Appl. Microbiol. 2018;125:1526–1533. doi: 10.1111/jam.14044. [DOI] [PubMed] [Google Scholar]
- 16.Hebrard M., Viala J.P., Meresse S., Barras F., Aussel L. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J. Bacteriol. 2009;191:4605–4614. doi: 10.1128/JB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu J., Qi L., Hu M., Liu Y., Yu K., Liu Q., Liu X. Salmonella proteomics under oxidative stress reveals coordinated regulation of antioxidant defense with iron metabolism and bacterial virulence. J. Proteom. 2017;157:52–58. doi: 10.1016/j.jprot.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Buckner M.M., Finlay B.B. Host-microbe interaction: Innate immunity cues virulence. Nature. 2011;472:179–180. doi: 10.1038/472179a. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal T., Davis P., Garrido-Lecca A. Operon and non-operon gene clusters in the C. elegans genome. WormBook. 2015:1–20. doi: 10.1895/wormbook.1.175.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eswarappa S.M., Panguluri K.K., Hensel M., Chakravortty D. The yejABEF operon of Salmonella confers resistance to antimicrobial peptides and contributes to its virulence. Microbiology. 2008;154:666–678. doi: 10.1099/mic.0.2007/011114-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q., Zhang Y., Zhang X., Zhan L., Zhao X., Xu S., Sheng X., Huang X. The novel cis-encoded antisense RNA AsrC positively regulates the expression of rpoE-rseABC operon and thus enhances the motility of Salmonella enterica serovar typhi. Front Microbiol. 2015;6:990. doi: 10.3389/fmicb.2015.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandis G., Bergman J.M., Hughes D. Autoregulation of the tufB operon in Salmonella. Mol. Microbiol. 2016;100:1004–1016. doi: 10.1111/mmi.13364. [DOI] [PubMed] [Google Scholar]
- 23.Qin T., Ken-Ichiro I., Ren H.Y., Zhou H.J., Yoshida S. Legionella dumoffii Tex-KL Mutated in an Operon Homologous to traC-traD is Defective in Epithelial Cell Invasion. Biomed. Environ. Sci. 2016;29:424–434. doi: 10.3967/bes2016.055. [DOI] [PubMed] [Google Scholar]
- 24.Godfrey R.E., Lee D.J., Busby S.J.W., Browning D.F. Regulation of nrf operon expression in pathogenic enteric bacteria: Sequence divergence reveals new regulatory complexity. Mol. Microbiol. 2017;104:580–594. doi: 10.1111/mmi.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui G., Wang J., Qi X., Su J. Transcription Elongation Factor GreA Plays a Key Role in Cellular Invasion and Virulence of Francisella tularensis subsp. novicida. Sci. Rep. 2018;8:6895. doi: 10.1038/s41598-018-25271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M., Qi L., Xiao Y., Wang M., Qin C., Zhang H., Sheng Y., Du H. SufC may promote the survival of Salmonella enterica serovar Typhi in macrophages. Microb. Pathog. 2015;85:40–43. doi: 10.1016/j.micpath.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Gong H., Vu G.P., Bai Y., Chan E., Wu R., Yang E., Liu F., Lu S. A Salmonella small non-coding RNA facilitates bacterial invasion and intracellular replication by modulating the expression of virulence factors. PLoS Pathog. 2011;7:e1002120. doi: 10.1371/journal.ppat.1002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.