Abstract
Bladder cancer is one of the most incident neoplasms worldwide, and its treatment remains a significant challenge, since the mechanisms underlying disease progression are still poorly understood. The epithelial to mesenchymal transition (EMT) has been proven to play an important role in the tumorigenic process, particularly in cancer cell invasiveness and metastatic potential. Several studies have reported the importance of epigenetic mechanisms and enzymes, which orchestrate them in several features of cancer cells and, specifically, in EMT. In this paper, we discuss the epigenetic enzymes, protein-coding and non-coding genes, and mechanisms altered in the EMT process occurring in bladder cancer cells, as well as its implications, which allows for improved understanding of bladder cancer biology and for the development of novel targeted therapies.
Keywords: bladder cancer, EMT, miRNA, lncRNA, epigenetics
1. Introduction
1.1. Bladder Cancer
Bladder cancer (BlCa) is the seventh most prevalent cancer worldwide and the second most frequent urological malignancy after prostate cancer. Incidence has been rising in most countries, with an estimated 549,393 new cases diagnosed in 2018 and 990,724 new cases expected in 2040. Therefore, this almost doubled the number. Moreover, BlCa constitutes an important cause of cancer-related death with 199,922 deaths estimated in 2018 and 387,232 predicted for 2040 [1,2]. There is a strong male predominance, approximating a 3:1 ratio, and epidemiological trends track closely the prevalence of tobacco smoking [3]. Similar to other urological malignancies, mortality-to-incidence ratios are higher in underdeveloped countries, which probably reflects different environmental exposures and/or inequalities in healthcare accessibility [4]. Importantly, due to its high prevalence, mortality and, particularly, the propensity for multiple recurrences and/or disease progression and consequent additional treatments, BlCa is the most costly neoplastic disease constituting an important financial burden (costs about €4.9 billion in the European Union, alone, in 2012) [5].
BlCa generally refers to a cancer derived from epithelial layer, the urothelium, which is shared with other organs of the urinary tract and it extends from the renal pelvis to the urethra. Hence, although other much rarer tumor formations occur, its major histological subtype is urothelial carcinoma, which will be the focus of this review. Two major forms of BlCa are acknowledged, differing clinically, pathologically, and molecularly. Non-muscle invasive BlCa (NMIBC, corresponding to 75% to 80% of all cases, disclosing papillary architecture, with the propensity to recur and eventually invade the bladder wall over time) and muscle-invasive BlCa (MIBC, 20% to 25% of all cases, mostly derived from urothelial carcinoma in situ, which constitutes an aggressive disease that invades locally and metastasizes systemically) [6,7].
1.2. Epigenetics
During many years, scientists believed that living organisms’ identity was defined by the genetic component of its cells, but, rapidly, it became clear that this could not explain how cells with the same genomic composition could disclose different phenotypes depending on different conditions. Now it is known that the identity of a cell is defined by both genetic and epigenetic patterns with the latter being crucial for fetal development in mammals, as well as cell and tissue differentiation [8,9,10,11]. Epigenetics is defined as the study of heritable modifications of DNA or associated proteins, which carry information related to gene expression during cell division, and currently encompasses all potentially reversible mechanisms that lead to changes in expression regulation without affecting the DNA sequence [12]. The most well-known epigenetic mechanisms comprise four major groups: DNA methylation, histone post-translational modifications or chromatin remodeling, histone variants, and non-coding RNAs’ regulation [11]. These modifications are tightly regulated by several enzymes, which may act isolated or in chromatin remodeling complexes, and grouped according to function. These epigenetic enzymes include: DNA methyltransferases (DNMTs) and demethylases (TETs), histone methyltransferases (HTMs) and demethylases (HDMs), histone acetyltransferases (HATs) and deacetylases (HDACs), and histone ubiquitin ligases (UbLs) and deubiquitinases (dUbs) [12].
Cancer cells exhibit a distinct epigenetic landscape and they take advantage of all of the previously mentioned mechanisms to acquire the characteristic malignant features, from transformation to progression [13]. BlCa is no exception. Several studies have associated epigenetic machinery deregulation and this cancer type. Moreover, the potential of epigenetic biomarkers to assist in clinical management of BlCa patients, not only for detection, but also for follow-up, treatment monitoring and prediction of recurrence/progression has been intensively investigated [14,15]. In parallel, efforts have been made to understand how epigenetic mechanisms are involved in the various steps of urothelial carcinogenesis [16,17]. One question remains mostly unanswered. What mechanisms distinguish neoplastic cells with the ability to invade the muscle layer of the bladder, and eventually metastasize, from those that do not have this ability? In fact, epigenetics may help answer this question.
1.3. Epithelial to Mesenchymal Transition
The epithelial to mesenchymal transition (EMT) is a multistep process in which epithelial cells acquire a range of mesenchymal characteristics, which enables cell motility and invasiveness [18]. Importantly, these mesenchymal characteristics are reversible, with cells resuming their epithelial phenotype, through a process named mesenchymal to epithelial transition (MET). Recently, the classic concept of EMT, which strictly pointed out to mutually exclusive epithelial or mesenchymal phenotypes, has been challenged by “partial EMT” in which cells may transiently display both epithelial and mesenchymal features, corresponding to an intermediate state of EMT [19,20]. The concept of a partial EMT may be explained by implicating epigenetic regulation of EMT/MET reversibility and cell plasticity. Various factors and cellular environmental conditions are known to induce EMT, by triggering a cascade of signalling pathways that lead to post-transcriptional modification of the most well-known EMT transcription factors (EMT-TFs): Snail, Slug, ZEB1, ZEB2, and TWIST [21,22]. The interplay between the EMT-TFs and various key regulatory proteins and epigenetic enzymes that regulate EMT-TFs themselves, results in overexpression or repression of well-described EMT effectors, such as the cadherin family (CDH1, CDH2, and CDH3) and vimentin [23,24,25,26].
1.4. Influence of EMT Major Players in Bladder Cancer
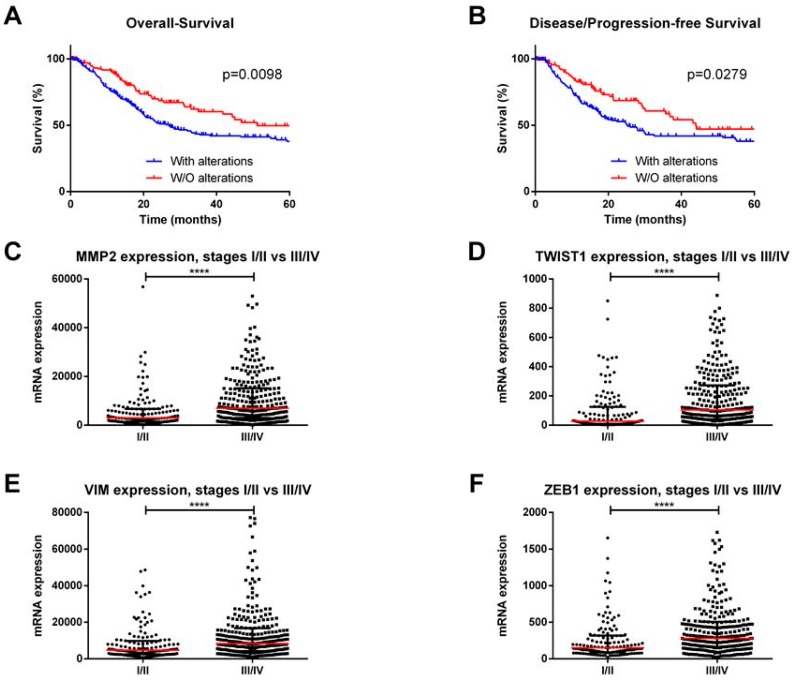
EMT is essential for various physiological processes, including early embryogenesis as well as in cancer. Accordingly, in vitro and in vivo studies implicated EMT in cell invasion and metastatic potential in several cancer types [19]. Intense research efforts uncovered the major EMT players in epithelial cancers, including BlCa. We performed an in silico analysis of The Cancer Genome Atlas (TCGA) database for BlCa (using the online resource cBioPortal for Cancer Genomics [27]), with a user-defined entry set of major EMT players (CDH1, CDH2, CDH3, CTNNB1, GSK3B, MMP2, MUC1, SNAI2, SNAI1, TWIST1, VIM, ZEB1, and ZEB2), and we found that these genes are deregulated in 272/413 (66%) tumors being significantly associated with reduced overall survival (p = 0.0098) and disease/progression-free survival (p = 0.0279) (Figure 1A,B). Furthermore, the expression levels of mesenchymal markers, like MMP2, VIM, TWIST1, ZEB1, and ZEB2, were significantly higher in stages III/IV when compared to stages I/II (p < 0.0001) (Figure 1C–F).
Figure 1.
In silico analysis of The Cancer Genome Atlas database for bladder cancer (using the online resource cBioPortal for Cancer Genomics). (A) Overall and (B) Disease/Progression-free survival curves according to major EMT players’ alterations. (C) MMP2, (D) TWIST1, (E) VIM, and (F) ZEB1 transcript levels in stages I/II vs. III/IV bladder cancer cases. **** p < 0.0001.
2. Epigenetic Enzymes and Mechanisms Altering EMT in Bladder Cancer
2.1. Protein-Coding Genes
DNA methylation and chromatin remodelling deregulation in cancer result from aberrant epigenetic enzymes’ activity that ultimately lead to abnormal gene expression, which empowers tumors to quickly evolve. It facilitates invasion and metastasis. Overall, while the importance of these epigenetic enzymes in promoting bladder cancer transformation has been already acknowledged, only a limited number of studies have characterized its role in the context of EMT process in this tumor model.
One of the epigenetic enzymes involved in EMT is the enhancer of zeste homolog 2 (EZH2), which is a core subunit of the polycomb repressive complex 2 (PRC2) that acts as a chromatin modifier by adding two or three methyl groups at H3K27 residues [28]. In several cancer models, EZH2 was proven to be associated with CDH1 transcriptional silencing and the mesenchymal phenotype [29,30,31]. Using chromatin immunoprecipitation (ChIP), Luo M. et al. demonstrated EZH2 and H3K23me3 enrichment within CDH1 promoter in BlCa cells even though no clues were yet provided on how PRC2 is specifically recruited to CDH1 [32]. Nonetheless, Kottakis et al. suggested that EZH2 might be regulated by FGF-2 upregulation in BlCa cells, which, in turn, upregulates the lysine demethylase 2B (KDM2B) and triggers EZH2 recruitment. The upregulation of these two enzymes is associated with miR-101 transcription repression, due to H3K36 demethylation by KDM2B, and H3K27 trimethylation by EZH2. As a result, and because EZH2 is also post-transcriptionally regulated by miR-101, these events ultimately contribute to EZH2 overexpression in a loop [33,34,35]. Moreover, several EMT-TFs were also found to be overexpressed in these cells, which further supports EZH2 implication in EMT [33,36]. The E2F1 transcription factor and the epigenetic reader BRD4 were also suggested as possible EZH2 regulators in BlCa, but its direct link with EMT and respective TFs is still elusive [37,38]. Importantly, because EZH2 overexpression is common to several tumors, inhibitors for this histone methyltransferase are under evaluation as potential anticancer drugs in phase one and two clinical trials [39]. Nevertheless, just one of the undergoing studies targets BlCa patients, and only those that have unresectable or metastatic disease [40]. The development of new therapies for BlCa is still an unmet need since these tumors have limited treatment options. Specifically, EZH2 inhibition might restrain the progression of non-muscle to muscle invasive disease.
DNA methylation—a covalent modification of DNA, in which a methyl group is transferred from S-adenosylmethionine (SAM) to the fifth carbon of a cytosine‒constitutes a stable and heritable mark frequently associated with the maintenance of a closed chromatin structure, which results in the silencing of repeat elements in the genome and genes’ transcriptional repression [41]. Across the genome, clustered regions of CpG dinucleotides, also known as CpG islands, are often found in genes’ promoter regions. Several cancer-related genes were reported to be regulated by promoter methylation, some of which were implicated in BlCa EMT (Table 1). Among these, serine protease PRSS8 was found to be downregulated by promoter methylation in high-grade BlCa tissues, and its overexpression in cell lines was associated with E-Cadherin upregulation, which suggests an interplay between these two proteins during epithelial differentiation [42,43].
Table 1.
Epigenetically modulated protein-coding genes implicated in Bladder Cancer EMT.
| Gene | Expression in BlCa | Effect on EMT | Epigenetic Regulation | Sample Type and Size | Author |
|---|---|---|---|---|---|
| MAEL | Upregulated | ↑EMT (↓ECAD, ↓β-catenin, ↑Fibronectin, ↑VIM) Recruitment of DNMT3B and HDAC1/2 to MTSS1 promoter) |
Downregulated by miR186 | 184 primary tumors, in vitro and in vivo assays | Li, X.D., 2016 [47] |
| GDF15 | Downregulated | ↓EMT (knockdown cells with ↓ECAD, ↑NCAD, ↑Snail, ↑Slug) |
Upregulated by demethylation | In vivo assays | Tsui, K.H. and Hsu, S.Y., 2015 [48] |
| KLF4 | Downregulated | ↓EMT (↑ECAD, ↓NCAD, ↓ β-catenin, ↓VIM, ↓Snail, ↓Slug) |
Promoter methylation; Upregulated by 5AZA treatment |
139 non-muscle invasive primary tumors, in vitro and in vivo assays | Li, H. and Wang, J., 2013 [49] |
| ↓EMT (Upregulation) |
Promoter methylation confirmed by BSP | In vitro assays | Xu, X., 2017 [50] |
||
| PRSS8 | Downregulated | ↓EMT (↑ECAD in cells with forced PRSS8 expression) |
Promoter methylation. Upregulated by 5AZA and TSA treatment |
40 primary tumors and in vivo assays | Chen, L.M., 2009 [43] |
| ELF5 | Downregulated | ↓EMT (↑ECAD, ↓NCAD, ↓VIM, ↓Snail, ↓ZEB1) |
Promoter methylation. Upregulated by 5AZA treatment |
182 FFPE + 50 FF primary tumors and in vivo assays | Wu, B., 2015 [46] |
Abbreviations: 5AZA—5-Azacytidine. BlCa—bladder cancer. BSP—Bisulfite sequencing. EMT—epithelial to mesenchymal transition. FF—Fresh-frozen. FFPE—Formalin-fixed paraffin-embedded. miR—microRNA. TSA—Trichostatin A.
Similarly, the Elf5 transcription factor, which is also regulated by methylation in several cellular developmental processes, was associated with EMT in primary BlCa and in vitro studies [44,45,46]. Elf5 reduced expression, both at mRNA and protein levels, is associated with disease progression, and, in BlCa cell lines, its downregulation is associated with increased mesenchymal markers, such as Snail, ZEB1, and vimentin. Furthermore, ELF5-silenced BlCa cells exhibited an invasive phenotype, and exposure to the demethylating agent 5-AZA restored ELF5 expression in the same cells, which attenuated its invasion capacity [46].
Furthermore, hypermethylation of the growth differentiation factor-15 (GDF15), which is a member of the TGF-β superfamily reported as an urothelial cancer biomarker [51,52], was found to be lower in BlCa cell lines derived from MIBC tumors. Moreover, GDF15-knockdown cells displayed E-Cadherin downregulation while several EMT-TFs were upregulated [48]. Thus, the discovery of epigenetically downregulated genes in MIBC provides new insights about BlCa progression and metastasis.
KLF4, which is a zinc finger transcription factor, is commonly downregulated in several cancers [53,54,55,56] including BlCa [49,50]. Specifically, KLF4 was found to be repressed by promoter methylation [49,50]. Furthermore, (CRISPR)-ON upregulation reduced BlCa cells’ migration, invasion and EMT abilities, which is paralleled by the growth inhibition of tumor xenografts and lung metastasis formation in mice. However, epigenetic editing (e.g., residue specific methylation or demethylation) would be more suitable for assessing the specific role of KLF4 promoter methylation in gene expression regulation [57,58]. The new epigenetic tools available would allow for the clarification of promoter methylation’s regulation of all the previously mentioned genes implicated in BlCa EMT and metastasis.
Several epigenetic mechanisms act synergistically to maintain the epigenetic landscape through a regulation loop in which they simultaneously control protein-coding genes’ expression and other epigenetic players at different regulation levels. Specifically, for BlCa, the oncogene maelstrom (MAEL), frequently upregulated in this malignancy, downregulates the metastasis suppressor MTSS1 gene by recruiting DNMT3B and HDAC1/2 to its promoter. Moreover, MAEL is also targeted by miR-186 and, possibly, by loss of promoter methylation, which constitutes an example of a gene that recruits epigenetic enzymes and is, in turn, regulated by epigenetic mechanisms [47].
2.2. Non-Coding RNAs
Non-coding RNAs (ncRNAs) are also involved in the dynamic regulation of EMT-related genes’ expression. There are several ncRNA categories, commonly classified according to their size, including the long ncRNAs (lncRNAs) with more than 200 nt and the small ncRNAs (sncRNAs), which present less than 200 nt [59,60]. ncRNAs, not only directly hinder messenger RNA (mRNA), but also interact (directly or indirectly) with DNMTs, various histone modifying enzymes, and remodelling complexes, which establishes important links between all epigenetic players that modulate gene expression. Therefore, ncRNAs have been implicated in a broad range of biological processes, including proliferation, adhesion, invasion, migration, metastasis, stemness, apoptosis, genomic instability, and, also, EMT, by mediating cell-cell communication (via ncRNA-containing extracellular vesicles), which binds to transcription factors and proteins, DNA methylation regulation, splicing, and scaffolding [61,62].
Among ncRNAs, sncRNAs have been considered the most biologically relevant in the context of EMT. They are involved in post-transcriptional regulation of target RNAs (by forming complexes with proteins of the Argonaute family) with microRNAs being the most intensively studied within this class. Their mature forms are single-stranded and have 20–25 nt in length, which constitutes the final product of a processing pathway involving DROSHA, DICER, and RISC [63]. In fact, in silico analysis has shown several up-regulated and downregulated microRNAs that target the most important EMT players associated with aggressive disease [64].
Our literature review disclosed 31 different microRNAs, which participate in BlCa EMT regulation [(Table 2), [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92]]. Most studies were performed in patients’ samples (n = 25) and/or in cell lines (n = 31), but some have also tested animal models (n = 9). For most microRNAs, the net effect was to counteract an EMT phenomenon (n = 25), while only miR92 family/miR92b, miR135a, miR221, miR224, and miR301b were reported to promote EMT. In addition, to a putative value as diagnostic markers, 13 microRNAs were shown to have prognostic and/or predictive value as well, associated with clinicopathological variables such as tumor grade, stage, occurrence of metastases, and patients’ survival.
Table 2.
Non-coding RNAs associated with EMT in bladder cancer.
| Non-Coding RNA | Effect on EMT (and Others) | Main Regulators | Main Targets/Pathways | Sample Type and Size | Author |
|---|---|---|---|---|---|
| Small Non-Coding RNAs | |||||
| miR22 | ↓EMT, diagnostic value (↓ in tumor, vs. normal) | Snail and MAPK/Slug/VIM | 13 primary tumors, in vitro and in vivo assays | Xu, M., 2018 [90] | |
| miR23b | ↓EMT, diagnostic (↓ in tumor vs. normal) and prognostic (↑OS) value | ZEB1 | 20 primary tumors and in vivo assays | Majid, S., 2013 [68] | |
| miR24 | ↓EMT, diagnostic value (↓ in tumor, vs. normal) | CARMA3 | In vitro assays | Zhang, S., 2015 [71] | |
| miR34a | ↓EMT, diagnostic value (↓ in tumor, vs. normal) | CD44 | 8 primary tumors, in vitro and in vivo assays | Yu, G., 2014 [72] | |
| miR92 (family) | ↑EMT, diagnostic (↑ in tumor vs. normal) value, induces cisplatin resistance | GSK-3β/ Wnt/c-myc/MMP7 | 20 primary tumors and In vitro assays | Wang, H., 2016 [79] | |
| miR92b | ↑EMT | DAB2IP | In vitro assays | Huang, J., 2016 [80] | |
| miR-124-3p | ↓EMT, diagnostic value (↓ in tumor, vs. normal) | ROCK1, MMP2, MMP9 | 13 primary tumors and in vitro assays | Xu, X., 2013 [66] | |
| miR135a | ↑EMT | GSK-3β | 165 primary tumors and in vitro assays | Mao, X.W., 2018 [91] | |
| miR141 | ↓EMT, prognostic value (LN metastases) | MMP2 and 9, Vimentin, N-Cadherin, E-Cadherin | 30 primary tumors, 78 urine samples and in vitro assays | Liu, W. and Qi, L., 2015 [93] | |
| miR-148a-3p | ↓EMT, diagnostic value (↓ in tumor, vs. normal) | ↓expression mediated by DNA methylation (DNMT1) – ↑expression with 5AZA | ERBB3-AKT2-c-myc/SNAIL axis | 59 primary tumors, in vitro and in vivo assays | Wang, X., 2016 [82] |
| miR186 | ↓EMT, diagnostic value (↓ in tumor, vs. normal) | NSBP1 | 20 primary tumors and in vitro assays | Yao, K., 2015 [73] | |
| miR-199a-5p | ↓EMT, diagnostic (↓ in tumor vs. normal) and prognostic (stage, grade) value | CCR7, MMP9 | 40 primary tumors and in vitro assays | Zhou, M., 2016 [81] | |
| miR200 (family) | ↓EMT, prognostic value (↑ survival) | ↓expression mediated by EZH2 and BMI-1 | BMI-1, ZEB1, ZEB2 | 87 primary tumors and in vitro assays | Martínez-Fernández, M. and Duenas, M., 2015 [74] |
| ↓EMT and proliferation, diagnostic (↓ in tumor vs. normal) and prognostic (↑ survival) value | BMI-1 and E2F3 | 15 primary tumors and in vitro assays | Liu, L., 2014 [69] | ||
| miR200b | ↓EMT, prognostic value (LN metastases) | MMP2 and 9, Vimentin, N-Cadherin, E-Cadherin | 30 primary tumors, 78 urine samples and in vitro assays | Liu, W. and Qi, L., 2015 [93] | |
| ↓EMT | ↓expression mediated by TGF-β1 | MMP16 | In vitro assays | Chen, M.F. and Zeng, F., 2014 [75] | |
| miR200c | ↓EMT, restores sensitivity to EGFR inhibitors | ZEB1, ZEB2 and ERRFI-1 | In vitro assays | Adam, L., 2009 [65] | |
| miR203 | ↓EMT, diagnostic value (↓ in tumor, vs. normal) | Twist1 | 24 primary tumors and in vitro assays | Shen, J., 2017 [85] | |
| miR205 | ↓EMT, poor prognosis | ↑expression mediated by p63 isoform ΔNp63α | ZEB1, ZEB2 | 98 primary tumors and in vitro assays | Tran, M.N., 2013 [67] |
| miR221 | ↑EMT | ↑expression mediated by TGF-β1 | STMN1 | In vitro assays | Liu, J., 2015 [76] |
| miR224 | ↑EMT, diagnostic (↑ in tumor vs. normal) and prognostic (stage, metastases, ↓survival) value | SUFU/Hedgehog pathway | 97 primary tumors, in vitro and in vivo assays | Miao, X., Gao, H. and Liu, S., 2018 [86] | |
| miR301b | ↑EMT, diagnostic value (↑ in tumor, vs. normal) | EGR1 | In vitro assays | Yan, L., 2017 [94] | |
| miR-323a-3p | ↓EMT, diagnostic (↓ in tumor vs. normal) and prognostic (↑OS) value | ↓expression mediated by methylation of IG-DMR | Met/SMAD3/Snail | 9 primary tumors and in vivo assays | Li, J., 2017 [87] |
| miR-370-3p | ↓EMT | Wnt7a | 41 primary tumors in vitro and in vivo assays | Huang, X. and Zhu, H., 2018 [92] | |
| miR-370-5p | ↓EMT | p21 | In vitro assays | Wang, C., 2016 [95] | |
| miR424 | ↓EMT, diagnostic (↓ in tumor vs. normal) and prognostic (stage, ↑OS and DFS) value | ↓expression mediated by DNMT1 | EGFR pathway | 124 primary tumors, in vitro and in vivo assays | Wu, C.T., 2015 [77] |
| miR429 | ↓EMT | ZEB1/βcatenin axis | In vitro assays | Wu, C.L., 2016 [83] | |
| miR433 | ↓EMT, diagnostic value (↓ in tumor, vs. normal) | c-Met/CREB1-Akt/GSK-3β/Snail | 13 primary tumors and in vitro assays | Xu, X., 2016 [84] | |
| miR451 | ↓EMT, diagnostic (↓ in tumor vs. normal) and prognostic (grade and stage) value | E-Cadherin, N-Cadherin | 40 primary tumors and in vitro assays | Zeng, T. and Peng, L., 2014 [70] | |
| miR-485-5p | ↓EMT, diagnostic value (↓ in tumor vs. normal) | HMGA2 | 15 primary tumors and in vitro assays | Chen, Z., 2015 [78] | |
| miR497 | ↓EMT, diagnostic (↓ in tumor vs. normal) and prognostic (stage, metastases) value | E-Cadherin, Vimentin | 50 primary tumors and in vitro assays | Wei, Z., 2017 [88] | |
| miR612 | ↓EMT, diagnostic (↓ in tumor vs. normal) and prognostic (stage, metastases) value | ME1 | 46 primary tumors and in vitro assays | Liu, M. and Chen, Y., 2018 [96] | |
| miR613 | ↓EMT, diagnostic value (↓ in tumor vs. normal) | SphK1 | 35 primary tumors and in vitro assays | Yu, H., 2017 [89] | |
| Long non-coding RNAs | |||||
| circRNA MYLK | ↑EMT, prognostic value (stage, grade) | miR29a/VEGFA/VEGFR2 axis | 32 primary tumors, in vitro and in vivo assays | Zhong, Z., 2017 [97] | |
| lncRNA GHET1 | ↑EMT, diagnostic (↑ in tumor vs. normal) and prognostic (grade, stage, metastases, ↓OS) value | E-Cadherin, Vimentin, Fibronectin, Slug, Twist, Snail, ZEB1 | 80 primary tumors and in vitro assays | Li, L.J., 2014 [98] | |
| lncRNA HOTAIR | ↑EMT | Various EMT players | 10 primary tumors and in vitro assays | Berrondo, C., 2016 [99] | |
| lncRNA H19 | ↑EMT, diagnostic value (↑ in tumor vs. normal) | miR-29b-3p/DNMT3B axis | 35 primary tumors, in vitro and in vivo assays | Lv, M., 2017 [100] | |
| lncRNA Malat1 | ↑EMT, poor prognosis | ↑expression mediated by TGF-β | suz12 | 95 primary tumors, in vitro and in vivo assays | Fan, Y., 2014 [101] |
| lncRNA ROR | ↑EMT, diagnostic value (↑ in tumor vs. normal) | ZEB1 | 36 primary tumors and in vitro assays | Chen, Y., 2017 [102] | |
| lncRNA TP73-AS1 | ↓EMT, diagnostic (↓ in tumor vs. normal) and prognostic (↑OS and PFS) value | Various EMT players | 128 primary tumors and in vitro assays | Tuo, Z., 2018 [103] | |
| lncRNA TUG1 | ↑EMT, diagnostic (↑ in tumor vs. normal) and prognostic (stage, ↓OS) value, promotes radio-resistance | miR145/ ZEB2 axis | 54 primary tumors, in vitro and in vivo assays | Tan, J., 2015 [104] | |
| lncRNA UCA1 | ↑EMT | miR145-ZEB1/2-FSCN1 axis | In vitro assays | Xue, M., 2016 [105] | |
| miR143/HMGB1 | 52 primary tumors and in vitro assays | Luo, J., 2017 [106] | |||
| lncRNA XIST | ↑EMT | miR200c | In vitro and in vivo assays | Xu, R., 2018 [107] | |
| lncRNA ZEB2NAT | ↑EMT, diagnostic value (↑ in tumor vs. normal) | ↑expression mediated by TGF-β1 | ZEB2 | 30 primary tumors and in vitro assays | Zhuang, J. and Lu, Q., 2015 [108] |
Abbreviations: DFS—disease-free survival. EMT—epithelial to mesenchymal transition. lncRNA—long non-coding RNA. miR—microRNA. OS—overall survival.
Some of the most well-studied microRNAs belong to the miR200 family. Their expression has been found to hamper EMT in different tumor models such as breast, prostate, ovarian, and endometrial carcinomas, in part by affecting different EMT players like ZEB1, ZEB2, and E-Cadherin [109,110,111,112,113]. Martínez-Fernández et al. [74] showed that PRC members EZH2 and BMI1 repress miR200 family, which results in EMT activation and aggressive disease, which is in accordance with the association of EZH2 overexpression with high risk for recurrence in NMIBC [114]. These findings support the dynamic regulation and cooperation between protein coding and non-coding RNAs in EMT. Since EZH2 pharmacological inhibition is already available and efficiently increases miR200 in BlCa cell lines, this might constitute a therapeutic opportunity for hindering cancer progression. It has also been reported that epidermal growth factor receptor (EGFR) inhibition may lead to therapeutic resistance due to mesenchymal features. Additionally, miR200c induction (which targets the ERBB receptor feedback inhibitor 1‒ERRFI-1) is effective in restoring sensitivity to EGFR inhibitors, which constitutes another example of pharmacological modulation of EMT that could be translated into clinical practice [65]. Lastly, another member of the miR200 family, miR200b, was demonstrated to target matrix metalloproteinase-16 (MMP16) in BlCa cell lines, which is downregulated by transforming growth factor beta 1 (TGF-β1), previously associated with metastatic potential acquisition. This leads to miR downregulation having a net effect of promoting EMT [75]. In fact, TGF-β1 also cooperates with several other miRs, including miR221. Liu et al. showed that, by targeting STMN1, miR221 facilitates TGF-β1-induced EMT, and that its inhibition resulted in increased levels of epithelial marker E-cadherin and reduction of mesenchymal markers such as vimentin, fibroactin, and N-cadherin [76].
A connection between microRNAs and methylation was also reported, which disclosed a feedback loop between DNMT1 and miR-148a-3p [79]. miR-148a-3p, a BlCa tumor suppressor, and an EMT inhibitor, by targeting the ERBB3/AKT2/c-MYC axis, was shown to be downregulated by DNMT1-induced methylation. Moreover, re-expression was observed after treatment with 5-Aza-2’-deoxycytidine (5AZA) [79]. Wu et al. obtained similar findings for miR424 in BlCa cell lines, in vivo models, and patient-derived specimens. DNMT1 inhibition resulted in substantial miR424 upregulation, which, in turn, promoted epithelial characteristics of BlCa cells (changing the relative expression levels of E-cadherin and Twist) and resulted in reduced invasion ability. Additionally, the same authors identified the EGFR-PI3K-AKT axis as the target of miR424, explaining its effect on EMT [77]. Lastly, miR-323a-3p was also implicated in EMT of BlCa cells by targeting MET and SMAD3, which interfered with their regulation of Snail and resulted in the net effect of repressing EMT. On the other hand, miR-323a-3p is downregulated by aberrant methylation of the intergenic differential methylated region (IG-DMR) [87]. In addition, miRs might also be regulated by methylation and this feature might be used for urothelial carcinoma detection in bodily fluids, such as urine [115].
EMT-related miRs have also been demonstrated to impact the resistance to cytotoxic drugs. Furthermore, miR-92 was found to promote EMT (changing the relative expression levels of two of its major players, E-cadherin, and vimentin) by activating glycogen synthase kinase 3 beta (GSK3B) and the Wnt signalling pathway, inducing resistance to cisplatin (increasing BlCa cells viability and decreasing apoptosis upon treatment with cisplatin) [79].
Most of the human genome is transcribed into structural ncRNAs. LncRNAs, which include both linear and circular forms (the latter being referred to as circRNAs), display different regulatory functions, according to their cellular location. Whereas nuclear lncRNAs can either sequester transcription factors and recruit chromatin-remodelling complexes to a cell-site (hence impeding transcription), or trigger chromatin-modifying complexes (thus, activating transcription), cytoplasmic lncRNAs modulate RNAs stability and translation, competing with endogenous RNAs (ceRNAs) for microRNA binding. Additionally, having a longer half-life than their linear counterparts, circRNAs may also act as microRNA “sponges” [116,117].
Eleven lncRNAs (ten linear and one circRNA) [97,98,99,100,101,102,103,104,105,106,107,108] have been reported to modulate EMT in BlCa. Contrary to microRNAs, only one lncRNA (TP73-AS1) was implicated in negative regulation of EMT, whereas all the remainder substances promoted its activation. Five of the lncRNAs (including circRNA MYLK and lncRNAs GHET1, Malat1, TP73-AS1, and TUG1) were explored as potential diagnostic and prognostic biomarkers.
CircRNA MYLK was found to function as ceRNA for miR-29a, which, in result, promotes EMT and activates the vascular endothelial growth factor receptor (VEGFR) pathway, which is associated with BlCa progression [97]. Thus, circRNA MYLK modulation might constitute a therapeutic target in combination with anti-VEGF drugs such as bevacizumab. Moreover, Lv et al. [100] have shown that lncRNA H19 also functions as a ceRNA for miR-29b-3p, which is another member of the miR29 family. Therefore, this allows for the expression of target DNMT3B, reprograms DNA methylation patterns, and promotes EMT (through Twist, vimentin, and MMP9 upregulation and E-cadherin downregulation) and metastasis.
Non-coding RNAs may modulate not only the response to systemic treatments, but also to local therapies such as radiotherapy. Tan et al. [104] showed that miR145’s downmodulation by lncRNA TUG1 associated with EMT and radio-resistance due to its action on the ZEB2 axis. Targeting this lncRNA might re-sensitize BlCa to radiotherapy, which results in a better patient response and outcome.
Furthermore, TGF-β1 leads to overexpression of lncRNA malat1, which is associated with suppressor of zeste 12 (suz12), decreases E-cadherin, and increases N-cadherin and fibronectin expression levels [101]. Moreover, another lncRNA-ZEB2NAT—was shown to be essential for the role of TGF-β1-secreting cancer associated fibroblasts (CAFs) in promoting EMT in BlCa cells. Zhuang et al. elegantly showed that CAFs induce EMT by activating the TGF-β1/ZEB2NAT/ZEB2 axis, whereas ZEB2NAT inhibition reduced ZEB2 expression levels and inhibited BlCa cells invasion capacity [108].
Several lncRNAs might target the same microRNA and the same lncRNA may influence more than one microRNA simultaneously. Such is the case of lncRNA UCA1, which induces EMT either by targeting miR145 or miR143 [105,106]. These studies suggest that lncRNAs might be implicated in EMT by interfering with several pathways through various regulatory functions, due to their redundancy.
3. Conclusions
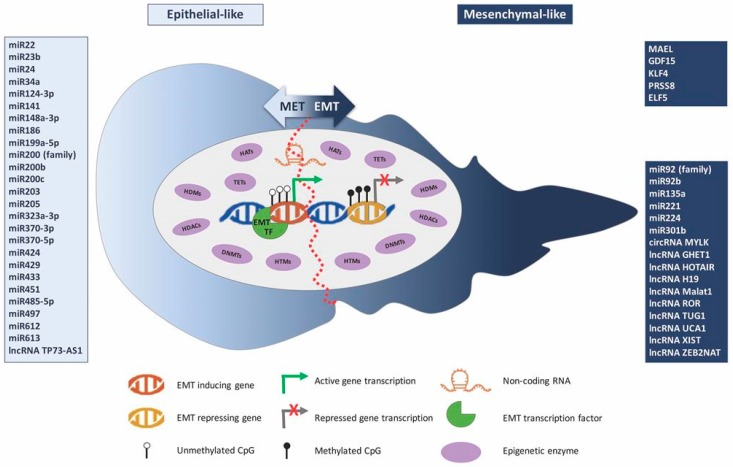
As discussed in this review, epigenetic mechanisms and connected enzymes are intrinsically involved in the various steps of EMT in BlCa cells, which acts in concert and controlling EMT-TFs as well as several upstream targets (Figure 2). All the studies published to the date illuminate the way for the development of specific anti-cancer drugs, which could abrogate EMT by targeting epigenetic enzymes and genes regulated by these reversible modifications.
Figure 2.
Epigenetic mechanisms’ interplay with the epithelial-to-mesenchymal transition process in bladder cancer.
Nevertheless, the epigenetic regulation of EMT requires further investigation to provide clinically useful information for BlCa patient management.
Abbreviations
| 5AZA | 5-Aza-2’-deoxycytidine |
| BlCa | Bladder cancer |
| CAF | Cancer associated fibroblast |
| ceRNA | Competing endogenous RNA |
| circRNA | Circular RNA |
| DNMT | DNA methyltransferase |
| dUb | Histone deubiquitinase |
| ECAD | Cadherin 1, E-Cadherin |
| ELF5 | E74 Like ETS Transcription Factor 5 |
| EMT | Epithelial to mesenchymal transition |
| EMT-TF | EMT transcription factor |
| GDF15 | Growth Differentiation Factor 15 |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylase |
| HDM | Histone demethylase |
| HTM | Histone methyltransferase |
| KLF4 | Kruppel Like Factor 4 |
| lncRNA | Long non-coding RNA |
| MAEL | Maelstrom Spermatogenic Transposon Silencer |
| MET | Mesenchymal to epithelial transition |
| MIBC | Muscle invasive bladder cancer |
| miR | Micro RNA |
| MTSS1 | Metastasis Supressor Protein 1 |
| NCAD | Cadherin 2, N-Cadherin |
| ncRNA | Non-coding RNA |
| NMIBC | Non-muscle invasive bladder cancer |
| PRC | Polycomb repressive complex |
| PRSS8 | Serine Protease 8 |
| Snail | Snail Family Transcriptional Repressor 1 |
| Slug | Snail Family Transcriptional Repressor 2 |
| sncRNA | Small non-coding RNA |
| TET | DNA demethylase |
| UbL | Histone ubiquitin ligase |
| VIM | Vimentin |
| ZEB1 | Zync Finger E-Box Binding Homeobox 1 |
| ZEB2 | Zync Finger E-Box Binding Homeobox 2 |
Funding
This research was funded by research grants from the Research Center of Portuguese Oncology Institute of Porto (CI-IPOP-FBGEBC-27) and (PI 74-CI-IPOP-19-2016). SR-M and JL are supported by FCT – Fundação para a Ciência e Tecnologia PhD fellowships (SFRH/BD/112673/2015 and SFRH/BD/132751/2017).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wong M.C.S., Fung F.D.H., Leung C., Cheung W.W.L., Goggins W.B., Ng C.F. The global epidemiology of bladder cancer: A joinpoint regression analysis of its incidence and mortality trends and projection. Sci. Rep. 2018;8:1129. doi: 10.1038/s41598-018-19199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Tomorrow. WHO; Geneva, Switzerland: 2018. [Google Scholar]
- 3.Antoni S., Ferlay J., Soerjomataram I., Znaor A., Jemal A., Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Greiman A.K., Rosoff J.S., Prasad S.M. Association of Human Development Index with global bladder, kidney, prostate and testis cancer incidence and mortality. BJU Int. 2017;120:799–807. doi: 10.1111/bju.13875. [DOI] [PubMed] [Google Scholar]
- 5.Leal J., Luengo-Fernandez R., Sullivan R., Witjes J.A. Economic Burden of Bladder Cancer Across the European Union. Eur. Urol. 2016;69:438–447. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Sanli O., Dobruch J., Knowles M.A., Burger M., Alemozaffar M., Nielsen M.E., Lotan Y. Bladder cancer. Nat. Rev. Dis. Primers. 2017;3:17022. doi: 10.1038/nrdp.2017.22. [DOI] [PubMed] [Google Scholar]
- 7.Moch H., Ulbright T., Humphrey P., Reuter V. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. IARC; Lyon, France: 2016. [DOI] [PubMed] [Google Scholar]
- 8.Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat. Biotechnol. 2010;28:1079–1088. doi: 10.1038/nbt.1684. [DOI] [PubMed] [Google Scholar]
- 9.Skinner M.K. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res. C Embryo Today. 2011;93:51–55. doi: 10.1002/bdrc.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saitou M., Kagiwada S., Kurimoto K. Epigenetic reprogramming in mouse pre-implantation development and primordial germ cells. Development. 2012;139:15–31. doi: 10.1242/dev.050849. [DOI] [PubMed] [Google Scholar]
- 11.Kelly A.D., Issa J.J. The promise of epigenetic therapy: Reprogramming the cancer epigenome. Curr. Opin. Genet. Dev. 2017;42:68–77. doi: 10.1016/j.gde.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Baylin S.B., Jones P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteller M. Epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 14.Mossanen M., Gore J.L. The burden of bladder cancer care: Direct and indirect costs. Curr. Opin. Urol. 2014;24:487–491. doi: 10.1097/MOU.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 15.Kojima T., Kawai K., Miyazaki J., Nishiyama H. Biomarkers for precision medicine in bladder cancer. Int. J. Clin. Oncol. 2017;22:207–213. doi: 10.1007/s10147-016-1068-8. [DOI] [PubMed] [Google Scholar]
- 16.Dudziec E., Goepel J.R., Catto J.W. Global epigenetic profiling in bladder cancer. Epigenomics. 2011;3:35–45. doi: 10.2217/epi.10.71. [DOI] [PubMed] [Google Scholar]
- 17.Schulz W.A., Koutsogiannouli E.A., Niegisch G., Hoffmann M.J. Epigenetics of urothelial carcinoma. Methods Mol. Biol. 2015;1238:183–215. doi: 10.1007/978-1-4939-1804-1_10. [DOI] [PubMed] [Google Scholar]
- 18.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Tam W.L., Weinberg R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013;19:1438–1449. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 22.De Craene B., Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 23.Van Roy F., Berx G. The cell-cell adhesion molecule E-cadherin. Cell. Mol. Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bracken C.P., Gregory P.A., Kolesnikoff N., Bert A.G., Wang J., Shannon M.F., Goodall G.J. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 25.Chaffer C.L., Brennan J.P., Slavin J.L., Blick T., Thompson E.W., Williams E.D. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: Role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 26.Choi J., Park S.Y., Joo C.K. Transforming growth factor-beta1 represses E-cadherin production via slug expression in lens epithelial cells. Investig. Ophthalmol. Vis. Sci. 2007;48:2708–2718. doi: 10.1167/iovs.06-0639. [DOI] [PubMed] [Google Scholar]
- 27.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Q., Yu J., Dhanasekaran S.M., Kim J.H., Mani R.S., Tomlins S.A., Mehra R., Laxman B., Cao X., Yu J., et al. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27:7274–7284. doi: 10.1038/onc.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiwari N., Tiwari V.K., Waldmeier L., Balwierz P.J., Arnold P., Pachkov M., Meyer-Schaller N., Schubeler D., van Nimwegen E., Christofori G. Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell. 2013;23:768–783. doi: 10.1016/j.ccr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Liu L., Xu Z., Zhong L., Wang H., Jiang S., Long Q., Xu J., Guo J. Enhancer of zeste homolog 2 (EZH2) promotes tumour cell migration and invasion via epigenetic repression of E-cadherin in renal cell carcinoma. BJU Int. 2016;117:351–362. doi: 10.1111/bju.12702. [DOI] [PubMed] [Google Scholar]
- 31.Liu X., Wang C., Chen Z., Jin Y., Wang Y., Kolokythas A., Dai Y., Zhou X. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem. J. 2011;440:23–31. doi: 10.1042/BJ20111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo M., Li Z., Wang W., Zeng Y., Liu Z., Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–221. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Kottakis F., Polytarchou C., Foltopoulou P., Sanidas I., Kampranis S.C., Tsichlis P.N. FGF-2 regulates cell proliferation, migration, and angiogenesis through an NDY1/KDM2B-miR-101-EZH2 pathway. Mol. Cell. 2011;43:285–298. doi: 10.1016/j.molcel.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman J.M., Liang G., Liu C.C., Wolff E.M., Tsai Y.C., Ye W., Zhou X., Jones P.A. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69:2623–2629. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 35.Varambally S., Cao Q., Mani R.S., Shankar S., Wang X., Ateeq B., Laxman B., Cao X., Jing X., Ramnarayanan K., et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNiel E.A., Tsichlis P.N. Analyses of publicly available genomics resources define FGF-2-expressing bladder carcinomas as EMT-prone, proliferative tumors with low mutation rates and high expression of CTLA-4, PD-1 and PD-L1. Signal Transduct. Target. Ther. 2017;2 doi: 10.1038/sigtrans.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S.R., Roh Y.G., Kim S.K., Lee J.S., Seol S.Y., Lee H.H., Kim W.T., Kim W.J., Heo J., Cha H.J., et al. Activation of EZH2 and SUZ12 Regulated by E2F1 Predicts the Disease Progression and Aggressive Characteristics of Bladder Cancer. Clin. Cancer Res. 2015;21:5391–5403. doi: 10.1158/1078-0432.CCR-14-2680. [DOI] [PubMed] [Google Scholar]
- 38.Wu X., Liu D., Tao D., Xiang W., Xiao X., Wang M., Wang L., Luo G., Li Y., Zeng F., et al. BRD4 Regulates EZH2 Transcription through Upregulation of C-MYC and Represents a Novel Therapeutic Target in Bladder Cancer. Mol. Cancer Ther. 2016;15:1029–1042. doi: 10.1158/1535-7163.MCT-15-0750. [DOI] [PubMed] [Google Scholar]
- 39.Jones B.A., Varambally S., Arend R.C. Histone Methyltransferase EZH2: A Therapeutic Target for Ovarian Cancer. Mol. Cancer Ther. 2018;17:591–602. doi: 10.1158/1535-7163.MCT-17-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ORIOn-E: A Study Evaluating CPI-1205 in Patients with Advanced Solid Tumors. [(accessed on 03 December 2018)]; Available online: https://clinicaltrials.gov/show/NCT03525795.
- 41.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 42.Leyvraz C., Charles R.P., Rubera I., Guitard M., Rotman S., Breiden B., Sandhoff K., Hummler E. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J. Cell Biol. 2005;170:487–496. doi: 10.1083/jcb.200501038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L.M., Verity N.J., Chai K.X. Loss of prostasin (PRSS8) in human bladder transitional cell carcinoma cell lines is associated with epithelial-mesenchymal transition (EMT) BMC Cancer. 2009;9:377. doi: 10.1186/1471-2407-9-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemberger M., Udayashankar R., Tesar P., Moore H., Burton G.J. ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum. Mol. Genet. 2010;19:2456–2467. doi: 10.1093/hmg/ddq128. [DOI] [PubMed] [Google Scholar]
- 45.Ng R.K., Dean W., Dawson C., Lucifero D., Madeja Z., Reik W., Hemberger M. Epigenetic restriction of embryonic cell lineage fate by methylation of Elf5. Nat. Cell Biol. 2008;10:1280–1290. doi: 10.1038/ncb1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu B., Cao X., Liang X., Zhang X., Zhang W., Sun G., Wang D. Epigenetic regulation of Elf5 is associated with epithelial-mesenchymal transition in urothelial cancer. PLoS ONE. 2015;10:e0117510. doi: 10.1371/journal.pone.0117510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X.D., Zhang J.X., Jiang L.J., Wang F.W., Liu L.L., Liao Y.J., Jin X.H., Chen W.H., Chen X., Guo S.J., et al. Overexpression of maelstrom promotes bladder urothelial carcinoma cell aggressiveness by epigenetically downregulating MTSS1 through DNMT3B. Oncogene. 2016;35:6281–6292. doi: 10.1038/onc.2016.165. [DOI] [PubMed] [Google Scholar]
- 48.Tsui K.H., Hsu S.Y., Chung L.C., Lin Y.H., Feng T.H., Lee T.Y., Chang P.L., Juang H.H. Growth differentiation factor-15: A p53- and demethylation-upregulating gene represses cell proliferation, invasion, and tumorigenesis in bladder carcinoma cells. Sci. Rep. 2015;5:12870. doi: 10.1038/srep12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H., Wang J., Xiao W., Xia D., Lang B., Wang T., Guo X., Hu Z., Ye Z., Xu H. Epigenetic inactivation of KLF4 is associated with urothelial cancer progression and early recurrence. J. Urol. 2014;191:493–501. doi: 10.1016/j.juro.2013.08.087. [DOI] [PubMed] [Google Scholar]
- 50.Xu X., Li J., Zhu Y., Xie B., Wang X., Wang S., Xie H., Yan H., Ying Y., Lin Y., et al. CRISPR-ON-Mediated KLF4 overexpression inhibits the proliferation, migration and invasion of urothelial bladder cancer in vitro and in vivo. Oncotarget. 2017;8:102078–102087. doi: 10.18632/oncotarget.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costa V.L., Henrique R., Danielsen S.A., Duarte-Pereira S., Eknaes M., Skotheim R.I., Rodrigues A., Magalhaes J.S., Oliveira J., Lothe R.A., et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin. Cancer Res. 2010;16:5842–5851. doi: 10.1158/1078-0432.CCR-10-1312. [DOI] [PubMed] [Google Scholar]
- 52.Monteiro-Reis S., Leca L., Almeida M., Antunes L., Monteiro P., Dias P.C., Morais A., Oliveira J., Henrique R., Jeronimo C. Accurate detection of upper tract urothelial carcinoma in tissue and urine by means of quantitative GDF15, TMEFF2 and VIM promoter methylation. Eur. J. Cancer. 2014;50:226–233. doi: 10.1016/j.ejca.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Wei D., Gong W., Kanai M., Schlunk C., Wang L., Yao J.C., Wu T.T., Huang S., Xie K. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65:2746–2754. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y., Goldstein B.G., Chao H.H., Katz J.P. KLF4 and KLF5 regulate proliferation, apoptosis and invasion in esophageal cancer cells. Cancer Biol. Ther. 2005;4:1216–1221. doi: 10.4161/cbt.4.11.2090. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Place R.F., Huang V., Wang X., Noonan E.J., Magyar C.E., Huang J., Li L.C. Prognostic value and function of KLF4 in prostate cancer: RNAa and vector-mediated overexpression identify KLF4 as an inhibitor of tumor cell growth and migration. Cancer Res. 2010;70:10182–10191. doi: 10.1158/0008-5472.CAN-10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu W., Hofstetter W.L., Li H., Zhou Y., He Y., Pataer A., Wang L., Xie K., Swisher S.G., Fang B. Putative tumor-suppressive function of Kruppel-like factor 4 in primary lung carcinoma. Clin. Cancer Res. 2009;15:5688–5695. doi: 10.1158/1078-0432.CCR-09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu X., Tao Y., Gao X., Zhang L., Li X., Zou W., Ruan K., Wang F., Xu G.L., Hu R. A CRISPR-based approach for targeted DNA demethylation. Cell Discov. 2016;2:16009. doi: 10.1038/celldisc.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen H., Kazemier H.G., de Groote M.L., Ruiters M.H., Xu G.L., Rots M.G. Induced DNA demethylation by targeting Ten-Eleven Translocation 2 to the human ICAM-1 promoter. Nucleic Acids Res. 2014;42:1563–1574. doi: 10.1093/nar/gkt1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattick J.S., Rinn J.L. Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol. 2015;22:5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]
- 60.Ramalho-Carvalho J., Fromm B., Henrique R., Jeronimo C. Deciphering the function of non-coding RNAs in prostate cancer. Cancer Metastasis Rev. 2016;35:235–262. doi: 10.1007/s10555-016-9628-y. [DOI] [PubMed] [Google Scholar]
- 61.Peschansky V.J., Wahlestedt C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics. 2014;9:3–12. doi: 10.4161/epi.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anfossi S., Babayan A., Pantel K., Calin G.A. Clinical utility of circulating non-coding RNAs—An update. Nat. Rev. Clin. Oncol. 2018;15:541–563. doi: 10.1038/s41571-018-0035-x. [DOI] [PubMed] [Google Scholar]
- 63.Ghildiyal M., Zamore P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falzone L., Candido S., Salemi R., Basile M.S., Scalisi A., McCubrey J.A., Torino F., Signorelli S.S., Montella M., Libra M. Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget. 2016;7:72758–72766. doi: 10.18632/oncotarget.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adam L., Zhong M., Choi W., Qi W., Nicoloso M., Arora A., Calin G., Wang H., Siefker-Radtke A., McConkey D., et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin. Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X., Li S., Lin Y., Chen H., Hu Z., Mao Y., Xu X., Wu J., Zhu Y., Zheng X., et al. MicroRNA-124-3p inhibits cell migration and invasion in bladder cancer cells by targeting ROCK1. J. Transl. Med. 2013;11:276. doi: 10.1186/1479-5876-11-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tran M.N., Choi W., Wszolek M.F., Navai N., Lee I.L., Nitti G., Wen S., Flores E.R., Siefker-Radtke A., Czerniak B., et al. The p63 protein isoform DeltaNp63alpha inhibits epithelial-mesenchymal transition in human bladder cancer cells: Role of MIR-205. J. Biol. Chem. 2013;288:3275–3288. doi: 10.1074/jbc.M112.408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majid S., Dar A.A., Saini S., Deng G., Chang I., Greene K., Tanaka Y., Dahiya R., Yamamura S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS ONE. 2013;8:e67686. doi: 10.1371/journal.pone.0067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu L., Qiu M., Tan G., Liang Z., Qin Y., Chen L., Chen H., Liu J. miR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J. Transl. Med. 2014;12:305. doi: 10.1186/s12967-014-0305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng T., Peng L., Chao C., Fu B., Wang G., Wang Y., Zhu X. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int. J. Clin. Exp. Pathol. 2014;7:7653–7662. [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang S., Zhang C., Liu W., Zheng W., Zhang Y., Wang S., Huang D., Liu X., Bai Z. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int. J. Oncol. 2015;47:1351–1360. doi: 10.3892/ijo.2015.3117. [DOI] [PubMed] [Google Scholar]
- 72.Yu G., Xu K., Xu S., Zhang X., Huang Q., Lang B. MicroRNA-34a regulates cell cycle by targeting CD44 in human bladder carcinoma cells. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:935–940. [PubMed] [Google Scholar]
- 73.Yao K., He L., Gan Y., Zeng Q., Dai Y., Tan J. MiR-186 suppresses the growth and metastasis of bladder cancer by targeting NSBP1. Diagn. Pathol. 2015;10:146. doi: 10.1186/s13000-015-0372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martinez-Fernandez M., Duenas M., Feber A., Segovia C., Garcia-Escudero R., Rubio C., Lopez-Calderon F.F., Diaz-Garcia C., Villacampa F., Duarte J., et al. A Polycomb-mir200 loop regulates clinical outcome in bladder cancer. Oncotarget. 2015;6:42258–42275. doi: 10.18632/oncotarget.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen M.F., Zeng F., Qi L., Zu X.B., Wang J., Liu L.F., Li Y. Transforming growth factorβ1 induces epithelialmesenchymal transition and increased expression of matrix metalloproteinase16 via miR200b downregulation in bladder cancer cells. Mol. Med. Rep. 2014;10:1549–1554. doi: 10.3892/mmr.2014.2366. [DOI] [PubMed] [Google Scholar]
- 76.Liu J., Cao J., Zhao X. miR-221 facilitates the TGFbeta1-induced epithelial-mesenchymal transition in human bladder cancer cells by targeting STMN1. BMC Urol. 2015;15:36. doi: 10.1186/s12894-015-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu C.T., Lin W.Y., Chang Y.H., Lin P.Y., Chen W.C., Chen M.F. DNMT1-dependent suppression of microRNA424 regulates tumor progression in human bladder cancer. Oncotarget. 2015;6:24119–24131. doi: 10.18632/oncotarget.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z., Li Q., Wang S., Zhang J. miR4855p inhibits bladder cancer metastasis by targeting HMGA2. Int. J. Mol. Med. 2015;36:1136–1142. doi: 10.3892/ijmm.2015.2302. [DOI] [PubMed] [Google Scholar]
- 79.Wang H., Ke C., Ma X., Zhao Q., Yang M., Zhang W., Wang J. MicroRNA-92 promotes invasion and chemoresistance by targeting GSK3β and activating Wnt signaling in bladder cancer cells. Tumour Biol. 2016 doi: 10.1007/s13277-016-5460-9. [DOI] [PubMed] [Google Scholar]
- 80.Huang J., Wang B., Hui K., Zeng J., Fan J., Wang X., Hsieh J.T., He D., Wu K. miR-92b targets DAB2IP to promote EMT in bladder cancer migration and invasion. Oncol. Rep. 2016;36:1693–1701. doi: 10.3892/or.2016.4940. [DOI] [PubMed] [Google Scholar]
- 81.Zhou M., Wang S., Hu L., Liu F., Zhang Q., Zhang D. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol. 2016;16:64. doi: 10.1186/s12894-016-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X., Liang Z., Xu X., Li J., Zhu Y., Meng S., Li S., Wang S., Xie B., Ji A., et al. miR-148a-3p represses proliferation and EMT by establishing regulatory circuits between ERBB3/AKT2/c-myc and DNMT1 in bladder cancer. Cell Death Dis. 2016;7:e2503. doi: 10.1038/cddis.2016.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu C.L., Ho J.Y., Chou S.C., Yu D.S. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget. 2016;7:26593–26603. doi: 10.18632/oncotarget.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu X., Zhu Y., Liang Z., Li S., Xu X., Wang X., Wu J., Hu Z., Meng S., Liu B., et al. c-Met and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3β/Snail signaling. Cell Death Dis. 2016;7:e2088. doi: 10.1038/cddis.2015.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen J., Zhang J., Xiao M., Yang J., Zhang N. MiR-203 Suppresses Bladder Cancer Cell Growth and Targets the Twist1. Oncol. Res. 2017;26:1155–1165. doi: 10.3727/096504017X15041934685237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Miao X., Gao H., Liu S., Chen M., Xu W., Ling X., Deng X., Rao C. Down-regulation of microRNA-224 -inhibites growth and epithelial-to-mesenchymal transition phenotype -via modulating SUFU expression in bladder cancer cells. Int. J. Biol. Macromol. 2018;106:234–240. doi: 10.1016/j.ijbiomac.2017.07.184. [DOI] [PubMed] [Google Scholar]
- 87.Li J., Xu X., Meng S., Liang Z., Wang X., Xu M., Wang S., Li S., Zhu Y., Xie B., et al. MET/SMAD3/SNAIL circuit mediated by miR-323a-3p is involved in regulating epithelial-mesenchymal transition progression in bladder cancer. Cell Death Dis. 2017;8:e3010. doi: 10.1038/cddis.2017.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei Z., Hu X., Liu J., Zhu W., Zhan X., Sun S. MicroRNA-497 upregulation inhibits cell invasion and metastasis in T24 and BIU-87 bladder cancer cells. Mol. Med. Rep. 2017;16:2055–2060. doi: 10.3892/mmr.2017.6805. [DOI] [PubMed] [Google Scholar]
- 89.Yu H., Duan P., Zhu H., Rao D. miR-613 inhibits bladder cancer proliferation and migration through targeting SphK1. Am. J. Transl. Res. 2017;9:1213–1221. [PMC free article] [PubMed] [Google Scholar]
- 90.Xu M., Li J., Wang X., Meng S., Shen J., Wang S., Xu X., Xie B., Liu B., Xie L. MiR-22 suppresses epithelial-mesenchymal transition in bladder cancer by inhibiting Snail and MAPK1/Slug/vimentin feedback loop. Cell Death Dis. 2018;9:209. doi: 10.1038/s41419-017-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mao X.W., Xiao J.Q., Li Z.Y., Zheng Y.C., Zhang N. Effects of microRNA-135a on the epithelial-mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway. Exp. Mol. Med. 2018;50:e429. doi: 10.1038/emm.2017.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang X., Zhu H., Gao Z., Li J., Zhuang J., Dong Y., Shen B., Li M., Zhou H., Guo H., et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J. Biol. Chem. 2018 doi: 10.1074/jbc.RA118.001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu W., Qi L., Lv H., Zu X., Chen M., Wang J., Liu L., Zeng F., Li Y. MiRNA-141 and miRNA-200b are closely related to invasive ability and considered as decision-making biomarkers for the extent of PLND during cystectomy. BMC Cancer. 2015;15:92. doi: 10.1186/s12885-015-1110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan L., Wang Y., Liang J., Liu Z., Sun X., Cai K. MiR-301b promotes the proliferation, mobility, and epithelial-to-mesenchymal transition of bladder cancer cells by targeting EGR1. Biochem. Cell Biol. 2017;95:571–577. doi: 10.1139/bcb-2016-0232. [DOI] [PubMed] [Google Scholar]
- 95.Wang C., Ge Q., Chen Z., Hu J., Li F., Ye Z. Promoter-associated endogenous and exogenous small RNAs suppress human bladder cancer cell metastasis by activating p21 (CIP1/WAF1) expression. Tumour Biol. 2016;37:6589–6598. doi: 10.1007/s13277-015-4571-z. [DOI] [PubMed] [Google Scholar]
- 96.Liu M., Chen Y., Huang B., Mao S., Cai K., Wang L., Yao X. Tumor-suppressing effects of microRNA-612 in bladder cancer cells by targeting malic enzyme 1 expression. Int. J. Oncol. 2018;52:1923–1933. doi: 10.3892/ijo.2018.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhong Z., Huang M., Lv M., He Y., Duan C., Zhang L., Chen J. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305–317. doi: 10.1016/j.canlet.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 98.Li L.J., Zhu J.L., Bao W.S., Chen D.K., Huang W.W., Weng Z.L. Long noncoding RNA GHET1 promotes the development of bladder cancer. Int. J. Clin. Exp. Pathol. 2014;7:7196–7205. [PMC free article] [PubMed] [Google Scholar]
- 99.Berrondo C., Flax J., Kucherov V., Siebert A., Osinski T., Rosenberg A., Fucile C., Richheimer S., Beckham C.J. Expression of the Long Non-Coding RNA HOTAIR Correlates with Disease Progression in Bladder Cancer and Is Contained in Bladder Cancer Patient Urinary Exosomes. PLoS ONE. 2016;11:e0147236. doi: 10.1371/journal.pone.0147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lv M., Zhong Z., Huang M., Tian Q., Jiang R., Chen J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim. Biophys. Acta. 2017;1864:1887–1899. doi: 10.1016/j.bbamcr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Fan Y., Shen B., Tan M., Mu X., Qin Y., Zhang F., Liu Y. TGF-beta-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin. Cancer Res. 2014;20:1531–1541. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 102.Chen Y., Peng Y., Xu Z., Ge B., Xiang X., Zhang T., Gao L., Shi H., Wang C., Huang J. LncROR Promotes Bladder Cancer Cell Proliferation, Migration, and Epithelial-Mesenchymal Transition. Cell. Physiol. Biochem. 2017;41:2399–2410. doi: 10.1159/000475910. [DOI] [PubMed] [Google Scholar]
- 103.Tuo Z., Zhang J., Xue W. LncRNA TP73-AS1 predicts the prognosis of bladder cancer patients and functions as a suppressor for bladder cancer by EMT pathway. Biochem. Biophys. Res. Commun. 2018;499:875–881. doi: 10.1016/j.bbrc.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 104.Tan J., Qiu K., Li M., Liang Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015;589:3175–3181. doi: 10.1016/j.febslet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 105.Xue M., Pang H., Li X., Li H., Pan J., Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107:18–27. doi: 10.1111/cas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo J., Chen J., Li H., Yang Y., Yun H., Yang S., Mao X. LncRNA UCA1 promotes the invasion and EMT of bladder cancer cells by regulating the miR-143/HMGB1 pathway. Oncol. Lett. 2017;14:5556–5562. doi: 10.3892/ol.2017.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu R., Zhu X., Chen F., Huang C., Ai K., Wu H., Zhang L., Zhao X. LncRNA XIST/miR-200c regulates the stemness properties and tumourigenicity of human bladder cancer stem cell-like cells. Cancer Cell Int. 2018;18:41. doi: 10.1186/s12935-018-0540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhuang J., Lu Q., Shen B., Huang X., Shen L., Zheng X., Huang R., Yan J., Guo H. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep. 2015;5:11924. doi: 10.1038/srep11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gregory P.A., Bert A.G., Paterson E.L., Barry S.C., Tsykin A., Farshid G., Vadas M.A., Khew-Goodall Y., Goodall G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 110.Shimono Y., Zabala M., Cho R.W., Lobo N., Dalerba P., Qian D., Diehn M., Liu H., Panula S.P., Chiao E., et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leskela S., Leandro-Garcia L.J., Mendiola M., Barriuso J., Inglada-Perez L., Munoz I., Martinez-Delgado B., Redondo A., de Santiago J., Robledo M., et al. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr. Relat. Cancer. 2011;18:85–95. doi: 10.1677/ERC-10-0148. [DOI] [PubMed] [Google Scholar]
- 112.Vallejo D.M., Caparros E., Dominguez M. Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J. 2011;30:756–769. doi: 10.1038/emboj.2010.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee J.W., Park Y.A., Choi J.J., Lee Y.Y., Kim C.J., Choi C., Kim T.J., Lee N.W., Kim B.G., Bae D.S. The expression of the miRNA-200 family in endometrial endometrioid carcinoma. Gynecol. Oncol. 2011;120:56–62. doi: 10.1016/j.ygyno.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 114.Santos M., Martinez-Fernandez M., Duenas M., Garcia-Escudero R., Alfaya B., Villacampa F., Saiz-Ladera C., Costa C., Oteo M., Duarte J., et al. In vivo disruption of an Rb-E2F-Ezh2 signaling loop causes bladder cancer. Cancer Res. 2014;74:6565–6577. doi: 10.1158/0008-5472.CAN-14-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Padrao N.A., Monteiro-Reis S., Torres-Ferreira J., Antunes L., Leca L., Montezuma D., Ramalho-Carvalho J., Dias P.C., Monteiro P., Oliveira J., et al. MicroRNA promoter methylation: A new tool for accurate detection of urothelial carcinoma. Br. J. Cancer. 2017;116:634–639. doi: 10.1038/bjc.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 117.Morris K.V., Mattick J.S. The rise of regulatory RNA. Nat. Rev. Genet. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]