Abstract
Alcyonacea (soft corals and gorgonia) are well known for their production of a wide array of unprecedented architecture of bioactive metabolites. This diversity of compounds reported from Alcyonacea confirms its productivity as a source of drug leads and, consequently, indicates requirement of further chemo-biological investigation. This review can be considered a roadmap to investigate the Alcyonacea, particularly those produce nitrogen-containing metabolites. It covers the era from the beginning of marine nitrogen-containing terpenoids isolation from Alcyonacea up to December 2018. One hundred twenty-one compounds with nitrogenous moiety are published from fifteen genera. Their prominent biological activity is evident in their antiproliferative effect, which makes them interesting as potential leads for antitumor agents. For instance, eleutherobin and sarcodictyins are in preclinical or clinical stages.
Keywords: Alcyonacea, sesquiterpenes, diterpenes, antitumor, cytotoxicity
1. Introduction
The marine biota is characterized by living under harsh environmental conditions (e.g., high salinity, variable pressures, hydrothermal vents and variable nutrient accessibility) [1,2]. The marine invertebrates, particularly soft corals, suffer from absence of mechanical defenses [3,4,5]. They produce chemical agents to maintain their life. These defenses can be represented by production of secondary metabolites that possess ecological functions including anti-predatory protection, thus several natural metabolites were discovered from marine sources [6]. Most of these substances have unprecedented structures with diversity of pharmacological application [7,8,9,10,11,12,13]. Alcyonacea comprises marine invertebrates that live ubiquitously in tropical sea waters, particularly intertidal zones or inner reefs below the stony corals, and are less prone to damage from collecting or shipping than the stony corals [14]. They are animals, provide stinging cells in the form of toxic stinging nematocysts with absence of the rigid protective skeleton of scleractinians, and possess allelopathic capabilities of chemical production [15,16]. This enables the sessile corals to strive and reduce their palatability, by reducing fouling effect through producing chemical substances, mucus or terpenoids, aiming at protecting them against predators [17,18,19]. Alcyonacea is well known for the production of terpenoids, while the occurrence of nitrogenous terpenoidal derivatives is rare. The bio-synthetic pathway of the nitrogenous moiety is unclear [6,20,21]. Conclusively, Alcyonacea has conceivable therapeutics, which includes immunomodulator, anticancer and useful antifouling agents.
Amazingly, 40% of the chemical frameworks that appear in different databases are natural compounds. Approximately, half of new drugs recently reported are of natural origin or constructed based on natural architectures [22]. A comparative analysis study indicates that marine products are superior to earthly metabolites in terms of chemical uniqueness [23]. An investigation has led to establishing that 70% of metabolites that appear in Dictionary of Marine Natural Products (DMNP) are completely exploited by marine invertebrates. Additionally, marine drugs have successfully launched in the market, and others are still in different phases of clinical trials. A recent review reports the marine pharmaceutical products [24,25,26].
In 1969, FDA approved cytarabine as an anticancer agent, while vidarabine was permitted in 1976 as an antiviral agent. Around fourteen years later, ziconotide was approval by FDA for treatment of severe chronic pain. Trabectedin was approved from FDA in 2010 for metastatic breast cancer [25]. On their way to the market, another twelve marine-derived drugs are being clinically investigated [26]; for instance, the bryostatin and dolastatin derivatives, soblidotin and synthadotin, respectively. A tricyclic diterpenoid, eleutherobin, has been reported from Eleutherobia sp., with potent inducer of tubulin polymerization in vitro, which mimics taxol-like effect [25,26,27,28].
In the current review, marine nitrogen-containing metabolites isolated from Alcyonacea are presented. These compounds show significant effects toward certain diseases and/or have a role in drug discovery. It is interesting to discuss the future perspectives of the structure–activity relationship of these metabolites. An extensive bibliographic literature survey was conducted employing different scientific databases including Scopus, Pubmed, SciFinder, Google scholar and Web of Science.
2. Chemical Constituents
2.1. N-Containing Sesquiterpenes
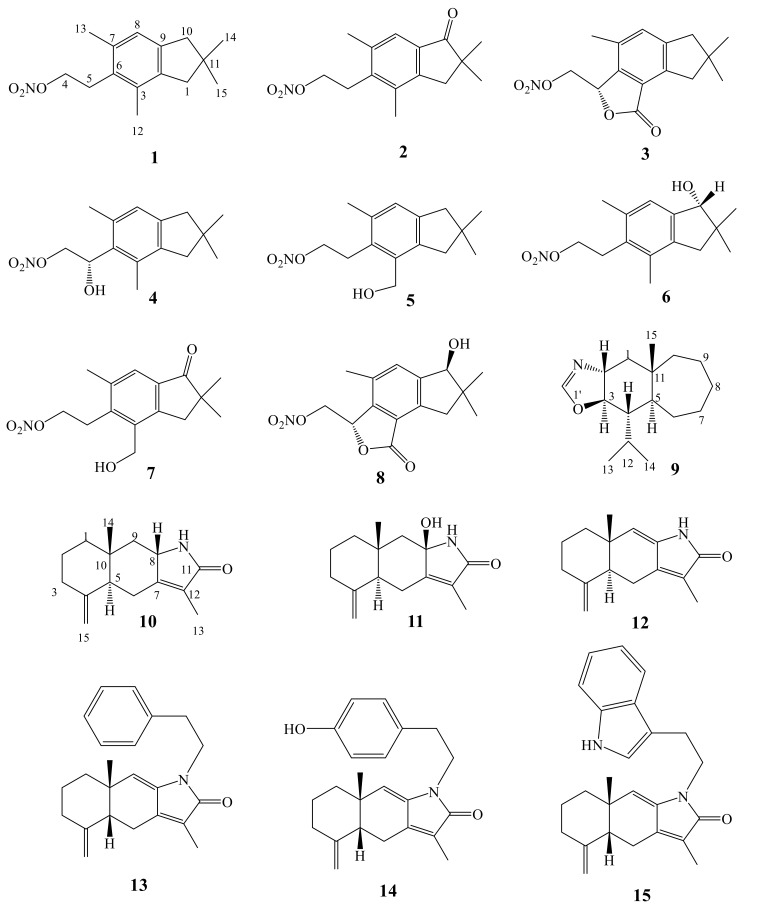
Sesquiterpenes are a class of widely distributed metabolites among marine soft corals. Although nitrogen-containing sesquiterpenoids are rare, they are produced by certain soft corals (e.g., Alcyonium sp.) [20]. Illudalane-type sesquiterpenoids (Table 1 and Figure 1), have limited distribution in nature and metabolites of this class are exclusively reported from ferns and fungi [29,30]. Congeners of illudalane-type sesquiterpenoids identified as alcyopterosins (B, C, E, F, G, H, J and M) (1–8) are reported from the sub-Antarctic globular spongy pink coral Alcyonium paessleri, gathered around South Georgia Islands. The combined extracts (EtOH and EtOAc) purified by employing different chromatographic techniques led to isolation of the congeners (1–8), which numerated as the first report of nitrogen-containing illudalane type sesquiterpenoids, as well as a first report of nitrate ester to be isolated from marine soft corals [20]. These metabolites represent a new trend in biosynthetic pathway of sesquiterpenoids. All reported sesquiterpenes may have sulfate, phosphate, or halogenic moieties, but finding nitrogen-containing sesquiterpenes is infrequent. Alcyopterosins (1–8) were evaluated for their cytotoxicity towards Human larynx carcinoma (Hep-2) and Human colon carcinoma (HT-29). Alcyopterosins E (5) showed cytotoxicity against Hep-2 with IC50 value of 13.5 µM, while Alcyopterosins C (2) and H (6) showed cytotoxicity against HT-29 with IC50 value of 10 µM [20]. Although nitrate ester derivatives are very rare, some synthetic nitrate esters are published. An acceptable speculation is that the hydrolysis of esters leads to the corresponding alcohols, which play roles in their cytotoxic effects [20].
Table 1.
Bioactivity of nitrogen containing metabolites from Alcyonacea.
| Cpd. No. | Species | Bioactivity | Ref. No. |
|---|---|---|---|
| 1–8 | Alcyonium paessleri | Cytotoxic | [20] |
| 9 | Cladiella sp. | Not published data | [21] |
| 10–12 | Cespitularia taeniata | Cytotoxic | [31,32] |
| 13–15 | Cespitularia taeniata | Cytotoxic | [33] |
| 16 | Eleutherobia sp. | Cytotoxic | [34] |
| 17–22 | Erythropodium caribaeorum | Cytotoxic | [35,36,37,38,39] |
| 23–28 | Sarcodictyon roseum | Cytotoxic | [40,41,42] |
| 29–33 | Alcyonium valdivae | Anti-inflammatory | [43] |
| 34–35 | Eleutherobia aurea | Cytotoxic | [44] |
| 36–37 | Erythropodium caribaeorum | Cytotoxic | [36] |
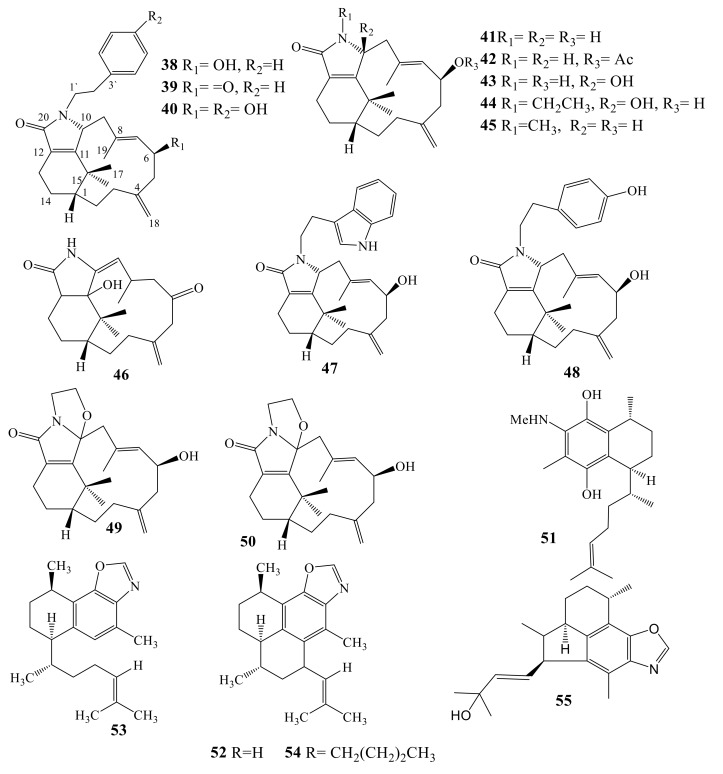
| 38–50 | Cespitularia taeniata | Cytotoxic and Antimicrobial | [45,46,47] |
| 51 | Pseudopterogorgia elisabethae | Cytotoxic | [48] |
| 52–54 | Pseudopterogorgia elisabethae | Cytotoxic | [49,50] |
| 55 | Pseudopterogorgia elisabethae | Cytotoxic | [51,52,53,54,55,56,57] |
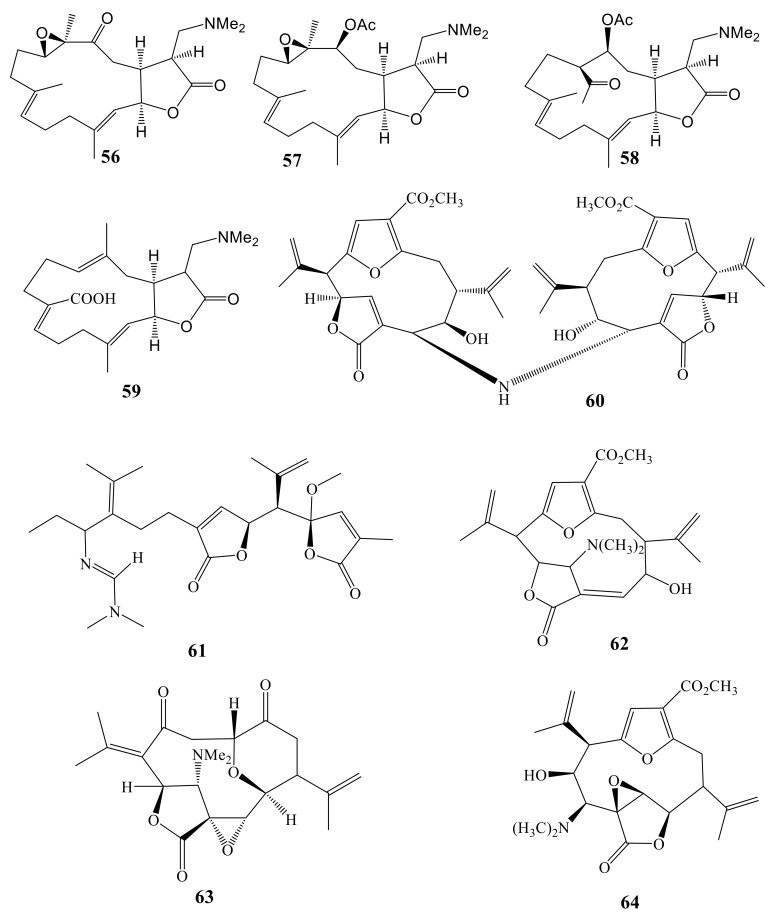
| 56–58 | Sinularia sp. | Antiproliferative | [58,59] |
| 59 | Lobophytum sp. | Cytotoxic | [60,61] |
| HIV inhibitory | |||
| 60 | Pseudopterogorgia acerosa | Cytotoxic | [62,63] |
| 61 | Pseudopterogorgia bipinnata | Antimalarial | [64] |
| 62 | Pseudopterogorgia acerosa | - | [65] |
| 63 | Pseudopterogorgia acerosa | - | [66] |
| 64 | Pseudopterogorgia spp. | Cytotoxic | [67,68] |
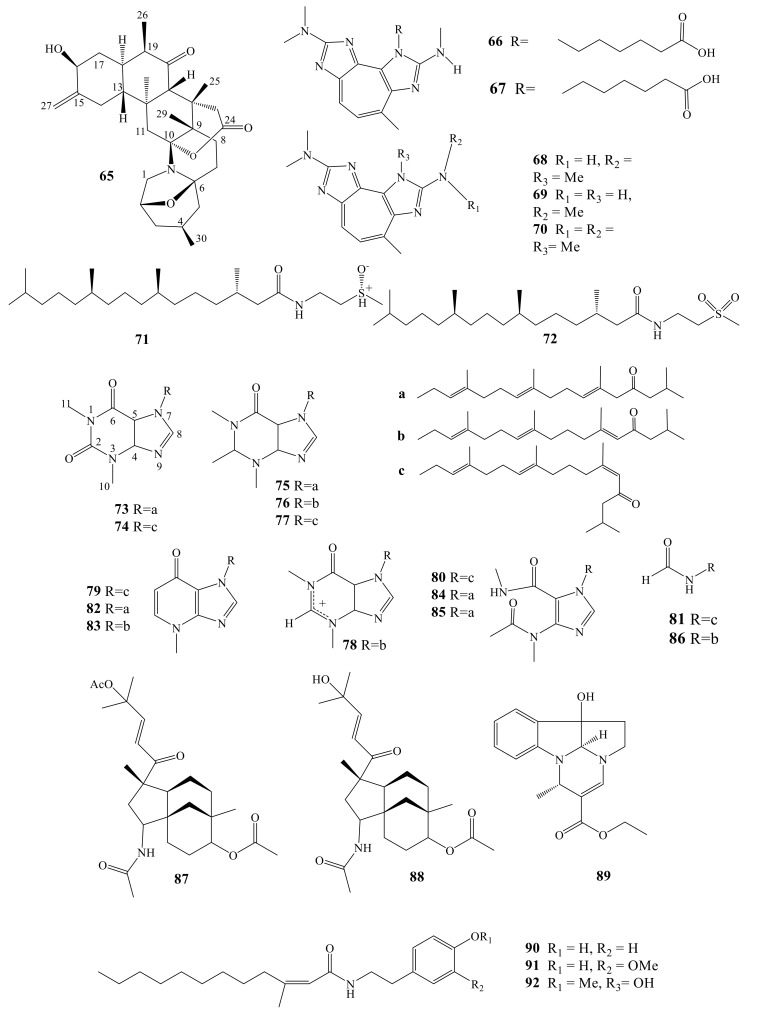
| 65 | Lobophytum sp. | Cytotoxic | [69,70,71] |
| 66–70 | Echinogorgia pseudossapo | Antiviral | [72] |
| 71–72 | Sinularia sp. | Inhibit LPS-induced NO release | [73,74] |
| 73–86 |
Euplexaura nuttingi and Leptogorgia gilchristi |
Cytotoxic | [75,76,77] |
| 87–88 | Sarcophyton infundibuliforme | Immunomodulator | [78] |
| 89 | Sinularia verruca | - | [79] |
| 90–92 | Sinularia flexibilis | Cytotoxic | [80] |
| 93–94 | Sinularia crassa | - | [81,82,83,84,85] |
| 95–96 | Sinularia leptoclados | Antibacterial | [86] |
| 97–98 | Lobophytum sp. | - | [87] |
| 99 | Sarcophyton auritum | Anxiolytic effect | [88] |
| 100–102 | Pseudopterogorgia australiensis | Antimicrobial | [89] |
| 103–108 | Sarcophyton ehrenbergi | Anti-inflammatory | [90] |
| 109–112 | Lobophytum sp. | Cytotoxic | [91] |
| 113 | Litophyton arboreum | HIV-1 PR inhibitory | [92] |
| 114 | Sinularia sp. | Anti-inflammatory | [93,94] |
| 115–116 | Sinularia brongersmai | Cytotoxic | [95] |
| 117 | Sinularia sp. | Gastric H, K-ATPase and cytotoxic | [96] |
| 118–119 | Sinularia sp. | Cytotoxic | [97] |
| 120 | Sinularia sp. | Cytotoxic | [98] |
| 121–123 | Clavularia viridis | Cytotoxic | [99,100] |
| 124 | Sinularia flexibilis | Cytotoxic | [101] |
| 125–126 | Eunicella granulate | Cytotoxic | [102] |
Figure 1.
N-Containing sesquiterpenes (1–15).
Cladioxazole (9) represents the first oxazole-derived terpenoid, isolated from Cladiella sp. which, collected from Andaman Island [21]. Unfortunately, no information about the biological activity of 9 has been published. Three sesquiterpene lactams, taenialactams A (10), B (11) and atractylenolactam (12), were isolated from Cespitularia taeniata collected from Taiwan [31]. The methanolic extract of C. taeniata has been partitioned between water and ethyl acetate. The EtOAc-soluble portion has been purified on silica gel columns and HPLC to yield 10–12. Atractylenolactam (12) was previously isolated from the rhizome of a terrestrial plant, Atractylodes macrocephala, a Chinese medicinal plant employed for the treatment of indigestion and anorexia [32]. Compounds 10–12 were evaluated towards KB, WiDr, and Daoy tumor cells, no effects were observed [31]. Fractionation of the organic extract of Taiwanese soft coral Cespitularia taeniata, by employing different chromatographic techniques including HPLC led to identification of ten compounds, three of which are nitrogenous sesquiterpenoids, cespilamides C–E (13–15, Figure 1). Cespilamide E (15) exhibited cytotoxicity against Hela, Daoy, WiDr and MCF-7 cells with IC50 of 24.7, 22.3, 34.1 and 17.5 μM, respectively. Cespilamide E (13) exhibited cytotoxicity against Hela, Daoy, WiDr and MCF-7 cells with IC50 of 30.9, 34.8, 49.5 and 30.6 μM, respectively, while cespilamide D (14) was inactive (>40 μM) [31,33].
2.2. N-Containing Diterpenes
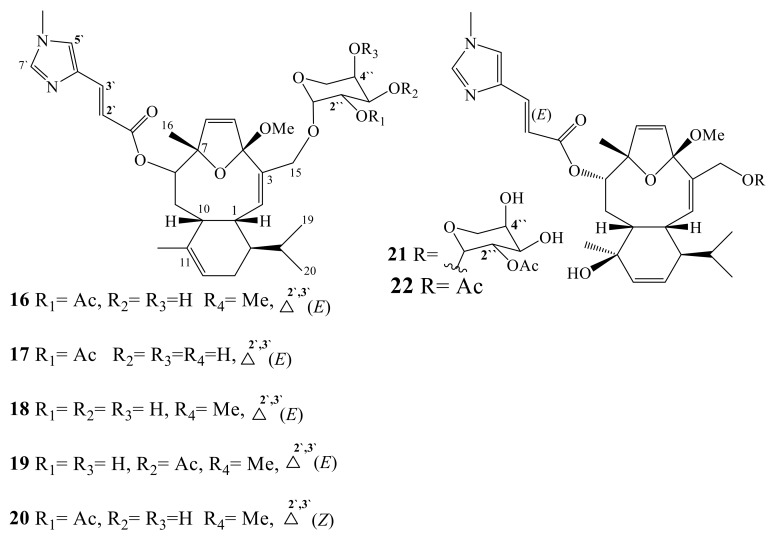
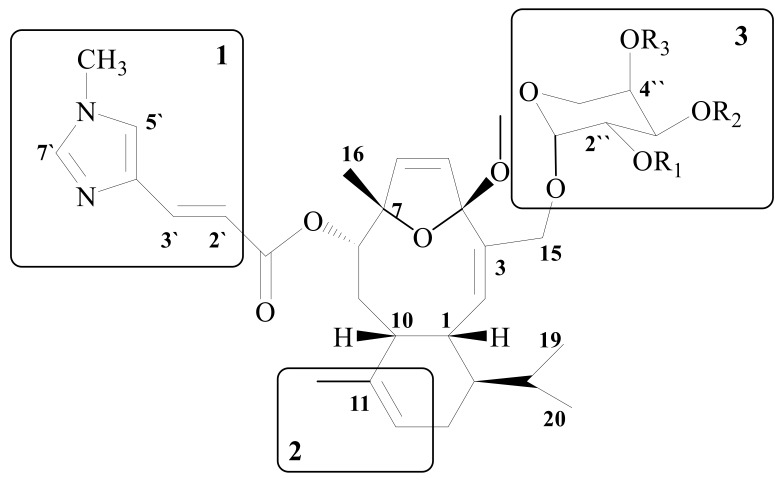
Eleutherobin (16) is unique nitrogen-containing diterpene glycoside, isolated from an Australian soft coral, identified as Eleutherobia sp. (Figure 2). The non-sugar moiety of eleutherobin was escalated by presence of eunicellane nucleus, a diterpene framework that is first reported from the gorgonian octocoral Eunicella stricta [34]. It possesses N-methylurocanic acid ester at the C-8 position, while the sugar moiety is acetyl-arabinose [26,34,35]. Eleutherobin possessed significant cytotoxic effect towards various cancer cells with IC50 range of 10–15 nM. National Cancer Institute measured the selectivity of Eleutherobin (16) on 60 diverse panels of cancer cells. Its potency was estimated as 100-fold more against selected types of cancer cell lines than paclitaxel [36,37,38,39]. Investigation of a Caribbean octocoral, namely Erythropodium caribaeorum, collected from shallow reefs near Dominica, led to isolation of seven compounds: eleutherobin (16), desmethyleleutherobin (17), desacetyleleutherobin (18), isoeleutherobin A (19), Z-eleutherobin (20), caribaeoside (21), and caribaeolin (22) (Figure 2) [36]. The reported pharmacological mechanism of eleutherobin is mimetic to the mode of action observed with paclitaxel [37,38,39]. This appeared through stabilization of microtubules and confirmed by employing the electron microscopy technique [37,38,39].
Figure 2.
N-Containing diterpenes (16–22).
This class of compounds (nitrogen-containing diterpenes, 16–22), was examined as pharmacophore models for microtubule stabilizing agents. Desacetyleleutherobin (18) possesses the arabinose, instead of 2′acetyl substituent, while isoeleutherobin A (19) has an acetyl group at the 3′ instead of the 2′. Z-eleutherobin (20) is a geometric isomer of eleutherobin at the C-2′ to C-3′ double bond of the C-8 N-(6′)-methylurocanic acid ester side chain. Desmethyleleutherobin (17) differs from eleutherobin (16) by the occurrence of a hydroxyl function instead of an OMe at C-4. Caribaeoside (21) differs from eleutherobin by the presence of a hydroxyl at C-11 of the tricyclic core, and a double bond at C-12 to C-13 instead of C-11 to C-12, significantly altering the cyclohexene ring. Caribaeolin (22) differs from caribaeoside only by the occurrence of a –CH2OCO-CH3 substituent in the C-3 terminal chain. Eleutherobin (16) had an IC50 of 100 nM. The activity of Z-eleutherobin (20) was close, with an IC50 of 250 nM. Desmethyleleutherobin (17) and isoeleutherobin A (19) were more potent than eleutherobin, with IC50 of 20 and 50 nM, respectively, while Desacetyleleutherobin (18) was less potent, with an IC50 of 400 nM [35,36,37,38,39].
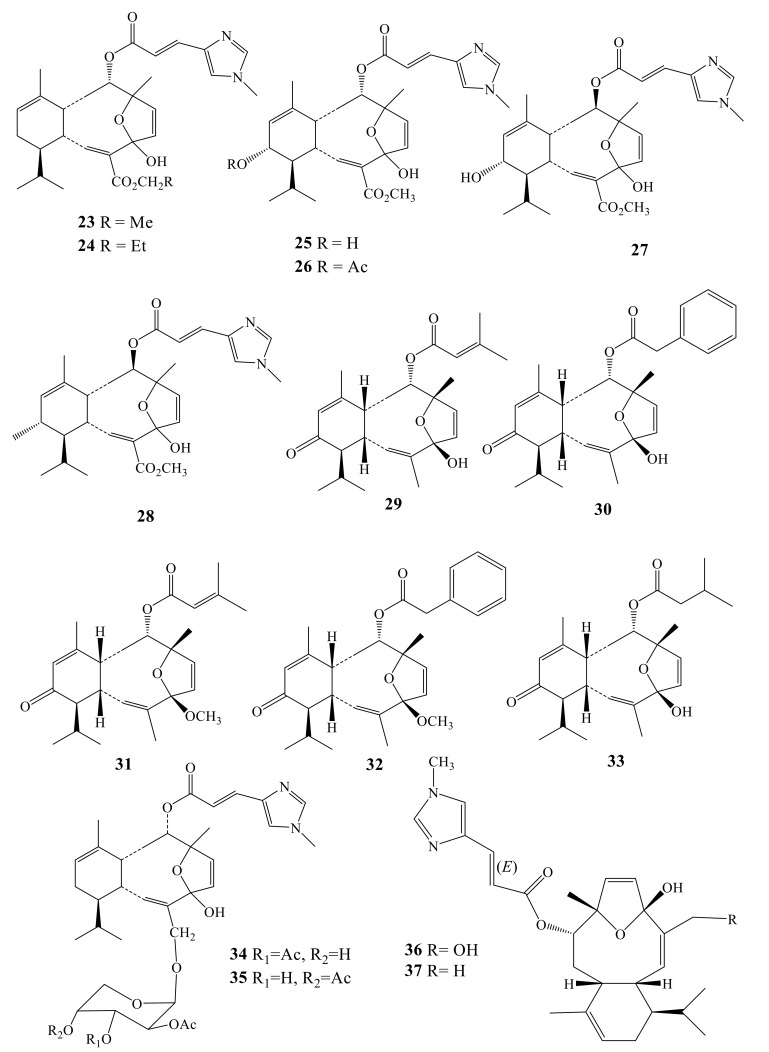
Sarcodictyins are congeners of eleutherobin and belong to a family of marine-derived diterpenoids with potent antitumor effects. They possess a rigid oxygen-bridged bi cyclo[8.4.0]tetradecatriene skeleton [40]. Sarcodictyins A and B (23–24) were isolated from stoloniferan coral Sarcodictyon roseum [41]. A year later, sarcodictyin C–F (25–28) were isolated from the same soft coral [42]. These metabolites showed their cytotoxic effects through tubulin binding in a mechanism similar to Taxol. Sarcodictyins A (23), B (24), C (25) and E (27) were proven to be more potent cytotoxic than the other sarcodictyins. Pharmacia-Upjohn′s researcher (1997) reported that sarcodictyins A–C (23–25) and E (27) exhibited cytotoxicity against Ll 210 murine leukemia (IC50 ranged between 408.5 ± 21.3 and 911.7 ± 393.5 nM; compare with taxol: IC50 = 16.6 ± 5.2 nM). The dose required to promote 90% tubulin polymerization ranged 1.7–6.6 µM, while 4.4 µM for taxol [40]. Five non-nitrogenous sarcodyctins have been isolated from Alcyonium valdivae: valdivone A (29), valdivone B (30), 4-O-Methyl valdivone A (31) 4-O-Methyl valdivone B (32), and dihydrovaldivone A (33) [43]. Compounds 29–30 were shown to inhibit the chemical induced anti-inflammatory. Sarcodictyin A (23) showed lower activity, with an IC50 of 2 μM, while caribaeoside (21) and caribaeolin (22) were considerably less potent, with an IC50 of 20 µM for both compounds [35,36,37,38,39].
Eleuthosides A (34) and B (35), along with sarcodictyin A (23) were reported from Eleutherobia aurea, gathered around Kwazulu-Natal Coast, South Africa [44]. Both 34 and 35 are novel diterpenoidal glycosides, and were proven to have potent microtubule stabilizing effect. The detailed information is registered in a patent with publication Number WO1999021862 A1.
Investigation of another Erythropodium species, E. caribaeorum, led to identification of two unprecedented natural metabolites, namely caribaeorane (36) and 15-hydroxycaribaeorane (37), which are characterized by the presence of C-4 methylketal (Figure 3) [36].
Figure 3.
N-Containing diterpenes (23–37).
Eleutherobin has potent inducing in vitro tubulin polymerization effect, although its cytotoxicity is less than those obtained from paclitaxel [35,36,37,38,39,44]. P-glycoprotein is a target substance of Eleutherobin, similar to paclitaxel. Both showed cross-resistance in MDR1-expressing lines. Sarcodictyins are reported from Sarcodictyon roseum and seemed to be more promising than eleutherobin, despite their lower effects. Eleutherobins and sarcodictyins have been extensively modified by employing conventional and combinatorial chemistry techniques, which have also allowed the formation of hybrid molecules of the two base structures. These investigations indicated their SARS (Figure 4) [36,44].
Figure 4.
The three possible domains for binding to tubulin.
The side chain and the imidazole ring are important.
Both OH and OCH3 groups are tolerated, with little difference in effects.
Elimination or alteration of the aglycone moiety of eleutherobin changes the cytotoxicity and resistance pattern.
Sarcodictyin esters are more potent than amides.
Cespitulactams A–C (38–40, Figure 5) are reported from Cespitularia taeniata, collected around Taiwan [45]. The obtained metabolites are nitrogenous tricyclic derivative, close to taxane diterpenes, particularly 3, 8-seco-taxoids and an infrequent N-phenylethyl-butyrolactamyl moiety. Four cancer cell lines, WiDr and Daoy, KB and Hepa59T/VGH, were tested. Cespitulactam A (38) exhibited potent toxicity against Widr and Daoy cancer cells (IC50 = 2.72 and 6.34 μg/mL, respectively) [46]. Further investigation of the organic extract of Cespitularia taeniata led to identification of eight more nitrogenous verticillene diterpenoids, cespitulactams (D–K) (41–48) [47]. These substances were evaluated against KB and murine L1210 leukemia. Compound 48 was active towards both cancer cells at 8.49 and 11.7 µM, respectively, and no effect was observed with the rest. Diterpene alkaloids (41–48) have been assayed for antimicrobial effects towards several microorganisms. Cespitulactam G (44) showed potent effect towards Trichophyton mentagrophytes (IFM45110) with MIC value of 11.56 µM. Compounds 41, 47, and 48 displayed significant effect towards Micrococcus luteus (IFM2066) and Cryptococcus neoformans (IFM46914) (46, 47 and 48) with MIC values of 12.53, 9.06 and 9.54 µM, respectively, and T. mentagrophytes (42 and 47) with MIC values of 11.62 and 9.06 µM. Unfortunately, all isolated Cespitulactams (41–48) were inactive against Staphylococcus aureus (209P) [46]. Another investigation on Cespitularia taeniata led to identification of two nitrogenous verticillene (Figure 5), cespilamides A and B (49 and 50). Neither compound exhibited cytotoxicity against Hela, Daoy, WiDr, or MCF-7 at concentration >40 μM [34,47].
Figure 5.
N-Containing diterpenes (38–55).
Diterpenoidal alkaloid, elisabethamine (51), has a serrulatane nucleus with methyl amino functionality, isolated from Pseudopterogorgia elisabethae [48]. Elisabethamine (51) had significant effect against LNCap and Calu cancer cell lines (IC50 = 10.35 and 20 μg/mL, respectively) [48]. Further investigation of the organic extract of Pseudopterogorgia elisabethae afforded pseudopteroxazole (52) and seco-pseudopteroxazole (53), having amphilectane skeleton and the uncommon benzoxazole function [49]. One more amphilectane derivative homopseudopteroxazole (54) was isolated from Pseudopetrospongia elisabethae collected from the Western Atlantic Ocean [50]. Pseudopteroxazole (52) showed potent inhibitory effect (97%) against Mycobacterium tuberculosis H37Rv at 40.45 µM, whereas seco-pseudopteroxazole (53) inhibited 66% of mycobacterial growth. Therefore, the potency effect of 52 might be attributed to the benzoxazole function. Remarkably, biological screening of 52 against 60 cancer cell lines designated potent in vitro cytotoxicity. Several approaches for syntheses of pseudopteroxazole are published [51,52,53,54]. Another diterpene, ileabethoxazole (55), was obtained as a light yellow oil from Pseudopterogorgia elisabethae collected near Providencia Island (Figure 5). It represents novel diterpenes carbon skeleton (Ileabethane), which appears to be biosynthetically related to the serrulatane skeleton. It displayed strong inhibitory effect against M. tuberculosis H37Rv [55,56,57].
Three new nitrogen containing cembranolide diterpenes, sinularamine I (56), sinularamine II (57), and sinularamine III (58), each possessing a dimethylamino group, were isolated from the Okinawan soft coral species of Sinularia genus and their structures were elucidated by spectroscopic analyses and chemical transformations [58]. Compounds 56 and 58 inhibited the proliferation of KB cells at concentrations of 2.0 and 1.65 µg/mL, respectively [58,59]. Modified structures of sinularamines I–III were isolated from Okinawan soft coral of Sinularia species [59,60].
Investigation on Lobophytum sp., collected from Philippine Islands, led to identification of a cembranoid diterpene (17-dimethylaminolobohedleolide, 59), which blocked the cytopathic effect of in vitro HIV-1 infection in a cell-based assay with EC50 = 8.78 µM [61].
Tinto et al. (1991) reported seven pseudopteranoids isolated from Pseudopterogorgia acerosa, including the rare bisditerpenoid amine bis(gorgiacerol)amine (60). It showed selective growth inhibition activity against cancer cell lines (HCT116 and HeLa) [62,63].
Caucanolide B (61), a possible pesudopterane-type diterpenoid successor through oxidation cleavage at C-2/C-3 together with five rare diterpenes, was isolated from Pseudoptergorgia bipinnata collected near the Colombian Southwestern Caribbean Sea. These caucanolides were evaluated against the malaria parasite, Plasmodium falciparum. Unfortunately, Caucanolide B showed no significant activity, although it is the only example from nature of a secondary metabolite possessing the N1,N1-dimethyl-N2- acylformamidine functionality [64].
Fenical and Clardy (1982) examined the constituents of Floridian specimens of Pseudopterogorgia acerosa, leading to the isolation of pseudopterolide, a remarkable metabolite based on the 12-membered carbocyclic pseudopterane skeleton [64]. Further examination of extracts of several Pseudopterogorgia spp. has since resulted in the isolation of other pseudopterane metabolites, many of which possess chemically unique structural features (Figure 6). Tobagolide (62), one such metabolite, is a rare nitrogen-containing diterpenoid isolated from a Trinidadian specimen of Pseudoptergorgia acerosa [65]. Another examination of Puerto Rican specimens of Pseudoptergorgia acerosa led to isolation and structural determination of alanolide (63), a novel tetracyclic norditerpene, which appears to be biogenetically related to tobagolide [66]. The structure of aceropterine (64), the first pseudopterane with a transposed lactone moiety, is closely related to that of tobagolide, which was isolated from a Trinidadian specimen of Pseudoptergorgia acerosa [67,68].
Figure 6.
N-Containing diterpenes (56–64).
2.3. Terpenoidal Alkaloids
Zoanthamine-type alkaloid (Lobozoantamine, 65) was identified from an Indonesian coral, Lobophytum sp. It belongs to a unique class of alkaloids, the precursor type of which is still ambiguous, whether it is a triterpenoid or a polyketide [68]. The first member of the zoanthamine-type alkaloid was isolated from Zoanthus sp. [69,70], followed by a series of analogs isolated from the genus Zoanthus, with the single exception of zooxanthellamine, which was isolated from the unicellular dinoflagellate Symbiodinium sp. [71]. Lobozoanthamine (65) was evaluated against AGS and C6, and showed cytotoxic effect with IC50 value > 50 μM on both cell lines.
Five zoanthoxanthin alkaloids (Figure 7) were reported from Chinese Sea gorgonian Echinogorgia pseudossapo, pseudozoanthoxanthins III (66), pseudozoanthoxanthins IV (67), Zoanthoxanthin (68), Paragracine (69) and Zoanthoxanthin (70) [72]. In vitro antiviral activity of 66–69 against HSV-1 was evaluated using plaque reduction assay. The completely non-toxic concentration of 66–69 and positive control ACV on Vero cells were tested to be 270.3, 523.6, 185.2, 195.3, and >7500 μM by MTT assay, respectively. Further antiviral studies, employing concentrations lower than the tested non-toxic concentration values. Compounds 66–69 exhibited anti-HSV-1 activity with EC50 values of 108.1, 471.2, 70.4, 117.2, and 6.08 μM, respectively. The results suggest that the side chain at the nitrogen N(3) in 66–69 could affect their antiviral activity. Although 66 and 68 showed mild anti-HSV-1 activity, their effects were less than that obtained with the positive control [72].
Figure 7.
Terpenoidal alkaloids (65–92).
Sinulasulfoxide (71) and sinulasulfone (72), two alkaloids with sulfur moiety, were reported from Indonesian Sinularia sp. [73]. Their skeleton is characterized by presence of a phytanic acid moiety connected to sulfated diterpene through an amide bond. Among marine metabolites, the 2-methylsulfinyl ethanamine moiety infrequently occurs in nature; however, it was isolated by Piper [74]. Another example, psammaplin N, was isolated from sponge Aplysinella rhax [72]. An analog, 3-methylsulfinyl propanamine (decarboxylated methionine sulfoxide), was also reported from the same sponge [74]. Both compounds were evaluated for anti-inflammatory activity. Sinulasulfoxide (71) showed moderate inhibition of NO2 production (22.6%) [72].
Nuttingins A–F (tetraprenylated purine alkaloids, 73–78) and malonganenones D–H (82–86) were reported from Euplexaura nuttingi [75], while malonganenones, A–C (79–81) were previously reported from Leptogorgia gilchristi [76]. Nuttingins A–E (73–77) and malonganenones D–G (82–84) showed inhibitory effect towards K562 and UT7 cancer cells (Figure 6).
Compounds 73–77 and 82–86 also induced apoptosis in transformed mammalian cells at 1.25 µg/mL [5,76]. Nuttingin A (73) is the first 3, 7-disubstituted hypoxanthine, while Nuttingin B (74) and Nuttingin C (75) represent the first formamides identified from soft corals. The anti-esophageal cancer effect of Nuttingins A–C (73–75) was compared with that of rietone, which was previously identified from L. gilchristi. Nuttingins A–C (73–75) were assessed towards several tumor cells (WHCO1, WHCO5, WHCO6, KYSE70, KYSE180, and KYSE520). Compounds 73–75 showed moderate cytotoxicity against seven cancer cells. Remarkably, Nuttingin A (73) exhibited toxicity against WHCO1 cells comparable with cisplatin (IC50 =15 μM). The anti-microbial effect of Nuttingins A (73) was evaluated against Staphylococcus aureus, Escherichia coli, and Aspergillus niger (20 and 100 mg/disk). Nuttingins A–E (73–77) showed no effect against E. coli and A. niger, while showed mild effect against S. aureus (100 mg/disk) [5,77].
Two unprecedented nitrogenous diterpenoidal metabolites, sarinfacetamides A (87) and B (88), were reported from the Chinese Sarcophyton infundibuliforme [78]. These compounds are members of uncommon tricycle-dodecane scaffold with an acetamide group (Figure 6). Compounds 87 and 88 were evaluated for different biological effects, including cytotoxicity and immunomodulatory effects. Unfortunately, when evaluated against HL-60, K562, MGC-803, BEL-7402, SH-SY5Y, HCT-116, MDAMB-231, A549, MCF-7/ADM, HO8910, U87, and NCIH1975 cell lines, they showed no effects at 10 μM. Interestingly, in the immunological assay, compound 87 was found to moderately promote the ConA-induced T lymphocytes at 10 μM with proliferation rate of 36.18% [78].
A rare pyrroloindoline alkaloid verrupyrroloindoline (89) was isolated from Sinularia verruca. It showed no protection towards the cytopathic effects of HIV-1 infection and no effect was observed against LPS-induced NO production [79].
4-(2-aminoethy1)-2-methoxyphenol (90), 5-(2-aminoethy1)-2-methoxyphenol (91) and (2)-3-methyldodec-2-enoic acid (92), three tyramine derivatives, were isolated from the soft coral Sinularia flexibilis (Figure 7). It is worth mentioning that total synthesis was the main tool for structural elucidation of these amides [80]. Compound 90 displayed potent anti-inflammatory effect in the inhibition of superoxide generation and elastase release in fMLP/CB-induced human neutrophils [80].
2.4. Ceramides and Cerebrosides
Cerebrosides are a group of metabolites holding a ceramide moiety and sugar units [81]. They have been published from different invertebrates including, sponges [82], sea anemones, [83], ascidians [84] and soft corals [85,86,87,88,89,90,91,92,93,94].
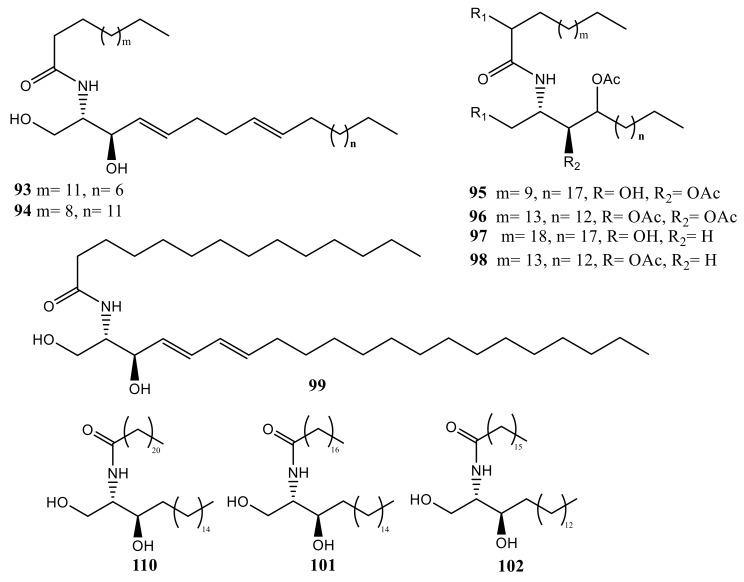
Investigation of Sinularia crassa led to identification of two new sphingosine derivatives, N-hexadecanoyl-1,3-dihydroxy-2-amino-4,8-octadecadiene (93) and N-heneicosanoyl-1,3,4-trihydroxy-2-aminotetradecane (94) [85]. Two sphingolipids, (2S,3S,4R)-1,3,4-trihydroxy-2-[((R)-2′-hydroxytetradecanoyl) amino] tricosane (95) and (2S,3S,4R)-1,3,4-triacetoxy-2-l((R)-2′–acetoxy octadecanoyl) amino] octadecane (96), were isolated from Sinularia leptoclados (Figure 8). They exhibited mild antibacterial effect against Gram-negative bacteria, while no activity was found against Gram-positive bacteria tested [86].
Figure 8.
Sphingolipids (93–102).
Investigations of Lobophytum sp. yielded a ceramide: (2S,3S,4R)-2-[(R)-2′-hy-droxytricosanoyl amino]-1,3,4-tridecanetriol (97). The length of the sphingosine chain of the fatty acid was determined by methanolysis, followed by acetylation of sphingamines to give 98 [87].
Investigation of Sarcophyton auritum led to identification ceramide N-((2S,3R,4E,6E)-1,3-dihydroxyhenicosa-4,6-dien-2-yl) tridecanamide (99), which showed antagonized effect the lethality of pentylenetetrazole in mice. In addition to this effect, compound 99 showed significant anxiolytic effect as well as CNS depressing activity, possibly through GABA and serotonin receptor modulation [88].
Three sphingosines, (2S,3R)-2-(docosanoyl amino)nonadecane-1,3-diol (100) and (2S,3S,4R)-2-[(29R)-29-hydroxynonadecanoylamino]nonadecane-1,3,4-triol (101), along with the known (2S,3R,4E)-2- (heptadecanoylamino)octadec-4-ene-1,3-diol (102), were isolated from Pseudopterogorgia australiensis collected from the Tuticorin Coast, India (Figure 7) [89]. Compounds 100–102 showed moderate antibacterial activity against Gram-positive bacteria Bacillus pumilis, B. subtilis, and S. aureus and Gram-negative bacteria E. coli, Proteus vulgaris, and Pseudomonas aeruginosa. Unfortunately, no significant effect was observed towards Candida albicans or Aspergillus niger [89].
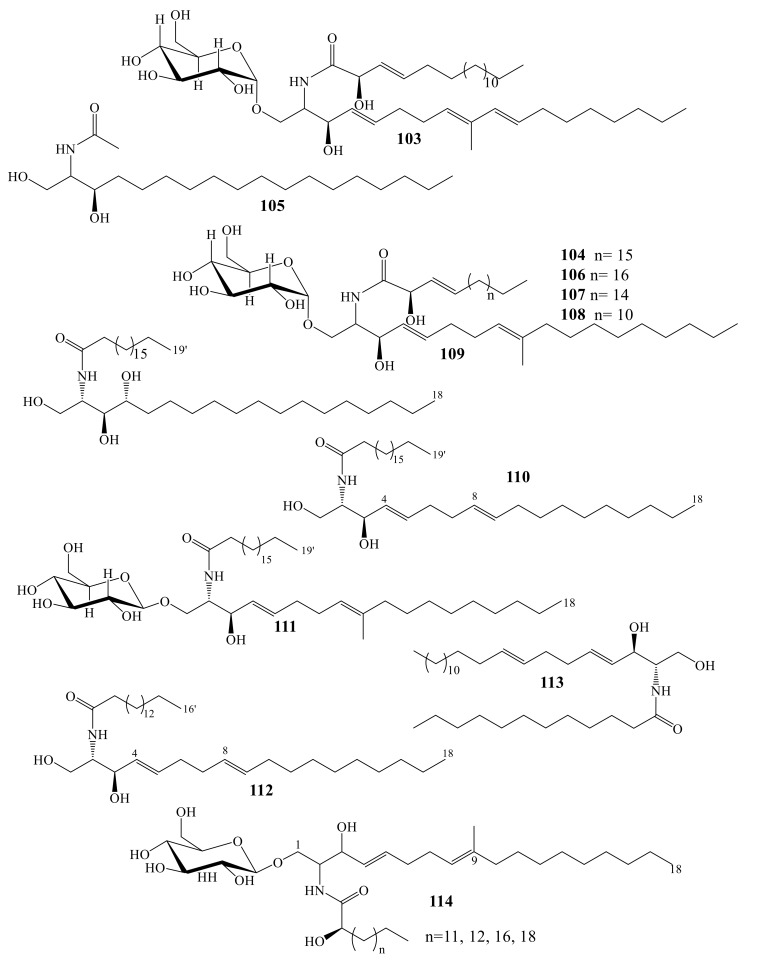
Investigation of Sarcophyton ehrenbergi collected from water around Taiwan led to cerebrosides sarcoehrenoside A (103), sarcoehrenoside B (104), and known ceramide (105), along with three known cerebrosides (106–108) [90]. Sarcoehrenoside A (103) has a characteristic β-glucose moiety, which differs from previous reported marine cerebrosides (Figure 8). Compounds 103–108 were evaluated toward their antimicrobial effects including Enterobacter aerogenes, Serratia marcescens, Salmonella enteritidis, Yersinia enterocolitica and Shigella sonnei. Unfortunately, they showed no antimicrobial effect (100 μg/disk). However, in-vitro anti-inflammatory effects of 103–108 were estimated [90]. Both 103 and 108 reduced the levels of iNOS to 46.9 ± 9.7% and 20.3 ± 6.8%, respectively, and of COX-2 to 77.2 ± 9.9% and 64.3 ±8.6%, respectively. Compounds 104, 105, and107 reduced iNOS protein expression to be ranged 25.8- 47.3%, did not inhibit COX-2 protein expression [90].
Four sphingolipids were isolated from Lobophytum sp. (Figure 9), identified as (2S,3S,4R)-2- nonadecanoylamino-octadecane-1,3,4-triol (109), (2S,3R,4E,8E)-[(2′R)-2′-hydroxyhepta decanoylamino]-4,8-octadecadiene-1,3-diol (110), 1-O-(β-d-glucopyranosyl)-(2S,3R,4E,8E)-2-[(2′R)-2′-hydroxynonadecanoyl amino]-9-methyl-4,8-octadecadiene-1,3-diol (111), and (2S,3R,4E,8E)-2-hexadecanoyl amino-4,8-octadecadien-1,3-diol (112). All these sphingolipids were evaluated for toxicity against human Peripheral Blood Mononuclear Cells (PBMC) [91].
Figure 9.
Ceramides and Cerebrosides (103–114).
Bioactivity directed purification of the Litophyton arboreum extract led to the identification of erythro-N-dodecanoyl-docosasphinga-(4E,8E)-dienine (113) and showed inhibitory effect against HIV-1 PR (IC50 = 4.80 ± 0.92 μM). Compound 113 showed potent HIV-1 PR inhibitory effect in the absence of cytotoxicity against the tested cell lines [92]. Compound 113, which was previously isolated from Anemonia sulcata, showed cytotoxicity (ED50 of 37.31 μM [83].
Anti-inflammatory cerebroside (114) was isolated from Indonesian Sinularia sp. [93]. The ESI-MS spectrum of 114 indicated it to be a mixture of homologs (Figure 8). Micro-scale chemical degradation of 114 allowed indicating the nature of the fatty acid moieties present [93,94]
2.5. Miscellaneous Nitrogen-Containing Metabolites
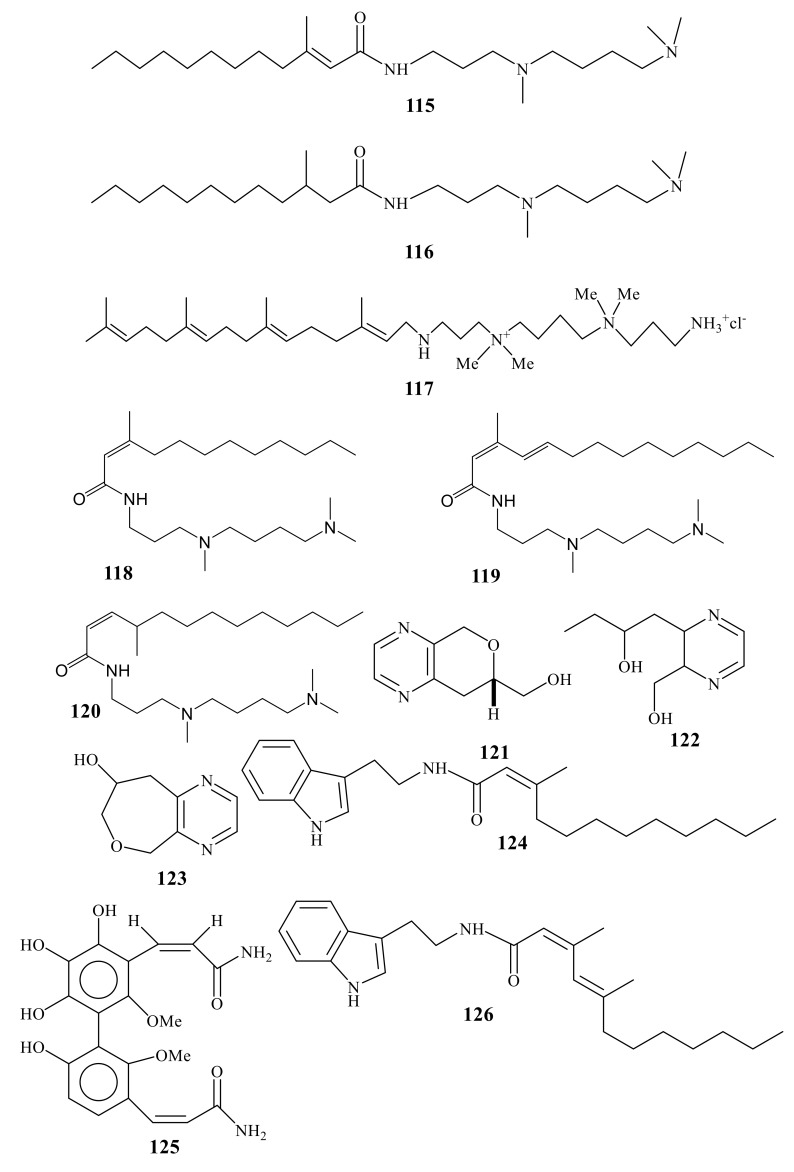
Spermidine is a polyamine found in ribosomes and living tissues. It has various metabolic functions within organisms and was isolated originally from semen. Spermidine is commonly used for in vitro molecular biology reactions, particularly in vitro transcription by Phage RNA polymerases, in vitro transcription by human RNA polymerase II, and in vitro translation. Spermidine increases specificity and reproducibility of Taq-mediated PCR by neutralizing and stabilizing the negative charge on DNA phosphate backbone. Spermidine is, at physiological pH, a polycationic reagent that aids in enzyme digestion by forcing apart DNA molecules. Two cytotoxic spermidine derivatives (115 and 116) (Figure 9) were reported from Pacific Sinularia brongersmai [95]. Sinulamide (117), a tetraprenylatedspermine derivative, was identified from Japanese Sinularia sp. Sinulamide (117) not only inhibits H, K-ATPase with an IC50 value of 5.5 µM, but also is cytotoxic against L1210 and P388 with IC50 values of 3.1 and 4.5 µg/mL, respectively [96].
Two N-methylated spermidine amides, 118 and 119, were isolated from Sinularia sp. [97]. An acylated spermidine (120) was identified from Sinularia sp. and was found to be cytotoxic against P-388 cells (ED50 0.04 µg/mL) [98].
A pyrazine congener, clavulazine (121), was first isolated from isolated from Okanawan Clavularia viridis [99], along with two marine pyrazine derivatives, Clavulazols A (122) and B (123) [100].
A unique cinnamide dimer (124) was isolated from Sinularia flexibilis [101]. Granulatamides A (125) and B (126) and a tryptamine derivative (Figure 10) were isolated from the Eunicella granulate (Figure 10). The cytotoxic effects of 125 and 126 were evaluated against certain cancer cell and GI50 values were in ranges 1.7–12.7 and 3.5–13.8 µM, respectively [102].
Figure 10.
Spermidines (115–126).
3. Summary and Conclusions
Natural products possess a characteristic chemical spatial orientation. This enables them to interact with their biological targets, which validates initial points for drug discovery. Recently, half of new drugs reported are naturally occurring or constructed on the basis of natural chemical frame. Forty percent of the bioactive compounds are natural metabolites and appear in the Dictionary of Natural Products. Chemical novelty of marine products is superior to terrestrial metabolites. Approximately 70% of the molecular skeletons that appear in databases are produced by marine organisms. Additionally, marine drugs have successfully been purchased and others are in different clinical phases.
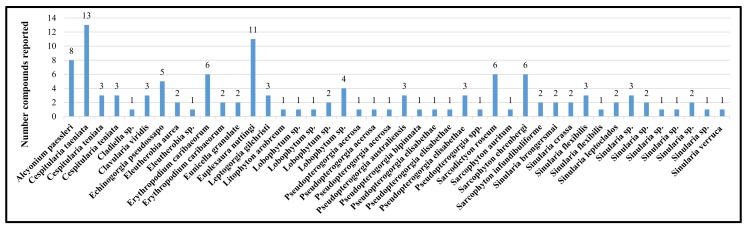
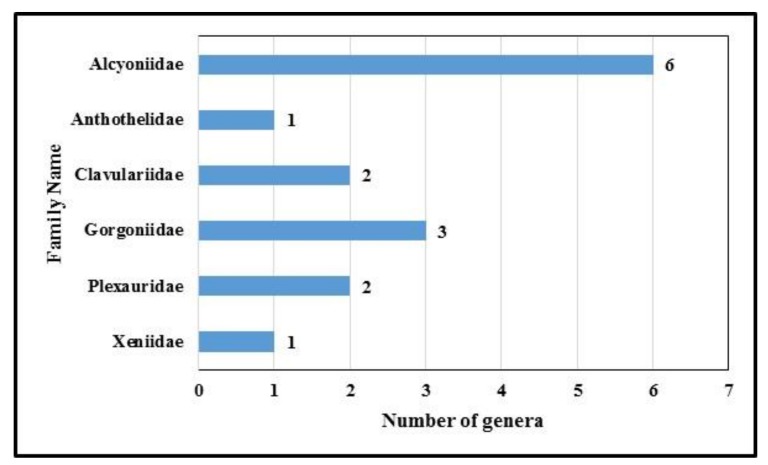
Alcyonacea will be considered as potential source of bioactive nitrogen containing metabolites. The engagement of different approaches played a significant role in the facilitation of the forthcoming drug discovery process. Noteworthy, many marine metabolites displaying fascinating molecular structures with diverse pharmacological effects have been reported from Alcyonacea during the last four decades (1978–2018). Of the 121 distinctive structures accounted for in this review, 61 (50.4%) are nitrogen-containing terpenoidal metabolites. Figure 11 illustrates the number of nitrogenous metabolites, as reported from 44 species. These species belong to six genera, as illustrated in Figure 12.
Figure 11.
Distribution of Nitrogen containing metabolites between Alcyonacea species.
Figure 12.
Relationship between Alcyonacea families and genera produce Nitrogen containing metabolites.
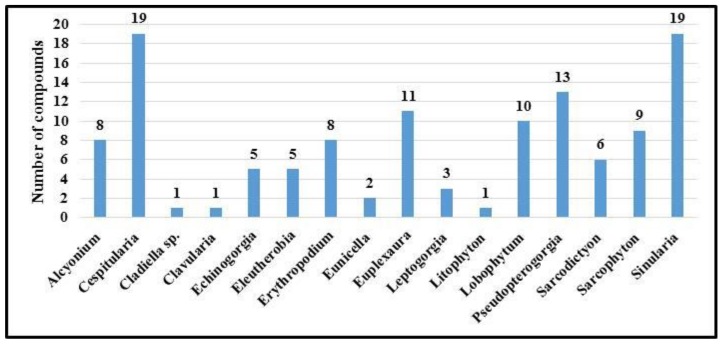
Figure 13 illustrates nitrogenous metabolites produced by 15 genera. The five most productive genera are: Cespitularia, Expeuxaura, Lobophytum, Pseudopterogorgonia and Sinularia, with 19 (15.7%), 11 (9%), and 10 (8%), 13 (9.9%) and 21 (17.3%), respectively.
Figure 13.
Distribution of nitrogen containing metabolites isolated from soft corals by genus.
A remarkable 121 metabolites reported from Alcyonacea are discussed in the current review. Of these distinctive structures, 121 (100%) are nitrogen-containing terpenes. The major classes of nitrogen-containing metabolites produced by Alcyonacea are sesquiterpenes, diterpenes and ceramides. The estimated analysis per class is as follows: 46 (38%) are diterpenes, 15 (12.4%) are sesquiterpenes, 24 (19.8%) are alkaloids and the remaining 36 (28.8%) are spermidins, cerebrosides, and ceramides, as appeared in Table 2.
Table 2.
Distribution of nitrogen-containing metabolites isolated from soft corals by skeletal class.
| Organism | Compounds | Chemical Class |
|---|---|---|
| Alcyonium paessleri | 1–8 | Illudalane-sesquiterpenoid |
| Cladiella sp. | 9 | Oxazole-derived sesquiterpene |
| Cespitularia taeniata | 10–15 | Eudesmane-sesquiterpenoid |
| 38–50 | Verticillane-diterpenoid | |
| Eleutherobia sp. and Eleutherobia aurea | 16,34, 35 | Eunicellane-diterpenoid |
| Erythropodium caribaeorum | 17–22, 36, 37 | Eunicellane-diterpenoid |
| Sarcodictyon roseum | 23–28 | Eunicellane-diterpenoid |
| Pseudopterogorgia elisabethae | 51 | Serrulatane-diterpenoid |
| 52–54 | Amphilectane-diterpenoid | |
| 55 | Ileabethane-diterpenoid | |
| Pseudopterogorgia acerosa | 60 | Pseudopteranoids bisditerpenoids |
| Pseudopterogorgia bipinnata | 63–64, 61 | Pesudopterane-diterpenoid |
| Pseudopterogorgia sp. | 62 | Pesudopterane-diterpenoid |
| Pseudopterogorgia australiensis | 100–102 | Ceramides |
| Sinularia sp. | 56–58 | Cembranoid-diterpenoid |
| 71–72 | Geranylgeraniane-diterpenoid | |
| Sinularia verruca | 89 | Pyrroloindoline alkaloid |
| Sinularia crassa, Sinularia leptoclados, and Sinularia sp. | 93–94,114 | Ceramides and cerebrosides |
| Sinularia brongersmai and Sinularia sp. | 115–120 | Spermidines |
| Sinularia flexibilis | 90–92 | Tyramine derivatives |
| Lobophytum sp. | 59 | Cembranoid-diterpenoid |
| 65 | Zoanthamine-type alkaloid | |
| 88–112 | Ceramides and cerebrosides | |
| Litophyton arboreum | 113 | Ceramides |
| Echinogorgia pseudossapo | 66–70 | Zoanthoxanthin alkaloids |
| Euplexaura nuttingi | 73–78 | Hypoxanthine |
| Leptogorgia gilchristi | 79–86 | Hypoxanthine and Seco-Hypoxanthine |
| Sarcophyton infundibuliforme | 87–88 | Tricyclic dodeca-diterpenoid |
| Sarcophyton auritum | 99 | Ceramides |
| Sarcophyton ehrenbergi | 103–108 | Ceramides and Cerebrosides |
| Clavularia viridis | 124 | Spermidines |
| Eunicella granulate | 125–126 | Spermidines |
Diterpenoids, the major division, are in turn further analyzed for each Alcyonacean species, and the distribution by skeleton classes of compounds in this group is shown in Table 3, which summarizes the impressive structural variety of terpenoid carbon skeletons found in these animals. The diterpene skeleton is most frequently elaborated by the genera Pseudopterogorgia, Erythropodium, Sarcodictyon, Lobophytom and Sinularia. These diterpenes have highly functionalized moieties with potent cytotoxicity that could be mimetic the mode of action of taxol. Some of them are currently in clinical trials.
Table 3.
Distribution of nitrogen-containing metabolites isolated from soft corals by genus and chemical classes.
| Genus | Family | Common N-Containing Chemical Class |
|---|---|---|
|
Alcyonium paessleri Cladiella sp. Eleutherobia Sinularia Lobophytum Sarcophyton |
Alcyoniidae | Illudalane-type sesquiterpenes Oxazole-derived sesquiterpene Eunicellane- diterpenoid Cembranoid-diterpenoid Geranylgeraniane-diterpenoid Pyrroloindoline alkaloid Zoanthamine-type alkaloid Tricyclic dodeca-diterpenoid Ceramides and cerebrosides Spermidines |
|
Pseudopterogorgia
Leptogorgia Eunicella |
Gorgoniidae | Serrulatane- diterpenoid Amphilectane- diterpenoid Ileabethane- diterpenoid Pseudopteranoids-bisditerpenoids Hypoxanthine and seco- Hypoxanthine Spermidines |
| Cespitularia | Xeniidae | Eudesmane- sesquiterpenoid Verticillane- diterpenoid |
| Erythropodium | Anthothelidae | Eunicellane- diterpenoid |
|
Sarcodictyon
Clavularia |
Clavulariidae | Eunicellane- diterpenoid Spermidines |
| Euplexaura | Plexauridae | Hypoxanthine |
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blunt J.W., Copp B.R., Munro M.H., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2011;28:196–268. doi: 10.1039/C005001F. [DOI] [PubMed] [Google Scholar]
- 2.Hegazy M.E., Mohamed T.A., Alhammady M.A., Shaheen A.M., Reda E.H., Elshamy A., Aziz M., Paul W. Molecular architecture and biomedical leads of terpenes from Red Sea marine invertebrates. Mar. Drugs. 2015;13:3154–3181. doi: 10.3390/md13053154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowski K., Schneider G. Properties and architecture of drugs and natural products revisited. Curr. Chem. Biol. 2007;1:115–127. [Google Scholar]
- 4.Datta D., Talapatra S.N., Swarnakar S. Bioactive compounds from marine invertebrates for potential medicines—An overview. Inter. Lett. Nat. Sci. 2015;34:42–61. doi: 10.18052/www.scipress.com/ILNS.34.42. [DOI] [Google Scholar]
- 5.Rocha J., Peixe L., Gomes N.C.M., Calado R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs. 2011;9:1860–1886. doi: 10.3390/md9101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrue F., Kerr R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009;26:681–710. doi: 10.1039/b821918b. [DOI] [PubMed] [Google Scholar]
- 7.Altmann K.H., Gertsch J. Anticancer drugs from nature--natural products as a unique source of new microtubule-stabilizing agents. Nat. Prod. Rep. 2007;24:327–357. doi: 10.1039/B515619J. [DOI] [PubMed] [Google Scholar]
- 8.Turner A.H., Graik D.J., Kaas Q., Schroeder C.I. Bioactive compounds isolated from neglected predatory marine gastropods. Mar. Drugs. 2018;16:118. doi: 10.3390/md16040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J., Wu J., An B., de Voogd N.J., Cheng W., Lin W. Bromopyrrole alkaloids with the inhibitory effects against the biofilm formation of gram negative bacteria. Mar. Drugs. 2018;16:9. doi: 10.3390/md16010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C., Zhu J., Wang Y., Chen Y., Song L., Zheng W., Li J. Antibacterial activity of Al-hemocidin 2, a novel N-terminal peptide of hemoglobin purified from arca inflate. Mar. Drugs. 2017;15:205. doi: 10.3390/md15070205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez N.G.M., Dasari R., Chandra S., Kiss R., Kornienko A. Marine invertebrate metabolites with anticancer activities: solutions to the “supply problem”. Mar. Drugs. 2016;14:98. doi: 10.3390/md14050098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putra M.Y., Murniasih T., Swasono R.T., Wibowo J.T., Saputri A.N.C., Widhiana M.R., Arlyza I.S. Secondary metabolites and their biological activities in Indonesian soft coral of the genus Lobophytum. Asian Pac. J. Trop. Biomed. 2016;6:909–913. doi: 10.1016/j.apjtb.2016.08.011. [DOI] [Google Scholar]
- 13.Sun Z.H., Cai Y.H., Fan C.Q., Tang G.H., Luo H.-B., Yin S. Six new tetraprenylated alkaloids from the south China Sea gorgonian Echinogorgia pseudossapo. Mar. Drugs. 2014;12:672–681. doi: 10.3390/md12020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanmethakul T., Chansang H., Watanasit S. Soft coral (Cnidaria: Alcyonacea) distribution patterns in thai waters. Zool. Stud. 2010;49:72–84. [Google Scholar]
- 15.Liang I.F., Guo Y.W. Terpenes from the soft corals of the genus Sarcophyton: chemistry and biological activities. Chem. Biodivers. 2013;10:2161–2196. doi: 10.1002/cbdv.201200122. [DOI] [PubMed] [Google Scholar]
- 16.Sammarco P.W., Coll J.C. Chemical adaptations in the octocorallia: evolutionary considerations. Mar. Ecol. Prog. Ser. 1992;88:93–104. doi: 10.3354/meps088093. [DOI] [Google Scholar]
- 17.Aceret T.L., Coll J.C., Uchio Y., Sammarco P.W. Antimicrobial activity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis Quoy and Gaimard 1833 (Coelenterata: Alcyonacea, Octocorallia) Comp. Biochem. Physiol. C. Pharmacol Toxicol. Endocrinol. 1998;120:121–126. doi: 10.1016/S0742-8413(98)00032-2. [DOI] [PubMed] [Google Scholar]
- 18.Valmsen K., Jarving I., Boeglin W.E., Varvas K., Koljak R., Pehk T., Brash A.R., Samel N. The origin of 15R- prostaglandins in the Caribbean coral Plexaura homomalla: Molecular cloning and expression of a novel cyclooxygenase. Proc. Natl. Acad. Sci. USA. 2001;98:7700–7705. doi: 10.1073/pnas.131022398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zubair M., Alarif W.M., Al-Footy Kh.O., PH M., Aly M., Basaif S., Al-Lihaibi S., Ayyad S.-E. New antimicrobial biscembrane hydrocarbon and cembranoid diterpenes from the soft coral Sarcophyton trocheliophorum. Turk. J. Chem. 2016;40:385–392. doi: 10.3906/kim-1502-82. [DOI] [Google Scholar]
- 20.Palermo J.A., Rodrıguez-Brasco M.F., Spagnuolo C., Seldes A.M. Illudalane sesquiterpenoids from the soft coral Alcyonium paessleri: the first natural nitrate esters. J. Org. Chem. 2000;65:4482–4486. doi: 10.1021/jo991740x. [DOI] [PubMed] [Google Scholar]
- 21.Ata A., Ackerman J., Radhika P. Cladixazole: a novel sesquiterpene from a marine soft coral of genus Cladiella. Tetrahedron Lett. 2003;44:6951–6953. doi: 10.1016/S0040-4039(03)01712-X. [DOI] [Google Scholar]
- 22.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 23.Kong D.X., Jiang Y.Y., Zhang H.Y. Marine natural products as sources of novel scaffolds: Achievement and concern. Drug Discov. Today. 2010;15:884–886. doi: 10.1016/j.drudis.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Mayer A.M.S., Glaser K.B., Cuevas C., Jacobs R.S., Kem W., Little R.D., McIntosh J.M., Newman D.J., Potts B.C., Shuster D.E. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol. Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Montaser R., Luesch H. Marine natural products: a new wave of drugs? Future Med. Chem. 2011;3:1475–1489. doi: 10.4155/fmc.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross H., Koenig G.M. Terpenoids from marine organisms: unique structures and their pharmacological potential. Phytochem. Rev. 2006;5:115–141. doi: 10.1007/s11101-005-5464-3. [DOI] [Google Scholar]
- 27.Faulkner D.J. Marine pharmacology. Antonie Van Leeuwenhoek. 2000;77:135–145. doi: 10.1023/A:1002405815493. [DOI] [PubMed] [Google Scholar]
- 28.Nakao Y., Yoshida S., Matsunaga S., Fusetani N. (Z)-sarcodictyin A, a new highly cytotoxic diterpenoid from the soft coral Bellonella albiflora. J. Nat. Prod. 2003;66:524–527. doi: 10.1021/np0205452. [DOI] [PubMed] [Google Scholar]
- 29.Ayer W.A., Browne L.M. Terpenoid metabolites of mushrooms and related basidiomycetes. Tetrahedron. 1981;37:2197–2248. doi: 10.1016/S0040-4020(01)97979-7. [DOI] [Google Scholar]
- 30.Ayer W.A., McCaskill R.H. The cybrodins: new seco-illudalanes from the bird’s nest fungus Cyathus bulleri brodie. Tetrahedron Lett. 1980;21:1917–1920. doi: 10.1016/S0040-4039(00)93643-8. [DOI] [Google Scholar]
- 31.Cheng Y.B., Chen C.Y., Kuo Y.H., Shen Y.C. New Nitrogen-containing sesquiterpenoids from the Taiwanese soft coral Cespitularia taeniata MAY. Chem. Biodiver. 2009;6:1266–1272. doi: 10.1002/cbdv.200800195. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z.L., Cao W.Y., Zhou G.X., Wichtl M. A sesquiterpene lactam from Artractylodes macrocephala. Phytochemistry. 1997;45:765–767. doi: 10.1016/S0031-9422(97)00036-8. [DOI] [Google Scholar]
- 33.Wang S.S., Cheng Y.B., Lin Y.C., Liaw C.C., Chang J.Y., Kuo Y.H., Shen Y.C. Nitrogen-containing diterpenoids, sesquiterpenoids, and nor-diterpenoids from Cespitularia taeniata. Mar. Drugs. 2015;13:5796–5814. doi: 10.3390/md13095796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindel T., Jensen P.R., Fenical W., Long B.H., Casazza A.M., Carboni J., Fairchild C.R. Eleutherobin, a new cytotoxin that mimics paclitaxel (taxol) by stabilizing microtubules. J. Am. Chem. Soc. 1997;119:8744–8745. doi: 10.1021/ja9717828. [DOI] [Google Scholar]
- 35.Cinel B., Roberge M., Behrisch H., van Ofwegen L., Castro C.B., Andersen R.J. Antimitotic diterpenes from Erythropodium caribaeorum test pharmacophore models for microtubule stabilization. J. Org. Lett. 2000;2:257–260. doi: 10.1021/ol9912027. [DOI] [PubMed] [Google Scholar]
- 36.Britton R., Roberge M., Berisch H., Andersen R.J. Antimitotic diterpenoids from Erythropodium caribaeorum: Isolation artifacts and putative biosynthetic intermediates. Tetrahedron Lett. 2001;42:2953–2956. doi: 10.1016/S0040-4039(01)00347-1. [DOI] [Google Scholar]
- 37.Roberge M., Cinel B., Anderson H.J., Lim L., Jiang X., Xu L., Kelly M.T., Andersen R.J. Cell-based screen for antimitotic agents and identification of analogues of rhizoxin, eleutherobin, and paclitaxel in natural extracts. Cancer Res. 2000;60:5052–5058. [PubMed] [Google Scholar]
- 38.Bollag D.M., McQueney P.A., Zhu J., Hansens O., Koupal L., Liesch J., Goetz M., Lazarides E., Woods C.M. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 39.Ganesh T.L., Guza R.C., Bane S., Ravindra R., Shanker N., Lakdawala A.S., Snyder J.P., Kingston D.G. The bioactive taxol conformation on beta-tubulin: experimental evidence from highly active constrained analogs. Proc. Natl. Acad. Sci. USA. 2004;101:10006–10011. doi: 10.1073/pnas.0403459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicolaou K.C., Pfefferkorn J., Xu J., Winssinger N., Ohshima T., Kim S., Hosokawa S., Vourloumis D., Van Delft F., Li T. Total synthesis and chemical biology of the sarcodictyins. Chem. Pharm. Bull. 1999;47:1199–1213. doi: 10.1248/cpb.47.1199. [DOI] [PubMed] [Google Scholar]
- 41.D’Ambrosio M., Guerriero A., Pietra F. Sarcodictyin A. and Sarcodictyin B. Novel diterpenoidic alcohols esterified by (E)-N(1)-Methylurocanic acid, isolation from the Mediterranean stolonifer Sarcodictyon roseum. Helvetica Chimica Acta. 1987;70:2019–2027. doi: 10.1002/hlca.19870700807. [DOI] [Google Scholar]
- 42.D’Ambrosio M., Guerriero A., Pietra F. Isolation from the Mediterranean stoloniferan coral Sarcodictyon roseurn of sarcodictyin C, D, E, and F, Novel diterpenoidic alcohols esterified by (E)-or (Z)-N(1)-methylurocanic acid. Failure of the carbon-skeleton type as a classification criterion. Helvetica Chimica Acta. 1988;71:964–976. doi: 10.1002/hlca.19880710504. [DOI] [Google Scholar]
- 43.Lin Y., Bewley C.A., Faulkner D.J. The Valdivones, anti-inflammatory diterpene esters from the South African soft coral Alcyonium valdivae. Tetrahedron. 1993;49:7977–7984. doi: 10.1016/S0040-4020(01)88021-2. [DOI] [Google Scholar]
- 44.Ketzinel S., Rudi A., Schleyer M., Benayahu Y., Kashman Y. Sarcodictyin A and two novel diterpenoid glycosides, eleuthosides A and B, from the Soft Coral Eleutherobia aurea. J. Nat. Prod. 1996;59:873–875. doi: 10.1021/np960361l. [DOI] [Google Scholar]
- 45.Shen Y.C., Lin Y.S., Kuo Y.H., Cheng Y. Cespitulactams A, B, and C, three new nitrogen-containing diterpenes from Cespitularia taeniata MAY. Tetrahedron Lett. 2005;46:7893–7897. doi: 10.1016/j.tetlet.2005.09.105. [DOI] [Google Scholar]
- 46.Shen Y.C., Cheng Y.B., Kobayashi J., Kubota T., Takahashi Y., Mikami Y., Ito J., Lin Y.S. Nitrogen-containing verticillene diterpenoids from the Taiwanese soft coral Cespitularia taeniata. J. Nat. Prod. 2007;70:1961–1965. doi: 10.1021/np078011u. [DOI] [PubMed] [Google Scholar]
- 47.Elshamy A.I., Nassar M.I., Mohamed T.A., Hegazy M.E.F. Chemical and biological profile of Cespitularia species: A mini review. J. Adv. Res. 2016;7:209–224. doi: 10.1016/j.jare.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ata A., Kerr R.G. Elisabethamine: a new diterpene alkaloid from Pseudopterogorgia elisabethae. Tetrahedron Lett. 2000;41:5821–5825. doi: 10.1016/S0040-4039(00)00980-1. [DOI] [Google Scholar]
- 49.Rodríguez A.D., Ramírez C., Rodríguez I.I., González E. Novel Antimycobacterial benzoxazole Alkaloids, from the West Indian Sea Whip Pseudopterogorgia elisabethae. Org. Lett. 1999;1:527–530. doi: 10.1021/ol9907116. [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez I.I., Rodríguez A.D. Homopseudopteroxazole, a new antimycobacterial diterpene alkaloid from Pseudopterogorgia elisabethae. J. Nat. Prod. 2003;66:855–857. doi: 10.1021/np030052c. [DOI] [PubMed] [Google Scholar]
- 51.Johnson T.W., Corey E.J. Enantiospecific synthesis of the proposed structure of the antitubercular marine diterpenoid pseudopteroxazole: Revision of stereochemistry. J. Am. Chem. Soc. 2001;123:4475–4479. doi: 10.1021/ja010221k. [DOI] [PubMed] [Google Scholar]
- 52.Davidson J.P., Corey E.J. First enantiospecific total synthesis of the antitubercular marine natural product pseudopteroxazole. Revision of assigned stereochemistry. J. Am. Chem. Soc. 2003;125:13486–13489. doi: 10.1021/ja0378916. [DOI] [PubMed] [Google Scholar]
- 53.Harmata M., Hong X., Barnes C.L. Benzothiazines in synthesis. Toward the synthesis of pseudopteroxazole. Org. Lett. 2004;6:2201–2203. doi: 10.1021/ol049334 . [DOI] [PubMed] [Google Scholar]
- 54.Harmata M., Hong X. Benzothiazines in synthesis. A total synthesis of pseudopteroxazole. Org. Lett. 2005;7:3581–3583. doi: 10.1021/ol0515412. [DOI] [PubMed] [Google Scholar]
- 55.Coleman A.C., Kerr R.G. Radioactivity-guided isolation and characterization of the bicyclic pseudopterosin diterpene cyclase product from Pseudopterogorgia elisabethae. Tetrahedron. 2000;56:9569–9574. doi: 10.1016/S0040-4020(00)00930-3. [DOI] [Google Scholar]
- 56.Kohl A.C., Kerr R.G. Pseudopterosin biosynthesis: aromatization of the diterpene cyclase product, elisabethatriene. Mar. Drugs. 2003;1:54–65. doi: 10.3390/md101054. [DOI] [Google Scholar]
- 57.Rodríguez A.D., Ramírez C. Serrulatane diterpenes with antimycobacterial activity isolated from the West Indian Sea whip Pseudopterogorgia elisabethae. J. Nat. Prod. 2001;64:100–102. doi: 10.1021/np000196g. [DOI] [PubMed] [Google Scholar]
- 58.Iguchi K., Nishimura K., Yamazaki K., Iwashima M., Yamada Y. New cembranolide diterpenes with a dimethylamino group from the Okinawan soft coral (Sinularia sp.) Chem. Lett. 1992;21:127–130. doi: 10.1246/cl.1992.127. [DOI] [Google Scholar]
- 59.Lakshmi V., Kumar R. Metabolites from Sinularia species. Nat. Prod. Res. 2009;23:801–850. doi: 10.1080/14786410802137135. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi M., Isizaka T. Marine terpenes and terpenoids, Part XV. Dimethylamine adducts of α-methylene-γ-lactonic marine cembranoids. J. Chem Res. 1992;10:340–341. [Google Scholar]
- 61.Rashid M.A., Gustafson K.R., Boyd M.R. HIV-Inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum Species. J. Nat. Prod. 2000;63:531–533. doi: 10.1021/np990372p. [DOI] [PubMed] [Google Scholar]
- 62.Kate A.S., Pearson J.K., Ramanathan B., Richard K., Kerr R.G. Biomimetic synthesis, and cytotoxic activity of bis (pseudopterane) amines. J. Nat. Prod. 2009;72:1331–1334. doi: 10.1021/np8008144. [DOI] [PubMed] [Google Scholar]
- 63.Tinto W.F., John L., Reynolds W.F., McLean S. Novel psendopteranoids of Pseudontenmowia acerosa. Tetrahedron. 1991;47:8679–8686. doi: 10.1016/S0040-4020(01)96190-3. [DOI] [Google Scholar]
- 64.Ospina C.A., Rodrıguez A.D., Sanchez J.A., Ortega-Barria E., Capson T.L., Mayer A.M.S. Caucanolides A-F, unusual antiplasmodial constituents from a colombian collection of the gorgonian coral Pseudopterogorgia bipinnata. J. Nat. Prod. 2005;68:1519–1526. doi: 10.1021/np050239z. [DOI] [PubMed] [Google Scholar]
- 65.Bandurraga M.M., Fenical W., Donovan S.F., Clardy J. Pseudopterolide, an irregular diterpenoid with unusual cytotoxic properties from the Caribbean Sea whip Pseudopterogorgia acerosa (Pallas) (Gorgonacea) J. Am. Chem. Soc. 1982;104:6463–6465. doi: 10.1021/ja00387a059. [DOI] [Google Scholar]
- 66.Tinto W.F., Chan W.R., Reynolds W.F., Mclean S. Tobagolide, a new pseudopterane diterpenoid of the octocoral Pseudopterogorgia acerosa. Tetrahedron Lett. 1990;31:465–468. doi: 10.1016/0040-4039(90)87009-O. [DOI] [Google Scholar]
- 67.Rodrıguez A.D., Soto J.J. A novel norditerpene from the Caribbean Sea plume Pseudopterogorgia acerosa (Pallas) J. Org. Chem. 1996;61:4487–4490. doi: 10.1021/jo951920g. [DOI] [PubMed] [Google Scholar]
- 68.Rodrıguez A.D., Soto J.J. Isolation and structure of aceropterine, a rare pseudopterane alkaloid from the Caribbean Sea plume Pseudopterogorgia acerosa (pallas) Tetrahedron Lett. 1996;37:2687–2690. doi: 10.1016/0040-4039(96)00392-9. [DOI] [Google Scholar]
- 69.Rao C.B., Anjaneyula A.S.R., Sarma N.S., Vankateswarlu Y., Rosser R.M., Faulkner D.J., Chen M.H.M., Clardy J. Zoanthamine: a novel alkaloid from a marine zoanthid. J. Am. Chem. Soc. 1984;106:7983–7984. doi: 10.1021/ja00337a062. [DOI] [Google Scholar]
- 70.Fattorusso E., Romano A., Taglialatela-Scafati O., Achmad M.J., Bavestrello G., Cerrano C. Lobozoanthamine, a new zoanthamine-type alkaloid from the Indonesian soft coral Lobophytum sp. Tetrahedron Lett. 2008;49:2189–2192. doi: 10.1016/j.tetlet.2008.02.028. [DOI] [Google Scholar]
- 71.Nakamura H., Kawase Y., Maruyama K., Murai A. Studies on polyketide metabolites of a symbiotic dinoflagellate, Symbiodinium sp.: a new C30 marine alkaloid, zooxanthellamine, a plausible precursor for zoanthid alkaloids. Bull. Chem. Soc. Jpn. 1998;71:781–787. doi: 10.1246/bcsj.71.781. [DOI] [Google Scholar]
- 72.Gao C.H., Wang Y.F., Li S., Qian P.-Y., Qi S.H. Alkaloids and sesquiterpenes from the South China Sea gorgonian Echinogorgia pseudossapo. Mar. Drugs. 2011;9:2479–2487. doi: 10.3390/md9112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Putra M.Y., Ianaro A., Panza E., Bavestrello G., Cerrano C., Fattorusso E., Taglialatela-Scafati O. Sinularioside, triacetylated glycolipid from the Indonesian soft coral Sinularia sp., is an inhibitor of NO release. Bioorg. Med. Chem. Lett. 2012;22:2723–2725. doi: 10.1016/j.bmcl.2012.02.102. [DOI] [PubMed] [Google Scholar]
- 74.Tang G.H., Chen D.M., Qiu B.Y., Sheng L., Wang Y.H., Hu G.W., Zhao F.W., Ma L.J., Wang H., Huang Q.-Q., Zu J.J., Long C.L., Li J. Cytotoxic amide alkaloids from piper Boehmeriae folium. J. Nat. Prod. 2011;74:45–49. doi: 10.1021/np100606u. [DOI] [PubMed] [Google Scholar]
- 75.Graham S.K., Lambert L.K., Pierens G.K., Hooper J.N.A., Garson M.J. Psammaplin metabolites new and old: a nmr study involving chiral sulfur chemistry. Aust. J. Chem. 2010;63:867–872. doi: 10.1071/CH09617. [DOI] [Google Scholar]
- 76.Sorek H., Rudi A., Benayahu Y., Ben-Califa N., Neumann D., Kashman Y. Nuttingins A-F and Malonganenones D-H, Tetraprenylated alkaloids from the Tanzanian gorgonian Euplexaura nuttingi. J. Nat. Prod. 2007;70:1104–1109. doi: 10.1021/np060642l. [DOI] [PubMed] [Google Scholar]
- 77.Keyzers R.A., Gray C.A., Schleyer M.H., Whibley C.E., Hendricks D.T., Davies-Coleman M.T. Malonganenones A–C, novel tetraprenylated alkaloids from the Mozambique gorgonian Leptogorgia gilchristi. Tetrahedron. 2006;62:2200–2206. doi: 10.1016/j.tet.2005.12.018. [DOI] [Google Scholar]
- 78.Ye F., Li J., Wu Y., Zhu Z.D., Mollo E., Gavagnin M., Gu Y.C., Zhu W.L., Li X.W., Guo Y.W. Sarinfacetamides A and B, nitrogenous diterpenoids with tricyclo [6.3.1.01,5]dodecane scaffold from the south china sea soft coral Sarcophyton infundibuliforme. Org. Lett. 2018;20:2637–2640. doi: 10.1021/acs.orglett.8b00842. [DOI] [PubMed] [Google Scholar]
- 79.Yuan W., Cheng S., Fu W., Zhao M., Li X., Cai Y., Dong J., Huang K., Gustafson K.R., Yan P. Structurally diverse metabolites from the soft coral Sinularia verruca collected in the South China Sea. J. Nat. Prod. 2016;79:1124–1131. doi: 10.1021/acs.jnatprod.6b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kazlauskas R., Marwood J.F., Wells R.J. 2-Phenylethylamides of a novel lipid acid; atrial stimulants from the soft coral Sinularia flexibilis. J. Aust. Chem. 1980;33:1799–1803. doi: 10.1071/CH9801799. [DOI] [Google Scholar]
- 81.Jin W., Rinehart K.L., Jares-Erijman E.A. Ophidiacerebrosides: cytotoxic glycosphingolipids containing a novel sphingosine from a sea star. J. Org. Chem. 1994;59:144–147. doi: 10.1021/jo00080a023. [DOI] [Google Scholar]
- 82.Zhang G.-W., Ma X.-Q., Zhang C.-X., Su J.-Y., Ye W.-C., Zhang X.-Q., Yao X.-S., Zeng L.-M. Two new ceramides from the marine sponge Ircinia fasciculate. Helv. Chim. Acta. 2005;88:885–890. doi: 10.1002/hlca.200590066. [DOI] [Google Scholar]
- 83.Chebaane K., Guyot W. Occurrence of eryzw-docosasphinga-4, 8-dienine, as an ester, in anemonia sulcata. Tetrahedron Lett. 1986;27:1495–1496. doi: 10.1016/S0040-4039(00)84294-X. [DOI] [Google Scholar]
- 84.Carter G.T., Rinehart K.L., Jr. Aplidiasphingosine, an antimicrobial and antitumor terpenoid from an Aplidium species (marine tunicate) J. Am. Chem. Soc. 1978;100:7441–7442. doi: 10.1021/ja00491a066. [DOI] [Google Scholar]
- 85.Anjaneyulu V., Radhika P. Two new sphingosine derivatives from Sinularia crassa Tixier-Durivault of the Andaman and Nicobar Islands. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1999;38:457–460. doi: 10.1002/chin.199941252. [DOI] [Google Scholar]
- 86.Bala S.R.G., Venkata R.D., Bheemasankara R.C., Dhananjaya N., Kuttan R., Babu T.D. Isolation and structural determination of new sphingolipids and pharmacological activity of africanene and other metabolites from Sinularia leptoclados. Chem. Pharm. Bull. 1999;47:1214–1220. doi: 10.1248/cpb.47.1214. [DOI] [PubMed] [Google Scholar]
- 87.Radhika P., Rao V.L., Laatsch H. Chemical constituents of a marine soft coral of the genus Lobophytum. Chem. Pharm. Bull. 2004;52:1345–1348. doi: 10.1248/cpb.52.1345. [DOI] [PubMed] [Google Scholar]
- 88.Eltahawy N.A., Ibrahim A.K., Radwan M.M., Zaitone S., Gomaa M., ElSohly M.A., Hassanean H.A., Ahmed S.A. Mechanism of action of antiepileptic ceramide from red sea soft coral Sarcophyton auritum. Bioorg. Med. Chem. Lett. 2015;25:5819–5824. doi: 10.1016/j.bmcl.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 89.Krishna N., Muralidhar P., Kumar M.M., Rao D.V., Rao C.B. New Sphingosines from a gorgonian, Pseudopterogorgia australiensis Ridley, of the Indian Ocean. J. Nat. Prod. 2004;67:1423–1425. doi: 10.1021/np040022p. [DOI] [PubMed] [Google Scholar]
- 90.Cheng S.Y., Wen Z.H., Chiou S.F., Tsai C.W., Wang S.K., Hsu C.H., Dai C.F., Chiang M.Y., Wang W.H., Duh C.Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009;72:465–468. doi: 10.1021/np800362g. [DOI] [PubMed] [Google Scholar]
- 91.Muralidhar P., Kumar M.M., Krishna N., Rao C.B., Rao D.V. New sphingolipids and a sterol from a Lobophytum species of the Indian Ocean. Chem. Pharm. Bull. 2005;53:168–171. doi: 10.1248/cpb.53.168. [DOI] [PubMed] [Google Scholar]
- 92.Ellithey M.S., Lall N., Hussein A.A., Meyer D. Cytotoxic, cytostatic and HIV-1 PR inhibitory activities of the soft coral Litophyton arboreum. Mar. Drugs. 2013;11:4917–4936. doi: 10.3390/md11124917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Putra M.Y., Ianaro A., Panza E., Bavestrello G., Cerrano C., Fattorusso E., Taglialatela-Scafati O. Sinulasulfoxide and sinulasulfone, sulfur containing alkaloids from the Indonesian soft coral Sinularia sp. Tetrahedron Lett. 2012;53:3937–3939. doi: 10.1016/j.tetlet.2012.05.095. [DOI] [Google Scholar]
- 94.Sata N.U., Sugano M., Matsunaga S., Fusetan N. Sinulamide: An H, K-ATPase inhibitor from a soft coral Sinularia sp. Tetrahedron Lett. 1999;40:719–722. doi: 10.1016/S0040-4039(98)02438-1. [DOI] [Google Scholar]
- 95.Schmitz F.J., Hollenbeak K.H., Prasad R.S. Marine Natural products: cytotoxic spermidine derivatives from the soft coral Sinularia brongersmai. Tetrahedron Lett. 1979;36:3387–3390. doi: 10.1016/S0040-4039(01)95416-4. [DOI] [Google Scholar]
- 96.Faulkner D.J. Marine natural products. Nat. Prod. Rep. 1997;14:259–302. doi: 10.1039/np9971400259. [DOI] [Google Scholar]
- 97.Kazlauskas R., Murphy P.T., Ravi B.N., Sanders R.L., Wells R. Spermidine derivative s and 9,11-secosteroids from a soft coral (Sinularia sp.) Aust. J. Chem. 1982;35:69–75. doi: 10.1071/CH9820069. [DOI] [Google Scholar]
- 98.Choi Y.H., Schmitz F.J. Cytotoxic acylated spermidine from a soft coral, Sinularia sp. J. Nat. Prod. 1997;60:495–496. doi: 10.1021/np960662v. [DOI] [PubMed] [Google Scholar]
- 99.Watanabe K., Iguchi K., Fujimori K. Clavulazine, a new marine pyrazine congener from the Okinawan soft coral Clavularia viridis. Heterocycles. 1998;49:269–274. doi: 10.1002/chin.199923272. [DOI] [Google Scholar]
- 100.Shen Y.C., Wang L.T., Cheng Y.B., Khalil A.T., Chen M.H., Lin Y.C. Clavulazols A and B, two new pyrazine derivatives from Clavularia viridis. J. Chin. Chem. Soc. 2004;51:1421–1424. doi: 10.1002/jccs.200400209. [DOI] [Google Scholar]
- 101.Anjaneyulu A.S.R., Sagar K.S., Rao N.S.K. A Novel cinnamide dimer from the Indian Ocean soft coral Sinularia flexibilis. Nat. Prod. Lett. 1997;11:5–11. doi: 10.1080/10575639708043750. [DOI] [Google Scholar]
- 102.Reyes F., Martin R., Fernandez R. Granulatamides A and B, cytotoxic tryptamine derivative from the soft coral Eunicella granulate. J. Nat. Prod. 2006;69:668–670. doi: 10.1021/np050382s. [DOI] [PubMed] [Google Scholar]