Abstract
The aim of the study was to determine the chemical composition of lemon, rosewood, geranium and rosemary oils, and compare their effect on the sensitivity of Fusarium graminearum ZALF 24 and Fusarium graminearum ZALF 339 isolated from infected cereals. The tested oils were added to Potato Dextrose Agar (PDA) medium at concentrations of 0.125%, 0.25%, 0.5%, 1.0% and 2.0%. The activity of the oils on inhibition of the linear growth of mycelium was evaluated by measuring the growth of fungal colonies (growth index), while the fungistatic activity was evaluated on the basis of the percentage growth inhibition of a fungal colony and calculated according to Abbott’s formula. The sensitivity of the test strains was variable and depended on the type and concentration of the tested oils. Geranium and rosewood oils in all of the concentrations completely inhibited the growth of the used isolates. In contrast, lemon oil relative to F. graminearum ZALF 339 showed the highest activity at a concentration of 1.0% and rosemary oil, 0.5%. The highest activity against F. graminearum ZALF 24 was shown by the oils of rosemary and lemon at concentrations from 1.0% to 2.0%. The susceptibility of Fusarium graminearum isolates was differentiated and depended on the type and concentration of tested oils.
Keywords: Fusarium graminearum, essential oils, geranium oil, rosewood oil, lemon oil, rosemary oil
1. Introduction
In Poland, since January 2014, there has been an obligation to apply the principles of integrated cropping systems in agriculture, resulting from the provisions of Art. 14 of Directive 2009/128/EC and Regulation No. 1107/2009. These principles involve a reduction in the consumption of chemical pesticides and fertilisers. Instead, appropriate agricultural practices, organic fertilisation, and biological methods, which use microbial activity as well as biologically active substances, should be applied.
Both in the case of an integrated system of cultivation and organic waste management, there are few technologies available that can improve productivity and increase competitiveness on the market. Chemical fungicides, such as Cuprate 50 HR, Amistar 250 SC, Tango Star 334 SE, Caramba 60 SL and Folicur Plus 375 EC Artea 330 EC [1], which are currently used in the cultivation of plants to combat phytopathogenic fungi of the genus Fusarium, despite their efficiency and simplicity of application, cause a risk to the health and safety of the consumer. The threat is caused by fungicide residues, increasing immunisation of fungal pathogens, and the reduction of beneficial organisms [2,3]. Synthetic fungicides, which are nonbiodegradable in the environment, have a negative impact on terrestrial ecosystems, which is why a biological control seems to be a good alternative to chemicals [4,5].
The alternative could be essential oils that show fungicidal activity [6] and are safe for people and the environment [7]. Numerous studies have shown that essential oils that are a mixture of monoterpenes, monoterpenoids, sesquiterpenes and fragrances (esters, ketones, phenols, alcohols, aldehydes, ethers, hydrocarbons, coumarins and organic acids) effectively limit the development of phytopathogenic fungi types: Fusarium (Fusarium oxysporum, Fusarium culmorum), Phytophthora, Stemphylium, Sphaerotheca, Botrytis, Erysiphe, Aspergillus, Mortierella, Sclerotinia, Sporotrichum, Penicillium and Alternaria [8,9,10] and yeast [11]. The effect of their biocidal action is dependent on their chemical composition and the essential oils’ concentration, as well as the sensitivity of phytopathogenic fungi strains; thus, research into the use of essential oils as effective biofungicides is constantly conducted.
The aim of the study was to determine the chemical composition of lemon, rosewood, geranium and rosemary oils and to compare their effect on the sensitivity of Fusarium graminearum ZALF 24 and Fusarium graminearum ZALF 339 isolated from infected cereals.
2. Results
In studies on the reduction of the development of phytopathogens, essential oils of different chemical composition were used. Table 1 and Table 2 present the main types of terpenes found in the tested oils and the details of the chemical composition. The chromatograms of the used essential oils are presented in Figure A1, Figure A2, Figure A3 and Figure A4. The rosemary and geranium oils contained mainly monoterpenoid (over 96% and over 88%, respectively), whereas lemon oil contained mainly monoterpenes (less than 86%) and only 13.8% monoterpenoids. The most varied in terms of chemical composition turned out to be rosemary oil, which contained both monoterpenoids (52.8%) and monoterpenes (35.3%) as well as sesquiterpenes (11.5%).
Table 1.
The contents of terpenes (%) in the tested essential oils.
| Area (%) of Terpenes in Etja Essential Oils | ||||
|---|---|---|---|---|
| Lemon | Rosewood | Geranium | Rosemary | |
| monoterpenes | 85.70 | 0.75 | 8.09 | 35.27 |
| oxygenated monoterpenes | 13.76 | 96.75 | 88.17 | 52.76 |
| sesquiterpenes | - | - | 1.17 | 11.54 |
| oxygenated sesquiterpenes | - | 0.95 | 1.34 | 0.30 |
The remainder up to 100% were non-terpenes compounds.
Table 2.
The terpene ingredients (%) of the tested essential oils.
| IR | Area (%) ± SD of Components in Etja Essential Oils | ||||
|---|---|---|---|---|---|
| Lemon | Rosewood | Geranium | Rosemary | ||
| MONOTERPENES | |||||
| tricyclene | 919 | 0.21 ± 0.04 | - | - | 0.48 ± 0.03 |
| α-pinene | 930 | 11.06 ± 0.14 | - | 0.05 ± 0.01 | 10.33 ± 0.03 |
| camphene | 946 | 0.35 ± 0.04 | - | 0.04 ± 0.01 | 8.18 ± 0.03 |
| β-citronellene | 948 | - | 0.02 ± 0.01 | - | - |
| β-thujene | 970 | - | - | - | 3.92 ± 0.20 |
| β-pinene | 973 | 15.14 ± 0.04 | - | 0.03 ± 0.01 | 7.62 ± 0.14 |
| 2,6-dimethyl-2,6-octadiene | 980 | - | - | 0.45 ± 0.01 | - |
| 3-menthene | 981 | - | - | 0.26 ± 0.02 | - |
| β-myrcene | 984 | 4.11 ± 0.06 | - | - | 1.72 ± 0.03 |
| 2-carene | 996 | - | - | 0.05 ± 0.01 | - |
| α-phellandrene | 999 | - | - | - | 0.11 ± 0.02 |
| α-terpinene | 1016 | 0.40 ± 0.12 | - | - | 0.06 ± 0.01 |
| p-cymene | 1019 | - | - | 0.19 ± 0.01 | - |
| limonene | 1024 | 48.27 ± 0.05 | 0.73 ± 0.02 | 6.86 ± 0.05 | - |
| trans-β-ocimene | 1040 | 0.13 ± 0.03 | - | - | - |
| α-ocimene | 1050 | - | - | 0.01 ± 0.01 | - |
| β-terpinene | 1053 | - | - | 0.01 ± 0.01 | - |
| γ-terpinene | 1059 | 4.85 ± 0.06 | - | - | 2.16 ± 0.02 |
| terpinolene | 1077 | 1.19 ± 0.03 | - | - | 0.68 ± 0.02 |
| Sum | - | 85.70 | 0.75 | 8.09 | 35.27 |
| OXYGENATED MONOTERPENES | |||||
| eucalyptol | 1030 | - | 1.55 ± 0.04 | - | 15.87 ± 0.12 |
| dehydrolinalool | 1072 | - | 0.11 ± 0.01 | - | - |
| cis-linalool oxide | 1073 | - | 2.07 ± 0.03 | 0.05 ± 0.01 | - |
| rose oil | 1087 | - | - | 3.05 ± 0.04 | - |
| trans-linalool oxide | 1089 | - | 1.56 ± 0.03 | 0.07 ± 0.01 | - |
| cis rose oxide | 1096 | - | - | 0.20 ± 0.01 | - |
| linalool | 1099 | 0.29 ± 0.06 | 79.51 ± 0.32 | 11.27 ± 0.06 | 1.83 ± 0.06 |
| myrcenol | 1099 | - | 0.06 ± 0.01 | - | - |
| trans rose oxide | 1113 | - | - | 0.09 ± 0.01 | - |
| 1-terpineol | 1121 | - | - | 0.05 ± 0.01 | - |
| α-pinene oxide | 1126 | 0.17 ± 0.02 | - | - | - |
| menthone | 1133 | - | - | 0.59 ± 0.02 | - |
| camphor | 1141 | - | - | - | 16.00 ± 0.11 |
| α-phellandren-8-ol | 1144 | - | 0.01 ± 0.01 | - | - |
| isomenthone | 1144 | - | - | 0.53 ± 0.03 | - |
| isoborneol | 1145 | - | - | 0.32 ± 0.01 | - |
| isopulegol | 1149 | - | - | 0.33 ± 0.01 | - |
| verbenol | 1154 | 0.08 ± 0.01 | - | - | - |
| menthol | 1164 | 0.12 ± 0.01 | - | - | - |
| borneol | 1168 | - | - | 0.49 ± 0.01 | 7.95 ± 0.10 |
| lavandulol | 1171 | - | 1.72 ± 0.01 | - | - |
| terpinen-4-ol | 1174 | - | - | - | 1.43 ± 0.03 |
| γ-terpineol | 1185 | - | - | 0.32 ± 0.03 | - |
| α-terpineol | 1197 | - | 8.27 ± 0.01 | 1.53 ± 0.03 | 5.62 ± 0.12 |
| fenchol | 1199 | - | - | - | 0.08 ± 0.01 |
| α-fenchyl acetate | 1202 | - | - | - | 0.50 ± 0.05 |
| linalyl formate | 1205 | - | - | 2.81 ± 0.09 | - |
| β-citronellol | 1208 | - | - | 30.99 ± 0.20 | - |
| α-citronellol | 1209 | 0.20 ± 0.02 | - | 4.73 ± 0.23 | - |
| nerol | 1232 | - | 1.64 ± 0.02 | - | - |
| geraniol | 1233 | - | - | 17.20 ± 0.10 | - |
| α-citral | 1247 | 7.14 ± 0.07 | 0.08 ± 0.01 | 0.32 ± 0.03 | - |
| 2,6-dimethyl-1,7-octadiene-3,6-diol | 1268 | - | 0.07 ± 0.01 | - | - |
| borneol acetate | 1270 | - | - | - | 3.47 ± 0.03 |
| β-citral | 1278 | 4.30 ± 0.14 | 0.05 ± 0.01 | 0.25 ± 0.11 | - |
| geranyl formate | 1294 | 0.91 ± 0.01 | - | 0.62 ± 0.03 | - |
| citronellol acetate | 1338 | - | - | 0.35 ± 0.01 | - |
| p-mentha-1-en-3,8-diol | 1351 | - | 0.03 ± 0.01 | - | - |
| neryl acetate | 1364 | 0.49 ± 0.06 | - | 0.51 ± 0.01 | - |
| geranyl acetate | 1383 | - | - | 8.56 ± 0.22 | - |
| cis-geranylacetone | 1418 | - | - | 0.17 ± 0.01 | - |
| carvone hydrate | 1427 | - | 0.02 ± 0.01 | - | - |
| citronellyl propionate | 1431 | - | - | 0.33 ± 0.02 | - |
| geranyl propionate | 1451 | - | - | 0.10 ± 0.01 | - |
| geranyl isobutyrate | 1494 | - | - | 0.70 ± 0.01 | - |
| geranyl butyrate | 1542 | - | - | 1.22 ± 0.03 | - |
| citronellyl tiglate | 1646 | - | - | 0.30 ± 0.03 | - |
| geranyl tiglate | 1657 | - | - | 0.12 ± 0.01 | - |
| sum | - | 13.76 | 96.75 | 88.17 | 52.76 |
| SESQUITERPENES | |||||
| α-cubebene | 1354 | - | - | - | 0.16 ± 0.01 |
| α-longipinene | 1359 | - | - | - | 0.07 ± 0.01 |
| α-copaene | 1379 | - | - | 0.03 ± 0.01 | 0.57 ± 0.02 |
| β-cubebene | 1384 | - | - | - | 0.20 ± 0.05 |
| β-bourbonene | 1388 | - | - | 0.10 ± 0.02 | - |
| y-langene | 1388 | - | - | - | 0.15 ± 0.02 |
| γ-maaliene | 1398 | - | - | 0.03 ± 0.01 | - |
| aristolene | 1402 | - | - | 0.38 ± 0.02 | - |
| longifolene | 1421 | - | - | 0.05 ± 0.00 | 0.57 ± 0.01 |
| caryophyllene | 1423 | - | - | 0.11 ± 0.01 | 7.58 ± 0.03 |
| alloaromadendrene | 1426 | - | - | 0.05 ± 0.01 | - |
| calarene | 1436 | - | - | 0.16 ± 0.01 | - |
| aromadendrene | 1443 | - | - | - | 0.08 ± 0.01 |
| humulene | 1452 | - | - | - | 1.36 ± 0.08 |
| ledene | 1470 | - | - | 0.22 ± 0.01 | - |
| γ-muurolene | 1479 | - | - | - | 0.11 ± 0.01 |
| isocaryophyllene | 1495 | - | - | - | 0.04 ± 0.01 |
| γ-cadinene | 1503 | - | - | - | 0.29 ± 0.03 |
| δ-selinene | 1510 | - | - | 0.04 ± 0.01 | - |
| δ-cadinene | 1525 | - | - | - | 0.37 ± 0.02 |
| Sum | - | - | - | 1.17 | 11.54 |
| OXYGENATED SESQUITERPENES | |||||
| elemol | 1535 | - | - | 0.03 ± 0.01 | - |
| nerolidol | 1540 | - | 0.11 ± 0.01 | - | - |
| cis-nerolidol | 1545 | - | 0.16 ± 0.02 | - | - |
| trans-nerolidol | 1551 | - | 0.68 ± 0.02 | - | - |
| guaiol | 1584 | - | - | 0.47 ± 0.02 | - |
| caryophyllene oxide | 1588 | - | - | - | 0.30 ± 0.02 |
| γ-eudesmol | 1620 | - | - | 0.04 ± 0.01 | - |
| bulnesol | 1650 | - | - | 0.54 ± 0.03 | - |
| methyl abietate | 2175 | - | - | 0.26 ± 0.03 | - |
| Sum | - | 0.95 | 1.34 | 0.30 | |
IR, Kovats retention index; SD, standard deviation. The remainder up to 100% were non-terpenes compounds.
The geranium and rosemary essential oils contained 56 and 33 terpenes and terpenoides, respectively. In contrast, the lemon and rosewood oils contained only 20 terpene compounds. On the basis of the GCMS analysis, it was found that β-citronellol, geraniol and linalool were present in geranium essential oils in the largest amounts, respectively 31%, 17.2% and 11.3%. The following compounds were the main components of the rosemary essential oil: α-phellandren-8-ol (16%), eucalyptol (15.9%) and α-pinene (10.3%). In the lemon essential oil, the main compounds were limonene (48,3%), β-pinene (15.1%) and α-pinene (11.1%). Linalool (79.5%) and α-terpineol (8,3%) were the main components of the rosewood essential oil (Table 2).
All tested oils, when compared to a relative control at concentrations of 0.125% to 2.0%, decreased the mycelial growth of F. graminearum. The growth index of F. graminearum ZALF 24 and F. graminearum ZALF 339 was differentiated and it depended on the type and concentration of the oil used (Table 3 and Table 4).
Table 3.
The index of linear growth (T) of Fusarium graminearum ZALF 24.
| Oil Concentration (%) | Etja Essential Oils | ||||
|---|---|---|---|---|---|
| Control | Lemon | Rosewood | Geranium | Rosemary | |
| 0.125 | 85.00 a | 69.00 b | 0.00 f | 0.00 f | 36.67 c |
| 0.25 | 85.00 a | 15.00 de | 0.00 f | 0.00 f | 20.67 d |
| 0.5 | 85.00 a | 0.00 f | 0.00 f | 0.00 f | 7.33 ef |
| 1.0 | 85.00 a | 0.00 f | 0.00 f | 0.00 f | 0.00 f |
| 2.0 | 85.00 a | 0.00 f | 0.00 f | 0.00 f | 0.00 f |
a–f—values denoted with the same letters do not differ statistically (p < 0.05).
Table 4.
The index of linear growth (T) of Fusarium graminearum ZALF 339.
| Oil Concentration (%) | Etja Essential Oils | ||||
|---|---|---|---|---|---|
| Control | Lemon | Rosewood | Geranium | Rosemary | |
| 0.125 | 85.00 a | 59.67 b | 0.00 f | 0.00 f | 54.00 bc |
| 0.25 | 85.00 a | 54.33 b | 0.00 f | 0.00 f | 19.00 de |
| 0.5 | 85.00 a | 35.67 cd | 0.00 f | 0.00 f | 4.17 ef |
| 1.0 | 85.00 a | 0.00 f | 0.00 f | 0.00 f | 0.00 f |
| 2.0 | 85.00 a | 0.00 f | 0.00 f | 0.00 f | 0.00 f |
a–f—values denoted with the same letters do not differ statistically (p < 0.05).
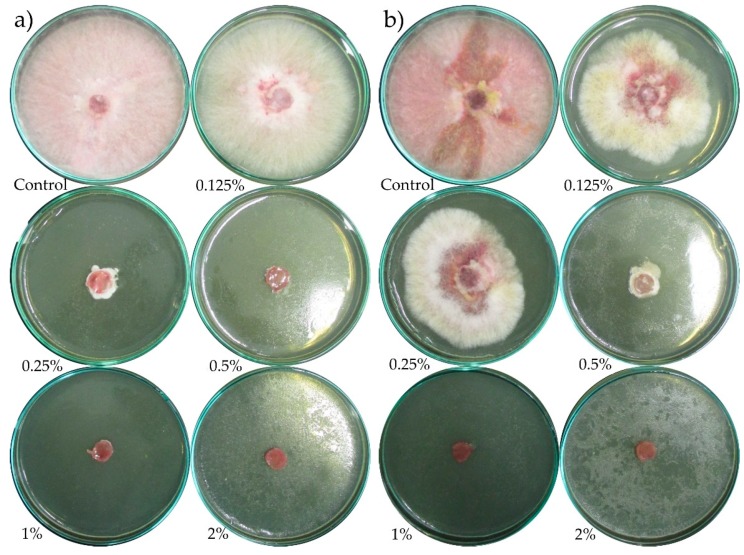
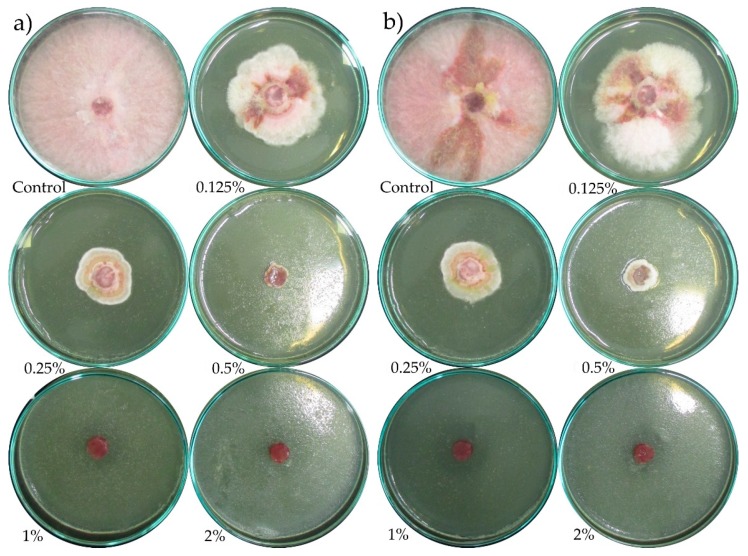
Complete inhibition of mycelium growth, regardless of the concentration used, was caused by the geranium and rosewood oils, the active substances of which were oxygenated monoterpenes: citronellol and geraniol in the geranium oil and linalool in the rosewood oil (Table 2 and Table 3, Figure A5a and Figure A6a).
A similar relationship for the geranium and rosewood oils was observed for the strain of F. graminearum ZALF 339 (Table 4, Figure A5b and Figure A6b).
After 11 days of incubation, the geranium oil completely inhibited the growth of the tested strains at all examined concentrations; the same relationship can be observed for the essential oil of rosewood.
The rosemary and lemon oils, despite the differences in the chemical composition, worked similarly on the tested Fusarium isolates. The total inhibition of linear mycelium growth was observed in both cases only after applying oils at 1 and 2% concentrations, for example (Table 3 and Table 4, Figure A7 and Figure A8).
In contrast, the index of linear growth of the test strains in the presence of lemon oil varied and depended on the concentration used. The oil at a concentration of 0.125% showed little effect on the inhibition of linear growth of the tested fungi. The increase of lemon oil concentration, depending on the strains, inhibited the growth of these fungi in different ways. The development of the mycelium F. graminearum ZALF 24 was completely inhibited by the oil at concentrations of 0.5% to 2.0% (Table 3) and F. graminearum ZALF 339 at a concentration of 1.0% to 2.0% (Table 4).
Fungistatic activity also depended on the oil concentration and strain sensitivity. The highest fungicidal activity with respect to both tested strains of F. graminearum, regardless of the concentration used, was shown by the geranium and rosewood oil. The total inhibition of the growth of tested fungi was observed in the entire concentration of both of these essential oils.
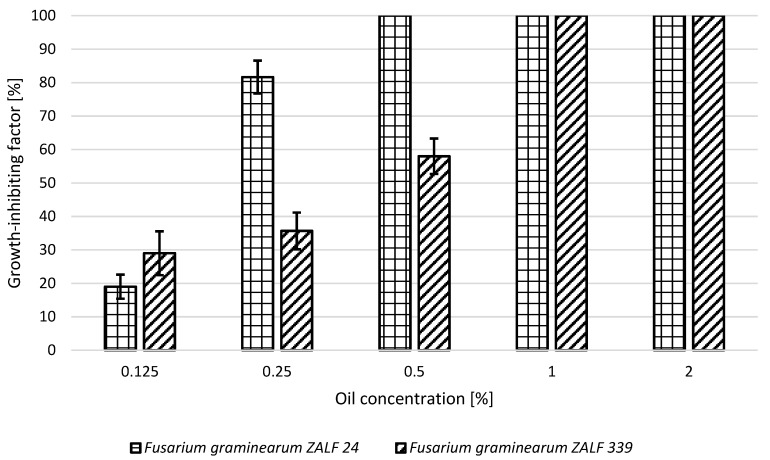
In contrast, the highest fungicidal activity of lemon oil was observed in F. graminearum ZALF 24 at a concentration of 0.5% to 2.0%, and in F. graminearum ZALF 339 at a concentration of 1.0% to 2.0%. At lower concentrations, the fungistatic activity of lemon oil ranged from 19% to 82% for F. graminearum ZALF 24 and from 29 to 58% for F. graminearum ZALF 339 (Figure 1).
Figure 1.
Growth-inhibiting factor of Fusarium graminearum ZALF 24 and Fusarium graminearum ZALF 339 strains in the presence of lemon oil.
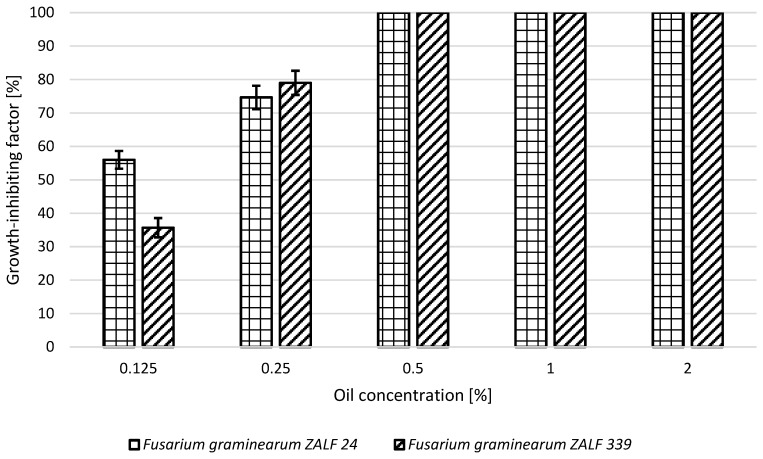
At lower concentrations, the fungistatic activity of the rosemary oil ranged from 56% to 75% for F. graminearum ZALF 24 and from 36% to 78% for F. graminearum ZALF 339. It showed the highest fungistatic activity at concentrations from 0.5% to 2.0% (Figure 2).
Figure 2.
Growth-inhibiting factor of Fusarium graminearum ZALF 24 and Fusarium graminearum ZALF 339 strains in the presence of rosemary oil.
3. Discussion
Phytopathogenic species of Fusarium graminearum are found in nature in the heterothallic and homothallic forms. Distinctive heterothallic strains create a fluffy and airy mycelium with colouring from white to yellow or light brown on Potato Dextrose Agar (PDA) medium and occur mostly in Australia and California. In contrast, homothallic strains, which are located in Europe, including Poland, and the eastern part of the United States, create a sparser airy mycelium of pink carmine colour with a shade of yellow [12]. The sensitivity of fungi strains of the Fusarium genus to essential oils may already vary within the species and may depend on both the strain and the chemical composition, as well as on the concentration of essential oils. The qualitative and quantitative composition of the active substance contained in the raw material decides the biological activity and efficacy of essential oils [13,14,15,16], but even a small percentage of another compound in the oil may affect the fungicidal activity [17]. Differentiation of the various components contained in the essential oils has an impact on their properties and bioactivity [18].
F. graminearum sensitivity to essential oils is the subject of many studies [19,20,21,22,23].
In our studies, we found that all tested essential oils had fungicidal activity, including the total inhibition of mycelial growth of F. graminearum ZALF 24 and F. graminearum ZALF 339, regardless of the concentration of the used geranium and rosewood oil. A high fungistatic activity of these oils was also exhibited by other researchers [24,25,26]. These oils worked similarly despite clear differences in their chemical composition. The active substances of geranium oil are citronellol and geraniol, while linalool is active in rosewood oil. For this reason, they can be the basis for constructing biofungicides.
Other tested oils showed the desired effect at higher concentrations. The highest biocidal activity of the rosemary oil on the studied strains (F. graminearum ZALF 24 and F. graminearum ZALF 339) was observed for a concentration higher than 0.5%. The fungicidal properties of rosemary oil with respect to Fusarium are confirmed by the studies of Dimitra et al. [18] and Surviliené et al. [10]. Dimitra et al. [18] suggested that borneol, not eucalyptol (the major component of this oil), is likely to be responsible for its fungistatic activity. However, Ćosić et al. [27] have shown that rosemary oil, as well as cinnamon, sage, pine, bitter orange, anise, cumin and lavender oils, did not inhibit the growth of an F. graminearum strain. Only a strong biocidal effect in relation to this fungus was observed in thyme oil, and a weak one in peppermint oil. It is likely that the obtained results depended on the methods used, the concentrations of essential oils and the form of the occurring strains (heterothallic and homothallic).
The studies also demonstrated the efficacy of lemon oil at concentrations of 0.5–2.0% in reducing the development of F. graminearum strains. However, this was not confirmed in the research of Gömöri et al. [28], although Viuda-Martos et al. [17] showed that this oil at the concentration of 0.94% completely inhibited the growth of Aspergillus niger, Aspergillus flavus, Penicillium verrucosum and Penicillium chrysogenum fungi. Antifungal properties of citrus oils are attributed to the presence of such components as D-limonene and linalool; however, even a small percentage of another compound in the oil may affect its fungicidal activity [29]. The main ingredient of the tested lemon oil was D-limonene; however, smaller quantities of citral, α-terpineol, α-pinene, β-pinene, citronellal, linalyl and geranyl acetate, p-cymene, γ-pinene, β-myrcene, coumarins and bioflavonoids occurring in this oil could have had an effect on the biocidal properties. A proper selection of essential oils exhibiting fungicidal activity even at low concentrations can be used in biological plant protection against phytopathogens of the Fusarium genus, and seem to be a good alternative to chemicals [4,5,6,7].
4. Materials and Methods
4.1. Materials
The research material was strains of Fusarium graminearum isolated on PDA medium from infected cereals. The strains were identified on the basis of morphological characteristics [12,30,31]. Commercial essential oils (produced by ETJA, Elbląg, Poland), such as geranium (Pelargonium graveolens), rosewood (Aniba Rosaeodora), rosemary (Rosmarinus officinalis) and lemon (Citrus limonum), which are widely available in the course of trade, were tested.
4.2. The Gas Chromatography Mass Spectrometry Analysis
The Hewlett Packard HP 6890 series GC system chromatograph (Hewlett Packard, WALDBRONN, Germany) was used for the study, which was coupled with the Hewlett Packard 5973 mass selective detector (Hewlett Packard, Waldbronn, Germany). The chromatograph was equipped with the non-polar, high-temperature ZB-5HT capillary column; length, 30 m; inner diameter, 0.32 mm; film thickness, 0.25 μm (Phenomenex Inc., Torrance, CA, USA). The on-column injector was used and 1 μm of a sample was introduced. The initial temperatures, both of the injector and the oven, were 60 °C, and the temperature was increased by 10 °C per minute up to 280 °C; the auxiliary temperature was 300 °C. Helium was used as the carrier gas and its flow was 2 mL/min. The components were identified by comparison of their mass spectra with the spectrometer database of the NIST 11 Library (National Institute of Standards and Technology, Gaithersburg, MD, USA) and by comparison of their retention index calculated against n-alkanes (C9–C20). Each chromatographic analysis was repeated three times. The average value of the relative composition of the essential oil percentage was calculated from the peak areas.
4.3. Biological
Tested oils were inserted into a PDA medium (Potato Dextrose Agar produced by BioMaxima S.A., Lublin, Poland) enriched with 0.01% Tween 80 (produced by BTL, Warsaw, Poland) in the following concentrations: 0.125; 0.25; 0.5; 1.0; 2.0%. The control was carried out through the growth of tested isolates in modified PDA medium (without oils).
The biotic activity of oils in reducing the linear growth of the fungus F. graminearum ZALF 24 and F. graminearum ZALF 339 was assessed by using the method of poisoned substrates [32]: cultures were grown in PDA medium for 14 days at 25 °C, and inoculum—the spore suspension of F. graminearum ZALF 24 and F. graminearum ZALF 339 in a 0.01% sterile solution of Tween 80 (produced by BTL, Warsaw, Poland)—was obtained from an 11-day-old culture. The haemocytometer Thoma was used to obtain a spore suspension of 1·106 CFU·cm3. Petri dishes (9 cm diameter) containing 20 cm3 PDA medium were used to inoculate this spore suspension and stored at 25 °C for 11 days. Inoculum rings with a diameter of 10 mm overgrown by mycelium were obtained. The absolute control was the culture of the fungus on modified PDA medium without oils.
On the basis of measurements of the fungal colony, the linear growth of mycelium index (T) and the fungistatic activity of essential oils were calculated.
The growth rates index of Fusarium strains was calculated using the following formula (1) [33]:
| (1) |
where:
T – index of linear growth
A – average measurement value of diameter colonies (mm)
D – duration of the experiment
b1….bx – increase in colonies diameter (mm)
d1..dx – number of days since last measurement
The fungistatic activity of the tested oils was assessed based on the percentage of the growth inhibition of fungus colonies and calculated using Abbott’s formula (2):
| (2) |
where:
I – fungus linear growth inhibition index (%)
C – fungus colony diameter in the control combination (mm)
M – fungus colony diameter on a control plate with a given oil in the combination containing a tested substance concentration in the medium [mm]
All analyses were performed with three repetitions. A two-way analysis of variance (ANOVA) was used to examine the effect of essential oils. A post-hoc test by Tukey’s HSD was used to determine significant differences at a level of P < 0.05. All statistical analyses were done using R.
5. Conclusions
The growth index of F. graminearum ZALF 24 and F. graminearum ZALF 339 varied and depended on the used oil and its concentration. The highest fungicidal activity with respect to both F. graminearum strains, regardless of the concentration, was shown by the geranium oil and the rosewood oil, which contain about 88–97% monoterpenoids. The development of the F. graminearum ZALF 24 strain was inhibited by the rosemary and lemon oils at concentrations from 0.5% to 2.0%, and F. graminearum ZALF 339 by a 1.0% concentration of lemon oil. All tested oils, compared to the control, reduced the rate of mycelial growth of Fusarium graminearum and showed fungistatic activity.
Appendix A
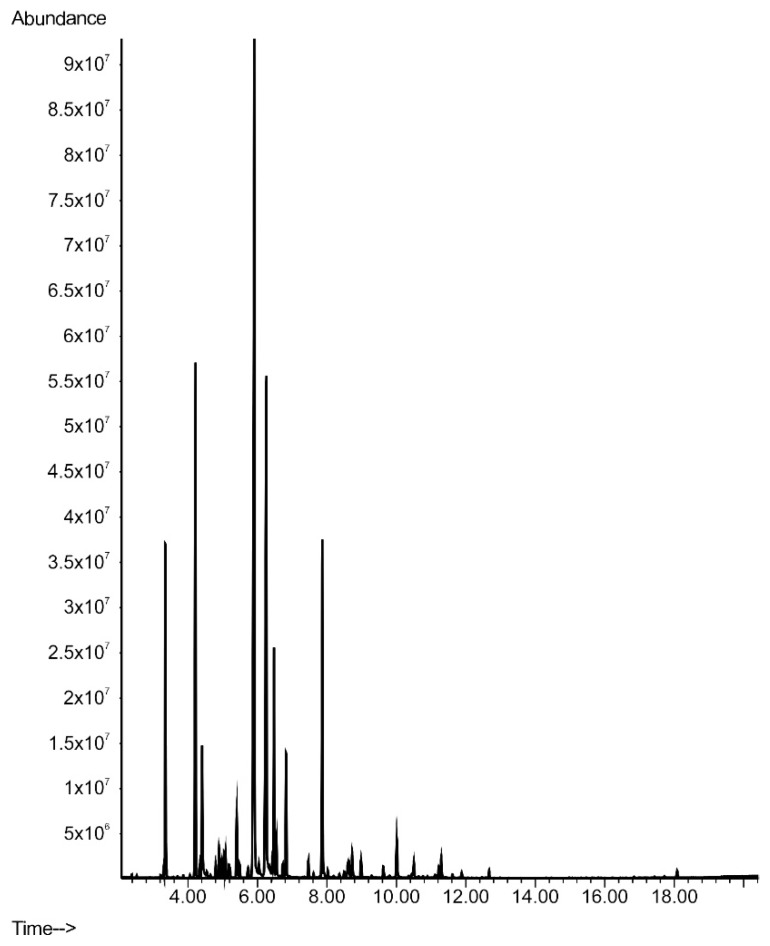
Figure A1.
The gas chromatogram of the lemon commercial oil. Dilution 1:40 (v/v) in dichloromethane.
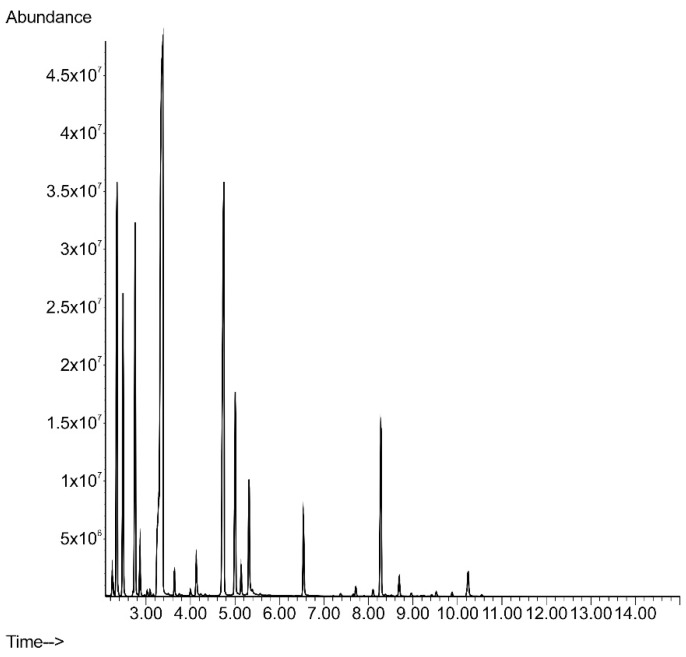
Figure A2.
The gas chromatogram of the rosewood commercial oil. Dilution 1:40 (v/v) in dichloromethane.
Figure A3.
The gas chromatogram of the geranium commercial oil. Dilution 1:40 (v/v) in dichloromethane.
Figure A4.
The gas chromatogram of the rosemary commercial oil. Dilution 1:40 (v/v) in dichloromethane.
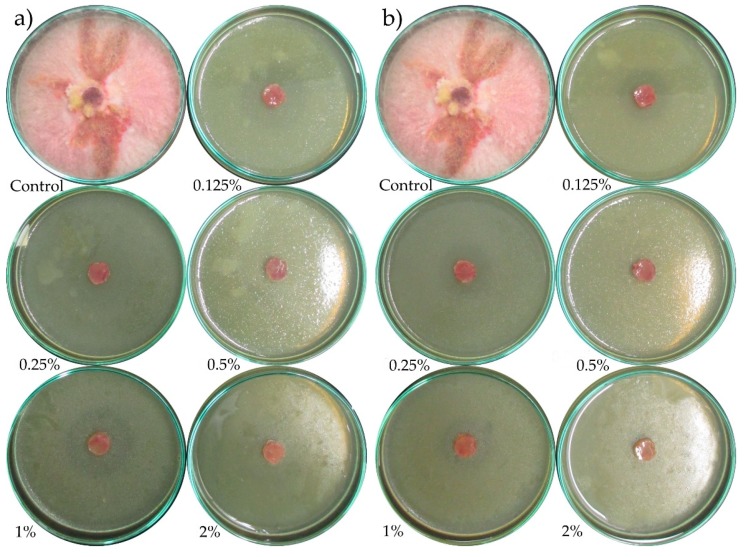
Figure A5.
The effect of geranium oil concentrations on the growth of Fusarium graminearum ZALF 24 (a) and Fusarium graminearum ZALF 339 (b).
Figure A6.
The effect of rosewood oil concentrations on the growth of Fusarium graminearum ZALF 24 (a) and Fusarium graminearum ZALF 339 (b).
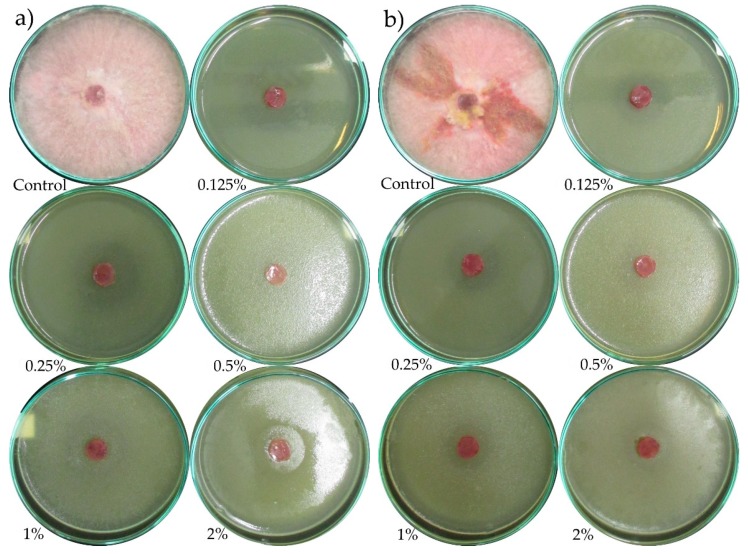
Figure A7.
The effect of lemon oil concentrations on the growth of Fusarium graminearum ZALF 24 (a) and Fusarium graminearum ZALF 339 (b).
Figure A8.
The effect of rosemary oil concentrations on the growth of Fusarium graminearum ZALF 24 (a) and Fusarium graminearum ZALF 339 (b).
Author Contributions
Conceptualization, T.K.Ł.; Methodology, T.K.Ł. and M.B.; Formal Analysis, M.B. and W.W.; Investigation, M.B. and W.W.; Data Curation, M.B.; Writing (Original Draft Preparation), T.K.Ł.; Writing (Review & Editing), M.B.; Visualization, M.B.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Walkowiak W., Krzyśko-Łupicka T. New solution in grain protection against fusariosis. Prog. Plant Prot. 2014;54:127–134. [Google Scholar]
- 2.Kordowska-Wiater M. Yeasts as biological control agents for plants. Post. Mikrobiol. 2011;50:107–119. [Google Scholar]
- 3.Nowak W., Sowiński J., Pietr S.J., Kita W. Influence of methods of winter wheat protection on the quality of consumer grain. Pam. Puł. 2005;139:117–127. [Google Scholar]
- 4.Mares D., Tosi B., Poli F., Andreotti E., Romagnoli C. Antifungal activity of Tagetes patula extracts on some phytopathogenic fungi: Ultrastructural evidence on Pythium ultimum. Microbiol. Res. 2004;159:295–304. doi: 10.1016/j.micres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Thomas M.B., Willis A.I. Bicontrol-risky but necessary. Tree. 1998;13:325–329. [Google Scholar]
- 6.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Hashem M., Moharam A.M., Zaied A.A., Saleh F.E.M. Efficacy of essential oils in the control of cumin root rot disease caused by Fusarium spp. Crop Prot. 2010;29:1111–1117. doi: 10.1016/j.cropro.2010.04.020. [DOI] [Google Scholar]
- 8.Orlikowski L.B. Development and spread of Phytophthora ramorum in the presence of grapefruit extract. J. Plant Prot. Res. 2003;43:213–218. [Google Scholar]
- 9.Burgieł Z.J., Smagłowski M. Fungistic properties of tea tree oil. Probl. J. Agric. Sci. 2008;529:13–18. [Google Scholar]
- 10.Survilienė E., Valiuškaitė A., Snieškienė V., Stankevičienė A. Effect of essential oils on fungi isolated from apples and vegetables. Lithuanianinstitute Horticult. Lithuannian Univ. Agric. 2009;28:227–234. [Google Scholar]
- 11.Białoń M., Krzyśko-Łupicka T., Koszałkowska M., Wieczorek P.P. Chemical composition of lemon essential oils and their fungicidal activity against Candida yeasts. Mycopathologia. 2014;177:29–39. doi: 10.1007/s11046-013-9723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwaśna H., Chełkowski J., Zajkowski P. Poland Flora, Fungi (Mycota), Volume XXII, Sierpik (Fusarium) PAN Publisher; Warsaw-Krakow, Poland: 1991. [Google Scholar]
- 13.Kędzia A. Antifungal activity of geranium oil (Oleum geranii) Adv. Phytoth. 2009;4:217–222. [Google Scholar]
- 14.Cavanagh H.M.A., Wilkinson J.M. Biological Activities of Lavender Essential Oil. Phytother. Res. 2002;16:301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- 15.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 16.Sienkiewicz M., Kalemba D., Wasiela M. The evaluation of the sensitivity of clinical strains of Escherichia coli to the action of thyme and lavender oil in relation to their drug resistance. Med. Exp. Microbiol. 2011;63:273–281. [PubMed] [Google Scholar]
- 17.Viuda-Martos M., Ruiz-Navajas Y., Fernaández-López J., Pêrez-Ălvarez J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control. 2007;19:1130–1138. doi: 10.1016/j.foodcont.2007.12.003. [DOI] [Google Scholar]
- 18.Dimitra J.D., Basil N.Z., Moschos G.P. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003;22:39–44. doi: 10.1016/S0261-2194(02)00095-9. [DOI] [Google Scholar]
- 19.Te Z., Fei G., Lin Z., Tian-you S. Essential Oil from Inula britannica extraction with SF-CO2 and its antifungal activity. J. Integr. Agric. 2013;12:1791–1798. doi: 10.1016/S2095-3119(13)60382-2. [DOI] [Google Scholar]
- 20.Riccioni L., Orzali L. Activity of Tea Tree (Melaleuca alternifolia, Cheel) and thyme (Thymus vulgaris, Linnaeus.) Essential Oils against Some Pathogenic Seed Borne Fungi. J. Essent. Oil Res. 2011;23:43–47. doi: 10.1080/10412905.2011.9712280. [DOI] [Google Scholar]
- 21.Amini M., Safaie N., Salmani M.J., Shams-Baksh M. Antifungal activity of three medicinal plant essential oils against some phytopathogenic fungi. Trakia J. Sci. 2012;10:1–8. [Google Scholar]
- 22.Samie A., Nefefe T. Antifungal activities of essential oils from Southern African medicinal plants against five Fusarium species. J. Med. Plant. Res. 2012;6:465–478. [Google Scholar]
- 23.Krifa M., Gharad T., Haouala R. Biological activities of essential oil, aqueous and organic extracts of Pituranthos tortuosus (Coss.) Maire. Sci. Hort. 2010;128:61–67. doi: 10.1016/j.scienta.2010.12.016. [DOI] [Google Scholar]
- 24.Simić A., Soković M.D., Ristić M., Grujić-Jovanović S., Vukojević J., Marin P.D. The Chemical Composition of some Lauraceae Essential Oils and Their Antifungal Activities. Phytother. Res. 2004;18:713–717. doi: 10.1002/ptr.1516. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Kader M., El-Mougy S., Lashin S. Essentials oils and Trichoderma harzianum as an integrated control measure against faba bean root rot pathogens. J. Plant Prot. Res. 2011;51:306–313. doi: 10.2478/v10045-011-0050-8. [DOI] [Google Scholar]
- 26.Khirsagar R.P., Kothamasu M.V., Patil M.A., Reddy B.G., Kumar B.D., Diwan P.V. Geranium oil ameliorates endothelial dysfunction in high fat high sucrose diet induced metabolic complications in rats. J. Funct. Foods. 2015;15:284–293. doi: 10.1016/j.jff.2015.03.029. [DOI] [Google Scholar]
- 27.Ćosić J., Vrandečić K., Postić J., Jurković D., Ravlić M. In vitro antifungal activity of essential oils on growth of phytopathogenic fungi. Poljoprivreda. 2010;16:25–28. [Google Scholar]
- 28.Gömöri C., Nacsa-Farkas E., Kerekes E.B., Kocsubé S., Vágvölgyi C., Krisch J. Evaluation of five essential oils for the control of foodspoilage and mycotoxin producing fungi. Acta Biol. Szeged. 2013;57:113–116. [Google Scholar]
- 29.Koshima C.C., Capellini M.C., Geramias I.M., Aracava K.K., Gonçalves C.B., Rodriques E.C. Fractionation of lemon essential oil by solvent extraction: Phase equilibrium for model systems at T = 298.2 K. J. Chem. Thermodyn. 2012;54:316–332. doi: 10.1016/j.jct.2012.05.011. [DOI] [Google Scholar]
- 30.Watanabe T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species. 3rd ed. CRC Press Taylor & Francis Group; Boca Raton, FL, USA: 2010. [Google Scholar]
- 31.Pląskowska E. Characteristics and taxonomy of fungi of the genus Fusarium. Med. Mycol. 2010;17:172–176. [Google Scholar]
- 32.Barbiel M. Sclerotium rolfsii and Thielaviopsis basicola to the Botanical Fungicide Timorex Gold (Melaleuca alternifolia) under the Paper Disk Diffusion and the Poisoning Agar Testing Methods. University of Florida-IFAS, Plant Medicine Program, Gainesville, Marcel Barbier; Gainesville, FL, USA: 2012. In vitro of Didymella bryoniae, Fusarium oxysporum, Trichoderma spp. [Google Scholar]
- 33.Gleń K., Boligłowa E. The effect of extracts from herbal plants on dominating species of fungi colonizing broad bean seeds. J. Res. Appl. Agric. Eng. 2012;57:98–103. [Google Scholar]