Abstract
Maternal obesity during pregnancy increases risk for neurodevelopmental disorders in offspring, although the underlying mechanisms remain unclear. Epigenetic deregulation associates with many neurodevelopmental disorders, and recent evidence indicates that maternal nutritional status can alter chromatin marks in the offspring brain. Thus, maternal obesity may disrupt epigenetic regulation of gene expression during offspring neurodevelopment. Using a C57BL/6 mouse model, we investigated whether maternal high fat diet (mHFD)-induced obesity alters the expression of genes previously implicated in the etiology of neurodevelopmental disorders within the Gestational Day 17.5 (GD 17.5) offspring hippocampus. We found significant two-fold upregulation of oxytocin receptor (Oxtr) mRNA in the hippocampus of male, but not female, GD 17.5 offspring from mHFD-induced obese dams (p < 0.05). To determine whether altered histone binding at the Oxtr gene promoter may underpin these transcriptional changes, we then performed chromatin immunoprecipitation (ChIP). Consistent with the Oxtr transcriptional changes, we observed increased binding of active histone mark H3K9Ac at the Oxtr transcriptional start site (TSS) in the hippocampus of mHFD male (p < 0.05), but not female, offspring. Together, these data indicate an increased vulnerability of male offspring to maternal obesity-induced changes in chromatin remodeling processes that regulate gene expression in the developing hippocampus, and contributes to our understanding of how early life nutrition affects the offspring brain epigenome.
Keywords: developmental programming, maternal nutrition, neurodevelopment, epigenetic modification, H3K9Ac, H3K9me3, oxytocin receptor, obesity
1. Introduction
The correct establishment of the epigenome is critical for driving the complex gene expression patterns that underpin normal brain development. Epigenetic regulation via histone modifications, DNA methylation, or non-coding RNA can remodel chromatin structure by changing interactions between DNA and histone proteins, or histone–histone dynamics, to enhance or reduce the accessibility of DNA for the transcriptional machinery. Increasing evidence suggests that these epigenetic mechanisms have a high degree of plasticity during development, and can be modulated by external factors in the in utero environment, including maternal nutrition [1,2]. In this way, the maternal environment may contribute to the programming of offspring disease risk. For example, pregnancies complicated by maternal obesity are associated with a range of poor health outcomes for offspring, including an increased risk of obesity and related metabolic disorders [3]. Animal models of maternal obesity can reliably reproduce the offspring phenotype of elevated obesity risk [4], and recent work from both animal models and human studies indicate that maternal obesity can indeed alter epigenetic regulation of genes related to metabolism and food-seeking behaviors in the brain of offspring [5,6].
Recently, studies have identified obesity during pregnancy also incurs a greater risk for neurodevelopmental disorders in offspring [7,8]. In humans, maternal obesity has been linked to increased incidence of anxiety, depression, learning disabilities, poor psychosocial development, attention deficit hyperactivity disorder (ADHD), and autism spectrum disorders (ASD) [7,8,9,10,11]. Rodent models of maternal obesity during pregnancy have largely reproduced these findings, including elevated risk for impaired social and cognitive behavior in the offspring of obese dams [12,13]. As epigenetic regulation underpins normal developmental gene expression in the brain, and epigenetic abnormalities are linked to the etiology of several neurodevelopmental disorders [14,15,16], the epigenome has surfaced as a potential molecular mediator of the association between maternal obesity and adverse neurodevelopmental programming of offspring.
When considering how maternal obesity might increase offspring risk for social and cognitive behavioral impairments, changes in the epigenetic regulation of arginine vasopressin (Avp) and oxytocin (Oxt) signaling pathways may be particularly important. These neuropeptides signal via their cognate G protein-coupled receptors, arginine vasopressin 1A (Avpr1a) and oxytocin receptor (Oxtr), to modulate diverse aspects of human social behavior, and dysfunction of these systems are well established in neurodevelopmental disorders [17,18,19]. In addition, there is evidence that epigenetic regulation of Avpr1a and Oxtr expression may be particularly sensitive to environmental disruption during critical periods of development [20,21], and post translational modifications to histone tails is one of the major epigenetic processes regulating the transcription of G-protein coupled receptors in the brain [22]. Late prenatal life (e.g., GD 17.5 in the mouse) is one such critical period where we and others have already observed several changes both to fetal physiology [23] and developmental changes to multiple cell types in multiple brain regions [12,24,25,26,27].
Here, we used a mouse model to test the hypothesis that maternal high fat diet (mHFD)-induced obesity programs the risk for neurodevelopmental disorders by altering epigenetic regulation and transcription of Oxtr and Avpr1a in the offspring brain during late prenatal development. We found that mHFD-induced obesity led to sex-specific programming effects on epigenetic control of gene expression in the offspring brain, with upregulation of Oxtr mRNA in the hippocampus of male mHFD offspring associated with increased binding of an active histone mark H3K9Ac at the Oxtr promoter. In the hippocampus of female mHFD offspring, Oxtr transcription was not altered, but H3K9me3 binding to the Oxtr promoter was decreased. Together, these data reveal sex differences in the vulnerability of offspring to maternal obesity-induced epigenetic programming, resulting in unbalanced expression of a key neuromodulatory signaling receptor in the developing hippocampus.
2. Results
2.1. Maternal High Fat Diet-Induced Obesity Modulates Expression of Oxtr mRNA in Hippocampus of Male Offspring
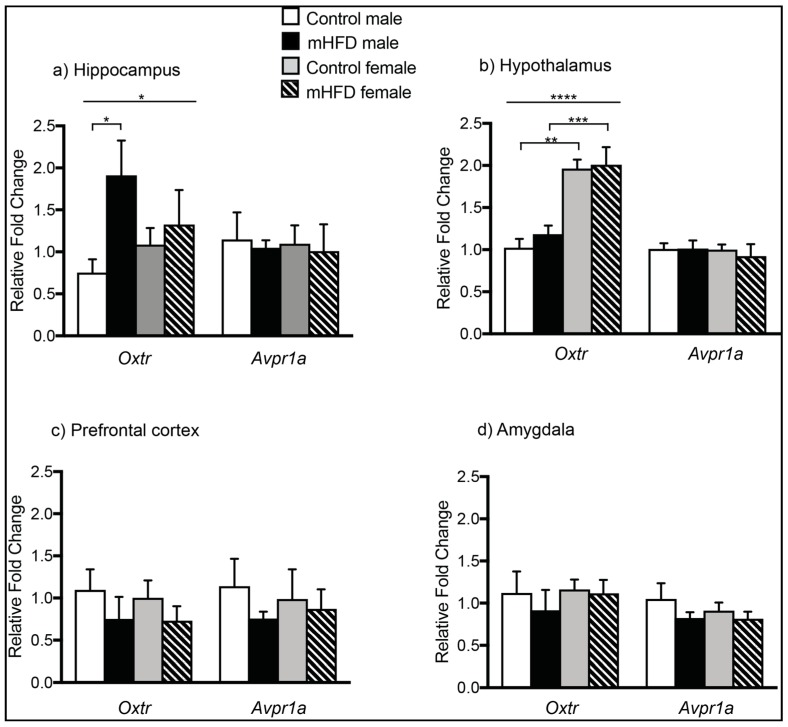
Quantitative PCR analysis indicated that mHFD-induced obesity resulted in male offspring-specific changes in Oxtr, but not Avpr1a expression, at GD 17.5 (Figure 1a–d). In offspring hippocampus, there was a significant main effect of maternal diet on Oxtr mRNA expression (main effect of diet, F(1, 16) = 4.85, p = 0.0427), with post-hoc comparisons revealing approximately two-fold upregulation of Oxtr in male mHFD offspring hippocampus compared to controls (Figure 1a) (Oxtr: mHFD males 1.912 ± 0.414, control males 0.753 ± 0.158, p = 0.0403). The maternal diet-induced change was specific to mHFD males only, with no differences in hippocampal Oxtr expression of mHFD female offspring compared to control females (Oxtr: mHFD females 1.087 ± 0.198, control females 1.327 ± 0.411, p > 0.05). By contrast, mHFD-induced obesity did not alter hippocampal expression of Avpr1a in male or female offspring (Avpr1a: mHFD males 1.05 ± 0.08, control males 1.47 ± 0.32; mHFD females 1.01 ± 0.32, control females 1.097 ± 0.22).
Figure 1.
Maternal high fat diet-induced obesity modulates expression of Oxtr mRNA in male offspring hippocampus at GD 17.5. Bar graphs depicting mRNA expression of Avpr1a and Oxtr in maternal high fat diet (mHFD) offspring relative to controls in: (a) hippocampus; (b) hypothalamus; (c) prefrontal cortex; and (d) amygdala. Data normalized to reference genes are expressed relative to control males as mean FC ± SEM, with p ≤ 0.05 *, p ≤ 0.01 **, p ≤ 0.001 ***, p ≤ 0.0001 ****.
Within offspring hypothalamus (Figure 1b), there was a statistically significant main effect of sex on offspring Oxtr transcript levels (ANOVA-2; sex F(1, 16) = 41.23, p < 0.0001), with post-hoc tests revealing significantly higher expression in females versus males at GD 17.5 irrespective of diet (control females 1.96 ± 0.11 vs. control males 1.03 ± 0.102, p = 0.0004; mHFD females 2.01 ± 0.21 vs. mHFD males 1.18 ± 0.103, p = 0.0012). There was no effect of diet on Oxtr expression within the hypothalamus for either sex (ANOVA-2; sex F(1, 16) = 0.5616, p = 0.4645). No differences were found between groups in hypothalamic levels of Avpr1a (mHFD males 1.013 ± 0.098, control males 1.01 ± 0.07; mHFD females 0.923 ± 0.142, control females 1.002 ± 0.06).
In the offspring prefrontal cortex (PFC), no differences in Oxtr (Oxtr: mHFD males 0.757 ± 0.082, control males 1.14 ± 0.327; mHFD females 0.871 ± 0.232, control females 0.988 ± 0.353) or Avpr1a expression were detected among any groups (Avpr1a: mHFD males 0.754 ± 0.26, control males 1.097 ± 0.245; mHFD females 0.732 ± 0.172, control females 1.004 ± 0.205) (Figure 1c). Similarly, in the amygdala, there was no effect of maternal diet or offspring sex on expression of Oxtr (Figure 1d) (mHFD males 0.92 ± 0.241, control males 1.122 ± 0.254; mHFD females 1.12 ± 0.158, control females 1.16 ± 0.118) or Avpr1a (mHFD males 0.824 ± 0.07, control males 1.05 ± 0.185; mHFD females 0.815 ± 0.084, control females 0.912 ± 0.096) (Figure 1d).
2.2. Maternal High Fat Diet-Induced Obesity Alters Histone H3 Acetyl Lysine Binding to the Promoter Region of Oxtr in Hippocampus of Male Offspring
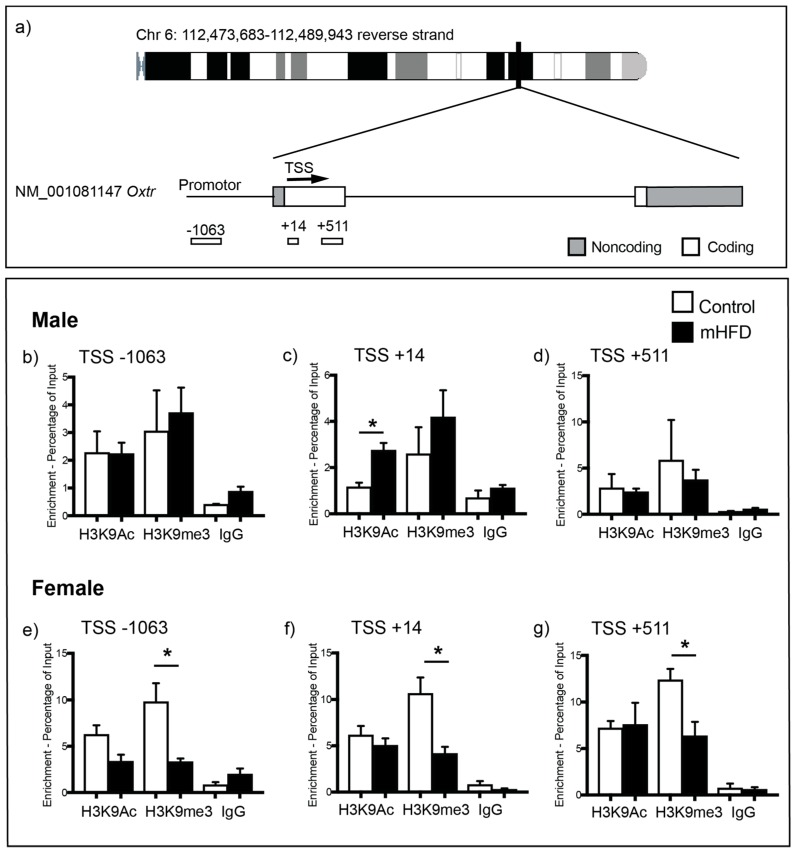
We next performed ChIP coupled with qPCR to investigate whether increased Oxtr gene expression in the hippocampus of male mHFD offspring could be underpinned by altered histone binding within the Oxtr promoter region (Figure 2a). We chose to examine acetylation of histone H3 lysine 9 (H3K9Ac) (Figure 2b–d), a marker associated with increased gene expression, and histone H3 lysine 9 trimethylation (H3K9me3) (Figure 2e–g), a marker associated with transcriptional repression, as these have previously been shown to be modulated by maternal nutrition [28,29]. The binding of H3K9Ac at the Oxtr promoter was significantly increased in male mHFD offspring hippocampus in comparison to male control offspring, with differential enrichment of H3K9Ac found specifically at the site nearest the transcription start site (TSS) of Oxtr (male mHFD 2.75 ± 0.37%; male control 1.16 ± 0.19%; p = 0.0123, Figure 2c). There were no differences in levels of H3K9Ac binding to the Oxtr promoter region either upstream (Figure 2b) or downstream (Figure 2d) of the Oxtr TSS in male hippocampus. In females, maternal diet had no effect on H3K9Ac enrichment at any targeted regions within the Oxtr promoter (p > 0.05, Figure 2e–g).
Figure 2.
(a) ChIP-qPCR primers targeting regions up and downstream of the Oxtr transcription start site (TSS). (b–d) Bar graphs show H3K9Ac, H3K9me3 and IgG (control) binding at the Oxtr promoter region of control and mHFD male offspring hippocampus at: (b) −1063; (c) +14; and (d) +501 base pairs from the TSS. (e–g) Graphs show H3K9Ac, H3K9me3 and IgG (control) binding at the Oxtr promoter region of control and mHFD female offspring hippocampus at: (e) −1063; (f) +14; and (g) +501 base pairs from the transcription start site. Data are expressed as mean log2 ChIP/input ± SEM of three independent experiments, as analyzed by unpaired t-test, with p ≤ 0.05 *.
We also found sexually dimorphic effects of maternal HFD-induced obesity on H3K9me3 binding at the Oxtr promoter. H3K9me3 binding was significantly reduced at all three targeted regions in mHFD female offspring hippocampus in comparison to control females (Figure 2e–g) (−1063; female mHFD 3.32 ± 0.34%; female control 9.795 ± 2.00%; p = 0.0332; +14; female mHFD 4.16 ± 0.72%; female control 10.65 ± 1.72%; p = 0.0251; +511; female mHFD 6.36 ± 1.51%; female control 12.38 ± 1.19%; p = 0.0351). No differences between male mHFD and control offspring were observed for H3K9me3 binding (Figure 2b–d).
Together, these observations are consistent with the idea that sex-specific effects of maternal diet on offspring Oxtr gene expression in the hippocampus may be underpinned by maternal diet-induced epigenetic change.
3. Discussion
We provide evidence that in utero exposure to mHFD-induced obesity alters Oxtr gene expression and histone binding at the Oxtr promoter in offspring hippocampus in a sexually dimorphic manner. In response to mHFD, Oxtr transcription increased and H3K9Ac binding was enriched at the Oxtr promoter in male offspring hippocampus. These observations are consistent with current understanding of the function of histone acetylation at the gene promoter, which typically correlates with transcriptional activation [30], including that of G-protein coupled receptors in the brain [31]. By contrast, mHFD was associated with decreased binding of the repressive histone mark H3K9me3 to the Oxtr promoter in female offspring hippocampus, with no downstream changes in Oxtr transcript levels.
The mHFD-induced changes in Oxtr mRNA levels concomitant with altered histone binding at the Oxtr promoter in male offspring hippocampus indicate that Oxtr transcriptional control is deregulated as a consequence of maternal diet-induced obesity. This altered Oxtr gene expression is likely to have downstream functional consequences on oxytocinergic signaling within the hippocampus, impacting hippocampal development and later-life function. Oxt surges in the maternal circulation in late gestation, in preparation for parturition and subsequent lactation [32]. In addition to modulating maternal behavior, elevated Oxt has been shown to reach the fetal brain where it plays an integral role in a number of processes related to the formation of neuronal circuitry and synaptic transmission within the hippocampus. Oxt signaling is important for the late gestation/early neonatal switch in GABAergic signaling from excitatory to inhibitory [33], and perturbations have been shown to lead to seizures [34]. Oxt signaling is also important for the maturation of the glutamatergic system within the hippocampus, through its ability to modulate dendrite branching and synapse development, synaptic transmission, and the synchronicity of neuronal networks [35]. Together, these data paint a picture wherein late gestational Oxt/Oxtr signaling is vital for the development of the balance of GABAergic and glutamatergic neural circuitry and transmission within the offspring hippocampus; and situations in which this is altered, such as maternal obesity, it would be expected to lead to hippocampal dysfunction. Indeed, maternal obesity is associated with altered GABAergic signaling in the developing hippocampus of offspring [36], impaired growth, cell division and neurogenesis [37], and hippocampal specific changes in expression of genes involved in synaptogenesis [38]. Thus, given the dependence of hippocampal neural circuitry development on Oxtr signaling, it seems likely that the epigenetic dysregulation of Oxtr expression reported here may underpin at least some of these deficits observed in the hippocampus of mHFD offspring.
Oxytocin signaling via the Oxtr is strongly implicated in modulating social behavior. Disrupted Oxtr function is implicated in a number of neurodevelopmental disorders, particularly those associated with core social deficits [39,40], and abnormal social behavior can be induced by modulating oxytocin systems in animal models [41]. Recent animal studies also suggest that some of the cognitive difficulties associated with ASD may result from disrupted hippocampal function and circuitry [42,43], and disrupting hippocampal-cortical signaling results in ASD-like behaviors [44]. The mHFD-induced deregulation of Oxtr signaling in the hippocampus of male offspring may therefore also have functional outcomes in terms of social impairments. Consistent with this, animal models of maternal obesity have reported social deficits in offspring [38,45], as well as altered Oxt circuitry [46].
Although limited in number, studies examining sex differences in offspring brain in response to maternal obesity indicate divergent developmental vulnerabilities might exist between the sexes. For example, male offspring are more susceptible to mHFD-induced changes in brain gene expression [47], and to deregulation of epigenetic regulators in the hippocampus, amygdala and PFC [12]. These sex-differences in vulnerability to mHFD-induced changes in the brain may contribute to the known male bias in the prevalence of some neurodevelopmental disorders such as ASD and ADHD [48,49].
It is unclear why changes to H3K9me3 binding at the Oxtr promoter of mHFD females were not predictive of gene expression. There is growing consensus that histone lysine methylation is interrelated with DNA methylation, reviewed in [50]. Decreased H3K9me3 binding at the Oxtr promoter in mHFD females might therefore reflect changes in methylation, however previous studies have found changes in methylation of the Oxtr promoter directly correlate with transcription, at least in the adult brain [17,51]. Thus, histone lysine methylation may be more important for transcriptional control of Oxtr in an adult rather than developmental context. Extensive examination of the transcriptional regulation of the Oxtr gene across development and into adulthood would provide clarity here.
Finally, the molecular changes that account for the link between maternal obesity and epigenetic changes remain obscure. There is a complex relationship between inflammatory cytokine signaling and histone deacetylase activity [52,53], thus the expression or activity of enzymes that catalyze epigenetic changes may be modulated by mHFD-associated gestational inflammation [23]. More work in the area of how histone modifying enzymes are regulated to affect epigenetic change would shed important light on this.
With growing evidence that maternal obesity is associated with an increased risk of offspring neurodevelopmental disorders [7,54,55,56,57], and an increasing global prevalence of pregnancies complicated by maternal obesity [58,59], understanding the impact of maternal obesity on epigenetic regulation of brain development will be critical for developing future interventions to protect the offspring brain.
4. Materials and Methods
4.1. Animal Housing and Maternal Diet
All experiments were performed using protocols approved by the Animal Ethics Committee of the University of Otago (ET 16/17; 19 February 2018), and in accordance with the guidelines of the National Animal Welfare Advisory Committee, NZ. Generation of the mHFD-induced obesity mouse model has been previously described in [12,27]. Briefly, 4-week-old female C57/BL6 mice were housed under a 12 h light–dark cycle at constant temperature (21 ± 2 °C), and randomly allocated to an ad libitum control diet (10% kcal fat; D12450B, Research Diets, NJ, USA), or a high-fat diet (45% kcal fat; D12451, Research Diets, NJ, USA). Respective diets were maintained for 8 weeks, and through mating and gestation. Bodyweight was monitored weekly, and only high-fat diet fed animals determined significantly heavier than controls (≥30%, t-test, p ≤ 0.05) were used to generate offspring. Females were timed-mated with 4-month-old males maintained on chow. Litters were collected by caesarean at GD 17.5 between the 09:00 and 10:00. Sex was determined by Sry PCR [12].
4.2. RNA Extraction
Brains were cryosectioned at 70 μm, and incubated in RNAlater-ICE (Thermo Fisher Scientific, Melbourne, Australia) at −20 °C overnight. Hippocampi were micro-dissected from sections using an Olympus SZ61 microscope and RNA extracted with ZR-Duet DNA/RNA MiniPrep kit (Zymo Research, Irvine, USA), and treated with DNase I. RNA purity was assessed by NanoDrop ND-1000, quantified using a Qubit 2.0 Fluorometer, and reverse transcribed with Quanta qScript XLT cDNA SuperMix.
4.3. Quantitative PCR (qPCR)
All qPCR assays were performed on the Viia7 Real-Time PCR System. Triplicate (10 µL) reactions contained 2× PowerUp SYBR Green, 1 µL cDNA and 250 nm forward and reverse primers. Each qPCR run included no template-control and RNA as template reactions, and dissociation analysis to ensure amplification specificity. Primers were designed as previously described [27,60], with sequences and Genbank Accession numbers listed in Table S1. Previously validated region-specific reference genes with stable expression irrespective of maternal diet were used (Gapdh, Pgk1, Tbp, Sdha) [12]. For each group, fetal tissue was gathered from n = 5 independent adult female pregnancies. For each pregnancy, fetal tissue from 4 fetuses per sex was pooled. Thus, each group consisted of data derived from tissue from 20 fetuses (4 per pregnancy × 5 pregnancies). Normalized fold change in comparison to control males was determined using 2−ΔΔCT with efficiency correction [61].
4.4. ChIP-qPCR
Dissected hippocampal tissue was crosslinked in 1% formaldehyde for 10 min, quenched with glycine [0.125 M], and stored at −80 °C until required. ChIP validated rabbit polyclonal antibodies [anti-H3K9me3 (Abcam, ab8898), anti-H3K9Ac (Abcam, ab4441), and preimmune IgG (Abcam, ab1220)] were conjugated to protein G Dynabeads in 0.5% BSA for 3 h at 4 °C. Chromatin was enzymatically sheared using Micrococcal Nuclease, extracted, and lysate was precleared for 1 h at 4 °C. Chromatin was immunoprecipitated with 2 µg of antibody overnight at 4 °C. Immunoprecipitated DNA was washed from Dynabeads, eluted in TE, reverse crosslinked for 4 h at 65 °C, and column purified. All ChIP-qPCR primer sequences are listed in Table S2. Animal numbers and study design were as in 4.3 qPCR above.
4.5. Statistical Analysis
Statistical analyses were performed using Prism (v7.0d) software. For gene expression qPCR, average fold change relative to control males was determined using the 2−ΔΔCT relative quantification method with efficiency correction [61], using a two-way ANOVA (diet × sex), followed by Bonferroni post hoc pairwise comparisons. For ChIP-qPCR, data were normalized to input chromatin using the ∆CT method, and analyzed by Student’s unpaired t-test. Results were considered statistically significant where p ≤ 0.05.
Abbreviations
| ADHD | attention deficit hyperactivity disorder |
| ASD | autism spectrum disorders |
| Avp | Arginine vasopressin |
| Avpr1a | Arginine vasopressin receptor 1A |
| ChIP | Chromatin Immunoprecipitation |
| GD | Gestational day |
| H3K9Ac | Histone 3 Lysine 9 Acetylation |
| H3K9me3 | Histone 3 Lysine 9 methylation |
| mHFD | Maternal high fat diet |
| Oxt | Oxytocin |
| Oxtr | Oxytocin receptor |
| qPCR | Quantitative PCR |
| TSS | Transcription start site |
Supplementary Materials
Supplementary Materials can be found at http://www.mdpi.com/1422-0067/20/2/329/s1.
Author Contributions
Conceptualization, K.G. and C.J.; methodology, K.G. and C.J.; formal analysis, K.G.; writing—original draft preparation, K.G. and C.J.; writing—review and editing, K.G. and C.J.; and funding acquisition, C.J.
Funding
This research was funded by The Health Research Council of New Zealand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Heijmans B.T., Tobi E.W., Stein A.D., Putter H., Blauw G.J., Susser E.S., Slagboom P.E., Lumey L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper W.N., Khulan B., Owens S., Elks C.E., Seidel V., Prentice A.M., Belteki G., Ong K.K., Affara N.A., Constancia M., et al. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: Results of a pilot randomized controlled trial. FASEB J. 2012;26:1782–1790. doi: 10.1096/fj.11-192708. [DOI] [PubMed] [Google Scholar]
- 3.Schack-Nielsen L., Michaelsen K.F., Gamborg M., Mortensen E.L., Sorensen T.I.A. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int. J. Obes. 2010;34:67–74. doi: 10.1038/ijo.2009.206. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson A.M., Matthews P.A., Argenton M., Christie M.R., McConnell J.M., Jansen E.H., Piersma A.H., Ozanne S.E., Twinn D.F., Remacle C., et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 5.Vucetic Z., Kimmel J., Totoki K., Hollenbeck E., Reyes T.M. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng J., Xiao X.H., Zhang Q., Yu M., Xu J.P., Wang Z.X., Qi C.J., Wang T. Maternal and post-weaning high-fat, high-sucrose diet modulates glucose homeostasis and hypothalamic POMC promoter methylation in mouse offspring. Metab. Brain Dis. 2015;30:1129–1137. doi: 10.1007/s11011-015-9678-9. [DOI] [PubMed] [Google Scholar]
- 7.Krakowiak P., Walker C.K., Bremer A.A., Baker A.S., Ozonoff S., Hansen R.L., Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child Psychol. Psychiatr. Allied Discip. 2010;51:134–143. doi: 10.1111/j.1469-7610.2009.02133.x. [DOI] [PubMed] [Google Scholar]
- 9.Basatemur E., Gardiner J., Williams C., Melhuish E., Barnes J., Sutcliffe A. Maternal prepregnancy BMI and child cognition: A longitudinal cohort study. Pediatrics. 2013;131:56–63. doi: 10.1542/peds.2012-0788. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann S., Schlesier-Michel A., Wendt V., Grube M., Keitel-Korndorfer A., Gausche R., von Klitzing K., Klein A.M. Maternal Weight Predicts Children’s Psychosocial Development via Parenting Stress and Emotional Availability. Front. Psychol. 2016;7:1156. doi: 10.3389/fpsyg.2016.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contu L., Hawkes C.A. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int. J. Mol. Sci. 2017;18:1093. doi: 10.3390/ijms18051093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glendining K.A., Fisher L.C., Jasoni C.L. Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology. 2018;96:132–141. doi: 10.1016/j.psyneuen.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Kang S.S., Kurti A., Fair D.A., Fryer J.D. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 2014;11:156. doi: 10.1186/s12974-014-0156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 15.Nagarajan R.P., Hogart A.R., Gwye Y., Martin M.R., LaSalle J.M. Reduced MeCP2 Expression is Frequent in Autism Frontal Cortex and Correlates with Aberrant MECP2 Promoter Methylation. Epigenetics-Us. 2006;1:172–182. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saradalekshmi K.R., Neetha N.V., Sathyan S., Nair I.V., Nair C.M., Banerjee M. DNA Methyl Transferase (DNMT) Gene Polymorphisms Could Be a Primary Event in Epigenetic Susceptibility to Schizophrenia. PLoS ONE. 2014;9:e98182. doi: 10.1371/journal.pone.0098182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory S.G., Connelly J.J., Towers A.J., Johnson J., Biscocho D., Markunas C.A., Lintas C., Abramson R.K., Wright H.H., Ellis P., et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller M., Bales K.L., Taylor S.L., Yoon J., Hostetler C.M., Carter C.S., Solomon M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Res. Off. J. Int. Soc. Autism Res. 2013;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rich M.E., Caldwell H.K. A Role for Oxytocin in the Etiology and Treatment of Schizophrenia. Front. Endocrinol. 2015;6:90. doi: 10.3389/fendo.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodden C., van den Hove D., Lesch K.P., Sachser N. Impact of varying social experiences during life history on behaviour, gene expression, and vasopressin receptor gene methylation in mice. Sci. Rep. 2017;7:8719. doi: 10.1038/s41598-017-09292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumsta R., Hummel E., Chen F.S., Heinrichs M. Epigenetic regulation of the oxytocin receptor gene: Implications for behavioral neuroscience. Front. Neurosci. 2013;7:83. doi: 10.3389/fnins.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dogra S., Sona C., Kumar A., Yadav P.N. Epigenetic regulation of G protein coupled receptor signaling and its implications in psychiatric disorders. Int. J. Biochem. Cell Biol. 2016;77:226–239. doi: 10.1016/j.biocel.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Kim D.W., Young S.L., Grattan D.R., Jasoni C.L. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol. Reprod. 2014;90:130. doi: 10.1095/biolreprod.113.117259. [DOI] [PubMed] [Google Scholar]
- 24.Kim D.W., Glendining K.A., Grattan D.R., Jasoni C.L. Maternal obesity in the mouse compromises the blood-brain barrier in the arcuate nucleus of offspring. Endocrinology. 2016;157:2229–2242. doi: 10.1210/en.2016-1014. [DOI] [PubMed] [Google Scholar]
- 25.Kim D.W., Glendining K.A., Grattan D.R., Jasoni C.L. Maternal obesity leads to increased proliferation and numbers of astrocytes in the developing fetal and neonatal mouse hypothalamus. Int. J. Dev. Neurosci. 2016;53:18–25. doi: 10.1016/j.ijdevneu.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Sanders T.R., Glendining K.A., Jasoni C.L. Obesity during pregnancy in the mouse alters the Netrin-1 responsiveness of foetal arcuate nucleus neuropeptide Y neurones. J. Neuroendocrinol. 2017;29 doi: 10.1111/jne.12556. [DOI] [PubMed] [Google Scholar]
- 27.Sanders T.R., Kim D.W., Glendining K.A., Jasoni C.L. Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology. 2014;155:2566–2577. doi: 10.1210/en.2013-1968. [DOI] [PubMed] [Google Scholar]
- 28.Li C., Guo S., Gao J., Guo Y., Du E., Lv Z., Zhang B. Maternal high-zinc diet attenuates intestinal inflammation by reducing DNA methylation and elevating H3K9 acetylation in the A20 promoter of offspring chicks. J. Nutr. Biochem. 2015;26:173–183. doi: 10.1016/j.jnutbio.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Yang K.F., Cai W., Xu J.L., Shi W. Maternal high-fat diet programs Wnt genes through histone modification in the liver of neonatal rats. J. Mol. Endocrinol. 2012;49:107–114. doi: 10.1530/JME-12-0046. [DOI] [PubMed] [Google Scholar]
- 30.Shahbazian M.D., Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 31.Tran L., Schulkin J., Ligon C.O., Greenwood-Van Meerveld B. Epigenetic modulation of chronic anxiety and pain by histone deacetylation. Mol. Psychiatry. 2015;20:1219–1231. doi: 10.1038/mp.2014.122. [DOI] [PubMed] [Google Scholar]
- 32.Pfaff D.W., Arnold A.P., Fahrbach S.A., Etgen A.M., Rubin R.T.E. Hormones, Brain, and Behavior. Elsevier Inc.; Amsterdam, The Netherlands: 2002. [Google Scholar]
- 33.Tyzio R., Cossart R., Khalilov I., Minlebaev M., Hubner C.A., Represa A., Ben-Ari Y., Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- 34.Sala M., Braida D., Lentini D., Busnelli M., Bulgheroni E., Capurro V., Finardi A., Donzelli A., Pattini L., Rubino T., et al. Pharmacologic Rescue of Impaired Cognitive Flexibility, Social Deficits, Increased Aggression, and Seizure Susceptibility in Oxytocin Receptor Null Mice: A Neurobehavioral Model of Autism. Biol. Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Ripamonti S., Ambrozkiewicz M.C., Guzzi F., Gravati M., Biella G., Bormuth I., Hammer M., Tuffy L.P., Sigler A., Kawabe H., et al. Transient oxytocin signaling primes the development and function of excitatory hippocampal neurons. eLife. 2017;6 doi: 10.7554/eLife.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peleg-Raibstein D., Luca E., Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav. Brain Res. 2012;233:398–404. doi: 10.1016/j.bbr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Tozuka Y., Wada E., Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009;23:1920–1934. doi: 10.1096/fj.08-124784. [DOI] [PubMed] [Google Scholar]
- 38.Page K.C., Jones E.K., Anday E.K. Maternal and postweaning high-fat diets disturb hippocampal gene expression, learning, and memory function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306:R527–R537. doi: 10.1152/ajpregu.00319.2013. [DOI] [PubMed] [Google Scholar]
- 39.Elagoz Yuksel M., Yuceturk B., Karatas O.F., Ozen M., Dogangun B. The altered promoter methylation of oxytocin receptor gene in autism. J. Neurogenet. 2016;30:280–284. doi: 10.1080/01677063.2016.1202951. [DOI] [PubMed] [Google Scholar]
- 40.LoParo D., Waldman I.D. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: A meta-analysis. Mol. Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- 41.Tyzio R., Nardou R., Ferrari D.C., Tsintsadze T., Shahrokhi A., Eftekhari S., Khalilov I., Tsintsadze V., Brouchoud C., Chazal G., et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 42.Hitti F.L., Siegelbaum S.A. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radwan B., Dvorak D., Fenton A.A. Impaired cognitive discrimination and discoordination of coupled theta-gamma oscillations in Fmr1 knockout mice. Neurobiol. Dis. 2016;88:125–138. doi: 10.1016/j.nbd.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson E.B., Grossrubatscher I., Frank L. Dynamic Hippocampal Circuits Support Learning- and Memory-Guided Behaviors. Cold Spring Harb. Symp. Quant. Biol. 2014;79:51–58. doi: 10.1101/sqb.2014.79.024760. [DOI] [PubMed] [Google Scholar]
- 45.Tozuka Y., Kumon M., Wada E., Onodera M., Mochizuki H., Wada K. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 2010;57:235–247. doi: 10.1016/j.neuint.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 46.Buffington S.A., Di Prisco G.V., Auchtung T.A., Ajami N.J., Petrosino J.F., Costa-Mattioli M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell. 2016;165:1762–1775. doi: 10.1016/j.cell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edlow A.G., Guedj F., Pennings J.L.A., Sverdlov D., Neri C., Bianchi D.W. Males are from Mars, and females are from Venus: Sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am. J. Obstet. Gynecol. 2016;214:623.e1–623.e10. doi: 10.1016/j.ajog.2016.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balint S., Czobor P., Komlosi S., Meszaros A., Simon V., Bitter I. Attention deficit hyperactivity disorder (ADHD): Gender- and age-related differences in neurocognition. Psychol. Med. 2009;39:1337–1345. doi: 10.1017/S0033291708004236. [DOI] [PubMed] [Google Scholar]
- 49.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr. Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 50.Rose N.R., Klose R.J. Understanding the relationship between DNA methylation and histone lysine methylation. BBA-Gene Regul. Mech. 2014;1839:1362–1372. doi: 10.1016/j.bbagrm.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kusui C., Kimura T., Ogita K., Nakamura H., Matsumura Y., Koyama M., Azuma C., Murata Y. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem. Biophys. Res. Commun. 2001;289:681–686. doi: 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- 52.Gonneaud A., Gagne J.M., Turgeon N., Asselin C. The histone deacetylase Hdac1 regulates inflammatory signalling in intestinal epithelial cells. J. Inflamm. (Lond.) 2014;11:43. doi: 10.1186/s12950-014-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leus N.G.J., Zwinderman M.R.H., Dekker F.J. Histone deacetylase 3 (HDAC 3) as emerging drug target in NF-kappa B-mediated inflammation. Curr. Opin. Chem. Biol. 2016;33:160–168. doi: 10.1016/j.cbpa.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinkle S.N., Schieve L.A., Stein A.D., Swan D.W., Ramakrishnan U., Sharma A.J. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int. J. Obes. 2012;36:1312–1319. doi: 10.1038/ijo.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones P.B., Rantakallio P., Hartikainen A.L., Isohanni M., Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: A 28-year follow-up of the 1966 North Finland general population birth cohort. Am. J. Psychiat. 1998;155:355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds L.C., Inder T.E., Neil J.J., Pineda R.G., Rogers C.E. Maternal obesity and increased risk for autism and developmental delay among very preterm infants. J. Perinatol. 2014;34:688–692. doi: 10.1038/jp.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaefer C.A., Brown A.S., Wyatt R.J., Kline J., Begg M.D., Bresnahan M.A., Susser E.S. Maternal prepregnant body mass and risk of schizophrenia in adult offspring. Schizophr. Bull. 2000;26:275–286. doi: 10.1093/oxfordjournals.schbul.a033452. [DOI] [PubMed] [Google Scholar]
- 58.Heslehurst N., Rankin J., Wilkinson J.R., Summerbell C.D. A nationally representative study of maternal obesity in England, UK: Trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int. J. Obes. 2010;34:420–428. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- 59.Gregor L., Remington P.L., Lindberg S., Ehrenthal D. Prevalence of Pre-pregnancy Obesity, 2011–2014. WMJ Off. Publ. State Med. Soc. Wis. 2016;115:228–232. [PMC free article] [PubMed] [Google Scholar]
- 60.Glendining K.A., Markie D., Gardner R.J., Franz E.A., Robertson S.P., Jasoni C.L. A novel role for the DNA repair gene Rad51 in Netrin-1 signalling. Sci. Rep. 2017;7:39823. doi: 10.1038/srep39823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.