Abstract
Transient Receptor Potential ion channels (TRPs) have been described as polymodal sensors, being responsible for transducing a wide variety of stimuli, and being involved in sensory functions such as chemosensation, thermosensation, mechanosensation, and photosensation. Mechanical and chemical stresses exerted on the membrane can be transduced by specialized proteins into meaningful intracellular biochemical signaling, resulting in physiological changes. Of particular interest are compounds that can change the local physical properties of the membrane, thereby affecting nearby proteins, such as TRP channels, which are highly sensitive to the membrane environment. In this review, we provide an overview of the current knowledge of TRP channel activation as a result of changes in the membrane properties induced by amphipathic structural lipidic components such as cholesterol and diacylglycerol, and by exogenous amphipathic bacterial endotoxins.
Keywords: cellular membranes, mechanosensation, TRP channels, LPS, lipophilic compounds
1. TRP Channels and Mechanosensitivity
Detecting and responding to mechanical stimuli such as thermal molecular agitation or osmotic pressure gradients is an indispensable part of any living system [1]. Many cells such as chondrocytes, endothelial and cochlear cells are continuously exposed to and regulated by mechanical stimuli such as shear and compressive forces [2,3,4]. As such, multiple proteins transduce mechanical stimuli exerted onto the membrane into an electrical and/or biochemical signal [5,6]. Mechanosensitive ion channels (MSCs) are a diverse population of proteins that can be divided into two categories. First, channels that respond to membrane tension due to the presence of specialized mechanosensing motifs/mechanical sensors or because their structure renders them susceptible to membrane tension [7]. Second, channels activated by stretch because the gating domain is intrinsically sensitive to membrane tension [8,9]. MSCs are fast transducers of mechanical stress and serve as both signaling sensors and effectors. They modify the electrical potential of cells and mediate the influx of specific ions, such as Ca2+, across the plasma membrane [7]. MSCs are implicated in a myriad of physiological processes, for example touch and pain sensation, hearing, blood pressure control and cell volume regulation [10].
Transient Receptor Potential channels (TRPs) are membrane-spanning proteins that form non-selective cation channels. TRP channels have been extensively studied and described as polymodal sensors, as they are responsible for transducing a wide variety of stimuli. TRPs are involved in chemosensation [11], thermosensation [12,13,14], mechanosensation [15] and photosensation [16,17]. For instance, several TRPs are highly sensitive to temperature changes and provide a broad thermosensitivity, extending from noxious cold to burning heat. How these channels are able to detect thermal stimuli remains a topic of investigation [12,18]. In addition, TRPs interact with a plethora of structurally unrelated exogenous and endogenous chemical ligands. In particular, some plant-derived chemicals favorably target the thermosensitive TRP channels, explaining why we attribute characteristic thermal traits with certain foods, such as a ‘cool’ mint and a ‘hot’ chili pepper [19,20,21]. In contrast to the huge number of exogenous compounds modulating TRPs, few endogenous ligands are known, yet the list is growing.
Nearly all cells in mammals express at least one member of the TRP channel family. Due to their widespread expression and localization, TRPs contribute to fundamental physiological processes, covering pure sensory functions (such as pheromone signaling, taste transduction, nociception and temperature sensation), homeostatic functions (such as Ca2+ and Mg2+ reabsorption and osmoregulation) and motile functions, such as muscle contraction and vasomotor control [13,22,23,24]. TRP channel activation leads to cation influx, membrane depolarization and the activation of Ca2+ dependent signaling pathways. Action potential firing in nociceptive sensory neurons generates the sensation of pain, while Ca2+ signaling activates intracellular pathways inducing altered membrane protein expression and triggering the release of signaling peptides [25,26].
The TRP channel family consists of 13 or 28 members depending on the species. Mammals contain 28 members divided in six subgroups according to amino acid sequence homology: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin) and TRPA (ankyrin). A seventh subgroup found in insects, nematodes, fish and amphibians is named TRPN, after the no-mechanoreceptor potential channel C (NOMP-C), a mechanotransduction channel required for gentle touch in Drosophila flies. For a detailed overview of the members of the different subgroups and their putative roles, we refer to other recent reviews and chapters [27,28]. Despite variations in sequence homology, TRP channels share a similar architecture. All TRP channels consist of identical or homologous tetramers. Each of the four subunits in the tetramer comprises six transmembrane (TM) domains, with intracellular carboxy and amino termini. TM5 and TM6 are linked by an extracellular loop and together they form the cation conduction pore.
TRP channels play a role in mechanotransduction pathways following fluid shear stress, and increased membrane tension and changes in cell volume or changes in osmolarity. The involvement of TRP channels in mechanosensation was discovered by a mutation in the osm-9 gene of Caenorhabditis elegans that encodes a TRPV-like channel. This mutation led to defects in the avoidance to high osmolarity and nose touch [29]. Later, it was shown that TRPV1 is required for the response of osmosensory neurons in the organum vasculosum lamina terminalis, the primary osmosensor in the brain [30]. A deletion of 60 amino acids in the N-terminal region of TRPV1 renders stretch-inhibited cation channels [30,31,32]. In addition, multiple members of the TRPV subgroup are both mechano- and osmo-sensitive. TRPV2 functions as a mechanosensor in vascular smooth muscle cells and appears to be activated by osmotic cell swelling [33,34]. TRPV2 membrane insertion is promoted by PI3-kinase [35] and altered cellular PtdIns(4,5)P2 levels were proposed to underlie TRPV2 mechanosensitivity [36]. The interaction of TRPV2 with protein kinase A (PKA) suggest that PKA modification could modulate TRPV2 activity similarly as previously described for TRPV1 and the heat-activated K+ channel, TREK [37]. TRPV4 is another TRPV channel which is activated by osmotic cell swelling. While swelling induced activation of TRPV4 is independent of N-terminal ankyrin repeats [38], TRPV4 activation is mediated by the arachidonic acid metabolite 5′,6′-epoxyeicosatrienoic acid (5′,6′-EET) [39,40,41], consistent with the previously demonstrated swelling-induced activation of phospholipase A2 (PLA2) [42,43].
Multiple TRPC channels were identified as a mechanosensors, for example TRPC6 is activated by mechanical or osmotic stimuli that directly depends on the lateral-lipid tension and an energetic mismatch between protein and bilayer [44]. TRPC6 activity appears to be important for controlling the vascular tone in response to increased intravascular pressure [33,45,46].
Members of the TRPM subgroup also display putative mechanosensitive roles. Human TRPM3 channels display activity upon hypotonic cell swelling [47]. TRPM4 is involved in the control of pressure-induced smooth muscle cell depolarization, myogenic vasoconstriction in cerebral arteries and isolated vascular smooth muscle cells [45,48,49,50]. TRPM7 was proposed to be directly activated by cell stretch and potentiated by hypotonic cell swelling [51]. TRPM7 may also play a role in responses to shear stress, as its expression levels and responses are highly increased after stimulation [52].
TRPA1 has been proposed to function as a mechanosensor, but despite a large number of studies, its role in mechanosensitivity remains contentious. At first, mechanosensitivity of TRPA1 was attributed to a long stretch of ANK repeats, which were proposed to act as gating spring [53,54,55]. In mammals, TRPA1 expression has been confirmed in hair cell epithelia [54], but TRPA1 ablation failed to show auditory deficits [56,57]. Then again, experiments using isolated neurons [58,59], cell lines [60] and mechanosensitive sensory afferent fibers [61,62] imply TRPA1 in mechanosensation. Finally, TRPA1 was shown to contribute to the most abundant type of mechanically activated current, intermediately adapting macroscopic currents (IAMCs), with 43% reduction in small-diameter neurons from trpa1 KO mice [63]. Other mechanically activated currents (such as Slow AMC and Rapid AMC) in small- and large-diameter neurons do not require TRPA1 for normal mechanosensory function [63]. This suggests a positive contribution of TRPA1 to mechanosensation in only a specific type of nociceptors. Additionally, increased expression of TRPA1 in the cellular membranes following acute activation might trigger chronic mechanical hypersensitivity. Nevertheless, it remains uncertain whether TRPA1 directly or indirectly contributes to mechanosensitivity.
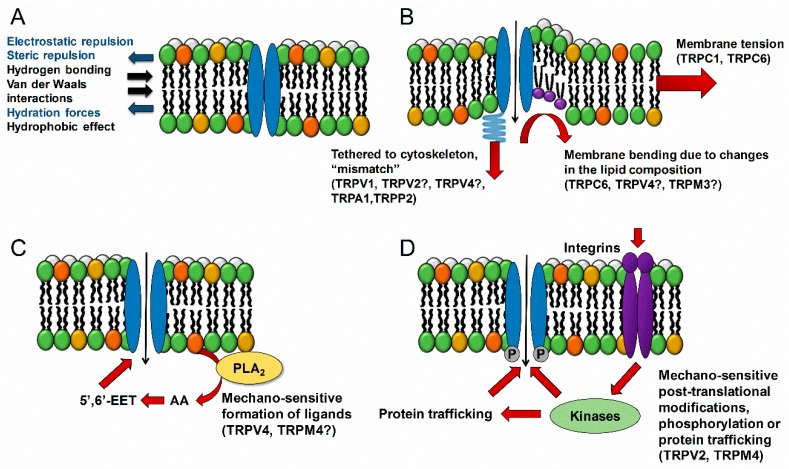
A question that arises is that of how mechanosensitive proteins detect changes in the membrane properties. Transmembrane proteins are exposed to negative and positive pressures produced by the bilayer. In normal conditions, the protein conformational energy matches the membrane energy profile. A change of that equilibrium, by membrane stretch or bending, results in modulation of the protein activity, as is the case for the ion channel conformational change and channel opening (Figure 1). This indicates that the insertion of lipids or membrane-modulating chemicals could induce channel gating. Other important factors involve accessory proteins for instance integrin clustering or cytoskeletal reorganization. Many TRPs are modulated by phospholipase C (PLC) activity, functionally linking them to GPCRs (e.g., Gq/11 linked to PLCβ) and tyrosine kinase receptors (via PLCγ). In addition, activity of TRP channels is modulated by intracellular Ca2+ changes and phosphorylation [64,65].
Figure 1.
Mechanisms of mechanosensing. (A) All transmembrane channels are exposed to lateral forces from the bilayer creating lateral pressure acting on the channel. If the equilibrium is changed, conformational protein changes will occur modulating channel activity. (B) Changes in the membrane tension such as membrane ordering can induce channel gating [1]. The reorganization of the cytoskeleton due to mechanical stress might induce a mismatch between the conformation energy of a cytoskeletal tethered channel and the intrinsic lipid tension, causing the channel to open [1]. Changes in the membrane composition due to the insertion of specific lipids (phosphatidylcholines, lysophosphatidylcholines, or arachidonic acid) or lipophilic chemicals induce changes in the bilayer curvature or order and can lead to channel gating [1]. (C) Mechanical stimulation of the membrane can trigger the production of intracellular messengers, for instance the activation of PLA2 results in the formation of arachidonic acid, which by itself or by subsequent metabolization products activates the channel, as was shown to be the case for TRPV4 [39]. (D) Activation of accessory proteins such as integrins or kinases can regulate both the activity, trafficking and expression levels of the channel.
2. TRP Channel Modulation by the Local Lipid Environment
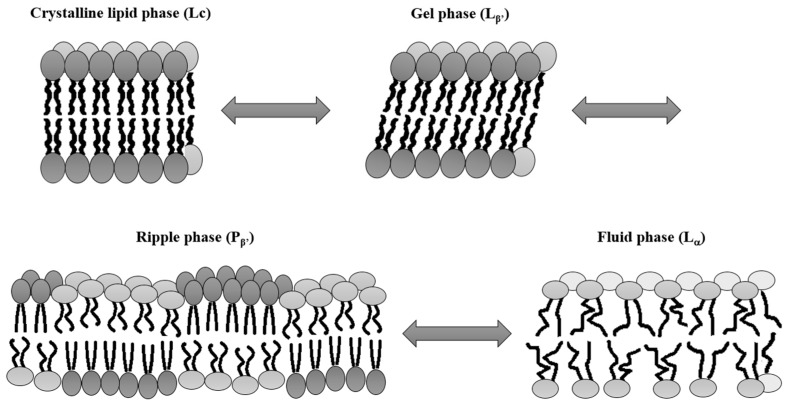
Biological membranes consist of a lipid bilayer and numerous proteins. This complex and dynamic system establishes a protective boundary around the cell’s interior [66]. Lipid bilayers are composed of two opposing leaflets made of multiple, asymmetrically distributed lipids species, belonging to the main three classes, including glycerophospholipids, sphingolipids and sterols [66,67,68]. Amphipathic lipids, consisting of polar, hydrophilic head groups and apolar, hydrophobic chains, tend to aggregate in the lipid-water systems into specific phases such as micelles, lamellar, cubic or inverse hexagonal phases [69,70]. This supramolecular phase organization is driven by the shape and concentration of the amphipathic molecule as well as the system’s temperature. The lipid phase organization reflects lipid packing at the lowest energy needed to balance repulsive head group interactions and hydrophobic chains effects. The most common lamellar, two-dimensional leaflets can exist in different states as crystalline phase (Lc), gel phase (Lβ’), ripple phase (Pβ’) and fluid phase (Lα) (Figure 2) [71,72,73,74].
Figure 2.
The lipid membrane phases. In the solid crystalline phase (Lc), lipids are highly ordered into all-trans chain conformation generating a compact lipid environment which is characterized by a large reduction in lateral lipid diffusion and low levels of membrane hydration. Lipids are tightly packed parallel to each other into a hexagonal lattice with a spacing of roughly 0.4 nm [79]. The lipid hydrocarbons chains can be tilted with respect to the crystalline phase, thereby forming a gel phase (Lβ’) reliant on lipid composition and hydration levels or the presence of other ions. [73]. Depending on the composition of the bilayer, the transition between gel and fluid phase (Lα) arises at a specific temperature called the thermotropic phase transition (Tm). This process is usually preceded by a so-called pre-transition inducing the formation of a ripple phase (Pβ’), characterized by periodic one-dimensional ripples. In the fluid phase, the lipids are highly disordered, and the triangular lattice order is lost with an increased lateral and rotational diffusion of the lipids (Modified from Heimburg [80]).
Lateral organization of biological membranes is influenced by temperature, pH, the intercalation of chemicals or simply the presence of a different lipid species such as cholesterol. Cholesterol is a vital sterol component of most animal cell membranes, which is crucial for maintaining membrane integrity. High concentrations of cholesterol induce conformational ordering of the lipid chains followed by stiffening of the membrane, thereby acting as a permeability barrier [75]. In contrast, cholesterol depletion significantly increases the bilayer permeability, affects transport functions, membrane enzyme activities and the conformation of membrane proteins [76].
In addition, cholesterol determines membrane thickness and fluidity. While the cholesterol content in eukaryotic plasma membranes usually exceeds 20 wt%, it is non-homogenously distributed in the membranes of intracellular organelles, varying between 3 and 8 wt% [77]. This amphipathic molecule has a non-polar, hydrophobic planar hydrocarbon ring, and an isooctyl tail and a hydroxyl (OH) group making it soluble in water. This chemical structure renders specific interactions between cholesterol and other membrane components with the cholesterol backbone inserted in the bilayer hydrocarbon core and its OH group penetrating into the polar headgroup region.
Cholesterol might influence ion channel activity either by direct interaction with ion channels, modifying the mechanical properties of the plasma membrane or facilitating the formation of lipid-enriched microdomains known as lipid rafts [78]. These are small (10 to 100 nm) signaling platforms which are highly enriched in cholesterol and sphingolipids in the outer exoplasmic leaflet and associated with phospholipids and cholesterol of the inner cytoplasmic leaflet of the membrane [67].
The local lipid environment plays a crucial role in the expression pattern and function of several TRP channels that preferably segregated into cholesterol-rich lipid rafts. These include members of the TRPC subfamily (TRPC1, TRPC3, TRPC4 and TRPC5) [81,82,83,84], TRPV1 [85], TRPV4 [86], TRPM8 [87] or the Drosophila photoreceptor TRP-like channel (dTRPL). Interestingly, cholesterol regulates these channels in distinct manners. For instance, depletion of cholesterol reduces the activity of TRPV1 [85,88,89,90], TRPV4 [91] and dTRPL [92], but increases stimulation of TRPM8 [87] and TRPM3 [93]. Moreover, there is increasing evidence that TRPA1 activation maybe induced by changes in the lipid environment [94,95,96].
The TRP channels present in lipid rafts would be highly influenced by changes in bilayer rigidity, thickness, fluidity, and phase. Nevertheless, further investigation is needed to fully understand the nature and the mechanisms by which changes in the membrane phase state affect ion channel properties.
3. TRP Channel Modulation by the Conically-Shaped Lipid Diacylglycerol
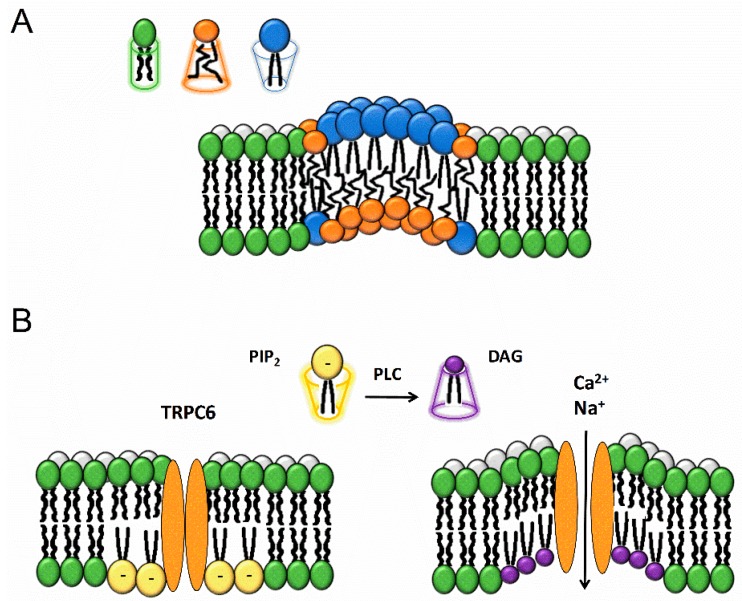
Biological membranes are asymmetric, with their individual leaflets having different properties that offer distinct microenvironments for different protein expression, trafficking and signaling processes. Furthermore, plasma membrane curvature defines the morphology of the cell and contributes to local membrane subdomains. The curvature-coupling hypothesis states that different lipids will induce specific local spontaneous curvatures (Figure 3A). Pure lipid bilayers stay flat, and upon stimulation can be curved towards the cytoplasm (positive curvature) or away from the cytoplasm (negative curvature) [97]. Fluctuations in one of the leaflets will induce changes in the second leaflet. For example, lipids with large, bulky heads and short chains will be preferentially present in the outer membrane while long chain, small heads lipids will be localized in the inner leaflet at the same position. Further, to achieve specific intrinsic curvature, membrane lipids are sorted in several ways. Lipids can be redistributed between opposing leaflets or between different regions, resulting in specific curvatures in distinct membrane regions [98].
Figure 3.
Lipids and membrane bending. (A) The membrane curvature can be altered by inserting lipids with specific shapes into the bilayer. Lipids can have a cylindrical (e.g., dioleoylphosphocholine), conical (e.g., dioleoylphosphotidylethanolamine, DAG and cholesterol) or inverse conical (e.g., lysophosphocholine) geometry [99]. Similar lipids tend to self-assemble in dynamic macrostructures depending on their lipid geometry and thus create asymmetric lipid bilayers. The increase in variability of pressure distribution along the membrane leaflets leads to different monolayer torques. The leaflet with the bigger torque bends to restore the system torque balance. (B) PLC-dependent breakdown of large-head, charged PIP2 to small-head, uncharged DAG occurs predominantly in the inner leaflet of the membrane in the close proximity to TRPC6. This causes inner leaflet deflection inducing changes in the curvature, resulting in bilayer stress and TRPC6 channel opening.
The conically shaped lipid diacylglycerol (DAG) has been shown to directly activate members of the TRPC3,6,7 subfamily of the TRP channels, either added exogenously to cells or generated in response to PLC-coupled receptors (Figure 3B). TRPC6, is activated by mechanically or osmotically induced membrane stretch/deformation in a manner inhibited by the tarantula peptide toxin GsMTx-4, an inhibitor of mechanosensitive ion channels. Further, PLC-independent mechanical stress causing membrane thinning can also trigger TRPC6 activation. This mechanism directly depends on the lateral-lipid tension and lipid-protein mismatch, in such a way that the stretch-induced reduction in membrane bilayer thickness shifts the channel conformation to the open state [44]. In this sense, DAG might act similarly by changing membrane curvature, whereas GsMTx-4 may relieve membrane lipid stress and inhibit channel activation [44].
In addition, changes in the membrane curvature can be also induced by the intercalation of chemicals. For instance, trinitrophenol is a negatively charged amphiphilic compound that accumulates in the outer leaflet of the plasma membrane, leading to crenation. Another chemical, chlorpromazine, inserts into the inner leaflet of the membrane and induces cup formation. In this regard, trinitrophenol has been shown to activate TRPA1, whereas chlorpromazine inhibits it, suggesting that this channel is sensitive to membrane deformation [94].
4. TRP Channel Modulation by the Natural Membrane Modulator Lipopolysaccharide
Lipopolysaccharides (LPS) are essential structural components of the outer membrane of gram-negative bacteria [100,101]. Due to their vital role in the bacterial growth and survival, endotoxins structures are highly conserved within all gram-negative species [102]. Either as an isolated compound or as part of the bacterial outer membrane, endotoxins play a crucial role in the pathogenesis and onset of symptoms of gram-negative infections. LPS are one of the most potent mediators inducing local or generalized inflammatory responses in humans, animals and even plants [103].
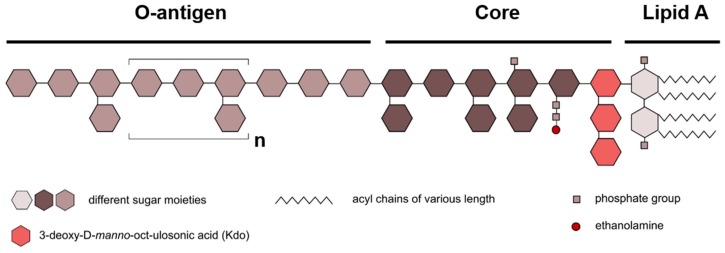
LPS from all gram-negative bacteria consist of three chemically distinct domains: the acylated and phosphorylated lipid A, the core oligosaccharide, and the O-antigen or O-specific polysaccharide (Figure 4). The O-antigen forms the outermost part of the LPS and is a major target for the host immune system. It consists of repeating sugar units (O-units), containing two to eight sugar residues. The types of sugar, the sequence and chemical linkage varies within and between O-units. For this reason, the O-antigen differs tremendously among bacterial strains [104]. The core oligosaccharide is an often-branched and phosphorylated hetero-oligosaccharide composed of up to 15 sugars and is divided to an inner and outer core. The inner core, proximal to the lipid A, contains residues of keto-deoxyoctulosonate (kDo) and heptose. Generally, the inner core tends to be well conserved, reflecting its importance for bacterial outer membrane integrity. The outer core is more variable, consistent with regions exposed to selective pressures of the environment (e.g., host responses). It typically accommodates several hexose residues such as glucose, mannose and galactose [105].
Figure 4.
Schematic diagram of the general LPS structure. Lipopolysaccharides consist of three main subunits: lipid A, the core region and the O-antigen. The number and chemical structure of the acyl chains can vary. Sugar moieties are depicted as hexagons in different colors. The number and chemical nature of these sugars can differ (Modified from Steimle et al. [102]).
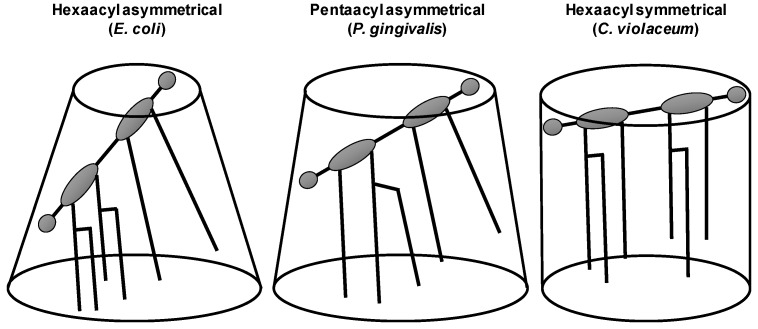
LPS and lipid A are amphiphilic molecules. The chemical variations of lipid A strongly influence its capacity to interact and activate receptors of the immune system. The intrinsic conformations have been demonstrated to be responsible for its agonistic and antagonistic activity [106]. Lipid A is responsible for the toxic effects associated with gram-negative bacteria infections [107]. It constitutes the minimal structural moiety required to trigger the activation of the canonical Toll-Like Receptor 4 (TLR4)-mediated pathway [108,109]. Usually lipid A is composed of a glucosamine disaccharide backbone attached to up to four fatty acid residues, which can be further substituted to provide up to seven acyl substituents. The number, position and length of these acyl chains and the presence of charged groups on the polar heads as well as the number of phosphate groups coupled to the disaccharide backbone can differ. Each glucosamine can carry the same number of acyl chains denoting it a ‘symmetrically’ acetylated lipid A. E. coli LPS is an example of ‘asymmetrically’ acetylated lipid A, since four of its six acyl chains are carried by one of the glucosamines [102,105]. Despite its general structural conservation, lipid A structures display considerable micro-heterogeneity among gram-negative bacterial species, directly resulting in altered endotoxic strength [103]. The bioactivity of lipid A is related to its three-dimensional shape and the biochemical composition (i.e., the chemical structure, the net charge and the inclination of the head group) [106]. Endotoxically active lipid A can be distinguished from endotoxically inactive lipid A (with antagonistic properties) by the intrinsic shape with a larger inclination of the diglucosamine ring plane with respect to the membrane surface and, concomitantly, a conical instead of a cylindrical shape [110,111] (Figure 5). For example, the hexa-acylated E. coli lipid A acts as a potent agonist for all mammalian cells.
Figure 5.
LPS macrostructure proposed for different lipid A moieties. (Modified from Seydel et al. [110]).
The detection of LPS leads to a strong host immune system activation. Classical immune pathways involve LPS recognition by pattern recognition receptors (PRRs) [112], of which the most studied is the TLR4 pathway [109]. Activation of PRRs triggers signaling cascades inducing the biosynthesis of inflammatory cytokines (TNF-α, IL-1 and IL-6), as well as ROS and antimicrobial peptides [109]. In addition to the classical TLR4-mediated pathway, other proteins have been shown to be involved in LPS recognition and bioactivity [113,114,115]. For example, LPS activates multiple TRP cation channels including TRPA1 [95], TRPV1 and TRPV4 [116] in a TLR4-independent manner. TRPA1 activity has been related to several acute LPS responses in mice such as hyperalgesia, edema, release of calcitonin gene-related peptide (CGRP) and arterial dilation establishing channel function in triggering acute inflammatory and pain responses [95]. TRPA1 activation by bacterial endotoxins produces avoidance during feeding and oviposition in Drosophila melanogaster [117]. Further, TRPV4 activation in airway epithelial cells induces protective responses such as nitric oxide production and increased ciliary beat frequency [116]. Thus, TRP channels are sensors of LPS, operating independently of classical TLR4 signaling.
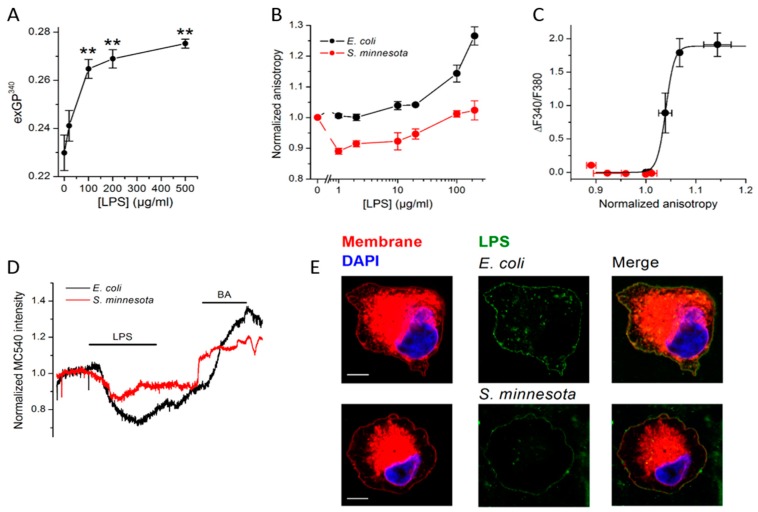
Yet, the mechanism of activation by LPS remained elusive. Interestingly, the most sensitive TRP channel for LPS, TRPA1, plays a role in mechanosensation [62,118] and can be activated by chemicals, which are known to alter membrane properties [94]. A recent study demonstrated that mechanical perturbations of the cellular membrane induced by E. coli LPS can trigger TRPA1 activation both in cellular and artificial membranes (Figure 6 A–D) [96]. These findings offer decisive support to previous reports on the ordering of lipid membranes by E. coli LPS [119,120,121,122,123]. LPS was shown to insert into 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) bilayers, prompting significant reduction of the elastic strength, subsequently reducing vesicles size [124].
Figure 6.
Effect of LPS insertion on membrane properties. (A) Determination of Laurdan excitation Generalized Polarization (exGP) is a useful tool in describing membrane fluidity as this probe displays a shift in the emission when in contact with ordered or disordered membranes [136,137]. The GP values recorded in one component lipid giant unilamellar vesicles (GUV) are between −0.3 to 0.3 corresponding to the fluid and between 0.5 and 0.6 in gel phase [138]. The exGP values determined in DPPC GUVs indicate membrane rigidification induced by E. coli LPS. (B) Fluorescence anisotropy of membrane sensitive dye diphenylhexatriene (DPH) changes upon alterations of the membrane fluidity. Intercalation of E. coli LPS into CHO-mTRPA1 membranes caused anisotropy increase demonstrating reduction in its fluidity. Contrary, S. minnesota LPS that did not prompt significant changes. (C) Relation between LPS-induced increases in intracellular Ca2+ levels caused by TRPA1 activation and change in DPH fluorescence anisotropy recorded in CHO-TRPA1 cells upon application of E. coli or S. minnesota LPS (0–100 µg/mL), clearly indicted differential effects of both LPS. (D) Merocyanine 540 (MC540), a lipophilic, viscosity probe which fluorescence increases upon binding to membranes in fluid-phase, has been used to further differentiate between effects induced by E. coli or S. minnesota LPS. Benzyl alcohol (BA), a well-described membrane fluidizer [139], has been used after LPS wash out. S. minnesota LPS prompted weaker effects in the plasma membrane mechanical properties that could be attributed to a lower efficiency of its insertion into the cellular membranes (E). Representative Airyscan confocal images of CHO-TRPA1 cells stained with membrane mask (red), DAPI (blue) and treated with Alexa Fluor 488 LPS (green) from E. coli (20 µg/mL top panel) or S. minnesota (20 µg/mL bottom panel). Scale bar, 5 µm. Reproduced with permission from Startek et al. [96].
E. coli LPS intercalation into the membrane of red blood cells caused volume decrease, increased membrane viscosity, as well as reduction of stress tolerance [120,121,122]. Similarly, E. coli LPS insertion into hepatocyte membranes produced an increased viscosity and delay in the membrane fluidization induced by heat [123]. Further, it was demonstrated that LPSs with different conformations of lipid A, such as LPS from S. minnesota, are less effective in inducing changes in the membrane order. The reduced membrane insertion efficiency of S. minnesota LPS compared to E. coli LPS (Figure 6E) indicates the importance of the shape of lipophilic lipid A [96]. These findings suggest that the conically-shaped lipid A moiety of E. coli alters membrane properties to a greater extent than the cylindrical ones such as those from S. minnesota. Further support for this idea came from the correlation between the lipid A shape and the ability of different LPS molecules to activate TRPA1 in vitro and to cause inflammation in vivo [95]. These effects follow studies reporting lower bioactivity of S. minnesota lipid A with respect to E. coli lipid A on TRPA1 channel activation and human-derived cells [95,125]. As the TRPA1 subunit organization in the cellular membranes indicates a large contact surface with membrane lipids [126], changes in the physical properties of the membrane such as order, tension, thickness and curvature resulting from LPS intercalation in the vicinity to the channel could alter the TRPA1-bilayer interaction and induce channel opening [127].
It should be noted that the E. coli LPS concentrations shown to activate TRPA1 (EC50 ~3 µg/mL) [95] are about 2 orders of magnitude higher than those activating the canonical TLR4 pathway in vitro. However, it was reported that during pathological conditions such as severe endotoxemia, plasma LPS levels can rise into the µg/mL range [128]. Furthermore, relatively high LPS concentrations are present in urine and locally infected tissues [129] and are used in current experimental models of endotoxin challenge in LPS-evoked pain behavior experiments [130] and LPS-induced airway inflammation [131,132,133]. Bacterial lysis during acute infection can also result in locally high concentrations especially in the very small volumes of acute dental abscesses and micrometer-thin mucosal layers at epithelial barriers.
Further, fever triggered by the stimulation of the immune system and local changes of temperature induced by neurogenic inflammation at the site of infection may influence the response of TRP channels to LPS. However, the outcome of these interactions is difficult to predict, because these channels are also strongly modulated by temperature. Although we recently reported that LPS-induced activation of TRPM8 by LPS was enhanced at cold temperatures [134], the underlying mechanism of this effect and the existence of such interactions for other sensory TRP channels (TRPA1, TRPV1, TRPV4 and TRPM3) remain unexplored.
As LPS insertion produces lateral membrane compression, the mechanism underlying TRPA1 activation by LPS is different from that operating in “classical” mechanosensitive channels e.g., membrane stretch [1,10,135]. Elucidation of TRPA1 activation mechanisms by LPS helps to understand novel protective defense mechanisms against microbial species where TRP channels activation triggers protective responses mediated by sensory neurons.
5. TRP Channel Modulation by Lipophilic Compounds
Local anesthetics (LAs) inhibit voltage-gated Na+ channels (VGSCs), thereby preventing the generation of neuronal action potentials and pain sensation [140,141]. Many widely used LAs, such as procaine, lidocaine and tetracaine interact with the membrane hydrophobic region and can alter its properties, resulting in the modulation of specific signaling events. It has been suggested that interactions between LAs and the membrane leads to changes in the bilayer bending rigidity and tension [142,143]. LAs were reported to rearrange the intermolecular hydrogen-bonded network among phospholipids and alter the orientation of their P–N dipole, inducing an increase in membrane fluidity [144]. VGSCs are expressed in lipid rafts and high cholesterol levels have been shown to promote LAs partitioning into bilayers [145]. Further, the artificial raft-mimicking phases are destabilized by inserting LAs [143]. Thus, if LAs destabilize specific membrane domains, nearby VGSCs may also be affected. Once lipid rafts are destabilized, the order, structure, and movement of the VGSC subunits forming the structure of the channel gates may be altered [146]. Different LAs have different effects on the membrane, as for instance, tetracaine induces higher miscibility temperature, line tension at the ordered/disordered phase interface, and lipid main transition temperature and phase separation than lidocaine [147]. Additionally, other molecules such as n-alcohol general anesthetics are reported to correlate increasing molecule hydrophobicity, producing a decrease in lipid transition temperature, with an increase in the strength of anesthesia [148,149].
Interestingly, in high concentrations, LAs have excitatory effects attributed to activation of TRPV1 and to a lesser extent of TRPA1 channels, resulting in a Ca2+-dependent release of CGRP and substance P [150,151,152]. This leads to pain, local tissue damage, inflammation and neurotoxicity [153]. Lidocaine not only activates both rodent TRPV1 and TRPA1 channels, but also inhibits them in a concentration-dependent manner [150]. Although the mechanism underlying TRP activation by LAs may involve changes in the membrane fluidity, binding to specific channels domains cannot be excluded. Activation of TRPV1 by lidocaine was reported to involve interactions with a vanilloid-like binding domain composed of amino acid residues located in TM3 and TM4 and the respective cytosolic interfaces [151]. It also requires an interaction of PIP2 with the proximal C-terminal TRP domain [151]. Capsazepine, the competitive TRPV1 antagonist, prevented activation of TRPV1 by lidocaine [151]. Lidocaine induced TRPV1 desensitization involves channel dephosphorylation through a mechanism involving calcineurin. Desensitization could be also reduced by PKA- and PKC-mediated phosphorylation and Ca2+-dependent inactivation involving calmodulin [151]. On the other hand, activation of TRPA1 by LAs does not involve either vanilloid-binding domain or a TRP domain. LAs were shown to interact with TM5 of TRPA1, which may explain the differences between rodent and human TRPA1 lidocaine-evoked currents [150]. Nevertheless, lidocaine activates the menthol-insensitive mutant mTRPA1-S876V/T877L [150,154]. The mechanism of TRPA1 activation may also involve direct stimulation by PLC and regulation by PIP2 [150]. Further, general anesthetics such as desflurane, isoflurane, sevoflurane and halothane were suggested to act in a membrane-delimited fashion. However, TRPA1 activation by these compounds did not correspond with their ability to partition into cellular membranes, arguing against induction of changes in the membrane fluidity as underlying mechanism of channel activation [155]. Furthermore, the response cutoff correlated with 8–10 carbon length chain of alcohols, indicating existence of a binding pocket within TRPA1 [155].
Another group of chemicals, primary alcohols, are TRPA1 activators inducing skin, eye and nasal irritation [156,157]. Potentiation of harmful effects and activation of TRPA1 was correlated with the increase in the length of a carbon chain (from C2OH to C8OH) [158]. The comparison between primary and secondary alcohols with different lengths of the carbon chains revealed that TRPA1 activation ability depends mainly on the length of the carbon chain and not on the position of their hydroxyl group [158]. These results strongly suggest that the octanol/water partition coefficient (lipophilicity) may be a critical determinant of TRPA1 activation. Similar effects were also reported for other TRP channels, e.g., TRPV1 [159,160], indicating a possible general activation mechanism.
Notably, a very large group of non-electrophilic TRPA1 agonists has, as a common feature, the ability to intercalate into cellular membranes, inducing mechanical alterations. The analysis of the chemical structures and properties of thymol and different alkyl-substituted phenol derivatives suggests for a correlation between the partition coefficient (logP) and the potency to activate the channel [161]. Similar conclusions could be made for a series of parahydroxybenzoates (parabens), for which an increase in calculated logP value correlated with a reduced EC50 value for TRPA1 activation [162]. Also, the lipophilic farnesyl thiosalicylic acid and its analogs [163] and 6-gingerol analogs [164,165] are able to activate TRPA1. Similarly, the highly lipophilic compounds leucettamols A and B and of their semi-synthetic analogues were shown to modulate TRPA1 activity [166]. Despite the increasing evidence that TRPA1 activation by lipophilic chemicals could be induced by changes in the channel local lipid environment caused by the compounds intercalation, the full mechanism of channel activation remains elusive.
Moreover, cells undergo changes in membrane composition and physical properties that tend to preserve specific membrane order in response to harmful environmental chemicals, such as crude oil [167], PCB-153 [168], hydrocarbons, alcohols and detergents [169]. The effects of temperature on the composition of the biological membranes are also well described in plants [170], poikilotherms [171], Archaea [172], zooplankton [173], fish [174,175] and mammals [176,177]. One of the mechanisms contributing to the regulation of cellular membrane order, known as homeoviscous adaptation (HVA), is based on the stabilization of membrane fluidity by changing the relative concentrations of saturated and unsaturated membrane fatty acids. Although originally the HVA theory was formulated for a temperature-dependent response in bacteria, it has been later shown to have a much broader evolutionary significance [176,178]. For instance, mammalian membrane order is preserved by biological membranes in response to hydrostatic and osmotic pressure [174,179], low magnetic field strength [180], chemicals [168] and lipids such as cholesterol content. Membrane fluidization by exogenous chemicals or heat [181,182] increases the concentration of intracellular Ca2+, which may act as a second messenger in the cellular pathways (e.g., activation of heat-shock proteins [183]). Because sensory TRPs can be activated by a wide variety of external stimuli, we hypothesize that these channels may be involved in the mechanisms underlying the remodeling of cellular membranes upon chemical and thermal challenge. In a broader context, cellular phospholipid homeostasis could help to understand pathophysiology of multiple diseases such as cancer [184], Alzheimer’s disease [185], liver disease [186,187] and diabetes [188] in which fine balance between in membrane properties is disturbed.
Taken together, evidence recently accumulated led us to conclude that the “mechanical detection” of chemical partitioning in cellular membranes by TRP channels might be part of a fundamental mechanism in which TRP channel activation by chemical and physical stimuli might result not only from classical compound-receptor interaction but also from their mechanosensory properties [15,94].
Author Contributions
All authors wrote, reviewed and edited the manuscript; V.M. and K.T. equally contributed to supervise the project.
Funding
This work was supported by grants of the Research Council of the KU Leuven (GOA/14/011 and C14/18/086), the Fund for Scientific Research Flanders (FWO: G070212N, G0C7715N and G0D0417N), SAF2017-83674-C2-2 Agencia Estatal de Investigación, Spain and ERDF, European Union; GV/2018//098 Generalitat Valenciana, Spain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pedersen S.F., Nilius B. Chapter ten—Transient receptor potential channels in mechanosensing and cell volume regulation. In: Häussinger D., Sies H., editors. Methods in Enzymology. Volume 428. Academic Press; New York, NY, USA: 2007. pp. 183–207. [DOI] [PubMed] [Google Scholar]
- 2.White C.R., Frangos J.A. The shear stress of it all: The cell membrane and mechanochemical transduction. Philos. Trans. R. Soc. B Biol. Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huiskes R., Ruimerman R., van Lenthe G.H., Janssen J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405:704–706. doi: 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie P.G., Müller U. Mechanotransduction by hair cells: Models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Añoveros J., Corey D.P. The molecules of mechanosensation. Ann. Rev. Neurosci. 1997;20:567–594. doi: 10.1146/annurev.neuro.20.1.567. [DOI] [PubMed] [Google Scholar]
- 6.Stukel J.M., Willits R.K. Mechanotransduction of neural cells through cell-substrate interactions. Tissue Eng. Part B Rev. 2016;22:173–182. doi: 10.1089/ten.teb.2015.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukharev S., Sachs F. Molecular force transduction by ion channels—Diversity and unifying principles. J. Cell Sci. 2012;125:3075–3083. doi: 10.1242/jcs.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagriantsev S.N., Peyronnet R., Clark K.A., Honoré E., Minor D.L. Multiple modalities converge on a common gate to control k(2p) channel function. EMBO J. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao J., Padilla F., Dandonneau M., Lavebratt C., Lesage F., Noël J., Delmas P. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77:899–914. doi: 10.1016/j.neuron.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Hamill O.P., Martinac B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 11.Boonen B., Startek J.B., Talavera K. Chemical activation of sensory trp channels. In: Krautwurst D., editor. Taste and Smell. Springer International Publishing; Cham, Switzerland: 2016. pp. 73–113. [Google Scholar]
- 12.Voets T., Droogmans G., Wissenbach U., Janssens A., Flockerzi V., Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive trp channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 13.Talavera K., Nilius B., Voets T. Neuronal trp channels: Thermometers, pathfinders and life-savers. Trends Neurosci. 2008;31:287–295. doi: 10.1016/j.tins.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Vriens J., Nilius B., Voets T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014;15:573–589. doi: 10.1038/nrn3784. [DOI] [PubMed] [Google Scholar]
- 15.Liu C., Montell C. Forcing open trp channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015;460:22–25. doi: 10.1016/j.bbrc.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montell C., Rubin G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-X. [DOI] [PubMed] [Google Scholar]
- 17.Hardie R.C., Minke B. The trp gene is essential for a light-activated ca2+ channel in drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-S. [DOI] [PubMed] [Google Scholar]
- 18.Clapham D.E., Miller C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (trp) channels. Proc. Natl. Acad. Sci. USA. 2011;108:19492–19497. doi: 10.1073/pnas.1117485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 20.McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for trp channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 21.Startek J.B., Voets T., Talavera K. To flourish or perish: Evolutionary trips into the sensory biology of plant-herbivore interactions. Pflugers Arch. 2018 doi: 10.1007/s00424-018-2205-1. [DOI] [PubMed] [Google Scholar]
- 22.Venkatachalam K., Montell C. Trp channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran M.M., McAlexander M.A., Biro T., Szallasi A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- 24.Alonso-Carbajo L., Kecskes M., Jacobs G., Pironet A., Syam N., Talavera K., Vennekens R. Muscling in on trp channels in vascular smooth muscle cells and cardiomyocytes. Cell Calcium. 2017;66:48–61. doi: 10.1016/j.ceca.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Laing R.J., Dhaka A. Thermotrps and pain. Neuroscientist. 2016;22:171–187. doi: 10.1177/1073858414567884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Y. Trps and pain. Semin. Immunopathol. 2016;38:277–291. doi: 10.1007/s00281-015-0526-0. [DOI] [PubMed] [Google Scholar]
- 27.Nilius B., Flockerzi V. Mammalian transient receptor potential (trp) cation channels. Preface. Handb. Exp. Pharmacol. 2014;223:v–vi. [PubMed] [Google Scholar]
- 28.Geffeney S. Frontiers in neuroscience sensory mechanotransduction and thermotransduction in invertebrates. In: Emir T.L.R., editor. Neurobiology of Trp Channels. Taylor & Francis Group, LLC.; Boca Raton, FL, USA: 2017. pp. 65–83. [PubMed] [Google Scholar]
- 29.Colbert H.A., Smith T.L., Bargmann C.I. Osm-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in caenorhabditis elegans. J. Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciura S., Bourque C.W. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J. Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharif Naeini R., Witty M.F., Séguéla P., Bourque C.W. An n-terminal variant of trpv1 channel is required for osmosensory transduction. Nat. Neurosci. 2006;9:93–98. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- 32.Lu G., Henderson D., Liu L., Reinhart P.H., Simon S.A. Trpv1b, a functional human vanilloid receptor splice variant. Mol. Pharmacol. 2005;67:1119–1127. doi: 10.1124/mol.104.009852. [DOI] [PubMed] [Google Scholar]
- 33.Beech D.J., Muraki K., Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of drosophila trp. J. Physiol. 2004;559:685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muraki K., Iwata Y., Katanosaka Y., Ito T., Ohya S., Shigekawa M., Imaizumi Y. Trpv2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 35.Penna A., Juvin V., Chemin J., Compan V., Monet M., Rassendren F.-A. Pi3-kinase promotes trpv2 activity independently of channel translocation to the plasma membrane. Cell Calcium. 2006;39:495–507. doi: 10.1016/j.ceca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M., Chen M.Z., Wang Y.J., Sun H.Q., Wei Y., Martinez M., Yin H.L. Hypertonic stress increases phosphatidylinositol 4,5-bisphosphate levels by activating pip5kibeta. J. Biol. Chem. 2006;281:32630–32638. doi: 10.1074/jbc.M605928200. [DOI] [PubMed] [Google Scholar]
- 37.Stokes A.J., Shimoda L.M.N., Koblan-Huberson M., Adra C.N., Turner H. A trpv2–pka signaling module for transduction of physical stimuli in mast cells. J. Exp. Med. 2004;200:137. doi: 10.1084/jem.20032082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liedtke W., Choe Y., Martí-Renom M.A., Bell A.M., Denis C.S., AndrejŠali, Hudspeth A.J., Friedman J.M., Heller S. Vanilloid receptor–related osmotically activated channel (vr-oac), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vriens J., Watanabe H., Janssens A., Droogmans G., Voets T., Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel trpv4. Proc. Natl. Acad. Sci. USA. 2004;101:396. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vriens J., Owsianik G., Fisslthaler B., Suzuki M., Janssens A., Voets T., Morisseau C., Hammock B.D., Fleming I., Busse R., et al. Modulation of the ca2 permeable cation channel trpv4 by cytochrome p450 epoxygenases in vascular endothelium. Circ. Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate trpv4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 42.Kinnunen P.K.J. Lipid bilayers as osmotic response elements. Cell. Physiol. Biochem. 2000;10:243–250. doi: 10.1159/000016360. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen S.F., Poulsen K.A., Lambert I.H. Roles of phospholipase a2 isoforms in swelling- and melittin-induced arachidonic acid release and taurine efflux in nih3t3 fibroblasts. Am. J. Physiol.-Cell Physiol. 2006;291:C1286–C1296. doi: 10.1152/ajpcell.00325.2005. [DOI] [PubMed] [Google Scholar]
- 44.Spassova M.A., Hewavitharana T., Xu W., Soboloff J., Gill D.L. A common mechanism underlies stretch activation and receptor activation of trpc6 channels. Proc. Natl. Acad. Sci. USA. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue R., Jensen L.J., Shi J., Morita H., Nishida M., Honda A., Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ. Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 46.Welsh D.G., Morielli A.D., Nelson M.T., Brayden J.E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 47.Grimm C., Kraft R., Sauerbruch S., Schultz G., Harteneck C. Molecular and functional characterization of the melastatin-related cation channel trpm3. J. Biol. Chem. 2003;278:21493–21501. doi: 10.1074/jbc.M300945200. [DOI] [PubMed] [Google Scholar]
- 48.Li Y., Baylie R.L., Tavares M.J., Brayden J.E. Trpm4 channels couple purinergic receptor mechanoactivation and myogenic tone development in cerebral parenchymal arterioles. J. Cereb. Blood Flow Metab. 2014;34:1706–1714. doi: 10.1038/jcbfm.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Earley S., Waldron B.J., Brayden J.E. Critical role for transient receptor potential channel trpm4 in myogenic constriction of cerebral arteries. Circ. Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich A., Chubanov V., Kalwa H., Rost B.R., Gudermann T. Cation channels of the transient receptor potential superfamily: Their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol. Ther. 2006;112:744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Numata T., Shimizu T., Okada Y. Trpm7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am. J. Physiol. Cell Physiol. 2007;292:C460–C467. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- 52.Oancea E., Wolfe J.T., Clapham D.E. Functional trpm7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 53.Corey D.P. New trp channels in hearing and mechanosensation. Neuron. 2003;39:585–588. doi: 10.1016/S0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- 54.Corey D.P., García-Añoveros J., Holt J.R., Kwan K.Y., Lin S.Y., Vollrath M.A., Amalfitano A., Cheung E.L., Derfler B.H., Duggan A., et al. Trpa1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 55.Sotomayor M., Corey D.P., Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure. 2005;13:669–682. doi: 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Bautista D.M., Jordt S.E., Nikai T., Tsuruda P.R., Read A.J., Poblete J., Yamoah E.N., Basbaum A.I., Julius D. Trpa1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 57.Kwan K.Y., Allchorne A.J., Vollrath M.A., Christensen A.P., Zhang D.S., Woolf C.J., Corey D.P. Trpa1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 58.Bhattacharya M.R.C., Bautista D.M., Wu K., Haeberle H., Lumpkin E.A., Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc. Natl. Acad. Sci. USA. 2008;105:20015. doi: 10.1073/pnas.0810801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vilceanu D., Stucky C.L. Trpa1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS ONE. 2010;5:e12177. doi: 10.1371/journal.pone.0012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rugiero F., Wood J.N. The mechanosensitive cell line nd-c does not express functional thermotrp channels. Neuropharmacology. 2009;56:1138–1146. doi: 10.1016/j.neuropharm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Brierley S.M., Hughes P.A., Page A.J., Kwan K.Y., Martin C.M., O’Donnell T.A., Cooper N.J., Harrington A.M., Adam B., Liebregts T., et al. The ion channel trpa1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095.e2083. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwan K.Y., Glazer J.M., Corey D.P., Rice F.L., Stucky C.L. Trpa1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brierley S.M., Castro J., Harrington A.M., Hughes P.A., Page A.J., Rychkov G.Y., Blackshaw L.A. Trpa1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J. Physiol. 2011;589:3575–3593. doi: 10.1113/jphysiol.2011.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nilius B., Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meents J.E., Fischer M.J., McNaughton P.A. Sensitization of trpa1 by protein kinase a. PLoS ONE. 2017;12:e0170097. doi: 10.1371/journal.pone.0170097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singer S.J., Nicolson G.L. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 67.Simons K., Sampaio J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011;3:a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simons K., Vaz W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 69.Lombardo D., Kiselev M.A., Magaz S., Calandra P. Amphiphiles self-assembly: Basic concepts and future perspectives of supramolecular approaches. Adv. Condens. Matter Phys. 2015;2015:22. doi: 10.1155/2015/151683. [DOI] [Google Scholar]
- 70.Garidel P., Kaconis Y., Heinbockel L., Wulf M., Gerber S., Munk A., Vill V., Brandenburg K. Self-organisation, thermotropic and lyotropic properties of glycolipids related to their biological implications. Open Biochem. J. 2015;9:49–72. doi: 10.2174/1874091X01509010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janiak M.J., Small D.M., Shipley G.G. Nature of the thermal pretransition of synthetic phospholipids: Dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976;15:4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- 72.Janiak M.J., Small D.M., Shipley G.G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J. Biol. Chem. 1979;254:6068–6078. [PubMed] [Google Scholar]
- 73.Tardieu A., Luzzati V., Reman F.C. Structure and polymorphism of the hydrocarbon chains of lipids: A study of lecithin-water phases. J. Mol. Biol. 1973;75:711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- 74.Eeman M., Deleu M. From biological membranes to biomimetic model membranes. BASE. 2010;14:719–736. [Google Scholar]
- 75.Bloom M., Evans E., Mouritsen O.G. Physical properties of the fluid lipid-bilayer component of cell membranes: A perspective. Q. Rev. Biophys. 1991;24:293–397. doi: 10.1017/S0033583500003735. [DOI] [PubMed] [Google Scholar]
- 76.Corvera E., Mouritsen O.G., Singer M.A., Zuckermann M.J. The permeability and the effect of acyl-chain length for phospholipid bilayers containing cholesterol: Theory and experiment. Biochim. Biophys. Acta (BBA) Biomembr. 1992;1107:261–270. doi: 10.1016/0005-2736(92)90413-G. [DOI] [PubMed] [Google Scholar]
- 77.Raffy S., Teissié J. Control of lipid membrane stability by cholesterol content. Biophys. J. 1999;76:2072–2080. doi: 10.1016/S0006-3495(99)77363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ciardo M.G., Ferrer-Montiel A. Lipids as central modulators of sensory trp channels. Biochim. Biophys. Acta Biomembr. 2017;1859:1615–1628. doi: 10.1016/j.bbamem.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Vaz W.L.C., Begley T.P. Wiley Encyclopedia of Chemical Biology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2007. Lipid bilayers: Properties. [Google Scholar]
- 80.Heimburg T. Thermal Biophysics of Membranes. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2007. Membrane structure. [Google Scholar]
- 81.Ambudkar I.S., Brazer S.C., Liu X., Lockwich T., Singh B. Plasma membrane localization of trpc channels: Role of caveolar lipid rafts. Nov. Found. Symp. 2004;258:63–70. discussion 70–64, 98–102, 263–106. [PubMed] [Google Scholar]
- 82.Lockwich T.P., Liu X., Singh B.B., Jadlowiec J., Weiland S., Ambudkar I.S. Assembly of trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 83.Brownlow S.L., Sage S.O. Transient receptor potential protein subunit assembly and membrane distribution in human platelets. Thromb. Haemost. 2005;94:839–845. doi: 10.1160/TH05-06-0391. [DOI] [PubMed] [Google Scholar]
- 84.Graziani A., Rosker C., Kohlwein S.D., Zhu M.X., Romanin C., Sattler W., Groschner K., Poteser M. Cellular cholesterol controls trpc3 function: Evidence from a novel dominant-negative knockdown strategy. Biochem. J. 2006;396:147–155. doi: 10.1042/BJ20051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Szoke E., Börzsei R., Tóth D.M., Lengl O., Helyes Z., Sándor Z., Szolcsányi J. Effect of lipid raft disruption on trpv1 receptor activation of trigeminal sensory neurons and transfected cell line. Eur. J. Pharmacol. 2010;628:67–74. doi: 10.1016/j.ejphar.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 86.Kumari S., Kumar A., Sardar P., Yadav M., Majhi R.K., Goswami C. Influence of membrane cholesterol in the molecular evolution and functional regulation of trpv4. Biochem. Biophys. Res. Commun. 2015;456:312–319. doi: 10.1016/j.bbrc.2014.11.077. [DOI] [PubMed] [Google Scholar]
- 87.Morenilla-Palao C., Pertusa M., Meseguer V., Cabedo H., Viana F. Lipid raft segregation modulates trpm8 channel activity. J. Biol. Chem. 2009;284:9215–9224. doi: 10.1074/jbc.M807228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu M., Huang W., Wu D., Priestley J.V. Trpv1, but not p2x, requires cholesterol for its function and membrane expression in rat nociceptors. Eur. J. Neurosci. 2006;24:1–6. doi: 10.1111/j.1460-9568.2006.04889.x. [DOI] [PubMed] [Google Scholar]
- 89.Sághy É., Szőke É., Payrits M., Helyes Z., Börzsei R., Erostyák J., Jánosi T.Z., Sétáló G., Szolcsányi J. Evidence for the role of lipid rafts and sphingomyelin in ca2+-gating of transient receptor potential channels in trigeminal sensory neurons and peripheral nerve terminals. Pharmacol. Res. 2015;100:101–116. doi: 10.1016/j.phrs.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 90.Saghy E., Payrits M., Biro-Suto T., Skoda-Foldes R., Szanti-Pinter E., Erostyak J., Makkai G., Setalo G., Jr., Kollar L., Koszegi T., et al. Carboxamido steroids inhibit the opening properties of transient receptor potential ion channels by lipid raft modulation. J. Lipid Res. 2018;59:1851–1863. doi: 10.1194/jlr.M084723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lakk M., Yarishkin O., Baumann J.M., Iuso A., Krizaj D. Cholesterol regulates polymodal sensory transduction in müller glia. Glia. 2017;65:2038–2050. doi: 10.1002/glia.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peters M., Katz B., Lev S., Zaguri R., Gutorov R., Minke B. Depletion of membrane cholesterol suppresses drosophila transient receptor potential-like (trpl) channel activity. Curr. Top. Membr. 2017;80:233–254. doi: 10.1016/bs.ctm.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 93.Naylor J., Li J., Milligan C.J., Zeng F., Sukumar P., Hou B., Sedo A., Yuldasheva N., Majeed Y., Beri D., et al. Pregnenolone sulphate- and cholesterol-regulated trpm3 channels coupled to vascular smooth muscle secretion and contraction. Circ. Res. 2010;106:1507–1515. doi: 10.1161/CIRCRESAHA.110.219329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hill K., Schaefer M. Trpa1 is differentially modulated by the amphipathic molecules trinitrophenol and chlorpromazine. J. Biol. Chem. 2007;282:7145–7153. doi: 10.1074/jbc.M609600200. [DOI] [PubMed] [Google Scholar]
- 95.Meseguer V., Alpizar Y.A., Luis E., Tajada S., Denlinger B., Fajardo O., Manenschijn J.A., Fernández-Peña C., Talavera A., Kichko T., et al. Trpa1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Startek J.B., Talavera K., Voets T., Alpizar Y.A. Differential interactions of bacterial lipopolysaccharides with lipid membranes: Implications for trpa1-mediated chemosensation. Sci. Rep. 2018;8:12010. doi: 10.1038/s41598-018-30534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McMahon H.T., Gallop J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 98.Callan-Jones A., Sorre B., Bassereau P. Curvature-Driven Lipid Sorting in Biomembranes. Cold Spring Harbor Perspectives in Biology. 2011:3. doi: 10.1101/cshperspect.a004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frolov V.A., Shnyrova A.V., Zimmerberg J. Lipid Polymorphisms and Membrane Shape. Cold Spring Harbor Perspectives in Biology. 2011:3. doi: 10.1101/cshperspect.a004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alexander C., Rietschel E.T. Invited review: Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001;7:167–202. doi: 10.1177/09680519010070030101. [DOI] [PubMed] [Google Scholar]
- 101.Raetz C.R.H., Whitfield C. Lipopolysaccharide endotoxins. Ann. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steimle A., Autenrieth I.B., Frick J.-S. Structure and function: Lipid a modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016;306:290–301. doi: 10.1016/j.ijmm.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Molinaro A., Holst O., Di Lorenzo F., Callaghan M., Nurisso A., D’Errico G., Zamyatina A., Peri F., Berisio R., Jerala R., et al. Chemistry of lipid a: At the heart of innate immunity. Chemistry. 2015;21:500–519. doi: 10.1002/chem.201403923. [DOI] [PubMed] [Google Scholar]
- 104.Wang L., Wang Q., Reeves P.R. The variation of o antigens in gram-negative bacteria. Subcell Biochem. 2010;53:123–152. doi: 10.1007/978-90-481-9078-2_6. [DOI] [PubMed] [Google Scholar]
- 105.Erridge C., Bennett-Guerrero E., Poxton I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002;4:837–851. doi: 10.1016/S1286-4579(02)01604-0. [DOI] [PubMed] [Google Scholar]
- 106.Schromm A.B., Brandenburg K., Loppnow H., Moran A.P., Koch M.H., Rietschel E.T., Seydel U. Biological activities of lipopolysaccharides are determined by the shape of their lipid a portion. Eur. J. Biochem. 2000;267:2008–2013. doi: 10.1046/j.1432-1327.2000.01204.x. [DOI] [PubMed] [Google Scholar]
- 107.Galanos C., Luderitz O., Rietschel E.T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H., et al. Synthetic and natural escherichia coli free lipid a express identical endotoxic activities. Eur. J. Biochem. 1985;148:1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- 108.Das S., Owen K.A., Ly K.T., Park D., Black S.G., Wilson J.M., Sifri C.D., Ravichandran K.S., Ernst P.B., Casanova J.E. Brain angiogenesis inhibitor 1 (bai1) is a pattern recognition receptor that mediates macrophage binding and engulfment of gram-negative bacteria. Proc. Natl. Acad. Sci. USA. 2011;108:2136–2141. doi: 10.1073/pnas.1014775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosadini C.V., Kagan J.C. Early innate immune responses to bacterial lps. Curr. Opin. Immunol. 2017;44:14–19. doi: 10.1016/j.coi.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seydel U., Oikawa M., Fukase K., Kusumoto S., Brandenburg K. Intrinsic conformation of lipid a is responsible for agonistic and antagonistic activity. Eur. J. Biochem. 2000;267:3032–3039. doi: 10.1046/j.1432-1033.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 111.Netea M.G., van Deuren M., Kullberg B.J., Cavaillon J.M., Van der Meer J.W. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002;23:135–139. doi: 10.1016/S1471-4906(01)02169-X. [DOI] [PubMed] [Google Scholar]
- 112.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 113.Boonen B., Alpizar Y.A., Meseguer V.M., Talavera K. Trp channels as sensors of bacterial endotoxins. Toxins. 2018;10:326. doi: 10.3390/toxins10080326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 115.Hagar J.A., Powell D.A., Aachoui Y., Ernst R.K., Miao E.A. Cytoplasmic lps activates caspase-11: Implications in tlr4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alpizar Y.A., Boonen B., Sanchez A., Jung C., Lopez-Requena A., Naert R., Steelant B., Luyts K., Plata C., De Vooght V., et al. Trpv4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells. Nat. Commun. 2017;8:1059. doi: 10.1038/s41467-017-01201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Soldano A., Alpizar Y.A., Boonen B., Franco L., Lopez-Requena A., Liu G., Mora N., Yaksi E., Voets T., Vennekens R., et al. Gustatory-mediated avoidance of bacterial lipopolysaccharides via trpa1 activation in drosophila. eLife. 2016;5 doi: 10.7554/eLife.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zappia K.J., O’Hara C.L., Moehring F., Kwan K.Y., Stucky C.L. Sensory neuron-specific deletion of trpa1 results in mechanical cutaneous sensory deficits. eNeuro. 2017;4 doi: 10.1523/ENEURO.0069-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ciesielski F., Davis B., Rittig M., Bonev B.B., O’Shea P. Receptor-independent interaction of bacterial lipopolysaccharide with lipid and lymphocyte membranes; the role of cholesterol. PLoS ONE. 2012;7:e38677. doi: 10.1371/journal.pone.0038677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brauckmann S., Effenberger-Neidnicht K., de Groot H., Nagel M., Mayer C., Peters J., Hartmann M. Lipopolysaccharide-induced hemolysis: Evidence for direct membrane interactions. Sci. Rep. 2016;6:35508. doi: 10.1038/srep35508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pöschl J.M., Leray C., Ruef P., Cazenave J.P., Linderkamp O. Endotoxin binding to erythrocyte membrane and erythrocyte deformability in human sepsis and in vitro. Crit. Care Med. 2003;31:924–928. doi: 10.1097/01.CCM.0000055366.24147.80. [DOI] [PubMed] [Google Scholar]
- 122.Todd J.C., Mollitt D.L. Sepsis-induced alterations in the erythrocyte membrane. Am. Surg. 1994;60:954–957. doi: 10.1097/00005373-199107000-00097. [DOI] [PubMed] [Google Scholar]
- 123.Portolés M.T., Pagani R., Díaz-Laviada I., Municio A.M. Effect of escherichia coli lipopolysaccharide on the microviscosity of liver plasma membranes and hepatocyte suspensions and monolayers. Cell Biochem. Funct. 1987;5:55–61. doi: 10.1002/cbf.290050107. [DOI] [PubMed] [Google Scholar]
- 124.Nagel M., Brauckmann S., Moegle-Hofacker F., Effenberger-Neidnicht K., Hartmann M., de Groot H., Mayer C. Impact of bacterial endotoxin on the structure of dmpc membranes. Biochim. Biophys. Acta. 2015;1848:2271–2276. doi: 10.1016/j.bbamem.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 125.Tanamoto K.-I., Azumi S. Salmonella-type heptaacylated lipid a is inactive and acts as an antagonist of lipopolysaccharide action on human line cells. J. Immunol. 2000;164:3149–3156. doi: 10.4049/jimmunol.164.6.3149. [DOI] [PubMed] [Google Scholar]
- 126.Paulsen C.E., Armache J.P., Gao Y., Cheng Y., Julius D. Structure of the trpa1 ion channel suggests regulatory mechanisms. Nature. 2015;525:552–557. doi: 10.1038/nature14871. [DOI] [PubMed] [Google Scholar]
- 127.Lee A.G. Lipid-protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta. 2003;1612:1–40. doi: 10.1016/S0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 128.Hurley J.C. Endotoxemia: Methods of detection and clinical correlates. Clin. Microbiol. Rev. 1995;8:268–292. doi: 10.1128/CMR.8.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacinto R.C., Gomes B.P., Shah H.N., Ferraz C.C., Zaia A.A., Souza-Filho F.J. Quantification of endotoxins in necrotic root canals from symptomatic and asymptomatic teeth. J. Med. Microbiol. 2005;54:777–783. doi: 10.1099/jmm.0.45976-0. [DOI] [PubMed] [Google Scholar]
- 130.Kanaan S.A., Saadé N.E., Haddad J.J., Abdelnoor A.M., Atweh S.F., Jabbur S.J., Safieh-Garabedian B. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: A new model for inflammatory pain. Pain. 1996;66:373–379. doi: 10.1016/0304-3959(96)03068-0. [DOI] [PubMed] [Google Scholar]
- 131.Okamoto T., Gohil K., Finkelstein E.I., Bove P., Akaike T., van der Vliet A. Multiple contributing roles for nos2 in lps-induced acute airway inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L198–L209. doi: 10.1152/ajplung.00136.2003. [DOI] [PubMed] [Google Scholar]
- 132.Speyer C.L., Neff T.A., Warner R.L., Guo R.F., Sarma J.V., Riedemann N.C., Murphy M.E., Murphy H.S., Ward P.A. Regulatory effects of inos on acute lung inflammatory responses in mice. Am. J. Pathol. 2003;163:2319–2328. doi: 10.1016/S0002-9440(10)63588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schuijs M.J., Willart M.A., Vergote K., Gras D., Deswarte K., Ege M.J., Madeira F.B., Beyaert R., van Loo G., Bracher F., et al. Farm dust and endotoxin protect against allergy through a20 induction in lung epithelial cells. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 134.Boonen B., Alpizar Y.A., Sanchez A., Lopez-Requena A., Voets T., Talavera K. Differential effects of lipopolysaccharide on mouse sensory trp channels. Cell Calcium. 2018;73:72–81. doi: 10.1016/j.ceca.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 135.Sachs F. Stretch-activated ion channels: What are they? Physiology (Bethesda) 2010;25:50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Parasassi T., Krasnowska E.K., Bagatolli L., Gratton E. Laurdan and prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 1998;8:365–373. doi: 10.1023/A:1020528716621. [DOI] [Google Scholar]
- 137.Parasassi T., Gratton E. Membrane lipid domains and dynamics as detected by laurdan fluorescence. J Fluoresc. 1995;5:59–69. doi: 10.1007/BF00718783. [DOI] [PubMed] [Google Scholar]
- 138.Parasassi T., Ravagnan G., Rusch R.M., Gratton E. Modulation and dynamics of phase properties in phospholipid mixtures detected by laurdan fluorescence. Photochem. Photobiol. 1993;57:403–410. doi: 10.1111/j.1751-1097.1993.tb02309.x. [DOI] [PubMed] [Google Scholar]
- 139.Friedlander G., Le Grimellec C., Giocondi M.C., Amiel C. Benzyl alcohol increases membrane fluidity and modulates cyclic AMP synthesis in intact renal epithelial cells. Biochim. Biophys. Acta. 1987;903:341–348. doi: 10.1016/0005-2736(87)90224-0. [DOI] [PubMed] [Google Scholar]
- 140.Scholz A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br. J. Anaesth. 2002;89:52–61. doi: 10.1093/bja/aef163. [DOI] [PubMed] [Google Scholar]
- 141.Ragsdale D.S., McPhee J.C., Scheuer T., Catterall W.A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated na+ channels. Proc. Natl. Acad. Sci. USA. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsuchiya H., Mizogami M. Interaction of local anesthetics with biomembranes consisting of phospholipids and cholesterol: Mechanistic and clinical implications for anesthetic and cardiotoxic effects. Anesthesiol. Res. Pract. 2013;2013:297141. doi: 10.1155/2013/297141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sugahara K., Shimokawa N., Takagi M. Thermal stability of phase-separated domains in multicomponent lipid membranes with local anesthetics. Membranes. 2017;7:33. doi: 10.3390/membranes7030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yun I., Cho E.-S., Jang H.-O., Kim U.-K., Choi C.-H., Chung I.-K., Kim I.-S., Wood W.G. Amphiphilic effects of local anesthetics on rotational mobility in neuronal and model membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2002;1564:123–132. doi: 10.1016/S0005-2736(02)00409-1. [DOI] [PubMed] [Google Scholar]
- 145.Pristerà A., Baker M.D., Okuse K. Association between tetrodotoxin resistant channels and lipid rafts regulates sensory neuron excitability. PLoS ONE. 2012;7:e40079. doi: 10.1371/journal.pone.0040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Catterall W.A. Structure and function of voltage-gated sodium channels at atomic resolution. Exp. Physiol. 2014;99 doi: 10.1113/expphysiol.2013.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sugahara K., Shimokawa N., Takagi M. Destabilization of phase-separated structures in local anesthetic-containing model biomembranes. Chem. Lett. 2015;44:1604–1606. doi: 10.1246/cl.150636. [DOI] [Google Scholar]
- 148.Strichartz G.R., Sanchez V., Arthur G.R., Chafetz R., Martin D. Fundamental properties of local anesthetics. Ii. Measured octanol:Buffer partition coefficients and pka values of clinically used drugs. Anesth. Analg. 1990;71:158–170. doi: 10.1213/00000539-199008000-00008. [DOI] [PubMed] [Google Scholar]
- 149.Gray E., Karslake J., Machta B.B., Veatch S.L. Liquid general anesthetics lower critical temperatures in plasma membrane vesicles. Biophys. J. 2013;105:2751–2759. doi: 10.1016/j.bpj.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leffler A., Lattrell A., Kronewald S., Niedermirtl F., Nau C. Activation of trpa1 by membrane permeable local anesthetics. Mol. Pain. 2011;7:62. doi: 10.1186/1744-8069-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leffler A., Fischer M.J., Rehner D., Kienel S., Kistner K., Sauer S.K., Gavva N.R., Reeh P.W., Nau C. The vanilloid receptor trpv1 is activated and sensitized by local anesthetics in rodent sensory neurons. J. Clin. Investig. 2008;118:763–776. doi: 10.1172/JCI32751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Eberhardt M., Stueber T., de la Roche J., Herzog C., Leffler A., Reeh P.W., Kistner K. Trpa1 and trpv1 are required for lidocaine-evoked calcium influx and neuropeptide release but not cytotoxicity in mouse sensory neurons. PLoS ONE. 2017;12:e0188008. doi: 10.1371/journal.pone.0188008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Butterworth J.F.T. Models and mechanisms of local anesthetic cardiac toxicity: A review. Reg. Anesth. Pain Med. 2010;35:167–176. doi: 10.1097/AAP.0b013e3181d231b9. [DOI] [PubMed] [Google Scholar]
- 154.Xiao B., Dubin A.E., Bursulaya B., Viswanath V., Jegla T.J., Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian trpa1 channels. J. Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matta J.A., Cornett P.M., Miyares R.L., Abe K., Sahibzada N., Ahern G.P. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc. Natl. Acad. Sci. USA. 2008;105:8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cometto-Muniz J.E., Cain W.S. Relative sensitivity of the ocular trigeminal, nasal trigeminal and olfactory systems to airborne chemicals. Chem. Senses. 1995;20:191–198. doi: 10.1093/chemse/20.2.191. [DOI] [PubMed] [Google Scholar]
- 157.Sato A., Obata K., Ikeda Y., Ohkoshi K., Okumura H., Ozawa N., Ogawa T., Katsumura Y., Kawai J., Tatsumi H., et al. Evaluation of human skin irritation by carboxylic acids, alcohols, esters and aldehydes, with nitrocellulose-replica method and closed patch testing. Contact Dermat. 1996;34:12–16. doi: 10.1111/j.1600-0536.1996.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 158.Komatsu T., Uchida K., Fujita F., Zhou Y., Tominaga M. Primary alcohols activate human trpa1 channel in a carbon chain length-dependent manner. Pflügers Arch. Eur. J. Physiol. 2012;463:549–559. doi: 10.1007/s00424-011-1069-4. [DOI] [PubMed] [Google Scholar]
- 159.Morita A., Iwasaki Y., Kobata K., Iida T., Higashi T., Oda K., Suzuki A., Narukawa M., Sasakuma S., Yokogoshi H., et al. Lipophilicity of capsaicinoids and capsinoids influences the multiple activation process of rat trpv1. Life Sci. 2006;79:2303–2310. doi: 10.1016/j.lfs.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 160.Ursu D., Knopp K., Beattie R.E., Liu B., Sher E. Pungency of trpv1 agonists is directly correlated with kinetics of receptor activation and lipophilicity. Eur. J. Pharmacol. 2010;641:114–122. doi: 10.1016/j.ejphar.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 161.Lee S.P., Buber M.T., Yang Q., Cerne R., Cortés R.Y., Sprous D.G., Bryant R.W. Thymol and related alkyl phenols activate the htrpa1 channel. Br. J. Pharmacol. 2008;153:1739–1749. doi: 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fujita F., Moriyama T., Higashi T., Shima A., Tominaga M. Methyl p-hydroxybenzoate causes pain sensation through activation of trpa1 channels. Br. J. Pharmacol. 2007;151:153–160. doi: 10.1038/sj.bjp.0707219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maher M., Ao H., Banke T., Nasser N., Wu N.T., Breitenbucher J.G., Chaplan S.R., Wickenden A.D. Activation of trpa1 by farnesyl thiosalicylic acid. Mol. Pharmacol. 2008;73:1225–1234. doi: 10.1124/mol.107.042663. [DOI] [PubMed] [Google Scholar]
- 164.Morera E., De Petrocellis L., Morera L., Moriello A.S., Nalli M., Di Marzo V., Ortar G. Synthesis and biological evaluation of [6]-gingerol analogues as transient receptor potential channel trpv1 and trpa1 modulators. Bioorg. Med. Chem. Lett. 2012;22:1674–1677. doi: 10.1016/j.bmcl.2011.12.113. [DOI] [PubMed] [Google Scholar]
- 165.Riera C.E., Menozzi-Smarrito C., Affolter M., Michlig S., Munari C., Robert F., Vogel H., Simon S.A., le Coutre J. Compounds from sichuan and melegueta peppers activate, covalently and non-covalently, trpa1 and trpv1 channels. Br. J. Pharmacol. 2009;157:1398–1409. doi: 10.1111/j.1476-5381.2009.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chianese G., Fattorusso E., Putra M.Y., Calcinai B., Bavestrello G., Moriello A.S., De Petrocellis L., Di Marzo V., Taglialatela-Scafati O. Leucettamols, bifunctionalized marine sphingoids, act as modulators of trpa1 and trpm8 channels. Mar. Drugs. 2012;10:2435–2447. doi: 10.3390/md10112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mazzella N., Syakti A.D., Molinet J., Gilewicz M., Doumenq P., Artaud J., Bertrand J.C. Effects of crude oil on phospholipid fatty acid compositions of marine hydrocarbon degraders: Estimation of the bacterial membrane fluidity. Environ. Res. 2005;97:300–311. doi: 10.1016/j.envres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 168.Gonzalez A., Odjélé A., Weber J.-M. Pcb-153 and temperature cause restructuring of goldfish membranes: Homeoviscous response to a chemical fluidiser. Aquat. Toxicol. 2013;144–145:11–18. doi: 10.1016/j.aquatox.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 169.Wieslander A., Rilfors L., Lindblom G. Metabolic changes of membrane lipid composition in acholeplasma laidlawii by hydrocarbons, alcohols, and detergents: Arguments for effects on lipid packing. Biochemistry. 1986;25:7511–7517. doi: 10.1021/bi00371a038. [DOI] [PubMed] [Google Scholar]
- 170.Davy De Virville J., Cantrel C., Bousquet A.L., Hoffelt M., Tenreiro A.M., Vaz Pinto V., Arrabaça J.D., Caiveau O., Moreau F., Zachowski A. Homeoviscous and functional adaptations of mitochondrial membranes to growth temperature in soybean seedlings. Plant Cell Environ. 2002;25:1289–1297. doi: 10.1046/j.1365-3040.2002.00901.x. [DOI] [Google Scholar]
- 171.Guschina I.A., Harwood J.L. Mechanisms of temperature adaptation in poikilotherms. FEBS Lett. 2006;580:5477–5483. doi: 10.1016/j.febslet.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 172.Oger P.M., Cario A. Adaptation of the membrane in archaea. Biophys. Chem. 2013;183:42–56. doi: 10.1016/j.bpc.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 173.Gladyshev M.I., Semenchenko V.P., Dubovskaya O.P., Fefilova E.B., Makhutova O.N., Buseva Z.F., Sushchik N.N., Razlutskij V.I., Lepskaya E.V., Baturina M.A., et al. Effect of temperature on contents of essential highly unsaturated fatty acids in freshwater zooplankton. Limnologica. 2011;41:339–347. doi: 10.1016/j.limno.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 174.Behan M.K., Macdonald A.G., Jones G.R., Cossins A.R. Homeoviscous adaptation under pressure: The pressure dependence of membrane order in brain myelin membranes of deep-sea fish. Biochim. Biophys. Acta (BBA) Biomembr. 1992;1103:317–323. doi: 10.1016/0005-2736(92)90102-R. [DOI] [PubMed] [Google Scholar]
- 175.Behan-Martin M.K., Jones G.R., Bowler K., Cossins A.R. A near perfect temperature adaptation of bilayer order in vertebrate brain membranes. Biochim. Biophys. Acta (BBA) Biomembr. 1993;1151:216–222. doi: 10.1016/0005-2736(93)90106-A. [DOI] [PubMed] [Google Scholar]
- 176.Cossins A.R., Prosser C.L. Evolutionary adaptation of membranes to temperature. Proc. Natl. Acad. Sci. USA. 1978;75:2040–2043. doi: 10.1073/pnas.75.4.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Anderson R.L., Minton K.W., Li G.C., Hahn G.M. Temperature-induced homeoviscous adaptation of chinese hamster ovary cells. Biochim. Biophys. Acta (BBA) Biomembr. 1981;641:334–348. doi: 10.1016/0005-2736(81)90490-9. [DOI] [PubMed] [Google Scholar]
- 178.Dymond Marcus K. Mammalian phospholipid homeostasis: Evidence that membrane curvature elastic stress drives homeoviscous adaptation in vivo. J. R. Soc. Interface. 2016;13:20160228. doi: 10.1098/rsif.2016.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Laroche C., Beney L., Marechal P., Gervais P. The effect of osmotic pressure on the membrane fluidity of saccharomyces cerevisiae at different physiological temperatures. Appl. Microbiol. Biotechnol. 2001;56:249–254. doi: 10.1007/s002530000583. [DOI] [PubMed] [Google Scholar]
- 180.Santoro N., Lisi A., Pozzi D., Pasquali E., Serafino A., Grimaldi S. Effect of extremely low frequency (elf) magnetic field exposure on morphological and biophysical properties of human lymphoid cell line (raji) Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1997;1357:281–290. doi: 10.1016/S0167-4889(97)00032-3. [DOI] [PubMed] [Google Scholar]
- 181.Balogh G., Horváth I., Nagy E., Hoyk Z., Benkõ S., Bensaude O., Vígh L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 182.Balogh G., Maulucci G., Gombos I., Horváth I., Török Z., Péter M., Fodor E., Páli T., Benko S., Parasassi T., et al. Heat stress causes spatially-distinct membrane re-modelling in k562 leukemia cells. PLoS ONE. 2011;6:e21182. doi: 10.1371/journal.pone.0021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Balogh G., Péter M., Glatz A., Gombos I., Török Z., Horváth I., Harwood J.L., Vígh L. Key role of lipids in heat stress management. FEBS Lett. 2013;587:1970–1980. doi: 10.1016/j.febslet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 184.Baenke F., Peck B., Miess H., Schulze A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Di Paolo G., Kim T.-W. Linking lipids to alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li Z., Vance D.E. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 187.Li Z., Agellon L.B., Vance D.E. Phosphatidylcholine homeostasis and liver failure. J. Biol. Chem. 2005;280:37798–37802. doi: 10.1074/jbc.M508575200. [DOI] [PubMed] [Google Scholar]
- 188.Rob N.M.W. Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Curr. Diabetes Rev. 2012;8:390–400. doi: 10.2174/157339912802083531. [DOI] [PMC free article] [PubMed] [Google Scholar]