Abstract
Background:
Tax-1 protein of Human T-cell Leukemia Virus type 1(HTLV-1) serves as a key transcriptional regulatory gene product and has a crucial role in transactivating genes of infected cells by employing their transcriptional factors. This modulation includes induction of genes which encode CC-chemokines and their receptors. In this study, a recombinant vector containing Tax-1 gene was made and tested for its ability to induce CCR5 (CC chemokine receptor 5) expression in K562 cell line.
Methods:
In order to perform this research, two blood samples of HTLV-1 positive were obtained from Urmia blood transfusion center. After DNA extraction, a complete sequence of Tax-1 gene was amplified by specific primers. Recombinant vectors carrying Tax-1 gene were synthesized and transformed into Escherichia coli (E. coli). After bacteria transformation, bacteria containing recombinant plasmid were selected and purified. Then, the recombinant shuttle vectors, pCDNA3.1-TAX, were transfected into the cell culture (K562 cell line). Expression of CCR5 was measured after 72 hr by Syber Green Real-Time PCR method compared to control cell culture. Normalization was done with GAPDH as a standard gene.
Results:
Cloning of Tax-1 gene in the vector, pCDNA3.1 was confirmed by colony PCR, restriction digestion, and sequencing methods. Expression of Tax-1 and CCR5 genes were confirmed by real time PCR and also, expression of CCR5 gene showed an 8-fold increase in comparison to mock-treated controls (p<0.05).
Conclusion:
Our data suggested that recombinant Tax-1 may have the enhancing effect on CCR5 expression rate at mRNA levels in K562 cell line. Further studies are necessary to evaluate the effect of pCDNA3.1-TAX on cell surface CCR5 expression.
Keywords: CCR5, Human T-cell Leukemia Virus, Tax-1 protein
Introduction
Human T-cell Leukemia Virus (HTLV-1) is the first discovered human retrovirus in 1980. It was isolated from a patient with cutaneous T-cell lymphoma 1. Worldwide distribution of infected individuals has suggested that 15 to 20 million people are infected with HTLV 2. Africa, southern Japan, Caribbean, Central and South America and the Middle East are the endemic areas for this infection. It is the etiological agent of Adult T-cell Leukemia/Lymphoma (ATL) and HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) 3. However, only about 1–5% of infected patients are thought to develop cancer as a result of the infection with HTLV-I over their lifetimes 2. Infection is possible through sexual contact, transfusion of contaminated blood products, and from mother to child via breastfeeding 4. Entry of HTLV-1 into the CD4+ T-cells occurs through a ubiquitous cell surface transporter called Glucose Transporter 1 (GLUT1) 5, which is present on a wide range of cell types; however, they have a preferential tropism for CD4+ T-cells 6.
HTLV-1 belongs to retroviridea family and therefore, shares similar transmission modes with the other member, HIV-1, thus, both can be found in a host in endemic areas 7. Human retroviruses have the worldwide distribution but because of the lack of HTLV screening in most of the countries, its coinfection rate with HIV-1 has been underestimated. HTLV-1 and HIV-1 both infect CD4+ T-cells but have different outcomes on the same cell type. In spite of the long-lasting history of investigation of HTLV-1/HIV-1 coinfection, whether HTLV-1 can accelerate or attenuate HIV-1 infection has not been completely clarified as yet. Studies of clinical outcomes of the coinfection show conflicting results 8. What accounts for most of HTLV-1 influence on a host cell is Tax-1. Tax-1 is the oncogenic transactivator agent of HTLV-1 which causes several changes to an infected cell by modulating the transcription factors and pathways and shifting the mechanisms in favor of the virus replication 9. Furthermore, an important role was suggested for Tax-1 in a clinical and immunological manifestation of the HIV/HTLV coinfection 10. Tax-1 may have the capability to increase CCR5 expression in the Tax-1 transfected cell line.
Previous studies on this subject have had conflicting results of the effect of Tax-1 on CCR5 expression 11,12. In this study, the effect of Tax-1 on CCR5 expression in K562 cell line was investigated.
Materials and Methods
DNA purification and amplification
In order to isolate genomic DNA, two blood samples of HTLV-1 positive were obtained from Urmia blood transfusion center. DNA extraction was performed according to the method of extraction kit protocol (Yekta Tajhiz, Iran). Specific primers were designed using NCBI/Primer-Blast for the complete length of Tax-1 gene in the NCBI nucleotide database as follows: A forward primer containing a restriction enzyme site of HindIII 5’ATAAGCTTATGGCCCACTT CCCAGGGTT3’ and reverse primer containing a restriction site of EcoR1 5’TAGAATTCTCATCATCTG CCTCTTTTTCGTT3’ with an amplicon size of 890 base pairs. The PCR amplification kit (TaKaRa bio kit, USA) is used in a total volume of 50 μl containing 25 μl of the master mix, 1 μl of isolated DNA and 1 μl of each primer (10 μM). PCR condition included a primary denaturation step of 5 min at 95°C followed by 35 cycles of 45 s at 95°C, 30 s at 58°C, and 45 s at 72°C and a final extension step at 72°C for 10 min using Palm cycler (Corbett, USA).
Recombinant DNA construction
In order to construct the recombinant DNA, Pc-DNA3.1 plasmid was used as the vector. The PcDNA-3.1 plasmid was purified using a plasmid DNA miniprep system (Viogene, USA) from the bacterial culture containing the plasmids stored in glycerol stock according to the supplier's instructions. PCR products and the vectors were digested by EcoR1 and Hind III producing sticky ends and ligation was done by T4 ligase overnight to yield in the recombinant PcDNA3.1-Tax-1 plasmid. In order to make competent Escherichia coli (E. coli), the TG1 bacterial culture was treated with CaCl2 100 mM solution. Transformation of the TG1 culture with the recombinant vector was done with heat shock method. For confirmation of the recombinant PcDNA3.1-Tax-1 plasmid, colony PCR was done to evaluate cloning. The accuracy of the recombinant Pc-DNA3.1-Tax-1 plasmid was evaluated by DNA sequencing.
Cell line transfection
K562 cell line was used as a myeloid cell line competent of producing CCR5 naturally. Cells were cultured in an RPMI medium with 10% FBS and 1% penicillin/streptomycin. Transfection was done with Pc-DNA3.1-Tax-1 plasmids using XtremeGene Transfection kit (Roche, Germany) according to the manufacturer’s protocol. As a control, K562s were transfected with PcDNA3.1 without the insert.
Total RNA extraction and cDNA synthesis
Total RNA was extracted with RNA extraction kit (Yekta Tajhiz Azma, Iran) from cell culture after 72 hr of transfection. cDNAs were generated using cDNA synthesize kit (Invitrogen, USA) according to the manufacturer’s protocol.
Quantitative real-time PCR
To indicate the level of the Tax-1 and CCR5 genes expression, quantitative real-time PCR was performed on cDNA samples using previously mentioned Tax-1 primers and CCR5 specific primers which are as follows: Forward primer: 5′CAAAAAGAAGGTCTTCA TTACACC3′ and reverse primer: 5′CCTGTGCCTCT TCTTCTCATTTCG3′ 13. In order to determine the optimal annealing temperature, thermal gradient series of PCR were performed and 58°C was determined as the proper annealing temperature for both CCR5 and Tax-1 genes. Quantitative real time PCR was performed using Syber Green qPCR master mix (Ampliqon, Denmark) with real-time PCR (Rotor-Gene, Q USA). PCR condition was a primary denaturation and enzyme activation step of 1 cycle of 15 min at 95°C which was followed by 40 cycles of amplification including a denaturation step of 45 s at 95°C, a 30 s at 58°C for annealing and a 45 s at 72°C for extension and a final cooling step of 30 s at 40°C. In order to control the specificity of the amplification products, a melting curve was generated with a temperature ramp from 60°C to 95°C at a rate of 0.01°C/s. Normalization was done with the internal control, GAPDH gene. Relative expression of each gene was calculated with the comparative ΔΔCt method 14, in which ΔCt=Ct (Tax-1 or CCR5)–Ct (GAPDH). The ΔΔCt method was used to find the difference between the mean value of the ΔCt of the transfected group and the mean value of the ΔCt of the control group (ΔΔCt = ΔCTtransfected - ΔCtcontrol) for each analyzed molecule. Fold changes in the expression of target mRNA was calculated as 2−ΔΔCt. PCR efficiency for the gene of interest and for internal control must be similar which allows us to use the comparative Ct method. To determine the efficiency, tenfold dilution series were prepared for Tax-1 and CCR5 cDNAs and after real time PCR, a standard curve was established by plotting the logarithm of copy numbers in dilutions against the ΔCt values. The difference in PCR efficiency was determined by calculating the slope of the line.
Results
Amplification of the Tax-1 gene
PCR products were electrophoresed on gel agarose and according to the 100 bp DNA ladder, an 890-bp PCR fragment was amplified (Figure 1).
Figure 1.
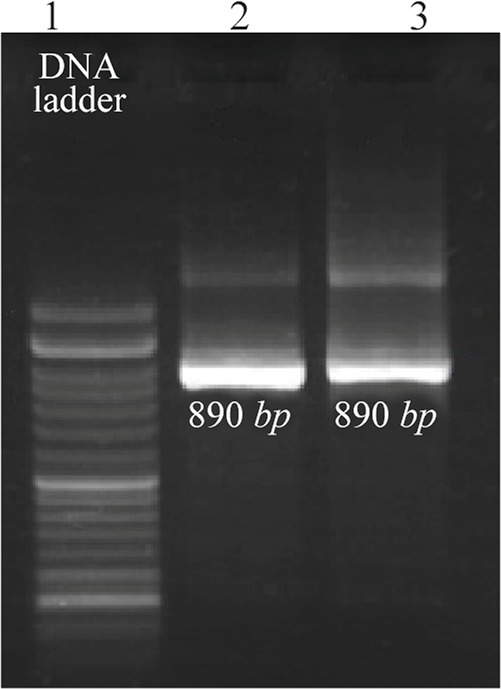
Agarose gel electrophoresis of PCR products of HTLV-1 positive samples. Line 1,100 bp DNA ladder. Lines 2 and 3, 890-bp PCR fragments of amplified Tax-1 gene.
Confirmation of recombinant vector PcDNA3.1-Tax-1
After colony PCR, products were electrophoresed on gel agarose and a fragment of about 890 base pairs was amplified using Tax-specific primers (Figure 2).
Figure 2.
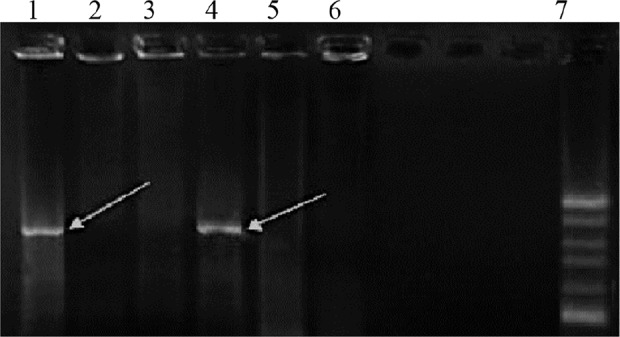
Agarose gel electrophoresis of colony PCR results of the selected clones containing recombinant plasmid PcDNA3.1-Tax-1. Lines 1 and 5, PCR products of the recombinant plasmid containing Tax-1 genes with an 890-bp fragment. Lines 2, 3 and 6, PCR products of plasmid PcDNA3.1 without the insert. Line 7,100 bp DNA ladder.
Also, the recombinant plasmid PcDNA3.1-Tax-1 was digested with EcoR1 and Hind III restriction enzymes that resulted in two fragments PcDNA3.1, 5428-bp, and Tax-1, 890-bp (Figure 3).
Figure 3.
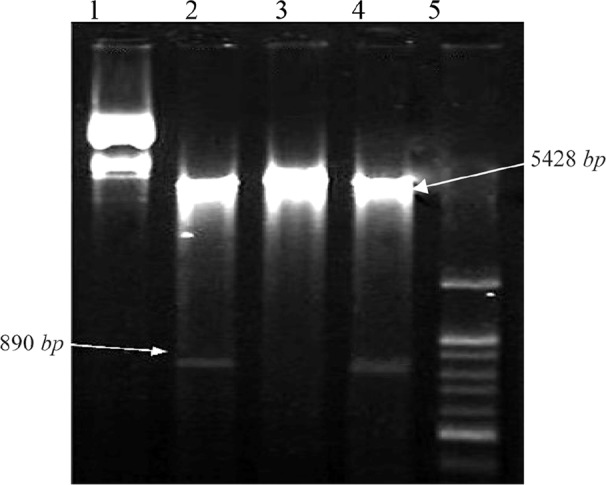
Agarose gel electrophoresis of double digestion of recombinant plasmid PcDNA3.1-Tax-1 containing Tax-1 gene. Lines 1 and 3, undigested recombinant plasmid PcDNA3.1-Tax-1, Lines 2 and 4, recombinant plasmid PcDNA3.1-Tax-1 that was digested with HindIII and EcoR1enzymes with two fragments (PcDNA3.1, 5428-bp, and Tax-1, 890-bp). Line 5, 100 bp DNA ladder.
The result of DNA sequencing showed that this isolate had 99% similarity with Tax-1 gene of the HTLV-1 virus with the accession number KF797883.1.
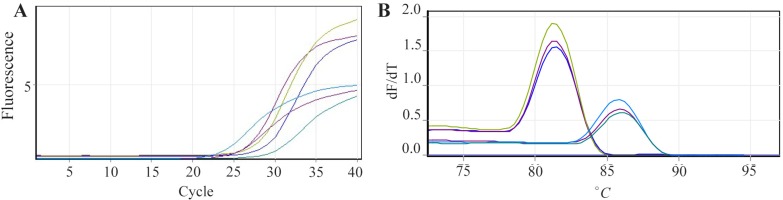
Analyzing real time PCR results after 72 hr of transfection with recombinant plasmid PcDNA3.1-Tax-1 indicated the expression of Tax-1 gene in comparison to the control group (p<0.001). After confirmation of Tax-1 gene expression in transfected cell line K562, samples were subjected to quantitative real time PCR to determine the effect of Tax-1 on CCR5 expression (Figure 4). According to the relative expression results which were analyzed by a Relative Expression Software Tool (REST) and ΔΔCt method, there is an over-expression rate of 8 fold in CCR5 expression in comparison to mock-treated group (p<0.001).
Figure 4.
A) Amplification curves and; B) melt curve analysis of CCR5 and GAPDH genes as target and control genes, respectively revealed melting peaks at 86°C for CCR5 gene and 83°C for GAPDH gene and the representative amplification with gene specific primers.
Discussion
HTLV-1 is one of the viruses with an incubation period of 10 to 40 years and in most of the patients, it has no manifestation during their lifetime, thus, patients in endemic areas with high risk behaviors are prone to coinfections with HIV-1 10. Although there has been a significant number of investigations on HTLV-1 infection throughout more than a quarter of a century, HTLV-1 significance in coinfection with HIV-1 has been underestimated due to lack of screening in most of the endemic areas 10. There have been reports of both enhancement and attenuation of HIV-1 replication in cases of coinfections with HTLV-1 15–20. Presence of HTLV-1 in the host can increase the chance of HIV-1 to get into the cells and therefore increasing the chance of HIV-1 infection. HTLV-1 has a transactivator agent named Tax-1, which can cause a series of transformations into the host cell. One of the modifications which is of importance in HTLV-1/HIV-1 coinfection is the modulation of CCR5 expression rate which is one of the co-receptors helping HIV-1’s entry into the cells 21.
There have been a few publications about the effect of Tax-1 gene on CCR5 expression rate. In a study, it was shown that a higher percentage of classical monocytes subset (CD14+ and CD16−) expressed chemokine receptor CCR5 among infected individuals with HT-LV-1 in comparison to uninfected individuals 22.
In another study, when healthy PBMC cultures were treated with different doses of Tax-1 protein, there was an increase in CC-chemokines such as CCL3, CCL4, and CCL5 and a reduction in the percentage of CCR5-positive cells compared to mock-treated lymphocytes which were evaluated by flow cytometry 11.
Studies showed that Tax1 protein is a potent trans-activator that regulates the expression of several cellular genes involved in cellular activation, proliferation, and transformation. For example, Tax-1 has been shown to be a relatively weak activator of the CXCR4 promoter 23. Also, in Twizere et al’s study, it was reported that Tax-1 would induce the expression of several proteins, including cytokines and chemokines after infection of T-cell by HTLV-1. But Tax-1 expression in primary T lymphocytes did not result in altered cell surface expression of CXCR4 in comparison with control cells and they demonstrated that Tax-1 could modify the binding properties of the CXCR4 chemokine receptor and induce CXCR4 receptor activation 24.
A possible explanation for the discrepancy of this study with previous studies can be due to evaluation of CCR5 expression rate by real time PCR which is an accurate and sensitive method for quantifying gene transcription. In this research, the expression of CCR5 gene was calculated at a transcriptional level without measuring cell surface CCR5 expression and the possible interaction with others proteins such as CC-chemokines or quantifying CC-chemokines expression.
The CCR5 expression is regulated at three levels: (a) genetic factors; (b) factors involved in activation, signaling, and trafficking of the receptors and; (c) environmental or other triggers 25. The CCR5 expression is mainly affected by the binding of its ligand; however, studying the direct effect of Tax-1 protein as a trans-activator on the CCR5 promoter and expression rate can be useful. It is reported that CCR5 expression rate increased autocrine effect of MIP1β expression which was induced by mitogens in a tax-1 transfected cell line 12. Furthermore, the rate of expression of the recombinant PcDNA3.1-Tax-1 plasmid and required time for its uptake by K562 cell line should be considered.
Conclusion
Our data suggested that recombinant Tax-1 may have the enhancing effect on CCR5 expression rate at mRNA levels in K562 cell line. Further studies are necessary to evaluate the effect of pCDNA3.1-TAX on cell surface CCR5 expression.
Acknowledgement
This study has been fulfilled with the financial support of the High Institute for Research and Education in Transfusion Medicine, Blood Transfusion Research Center. We gratefully acknowledge the advice and technical support of the staff of Blood Transfusion Research Centre who contributed to this work.
Footnotes
Conflict of Interest
None of the authors have any conflicts of interest to declare.
References
- 1.Gallo RC. The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology 2005;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 2012; 3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giam CZ, Jeang KT. HTLV-1 Tax and adult T-cell leukemia. Front Biosci 2007;12:1496–1507. [DOI] [PubMed] [Google Scholar]
- 4.Clark J, Saxinger C, Gibbs WN, Lofters W, Lagranade L, Deceulaer K, et al. Seroepidemiologic studies of human T-cell leukemia/lymphoma virus type I in Jamaica. Int J Cancer 1985;36(1):37–41. [DOI] [PubMed] [Google Scholar]
- 5.Yasunaga J, Matsuoka M. Molecular mechanisms of HTLV-1 infection and pathogenesis. Int J Hematol 2011; 94(5):435–442. [DOI] [PubMed] [Google Scholar]
- 6.Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis 2007;7(4):266–281. [DOI] [PubMed] [Google Scholar]
- 7.Pilotti E, Bianchi MV, De Maria A, Bozzano F, Romanelli MG, Bertazzoni U, et al. HTLV-1/-2 and HIV-1 co-infections: retroviral interference on host immune status. Front Microbiol 2013;4:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casoli C, Pilotti E, Bertazzoni U. Molecular and cellular interactions of HIV-1/HTLV coinfection and impact on AIDS progression. AIDS Rev 2007;9(3):140–149. [PubMed] [Google Scholar]
- 9.Grassmann R, Aboud M, Jeang K-T. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 2005;24(39):5976–5985. [DOI] [PubMed] [Google Scholar]
- 10.Beilke MA. Retroviral coinfections: HIV and HTLV: taking stock of more than a quarter century of research. AIDS Res Hum Retroviruses 2012;28(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrios CS, Abuerreish M, Lairmore MD, Castillo L, Giam CZ, Beilke MA. Recombinant human T-cell leukemia virus types 1 and 2 Tax proteins induce high levels of CC-chemokines and downregulate CCR5 in human peripheral blood mononuclear cells. Viral Immunol 2011;24(6):429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma V, Lorey SL. Autocrine role of macrophage inflammatory protein-1 beta in human T-cell lymphotropic virus type-I tax-transfected Jurkat T-cells. Biochem Biophys Res Commun 2001;287(4):910–913. [DOI] [PubMed] [Google Scholar]
- 13.Woitas RP, Ahlenstiel G, Iwan A, Rockstroh JK, Brackmann HH, Kupfer B, et al. Frequency of the HIV-protective CC chemokine receptor 5-delta32/delta32 genotype is increased in hepatitis C. Gastroenterology 2002; 122(7):1721–1728. [DOI] [PubMed] [Google Scholar]
- 14.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brites C, Alencar R, Gusmao R, Pedroso C, Netto EM, Pedral-Sampaio D, et al. Co-infection with HTLV-1 is associated with a shorter survival time for HIV-1-infected patients in Bahia, Brazil. AIDS 2001;15(15):2053–2055. [DOI] [PubMed] [Google Scholar]
- 16.Pedroso C, Netto EM, Weyll N, Brites C. Coinfection by HIV-1 and human lymphotropic virus type 1 in Brazilian children is strongly associated with a shorter survival time. J Acquir Immune Defic Syndr 2011;57 Suppl 3: S208–211. [DOI] [PubMed] [Google Scholar]
- 17.Tulius Silva M, de Melo Espindola O, Bezerra Leite AC, Araujo A. Neurological aspects of HIV/human T lymphotropic virus coinfection. AIDS Rev 2009;11(2):71–78. [PubMed] [Google Scholar]
- 18.Beilke MA, Theall KP, O'Brien M, Clayton JL, Benjamin SM, Winsor EL, et al. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin Infect Dis 2004;39(2):256–263. [DOI] [PubMed] [Google Scholar]
- 19.Chavance M, Neisson-Vernant C, Quist D, Monplaisir N, Armengaud B, Chout R. HIV/HTLV-I coinfection and clinical grade at diagnosis. J Acquir Immune Defic Syndr Hum Retrovirol 1995;8(1):91–95. [PubMed] [Google Scholar]
- 20.Shibata D, Brynes RK, Rabinowitz A, Hanson CA, Slovak ML, Spira TJ, et al. Human T-cell lymphotropic virus type I (HTLV-I)-associated adult T-cell leukemia-lymphoma in a patient infected with human immunodeficiency virus type 1 (HIV-1). Ann Intern Med 1989;111 (11):871–875. [DOI] [PubMed] [Google Scholar]
- 21.Lasky LA, Nakamura G, Smith DH, Fennie C, Shimasaki C, Patzer E, et al. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell 1987; 50(6):975–985. [DOI] [PubMed] [Google Scholar]
- 22.de Castro-Amarante MF, Pise-Masison CA, McKinnon K, Washington Parks R, Galli V, Omsland M, et al. Human T cell leukemia virus type 1 infection of the three monocyte subsets contributes to viral burden in humans. J Virol 2016;90(5):2195–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriuchi M, Moriuchi H, Fauci AS. HTLV type I Tax activation of the CXCR4 promoter by association with nuclear respiratory factor 1. AIDS Res Hum Retroviruses 1999;15(9):821–827. [DOI] [PubMed] [Google Scholar]
- 24.Twizere JC, Springael JY, Boxus M, Burny A, Dequiedt F, Dewulf JF, et al. Human T-cell leukemia virus type-1 Tax oncoprotein regulates G-protein signaling. Blood 2007;109(3):1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barmania F, Pepper MS. C-C chemokine receptor type five (CCR5): an emerging target for the control of HIV infection. Appl Transl Genom 2013;2:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]