Abstract
We have systematically assessed published cell studies and animal experimental reports on the efficacy of selected biophysical energies (BPEs) in the treatment of diabetic foot ulcers. These BPEs include electrical stimulation (ES), pulsed electromagnetic field (PEMF), extracorporeal shockwave (ECSW), photo energies and ultrasound (US). Databases searched included CINAHL, MEDLINE and PubMed from 1966 to 2018. Studies reviewed include animal and cell studies on treatment with BPEs compared with sham, control or other BPEs. Information regarding the objective measures of tissue healing and data was extracted. Eighty-two studies were eventually selected for the critical appraisal: five on PEMF, four each on ES and ECSW, sixty-six for photo energies, and three about US. Based on the percentage of original wound size affected by the BPEs, both PEMF and low-level laser therapy (LLL) demonstrated a significant clinical benefit compared to the control or sham treatment, whereas the effect of US did not reveal a significance. Our results indicate potential benefits of selected BPEs in diabetic wound management. However, due to the heterogeneity of the current clinical trials, comprehensive studies using well-designed trials are warranted to confirm the results.
Keywords: biophysical energies, skin wounds, diabetes mellitus, cell, experimental models, systematic review
1. Introduction
Thirty million children and adults in the United States have diabetes [1]. The incidence rate of diabetic foot ulcer is 6% [2], and 45% of diabetic patients die during the first year after the initial amputation [3]. Neuropathy, peripheral vascular disease and infection are the major risk factors for non-healing foot ulceration in patients with diabetes [4]. Increased inflammation and expression of matrix metalloprotiase-9, protein tyrosine phosphatase-1B in wound tissue and elevated level of serum growth factors were also found as the main factors associated with failure to heal diabetic foot ulcers [5]. Thus, treatments that manage neuropathy, ameliorate microcirculation and promote growth factor release may be helpful in treating chronic wounds or reducing their recurrence.
Biophysical energies (BPEs) are commonly used in physiotherapy daily practice [6]. BPE options for treating diabetic foot ulcers have included electrical stimulation (ES), MHz or kHz ultrasound (US), extracorporeal shockwave (ECSW), photo energies and pulsed electromagnetic field (PEMF). A systematic review reports positive findings on the use of the BPEs (ES, photo energies, and US) in managing foot ulcers [7] and peripheral neuropathy [8] in patients with diabetes. BPEs have been used to accelerate healing of chronic diabetic foot ulcers [9] and venous ulcers [10]. Moreover, BPEs may restore diabetes-associated microvascular [9] and neurological changes [11] that are important risk factors for delayed wound healing in patients with diabetes.
Despite the positive findings reported in some clinical studies, it is almost impossible to recruit homogeneous groups of patients in practice. Patients may respond differently to the same intervention due to variations in the severity of wound, location or chronicity. In contrast, the homogeneity in both experimental and control groups can be achieved in studies utilizing cell or animal models, and they also provide more insights into the mechanisms by which BPEs promote wound healing. Previous animal studies have shown that BPEs enhance macrophage migration [12] and antibacterial effects on ulcers [13]. In addition, BPEs have been shown to accelerate collagen deposition and enhance wound contraction in healthy Sprague-Dawley rats [14]. These animal model-based pre-clinical studies have brought some insights into the mechanisms of BPEs. However, it is important to note that rodent models cannot fully recapitulate human responses to BPEs due to mechanistic differences in wound healing, so findings from such studies may not be directly translated into clinical practice.
Thus far, there is a lack of updated review in the literature that evaluates the efficacy of BPEs for wound healing in cellular or animal models. The purpose of this review is to survey the current literature for studies that use cell culture and animal models to evaluate the efficacy of BPEs on diabetic wound healing, and to infer the underlying mechanisms of how BPEs promote wound healing.
2. Methods
This study followed the guidelines suggested by de Vries and co-worker [15] for reporting systematic reviews of animal studies.
2.1. Data Sources and Searches
The literature search for this review was restricted to published results of cellular studies and animal experiments. Databases including MEDLINE, CINAHL and PubMed were searched, covering the period from their inception to December 2018. This review was also restricted to articles published in English. Published review articles were also excluded. Keywords and Medical Subject Headings (MeSH) including PEMF, US, ECSW, ES, and LLL were combined with wound healing (limited to “cell” and “animal”) (Appendix A). A manual search of bibliographic references of relevant articles and existing reviews was also conducted to identify studies not captured by the electronic database search.
2.2. Study Selection
Published studies that reported the efficacy of BPEs in treating diabetic wounds were eligible for inclusion. The inclusion criteria were as follows:
Biophysical energies
Diabetic wound
Cell or animal experiments
The exclusion criteria were as follows:
Co-interventions (e.g., co-medication)
No diabetic wounds
Human studies
Systematic review or meta-analysis
2.3. Data Extraction and Quality Assessment
Literature search was conducted independently by two reviewers (RK and MC). Articles were screened according to the title, the abstract, followed by the full paper if necessary. Duplicates were checked and removed after excluding the publications that were clearly unrelated to the purpose of this study. The full text of publications satisfying the inclusion criteria was obtained for review. At all stages, whenever there were disagreements between the two reviewers, they were resolved by discussing between themselves, sometimes with a senior and experienced reviewer (GC) or the corresponding author when necessary.
Each included experimental animal study was assessed for methodological quality by the same two reviewers independently, using SYRCLE’s risk of bias tool [16]. The checklist consists of: (1) sequence generation; (2) baseline characteristics; (3) allocation concealment; (4) random housing; (5) investigator blinding; (6) random outcome assessment; (7) assessor blinding; (8) incomplete outcome data addressed; (9) selective outcome reporting; and (10) other source of bias.
Details of the studies were extracted and summarized using a data extraction sheet. Attempts were made to obtain any missing data by contacting the authors of the studies. Data from studies published in duplicate were included only once. The data collection form consisted of demographic data (author and year published), study design characteristics (experimental groups and number of animals), animal model characteristics (species, gender, and disease etiology), intervention characteristics (dosage, timing, and duration), outcomes measures and other (dropouts).
2.4. Primary Outcomes
Objective measures of healing were investigated, including the healing rate of diabetic wounds, the time for complete closure, and the proportion of subjects with wound closure within the trial period.
3. Results
3.1. Search Results
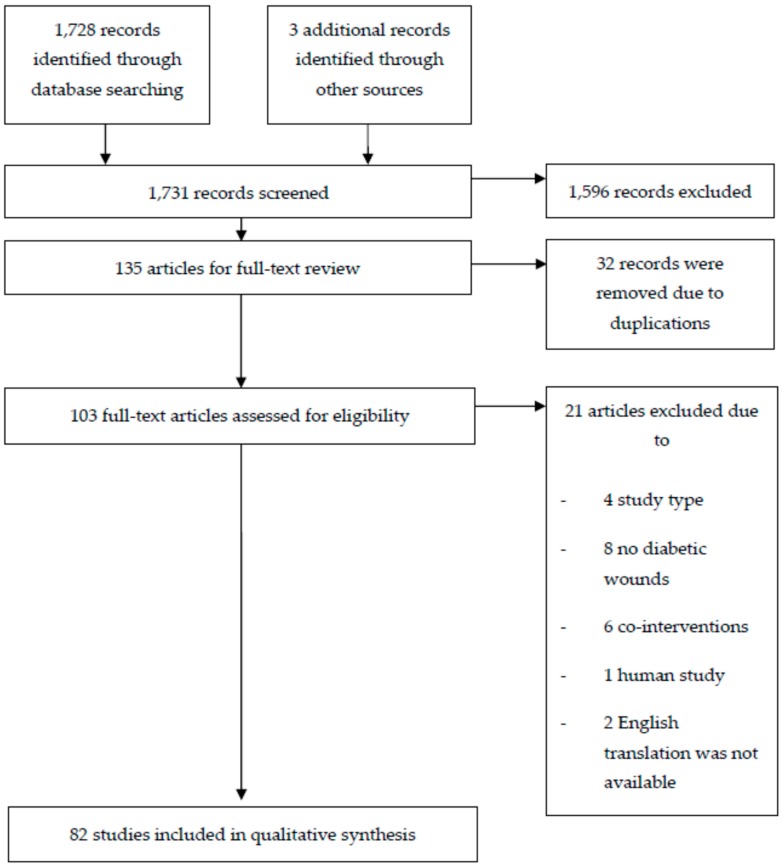
Using the pre-defined keywords and MeSH, we identified 1731 publications pertaining to the use of BPEs for diabetic wound treatment in animal and cellular models. By screening the title and abstract, we obtained 135 relevant articles and retrieved the full text for 103 publications after removing 32 duplicated articles. Of the 103 articles, 21 were excluded for reasons related to the study design (n = 4), not diabetic wounds (n = 8), with co-interventions (n = 6) or human study (n = 1). Two articles were also not included due to the lack of English version [17,18]. Finally, 82 studies that specifically examined the effects of BPEs on diabetic wound healing were critically appraised. Figure 1 illustrates the trial selection process.
Figure 1.
Systematic reviews flow diagram of the selected BPEs literature search.
3.2. Characteristics of Studies
Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6 present the descriptive information on each of the studies reviewed. The trials were conducted between 1984 and 2018. Overall, there were five trials on PEMF [19,20,21,22,23], three trials on US [24,25,26], four trials on ECSW [27,28,29,30], four trials on ES [31,32,33,34] and sixty-six trials on LLL [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100]. The majority of them (60/82; 73%) were published after 2008.
Table 1.
Outcomes of PEMF energy for treating diabetic ulcers.
| Reference | Study Type | Sample Type | Parameters | Outcome Measure | Main Results |
|---|---|---|---|---|---|
| Callaghan et al., 2008 [19] | In vivo | db/db mice (n = 6 in each group) | E: Asymmetric; 4.5 ms pulses; 15 Hz; magnetic flux density increased from 0 to 12 G in 200 μs and return to 0 in 24 μs; custom designed cage; 8 hrs daily C: Identical cages with inactive generators |
|
|
| C57BL6 mice (n = 6 in each group) | |||||
| FGF-2 knockout mice (n = 6) | |||||
| In vitro | Human umbilical vein endothelial cells | (No of plates = 6) 50 Hz inside the incubators measured less than 2 mG; harvested at each time point (0 to 12 h) |
|
|
|
| Goudarzi et al., 2010 [20] | In vivo | Male Wistar rats | E (n = 7): 20 Hz, 4 ms, 8 mT, 1 h/day for 10 days, with restrainer in energized coil C (n = 7): caged for same time without exposure to electromagnetic fields |
On Days 0, 4, 8, 12, and 16
|
|
| Cheing et al., 2014 [21] | In vivo | Male Sprague-Dawley rats | E (n = 28): 5 mT, 25 Hz, 1 h daily, sinusoidal pulses, 40 ms, in plastic cylindrical container C (n = 28): in plastic cylindrical container without exposure to electromagnetic fields |
|
|
| Choi et al., 2016 [22] | In vivo | Male Sprague-Dawley rats | E (n = 20): 5 mT, 25 Hz, 1 h daily, sinusoidal pulses, 40 ms, in plastic cylindrical container C (n = 20): in plastic cylindrical container without exposure to electromagnetic fields |
|
|
| Choi et al., 2018 [23] | In vivo | Male Sprague-Dawley rats | E1: 2 mT, 25 Hz, 1 h daily E2: 10 mT, 25 Hz, 1 h daily C: in plastic restrainer bag without exposure to electromagnetic fields |
|
|
E, Experimental group; C, Control group.
Table 2.
Outcomes of US energy for treating diabetic ulcers.
| Reference | Study Type | Sample Type | Parameters | Outcome Measure | Main Results |
|---|---|---|---|---|---|
| Thawer et al., 2004 [24] | In vivo | Male CD-1 mice | E (n = 27): alternate days, via vapor of 15 mL prewarmed saline, perpendicular for no more than 1 cm from wound bed, 1.5 min, 5 treatments over 10 days, 45 kHz, 0.1 Watt/cm2 C (n = 23): via intravenous drip of 15 mL prewarmed saline, perpendicular for no more than 1 cm from wound bed, 1.5 min, 5 treatments over 10 days, |
|
|
| Mann et al., 2014 [25] | In vivo | Male BKS.Cg-Dock7m +/+ Leprdb /J) mice (n = 3 mice and n = 6 wound per group per time point) | E: 40 kHz with saline vapor, at distance 5 to 15 mm, 3 min, 3 times/week C: Change dressing |
|
|
| In vitro | Dermal fibroblasts from db/db mice | Cell proliferation | Increased fibroblast proliferation (E: 42 ± 2 vs C: 22 ± 2; p < 0.001) | ||
| Roper et al., 2015 [26] | In vivo | Male Syndecan-4 wild-type; knockout C57BL/6J mice | E: 2.5 cm diameter transducer; water-based gel; 30 mWcm−2; 1.5 MHz; pulsed at 1 kHz, 20 min C: transducer applied but not activated |
|
|
| In vitro | Fibroblasts from wound tissue | Speed and persistent migration | Ultrasound switched the random migration to persistent migration in Sdc4 -/- fibroblasts. |
E, Experimental group; C, Control group.
Table 3.
Outcomes of ECSW energy for treating diabetic ulcers.
| Reference | Study Type | Sample Type | Parameters | Outcome Measure | Main Results |
|---|---|---|---|---|---|
| Kuo et al., 2009 [27] | In vivo | Male Wistar Rats | E1 (n = 10): 1 session of defocused ESWT on postoperative Day 3 E2 (n = 10): 2 sessions of defocused ESWT on postoperative Day 3 and 7 E3 (n = 10): 3 sessions of defocused ESWT on postoperative Day 3, 7 and 10 C1 (n = 10): normal control without shockwave C2 (n = 10): diabetic control without shockwave [E1–E3: 100 impulses/area, 8 areas in all wound edges] |
|
|
| Zins et al., 2010 [30] | In vivo | Female BALB/c, homozygous Bk.Cg-m Lepr db+/db+ | E: 200 impulses, 0.1 mJ/mm2, 5 pulses per second, 45 s C: sham treatment |
|
|
| Yang et al., 2011 [28] | In vivo | Male Sprague-Dawley rats | E1 (n = 12): 1 session of ECSW on Day 1 E2 (n = 12): 3 sessions of ECSW on Days 1, 3 and 5 C1 (n = 12): normal control without shockwave C2 (n = 12): diabetic control without shockwave [E1–E2: 100 impulses per cm wound length; 0.11 mJ/mm2; 3 Hz] |
|
|
| Hayashi et al., 2012 [29] | In vivo | Endothelial nitric oxide synthase-knockout (eNOS-KO) mice; C578l/6 mice | E1 (n = 7): eNOS-KO E2 (n = 11): C578l/6 C1 (n = 6): eNOS-KO, sham C2 (n = 8): C578l/6, sham [E1–E2: 70.25 mJ/mm2; 4 Hz; 100 impulses on surface of 4 cm2 per side] |
|
|
E, Experimental group; C, Control group; ECSW, extracorporeal shock-wave.
Table 4.
Outcomes of ES energy for treating diabetic ulcers.
| Reference | Study Type | Sample Type | Parameters | Outcome Measure | Main Results |
|---|---|---|---|---|---|
| Smith et al., 1984 [31] | In vivo | Male mice | E1 (n = 15): Diabetic mice, 20 volt, 20 ma E2 (n = 10): Diabetic mice, 1 volt, 10 ma E3 (n = 10): Normal mice, 20 volt, 20 ma E4 (n = 10): Normal mice, 1 volt, 10 ma C1 (n = 10): Diabetic mice, no charge C2 (n = 10): Normal mice without ES [E1–E3: Daily, 1 min interval, 5 days a week for 2 weeks; C1–C2: Electrode placement without charge] |
|
|
| Thawer et al., 2001 [32] | In vivo | CD-1 mice (n = 55) | E1: Diabetic 12.5 V E2: Normal 12.5 V C1: Diabetic 0 V C2: Normal 0 V [E1–E2: restrained by flexible fiberglass narrow cone; monophasic pulsed current; pulse duration 200 ms, 200 Hz; negative electrode as treatment probe soaked in saline; 15 mins; alternate days; C1–C2: same setting except the electrode was not activated |
|
|
| Kim et al., 2014 [33] | In vivo | Male Sprague-Dawley rats | E (n = 10): diabetic rats with high voltage pulsed current stimulation daily, 100 pps, 40 min, monophasic, twin-peak pulses for 140 μs, voltage from 35 to 50 V; negative pole for first 3 days and positive for next 4 days C1 (n = 10): diabetic rats with sham stimulation C2 (n = 10): normal rats with sham stimulation |
|
|
| Langoni Cassettari et al., 2014 [34] | In vivo | Male Wistar rats | E1 (n = 20): normal with continuous ES E2 (n = 20): diabetic with continuous ES C1 (n = 20): normal without stimulation C2 (n = 20): diabetic without stimulation C3 (n = 20): normal with zinc sulfate by transdermal iontophoresis C4 (n = 20): diabetic with zinc sulfate by transdermal iontophoresis [E1–E2: 2 mA, 10 min; at immediate after surgical incision, Days 1, 2 and 3] |
|
|
E, Experimental group; C, Control group; ES, Electrical stimulation.
Table 5.
Outcomes of in vivo studies on photo energies (PE) for treating diabetic ulcers.
| Reference | Sample Type | Parameters | Outcome Measure | Main Results |
|---|---|---|---|---|
| Low-level laser | ||||
| Yu et al., 1997 [35] | C57BL/Ksj/db/db mice (n = 40, wound = 80) | 630 nm, 20 ± 8 mW/cm2, 2 cm diameter, 250 s at each treatment session and received fluence of 5 J/cm2 |
|
|
| Reddy et al., 2001 [37] | Male Sprague-Dawley rats | Left side wounds; 1.0 J/cm2 He-Ne laser at 632.8 nm; 5 days/week until wound closed |
|
|
| Reddy, 2003 [40] | Male Sprague-Dawley rats (n = 15) | Continuous infrared radiation at 904 nm produced by Ga-As laser, 7 mW, 1.0 J/cm2, once a day, 5 days/week until wound closed |
|
|
| Danno et al., 2001 [36] | Male ICR mice (n = 20); Female C57BL/KsJ-db/db mice (n = 20) | Daily, 30 min, at distance of 20 cm, 54 J/cm2 | Wound area | The rate of wound closure significantly accelerated. |
| Stadler et al., 2001 [38] | C57BL/Ksj/db/db mice; Heterozygous littermates as control (n = 20) | Class IIIb 830 nm laser; 79 mW/cm2, daily, 5 J/cm2/wound; 5 consecutive days; 0–4 days or 3–7 days | Tensile strength | Tensile strength at 11 days was significant between diabetic laser group (2.16 ± 0.47 g/mm2) and sham (1.28 ± 0.32 g/mm2). Tensile strength at 23 days E than in C (2.72 ± 0.56 g/mm2 vs 1.5 ± 0.3 g/mm2). |
| Byrnes et al., 2004 [42] | Psammomys obesus (Sand rats) | Diabetic, 4 J/cm2, He-Ne gas laser: 632.8 nm, daily for 3 consecutive days, at left wound |
|
|
| Kawalec et al., 2004 [43] | C57BLKS/J mice (n = 56) | E1: 5 W every 2 days, 18 J/cm2 E2: 5 W every 4 days, 18 J/cm2 E3: 10 W every 2 days, 36 J/cm2 E4: 10 W every 4 days, 36 J/cm2 GaAIAs diode laser, 980 nm, 1 s |
|
|
| Maiya et al., 2005 [44] | Male Wistar rats (n = 48) | 632.8 nm, 4.8 J/cm2, He-Ne laser, 5 days per week until closed |
|
|
| Carvalho et al., 2006 [47] | Male Wistarrats | 632.8 nm HeNe laser, 4 J/cm2, 60 s/wound, continuous, 5 mW | Histology | Significant difference in collagen. |
| Rabelo et al., 2006 [48] | Male Wistar rats (n = 50) | 3 times/week, continuous, 632.8 nm HeNe laser, 10 J/cm2, 17 s |
|
|
| AI-Watban et al., 2007 [49] | Male Sprague-Dawley rats (n = 52) | E1: 532 nm, 5 J/cm2 E2: 633 nm, 5 J/cm2 E3: 810 nm, 5 J/cm2 E4: 980 nm, 5 J/cm2 E5: 532 nm, 10 J/cm2 E6: 633 nm, 10 J/cm2 E7: 810 nm, 10 J/cm2 E8: 980 nm, 10 J/cm2 E9: 532 nm, 20 J/cm2 E10: 633 nm, 20 J/cm2 E11: 810 nm, 20 J/cm2 E12: 980 nm, 20 J/cm2 E13: 532 nm, 30 J/cm2 E14: 633 nm, 30 J/cm2 E15: 810 nm, 30 J/cm2 E16: 980 nm, 30 J/cm2 |
Wound healing percentage | The percentage of wound healing acceleration is higher in all treatment groups than the control groups. The optimum wavelength and incident dose was at E6. |
| Meireles et al., 2008 [55] | Male Wistar rats (n = 55) | E1: 660 nm, 20 J/cm2 E2: 780 nm, 20 J/cm2 |
Histology | At Day 7, E1 as necrosis extended down to epidermis, and E2 has extending down to dermis. On Day 14, E1 and E2 showed moderate amount of neo-angiogenesis. On Day 21, E1 showed advanced re-epithelialization, but E2 showed no epithelialization. |
| Gungormus and Akyol, 2009 [59] | Female Wistar rats | Class IV, medical class IIB, 20 W, 50 Hz, GaA1As 808 nm, continuous, 0.1 W/cm2, 10 J/cm2, on Days 2, 4, 6, and 8 | Degree of re-epithelialization and inflammation | Significant between-group difference was found in re-epithelialization and inflammation on Day 10, but not on Day 20. |
| Akyol and Gungӧrmuş, 2010 [60] | Wistar rats (n = 54) | Diode laser; 808 nm, 0.1 W/cm2, Day 0,2,4,6 and 8, 10 J/cm2, 20 s per session | Histology analysis | Significant difference found in post hoc analysis between E and C in re-epithelialization and inflammation on Day 10. |
| Carvalho pde et al., 2010 [61] | Male Wistar rats | InGaA1P diode laser, continuous, 100 mW, 660 nm, 10 J/cm2 |
|
|
| Chung et al., 2010a [63] | BKS.Cg-m+/+Leprdb/J (n = 47) | E1: 660 nm, 20 s, 18 mW, 7 consecutive days, 0.36 J/day E2: 660 nm, 20 s, 80 mW, 7 consecutive days, 1.6 J/day |
|
|
| Chung et al., 2010b [62] | BKS.Cg-m+/+Leprdb/J | E1: 660 nm, 0 s, 80 mW, 7 consecutive days, 0 J/day E2: 660 nm, 10 s, 80 mW, 7 consecutive days, 0.8 J/day E3: 660 nm, 20 s, 80 mW, 7 consecutive days, 1.6 J/day E4: 660 nm, 30 s, 80 mW, 7 consecutive days, 3.2 J/day |
Histological analysis | In splinted wound, the mean dermal gap and epithelial gap for E3 was significantly different from E1, 2 and 3. All wounds in E3 completely re-epithelized, and granulation tissue with collagen fibers filled or almost filled the whole of wound bed in splinted wound. |
| Jahangiri Noudeh et al., 2010 [66] | Male Wistar rats (n = 19) | GaA1InP laser, 670 nm, 10 J/cm2; combined with 810 nm GaA1As laser, 250 mW, 12 J, 50 s, 1.33 J/cm2, performed every 3 days | Wound area | No statistical significance in wound area throughout repeated measurements in the study time period. |
| Santos et al., 2010 [68] | Male Wistar rats (n = 12) | E1: 680 nm, 40 J/cm2 per session E2: 790 nm, 40 J/cm2 per session |
Histological analysis | Fibroblast number and angiogenesis was higher in E2. Necrosis was more evident in E1. |
| Hegde et al., 2011 [69] | Male Swiss albino mice | E1: 4 min, 15 s−1 J cm−2 E2: 8 min, 32 s−2 J cm−2 E3: 12 min, 46 s−3 J cm−2 E4: 17 min, 3 s−4 J cm−2 E5: 21 min, 17 s−5 J cm−2 [E1–E5: 632.8 nm HeNe laser] |
Biochemical analysis | Hydroxyproline content in granulation tissue on Day 6 and Day 12 revealed a significant increase in the collagen content in all treatment groups. Rise in glucosamine levels was observed in all experimental groups on Day 6 but subsequently decreased linearly. |
| Peplow et al., 2011 [71] | BKS.Cg-m+/+Leprdb/J | E1: 100 mW, 233–313 mW/cm2 E2: 50 mW, 116–156 mW/cm2 E3: 25 mW, 58–78 mW/cm2 [E1–E3: 660 nm] |
Histological analysis | All splinted wounds were completely re-epithelized, and granulation tissue with collage fibers filled or almost filled the whole wound bed. |
| Dadpay et al., 2012 [74] | Male Wistar rats (n = 18) | 0.2 J/cm2, pulsed infrared diode laser, 1.08 W/cm−2, 890 nm, 80 Hz | Biomechanical examination | Significant increases in maximum load and accelerate wound healing. |
| Park and Kang, 2012 [89] | Male Sprague-Dawley rats (n = 48) | 980 GaA1As diode laser, 60 s every day, 0.01 W, 13.95 J/cm2 |
|
Histological observations and gene expression analyses revealed a faster initial healing and more alveolar bone formation. |
| Peplow et al., 2012 [76] | BKS.Cg-m+/+Leprdb/J | 660 nm, 100 mW, 20 s/day, 7 days |
|
|
| Aparecida Da Silva et al., 2013 [77] | Male Wistar rats (n = 120) | InGaA1P, 50 mW, 660 nm, 4 J/cm2 |
|
|
| Fathabadie et al., 2013 [78] | Male Wistar rats (n = 72) | Once daily for 6 days a week, pulsed infrared laser, 75 W, 1.08 W/cm2, 890 nm, 80 Hz, 180 ns pulse duration, 200 s, 0.2 J/cm2 | Morphometric examination | Significantly increased the number of mast cells on Days 4 and 15 after surgery. |
| Firat et al., 2013 [86] | Male Wistar rats (n = 42) | GaA1As laser, 940 nm, 10 J/cm2, 0.1 W, continuous for 9 s, first dose at 2 h after wounding, then at 2 days interval for 4 sessions |
|
|
| Franca et al., 2013 [87] | Male Wistar rats (n = 65) | 780 nm, 5 J/cm2, 10 s/point, 0.2 J |
|
|
| Dancáková et al., 2014 [80] | Male Sprague-Dawley rats (n = 21) | 810 nm laser |
|
|
| Kilík et al., 2014 [82] | Male Sprague-Dawley rats (n = 48) | GaA1As 635 nm, three times daily, 5 J/cm2; 1st wound: 1 mW/cm2; 2nd wound: 5 mW/cm2; 3rd wound: 15 mW/cm2 | Histopathological evaluation | The synthesis and organization of collagen fibers were consecutively enhanced in the 15 mW/cm2 group. A significant difference in the number of newly formed capillaries. |
| Sharifian et al., 2014 [83] | Male Wistar rats (n = 24) | 890 nm, 6 days per week, pulsed infrared laser, 80 Hz, 0.2 J/cm2 |
|
|
| De Loura Santana et al., 2015 [84] | Female Wistar rats (n = 90) | E1: laser 1 J/cm2, 26 s, 4 times E2: laser 4 J/cm2, 26 s, 1 time Gallium-aluminum-arsenide diode laser, 660 nm |
|
|
| Lau et al., 2015 [85] | Male Sprague Dawley rats (n = 120) | E1: 100 mW, 50 s, 0.1 W/cm2 E2: 200 mW, 25 s, 0.2 W/cm2 E3: 300 mW, 17 s, 0.3 W/cm2 808 nm diode laser, continuous mode, 5 J/cm2, once daily |
|
|
| Lau et al., 2015 [90] | Male rats (n = 21) | E1: 110 mW, 30 s E2: 110 mW, 60 s E3: 110 mW, 120 s E4: 510 mW, 30 s E5: 510 mW, 60 s E6: 510 mW, 120 s 808 nm diode laser, continuous mode |
|
|
| Fekrazad et al., 2015 [92] | Male Wistar rats (n = 40) | E1: blue (425 nm) laser, 50 mW/cm2, 2 J/cm2 E2: green (532 nm) laser, 55 mW/cm2, 2 J/cm2 E3: red (630 nm) laser, 50 mW/cm2, 2 J/cm2 |
Wound healing | Significant difference in the mean slope of wound healing between E and C. |
| de Loura Santana et al., 2016 [95] | Female Wistar rats (n = 90) | E1: Single dose laser, 4 J/cm2, 104 s, 3.12 J, Day 1 E2: Fractionated-dose laser, 1 J/cm2, 26 s, 0.78 J, Days 1, 3, 8 and 10 660 nm, 30 mW, 38 mW/cm2 |
|
|
| Ranjbar et al., 2016 [99] | Male Wistar rats (n = 30) | 685 nm InGaA1P laser, 15 mW, 3 J/cm2, 0.028 cm2 |
|
|
| Tatmatsu Rocha et al., 2016 [100] | Male Swiss mice (n = 20) | 904 nm GaAs diode laser, 5 days, 40 mW, 60 s |
|
|
| Denadai et al.,2017 [96] | Wistar rats (n = 36) | 660 nm InGaAlP, 100 mW, 60 s, 6 J/cm2, 0.028 cm2 | Malondialdehyde levels | Significant lower level of malondialdehyde. |
| Eissa and Salih, 2017 [97] | Wistar rats (n = 14; 6 males, 8 females) | 632.8 nm He-Ne laser, continuous, aperture ~2.3 × 10−6 mm, 4 mW/cm2, 4 min, 6 mm away from skin, 5 days/week until wound healed | Wound diameter | E healed on average on Day 21, whereas C healed after 40 days of 60 days. |
| Polychromatic light emitting diodes (LED) energy | ||||
| AI-Watban and Andres, 2003 [39] | Sprague-Dawley rats (n = 30) | E1: 5 J/cm2 E2: 10 J/cm2 E3: 20 J/cm2 E4: 30 J/cm2 25-LED array (510–543 nm; 594–599 nm; 626–639 nm; 640–670 nm; 842–872 nm); 13.6 mW/cm2; 3 times/week; 3 consecutive weeks |
Healing rate | Healing accelerated at 5 and 10 J/cm2, but no significant inhibition seen at 20 and 30 J/cm2. |
| Whelan et al., 2003 [41] | BKS.Cg-m +/+Leprdb (n = 80) | 670 nm LED with restrainer; daily for 14 days; 4 J/cm2; 28 mW/cm2 for 2 min and 24 s |
|
|
| Oliveira et al., 2010 [67] | Male Wistar rats (n = 30) | E1: Polarized light 400–2000 nm, 20 J/cm2 E2: Polarized light 400–2000 nm, 40 J/cm2 |
Histological analysis | Significant difference in revascularization and re-epithelialization. |
| Oliveira et al., 2011 [70] | Male Wistar rats (n = 90) | E1: polarized light 400–2000 nm, 10.2 J/cm2 E2: polarized light 400–2000 nm, 20.4 J/cm2 |
Histological analysis | 10.2 J/cm2 caused higher deposition of collagen, quicker inflammatory reaction and improved revascularization than 20.4 J/cm2. |
| Monochromatic infrared energy (MIRE) | ||||
| He et al., 2013 [79] | Male Sprague-Dawley rats (n = 30) | 890 nm, intensity set at level 6, 85% of full power, 30 min, three times a week before euthanized |
|
|
| Comparing different photo energies | ||||
| AI-Watban and Andres, 2006 [46] | Male Sprague-Dawley rats (n = 61) | E1: 5 J/cm2 E2: 10 J/cm2 E3: 20 J/cm2 E4: 30 J/cm2 25-LED array (510–543 nm; 594–599 nm; 626–639 nm; 640–670 nm; 842–872 nm); 13.6 mW/cm2; 3 times/week; 3 consecutive weeks |
Wound healing percentage | Wound healing percentage was significant for E1 (16 ± 3.1%, p = 0.01) but not significant for E2, 3 and 4 (7 ± 3.4, 3.4 ± 3.5, 0.9 ± 3.6%). |
| AI-Watban, 2009 [56] | Sprague-Dawley rats (n = 893) | E1: 5 J/cm2 E2: 10 J/cm2 E3: 20 J/cm2 E4: 30 J/cm2 [E1–E4: laser 532 nm, 143 mW, 20.4 mW/cm2] E5: 5 J/cm2 E6: 10 J/cm2 E7: 20 J/cm2 E8: 30 J/cm2 [E5–E8: laser 633 nm, 140 mW, 15.56 mW/cm2] E9: 5 J/cm2 E10: 10 J/cm2 E11: 20 J/cm2 E12: 30 J/cm2 [E9–E12: laser 810 nm, 200 mW, 22.22 mW/cm2] E13: 5 J/cm2 E14: 10 J/cm2 E15: 20 J/cm2 E16: 30 J/cm2 [E13–E16: laser 980 nm, 200 mW, 22.22 mW/cm2] E17: 5 J/cm2 E18: 10 J/cm2 E19: 20 J/cm2 E20: 30 J/cm2 [E17–E20: laser 10,600 nm, 300 mW, 66.37 mW/cm2] E21: 5 J/cm2 E22: 10 J/cm2 E23: 20 J/cm2 E24: 30 J/cm2 [E21–E24: Polychromatic LEDs 510–872 nm, 272 mW, 13.6 mW/cm2] [three times per week] |
Wound area | The best effects on wound healing was shown in E5–E8, followed by E1–E4 > E13–E16 > E9–E12 > E21–E24 > E17–E20. |
| AI-Watban et al., 2009 [57] | Male Sprague-Dawley rats | E1: 5 J/cm2 E2: 10 J/cm2 E3: 20 J/cm2 E4: 30 J/cm2 [E1–E4: laser 532 nm, 143 mW, 20.4 mW/cm2] E5: 5 J/cm2 E6: 10 J/cm2 E7: 20 J/cm2 E8: 30 J/cm2 [E5–E8: laser 633 nm, 140 mW, 15.56 mW/cm2] E9: 5 J/cm2 E10: 10 J/cm2 E11: 20 J/cm2 E12: 30 J/cm2 [E9–E12: laser 670 nm, 120 mW, 22.86 mW/cm2] E13: 5 J/cm2 E14: 10 J/cm2 E15: 20 J/cm2 E16: 30 J/cm2 [E13–E16: laser 810 nm, 200 mW, 22.22 mW/cm2] E17: 5 J/cm2 E18: 10 J/cm2 E19: 20 J/cm2 E20: 30 J/cm2 [E17–E20: laser 980 nm, 200 mW, 22.22 mW/cm2] [three times per week] |
Relative healing | Significant difference in the mean percentage of healing acceleration between the visible laser and invisible laser. |
| Dall Agnol et al., 2009 [58] | Male Wistarrats | E1: GaA1As LED, 40 nm bandwidth centered at 640 nm, 30 mW E2: indium-gallium-aluminum-phosphide (InGaA1P) laser, 660 nm, 30 mW, 6 J/cm2 |
|
|
| Wu et al., 2015 [91] | Male Zucker Diabetic Fatty rats (n = 30) | E1: Organic light-emitting diode E2: 635 nm laser [10 mW/cm2, 5 J/cm2, 8 mins 20 s, Daily for 7 consecutive days] |
|
|
E, Experimental group; C, Control group.
Table 6.
Outcomes of in vitro studies on photo energies for treating diabetic ulcers.
| Reference | Sample Type | Parameters | Outcome Measure | Main Results |
|---|---|---|---|---|
| Low-level laser | ||||
| Houreld and Abrahamse, 2007a [50] | Human skin fibroblast cells | E1: 26 min 33 s, 5 J/cm2 E2: 84 min 23 s, 16 J/cm2 Exposed once on Days 1 and 4, HeNe laser 632.8 nm, 3 mW/cm2 |
|
|
| Houreld and Abrahamse, 2007b [51] | Human skin fibroblast cells | E1: 37 min, 5 J/cm2 E2: 2 h, 16 J/cm2 HeNe laser 632.8 nm, 2.206 mW/cm2 |
|
|
| Houreld and Abrahamse, 2007c [52] | Human skin fibroblast cells | E1: 27 min 46 s, 5 J/cm2, at 30 min and 24 h E2: 2 h, 16 J/cm2, at 30 min and 72 h HeNe laser 632.8 nm, 3.034 mW/cm2 |
|
|
| Mirzaei et al., 2007 [53] | Cultures of fibroblast-like cells from Wistar rats | E1 (wells n = 10): 0.09 J/cm2, 30 s, 4 times/day E2 (wells n = 10): 0.09 J/cm2, 30 s, 4 times at 2 days E3 (wells n = 10): 1 J/cm2, 330 s, 4 times at 2 days E4 (wells n = 10): 1 J/cm2, 100 s, 4 times at 4 days E5 (wells n = 10): 4 J/cm2, 1320 s, 4 times at 4 days [E1–E5: HeNe laser 632.8 nm, 0.6 mW] |
|
|
| Houreld and Abrahamse, 2008 [54] | Human skin fibroblast cells | E1: HeNe 632.8 nm, 5 J/cm2, 23 mW, 2.206 mW/cm2 E2: HeNe 632.8 nm, 16 J/cm2, 23 mW, 2.206 mW/cm2 E3: diode 830 nm, 5 J/cm2, 55 mW, 6 mW/cm2 E4: diode 830 nm, 16 J/cm2, 55 mW, 6 mW/cm2 E5: Nd:YAG 1064 nm, 5 J/cm2, 1 W, 12.7 mW/cm2 E6: Nd:YAG 1064 nm, 16 J/cm2, 1 W, 12.7 mW/cm2 |
|
|
| Houreld and Abrahamse, 2010 [64] | Human skin fibroblast cells | E1: HeNe 632.8 nm, 5 J/cm2, 23 mW, 2.206 mW/cm2 E2: diode 830 nm, 5 J/cm2, 55 mW, 6 mW/cm2 E3: Nd:YAG 1064 nm, 5 J/cm2, 1 W, 12.7 mW/cm2 |
|
|
| Houreld et al., 2010 [65] | Human skin fibroblast cells (n = 6) | 830 nm, 40 mW, 5 J/cm2 |
|
|
| Sekhejane et al., 2011 [72] | Diabetic wounded and hypoxic human skin fibroblast cells (WS1) | 636 nm, continuous, 5 J/cm2, 476 s and incubated for 1 or 24 h |
|
|
| Ayuk et al., 2012 [73] | Diabetic wounded human skin fibroblast | 660 nm, continuous, 10.22 mW/cm2, 5 J/cm2, 8 min 9 s and incubated for 48 or 72 h |
|
Significant increase in cell migration, viability, proliferation and collagen production. |
| Houreld et al., 2012 [75] | Human skin fibroblast | E1: 5 J/cm2 E2: 15 J/cm2 660 nm, continuous, 11 mW/cm2 |
|
|
| Esmaeelinejad et al., 2014 [81] | Human skin fibroblasts | E1: 757 s, 0.5 J/cm2 E2: 1512 s, 1 J/cm2 E3: 3024 s, 2 J/cm2 HeNe laser, 1.5 mW, 632.8 nm, 0.66 mW/cm2 |
|
|
| Masha et al., 2013 [88] | Human skin fibroblast cells (WS1) | 660 nm, continuous, 100 mW, 11 mW/cm2, 5 J/cm2, 7 min 35 s | Gene expression | Upregulated the expression of mitochondrial genes COX6B2 (complex IV), COX6C (complex IV), PPA1 (complex V), ATP4B (complex V) and ATP5G2 (complex V), ATP5F1 (complex V), NDUFA11 (complex I), and NDUFS7 (complex I). |
| Goralczyk et al., 2016 [98] | Human umbilical vein endothelial cells | E1: 635 nm, 30 mW, 1066 s, 1.875 mW/cm2 E2: 830 nm, 60 mW, 533 s, 3.75 mW/cm2 80 cm2 irradiated area, 10 cm distance |
|
|
| Ayuk et al., 2016 [93] | Human skin fibroblasts | 830 nm, 5 J/cm2, continuous, 98 mW, 9.1 cm2, 10.76 mW/cm2, 7 min 43 s | Gene expression profiling | Stimulatory effect on cadherins, integrins, selectins and immunoglobulins. |
| Ayuk et al., 2018 [94] | Human skin fibroblast cells (WS1) | 660 nm, 5 J/cm2, continuous, 102 mW, 9.1 cm2, 11.23 mW/cm2, 7 min 25 s |
|
|
| Near-infrared | ||||
| Danno et al., 2001 [36] | Human foreskin keratinocytes; human foreskin microvascular endothelial cells; human newborn foreskin fibroblasts | Halolamps with 0.7–1.3 μm near infrared, 30 mW/cm2, 20–60 min at distance of 20 cm |
|
|
| Polychromatic light emitting diode (LED) energy | ||||
| Vinck et al., 2005 [45] | Chicken embryos fibroblast cultures (n = 256) | Green light of 570 nm, continuous mode, 0.1 J/cm2, 3 min, 10 mW, once per day for 3 days | Fibroblast survival and proliferation | Significantly higher rate of proliferation in hyperglycemia circumstances after irradiation. |
| Wu et al., 2015 [91] | Primary human dermal fibroblasts in 180 mM glucose concentration | Organic light-emitting diode, 623 nm peak wavelength; 7 or 10 mW/cm2, 0.2, 1 or 5 J/cm2 |
|
|
E, Experimental group; C, Control group.
3.3. Methodological Characteristics
The summary of methodological quality in animal studies is presented in Table 7. Only two trials have a detailed explanation of how randomization was carried out and provide an adequate report on the assignment of samples [39,46]. All trials provide baseline clinical characteristics including gender, age or weight of the subjects. In addition, all expected outcomes are reported [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,46,47,48,49,55,56,57,58,59,60,61,62,63,66,67,68,69,70,71,74,76,77,78,79,80,82,83,84,85,90,91,92,95,96,97,99,100]. Only one trial provides an adequate report on allocation concealment [24]. Five trials report the non-random approach when placing the animals within the facility [62,63,66,71,92]. None of the trials provide information about investigator blinding, but twenty trials report outcome assessor blinding [21,24,25,42,43,44,60,62,67,68,70,78,79,84,86,87,92,95,99,100]. Three trials report random outcome assessment, although no detailed method of randomization is provided [23,86,87]. Three trials did not include all subjects in the analysis [29,31,37]. Ten studies applied interventions to parts of the body in a single animal, accounting for the analysis bias [37,40,42,59,60,74,78,82,83,89,91].
Table 7.
Characteristics of animal experimental studies.
| Reference | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pulsed electromagnetic field | ||||||||||
| Callaghan et al., 2008 [19] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Goudarzi et al., 2010 [20] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Cheing et al., 2014 [21] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Choi et al., 2016 [22] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Choi et al., 2018 [23] | Yes | Yes | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes | Yes |
| Ultrasound | ||||||||||
| Thawer et al., 2004 [24] | Unclear | Yes | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Mann et al., 2014 [25] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Roper et al., 2015 [26] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Shockwave | ||||||||||
| Kuo et al., 2009 [27] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Zins et al., 2010 [30] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Yang et al., 2011 [28] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Hayashi et al., 2012 [29] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | No | Yes | Yes |
| Electrical stimulation | ||||||||||
| Smith et al., 1984 [31] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | No | Yes | Yes |
| Thawer et al., 2001 [32] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Kim et al., 2014 [33] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Langoni et al., 2014 [34] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Photo energy | ||||||||||
| Yu et al., 1997 [35] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Danno et al., 2001 [36] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Reddy et al., 2001 [37] | No | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | No |
| Stadler et al., 2001 [38] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| AI-Watban and Andres, 2003 [39] | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Reddy, 2003 [40] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No |
| Whelan et al., 2003 [41] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Byrnes et al., 2004 [42] | No | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | No |
| Kawalec et al., 2004 [43] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Maiya et al., 2005 [44] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| AI-Watban and Andres, 2006 [46] | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Carvalho et al., 2006 [47] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Rabelo et al., 2006 [48] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| AI-Watban et al., 2007 [49] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Meireles et al., 2008 [55] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| AI-Watban, 2009 [56] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| AI-Watban et al., 2009 [57] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Dall Agnol et al., 2009 [58] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Gungormus and Akyol, 2009 [59] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No |
| Akyol and Gungӧrmuş, 2010 [60] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | No |
| Carvalho pde et al., 2010 [61] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Chung et al., 2010a [63] | Unclear | Yes | Unclear | No | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Chung et al., 2010b [62] | Unclear | Yes | Unclear | No | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Jahangiri Noudeh et al., 2010 [66] | Unclear | Yes | Unclear | No | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Oliveira et al., 2010 [67] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Santos et al., 2010 [68] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Hegde et al., 2011 [69] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Oliveira et al., 2011 [70] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Peplow et al., 2011 [71] | Unclear | Yes | Unclear | No | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Dadpay et al., 2012 [74] | No | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No |
| Park and Kang, 2012 [89] | No | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No |
| Peplow et al., 2012 [76] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Aparecida Da Silva et al., 2013 [77] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Firat et al., 2013 [86] | Unclear | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes |
| Franca et al., 2013 [87] | Unclear | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes |
| He et al., 2013 [79] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Masha et al., 2013 [88] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Dancáková et al., 2014 [80] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Kilík et al., 2014 [82] | No | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No |
| Sharifian et al., 2014 [83] | No | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No |
| De Loura Santana et al., 2015 [84] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| Fekrazad et al., 2015 [92] | Unclear | Yes | Unclear | No | Unclear | Unclear | Yes | Unclear | Yes | Yes |
| Lau et al., 2015 [85] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Lau et al., 2015 [90] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes |
| Wu et al., 2015 [91] | No | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes | No |
| de Loura Santana et al., 2016 [95] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes |
| Ranjbar et al., 2016 [99] | Unclear | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes |
| Tatmatsu Rocha et al., 2016 [100] | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Yes | Yes |
| Denadai et al.,2017 [96] | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes |
| Eissa and Salih, 2017 [97] | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Yes |
Studies fulfilling the criteria of: (1) sequence generation; (2) baseline characteristics; (3) allocation concealment; (4) random housing; (5) investigator blinding; (6) random outcome assessment; (7) assessor blinding; (8) incomplete outcome data addressed; (9) free of selective outcome reporting; and (10) free of other source of bias.
3.4. Efficacy of Biophysical Energy (BPEs) Stimulation
3.4.1. Pulsed Electromagnetic Field (PEMF)
The five PEMF trials compared pulsed electromagnetic fields with the sham treatment [19,20,21,22,23] (Table 1). Three trials conducted by the same researchers compared 2 mT, 5 mT and 10 mT of 25 Hz sinusoidal PEMF in male SD rats with the sham treatment [21,22,23]. One trial compared 8 mT, 20 Hz PEMF in male Wistar rats with the sham treatment [20]. Another trial involved both in vitro and in vivo studies using human umbilical vein endothelial cells, db/db mice, C57BL6 mice and FGF-2 knockout mice [19].
Wound closure percentage was the main outcome measure for all five trials. Other measures included overall wound closure time, cell proliferation, vascularity, murine endothelial cell culture, FGF-2 secretion, wound tensile strength, myofibroblast production, type 1 collagen fiber deposition, collagen fibril alignment, collagen fiber anisotropy and orientation, energy absorption capacity, Young’s modulus, wound thickness, and maximum stress of wound tissue. Four trials report significant between-group difference in the percentage of original wound size, and the experimental groups in all these studies demonstrated improved wound healing compared to the control groups [19,20,21,23].
3.4.2. Ultrasound (US)
Two trials compared ultrasound with sham treatment [24,26], whereas one trial compared ultrasound with dressing changing [25]. The wound size was the main outcome measure for all three ultrasound trials. Other measures included wound closure duration, granulation tissue, collagen deposition, angiogenesis, VEGF expression, SDF-1 expression, fibroblast proliferation, speed and persistency of fibroblast migration (Table 2).
Male CD-1 mice, BKS.Cg-Dock7m+/+Leprdb/J mice, Syndecan-4 wild-type and knockout C57BL/6J mice were used in the animal models. Fibroblasts from wound tissues and db/db mouse skins were used as the cellular model. Thawer et al. and Mann et al. delivered ultrasound with saline vapor at 45 kHz and 40 kHz, respectively, while Roper et al. delivered 1 kHz ultrasound through water-based gel. Two out of three trials revealed significant between-group differences in wound size in favor of the experimental groups over the control groups in these studies [25,26]. The exception was the trial reported by Thawer and collaborators, which showed no significant between-group differences in wound size after ultrasound treatment.
3.4.3. Extracorporeal Shockwave (ECSW)
Four trials on the efficacy of shock wave used male Wistar rats, SD rats, endothelial nitric oxide synthase-knockout mice, C5781/6 mice BALB/c and Bk.Cg-m Lepr (db+/db+) mice. Outcome measures included wound healing area, topical blood perfusion, leukocyte infiltration, cell proliferation, angiogenesis, wound breaking strength, collagen content, fibroblast proliferation, TGF-β1 expression in fibroblasts, myofibroblast accumulation, eNOS expression and angiogenic gene expression (Table 3).
Kuo and colleagues compared three different protocols of shockwave with the control group receiving no shockwave energy and reported a significant acceleration in wound healing (p < 0.05). The perfusion in wound area was significantly higher in the experimental group treated with two sessions of defocused shockwave (on postoperative Days 3 and 7) than the diabetic control group (p = 0.023). In addition, fibroblast count and VEGF level were upregulated in experimental groups compared to control groups. The authors concluded that treatment with an optimal session of ECSW significantly enhanced diabetic wound healing associated with increased neo-angiogenesis, tissue regeneration and topical anti-inflammatory response. However, they did not provide details on the randomization method, allocation concealment, random housing, outcome assessment, and investigator and assessor blinding [27].
Yang and colleagues compared two different protocols of shockwave with the control groups, and they reported a significant improvement evident by increased wound breaking strength, number of fibroblasts and collagen fibers. The authors concluded that low energy ECSW can improve the healing of incisional wound in diabetic rats [28]. Zins et al. investigated the angiogenic gene expressions and wound closure kinetics during diabetic wound healing with or without ECSW therapy. The expression of certain genes in the diabetic wound was augmented by shockwave, especially PECAM-1; however, they found that shockwave had no effect on wound closure in both normal and diabetic models [30].
Hayashi et al. investigated the role of endothelial nitric oxide synthase with shockwave energy for diabetic wounds. A single session of ECSW accelerated wound healing in a streptozotocin-induced diabetic mouse model, accompanied by an increased expression of eNOS and vascular endothelial growth factor (VEGF). However, the efficacy of ECSW was attenuated in eNOS-KO mice. The authors concluded that eNOS played a critical role in the therapeutic effects of shockwave by accelerating the wound healing through VEGF upregulation and neovascularization [29].
3.4.4. Electrical Stimulation (ES)
The four ES trials used different types of protocols. Two trials compared ES with sham treatment [32,33]. One trial compared two different ES protocols with control receiving no ES [31]. Another trial compared ES with the control group receiving no ES or with transdermal iontophoresis by zinc sulfate [34]. None of these studies provided information about randomization, allocation concealment, investigator and assessor blinding, random housing and outcome assessment (Table 4).
Monophasic pulse wave is reported in two trials [32,33]. The outcome measures included wound healing rate, wound contraction, tensile strength, histology, collagen deposition, fibroblast proliferation and morphological analysis. Smith et al. classified the tensile strength into “poor”, “moderate” and ”good” after 10 days of stimulation, and they showed that ES enhanced diabetic wound healing. However, no statistical analysis is provided in their study [31].
Thawer et al. compared wound healing in diabetic mice with ES at 12.5 V and sham treatment (0 V). No statistical difference was found in epidermis thickness between groups. The authors suggested that ES at a high dose can alter collagen deposition in excisional wounds of diabetic mice; however, they found the effect of ES on wound healing to be disease-specific [32]. Kim and colleagues compared experimental groups receiving ES at 35–50 V with a control group receiving sham ES. Significant difference was found in wound healing rate between groups. In addition, elevated levels of collagen-I, α-SMA and TGF-β1 were found in experimental groups (all p < 0.05) [33].
Langoni Cassettari and collaborators divided the normal and diabetic Wistar rats into six experimental groups to study the effect of ES with direct current (DC) and zinc sulfate treatment by transdermal iontophoresis. The authors concluded that DC alone or used in association with zinc by transdermal iontophoresis was able to induce the morphological and ultrastructural changes observed during surgical wound healing in diabetic animals [34].
3.4.5. Photo Energies (PE)
The photo energies reported for treating diabetic wounds encompass low-level laser energy [35,37,38,40,42,43,44,47,48,49,50,51,52,53,54,55,59,60,61,62,63,64,65,66,68,69,71,72,73,74,75,76,77,78,80,81,82,83,84,85,86,87,88,89,90,92,93,97,99,100], near-infrared [36], polychromatic light emitting diodes [39,41,45,67,70] and monochromatic infrared energy [79]. Some studies also compared different types of photo energy [46,56,57,58] (Table 5 and Table 6).
Low Level Laser Therapy (LLL)
A broad spectrum of laser wavelengths has been reported by different studies, whereas wavelengths in the visible red range (630–685 nm) were most commonly investigated either in isolation or in combination with other wavelengths ranging from 425 nm to 1064 nm. Power density in mWcm2 was not specified in some of the reviewed studies, even though this represents an important parameter. The irradiance ranged widely from 4 to 79 mWcm2. Peplow et al. reported a range of irradiance instead of a specific density [71]. Similarly, a large variety of animal models have been used, including C57BL/Ksj/db/db mice, SD rats, Sand rats, Wistar rats, BKS.Cg-m+/+Leprdb/J mice, Zucker diabetic rats and Swiss albino mice. Several wound healing outcomes were measured using various techniques, most commonly wound size and histology. However, nine of our surveyed trials applied laser to parts of the body of a single animal for both experiment and control, and analysis was conducted as if every single wound were from an individual animal [37,40,42,59,60,74,78,82,83,89].
Polychromatic Light Emitting Diodes (LED)
In six trials that investigated effects of polychromatic light emitting diodes (LED), three trials studied burn healing in diabetic rats [39,67,70]. Al-Watban et al. compared the efficacy of LED (wavelength 510–543, 594–599, 626–639, 640–670 and 842–879 nm) on burn wound at four different doses with the sham treatment. Significant burn healing was found from 48.77% to 76.77% after LED stimulation at different doses in diabetic rats [39]. The same research group also compared the efficacy of laser of different wavelengths (532, 633, 810, 980, 10600 nm) to LED clusters (510–872 nm) with incident doses of 5, 10, 20 and 30 J/cm2 in SD rats (n = 893) [56]. Their results showed that phototherapy at 633 nm should be given three times a week at a fluence of 2.35 J/cm2 each time for diabetic wound treatment. Wu et al. [91] compared the 635 nm laser with organic LED and showed that the organic LED significantly increased fibroblast growth factor-2 expression and macrophage activation during the initial stages of wound healing. In addition, they also found that organic LED and laser had comparative effects on promoting diabetic wound healing in rats.
Infrared (IR)
Danno and colleagues conducted both in vitro and in vivo studies to compare the infrared irradiation treatment with sham irradiation control or thermal control [36]. The TGF-β1 and MMP-2 content in the medium of cultured cells was significantly elevated after irradiation. Negative results in thermal controls suggested that the action of the light was athermic in nature. In animal models, the rate of wound closure was significantly accelerated after repeated exposures. Cheing and collaborators compared the efficacy of managing acute wounds in male diabetic SD rats between groups of monochromatic infrared energy (MIRE) at 890 nm and the sham group without receiving infrared energy [79]. Both experimental and sham groups showed improvement in terms of wound closure percentage; however, no statistical difference was found between groups.
4. Discussion
Preclinical research is important for expanding knowledge and provides insights into the cellular and physiological mechanisms on how BPEs enhance diabetic wound healing. Two trials have investigated how cells respond when exposed to electrical currents [101,102]; however, research evidence showing its effects on diabetic wound healing is limited. Four in vivo studies described here present inconsistent results regarding the value of ES in acute diabetic wound healing in animals. Thawer et al. showed no statistical difference in epidermis thickness between groups, but they did find a significant increase in collage deposition [32]. Findings reported by Kim et al. are consistent with those found by Thawer’s team, in which collagen-I expression was higher after ES. In addition, α-SMA and TGF-β1 expression were also enhanced after daily ES [33]. Langoni Cassettari et al. found accelerated wound contraction, but the morphology of inflammation was not altered after ES [34]. Statistical analysis was not available in one of the studies examined [31], making it difficult to draw conclusions on the ES’ benefits in diabetic wound healing from this animal study.
Extracorporeal shockwave (ECSW) has been used clinically for treating musculoskeletal disorders and diabetic ulcers for some years [103]. However, preclinical studies examined in this review reported contradictory findings in supporting the use of ECSW on diabetic wound healing. Two studies showed that ECSW significantly reduced wound size compared to sham treatment groups in diabetic rats [27,29]. On the contrary, a recent study by Zins et al. found that ECSW did not accelerate wound closure in wildtype (nob-diabetic) mice or db/db diabetic mice [30]. Another study found that diabetic mice treated with ECSW significantly increased the wound breaking strength and the collagen fiber content [28]. However, this effect was attenuated in endothelial nitric oxide synthase-knockout mice, suggesting that nitric oxide synthesis plays a critical role in the therapeutic effects of ECSW in diabetic wound healing [29].
Pulsed electromagnetic field (PEMF) energy has been used to treat diabetic stump wounds [104] and chronic diabetic ulcers [9]. All five studies included in our review showed positive findings that supported the use of PEMF in promotion of diabetic wound healing in animal models [19,20,21,22,23]. However, when Callaghan et al. repeated the same protocol on FGF-2 knockout mice, there was no significant improvement found in wound closure rate, suggesting FGF-2 might be a crucial factor in PEMF stimulated diabetic wound healing [19].
Sixty-six studies concerning photo energies are included in the present review. Different types of photo energies with different frequencies have been used in various studies. The wavelengths used range from visible red to infrared, power values from milliwatt to watt, and irradiation from seconds to hours. The wide range of irradiation parameters from the current review suggests the bio-modulatory potential of laser therapy [105]. In addition, these studies were conducted using various diabetic wound models, and different outcome measures were used. The findings show that irradiation by laser accelerated wound closure and collagen production, and there were increases in cellular migration, tissue viability, growth factors and gene expression. Histopathological analysis also showed a decrease in inflammatory cells and an increase in vascularization after irradiation compared to the sham control. Most trials report positive results, except Jahangiri Noudeh et al. who found no statistical significance by repeated measurements throughout the entire study period when a combined 670 nm and 810 nm laser was applied to wound areas [66]. Histological analysis revealed that there was an increase in macrophages [61,95,99], fibroblasts [47,53,63,67,68,81,84,99,100], neutrophils [95], T lymphocyte [95], collagen deposition [37,40,70,77,82,85,99,100], nitrite [100] and nitric oxide level [65], catalase activity [100], thiobarbituric acid reactive substances [100] and vascularization [44,68,70,99] after irradiation. Chung et al. adopted a splinted diabetic wound model to minimize mouse skin contraction during wound healing [62]. Seven-day treatment of 3.7–5.0 J/cm2 caused maximum stimulation of wound healing in diabetic mice compared to the mice receiving no irradiation. Laser irradiation of wavelength at 780 nm improved muscle repair by enhancing reorganization of myofibers and perimysium in cryoinjured diabetic rats [87]. However, not all studies demonstrated a positive result due to the specificity of absorption spectrum and laser intensity. For instance, higher frequencies might cause a negative effect on cells. Houreld and Abrahamse compared the cell morphology and expression of human IL-6 between groups receiving 5 and 16 J/cm2. They found that subjects treated with 16 J/cm2 demonstrated signs of stress without a significant increase in IL-6 expression [51]. Therefore, the optimal protocol of laser therapy for enhancing diabetic wound healing should be further investigated.
The present review does not support the use of ultrasound (US) in promoting diabetic wound healing using animal models [24,25,26]. Thawer and collaborators [24] demonstrated no significant between-group difference in wound size reduction after US, however, a significant improvement was shown by Mann et al. and Roper et al. after treatment [25,26]. Fibroblast migration and proliferation [24,25,26], as well as vascular density [24,25], were enhanced by the use of US compared to the sham groups. Interestingly, these two studies applied 40 and 45 kHz US to wounds through saline vap or or mist (as the coupling medium) for 1.5 and 3 min, respectively [24,25]. Another study utilized US at 1.5 MHz applied via traditional coupling gel for 20 min [26]. The optimal protocol for using ultrasound for enhancing diabetic wound healing should be further evaluated in future studies.
Most research on BPEs have been conducted on animal models consisting of surgically excised skin or burn wounds. However, no animal tissue model could possibly replicate the clinical situation in humans because different species may involve different healing mechanisms in skin wound, therefore, treatments with different BPEs are likely to yield different cellular responses when compared to human skin [106]. These experimental wounds excluded common problems associated with delays in healing including ischemia and infection, thus they might not present the real situation in humans [107]. In addition, Wang et al. commented that most in vitro data derived from fibroblasts of abnormal wound lesions only represent the terminal stage of the disease [107]. Therefore, these wound models may not be ideal to study the effect of BPEs on human diabetic ulcer healing. Recently, a reproducible chronic diabetic wound model that had low mortality rate was established by using Pseudomonas aeruginosa biofilm in db/db mice [108,109]. This model could be adopted in future studies to evaluate the antibiofilm effectiveness of BPEs in chronic wounds, which simulate infected diabetic ulcerations commonly seen in clinical settings. It should be noted that humane issue is always a concern of animal studies, in particular for experiments involving burn and wound. Therefore, in vitro methods might be an alternative because not only the humane concerns are circumvented but also the human cells instead of animal cells can be directly tested. Due to the shortcomings of animal studies, well-designed human studies are still the gold standard in clinical practice.
5. Conclusions
The present review demonstrates methodological shortcomings in animal studies that have studied the efficacy of BPEs in diabetic wound healing. One major limitation exhibited in animal experiments is that random allocation of animals to experimental and control groups and blinding is not yet a standard practice [110]. In addition, critical information for animal housing conditions and dropouts are unreported. Investigators should consider the findings of this systematic review when designing future studies and attempting to improve the internal validity of the studies by using true randomization in group allocation and outcome assessment, investigator and assessor blinding, allocation concealment, random housing, and reporting accurately on the number of animals used. In this review, the search was restricted to English publications as the translation was not available for full text review, which may have resulted in language bias. Notably, a variety of animal models were used for in vivo wound healing studies, but the physiology and healing mechanisms may not be the same across different species, and they are even more distinct compared to humans. There was considerable variation in research design, methodology, and parameters which limited comparison of research findings between studies. Therefore, findings obtained from even well-controlled animal studies may not be readily translated into clinical practice for people with diabetes management. Based on positive effects of PEMF and photo energies towards diabetic wound healing, more high-quality human clinical trials to assess the effects of those biophysical energies are warranted in the future.
Abbreviations
| BPEs | biophysical energies |
| ES | electrical stimulation |
| PEMF | pulsed electromagnetic field |
| ECSW | extracorporeal shockwave |
| LLL | low-level laser therapy |
| US | ultrasound |
| LED | light emitting diode |
| NIR | infrared |
| E | experimental group |
| C | control group |
Appendix A
Detailed search strings
Basic search was combined with searches for interventions by adding the search term AND.
Basic search
(Diabetes Mellitus [MeSH]) OR (Diabetes Mellitus) OR (Diabetes) OR (Diabetic) OR (Diabetes Mellitus, Type 2)) OR (Diabetes Complications [MeSH]) AND (ulcer [MeSH]) OR ((Foot ulcer) OR (diabetic foot) OR (wound) OR (wound healing [MeSH]) OR (wounds and injuries [MeSH]).
Model
(Animal) OR (Animals [MeSH]) OR (mouse) OR (Mice [MeSH]) OR (murine) OR (Rats [MeSH]) OR (rodent) OR (Hamster) OR (Cricetulus [MeSH]) OR (Rabbits [MeSH]) OR (Guinea pigs [MeSH]) OR (Swine [MeSH]) OR (dog) OR (porcine) OR (Sprague-Dawley) OR (Transgenic) OR (Sheep [MeSH]) OR (pig) OR (In Vitro [MeSH]) OR (In vivo) OR (Cells [MeSH]) OR (macrophages) OR (fibroblasts) OR (Adenosine triphosphate) OR (Collagen).
Electrical stimulation
(Physical therapy modalities [MeSH]) OR (Electric stimulation therapy [MeSH]) OR (Electric* therapy) OR (Microamperage stimulation) OR (Low intensity direct current) OR (High voltage) OR (electrotherapy) OR (direct current) OR (microcurrent).
Electromagnetics
(Electromagnetic*) OR (Electromagnetic Fields [MeSH]) OR (Magnetic Field Therapy [MeSH]) OR (Pulsed electromagnetic therapy) OR (diathermy) OR (shortwave).
Phototherapy
(Ultraviolet rays [MeSH]) OR (Lasers [MeSH]) OR (Laser Therapy [MeSH]) OR (Laser Therapy, Low-Level [MeSH]) OR (MIRE) OR (monochromatic infrared energy) OR (Phototherapy [MeSH]) OR (Infrared Rays [MeSH]) OR (Anodyne) OR (near infrared) OR (near-infrared).
Ultrasound
(Ultrasound [MeSH]) OR (Ultrasonic Therapy [MeSH]) OR (Ultrasonic Therap*) OR (ultrasonic).
Extracorporeal shockwave therapy
(extracorporeal shockwave) OR (shockwave).
Filter
NOT (“review” [Publication Type]) OR (review literature as topic [MeSH]) OR (reviews).
Author Contributions
Conceptualization, R.L.-C.K. and G.L.-Y.C.; Methodology, R.L.-C.K.; Formal Analysis, R.L.-C.K.; Investigation, R.L.-C.K. and H.M.-C.C.; Resources, R.L.-C.K.; Data Curation, R.L.-C.K. and H.M.-C.C.; Writing—Original Draft Preparation, R.L.-C.K.; Writing—Review and Editing, R.L.-C.K., S.L., H.M.-C.C., L.C.K. and G.L.-Y.C.; Supervision, L.C.K and G.L.-Y.C.; Project Administration, R.L.-C.K. and G.C.; and Funding Acquisition, G.L.-Y.C.
Funding
This research was funded by the General Research Fund provided by the Research Grants Council of the Hong Kong SAR Government grant number (PolyU151003/14M).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.American Diabetes Association FAST FACTS—Data and Statistics about Diabetes. [(accessed on 18 December 2017)]; Available online: http://professional.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/Fast_Facts_3-2015.pdf.
- 2.Margolis D.J., Malay D.S., Hoffstad O.J., Leonard C.E., MaCurdy T., de Nava K.L., Tan Y., Molina T., Siegel K.L. Incidence of Diabetic Foot Ulcer and Lower Extremity Amputation among Medicare Beneficiaries, 2006 to 2008: Data Points #2. [(accessed on 18 December 2017)]; Available online: http://www.ncbi.nlm.nih.gov/books/NBK65149/
- 3.Johannesson A., Larsson G.U., Ramstrand N., Turkiewicz A., Wirehn A.B., Atroshi I. Incidence of lower-limb amputation in the diabetic and nondiabetic general population: A 10-year population-based cohort study of initial unilateral and contralateral amputations and reamputations. Diabetes Care. 2009;32:275–280. doi: 10.2337/dc08-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubský M., Jirkovská A., Bem R., Fejfarová V., Skibová J., Schaper N.C., Lipsky B.A. Risk factors for recurrence of diabetic foot ulcers: Prospective follow-up analysis in the Eurodiale subgroup. Int. Wound J. 2013;10:555–561. doi: 10.1111/j.1742-481X.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinh T., Tecilazich F., Kafanas A., Doupis J., Gnardellis C., Leal E., Tellechea A., Pradhan L., Lyons T.E., Giurini J.M., et al. Mechanisms Involved in the Development and Healing of Diabetic Foot Ulceration. Diabetes. 2012;61:2937–2947. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloth L.C. Electrical Stimulation Technologies for Wound Healing. Adv. Wound Care. 2014;3:81–90. doi: 10.1089/wound.2013.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwan R.L., Cheing G.L., Vong S.K., Lo S.K. Electrophysical therapy for managing diabetic foot ulcers: A systematic review. Int. Wound J. 2013;10:121–131. doi: 10.1111/j.1742-481X.2012.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakral G., Kim P.J., LaFontaine J., Menzies R., Najafi B., Lavery L.A. Electrical Stimulation as an Adjunctive Treatment of Painful and Sensory Diabetic Neuropathy. J. Diabetes Sci. Technol. 2013;7:1202–1209. doi: 10.1177/193229681300700510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan R.L., Wong W.C., Yip S.L., Chan K.L., Zheng Y.P., Cheing G.L. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers: A pilot study. Adv. Skin Wound Care. 2015;28:212–219. doi: 10.1097/01.ASW.0000462012.58911.53. [DOI] [PubMed] [Google Scholar]
- 10.Santamato A., Panza F., Fortunato F., Portincasa A., Frisardi V., Cassatella G., Valente M., Seripa D., Ranieri M., Fiore P. Effectiveness of the frequency rhythmic electrical modulation system for the treatment of chronic and painful venous leg ulcers in older adults. Rejuvenation Res. 2012;15:281–287. doi: 10.1089/rej.2011.1236. [DOI] [PubMed] [Google Scholar]
- 11.Musaev A.V., Guseinova S.G., Imamverdieva S.S. The use of pulsed electromagnetic fields with complex modulation in the treatment of patients with diabetic polyneuropathy. Neurosci. Behav. Physiol. 2003;33:745–752. doi: 10.1023/A:1025184912494. [DOI] [PubMed] [Google Scholar]
- 12.Cho M.R., Thatte H.S., Lee R.C., Golan D.E. Integrin-dependent human macrophage migration induced by oscillatory electrical stimulation. Ann. Biomed. Eng. 2000;28:234–243. doi: 10.1114/1.263. [DOI] [PubMed] [Google Scholar]
- 13.Ercan B., Kummer K.M., Tarquinio K.M., Webster T.J. Decreased Staphylococcus aureus biofilm growth on anodized nanotubular titanium and the effect of electrical stimulation. Acta Biomater. 2011;7:3003–3012. doi: 10.1016/j.actbio.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.-H., Jekal S.-J., Kwon P.-S. 630 nm Light Emitting Diode Irradiation Improves Dermal Wound Healing in Rats. JKPT. 2015;27:140–146. doi: 10.18857/jkpt.2015.27.3.140. [DOI] [Google Scholar]
- 15.de Vries R.B.M., Hooijmans C.R., Langendam M.W., van Luijk J., Leenaars M., Ritskes-Hoitinga M., Wever K.E. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid. Based Preclin. Med. 2015;2:1–9. doi: 10.1002/ebm2.7. [DOI] [Google Scholar]
- 16.Hooijmans C.R., Rovers M.M., de Vries R.B., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hass H.L. The therapeutic activity of the BIOPTRON-lamp in the treatment of disorders of wound healing. Diabetic gangrene. Krankenpfl. J. 1998;36:494–496. [PubMed] [Google Scholar]
- 18.Kilik R., Bober J., Gal P., Vidinsky B., Mokry M., Longauer F., Sabo J. The influence of laser irradiation with different power densities on incisional wound healing in healthy and diabetic rats. Rozhl Chir. 2007;86:384–387. [PubMed] [Google Scholar]
- 19.Callaghan M.J., Chang E.I., Seiser N., Aarabi S., Ghali S., Kinnucan E.R., Simon B.J., Gurtner G.C. Pulsed electromagnetic fields accelerate normal and diabetic wound healing by increasing endogenous FGF-2 release. Plast. Reconstr. Surg. 2008;121:130–141. doi: 10.1097/01.prs.0000293761.27219.84. [DOI] [PubMed] [Google Scholar]
- 20.Goudarzi I., Hajizadeh S., Salmani M.E., Abrari K. Pulsed electromagnetic fields accelerate wound healing in the skin of diabetic rats. Bioelectromagnetics. 2010;31:318–323. doi: 10.1002/bem.20567. [DOI] [PubMed] [Google Scholar]
- 21.Cheing G.L., Li X., Huang L., Kwan R.L., Cheung K.K. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics. 2014;35:161–169. doi: 10.1002/bem.21832. [DOI] [PubMed] [Google Scholar]
- 22.Choi M.C., Cheung K.K., Li X., Cheing G.L. Pulsed electromagnetic field (PEMF) promotes collagen fibre deposition associated with increased myofibroblast population in the early healing phase of diabetic wound. Arch. Dermatol. Res. 2016;308:21–29. doi: 10.1007/s00403-015-1604-9. [DOI] [PubMed] [Google Scholar]
- 23.Choi H.M.C., Cheing A.K.K., Ng G.Y.F., Cheing G.L.Y. Effects of pulsed electromagnetic field (PEMF) on the tensile biomechanical properties of diabetic wounds at different phases of healing. PLoS ONE. 2018;13:e0191074. doi: 10.1371/journal.pone.0208475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thawer H.A., Houghton P.E. Effects of ultrasound delivered through a mist of saline to wounds in mice with diabetes mellitus. J. Wound Care. 2004;13:171–176. doi: 10.12968/jowc.2004.13.5.26615. [DOI] [PubMed] [Google Scholar]
- 25.Maan Z.N., Januszyk M., Rennert R.C., Duscher D., Rodrigues M., Fujiwara T., Ho N., Whitmore A., Hu M.S., Longaker M.T., et al. Noncontact, low-frequency ultrasound therapy enhances neovascularization and wound healing in diabetic mice. Plast. Reconstr. Surg. 2014;134:402e–411e. doi: 10.1097/PRS.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roper J.A., Williamson R.C., Bally B., Cowell C.A., Brooks R., Stephens P., Harrison A.J., Bass M.D. Ultrasonic Stimulation of Mouse Skin Reverses the Healing Delays in Diabetes and Aging by Activation of Rac1. J. Investig. Dermatol. 2015;135:2824–2851. doi: 10.1038/jid.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo Y.R., Wang C.T., Wang F.S., Chiang Y.C., Wang C.J. Extracorporeal shock-wave therapy enhanced wound healing via increasing topical blood perfusion and tissue regeneration in a rat model of STZ-induced diabetes. Wound Repair Regen. 2009;17:522–530. doi: 10.1111/j.1524-475X.2009.00504.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang G., Luo C., Yan X., Cheng L., Chai Y. Extracorporeal shock wave treatment improves incisional wound healing in diabetic rats. Tohoku J. Exp. Med. 2011;225:285–292. doi: 10.1620/tjem.225.285. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi D., Kawakami K., Ito K., Ishii K., Tanno H., Imai Y., Kanno E., Maruyama R., Shimokawa H., Tachi M. Low-energy extracorporeal shock wave therapy enhances skin wound healing in diabetic mice: A critical role of endothelial nitric oxide synthase. Wound Repair Regen. 2012;20:887–895. doi: 10.1111/j.1524-475X.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 30.Zins S.R., Amare M.F., Tadaki D.K., Elster E.A., Davis T.A. Comparative analysis of angiogenic gene expression in normal and impaired wound healing in diabetic mice: Effects of extracorporeal shock wave therapy. Angiogenesis. 2010;13:293–304. doi: 10.1007/s10456-010-9186-9. [DOI] [PubMed] [Google Scholar]
- 31.Smith J., Romansky N., Vomero J., Davis R.H. The effect of electrical stimulation on wound healing in diabetic mice. J. Am. Podiatry Assoc. 1984;74:71–75. doi: 10.7547/87507315-74-2-71. [DOI] [PubMed] [Google Scholar]
- 32.Thawer H.A., Houghton P.E. Effects of electrical stimulation on the histological properties of wounds in diabetic mice. Wound Repair Regen. 2001;9:107–115. doi: 10.1046/j.1524-475x.2001.00107.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim T.H., Cho H.Y., Lee S.M. High-voltage pulsed current stimulation enhances wound healing in diabetic rats by restoring the expression of collagen, alpha-smooth muscle actin, and TGF-beta1. Tohoku J. Exp. Med. 2014;234:1–6. doi: 10.1620/tjem.234.1. [DOI] [PubMed] [Google Scholar]
- 34.Langoni Cassettari L., Colli Rocha Dias P., Natalia Lucchesi A., Ferraz de Arruda M., Veruska Paiva Ortolan E., Marques M.E., Spadella C.T. Continuous electrical current and zinc sulphate administered by transdermal iontophoresis improves skin healing in diabetic rats induced by alloxan: Morphological and ultrastructural analysis. J. Diabetes Res. 2014;2014:980232. doi: 10.1155/2014/980232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu W., Naim J.O., Lanzafame R.J. Effects of photostimulation on wound healing in diabetic mice. Lasers Surg. Med. 1997;20:56–63. doi: 10.1002/(SICI)1096-9101(1997)20:1<56::AID-LSM9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 36.Danno K., Mori N., Toda K., Kobayashi T., Utani A. Near-infrared irradiation stimulates cutaneous wound repair: Laboratory experiments on possible mechanisms. Photodermatol. Photoimmunol. Photomed. 2001;17:261–265. doi: 10.1034/j.1600-0781.2001.170603.x. [DOI] [PubMed] [Google Scholar]
- 37.Reddy G.K., Stehno-Bittel L., Enwemeka C.S. Laser photostimulation accelerates wound healing in diabetic rats. Wound Repair Regen. 2001;9:248–255. doi: 10.1046/j.1524-475x.2001.00248.x. [DOI] [PubMed] [Google Scholar]
- 38.Stadler I., Lanzafame R.J., Evans R., Narayan V., Dailey B., Buehner N., Naim J.O. 830-nm irradiation increases the wound tensile strength in a diabetic murine model. Lasers Surg. Med. 2001;28:220–226. doi: 10.1002/lsm.1042. [DOI] [PubMed] [Google Scholar]
- 39.Al-Watban F.A., Andres B.L. Polychromatic LED therapy in burn healing of non-diabetic and diabetic rats. J. Clin. Laser Med. Surg. 2003;21:249–258. doi: 10.1089/104454703322564451. [DOI] [PubMed] [Google Scholar]
- 40.Reddy G.K. Comparison of the photostimulatory effects of visible He-Ne and infrared Ga-As lasers on healing impaired diabetic rat wounds. Lasers Surg. Med. 2003;33:344–351. doi: 10.1002/lsm.10227. [DOI] [PubMed] [Google Scholar]
- 41.Whelan H.T., Buchmann E.V., Dhokalia A., Kane M.P., Whelan N.T., Wong-Riley M.T., Eells J.T., Gould L.J., Hammamieh R., Das R., et al. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J. Clin. Laser Med. Surg. 2003;21:67–74. doi: 10.1089/104454703765035484. [DOI] [PubMed] [Google Scholar]
- 42.Byrnes K.R., Barna L., Chenault V.M., Waynant R.W., Ilev I.K., Longo L., Miracco C., Johnson B., Anders J.J. Photobiomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomed. Laser Surg. 2004;22:281–290. doi: 10.1089/pho.2004.22.281. [DOI] [PubMed] [Google Scholar]
- 43.Kawalec J.S., Hetherington V.J., Pfennigwerth T.C., Dockery D.S., Dolce M. Effect of a diode laser on wound healing by using diabetic and nondiabetic mice. J. Foot Ankle Surg. 2004;43:214–220. doi: 10.1053/j.jfas.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Maiya G.A., Kumar P., Rao L. Effect of low intensity helium-neon (He-Ne) laser irradiation on diabetic wound healing dynamics. Photomed. Laser Surg. 2005;23:187–190. doi: 10.1089/pho.2005.23.187. [DOI] [PubMed] [Google Scholar]
- 45.Vinck E.M., Cagnie B.J., Cornelissen M.J., Declercq H.A., Cambier D.C. Green light emitting diode irradiation enhances fibroblast growth impaired by high glucose level. Photomed. Laser Surg. 2005;23:167–171. doi: 10.1089/pho.2005.23.167. [DOI] [PubMed] [Google Scholar]
- 46.Al-Watban F.A., Andres B.L. Polychromatic LED in oval full-thickness wound healing in non-diabetic and diabetic rats. Photomed. Laser Surg. 2006;24:10–16. doi: 10.1089/pho.2006.24.10. [DOI] [PubMed] [Google Scholar]
- 47.Carvalho P.T., Mazzer N., dos Reis F.A., Belchior A.C., Silva I.S. Analysis of the influence of low-power HeNe laser on the healing of skin wounds in diabetic and non-diabetic rats. Acta Cir. Bras. 2006;21:177–183. doi: 10.1590/S0102-86502006000300010. [DOI] [PubMed] [Google Scholar]
- 48.Rabelo S.B., Villaverde A.B., Nicolau R., Salgado M.C., Melo Mda S., Pacheco M.T. Comparison between wound healing in induced diabetic and nondiabetic rats after low-level laser therapy. Photomed. Laser Surg. 2006;24:474–479. doi: 10.1089/pho.2006.24.474. [DOI] [PubMed] [Google Scholar]
- 49.Al-Watban F.A., Zhang X.Y., Andres B.L. Low-level laser therapy enhances wound healing in diabetic rats: A comparison of different lasers. Photomed. Laser Surg. 2007;25:72–77. doi: 10.1089/pho.2006.1094. [DOI] [PubMed] [Google Scholar]
- 50.Houreld N., Abrahamse H. Irradiation with a 632.8 nm helium-neon laser with 5 J/cm2 stimulates proliferation and expression of interleukin-6 in diabetic wounded fibroblast cells. Diabetes Technol. Ther. 2007;9:451–459. doi: 10.1089/dia.2007.0203. [DOI] [PubMed] [Google Scholar]
- 51.Houreld N., Abrahamse H. In vitro exposure of wounded diabetic fibroblast cells to a helium-neon laser at 5 and 16 J/cm2. Photomed. Laser Surg. 2007;25:78–84. doi: 10.1089/pho.2006.990. [DOI] [PubMed] [Google Scholar]
- 52.Houreld N.N., Abrahamse H. Effectiveness of helium-neon laser irradiation on viability and cytotoxicity of diabetic-wounded fibroblast cells. Photomed. Laser Surg. 2007;25:474–481. doi: 10.1089/pho.2007.1095. [DOI] [PubMed] [Google Scholar]
- 53.Mirzaei M., Bayat M., Mosafa N., Mohsenifar Z., Piryaei A., Farokhi B., Rezaei F., Sadeghi Y., Rakhshan M. Effect of low-level laser therapy on skin fibroblasts of streptozotocin-diabetic rats. Photomed. Laser Surg. 2007;25:519–525. doi: 10.1089/pho.2007.2098. [DOI] [PubMed] [Google Scholar]
- 54.Houreld N.N., Abrahamse H. Laser light influences cellular viability and proliferation in diabetic-wounded fibroblast cells in a dose- and wavelength-dependent manner. Lasers Med. Sci. 2008;23:11–18. doi: 10.1007/s10103-007-0445-y. [DOI] [PubMed] [Google Scholar]
- 55.Meireles G.C., Santos J.N., Chagas P.O., Moura A.P., Pinheiro A.L. Effectiveness of laser photobiomodulation at 660 or 780 nanometers on the repair of third-degree burns in diabetic rats. Photomed. Laser Surg. 2008;26:47–54. doi: 10.1089/pho.2007.2051. [DOI] [PubMed] [Google Scholar]
- 56.Al-Watban F.A. Laser therapy converts diabetic wound healing to normal healing. Photomed. Laser Surg. 2009;27:127–135. doi: 10.1089/pho.2008.2406. [DOI] [PubMed] [Google Scholar]
- 57.Al-Watban F.A., Zhang X.Y., Andres B.L., Al-Anize A. Visible lasers were better than invisible lasers in accelerating burn healing on diabetic rats. Photomed. Laser Surg. 2009;27:269–272. doi: 10.1089/pho.2008.2310. [DOI] [PubMed] [Google Scholar]
- 58.Dall Agnol M.A., Nicolau R.A., de Lima C.J., Munin E. Comparative analysis of coherent light action (laser) versus non-coherent light (light-emitting diode) for tissue repair in diabetic rats. Lasers Med. Sci. 2009;24:909–916. doi: 10.1007/s10103-009-0648-5. [DOI] [PubMed] [Google Scholar]
- 59.Gungormus M., Akyol U.K. Effect of biostimulation on wound healing in diabetic rats. Photomed. Laser Surg. 2009;27:607–610. doi: 10.1089/pho.2008.2349. [DOI] [PubMed] [Google Scholar]
- 60.Akyol U., Gungormus M. The effect of low-level laser therapy on healing of skin incisions made using a diode laser in diabetic rats. Photomed. Laser Surg. 2010;28:51–55. doi: 10.1089/pho.2008.2425. [DOI] [PubMed] [Google Scholar]
- 61.Carvalho Pde T., Silva I.S., Reis F.A., Perreira D.M., Aydos R.D. Influence of ingaalp laser (660 nm) on the healing of skin wounds in diabetic rats. Acta Cir. Bras. 2010;25:71–79. doi: 10.1590/S0102-86502010000100016. [DOI] [PubMed] [Google Scholar]
- 62.Chung T.Y., Peplow P.V., Baxter G.D. Laser photobiostimulation of wound healing: Defining a dose response for splinted wounds in diabetic mice. Lasers Surg. Med. 2010;42:656–664. doi: 10.1002/lsm.20981. [DOI] [PubMed] [Google Scholar]
- 63.Chung T.Y., Peplow P.V., Baxter G.D. Laser photobiomodulation of wound healing in diabetic and non-diabetic mice: Effects in splinted and unsplinted wounds. Photomed. Laser Surg. 2010;28:251–261. doi: 10.1089/pho.2009.2493. [DOI] [PubMed] [Google Scholar]
- 64.Houreld N., Abrahamse H. Low-intensity laser irradiation stimulates wound healing in diabetic wounded fibroblast cells (WS1) Diabetes Technol. Ther. 2010;12:971–978. doi: 10.1089/dia.2010.0039. [DOI] [PubMed] [Google Scholar]
- 65.Houreld N.N., Sekhejane P.R., Abrahamse H. Irradiation at 830 nm stimulates nitric oxide production and inhibits pro-inflammatory cytokines in diabetic wounded fibroblast cells. Lasers Surg. Med. 2010;42:494–502. doi: 10.1002/lsm.20812. [DOI] [PubMed] [Google Scholar]
- 66.Jahangiri Noudeh Y., Shabani M., Vatankhah N., Hashemian S.J., Akbari K. A combination of 670 nm and 810 nm diode lasers for wound healing acceleration in diabetic rats. Photomed. Laser Surg. 2010;28:621–627. doi: 10.1089/pho.2009.2634. [DOI] [PubMed] [Google Scholar]
- 67.Oliveira P.C., Pinheiro A.L., Reis Junior J.A., de Castro I.C., Gurgel C., Noia M.P., Meireles G.C., Cangussu M.C., Ramalho L.M. Polarized light (lambda400-2000 nm) on third-degree burns in diabetic rats: Immunohistochemical study. Photomed. Laser Surg. 2010;28:613–619. doi: 10.1089/pho.2009.2675. [DOI] [PubMed] [Google Scholar]
- 68.Santos N.R., dos Santos J.N., dos Reis J.A., Jr., Oliveira P.C., de Sousa A.P., de Carvalho C.M., Soares L.G., Marques A.M., Pinheiro A.L. Influence of the use of laser phototherapy (lambda660 or 790 nm) on the survival of cutaneous flaps on diabetic rats. Photomed. Laser Surg. 2010;28:483–488. doi: 10.1089/pho.2009.2500. [DOI] [PubMed] [Google Scholar]
- 69.Hegde V.N., Prabhu V., Rao S.B., Chandra S., Kumar P., Satyamoorthy K., Mahato K.K. Effect of laser dose and treatment schedule on excision wound healing in diabetic mice. Photochem. Photobiol. 2011;87:1433–1441. doi: 10.1111/j.1751-1097.2011.00991.x. [DOI] [PubMed] [Google Scholar]
- 70.Oliveira P.C., Pinheiro A.L., de Castro I.C., Reis J.A., Jr., Noia M.P., Gurgel C., Teixeira Cangussu M.C., Pedreira Ramalho L.M. Evaluation of the effects of polarized light (lambda400-200 nm) on the healing of third-degree burns in induced diabetic and nondiabetic rats. Photomed. Laser Surg. 2011;29:619–625. doi: 10.1089/pho.2010.2914. [DOI] [PubMed] [Google Scholar]
- 71.Peplow P.V., Chung T.Y., Ryan B., Baxter G.D. Laser photobiostimulation of wound healing: Reciprocity of irradiance and exposure time on energy density for splinted wounds in diabetic mice. Lasers Surg. Med. 2011;43:843–850. doi: 10.1002/lsm.21094. [DOI] [PubMed] [Google Scholar]
- 72.Sekhejane P.R., Houreld N.N., Abrahamse H. Irradiation at 636 nm positively affects diabetic wounded and hypoxic cells in vitro. Photomed. Laser Surg. 2011;29:521–530. doi: 10.1089/pho.2010.2877. [DOI] [PubMed] [Google Scholar]
- 73.Ayuk S.M., Houreld N.N., Abrahamse H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 nm. Diabetes Technol. Ther. 2012;14:1110–1117. doi: 10.1089/dia.2012.0125. [DOI] [PubMed] [Google Scholar]
- 74.Dadpay M., Sharifian Z., Bayat M., Bayat M., Dabbagh A. Effects of pulsed infra-red low level-laser irradiation on open skin wound healing of healthy and streptozotocin-induced diabetic rats by biomechanical evaluation. J. Photochem. Photobiol. Bbiol. 2012;111:1–8. doi: 10.1016/j.jphotobiol.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Houreld N.N., Masha R.T., Abrahamse H. Low-intensity laser irradiation at 660 nm stimulates cytochrome c oxidase in stressed fibroblast cells. Lasers Surg. Med. 2012;44:429–434. doi: 10.1002/lsm.22027. [DOI] [PubMed] [Google Scholar]
- 76.Peplow P.V., Chung T.Y., Baxter G.D. Laser photostimulation (660 nm) of wound healing in diabetic mice is not brought about by ameliorating diabetes. Lasers Surg. Med. 2012;44:26–29. doi: 10.1002/lsm.21133. [DOI] [PubMed] [Google Scholar]
- 77.Aparecida Da Silva A., Leal-Junior E.C., Alves A.C., Rambo C.S., Dos Santos S.A., Vieira R.P., De Carvalho Pde T. Wound-healing effects of low-level laser therapy in diabetic rats involve the modulation of MMP-2 and MMP-9 and the redistribution of collagen types I and III. J. Cosmet. Laser Ther. 2013;15:210–216. doi: 10.3109/14764172.2012.761345. [DOI] [PubMed] [Google Scholar]
- 78.Fathabadie F.F., Bayat M., Amini A., Bayat M., Rezaie F. Effects of pulsed infra-red low level-laser irradiation on mast cells number and degranulation in open skin wound healing of healthy and streptozotocin-induced diabetic rats. J. Cosmet. Laser. 2013;15:294–304. doi: 10.3109/14764172.2013.764435. [DOI] [PubMed] [Google Scholar]
- 79.He Y., Yip S.L., Cheung K.K., Huang L., Wang S., Cheing G.L. The effect of monochromatic infrared energy on diabetic wound healing. Int. Wound J. 2013;10:645–652. doi: 10.1111/j.1742-481X.2012.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dancakova L., Vasilenko T., Kovac I., Jakubcova K., Holly M., Revajova V., Sabol F., Tomori Z., Iversen M., Gal P., et al. Low-level laser therapy with 810 nm wavelength improves skin wound healing in rats with streptozotocin-induced diabetes. Photomed. Laser Surg. 2014;32:198–204. doi: 10.1089/pho.2013.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Esmaeelinejad M., Bayat M., Darbandi H., Bayat M., Mosaffa N. The effects of low-level laser irradiation on cellular viability and proliferation of human skin fibroblasts cultured in high glucose mediums. Lasers Med. Sci. 2014;29:121–129. doi: 10.1007/s10103-013-1289-2. [DOI] [PubMed] [Google Scholar]
- 82.Kilik R., Lakyova L., Sabo J., Kruzliak P. Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and diabetic rats. Biomed. Res. Int. 2014;2014:269253. doi: 10.1155/2014/269253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharifian Z., Bayat M., Alidoust M., Farahani R.M., Bayat M., Rezaie F., Bayat H. Histological and gene expression analysis of the effects of pulsed low-level laser therapy on wound healing of streptozotocin-induced diabetic rats. Lasers Med. Sci. 2014;29:1227–1235. doi: 10.1007/s10103-013-1500-5. [DOI] [PubMed] [Google Scholar]
- 84.de Loura Santana C., Silva Dde F., Deana A.M., Prates R.A., Souza A.P., Gomes M.T., de Azevedo Sampaio B.P., Shibuya J.F., Bussadori S.K., Mesquita-Ferrari R.A., et al. Tissue responses to postoperative laser therapy in diabetic rats submitted to excisional wounds. PLoS ONE. 2015;10:e0122042. doi: 10.1371/journal.pone.0122042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lau P., Bidin N., Krishnan G., AnaybBaleg S.M., Sum M.B., Bakhtiar H., Nassir Z., Hamid A. Photobiostimulation effect on diabetic wound at different power density of near infrared laser. J. Photochem. Photobiology. B Biol. 2015;151:201–207. doi: 10.1016/j.jphotobiol.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 86.Firat E.T., Dag A., Gunay A., Kaya B., Karadede M.I., Kanay B.E., Ketani A., Evliyaoglu O., Uysal E. The effects of low-level laser therapy on palatal mucoperiosteal wound healing and oxidative stress status in experimental diabetic rats. Photomed. Laser Surg. 2013;31:315–321. doi: 10.1089/pho.2012.3406. [DOI] [PubMed] [Google Scholar]
- 87.Franca C.M., de Loura Santana C., Takahashi C.B., Alves A.N., De Souza Mernick A.P., Fernandes K.P., de Fatima Teixeira da Silva D., Bussadori S.K., Mesquita-Ferrari R.A. Effect of laser therapy on skeletal muscle repair process in diabetic rats. Lasers Med Sci. 2013;28:1331–1338. doi: 10.1007/s10103-012-1249-2. [DOI] [PubMed] [Google Scholar]
- 88.Masha R.T., Houreld N.N., Abrahamse H. Low-intensity laser irradiation at 660 nm stimulates transcription of genes involved in the electron transport chain. Photomed. Laser Surg. 2013;31:47–53. doi: 10.1089/pho.2012.3369. [DOI] [PubMed] [Google Scholar]
- 89.Park J.J., Kang K.L. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: A pilot study. Lasers Med. Sci. 2012;27:223–230. doi: 10.1007/s10103-011-0944-8. [DOI] [PubMed] [Google Scholar]
- 90.Lau P.S., Bidin N., Krishnan G., Nassir Z., Bahktiar H. Biophotonic effect of diode laser irradiance on tensile strength of diabetic rats. J. Cosmet. Laser Ther. 2015;17:86–89. doi: 10.3109/14764172.2014.968587. [DOI] [PubMed] [Google Scholar]
- 91.Wu X., Alberico S., Saidu E., Rahman Khan S., Zheng S., Romero R., Sik Chae H., Li S., Mochizuki A., Anders J. Organic light emitting diode improves diabetic cutaneous wound healing in rats. Wound Repair Regen. 2015;23:104–114. doi: 10.1111/wrr.12258. [DOI] [PubMed] [Google Scholar]
- 92.Fekrazad R., Mirmoezzi A., Kalhori K.A.M., Arany P. The effect of red, green and blue lasers on healing of oral wounds in diabetic rats. J. Photochem. Photobiol. B Biol. 2015;148:242–245. doi: 10.1016/j.jphotobiol.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 93.Ayuk S.M., Abrahamse H., Houreld N.N. The role of photobiomodulation on gene expression of cell adhesion molecules in diabetic wounded fibroblasts in vitro. J. Photochem. Photobiol. B Biol. 2016;161:368–374. doi: 10.1016/j.jphotobiol.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 94.Ayuk S.M., Houreld N.N., Abrahamse H. Effect of 660 nm visible red light on cell proliferation and viability in diabetic models in vitro under stressed conditions. Lasers Med. Sci. 2018;33:1085–1093. doi: 10.1007/s10103-017-2432-2. [DOI] [PubMed] [Google Scholar]
- 95.de Loura Santana C., de Fatima Teixeira Silva D., de Souza A.P., Jacinto M.V., Bussadori S.K., Mesquita-Ferrari R.A., Fernandes K.P., Franca C.M. Effect of laser therapy on immune cells infiltrate after excisional wounds in diabetic rats. Lasers Surg. Med. 2016;48:45–51. doi: 10.1002/lsm.22445. [DOI] [PubMed] [Google Scholar]
- 96.Denadai A.S., Aydos R.D., Silva I.S., Olmedo L., de Senna Cardoso B.M., da Silva B.A.K., de Carvalho P.T.C. Acute effects of low-level laser therapy (660 nm) on oxidative stress levels in diabetic rats with skin wounds. J. Exp. Ther. Oncol. 2017;11:85–89. [PubMed] [Google Scholar]
- 97.Eissa M., Salih W.H.M. The influence of low-intensity He-Ne laser on the wound healing in diabetic rats. Lasers Med. Sci. 2017;32:1261–1267. doi: 10.1007/s10103-017-2230-x. [DOI] [PubMed] [Google Scholar]
- 98.Goralczyk K., Szymanska J., Szot K., Fisz J., Rosc D. Low-level laser irradiation effect on endothelial cells under conditions of hyperglycemia. Lasers Med. Sci. 2016;31:825–831. doi: 10.1007/s10103-016-1880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ranjbar R., Takhtfooladi M.A. The effects of photobiomodulation therapy on Staphylococcus aureus infected surgical wounds in diabetic rats. A microbiological, histopathological, and biomechanical study. Acta Cir. Bras. 2016;31:498–504. doi: 10.1590/S0102-865020160080000001. [DOI] [PubMed] [Google Scholar]
- 100.Tatmatsu-Rocha J.C., Ferraresi C., Hamblin M.R., Damasceno Maia F., do Nascimento N.R., Driusso P., Parizotto N.A. Low-level laser therapy (904 nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J. Photochem. Photobiol. B Biol. 2016;164:96–102. doi: 10.1016/j.jphotobiol.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang Y.-J., Wu H.-C., Tai N.-H., Wang T.-W. Carbon Nanotube Rope with Electrical Stimulation Promotes the Differentiation and Maturity of Neural Stem Cells. Small. 2012;8:2869–2877. doi: 10.1002/smll.201200715. [DOI] [PubMed] [Google Scholar]
- 102.Langelaan M.L.P., Boonen K.J.M., Rosaria-Chak K.Y., van der Schaft D.W.J., Post M.J., Baaijens F.P.T. Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J. Tissue Eng. Regen. Med. 2011;5:529–539. doi: 10.1002/term.345. [DOI] [PubMed] [Google Scholar]
- 103.Omar M.T., Alghadir A., Al-Wahhabi K.K., Al-Askar A.B. Efficacy of shock wave therapy on chronic diabetic foot ulcer: A single-blinded randomized controlled clinical trial. Diabetes Res. Clin. Pract. 2014;106:548–554. doi: 10.1016/j.diabres.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 104.Isakov E., Ring H., Mendelevich I., Boduragin N., Susak Z., Kupfert Y., Marchetti N. Electromagnetic stimulation of stump wounds in diabetic amputees. J. Rehabil. Sci. 1996;9:46–48. [Google Scholar]
- 105.Peplow P.V., Chung T.Y., Baxter G.D. Laser photobiomodulation of wound healing: A review of experimental studies in mouse and rat animal models. Photomed. Laser Surg. 2010;28:291–325. doi: 10.1089/pho.2008.2446. [DOI] [PubMed] [Google Scholar]
- 106.Ud-Din S., Bayat A. Non-animal models of wound healing in cutaneous repair: In silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen. 2017;25:164–176. doi: 10.1111/wrr.12513. [DOI] [PubMed] [Google Scholar]
- 107.Wang P.H., Huang B.S., Horng H.C., Yeh C.C., Chen Y.J. Wound healing. J. Chin. Med. Assoc. 2018;81:94–101. doi: 10.1016/j.jcma.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 108.Zhao G., Hochwalt P.C., Usui M.L., Underwood R.A., Singh P.K., James G.A., Stewart P.S., Fleckman P., Olerud J.E. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: A model for the study of chronic wounds. Wound Repair Regen. 2010;18:467–477. doi: 10.1111/j.1524-475X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao G., Usui M.L., Underwood R.A., Singh P.K., James G.A., Stewart P.S., Fleckman P., Olerud J.E. Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model. Wound Repair Regen. 2012;20:342–352. doi: 10.1111/j.1524-475X.2012.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kilkenny C., Parsons N., Kadyszewski E., Festing M.F., Cuthill I.C., Fry D., Hutton J., Altman D.G. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE. 2009;4:e7824. doi: 10.1371/journal.pone.0007824. [DOI] [PMC free article] [PubMed] [Google Scholar]