Abstract
The pathology of Charcot-Marie-Tooth (CMT), a disease arising from mutations in different genes, has been associated with an impairment of mitochondrial dynamics and axonal biology of mitochondria. Mutations in ganglioside-induced differentiation-associated protein 1 (GDAP1) cause several forms of CMT neuropathy, but the pathogenic mechanisms involved remain unclear. GDAP1 is an outer mitochondrial membrane protein highly expressed in neurons. It has been proposed to play a role in different aspects of mitochondrial physiology, including mitochondrial dynamics, oxidative stress processes, and mitochondrial transport along the axons. Disruption of the mitochondrial network in a neuroblastoma model of GDAP1-related CMT has been shown to decrease Ca2+ entry through the store-operated calcium entry (SOCE), which caused a failure in stimulation of mitochondrial respiration. In this review, we summarize the different functions proposed for GDAP1 and focus on the consequences for Ca2+ homeostasis and mitochondrial energy production linked to CMT disease caused by different GDAP1 mutations.
Keywords: GDAP1, recessive mutations, store operated calcium entry, mitochondrial location, calcium regulated cell respiration
1. Introduction
Charcot-Marie-Tooth (CMT) disease is one of the most common inherited peripheral neuropathies, with an overall population prevalence estimated at 10-28/100,000 in Europe [1]. It is characterized by a degeneration of motor and sensory fibers that progresses slowly in a length-dependent manner. In general terms, CMT disease is classified into demyelinating (CMT1) or axonal (CMT2) forms, depending on the nerve conduction velocities in motor fibers of the median nerve [2]. More than 80 genes encoding proteins with very different functions have been linked to CMT disease and related neuropathies [3]. One of them is ganglioside-induced differentiation-associated protein 1 (GDAP1), of which more than 80 mutations have been found in CMT patients [4,5,6].
Mutations in GDAP1 show a wide range of severity and Mendelian heterogeneity [7], leading to several forms of CMT disease including: recessive axonal (AR-CMT2K) [6]; recessive demyelinating (CMT4A) [5]; recessive with intermediate clinical features (CMTRIA) [8]; and dominant with axonal features (CMT2K) [9,10].
GDAP1 is an outer mitochondrial membrane protein that is ubiquitously expressed with predominant expression in nervous tissues [6]. In the peripheral nervous system, GDAP1 has been found to be expressed mainly in axons rather than Schwann cells [11], but another study also reported the presence of GDAP1 in myelinating Schwann cells [12]. This protein contains two glutathione-S-transferase (GST) type domains [13] separated by an α-loop region, a C-proximal hydrophobic domain, and a C-terminal transmembrane domain which is critical for its targeting to the outer mitochondrial membrane [14]. GDAP1 has been proposed to play a role in a number of mitochondrial functions, including mitochondrial dynamics [12,15,16,17], redox processes [18,19] or mitochondrial transport, calcium homeostasis, and energy production [20,21].
Mitochondrial dysfunction has been shown to underlie numerous neurodegenerative diseases, including Alzheimer´s (AD), Parkinson´s (PD), Huntington´s disease (HD), and also other forms of CMT, such as CMT2A [22,23,24,25]. With this in mind, the implication of GDAP1 in CMT disease is not surprising, since GDAP1 is involved in many mitochondrial functions. At the present, the molecular pathogenesis of CMT disease caused by mutations in GDAP1 remains unclear. One reason may be that the primary effect on mitochondria depends on the location of each mutation, and consequently on the specific protein domain and function affected. However, a cell-specific effect of each of these mutations cannot be excluded, given that GDAP1 is expressed in both peripheral neurons and in lower levels in Schwann cells [6,18].
2. Proposed Roles of Ganglioside-Induced Differentiation-Associated Protein 1 (GDAP1) in Mitochondrial Physiology
Different roles of GDAP1 have been proposed, most of which are related to mitochondrial functions. Several studies have suggested an involvement of GDAP1 in mitochondrial dynamics. Specifically, GDAP1 has been described as a fission factor whose activity depends on the fission factors Drp1 and Fis1 [15]. Expression of human GDAP1 in Fis1 mutants in S. cerevisiae recovers cell size and mitochondrial network morphology [26]. GDAP1 overexpression induced fragmentation of the mitochondrial network, however, GDAP1 silencing enhanced the tubular aspect of mitochondria in some studies [12,27] but not in others [20]. In this context, the effect of missense mutations in GDAP1 found in CMT patients on mitochondrial dynamics has been also addressed. Some studies have shown that overexpression of recessive GDAP1 mutations was associated with a decrease in fission activity, but not all mutants reduced fission activity to the same degree [15]. However, other overexpression studies showed that these recessive mutations resulted in a fragmented mitochondrial network, with no differences compared to the wild type protein, whose overexpression also caused mitochondrial fragmentation [11,20]. Altogether, it is not clear how GDAP1 contributes to mitochondrial fission and this suggests that GDAP1 must have another function rather than mitochondrial fission.
When GDAP1 was identified, phylogenetic and structural analysis suggested that GDAP1 belongs to a GST family [6,13], but early studies did not show any evidence of glutathione-dependent activity [16,28]. However, a role of GDAP1 in regulation of cellular glutathione (GSH) content has been shown, in which it confers a protective response against oxidative stress conditions [18,19,29]. Thus, mouse hippocampal neural cells selected as resistant to oxidative stress showed increased GDAP1 levels and GDAP1 upregulation resulted in resistance against oxidative stress caused by GSH depletion. [18]. Loss of GDAP1 also caused mild oxidative stress in mouse hippocampal neural cells and in peripheral nerves in a mouse model of GDAP1-related CMT [18,19]. These studies suggest that GDAP1 acts as a sensor of the reduced to oxidized glutathione ratio (GSH/GSSG) participating in the release of an antioxidative response causing the recovery of GSH/GSSG. These results suggest a possible role of oxidative stress and chronic inflammation in the pathogenesis of GDAP1-related CMT, as shown to occur in other forms of CMT such as CMT1A [30] or CMT1C [31].
Another relevant finding to understand the cellular role of GDAP1 is the fact that it localizes to mitochondrial-associated membranes (MAMs) and interacts with trafficking-associated proteins [20]. MAMs are the place where physical communication between the endoplasmic reticulum (ER) and mitochondria takes place, with both organelles 10–30 nm apart [32,33]. This finding suggests participation of GDAP1 in the mitochondria-ER interface. Indeed, GDAP1 deficiency reduced contacts between mitochondria and ER in neuroblastoma cells, and overexpression of GDAP1 in HeLa cells increased co-localization between both organelles [20]. GDAP1 has also been involved in mitochondrial transport within the cell, based on the interaction found between GDAP1 and β-tubulin, and the trafficking-associated proteins, RAB6B and caytaxin [20]. The GDAP1 domain responsible for this interaction is the α-loop, which is located between the two GST domains. In CMT mutations of this domain, abnormal protein interactions between GDAP1 and the trafficking-associated proteins have been found [20]. Anomalous distribution of the mitochondrial network has also been found in mouse models lacking Gdap1 [34]. Together, these results suggest that a failure in mitochondrial movement (toward the ER or other cell locations) that alters the distribution of the mitochondrial network may be relevant to the pathogenesis of GDAP1-related CMT.
In addition to its role in mitochondrial functions, GDAP1 is also expressed in peroxisomes, where it is involved in the regulation of peroxisomal morphology [17]. In N1E-115 cells, reduced levels of GDAP1 led to elongated peroxisomes, while overexpression triggered peroxisomal fragmentation [17]. Patients suffering from CMT disease caused by mutations in GDAP1 show a wide range of clinical manifestations, with the recessive forms of the disease (CMT4A and AR-CMT2) much more severe than the dominant form (CMT2K) [7]. This fact points out that different molecular mechanisms may underlie the pathology of CMT due to different mutations. It is possible that the clinical phenotype of CMT disease caused by missense mutations depends on the specific protein domain affected and the function associated with it. The variability of clinical features in these patients can also be explained by the presence of modifier genes, whose influence on phenotype has been shown in several neurological disorders [35,36]. Recently, juntophilin-1 has been described to act as a negative modifier of GDAP1 [37], and the presence of the dominant GDAP1 p.R120W mutation along with the JPH1 p.R213P mutation in a CMT patient led to a more severe form of the disease [37].
3. Role of Mitochondrial Traffic and Location in Charcot-Marie-Tooth (CMT) Disease
Mitochondria carry out many essential functions in the cell; they are the major cellular source of ATP, obtained via oxidative phosphorylation, but also host several metabolic pathways, such as the citric acid cycle, β-oxidation of fatty acids, and pyrimidine metabolism, among others [38]. Mitochondria are the main site where reactive oxygen species (ROS) are generated [39], and they are also a key component of Ca2+ homeostasis in the cell, being able to modulate the dynamic of cytosolic calcium signals by buffering Ca2+ flux from the ER or the plasma membrane [40,41,42]. Ca2+ flux between the ER and mitochondria depends on the correct formation of MAMs [33]. Consequently, mitochondria are fundamental for several biological processes, such as cell proliferation and necrosis or apoptotic cell death [38].
Mitochondrial structure and localization are associated with their functionality. These two aspects become more important in cells with high energy requirements such as neurons, particularly those with long axons, in which mitochondrial positioning and turnover are more relevant due to the greater energy demand that occurs in axonal terminals. Specifically, synaptic transmission [43] and vesicle recycling [44] require larger amounts of energy. In sensory and motor neurons of the peripheral nervous system (which are the cells affected by CMT disease), the regions with the highest energy demands, and thus where mitochondria are concentrated, are: the distal region of the initial segment of the axon; the nodes of Ranvier; and the neuromuscular junctions (in motor neurons) or sensory end terminals (in sensory neurons) [45]. Interestingly, mitochondrial shape and localization are highly regulated and depend on mitochondrial dynamics, motility, and tethering, all processes that have been shown to be disrupted in GDAP1-related CMT models.
Defects in axonal transport or abnormal mitochondrial distribution in axons have been described in CMT arising from mutations in other genes, and other peripheral neuropathies involving mutations in mitochondrial proteins or in proteins with roles in mitochondrial transport [46,47]. For example, mutations in Mitofusin 2 (MFN2), which encodes an outer mitochondrial membrane protein involved in mitochondrial fusion [48], are the primary cause of CMT2A, the most common form of the autosomal dominant axonal CMT disease [22]. Dorsal root ganglion (DRG) neurons expressing clinical MFN2 mutations showed impairment in transport of mitochondria along the axons [49]. An abnormal mitochondrial distribution was observed in a different mouse model of CMT2A, which presented significant mitochondrial accumulation specifically in the distal axons [50]. Interestingly, the accumulation of axonal mitochondria in the distal part of the sural nerve has also been observed in CMT2A2 patients [51]. These results, together with a study that shows evidence for a role of MFN2 in mitochondrial transport through a direct interaction with the Miro/Milton complex [52], reinforce the hypothesis that a failure in mitochondrial transport along the axons is the main pathological mechanism involved in CMT2A2. Deficits in mitochondrial transport have been linked to other forms of CMT disease. Mutations in HSPB1 and HSPB8 cause CMT2F and CMT2L respectively, and in both situations a disruption of the cytoskeletal and axonal architecture, preventing mitochondrial movement along the axons, has been found [53,54,55,56]. Taking all this together, axonal transport as a therapeutic target in mitochondria-related CMT diseases has been proposed and it has been already addressed in different models of CMT2 associated with HSPB1 mutations. Inhibition of histone deacetylase 6 (HDAC6), the major deacetylating enzyme of α-tubulin [57] has been tested in order to increase the acetylation of α-tubulin and so facilitate the binding of molecular motor proteins (dynein and kinesin family) to the microtubules [58]. Results obtained in several studies showed that the use of different selective inhibitors of HDAC6 reverted mitochondrial transport defects in both motoneurons obtained by induced pluripotent stem cells (iPSCs) differentiation from two patients with different HPSB1 mutations [59] and in cultured DRG neurons expressing HSPB1 mutations [60,61]. Remarkably, the restored motor and sensory problems have been observed in mutant HSPB1 mice in vivo [61].
4. Ca2+ Deregulation in GDAP1-Related CMT Disease
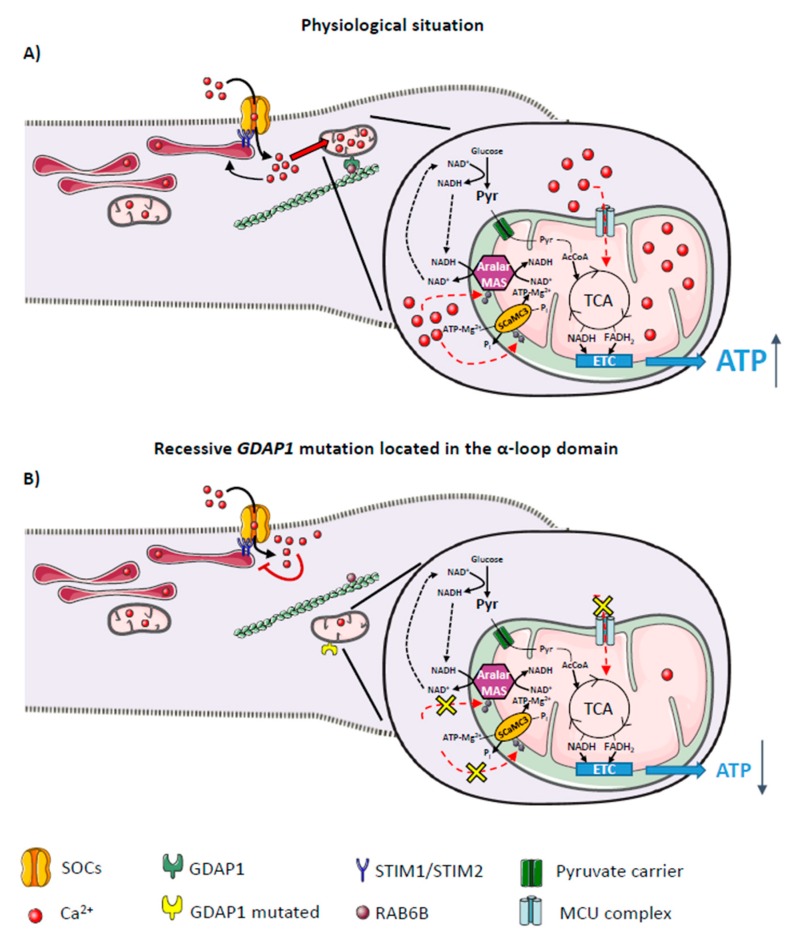
Impaired axonal mitochondrial movement and positioning found in different models of CMT disease [20,49,55,56] may affect multiple mitochondrial functions. GDAP1 silencing in neuroblastoma cells or genetic disruption in mice caused a similar change in Ca2+ homeostasis: a failure to activate the store-operated calcium entry (SOCE) process [20,34], a cytosolic Ca2+ entry mechanism activated by a decrease in ER- Ca2+ levels [62]. Activation of SOCE is a dynamic process; store depletion is sensed by the ER stromal interaction molecule (STIM) proteins, STIM1 and STIM2, which oligomerize and migrate to the ER regions juxtaposed to the plasma membrane [63,64]. At the same time, proteins from the ORAI and transient receptor potential cation (TRPC) families which form the Ca2+ channel accumulate in the plasma membrane directly opposite of the STIM clusters [65,66]. Interaction between the channel proteins and the STIM clusters activates the store-operated calcium channels (SOCs), allowing Ca2+ influx into the cytosol [67]. This process is highly regulated by Ca2+, with two different mechanisms: a fast Ca2+-dependent inactivation mediated by Ca2+ near the mouth of the channel; and a slow Ca2+-dependent inactivation mainly performed by mitochondria [68]. The failure in SOCE found in GDAP1-deficient cells has been linked to a mislocalization of mitochondria at SOCE sites (Figure 1). These cells showed a smaller number of mitochondria close to the plasma membrane after SOCE activation, preventing proper mitochondrial Ca2+ uptake in the proximity of SOCs [20,21]. Mitochondrial Ca2+ uptake has been shown to facilitate SOCE by preventing its slow Ca2+-dependent inactivation in several cell types [68,69,70,71,72,73].
Figure 1.
Effects of ganglioside-induced differentiation-associated protein 1 (GDAP1) on mitochondrial bioenergetic functions. (A) Interaction between GDAP1 and the trafficking proteins allows mitochondria to be located close to the plasma membrane after store-operated calcium entry (SOCE) activation, preventing its Ca2+-dependent inactivation. Ca2+ uptake by mitochondria facilitates SOCE but also regulates ATP production by oxidative phosphorylation. Ca2+-dependent regulation of OXPHOS involves two main mechanisms (dotted red arrows); (i) Ca2+ entry through the mitochondrial Ca2+ uniporter complex (MCUc) and the activation of dehydrogenases of the tricarboxylic acid cycle (TCA), and (ii) activation of the neuronal Ca2+-dependent mitochondrial transporters of aspartate/glutamate (Aralar) or ATP-Mg/Pi (SCaMC-3). Aralar activation increases Malate/Aspartate shuttle (MAS) activity, transferring reducing equivalents from NADH to mitochondria and thereby increasing pyruvate (Pyr) supply to mitochondria to enhance mitochondrial respiration. SCaMC-3 activation increases mitochondrial adenine nucleotide pool (solid and dotted black arrows); (B) Mitochondrial movement might be affected by recessive mutations located in the α-loop of GDAP1 causing the loss of interaction with trafficking proteins RAB6B and caytaxin which might affect the proper mitochondrial localization at the subplasmalemmal microdomains and disturb SOCE activity (red T bar). Subsequently, this will also impair mitochondrial bioenergetic functions by either decreasing Ca2+ uptake by MCUc and activation of matrix dehydrogenases, and/or by decreasing the activation of Ca2+-dependent mitochondrial transporters.
Interestingly, missense mutations in GDAP1 seem to have different effects on Ca2+ homeostasis, depending on their pattern of inheritance [21]. Overexpression of dominant GDAP1 mutations in GDAP1-silenced cells resulted in an increase in SOCE activity above control levels, while the effect of overexpression of recessive GDAP1 mutations depended on the location of the mutation in the protein. Cells carrying recessive GDAP1 mutations located in the GST or transmembrane domains showed no failure in SOCE, but those located inside the α-loop domain (the region involved in protein-protein interactions) mimicked the SOCE defect seen in GDAP1 deficiency [21]. Analysis of mitochondrial distribution in cells expressing these mutations may explain these differences in SOCE. Mitochondria carrying recessive GDAP1 mutations located in the α-loop domain did not relocate to the plasma membrane under SOCE-activated conditions (Figure 1), a likely cause for SOCE impairment that is similar to previous descriptions in GDAP1-deficient cells [20]. However, in cells expressing dominant GDAP1 mutations, mitochondria were already in close proximity to the plasma membrane in basal conditions [21]. This bias in basal mitochondrial distribution may be related to the increase in SOCE activity found in these mutants, whose Ca2+-inactivation may be prevented by resident mitochondria at the plasma membrane.
The decrease in SOCE activity in recessive mutations in GDAP1 α-loop domain has been linked to a decrease in ER- Ca2+ levels [21], consistent with the well-known function of SOCE in replenishing the intracellular Ca2+ stores [74]. Lower ER-Ca2+ levels have also been observed in GDAP1-deficient cells and in motor neurons from Gdap1-KO mice [34]. In neurons, ER- Ca2+ plays a relevant role in synaptic activity, regulating several processes including synaptic plasticity [75], spontaneous neurotransmitter release in synaptic boutons [76,77], and metabotropic glutamate receptor-dependent synaptic transmission [78]. Disruption of ER- Ca2+ levels also leads to activation of ER stress coping responses [79], such as the unfolded protein response (UPR), which activates apoptotic pathways if ER- Ca2+ homeostasis cannot be restored [80]. These processes may be triggered by disturbed Ca2+ homeostasis caused by the recessive mutations described above, but more studies are needed to elucidate the real consequences of the decreased ER- Ca2+ levels in neurons affected by CMT disease.
In conclusion, although alterations in SOCE have been described in dominant GDAP1 mutations and recessive mutations located in the α-loop domain, the mechanism of CMT pathogenesis of each group of mutations seems to differ. These results suggest that decreased Ca2+ entry through SOCs and lower ER- Ca2+ levels, probably due to a failure of SOCE Ca2+ uptake caused by mislocalized mitochondria, may contribute to the pathogenesis of CMT patients carrying a recessive GDAP1 mutation inside the α-loop domain (Figure 1). On the other hand, dominant mutations may act through a gain of function mechanism, which could be related to an increase in ROS production and apoptosis, as reported previously [15]. Interestingly, an increase in SOCE activity has been found to be detrimental in astrocytes in a mouse model of amyotrophic lateral sclerosis (ALS) [81], and in striatal neurons in a model of HD [82].
5. Neuronal Store-Operated Calcium Entry (SOCE) and Its Role in Neurodegenerative Diseases
Ca2+ is a major second messenger in cells [83,84] of critical importance in neurons since it is a key component of neurotransmission, synaptic plasticity, and gene transcription. Deregulation of Ca2+ homeostasis has been shown to be a common underlying mechanism of several major neurological disorders including PD [85], HD [86], AD [87,88], or ALS [89]. Therefore, control of Ca2+ homeostasis is essential for proper function of neurons. Neurons possess many different specialized Ca2+ channels and a collection of Ca2+ handling proteins, several of them specific to neurons [90,91]. SOCE is the main Ca2+ entry pathway in non-excitable cells [92], but its existence in neurons has been classically debated due to the presence of other major Ca2+ influx pathways [93,94]. However, SOCE activity has been found in different types of neurons both in the central [78,95,96,97,98,99] and peripheral nervous system [100]. Neuronal SOCE has been shown to perform different functions in resting neurons: refilling of Ca2+ stores [97]; control of spine morphology [101,102]; and regulation of neuronal gene expression [103]. Moreover, an increasing number of studies have also proposed a role of SOCE in neuronal synaptic activity by controlling Ca2+ dynamics [78,104,105,106] and different forms of synaptic plasticity [75,107,108,109], including long term depression [95].
Considering all these functions, it is not surprising that disruption of neuronal SOCE has been linked to major neurological disorders [110]. Different studies of familial AD caused by mutations in presenilin genes reported general alterations in Ca2+ homeostasis, and specifically an impairment in SOCE activity [101,111,112,113,114]. Additional evidence suggests that the molecular players of SOCE may also be involved in the pathogenesis of AD. Changes in STIM1 and STIM2, the sensors of intraluminal Ca2+ levels that transport information about Ca2+ load of the stores to channel proteins at the plasma membrane [67], have been found in cases of sporadic [101] and familial AD [115]. Decreased STIM2 levels caused an impaired neuronal SOCE in two different mouse models of familial AD, presenilin [101] and amyloid precursor protein knock-in (APPKI) mouse models [116], leading to a disruption of SOCE-Ca2+/calmodulin-dependent protein kinase II (CaMKII) pathway and finally, to mature spine loss. Importantly, overexpression of STIM2 in hippocampal neurons rescued the downstream signaling cascade and dendritic spine morphology in both presenilin [101] and APPKI mouse models [116]. The lack of STIM1 in differentiated neuroblastoma cells triggered a pathological increase in Ca2+ entry through L-type voltage-operated Ca2+ channels in response to depolarization [115], in agreement with the modulating role of STIM1 on these channels [117,118].
Similarly, a failure in SOCE activation may also play a role in the pathogenesis of PD. The loss of TRPC1 or STIM1, molecules involved in SOCE, has been linked to death of dopaminergic neurons due to an increase in L-type Ca2+ currents [119,120]. Moreover, skin fibroblasts from idiopathic and familial PD patients have been shown to have an impairment of SOCE activation not associated with reduction in Orai1, TRPC1, STIM1 or STIM2 expression, and depleted ER-Ca2+ stores [121].
On the contrary, hyperactive SOCE has been linked to HD. Studies using the transgenic YAC128 HD mouse model found an enhanced SOCE in striatal medium spiny neurons (MSNs) [122,123,124], which seems to compensate for an increase in Ca2+ leakage from the ER [122]. This abnormal activation of SOCE may underlie the striatal synaptic loss found in YAC128 mice [122]. In this HD mouse model, an increase in expression of the ER protein STIM2 has also recently been reported in cultured MSNs and the striatum. Accordingly, downregulation of STIM2 rescued the loss of dendritic spines [122]. Modulation of all molecular players of SOCE may have a neuroprotective role not only in HD [125], but also in other disorders where altered expression of these molecules has been found, such as epilepsy and traumatic brain injury (TBI). STIM1 and STIM2 are upregulated in the hippocampus of chronic epileptic mice and were found to be strongly expressed in hippocampal samples from a patient suffering from medial temporal lobe epilepsy [126]. This highlights a role for SOCE in somehow controlling neuronal network excitability, as proposed for dorsal horn neurons [127]. Expression of these two ER proteins was also found to be upregulated after induction of TBI in two different studies. Upregulation of STIM2, both in vitro and in vivo, triggered an increased SOCE, mitochondrial Ca2+ overload and deregulation of mitochondrial functions, such as ROS production. Interestingly, all these functions were recovered upon downregulation of STIM2 [128]. A different group showed STIM1 upregulation in response to neuronal injury, finding that Stim1 knockdown improved neuronal survival and reduced neuronal apoptosis after TBI [105].
All these studies suggest that SOCE may be a common mechanism involved in Ca2+ deregulation in many neurological disorders, which may include CMT. However, the role and relevance of Ca2+ influx by SOCs in the neurons that are affected by CMT, i.e., the sensory and motor neurons of the peripheral nervous system, is yet to be determined.
6. Impact of Ca2+ Deregulation on Mitochondrial Bioenergetics in GDAP1-CMT Model
Mitochondria use Ca2+ uptake and release to modulate cytosolic Ca2+ signals, thereby regulating numerous processes in cells [42] including SOCE [129]. Mitochondrial Ca2+ handling is a highly regulated process [130]. Permeability of the outer mitochondrial membrane is attributed to the abundant expression of porins [131], whereas in the inner mitochondrial membrane, Ca2+ influx into the matrix is mediated by the mitochondrial calcium uniporter (MCU) [132,133]. The properties of the MCU are determined by several proteins that interact with it to form the MCU complex (MCUc) [42], including the Ca2+ sensitivity modulators MICU1, MICU2, and MICU3 [134,135,136], the essential MCU regulator (EMRE) [137], the dominant negative subunit MCUb [138] and the regulator (MCUR) [139]. Additionally, there are other routes of Ca2+ influx which are under debate [140]. The major Ca2+ efflux pathway is the Na+/Ca2+ exchanger (NCLX) [141]. Ca2+ uptake by mitochondria not only serves as a Ca2+ buffering system, but also as a pathway to regulate intrinsic functions, including the main mitochondrial task: ATP production by oxidative phosphorylation [142,143].
Ca2+ influx through SOCs has been shown to stimulate mitochondrial respiration coupled to ATP synthesis in human neuroblastoma cells [21]. Previous work in these cells found that SOCE stimulation by muscarinic receptor activation induced glucose uptake by activation of AMP kinase subsequent to CaMKKβ activation [144], which may provide respiratory substrates for SOCE-stimulated respiration. Importantly, SOCE-induced upregulation of mitochondrial respiration is impaired by GDAP1 deficiency or by the presence of recessive GDAP1 mutations located inside the α-loop domain in these cells [21].
In excitable cells, the role of Ca2+ in tuning energy supply to demand has long been known [145,146,147]. Ca2+ regulates ATP homeostasis both by increasing ATP consumption via activation of several ATP-dependent processes, and by promoting ATP synthesis through activation of mitochondrial dehydrogenases and direct stimulation of oxidative phosphorylation (OXPHOS) [148]. Neuroblastoma cell experiments using BAPTA-AM, a calcium chelator that prevents Ca2+ signaling but not changes in workload [149], showed that Ca2+ signaling itself is needed to couple SOCE activity to mitochondrial respiration [21].
Ca2+-dependent regulation of OXPHOS involves two main mechanisms (Figure 1). On the one hand, Ca2+ entry into mitochondria through the MCUc [42] is known to activate different enzymes in the matrix, including the citric acid dehydrogenases: isocitrate dehydrogenase and α-ketoglutarate dehydrogenase, which directly bind Ca2+; and pyruvate dehydrogenase, which is activated by Ca2+-sensitive phosphatase activity [150,151]. Matrix Ca2+ has also been proposed to regulate both F1-F0ATPase activity, by reducing its resistance to ATP production, and some other respiratory complexes [148,151]. The second mechanism involved in Ca2+-dependent regulation of OXPHOS does not require Ca2+ entry into mitochondria, rather it depends on the activity of calcium binding mitochondrial carriers (CaMCs) (Figure 1). Two groups of carriers form this family: the aspartate-glutamate carriers (AGC); and the ATP-Mg/Pi carriers, also named SCaMC (for short CaMC) [152,153,154,155,156]. Both types of CaMCs are characterized by the presence of EF-hand domains that are oriented toward the intermembrane space and activated by cytosolic Ca2+ [157,158,159,160]; they both function along with the MCU to decode the Ca2+ signal into a mitochondrial response.
In neuroblastoma cells, a rise of mitochondrial Ca2+ levels has been shown upon SOCE activation, suggesting that both mechanisms, matrix and extramitochondrial Ca2+ signaling pathways, may be implied to boost respiration [21]. Establishing which of these mechanisms is involved will aid in identifying potential targets in GDAP1-related CMT diseases that may lead to a failure in mitochondrial energy metabolism. Disruption of any of these mechanisms has been found to impair mitochondrial bioenergetics in different cell types. For example, in cardiomyocytes, the MCU selectively mediates acute mitochondrial Ca2+ loading to augment ATP production [161,162], and a lack of MCU in skeletal muscle led to an impaired oxidative metabolism and a shift toward fatty acid metabolism [163,164]. On the other hand, in cortical neurons Aralar/AGC1 is required to adjust coupled respiration to ATP demand under different workloads by providing pyruvate to mitochondria [147,149,165]. These results indicate that each Ca2+-dependent regulation of OXPHOS may have different relevance depending on cell type.
In conclusion, in neuroblastoma cell lines, SOCE deregulation affects cellular respiration in cells carrying recessive GDAP1 mutations inside the α-loop domain (Figure 1) [21]. This suggests that a failure in mitochondrial bioenergetics may contribute to the molecular pathogenesis of CMT disease caused by these mutations. It will be interesting to know whether a similar deregulation and failure in mitochondrial bioenergetics occurs in sensory and motor neurons in other types of GDAP1-related and other mouse models of CMT disease.
7. Concluding Remarks
An impairment of axonal mitochondrial transport has been proposed to underlie the pathology of CMT due to mutations in different genes, as CMT2A2 [49], CMT2F [55,56], and CMT2L [53]. Studies of the effect of different CMT mutations in GDAP1 showed changes in mitochondrial dynamics and distribution within the cell and may be across the axon, leading to a general disruption of the mitochondrial network [12,15,16,20,21]. As alteration of a mitochondrial process can also affect other cellular functions, it can be challenging to identify the true cause of the pathology. A failure in Ca2+ homeostasis as a consequence of mitochondrial mislocalization has been found in cells carrying dominant GDAP1 mutations and recessive mutations located inside the α-loop domain (Figure 1), with an associated augmentation or decrease of SOCE activity, respectively [21]. Interestingly, no Ca2+ alterations have been found in recessive mutations located outside the α-loop domain of GDAP1, which may act by a completely different mechanism. Recessive GDAP1 mutations inside the α-loop domain cause a failure in stimulation of mitochondrial respiration associated with an impairment in SOCE activity (Figure 1) [21], but this may also take place in other GDAP1-related CMT diseases. For example, the prolonged elevated ROS production described in dominant mutations [15] may trigger permanent damage to mitochondria, leading to an impairment in bioenergetics [166].
These results suggest that a failure in mitochondrial control of Ca2+ entry by SOCs could be a possible mechanism underlying the pathology of GDAP1-related CMT, although additional defects in other Ca2+ entry mechanisms, such as voltage gated Ca2+ channels, cannot be excluded, particularly those in neuromuscular junctions [167,168,169,170]. More studies are necessary to identify which Ca2+ mechanisms are altered in the affected neurons of GDAP1-related CMT patients and Gdap1 mouse models in comparison with other mouse models of CMT.
Author Contributions
All authors contributed to the preparation of this review. Writing—original draft P.G.-S. and J.S.; Writing—review and editing P.G.-S., J.S., F.P. and A.d.A.
Funding
This work has been funded by grants from the Spanish Ministry of Science, Innovation and Universities SAF2014-56929-R (to JS), SAF2017-82560-R (to AdelA) and SAF2015–66625-R (FP). By the Collaborative Joint Project awarded by IRDiRC and funded by the Instituto de Salud Carlos III (ISCIII) IR11/TREAT-CMT (JS, FP), and grants from Fundación Ramón Areces (to JS) and the Generalitat de Catalunya 2015 FEDER/S-21 (FP). This work has also been funded by the CIBERER, an initiative from the ISCIII, and by an institutional grant from the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa. P G-S is the recipient of a postdoctoral research contract from Comunidad de Madrid.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pareyson D., Saveri P., Pisciotta C. New developments in Charcot-Marie-Tooth neuropathy and related diseases. Curr. Opin. Neurol. 2017;30:471–480. doi: 10.1097/WCO.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 2.Juarez P., Palau F. Neural and molecular features on Charcot-Marie-Tooth disease plasticity and therapy. Neural Plast. 2012;2012:171636. doi: 10.1155/2012/171636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossor A.M., Polke J.M., Houlden H., Reilly M.M. Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat. Rev. Neurol. 2013;9:562–571. doi: 10.1038/nrneurol.2013.179. [DOI] [PubMed] [Google Scholar]
- 4.Rzepnikowska W., Kochanski A. A role for the GDAP1 gene in the molecular pathogenesis of Charcot Marie Tooth disease. Acta Neurobiol. Exp. 2018;78:1–13. doi: 10.21307/ane-2018-002. [DOI] [PubMed] [Google Scholar]
- 5.Baxter R.V., Ben Othmane K., Rochelle J.M., Stajich J.E., Hulette C., Dew-Knight S., Hentati F., Ben Hamida M., Bel S., Stenger J.E., et al. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat. Genet. 2002;30:21–22. doi: 10.1038/ng796. [DOI] [PubMed] [Google Scholar]
- 6.Cuesta A., Pedrola L., Sevilla T., Garcia-Planells J., Chumillas M.J., Mayordomo F., LeGuern E., Marin I., Vilchez J.J., Palau F. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat. Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 7.Cassereau J., Chevrollier A., Gueguen N., Desquiret V., Verny C., Nicolas G., Dubas F., Amati-Bonneau P., Reynier P., Bonneau D., et al. Mitochondrial dysfunction and pathophysiology of Charcot-Marie-Tooth disease involving GDAP1 mutations. Exp. Neurol. 2011;227:31–41. doi: 10.1016/j.expneurol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Senderek J., Bergmann C., Ramaekers V.T., Nelis E., Bernert G., Makowski A., Zuchner S., De Jonghe P., Rudnik-Schoneborn S., Zerres K., et al. Mutations in the ganglioside-induced differentiation-associated protein-1 (GDAP1) gene in intermediate type autosomal recessive Charcot-Marie-Tooth neuropathy. Brain. 2003;126:642–649. doi: 10.1093/brain/awg068. [DOI] [PubMed] [Google Scholar]
- 9.Claramunt R., Pedrola L., Sevilla T., Lopez de Munain A., Berciano J., Cuesta A., Sanchez-Navarro B., Millan J.M., Saifi G.M., Lupski J.R., et al. Genetics of Charcot-Marie-Tooth disease type 4A: Mutations, inheritance, phenotypic variability, and founder effect. J. Med. Genet. 2005;42:358–365. doi: 10.1136/jmg.2004.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivera R., Espinos C., Vilchez J.J., Mas F., Martinez-Rubio D., Chumillas M.J., Mayordomo F., Muelas N., Bataller L., Palau F., et al. Phenotypical features of the p.R120W mutation in the GDAP1 gene causing autosomal dominant Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 2010;15:334–344. doi: 10.1111/j.1529-8027.2010.00286.x. [DOI] [PubMed] [Google Scholar]
- 11.Pedrola L., Espert A., Valdes-Sanchez T., Sanchez-Piris M., Sirkowski E.E., Scherer S.S., Farinas I., Palau F. Cell expression of GDAP1 in the nervous system and pathogenesis of Charcot-Marie-Tooth type 4A disease. J. Cell. Mol. Med. 2008;12:679–689. doi: 10.1111/j.1582-4934.2007.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niemann A., Ruegg M., La Padula V., Schenone A., Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: New implications for Charcot-Marie-Tooth disease. J. Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marco A., Cuesta A., Pedrola L., Palau F., Marin I. Evolutionary and structural analyses of GDAP1, involved in Charcot-Marie-Tooth disease, characterize a novel class of glutathione transferase-related genes. Mol. Biol. Evol. 2004;21:176–187. doi: 10.1093/molbev/msh013. [DOI] [PubMed] [Google Scholar]
- 14.Wagner K.M., Ruegg M., Niemann A., Suter U. Targeting and function of the mitochondrial fission factor GDAP1 are dependent on its tail-anchor. PLoS ONE. 2009;4:e5160. doi: 10.1371/journal.pone.0005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemann A., Wagner K.M., Ruegg M., Suter U. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol. Dis. 2009;36:509–520. doi: 10.1016/j.nbd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Pedrola L., Espert A., Wu X., Claramunt R., Shy M.E., Palau F. GDAP1, the protein causing Charcot-Marie-Tooth disease type 4A, is expressed in neurons and is associated with mitochondria. Hum. Mol. Genet. 2005;14:1087–1094. doi: 10.1093/hmg/ddi121. [DOI] [PubMed] [Google Scholar]
- 17.Huber N., Guimaraes S., Schrader M., Suter U., Niemann A. Charcot-Marie-Tooth disease-associated mutants of GDAP1 dissociate its roles in peroxisomal and mitochondrial fission. EMBO Rep. 2013;14:545–552. doi: 10.1038/embor.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noack R., Frede S., Albrecht P., Henke N., Pfeiffer A., Knoll K., Dehmel T., Meyer Zu Horste G., Stettner M., Kieseier B.C., et al. Charcot-Marie-Tooth disease CMT4A: GDAP1 increases cellular glutathione and the mitochondrial membrane potential. Hum. Mol. Genet. 2012;21:150–162. doi: 10.1093/hmg/ddr450. [DOI] [PubMed] [Google Scholar]
- 19.Niemann A., Huber N., Wagner K.M., Somandin C., Horn M., Lebrun-Julien F., Angst B., Pereira J.A., Halfter H., Welzl H., et al. The Gdap1 knockout mouse mechanistically links redox control to Charcot-Marie-Tooth disease. Brain. 2014;137:668–682. doi: 10.1093/brain/awt371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pla-Martin D., Rueda C.B., Estela A., Sanchez-Piris M., Gonzalez-Sanchez P., Traba J., de la Fuente S., Scorrano L., Renau-Piqueras J., Alvarez J., et al. Silencing of the Charcot-Marie-Tooth disease-associated gene GDAP1 induces abnormal mitochondrial distribution and affects Ca2+ homeostasis by reducing store-operated Ca2+ entry. Neurobiol. Dis. 2013;55:140–151. doi: 10.1016/j.nbd.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Sanchez P., Pla-Martin D., Martinez-Valero P., Rueda C.B., Calpena E., Del Arco A., Palau F., Satrustegui J. CMT-linked loss-of-function mutations in GDAP1 impair store-operated Ca2+ entry-stimulated respiration. Sci. Rep. 2017;7:42993. doi: 10.1038/srep42993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuchner S., Mersiyanova I.V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E.L., Zappia M., Nelis E., Patitucci A., Senderek J., et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 23.Pathak D., Berthet A., Nakamura K. Energy failure: Does it contribute to neurodegeneration? Ann. Neurol. 2013;74:506–516. doi: 10.1002/ana.24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panchal K., Tiwari A.K. Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion. 2018 doi: 10.1016/j.mito.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Murphy M.P., Hartley R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018 doi: 10.1038/nrd.2018.174. [DOI] [PubMed] [Google Scholar]
- 26.Estela A., Pla-Martin D., Sanchez-Piris M., Sesaki H., Palau F. Charcot-Marie-Tooth-related gene GDAP1 complements cell cycle delay at G2/M phase in Saccharomyces cerevisiae fis1 gene-defective cells. J. Biol. Chem. 2011;286:36777–36786. doi: 10.1074/jbc.M111.260042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez Del Amo V., Seco-Cervera M., Garcia-Gimenez J.L., Whitworth A.J., Pallardo F.V., Galindo M.I. Mitochondrial defects and neuromuscular degeneration caused by altered expression of Drosophila Gdap1: Implications for the Charcot-Marie-Tooth neuropathy. Hum. Mol. Genet. 2015;24:21–36. doi: 10.1093/hmg/ddu416. [DOI] [PubMed] [Google Scholar]
- 28.Shield A.J., Murray T.P., Board P.G. Functional characterisation of ganglioside-induced differentiation-associated protein 1 as a glutathione transferase. Biochem. Biophys. Res. Commun. 2006;347:859–866. doi: 10.1016/j.bbrc.2006.06.189. [DOI] [PubMed] [Google Scholar]
- 29.Huber N., Bieniossek C., Wagner K.M., Elsasser H.P., Suter U., Berger I., Niemann A. Glutathione-conjugating and membrane-remodeling activity of GDAP1 relies on amphipathic C-terminal domain. Sci. Rep. 2016;6:36930. doi: 10.1038/srep36930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chahbouni M., del Señor-Lopez M., Molina-Carballo A., de Haro T., Muñoz-Hoyos A., Fernandez-Ortiz M., Guerra-Librero A., Acuña-Castroviejo D. Melatonin treatment reduces oxidative damage and normalizes plasma pro-inflammatory cytokines in patients suffering from Charcot-Marie-Tooth neuropathy: A pilot study in three children. Molecules. 2017;22:1728. doi: 10.3390/molecules22101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W., Zhu H., Zhao X., Brancho D., Liang Y., Zou Y., Bennet C., Chow C.W. Dysregulated inflammatory signaling upon Charcot-Marie-Tooth type 1C mutation of SIMPLE protein. Mol. Cell. Biol. 2015;35:2464–2478. doi: 10.1128/MCB.00300-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi T., Rizzuto R., Hajnoczky G., Su T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vance J.E. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta. 2014;1841:595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Barneo-Munoz M., Juarez P., Civera-Tregon A., Yndriago L., Pla-Martin D., Zenker J., Cuevas-Martin C., Estela A., Sanchez-Arago M., Forteza-Vila J., et al. Lack of GDAP1 induces neuronal calcium and mitochondrial defects in a knockout mouse model of charcot-marie-tooth neuropathy. PLoS Genet. 2015;11:e1005115. doi: 10.1371/journal.pgen.1005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venturini G., Rose A.M., Shah A.Z., Bhattacharya S.S., Rivolta C. CNOT3 is a modifier of PRPF31 mutations in retinitis pigmentosa with incomplete penetrance. PLoS Genet. 2012;8:e1003040. doi: 10.1371/journal.pgen.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamar K.M., McNally E.M. Genetic Modifiers for Neuromuscular Diseases. J. Neuromuscul. Dis. 2014;1:3–13. doi: 10.3233/JND-140023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pla-Martin D., Calpena E., Lupo V., Marquez C., Rivas E., Sivera R., Sevilla T., Palau F., Espinos C. Junctophilin-1 is a modifier gene of GDAP1-related Charcot-Marie-Tooth disease. Hum. Mol. Genet. 2015;24:213–229. doi: 10.1093/hmg/ddu440. [DOI] [PubMed] [Google Scholar]
- 38.Nunnari J., Suomalainen A. Mitochondria: In sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D., Giorgi C., Leo S., Rimessi A., Siviero R., et al. Ca2+ transfer from the ER to mitochondria: When, how and why. Biochim. Biophys. Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carafoli E. The interplay of mitochondria with calcium: An historical appraisal. Cell Calcium. 2012;52:1–8. doi: 10.1016/j.ceca.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 42.De Stefani D., Rizzuto R., Pozzan T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annu. Rev. Biochem. 2016;85:161–192. doi: 10.1146/annurev-biochem-060614-034216. [DOI] [PubMed] [Google Scholar]
- 43.Harris J.J., Jolivet R., Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 44.Rangaraju V., Calloway N., Ryan T.A. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baloh R.H. Mitochondrial dynamics and peripheral neuropathy. Neuroscientist. 2008;14:12–18. doi: 10.1177/1073858407307354. [DOI] [PubMed] [Google Scholar]
- 46.Palau F., Estela A., Pla-Martin D., Sanchez-Piris M. The role of mitochondrial network dynamics in the pathogenesis of Charcot-Marie-Tooth disease. Adv. Exp. Med. Biol. 2009;652:129–137. doi: 10.1007/978-90-481-2813-6_9. [DOI] [PubMed] [Google Scholar]
- 47.Pareyson D., Saveri P., Sagnelli A., Piscosquito G. Mitochondrial dynamics and inherited peripheral nerve diseases. Neurosci. Lett. 2015;596:66–77. doi: 10.1016/j.neulet.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baloh R.H., Schmidt R.E., Pestronk A., Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J. Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cartoni R., Arnaud E., Medard J.J., Poirot O., Courvoisier D.S., Chrast R., Martinou J.C. Expression of mitofusin 2(R94Q) in a transgenic mouse leads to Charcot-Marie-Tooth neuropathy type 2A. Brain. 2010;133:1460–1469. doi: 10.1093/brain/awq082. [DOI] [PubMed] [Google Scholar]
- 51.Vallat J.M., Ouvrier R.A., Pollard J.D., Magdelaine C., Zhu D., Nicholson G.A., Grew S., Ryan M.M., Funalot B. Histopathological findings in hereditary motor and sensory neuropathy of axonal type with onset in early childhood associated with mitofusin 2 mutations. J. Neuropathol. Exp. Neurol. 2008;67:1097–1102. doi: 10.1097/NEN.0b013e31818b6cbc. [DOI] [PubMed] [Google Scholar]
- 52.Misko A., Jiang S., Wegorzewska I., Milbrandt J., Baloh R.H. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J. Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irobi J., Van Impe K., Seeman P., Jordanova A., Dierick I., Verpoorten N., Michalik A., De Vriendt E., Jacobs A., Van Gerwen V., et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat. Genet. 2004;36:597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- 54.Evgrafov O.V., Mersiyanova I., Irobi J., Van Den Bosch L., Dierick I., Leung C.L., Schagina O., Verpoorten N., Van Impe K., Fedotov V., et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat. Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- 55.Ackerley S., James P.A., Kalli A., French S., Davies K.E., Talbot K. A mutation in the small heat-shock protein HSPB1 leading to distal hereditary motor neuronopathy disrupts neurofilament assembly and the axonal transport of specific cellular cargoes. Hum. Mol. Genet. 2006;15:347–354. doi: 10.1093/hmg/ddi452. [DOI] [PubMed] [Google Scholar]
- 56.Kalmar B., Innes A., Wanisch K., Kolaszynska A.K., Pandraud A., Kelly G., Abramov A.Y., Reilly M.M., Schiavo G., Greensmith L. Mitochondrial deficits and abnormal mitochondrial retrograde axonal transport play a role in the pathogenesis of mutant Hsp27-induced Charcot Marie Tooth Disease. Hum. Mol. Genet. 2017;26:3313–3326. doi: 10.1093/hmg/ddx216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.F., Yao T.P. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 58.Reed N.A., Cai D., Blasius T.L., Jih G.T., Meyhofer E., Gaertig J., Verhey K.J. Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 59.Kim J.Y., Woo S.Y., Hong Y.B., Choi H., Kim J., Choi H., Mook-Jung I., Ha N., Kyung J., Koo S.K., et al. HDAC6 Inhibitors Rescued the Defective Axonal Mitochondrial Movement in Motor Neurons Derived from the Induced Pluripotent Stem Cells of Peripheral Neuropathy Patients with HSPB1 Mutation. Stem Cells Int. 2016;2016:9475981. doi: 10.1155/2016/9475981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen S., Benoy V., Bergman J.A., Kalin J.H., Frojuello M., Vistoli G., Haeck W., Van Den Bosch L., Kozikowski A.P. Bicyclic-Capped Histone Deacetylase 6 Inhibitors with Improved Activity in a Model of Axonal Charcot-Marie-Tooth Disease. ACS Chem. Neurosci. 2016;7:240–258. doi: 10.1021/acschemneuro.5b00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benoy V., Vanden Berghe P., Jarpe M., Van Damme P., Robberecht W., Van Den Bosch L. Development of Improved HDAC6 Inhibitors as Pharmacological Therapy for Axonal Charcot-Marie-Tooth Disease. Neurotherapeutics. 2017;14:417–428. doi: 10.1007/s13311-016-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Putney J.W., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 63.Roos J., Digregorio P.J., Yeromin A.V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J.A., Wagner S.L., Cahalan M.D., et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brandman O., Liou J., Park W.S., Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosado J.A., Diez R., Smani T., Jardin I. STIM and Orai1 Variants in Store-Operated Calcium Entry. Front. Pharmacol. 2015;6:325. doi: 10.3389/fphar.2015.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Worley P.F., Zeng W., Huang G.N., Yuan J.P., Kim J.Y., Lee M.G., Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205–211. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis R.S. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 68.Parekh A.B. Regulation of CRAC channels by Ca2+-dependent inactivation. Cell Calcium. 2017;63:20–23. doi: 10.1016/j.ceca.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Varadi A., Cirulli V., Rutter G.A. Mitochondrial localization as a determinant of capacitative Ca2+ entry in HeLa cells. Cell Calcium. 2004;36:499–508. doi: 10.1016/j.ceca.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Nunez L., Valero R.A., Senovilla L., Sanz-Blasco S., Garcia-Sancho J., Villalobos C. Cell proliferation depends on mitochondrial Ca2+ uptake: Inhibition by salicylate. J. Physiol. 2006;571:57–73. doi: 10.1113/jphysiol.2005.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quintana A., Schwarz E.C., Schwindling C., Lipp P., Kaestner L., Hoth M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J. Biol. Chem. 2006;281:40302–40309. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 72.Quintana A., Schwindling C., Wenning A.S., Becherer U., Rettig J., Schwarz E.C., Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc. Natl. Acad. Sci. USA. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samanta K., Douglas S., Parekh A.B. Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store-operated Ca2+ entry and Ca2+-dependent gene expression in response to receptor stimulation. PLoS ONE. 2014;9:e101188. doi: 10.1371/journal.pone.0101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parekh A.B., Putney J.W., Jr. Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 75.Segal M., Korkotian E. Roles of Calcium Stores and Store-Operated Channels in Plasticity of Dendritic Spines. Neuroscientist. 2016;22:477–485. doi: 10.1177/1073858415613277. [DOI] [PubMed] [Google Scholar]
- 76.Emptage N.J., Reid C.A., Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/S0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 77.de Juan-Sanz J., Holt G.T., Schreiter E.R., de Juan F., Kim D.S., Ryan T.A. Axonal Endoplasmic Reticulum Ca2+ Content Controls Release Probability in CNS Nerve Terminals. Neuron. 2017;93:867–881.e6. doi: 10.1016/j.neuron.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hartmann J., Karl R.M., Alexander R.P., Adelsberger H., Brill M.S., Ruhlmann C., Ansel A., Sakimura K., Baba Y., Kurosaki T., et al. STIM1 controls neuronal Ca2+ signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron. 2014;82:635–644. doi: 10.1016/j.neuron.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 79.Krebs J., Agellon L.B., Michalak M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015;460:114–121. doi: 10.1016/j.bbrc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 80.Shore G.C., Papa F.R., Oakes S.A. Signaling cell death from the endoplasmic reticulum stress response. Curr. Opin. Cell Biol. 2011;23:143–149. doi: 10.1016/j.ceb.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawamata H., Ng S.K., Diaz N., Burstein S., Morel L., Osgood A., Sider B., Higashimori H., Haydon P.G., Manfredi G., et al. Abnormal intracellular calcium signaling and SNARE-dependent exocytosis contributes to SOD1G93A astrocyte-mediated toxicity in amyotrophic lateral sclerosis. J. Neurosci. 2014;34:2331–2348. doi: 10.1523/JNEUROSCI.2689-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu J., Ryskamp D.A., Liang X., Egorova P., Zakharova O., Hung G., Bezprozvanny I. Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington’s Disease Mouse Model. J. Neurosci. 2016;36:125–141. doi: 10.1523/JNEUROSCI.1038-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 84.Brini M., Cali T., Ottolini D., Carafoli E. Intracellular calcium homeostasis and signaling. Met. Ions Life Sci. 2013;12:119–168. doi: 10.1007/978-94-007-5561-1_5. [DOI] [PubMed] [Google Scholar]
- 85.Pfeiffer R.F. Parkinson disease: Calcium channel blockers and Parkinson disease. Nat. Rev. Neurol. 2010;6:188–189. doi: 10.1038/nrneurol.2010.31. [DOI] [PubMed] [Google Scholar]
- 86.Lim D., Fedrizzi L., Tartari M., Zuccato C., Cattaneo E., Brini M., Carafoli E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J. Biol. Chem. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 87.Berridge M.J. Calcium regulation of neural rhythms, memory and Alzheimer’s disease. J. Physiol. 2014;592:281–293. doi: 10.1113/jphysiol.2013.257527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Popugaeva E., Pchitskaya E., Bezprozvanny I. Dysregulation of neuronal calcium homeostasis in Alzheimer’s disease—A therapeutic opportunity? Biochem. Biophys. Res. Commun. 2017;483:998–1004. doi: 10.1016/j.bbrc.2016.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leal S.S., Gomes C.M. Calcium dysregulation links ALS defective proteins and motor neuron selective vulnerability. Front. Cell. Neurosci. 2015;9:225. doi: 10.3389/fncel.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grienberger C., Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Brini M., Cali T., Ottolini D., Carafoli E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prakriya M., Lewis R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Putney J.W., Jr. Capacitative calcium entry in the nervous system. Cell Calcium. 2003;34:339–344. doi: 10.1016/S0143-4160(03)00143-X. [DOI] [PubMed] [Google Scholar]
- 94.Lu B., Fivaz M. Neuronal SOCE: Myth or Reality? Trends Cell Biol. 2016;26:890–893. doi: 10.1016/j.tcb.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 95.Gonzalez-Sanchez P., Del Arco A., Esteban J.A., Satrustegui J. Store-Operated Calcium Entry Is Required for mGluR-Dependent Long Term Depression in Cortical Neurons. Front. Cell. Neurosci. 2017;11:363. doi: 10.3389/fncel.2017.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kann O., Taubenberger N., Huchzermeyer C., Papageorgiou I.E., Benninger F., Heinemann U., Kovacs R. Muscarinic receptor activation determines the effects of store-operated Ca2+-entry on excitability and energy metabolism in pyramidal neurons. Cell Calcium. 2012;51:40–50. doi: 10.1016/j.ceca.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Samtleben S., Wachter B., Blum R. Store-operated calcium entry compensates fast ER calcium loss in resting hippocampal neurons. Cell Calcium. 2015;58:147–159. doi: 10.1016/j.ceca.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 98.Berna-Erro A., Braun A., Kraft R., Kleinschnitz C., Schuhmann M.K., Stegner D., Wultsch T., Eilers J., Meuth S.G., Stoll G., et al. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci. Signal. 2009;2:ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- 99.Xia J., Pan R., Gao X., Meucci O., Hu H. Native store-operated calcium channels are functionally expressed in mouse spinal cord dorsal horn neurons and regulate resting calcium homeostasis. J. Physiol. 2014;592:3443–3461. doi: 10.1113/jphysiol.2014.275065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gemes G., Bangaru M.L., Wu H.E., Tang Q., Weihrauch D., Koopmeiners A.S., Cruikshank J.M., Kwok W.M., Hogan Q.H. Store-operated Ca2+ entry in sensory neurons: Functional role and the effect of painful nerve injury. J. Neurosci. 2011;31:3536–3549. doi: 10.1523/JNEUROSCI.5053-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun S., Zhang H., Liu J., Popugaeva E., Xu N.J., Feske S., White C.L., 3rd, Bezprozvanny I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron. 2014;82:79–93. doi: 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Korkotian E., Segal M. Orai1 regulates calcium entry into dendritic spines. Channels. 2017;11:99–100. doi: 10.1080/19336950.2016.1247528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lalonde J., Saia G., Gill G. Store-operated calcium entry promotes the degradation of the transcription factor Sp4 in resting neurons. Sci. Signal. 2014;7:ra51. doi: 10.1126/scisignal.2005242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baba A., Yasui T., Fujisawa S., Yamada R.X., Yamada M.K., Nishiyama N., Matsuki N., Ikegaya Y. Activity-evoked capacitative Ca2+ entry: Implications in synaptic plasticity. J. Neurosci. 2003;23:7737–7741. doi: 10.1523/JNEUROSCI.23-21-07737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hou P.F., Liu Z.H., Li N., Cheng W.J., Guo S.W. Knockdown of STIM1 improves neuronal survival after traumatic neuronal injury through regulating mGluR1-dependent Ca2+ signaling in mouse cortical neurons. Cell. Mol. Neurobiol. 2015;35:283–292. doi: 10.1007/s10571-014-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gruszczynska-Biegala J., Sladowska M., Kuznicki J. AMPA Receptors Are Involved in Store-Operated Calcium Entry and Interact with STIM Proteins in Rat Primary Cortical Neurons. Front. Cell. Neurosci. 2016;10:251. doi: 10.3389/fncel.2016.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Majewski L., Kuznicki J. SOCE in neurons: Signaling or just refilling? Biochim. Biophys. Acta. 2015;1853:1940–1952. doi: 10.1016/j.bbamcr.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 108.Moccia F., Zuccolo E., Soda T., Tanzi F., Guerra G., Mapelli L., Lodola F., D’Angelo E. Stim and Orai proteins in neuronal Ca2+ signaling and excitability. Front. Cell. Neurosci. 2015;9:153. doi: 10.3389/fncel.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wegierski T., Kuznicki J. Neuronal calcium signaling via store-operated channels in health and disease. Cell Calcium. 2018;74:102–111. doi: 10.1016/j.ceca.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 110.Pchitskaya E., Popugaeva E., Bezprozvanny I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium. 2018;70:87–94. doi: 10.1016/j.ceca.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Herms J., Schneider I., Dewachter I., Caluwaerts N., Kretzschmar H., Van Leuven F. Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. J. Biol. Chem. 2003;278:2484–2489. doi: 10.1074/jbc.M206769200. [DOI] [PubMed] [Google Scholar]
- 112.Leissring M.A., Akbari Y., Fanger C.M., Cahalan M.D., Mattson M.P., LaFerla F.M. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J. Cell Biol. 2000;149:793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoo A.S., Cheng I., Chung S., Grenfell T.Z., Lee H., Pack-Chung E., Handler M., Shen J., Xia W., Tesco G., et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–572. doi: 10.1016/S0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 114.Tong B.C., Lee C.S., Cheng W.H., Lai K.O., Foskett J.K., Cheung K.H. Familial Alzheimer’s disease-associated presenilin 1 mutants promote gamma-secretase cleavage of STIM1 to impair store-operated Ca2+ entry. Sci. Signal. 2016;9:ra89. doi: 10.1126/scisignal.aaf1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pascual-Caro C., Berrocal M., Lopez-Guerrero A.M., Alvarez-Barrientos A., Pozo-Guisado E., Gutierrez-Merino C., Mata A.M., Martin-Romero F.J. STIM1 deficiency is linked to Alzheimer’s disease and triggers cell death in SH-SY5Y cells by upregulation of L-type voltage-operated Ca2+ entry. J. Mol. Med. 2018;96:1061–1079. doi: 10.1007/s00109-018-1677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang H., Wu L., Pchitskaya E., Zakharova O., Saito T., Saido T., Bezprozvanny I. Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-In Mouse Model of Alzheimer’s Disease. J. Neurosci. 2015;35:13275–13286. doi: 10.1523/JNEUROSCI.1034-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park C.Y., Shcheglovitov A., Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y., Deng X., Mancarella S., Hendron E., Eguchi S., Soboloff J., Tang X.D., Gill D.L. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arshad A., Chen X., Cong Z., Qing H., Deng Y. TRPC1 protects dopaminergic SH-SY5Y cells from MPP+, salsolinol, and N-methyl-(R)-salsolinol-induced cytotoxicity. Acta Biochim. Biophys. Sin. 2014;46:22–30. doi: 10.1093/abbs/gmt127. [DOI] [PubMed] [Google Scholar]
- 120.Sun Y., Zhang H., Selvaraj S., Sukumaran P., Lei S., Birnbaumer L., Singh B.B. Inhibition of L-Type Ca2+ Channels by TRPC1-STIM1 Complex Is Essential for the Protection of Dopaminergic Neurons. J. Neurosci. 2017;37:3364–3377. doi: 10.1523/JNEUROSCI.3010-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Q., Yen A., Rymarczyk G., Asai H., Trengrove C., Aziz N., Kirber M.T., Mostoslavsky G., Ikezu T., Wolozin B., et al. Impairment of PARK14-dependent Ca2+ signalling is a novel determinant of Parkinson’s disease. Nat. Commun. 2016;7:10332. doi: 10.1038/ncomms10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu J., Shih H.P., Vigont V., Hrdlicka L., Diggins L., Singh C., Mahoney M., Chesworth R., Shapiro G., Zimina O., et al. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington’s disease treatment. Chem. Biol. 2011;18:777–793. doi: 10.1016/j.chembiol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vigont V., Kolobkova Y., Skopin A., Zimina O., Zenin V., Glushankova L., Kaznacheyeva E. Both Orai1 and TRPC1 are Involved in Excessive Store-Operated Calcium Entry in Striatal Neurons Expressing Mutant Huntingtin Exon 1. Front. Physiol. 2015;6:337. doi: 10.3389/fphys.2015.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Czeredys M., Maciag F., Methner A., Kuznicki J. Tetrahydrocarbazoles decrease elevated SOCE in medium spiny neurons from transgenic YAC128 mice, a model of Huntington’s disease. Biochem. Biophys. Res. Commun. 2017;483:1194–1205. doi: 10.1016/j.bbrc.2016.08.106. [DOI] [PubMed] [Google Scholar]
- 125.Wu J., Ryskamp D., Birnbaumer L., Bezprozvanny I. Inhibition of TRPC1-Dependent Store-Operated Calcium Entry Improves Synaptic Stability and Motor Performance in a Mouse Model of Huntington’s Disease. J. Huntington’s Dis. 2018;7:35–50. doi: 10.3233/JHD-170266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Steinbeck J.A., Henke N., Opatz J., Gruszczynska-Biegala J., Schneider L., Theiss S., Hamacher N., Steinfarz B., Golz S., Brustle O., et al. Store-operated calcium entry modulates neuronal network activity in a model of chronic epilepsy. Exp. Neurol. 2011;232:185–194. doi: 10.1016/j.expneurol.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 127.Dou Y., Xia J., Gao R., Gao X., Munoz F.M., Wei D., Tian Y., Barrett J.E., Ajit S., Meucci O., et al. Orai1 Plays a Crucial Role in Central Sensitization by Modulating Neuronal Excitability. J. Neurosci. 2018;38:887–900. doi: 10.1523/JNEUROSCI.3007-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rao W., Zhang L., Peng C., Hui H., Wang K., Su N., Wang L., Dai S.H., Yang Y.F., Chen T., et al. Downregulation of STIM2 improves neuronal survival after traumatic brain injury by alleviating calcium overload and mitochondrial dysfunction. Biochim. Biophys. Acta. 2015;1852:2402–2413. doi: 10.1016/j.bbadis.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 129.Fonteriz R., Matesanz-Isabel J., Arias-Del-Val J., Alvarez-Illera P., Montero M., Alvarez J. Modulation of Calcium Entry by Mitochondria. Adv. Exp. Med. Biol. 2016;898:405–421. doi: 10.1007/978-3-319-26974-0_17. [DOI] [PubMed] [Google Scholar]
- 130.Szabadkai G., Duchen M.R. Mitochondria: The hub of cellular Ca2+ signaling. Physiology. 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 131.Blachly-Dyson E., Forte M. VDAC channels. IUBMB Life. 2001;52:113–118. doi: 10.1080/15216540152845902. [DOI] [PubMed] [Google Scholar]
- 132.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., Mccombs J.E., Palmer A.E., Mootha V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patron M., Checchetto V., Raffaello A., Teardo E., Vecellio Reane D., Mantoan M., Granatiero V., Szabo I., De Stefani D., Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patron M., Granatiero V., Espino J., Rizzuto R., De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2018;26:179–195. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sancak Y., Markhard A.L., Kitami T., Kovacs-Bogdan E., Kamer K.J., Udeshi N.D., Carr S.A., Chaudhuri D., Clapham D.E., Li A.A., et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raffaello A., De Stefani D., Sabbadin D., Teardo E., Merli G., Picard A., Checchetto V., Moro S., Szabo I., Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mallilankaraman K., Cardenas C., Doonan P.J., Chandramoorthy H.C., Irrinki K.M., Golenar T., Csordas G., Madireddi P., Yang J., Muller M., et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Elustondo P.A., Nichols M., Robertson G.S., Pavlov E.V. Mitochondrial Ca2+ uptake pathways. J. Bioenerg. Biomembr. 2017;49:113–119. doi: 10.1007/s10863-016-9676-6. [DOI] [PubMed] [Google Scholar]
- 141.Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 143.Llorente-Folch I., Rueda C.B., Pardo B., Szabadkai G., Duchen M.R., Satrustegui J. The regulation of neuronal mitochondrial metabolism by calcium. J. Physiol. 2015;593:3447–3462. doi: 10.1113/JP270254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Olianas M.C., Dedoni S., Onali P. Involvement of store-operated Ca2+ entry in activation of AMP-activated protein kinase and stimulation of glucose uptake by M3 muscarinic acetylcholine receptors in human neuroblastoma cells. Biochim. Biophys. Acta. 2014;1843:3004–3017. doi: 10.1016/j.bbamcr.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 145.Denton R.M., McCormack J.G. The calcium sensitive dehydrogenases of vertebrate mitochondria. Cell Calcium. 1986;7:377–386. doi: 10.1016/0143-4160(86)90040-0. [DOI] [PubMed] [Google Scholar]
- 146.Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hayakawa Y., Nemoto T., Iino M., Kasai H. Rapid Ca2+-dependent increase in oxygen consumption by mitochondria in single mammalian central neurons. Cell Calcium. 2005;37:359–370. doi: 10.1016/j.ceca.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 148.Glancy B., Balaban R.S. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Llorente-Folch I., Rueda C.B., Amigo I., del Arco A., Saheki T., Pardo B., Satrustegui J. Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J. Neurosci. 2013;33:13957–13971. doi: 10.1523/JNEUROSCI.0929-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 151.Balaban R.S. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.del Arco A., Satrustegui J. Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J. Biol. Chem. 1998;273:23327–23334. doi: 10.1074/jbc.273.36.23327. [DOI] [PubMed] [Google Scholar]
- 153.Palmieri L., Pardo B., Lasorsa F.M., del Arco A., Kobayashi K., Iijima M., Runswick M.J., Walker J.E., Saheki T., Satrustegui J., et al. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.del Arco A., Satrustegui J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J. Biol. Chem. 2004;279:24701–24713. doi: 10.1074/jbc.M401417200. [DOI] [PubMed] [Google Scholar]
- 155.Fiermonte G., De Leonardis F., Todisco S., Palmieri L., Lasorsa F.M., Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J. Biol. Chem. 2004;279:30722–30730. doi: 10.1074/jbc.M400445200. [DOI] [PubMed] [Google Scholar]
- 156.Satrustegui J., Pardo B., Del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol. Rev. 2007;87:29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- 157.Pardo B., Contreras L., Serrano A., Ramos M., Kobayashi K., Iijima M., Saheki T., Satrustegui J. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J. Biol. Chem. 2006;281:1039–1047. doi: 10.1074/jbc.M507270200. [DOI] [PubMed] [Google Scholar]
- 158.Contreras L., Gomez-Puertas P., Iijima M., Kobayashi K., Saheki T., Satrustegui J. Ca2+ Activation kinetics of the two aspartate-glutamate mitochondrial carriers, aralar and citrin: Role in the heart malate-aspartate NADH shuttle. J. Biol. Chem. 2007;282:7098–7106. doi: 10.1074/jbc.M610491200. [DOI] [PubMed] [Google Scholar]
- 159.Traba J., Froschauer E.M., Wiesenberger G., Satrustegui J., Del Arco A. Yeast mitochondria import ATP through the calcium-dependent ATP-Mg/Pi carrier Sal1p, and are ATP consumers during aerobic growth in glucose. Mol. Microbiol. 2008;69:570–585. doi: 10.1111/j.1365-2958.2008.06300.x. [DOI] [PubMed] [Google Scholar]
- 160.Traba J., Del Arco A., Duchen M.R., Szabadkai G., Satrustegui J. SCaMC-1 promotes cancer cell survival by desensitizing mitochondrial permeability transition via ATP/ADP-mediated matrix Ca2+ buffering. Cell Death Differ. 2012;19:650–660. doi: 10.1038/cdd.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luongo T.S., Lambert J.P., Yuan A., Zhang X., Gross P., Song J., Shanmughapriya S., Gao E., Jain M., Houser S.R., et al. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kwong J.Q., Lu X., Correll R.N., Schwanekamp J.A., Vagnozzi R.J., Sargent M.A., York A.J., Zhang J., Bers D.M., Molkentin J.D. The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kwong J.Q., Huo J., Bround M.J., Boyer J.G., Schwanekamp J.A., Ghazal N., Maxwell J.T., Jang Y.C., Khuchua Z., Shi K., et al. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight. 2018;3 doi: 10.1172/jci.insight.121689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gherardi G., Nogara L., Ciciliot S., Fadini G.P., Blaauw B., Braghetta P., Bonaldo P., De Stefani D., Rizzuto R., Mammucari C. Loss of mitochondrial calcium uniporter rewires skeletal muscle metabolism and substrate preference. Cell Death Differ. 2018 doi: 10.1038/s41418-018-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rueda C.B., Llorente-Folch I., Amigo I., Contreras L., Gonzalez-Sanchez P., Martinez-Valero P., Juaristi I., Pardo B., del Arco A., Satrustegui J. Ca2+ regulation of mitochondrial function in neurons. Biochim. Biophys. Acta. 2014;1837:1617–1624. doi: 10.1016/j.bbabio.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 166.Requejo-Aguilar R., Bolanos J.P. Mitochondrial control of cell bioenergetics in Parkinson’s disease. Free Radic. Biol. Med. 2016;100:123–137. doi: 10.1016/j.freeradbiomed.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Verstreken P., Ly C.V., Venken K.J., Koh T.W., Zhou Y., Bellen H.J. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 168.Trotta N., Rodesch C.K., Fergestad T., Broadie K. Cellular bases of activity-dependent paralysis in Drosophila stress-sensitive mutants. J. Neurobiol. 2004;60:328–347. doi: 10.1002/neu.20017. [DOI] [PubMed] [Google Scholar]
- 169.Guo X., Macleod G.T., Wellington A., Hu F., Panchumarthi S., Schoenfield M., Marin L., Charlton M.P., Atwood H.L., Zinsmaier K.E. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 170.Ly C.V., Verstreken P. Mitochondria at the synapse. Neuroscientist. 2006;12:291–299. doi: 10.1177/1073858406287661. [DOI] [PubMed] [Google Scholar]