Abstract
Background
Bartonella henselae, Bartonella clarridgeiae and the rare Bartonella koehlerae are zoonotic pathogens, with cats being regarded as the main reservoir hosts. The spread of the infection among cats occurs mainly via fleas and specific preventive measures need to be implemented. The effectiveness of a 10% imidacloprid/4.5% flumethrin polymer matrix collar (Seresto®, Bayer Animal Health), registered to prevent flea and tick infestations, in reducing the risk of Bartonella spp. infection in privately owned cats, was assessed in a prospective longitudinal study.
Methods
In March-May 2015 [Day 0 (D0)], 204 privately-owned cats from the Aeolian Islands (Sicily) were collared (G1, n = 104) or left as controls (G2, n = 100). The bacteraemia of Bartonella spp. was assessed at enrolment (D0) and study closure (D360) by PCR and DNA sequencing both prior to and after an enrichment step, using Bartonella alpha proteobacteria growth medium (BAPGM).
Results
A total of 152 cats completed the study with 3 in G1 and 10 in G2 being positive for Bartonella spp. Bartonella henselae genotype I ZF1 (1.35%) and genotype II Fizz/Cal-1 (6.76%) as well as B. clarridgeiae (5.41%) were detected in cats of G2. Bartonella clarridgeiae was the only species detected in G1. Based on the yearly crude incidence of Bartonella spp. infection (i.e. 3.85% in G1 and 13.51% in G2; P = 0.03) the Seresto® collar achieved a preventative efficacy of 71.54%. The incidence of Bartonella spp. infection was more frequent in flea-infested cats (6/33, 18.18%) than in uninfested ones (7/112, 5.88%) (P = 0.036).
Conclusions
Cats living in the Aeolian Islands are exposed to B. henselae and B. clarridgeiae. The Seresto® collar provided significant risk reduction against Bartonella spp. infection in outdoor cats under field conditions. Such a preventative tool could be a key contribution for decreasing the risk of Bartonella spp. infection in cats and thus ultimately to humans.
Keywords: Bartonella henselae, Bartonella clarridgeiae, Phylogeny, ZF1, Fizz/Cal1, Genotype, Feline VBDs, Incidence, Prevention, Pyrethroids, Flumethrin
Background
About 35 species of Bartonella, small intracellular, fastidious, Gram-negative alpha-proteobacteria, have been described so far [1]. These bacteria are transmitted by a number of vectors, including fleas, and are highly adapted to one or more mammalian hosts, often causing a long-lasting intra-erythrocytic bacteraemia as a hallmark of infection [2, 3]. Cats are the natural reservoir of Bartonella henselae and, less commonly, of Bartonella clarridgeiae and the rare Bartonella koehlerae [4–6].
Bartonella henselae exhibits heterogeneity with two serotypes (Houston-1 and Marseille) [7, 8] which overlap genotypes I (Houston I) and genotype II (Marseille), identified according to 16S rRNA gene sequences [8–11]. In addition, within the above genotypes, two genogroups (Houston I and Marseille II) with more genotypes were delineated based on the heme-binding protein (Pap31) encoding gene [10]. The two genogroups above displayed major variations according to animal host species (i.e. humans and domestic cats) and, within the same host species, according to the geographical origin. For example, B. henselae genotype II has been predominantly detected in cat populations from western USA, western Europe (France, Germany, Italy, Netherlands, UK) and Australia, whereas genotype I is dominant in Asia (Japan and the Philippines) [12, 13]. The spread of the Bartonella spp. infection among cats occurs mainly via Ctenocephalides felis, the cat flea [4, 14], in whose digestive system they multiply, surviving several days in the faeces [15, 16]. Indeed, B. henselae was experimentally transmitted among cats by transferring fleas fed on naturally infected cats to specific pathogen-free (SPF) cats, and by intradermal injection of excrement collected from fleas fed on B. henselae-infected cats [17–20]. Additionally, successful transmission of infection was demonstrated after intradermal injection of blood from cats that had been infected by a virulent field strain of B. henselae [20]. Accordingly, possible routes of transmission of Bartonella spp. amongst cats include flea bites, contamination of open wounds with excrement of infected fleas, and ingestion of infected fleas or flea faeces [16]. One to three weeks after infection by B. henselae cats develop an apparently asymptomatic relapsing bacteraemia, which may persist for months or years [20, 21].
For humans, the main mode of transmission of both B. henselae and B. clarridgeiae and putatively B. koehlerae is via contaminated scratches and/or bites of infected cats [22]. Incidental transmission of B. henselae to humans can result in a wide range of clinical manifestations also according to the patient’s immune status [23]. In immuno-competent individuals cat-scratch disease (CSD) is characterized by a self-limiting but long-lasting swelling of the lymph node(s) draining the primary site of infection, often associated with high fever and, only rarely, endocarditis, neuroretinitis, uveitis and hepato-splenic abscesses [23–26]. Conversely, immunocompromised patients, such as AIDS patients, may develop bacillary angiomatosis or bacillary peliosis, which are characterized by tumour-like vasoproliferative lesions of the skin or the inner organs, respectively [27]. Similarly, B. clarridgeiae is a zoonotic pathogen which may cause asymptomatic haemotropic infection in cats and fever, lymphadenopathy and inoculation papules in humans [28]. Additional, B. koehlerae was found associated to culture-negative endocarditis in a human patient [29].
As domestic cats play a central role in the transmission of Bartonella to humans, measures for preventing the infection in these pets should be implemented. Healthy Bartonella carriers are usually treated with antibiotics [28, 30, 31], but a complete clearance of bacteraemia cannot be guaranteed, presenting the risk of antimicrobial resistance as well as of zoonotic transmission to humans [28, 30, 31]. Consequently, the best approach for preventing Bartonella infection in cats relies on the control of flea infestations by using ectoparasitic treatment [32]. A 10% imidacloprid/4.5% flumethrin polymer matrix collar (Seresto®, Bayer Animal Health) with both repellent (anti-feeding) and rapid killing efficacy [33, 34] has been licensed for preventing flea and tick infestations in cats. Studies demonstrated the efficacy of the collar for preventing transmission of vector-borne diseases under experimental and field conditions [35–37]. However, the efficacy against B. henselae was exclusively tested under experimental conditions in a restricted number of cats [38] and field studies are lacking. Therefore, the protective effectiveness of the Seresto® collar against feline Bartonella spp. infection was assessed in a cohort of privately-owned cats with regular outdoor access, from the Aeolian Islands (Sicily, southern Italy). In that area the occurrence of both B. henselae (2.73%, 95% CI: 0.97–4.48), and B. clarridgeiae (1.21%, 95% CI: 0.03–2.39) has been reported [39]. Additionally, as more variants of B. henselae have been diagnosed in geographical areas where CSD cases have been reported [9, 10, 13], we genetically characterized the population of B. henselae in cats involved in this study.
Methods
Study site and design
Samples used were collected under the frame of a previous prospective longitudinal, partly blinded, randomized field study aiming at evaluating the protective effectiveness of the collar against Leishmania infantum infection in cats [37, 40].
The cohort study was conducted in Lipari and Vulcano, two of the seven islands of the Aeolian archipelago (Tyrrhenian Sea, Sicily, Italy). Briefly, a total of 204 cats belonging to 80 owners were visited and assigned to group 1 (G1, n = 104) or group 2 (G2, n = 100) following a “per household” assignment random plan in March-May 2015 (D0). The enrolled animals, aged from 6 months to 15 years, were healthy based on clinical examination performed by a veterinarian. The cats in the G1 were treated with the Seresto® collar while those in G2 were left untreated (i.e. no ectoparasiticides were allowed) as controls. Data for fleas were recorded at the enrolment (D0), on D210 (at replacement of collars) and on D360 (study closure) while blood samples were collected on D0 and D360 from the jugular vein. Details on blood sampling as well as ectoparasite collection and processing have been described elsewhere [37, 40, 41]. During the study, cats remained with their owners and were managed as per normal routine without any containment measure or restriction.
Laboratory procedures
The bacteraemia of Bartonella spp. in cats was determined using PCR both prior to and after an enrichment step using Bartonella alpha proteobacteria growth medium (BAPGM), and isolation on blood agar slants, as previously described [42]. After thawing, an aliquot of 1 ml of EDTA whole blood was inoculated into 10 ml of BAPGM, after which the cultures were maintained at 35 °C in a 5% CO2, water-saturated atmosphere. After a 14-day incubation period, samples were tested by ITS-PCR. A 0.2 ml aliquot of the enrichment BAPGM which tested ITS-PCR positive was inoculated onto blood agar slants and incubated at 35 °C, in a 5% CO2, water-saturated atmosphere. Slants were checked for colony formation at 7, 14 and 21 days after inoculation.
Whole genomic DNA for Bartonella spp. PCR assays was extracted from EDTA blood as well as BAPGM enrichment liquid blood samples, and from blood agar if colonies were visible. The QIAamp DNA Micro Kit (Qiagen, Milan, Italy) was used according to the manufacturer’s instructions. Reference strains B. henselae (PV 252926) and B. clarridgeiae (PV85065) were used as positive controls.
Bartonella spp. detection was performed using the primers 325s and 1100as designed to amplify the Bartonella 16S-23S internal transcribed spacer (ITS) region as described previously [43]. The amplicon size generated is species-dependent, allowing preliminary B. henselae, B. clarridgeiae and B. koehlerae identification.
For B. henselae genotyping, all B. henselae-positive samples underwent a second step PCR targeting the Pap-31 gene by using the species-specific primers Pap31-BHSs and Pap31-688as, according to previously reported methodology [43]. The PCR assays were performed using a DNA Thermal Cycler Gene AMP 9600 (Applied Biosystem, Milan, Italy) in a 25 μl final volume containing 2 μl of DNA, 12.5 ml of AccuPrimeTM SuperMixII mix (Invitrogen, Milan, Italy) (40 mM Tris-HCl pH 8.4, 3 mM MgCl2, 100 mM KCl, 400 mM of each dNTP, AccuPrimeTM Taq DNA Polymerase), 200 pM of each primer and DNase-free H2O up to 25 μl. PCR products were examined on 2% agarose gels stained with GelRed (VWR International PBI, Milano, Italy) and visualized on a GelLogic 100 gel documentation system (Kodak, New York, USA).
All of the generated ITS and Pap31 PCR products were sequenced in both directions using the same primers as for PCR by Eurofins Genomics (Vimodrone, Italy) for B. clarridgeiae/B. koehlerae species identification and for B. henselae genotyping, respectively. The newly-generated sequences were edited, aligned and compared to reference GenBank sequences by nucleotide BLASTN program (https://blast.ncbi.nlm.nih.gov). Phylogenetic analyses were carried out using the software package Geneious version 10.1.3 (Biomatters Ltd., Auckland, New Zealand). For phylogenetic tree construction, a 400-nt fragment of the B. henselae Pap31 gene was analysed using Geneious Tree Builder. The Neighbor-Joining method was used with data resampled 1000 times to estimate the confidence of branching patterns. Representative sequences obtained in the study were submitted to the GenBank database.
Additionally, cats were also screened for feline haemoplasma bacteraemia. DNA of three feline haemoplasma species, Mycoplasma haemofelis (Mhf), “Candidatus Mycopasma haemominutum” (CMhm) and “Candidatus Mycoplasma turicensis” (CMt), were molecularly detected in blood samples by three specific real-time PCR assays as previously described [44]. Real-time PCR was performed in a 25-μl reaction mixture containing 12.5 μl of iTaqTM Universal Probes Supermix (Bio-Rad Laboratories Srl, Segrate, Milan, Italy), 600 nM of primers, 200 nM of probe and 10 μl of DNA. For each assay, positive controls included DNA extracted from blood samples of cats naturally infected with each haemoplasma species (laboratory collection). The thermal protocol consisted of activation of iTaq DNA polymerase at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 15 s, annealing at 48 °C for 30 s and extension at 60 °C for 1 min.
Data management and statistical analyses
A minimum sample size of 70 cats was estimated for each group, based on the assumptions of confidence level of 95%, power of 80% and expected incidence of Bartonella spp. infection of 1% and 13% in treated and untreated cats, respectively. A cat was considered infected with Bartonella spp. if it tested positive in at least one of the diagnostic tests employed (PCR on EDTA blood, PCR on BAPGM and on blood agar colonies). The year-crude incidence (YCI) of infected/infested cats in each group on D360 was calculated as follows:
The efficacy in preventing Bartonella spp. infection and flea infestation was based on YCI and calculated as follows:
The differences between YCI in G1 and G2 were tested for statistical significance using Fisher’s exact test or Chi-square test, where appropriate.
Associations between variables (age, sex, flea infestation, lymph node enlargement, Leishmania infantum infection, haemotropic Mycoplasma) and Bartonella spp. infection were statistical analysed using a Chi-square or Fisher’s exact test, where appropriate. Relative risk (RR) at a 95% confidence interval (CI) was used to determine the magnitude of associations. The software used was WinEpi [45] and the statistical significance threshold for two-tailed tests was set at P < 0.05.
Results
Of the 204 cats enrolled, 152 animals (78 from G1 and 74 from G2) were available for assessment of Bartonella spp. whereas the remaining animals were excluded for different reasons (Table 1). Amongst the excluded cats, 13 (seven from G1 and six from G2) were removed after the enrolment because they were identified as already infected with Bartonella spp. at inclusion on D0. The two groups of cats included in the analysis were not statistically different for sex (χ2 = 0.696, df = 1, P = 0.4) and age (χ2 = 0.008, df = 1, P = 0.93) at the time of inclusion (D0) (Table 1).
Table 1.
Number and characteristics of cats treated with the Seresto® collar (G1) and untreated controls (G2) that either completed or were excluded from the study
| G1 | G2 | Total | |
|---|---|---|---|
| Completed the study | |||
| No. of cats | 78 | 74 | 152 |
| Median age in months (interquartile range) | 24 (10–48) | 18 (10–36) | 18 (10–36) |
| Sex n (%) female/male | 39/39 (50) | 42 (56.8)/32 (43.2) | 81 (52.29)/71 (46.71) |
| Excluded from the study | |||
| Number of cats | 26 | 26 | 52 |
| Infected with B. henselae at the inclusion | 2 | 5 | 7 |
| Infected with B. clarridgeiae at the inclusion | 5 | 1 | 6 |
| Deceaseda | 6 | 9 | 15 |
| Suspected adverse drug reaction | 1 | – | 1 |
| Lost to follow up | 12 | 11 | 23 |
aCar trauma (n = 4); suspected infectious disease (n = 3); respiratory failure (n = 1); aortic thromboembolism (n = 1)
On samples collected on the final study day (D360), ITS-PCR assays performed on both DNA extracted from EDTA blood samples and after BAPGM enrichment step yielded identical results. Out of 78 cats from G1 and 74 cats from G2 tested, 3 (3.85%) and 10 (13.51%) were found positive, respectively; among those from G2, 3 were also found positive by blood agar slant sub-cultures (Table 2, Fig. 1). Using ITS and Pap31 gene combined PCR assays, 3 out of 78 cats (3.85%) in G1 and 4 out of 74 cats (5.41%) in G2 were infected by B. clarridgeiae and 6 out of 74 cats (8.11%) in G2 scored positive to B. henselae (Table 2). Out of three cats infected by B. clarridgeiae in G1 two (# G1_43 and G1_76, Table 2) were found infested by fleas at D 210.
Table 2.
Results of PCR from both whole blood samples and after BAPGM enrichment and from isolates on blood agar for B. henselae and B. clarridgeiae in cats treated with the Seresto® collar (G1) or in untreated controls (G2) after being exposed to one transmission season in a highly endemic area (D360)
| Group (n) | Bartonella spp., n (%)a | B. clarridgeiae, n (%)b | B. henselae, n (%)c | B. henselae isolates, n (%)d |
|---|---|---|---|---|
| G1 (78) | 3/78 (3.85) | 3/78 (3.85) | 0 | 0/3 |
| G2 (74) | 10/74 (13.51) | 4/74 (5.41) | 6/74 (8.11) | 3/10 |
| Total (152) | 13/152 (8.55) | 7/152 (4.6)e | 6/152 (3.9)f | 3/13 (23)g |
aITS PCR from whole blood and enrichment BAPGM
bITS sequence analysis
cPap31 PCR
dB. henselae isolated on agar blood culture after enrichment BAPGM step
eSample IDs: G1_43, G1_56, G1_76, G2_132, G2_147, G2_172 and G2_173
fSample IDs: G2_27, G2_60, G2_72, G2_90, G2_106 and G2_149
gSample IDs: G2_60, G2_90 and G2_149
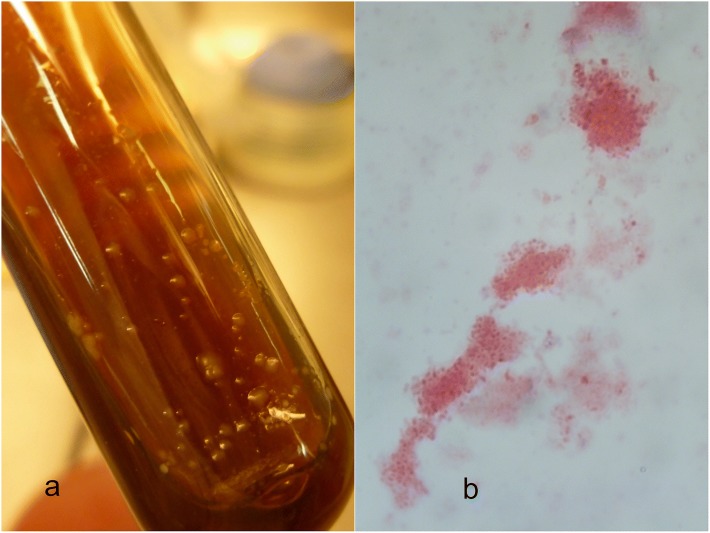
Fig. 1.
a Growth on blood agar slant (Oxoid®) supplemented with 5% defibrinated sheep blood. First-passage 21-day-old LipBh_4 isolate of B. henselae genogroup II, genotype Fizz/Cal1. Slants had been kept at 35 °C in a 5% CO2 atmosphere. B. henselae appears as circular brownish colonies, with humid aspect, sticking to the agar. b Light microscopy images (100×) of Gimenez-stained LipBh_4 isolate of B. henselae genotype II
All seven partial ITS B. clarridgeiae sequences obtained in the study showed 100% homology to sequences of B. clarridgeiae reference strain AF167989. Due to the 100% identity of the sequences obtained in this study only two representative sequences were deposited in the GenBank database with the corresponding accession numbers reported in Table 3.
Table 3.
Reference and field strains of Bartonella spp. used for sequence and phylogenetic analyses
| Bartonella species | 16S genogroup/serotype | Pap31 genotype | Strain | Sample ID | GenBank IDa | Reference (R) / Field strains (F) |
|---|---|---|---|---|---|---|
| B. henselae | I | Houston I | Houston I | – | AF001274 | R |
| B. henselae | I | PV262926 | PV_1 | – | ns | R |
| B. henselae | I | ZF1 | 60457 | – | AF321116 | R |
| B. henselae | I | ZF1 | LipBh_3 | G2_27 | MH350808 | F |
| B. henselae | II | Marseille | Marseille | – | AF308169 | R |
| B. henselae | II | Fizz | – | AF308167 | R | |
| B. henselae | II | Fizz/Cal1 | LipBh_4 | G2_60 | MH350809 | F |
| B. henselae | II | Fizz/Cal1 | LipBh_5 | G2_72 | MH350810 | F |
| B. henselae | II | Fizz/Cal1 | LipBh_6 | G2_90 | MH350811 | F |
| B. henselae | II | Fizz/Cal1 | LipBh_7 | G2_106 | MH350812 | F |
| B. henselae | II | Fizz/Cal1 | LipBh_8 | G2_149 | MH350813 | F |
| B. clarridgeiae | 16S-23S ITS | – | – | AF167989 | R | |
| B. clarridgeiae | 16S-23S ITS | LipBc_1 | G1_43 | MH348146 | F | |
| B. clarridgeiae | 16S-23S ITS | LipBc_2 | G2_132 | MH348147 | F | |
Four B. henselae reference strains, one for each known Pap-31 genotype, were included in the analyses (Table 3). BLAST search and nucleotide alignment with B. henselae reference sequences showed that B. henselae strains detected in this study belonged to Pap31 genogroup I (n = 1) and genogroup II (n = 5) (Fig. 2, Table 3). The percentages of nucleotide identity of the sequences obtained in this study with the closest reference strains available in the GenBank database were as follows: LipBh_3, 99.77% nucleotide identity with B. henselae ZF1 (AF321116) strain of genogroup I; LipBh_4 to _8 strains, 99.77% nucleotide identity with strain Fizz/Cal1 (AF308167) of the B. henselae genogroup II. Five B. henselae genogroup II strains (LipBh_4 to _8) displayed a 100% nucleotide identity with each other.
Fig. 2.
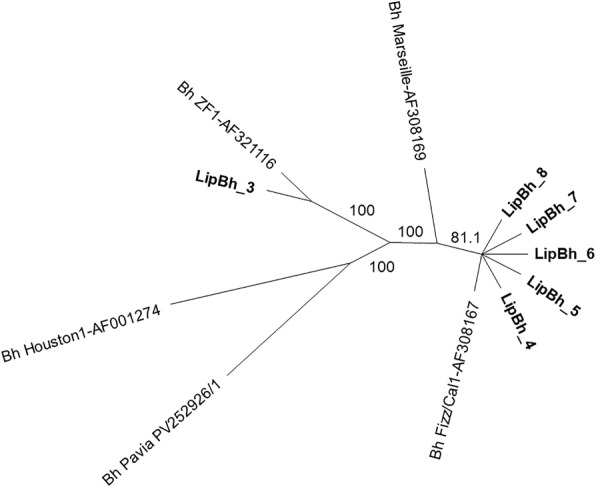
Neighbor-joining unrooted tree (obtained by using the Geneious® 10.3.1 software package, Biomatters Ltd, Aukland, New Zealand) generated with pap31-based sequencing data for 5 reference and 6 field B. henselae variants. The numbers at the nodes indicate the percentages of occurrence of the branching order in 100 bootstrapped trees for the neighbour-joining trees. The strains generated in this study are indicated in bold. All field strains were from the cats of the G2 group (unprotected control animals). The B. henselae strain LipBh_3 (GenBank: MH350808) detected from the cat G2_27 was 99.77% identical to the genotype Bh ZF1 (GenBank: AF321116), inside genogroup I. Strains LipBh_4 to LipBh_8 (GenBank: MH350809-MH350813) isolated from the cats G2_60, _72, _90, _106 and _149 clustered into the genotype Fizz/Cal1 (GenBank: AF308167) of the genogroup II Marseille
The analysis confirmed the subtyping obtained by sequence analysis. Bartonella henselae genotype I ZF1 (n = 1, 1.35%) and B. henselae genotype II Fizz/Cal-1 (n = 5, 6.76%) were detected in cats from G2.
Both B. henselae- and B. clarridgeiae-infected cats were evaluated together in a risk factor analysis. Bartonella spp. infection was more frequent in cats younger than 12 months-old than in elder ones (11.86 vs 6.65%; χ2 = 1.35, df = 1, P = 0.25) and equally distributed between male and female cats (9.86 vs 7.41%; χ2 = 0.29, df = 1, P = 0.59). The YCI was 3.85% in G1 and 13.51% in G2 (P = 0.03) leading to 71.54% efficacy of the collar in preventing Bartonella spp. infection.
Of the 152 cats analysed for Bartonella spp., 126 animals (67 from G1 and 59 from G2) were available for assessment of haemotropic Mycoplasma incidence whereas the 26 remaining were excluded from the analysis because they were identified to be already infected with haemotropic Mycoplasma at inclusion on D0. Out of 67 cats from G1 and 59 cats from G2 tested, 7 (10.45%) and 8 (13.56%) were found haemotropic Mycoplasma spp.-positive, with CMhm being more prevalent; among those from G2, two were co-infected with B. henselae or B. clarridgeiae. Only one cat in G2 was infected with M. haemofelis. No significant difference of incidence (χ2 = 0.29, df = 1, P = 0.59) for Mycoplasma spp. was detected between the two groups.
At enrolment (D0), G1 and G2 had a comparable percentage of cats infested by fleas (24.35 vs 27.02%; χ2 = 0.142, df = 1, P = 0.71) (Table 4). During the course of the study, the percentage of flea infested cats in G1 was reduced to 4 cats (5.1%, χ2 = 19.638, df = 1, P = 0.0001) at D210 and none (0%, χ2 = 42.266, df = 1, P = 0.0001) at D360 (Table 4). Conversely, for G2, flea infestation was observed in 24 (32.4%) cats at D210 and 33 (44.6%) cats at D360, with no significant prevalence variation in the infestation rates (χ2 = 5.295, df = 3, P = 0.0708). This resulted in efficacies against fleas of 84.26 and 100% on D210 and D360, respectively. At the study closure all cats were healthy based on clinical examination; however, 11 (14.10%) cats in G1 and 28 (37.84%) in G2 showed peripheral lymph node enlargement being more frequent in animals of the G2 group than in those of G1 (χ2 = 11.21, df = 1, P = 0.0008). Of the 152 cats included in the analysis three (3.85%) in G1 and 20 (23.03%) in G2 tested Leishmania spp. positive, as reported in a previous study [37].
Table 4.
Number and percentages of flea infested cats from treated (G1) or untreated (G2) groups, at the enrolment (D0), during treatment (D210) and at the end of experiment (D360) with Seresto® collar
| Study day (D) | D0 | D210 | D360 | |||
|---|---|---|---|---|---|---|
| Group | G1 (n = 78) | G2 (n = 74) | G1 (n = 78) | G2 (n = 74) | G1 (n = 78) | G2 (n = 74) |
| Flea infestation, n (%) | 19 (24.35) | 20 (27.02) | 4 (5.1)a | 24 (32.4)a | 0b | 33 (44.59)b |
Significant differences are marked with equal letters
aχ2 = 18.839, df = 1, P < 0.0001
bχ2 = 44.430, df =1, P < 0.0001
While no significant association was detected between Bartonella spp. and Leishmania infantum (χ2 = 0.001, df = 1, P = 0.98) or Bartonella spp. and peripheral lymph node enlargement (χ2 = 3.131, df = 1, P = 0.08), significant association was recorded between Leishmania infantum and peripheral lymph node enlargement (χ2 =13.53, df = 1, P = 0.0002; RR = 2.8, 95% CI: 1.62–4.87).
Discussion
Cats of the Aeolian Islands are exposed to B. clarridgeiae, B. henselae and haemotropic Mycoplasma. The Seresto® collar proved to be effective in reducing the risk of Bartonella infection in cats under natural field conditions with an overall efficacy of 71.54%.
The PCR on whole blood samples prior to and after BAPGM enrichment step, blood agar slant culture and DNA sequencing approach used in this study detected B. clarridgeiae and variants of B. henselae infections in the feline population examined. Both bacterial culture and PCR amplification from blood were used as elective diagnosis supporting an infection at the time of sampling [46], instrumental to the assessment of the prevention efficacy of the collar against Bartonella spp. infection in cats.
In our study 13 out 152 (8.55%) blood samples tested positive, thus revealing the Bartonella spp. infection in cats from the Aeolian Islands. No differences were observed for yield of Bartonella spp. detection by using PCR assay after BAPGM enrichment step compared with PCR results obtained from whole blood samples without enrichment, according to a previous study [47]. Additionally, BAPGM enrichment step did not improve the isolation rate, with only 3 out of 13 (23%) PCR positive samples yielding growth of Bartonella spp. colonies on blood agar slants. Several factors may affect the isolation of Bartonella spp. in culture, including the species of Bartonella, the magnitude and the duration of bacteraemia and the maintenance of organism viability from the sample collection time to its cultivation [48]. The higher number of BAPGM enrichment-PCR positive samples (n = 13) as compared with agar slant sub-cultures (n = 3) may indicate both the amplification of non-viable bacteria in blood samples that failed to grow in solid cultures and the difficulty in cultivating and isolating the Bartonella spp. from reservoir and non-reservoir patients [42]. Therefore, PCR assay on ITS of blood may be the best approach for screening population animals, being faster than BAPGM enrichment liquid culture which takes at least four weeks to yield definitive results [48].
The overall incidence of Bartonella spp. was 8.55% (13/152), being 3.85% (3/78) in treated animals compared to 13.51% (10/74) in untreated control animals. Of the two species, B. henselae was more frequent (6/74, 8.11%) than B. clarridgeiae (4/74, 5.41%) in the G2 group kept under natural conditions. This is similar to data found in a recent study in the area and in different European countries [49–53].
Two main pathogenic B. henselae variants belonging to Houston I and Marseille II genogroups have been identified in both animals and humans, and both of these variants are involved in CSD. B. henselae genogroup II-genotype Fizz/Cal-1 was predominant in Sicily being detected with the highest frequency (6.76%) in G2 cats and similar to findings in cats from north Italy [12], France [17] and Germany [23]. Comparatively, only one cat in G2 harboured the less frequent B. henselae genogroup I-genotype ZF1.
Although no significant association was observed between incidence and sex (P = 0.25) or age (P = 0.08) of the cats examined, cats younger than one year-old showed a higher prevalence for Bartonella spp. than elder ones, as already demonstrated in the USA and the Netherlands [9]. These data further support the hypothesis that juvenile cats, rather than adults, may be more efficient reservoirs of CSD for humans with an association between owning a kitten and CSD [22]. Clinical signs were not related to Bartonella spp. infection (P = 0.08) according to previous studies [54], whereas cats infected with Leishmania infantum were nearly three times more at risk to develop peripheral lymph node enlargement (χ2 = 13.53, df = 1, P = 0.0002; RR = 2.8, 95% CI: 1.62–4.87).
Haemoplasma infection is common in cats worldwide [49] and these infections are recognized in Italy [55]. In the present study, 11.9% of the cats were positive for Mycoplasma spp., with CMhm being the most prevalent species, similar to previous studies [53]. Although it is still unknown how feline hemoplasmas are transmitted, vector transmission through fleas [56] or ticks [57] has been suggested. In the present study, no difference in incidence (χ2 = 0.29, df = 1, P = 0.59) was recorded in cats treated with the Seresto® collar (G1) or in untreated controls (G2) after being exposed to one transmission season in highly endemic area (D360) thus supporting a major role of direct transmission (e.g. aggressive interactions) among cats.
By virtue of its anti-flea activity the Seresto® collar significantly decreased the incidence of Bartonella spp. infection (χ2 = 4.54, df = 1, P = 0.03; RR = 3.51, 95% CI: 1.0062–12.69) in cats with an overall efficacy of 71.54%. Cats included in this trial were at high risk of Bartonella infection with the study being carried out in a highly endemic area for Bartonella spp. [36, 55, 58]. The vast majority of cats lived constantly outdoors in suburban or rural areas, with a lifestyle at high risk of flea infestation, as previously reported [40]. The collar was efficacious in controlling flea infestation in the population of cats (χ2 = 29.027, df = 3, P < 0.0001), thus reducing the risk of infection in collared cats as compared to non-collared cats. This result mirrors a previous study performed in Japan [59] where a significantly higher seroprevalence of B. henselae was observed in cats with flea infestations than that in flea-free cats, thus highlighting the main role of fleas for the infection under natural conditions.
The YCI of B. clarridgeiae (i.e. 3.85%) in cats negative for fleas in the collared cats only apparently conflicts with the epidemiological and pathogenic mechanisms of Bartonella spp. infection in cats, which are strictly related to the contact with fleas or their excrement [17–20]. Indeed, two of the three collared cats infected by B. clarridgeiae, which were older than two years at the enrolment (D0), had already been found infested by fleas at D210 [37], although we cannot state the role of those fleas as we did not test Bartonella spp. infection either in fleas or cats at D210. Furthermore, we cannot exclude that those three cats were already infected with B. clarridgeiae at enrolment. Indeed, after the infection exposure, bacteria colonize the vascular endothelial cells (primary niche) and the bacteraemia only begins four to five days later [2]. Accordingly, those cats could have tested negative as the level of bacteraemia may have been at low level at D0 and thus undetectable, or they had only just been infected.
Conclusions
Cats living in the Aeolian Islands are exposed to B. henselae, B. clarridgeiae, haemotropic Mycoplasma and Leishmania infantum. The Seresto® collar represents a useful preventive measure against infections with both B. henselae and B. clarridgeiae, in addition to Leishmania infantum [40], therefore providing individual protection for cats living in Bartonella endemic areas that could potentially act as reservoirs of the pathogen to humans.
Acknowledgements
Authors are grateful to Dr Massimo Fabbi, Sezione Diagnostica di Pavia, Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna “Bruno Ubertini” Pavia, Italy, for providing the strains of B. clarridgeiae and B. henselae.
Funding
The study was partially funded by Bayer Animal Health (cost of consumables and publication fees).
Availability of data and materials
All data generated or analysed during this study are included in this published article. Sequences obtained during the present study are available in GenBank with accession numbers MH350808-MH350813 and MH348146-MH348147 (Table 3).
Abbreviations
- AIDS
Acquired immune deficiency syndrome
- BAPGM
Bartonella alpha proteobacteria growth medium
- CSD
Cat scratch disease
- D
Study day
- ITS
Internal transcribed spacer
- RR
Relative risk
- YCI
Year crude incidence
Authors’ contributions
DO, EB, GG, MP and BS conceived and designed the study. EB, DO and FDT carried out the field activities. GG and GD carried out the laboratory work. GG carried out phylogenetic and statistical analyses. GG and DO drafted the first version of the manuscript. EB, CB, GC, MP, BS, GC and FDT critically reviewed the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study complied with Good Clinical Practice (VICH GL9 GCP). The Italian Ministry of Health approved both protocol and procedures (authorization no. 0006088-10/03/2015-DGSAF-COD_UO- P). Animals were enrolled in the study after their owners signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
MP and BS are employees of Bayer Animal Health GmbH, which funded the study. GG, EB, CB, GC, GD, GC, FDT and DO declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Grazia Greco, Email: grazia.greco@uniba.it.
Emanuele Brianti, Email: ebrianti@unime.it.
Canio Buonavoglia, Email: canio.buonavoglia@uniba.it.
Grazia Carelli, Email: grazia.carelli@uniba.it.
Matthias Pollmeier, Email: matthias.pollmeier@bayer.com.
Bettina Schunack, Email: bettina.schunack@bayer.com.
Giulia Dowgier, Email: giulia.dowgier@pirbright.ac.uk.
Gioia Capelli, Email: gcapelli@izsvenezie.it.
Filipe Dantas-Torres, Email: filipe.dantas@cpqam.fiocruz.br.
Domenico Otranto, Email: domenico.otranto@uniba.it.
References
- 1.Welch DF, et al. Bartonella. In: Whitman WB, Rainey F, Kämpfer P, Trujillo M, Chun J, DeVos P, et al., editors. Bergey’s Manual of Systematic of Archaea and Bacteria. Chichester: John Wiley & Sons, Ltd; 2015. pp. 1–15. [Google Scholar]
- 2.Schülein R, Seubert A, Gille C, Lanz C, Hansmann Y, Piémont Y, et al. Invasion and persistent intracellular colonization of erythrocytes. J Exp Med. 2001;193:1077–1086. doi: 10.1084/jem.193.9.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dehio C. Molecular and cellular basis of Bartonella pathogenesis. Annu Rev Microbiol. 2004;58:365–390. doi: 10.1146/annurev.micro.58.030603.123700. [DOI] [PubMed] [Google Scholar]
- 4.Breitschwerdt EB, Kordick DL. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–438. doi: 10.1128/CMR.13.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Hamzaoui B, Laroche M, Almeras L, Bérenger J-M, Raoult D, Parola P. Detection of Bartonella spp. in fleas by MALDI-TOF MS. PLoS Negl Trop Dis. 2018;12:e0006189. [DOI] [PMC free article] [PubMed]
- 6.Rolain JM, Fournier PE, Raoult D, Bonerandi JJ. First isolation and detection by immunofluorescence assay of Bartonella koehlerae in erythrocytes from a French cat. J Clin Microbiol. 2003;41:4001–4002. doi: 10.1128/JCM.41.8.4001-4002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drancourt M, Birtles R, Chaumentin G, Vandenesch F, Etienne J, Raoult D. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet. 1996;347:441–443. doi: 10.1016/S0140-6736(96)90012-4. [DOI] [PubMed] [Google Scholar]
- 8.Scola BL, Liang Z, Zeaiter Z, Houpikian P, Grimont PAD, Raoult D. Genotypic characteristics of two serotypes of Bartonella henselae. J Clin Microbiol. 2002;40:2002–2008. doi: 10.1128/JCM.40.6.2002-2008.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmans AM, Schellekens JF, van Embden JD, Schouls LM. Predominance of two Bartonella henselae variants among cat-scratch disease patients in the Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeaiter Z, Fournier P-E, Raoult D. Genomic variation of Bartonella henselae strains detected in lymph nodes of patients with cat scratch disease. J Clin Microbiol. 2002;40:1023–1030. doi: 10.1128/JCM.40.3.1023-1030.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woudstra C, Fach P, Chomel BB, Haddad N, Boulouis H-J. Draft genome sequences of 12 feline Bartonella henselae isolates. Genome Announc. 2017;5:e00075–e00017. doi: 10.1128/genomeA.00075-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabbi M, Vicari N, Tranquillo M, Pozzi C, Prati P, De Meneghi D, et al. Prevalence of Bartonella henselae in stray and domestic cats in different Italian areas: evaluation of the potential risk of transmission of Bartonella to humans. Parassitologia. 2004;46:127–129. [PubMed] [Google Scholar]
- 13.Boulouis H-J, Chao-Chin C, Henn JB, Kasten RW, Chomel BB. Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet Res. 2005;36:383–410. doi: 10.1051/vetres:2005009. [DOI] [PubMed] [Google Scholar]
- 14.Rolain JM, Franc M, Davoust B, Raoult D. Molecular detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in cat fleas, France. Emerg Infect Dis. 2003;9:338–342. doi: 10.3201/eid0903.020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra S, Forsyth M, Lawrence AL, Emery D, Šlapeta J. Cat fleas (Ctenocephalides felis) from cats and dogs in New Zealand: molecular characterisation, presence of Rickettsia felis and Bartonella clarridgeiae and comparison with Australia. Vet Parasitol. 2017;234:25–30. doi: 10.1016/j.vetpar.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Bouhsira E, Ferrandez Y, Liu M, Franc M, Boulouis H-J, Biville F. Ctenocephalides felis an in vitro potential vector for five Bartonella species. Comp Immunol Microbiol Infect Dis. 2013;36:105–111. doi: 10.1016/j.cimid.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Abbott RC, Chomel BB, Kasten RW, Floyd-Hawkins KA, Kikuchi Y, Koehler JE, et al. Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immunol Microbiol Infect Dis. 1997;20:41–51. doi: 10.1016/S0147-9571(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 18.Chomel BB, Kasten RW, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, et al. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foil L, Andress E, Freeland RL, Roy AF, Rutledge R, Triche PC, et al. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J Med Entomol. 1998;35:625–628. doi: 10.1093/jmedent/35.5.625. [DOI] [PubMed] [Google Scholar]
- 20.O’Reilly KL, Bauer RW, Freeland RL, Foil LD, Hughes KJ, Rohde KR, et al. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU16) Infect Immun. 1999;67:3066–3072. doi: 10.1128/iai.67.6.3066-3072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guptill L, Slater L, Wu CC, Lin TL, Glickman LT, Welch DF, et al. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 22.Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, Plikaytis BD, Hadler JL, et al. Cat scratch disease in Connecticut - epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993;329:8–13. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]
- 23.Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics. 2008;121:e1413–e1425. doi: 10.1542/peds.2007-1897. [DOI] [PubMed] [Google Scholar]
- 24.Burzo ML, Antonelli M, Pecorini G, Favuzzi AMR, Landolfi R, Flex A. Fever of unknown origin and splenomegaly: a case report of blood culture negative endocarditis. Medicine (Baltimore) 2017;96:e9197. doi: 10.1097/MD.0000000000009197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picascia A, Pagliuca C, Sommese L, Colicchio R, Casamassimi A, Labonia F, et al. Seroprevalence of Bartonella henselae in patients awaiting heart transplant in southern Italy. J Microbiol Immunol Infect. 2017;50:239–244. doi: 10.1016/j.jmii.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Scolfaro C, Mignone F, Gennari F, Alfarano A, Veltri A, Romagnoli R, et al. Possible donor-recipient bartonellosis transmission in a pediatric liver transplant. Transpl Infect Dis. 2008;10:431–433. doi: 10.1111/j.1399-3062.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 27.Wong MT, Dolan MJ, Lattuada CP, Regnery RL, Garcia ML, Mokulis EC, et al. Neuroretinitis, aseptic meningitis, and lymphadenitis associated with Bartonella (Rochalimaea) henselae infection in immunocompetent patients and patients infected with human immunodeficiency virus type 1. Clin Infect Dis. 1995;21:352–360. doi: 10.1093/clinids/21.2.352. [DOI] [PubMed] [Google Scholar]
- 28.Kordick DL, Hilyard EJ, Hadfield TL, Wilson KH, Steigerwalt AG, Brenner DJ, et al. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avidor B, Graidy M, Efrat G, Leibowitz C, Shapira G, Schattner A, et al. Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. J Clin Microbiol. 2004;42:3462–3468. doi: 10.1128/JCM.42.8.3462-3468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greene CE, McDermott M, Jameson PH, Atkins CL, Marks AM. Bartonella henselae infection in cats: evaluation during primary infection, treatment, and rechallenge infection. J Clin Microbiol. 1996;34:1682–1685. doi: 10.1128/jcm.34.7.1682-1685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regnery RL, Rooney JA, Johnson AM, Nesby SL, Manzewitsch P, Beaver K, et al. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996;57:1714–1719. [PubMed] [Google Scholar]
- 32.Pennisi MG, Marsilio F, Hartmann K, Lloret A, Addie D, Belák S, et al. Bartonella species infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2013;15:563–569. doi: 10.1177/1098612X13489214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanneck D, Rass J, Radeloff I, Kruedewagen E, Le Sueur C, Hellmann K, et al. Evaluation of the long-term efficacy and safety of an imidacloprid 10%/flumethrin 4.5% polymer matrix collar (Seresto®) in dogs and cats naturally infested with fleas and/or ticks in multicentre clinical field studies in Europe. Parasit Vectors. 2012;5:66. doi: 10.1186/1756-3305-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanneck D, Kruedewagen EM, Fourie JJ, Horak IG, Davis W, Krieger KJ. Efficacy of an imidacloprid/flumethrin collar against fleas, ticks, mites and lice on dogs. Parasit Vectors. 2012;5:102. doi: 10.1186/1756-3305-5-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichard MV, Thomas JE, Arther RG, Joseph AH, Raetzel KL, Meinkoth JH, et al. Efficacy of an imidacloprid 10% / flumethrin 4.5% collar (Seresto®, Bayer) for preventing the transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum. Parasitol Res. 2013;112(Suppl. 1):11. doi: 10.1007/s00436-013-3277-7. [DOI] [PubMed] [Google Scholar]
- 36.Fourie JJ, Crafford D, Horak IG, Stanneck D. Prophylactic treatment of flea-infested cats with an imidacloprid/flumethrin collar to forestall infection with Dipylidium caninum. Parasit Vectors. 2012;5:151. doi: 10.1186/1756-3305-5-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otranto D, Dantas-Torres F, Napoli E, Solari Basano F, Deuster K, Pollmeier M, et al. Season-long control of flea and tick infestations in a population of cats in the Aeolian archipelago using a collar containing 10% imidacloprid and 4.5% flumethrin. Vet Parasitol. 2017;248:80–83. doi: 10.1016/j.vetpar.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Lappin MR, Davis WL, Hawley JR, Brewer M, Morris A, Stanneck D. A flea and tick collar containing 10% imidacloprid and 4.5% flumethrin prevents flea transmission of Bartonella henselae in cats. Parasit Vectors. 2013;6:26. doi: 10.1186/1756-3305-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otranto D, Napoli E, Latrofa MS, Annoscia G, Tarallo VD, Greco G, et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: pathogen and vector circulation in a confined environment. Vet Parasitol. 2017;236:144–151. doi: 10.1016/j.vetpar.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Brianti E, Falsone L, Napoli E, Gaglio G, Giannetto S, Pennisi MG, et al. Prevention of feline leishmaniosis with an imidacloprid 10%/flumethrin 4.5% polymer matrix collar. Parasit Vectors. 2017;10:334. doi: 10.1186/s13071-017-2258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berlinguer G. Aphaniptera d’Italia: Studio Monografico. Rome: Il Pensiero Scientifico; 1964. [Google Scholar]
- 42.Maggi RG, Duncan AW, Breitschwerdt EB. Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J Clin Microbiol. 2005;43:2651–2655. doi: 10.1128/JCM.43.6.2651-2655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diniz PPVDP, Maggi RG, Schwartz DS, Cadenas MB, Bradley JM, Hegarty B, et al. Canine bartonellosis: serological and molecular prevalence in Brazil and evidence of co-infection with Bartonella henselae and Bartonella vinsonii subsp. berkhoffii. Vet Res. 2007;38:697–710. doi: 10.1051/vetres:2007023. [DOI] [PubMed] [Google Scholar]
- 44.Peters IR, Helps CR, Willi B, Hofmann-Lehmann R, Tasker S. The prevalence of three species of feline haemoplasmas in samples submitted to a diagnostics service as determined by three novel real-time duplex PCR assays. Vet Microbiol. 2008;126:142–150. doi: 10.1016/j.vetmic.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 45.WinEpi: Working IN EPIdemiology. http://winepi.net/.
- 46.Bergmans AM, Peeters MF, Schellekens JF, Vos MC, Sabbe LJ, Ossewaarde JM, et al. Pitfalls and fallacies of cat scratch disease serology: evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J Clin Microbiol. 1997;35:1931–1937. doi: 10.1128/jcm.35.8.1931-1937.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleischman DA, Chomel BB, Kasten RW, Stuckey MJ, Scarlet J, Liu H, et al. Bartonella infection among cats adopted from a San Francisco shelter, revisited. Appl Environ Microbiol. 2015;81:6446–6450. doi: 10.1128/AEM.01864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenner SA, Rooney JA, Manzewitsch P, Regnery RL. Isolation of Bartonella (Rochalimaea) henselae: effects of methods of blood collection and handling. J Clin Microbiol. 1997;35:544–547. doi: 10.1128/jcm.35.3.544-547.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attipa C, Papasouliotis K, Solano-Gallego L, Baneth G, Nachum-Biala Y, Sarvani E, et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vectors. 2017;10:130. doi: 10.1186/s13071-017-2063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maia C, Ramos C, Coimbra M, Bastos F, Martins A, Pinto P, et al. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors. 2014;7:115. doi: 10.1186/1756-3305-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergmann M, Hartmann K. Vector-borne diseases in cats in Germany. Tierarztl Prax Ausg K Klientiere Heimtiere. 2017;45:329–335. doi: 10.15654/TPK-160874. [DOI] [PubMed] [Google Scholar]
- 52.Tabar M-D, Altet L, Francino O, Sánchez A, Ferrer L, Roura X. Vector-borne infections in cats: molecular study in Barcelona area (Spain) Vet Parasitol. 2008;151:332–336. doi: 10.1016/j.vetpar.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Persichetti MF, Pennisi MG, Vullo A, Masucci M, Migliazzo A, Solano-Gallego L. Clinical evaluation of outdoor cats exposed to ectoparasites and associated risk for vector-borne infections in southern Italy. Parasit Vectors. 2018;11:136. doi: 10.1186/s13071-018-2725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guptill L. Feline bartonellosis. Vet Clin North Am Small Anim Pract. 2010;40:1073–1090. doi: 10.1016/j.cvsm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Persichetti M-F, Solano-Gallego L, Serrano L, Altet L, Reale S, Masucci M, et al. Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasit Vectors. 2016;9:247. doi: 10.1186/s13071-016-1534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lappin MR, Griffin B, Brunt J, Riley A, Burney D, Hawley J, et al. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J Feline Med Surg. 2006;8:85–90. doi: 10.1016/j.jfms.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willi B, Boretti FS, Meli ML, Bernasconi MV, Casati S, Hegglin D, et al. Real-time PCR investigation of potential vectors, reservoirs, and shedding patterns of feline hemotropic mycoplasmas. Appl Environ Microbiol. 2007;73:3798–3802. doi: 10.1128/AEM.02977-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pennisi MG, La Camera E, Giacobbe L, Orlandella BM, Lentini V, Zummo S, et al. Molecular detection of Bartonella henselae and Bartonella clarridgeiae in clinical samples of pet cats from southern Italy. Res Vet Sci. 2010;88:379–84. [DOI] [PubMed]
- 59.Maruyama S, Kabeya H, Nakao R, Tanaka S, Sakai T, Xuan X, et al. Seroprevalence of Bartonella henselae, Toxoplasma gondii, FIV and FeLV infections in domestic cats in Japan. Microbiol Immunol. 2003;47:147–153. doi: 10.1111/j.1348-0421.2003.tb02798.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article. Sequences obtained during the present study are available in GenBank with accession numbers MH350808-MH350813 and MH348146-MH348147 (Table 3).