Abstract
Studies investigating oviposition preference of Ceratitis capitata (Wiedemann, 1824) in table grapes (Vitis vinifera L.) cultivated in the São Francisco River Valley can provide important information to guide control measures, therefore minimizing damage caused by this species in the region. The aim of this study was to evaluate the oviposition preference of C. capitata females for berries cv. ‘Italia’ collected at five different times (60, 70, 80, 90, and 100 d after production pruning [DAPP]), representing five grapevine growth stages. We also assessed the performance of immature stages of C. capitata regarding the physiological development of the berry. The total soluble solid content was significantly higher in berries at more advanced physiological developmental stages (90 and 100 DAPP). Conversely, these berries showed the lowest values of firmness and titratable acidity. With the onset of physiological development, the average number of punctures per berry increased and reached 5.2 per berry in 100 DAPP berries. The infestation level and pupal weight showed a positive correlation with the growth stage. The highest recovery of pupae was observed in 100 DAPP berries (1.54). Pupal viability values ranged from 50.9 to 64.7% independent of berry maturity stage. The physiological developmental stage of the berry did not affect offspring sex ratio. Results obtained suggest that regardless of the female preference for laying eggs on berries in a more advanced physiological developmental stage, females can initiate the attack to bunches of this cultivar at 60 DAPP, even if the berries have unfavorable physicochemical parameters for oviposition and development of larvae.
Keywords: Mediterranean fruit fly, development, Vitis vinifera, ripening
The Mediterranean fruit fly, Ceratitis capitata (Wiedemann, 1824), is among the most destructive agricultural pests worldwide due to high polyphagy, multivoltinism, severe direct damage caused by feeding larvae, indirect damage caused by quarantine restrictions, and its biological success in invading and adapting to diverse habitats (Liquido et al. 1991, Yuval and Hendrichs 1999, Gasperi et al. 2002, Malacrida et al. 2007, De Meyer et al. 2008). Several morphological, physiological, and behavioral traits at different life cycle stages have promoted the invasive and adaptive success of C. capitata (Yuval and Hendrichs 1999, Malacrida et al. 2007). As a result of its ability to occupy diverse ecological niches throughout tropical and subtropical regions, C. capitata infests a wide variety of hosts around the world depending on their availability (Bateman 1976, Fletcher 1989, Gasperi et al. 1991, Malacrida et al. 1992, Papanicolaou et al. 2016). Ceratitis capitata is endemic to sub-Saharan Africa, but a global invasion process has taken place over the past 200 yr, which has led to its colonization of tropical, subtropical, and warm temperate areas (Gasparich et al. 1997, Meixner et al. 2002, Silva et al. 2003, Malacrida et al. 2007). There are established populations of C. capitata throughout Africa, the Middle East, the Mediterranean region and other adjacent European countries, the Hawaiian Islands, Australia, and Central and South America (White and Elson-Harris 1992; De Meyer 1998, 1999; Silva et al. 2003; Malacrida et al. 2007; Diamantidis et al. 2009; Carey 2011).
In Brazil, C. capitata was reported for the first time in the early 1900s (Ihering 1901). This species was limited initially to the southern and southeastern regions of the country, with the Recôncavo Baiano region in the state of Bahia, as its northernmost limit until the 1980s (Malavasi et al. 1980, Nascimento and Zucchi 1981). In the early 1990s, C. capitata was reported further north in the northeastern region (Morgante 1991) and in the late 1990s in the Amazon (Ronchi-Teles and Da Silva 1996, Silva et al. 1998). At present, this species occurs in 22 of 27 states in Brazil ranging from the state of Rio Grande do Sul in the southern region to states in the North and Northeast regions. In Brazil, C. capitata infests 93 hosts in 27 plant families (Zucchi and Moraes 2012). Ceratitis capitata is a major quarantine pest in Brazil infesting native and introduced hosts, such as fine table grapes.
The submedium region of the São Francisco River Valley (SFRV), at latitude 8–9°S and longitude 40°W, has more than 360,000 ha of irrigable land, a tropical semiarid climate, and encompasses the states of Bahia and Pernambuco in the Northeast region of Brazil (Lima et al. 2014, Cassundé et al. 2017). This region is the largest producer of fine table grapes (Vitis vinifera L.) for export in Brazil, and grapes are the most economically important crop in this region with approximately 13,000 ha planted (Brasil 2016). The first V. vinifera cultivar introduced in the region was cv. ‘Italia’, which is the most widely cultivated variety in the region due to its agronomic characteristics, such as vigor, high average production of 30 t ha−1 yr−1, reaching up to 50 t ha−1 yr−1 with proper management (De Souza Leão 2004).
In the last decade, C. capitata was reported infesting fine table grapes in vineyards in the SFRV. At that time, the infestation level was low 0.05 pupae per berry (Botton et al. 2005). Recent monitoring data indicated an increase in C. capitata populations in vineyards in the region with an average number of flies per trap per day of 2.7 from January to December 2015 (BMB 2016).
One of the challenges faced by Mediterranean fruit fly females is finding adequate oviposition sites. The reproductive success of holometabolous phytophagous insects, such as Mediterranean fruit fly, especially those whose larvae have low mobility, are largely dependent on the resources selected by females via oviposition. Thus, parental traits and decisions, such as the choice of an adequate oviposition site, are critical to offspring survival and success (Yuval et al. 2002, Segura et al. 2007, Dias et al. 2017). Several studies on the host preference and acceptance of C. capitata have been carried out to better understand how females select the proper host for oviposition (Joachim-Bravo and Silva-Neto 2004, Diamantidis et al. 2008, Papachristos and Papadopoulos 2009). It has been found that a complex balance of plant physical and chemical factors can affect both the final selection of hosts and tephritid larval development (Joachim-Bravo et al. 2001, Aluja and Mangan 2008, Papachristos et al. 2008, Dias et al. 2017). Studies regarding the effect of different larval host on the offspring have been carried out on C. capitata and other polyphagous tephritids. These studies reported that larval development time and survival rates are parameters strongly influenced by hosts (Carey 1984, Krainacker et al. 1987, Liedo and Carey 1996, Vargas et al. 2000, Papachristos et al. 2008). Little or no effect was observed on egg and pupal developmental stage and survival rates (Carey 1984, Krainacker et al. 1987, Celedonio-Hurtado et al. 1988, Liedo and Carey 1996).
Host quality (e.g., size, color, penetrability, fruit maturity) is among the most important extrinsic factor that influences fruit fly oviposition behavior (Aluja and Mangan 2008). Because changes occur in the physicochemical properties of fruit throughout development, it is paramount to identify the developmental stage of the grape berry in which the onset of infestation by C. capitata takes place to most effectively prevent attacks in vineyards. Therefore, this study aimed to determine the preference of C. capitata females for table grape berries of cv. Italia at five different grapevine growth stages based on the number of days after production pruning (DAPP). As well, we tested whether the grape cv. Italia is an appropriate host for the development of the immature stages.
Materials and Methods
Study Site
The study was performed at the Biofábrica Moscamed Brasil (BMB) located in Juazeiro, Bahia, Brazil.
Insects
The insects used in the experiments were recovered as pupae from guava fruits (Psidium guajava L.) collected in an orchard located in the irrigated perimeter of Maria Teresa, Petrolina (9° 8′ 46.36″ S, 40° 33′ 28.33″ W), in the state of Pernambuco. The collected fruit were placed in plastic trays (38 × 27 × 10 cm) with a layer of vermiculite, covered with voile cloth until larval emergence and pupation and kept in acclimatized rooms under controlled conditions (25 ± 2°C, 60 ± 10% RH, and a photoperiod of 14:10 [L:D] h). After 7 and 14 d, the vermiculite was sieved, and all puparia obtained were placed in acrylic cages (28 × 12 × 12 cm) with a side opening for maintenance. Upon fly emergence, males and females were separated using an aspirator and placed in new cages. This procedure ensured that insects were virgin for the experiments. The adults were fed with a solid diet of sugar and hydrolyzed yeast (Biones Quatá, São Paulo, Brazil) at ratio 3:1 (Silva-Neto et al. 2012) and filtered water ad libitum.
Grape Samples
All grapes used in the experiments were of the cv. Italia and were from a commercial vineyard located in the irrigated perimeter of Nilo Coelho (9° 18′ 16.64″ S, 40° 25′ 57.11″ W) in the state of Pernambuco. The selection criteria were no previous use of insecticides and absence of the pest in the vineyard according to the monitoring reports from the previous week. The grape bunches were collected at 60, 70, 80, 90, and 100 DAPP, thus representing the following grapevine growth stages: 32, 33, 34, 35, and 36, respectively, according to the system proposed by Coombe (1995). These grapevine growth stages were described as follows: 32 (beginning of bunch closure, berries touching—if bunches are tight), 33 (berries still hard and green), 34 (berries begin to soften, and soluble solid contents start increasing), 35 (berries begin to color and enlarge), and 36 (berries with intermediate soluble solid values). The bunches were bagged in paper bags properly identified according to the developmental stage and taken to the Laboratory of Entomology at the BMB. We started the study with ʻItalia’ grapes at 60 DAPP, based on preliminary observations by BMB technicians, who had detected the presence of C. capitata in Jackson traps on vineyards and signs of infestation in bunches at 80 DAPP.
The grape bunches were examined under a stereoscopic microscope (Leica, model EZ4D, at 40× magnification) to check for the presence of oviposition scars by C. capitata, and any infested grapes were discarded. After this, the bunches were selected for the number of berries (on average 21 berries per bunch), rinsed with water, and placed in plastic trays (38 × 27 × 10 cm) with a layer of paper towel at the bottom and covered with voile cloth. Subsequently, the trays were kept at 5°C in a refrigerator until the beginning of the experiment (24 h later).
Berry Physicochemical Parameters
Physicochemical parameters were determined at the Postharvest Physiology Laboratory of Embrapa Semiárido. For each grapevine growth stage evaluated, a sample of 2 kg of grapes was used. We determined titratable acidity (TA), total soluble solid content (SS), and firmness (resistance of the berry under pressure). The firmness was determined using an electronic fruit texture analyzer (Stable Micro System, model TAXT. Plus; 2-mm-diameter tip). We used five replicates of 20 berries for each grapevine growth stage and one measurement per berry. The SS content was determined by direct reading of the pulp berry extract with a digital refractometer (ATAGO, model PAL-1), and the results were expressed in ºBrix (AOAC 2002). TA was estimated using titration with 0.1 molar solution of sodium hydroxide (NaOH) and expressed as grams of tartaric acid/100 ml of juice (AOAC 2002). For the SS and TA evaluations, five replicates of juice obtained from 20 grape berries were used for each grapevine growth stage.
Oviposition Preference of C. capitata Females: Choice Test
The experiment was carried out in field cages (2.80 × 2.90 × 2.0 m) containing two potted Ficus sp. plants to provide shade and mating surfaces for insects and support for the grape bunches. In total, 100 couples, sexually mature (10–12 d old), virgin, and without previous oviposition experience, were released in each cage. One day later, five grape bunches, one of each growth stage, were offered simultaneously as oviposition substrate to females. After 24 h, the fruits were removed from the cages and taken to the laboratory to determine the presence of oviposition scars by C. capitata and the percentage of infested berries per bunch. During the experiment, adults were provided food (filter paper strips with sugar) and water ad libitum in 10-ml glass vials. This study was carried out in a randomized block design with five treatments (grapevine growth stages-DAPP), three cages concomitantly (blocks), and was repeated four times. In total, 12 repetitions (bunches) were used for each stage.
Development of C. capitata in Grape Berries
We evaluated the influence of grapevine growth stage on immature development and survival rates. For this, we used 12 bunches of each growth stage, previously exposed as oviposition substrate to females of C. capitata during the choice test (see experiment above). Each bunch was weighed, placed into a plastic container (500 ml) with vermiculite as pupation substrate, covered with voile cloth, and kept in a room under controlled environmental conditions (25 ± 2°C, 65 ± 10% RH, and a photoperiod of 14:10 [L:D] h). Pupae were collected at 14, 21, and 28 d after grapes infestation. Pupae collection times were selected according to results of preliminary experiments with C. capitata larvae on grapes under laboratory conditions, which showed that larval development was slower on this host. Pupae were placed into plastic cups (100 ml) until adult emergence. To evaluate the influence of the host on puparium weight, 1 d after pupation, 50 pupae of each grapevine growth stage were weighed individually on an analytical balance (OHAUS Adventurer, model AR3130, precision of 0.0001 g). After emergence, the adults were sexed. The following parameters were evaluated: number of pupae per bunch, infestation level (pupae per berry), pupal viability, pupal weight, and offspring sex ratio. The experiment was carried out in completely a random design with five treatments (grapevine growth stages–DAPP) and 12 repetitions (bunches) for each biological parameter mentioned above. For pupal weight, we used 50 pupae for each grapevine growth stage.
Statistical Analysis
Prior to statistical analysis, the Kolmogorov–Smirnov test for normality and Bartlett test for homogeneity of variances were applied to all data. When these assumptions were not met, the mathematical transformations √x + 1 and arcsine x/100 were used, the latter for the data to be expressed as percentage. Physicochemical differences among the berries from the five grapevine growth stages tested were determined by one-way analysis of variance (ANOVA) followed by Tukey’s test to compare means. To determine the effect of grapevine growth stage on female oviposition preference, data from number of ovipositions per berry and percentage of infested berries per bunch were submitted to one-way ANOVA followed by Tukey’s test (HSD). Data regarding number of pupae recovered per bunch, infestation level (pupae per berry), viability of the pupal stage, and pupal weight were also subjected to one-way ANOVA, followed by Tukey’s test. For each growth stage of the berries, differences between the number of pupae recovered at 14, 21, and 28 d were determined with one-way ANOVA, and means were separated by Tukey’s test. The number of male and female progeny recovered was evaluated with the Student’s t-test. The relationship between the number of punctures per bunch and recovered pupae was determined by the Pearson correlation. All data were analyzed with the STATISTICA program v.10.0 (StatSoft Inc. 2001, Tulsa, OK).
Results
Berry Physicochemical Characteristics
The results of the berry physicochemical analysis are shown in Table 1. Firmness and TA were significantly lower in berries at more advanced physiological developmental stages (at 100 and 90 DAPP; firmness: F(4, 25) = 165.48; P < 0.05; acidity: F(4, 25) = 216.17; P < 0.05). These two parameters were similar in berries at 60, 70, and 80 DAPP. Conversely, the opposite was observed for the total soluble solid content (SS). This chemical parameter was lower in berries at 60, 70, and 80 DAPP than in berries at 90 and 100 DAPP, showing a monotonic increase in the level of SS content with physiological development of the berry (F(4, 25) = 277.69; P < 0.05; Table 1).
Table 1.
Mean values (± SE) of physicochemical characteristics of grapes of cv. Italia at five grapevine growth stages
| Stagea | Firmnessb | TAc | SSd |
|---|---|---|---|
| 60 | 11.21 ± 0.11b (10.86 –11.48) | 1.93 ± 0.02a (2.01–1.86) | 3.30 ± 0.08d (4.00–4.40) |
| 70 | 12.28 ± 0.44ab (10.89–13.36) | 1.65 ± 0.03b (1.59 –1.73) | 4.10 ± 0.07d (4.00 –4.40) |
| 80 | 12.45 ± 0.13a (12.14–12.87) | 1.33 ± 0.03c (1.26 –1.40) | 5.80 ± 0.11c (4.60 –5.20) |
| 90 | 5.13 ± 0.27c (4.27–5.78) | 1.39 ± 0.06c (1.20 –1.53) | 10.00 ± 0.18b (7.30 –8.20) |
| 100 | 5.21 ± 0.37c (4.76 –5.04) | 0.49 ± 0.01d (0.45 –0.53) | 12.00 ± 0.40a (11.00–13.40) |
Values within a column sharing a letter did not differ significantly by Tukey’s test (P < 0.05). Values in parentheses indicate the range of variation of the biological parameter evaluated.
aDays after production pruning (DAPP).
bFirmness expressed in Newton.
cTitratable acidity expressed as grams of tartaric acid per 100 ml.
dSoluble solid content, expressed in °Brix.
Oviposition Preference of C. capitata Females: Choice Test
Oviposition was detected in berries of all five growth stages of the ʻItalia’ grapes evaluated (Table 2). The number of ovipositions per berry (F(4, 55) = 18.13; P < 0.05) and ovipositions per bunch (F(4, 55) = 15.23; P < 0.05) were highest in berries at 100 DAPP, reaching averages values of 5.24 ± 0.52 and 105.25 ± 9.81, respectively. The maximum number of ovipositions per berry was detected in berries at 100 DAPP (18 ovipositions per berry). The berries at 60, 70, and 80 DAPP were less preferred by the females as oviposition substrate. The percentage of infested berries was, on average, higher than 85% for the bunches at the five grapevine growth stages (DAPP), and no significant statistical differences were detected among the five treatments evaluated (F(4, 55) = 0.34; P = 0.8504; Table 2).
Table 2.
Mean values (± SE) of number of berries per bunch, punctures per berry, and the infestation index of berries per bunch of C. capitata ovipositing on grapes of cv. Italia at five grapevine growth stages
| Stagea | Berries per bunch (no.) | Punctures per berry (no.) | Ovipositions per bunch (no.) | Infested berries per bunch (%) |
|---|---|---|---|---|
| 60 | 21.83 ± 0.49 (20–23) | 1.82 ± 0.18c (1–6) | 39.50 ± 3.80c (23–64) | 84.65 ± 3.76a (61–100) |
| 70 | 22.00 ± 0.55 (20–24) | 2.35 ± 0.30bc (1–0) | 51.08 ± 6.72bc (25–80) | 89.90 ± 3.20a (70–100) |
| 80 | 21.50 ± 0.60 (20–23) | 2.33 ± 0.22bc (1–8) | 49.67 ± 4.24bc (24–79) | 90.64 ± 3.97a (50–100) |
| 90 | 20.42 ± 0.48 (18–23) | 3.52 ± 0.26b (1–11) | 72.25 ± 5.99b (24–102) | 93.87 ± 3.41a (61–100) |
| 100 | 20.20 ± 0.46 (18–21) | 5.24 ± 0.52a (1–18) | 105.25 ± 9.81a (44–163) | 95.74 ± 1.60a (84–100) |
Values within a column sharing a letter did not differ significantly by Tukey’s test (P < 0.05). Values in parentheses indicate the range of variation of the biological parameter evaluated.
aDays after the production pruning (DAPP).
Development of C. capitata in Grape Berries
The average number of pupae recovered per bunch rose with increasing physiological development of the berries, and significant differences were detected among the grapevine growth stages (DAPP; F(4, 55) = 6.62; P < 0.05). The lowest average number of recovered pupae was observed in bunches from plants at 60 DAPP (Table 3). No correlation was found between the number of recovered pupae and number of ovipositions per bunch at 60 DAPP (r = −0.1348; P = 0.6762), 70 DAPP (r = −0.3426; P = 0.2756), 80 DAPP (r = 0.1091; P = 0.7358), and 90 DAPP (r = −0.3190, P = 0.3122). However, a positive correlation was observed between these two parameters for berries at 100 DAPP (r = 0.6812; P < 0.05).
Table 3.
Mean values (± SE) of biological parameters of C. capitata immatures and adults in grapes of cv. Italia at five grapevine growth stages
| Stagesa | Pupae per bunch (no.) | Infestation indexb (no.) | Pupa wt (mg) | Adults recovered per bunch (no.) | Offspring sex ratio (♀/(♀+♂) | |
|---|---|---|---|---|---|---|
| ♀ (no.) | ♂ (no.) | |||||
| 60 | 9.58 ± 1.53b (1–17) | 0.44 ± 0.08b (0.04–0.85) | 4.04 ± 0.28d (1–7) | 2.45 ± 0.53A (0–6) | 2.55 ± 0.64A (0–7) | 0.53 ± 0.10a (0–1) |
| 70 | 14.00 ± 2.57b (2–33) | 0.63 ± 0.12b (0.0–1.57) | 4.10 ± 0.23d (1–8) | 4.75 ± 1.46A (0–15) | 3.25 ± 0.78A (0–9) | 0.54 ± 0.08a (0–1) |
| 80 | 10.83 ± 2.22b (1–21) | 0.50 ± 0.10b (0.04–1.00) | 5.68 ± 0.24c (1–9) | 4.42 ± 0.99A (0–11) | 2.17 ± 0.68A (0–7) | 0.69 ± 0.06a (0–1) |
| 90 | 15.75 ± 2.83b (7–39) | 0.76 ± 0.13b (0.32–1.86) | 7.54 ± 0.23b (3–11) | 5.67 ± 1.48A (0–19) | 5.25 ± 1.27A (0–14) | 0.57 ± 0.08a (0–1) |
| 100 | 30.55 ± 5.38a (8–69) | 1.54 ± 0.30a (0.38–3.83) | 10.12 ± 0.27a (6–14) | 8.67 ± 1.56A (2–17) | 6.67 ± 1.66A (0–21) | 0.56 ± 0.07a (0.38–1) |
Values followed by the same lowercase letter in the column or uppercase letter in the same line did not differ significantly by Tukey’s test (P < 0.05) and Student’s t-test (P < 0.05), respectively. Values in parentheses indicate the range of variation of the biological parameter evaluated.
aDays after the production pruning (DAPP).
bNumber of pupae per berry.
The infestation level was also correlated with the development physiological stage of the berry (DAPP; F(4, 55) = 7.19; P < 0.05), and differences were detected between the berries that correspond to the most advanced grapevine growth stages (90 and 100 DAPP) and the initial growth stages (60, 70, and 80 DAPP). On average, the highest number of pupae per berry was detected in bunches at 90 DAPP (15.75) and 100 DAPP (30.55; Table 3).
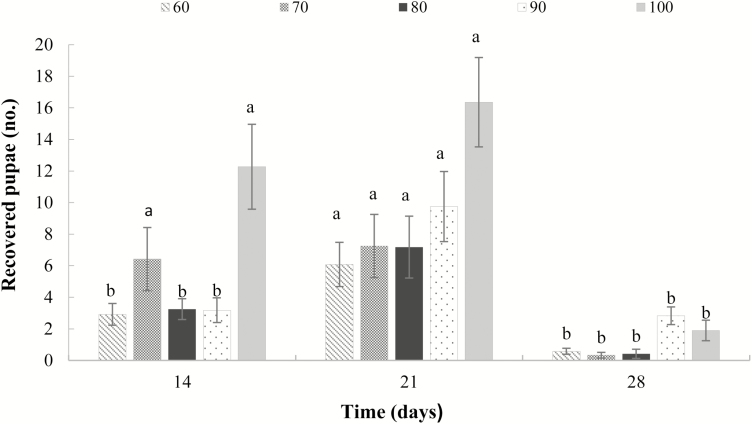
The recovery of pupae at 14, 21, and 28 d after the exposure of bunches to infestation is shown in Fig. 1. For each grapevine growth stage tested, significant differences were detected between the three time intervals evaluated (60 DAPP: F(2, 33) = 9.90; 70 DAPP: F(2, 33) = 9.49; 80 DAPP: F(2, 33) = 7.89; 90 DAPP: F(2, 33) = 9.53; 100 DAPP: F(2, 32) = 4.63; P < 0.05). Pupae recovery was much higher at 21 d after infestation for berries at 60, 80, and 90 DAPP. However, a different behavior was observed for berries at 70 and 100 DAPP, with a similar number of pupae recovered at 14 and 21 d after infestation. For all five grapevine growth stages, pupae of C. capitata were recovered at 14, 21, and 28 d after infestation, which shows intrapopulational variation in the rate of larval development.
Fig. 1.
Mean values (± SE) of pupae recovered per grape bunch of cv. Italia at 14, 21, and 28 d post-oviposition. Columns of the fill type capped by the same letters do not differ significantly by Tukey’s test (P < 0.05).
Pupal weight was influenced by the development physiological stage of the berry (F(4, 245) = 87.34; P < 0.05; Table 3). The lowest average pupal weight was observed in berries at 60 and 70 DAPP and the highest average pupal weight in berries at 100 DAPP (10.12 ± 0.27 mg). No effect from the developmental stage was observed on pupal viability (F(4, 55) = 0.68; P = 0.6072). Average values for this parameter were as follows: 51.31 ± 9.41; 50.98 ± 5.82; 53.42 ± 9.36; 64.68 ± 3.86, and 52.82 ± 3.62, for berries at 60, 70, 80, 90, and 100 DAPP, respectively.
No significant differences were detected regarding female and male emergence in the offspring recovered from the bunches of the five grapevine growth stages evaluated (60 DAPP: t = 0.01; df = 22; P = 0.6610; 70 DAPP: t = 0.66; df = 22; P = 0.1589; 80 DAPP: t = 1.78; df = 22; P = 0.4992; 90 DAPP: t = 0.35; df = 22; P = 0.7199; 100 DAPP: t = 1.09; df = 22; P = 0.4842). The physiological developmental stage of the berry had no effect on the sex ratio of the progenies obtained in the experiment (F(4, 52) = 1.08; P = 0.3767), which ranged from 0.53 to 0.70 (Table 3).
Discussion
The location and acceptance of host fruits by tephritid females is a complex process in which both physical and chemical signals from the host plant are involved (Vargas et al. 1991, Aluja and Prokopy 1992, Aluja and Mangan 2008). Among the important visual and tactile stimuli are color, shape, and size of fruit (McInnis 1989; Levinson et al. 2003). In addition, among the important chemical stimuli are semiochemicals produced and released by the host plant, host-marking pheronomes, and volatiles released during fruit ripening (Hernandez et al. 1999, Rattanapun et al. 2009). The oviposition preference hierarchy of C. capitata females in the present study regarding the physiological developmental stage (DAPP) of the grape berries is likely due to the changes in the physicochemical composition during ripening. Our physicochemical results indicated that berries at 90 and 100 DAPP were less firm, had lower acidity and higher soluble solid content, and showed a higher number of ovipositions per berry. These changes in berry physicochemical composition are consistent with grape ripening. The observed values of SS, acidity, and firmness are expected for the cv. Italia in the physiological developmental stage studied.
Several studies have reported the preference of tephritid females to oviposit in ripe or ripening fruit, which have a softer pericarp (Oi and Mau 1989, Jang and Light 1991, Hernandez et al. 1996, Rattanapun et al. 2009). Rattanapun et al. (2009) observed a preference of Bactrocera dorsalis (Hendel, 1912) (Diptera: Tephritidae) females to oviposit on softer parts of mangoes (Mangifera indica L.), which had higher levels of soluble solid content. Regarding C. capitata, Joachim-Bravo et al. (2001) registered higher oviposition activity on the ripest parts of papayas (Carica papaya L.). Santana (2012) reported a preference gradient of oviposition of C. capitata females on Barbados cherry (Malpighia glabra L.) in distinct maturation stages and the highest number of ovipositions on ripe fruit. These results corroborate our findings on the oviposition preference of C. capitata females for berries in initial maturity stages (90 and 100 DAPP).
However, it is noteworthy that we also detected ovipositions on berries at 60, 70, and 80 DAPP. The presence of eggs in less preferred fruit free choice conditions in the laboratory may be due to the presence of host-marking pheromone deposited by C. capitata in highly infested fruit (Prokopy et al. 1978, Silva et al. 2012). Conversely, Krainacker et al. (1987) suggested that this kind of behavior could also be related to the lack of fine host discrimination in C. capitata, which could also contribute to its polyphagy.
The number of pupae recovered per bunch was highest at more advanced physiological developmental stages, which guajava ted the influence of chemical characteristics of the substrate (SS and acidity) on larval development and survival. During ripening, grape berries undergo several structural changes, such as thinning of the cell wall in the mesocarp cells, expansion of the cellular volume, changes in the exocarp and vascular tissues, among others. These changes make the berries less firm (Keller 2010), which could make larval development and movement easier inside the berries. Acidity and soluble solids content also change with fruit ripening. A decrease in acidity may be due to the dilution of organic acids as a result of the increase in the berry volume (Rizzon et al. 2000). An increase in the level soluble solids content is linked to an increase in the synthesis of fructose and glucose.
In our study, pupae of C. capitata were recovered from infested berries in the five developmental stages of ʻItalia’ grapes tested. Our results contrast with those of Zart et al. (2011) for Anastrepha fraterculus (Wiedemann, 1830) (Diptera: Tephritidae) wine grapes. These authors did not find well-developed larvae in berries of the grape cv. ‘Moscato Embrapa’ when bunches were exposed to infestation by A. fraterculus during the phenological subperiod of pea and bunch closure. In the cv. ‘Niagara Rosada’, these authors found ovipositions in bunches at the beginning of the ripening process and also at the fully ripened stage. However, larvae did not pupate under laboratory conditions for the latter cultivar.
The berry physiological developmental stage also influenced pupal weight of C. capitata. This is an important parameter because it directly interferes with adult survival of C. capitata (Krainacker et al. 1989, Liedo and Carey 1996). The success of the metamorphosis from pupa to adult depends mostly on a proper larval development, which depends on the quality of the feeding substrate because this is the trophic stage during which food is ingested and stored for pupation and adult emergence (Cruz et al. 2000, Labandeira 2005). However, our results showed that about 50% of the recovered pupae hatched regardless of the berry developmental stage. Moreover, a decrease in larval development was observed in this host as pupae were recovered at 21 and 28 d after infestation in berries at 60, 70, 80, 90, and 100 DAPP. Our results contrast with those for C. capitata on other hosts (Krainacker et al. 1987, Fernandes-da-Silva and Zucoloto 1993, Costa et al. 2011). Our results on ʻItalia’ grapes suggest that factors other than the physicochemical composition of the grapes influenced the adequacy of this substrate for the larval development of C. capitata. Papachristos et al. (2008) did not observe a correlation between SS content, pH, acidity, and larval and pupal survival of C. capitata in different Citrus species either. According to these authors, the changes in other compounds of the fruit pulp (e.g., fatty acids, aminoacids, monoterpens, and glycosylate flavonoids) could play a determinant role in the development and survival of immature stages of C. capitata in citric species.
Our results showed that C. capitata females preferred to oviposit on ʻItalia’ grapes during the initial maturity stages (when berries begin to color, enlarge, and with intermediate soluble solid values), which is associated with choosing a better substrate, sweeter, and less acid for the proper development of the immature stages. However, females can initiate infestation in vineyards of this cultivar at 60 DAPP, when fruits still have physicochemical characteristics that are unfavorable for oviposition and development of immature stages. The fact that females oviposited on a nutritiously unfavorable host may represent an advantage for C. capitata when preferred hosts are scarce. Moreover, our results also help to better understand vineyard infestation rates and the susceptibility of different physiological developmental stages of Italia berries to C. capitata in the region of the São Francisco River Valley.
Acknowledgments
We thank Carter Robert Miller and two anonymous reviewers for their suggestions on an earlier version of the manuscript. We also thank Moscamed laboratory staff for their technical assistance during the experiments and Moscamed Brazil for the facilities to carry out this study. We are also grateful to growers from the San Francisco River Valley region for gently providing us the fruits used in this study. Thanks are also due to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the Ph.D. fellowship granted to M.G. and the research fellowship granted to J.G.S. is a CNPq research fellow.
References Cited
- Aluja M., and Mangan R. L.. . 2008. Fruit fly (Diptera: Tephritidae) host status determination: critical conceptual, methodological, and regulatory considerations. Ann. Rev. Entomol. 53: 246–247. [DOI] [PubMed] [Google Scholar]
- Aluja M., and Prokopy J. R.. . 1992. Host search behaviour by Rhagoletis pomonella flies: inter-tree movement patterns in response to wind-borne fruit volatiles under field conditions. Physiol. Entomol. 17: 1–8. [Google Scholar]
- (AOAC) Association on Official Agricultural Chemists 2002. Official methods of analysis of the AOAC International. AOAC, Washington, DC. [Google Scholar]
- Bateman M. A. 1976. Fruit flies, pp. 11–49. In V. L., DeLuchi (eds.), Studies in biological control. Cambridge University Press, London, United Kingdom. [Google Scholar]
- (BMB) Biofabrica Moscamed Brasil 2016. Banco de dados em Monitor XYZtemas. (http://xxxcnn8013.hospedagemdesites.ws/apps/moscamed/MonitorAgro2/selecao_usuario.jsp).
- Botton M., Haji F. N. P., Hickel E. R., and Soria S. J.. . 2005. Cachos arruinados. A ação de pragas-insetos nos frutos da videira compromete a produção in natura e de vinho. Conheça as estratégias de controle. Cultiv. Hortal. Frut. 6: 1–6. [Google Scholar]
- BRASIL 2016. Ministério do Desenvolvimento, Indústria e Comércio Exterior – MDIC. Secretaria de Comercio Exterior – SECEX Estatísticas brasileiras de exportações e importações, Brasília, DF: (http://aliceweb.mdic.gov.br) (accessed 10 September 2016). [Google Scholar]
- Carey J. R. 1984. Host-specific demographic studies of the Mediterranean fruit fly Ceratitis capitata. Ecol. Entomol. 9: 261–270. [Google Scholar]
- Carey J. R. 2011. Biodemography of the Mediterranean fruit fly: aging, longevity and adaptation in the wild. Exp. Gerontol. 46: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassundé N. F. Jr., Araújo F. R. S., and Lima R. C.. . 2017. Estratégias alternativas para o comércio internacional da manga brasileira. (https://www.researchgate.net/publication/238758570).
- Celedonio-Hurtado H., Liedo P., Aluja M., Guillen J., Berrigan D., and Carey J.. . 1988. Demography of Anastrepha ludens, A. obliqua and A. serpentina (Diptera: Tephritidae) in Mexico. Fla. Entomol. 71: 111–120. [Google Scholar]
- Coombe B. G. 1995. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1: 100–110. [Google Scholar]
- Costa A. M., Amorin F. O., Anjos-Duarte C. S., and Joachim-Bravo I. S.. . 2011. Influence of different tropical fruits on biological and behavioral aspects of the Mediterranean fruit fly Ceratitis capitata (Wiedemann) (Diptera, Tephritidae). Rev. Bras. Entomol. 55: 355–360. [Google Scholar]
- Cruz I. B. M., Cruz J. N., Taufer M., and Kalisz A. O.. . 2000. Morfologia do aparelho reprodutor e biologia do desenvolvimento, pp. 55–56. InMalavasi A. and R. A. Zucchi (eds.), Moscas-das-frutas de importância econômica no Brasil (conhecimento básico e aplicado). FAPESP-Holos, Ribeirão Preto, SP. [Google Scholar]
- De Meyer M. 1998. Revision of the subgenus Ceratitis (Ceratalaspis) Hancock (Diptera: Tephritidae). Bull. Entomol. Res. 88: 257–290. [Google Scholar]
- De Meyer M. 1999. Phylogeny of the genus Ceratitis (Dacinae: Ceratitidini), pp. 409–428. InAluja M. and A. L. Norrbom (eds.), Fruit flies (Tephritidae): phylogeny and evolution of behavior. CRC, Boca Raton, FL. [Google Scholar]
- De Meyer M., Robertson M. P., Peterson A. T., and Mansell M. W.. . 2008. Ecological niches and potential geographical distributions of Mediterranean fruit fly (Ceratitis capitata) and natal fruit fly (Ceratitis rosa). J. Biogeogr. 35: 270–281. [Google Scholar]
- De Souza Leão P. C. 2004. Cultivo da videira. (http://ainfo.cnptia.embrapa.br/digital/bitstream/item/112196/1/Cultivo-da-videira-32070.pdf).
- Diamantidis A. D., Carey J. R., and Papadopoulos N. T.. . 2008. Life history evolution of an invasive tephritid. J. Appl. Entomol. 132: 695–705. [Google Scholar]
- Diamantidis A. D., Papadopoulos N. T., Nakas C. T., Wu S., Muller H., and Carey J. R.. . 2009. Life history evolution in a globally invading tephritid: patterns of survival and reproduction in medflies from six world regions. Biol. J. Linn. Soc. 97: 106–117. [Google Scholar]
- Dias N. P., Ongaratto S., Garcia M. S., and Nava D. E.. . 2017. Oviposition of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in citrus fruits, and development in relation to maturity of orange fruits. Fla. Entomol. 100: 468–473. [Google Scholar]
- Fernandes-da-Silva P. G., and Zucoloto F. S.. . 1993. The influence of host nutritive value on the performance and food selection in Ceratitis capitata (Diptera, Tephritidae). J. Insect. Physiol. 39: 883–887. [Google Scholar]
- Fletcher B. S. 1989. Life history strategies of tephritid fruit flies, pp. 195–208. InRobinson A. S., and G. hooper (eds.), Fruit flies, their biology, natural enemies and control. Elsevier Science Publication, Amsterdam, The Netherlands. [Google Scholar]
- Gasparich G. E., Silva J. G., Han H-Y., McPheron B. A., Steck G. J., and Sheppard W. S.. . 1997. Population genetic structure of Mediterranean fruit fly (Diptera: Tephritidae) and implications for worldwide colonization patterns. Ann. Entomol. Soc. Am. 90: 790–797. [Google Scholar]
- Gasperi G., Guglielmino C. R., Malacrida A. R., and Milani R.. . 1991. Genetic variability and gene flow in geographical populations of Ceratitis capitata (Wied.) (medfly). Heredity (Edinb) 67(Pt 3): 347–356. [DOI] [PubMed] [Google Scholar]
- Gasperi G., Bonizzoni M., Gomulski L. M., Murelli V., Torti C., Malacrida A. R., and Guglielmino C. R.. . 2002. Genetic differentiation, gene flow and the origin of infestations of the medfly, Ceratitis capitata. Genetica 116: 125–135. [DOI] [PubMed] [Google Scholar]
- Hernandez M. M., Sanz I., Adelantado M., Ballach S., and Primo E.. . 1996. Electroantennogram activity from antennae of Ceratitis capitata (Wied.) to fresh orange airborne volatiles. j. Chem. Ecol. 22: 1607–1619. [DOI] [PubMed] [Google Scholar]
- Hernandez M. M., Vargas-Arispuro I., Sanz I., Adelantado M., and Primo-Yufera E.. . 1999. Electroantennogram activity and attraction assay of Ceratitis capitata (Wied.) to airborne volatiles from peach at three ripeness stages. Southwest. Entomol. 24: 133–142. [Google Scholar]
- Ihering H. V. 1901. Laranjas bichadas. Rev. Agríc. 70: 179–181. [Google Scholar]
- Jang E. B., and Light D. M.. . 1991. Behavioral responses of female Oriental fruit flies to the odor of papayas at three ripeness stages in a laboratory flight tunnel (Diptera: Tephritidae). J. Insect Behav. 4: 751–762. [Google Scholar]
- Joachim-Bravo I. S., and Silva-Neto A. M.. . 2004. Aceitação e preferência de frutos para oviposição em duas populações de Ceratitis capitata (Diptera: Tephritidae). Iheringia Sér. Zool. 94: 171–176. [Google Scholar]
- Joachim-Bravo I. S., Fernandez O. A., Bortoli S. A., and Zucoloto F. S.. . 2001. Oviposition behavior of Ceratitis capitata Wiedemann (Diptera: Tephritidae): association between oviposition preference and larval performance in individual females. Neotrop. Entomol. 30: 559–564. [Google Scholar]
- Keller M. 2010. The science of grapevines. Anatomy & physiology. Academic Press, Elsevier, Burlington, MA. [Google Scholar]
- Krainacker D. A., Carey J. R., and Vargas R. I.. . 1987. Effect of larval host on life history traits of the Mediterranean fruit fly, Ceratitis capitata. Oecologia 73: 583–590. [DOI] [PubMed] [Google Scholar]
- Krainacker D. A., Carey J. R., and Vargas R. I.. . 1989. Size-specific survival and fecundity for laboratory strains of two Tephritid (Díptera: Tephritidae) species: implications for mass rearing. J. Econ. Entomol. 82: 104–108. [Google Scholar]
- Labandeira C. C. 2005. Fossil history and evolutionary ecology of Diptera and their associations with plants, pp. 217–273InYeates D. K. and B. M. Wiegmann (eds.), The evolutionary biology of flies. Columbia University Press, New York. [Google Scholar]
- Levinson H., Levinson A., and Osterried E.. . 2003. Orange-derived stimuli regulating oviposition in the Mediterranean fruit fly. J. Appl. Entomol. 127: 269–275. [Google Scholar]
- Liedo P., and Carey J. R.. . 1996. Demography of fruit flies and implications to action programs, pp. 299–308. InMcPheron B. A. and G. J. Steck (eds.), Fruit fly pests. A world assessment of their biology and management. St. Lucie Press, Delray Beach, FL. [Google Scholar]
- Lima M. S., Silani I. S. V., Toaldo I. M., Corrêa L. C., Biasoto A. C. T., Pereira G. E., Bordignon-Luiz M. T., and Ninow J. L.. . 2014. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Foo Chem. 161: 94–103. [DOI] [PubMed] [Google Scholar]
- Liquido N. J., Shinoda L. A., and Cunningham R. T.. . 1991. Host plants of the Mediterranean fruit fly (Diptera: Tephritidae): an annotated world review. Misc. Publ. Entomol. Soc. Am. 77: 1–52. [Google Scholar]
- Malacrida A. R., Guglielmino C. R., Gasperi G., Baruffi L., and Milani R.. . 1992. Spatial and temporal differentiation in colonizing populations of Ceratitis capitata. Heredity 69: 101–111. [Google Scholar]
- Malacrida A. R., Gomulski L. M., Bonizzoni M., Bertin S., Gasperi G., and Guglielmino C. R.. . 2007. Globalization and fruitfly invasion and expansion: the medfly paradigm. Genetica 131: 1–9. [DOI] [PubMed] [Google Scholar]
- Malavasi A., Morgante J. S., and Zucchi R. A.. . 1980. Biologia de moscas-das-frutas (Diptera: Tephritidae). I: Lista de hospedeiros e ocorrência. Rev. Bras. Biol. 40: 9–16. [Google Scholar]
- McInnis D. O. 1989. Artificial oviposition sphere for Mediterranean fruit flies (Diptera: Tephritidae) in field cages. J. Econ. Entomol. 82: 1382–1385. [Google Scholar]
- Meixner M. D., B. A. McPheron J. G. Silva G. E. Gasparich, and Sheppard W. S.. 2002. The Mediterranean fruit fly in California: evidence for multiple introductions and persistent populations based on microsatellite and mitochondrial DNA variability. Mol. Ecol. 11: 891–899. [DOI] [PubMed] [Google Scholar]
- Morgante J. S. 1991. Moscas-das-frutas (Tephritidae): características biológicas, detecção e controle. SENIR, Boletim Técnico, 2, Brasília: 19 p. [Google Scholar]
- Nascimento A. S. D., and Zucchi R. A.. . 1981. Dinâmica populacional das moscas-das-frutas do gênero Anastrepha (Dip., Tephritidae) no Recôncavo Baiano. I – Levantamento das espécies. Pesq. Agropec. Bras. 16: 763–767. [Google Scholar]
- Oi D. H., and Mau R. F. L.. . 1989. Relationship of fruit ripeness to infestation in “Sharwil” avocados by the Mediterranean fruit fly and oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 82: 556–560. [Google Scholar]
- Papachristos D. P., and Papadopoulos N. T.. . 2009. Are citrus species favorable hosts for the Mediterranean fruit fly? A demographic perspective. Entomol. Exp. Appl. 132:1–12. [Google Scholar]
- Papachristos D. P., Papadopoulos N. T., and Nanos G. D.. . 2008. Survival and development of immature stages of the Mediterranean fruit fly (Diptera: Tephritidae) in citrus fruit. J. Econ. Entomol. 101: 866–872. [DOI] [PubMed] [Google Scholar]
- Papanicolaou A., Schetelig M. F., Arensburger P., Atkinson P. W., Benoit J. B., Bourtzis K., Castañera P., Cavanaugh J. P., Chao H., Childers C., . et al. 2016. The whole genome sequence of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), reveals insights into the biology and adaptive evolution of a highly invasive pest species. Genome Biol. 17: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopy R. J., Zeigler J. R., and Wong T.. . 1978. Deterrence of repeated oviposition by fruit marking pheromone in Ceratitis capitata (Diptera: Tephritidae). J. Chem. Ecol. 4: 55–63. [Google Scholar]
- Rattanapun W., Amornsak W., and Clarke A. R.. . 2009. Bactrocera dorsalis: preference and performance on two mango varieties at three stages of ripeness. Entomol. Exp. Appl. 131: 243–253. [Google Scholar]
- Rizzon L. A., Miele A., and Meneguzzo J.. . 2000. Avaliação da uva cv. Isabel para elaboração de vinho tinto. Ciên. Tecnol. Aliment. 20: 115–121. [Google Scholar]
- Ronchi-Teles B., and Da Silva N. M.. . 1996. Primeiro registro de ocorrência da mosca-do mediterrâneo, Ceratitis capitata (Wied.) (Diptera: Tephritidae) na Amazônia Brasileira. An. Soc. Entomol. Bras. 25: 569–570. [Google Scholar]
- Santana M. R. S. P. 2012. Relação moscas-das-frutas e acerola: clima, fenologia e nível de infestação no Vale do Submédio São Francisco. Dissertação de Mestrado, U niversidade Estadual da Bahia, Juazeiro, BA. [Google Scholar]
- Segura D. F., Viscarret M. M., Paladino L. Z. C., Ovruski S. M., and Cladera J. L.. . 2007. Role of visual information and learning in habitat selection by a generalist parasitoid foraging for concealed hosts. Anim. Behav. 74: 131–142. [Google Scholar]
- Silva J. G., Uramoto K., and Malavasi A.. . 1998. First report of Ceratitis capitate (Diptera: Tephritidae) in the eastern Amazon, Pará, Brazil. Fla. Entomol. 81: 574–577. [Google Scholar]
- Silva J. G., Meixner M. D., Mcpheron B. A., Steck G. J., and Sheppard W. S.. . 2003. Recent Mediterranean fruit fly (Diptera: Tephritidae) infestations in Florida – a genetic perspective. J. Econ. Entomol. 96: 1711–1718. [DOI] [PubMed] [Google Scholar]
- Silva M. A., Bezerra-Silva G. C. D., and Mastrangelo T.. . 2012. The host marking pheromone application on the management of fruit flies – a review. Braz. Arch. Biol. Technol. 55: 835–842. [Google Scholar]
- Silva-Neto A. M., Santos T. R. O., Dias V. S., Joaquim-Bravo I. S., Benevides L. J., Benevides C. M. J., Silva M. V. L., Santos D. C. C., Virginio J., Oliveira G. B., . et al. 2012. Mass-rearing of Mediterranean fruit fly using low-cost yeast products produced in Brazil. Sci. Agric. 69: 364–369. [Google Scholar]
- StatSoft Inc 2001. Statistica (data analysis software system), versão 13. São Caetano do Sul, 2015 (www.statsoft.com). [Google Scholar]
- Vargas R. I., Stark J. D., Prokopy J. R., and Green T. A.. . 1991. Response of oriental fruit fly and associated parasitoids to different color spheres. J. Econ. Entomol. 84: 1503–1507. [Google Scholar]
- Vargas R. I., Walsh W. A., Kanehisa D., Stark J. D., and Nishida T.. . 2000. Comparative demography of three Hawaiian fruit flies (Diptera: Tephritidae) alternating temperatures. Ann. Entomol. Soc. Am. 93: 75–81. [Google Scholar]
- White I. M., and Elson-Harris M. M.. . 1992. Fruit flies of economic significance: their identification and bionomics. Environ. Entomol. 22: 1408. [Google Scholar]
- Yuval B., and Hendrichs J.. . 1999. Behavior of flies in the genus Ceratitis (Dacinae: Ceratidini), pp. 429–458. InAluja M. and A. L. Norrbom (eds.), Fruit flies (Tephritidae): phylogeny and evolution of behavior. CRC Press, Boca Raton, FL. [Google Scholar]
- Yuval B., Kaspi R., Field S. A., Blay S., and Taylor P.. . 2002. Effects of post-teneral nutrition on reproductive success of male Mediterranean fruit flies (Diptera: Tephritidae). Fla. Entomol. 85: 165–170. [Google Scholar]
- Zart M., Botton M., and Fernandes O. A.. . 2011. Injúrias causadas por mosca-das-frutas sul-americana em cultivares de videira. Bragantia 70: 64–71. [Google Scholar]
- Zucchi R. A., and Moraes R. C. B.. . 2012. Fruit flies in Brazil – hosts and parasitoids of the Mediterranean fruit fly (www.lea.esalq.usp.br/ceratitis/) (updated 17 September 2018, accessed 13 October 2018).