Abstract
Background
Idiopathic pulmonary arterial hypertension (IPAH) patients are characterized by elevated triglyceride (TG)-to-HDL cholesterol (HDL-C) ratio, which has been proposed to be an important prognostic factor in this population. The mechanism of this phenomenon remains unknown. We therefore investigated the potential determinants of increased TG/HDL-C ratio in IPAH patients.
Material/Methods
We prospectively recruited consecutive clinically stable IPAH patients between January 2016 and February 2017. Patients with diabetes or using statins were excluded. Anthropometric measurements included body mass index (BMI) and skinfold thickness; body fat mass was calculated using age and sex-specific equations. We assessed lipid profile, homeostatic model assessment of insulin resistance (HOMA-IR), serum adipokine levels (adiponectin, resistin, leptin, and visfatin), and circulating cytokines (IL-1β, IL-6, MCP-1, and TNF-α).
Results
We assessed 47 IPAH patients: 9 of them had been diagnosed with diabetes and 10 were treated with statins; therefore, were excluded them from further analysis. Age, sex distribution, and BMI were similar irrespectively of TG/HDL-C ratio. Patients with increased TG/HDL-C ratio (>3) as compared to patients with TG/HDL-C ≤3 were characterized by higher levels of IL-1β, MCP-1, and IL-6. TG level was correlated with IL-1β (R=0.76, p<0.001), IL-6 (R=0.52, p=0.005), TNF-α (R=0.62, p<0.001), and MCP-1 (R=0.63, p<0.001). IL-1β was also inversely correlated with HDL-C (R=−0.44, p=0.02). We found no differences in concentration of fasting glucose, insulin, HOMA-IR, body fat content, or adipokine levels between patients with higher and lower TG/HDL-C ratios.
Conclusions
In IPAH patients, elevated TG/HDL-C ratio is a marker of systemic inflammation.
MeSH Keywords: Hypertension, Pulmonary; Inflammation; Insulin Resistance; Lipid Metabolism
Background
Idiopathic pulmonary arterial hypertension (IPAH) is a severe disease in which pulmonary vascular cells undergo uncontrolled hyperplasia, leading to narrowing and obliteration of small pulmonary arteries. This results in an increase of pulmonary vascular resistance, elevation of pulmonary artery pressure, and, eventually, heart failure [1]. The etiology of IPAH is considered to be multifactorial, but the exact mechanisms resulting in disease onset and progression are unclear. Most recently, IPAH has been considered as a systemic disease due to involvement of many organs and tissues, as well as inflammatory and metabolic pathways [2,3].
Recent clinical studies found alterations in lipid profile, such as elevated triglyceride (TG)-to-high-density lipoprotein cholesterol (HDL-C) ratio and lowered HDL-C, to be more prevalent in pulmonary arterial hypertension (PAH) patients than in age-matched controls [4,5]. Both of them were associated with poor survival [4] and worse clinical status in this population [6]. However, the mechanism of the increase in TG/HDL-C ratio in IPAH patients is not fully understood.
The mechanisms of increased TG and decreased HDL-C were investigated in different populations, including healthy subjects, patients with metabolic syndrome, and patients with chronic inflammatory disorders. In healthy adult and adolescent populations, elevated TG/HDL-C ratio was correlated with higher body weight and abdominal obesity [7,8]. Both increased TG and lower HDL-C concentrations were also associated with insulin levels and were described as characteristic lipid alterations associated with the defect in insulin action [9,10]. For this reason, TG/HDL-C was used as a marker of adiposity and insulin resistance in some populations. Patients with pulmonary hypertension, however, have similar or lower body mass index (BMI) than in the general population, and a recent study found that an increased TG/HDL-C ratio in this group was independent of the degree of obesity [4]. Therefore, the increased TG/HDL-C ratio cannot be simply explained by obesity or metabolic syndrome. Other studies show that multiple inflammatory cytokines can also increase serum TG levels [11] and decrease HDL-C concentration [12]. However, the mechanisms of increased TG/HDL-C ratio in the IPAH population have not yet been investigated.
In the present study we investigated the potential determinants of increased TG/HDL-C ratio in patients with IPAH. Based on the current literature, we analyzed body fat content and its endocrine function, insulin resistance, and β-cell function, as well as systemic inflammation.
Material and Methods
Study population
We prospectively recruited consecutively hospitalized IPAH patients from a single pulmonary hypertension reference center between January 2016 and February 2017. All hospitalizations were for diagnostic purposes. We included patients with precapillary pulmonary hypertension with elevated pulmonary vascular resistance (>3 Wood units) without other known causes of PAH or chronic thromboembolic pulmonary hypertension [1]. Patients included in the study were clinically stable. Due to the known significant interactions with TG/HDL-C ratio or anthropometric measurements, we excluded subjects with heart failure decompensation, signs of peripheral edema or infection, patients with diabetes, and those treated with antidiabetic drugs or lipid-lowering drugs. All patients signed an informed consent before participation in the study. The local ethics committee revised and approved the study protocol, which conforms to the guidelines of the Declaration of Helsinki.
Evaluation of patients and anthropometric measurements
Patient assessment, laboratory tests, and anthropometric measurements were performed on the same day at our center. We collected a medical history, including use of statin, fibrates, niacin, and estrogen hormonal therapy. Diabetes was diagnosed according to current guidelines [13]. Anthropometric measurements included weight, height, and body fat mass (FM). Body weight was measured by using an electronic scale (WPT 60/150 OW, RADWAG, Radom, Poland). BMI was calculated by indexing body mass to the square of body height. Subjects with BMI of ≥25 kg/m2 were considered as overweight and those with a BMI ≥30 kg/m2 as obese.
Body FM was calculated according to Equation 1 [14]:
| (1) |
where BD is the body density.
BD was calculated using skinfold thickness measurements with age- and sex-specific Durnin and Womersley equations [14]. Skinfold thickness was measured using the Harpenden Skinfold Caliper (Baty International, West Sussex, UK) at 4 sites: biceps, triceps, subscapular, and suprailiac. Each measurement was performed 3 times and the final outcome was calculated as an average of the readings.
Fat mass index (FMI) was obtained by indexing FM to the square of body height [15]:
| (2) |
Fat-free mass index (FFMI) was calculated:
| (3) |
Laboratory tests
We collected venous blood from each participant after 12 h of fasting on the day of hemodynamic assessment. Complete lipid profile was assessed using the direct colorimetric method, as described previously [5]. Insulin concentration was assessed using electro-chemiluminescence immunoassay (Roche, Switzerland). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using Equation 4 as the product of fasting glucose (mmol/l) and fasting insulin (mU/l) [16]:
| (4) |
The β cell function (%B) was calculated as shown in Equation 5 [16]:
| (5) |
The function of adipose tissue was assessed by measurement of serum adipokine levels: adiponectin, resistin, leptin (ELISA, Wuhan Fine Biotech, China), and visfatin (ELISA, Elabscience, USA). Systemic inflammation was assessed by measuring serum markers: IL-1β, IL-6, MCP-1, and TNF-α (ELISA, Wuhan Fine Biotech, China). The TG/HDL-C ratio was previously used as a surrogate measure of insulin resistance and a predictor of poor prognosis in numerous studies. Individuals with TG/HDL-C ratio >3 were described as insulin-resistant [4]. In our study, we used this threshold to compare patients with elevated TG/HDL-C ratio to other subjects.
Statistical analysis
We used median (interquartile range) to present continuous variables and counts (%) for categorical variables. Comparison of continuous variables between groups was performed using the t test or Mann-Whitney U test, according to data distribution. Comparison of categorical variables was performed using the chi-squared test. To assess correlations between continuous variables, we used Spearman rank-correlation. The alpha level was set as 0.05. Statistical analysis was performed with the use of the Dell Statistica data analysis software system (2016) version 13 (software.dell.com).
Results
Study population
Between January 2016 and February 2017, we assessed 47 clinically stable caucasian IPAH patients. Nine of them had been previously diagnosed with diabetes or received antidiabetic drugs and 10 patients were treated with statins; therefore, were excluded them from the study, as shown in the study flowchart (Figure 1). From the group of 28 patients included in the final analysis, 22 (79%) were treated with PAH-specific therapy: 9 (32%) with monotherapy, 9 (32%) with dual therapy, and 4 (14%) with triple combination therapy. Four (14%) patients were treated with calcium channel blockers. Daily activities of study patients were significantly limited, as half (n=14) of them were in the World Health Organization functional class III. At the time of diagnosis, all patients were told to avoid excessive physical activity, which could lead to distressing symptoms. Further details of the study group are shown in Table 1.
Figure 1.
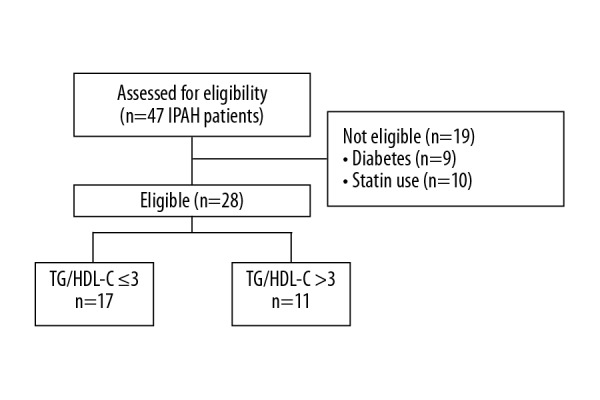
Study flowchart of participant selection. IPAH – idiopathic pulmonary arterial hypertension; TG/HDL-C – triglyceride-to-high-density lipoprotein cholesterol ratio.
Table 1.
Group characteristics.
| Age (y) | 43 (39.0–54.0) |
| Sex (female) | 24 (86%) |
| WHO-FC (II/III) | 14 (50%)/14 (50%) |
| NT-proBNP [pg/ml] | 785.5 (85.5–1723.0) |
| 6-MWD [m] | 422.5 (370.0–505.0) |
| mPAP [mmHg] | 47.5 (37.0–60.5) |
| CI [l/min/m2] | 2.3 (2.0–2.8) |
| PVR [WU] | 9.85 (6.43–13.1) |
| PAH-specific therapies | |
| ERA | 9 (32%) |
| PDE5i | 17 (61%) |
| Prostacyclin | 9 (32%) |
| ERA+ PDE5i | 4 (14%) |
| PDE5i + prostacyclin | 5 (18%) |
| Triple therapy | 4 (14%) |
Continuous variables are presented as median (interquartile range). 6-MWD – six-minute walk distance; CI – cardiac index; ERA – endothelin receptor antagonists; NT-proBNP – N-terminal pro-brain natriuretic peptide; mPAP – mean pulmonary artery pressure; PAH – pulmonary arterial hypertension; PDE-5i – phosphodiesterase type 5 inhibitors; PVR – pulmonary vascular resistance; WHO-FC – WHO Functional Class.
After dividing the study population using TG/HDL-C ≤3 and >3 cutoff levels, we found significant differences in TG (1.0 vs. 1.7 mmol/l, p<0.001, respectively) and HDL-C (1.6 vs. 0.9 mmol/l, p<0.001, respectively) levels between groups. We found no differences in LDL-C levels (3.0 vs. 3.4mmol/l, p=0.6 respectively). Patients with TG/HDL-C >3 had similar age (44.0 vs. 42.0 years, p=0.6) and proportion of female sex (94 vs. 73%, p=0.1) compared with patients with lower TG/HDL-C. We found no differences in established clinical, laboratory, and hemodynamic markers of disease severity between patients with TG/HDL-C ≤3 and those with TG/HDL-C >3: the N-terminal pro-brain natriuretic peptide concentration was 867 (84–1731) vs. 704 (97–1428) pg/ml; p=0.8, the proportion of WHO Functional Class III was 59 vs. 36%; p=0.3, the six-minute walk distance was 405 (360–500) vs. 440 (400–513) m; p=0.2, the mean pulmonary arterial pressure was 48 (35–61) vs. 47 (43–60) mmHg; p=0.7, the right atrial pressure was 4 (3–6) vs. 4 (3–9) mmHg; p=0.6, the cardiac index was 2.4 (2.1–2.9) vs. 2.11 (2.0–2.7) l/min/m2; p=0.5), and the mixed venous saturation was 67.7 (64.2–71.9) vs. 68.5 (65.8–70.1)%; p=0.6.
TG/HDL-C ratio and body fat
TG/HDL-C in IPAH patients was not associated with parameters of body composition, including BMI (R=0.14, p=0.5) and FMI (R=0.03, p=0.9), as shown in Table 2. Additionally, no association between fat tissue function and TG/HDL-C ratio was found. BMI and FMI were, however, associated with higher HOMA-IR (R=0.55, p=0.003 and R=0.7, p<0.001, respectively). Overweight patients (n=15) were characterized by higher HOMA-IR than patients with normal BMI (3.5±2.2 vs. 1.67±0.96, p=0.008). Additionally, we noted a strong association between HOMA-IR and adipokines: visfatin (R=0.8, p<0.001) and leptin (R=0.76, p<0.001). We found no correlation between HOMA-IR and inflammatory cytokines.
Table 2.
Adipose tissue content and function in patients with TG/HDL-C >3 and TG/HDL-C ≤3.
| TG/HDL-C ≤3 (n=17) | TG/HDL-C >3 (n=11) | p | |
|---|---|---|---|
| Sex (Female) | 16 (94%) | 8 (73%) | 0.1 |
| Age (y) | 44.0 (39.0–54.0) | 42.0 (38.0–54.0) | 0.6 |
| BMI (kg/m2) | 23.6 (22.0–27.5) | 26.5 (23.2–29.4) | 0.1 |
| FMI (kg/m2) | 7.7 (7.0–9.7) | 8.7 (8.1–9.7) | 0.5 |
| FFMI (kg/m2) | 16.3 (15.0–17.9) | 17.5 (16.9–20.9) | 0.1 |
| % of fat | 33.4 (31.9–35.0) | 33.1 (27.8–36.0) | 0.8 |
| Resistin (ng/ml) | 4.0 (3.5–6.6) | 6.6 (5.1–17.5) | 0.03 |
| Leptin (ng/ml) | 14.1 (8.5–26.9) | 17.6 (6.9–27.0) | 0.9 |
| Adiponectin (ng/ml) | 448.0 (360.0–519.0) | 334.0 (259.0–463.0) | 0.1 |
| Visfatin (ng/ml) | 61.1 (45.7–113.1) | 101.4 (23.0–136.9) | 1.0 |
Continuous variables are presented as median (interquartile range). BMI – body mass index; FFMI – fat-free mass index; FMI – fat mass index; TG/HDL-C – triglyceride-to-high-density lipoprotein cholesterol ratio.
TG/HDL-C ratio, systemic inflammation, and insulin resistance
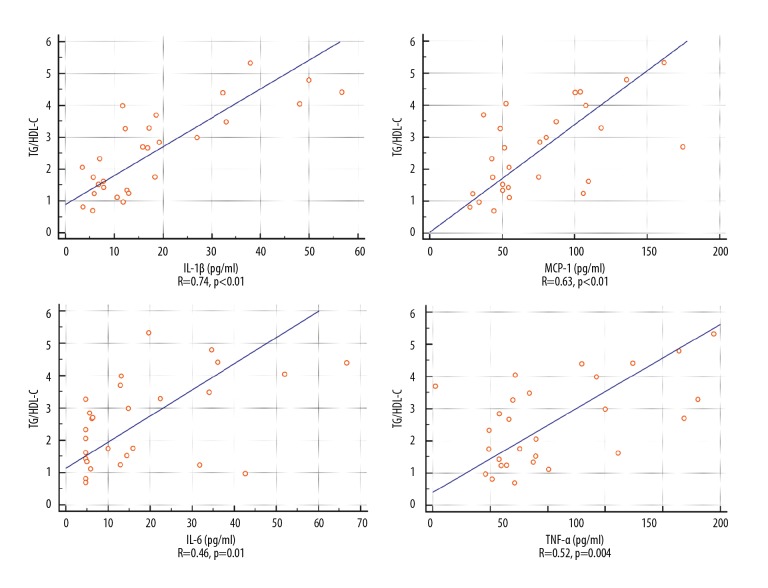
Patients with elevated TG/HDL-C had higher levels of IL-1β, MCP-1, and IL-6 than found in other individuals (Table 3). Graphic representation of the correlation between TG/HDL-C and inflammatory cytokines is shown in Figure 2.
Table 3.
Comparison of inflammatory markers between patients with TG/HDL-C >3 and TG/HDL-C ≤3.
| TG/HDL-C ≤3 (n=17) | TG/HDL-C >3 (n=11) | p | |
|---|---|---|---|
| IL-1β (pg/ml) | 7.8 (5.9–12.9) | 32.3 (15.8–48.0) | <0.001 |
| MCP-1 (pg/ml) | 51.5 (43.3–75.0) | 103.9 (52.4–135.8) | 0.01 |
| TNFα (pg/ml) | 66.1 (57.7–89.5) | 142.4 (71.6–218.5) | 0.03 |
| IL-6 (pg/ml) | 5.8 (4.7–14.4) | 22.4 (12.9–36.0) | 0.009 |
Continuous variables are presented as median (interquartile range). Il-1β – interleukin 1β; Il-6 – interleukin 6; MCP-1 – monocyte chemoattractant protein 1; TG/HDL-C – triglyceride-to-high-density lipoprotein cholesterol ratio; TNFα – tumor necrosis factor α.
Figure 2.
Association between TG/HDL-C and inflammatory cytokines. Il-1β – interleukin 1β; Il-6 – interleukin 6; MCP-1 – monocyte chemoattractant protein 1; TG/HDL-C – triglyceride-to-high-density lipoprotein cholesterol ratio; TNFα – tumor necrosis factor α.
Similar to TG/HDL-C ratio, TG level was correlated with IL-1β (R=0.76, p<0.001), IL-6 (R=0.52, p=0.005), TNF-α (R=0.62, p<0.001), and MCP-1(R=0.63, p<0.001). IL-1β was also correlated with HDL-C (R=-0.44, p=0.02). There were no significant differences in concentration of fasting glucose, insulin, HOMA-IR, or %B between patients with TG/HDL-C >3 and those with TG/HDL-C ≤3 (Table 4).
Table 4.
Insulin resistance and beta cell function in patients with TG/HDL-C >3 and TG/HDL-C ≤3.
| TG/HDL-C ≤3 (n=17) | TG/HDL-C >3 (n=11) | p | |
|---|---|---|---|
| Insulin (uIU/ml) | 9.8 (5.4–14.6) | 11.5 (4.4–16.2) | 0.8 |
| HOMA-IR | 2.0 (1.3–3.6) | 2.4 (1.0–4.2) | 0.8 |
| %B | 126.7 (78.8–178.6) | 141.7 (64.6–214.0) | 0.6 |
| Glucose (mmol/l) | 5.2 (4.9–5.4) | 5.1 (4.6–6.0) | 0.8 |
Continuous variables are presented as median (interquartile range). %B – pancreatic β-cell function; HDL-C – high-density lipoprotein cholesterol; HOMA-IR – homeostatic model assessment of insulin resistance; LDL-C – low-density lipoprotein cholesterol; TG – triglyceride; TG/HDL-C – triglyceride-to-high-density lipoprotein cholesterol ratio.
Discussion
The present study shows for the first time that in a selected population of clinically stable IPAH patients without diabetes or lipid-lowering therapies, TG/HDL-C ratio was strongly associated with systemic inflammation. Inflammatory cytokines were correlated with higher TG and lower HDL-C. However, we did not find any link between TG/HDL-C ratio and body fat content and function, or between TG/HDL-C ratio and HOMA-IR or beta cell function.
In the general population, elevated TG/HDL-C ratio is used as an accessible and easy to apply marker of insulin resistance, and was associated with age and the degree of obesity [17]. Increased TG/HDL-C ratio was also found in PAH patients but not in age-matched healthy adults, and was established as an important prognostic marker. In this group of patients, increased TG/HDL-C ratio independent of BMI was able to predict worse clinical outcome and poor survival [4]. The mechanisms of increased TG/HDL-C ratio in the PAH population are still not well understood, especially when one considers that PAH patients are not more obese than their healthy counterparts. Considering the distinct clinical characteristics of patients with IPAH, we reviewed potential determinants of elevated TG and decreased HDL-C levels in this population, including inflammation, body fat content, and altered insulin-glucose homeostasis.
Chronic inflammation was recently proposed as an important factor in the progression of pulmonary vascular disease. In experimental studies, upregulated inflammatory response precedes vascular remodeling and is considered to cause altered vascular cell metabolism and consequent smooth-muscle cells proliferation [3,18–21]. In IPAH patients, levels of circulating cytokines such as interleukin-1β, IL-2, IL-6, IL-8, IL-10, TNF-α, and MCP-1 are elevated and can predict poor prognosis, including increased mortality [22–24]. In our study, we found that in IPAH patients, inflammation is associated with elevated level of TG and decreased HDL-C. This kind of dyslipidemia was also described in other inflammatory diseases, including rheumatoid arthritis [25], lupus [26], and psoriasis [27]. It was shown that inflammatory cytokines or bacterial lipopolysaccharide increased TG levels by upregulated hepatic fatty acid synthesis and increase in adipose tissue lipolysis [11,28]. Inflammation also increased expression of the angiopoietin-like protein 4, an inhibitor of lipoprotein lipase activity, which further inhibited the metabolism of TG-rich lipoproteins [29]. In another study, modification of the TG lipases by cytokines decreased HDL-C concentration [12]. The causative role of inflammation in lipid abnormalities was confirmed by a study of rheumatoid arthritis patients treated with anti-TNFα antibodies, which resulted in a decrease of IL-6 and elevation of HDL-C, independent of other lipid fractions [30].
Most recent studies have proposed the integrated theory of PAH pathogenesis, incorporating both the role of inflammation and metabolic alteration in vascular smooth-muscle cells proliferation [3,31]. In this theory, the central role is played by the altered bone morphogenetic protein type 2 receptor (BMPR2)/peroxisome proliferator-activated receptor γ (PPARγ) signaling. Mutation of the BMPR2 gene is the most common cause of heritable PAH, and depressed BMPR2 expression was described in the lungs of patients with other forms of PAH as well [1,32]. Decreased activity of PPARγ can lead to increased inflammatory response and elevation of TG/HDL ratio [33–37] and thus at least partly explains the coexistence of inflammation and lipid abnormalities in our study patients.
In the general population, low plasma HDL-C and elevated TG traditionally have been associated with metabolic syndrome, increased adiposity, and insulin resistance. Visceral fat can supply the liver with free fatty acids, leading to changes in TG secretion [8]. Insulin is able to stimulate de novo synthesis of fatty acids, which is an important step in hepatic TG synthesis [38]. Hyperinsulinemia was also shown to stimulate hepatic TG secretion and apolipoprotein A1(ApoA1) catabolism, which results in decreased HDL-C levels [39].
In contrast to the abovementioned studies performed in the general population, lipid alterations in IPAH patients in our study were not associated with adiposity or elevated fasting glucose and insulin levels. Therefore, we conclude that the mechanisms of TG/HDL-C ratio alterations described in the general population may not play the leading role in IPAH patients. It seems possible that in this group, elevated TG/HDL-C ratio primarily reflects altered inflammation rather than the dysfunction of insulin-mediated signaling itself. In fact, in our study we did not found any association between TG/HDL-C and HOMA-IR or between TG/HDL-C and body fat content or levels of adipokines. Interestingly, a recent study by Heresi et al. showed that fasting insulin and HOMA-IR levels in IPAH patients were lower than in age-, sex-, and BMI-matched controls, which casts doubt on the role of fasting indices of insulin resistance in lipid abnormalities in this population. The higher prevalence of impaired glucose tolerance with decreased fasting indices of IR suggests a significant role of pancreatic β cell dysfunction, which may affect metabolic changes in IPAH patients [40].
The present study has several important strengths. For the first time we described an association between TG/HDL-C and inflammatory cytokines in patients with IPAH. Second, we rigorously selected a subgroup of patients with stable disease without diabetes or statin use, which could potentially confound the study results. Third, understanding determinants of increased TG/HDL-C ratio in IPAH is clinically significant since it has been shown to be an important predictor of poor prognosis in this population. Additionally, our data provide potential targets for the therapy of lipid abnormalities in IPAH patients.
Our study has also some limitations. First, this was a single-center study and, due to strict patient selection, the number of enrolled patients was limited. Therefore, the present study may have lacked sufficient statistical power to reveal some less prominent associations between TG/HDL-C and other anthropometric or metabolic markers. We were also unable to adjust our association for potential confounders. We were, however, able to show that TG/HDL-C is strongly related to inflammation in IPAH patients. Second, due to the observational design of this study, we could not determine if the relationship between TG/HDL-C and inflammation is causal. Based on previous studies and experimental models, we were, however, able to propose potential mechanisms underlying the observed findings.
Conclusions
In IPAH patients, elevated TG/HDL-C ratio is a marker of systemic inflammation.
Footnotes
Conflicts of Interest
None.
Source of support: The study was supported by the National Science Center (grant no 2017/26/E/NZ5/01223)
References
- 1.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–64. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–75. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–24. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopec G, Waligora M, Tyrka A, et al. Low-density lipoprotein cholesterol and survival in pulmonary arterial hypertension. Sci Rep. 2017;7:41650. doi: 10.1038/srep41650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner NW, Skhiri M, Fortenko O, et al. Impact of insulin resistance on ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant. 2014;33:721–26. doi: 10.1016/j.healun.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Berchtold P, Berger M, Jorgens V, et al. Cardiovascular risk factors and HDL-cholesterol levels in obesity. Int J Obes. 1981;5:1–10. [PubMed] [Google Scholar]
- 8.Tresaco B, Moreno LA, Ruiz JR, et al. Truncal and abdominal fat as determinants of high triglycerides and low HDL-cholesterol in adolescents. Obesity. 2009;17:1086–91. doi: 10.1038/oby.2008.626. [DOI] [PubMed] [Google Scholar]
- 9.Zavaroni I, Bonora E, Pagliara M, et al. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N Engl J Med. 1989;320:702–6. doi: 10.1056/NEJM198903163201105. [DOI] [PubMed] [Google Scholar]
- 10.Tobey TA, Greenfield M, Kraemer F, Reaven GM. Relationship between insulin resistance, insulin secretion, very low density lipoprotein kinetics, and plasma triglyceride levels in normotriglyceridemic man. Metabolism. 1981;30:165–71. doi: 10.1016/0026-0495(81)90167-0. [DOI] [PubMed] [Google Scholar]
- 11.Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–96. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Zuliani G, Volpato S, Ble A, et al. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: the InChianti study. Atherosclerosis. 2007;192:384–90. doi: 10.1016/j.atherosclerosis.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(Suppl 1):S13–22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 14.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 15.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26:953–60. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–19. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin T, Abbasi F, Cheal K, et al. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139:802–9. doi: 10.7326/0003-4819-139-10-200311180-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wallace RG, Twomey LC, Custaud MA, et al. Potential diagnostic and prognostic biomarkers of epigenetic drift within the cardiovascular compartment. Biomed Res Int. 2016;2016 doi: 10.1155/2016/2465763. 2465763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamosiuniene R, Tian W, Dhillon G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res. 2011;109:867–79. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopec G, Podolec P. Clinical significance of measuring inflammatory markers in patients with pulmonary arterial hypertension. Pol Arch Med Wewn. 2015;125:215–16. [PubMed] [Google Scholar]
- 21.Huang AX, Lu LW, Liu WJ, Huang M. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-alpha levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (COPD) overlap syndrome. Med Sci Monit. 2016;22:2800–8. doi: 10.12659/MSM.896458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–27. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 23.Katsushi H, Kazufumi N, Hideki F, et al. Epoprostenol therapy decreases elevated circulating levels of monocyte chemoattractant protein-1 in patients with primary pulmonary hypertension. Circ J. 2004;68:227–31. doi: 10.1253/circj.68.227. [DOI] [PubMed] [Google Scholar]
- 24.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–31. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 25.Choi HK, Seeger JD. Lipid profiles among US elderly with untreated rheumatoid arthritis – the Third National Health and Nutrition Examination Survey. J Rheumatol. 2005;32:2311–16. [PubMed] [Google Scholar]
- 26.de Carvalho JF, Bonfa E, Borba EF. Systemic lupus erythematosus and “lupus dyslipoproteinemia”. Autoimmun Rev. 2008;7:246–50. doi: 10.1016/j.autrev.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Feingold KR, Grunfeld C. Psoriasis: It’s more than just the skin. J Lipid Res. 2012;53:1427–29. doi: 10.1194/jlr.E029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bi X, Ai H, Wu Q, et al. Insulin resistance is associated with interleukin 1beta (IL-1beta) in non-diabetic hemodialysis patients. Med Sci Monit. 2018;24:897–902. doi: 10.12659/MSM.906269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu B, Moser A, Shigenaga JK, et al. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun. 2010;391:1737–41. doi: 10.1016/j.bbrc.2009.12.145. [DOI] [PubMed] [Google Scholar]
- 30.Popa C, Netea MG, Radstake T, et al. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:303–5. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122:4306–13. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atkinson C, Stewart S, Upton PD, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–78. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 33.Hansmann G, de Jesus Perez VA, Alastalo TP, et al. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–57. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansmann G, Wagner RA, Schellong S, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115:1275–84. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 35.Lawrie A, Hameed AG, Chamberlain J, et al. Paigen diet-fed apolipoprotein E knockout mice develop severe pulmonary hypertension in an interleukin-1-dependent manner. Am J Pathol. 2011;179:1693–705. doi: 10.1016/j.ajpath.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhee EJ, Oh KW, Lee WY, et al. Effects of two common polymorphisms of peroxisome proliferator-activated receptor-gamma gene on metabolic syndrome. Arch Med Res. 2006;37:86–94. doi: 10.1016/j.arcmed.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007;1771:999–1013. doi: 10.1016/j.bbalip.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartlett SM, Gibbons GF. Short- and longer-term regulation of very-low-density lipoprotein secretion by insulin, dexamethasone and lipogenic substrates in cultured hepatocytes. A biphasic effect of insulin. Biochem J. 1988;249:37–43. doi: 10.1042/bj2490037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golay A, Zech L, Shi MZ, et al. Role of insulin in regulation of high density lipoprotein metabolism. J Lipid Res. 1987;28:10–18. [PubMed] [Google Scholar]
- 40.Heresi GA, Malin SK, Barnes JW, et al. Abnormal glucose metabolism and high-energy expenditure in idiopathic pulmonary arterial hypertension. Ann Am Thorac Soc. 2017;14:190–99. doi: 10.1513/AnnalsATS.201608-605OC. [DOI] [PMC free article] [PubMed] [Google Scholar]