Abstract
Objectives
Many in vitro studies have investigated the mechanism by which mechanical signals are transduced into biological signals that regulate bone homeostasis via periodontal ligament fibroblasts during orthodontic treatment, but the results have not been systematically reviewed. This review aims to do this, considering the parameters of various in vitro mechanical loading approaches and their effects on osteogenic and osteoclastogenic properties of periodontal ligament fibroblasts.
Methods
Specific keywords were used to search electronic databases (EMBASE, PubMed, and Web of Science) for English-language literature published between 1995 and 2017.
Results
A total of 26 studies from the 555 articles obtained via the database search were ultimately included, and four main types of biomechanical approach were identified. Compressive force is characterized by static and continuous application, whereas tensile force is mainly cyclic. Only nine studies investigated the mechanisms by which periodontal ligament fibroblasts transduce mechanical stimulus. The studies provided evidence from in vitro mechanical loading regimens that periodontal ligament fibroblasts play a unique and dominant role in the regulation of bone remodelling during orthodontic tooth movement.
Conclusion
Evidence from the reviewed studies described the characteristics of periodontal ligament fibroblasts exposed to mechanical force. This is expected to benefit subsequent research into periodontal ligament fibroblasts and to provide indirectly evidence-based insights regarding orthodontic treatment. Further studies should be performed to explore the effects of static tension on cytomechanical properties, better techniques for static compressive force loading, and deeper analysis of underlying regulatory systems.
Cite this article: M. Li, C. Zhang, Y. Yang. Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts: A systematic review of in vitro studies. Bone Joint Res 2019;8:19–31. DOI: 10.1302/2046-3758.81.BJR-2018-0060.R1.
Keywords: Periodontal ligament fibroblasts, Mechanical forces, Osteogenesis, Osteoclastogenesis
Article focus
The effects of mechanical force on osteogenesis and osteoclastogenesis in periodontal ligament fibroblasts.
The characteristics of different types of in vitro mechanical force loading models.
Key messages
This study is expected to benefit subsequent research into periodontal ligament fibroblasts and to provide indirectly evidence-based insights regarding orthodontic treatment.
Further studies should be performed to explore the effects of static tension on cytomechanical properties, better techniques for static compressive force loading, and deeper analysis of underlying regulatory systems.
Strengths and limitations
This study systematically reviewed the mechanism by which mechanical signals are transduced into biological signals that regulate bone homeostasis via periodontal ligament fibroblasts during orthodontic treatment.
Due to the lack of a consensus standard for the quality assessment of in vitro studies, we could only refer to the modified Animal Research: Reporting of In Vivo Experiment (ARRIVE) guidelines.
Introduction
The orthodontic movement of teeth is governed by deposition of bone on the tension side and resorption on the compression side of the tooth. During this movement, bone remodelling is initiated via the periodontal ligament (PDL), through which forces are transmitted from the teeth to the supporting alveolar bone. Within this ligament, the predominant cell type is the mechanosensitive periodontal ligament fibroblast, which converts the mechanical stimuli of stretching or compression to biological signals regulating osteoblastogenesis and osteoclastogenesis.1
Periodontal ligament fibroblasts can be easily isolated from the roots of extracted teeth and cultured for a number of research purposes. They exhibit many osteoblast-like properties in vitro, including the ability to express osteogenic markers, induce alkaline phosphatase (ALP) activity, and form mineralized nodules.2,3 They also contribute to the differentiation of osteoclasts; the co-culture of PDL fibroblasts and osteoclast precursors leads to the formation of tartrate-resistant acid phosphatase (TRAP)-positive cells in vitro and the concomitant capacity to synthesize osteoclastogenesis-stimulating molecules.4
Because of their role in the biomechanics of orthodontic loading, in vitro models have subjected primary cultures of these cells to mechanical forces in order to investigate their molecular-level responses and functions. The results of these studies have not, however, been subjected to systematic review. Heterogeneous approaches to magnitude and directions of loading mean comparisons between studies may not be straightforward. In this systematic review we address the parameters of in vitro compressive and tensile force loading approaches, osteogenic and osteoclastogenic effects of mechanical forces, and the regulatory bioprocesses occurring in periodontal ligament fibroblasts exposed to mechanical stimuli.
Materials and Methods
Search strategy
A search of the EMBASE, PubMed, and Web of Science databases was performed using the following keywords: periodontal ligament cells or periodontal ligament fibroblasts; mechanical, force, compression, compressive, tension, tensile, stress, stretch, strain or shear; bone remodelling, bone homeostasis, bone metabolism, bone resorption, bone formation, bone deposition, ‘osteogenesis’ or ‘osteogenic’ or ‘osteoblastogenesis’ or ‘osteoclastogenesis’ or ‘osteoclastogenic’. The year of publication was limited from 1995 to 2017. The references cited by the papers were also reviewed to identify potential additional publications.
Eligibility criteria
Full-text articles were considered eligible if they satisfied the following criteria: 1) cell type, human periodontal ligament fibroblasts; 2) primary intervention, mechanical loading of the cells (but not by vibration or hydrostatic loading); 3) outcome measurements, osteogenesis- and osteoclastogenesis-related gene expression in with objective measurement of levels of bone-forming or resorbing activities; and 4) English-language articles. Any discrepancies between the two reviewers were resolved by consensus or discussion with a third reviewer.
Quality assessment of the included studies
In the absence of a consensus standard for the quality assessment of in vitro studies, we referred to the modified Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Supplementary Table i), previously used in related systematic reviews.5 A study was defined as having a low risk of bias if no more than two items received a rating of ‘no’, ‘clearly inaccurate’, or ‘clearly insufficient’. A study was defined as having a high risk of bias if less than half of the items received a rating of ‘yes’, ‘clearly accurate’, or ‘clearly sufficient’.
Results
Search results
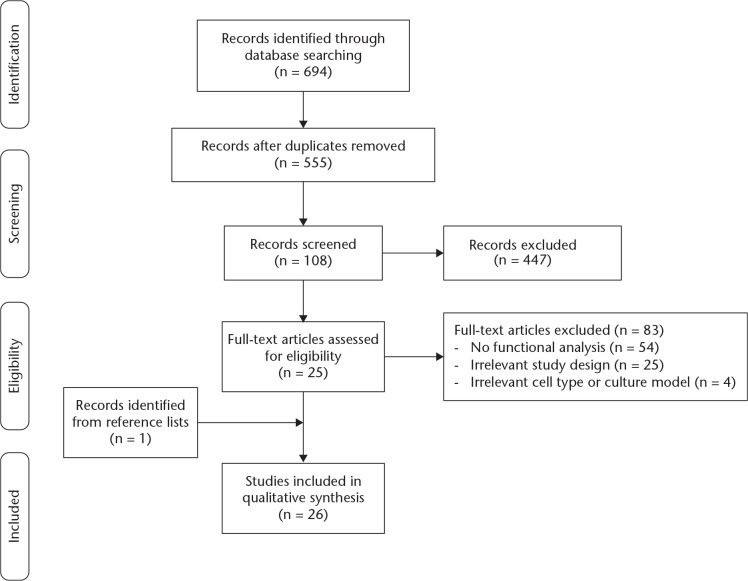
After removing duplicates from a total of 694 records identified from three databases, 555 citations were screened. Of 108 studies fulfilling the inclusion criteria, 83 were then excluded for lack of functional analysis (54 studies), inappropriate study design or outcomes (25 studies), or inappropriate cell types (four studies; two each with immortalized cell lines and rat-derived cells). Of those 25 studies with an inappropriate study design, one evaluated tooth movement in mice, five investigated periodontal ligament and collagen metabolism, four investigated the extracellular matrix, four investigated inflammation, three had irrelevant study objectives, and one study each investigated apoptosis, cell adhesion, mechano-transduction, cytoskeletal rearrangement, angiogenesis, enamel matrix derivative, hyper-occlusion, and pain induction, respectively. Together with one article identified from the references of another, we eventually included 26 publications in the review (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of the literature search.
Study quality assessment
All included studies were assessed to have a low risk of bias (Table I).6-31 The title of one study10 was considered inaccurate, making reference to ‘during tooth movement’ in the absence of any biomechanical or in vivo analysis.
Table I.
Evaluation of selected studies by Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines
| Author (year) | Item score |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Item 12 | |
| Asano et al6 (2011) | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Cao et al7 (2014) | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Chang et al8 (2017) | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Chen et al9 (2014) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Diercke et al10 (2011) | 0 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Fujihara et al11 (2010) | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 0 |
| Jin et al12 (2015) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 2 |
| Kanzaki et al13 (2002) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Kanzaki et al14 (2006) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Kikuta et al15 (2015) | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Kook et al16 (2009) | 1 | 2 | 1 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Kook et al17 (2011) | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Kunii et al18 (2013) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 2 |
| Mayahara et al19 (2012) | 1 | 2 | 1 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 2 |
| Monnouchi et al20 (2015) | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Qi and Zhang21 (2014) | 1 | 2 | 1 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Ren et al22 (2015) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 2 |
| Römer et al23 (2013) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Sun et al24 (2016) | 1 | 2 | 1 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 2 |
| Sun et al25 (2017) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Tang et al26 (2014) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Ueda et al27 (2016) | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 |
| Ueda et al28 (2016) | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 |
| Yamaguchi et al29 (2006) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 0 |
| Yang et al30 (2016) | 1 | 2 | 2 | 1 | 2 | 2 | N/A | 2 | 2 | 2 | 2 | 2 |
| Yoshino et al31 (2014) | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
N/A, not applicable
Characteristics of cell cultures
All periodontal ligament fibroblasts were from primary cell lines derived from the PDL tissues of human teeth. We excluded those based on periodontal ligament stem cells as, while of orthopaedic interest, they are obtained and investigated in a different fashion. We also excluded periodontal ligament tissue models because the force magnitudes and vectors used in multilayer cultures differ from those used in monolayer cultures.32
Types and parameters of mechanical loading models
Of the forces used to investigate the tissues, 13 studies investigated compressive force only,6,7,12,13,15,16,18,19,23,27,28,29,31 whereas ten focused on tensile force8-11,14,20,22,24,25,30 and two focused on fluid shear stress.21,26 Another study used both compression and tension approaches17 (Table II).
Table II.
Characteristics of mechanical loading models
| Loading models | Characteristics | Advantages | Disadvantages |
|---|---|---|---|
| Compressive force | |||
| Uniform weight method | Static/continuous | Adjustable and simple; the most appropriate to mimic orthodontic pressure | Produces limited amount of compression |
| Flexcell compression system | Cyclic | Computer-controlled | Costly |
| Centrifugal force: centrifuging of culture plates | Continuous | Considered a special type of compression | Performed in a fast-moving rather than a static status |
| Tensile force | |||
| Flexcell strain unit | Cyclic | Computer-controlled | Costly |
| Other substrate deformation-based approaches | Cyclic | Similar tensile force to Flexcell | Different approaches cannot be compared |
| Tension incubator (-100kPa) | Continuous | ||
| Fluid shear stress | |||
| Parallel-plate flow system | N/A | Simulates the force generated by interstitial fluid within PDL space during orthodontic loading | Mainly sensed by osteocytes rather than PDLFs |
PDL, periodontal ligament; PDLFs, periodontal ligament fibroblasts; N/A, not applicable
The uniform weight method was the most commonly used compression method, used by ten of the 14 studies applying compressive forces.6,7,12,13,15,18,19,23,29,31 In this technique, a glass cylinder or cover glass containing metal weights is placed over the cultured cell layers such that gravity generates a static and unidirectional compressive force. The magnitude of the force can be adjusted by increasing and decreasing the amount of weight. Another type of compression loading used centrifugation to apply centrifugal forces, achieving compression.16,27 One study used the substrate deformation-based Flexcell compression system (Flexcell, Inc., Hillsborough, North Carolina).17 Briefly, this system applies force via air pressure to elicit the bulging of flexible-bottomed culture plates, thereby compressing the cells grown on them.17
By contrast, 11 studies used tensile force models. Of these, six used the Flexcell strain unit, which exerts a vacuum on the underside of culture plates, thereby stretching the plate and the attached cells.14,17,22,24,25,30 Four studies also applied substrate deformation-based approaches using other stretching apparatuses with mechanisms similar to that of the Flexcell system.8,10,11,20 One study used a tension incubator to exert a continuous or intermittent tensile force.9
Finally, two studies used a parallel-plate flow system to create fluid shear stresses.21,26 The different loading models are summarized in Table III.6-31,33-37
Table III.
Details of mechanical loading models
| Study | Status of force | Loading methods | Magnitude | Loading time | Related molecules |
|---|---|---|---|---|---|
| Compressive force | |||||
| Studies investigating osteoclastogenesis | |||||
| Asano et al6 (2011) | Static | Uniform compression method (Nakajima et al33 (2008)): by a glass cylinder with lead granules in a 90 mm dish | 1.0, 2.0, 3.0, 4.0 g/cm2 | 3, 6, 9, 12, 24 hrs | IL-8, MCP-1, TRAP staining; pit formation assay |
| Cao et al7 (2014) | Static | Uniform method (Mitsui et al34 (2005)): layer of glass with additional metal weights | 0.5, 1.0, 1.5, 2.0 g/cm2 | 2, 4, 6, 8, 12 hrs | Adrb2, RANKL, OPG, TRAP staining |
| Jin et al12 (2015) | Static | Uniform compression method (Kanzaki et al13 (2002); Nakajima et al33 (2008)): cover glass and glass bottle with lead granules | 2.0 g/cm2 | 0.5, 3, 6, 12 hrs | Piezo 1, RANKL, OPG, PGE2, COX-2, NF-κB, histone 3, TRAP staining |
| Kanzaki et al13 (2002) | Static | Uniform compression method: glass cylinder with lead granules | 0.5, 1.0, 2.0, 3.0 g/cm2 | 0.5, 1.5, 6, 24, 48 hrs | PGE2, COX-1, COX-2, RANKL, OPG, TRAP staining |
| Kikuta et al15 (2015) | Static | Uniform compression method (Nakajima et al33 (2008)) | 4.0 g/cm2 | 1, 3, 6, 9, 12, 24 hrs | Jagged1 (Notch signalling), RANKL, sRANKL, IL-6, TRAP staining; pit formation assay |
| Kook et al17 (2011) | - | FX-4000 Compression System | 0.5% elongation (compression) | 1, 3, 6, 24 hrs | OPG, RANKL, TNF-α, TRAP staining; bone resorption assay |
| Kunii et al18 (2013) | Static | Uniform weight method (Yamaguchi et al29 (2006)) | 1.0, 2.0, 4.0 g/cm2 | 3, 6, 8, 12, 24, 48, 72 hrs | IL-6; pit formation assay |
| Mayahara et al19 (2012) | Static | Cell layer covered by thin glass plate | 2.0 g/cm2 | 24 hrs | COX-2, RANKL, OPG, TRAP staining |
| Römer et al23 (2013) | Static | Glass cylinder upon cells (Kanzaki et al13 (2002)) | 2.0 g/cm2 | 24 hrs | PGE2, COX-2, RANKL, OPG, TRAP staining |
| Yamaguchi et al29 (2006) | Static | Uniform compression method (Kanzaki et al13 (2002)) | 0.5, 1.0, 1.5, 2.0, 3.0 g/cm2 | 3, 6, 9, 12, 24, 48 hrs | sRANKL, RANKL, OPG, TRAP staining; pit formation assay |
| Yoshino et al31 (2014) | Static | Uniform compression method (Yamaguchi et al29 (2006)) | 4.0 g/cm2 | 1, 3, 6, 9, 12, 24, 48 hrs | RANKL, sRANKL, TNF-α, TRAP staining; pit formation assay |
| Centrifugal force | |||||
| Studies investigating osteoclastogenesis | |||||
| Kook et al (2009)16 | - | Centrifugation: horizontal microplate rotor (Universal 32 R, Hettich, Germany) | 50 g/cm2 | 30, 60, 90 mins | RANKL, OPG, ERK1/2, p-ERK1/2, TRAP staining; bone resorption assay |
| Studies investigating osteogenesis | |||||
| Ueda et al27 (2016) | - | Centrifugation (Redlich et al35 (1998)) | 40, 90, 135, and 160 × g corresponded to 16.0, 36.0, 53.9, and 63.9 g/cm2 | 24 hrs | Asporin (ASPN), sclerostin (SOST), bone formation assay (Von Kossa staining) |
| Ueda et al28 (2016) | - | Centrifugation (Redlich et al35 (1998)) | 40 and 90 × g corresponded to 16.1 and 36.2 g/cm2 | 24 hrs | SOST/sclerostin, Von Kossa staining for mineralization |
| Tensile force | |||||
| Studies investigating osteogenesis | |||||
| Chang et al8 (2017) | Cyclic | Custom-made strain device Tension Plus System (Chang et al36 (2015)) | 12% elongation at 0.1 Hz (5 sec stretch, 5 sec relaxation) | 24, 48, 72 hrs | MicroRNA-195-5p, Wnt, FGF2, BMP ALP assay; Alizarin red staining |
| Chen et al9 (2014) | - | Tension incubator (TI, Model 3618P; Lab-Line Instruments, Inc., Thermolyne Co., Melrose Park, Illinois) Normal incubator as controls: 101.3 kPa | -100 kPa (1 Pa = 10-5 kg/cm2, equal to a negative force of 101 g/mm2) | 3, 6, 12, 24 hrs; 1, 3, 7, 15 days | FAK, ERK1/2, p-FAK, p-ERK1/2, COL, ALP, OCN, IL-1, TNF-α, ALP assay; Alizarin red staining |
| Diercke et al10 (2011) | Continuous | Method described by Hasegawa et al37 (1985): cells are seeded on flexible bottomed dishes, on top of which is placed a lead weight, causing the dish bottom and attached cells to be stretched | 2.5 % | 5, 15, 30 mins; 1, 4, 24, 48, 72 hrs | FAK, p-FAK, ERK1/2, p-ERK1/2, ephrinB2, EphB4, Alizarin red staining |
| Fujihara et al11 (2010) | Cyclic | Stretch apparatus Scholertec NS-350 (Scholertec, Osaka, Japan) | 110% elongation (30 cycles/min; 0.5 Hz) | 24, 48 hrs | DNA chip analysis, c-Fos, Runx2, ALP Glutamate signalling, ALP activity; Alizarin red Staining |
| Monnouchi et al20 (2015) | Cyclic uniaxial | STB-140 (STREX Inc., Osaka, Japan) | 8% elongation (0.5 sec stretch and 0.5 sec relaxation per cycle) | 1 hr | IL-11, Ang II pathway, Alizarin red staining |
| Ren et al22 (2015) | Cyclic | Flexcell FX-4000 Strain Unit (Flexcell International Corp., Hillsborough, North Carolina) | 10% deformation at 0.5 Hz (30 cycles/min) | 1, 3, 6, 12, 18, 24 hrs | Runx2, ATF4, SP7, OCN, BSP, ERK, p-ERK; Alizarin red staining |
| Sun et al24 (2016) | Cyclic intermittent | FX-5000T Flexcell Tension Plus unit (Flexcell) | 12% elongation at 0.5 Hz | 4 hrs per day for 1 and 5 days | IL-1β, TNF- α, Runx2, COL1, ALP activity; Alizarin red staining |
| Sun et al25 (2017) | Cyclic | FX-5000T Flexcell Tension Plus unit (Flexcell) | 12% elongation at 0.5 Hz | 12, 24, 48 hrs | IL-1β, TNF- α, Runx2, COL1, ALP activity; Mineralization assay |
| Yang et al30 (2016) | Cyclic | Flexcell FX-5000 Tension System (Flexcell) | 10% deformation at 0.5 Hz (30 cycles/min) | 1, 3, 6, 12, 24 hrs | ATF4, OCN, BSP ERS-related genes: Bip, Xbp1, PERK, eIF2α; Alizarin red staining |
| Studies investigating osteoclastogenesis | |||||
| Kanzaki et al14 (2006) | Cyclic | Flexercell Strain Unit (Flexcell) | 15% elongation (1 sec stretch/1 sec relaxation) | 0.5, 1.5, 6, 24, 48, 72 hrs | RANKL, OPG, TGF-β1, TRAP staining; pit formation assay |
| Kook et al17 (2011) | Cyclic | FX-4000 Tension System | 1.5% elongation | 1, 3, 6, 24 hrs | OPG, RANKL, TNF-α, TRAP staining; bone resorption assay |
| Fluid shear stress | |||||
| Studies investigating osteogenesis | |||||
| Qi and Zhang21 (2014) | - | Parallel-plate flow chamber using a closed loop | 3, 6, 9, 12, and 15 dynes/cm2 | 6 hrs | microRNA assay; ALP activity; mineralization assay, miRNA-132, ALP, OCN, OPN, PON, PI3K, Akt1, mTOR |
| Tang et al26 (2014) | - | A parallel-plate flow system | 12 dynes/cm2 | 15, 30, 60 mins; 6, 12, 24, 48 hrs; 7, 14, 21 days | COLI, ALP, Runx2, SP7, BMP2, p38, ERK1/2, ALP activity; calcium deposition (Alizarin red staining) |
IL, interleukin; MCP-1, monocyte chemotactic protein; TRAP, tartrate-resistant acid phosphatase; Adrb2, β-2 adrenergic receptor; RANKL, receptor activator of nuclear factor kappa-B ligand; OPG, osteoprotegerin; PGE2, prostaglandin E2; COX-2, cyclooxygenase-2; NF-κB, nuclear factor-κB; TNF-α, tumour necrosis factor-α; sRANKL, soluble RANKL; ERK1/2, extracellular signal-regulated kinase 1/2; p-ERK1/2, phospho-ERK1/2; FGF2, fibroblast growth factors; BMP, bone morphogenetic proteins; ALP, alkaline phosphatase; FAK, focal adhesion kinase; p-FAK, phospho-FAK; COL, collagen; OCN, osteocalcin; Runx2, runt-related transcription factor 2; Ang II, angiotensin II; ATF4, activating transcription factor 4; SP7, osteriox; BSP, sialoprotein; ERS, endoplasmic reticulum stress; Bip, binding immunoglobulin protein; Xbp1, X-box binding protein 1; PERK, protein kinase R like endoplasmic reticulum kinase; eIF2α, α-subunit of eukaryotic initiation factor 2; TGF-β1, transforming growth factor-β1; OPN, osteopontin; PON, periostin; PI3K, phosphoinositide 3-kinase; mTOR, mechanistic target of rapamycin
Evidence from reviewed studies
The studies meeting inclusion criteria are summarized in Table IV.6-31 The roles of osteogenic and osteoclastogenic genes, and some indirectly related molecules, in mechanical force-related bone resorption and formation are discussed in the next section.
Table IV.
Major information summarized from included studies
| Study | Cell culture | Type of force | Grouping and intervention | Conclusions |
|---|---|---|---|---|
| Asano et al (2011)6 | PDLFs were taken from 6 healthy young volunteers (3 boys, 3 girls; aged 14 to 16 years) during orthodontic treatment; cultured in α-MEM. | Compressive force (CF) | a) Control cells covered with 0.032 g/cm2 CF; b) cells loaded with CF. | The expression of IL-8 (CINC-1) and MCP-1 was increased in a time- and magnitude-dependent manner after CF. CINC and MCP-1 treatment increased the number of TRAP-positive cells and resorption pits. |
| Cao et al (2014)7 | PDLFs were isolated from normal orthodontic extracted bicuspids from 3 individuals; 3 to 4 passages. | Compressive force | a) CF; b) treating cells with Ca2+; c) Ca2+ + CF; d) selective (ICI) or non-selective (ISO) Adrb2 antagonist; e) Adrb2 knockdown with siRNA transfection. | CF upregulated Adrb2 expression through the CF-induced increase in the intracellular Ca2+ concentration. The activation of Adrb2 contributed to osteoclast differentiation through the RANKL/OPG system and promoted SNS-regulated orthodontic tooth movement. |
| Jin et al (2015)12 | PDLFs were isolated from healthy extracted premolars for orthodontic reasons; cultured in α-MEM; between 3rd and 5th passages. | Compressive force | a) Unloaded control; b) cells subjected to CF for 0, 0.5, 3, 6, or 12 hrs; c) cells loaded with CF for 12 hrs and added with GsMTx4 (inhibitor of Piezo1 ion channels). | RANKL, COX-2, PGE2 synthesis and osteoclast differentiation induced by CF is transduced by Piezo1 ion channel and mediated by NF-κB signalling pathway. |
| Kanzaki et al (2002)13 | PDLFs were obtained from the teeth extracted for orthodontic reasons; cultured in α-MEM; 4th to 8th passages. | Compressive force | a) Cells were subjected to CF for 0, 0.5, 1.5, 6, 24, or 48 hrs; b) cells were treated with indomethacin (10-6 M) + CF; c) exogenous PGE2 was added to the cells. | CF promotes osteoclastogenesis through the induction of COX-2 (but not COX-1), promoting PGE2 production that results in the upregulation of RANKL expression in PDLFs. RANKL upregulation in PDLFs was dependent on PGE2. |
| Kikuta et al (2015)15 | PDLFs were collected from premolars extracted from 6 healthy young volunteers (3 boys, 3 girls; aged 14 to 16 years) during the course of orthodontic treatment. | Compressive force | a) Cells without CF; b) cells subjected to CF; c) cells subjected to CF and treated with rhJagged 1; c) cells subjected to CF with GSI (inhibitor of Notch signalling). | Jagged 1, RANKL and IL-6 expression increased upon CF. RANKL and IL-6 mRNA were decreased in CF+GSI group. The Notch signalling response to CF may stimulate the process of osteoclastogenesis via RANKL and IL-6 production. |
| Kunii et al (2013)18 | PDLFs were obtained from premolars extracted from 6 healthy young patients (3 male and 3 female adolescents; 14 to 16 yrs of age) during orthodontic treatment; cultured in α-MEM. | Compressive force | a) Controls with CF of 0.032 g/cm2; b) cells with CF for up to 72 hrs; c) cells treated with rhIL-6. | CF induced IL-6 production and stimulated osteoclastogenesis. Resorbed areas were greater in rhIL-6-treated group. |
| Mayahara et al (2012)19 | PDLFs were obtained from the healthy premolars of 14 patients aged 11 to 50 years in orthodontic treatment; cultured in α-MEM; passaged 5 to 6 times. | Compressive force | a) Cells with no CF; b) cells were subjected to CF for 24 hrs; c) cells were treated with 0 (control), 10-11, 10-10, 10-9, 10-8, 10-7, 10-6, or 10-5 M PGE2 for 48 hrs. | COX-2 expression increased by CF, but RANKL was almost undetected. RANKL expression was increased by low-concentration PGE2 but was decreased by high-concentration PGE2. OPG and M-CSF were decreased by PGE2. Only a few TRAP-positive cells were detected and unaffected by PGE2. |
| Römer et al (2013)23 | PDLFs were scraped from caries-free third molars from 3 donors (male, aged 18 to 25 years) extracted for orthodontic reasons; cultured in DMEM; 4th passage. | Compressive force | a) Untreated control group for 4 days; b) cells were grown for 3 days then CF applied for 24 hrs; c) cells were stressed with Aggregatibacter actinomycetemcomitans for 4 days; d) cells were stressed with A. actinomycetemcomitans for 3 days then with CF load simultaneously; e) COX-2 inhibition + A. actinomycetemcomitans + CF. | The synergistic action of pathogens and CF leads to an increased expression of COX-2 and PGE2 that intensifies the RANKL production, which, in turn, induces deteriorated osteoclast differentiation and subsequent osteoclastogenesis. |
| Yamaguchi et al (2006)29 | PDLFs were obtained from premolars of 10 patients during orthodontic treatment, designated to severe root resorption group (2 male, 3 female; mean 22.5 years (sd 2.8)) and non-resorption group (2 male, 3 female; 23.2 years (sd 3.3); cultured in α-MEM. | Compressive force | a) Unloaded cells; b) 2.0 g/cm2 CF for 3 to 48 hrs; c) 0.5 g/cm2 to 2.0 g/cm2 CF for 12 hrs. | Compressed PDL cells obtained from patients with severe external apical root resorption produced large amounts of RANKL and decreased the production of OPG, and also stimulated osteoclast formation. |
| Yoshino et al (2014)31 | PDLFs were collected from the premolars extracted from 6 healthy young volunteers (3 boys, 3 girls; aged 14 to 16 years); cultured in α-MEM. | Compressive force | a) Control cells with 0.032 g/cm2 CF; b) cells were subjected to CF for 1, 3, 6, 9, 12, 24, or 48 hrs; c) conditioned medium (CM) with CF + rhTNF-α or TNF-α antibody. | The expression of TNF-α and RANKL increased after CF. The combined effects of TNF-α and RANKL on osteoclast formation are more significant than those of RANKL alone. |
| Kook et al (2009)16 | PDLFs were obtained from healthy male individuals aged 20 to 30 years who had molar extraction procedures; cultured in DMEM; at passages 4 to 7. | Centrifugal force | a) Non-centrifuged cells; b) centrifuged for 30, 60, and 90 mins; c) anti-OPG antibody + centrifuge force; d) siRNAs against ERK1/2 transfection + centrifuge force. | OPG and RANKL were increased after mechanical force. Centrifugal force inhibits the formation of osteoclast-like cells through production of OPG, where ERK-mediated signalling is closely involved. |
| Ueda et al (2016)27 | PDLFs were isolated from healthy PDL of premolars extracted for orthodontic reasons; cultured in α-MEM; 3 to 8 passages. | Centrifugal compression | a) Untreated cells; b) cells treated with 40, 90, 135, 160 × g centrifugal compression. | PDLFs subjected to an optimal compression (90 × g) induce the expression and release of Asporin (ASPN) but decrease SOST expression, which inhibits bone formation. |
| Ueda et al (2016)28 | PDLFs were isolated from healthy premolars extracted for orthodontic reasons; cultured in α-MEM; 3 to 8 passages. | Centrifugal compression | a) Control cells; b) CF-treated cells of 40 or 90 g; c) hOBs treated with supernatant from PDLFs with CF; d) hOBs treated with sclerostin peptide. | PDLFs subjected to light CF exhibited increased expression of SOST/sclerostin, which inhibited bone formation. |
| Chang et al (2017)8 | PDLFs were obtained from premolars extracted from 9 healthy individuals (aged 14 to 20 years) for orthodontic purpose; cultured in α-MEM; passages < 5. | Tensile force (TF) | a) Unloaded controls; b) 12% tension strain for 24, 48, 72 hrs; c) miR-195-5p transfection + tension; d) miR-195 inhibitors + tension. | MicroRNA-195-5p, which decreased by tension, inhibited osteogenic differentiation of PDLFs under tension. Wnt3A, FGF2, and BMPR1A increased by tension and were targets of miRNA-195-5p. |
| Chen et al (2014)9 | PDLFs were derived from third premolar extracted for orthodontic reasons; cultured in DMEM; 3rd to 9th passages. | Tensile force | a) Cells at 101.3 kPa as controls (normal incubator); b) -100 kPa (tension incubator); c) FAK siRNA knockdown + tension. | Tensile force activated FAK pathways and ERK expression in PDL cells, and also downregulated immune cytokine (IL-1 and TNF-α) and upregulated osteogenic protein (ALP and OCN). FAK knockdown attenuates tensile force-induced ALP and OCN as downstream effectors. |
| Diercke et al (2011)10 | PDLFs were obtained from juvenile patients (aged 12 to 20 years) following premolar extraction during orthodontic treatment; cultured in DMEM; between passages 3 and 9. | Mechanical strain | a) Cells with no strain; b) mechanical strain; b) siRNA-attenuated FAK expression + mechanical strain; d) PDLFs treated with 2 μg/ml or 4 μg/ml ephrinB2-Fc chimeras. | PDLFs exposed to tensile strain induce the expression of ephrin-B2 via a FAK-, Ras-, ERK1/2-, and SP1-dependent pathway. Ephrin-B2-Fc induces osteoblast differentiation. |
| Fujihara et al (2010)11 | PDLFs were isolated as the literature describes; cultured in α-MEM. | Mechanical stretch | a) Cells without stretching; b) cells with stretching; c) cells with glutamate; d) glutamate inhibitor (riluzole) without stretching. | Mechanical stress induces glutamate signalling in the PDL, resulting in enhancement of c-Fos, Runx2 expression, ALP activity, and mineralization of PDL cells. |
| Kanzaki et al (2006)14 | PDLFs were obtained from teeth extracted for orthodontic reasons; cultured in α-MEM; from 4th to 8th passages. | Tensile force | a) Unloaded controls; b) PDLFs loaded with cyclical tensile force for 0.5 to 72 hrs; c) TGF-β2 neutralizing antibody + tension for 72 hrs. | Tension upregulated OPG, RANKL, and TGF-β expression in PDLFs, and inhibited osteoclastogenesis from PBMCs. The upregulation of OPG by tension was partially dependent on TGF-β. |
| Monnouchi et al (2015)20 | PDLFs were obtained from 2 women, aged 30 and 39 years, and a 26-year-old man; culture in α-MEM; 5 to 7 passages. Human PDL stem/progenitor cell line 1-17. | Mechanical stretch | a) Non-loading cells; b) stretch loading for 1 hr; c) cells were transfected with siRNA targeting AT2 receptor, then stretched. | Mechanical stretch appears to control IL-11 expression in PDLFs through the regulation of Ang II and AT2 receptor. Mechanical induction of IL-11 regulates osteoblastic differentiation of PDLSCs. |
| Ren et al (2015)22 | PDLFs were scraped from healthy premolars extracted from donors aged between 12 and 18 for orthodontic reasons; cultured in α-MEM; passages 4 to 6. | Tensile force | a) Non-loaded control cells; b) tension applied for 1 to 24 hrs; c) cells transfected with LV-Runx2 (overexpression); d) tension + U0126 (block ERK1/2) for 3 hrs. | Tension activated ERK1/2 and resulted in elevated Runx2 level, as well as enhanced ATF4, SP7, OCN, and BSP expressions. Phosphorylation of Runx2 induced by tension via ERK1/2 pathway contributes to osteodifferentiation of PDLFs. |
| Sun et al (2016)24 | PDLFs were prepared from premolar and wisdom teeth extracted for orthodontic and impacted reasons; cultured in α-MEM; 3-5 passages. | Mechanical strain | a) Unstimulated controls; b) 12% strain; c) IL-1β and TNF-α; d) IL-1β, TNF-α + strain. | Intermittent strain increased the expression of IL-1β and TNF-α. Strain impaired the osteogenic capacity of PDLFs when incubated with pro-inflammatory cytokines, as evidenced by low ALP activity, as well as reduced Alizarin red staining and Runx2 and COL1. |
| Sun et al (2017)25 | PDLFs were harvested from 6 teeth from 4 systemically healthy patients (aged 18 to 28 years); cultured in α-MEM; used at passages 3 to 5. | Tensile force | a) Untreated cells; b) tension alone; c) IL-1β/TNF-α alone; d) tension and IL-1β/TNF-α. | Tension increased the expression of IL-1β and TNF-α. PDLFs exposed to tension in inflammatory environment exhibited reduced proliferation and mineralization potential. Runx2 and COL1 expression were also decreased. |
| Yang et al (2016)30 | PDLFs were isolated from healthy premolars of teenagers (12 to 16 years old) undergoing extraction for orthodontic reasons; cultured in α-MEM; passages 3 and 4. | Tensile force | a) Unloaded cells; b) tension was applied for up to 24 hrs; c) PERK transfected cells (PERK+/+) + tension; d) PERK knockdown cells (PERK−/−) + tension. | The tension-induced PERK-eIF2α-ATF4 signalling pathway mediated by ERS may exert an influence on osteogenesis differentiation (OCN and BSP expression, and mineralization) of hPDLFs during the process of orthodontic tooth movement. |
| Kook et al (2011)17 | PDLF were obtained from healthy male individuals aged 20 to 30 years; cultured in DMEM; at passages 4 through 7. | Tensile/compressive force | a) Cells subjected to tension for 0 to 6 hrs; b) cells subjected to CF for 0 to 6 hrs; c) co-culture of BMM with PLF without anti-OPG antibody then subjected to mechanical forces. | With the cooperation of RANKL, CF increased the production of TNF-α, which stimulates osteoclastic differentiation and pit formation, while tension reduced TNF-α expression. |
| Qi and Zhang (2014)21 | PDLFs were isolated from patients under 25 years of age undergoing orthodontic treatment; immortalization of the PDLFs. | Fluid shear stress (FSS) | a) Untreated controls; b) FSS; c) FSS + miRNA-132 knockdown; d) FSS + PI3K/mTOR inhibitor. | microRNA-132 regulated the differentiation of PDLFs, which was induced by FSS, through PI3K/ATK/mTOR signalling axis. |
| Tang et al (2014)26 | PDLFs were obtained from the healthy premolars extracted from donors (3 male, 3 female, aged 11 to 28 years) for orthodontic reasons; cultured in α-MEM; passages 3 to 8. | Fluid shear stress | a) Control group; b) PDLFs subjected to FSS; c) PDLFs treated with ERK1/2 and/or p38 inhibitor during 2-hr FSS. | Appropriate FSS promotes the osteogenic differentiation (Runx2, SP7, BMP2, and COLI expression, ALP activity, and mineralization) of PDLFs. The ERK1/2 and p38 MAPK signalling pathways contribute to this cellular process. |
PDLFs, periodontal ligament fibroblasts; α-MEM, minimum essential medium Eagle – alpha modification; IL, interleukin; CINC-1, cytokine-induced neutrophil chemoattractant 1; MCP-1, monocyte chemotactic protein; TRAP, tartrate-resistant acid phosphatase; Adrb2, β-2 adrenergic receptor; siRNA, small interfering RNA; RANKL, receptor activator of nuclear factor kappa-B ligand; OPG, osteoprotegerin; SNS, sympathetic nervous system; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; NF-κB, nuclear factor-κB; GSI, glutamine synthetase type I; TNF-α, tumour necrosis factor-α; ERK1/2, extracellular signal-regulated kinase 1/2; ASPN, aspirin; SOST, sclerostin; FGF2, fibroblast growth factors; BMPR1A, bone morphogenetic protein receptor type 1A; DMEM, Dulbecco's modified Eagle's medium; FAK, focal adhesion kinase; PDL, periodontal ligament; ALP, alkaline phosphatase; OCN, osteocalcin; TGF-β1, transforming growth factor-β1; PBMCs, peripheral blood mononuclear cells; AT2 receptor, angiotensin II receptor; Ang II, angiotensin II; PDLSCs, stem cells in periodontal ligament tissues; Runx2, runt-related transcription factor 2; ATF4, activating transcription factor 4; SP7, osteriox; BSP, sialoprotein; COL1, collagen α1; PERK, protein kinase R like endoplasmic reticulum kinase; eIF2α, α-subunit of eukaryotic initiation factor 2; ERS, endoplasmic reticulum stress; BMM, bone marrow macrophage; mTOR, mechanistic target of rapamycin; PI3K, phosphoinositide 3-kinase; MAPK, mitogen-activated protein kinases
Discussion
Periodontal ligament fibroblasts, the most abundant cells in the periodontal ligament, are known to respond to the mechanical forces generated by orthodontic treatment and thereby to regulate the differentiation of osteoblasts and osteoclasts required for tooth repositioning. In vitro studies of primary periodontal ligament fibroblasts derived from periodontal ligament tissue have demonstrated intracellular signals in response to biomechanical stimuli. This systematic review was conducted to address the details underlying the mechanism by which mechanical loading affects human periodontal ligament fibroblasts. The capacities of periodontal ligament fibroblasts to produce osteogenic- and osteoclastogenic-associated molecules during alveolar bone metabolism are regulated by mechanical forces and depend on the type of force-loading model.
In vitro mechanical loading models of 2D cultured periodontal ligament fibroblasts
This review addresses four conventional types of mechanical force approach: the static weight approach; substrate deformation-based approach; centrifugation approach; and fluid shear stress approach. The vibration approach was excluded because it is used to simulate physiological conditions under masticatory force rather than orthodontic force.38 During orthodontic treatment, water is completely extruded from the periodontal ligament on the pressure side after sustained force loading and does not exert a force equivalent to orthodontic force; we did not include experiments conducted with hydrostatic pressure methods in our review. There is a striking disparity between the forces calculated in finite element analysis and those used in the weight method. The finite element method is a software model used to analyze stress distribution in periodontal ligament and alveolar bone by simulating real objects in a complex mesh of points. According to this, for loading with a lingually directed 100 g horizontal force, the highest stress in the periodontal area is approximately 120 g/cm2, which differs from the force magnitude used in in vitro studies.39 The in vitro studies, however, predominantly focus on mechanical interactions at the cellular level, rather than the interactions between a larger structure and its environment. The contact between glass plates and cells may itself alter their function, by altering electrical charges or gas exchange, and so some argue that control, unweighted plates should be used on the control cell layers.6,18,31 Future studies are needed in order to explore more physiological models of compressive force loading on cells.
Cells are theoretically compressed in a perpendicular direction in a centrifugal force model and there is broadly good evidence to support this method of loading as a means of facilitating osteoclastogenesis and inhibiting osteoblast mineralization.27 One study, by contrast, reported that centrifugal force inhibits osteoclast formation.16
The different devices used to exert tensile strain in various studies have led to varied amplitudes of cellular deformation on the silicone membranes, and so comparison of results is challenging. Both the degree of deformation and the frequency at which it is applied in this normally cyclical method are important. Only one study, excluded from the systematic review due to a lack of functional evaluation, employed static tension on periodontal ligament fibroblasts.40 The differences between static and cyclic tension have not been compared or discussed in the literature. Future studies are needed to investigate the effects of static tensile force on periodontal ligament fibroblasts and how these differ from cyclically applied force.
Roles of osteoclast differentiation-related factors in mechanical force-induced bone resorption
A total of 13 studies examined osteoclastogenesis at a functional level in that they assessed the differentiation of osteoclastic multinucleated cells. In five studies, the osteoclast precursors were treated with the conditioned medium obtained from periodontal ligament fibroblasts subjected to mechanical force with or without stimulation of target molecules.14,15,18,29,31 The other eight studies directly co-cultured osteoclast progenitors with pre-loaded or pre-treated periodontal ligament fibroblasts to investigate how the periodontal ligament fibroblasts, under mechanical force, influence osteoclastogenesis.6,7,12,13,16,17,19,23 Contact of the cell bodies between periodontal ligament fibroblasts and osteoclast precursors is required in direct co-culture, while indirect co-culture only requires the supernatant containing the proteins secreted by periodontal ligament fibroblasts.
RANKL/OPG system
A total of 11 studies investigated the receptor activator of nuclear factor kappa-B ligand (RANKL)/osteoprotegerin (OPG) system,7,12-17,19,23,29,31 including one study that used both compressive and tensile forces (Table V).17 Periodontal ligament fibroblasts can secrete soluble factors that interact with receptors expressed by osteoclast precursors and thus further regulate osteoclast formation.4 RANKL binds to its receptor RANK to stimulate osteoclast maturation, while OPG functions as a decoy receptor for RANKL to prevent RANKL-RANK binding, which in turn inhibits osteoclastogenesis.41 RANKL/OPG signalling between periodontal ligament fibroblasts and osteoclast precursors can thus transduce cytomechanical signals that activate bone resorption. These studies demonstrated that both compressive and centrifugal force activate the RANKL-RANK axis to facilitate TRAP-positive osteoclast formation when progenitor cells are co-cultured with periodontal ligament fibroblasts. Among those experiments, the most favourable and efficient static compression magnitude was 2.0 g/cm2 (0.5 g/cm2 to 4.0 g/cm2). An excessive compressive force is thought to decrease cell viability and thus a weak force may effectively induce RANKL production in the periodontal ligament on the pressure side, leading to bone resorption.42 This is consistent with the natural history of relatively light static forces applied therapeutically achieving significant repositioning of teeth. Greater forces should be avoided, as they do not offer reduced duration of treatment but may induce necrosis (hyalinization) of the periodontal ligament and hence risk bone destruction and root resorption.43,44 By contrast, tensile forces tend to increase OPG expression or reduce the RANKL/OPG ratio. Different durations of effect have been noted (from 30 minutes to 72 hours) and may depend largely on variations in other target genes, study objectives, and detection methods.
Table V.
Regulation of mechanical force on the receptor activator of nuclear factor kappa-B ligand (RANKL)/osteoprotegerin (OPG) system
| Type of force | Major findings | Study | Total studies |
|---|---|---|---|
| Compression | Increased RANKL; decreased or unaltered OPG; increased RANKL/OPG ratio | Jin et al12 (2015); Kanzaki et al13 (2002); Römer et al23 (2013); Yamaguchi et al29 (2006) | 4 |
| Compression | Increased RANKL; no evaluation of OPG | Cao et al7 (2014); Kikuta et al15 (2015); Yoshino et al31 (2014) | 3 |
| Compression | Increased RANKL and OPG | Kook et al17 (2011) | 1 |
| Compression | Unaltered RANKL | Mayahara et al19 (2012) | 1 |
| Centrifugal force | Increased RANKL and OPG | Kook et al16 (2009) | 1 |
| Tension | Decreased or unaltered RANKL; increased OPG; decreased RANKL/OPG ratio | Kook et al17 (2011) | 1 |
| Tension | Increased RANKL and OPG | Kanzaki et al14 (2006) | 1 |
The reviewed studies have demonstrated that the RANKL/OPG system serves as a downstream target of many genes expressed and osteoclastogenic signalling pathways seen in periodontal ligament fibroblasts exposed to mechanical force. Among these pathways, prostaglandin (PG) E2/cyclooxygenase (COX)-2,13,23 β-2 adrenergic receptor (Adrb2),7 Piezo1 channel,12 Notch signalling,15 and extracellular signal-regulated kinase (ERK) signalling16 are activated by mechanical stimuli and regulate the bone homeostasis via stimulation of the RANKL/OPG system. Four studies investigated the RANKL/OPG-dependent mechanism,7,12,15,16 while seven other studies were purely descriptive.13,14,17,19,23,29,31 Mechanical compressive force upregulates Adrb2 expression by increasing the intracellular calcium concentration.7 The Piezo1 channel influences transduction in mechanically stimulated periodontal ligament fibroblasts via nuclear factor (NF)-κB.12 Altered expression of Jagged 1 in periodontal ligament fibroblasts subjected to mechanical loading leads to Notch signalling pathway activation.15 Centrifugal force increased the expression of RANKL and OPG via the activation of ERK signalling.16 All of these factors contribute to compression-induced bone resorption and act as upstream regulators and mediators of the production of RANKL/OPG that subsequently accelerate the differentiation of progenitor cells into osteoclast-like cells. These studies elucidate the molecular mechanism underlying the stress-induced osteoclastogenesis and the complex network between RANKL/OPG system and other mechanically sensitive factors. By contrast, the tension-mediated upregulation of OPG, which inhibits osteoclastogenesis, depends partly on transforming growth factor-beta (TGF-β).14
PGE2/COX-2 signalling
Prostaglandin E2 (PGE2) has been described as a potent stimulator of osteoclastogenesis. Cyclooxygenase-2 is responsible for the biosynthesis of PGE2, which involves the catalysis of arachidonic acids.45 Dysregulation of the PGE2/COX-2 pathway affects bone resorption through a number of specific mechanisms. In the four studies that evaluated PGE2/COX-2 signalling, compressive force was found to augment PGE2 synthesis and COX-2 production in periodontal ligament fibroblasts.12,13,19,23 This response further upregulated RANKL expression and promoted osteoclastogenesis.13 It appears, therefore, that PGE2, a potent pro-inflammatory factor, also participates in compression-related inflammation as well as bone resorption. The synergistic action of pathogens and compressive force upregulates the expression of PGE2/COX-2 to enhance RANKL production and, subsequently, suppress osteoclast differentiation.23 The relationship between PGE2/COX-2 signalling and the RANKL/OPG system is, however, intricate. Although indomethacin greatly reduces the compression-enhanced expression of RANKL, PGE2 may not always stimulate RANKL activity in periodontal ligament fibroblasts because its effect is concentration-dependent.13,19,23 These findings echo, to some extent, those of other studies in which an increase in RANKL expression in bone marrow cells was found to be accompanied by excessive PGE2 expression.46 As with the RANKL/OPG system, PGE2/COX-2 signalling serves as a downstream effector of the Piezo1 channel.12
Other factors related to osteoclast differentiation
Eight studies included in this review described additional stimulators of osteoclastogenesis, including interleukin (IL)-1 (three studies), IL-6 (two studies), IL-8 (one study), and tumour necrosis factor alpha (TNF-α) (four studies). Interleukin-1β regulates inflammatory responses and potently induces bone resorption. Two studies reported upregulated IL-1β production and reduced osteogenesis in periodontal ligament fibroblasts subjected to tensile strain.24,25 Tensile force induces an inflammatory response during orthodontic tooth movement, as shown by increased IL-1β levels at sites of periodontal inflammation. This phenomenon is consistent with the finding that the IL-1β level in gingival crevicular fluid increases one hour after the application of orthodontic force.47 Interleukin-1 includes both IL-1α and IL-1β, and has been identified as an essential pro-inflammatory mediator that can be downregulated by tensile force.9 The discrepancies in IL-1β expression may relate to the different experimental approaches used by previous studies. Notably, the tensile force created by a negative pressure model differs from that of a substrate deformation-based model and the two are not comparable.9,24,25
When considering the differentiation of osteoclasts, TNF-α is a bone-resorbing stimulus associated with intracellular RANKL mechanisms. It has been shown to promote RANKL expression in osteoblasts and marrow stromal cells, and to augment the responses of osteoclast precursors to RANKL.48 Concomitantly, this mechanical compression-induced upregulation of TNF-α in RANKL-positive periodontal ligament fibroblasts stimulates osteoclastogenesis, as demonstrated by an increase in the TRAP-positive cell population in a co-culture system.17,31 These findings suggest that the combined effect of TNF-α and RANKL is more significant than RANKL alone in terms of force-mediated bone resorption. Likewise, TNF-α expression in periodontal ligament fibroblasts is also reduced by exposure to tensile force.9,17 Other studies have shown, however, that periodontal ligament fibroblasts exposed to tensile strain exhibited higher levels of TNF-α expression.24,25 An increased tensile force resulting in 12% elongation was shown to induce TNF-α and IL-1β production, whereas a 1.5% elongation decreased TNF-α production. Although orthodontic tooth movement-induced bone remodelling is associated with an inflammatory process, the inflammatory responses of periodontal ligament fibroblasts to tensile force may be strain magnitude-dependent because low magnitudes were found to have an anti-inflammatory effect.49 As TNF-α is among the cytokines responsible for immune responses, its expression might be inhibited by a lower-magnitude tensile force but enhanced by a greater force. Both IL-6 and IL-8 were upregulated by compressive force in a time- and magnitude-dependent manner, and treatment with either cytokine enhanced osteoclast differentiation.6,15,18
Roles of osteoblast differentiation-related factors in mechanical force-induced bone formation
When considering osteogenesis, studies with no functional analysis have been excluded from this systematic review and hence all of the studies addressing osteogenesis investigated the formation of calcific nodules.
While the presence of osteoclastogenesis-stimulating factors necessitates osteoclast differentiation on the compression side, more attention has been given to the potential roles of osteogenic substances on the tension side in bone formation. Prolonged studies of periodontal ligament fibroblasts have focused largely on osteogenic gene expression and bone-forming activities. Ten papers reported the regulatory effects of mechanical force on the expression of specific osteoblastogenic factors, including ALP, osteocalcin (OCN), runt-related transcription factor 2 (Runx2), bone morphogenetic protein (BMP), fibroblast growth factor (FGF)-2, SP7, osteopontin (OPN), bone sialoprotein (BSP), TGF-β1, and collagen α1 (COL1). Eight experiments applied tensile force, and these substances were mainly found to be upregulated in periodontal ligament fibroblasts.8,9,11,14,22,24,25,30 The fluctuations in the expression of these osteogenic differentiation factors in response to tensile strain depended on different factors (Table VI). Two other studies used a fluid shear stress approach and noted trends towards increased expression of ALP, Runx2, COL1, BMP-2, SP7, and OPN when force was applied, which were further mediated by the ERK1/2 and p38 MAPK signalling pathways and the PI3K/ATK/mTOR signalling axis.21,26
Table VI.
Mechanisms of tension regulation of osteogenic genes
| Genes | Dependent signalling and pathway | Other related molecules |
|---|---|---|
| ALP | FAK pathway9 or glutamate signalling11 | Suppressed by IL-1β and TNF-α24,25 |
| OCN | FAK pathway9 or PERK-eIF2α-ATF4 pathway30 | Regulated by Runx222 |
| Runx2 | Glutamate signalling11 or ERK1/2 pathway22 | Suppressed by IL-1β and TNF-α24,25 |
| BMPR1A | N/A | Coordinated with microRNA-195-5p8 |
| FGF2 | N/A | Coordinated with microRNA-195-5p8 |
| SP7 | N/A | Regulated by Runx222 |
| BSP | PERK-eIF2α-ATF4 pathway30 | Regulated by Runx222 |
| TGF-β1 | N/A | Influenced OPG14 |
| COL1 | N/A | Suppressed by IL-1β and TNF-α24,25 |
ALP, alkaline phosphatase; FAK, focal adhesion kinase; IL, interleukin; TNF-α, tumour necrosis factor-α; OCN, osteocalcin; PERK, protein kinase R like endoplasmic reticulum kinase; eIF2α, α-subunit of eukaryotic initiation factor 2; ATF4, activating transcription factor 4; Runx2, runt-related transcription factor 2; ERK1/2, extracellular signal-regulated kinase 1/2; BMPR1A, bone morphogenetic protein receptor type 1A; N/A, not applicable; FGF2, fibroblast growth factors; BSP, sialoprotein; TGF-β1, transforming growth factor-β1; OPG, osteoprotegerin; COL1, collagen α1
Alveolar remodelling during orthodontic tooth movement tends to improve bone deposition on the tension side. Alkaline phosphatase is involved in the process of hard tissue mineralization, and, accordingly, ALP activity has been used as an indicator of osteoblastic differentiation in bone cells.50,51 Periodontal ligament fibroblasts exhibit high levels of ALP activity, suggestive of an osteoblastic phenotype. Accumulating evidence suggests that the integrin-focal adhesion kinase (FAK) complex acts as a mechanoreceptor in periodontal ligament fibroblasts.52 Upon phosphorylation, FAK contributes to ERK1/2/MAPK pathway activation, which is associated with ALP activity in stretched periodontal ligament fibroblasts.9,22 The relationships among various osteogenic genes and factors are complex. Runx2, for example, serves as an upstream effector of several osteoblast markers, including OCN, BSP, and SP7.22 The tensile induction of OPG is partially dependent on TGF-β.14 Interactions among these different osteogenic molecules contribute to the tensile force-mediated upregulation of osteodifferentiation and downregulation of osteoclastogenesis from periodontal ligament fibroblasts.
Some studies described other molecules involved in tensile force and fluid shear stress-induced calcium deposition. Activating transcription factor 4 (ATF4), another important factor, may regulate bone formation in response to tensile force loading. Osteocalcin and BSP are the target genes of ATF4,30 while Runx2 transfection decreases the ATF4 expression in periodontal ligament fibroblasts.22 Activating transcription factor 4 also can be activated by protein kinase R-like endoplasmic reticulum kinase (PERK) and the ERK1/2 pathway. MicroRNAs are small, non-coding RNAs that play critical roles in force-related osteodifferentiation of periodontal ligament fibroblasts. Fluid shear stress increases the expression of miRNA-132, which further enhances the mineralization of periodontal ligament fibroblasts, while tensile force decreases the expression of miRNA-195-5p, which suppresses mineralized nodules.8,21
Five studies offered a basis for the mechanism by which tensile force and fluid shear stress affect osteodifferentiation,9,21,22,26,30 while others merely observed a relationship.
Molecules indirectly related to bone remodelling
Other mechanical stress-associated molecules indirectly affect osteogenesis and osteoclastogenesis in periodontal ligament fibroblasts, as outlined in Table VII.
Table VII.
Regulation of mechanical force on indirect-related molecules
| Molecules | Stimulation | Changes | Effect on other factors | Dependent signalling pathway |
|---|---|---|---|---|
| MCP-16 | Compression | Increased | Contributed to osteoclast differentiation and pit formation | - |
| Sclerostin27 | Centrifugation | Increased | Inhibitory effect on osteodifferentiation | - |
| Asporin27 | Centrifugation | Increased | Inhibitory effect on osteodifferentiation | - |
| EphB4-ephrinB210 | Tension | Increased | Induced ALP and Runx2 transcription, and osteodifferentiation | FAK pathway |
| IL-1120 | Tension | Increased | Stimulated CP-23, OPN, BSP, and osteodifferentiation | AT2 receptor |
MCP-1, monocyte chemotactic protein; ALP, alkaline phosphatase; Runx2, runt-related transcription factor 2; IL, interleukin; OPN, osteopontin; BSP, bone sialoprotein
The ephrin ligands and Eph receptors are thought to regulate the processes of cell-cell adhesion and migration in bone biology.53 The EphB4-ephrinB2 axis has been characterized as a coupling factor in bidirectional osteoblast-osteoclast communication.54 It has been shown that the activation of EphB4-ephrinB2 signalling stimulates osteoblast differentiation while suppressing osteoclastogenesis, thereby yielding bone formation. In a study of stretched periodontal ligament fibroblasts, Diercke et al10 reported an osteogenic interaction between ephrinB2-expressing periodontal ligament fibroblasts and EphB4-expressing osteoblasts.
Interleukin-11, a member of the IL-6 family, can act as a pro-inflammatory cytokine and has osteogenic effects in stretched periodontal ligament fibroblasts. Tensile strain and a subsequent upregulation in angiotensin (Ang) II expression promotes IL-11 production via Ang II type 2 (AT2) receptors and the renin-angiotensin system (RAS).20 Asporin and sclerostin, both of which may suppress osteoblasts, are responsive to compression and their expression is related to the magnitude of force. Light force upregulates the synthesis of sclerostin, whereas higher magnitudes of force increase the release of asporin but decrease that of sclerostin.27 The molecular mechanism by which compression inhibits bone formation therefore varies according to the magnitude of force.
Bone cells within the skeleton, including osteoclasts, osteoblasts, and osteocytes, are responsive to mechanical signals, facilitating bone remodelling.55 A different situation exists with alveolar bone, which mechanocouples with the teeth via PDLFs and hence has a different form of control mechanism.
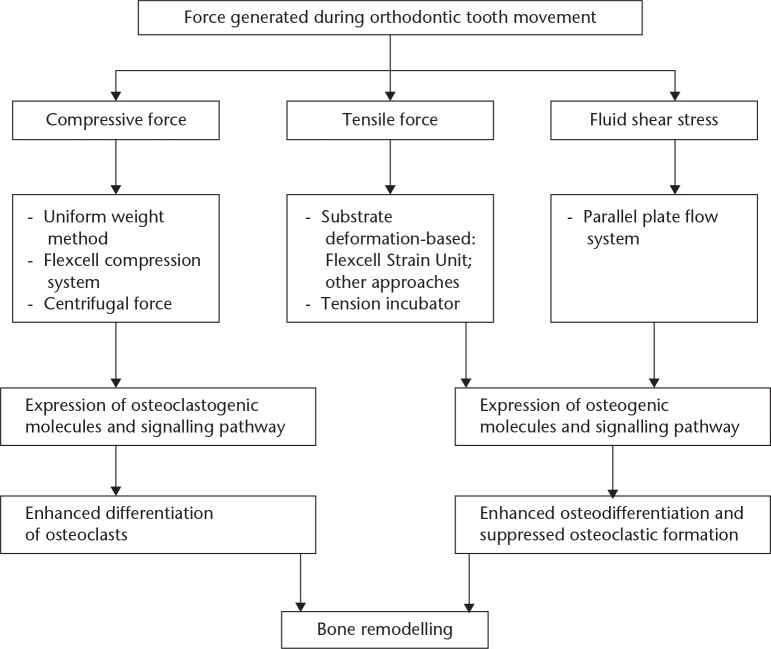
In conclusion, periodontal ligament fibroblasts, which interface with osteoblasts and osteoclasts in alveolar bone, are the targets of the mechanical forces applied in orthodontic settings. A number of substances have been proposed as links between mechanostimulation of the periodontium and the initiation of bone modelling. In vitro force application models that simulate clinical orthodontic force loading have enabled researchers to elucidate the biological behaviour of periodontal ligament fibroblasts in response to mechanical stimuli. Our systematic review summarizes the suitable loading approaches and data pertaining to molecules that regulate osteogenesis and osteoclastogenesis (Fig. 2). An improved understanding of this topic might facilitate the clinical development of novel therapeutic strategies aimed at accelerating orthodontic tooth movement and preventing tooth overloading. There is merit in carrying out future studies to investigate how the static tension affects the cytomechanical properties of periodontal ligament fibroblasts, and to explore a better mode of static compressive force loading. In addition, the clarification of some of the underlying regulatory systems is yet to occur, and further studies are needed to facilitate this.
Fig. 2.
Schematic diagram of the mechanical function of periodontal ligament fibroblasts
Footnotes
Author contributions: M. Li: Collected the data, Wrote the manuscript.
C. Zhang: Reviewed the literature.
Y. Yang: Designed the study.
Follow us @BoneJointRes
Funding statement
This work was supported by the University of Hong Kong Seed Funding Programme for Basic Research (grant number: 201409176098).
References
- 1. Lekic P, McCulloch CA. Periodontal ligament cell population: the central role of fibroblasts in creating a unique tissue. Anat Rec 1996;245:327-341. [DOI] [PubMed] [Google Scholar]
- 2. Basdra EK, Komposch G. Osteoblast-like properties of human periodontal ligament cells: an in vitro analysis. Eur J Orthod 1997;19:615-621. [DOI] [PubMed] [Google Scholar]
- 3. Lekic P, Rojas J, Birek C, Tenenbaum H, McCulloch CA. Phenotypic comparison of periodontal ligament cells in vivo and in vitro. J Periodontal Res 2001;36:71-79. [DOI] [PubMed] [Google Scholar]
- 4. Sokos D, Everts V, de Vries TJ. Role of periodontal ligament fibroblasts in osteoclastogenesis: a review. J Periodontal Res 2015;50:152-159. [DOI] [PubMed] [Google Scholar]
- 5. Xu JG, Gong T, Heng BC, Zhang CF. A systematic review: differentiation of stem cells into functional pericytes. FASEB J 2017;31:1775-1786. [DOI] [PubMed] [Google Scholar]
- 6. Asano M, Yamaguchi M, Nakajima R, et al. IL-8 and MCP-1 induced by excessive orthodontic force mediates odontoclastogenesis in periodontal tissues. Oral Dis 2011;17:489-498. [DOI] [PubMed] [Google Scholar]
- 7. Cao H, Kou X, Yang R, et al. Force-induced Adrb2 in periodontal ligament cells promotes tooth movement. J Dent Res 2014;93:1163-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang M, Lin H, Fu H, et al. MicroRNA-195-5p regulates osteogenic differentiation of periodontal ligament cells under mechanical loading. J Cell Physiol 2017;232:3762-3774. [DOI] [PubMed] [Google Scholar]
- 9. Chen YJ, Shie MY, Hung CJ, et al. Activation of focal adhesion kinase induces extracellular signal-regulated kinase-mediated osteogenesis in tensile force-subjected periodontal ligament fibroblasts but not in osteoblasts. J Bone Miner Metab 2014;32:671-682. [DOI] [PubMed] [Google Scholar]
- 10. Diercke K, Kohl A, Lux CJ, Erber R. Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement. J Biol Chem 2011;286:37651-37664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujihara C, Yamada S, Ozaki N, et al. Role of mechanical stress-induced glutamate signaling-associated molecules in cytodifferentiation of periodontal ligament cells. J Biol Chem 2010;285:28286-28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin Y, Li J, Wang Y, et al. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod 2015;85:87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res 2002;17:210-220. [DOI] [PubMed] [Google Scholar]
- 14. Kanzaki H, Chiba M, Sato A, et al. Cyclical tensile force on periodontal ligament cells inhibits osteoclastogenesis through OPG induction. J Dent Res 2006;85:457-462. [DOI] [PubMed] [Google Scholar]
- 15. Kikuta J, Yamaguchi M, Shimizu M, Yoshino T, Kasai K. Notch signaling induces root resorption via RANKL and IL-6 from hPDL cells. J Dent Res 2015;94:140-147. [DOI] [PubMed] [Google Scholar]
- 16. Kook SH, Son YO, Hwang JM, et al. Mechanical force inhibits osteoclastogenic potential of human periodontal ligament fibroblasts through OPG production and ERK-mediated signaling. J Cell Biochem 2009;106:1010-1019. [DOI] [PubMed] [Google Scholar]
- 17. Kook SH, Jang YS, Lee JC. Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-α-mediated activation of CD4+ T cells. J Cell Biochem 2011;112:2891-2901. [DOI] [PubMed] [Google Scholar]
- 18. Kunii R, Yamaguchi M, Tanimoto Y, et al. Role of interleukin-6 in orthodontically induced inflammatory root resorption in humans. Korean J Orthod 2013;43:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayahara K, Yamaguchi A, Takenouchi H, et al. Osteoblasts stimulate osteoclastogenesis via RANKL expression more strongly than periodontal ligament cells do in response to PGE(2). Arch Oral Biol 2012;57:1377-1384. [DOI] [PubMed] [Google Scholar]
- 20. Monnouchi S, Maeda H, Yuda A, et al. Mechanical induction of interleukin-11 regulates osteoblastic/cementoblastic differentiation of human periodontal ligament stem/progenitor cells. J Periodontal Res 2015;50:231-239. [DOI] [PubMed] [Google Scholar]
- 21. Qi L, Zhang Y. The microRNA 132 regulates fluid shear stress-induced differentiation in periodontal ligament cells through mTOR signaling pathway. Cell Physiol Biochem 2014;33:433-445. [DOI] [PubMed] [Google Scholar]
- 22. Ren D, Wei F, Hu L, et al. Phosphorylation of Runx2, induced by cyclic mechanical tension via ERK1/2 pathway, contributes to osteodifferentiation of human periodontal ligament fibroblasts. J Cell Physiol 2015;230:2426-2436. [DOI] [PubMed] [Google Scholar]
- 23. Römer P, Köstler J, Koretsi V, Proff P. Endotoxins potentiate COX-2 and RANKL expression in compressed PDL cells. Clin Oral Investig 2013;17:2041-2048. [DOI] [PubMed] [Google Scholar]
- 24. Sun C, Chen L, Shi X, et al. Combined effects of proinflammatory cytokines and intermittent cyclic mechanical strain in inhibiting osteogenicity in human periodontal ligament cells. Cell Biol Int 2016;40:999-1007. [DOI] [PubMed] [Google Scholar]
- 25. Sun C, Liu F, Cen S, et al. Tensile strength suppresses the osteogenesis of periodontal ligament cells in inflammatory microenvironments. Mol Med Rep 2017;16:666-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang M, Peng Z, Mai Z, et al. Fluid shear stress stimulates osteogenic differentiation of human periodontal ligament cells via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling pathways. J Periodontol 2014;85:1806-1813. [DOI] [PubMed] [Google Scholar]
- 27. Ueda M, Goto T, Kuroishi KN, et al. Asporin in compressed periodontal ligament cells inhibits bone formation. Arch Oral Biol 2016;62:86-92. [DOI] [PubMed] [Google Scholar]
- 28. Ueda M, Kuroishi KN, Gunjigake KK, Ikeda E, Kawamoto T. Expression of SOST/sclerostin in compressed periodontal ligament cells. J Dent Sci 2016;11:272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamaguchi M, Aihara N, Kojima T, Kasai K. RANKL increase in compressed periodontal ligament cells from root resorption. J Dent Res 2006;85:751-756. [DOI] [PubMed] [Google Scholar]
- 30. Yang SY, Wei FL, Hu LH, Wang CL. PERK-eIF2α-ATF4 pathway mediated by endoplasmic reticulum stress response is involved in osteodifferentiation of human periodontal ligament cells under cyclic mechanical force. Cell Signal 2016;28:880-886. [DOI] [PubMed] [Google Scholar]
- 31. Yoshino T, Yamaguchi M, Shimizu M, Yamada K, Kasai K. TNF-alpha aggravates the progression of orthodontically-induced inflammatory root resorption in the presence of RANKL. J Hard Tissue Biol 2014;23:155-162. [Google Scholar]
- 32. Li Y, Zheng W, Liu JS, et al. Expression of osteoclastogenesis inducers in a tissue model of periodontal ligament under compression. J Dent Res 2011;90:115-120. [DOI] [PubMed] [Google Scholar]
- 33. Nakajima R, Yamaguchi M, Kojima T, Takano M, Kasai K. Effects of compression force on fibroblast growth factor-2 and receptor activator of nuclear factor kappa B ligand production by periodontal ligament cells in vitro. J Periodontal Res 2008;43:168-173. [DOI] [PubMed] [Google Scholar]
- 34. Mitsui N, Suzuki N, Maeno M, et al. Optimal compressive force induces bone formation via increasing bone sialoprotein and prostaglandin E(2) production appropriately. Life Sci 2005;77:3168-3182. [DOI] [PubMed] [Google Scholar]
- 35. Redlich M, Palmon A, Zaks B, et al. The effect of centrifugal force on the transcription levels of collagen type I and collagenase in cultured canine gingival fibroblasts. Arch Oral Biol 1998;43:313-316. [DOI] [PubMed] [Google Scholar]
- 36. Chang M, Lin H, Luo M, Wang J, Han G. Integrated miRNA and mRNA expression profiling of tension force-induced bone formation in periodontal ligament cells. In Vitro Cell Dev Biol Anim 2015;51:797–807. [DOI] [PubMed] [Google Scholar]
- 37. Hasegawa S, Sato S, Saito S, Suzuki Y, Brunette DM. Mechanical stretching increases the number of cultured bone cells synthesizing DNA and alters their pattern of protein synthesis. Calcif Tissue Int 1985;37:431-436. [DOI] [PubMed] [Google Scholar]
- 38. Yang L, Yang Y, Wang S, Li Y, Zhao Z. In vitro mechanical loading models for periodontal ligament cells: from two-dimensional to three-dimensional models. Arch Oral Biol 2015;60:416-424. [DOI] [PubMed] [Google Scholar]
- 39. Tanne K, Sakuda M, Burstone CJ. Three-dimensional finite element analysis for stress in the periodontal tissue by orthodontic forces. Am J Orthod Dentofacial Orthop 1987;92:499-505. [DOI] [PubMed] [Google Scholar]
- 40. Jacobs C, Grimm S, Ziebart T, Walter C, Wehrbein H. Osteogenic differentiation of periodontal fibroblasts is dependent on the strength of mechanical strain. Arch Oral Biol 2013;58:896-904. [DOI] [PubMed] [Google Scholar]
- 41. Theoleyre S, Wittrant Y, Tat SK, et al. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev 2004;15:457-475. [DOI] [PubMed] [Google Scholar]
- 42. Nakao K, Goto T, Gunjigake K, et al. Neuropeptides modulate RANKL and OPG expression in human periodontal ligament cells. Orthod Waves 2007;66:33-40. [Google Scholar]
- 43. Gonzales C, Hotokezaka H, Yoshimatsu M, et al. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod 2008;78:502-509. [DOI] [PubMed] [Google Scholar]
- 44. Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop 2006;129:469.e1-e32. [DOI] [PubMed] [Google Scholar]
- 45. Shimizu N, Ozawa Y, Yamaguchi M, et al. Induction of COX-2 expression by mechanical tension force in human periodontal ligament cells. J Periodontol 1998;69:670-677. [DOI] [PubMed] [Google Scholar]
- 46. Kanematsu M, Sato T, Takai H, et al. Prostaglandin E2 induces expression of receptor activator of nuclear factor-kappa B ligand/osteoprotegrin ligand on pre-B cells: implications for accelerated osteoclastogenesis in estrogen deficiency. J Bone Miner Res 2000;15:1321-1329. [DOI] [PubMed] [Google Scholar]
- 47. Kapoor P, Kharbanda OP, Monga N, Miglani R, Kapila S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: a systematic review. Prog Orthod 2014;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takayanagi H. Inflammatory bone destruction and osteoimmunology. J Periodontal Res 2005;40:287-293. [DOI] [PubMed] [Google Scholar]
- 49. Long P, Hu J, Piesco N, Buckley M, Agarwal S. Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J Dent Res 2001;80:1416-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kondo Y, Irie K, Ikegame M, et al. Role of stromal cells in osteoclast differentiation in bone marrow. J Bone Miner Metab 2001;19:352-358. [DOI] [PubMed] [Google Scholar]
- 51. Yamashita Y, Sato M, Noguchi T. Alkaline phosphatase in the periodontal ligament of the rabbit and macaque monkey. Arch Oral Biol 1987;32:677-678. [DOI] [PubMed] [Google Scholar]
- 52. Kim SJ, Park KH, Park YG, Lee SW, Kang YG. Compressive stress induced the up-regulation of M-CSF, RANKL, TNF-α expression and the down-regulation of OPG expression in PDL cells via the integrin-FAK pathway. Arch Oral Biol 2013;58:707-716. [DOI] [PubMed] [Google Scholar]
- 53. Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adhes Migr 2012;6:148-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao C, Irie N, Takada Y, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 2006;4:111-121. [DOI] [PubMed] [Google Scholar]
- 55. Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene 2012;503:179-193. [DOI] [PMC free article] [PubMed] [Google Scholar]