Abstract
Objectives
Tranexamic acid (TXA) is an anti-fibrinolytic medication commonly used to reduce perioperative bleeding. Increasingly, topical administration as an intra-articular injection or perioperative wash is being administered during surgery. Adult soft tissues have a poor regenerative capacity and therefore damage to these tissues can be harmful to the patient. This study investigated the effects of TXA on human periarticular tissues and primary cell cultures using clinically relevant concentrations.
Methods
Tendon, synovium, and cartilage obtained from routine orthopaedic surgeries were used for ex vivo and in vitro studies using various concentrations of TXA. The in vitro effect of TXA on primary cultured tenocytes, fibroblast-like synoviocytes, and chondrocytes was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assays, fluorescent microscopy, and multi-protein apoptotic arrays for cell death.
Results
There was a significant (p < 0.01) increase in cell death within all tissue explants treated with 100 mg/ml TXA. MTT assays revealed a significant (p < 0.05) decrease in cell viability in all tissues following treatment with 50 mg/ml or 100 mg/ml of TXA within four hours. There was a significant (p < 0.05) increase in cell apoptosis after one hour of exposure to TXA (100 mg/ml) in all tissues.
Conclusion
The current study demonstrates that TXA caused significant periarticular tissue toxicity ex vivo and in vitro at commonly used clinical concentrations.
Cite this article: M. McLean, K. McCall, I. D. M. Smith, M. Blyth, S. M. Kitson, L. A. N. Crowe, W. J. Leach, B. P. Rooney, S. J. Spencer, M. Mullen, J. L. Campton, I. B. McInnes, M. Akbar, N. L. Millar. Tranexamic acid toxicity in human periarticular tissues. Bone Joint Res 2019;8:11–18. DOI: 10.1302/2046-3758.81.BJR-2018-0181.R1.
Keywords: Tranexamic acid, Apoptosis, Periarticular tissues, Tendon, Cartilage, Synovium
Article focus
This study investigated the effects of tranexamic acid (TXA) toxicity on human periarticular tissues including tendon, synovium, and cartilage.
Key messages
TXA caused significant periarticular tissue toxicity ex vivo and in vitro at commonly used clinical concentrations.
We suggest clinicians show caution when considering topical TXA treatments, particularly on soft-tissue orthopaedic pro-cedures.
Further human clinical trials are required to confirm the safety of TXA before it can be recommended for standard topical practice.
Strengths and limitations
While our study shows increased cell death, this may not necessarily translate into poor clinical outcomes to the patient and we recognize that studies evaluating early and longer-term clinical follow-up of topical TXA are required.
Introduction
Surgeons continually strive to improve post-procedural rehabilitation in their patients, with the hope and intention of enhancing patient satisfaction and surgical outcomes. One such area that has been targeted across the surgical specialties is intra- and postoperative blood loss.1 Within orthopaedic surgery, this is particularly relevant in patients undergoing hip and knee arthroplasty, commonly performed procedures that are frequently associated with considerable blood loss.2 Postoperative anaemia may predispose patients to an increased risk of cardiopulmonary events and adverse transfusion reactions,3 while prolonged hospitalization associated with significant blood loss may result in an increased risk of hospital-acquired infections.4,5 Ultimately, there may be delayed patient rehabilitation and an escalation in healthcare costs.
Activation of fibrinolysis during and after surgery is a phenomenon considered to contribute significantly to intra- and postoperative blood loss.6 Intravenous (IV) administration of the anti-fibrinolytic agent (TXA) is an established off-licence perioperative haemostatic treatment.7 Tranexamic acid is a synthetic derivative of the amino acid lysine that inhibits fibrinolysis by competitively blocking the lysine binding site on plasminogen and subsequently inhibiting its conversion to plasmin, a fibrinolytic protease that degrades fibrin clots. It has been shown to reduce postoperative bleeding and blood transfusion requirements in patients undergoing hip and knee arthroplasty.3,8 Despite its proven benefits, concern remains about the safety of its intravenous administration, given the documented risk of thromboembolic events and acute renal impairment.3,9
In view of the safety concerns relating to the intravenous administration of TXA, it has been hypothesized that topical administration (intra-/periarticular) may avoid the risks of systemic administration. Recent randomized controlled trials investigating topical administration during hip and knee arthroplasty have demonstrated significantly lower blood loss and transfusion rates in comparison with control groups with no apparent significant systemic side effects.10,11 Topical TXA solution concentrations have ranged from 10 mg/ml to 200 mg/ml, with application strategies varying between studies.3 Most topical administration studies to date have focused primarily on hip and knee arthroplasty, although it is becoming increasingly popular during soft-tissue reconstructions such as anterior cruciate ligament (ACL) reconstruction.12 However, unlike the majority of hip and knee arthroplasties, this involves the direct exposure of healthy cartilage, tendon, and ligament to TXA. At present, the interaction between these important periarticular tissues and TXA remains largely unknown, with rodent and human in vitro studies on chondrocytes providing conflicting results.13-17 Adult tendon and, in particular, cartilage have a poor regenerative capacity and therefore significant damage to these tissues may be devastating to the patient.18,19
This study was designed to test the hypothesis that TXA had no cytotoxic effects on human periarticular tissues including tendon, synovium, and cartilage.
Materials and Methods
Study design
All procedures and protocols were approved by the Ethics Committee under approval number REC14/WS/1035 with informed consent obtained and carried out in accordance with standard operative procedures.
Tissue collection and preparation
Human tendon tissue was harvested during hamstring tendon ACL. Synovial tissue was harvested during total hip arthroplasty (THA) or total knee arthroplasty (TKA). Cartilage tissue was harvested from femoral heads obtained during hip hemiarthoplasty for femoral neck fracture. Tissues were then separated for either ex vivo or in vitro experiments as outlined below. All patients were screened and excluded if there was a prior history of surgery affecting the same joint, malignancy, or infection. Cartilage was excluded if there was any macroscopic evidence of osteoarthritis, and only Outerbridge grade 0 cartilage was used.20
Tissue culture
Human tendon-derived tenocytes were explanted from hamstring tendons and fibroblast-like synoviocytes (FLS) from synovial tissue, as described previously.21 Cultures were maintained in a humidified environment at 37°C, 5% CO2 in Roswell Park Memorial Institute (RPMI) 1640, supplemented with 10% foetal bovine serum (FBS), 1% penicillin-streptomyocin, and L-glutamine (Life Technologies). Cells were grown to subconfluency and passaged using trypsin ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, Gillingham, United Kingdom). Cells from the third and fourth passages were seeded two days prior to experimentation and no phenotypic drift was confirmed using polymerase chain reaction (PCR; Scleraxis, Aggregan, SOX9 genes).
Human chondrocytes were isolated by enzymatic digestion of intact femoral head articular cartilage.22 Chondrocytes were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% FBS, 1% penicillin-streptomycin, 1% amphotericin, and 10% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). Cells were grown to subconfluency and detached for seeding using Accutase (Sigma-Aldrich). Cells from the initial explantation were not passaged. They were seeded two days prior to experimentation. Tranexamic acid was added to concentrations of 0 mg/ml, 1 mg/ml, 50 mg/ml, or 100 mg/ml. Dilution groups were pH-monitored to ensure effects were not related to acidity.
Confocal microscopy
Tissue samples were cut into 1 cm2 quadruplicate replicate pieces and treated with 0 mg/ml or 100 mg/ml TXA in RPMI, supplemented with 10% FBS and 1% penicillin-streptomycin, at 37°C and 5% CO2 for 16 hours. They were then washed in RPMI and stained with 12.5 µM 5-chloromethylfluorescein diacetate (CMFDA) and 10 μMl propidium iodide23 for two hours at 37°C, then fixed in 4% formaldehyde. They were imaged using confocal microscopy (LSM 800; Zeiss, Cambridge, United Kingdom), and the proportion of live (green) and dead (red) cells was counted using Imaris x64 (8.4.1) software (Bitplane Ltd, Zurich, Switzerland). Three individual donor tissues were used for the tendon and synovium, and four donors were used for the cartilage tissue.
Cell viability assays
The in vitro effect of TXA at concentrations of 0 mg/ml, 1 mg/ml, 50 mg/ml, and 100 mg/ml over 1, 4, and 24 hours on primary human cell cultures was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assays (Sigma-Aldrich). 2.5 × 104 cells were seeded in each well of a 12-well plate in a volume of 500 μl media per well. Once attached, cells were treated with TXA. Culture medium was aspirated, and sterile-filtered MTT was added to each well. Cells were washed with phosphate-buffered saline (PBS) and incubated with dimethyl sulfoxide (DMSO) for ten minutes to dissolve the formazan product. This was then transferred into a 96-well plate (100 μl per well in duplicate) and read on a microplate reader at 540 nm.
Mitochondrial membrane potential assays
The mitochondrial membrane potential was measured according to the manufacturer’s instructions (DePsipher; R&D Systems, Inc., Minneapolis, Minnesota) by fluorescent microscopy using a fluorescent cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1). 5 ×104 cells/well were cultured in 12-well plates for 48 hours in the presence of increasing concentrations of TXA with medium (0 mg/ml, 1 mg/ml, 50 mg/ml, or 100 mg/ml). The cells were then washed with PBS, and reaction buffer was added with DePsipher (5 µl per sample) and stabilizer solution. The cells were incubated at room temperature, in the dark, for a minimum of 15 minutes. The cells were analyzed within one hour by fluorescent microscopy using a fluorescein long-pass filter (EVOS FL Auto 2 Imaging System; Thermo Fisher Scientific, Waltham, Massachusetts). Using this system, cells undergoing apoptosis should appear green (585/590 nm), while healthy cells appear red (510/527 nm). Four corner random field images were saved and the number of apoptotic (green) and live (red) cells counted per image. The percentage of apoptotic cells as a proportion of total cells was calculated for each image and the mean percentage calculated for each sample.
Cellular apoptosis
Multi-apoptotic protein detection arrays (Human Apoptosis Antibody Array Kit; R&D Systems, Inc.) were used to determine possible apoptotic pathways involved in cell death according to the manufacturer’s instructions. Cells were treated with 0 mg/ml, 1 mg/ml, 50 mg/ml, or 100 mg/ml TXA for one hour. The protein content of each well was quantified using a Micro bicinchoninic acid assay (BCA) Protein Assay Kit (Thermo Fisher Scientific) and read on a microplate reader at 540 nm. Lysate protein concentrations were then normalized in lysis buffer. Cells were cultured in 12-well plates for one hour, with increasing concentrations of TXA with medium (0 mg/ml, 1 mg/ml, 50 mg/ml, or 100 mg/ml). Cell supernatants were aspirated and discarded. After blocking of non-specific binding at room temperature for one hour, antibody array membranes were incubated with the cell lysate (1.5 ml) at 4°C overnight and then with a diluted solution of horseradish peroxidase (HRP)-conjugated streptavidin at room temperature for 30 minutes. Visualization of protein expression was carried out by chemiluminescence and signal intensity was quantified using Luminex (Azure c500; Azure Biosystems, Dublin, California). The immunoreactive band density was analyzed using WinImage studio version 5.2 (LI-COR Biosciences, Lincoln, Nebraska).
Statistical analysis
All results are shown as mean ± standard error of the mean, and all statistical analysis was performed using Student’s paired t-test or analysis of variance (ANOVA) test with Dunnett’s post hoc analysis, as shown in the figures, using the GraphPad Prism 5 software (GraphPad Software, San Diego, California). A p-value of < 0.05 was considered statistically significant.
Results
Tranexamic acid toxicity in periarticular tissues ex vivo
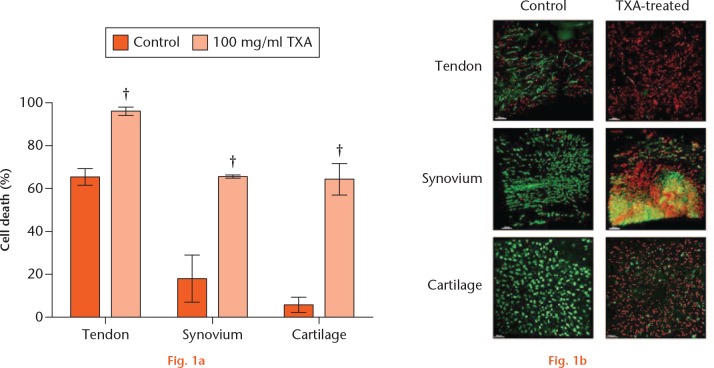
We first investigated whether ex vivo tissues (n = 3 tendon, synovium; n = 4 cartilage) treated with TXA displayed evidence of cytotoxicity. Treatment with 100 mg/ml of TXA for 16 hours caused a significant increase in cell death within tendon, synovium, and cartilage tissues compared with control (Fig. 1 and Table I).
Tranexamic acid (TXA) toxicity in periarticular tissues ex vivo. a) Percentage of dead cells in control (0 mg/ml) and TXA-treated (100 mg/ml) cells in each periarticular tissue (n = 3 tendon, synovium; n = 4 cartilage) after 16 hours of treatment. Mean ± standard error of the mean; Student’s paired t-test. †p < 0.01. b) Tendon, synovium, ligament, and cartilage tissue imaged by confocal microscopy with 5-chloromethylfluorescein diacetate (CMFDA) and propidium iodide (PI) stain at 16 hours of treatment in control and TXA-treated (100 mg/ml TXA cells). Green stained cells counted as live and red stained cells counted as dead.
Table I.
Numerical representation of the data shown in Figure 1
| 0 mg/ml |
100 mg/ml |
p-value | |||
|---|---|---|---|---|---|
| Mean (sd) | n | Mean (sd) | n | ||
| Cartilage | 5.5 (4.1) | 4 | 65.0 (14.3) | 4 | 0.005 |
| Tendon | 65.0 (4.5) | 3 | 96.3 (3.2) | 3 | 0.002 |
| Synovium | 18.0 (11.1) | 3 | 66.0 (1.0) | 3 | 0.02 |
Tranexamic acid toxicity in vitro
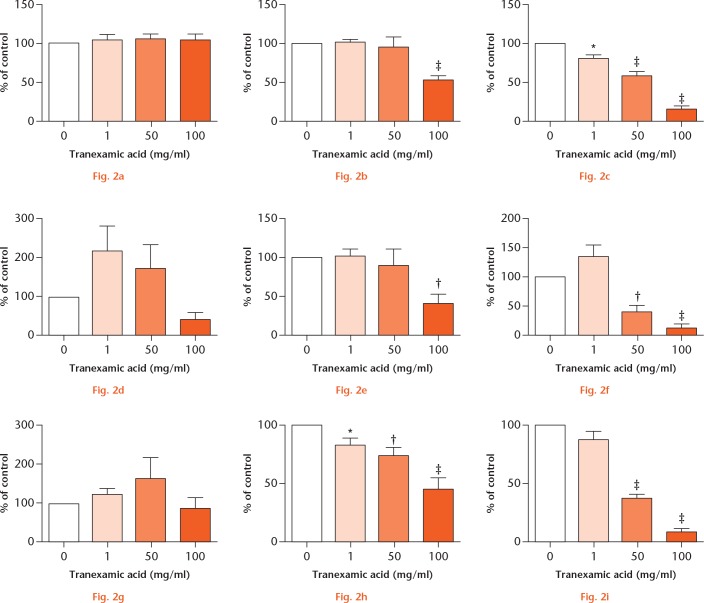
Following ex vivo experiments, we performed time and dose curves to determine the concentration and duration of exposure to TXA at which a significant reduction in cell viability occurred. MTT assays measuring cell metabolic activity acted as an indirect measurement of cell viability. A significant fall in cell viability occurred in all cell types at higher doses over a four-hour duration or low concentrations following 24 hours of treatment.
Tenocyte viability was significantly reduced when treated with TXA at 100 mg/ml for four hours (Fig. 2). Additionally, concentrations of 1 mg/ml, 50 mg/ml, or 100 mg/ml of TXA for 24 hours also caused a significant reduction in cell viability. Fibroblast-like synoviocyte viability was significantly reduced when treated with TXA at 1 mg/ml, 50 mg/ml, or 100 mg/ml for four hours and 50 mg/ml or 100 mg/ml for 24 hours (Fig. 2). Furthermore, chondrocyte viability was significantly reduced when treated with TXA at 100 mg/ml for four hours and 50 mg/ml or 100mg/ml for 24 hours. There were no significant differences at one hour or with lower concentrations of TXA.
Tranexamic acid (TXA) toxicity in vitro. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay of: a) to c) tenocyte (n = 11); d) to f) fibroblast-like synoviocyte (FLS; n = 5); and g) to i) chondrocyte (n = 5) viability at: a), d), g) one; b), e), h) four; and c), f), i) 24 hours. Values were measured as luminescence and expressed as percentage change from untreated (control) wells. *p < 0.05; †p < 0.01; ‡p < 0.001. Data are means ± standard error of the mean; one-way analysis of variance; Dunnett’s Multiple Comparison Test.
Cellular death
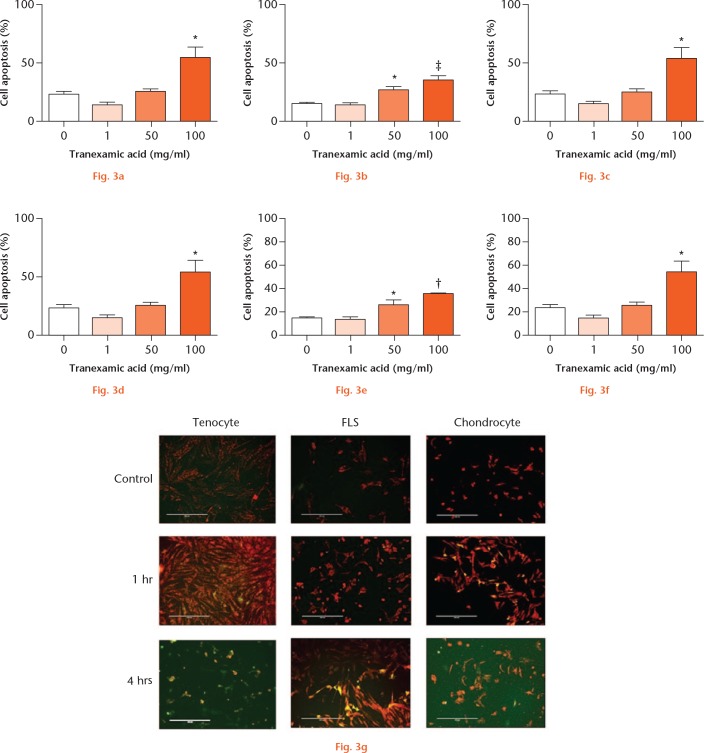
We subsequently measured mitochondrial membrane potential as a direct measure of cellular death (apoptosis, Fig. 3). Tenocyte apoptosis was significantly increased when cells were treated with 100 mg/ ml for one hour or 50 mg/ml to 100 mg/ml TXA for four hours. Fibroblast-like synoviocyte and chondrocyte cell apoptosis was significantly increased when cells were exposed to 50 mg/ml or 100 mg/ml for one hour and 100 mg/ml for four hours.
Tranexamic acid (TXA)-associated periarticular apoptosis. Percentage of: a) and b) tenocyte (n = 3); c) and d) fibroblast-like synoviocyte (FLS; n = 3); and e) and f) chondrocyte (n = 4) death at a), c), e) one and b), d), f) four hours with 0 mg/ml, 1 mg/ml, 50 mg/ml, or 100 mg/ml TXA treatment. Dying cells (green) were calculated as a proportion of healthy (red) cells. *p < 0.05; †p < 0.01. Data represent mean ± standard error of the mean; one-way analysis of variance; Dunnett’s Multiple Comparison Test. g) Fluorescent microscopy images show tenocytes, FLS, and chondrocytes at: one hour, 0 mg/ml TXA; one hour, 100 mg/ml TXA; and four hours, 100 mg/ml TXA.
Multi-protein apoptotic arrays
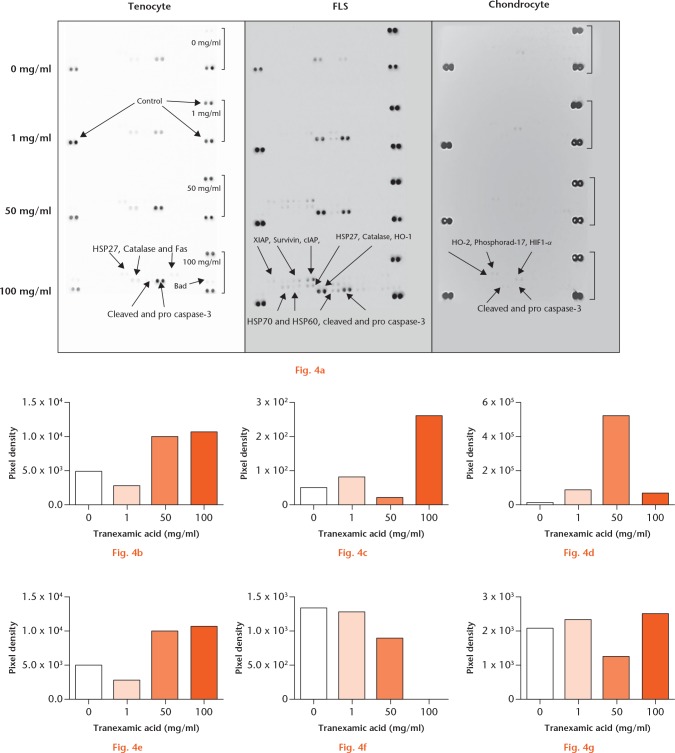
As fluorescent microscopy identified increased cell death at one and four hours, we carried out single-sample, multi-protein, apoptotic arrays to identify potential mechanisms that may be involved (Fig. 4). Treatment with 0 mg/ml, 1 mg/ml, 50 mg/ml, and 100 mg/ml of TXA for one hour caused an increase in apoptotic protein expression in tenocytes (pro caspase-3, cleaved caspase-3, Catalase, heat shock protein (HSP) 27, FLS cells (X-linked inhibitor of apoptosis protein (XIAP), Survivin, cellular inhibitor of apoptosis protein (cIAP), HSP27, Catalase, heme oxygenase 1 (HO-1), HSP70 and HSP60, cleaved and pro caspase-3) and chondrocytes (HO-2, Phosphorad-17, hypoxia-inducible factor 1-alpha (HIF1-α), cleaved and pro caspase-3). This suggests that TXA-mediated cell death may be orchestrated in part via a caspase-3-dependent apoptotic mechanism across all cell types.
a) Tranexamic acid (TXA)-associated periarticular apoptosis pathways. Caspase-3 activation induced by tranexamic acid in vitro in periarticular tissues: b) and c) tenocyte; d) and e) fibroblast-like synoviocyte (FLS); and f) and g) chondrocyte single-sample multi-protein apoptotic arrays. b), d), f) Pro caspase-3 and c), e), g) cleaved caspase-3 samples were treated with 0 mg/ml, 1 mg/ml, 50 mg/ml, or 100 mg/ml of TXA for one hour. Pro and cleaved forms of caspase-3 signal pixel density plotted relative to control spots. HSP, heat shock protein; XIAP, X-linked inhibitor of apoptosis protein; cIAP, cellular inhibitor of apoptosis protein; HO-1, heme oxygenase 1; HIF1-α, hypoxia-inducible factor 1-alpha.
Discussion
Our study provides evidence of TXA cytotoxicity to human periarticular tissues ex vivo and in vitro at concentrations and durations of treatment routinely used in a clinical environment. Human tendon, synovium, and cartilage tissues all showed significantly higher rates of cell death when treated with TXA over a clinically relevant time period when considering the tissue half-life of TXA following IV administration.6
The use of TXA as an intravenous haemostatic agent during hip and knee arthroplasty is well established, with proven benefits in reduction of peri- and postoperative blood loss.3,6 Increasingly, its use is being expanded to topical application in orthopaedic surgical procedures,23-25 which has been shown to deliver similar outcomes postoperatively to those of intravenous administration.11,26-29 Furthermore, its utilization has now expanded into topical application during soft-tissue surgery including arthroscopic ACL reconstruction.30,31 There are various methods of topical administration described in the literature, including, but not limited to, pre- or postoperative injection, intraoperative wash, postoperative intra-articular infusion through a surgical drain, and direct tissue impregnation.11,28,29,32-36 Most literature describes the administration of between 10 mg/ml and 100 mg/ml of TXA; however, significantly larger doses have been reported.33 Indeed, treatment with high-dose TXA (100 mg/ml) into tissues or a closed joint space may result in concentrations remaining within the synovial fluid and tissues36 of over 1 mg/ml at up to 24 hours or longer. Our findings suggest that caution should be used when considering exposing periarticular tissues to TXA either at high concentrations or for durations even as short as one hour. The practice of intra-articular injection or infusion, prolonged surgical soaking, or tissue impregnation, particularly when into an enclosed joint or space, should be undertaken with the knowledge of the current study and other studies that show increased cell death.
Previous studies investigating TXA cytotoxicity to resident periarticular tissues have produced variable results depending on animal versus human tissue and dosing regime utilized.13-17 Tuttle et al17 found that treatment with 100 mg/ml of TXA was cytotoxic to bovine cartilage explants, and 50 mg/ml to murine chondrocytes from as early as eight hours. Ambra et al14 treated porcine cartilage explants with 0 mg/ml to 4 mg/ml of TXA for up to six hours and found no significant increase in cytotoxicity with live/dead cell staining and confocal microscopy. Sitek et al13 found no obvious effect on human chondrocyte culture within gel grafts when grown in culture media containing 10 mg/ml or 20 mg/ml of TXA. More recently, Parker et al16 described cytotoxicity in human chondrocytes in vivo and cartilage explants ex vivo at concentrations over 20 mg/ml at 12 hours’ exposure. We noted decreased cell viability in tenocytes, FLS, and chondrocytes when exposed to high concentrations (100 mg/ml) over an intermediate duration (four hours), but also even at the lowest concentration (1 mg/ml) over a longer duration (24 hours). Furthermore, our study directly addresses the mechanism underlying TXA-induced cell death whereby we implicate caspase-dependent apoptosis.
There are limitations inherent in our study. None of our experiments take into account the TXA being washed away in vivo; the joint space is a dynamic environment and other cell types (e.g. inflammatory cells and platelets) could potentially have a role in TXA clearance. We do, however, know that the half-life of TXA in a closed joint following intravenous administration is around three hours and thus we feel confident that our in vitro experiments are representative.
With our ex vivo model, we had a high percentage of cell death in our tendon control. This is likely due to the effect of tissue drying between surgical harvesting and completion of graft preparation. However, this may actually mask the true extent of TXA toxicity in tendon tissue. It may also have been beneficial to study the ex vivo effects of TXA at additional timepoints and TXA concentrations, as was carried out with our in vitro experimentation. In addition, we acknowledge that age-related degenerative changes may have had an influence on cell viability with regard to the synovium/cartilage obtained at THA/TKA, as they were from older patients but were constrained by our local ethics consent for obtaining tissue samples. While the current study shows increased cell death, this may not necessarily translate into poor clinical outcomes to the patient, and we recognize that studies evaluating early and longer-term clinical follow-up of topical TXA-treated patients, particularly in soft-tissue orthopaedic surgery, should be undertaken in order to understand the translational potential of this basic science study.
On the basis of our findings, we would suggest that clinicians should show caution when considering topical TXA treatments, particularly on soft-tissue orthopaedic procedures. There is a need for further human clinical trials in order to clarify the safety of this before it can be recommended for standard topical practice.
Acknowledgments
The authors wish to thank: Dr Gary Litherland (University of the West of Scotland) for providing support with chondrocyte harvest and culture; and Dr Anisha Kubasik-Thayil at the Image Analysis, Multi-Photon and Confocal Technologies (IMPACT) Centre for Integrative Physiology, Edinburgh University.
Footnotes
Author contributions: M. McLean: Collected and analyzed the data, Wrote and edited the manuscript.
K. McCall: Collected and analyzed the data, Wrote and edited the manuscript.
I. D. M. Smith: Collected and analyzed the data, Wrote and edited the manuscript.
M. Blyth: Designed and supervised the study.
S. M. Kitson: Collected and analyzed the data.
L. A. N. Crowe: Collected and analyzed the data.
W. J. Leach: Designed and supervised the study.
B. P. Rooney: Designed and supervised the study.
S.J. Spencer: Designed and supervised the study.
M. Mullen: Designed and supervised the study.
J.L. Campton: Designed and supervised the study.
I. B. McInnes: Supervised the study.
M. Akbar: Collected and analyzed the data, Wrote and edited the manuscript.
N. L. Millar: Collected and analyzed the data, Supervised the study, Wrote and edited the manuscript.
Conflict of interest statement: None declared
Data sharing statement: M. McLean has access to all the data and data are available upon request.
Ethical review statement: All procedures and protocols were approved by the Ethics Committee under approval number REC14/WS/1035 with informed consent obtained and carried out in accordance with standard operative procedures.
Follow us @BoneJointRes
Funding statement
This work was supported by the Royal College of Surgeons (Edinburgh) through a pump priming small research grant and Arthritis Research UK (21346).
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Neveleff DJ, Kraiss LW, Schulman CS. Implementing methods to improve perioperative hemostasis in the surgical and trauma settings. AORN J 2010;92:S1-S15. [DOI] [PubMed] [Google Scholar]
- 2. Carling MS, Jeppsson A, Eriksson BI, Brisby H. Transfusions and blood loss in total hip and knee arthroplasty: a prospective observational study. J Orthop Surg Res 2015;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes 2013;6:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev 1993;6:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raut S, Mertes SC, Muniz-Terrera G, Khanduja V. Factors associated with prolonged length of stay following a total knee replacement in patients aged over 75. Int Orthop 2012;36:1601-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jahanshahi J, Hashemian F, Pazira S, et al. Effect of topical tranexamic acid on bleeding and quality of surgical field during functional endoscopic sinus surgery in patients with chronic rhinosinusitis: a triple blind randomized clinical trial. PLoS One 2014;9:e104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349:g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrison RJM, Tsang B, Fishley W, et al. Dose optimisation of intravenous tranexamic acid for elective hip and knee arthroplasty: the effectiveness of a single pre-operative dose. Bone Joint Res 2017;6:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dyba J, Chan FC, Lau KK, Chan AK, Chan HW. Tranexamic acid is a weak provoking factor for thromboembolic events: a systematic review of the literature. Blood 2013;122:3629. [Google Scholar]
- 10. Wong MW, Lui WT, Fu SC, Lee KM. The effect of glucocorticoids on tendon cell viability in human tendon explants. Acta Orthop 2009;80:363-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilbody J, Dhotar HS, Perruccio AV, Davey JR. Topical tranexamic acid reduces transfusion rates in total hip and knee arthroplasty. J Arthroplasty 2014;29:681-684. [DOI] [PubMed] [Google Scholar]
- 12. Kjolseth D, Frank JM, Barker JH, et al. Comparison of the effects of commonly used wound agents on epithelialization and neovascularization. J Am Coll Surg 1994;179:305-312. [PubMed] [Google Scholar]
- 13. Sitek P, Wysocka-Wycisk A, Ke˛pski F, et al. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank 2013;14:133-140. [DOI] [PubMed] [Google Scholar]
- 14. Ambra LF, de Girolamo L, Niu W, et al. No effect of topical application of tranexamic acid on articular cartilage. Knee Surg Sports Traumatol Arthrosc 2017. (Epub ahead of print). PMID: 29119286. [DOI] [PubMed] [Google Scholar]
- 15. Marmotti A, Mattia S, Mangiavini L, et al. Tranexamic acid effects on cartilage and synovial tissue: an in vitro study for a possible safe intra-articular use. J Biol Regul Homeost Agents 2016;30(Suppl 1):33-40. [PubMed] [Google Scholar]
- 16. Parker JD, Lim KS, Kieser DC, Woodfield TBF, Hooper GJ. Is tranexamic acid toxic to articular cartilage when administered topically? Bone Joint J 2018:100-B:404-412. [DOI] [PubMed] [Google Scholar]
- 17. Tuttle JR, Feltman PR, Ritterman SA, Ehrlich MG. Effects of tranexamic acid cytotoxicity on in vitro chondrocytes. Am J Orthop 2015;44:E497-E502. [PubMed] [Google Scholar]
- 18. Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol 2010;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muir H. The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. BioEssays 1995;17:1039-1048. [DOI] [PubMed] [Google Scholar]
- 20. Outerbridge RE, Dunlop JA. The problem of chondromalacia patellae. Clin Orthop Relat Res 1975;110:177-196. [DOI] [PubMed] [Google Scholar]
- 21. Millar NL, Gilchrist DS, Akbar M, et al. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun 2015;6:6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hui W, Litherland GJ, Jefferson M, et al. Lithium protects cartilage from cytokine-mediated degradation by reducing collagen-degrading MMP production via inhibition of the P38 mitogen-activated protein kinase pathway. Rheumatology (Oxford) 2010;49:2043-2053. [DOI] [PubMed] [Google Scholar]
- 23. Karaaslan F, Seijas R, Sallent A, et al. Tranexamic acid in bolus alone vs bolus and continuous infusion in hip arthroscopy. Int J Orthop 2017;4:749-752. [Google Scholar]
- 24. Yu CC, Gao WJ, Yang JS, et al. Can tranexamic acid reduce blood loss in cervical laminectomy with lateral mass screw fixation and bone grafting: a retrospective observational study. Medicine (Baltimore) 2017;96:e6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karaaslan F, Karaoğlu S, Yurdakul E. Reducing intra-articular hemarthrosis after arthroscopic anterior cruciate ligament reconstruction by the administration of intravenous tranexamic acid: a prospective, randomized controlled trial. Am J Sports Med 2015;43:2720-2726. [DOI] [PubMed] [Google Scholar]
- 26. Patel JN, Spanyer JM, Smith LS, et al. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty 2014;29:1528-1531. [DOI] [PubMed] [Google Scholar]
- 27. Drosos GI, Ververidis A, Valkanis C, et al. A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. J Orthop 2016;13:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop 2011;35:1639-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg [Am] 2010;92-A:2503-2513. [DOI] [PubMed] [Google Scholar]
- 30. Chiang E-R, Ma H-L. Intra-articular injection of tranexamic acid for reducing post-operative hemarthrosis in arthroscopic anterior cruciate ligament reconstruction [poster]. ISAKOS Congress, 2017. [Google Scholar]
- 31. Kunugiza Y, Nakamura Y, Mikami K, Suzuki S. Warfarin-related recurrent knee haemarthrosis treated with arterial embolisation and intra-articular injection of tranexamic acid. BMJ Case Rep 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mutsuzaki H, Ikeda K. Intra-articular injection of tranexamic acid via a drain plus drain-clamping to reduce blood loss in cementless total knee arthroplasty. J Orthop Surg Res 2012;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sabatini L, Atzori F. Topical intra-articular and intravenous tranexamic acid to reduce blood loss in total knee arthroplasty. Ann Transl Med 2015;3(Suppl 1):S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tahmasebi MN, Bashti K, Ghorbani G, Sobhan MR. Intraarticular administration of tranexamic acid following total knee arthroplasty: a case-control study. Arch Bone Jt Surg 2014;2:141-145. [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Fu X, Liu WX, et al. Safety and efficacy of intra-articular injection of tranexamic acid in total knee arthroplasty. Orthopedics 2014;37:e775-e782. [DOI] [PubMed] [Google Scholar]
- 36. Ahlberg A, Eriksson O, Kjellman H. Diffusion of tranexamic acid to the joint. Acta Orthop Scand 1976;47:486-488. [DOI] [PubMed] [Google Scholar]