Abstract
Children are generally more sensitive to toxicants than adults, including an increased sensitivity to genotoxic carcinogens. We previously demonstrated that neonatal mice are also more sensitive to the mutagenic effects of the direct alkylating agents N-ethyl-N-nitrosoamine and the arylamine 4-aminobiphenyl than adult mice. In this study, we have evaluated the effect of age on the mutagenicity of the fungal toxin and liver carcinogen aflatoxin B1 (AFB1). Neonatal Big Blue transgenic mice were treated with 6 mg/kg AFB1, α treatment that produces liver tumors, while adult mice were treated with 6 and 60 mg/kg AFB1, treatments that do not result in tumors. The CII liver mutant frequency (MF) in mice treated with AFB1 as neonates was 22-fold higher than in control neonatal mice, whereas the treatment of adult mice with either dose of AFB1 did not significantly increase the liver MF over the controls. In AFB1-treated neonatal mice, the frequency of G:C → T:A transversion, a major type of mutation induced by AFB1, was about 82-fold higher than for the control and 31-fold higher than for adult mice treated with 60 mg/kg AFB1. Our mutagenicity findings parallel the relative carcinogenicity of AFB1 in neonatal and adult mice, and are consistent with previous observations of the lower level of hepatic glutathione S-transferase and higher level of hepatic AFB1-DNA adduction in neonatal mice compared to adult mice. Environ. Mol. Mutagen. 51:156–163, 2010.Published 2009 by Wiley-Liss, Inc.†
Keywords: aflatoxin B1, neonate, mutagenicity, mutation, glutathione S-transferase
INTRODUCTION
Cancer is the second leading cause of death among 1- to 14-year-old children in the United States, following only accidents as a cause of death in this age group. Although there have been significant improvements in the relative survival rate for many childhood cancers, the overall cancer incidence in children continues to increase slowly [Jemal et al., 2006]. The reason for this trend is not clear, although many possibilities exist. Infants and children may be more vulnerable to the toxicity of carcinogenic chemicals than adults because of unique routes and types of exposure, unique susceptibilities, physiological and metabolic factors, pharmacokinetics, diet, and behavior.
Our previous studies with mice demonstrated one possible factor promoting the higher incidence of childhood cancer that DNA damage induced by carcinogens is more efficiently converted into mutations in neonates than in adults [Slikker et al., 2004; Chen et al., 2005; Mei et al., 2005]. These results were attributed to faster cell turnover rates in neonates than adults. In addition, it has been recognized that, for some agents, neonatal mice may have lower activity than adults for the metabolic enzymes that convert carcinogenic metabolites into inactive conjugates [Gorrod et al., 1968]. The resulting increased levels of carcinogenic metabolites, coupled with rapid cell division, could induce more mutations in neonates than adults. Aflatoxin B1 (AFB1) is an example of an agent that may be conjugated poorly by neonates.
AFB1, a metabolite of the grain mold Aspergillus flavus, is a potent human hepatocarcinogen and widespread contaminant of human food supplies. AFB1 also induces tumors or preneoplastic lesions in experimental animals [IARC, 1990, 1993]. AFB1, however, displays striking interspecies variation in carcinogenic potency, with rats being the most sensitive species and mice being refractory to dietary levels three orders of magnitude higher than those carcinogenic in rats. An efficient conjugation with glutathione, catalyzed by glutathione S-transferase (GST), confers AFB1 resistance in mice [Hengstler et al., 1999]. Species-specific differences in hepatic mutations among adult lambda/lacI transgenic rats and mice following exposure to AFB1 also have been reported [Dycaico et al., 1996]. A single dose of 2.5 mg/kg AFB1 did not increase liver mutant frequency (MF) relative to vehicle-treated controls in adult mice, while rats subjected to one-tenth of this dose responded with an ~20-fold induction in liver MF over background. The mutants were sequenced and the mutational spectra were different between AFB1-treated mice and rats. A large increase in G:C → T:A transversions was observed among the mutations isolated from the AFB1-treated rats while the major mutation type for AFB1-treated mice was G:C → A:T transition. In contrast to the resistance of adult mice to AFB1 carcinogenesis, AFB1 is a strong carcinogen in the neonatal mouse tumorigenicity assay. The liver of neonatal mice is known to be relatively deficient in its ability to conjugate AFB1, perhaps accounting for the sensitivity of neonatal mice to AFB1 tumorigenicity [Vesselinovitch et al., 1972; Flammang et al., 1997]. Therefore, AFB1 should be a good chemical for testing the hypothesis that a lower conjugating activity in neonatal mice might be a factor responsible for the greater sensitivity of neonates than adults to the carcinogenicity of certain agents.
The Big Blue transgenic mouse assay provides a unique opportunity for studying the induction of tissue-specific mutation. In this in vivo model, reporter genes are located on shuttle vectors that are derivatives of the bacteriophage lambda (λ). Multiple genomic copies of the phage are contained within the genome of each transgenic animal cell as stably integrated concatamers. After exposure of transgenic animals to a test substance, DNA can be isolated from individual organs, such as liver, and single copies of the phage genome can be excised from high molecular weight DNA and packaged into infectious particles with the help of a packaging mix [Kohler et al., 1990]. If appropriate E. coli host cells are infected, plated, and incubated, plaques become visible on the plates within hours. Mutant phage can be isolated from the plates and mutations identified in the target gene, most often the cII gene. The cII gene encodes a protein that activates transcriptional promoters in λ that are essential for lysogenization. Mutations in the cII region that lower the levels of the cII protein result in a decreased ability of λ to lysogenize. When grown under conditions that favor lysogeny, λ prophages carrying cII mutations (λcII–) survive only by entering the lytic pathway of development, forming plaques. Prophages that are wild-type for the cII region (λcII+) integrate into the host genome and become part of the developing bacterial lawn [Banuett et al., 1986; Jakubczak et al., 1996]. In this study, we have continued our examination of the sensitivity of adult and neonatal mice to the mutagenicity of genotoxic carcinogens by measuring the mutagenicity of AFB1 in neonatal and adult Big Blue transgenic mice.
MATERIALS AND METHODS
Animals and AFB1Treatment
Big Blue B6C3F1 transgenic mice were obtained from Taconic Laboratories (Germantown, NY). We followed the recommendations of our Institutional Animal Care and Use Committee for the handling, maintenance, treatment, and sacrifice of the animals. AFB1 was purchased from Sigma (St. Louis, MO). Male neonatal and adult Big Blue B6C3F1 mice were divided into five groups, with each group containing five mice. Table I shows the dose levels of AFB1 and treatment schedule for the various experimental groups. The mice in the two neonatal groups were injected i.p. with total doses of 0 or 6 mg/kg body weight AFB1 at the age of 4, 7, and 10 days. This three-dose, every third-day treatment schedule has also been used for AFB1 liver tumor induction in adult male mice [Wogan, 1969]. Thus, mice in the three adult groups in our study were injected i.p. with total doses of 0, 6, or 60 mg/kg body weight AFB1 in three split-doses at 120, 123, and 126 days of age. AFB1 was dissolved in DMSO for treatment and the volume of the chemical administration was 2 μl/g body weight. The animals were sacrificed 6 weeks after the treatment. The choice of a 6-week mutant manifestation time was based on the recommendations for adult mice by Thybaud et al. [2003] and our observations on mutant manifestation following transplacental exposure [Mei et al., 2005]. The livers were isolated, frozen quickly by using liquid nitrogen, and stored at −80°C.
Table I.
Schedule for AFB1 Treatment and Sacrifice of Big Blue B6C3F1 Mice
| Groupa | Age at dosing (days) | Dose at each treatment time (mg/kg bw) | Total dose (mg/kg bw) | Age at sacrifice (weeks) |
|---|---|---|---|---|
| I | 4, 7, and 10 | 0 | 0 | 8 |
| II | 4, 7, and 10 | 2 | 6 | 8 |
| III | 120, 123, and 126 | 0 | 0 | 24 |
| IV | 120, 123, and 126 | 2 | 6 | 24 |
| V | 120, 123, and 126 | 20 | 60 | 24 |
bw, body weight.
Each group contained five male mice.
Isolation of DNA from Liver Tissue
Liver DNA was isolated with a RecoverEase DNA isolation kit (Stratagene, La Jolla, CA). Each DNA sample was isolated from ~100 mg of mouse liver tissue. Liver tissue was gently homogenized in 5 ml of cold lysis buffer to disaggregate the cells, then sent through a sterile cell strainer into a 50 ml conical tube and centrifuged at 1,100g for 12 min at 4°C. The supernatant fluid was discarded and any residual droplets were removed from the tube with a sterile applicator. One milliliter of digestion solution from the DNA isolation kit was supplemented with 20 μl RNace-It ribonuclease; 70 μl of this digestion solution and 70 μl of warmed proteinase K solution were mixed and added to each liver cell pellet. The conical tube was then placed in a 50°C water bath for 45 min. The mixture was transferred to a dialysis cup floating on TE buffer, and left to dialyze at room temperature for ~42 hr. Fully hydrated genomic DNA was then removed from the dialysis cup and stored at 4°C until use.
cII Mutation Assay
The packaging of λ phage, plating of the packaged DNA samples, and determination of MF were conducted following Stratagene’s procedure for the λ select-cII mutation detection system for Big Blue rodents. The λ shuttle vector containing the cII target gene was rescued from total genomic DNA with λ phage packaging extract (Transpack; Stratagene). Plating was performed with the Escherichia coli host strain G1250. The bacteria were grown in TB1 liquid medium with 1% maltose-MgSO4 (1 M) overnight at 308C in preparation for the experiment. To determine the total titer of packaged phages, G1250 bacteria were mixed with a 1:3,000 dilution of packaged phage, plated on TB1 plates, and incubated overnight at 37°C (nonselective conditions). For mutant selection, the packaged phages were mixed with G1250 cells, plated on TB1 plates, and incubated at 24°C for about 42 hr (conditions for λ cII–selection). The cII MF was calculated as the ratio of the total number of mutant plaques (as determined at 24°C) to the total number of plaques screened (as determined at 37°C). Statistical analyses of MFs were performed using SigmaStat (SPSS Science, Chicago, IL). All MF data are expressed as the mean ± standard deviation (SD) from five animals. Statistical significance was determined by one-way analysis of variance (ANOVA), followed by Student-Newman-Keuls test for comparison of multiple treatment groups.
Sequence Analysis of Mutation Types in the cII Gene
Mutants for sequencing were selected from mutant plaques recovered from mice in the 6 mg/kg neonate and 60 mg/kg adult AFB1 treatment groups. The sequencing protocol was adapted from a previous procedure [Chen et al., 2002], with minor modifications. The cII mutant plaques were selected at random from selection plates assaying liver DNA from different animals and replated at low density to verify the mutant phenotype. Single, well-isolated plaques were selected from these plates and transferred to microcentrifuge tubes containing 100 μl of autoclaved distilled water. The tubes were placed in a thermocycler at 99.9°C for 10 min and centrifuged at 1,500g for 5 min immediately after the heating. The cII target DNA was amplified by PCR with primers 5′-AAAAA GGGCATCAAATTAACC-3′ and 5′-CCGAAGTTGAGTATTTTTGCTG-3′. For PCR amplification, 10 μl of the supernatant and 0.03 μl of the each primer (148 μM) were added to 10 μl of 2× PCR Master Mix (Promega, Madison, WI). The PCR reaction was carried out with the following cycling parameters: a 3 min denaturation at 95°C; followed by 30 cycles of 30 sec at 95°C, 1 min at 60°C, and 1 min at 72°C; with a final extension of 10 min at 72°C. The PCR products were purified using QiaQuick PCR purification kits (Qiagen, Chatsworth, CA). The cII mutant DNA was sequenced and analyzed with a CEQ DTCS-Quick Start Kit and a CEQ 8000 Genetic Analysis System (Beckman Coulter, Fullerton, CA), according to the manufacturer’s instructions. The primers for cII mutation sequencing were the same as those used for the PCR. The differences in mutational types detected in different groups were tested statistically using Monte Carlo analysis [Adams and Skopek, 1987].
Mutation Frequency Calculation
MF was defined as the frequency of plaques that were phenotypically mutants in the cII mutant assay, while mutation frequency was the frequency of mutant plaques carrying independent mutations and therefore requires correction for clonal expansion. In this calculation, mutations found more than once in the mutants isolated from a single animal were assumed to be siblings and were considered to represent a single independent mutation. The total mutation frequency was calculated as MF × the ratio of independent mutations to the total number of analyzed mutations. The mutation frequency for each type of mutation was determined as the total mutation frequency × the percentage of the mutation type among all independent mutations.
RESULTS
Mutant Frequency
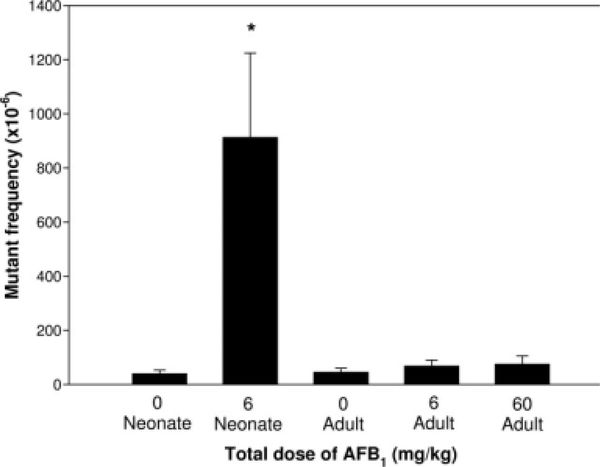
Groups of five neonatal and adult male Big Blue mice were administered AFB1 and sacrificed 6 weeks later along with corresponding controls. The MFs in the liver cII genes from the different treatment groups and their concurrent controls are compared in Figure 1. The mice treated as neonates with 6 mg/kg AFB1 had a significant, ~22-fold increase in liver MF (P < 0.001; 914 ± 311 × 10−6 in mice treated with AFB1 vs. 41 ± 13 × 10−6 in the neonatal control). In addition, the liver cII MF in mice treated with AFB1 as neonates was significantly greater than the MFs in mice treated with AFB1 as adults (neonate vs. 6 mg/kg AFB1 adult, P < 0.001 and neonate vs. 60 mg/kg AFB1 adult, P < 0.001). Although AFB1 treatment of adults produced a dose-related increase in MF, the MFs in the treated mice were not significantly increased compared to the concurrent control group (46 ± 14 × 10−6 in the adult control vs. 69 ± 22 × 10−6 in 6 mg/kg AFB1 adult group, P = 0.800; 76 ± 30 × 10−6 in 60 mg/kg AFB1 adult group vs. the adult control, P = 0.941). Also, there was no significant difference between the liver MFs in the two adult AFB1-treatment groups (P = 0.941).
Fig. 1.
Comparison of MFs in the liver cII gene of mice treated with AFB1 as neonates and adults. Data are the mean ± SD for five mice per group. The asterisk indicates a significant difference in MF between the neonatal AFB1-treatment group and other groups. There was a significant difference between the MFs induced by AFB1 in the neonatal and adult treatment groups (P < 0.001). While the MF in the neonatal treatment group was about 22-fold higher than that in the concurrent control, the MFs in the adult AFB1-treatment groups were only marginally higher than that in their concurrent control.
Mutation Types
AFB1-induced liver cII mutations were evaluated by DNA sequence analysis of 93 and 63 mutants isolated from the neonatal and adult AFB1-treated sample groups, respectively (Table II). Fewer mutants were sequenced in the adult group because the low mutant frequency provided a relatively small number of mutants for analysis. A total of 70 independent mutations from neonatal and 55 from adult mice were identified (Table III). The mutation spectrum for control mice was obtained from our previous study [Chen et al., 2005] because the ages of the control neonates and adults, as well as the type of vehicle used, were the same in the previous study and in this study. Table III summarizes the types of mutations in the liver cII gene from mice treated with AFB1 as neonates and adults, as well as the control mice. The most common type of mutation detected in AFB1-treated neonates was G:C → T:A transversion (76%), while G:C → T:A transversion (51%) and G:C → A:T transition (32%) were the predominant mutations found in AFB1-treated adults. The main type of mutation for the control groups was G:C → A:T transition. The mutation spectra in the neonatal and adult AFB1-treatment groups were significantly different from their respective controls (P < 0.0001). Also, there was a significant difference between the AFB1-treated neonatal and adult mouse spectra (P < 0.01) while there was no significant difference between the spectra for neonatal and adult control mice.
Table II.
Mutations in the Liver cII Gene of Big Blue Mice Treated With AFB1 as Neonates and Adults
| Positiona | Mutationb | Amino acid change | Sequence context 5′ → 3′c | Number of mutationsn (number of independent mutations when different from mutants) |
|
|---|---|---|---|---|---|
| Neonate | Adult | ||||
| −14 | G → T | N/A | ctaAGGaaa | 1 | 1 |
| −13 | G → T | N/A | ctaAGGaaa | 2 | |
| 3 | G → T | Met → Ile | catATGgtt | 1 | |
| 4 | G → T | Val → Phe | atgGTTcgt | 1 | |
| 24/25 | CG → AA | AsnGlu → LysLys | cgcAACGAGgct | 1 | |
| 25 | G → A | Glu → Lys | aacGAGgct | 1 | |
| G → T | Glu → Stop | aacGAGgct | 2 | ||
| 28 | G → T | Ala → Ser | gagGCTcta | 2 | |
| 29 | C → A | Ala → Asp | gagGCTcta | 2(1) | |
| 34 | C → T | Arg → Stop | ctaCGAatc | 2 | |
| 40 | G → T | Glu → Stop | atcGAGagt | 1 | 1 |
| 47 | C → A | Ala → Glu | agtGCGttg | 1 | |
| C → G | Ala → Gly | agtGCGttg | 1 | ||
| 51 | G → T | Leu → Phe | gcgTTGctt | 2(1) | 1 |
| 64 | G → A | Ala → Thr | atcGCAatg | 3 | |
| 73 | G → T | Gly → Stop | cttGGAact | 1 | 1 |
| 74 | G → T | Gly → Val | cttGGAact | 2 | |
| 89 | C → G | Ala → Gly | acaGCGgaa | 1 | |
| C → A | Ala → Glu | acaGCGgaa | 8 (5) | 3 (2) | |
| C → T | Ala → Val | acaGCGgaa | 1 | 2 | |
| 91 | G → T | Glu → Stop | gcgGAAgct | 1 | |
| 94 | G → C | Ala → Pro | gaaGCTgtg | 1 | |
| 95 | C → A | Ala → Asp | gaaGCTgtg | 1 | |
| 101 | G → T | Gly → Val | gtgGGCgtt | 1 | |
| 103 | G → A | Val → Ile | ggcGTTgat | 1 | |
| G → T | Val → Phe | ggcGTTgat | 5 (3) | 4 (2) | |
| 108 | T → A | Asp → Glu | gttGATaag | 1 | |
| 113 | C → A | Ser → Stop | aagTCGcag | 1 | |
| 122 | G → T | Ser → Ile | atcAGCagg | 4 (3) | |
| 123 | C → A | Ser → Arg | atcAGCagg | 2 | 2 |
| 125 | G → T | Arg → Met | agcAGGtgg | 2 | |
| 128 | G → T | Trp → Leu | aggTGGaag | 1 | |
| 129 | G → A | Trp → Stop | aggTGGaag | 1 | |
| 131 | A → T | Lys → Met | tggAAGagg | 1 | |
| 134 | G → T | Arg → Met | aagAGGgac | 1 | |
| G → C | Arg → Thr | aagAGGgac | 1 | ||
| 135 | G → T | Arg → Ser | aagAGGgac | 1 | 1 |
| 135–136 | GG → TT | ArgAsp → SerTry | aagAGGGACtgg | 1 | |
| 140 | G → C | Trp → Ser | gacTGGatt | 1 | |
| 141 | G → T | Trp → Cys | gacTGGatt | 2(1) | |
| 145 | C → A | Pro → Thr | attCCAaag | 1 | 1 |
| 145–146 | CC → AA | Pro → Lys | attCCAaag | 1 | |
| 145/146 | −C | Frameshift | attCCAaag | 1 | |
| 150 | G → T | Lys → Asn | ccaAAGttc | 1 | |
| 155 | C → T | Ser → Leu | ttcTCAatg | 1 | |
| 158 | −T | Frameshift | tcaATGctg | 1 | |
| 159 | G → A | Met → Iie | tcaATGctg | 1 | |
| 159–160 | GC → TG | MetLeu → IieVal | tcaATGCTGctt | 1 | |
| 160 | C → A | Leu → Met | atgCTGctt | 2 | 1 |
| 161 | T → A | Leu → Gln | atgCTGctt | 1 | |
| 163 | C → A | Leu → Ile | ctgCTTgct | 2(1) | |
| C → T | Leu → Phe | ctgCTTgct | 1 | ||
| 166 | G → T | Ala → Ser | cttGCTgtt | 2 | |
| 167 | C → A | Ala → Asp | cttGCTgtt | 2 | |
| 173/174 | +GTTC | Frameshift | gttCTTgaa | 1 | |
| 178/185 | +G | Frameshift | gaaTGGGGGGTCgtt | 1 | |
| 179 | G → T | Trp → Leu | gaaTGGggg | 1 | |
| 179–184 | −G | Frameshift | gaaTGGGGGGTCgtt | 1 | |
| 181 | G → T | Gly → Trp | tggGGGgtc | 2(1) | |
| 190 | G → T | Asp → Tyr | gttGACgac | 1 | |
| 193 | G → T | Asp → Tyr | gacGACgac | 1 | 1 |
| 196 | G → A | Asp → Asn | gacGACatg | 1 | 1 |
| G → C | Asp → His | gacGACatg | 1 | ||
| G → T | Asp → Tyr | gacGACatg | 1 | ||
| 212 | C → A | Ala → Glu | ttgGCGcga | 18(6) | 7 (4) |
| C → T | Ala → Val | ttgGCGcga | 3 (2) | 5 (4) | |
| 214 | C → T | Arg → Stop | gcgCGAcaa | 2 | |
| 217 | C → A | Gln → Lys | cgaCAAgtt | 1 | |
| 224 | C → A | Ala → Asp | gttGCTgcg | 3 | |
| 232 | C → A | Leu → Ile | attCTCacc | 1 | |
| 256 | −G | Frameshift | gcgGCAacc | 1 | |
| 285/286 | −G | Frameshift | cagATGGAGttc | 1 | |
| Total | 93 (70) | 63 (55) | |||
−, deletion; +, insertion.
Position 1 is the first base of the start codon in the cII coding sequence.
Presented in term of sequence change on nontranscribed DNA strand.
Uppercase indicates target codon and target bases are underlined.
Table III.
Summary of the Independent Mutations in the Liver cII Gene of Big Blue Mice Treated With AFB1 as Neonates and Adults
| AFB1-treateda |
Controlb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Neonatal |
Adult |
Neonatal |
Adult |
|||||
| Type of mutation | Number | % | Number | % | Number | % | Number | % |
| G:C → C:G | 5 | 7 | 1 | 2 | 3 | 5 | 3 | 9 |
| G:C → A:T | 6 | 9 | 18 | 32 | 28 | 50 | 13 | 41 |
| G:C → T:A | 53 | 76 | 28 | 51 | 12 | 21 | 2 | 6 |
| A:T → T:A | 1 | 1 | 2 | 4 | 0 | 0 | 0 | 0 |
| A:T → C:G | 0 | 0 | 0 | 0 | 5 | 9 | 3 | 9 |
| A:T → G:C | 0 | 0 | 0 | 0 | 1 | 2 | 3 | 9 |
| Frameshift | 3 | 4 | 4 | 7 | 7 | 13 | 8 | 25 |
| Tandem | 2 | 3 | 2 | 4 | 0 | 0 | 0 | 0 |
| Total mutants screened | 70 | 100 | 55 | 100 | 56 | 100 | 32 | 100 |
The mutational spectra for AFB1-treated neonatal and adult mice were significantly different from their respective concurrent controls (P < 0.0001). Also, there was a significant difference between the AFB1-treated neonatal and adult mice (P < 0.01). There was no significant difference between the two control groups.
Data for controls were obtained from our previous study [Chen et al., 2005]. The control animals were treated with DMSO at 2 μl/g body weight.
Mutation Frequency
Table IV shows the mutation frequencies that were detected for each of the AFB1-treated and control groups of mice. Mutation frequencies are given that include all types of mutations, along with mutation frequencies for each of the major types of mutation that were detected, G:C → T:A, G:C → A:T, and frameshifts. The total mutation frequencies in the different groups were very similar to the corresponding MFs for these groups, indicating that clonal expansion was not a factor in producing the high MFs induced by AFB1 in the neonatal mice. The mutation frequencies for G:C → A:T and frameshifts showed relatively small differences among the treatment groups. In contrast, the mutation frequencies for G:C → T:A differed greatly between the treatment and control groups, 82-fold and up to fivefold changes for neonatal and adult mice, respectively, indicating that AFB1 mainly induced a G:C → T:A transversion in mouse liver.
Table IV.
Comparison of the Mutation Frequencies for All Mutation Types and for Three Major Types of Mutations in the Liver cII Gene of Mice Treated With AFB1 as
| Treatment group | Total dose | Mutation frequencies (X10−6) |
|||
|---|---|---|---|---|---|
| All types | G:C → A:T | G:C → T:A | Frameshift | ||
| Neonate | 0 | 28 | 14 | 6 | 4 |
| 6 | 688 | 62 | 489 | 28 | |
| Adult | 0 | 42 | 17 | 3 | 10 |
| 60 | 66 | 21 | 16 | 5 | |
The unit for total dose is mg AFB1/kg body weight.
DISCUSSION
Mutagenicity of AFB1Correlates With the Carcinogenicity of AFB1in Neonatal and Adult Mice
AFB1 is an extremely potent hepatocarcinogen in neonatal mice, but not in adult mice. The same protocol used for treating neonatal mice with 6 mg/kg AFB1 in this study resulted in liver tumor incidences of 70% at 52 weeks of age, and 100% at 82 weeks of age, whereas treatment of adult mice with 60 mg/kg AFB1 did not produce significant toxic or carcinogenic responses [Wogan, 1969]. Carcinogenesis is commonly broken down into three main stages: initiation, promotion, and progression. Initiation can result from carcinogen-induced mutations in critical genes involved in carcinogenesis. If initiation is the main reason for the sensitivity of neonates to AFB1 carcinogenicity, a high MF in liver would be expected in the neonatal, but not in the adult, AFB1-treatment group. In this study, the cII liver MF in neonatal mice treated with 6 mg/kg AFB1 was 22-fold higher than that of the concurrent control, whereas treatment of adult mice with 6 and 60 mg/kg AFB1 did not significantly increase the MFs over the control (Fig. 1, about 1.5-fold and 1.7-fold more than the MF of the control, respectively). A previous study also showed that a single dose of 2.5 mg/kg AFB1 did not increase liver lacI MF in adult Big Blue mice (1.3-fold increase, P = 0.25) [Dycaico et al., 1996]. These results suggest that neonatal mice are much more sensitive to the mutagenicity of AFB1 than adult mice. This correlation between the mutagenicity and carcinogenicity of AFB1 in neonatal and adult mice is consistent with mutagenicity being an important factor for AFB1 carcinogenesis.
The Mutational Spectra in Neonatal and Adult Mice Reflect the Mutational Specificity of AFB1-DNA Adducts
The spectra of cII gene mutations in liver from AFB1-treated neonatal and adult mice were significantly different from their respective controls. Also, there was a significant difference between the spectra in AFB1-treated neonatal and adult mice (Table III). Relative to the mutational spectrum in control mice, AFB1-treated neonatal mice had a dramatic increase in the proportion of G:C → T:A transversions, to 76% of all mutations, and a large reduction in the proportion of G:C → A:T transitions, the major mutation in control mice, to only 9%. In the AFB1-treated adult mice, the changes in the proportions of G:C → T:A transversions and G:C → A:T transitions (to 51% and 32%, respectively) were not as great, even though the overall spectrum was significantly different from the control. These more modest changes indicate that AFB1 did induce mutations in adult mice, but that the induction was relatively low, and not sufficient to alter the MF significantly. The difference between the mutational spectra in the AFB1-treated neonatal and adult mice resulted from the different percentages of G:C → T:A transversions and G:C → A:T transitions, and reflected a lower impact of AFB1-specific mutation in the adult mice.
AFB1 is bioactivated by epoxidation of the terminal furan ring double bond to form AFB1 exo-8,9-epoxide, which reacts covalently with DNA [Miller, 1978; Essigmann et al., 1983]. The major DNA adduct induced by AFB1 is 8,9-dihydro-8-(N7-guanyl)29-hydroxyaflatoxin B1 (AFB1-N7-Gua) [Essigmann et al., 1977; Lin et al., 1977; Martin and Garner, 1977; Swenson et al., 1977; Croy et al., 1978; Croy and Wogan, 1981a,b; Groopman et al., 1981]. This DNA lesion induces mainly G:C → T:A transversions in a variety of biological systems. AFB1 is known to produce G:C → T:A transversion in bacteria [Foster et al., 1983; Bailey et al., 1996], in Big Blue mice treated with a glutathione depleting agent [Autrup et al., 1996], and in Big Blue rats [Dycaico et al., 1996]. This mutation is also the principal mutation detected in human liver tumors that are believed to be induced by aflatoxin. A high proportion of liver cancers in populations exposed to high levels of dietary aflatoxin have p53 codon 249 G:C → T:A transversions [Bressac et al., 1991; Hsu et al., 1991; Li et al., 1993]. Analysis of mutation frequencies in mice from our study showed large AFB1-induced increases in G:C → T:A transversion in both neonatal and adult mice. Therefore, the mutational spectra induced by AFB1 in our study are largely consistent with the reported mutational specificity of AFB1 in other systems. The spectra reflect a common biological activity of AFB1 in neonatal and adult mice, with a much higher sensitivity to mutation induction by AFB1 in neonatal mice.
The high MFs in AFB1-treated neonatal mice could, in theory, result from clonal expansion due to rapid division of a relatively few cells containing cII mutations. In that case, a low induced mutation frequency would be found in the AFB1-treated neonatal mice. Our analysis of mutation frequencies showed that the total mutation frequencies in the different treatment groups, including the AFB1-treated neonatal mouse group (Table IV), were similar to the MFs for these groups (Fig. 1). This observation indicates that clonal expansion was not a factor in producing the elevated MFs detected in AFB1-treated neonatal mice.
The Mutagenicity of AFB1 Is Consistent With GST Enzyme Activity in Neonatal and Adult Mice
In mammalian cells, the primary pathway for AFB1 detoxification is through GST-mediated conjugation of the exo-8,9-epoxide with reduced glutathione (GSH), with the level of GST conjugation for a species being inversely related to the susceptibility of the species to AFB1-induced hepatocarcinogenesis [Hengstler et al., 1999]. Adult mice are comparatively resistant to AFB1-induced toxicity and carcinogenicity and this resistance is thought to be mainly due to their relatively high levels of GST [Eaton et al., 1993].
A previous study [Autrup et al., 1996] investigated the mutagenic activity of AFB1 in the liver of adult mice co-treated with phorone, a GSH depleting agent. While AFB1 alone did not result in a significant increase in liver lacI MF, co-treatment of AFB1 and phorone resulted in a significant increase in MF (four-fold over the control). These results suggest that the GST levels in liver could be a major factor for the differential susceptibility of neonatal and adult mice for mutation induction by AFB1. It is theoretically possible that DNA repair in neonatal mice is not as efficient as in adult mice, and may account for some of the sensitivity of neonatal mice to AFB1. Some studies indicate that differences in DNA repair are an important determinant in tissue- and species-related differences in susceptibility to AFB1 [Bedard et al., 2005]. However, there are no reports indicating that neonatal mice have a lower DNA repair capacity for AFB1-induced DNA damage than adults.
Shupe and Sell [2004] measured AFB1-DNA adduct formation in relation to GST levels in murine liver. Based on their results, it is expected that during the first 10 days of age, when we treated neonatal mice with AFB1 in this study, GST levels in murine liver were about 0.05 mmol/(mg protein min), while at 120 days of age, when we treated our adult mice, liver GST levels were 0.26 mmol/(mg protein min). Shupe and Sell [2004] also reported that AFB1-DNA adduct formation was inversely correlated with GST levels at the different ages. DNA adducts were 13-fold higher in the 10-day-old neonatal mice than the 120-day-old adult mice. The results of our study are consistent with these data. The MF induced by AFB1 in the neonatal mice was about 13-fold higher than that in the treated adults.
In summary, AFB1 treatment of neonatal and adult mice resulted in very different liver MFs and mutation frequencies. Neonatal treatment with AFB1 resulted in a significant induction of liver cII MF, whereas AFB1 treatment of adults had no significant effect on MF. The differential mutagenicities of AFB1 in mouse liver correlate well with the carcinogenicities resulting from neonatal and adult exposure to AFB1. A dramatic increase in G:C → T:A transversion frequency in mice treated with AFB1 as neonates is consistent with the reported mutational specificity of AFB1 in other systems. These observations indicate that the sensitivity of neonatal mice to AFB1-induced G:C → T:A transversions plays a critical role in their sensitivity to AFB1 carcinogenicity. Our results suggest that young children might be more sensitive to AFB1 mutagenicity and have a higher risk to AFB1 exposure.
ACKNOWLEDGMENTS
The views presented in this article do not necessarily reflect those of the Food and Drug Administration.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- Adams WT, Skopek TR. 1987. Statistical test for the comparison of samples from mutational spectra. J Mol Biol 194:391–396. [DOI] [PubMed] [Google Scholar]
- Autrup H, Jorgensen EC, Jensen O. 1996. Aflatoxin B1 induced lacI mutation in liver and kidney of transgenic mice C57BL/6N: Effect of phorone. Mutagen 11:69–73. [DOI] [PubMed] [Google Scholar]
- Bailey EA, Iyer RS, Stone MP, Harris TM, Essigmann JM. 1996. Mutational properties of the primary aflatoxin B1-DNA adduct. Proc Natl Acad Sci USA 93:1535–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F, Hoyt MA, McFarlane L, Echols H, Herskowitz I. 1986. hflB, a new Escherichia coli locus regulating lysogeny and the level of bacteriophage lambda cII protein. J Mol Biol 187:213–224. [DOI] [PubMed] [Google Scholar]
- Bedard LL, Alessi M, Davey S, Massey TE. 2005. Susceptibility to aflatoxin B1-induced carcinogenesis correlates with tissue-specific differences in DNA repair activity in mouse and in rat. Cancer Res 65:1265–1270. [DOI] [PubMed] [Google Scholar]
- Bressac B, Kew M, Wands J, Ozturk M. 1991. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature 350:429–431. [DOI] [PubMed] [Google Scholar]
- Chen T, Gamboa da Costa G, Marques MM, Shelton SD, Beland FA, Manjanatha MG. 2002. Mutations induced by alpha-hydroxytamoxifen in the lacI and cII genes of Big Blue transgenic rats. Carcinogenesis 23:1751–1757. [DOI] [PubMed] [Google Scholar]
- Chen T, Mittelstaedt RA, Beland FA, Heflich RH, Moore MM, Parsons BL. 2005. 4-Aminobiphenyl induces liver DNA adducts in both neonatal and adult mice but induces liver mutations only in neonatal mice. Int J Cancer 117:182–187. [DOI] [PubMed] [Google Scholar]
- Croy RG, Wogan GN. 1981a. Quantitative comparison of covalent aflatoxin-DNA adducts formed in rat and mouse livers and kidneys. J Natl Cancer Inst 66:761–768. [PubMed] [Google Scholar]
- Croy RG, Wogan GN. 1981b. Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Res 41:197–203. [PubMed] [Google Scholar]
- Croy RG, Essigmann JM, Reinhold VN, Wogan GN. 1978. Identification of the principal aflatoxin B1-DNA adduct formed in vivo in rat liver. Proc Natl Acad Sci USA 75:1745–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dycaico MJ, Stuart GR, Tobal GM, de Boer JG, Glickman BW, Provost GS. 1996. Species-specific differences in hepatic mutant frequency and mutational spectrum among lambda/lacI transgenic rats and mice following exposure to aflatoxin B1. Carcinogenesis 17:2347–2356. [DOI] [PubMed] [Google Scholar]
- Eaton D,Van Ness K,Beutler T, editors. 1993. GST Mediated Protection Against Carcinogens: Aflatoxin B1 as an Example. Boca Raton: CRC Press; pp 187–198. [Google Scholar]
- Essigmann JM, Croy RG, Nadzan AM, Busby WF Jr, Reinhold VN, Buchi G, Wogan GN. 1977. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci USA 74:1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essigmann JM, Green CL, Croy RG, Fowler KW, Buchi GH, Wogan GN. 1983. Interactions of aflatoxin B1 and alkylating agents with DNA: Structural and functional studies. Cold Spring Harb Symp Quant Biol 47 (Part 1):327–337. [DOI] [PubMed] [Google Scholar]
- Flammang TJ, Tungeln LS, Kadlubar FF, Fu PP. 1997. Neonatal mouse assay for tumorigenicity: Alternative to the chronic rodent bioassay. Regul Toxicol Pharmacol 26:230–240. [DOI] [PubMed] [Google Scholar]
- Foster PL, Eisenstadt E, Miller JH. 1983. Base substitution mutations induced by metabolically activated aflatoxin B1. Proc Natl Acad Sci USA 80:2695–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrod JW, Carter RL, Roe FJ. 1968. Induction of hepatomas by 4-aminobiphenyl and three of its hydroxylated derivatives administered to newborn mice. J Natl Cancer Inst 41:403–410. [PubMed] [Google Scholar]
- Groopman JD, Croy RG, Wogan GN. 1981. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci USA 78:5445–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstler JG, Van der Burg B, Steinberg P, Oesch F. 1999. Interspecies differences in cancer susceptibility and toxicity. Drug Metab Rev 31:917–970. [DOI] [PubMed] [Google Scholar]
- Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. 1991. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature 350:427–428. [DOI] [PubMed] [Google Scholar]
- IARC. 1990. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Pharmaceutical Drugs. Lyon: IARC; pp 123–141. [Google Scholar]
- IARC. 1993. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Lyon: IARC; pp 197–572. [Google Scholar]
- Jakubczak JL, Merlino G, French JE, Muller WJ, Paul B, Adhya S, Garges S. 1996. Analysis of genetic instability during mammary tumor progression using a novel selection-based assay for in vivo mutations in a bacteriophage lambda transgene target. Proc Natl Acad Sci USA 93:9073–9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. 2006. Cancer statistics, 2006. CA Cancer J Clin 56:106–130. [DOI] [PubMed] [Google Scholar]
- Kohler SW, Provost GS, Kretz PL, Fieck A, Sorge JA, Short JM. 1990. The use of transgenic mice for short-term, in vivo mutagenicity testing. Genet Anal Tech Appl 7:212–218. [DOI] [PubMed] [Google Scholar]
- Li D, Cao Y, He L, Wang NJ, Gu JR. 1993. Aberrations of p53 gene in human hepatocellular carcinoma from China. Carcinogenesis 14:169–173. [DOI] [PubMed] [Google Scholar]
- Lin JK, Miller JA, Miller EC. 1977. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res 37:4430–4438. [PubMed] [Google Scholar]
- Martin CN, Garner RC. 1977. Aflatoxin B-oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature 267:863–865. [DOI] [PubMed] [Google Scholar]
- Mei N, Heflich RH, Moore MM, Chen T. 2005. Age-dependent sensitivity of Big Blue transgenic mice to the mutagenicity of N-ethyl-N-nitrosourea (ENU) in liver. Mutat Res 572:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EC. 1978. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res 38:1479–1496. [PubMed] [Google Scholar]
- Shupe T, Sell S. 2004. Low hepatic glutathione S-transferase and increased hepatic DNA adduction contribute to increased tumorigenicity of aflatoxin B1 in newborn and partially hepatectomized mice. Toxicol Lett 148:1–9. [DOI] [PubMed] [Google Scholar]
- Slikker W III, Mei N, Chen T. 2004. N-ethyl-N-nitrosourea (ENU) increased brain mutations in prenatal and neonatal mice but not in the adults. Toxicol Sci 81:112–120. [DOI] [PubMed] [Google Scholar]
- Swenson DH, Lin JK, Miller EC, Miller JA. 1977. Aflatoxin B1–2,3-oxide as a probable intermediate in the covalent binding of aflatoxins B1 and B2 to rat liver DNA, ribosomal RNA in vivo. Cancer Res 37:172–181. [PubMed] [Google Scholar]
- Thybaud V, Dean S, Nohmi T, de Boer J, Douglas GR, Glickman BW, Gorelick NJ, Heddle JA, Heflich RH, Lambert I, Martus H-J, Mirsalis JC, Suzuki T, Yajima N. 2003. In vivo transgenic mutation assays. Mutat Res 540:141–151. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch SD, Mihailovich N, Wogan GN, Lombard LS, Rao KV. 1972. Aflatoxin B 1, a hepatocarcinogen in the infant mouse. Cancer Res 32:2289–2291. [PubMed] [Google Scholar]
- Wogan GN. 1969. Metabolism and biochemical effects of aflatoxins In: Goldblatt LA, editor. Aflatoxin-Scientific Background Control and Implications. New York: Wiley; pp 321–350. [Google Scholar]