Abstract
Cigarette smoke condensate (CSC) is genotoxic in nearly all assays in which it has been tested. In this study, we investigated the mutagenicity of 11 CSCs using the microwell and soft-agar versions of the mouse lymphoma assay (MLA). These CSCs were prepared from commercial or experimental cigarettes, 10 of them were produced using International Organisation for Standardisation (ISO) conditions and one CSC was generated using intense Massachusetts Department of Public Health (MDPH) conditions. In the presence of rat liver S9, the L5178Y/Tk+/− mouse lymphoma cells were treated with 11 CSCs at different concentrations (25–200 μg/ml) for 4 h. All CSCs resulted in dose-dependent increases of both cytotoxicity and mutagenicity in both versions of the MLA. The mutagenic potencies of the CSCs were calculated as mutant frequency per microgram CSC from the slope of the linear regression of the dose–response curves and showed no correlations with the tar yield of the cigarette or nicotine concentrations of the CSCs. Comparing two CSCs produced from the same commercial cigarettes using two different smoking conditions, the one generated under ISO conditions was more mutagenic than the other generated under intense conditions on a per microgram CSC basis. We also examined the loss of heterozygosity (LOH) at four microsatellite loci spanning the entire chromosome 11 for the mutants induced by 11 CSCs. The most common type of mutation observed was LOH with chromosome damage spanning less than ~34 Mbp. These results indicate that the MLA identifies different genotoxic potencies among a variety of CSCs and that the results from both versions of the assay are comparable.
Introduction
Tobacco smoking remains a major public health problem by threatening the lives of 1 billon people during this century. The World Health Organisation Report on the Global Tobacco Epidemic showed that it is a risk factor for six of the eight leading causes of death in the world. Tobacco use is estimated to kill >5 million people each year worldwide, and by 2030, there will be >8 million deaths every year unless urgent action is taken (1). There is a causal relationship of cigarette smoking in the aetiology of heart disease and cancers at 11 organ sites in humans, and therefore tobacco smoking is considered as the most extreme example of a systemic human mutagen and carcinogen (2,3).
Research on smoking behaviour and pharmacology has demonstrated that most smokers smoke to maintain nicotine levels in the brain to avoid the negative effects of nicotine withdrawal and to modulate mood (2). Unfortunately, using cigarettes as a nicotine delivery system exposes humans to many cytotoxic and carcinogenic chemicals because cigarette mainstream smoke is comprised >4000 chemicals. At least 200 of them are toxic to humans and/or experimental animals, and >50 are recognised as known or probable human carcinogens (4,5). Short-term in vitro genotoxicity assays have been widely conducted over decades to evaluate the toxicity of cigarette mainstream smoke, with a large number of studies using bacteria cells (6–8) and a relatively small number of studies using mammalian cells (9–11). Recently, several comparative studies have been performed confirming that cigarette mainstream smoke from a variety of cigarette types is mutagenic. Total particulate matter (TPM) from the smoke of 10 blended cigarettes was mutagenic in the Ames test (12) and mouse lymphoma assay (MLA) (13). The cigarette smoke condensates (CSCs) from 12 brands of cigarettes produced positive responses in human B lymphoblastoid cells for DNA damage using the comet assay and for micronuclei using the cytokinesis-block assay (14). Ten CSCs from commercial or experimental cigarettes were positive in the Ames test using two tester strains, in the comet assay and micronucleus assay using mouse lymphoma cells, and in the chromosome aberration assay using Chinese hamster ovary cells (15). Different genotoxicity assays have different sensitivities, specificities and genotoxic end points. Therefore, more comparative genotoxic studies as well as biological and chemical studies of cigarette mainstream smoke are needed to expand the available database and to evaluate the mutagenic potency of TPM/CSC and the quantitative detection of the overall toxicity of cigarette smoke, which will give more confidence in the interpretation of the results.
The MLA using the thymidine kinase (Tk) gene of the L5178Y/Tk+/−-3.7.2C mouse lymphoma cell line is used internationally as an in vitro mammalian genetic toxicology assay for regulatory decision-making (16,17). The MLA was originally developed using cloned cells immobilised in soft agar to enumerate mutants (18). In 1983, an alternative method of cloning with medium in 96-well microwell plates was developed for mutant frequency (MF) determination (19). During the last decade, the MLA Workgroup, as a part of the International Workshop for Genotoxicity Testing (IWGT), has reached consensus on a number of important issues for the appropriate approach to data evaluation and has developed acceptance criteria for both the microwell and soft agar versions of the MLA assay (20–24). In this study, we investigated the cytotoxicity and mutagenicity of 11 CSCs (including the 2R4F Kentucky reference cigarette) using both the microwell and soft agar versions of the MLA. To our knowledge, it is the first time for these CSCs (Table I), except 2R4F, were tested in the MLA and there is no mutagenicity data available for two CSCs (LVB and LCF) from other assays in the published literature. In addition, the underlying mechanisms for mutation induction by these CSCs were explored by the loss of heterozygosity (LOH) analysis of the induced mutants.
Table I.
Tar and nicotine concentrations in 11 CSCs
| No. | CSC | Tar (mg/cigarette) | Nicotine (mg/cigarette) | Tar (mg/ml) | Nicotine (mg/ml) | Descriptiona |
|---|---|---|---|---|---|---|
| 1 | 2R4F | 8.9 | 0.75 | 20 | 0.91 | 2R4F Kentucky Reference cigarette |
| 2 | LT | 10.0 | 0.8 | 20 | 1.15 | Commercial US light non-menthol brand cigarette |
| 3 | LTMAS | 21.7 | 1.6 | 20 | 1.16 | Commercial US light non-menthol brand cigarette |
| 4 | FF | 16.0 | 0.9 | 20 | 0.98 | Commercial US full-flavour non-menthol brand cigarette |
| 5 | LIP | 10.0 | 0.9 | 20 | 1.16 | Commercial US light low ignition propensity non-menthol brand cigarette |
| 6 | LVB | 10.0 | 1.0 | 20 | 0.98 | Commercial US light Virginia blend non-menthol brand cigarette |
| 7 | LCF | 12.0 | 1.0 | 20 | 1.15 | Commercial US light charcoal filtered non-menthol brand cigarette |
| 8 | BUR | 23.3 | 7.5 | 20 | 2.33 | Experimental 100% burley tobacco cigarette |
| 9 | BRI | 34.6 | 5.0 | 20 | 2.34 | Experimental 100% flue-cured tobacco cigarette |
| 10 | REC | 11.8 | 0.8 | 20 | 0.64 | Experimental 100% paper reconstituted tobacco cigarette |
| 11 | UL | 5.0 | 0.5 | 20 | 1.01 | Commercial US ultra light non-menthol brand cigarette |
While No. 3 of LTMAS was smoked under Massachusetts Department of Public Health (MDPH) conditions, all others were generated under ISO conditions. LT and LTMAS were generated from the same commercial US light non-menthol cigarette.
Materials and methods
Materials
Trifluorothymidine (TFT) and dimethyl sulfoxide (DMSO) were purchased from the Sigma Chemical Company (St Louis, MO, USA). Fischer’s medium was obtained from Quality Biological Inc. (Gaithersburg, MD, USA), and all other cell culture supplies were acquired from Invitrogen Life Technologies (Carlsbad, CA, USA). The Difco Noble Agar used for cloning was obtained from Becton Dickinson (Sparks, MD, USA). Aroclor 1254 induced male Sprague–Dawley rats liver post-mitochondrial fraction (S9) was purchased from Moltox (Boone, NC, USA). PCR Master Mix was from Promega Company (Madison, WI, USA). The primers used for detection of LOH at the Tk locus and the D11Mit36, D11Mit20 and D11Mit74 loci were synthesised by Invitrogen Life Technologies.
Preparation of the CSCs
Detailed information about cigarette collection, smoking conditions and preparation of CSCs was described previously (15). Briefly, the commercial cigarettes were purchased at retail outlets in Atlanta, GA, and the 2R4F reference cigarettes were purchased from the University of Kentucky Tobacco and Health Research Institute (Lexington, KY, USA). Custom unfiltered research cigarettes containing 100% reconstituted tobacco, burley or flue-cured tobaccos were obtained from Murty Pharmaceuticals (Lexington, KY, USA). Cigarette smoke was generated by automated smoking on a linear 16-port ASM 500 smoking machine (Cerulean, Milton Keynes, UK). Ten cigarettes (Table I) were smoked according to the US Federal Trade Commission/International Organisation for Standardisation (ISO) conditions (35 ml puff volume, 2 sec duration, 60-sec puff interval, with the ventilation holes unblocked). One commercial US light non-menthol cigarette brand was also smoked according to the Massachusetts Department of Public Health (MDPH) intense conditions (45 ml volume, 2 sec duration, 30-sec puff interval and with 50% blockage of filter ventilation holes) to generate LTMAS (Table I). After smoking, the cigarette filter pads were extracted with DMSO to yield a concentration of 20 mg tar/ml DMSO. Tar is defined as the mass of wet total particulate matter (TPM) minus the mass of nicotine and water. All CSC samples were generated at the US Centers for Disease Control and Prevention (CDC) (Atlanta, GA, USA), shipped on dry ice to the National Center for Toxicological Research (Jefferson, AR, USA) and stored at -70°C until analysis. The concentrations of smoke tar and CSC nicotine of the 11 CSCs are shown in Table I.
Cells and culture conditions
The L5178Y/Tk+/–-3.7.2C mouse lymphoma cell line was used for the mutation assay. Cells were grown according to the methods described previously (25). The basic medium was Fischer’s medium for leukaemic cells of mice with L-glutamine supplemented with pluronic F68 (0.1%), sodium pyruvate (1 mM), penicillin (100 U/ml) and streptomycin (100 μg/ml). The treatment medium (F5p), growth medium (F10p) and cloning medium (F20p) were the basic medium supplemented with 5, 10 and 20% heat-inactivated horse serum, respectively. The cultures were gassed with 5% (v/v) CO2 in air and were maintained in a shaker incubator at 37°C.
S9 mix preparation
All CSCs were studied in the presence of metabolic activation. The S9 fraction of livers from male Sprague–Dawley rats treated with Aroclor 1254 (Moltox) was mixed with a nicotinamide adenine dinucleotide phosphate-generating system. Glucose-6-phosphate (180 mg/ml), NADP+ (25 mg/ml), 150 mM KCl and rat liver S9 were mixed in the ratio 1:1:1:2, and 250 μl of S9 mix were added to 10 ml of treatment medium. In all cases, including the solvent controls (DMSO) and positive controls [0.3 μg/ml benzo[a]pyrene (BP)], the final S9 concentration in the treatment medium was 1%.
Cell treatments with CSCs
The CSC working solution (100×) was prepared just prior to use by a series of dilutions using DMSO from original stocks generated at CDC. The mouse lymphoma cells were suspended in 50-ml centrifuge tubes containing 6 × 106 cells in 10 ml of treatment medium. In the dose range finding tests, the cells were exposed to each CSC at the concentrations of 25, 50, 75 and 100 μg/ml. In the mutation assays, four or five concentrations (25–200 μg/ml) were selected for each CSC based on the dose range finding tests. One hundred microlitres of CSC working solutions and 250 μl of S9 mix were added to the cell cultures and then the cells were gassed with 5% (v/v) CO2 in air and placed on a roller drum (15 r.p.m.) in a 37°C incubator for 4 h. In all cases, the final concentration of DMSO in the medium was 1%, including the solvent controls (DMSO) and positive controls (BP). After treatment, the cells were centrifuged and washed twice with fresh medium and then were resuspended in growth medium at a density of 3 × 105 cells/ml. The culture tubes were placed on a roller drum in a 37°C incubator to begin the 2-day phenotypic expression.
The microwell and soft-agar versions of the MLA
Following the 2-day expression period, the treated cultures were divided to perform mutant selection using both the microwell and soft-agar versions of the MLA (25). In the microwell version, 3 μg/ml of TFT was added to the cells in the cloning medium for mutant enumeration, and the cells were seeded into four 96-well flat-bottom microtitre plates using 200 μl/well and a final density of 2000 cells per well. For the determination of plating efficiency, the cultures were adjusted to 8 cells/ml medium and aliquoted in 200 μl/well into two 96-well flat-bottom microtitre plates. In the soft-agar version, 1 μg/ml of TFT was added in the cloning medium to enumerate the mutants. Cells were suspended in 100 ml of cloning medium with 0.28% agar and poured onto three 100-mm diameter tissue culture dishes to give a final density of 30 000 cells/ml. Six hundreds cells were suspended in 100 ml of 0.28% soft agar cloning medium for determining plating efficiency.
All 96-well plates and 100-mm tissue culture dishes were incubated at 37°C in a humidified incubator with 5% CO2 in air. After 11 days of incubation, colonies were counted and mutant colonies were categorised as small or large (25). For the microwell version, counting was done visually and the small colonies were defined as those smaller than 25% of the diameter of the well. MFs were calculated using the Poisson distribution (25). For the soft-agar version, colony counting and sizing was performed using an automatic colony counter (Microbiology International, Frederick, MD, USA) fitted with the capability to evaluate the size of the colonies. Mutant colonies approximately <0.6 mm in diameter were considered to be small-colony mutants. Cytotoxicity was measured using relative total growth (RTG) that includes a measure of growth during treatment, expression and cloning (25). The potencies of the CSCs to induce MF were calculated from the slope of the linear regression of the dose–response curves (15).
LOH evaluation of the Tk mutants
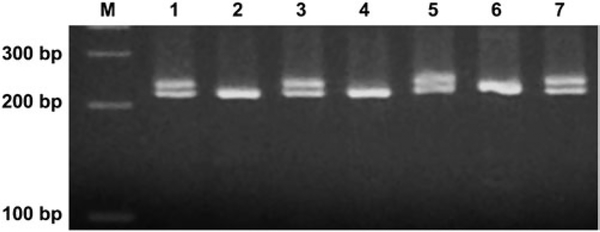
The mutant colonies for the LOH analysis were collected from each of the CSC treatment cultures giving an RTG of about 10–20%. Forty-eight large and 48 small mutant colonies from each CSC treatment and negative control (DMSO) were analysed. Mutant clones were directly taken from the TFT-selection 96-well plates and washed once with 200 μl of phosphate-buffered saline by centrifugation. The cell pellets were quickly frozen and stored at -20°C until analysis. Genomic DNA was extracted by digesting the cells in lysis buffer [10 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 1% (v/v) Triton X-100 and 1% (v/v) Tween 20] with 200 μg/ml of proteinase K at 60°C for 90 min, followed by inactivation of proteinase K at 95°C for 10 min. The procedure for the polymerase chain reaction (PCR) analysis of LOH using allele-specific PCR at the Tk locus and other three microsatellite loci (D11Mit36, D11Mit20 and D11Mit74) spanning the entire chromosome 11 was previously described (26). The amplification reactions were carried out in a total volume of 20 μl using 2× PCR Master Mix, and pairs of primer sequences for specific loci showed in Table II. The thermal cycling conditions were as follows: initial incubation at 94°C for 3 min, 35 cycles of 94°C denaturation for 30 sec, 55°C annealing for 30 sec and 72°C extension for 30 sec and a final extension at 72°C for 7 min. The amplification products were analysed by 3.5% agarose gel electrophoresis for D11Mit36 locus or 2% gel electrophoresis for the other three loci, stained with 1 μg/ml of ethidium bromide and scored as LOH (presence of one band) or non-LOH (retention of two bands at the given locus). Figure 1 shows a representative electrophoresis image of PCR analysis of LOH.
Table II.
Primer pairs used for PCR amplification to determine LOH at four loci along chromosome 11
| Locus | Position (Mbp)a | Sequences of forward and reverse primers |
|---|---|---|
| D11Mit74 | 5.2 | 5ˊ-AAAACCTGAGTTCGACCCCT-3ˊ |
| 5ˊ-ATAAAGCCTCATCTACATGGGC-3ˊ | ||
| D11Mit20 | 44.6 | 5ˊ-CCTGTCCAGGTTTGAGAGGA-3ˊ |
| 5ˊ-CTTGGGAGCCTCTTCGGT-3ˊ | ||
| D11Mit36 | 83.7 | 5ˊ-CCAGAACTTTTGCTGCTTC-3ˊ |
| 5ˊ-GTGAGCCCTAGGTCCAGTGA-3ˊ | ||
| Tk1 | 117.7 | 5ˊ-AGGGAGGTGCCTGGCTAACTGACCGCA-3ˊ |
| 5ˊ-GCGGGACACGGAGTGATACTTGTCGGC-3ˊ |
Locus positions on chromosome 11 in megabase pairs according to the database (http://www.informatics.jax.org), and the chromosome 11 has 122 Mbp (http://www.ncbi.nlm.nih.gov/mapview/maps.cgi?TAXID=10090&MAPS=assembly,cntg-r,ugMm,genes&CHR=11).
Fig. 1.
Representative electrophoresis image of PCR analysis of LOH at the D11Mit36 microsatellite locus of mouse lymphoma cells. While the Lanes 2, 4 and 6 show LOH (presence of one band), and the others indicate non-LOH (retention of two bands at the given locus).
Data analysis
The data evaluation criteria developed by the MLA Expert Workgroup of IWGT were used to determine whether a specific treatment condition was positive. Positive responses were defined as those where the induced MF in one or more treated cultures exceeded the global evaluation factor (126 or 90 mutants per 106 cells for the microwell or soft agar version, respectively) and where there was also a dose-related increase with MF (22). LOH patterns of mutants obtained from each CSC were compared using the computer program written by Cariello et al. (27) for the Monte Carlo analysis developed by Adams and Skopek (28).
Results
Dose range finding tests for each CSC were conducted using the same dose range (25, 50, 75 and 100 μg/ml) in both the microwell and soft agar versions. The 11 CSCs displayed different cytotoxic and mutagenic potencies in both versions, and the dose-related increases in MF were associated with the dose-related increases in cytotoxicity in all CSCs (data not shown). When expressed on a per condensate basis, three CSCs (LT, BRI and REC) exhibited greater cytotoxic and mutagenic effects than the other CSCs in both the microwell and soft agar versions of the assay, with RTGs <10% at the concentration of 100 μg/ml. LIP showed the least cytotoxic and mutagenic potency among the 11 CSCs, with an RTG of 56–60% at a concentration of 100 μg/ml in both versions of the assay.
The main experiments were conducted with each CSC using four or five selected concentrations based on the dose range finding tests. Table III shows the detailed information for RTG, MF and the percentage of small colonies in both the microwell and soft agar versions. Using the data evaluation criteria developed by the IWGT–MLA workgroup (22), all 11 CSCs induced dose-related cytotoxic and mutagenic effects in mouse lymphoma cells in both the microwell and soft agar versions. The lowest concentration that resulted in a positive response was 40 μg/ml in three CSCs (LT, LCF and REC) with an RTG of 54–67% for the microwell version and in two CSCs (BRI and REC) with an RTG of 54–56% for the soft agar version. On the other hand, LIP required the highest concentration (125 μg/ml), which began to give a positive response with an RTG of 44–47% in both versions. Based on the concentrations required to give an RTG between 20 and 10% in both versions, the highest concentration ranged from 70 to 200 μg/ml and the 11 CSCs were divided into three groups. Three CSCs (LT, BRI and REC) induced higher MFs at lower concentrations (70–80 μg/ml), four CSCs (2R4F, LTMAS, LVB and LCF) showed moderate mutagenicity at the concentrations around 100–125 μg/ml and the other four CSCs (FF, LIP, BUR and UL) displayed relatively lower MFs at higher concentrations (150–200 μg/ml). All 11 CSCs induced both large-colony mutants and small-colony mutants in both versions of the MLA, and the proportion of small-colony mutants increased with increasing CSC concentrations (Table III).
Table III.
RTG, MF and the percentage of small colonies induced by 11 CSCs
| CSC | Dose (μg/ml) | Nicotine (μg/ml)a | Microwell version |
Soft- agar version |
||||
|---|---|---|---|---|---|---|---|---|
| RTG (%) | MF (×10−6) | % of small colony | RTG (%) | MF (×10−6) | % of small colony | |||
| 2R4F | 0 | 0 | 100 | 76 | 50 | 100 | 50 | 43 |
| 25 | 1.14 | 99 | 150 | 53 | 100 | 70 | 43 | |
| 50 | 2.28 | 68 | 231 | 62 | 63 | 129 | 64 | |
| 75 | 3.41 | 37 | 506 | 65 | 41 | 252 | 68 | |
| 100 | 4.55 | 19 | 972 | 60 | 21 | 451 | 82 | |
| PC | 43 | 874 | 53 | 42 | 387 | 62 | ||
| LT | 0 | 0 | 100 | 100 | 65 | 100 | 80 | 57 |
| 30 | 1.73 | 76 | 221 | 66 | 86 | 126 | 68 | |
| 40 | 2.30 | 67 | 319 | 71 | 84 | 145 | 74 | |
| 50 | 2.88 | 44 | 617 | 70 | 48 | 271 | 80 | |
| 60 | 3.45 | 39 | 772 | 71 | 39 | 355 | 83 | |
| 70 | 4.03 | 27 | 1204 | 65 | 24 | 399 | 71 | |
| PC | 30 | 1167 | 64 | 43 | 349 | 65 | ||
| LTMAS | 0 | 0 | 100 | 69 | 40 | 100 | 70 | 54 |
| 50 | 2.90 | 75 | 186 | 60 | 96 | 123 | 65 | |
| 75 | 4.35 | 54 | 334 | 59 | 65 | 260 | 76 | |
| 100 | 5.80 | 35 | 610 | 57 | 48 | 362 | 79 | |
| 125 | 7.25 | 17 | 1172 | 63 | 26 | 594 | 87 | |
| PC | 26 | 825 | 57 | 30 | 695 | 76 | ||
| FF | 0 | 0 | 100 | 88 | 26 | 100 | 87 | 33 |
| 50 | 2.45 | 92 | 191 | 46 | 78 | 147 | 33 | |
| 75 | 3.68 | 72 | 279 | 50 | 67 | 159 | 47 | |
| 100 | 4.90 | 42 | 517 | 62 | 41 | 252 | 38 | |
| 125 | 6.13 | 29 | 581 | 64 | 26 | 301 | 51 | |
| 150 | 7.35 | 16 | 1003 | 70 | 16 | 420 | 54 | |
| PC | 64 | 952 | 52 | 62 | 356 | 37 | ||
| LIP | 0 | 0 | 100 | 87 | 29 | 100 | 67 | 39 |
| 100 | 5.80 | 52 | 203 | 59 | 59 | 153 | 67 | |
| 125 | 7.25 | 47 | 324 | 56 | 44 | 284 | 68 | |
| 150 | 8.70 | 34 | 522 | 67 | 35 | 363 | 75 | |
| 175 | 10.15 | 23 | 761 | 60 | 22 | 544 | 75 | |
| 200 | 11.60 | 19 | 771 | 61 | 19 | 566 | 84 | |
| PC | 40 | 1290 | 48 | 39 | 664 | 72 | ||
| LVB | 0 | 0 | 100 | 100 | 65 | 100 | 80 | 57 |
| 25 | 1.23 | 89 | 134 | 65 | 99 | 99 | 51 | |
| 50 | 2.45 | 79 | 199 | 68 | 71 | 111 | 54 | |
| 75 | 3.68 | 43 | 497 | 76 | 39 | 222 | 76 | |
| 100 | 4.90 | 14 | 1151 | 73 | 15 | 368 | 80 | |
| PC | 30 | 1167 | 64 | 43 | 349 | 65 | ||
| LCF | 0 | 0 | 100 | 80 | 36 | 100 | 62 | 26 |
| 40 | 2.30 | 54 | 229 | 48 | 63 | 106 | 29 | |
| 60 | 3.45 | 50 | 276 | 63 | 45 | 133 | 54 | |
| 80 | 4.60 | 34 | 434 | 64 | 35 | 220 | 57 | |
| 100 | 5.75 | 13 | 772 | 67 | 17 | 277 | 70 | |
| PC | 55 | 832 | 50 | 52 | 373 | 38 | ||
| BUR | 0 | 0 | 100 | 93 | 33 | 100 | 87 | 32 |
| 50 | 5.83 | 75 | 235 | 38 | 84 | 144 | 45 | |
| 75 | 8.74 | 47 | 239 | 53 | 48 | 157 | 52 | |
| 100 | 11.65 | 28 | 276 | 59 | 29 | 234 | 58 | |
| 125 | 14.56 | 20 | 612 | 68 | 25 | 260 | 72 | |
| 150 | 17.48 | 13 | 699 | 60 | 13 | 471 | 77 | |
| PC | 37 | 1396 | 39 | 56 | 398 | 76 | ||
| BRI | 0 | 0 | 100 | 96 | 35 | 100 | 53 | 30 |
| 40 | 4.68 | 64 | 202 | 45 | 54 | 170 | 59 | |
| 50 | 5.85 | 37 | 444 | 62 | 39 | 245 | 73 | |
| 60 | 7.02 | 18 | 885 | 59 | 23 | 343 | 75 | |
| 70 | 8.19 | 11 | 996 | 60 | 12 | 467 | 81 | |
| PC | 45 | 999 | 40 | 41 | 482 | 61 | ||
| REC | 0 | 0 | 100 | 96 | 35 | 100 | 53 | 30 |
| 40 | 1.28 | 58 | 255 | 52 | 56 | 157 | 56 | |
| 60 | 1.92 | 33 | 584 | 54 | 31 | 325 | 72 | |
| 70 | 2.24 | 17 | 1036 | 53 | 16 | 558 | 80 | |
| 80 | 2.56 | 12 | 1319 | 56 | 10 | 701 | 85 | |
| PC | 45 | 999 | 40 | 41 | 482 | 61 | ||
| UL | 0 | 0 | 100 | 93 | 33 | 100 | 87 | 32 |
| 75 | 3.79 | 62 | 273 | 50 | 67 | 180 | 53 | |
| 100 | 5.05 | 45 | 344 | 50 | 46 | 254 | 65 | |
| 125 | 6.31 | 21 | 602 | 68 | 22 | 403 | 80 | |
| 150 | 7.58 | 14 | 892 | 66 | 16 | 470 | 75 | |
| PC | 37 | 1396 | 39 | 56 | 398 | 76 | ||
RTG, relative total growth that includes a measure of growth during treatment, expression and cloning; MF, mutant frequency per 106 cells; PC, positive control of 0.3 μ/ml benzo[a]pyrene in each experiment.
Converted from CSC mass concentration according to the nicotine concentration in each CSC shown in Table I, in order to adjust the toxicity value for compensatory smoking.
In order to confirm the results shown in Table III, additional experiments using five CSCs (BRI, BUR, LIP, LTMAS and REC) were conducted using both versions of the MLA. These five CSCs resulted in dose-dependent cytotoxicity and mutagenicity (data not shown), which were similar to the main experiments shown in Table III.
The mutagenic potencies of the CSCs were calculated using the slope of the linear regression of the dose–response curves (R2 = 0.786–0.911), based on the data in Table III. The genotoxic potencies (ranking) of 11 CSCs as an MF per microgram of CSC in both versions of the MLA are listed in Table IV. The potency values are also expressed per microgram of nicotine (Table IV). Among the 11 CSCs, LT, BRI and REC were the three most potent CSCs in both versions when calculated per microgram of CSC. LT and REC remained among the top three most potent after adjusting for the nicotine content of the CSCs. BUR was the least mutagenic in both versions when calculated per microgram of nicotine. Expressed per microgram of CSC, LIP and FF were the least mutagenic in the microwell or soft agar version, respectively. In both versions, the mutagenic potencies spanned a 3.5- to 4.1-fold or a 12.3- to 13.1-fold range when expressed as MF per microgram CSC or per microgram nicotine, respectively (Table IV). In addition, the mutagenic potencies of the CSCs did not show a correlation with the tar or nicotine concentrations in the cigarettes.
Table IV.
Mutagenic potencies (rankings) of 11 CSCs expressed per microgram of CSC and per microgram of nicotine a
| CSC | MF ×10−6 per microgram of CSC |
MF ×10−6 per microgram of nicotine |
||||||
|---|---|---|---|---|---|---|---|---|
| Microwell | Ranking | Soft agar | Ranking | Microwell | Ranking | Soft agar | Ranking | |
| 2R4F | 8.6 | 5 | 3.9 | 5 | 188.8 | 4 | 86.5 | 2 |
| LT | 15.0 | 1 | 4.9 | 3 | 260.2 | 2 | 84.8 | 3 |
| LTMAS | 8.2 | 6 | 4.1 | 4 | 141.4 | 5 | 69.8 | 4 |
| FF | 5.5 | 8 | 2.1 | 11 | 112.4 | 7 | 42.4 | 9 |
| LIP | 3.7 | 11 | 2.6 | 7 | 63.1 | 10 | 44.8 | 8 |
| LVB | 9.9 | 4 | 2.8 | 6 | 201.2 | 3 | 57.1 | 6 |
| LCF | 6.3 | 7 | 2.2 | 9 | 109.7 | 8 | 37.7 | 10 |
| BUR | 4.0 | 10 | 2.2 | 10 | 34.2 | 11 | 18.9 | 11 |
| BRI | 14.7 | 2 | 7.2 | 2 | 125.9 | 6 | 61.9 | 5 |
| REC | 14.3 | 3 | 7.4 | 1 | 447.5 | 1 | 232.2 | 1 |
| UL | 4.9 | 9 | 2.5 | 8 | 96.4 | 9 | 50.3 | 7 |
The potencies of the CSCs to induce MF ×10−6 were calculated from the slope of the linear regression of the dose-response curves (R2 = 0.786–0.911).
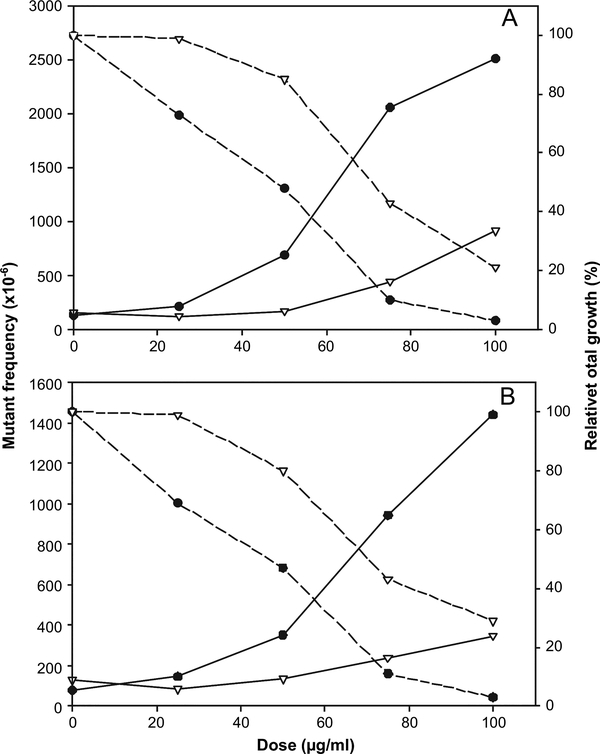
Comparing two CSCs produced from the same commercial cigarette (US light non-menthol brand) under two sets of smoking conditions, the smoke condensate of LT smoked under ISO conditions was more cytotoxic and mutagenic than LTMAS produced under MDPH conditions in both versions of the MLA on a per microgram CSC basis (Figure 2). Expressed per microgram of CSC, the potency of LT was ranked first and third and the potency of LTMAS was sixth and fourth in the microwell and soft agar versions, respectively. Expressed per microgram of nicotine, the potency of LT was ranked second and third and the potency of LTMAS was fifth and fourth in the microwell and soft agar versions, respectively. Most of the CSC rankings did not change when comparing the MF potency expressed per microgram of CSC with the MF potency expressed per microgram of nicotine. Possible exceptions are BRI that decreased in potency when expressed per microgram of nicotine in both versions and 2R4F that increased in potency ranking in the soft agar version when expressed per microgram of nicotine.
Fig. 2.
Comparison of cytotoxic and mutagenic effects in mouse lymphoma cells treated with CSCs from same cigarette machine smoked under the different conditions. The CSCs of US ‘light’ non-menthol brand cigarettes were produced under ISO conditions (LT) or MDPH conditions (LTMAS), respectively. (A) the microwell version of the MLA; (B) the soft agar version of the MLA; (filled circles) LT; (inverted triangles), LTMAS; solid line, mutagenic effects presented as mutant frequency per million cells; dashed line, cytotoxic effects presented as relative total growth (%).
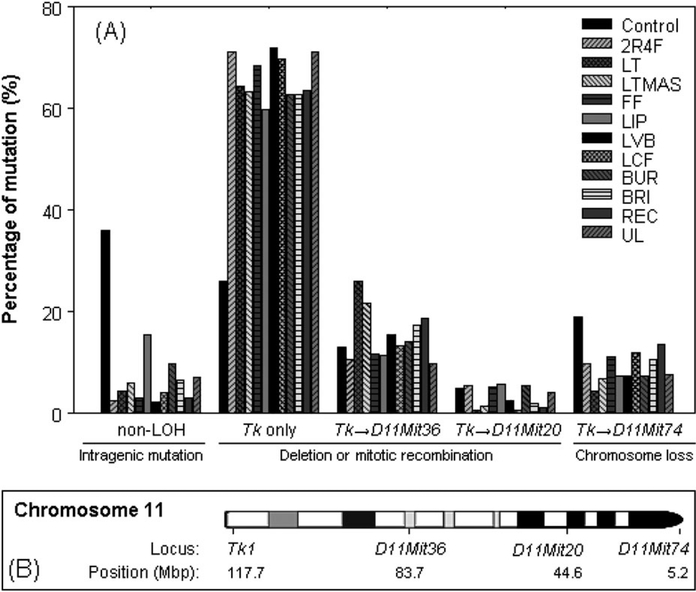
LOH analysis of the mutants was conducted using four microsatellite loci, Tk locus, D11Mit36, D11Mit20 and D11Mit74, to determine the types of the mutations. These four polymorphic markers were almost evenly distributed along the full length of chromosome 11. DNA samples were isolated from 48 large and 48 small-mutant colonies for each CSC treatment using the highest dose (see Table III) and DMSO-negative control. More than 900 mutants (92%) from 11 CSC treatments lost heterozygosity at the Tk locus. The percentages of the different types of mutations in all (large and small) mutant colonies are displayed in Figure 3. The mutational spectra induced by each CSC treatment were significantly different than the negative control. The most common type of mutation for all the CSCs was LOH involving only the Tk locus, indicating most mutants had chromosome damage less than ~34 Mbp of chromosome 11. The second most common type of mutation was LOH involving both Tk and D11Mit36 loci, suggesting chromosome damage between 34 and 73 Mbp (Figure 3).
Fig. 3.
(A) Comparison of the percentage of mutational types for all (large and small) colonies produced in mouse lymphoma cells treated with 11 CSCs or control (DMSO). The mutants were obtained from the highest dose of 11 CSCs with RTG of 12–27% in Table III. The data are the weighted sum of mutation percentages from large and small colonies (see Table III for the proportion of small-colony mutants). The ‘→’ indicates the LOH extends from Tk locus to other three loci (D11Mit36, D11Mit20 and D11Mit74). (B) The loci that were analysed for LOH (Tk1, D11Mit36, D11Mit20 and D11Mit74) are marked on the chromosome 11, and the locus of D11Mit74 is located at the top of the chromosome 11.
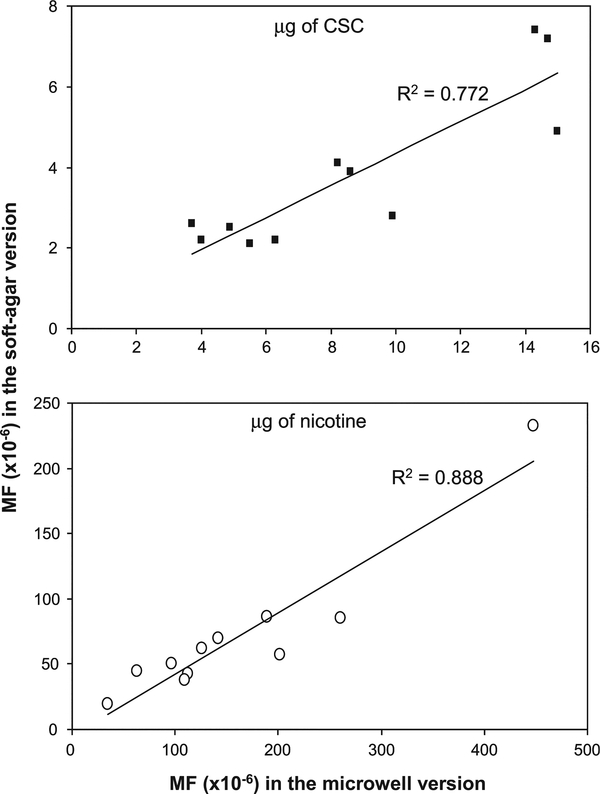
To compare the results of the two equally acceptable MLA methodologies, both the microwell and soft agar versions were conducted for all 11 CSCs. It should be noted that in all cases, the cell treatment and mutant expression were conducted using a single culture. Each CSC-treated cell culture was used for the two different selection methods, 96-microwell plates and soft agar petri dishes. Therefore, the relative suspension growth calculated during the 2-day phenotypic expression in both versions of the MLA was same for each CSC. As seen in Table III, the RTG of the two versions were similar. Although the absolute values of MFs (especially in high doses) in the microwell version were higher than those in the soft agar version at the same dose-treated cultures, dose-related increases in MFs were seen for all tested CSCs in both versions of the assay (Table III). While the order of genotoxic potencies appears a little bit different in the two versions of the MLA expressed either as MF per microgram of CSC or nicotine, the mutagenic potencies of all CSCs were strongly correlated (R2 = 0.772 or 0.888, respectively) between the two versions of the MLA (Figure 4), indicating that the two versions give comparable results.
Fig. 4.
Correlations between the mutagenic potencies of 11 CSCs. The data for mutagenic potencies were shown in Table IV. The top panel, the potencies per microgram of CSC; the bottom panel, the potencies per microgram of nicotine.
Discussion
Tobacco smoke, including involuntary smoking, is classified by the International Agency for Research on Cancer as a Group 1 carcinogen (sufficient evidence of carcinogenicity) (29). Smoking is the single most preventable cause of disease, disability and death in the USA, and >400 000 people die prematurely from smoking or exposure to second-hand smoke each year (30). Cigarettes deliver toxic chemicals to smokers and non-smokers exposed to second-hand smoke. In this regard, it is appropriate to evaluate standardised toxicity tests for the ability to distinguish between cigarettes with differing characteristics (e.g. charcoal filters) and also to investigate the contribution of different tobacco types to the overall toxicity of the product. In vitro short-term tests have always been considered prior to other assays because of their rapid, inexpensive yet standardised data. Previous studies demonstrated that in vitro toxicology tests have been successfully used for better understanding of the biological activity of CSCs (5).
The MLA has been widely used for short-term mutagenicity tests (16,17) and is capable of evaluating the ability of mutagens to induce a wide variety of mutational events, i.e. point mutations, large-scale chromosomal changes, recombination and mitotic non-disjunction (31–35). This assay has been considered as the most sensitive in vitro mammalian cell gene mutation assay and therefore is especially useful in evaluating substances with unknown or multiple genotoxic mechanisms (13).
When the mouse lymphoma cells were exposed to CSC, all 11 CSCs were cytotoxic and mutagenic (Tables III) by causing LOH involving chromosome 11, the location of the Tk gene (Figure 3). About 60–87% of mutants induced by 11 CSCs were small colonies in both versions. The dose-related increases in MFs were associated with dose-related increases in cytotoxicity for all tested CSCs. The mutagenic potencies observed among the 11 CSCs produced from 10 diverse cigarette types varied by 12- to 13-fold when expressed per microgram of nicotine allowing better separation than the potencies when expressed on a per microgram of CSC basis (Table IV). In the soft agar version, the potencies of CSCs as MF per microgram of CSC basis suggested three groups of cigarettes, REC and BRI > LT, LTMAS and 2R4F > LVB, LIP, UL, BUR, LCF and FF. These results suggest that the MLA is capable of discriminating the mutagenic activity of different CSCs. It is worth noting that these differences were not related to the tar concentrations in the cigarettes. For example, UL contained the lowest tar of 5 mg/cigarette and showed even higher cytotoxicity and mutagenicity than LT or 2R4F with 10 and 8.9 mg of tar/cigarette. This finding is supported by epidemiologic studies that demonstrate no difference in uptake of lung carcinogens and lung cancer risk among smokers of cigarettes having tar levels of regular (>14.5 mg), light (6.5–14.5 mg) and ultralight (<6.5 mg) (36,37), indicating that cigarette properties other than a single tar value influence the genotoxicity of the cigarettes. In fact, there are >4000 individual constituents identified in cigarette smoke and the chemical composition of the tar likely varies based on the tobacco blend, physical and chemical characteristics of the cigarette and smoking topography (i.e. how a person smokes a cigarette including the number of puffs, puff volume and puff duration) (38).
The MLA results presented here indicate some differences in the mutagenic potencies of the 11 CSCs (Table IV) than the genotoxic potencies found in the Ames test, the comet assay, the micronucleus assay and the chromosome aberration assay (15). However, there is some agreement between the two studies. For example, when expressed on a per microgram of CSC basis, the potency rankings of four CSCs (UL, FF, LT and LTMAS) were similar to their potency rankings in the micronucleus assay and of REC, FF, LT and LTMAS with the potency rankings in the chromosomal aberration assay in the DeMarini study (15). The biological relevance of the similarities and differences across assays warrant further study. It has long been recognised that the in vitro genotoxic test systems measure different biological end points and therefore would be expected to respond differently to different chemical classes or constituents of cigarette smoke. There are at least 106 chemicals present in tobacco that have been tested in the MLA. Sixty of them belonging to several different chemical classes were evaluated as limited or definitive positives in the MLA assay (13,39). Due to the chemical complexity of tobacco smoke, the quantitative contribution of any particular smoke constituent and their contribution to the overall mutagenicity and carcinogenicity of cigarette smoke remain to be elucidated.
It is recognised that not all smokers smoke their cigarettes in the same way. Numerous studies have shown that some smokers take larger puffs and/or more frequent puffs than the machine smoked under ISO conditions (40–42). Therefore, a CSC produced from a cigarette machine smoked under more intense conditions was included in this study to compare the mutagenicity of cigarette smoke under both standard and more intense smoking conditions. LT and LTMAS were produced from the same US ‘light’ non-menthol brand. LTMAS generated under MDPH condition had a higher tar concentration than LT generated under ISO conditions (21.7 versus 10 mg/cigarette, Table I). LT showed more cytotoxicity and mutagenicity than LTMAS in both versions of the MLA (Figure 2), with ~1.8-fold and 1.2-fold higher MFs in the microwell and soft agar versions, respectively (Table IV). Similarly, in a previous study which tested the mutagenicity of cigarette mainstream smoke particulate phase from eight US commercial cigarettes plus two reference cigarettes using the MLA, the mutagenic activity expressed on a per milligram TPM basis was in general slightly lower under MDPH conditions than those under ISO conditions, especially ‘Parliament Lights 100’s and Marlboro’ (13). Roemer et al. (12) also reported that the mutagenic activity as a function of TPM delivery was lower using MDPH conditions when compared to ISO conditions in the Ames assay.
While there have been a few previous studies using the MLA to evaluate cigarette mainstream smoke with one or more CSC/ TPM samples (13,43–45), none has analysed the mutants for LOH beyond the Tk locus on chromosome 11. In our study, four microsatellite loci (Tk locus, D11Mit36, D11Mit20 and D11Mit74) were selected that were almost evenly distributed along the full length of chromosome 11. The mutational spectra induced by each CSC treatment were significantly different from the negative control (Figure 3), with the most common type of mutation for all CSCs being LOH at the Tk locus alone. That is, the LOH did not extend to D11Mit36 so the chromosome damage involved <34 Mbp of chromosome 11. These results indicate that CSC-induced mutagenicity likely occurs through a clastogenic mode of action. Massey et al. (46) observed that micronuclei induced by whole cigarette smoke occurred primarily from whole chromosome loss (aneuploidy). Recently, DeMarini et al. (15) reported chromosomal aberration data using CHO cells for 10 CSCs and the clastogenic potencies ranged 4-fold based on per microgram CSC. LOH is frequently observed in a variety of human cancers at loci that are tumour suppressor genes and thus is an important mutational event in tumorigenesis. LOH can result from any of the several mechanisms, including large deletions, mitotic recombination and whole chromosome loss (34).
In this study, both the microwell and soft agar versions of the MLA were performed with 11 CSCs. These results (Table III) demonstrated that the cytotoxic and mutagenic effects of 11 CSCs were comparable in both versions. The RTG of the two versions were similar, suggesting that these two versions have the same potential to evaluate the cytotoxicity of the chemicals. With regard to the MFs, the microwell procedure yielded slightly higher background MFs than the soft agar version. The CSC-induced MFs in the microwell version were somewhat greater than those in the soft agar version at the lower treatment concentrations and were about two times greater in the higher treatment concentrations (Table III). These results are in agreement with a previous study, which compared the two methodologies using model chemical compounds, such as ethyl methanesulfonate, methyl methanesulfonate and dimethylbenzanthracene (47). The difference between the two versions of the MLA probably results from the cell culture medium, particularly the presence or absence of agar, and the methods for calculating MF. The cell growth is likely a little bit faster in the cloning medium without agar (microwell version) than those growing in the cloning medium including agar (soft agar version). The Poisson distribution is used to calculate the plating efficiencies and MFs in the microwell version of the MLA, while the absolute colony numbers are employed to directly calculate MFs in the soft agar version (25). We plotted the mutagenic potencies of the 11 CSCs generated using the two versions of the MLA either in MF per microgram of CSC or nicotine (Table IV). Although the order of genotoxic potencies showed some differences in both versions of the MLA, the mutagenic potencies of all CSCs were strongly correlated (Figure 4) between the two versions of the MLA when expressed as MF per microgram of CSC (R2 = 0.772) or nicotine (R2 = 0.888), indicating that the two versions give comparable results.
In summary, the results of this study demonstrate that CSCs produce dose-related cytotoxic and mutagenic effects in mouse lymphoma cells using both the microwell and soft agar versions of the MLA, with the mutagenic potencies spanning about a 4-fold or a 12- to 13-fold range when expressed as MF per microgram CSC or per microgram nicotine, respectively. The major type of the mutations induced by the 11 CSCs was LOH involving <34 Mbp of chromosome 11, indicating a clastogenic mode of action. The MLA is capable of assessing and comparing the cytotoxic and mutagenic activities of complex mixtures of chemicals such as CSC, and the results from both versions of this assay are comparable.
Acknowledgements
Use of trade names is for informational purposes only and in no way implies endorsement by the US Government, the US Department of Health and Human Services or CDC. This manuscript has been reviewed by the NCTR and the CDC. The views presented in this paper do not necessarily reflect those of the US FDA or the CDC.
Funding
This work was supported by internal funds of the US CDC through an interagency agreement between the CDC and the U S Food and Drug Administration (FDA)/National Center for Toxicological Research (NCTR) and also partly supported by an appointment (X.G.) to the Postgraduate Research Program at the NCTR administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the FDA.
Footnotes
Conflict of interest statement: None declared.
Contributor Information
Xiaoqing Guo, Division of Genetic and Molecular Toxicology, National Center for Toxicological Research, Food and Drug Administration, 3900 NCTR Road, Jefferson, AR 72079, USA.
Tracie L. Verkler, Division of Genetic and Molecular Toxicology, National Center for Toxicological Research, Food and Drug Administration, 3900 NCTR Road, Jefferson, AR 72079, USA
Ying Chen, Division of Genetic and Molecular Toxicology, National Center for Toxicological Research, Food and Drug Administration, 3900 NCTR Road, Jefferson, AR 72079, USA.
Patricia A. Richter, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, 4770 Buford Highway, Atlanta, GA 30341, USA
Gregory M. Polzin, Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, 4770 Buford Highway, Atlanta, GA 30341, USA
Martha M. Moore, Division of Genetic and Molecular Toxicology, National Center for Toxicological Research, Food and Drug Administration, 3900 NCTR Road, Jefferson, AR 72079, USA
Nan Mei, Division of Genetic and Molecular Toxicology, National Center for Toxicological Research, Food and Drug Administration, 3900 NCTR Road, Jefferson, AR 72079, USA.
References
- 1.WHO (2009) WHO Report on the Global Tobacco Epidemic. World Health Organization, http://whqlibdoc.who.int/publications/2009/9789241563918_eng_full.pdf (accessed October 5, 2010). [Google Scholar]
- 2.Bergen AW and Caporaso N (1999) Cigarette smoking. J. Natl Cancer Inst., 91, 1365–1375. [DOI] [PubMed] [Google Scholar]
- 3.DeMarini DM (2004) Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat. Res., 567, 447–474. [DOI] [PubMed] [Google Scholar]
- 4.Husgafvel-Pursiainen K (2004) Genotoxicity of environmental tobacco smoke: a review. Mutat. Res., 567, 427–445. [DOI] [PubMed] [Google Scholar]
- 5.Andreoli C, Gigante D and Nunziata A (2003) A review of in vitro methods to assess the biological activity of tobacco smoke with the aim of reducing the toxicity of smoke. Toxicol. In Vitro, 17, 587–594. [DOI] [PubMed] [Google Scholar]
- 6.Steele RH, Payne VM, Fulp CW, Rees DC, Lee CK and Doolittle DJ (1995) A comparison of the mutagenicity of mainstream cigarette smoke condensates from a representative sample of the U.S. cigarette market with a Kentucky reference cigarette (K1R4F). Mutat. Res., 342, 179–190. [DOI] [PubMed] [Google Scholar]
- 7.Chepiga TA, Morton MJ, Murphy PA, Avalos JT, Bombick BR, Doolittle DJ, Borgerding MF and Swauger JE (2000) A comparison of the mainstream smoke chemistry and mutagenicity of a representative sample of the US cigarette market with two Kentucky reference cigarettes (K1R4F and K1R5F). Food Chem. Toxicol., 38, 949–962. [DOI] [PubMed] [Google Scholar]
- 8.Foy JW, Bombick BR, Bombick DW, Doolittle DJ,Mosberg AT and Swauger JE (2004) A comparison of in vitro toxicities of cigarette smoke condensate from Eclipse cigarettes and four commercially available ultra low-"tar" cigarettes. Food Chem. Toxicol., 42, 237–243. [DOI] [PubMed] [Google Scholar]
- 9.Lou J, Chu G, Zhou G et al. (2010) Comparison between two kinds of cigarette smoke condensates (CSCs) of the cytogenotoxicity and protein expression in a human B-cell lymphoblastoid cell line using CCK-8 assay, comet assay and protein microarray. Mutat. Res., 697, 55–59. [DOI] [PubMed] [Google Scholar]
- 10.Jianlin L, Guohai C, Guojun Z et al. (2009) Assessing cytogenotoxicity of cigarette smoke condensates using three in vitro assays. Mutat. Res., 677, 21–26. [DOI] [PubMed] [Google Scholar]
- 11.Jongen WM, Hakkert BC and van der Hoeven JC (1985) Genotoxicity testing of cigarette-smoke condensate in the SCE and HGPRT assays with V79 Chinese hamster cells. Food Chem. Toxicol., 23, 603–607. [DOI] [PubMed] [Google Scholar]
- 12.Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ,Reininghaus W, Carchman RA, Gaworski CL and Podraza KF (2004) Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology, 195, 31–52. [DOI] [PubMed] [Google Scholar]
- 13.Schramke H, Meisgen TJ, Tewes FJ, Gomm W and Roemer E (2006) The mouse lymphoma thymidine kinase assay for the assessment and comparison of the mutagenic activity of cigarette mainstream smoke particulate phase. Toxicology, 227, 193–210. [DOI] [PubMed] [Google Scholar]
- 14.Lou J, Zhou G, Chu G, Jiang J, Huang F, Zheng S, Lu Y, Li Xand He J. (2010) Studying the cyto-genotoxic effects of 12 cigarette smoke condensates on human lymphoblastoid cell line in vitro. Mutat. Res., 696, 48–54. [DOI] [PubMed] [Google Scholar]
- 15.DeMarini DM, Gudi R, Szkudlinska A, Rao M, Recio L, Kehl M,Kirby PE, Polzin G and Richter PA (2008) Genotoxicity of 10 cigarette smoke condensates in four test systems: comparisons between assays and condensates. Mutat. Res., 650, 15–29. [DOI] [PubMed] [Google Scholar]
- 16.Dearfield KL, Auletta AE, Cimino MC and Moore MM (1991) Considerations in the U.S. Environmental Protection Agency’s testing approach for mutagenicity. Mutat. Res., 258, 259–283. [DOI] [PubMed] [Google Scholar]
- 17.Muller L, Kikuchi Y, Probst G, Schechtman L, Shimada H,Sofuni T and Tweats D (1999) ICH-harmonised guidances on genotoxicity testing of pharmaceuticals: evolution, reasoning and impact. Mutat. Res., 436, 195–225. [DOI] [PubMed] [Google Scholar]
- 18.Clive D, Flamm WG, Machesko MR and Bernheim NJ (1972) A mutational assay system using the thymidine kinase locus in mouse lymphoma cells. Mutat. Res., 16, 77–87. [DOI] [PubMed] [Google Scholar]
- 19.Cole J, Arlett CF, Green MH, Lowe J and Muriel W (1983) A comparison of the agar cloning and microtitration techniques for assaying cell survival and mutation frequency in L5178Y mouse lymphoma cells. Mutat. Res., 111, 371–386. [DOI] [PubMed] [Google Scholar]
- 20.Moore MM, Honma M, Clements J et al. (2000) Mouse lymphoma thymidine kinase locus gene mutation assay: nternational Workshop on Genotoxicity Test Procedures Workgroup Report. Environ. Mol. Mutagen., 35, 185–190. [DOI] [PubMed] [Google Scholar]
- 21.Moore MM, Honma M, Clements J et al. (2007) Mouse lymphoma thymidine kinase gene mutation assay: meeting of the International Workshop on Genotoxicity Testing, San Francisco, 2005, recommendations for 24-h treatment. Mutat. Res., 627, 36–40. [DOI] [PubMed] [Google Scholar]
- 22.Moore MM, Honma M, Clements J et al. (2006) Mouse lymphoma thymidine kinase gene mutation assay: follow-up meeting of the International Workshop on Genotoxicity Testing—Aberdeen, Scotland, 2003—Assay acceptance criteria, positive controls, and data evaluation. Environ. Mol. Mutagen., 47, 1–5. [DOI] [PubMed] [Google Scholar]
- 23.Moore MM, Honma M, Clements J et al. (2003) Mouse lymphoma thymidine kinase gene mutation assay: nternational Workshop on Genotoxicity Tests Workgroup report—Plymouth, UK 2002. Mutat. Res., 540, 127–140. [DOI] [PubMed] [Google Scholar]
- 24.Moore MM, Honma M, Clements J et al. (2002) Mouse lymphoma thymidine kinase gene mutation assay: follow-up International Workshop on Genotoxicity Test Procedures, New Orleans, Louisiana, April 2000. Environ. Mol. Mutagen., 40, 292–299. [DOI] [PubMed] [Google Scholar]
- 25.Chen T and Moore MM (2004) Screening for chemical mutagens using the mouse lymphoma assay In Yan Z and Caldwell GW (eds), In Optimization in Drug Discovery: In-vitro Methods. Humana Press, Totowa, NJ, USA, pp. 337–352. [Google Scholar]
- 26.Mei N, Hu J, Xia Q, Fu PP, Moore MM and Chen T (2010) Cytotoxicity and mutagenicity of retinol with ultraviolet A irradiation in mouse lymphoma cells. Toxicol. In Vitro, 24, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cariello NF, Piegorsch WW, Adams WT and Skopek TR (1994) Computer program for the analysis of mutational spectra: application to p.53 mutations. Carcinogenesis, 15, 2281–2285. [DOI] [PubMed] [Google Scholar]
- 28.Adams WT and Skopek TR (1987) Statistical test for the comparison of samples from mutational spectra. J. Mol. Biol., 194, 391–396. [DOI] [PubMed] [Google Scholar]
- 29.International Agency for Research on Cancer (2003) Tobacco smoke and involuntary smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 83, Lyon, France. http://monographs.iarc.fr/ENG/Monographs/vol83/volume83.pdf (accessed October 5, 2010). [PMC free article] [PubMed] [Google Scholar]
- 30.CDC (2010) Tobacco Use—Targeting the Nation’s Leading Killer. Centers for Disease Control and Prevention, Atlanta, GA, USA, http://www.cdc.gov/chronicdisease/resources/publications/aag/osh.htm (accessed October 5, 2010). [Google Scholar]
- 31.Applegate ML, Moore MM, Broder CB, Burrell A, Juhn G,Kasweck KL, Lin PF, Wadhams A and Hozier JC (1990) Molecular dissection of mutations at the heterozygous thymidine kinase locus in mouse lymphoma cells. Proc. Natl Acad. Sci. USA, 87, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clements J (2000) The mouse lymphoma assay. Mutat. Res., 455, 97–110. [DOI] [PubMed] [Google Scholar]
- 33.Liechty MC, Scalzi JM, Sims KR, Crosby H Jr, Spencer DL,Davis LM, Caspary WJ and Hozier JC (1998) Analysis of large and small colony L5178Y tk-/- mouse lymphoma mutants by loss of heterozygosity (LOH) and by whole chromosome 11 painting: detection of recombination. Mutagenesis, 13, 461–474. [DOI] [PubMed] [Google Scholar]
- 34.Honma M, Momose M, Sakamoto H, Sofuni T and Hayashi M (2001) Spindle poisons induce allelic loss in mouse lymphoma cells through mitotic non-disjunction. Mutat. Res., 493, 101–114. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Sawyer JR, Chen L, Chen T, Honma M, Mei N and Moore MM (2009) The mouse lymphoma assay detects recombination, deletion, and aneuploidy. Toxicol. Sci., 109, 96–105. [DOI] [PubMed] [Google Scholar]
- 36.Harris JE, Thun MJ, Mondul AM and Calle EE (2004) Cigarette tar yields in relation to mortality from lung cancer in the cancer prevention study II prospective cohort, 1982–8. BMJ, 328, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hecht SS, Murphy SE, Carmella SG, Li S, Jensen J, Le C, Joseph AM and Hatsukami DK (2005) Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol. Biomarkers Prev., 14, 693–698. [DOI] [PubMed] [Google Scholar]
- 38.Burns DM (1991) Cigarettes and cigarette smoking. Clin. Chest Med., 12, 631–642. [PubMed] [Google Scholar]
- 39.Mitchell AD, Auletta AE, Clive D, Kirby PE, Moore MM and Myhr BC (1997) The L5178Y/tk+/− mouse lymphoma specific gene and chromosomal mutation assay a phase III report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat. Res., 394, 177–303. [DOI] [PubMed] [Google Scholar]
- 40.Ding YS, Ashley DL and Watson CH (2007) Determination of 10 carcinogenic polycyclic aromatic hydrocarbons in mainstream cigarette smoke. J. Agric. Food Chem., 55, 5966–5973. [DOI] [PubMed] [Google Scholar]
- 41.Kozlowski LT and O’Connor RJ (2002) Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tob. Control, 11 (Suppl. 1), I40–I50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rickert WS, Trivedi AH, Momin RA, Wright WG and Lauterbach JH (2007) Effect of smoking conditions and methods of collection on the mutagenicity and cytotoxicity of cigarette mainstream smoke. Toxicol. Sci., 96, 285–293. [DOI] [PubMed] [Google Scholar]
- 43.Clive D, Johnson KO, Spector JF, Batson AG and Brown MM (1979) Validation and characterization of the L5178Y/TK+/− mouse lymphoma mutagen assay system. Mutat. Res., 59, 61–108. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell AD, Evans EL, Jotz MM, Riccio ES, Morelmans KE and Simmon VF (1981) Mutagenic and carcinogenic potency of extracts of diesel and related environmental emissions: in vitro mutagenesis and DNA damage. Environ. Int., 5, 393–401. [Google Scholar]
- 45.Cobb RR, Martin J, Korytynski E, Monteith L and Hughes TJ (1989) Preliminary molecular analysis of the TK locus in L5178Y large-and small-colony mouse lymphoma cell mutants. Mutat. Res., 226, 253–258. [DOI] [PubMed] [Google Scholar]
- 46.Massey E, Aufderheide M, Koch W, Lodding H, Pohlmann G,Windt H, Jarck P and Knebel JW (1998) Micronucleus induction in V79 cells after direct exposure to whole cigarette smoke. Mutagenesis, 13, 145–149. [DOI] [PubMed] [Google Scholar]
- 47.Oberly TJ, Yount DL and Garriott ML (1997) A comparison of the soft agar and microtitre methodologies for the L5178Y tk+/− mouse lymphoma assay. Mutat. Res., 388, 59–66. [DOI] [PubMed] [Google Scholar]