Summary
Background
Therapeutic cancer vaccines have shown activity in metastatic castration-resistant prostate cancer (mCRPC), and methods are being assessed to enhance their efficacy. Ipilimumab is an antagonistic monoclonal antibody that binds cytotoxic T-lymphocyte-associated protein 4, an immunomodulatory molecule expressed by activated T cells, and to CD80 on antigen-presenting cells. We aimed to assess the safety and tolerability of ipilimumab in combination with a poxviral-based vaccine targeting prostate-specific antigen (PSA) and containing transgenes for T-cell co-stimulatory molecule expression, including CD80.
Methods
We did a phase 1 dose-escalation trial, with a subsequent expansion phase, to assess the safety and tolerability of escalating doses of ipilimumab in combination with a fixed dose of the PSA-Tricom vaccine. Patients with mCRPC received 2×109 plaque-forming units of recombinant vaccinia PSA-Tricom subcutaneously on day 1 of cycle 1, with subsequent monthly boosts of 1×109 plaque-forming units, starting on day 15. Intravenous ipilimumab was given monthly starting at day 15, in doses of 1, 3, 5, and 10 mg/kg. Our primary goal was to assess the safety of the combination. This study is registered with ClinicalTrials.gov, number NCT00113984.
Findings
We completed enrolment with 30 patients (24 of whom had not been previously treated with chemotherapy) and we did not identify any dose-limiting toxic effects. Grade 1 and 2 vaccination-site reactions were the most common toxic effects: three of 30 patients had grade 1 reactions and 26 had grade 2 reactions. 21 patients had grade 2 or greater immune-related adverse events. Grade 3 or 4 immune-related adverse events included diarrhoea or colitis in four patients and grade 3 rash (two patients), grade 3 raised aminotransferases (two patients), grade 3 endocrine immune-related adverse events (two patients), and grade 4 neutropenia (one patient). Only one of the six patients previously treated with chemotherapy had a PSA decline from baseline. Of the 24 patients who were chemotherapy-naive, 14 (58%) had PSA declines from baseline, of which six were greater than 50%.
Interpretation
The use of a vaccine targeting PSA that also enhances co-stimulation of the immune system did not seem to exacerbate the immune-related adverse events associated with ipilimumab. Randomised trials are needed to further assess clinical outcomes of the combination of ipilimumab and vaccine in mCRPC.
Introduction
Until recently, docetaxel with prednisone was the only standard treatment for patients with metastatic castration-resistant prostate cancer (mCRPC). This cancer results in more than 250 000 deaths worldwide each year, highlighting the need for additional treatments.1 However, several additional agents have recently emerged that improve survival in mCRPC.
One such agent, sipuleucel-T (Dendreon, Seattle, WA, USA), is a vaccine based on antigen-presenting cells that has shown an overall survival benefit in two phase 3 trials.2,3 On the basis of these findings, the US Food and Drug Administration (FDA) approved sipuleucel-T for the treatment of mCRPC.4
Another vaccine that has shown a survival advantage in mCRPC is PSA-Tricom (Prostvac, developed by the US National Cancer Institute [NCI], licensed to BN Immuno-therapeutics, Mountain View, CA, USA). This vector-based vaccine, which expresses transgenes for prostate-specific antigen (PSA) and three T-cell co-stimulatory molecules, showed an 8·5 month improvement in overall survival relative to placebo (p=0·006) in a multicentre randomised phase 2 trial.5 In a similar but smaller trial at the NCI, PSA-Tricom was shown to generate an antigen-specific immune response, which was associated with favourable survival outcomes.6 A multicentre phase 3 trial of PSA-Tricom in mCRPC is underway.7
On the basis of these findings, investigations are underway to further augment the immune response generated by these vaccines, with the goal of substantially enhancing clinical outcome. Among the strategies being assessed is combination therapy with agents that inhibit immune checkpoints that serve as the body’s natural mediators of immune response. Although cancer vaccines might induce an antigen-specific T-cell response, once activated, T cells upregulate cytotoxic T-lymphocyte-associated protein 4 (CTLA4), a negative regulatory molecule. Preclinical studies in mice have shown that CTLA4 blockade can delay turning off an immune response and increase T-cell avidity, leading to enhanced T-cell-mediated immune responses to the vaccine.8–10 The key role of CTLA4 in regulating immune response is evident in CTLA4 knockout mice, which cannot modulate immune responses. These animals live only 3–4 weeks before succumbing to massive organ infiltration by unchecked autoreactive T cells.11
Ipilimumab (Bristol-Myers Squibb, New York, NY, USA) is an antagonistic anti-CTLA4 monoclonal antibody that blocks the activity of CTLA4. Ipilimumab has been extensively studied in melanoma and has also been assessed in the treatment of prostate cancer, in which a minority (about 20%) of patients had significant PSA declines.12–14 A phase 3 randomised trial of ipilimumab in patients with metastatic melanoma showed a significant improvement in overall survival relative to an active control group.15
The PSA-Tricom vaccine is designed to enhance T-cell co-stimulation through enhanced expression of the transgenes of PSA and three T-cell co-stimulatory molecules (CD58, CD80, and ICAM1) on antigen-presenting cells engaging their respective ligands on T cells. CD80 is known to react with CD28 on T cells for positive co-stimulation, and CTLA4 for negative immune checkpoint inhibition. The antagonist monoclonal antibody anti-CTLA4 was designed to interrupt this negative signal and enhance immunity. It is thus unclear how a vaccine such as PSA-Tricom, with its positive co-stimulation, would interact in terms of safety and efficacy with an anti-CTLA4 monoclonal antibody designed to block negative co-stimulatory signals, especially in view of the immune-related adverse events noted in patients receiving anti-CTLA4 alone. Therefore, we designed a study to assess fixed doses of PSA-Tricom with escalating doses of ipilimumab, with the aim of establishing the safety and tolerability of these combined treatments.
Methods
Participants
Between August, 2005, and July, 2008, we did a phase 1 trial to assess the safety and tolerability of escalating doses of ipilimumab in combination with a fixed dose of the PSA-Tricom vaccine. All patients we enrolled had a histologically confirmed diagnosis of mCRPC. Patients had no bone pain that needed treatment with narcotics and we required them to have a life expectancy greater than 6 months and an Eastern Cooperative Oncology Group performance status of 0–1. We also required patients to have normal hepatic, renal, and haematological characteristics. Our exclusion criteria were any evidence or history of autoimmune disease, HIV or hepatitis B or C positivity, diseases needing systemic treatment with steroids, allergy to eggs, congestive heart failure, and known brain metastasis. Although we initially enrolled patients who had been previously treated with docetaxel, we restricted enrolment to chemotherapy-naive patients after the first six patients because of emerging data suggesting that patients treated with chemotherapy would be less likely to respond to the vaccine.16 All patients reviewed and signed an informed consent form approved by the NCI’s institutional review board.
Procedures
Our primary objective was to establish the safety and tolerability of the combination of a fixed dose of vaccine with an escalating dose of ipilimumab (1, 3, 5, and 10 mg). We used NCI Common Terminology Criteria for Adverse Events 3.0 to grade all toxic effects.17 We defined dose-limiting toxic effects in the dose-escalation phase as any grade 4 toxic effect, any grade 3 autoimmune event that did not resolve to a grade 2 or less within 21 days, or a grade 3 toxic effect that did not improve with adrenal corticosteroids or surgery. Our secondary objective was to assess immunological response (which we defined as an increase in PSA-specific T cells measured by enzyme-linked immunosorbent spot assay [ELISPOT] in patients with the HLA-A2 serotype) and clinical response (measured with Response Evaluation Criteria In Solid Tumors [version 1.0] and PSA consensus criteria).
A dose of 2×108 plaque-forming units of recombinant vaccinia PSA-Tricom was given subcutaneously as the initial priming vaccine on day 1 of cycle one, with subsequent monthly boosts of 1×109 plaque-forming units, starting on day 15. 100 µg per day of sargramostim (recombinant granulocyte-macrophage colony-stimulating factor [GM-CSF]) was given subcutaneously at the vaccination site for four consecutive daily doses starting the day of each vaccination. Ipilimumab was given at the assigned dose as a 90 min intravenous infusion on the same day as the recombinant fowlpox PSA-Tricom boosts. Our protocol initially allowed for only six courses of ipilimumab; however, a protocol amendment (approved Nov 26, 2007) gave patients with stable disease the option of additional ipilimumab every 3 months for a maximum of four additional doses. The maintenance dose of monthly vaccine could continue until there was evidence of disease progression on imaging studies or toxic effects that required discontinuation. All injections were given at the NIH Clinical Center (Bethesda, MD, USA).
We monitored patients with physical examination and laboratory tests of serum chemistries, complete blood count, and PSA on days 1 and 15, then monthly. Radiological studies including bone scan and CT scan of the chest, abdomen, and pelvis were done on about days 99 and 183, then every 3 months thereafter while the patient remained on study. We established progression on the basis of imaging alone, including a 20% increase in measurable lesions (longest dimension) on CT scans, or the development of one or more new lesions on CT or bone scan.
We collected immunological samples from patients with the HLA-A2 serotype via leucapheresis before vaccination and about 3 months after the start of treatment. We assessed the samples with ELISPOT for T-cell responses to tumour-associated antigens using a modification of the procedure described elsewhere,18 with K562/A*0201 as antigen-presenting cells. We used T-cell responses to influenza and HIV as positive and negative controls, respectively. We also assessed T-cell responses to the tumour-associated antigens PSA, MUC1, ANO7, and brachyury peptide RP2.
We assessed the presence of anti-PSA antibodies in the sera of patients before and after vaccination with ELISA. We incubated polyvinyl chloride 96-well microtitre plates (BD Biosciences, Franklin Lakes, NJ, USA) overnight at 4°C with a purified preparation of PSA (AspenBio Pharma, Castle Rock, CO, USA) and plates containing bovine serum albumin (BSA; pH 7 2) as negative controls. The wells were blocked for 1 h with phosphate-buffered saline (PBS) containing 5% BSA and then washed once with PBS containing 1% BSA (assay buffer). Patient sera and normal human serum were diluted starting at 1:50; three serial dilutions of 1:5 were done, with a final dilution of 1:6250. We used purified mouse anti-PSA immunoglobulin (IgG1) antibody (Fitzgerald Industries, Concord, MA, USA) as a positive control for PSA binding. We used an isotype-matched (MOPC-21) IgG1 antibody (Sigma-Aldrich, St Louis, MO, USA) as a negative control.
After incubation overnight at room temperature, we washed the wells four times with assay buffer, and we added 50 µL of a 1:4000 dilution of peroxidase-conjugated goat anti-human IgA plus IgG plus IgM (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA) to each well. We used a 1:4000 dilution of peroxidase-conjugated goat anti-mouse IgG (Kirkegaard and Perry) for the PSA antibody controls. After incubation at 37°C for 1 h, we washed the wells four times with assay buffer and added 100 µL of TMB substrate to each well. After a 15 min incubation at room temperature (in the dark), we stopped the reaction with 25 µL of 4N H2SO4. We measured the absorbance of each well at 450 nm with an ELISA microplate autoreader (Bio-Tek Instruments, Winooski, VT, USA).
Statistical analysis
We designed our trial as a dose-escalation study with standard methods: enrolling three patients at a dose if there were no patients with a dose-limiting toxic effect, or enrolling a total of six at a dose to ensure no more than one of six with a dose-limiting toxic effect. Our criteria for decision making regarding the escalation to a subsequent dose used any dose-limiting toxic effect from enrolment to 2 weeks after the second dose of ipilimumab (about day 56). We planned to assess four dose levels.
Assessment of safety was the primary outcome until we reached the 10 mg dose (our highest planned dose), but once at that dose we expanded the cohort to allow for a total of 15 patients to permit a determination, in a pilot fashion, that this dose was associated with a potentially useful clinical benefit. With 15 patients at this dose, the 95% CIs associated with two to five patients without progression by 6 months ranged from 1·7–40·5% (two of 15 without progression by 6 months) to 11·8–61·6% (five of 15 without progression by 6 months). As the purpose of this portion of our study was to estimate the proportion of patients without progression by 6 months, without formal hypothesis testing, we did not do an explicit power calculation. We did all analyses with SAS version 9.2.
We monitored toxic effects every 2 weeks for 1 month and then monthly. At the 10 mg dose, we monitored patients in groups of three for the cumulative incidence of dose-limiting toxic effects. If the cumulative incidence equalled or exceeded 33%, no further patients were to be enrolled at the 10 mg dose.
We deemed all other endpoints secondary, and we present these results as exploratory and hypothesis generating. The probabilities of survival and progression-free survival as a function of time were established with the Kaplan-Meier method, with 95% CIs around the median established with a reflected CI approach. All patients were included in the safety and efficacy analyses. This study is registered with ClinicalTrials.gov, number NCT00113984.
Role of the funding source
The sponsor of the study approved the study design, but had no role in data collection, data analysis, data interpretation, or writing of the report. RAM, MM, SMS, and JLG had access to the raw data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We enrolled 30 patients in four cohorts over the course of our study (table 1). Their baseline characteristics are shown in table 2. We did not record any dose-limiting toxic effects during the period we assessed dose-limiting toxic effects (to 2 weeks after the second infusion of ipilimumab) and thus did not expand any cohort for this reason. Toxic effects for our combination regimen were primarily local grade 1 and 2 injection-site reactions (three patients with grade 1 events, 26 with grade 2) and immune-related adverse events (table 3); the maximum tolerated dose was not exceeded in our study. Rash was the most common immune-related adverse event, noted mostly in patients receiving 10 mg/kg ipilimumab. Endocrine immune-related adverse events were more common at 5 and 10 mg/kg doses. However, we consistently noted grade 2 and 3 diarrhoea or colitis at all doses beyond the lowest (1 mg/kg). Other uncommon immune-related adverse events included raised concentrations of aminotransferases and neutropenia or leucopenia. Overall, 21 patients (70%) had a grade 2 or greater immune-related adverse event, and eight patients (27%) had grade 3 or 4 immune-related adverse events.
Table 1:
Recruitment of patients by cohort
| Dose of ipilimumab | Number of patients (number previously treated with chemotherapy) | |
|---|---|---|
| 1 | 1 mg/kg | 3 (3) |
| 2 | 3 mg/kg | 6 (3)* |
| 3 | 5 mg/kg | 6 (0)* |
| 4 | 10 mg/kg | 15 (0) |
Cohorts expanded to obtain more clinical and immunological data at these doses.
Table 2:
Baseline patient characteristics
| Characteristics | |
|---|---|
| Age (years) | 69(49–81) |
| Ethnic origin | |
| White | 28(93%) |
| African American | 2(7%) |
| On-study PSA (µg/L) | 49(3–729) |
| On-study PSA doubling time (months) | 2.3 (0.8–10.4) |
| Gleason score | 8 |
| Gleason 4–6 | 4(13%) |
| Gleason 7 | 6 (20%) |
| Gleason 8–10 | 20 (67%) |
| Alkaline phosphatase (U/L) | 78 5 (35–587) |
| Lactate dehydrogenase (U/L) | 183.5 (72–380) |
| Haemoglobin (g/L) | 130 (98–164) |
| Patients with visceral disease | 6 (20%) |
| Patients with bone disease | 28(93%) |
Data are n (%) or median (range). PSA=prostate-specific antigen.
Table 3:
Immune-related adverse events by dose and grade
| 1 mg/kg (n=3) |
3 mg/kg (n=6) |
5 mg/kg (n=6) |
10 mg/kg (n=15) |
Total (n=30) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 2 | Grade 3/4 | Grade 2 | Grade 3 | Grade 2 | Grade 3/4 | Grade 2–4 | |
| Diarrhoea or colitis | 0 | 0 | 1 | 1 (grade 3) | 1 | 1 | 2 | 2 (1 grade 3 plus 1 grade 4) | 8 |
| Rash | 0 | 0 | 1 | 0 | 1 | 0 | 6 | 2 (grade 3) | 10 |
| Panhypophysitis | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 (grade 3) | 4 |
| Hypothyroidism | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 4 |
| Adrenal insufficiency | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 (grade 3) | 3 |
| Raised aminotransferases | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 (grade 3) | 3 |
| Neutropenia | 0 | 0 | 0 | 1 (grade 4) | 1 | 0 | 0 | 0 | 2 |
| Total (proportion of ipilimumab cycles) | 0 (0%) | 0 (0%) | 2 (14%) | 2 (14%) | 7 (41%) | 2 (12%) | 13 (28%) | 8 (18%) | ·· |
| Total for grades 2–4 (proportion of ipilimumab cycles | ·· | 0 (0%) | ·· | 4 (28%) | ·· | 9 (53%) | ·· | 21 (46%) | 34 (40%) |
Data are number of events; some patients had more than one event.
There were no other non-immune-related grade 4 toxic effects, and the remaining grade 3 toxic effects were probably related to the accompanying immune-related adverse events in the same patient, including dehydration (two patients), hypotension (two patients), fatigue (two patients), hyponatraemia (one), hypophosphataemia (one), and fever (one). We recorded a grade 3 thrombocytopenia in a patient with colitis.
Overall, 14 patients discontinued ipilimumab because of disease progression (progressed before the planned initial six doses of ipilimumab), including seven of the nine patients in cohorts 1 and 2 (median 3 doses, range 1–4); 13 discontinued ipilimumab because of immune-related adverse events (median 2 doses, range 1–3); and three received all six planned initial doses. Of these toxic effects, six patients had immune-related adverse events in the first month of treatment. These adverse events included rashes in four patients (all grade 2) and two cases of diarrhoea or colitis (grade 2 and 3). With the exception of a rash at the 3 mg/kg dose, all other immune-related adverse events in the first month were at the 10 mg/kg dose.
The duration of all immune-related adverse events varied on the basis of toxic effects and individual patients. For endocrine-related toxic effects, we placed patients indefinitely on replacement hormones. Two patients were weaned off these treatments. We treated rashes with supportive measures for a median of 27 days (range 6–138) for ten patients who could be assessed. The duration of diarrhoea or colitis varied from 2 to 98 days, with a median of 32 days until resolution of symptoms to grade 1 or less. The three episodes of raised aminotransferases lasted for 3, 25, and 28 days, respectively, and the single episode of grade 4 neutropenia lasted 6 days.
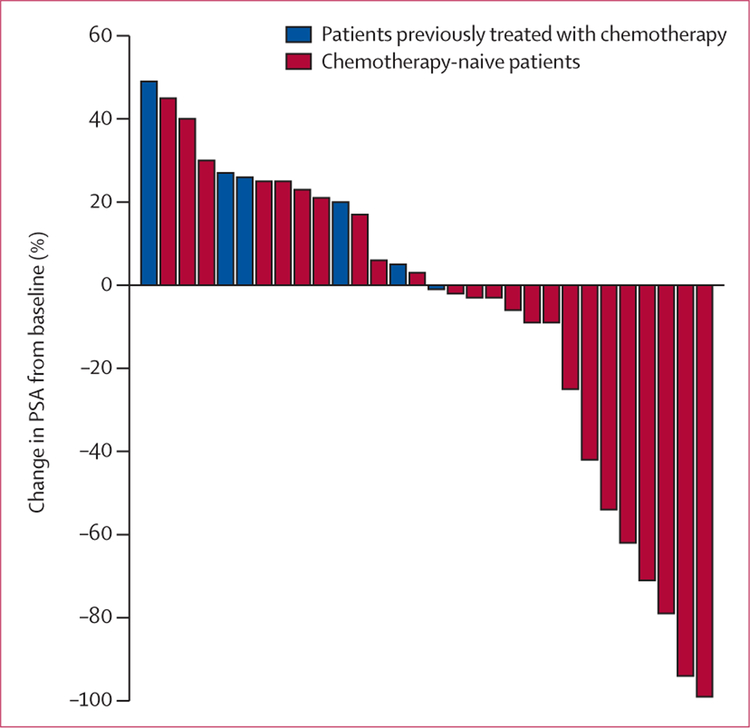
Only one of the six patients previously treated with chemotherapy (in cohorts 1 and 2) had a PSA decline. Of the remaining 24 patients who were chemotherapy-naive and treated with doses of 3, 5, and 10 mg/kg, 14 (58%) had PSA declines. Of these 24 patients, six (25%) had PSA declines greater than 50% (figure). To establish whether any changes in PSA were caused by production of PSA antibodies, we analysed serum before and after vaccination. No patients in our study produced detectable titres of anti-PSA antibodies. Although we cannot categorically exclude endocrine toxic effects from affecting serum PSA values, two patients with greater than 50% PSA declines had no endocrine immune-related adverse events (one patient had colitis and the other had no immune-related adverse events).
Figure: Best PSA response after treatment.
14 (58%) of 24 patients who were chemotherapy-naive had PSA declines; six (25%) had PSA declines greater than 50%. PSA=prostate-specific antigen.
Median progression-free survival for chemotherapy-naive patients was 5·9 months (95% CI 3·4–8·8), with a 1 year progression-free survival of 13·5% (4·7–33·0). For patients who had been previously treated with chemotherapy (n=6), median progression-free survival was 2·4 months (1·5–3·7); for all patients median progression-free survival was 3·9 months (3·3–6·3) and 1 year progression-free survival was 10·8% (3·8–27·3). There was no statistically significant difference in progression-free survival between chemotherapy-naive patients treated at the three lower doses (1, 3, and 5 mg/kg) compared with patients treated with 10 mg/kg (p=0·19; appendix). There was no apparent association between immune-related adverse events and progression-free survival.
Although patterns of radiographic response are unclear in immunological therapies, we identified three of 12 patients with measurable disease to have unconfirmed partial responses on CT imaging. Patient 4 (3 mg/kg) had a 30% reduction in target lesions at first restaging, patient 11 (3 mg/kg) had a 42% reduction at first restaging, and patient 12 (5 mg/kg) had a 36% reduction at second restaging. 18 patients developed new bone lesions while on study; seven of these patients also had new or increasing soft-tissue lesions. Nine patients had disease progression only in soft-tissue lesions while on study.
Median overall survival for all patients was 34·4 months (95% CI 29·6 to >41), with a 2 year overall survival of 73% (55·6–85·8). There was no significant difference in overall survival on the basis of dose (appendix). For the 24 chemotherapy-naive patients, median overall survival has not been reached and 3 year overall survival was 52·6% (31·4–72·9; appendix). For the six patients previously treated with chemotherapy, median overall survival was 31·3 months (4·8–41·4) and 3 year overall survival was 16·7% (3·0–56·4). There was no apparent association between immune-related adverse events and overall survival.
Nine patients who received doses of 3, 5, and 10 mg/kg of ipilimumab were positive for the HLA-A2 serotype and thus could be assessed for tumour-associated-antigen-specific ELISPOT T-cell responses (table 4). Two of three patients that could be assessed in the 10 mg/kg cohort had increased responses to PSA peptide after vaccination. The third patient in this cohort had PSA responses before and after vaccination.
Table 4:
T-cell-specific responses in patients positive for the HLA-A2 serotype by dose
| Influenza (control) | PSA peptide | MUC1 | Brachyury | ANO7 | HIV peptide | |
|---|---|---|---|---|---|---|
| 3 mg/kg | ||||||
| Patient 5 | ||||||
| Before | 1/7595 | <1/200000 | <1/200 000 | NA | NA | <1/200000 |
| Day 102 | 1/22 222 | <1/200000 | <1/200000 | NA | NA | <1/200000 |
| Patient 6 | ||||||
| Before | 1/9434 | <1/200000 | <1/200 000 | 1/120000 | NA | <1/200000 |
| Day 78 | 1/16667 | <1/200000 | 1/100000 | 1/46154 | NA | <1/200000 |
| Patient 7 | ||||||
| Before | 1/35294 | <1/200000 | <1/200 000 | NA | NA | <1/200000 |
| Day 70 | 1/17143 | <1/200000 | <1/200000 | NA | NA | <1/200000 |
| 5 mg/kg | ||||||
| Patient 8 | ||||||
| Before | 1/7692 | <1/200000 | <1/200 000 | 1/33333 | NA | <1/200000 |
| Day 93 | 1/10 638 | <1/200000 | <1/200000 | <1/200000 | NA | <1/200000 |
| Patient 10 | ||||||
| Before | 1/3226 | <1/200000 | <1/200 000 | <1/200000 | NA | <1/200000 |
| Day 118 | 1/4615 | <1/200000 | 1/85714 | <1/200000 | NA | <1/200000 |
| Patient 11 | ||||||
| Before | 1/50000 | <1/200000 | <1/200 000 | NA | NA | <1/200000 |
| Day 78 | 1/16667 | <1/200000 | <1/100000 | NA | NA | <1/200000 |
| 10 mg/kg | ||||||
| Patient 20 | ||||||
| Before | 1/25000 | <1/200000 | <1/200 000 | <1/200000 | <1/200 000 | <1/200000 |
| Day 94 | 1/30 000 | 1/150000 | 1/66 667 | 1/46154 | 1/26087 | <1/200000 |
| Patient 21 | ||||||
| Before | 1/62 500 | <1/200000 | <1/200 000 | <1/200000 | <1/200 000 | <1/200000 |
| Day 97 | 1/15625 | 1/40000 | 1/100000 | 1/41667 | 1/125000 | <1/200000 |
| Patient 22 | ||||||
| Before | 1/19231 | 1/29412 | <1/200 000 | 1/83333 | <1/200 000 | <1/200000 |
| Day 78 | 1/10714 | 1/46154 | <1/200000 | <1/200000 | <1/200 000 | <1/200000 |
Dose refers to dose of ipilimumab. PSA=prostate-specific antigen.
We and others have previously described antigen cascade or epitope spreading whereby hosts develop post-vaccination immune responses to tumour-associated antigens that are not present in the vaccine, probably through the process of cross-priming.18–20 One patient at the 3 mg/kg dose had a post-vaccination increase in T-cell-specific responses to the tumour-associated antigens MUC1 and brachyury, a transcription factor implicated in the epithelial-to-mesenchymal transition pathway.21,22 Two patients at the 5 mg/kg dose had increases in antigen-specific responses to MUC1, another antigen overexpressed in most patients with prostate cancer.23 Two of three patients at the 10 mg/kg dose had broad responses to all four tumour-associated antigens that we assessed. We assessed sera for anti-PSA antibodies by ELISA from 29 patients obtained before vaccination and about 3 months after the start of treatment, and all were negative.
Discussion
As expected, the range of toxic effects we identified exceeded those in single-agent studies involving PSA-Tricom, where grade 1 or 2 local injection-site reactions were most common.2,6,24 However, the proportion of patients affected by grade 3–4 toxic effects in our study (eight [27%] of 30 patients) was similar to previous phase 1 trials involving ipilimumab (panel).25–27 Our data suggest that the combination of a vaccine that enhances immune co-stimulation with an immune checkpoint inhibitor does not seem to be associated with increased immune-related adverse events compared with ipilimumab alone.
In total, 21 patients in our study had grade 2 or greater immune-related adverse events, with eight patients having grade 3 or 4 events. The most common immune-related adverse event was rash, which was more common at the highest doses (5 and 10 mg/kg). Although the rash was often self-limiting, topical or oral steroids were sometimes needed. Colitis, usually presenting as diarrhoea, was the most challenging immune-related adverse event, in view of the risk of perforation. We admitted patients who presented with clinical signs of colitis for observation, maintained without oral intake, and treated with oral or intravenous steroids (≤1 mg methylprednisolone twice daily) until symptoms resolved. If symptoms persisted beyond 2 or 3 days, we did a colonoscopy to confirm diagnosis.
Endocrine-related side-effects were generally diagnosed clinically and with diagnostic laboratory tests. Subsequent appropriate replacement therapy rectified the symptoms within days for most patients. Less common immune-related adverse events included hepatotoxicity and neutropenia. We doubt that the one case of grade 4 transient neutropenia was attributable to treatment, having happened 3 months after ipilimumab was discontinued for suspected colitis. However, we did not establish any other clearly attributable cause after thorough assessment, including bone-marrow biopsy.
We allowed patients who needed systemic steroids for immune-related adverse events to resume vaccine alone 4 weeks after steroids were discontinued. We discontinued ipilimumab for all immune-related adverse events except mild rash or those of endocrine origin that were clinically stable on endocrine replacement. Since the treatment for endocrine immune-related adverse events consisted of indefinite replacement therapy, we deemed it unlikely that ipilimumab would cause any additional clinical symptoms relative to the affected organ; therefore, we gave patients the option to continue ipilimumab if they were clinically stable on a replacement dose of hormones (eg, levothyroxine). Grade 2 rashes often abated without recurrence on repeat exposure to ipilimumab. Although systemic steroids could have affected PSA in patients with immune-related adverse events, such brief treatments would not have had a meaningful effect on survival.
A similar study of a whole-tumour cell cancer vaccine (GVAX) plus ipilimumab for the treatment of mCRPC also yielded immune-related adverse events, including hypophysitis.28 There was a suggestion that the immune-related adverse events in the GVAX study were associated with clinical responses; however, no distinct similarity was evident in our study. Nevertheless, the median overall survival of longer than 34 months in our study is noteworthy, but because two-thirds of patients experienced a grade 2 or greater immune-related adverse event, an association between overall survival and immune-related adverse events could not be identified.
The magnitude of immune response and PSA declines in our study is similar to what has been previously reported in trials with PSA-Tricom. We did not detect any anti-PSA antibodies, suggesting that this did not affect PSA declines. Indeed, this vaccine, with multiple T-cell co-stimulatory molecules, is designed to generate a cellular immune response. Only nine patients were positive for the HLA-A2 serotype and thus could be assessed for antigen-specific immune responses. Six of those nine patients had antigen-specific T-cell responses, which is similar to a previous phase 2 study in chemotherapy-naive patients,6 where 12 of 29 patients that could be assessed had PSA-specific immune responses. Also consistent with previous studies of this and other therapeutic cancer vaccines, only a minority of patients had significant PSA declines, which might be a class effect due to the delayed effects of immune-based therapies.3,5,6,29
Previous studies have reported that patients who have recently had chemotherapy are less likely to mount immune responses after treatment with a therapeutic cancer vaccine.16 Thus, it is possible that patients in our study who had received previous chemotherapy and were then treated at the lower doses were less likely to respond to an immune-based regimen. Chemotherapy-naive patients enrolled at the higher doses (5 and 10 mg/kg) were more likely to have PSA declines and longer progression-free and overall survival than were patients who had previously received chemotherapy, although these findings were not statistically significant. Furthermore, the broadest tumour-associated-antigen-specific T-cell responses, and those of the greatest magnitude, were at the 10 mg/kg dose. It is also difficult to establish if higher doses induced a greater immune response, because we assessed a few patients for T-cell responses. Consistent with previous studies,12 more toxic effects were evident at higher doses, although, unlike other immune-related adverse events, diarrhoea or colitis was consistently evident at doses of 3, 5, and 10 mg/kg.
In our trial, we also gave 100 µg per day GM-CSF at the vaccine site as an immune adjuvant. A previous study in patients with biochemical recurrence and non-metastatic disease gave 250 µg/m2 daily for 14 of every 28 days. Although a few patients might have clinically benefited on the basis of stable or decreased PSA concentrations, in view of the lower dose and brief administration, we do not feel that GM-CSF had a substantial, independent therapeutic role.30 The ultimate value of GM-CSF with PSA-Tricom will be assessed prospectively in a phase 3 trial.7
Our findings provide the proof of concept for use of an immune checkpoint inhibitor in combination with a therapeutic cancer vaccine designed to enhance co-stimulation of the immune system. Although our trial was small and non-randomised, the median overall survival noted raises the possibility that the combination of fixed initial doses of ipilimumab and PSA-Tricom vaccine might result in prolonged overall survival. Furthermore, this study might provide the rationale for the combination of vaccine and monoclonal antibodies against PD-1, an immune checkpoint inhibitor that might have fewer associated toxic effects than ipilimumab, as suggested in preliminary trials.31 Randomised trials are needed to confirm the preliminary evidence of benefit of this type of combined immunotherapy.
Supplementary Material
Panel: Research in context.
Systematic review
We systematically reviewed published work (via PubMed) as part of the planning of our trial. Our search terms were “anti-CTLA4”, “vaccine”, and “immune check-point”; we did our search up to August, 2005, and it was not limited by language. Ipilimumab was identified as a potential immune-stimulating agent that could be complemented by an immune-stimulating therapeutic cancer vaccine, such as PSA-Tricom, with a non-overlapping profile of toxic effects. Our laboratory previously reported on the combination of poxviral vaccines containing multiple co-stimulatory molecules (Tricom) along with CTLA4 blockade in mice. This showed improved antitumour effect and improved avidity of antigen-specific T cells. Furthermore, therapeutic cancer vaccines have yielded improved survival in metastatic prostate cancer in phase 2 and phase 3 studies. Ipilimumab has been shown to improve survival in melanoma.
Interpretation
Our trial provides safety data and preliminary evidence of clinical benefit that could be the rationale to combine two forms of modern immune-based therapies. Our findings suggest that a vaccine designed to enhance positive co-stimulation can be combined with a therapy that blocks negative co-stimulation without more clinically significant or synergistic toxic effects. A large, randomised trial assessing the combination of a therapeutic cancer vaccine and an immune checkpoint inhibitor is still needed before the standard of care will be changed for patients with prostate cancer.
Acknowledgments
We acknowledge the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, for its support of this study. We also express appreciation to the professionals at the NIH Clinical Center Blood Bank for their part in apheresis procedures for study patients, and to the medical oncology fellows at the National Cancer Institute for their attention to patient care. We thank Bonnie L Casey and Debra Weingarten for their editorial assistance in the preparation of this report.
Funding US National Institutes of Health.
Footnotes
Conflicts of interest
We declare that we have no conflicts of interest.
See Online for appendix
Contributor Information
Ravi A Madan, Laboratory of Tumor Immunology and Biology, Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Mahsa Mohebtash, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Philip M Arlen, Laboratory of Tumor Immunology and Biology, Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Matteo Vergati, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Myrna Rauckhorst, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Seth M Steinberg, Biostatistics and Data Management Section, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Kwong YTsang, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Diane J Poole, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Howard L Parnes, Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
John J Wright, Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
William L Dahut, Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Jeffrey Schlom, Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
James L Gulley, Laboratory of Tumor Immunology and Biology, Medical Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006; 24: 3089–94. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–22. [DOI] [PubMed] [Google Scholar]
- 4.FDA. Highlights of prescribing information for PROVENGE (sipuleucel-T) http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM210031.pdf (accessed Jan 24, 2012).
- 5.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28: 1099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010; 59: 663–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bavarian Nordic. Prostvac: therapeutic vaccine candidate for the treatment of advanced prostate cancer http://www.bavarian-nordic.com/pipeline/prostvac.aspx (accessed Jan 24, 2012).
- 8.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002; 3: 611–18. [DOI] [PubMed] [Google Scholar]
- 9.Allison JP, Chambers C, Hurwitz A, et al. A role for CTLA-4-mediated inhibitory signals in peripheral T cell tolerance? Novartis Found Symp 1998; 215: 92–98. [DOI] [PubMed] [Google Scholar]
- 10.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol 2005; 174: 5994–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science 1995; 270: 985–88. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11: 155–64. [DOI] [PubMed] [Google Scholar]
- 13.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 2007; 13: 1810–15. [DOI] [PubMed] [Google Scholar]
- 14.Beer T, Slovin S, Higano CS, et al. Phase I trial of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration resistant prostate cancer (mCRPC). Proc Soc Am Clin Oncol 2008; 26 (suppl): abstr 5004. [Google Scholar]
- 15.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res 2001; 7: 1181–91. [PubMed] [Google Scholar]
- 17.NCI. Common terminology criteria for adverse events v3.0 http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed Jan 11, 2012).
- 18.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res 2005; 11: 3353–62. [DOI] [PubMed] [Google Scholar]
- 19.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol 2009; 27: 4685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res 2005; 11: 2416–26. [DOI] [PubMed] [Google Scholar]
- 21.Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res 2007; 13: 2471–78. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar D, Shields B, Davies ML, Müller J, Wakeman JA. Brachyury confers cancer stem cell characteristics on colorectal cancer cells. Int J Cancer 2012; 130: 328–37. [DOI] [PubMed] [Google Scholar]
- 23.Kirschenbaum A, Itzkowitz SH, Wang JP, Yao S, Eliashvili M, Levine AC. MUC1 expression in prostate carcinoma: correlation with grade and stage. Mol Urol 1999; 3: 163–68. [PubMed] [Google Scholar]
- 24.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 2007; 178: 1515–20. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol 2005; 23: 741–50. [DOI] [PubMed] [Google Scholar]
- 26.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 2003; 100: 8372–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008; 26: 5950–56. [DOI] [PubMed] [Google Scholar]
- 28.van den Eertwegh AJM, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012; published online Feb 10. DOI: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 29.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 2010; 15: 969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rini BI, Fong L, Weinberg V, Kavanaugh B, Small EJ. Clinical and immunological characteristics of patients with serologic progression of prostate cancer achieving long-term disease control with granulocyte-macrophage colony-stimulating factor. J Urol 2006; 175: 2087–91. [DOI] [PubMed] [Google Scholar]
- 31.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.