Abstract
Background:
Attention Deficit Hyperactivity Disorder (ADHD) is the most common neurobehavioral disorder in children, yet its etiology is poorly understood. Early thyroid hormone disruption may contribute to the development of ADHD. Disrupted maternal thyroid hormone function has been associated with adverse neurodevelopmental outcomes in children. Among newborns, early-treated congenital hypothyroidism has been consistently associated with later cognitive deficits.
Methods:
We systematically reviewed literature on the association between maternal or neonatal thyroid hormones and ADHD diagnosis or symptoms. We searched Embase, Pubmed, Cinahl, PsycInfo, ERIC, Medline, Scopus, and Web of Science for articles published or available ahead of print as of April 2018.
Results:
We identified 28 eligible articles: 16 studies of maternal thyroid hormones, seven studies of early-treated congenital hypothyroidism, and five studies of neonatal thyroid hormones. The studies provide moderate evidence for an association between maternal thyroid hormone levels and offspring ADHD, some evidence for an association between early-treated congenital hypothyroidism and ADHD, and little evidence for an association between neonatal thyroid hormone levels and later ADHD.
Conclusions:
The reviewed articles suggest an association between maternal thyroid function and ADHD, and possibly between early-treated congenital hypothyroidism and ADHD. Study limitations, however, weaken the conclusions in our systematic review, underlining the need for more research. Importantly, there was much variation in measurement of thyroid hormone function and of ADHD symptoms. Recommendations for future research include using population-based designs, attending to measurement issues for thyroid hormones and ADHD, considering biologically relevant covariates (e.g., iodine intake), and assessing non-linear dose–responses.
Keywords: Attention Deficit Hyperactivity Disorder, Thyroid Hormones, Congenital Hypothyroidism, Child Development, Maternal-Fetal Exchange, Thyroid Diseases, Systematic Review
INTRODUCTION
Attention-Deficit Hyperactivity Disorder (ADHD), characterized by impulsivity, inattention, and hyperactivity1, is the most common childhood neurobehavioral disorder, affecting approximately 136 million children and adolescents worldwide (5.6%).2 Associated with significant distress and pervasive social and academic impairment, ADHD can persist into adulthood, resulting in higher risks for incarceration, employment difficulties, and substance use disorders.3–5
ADHD has a strong genetic component, with heritability estimates of 70%−90%.6,7 However, substantial variation in the disorder may be attributable to non-genetic (environmental) risk factors and their interplay with genetic risk factors. There is not yet strong evidence identifying non-genetic risk factors as causal.8 Characterizing non-genetic causal pathways to ADHD is crucial because some may be preventable. This systematic review focused on one hypothesized non-genetic pathway to ADHD: the influence of thyroid hormones on brain development.
Variation in the lower and higher range of maternal and neonatal thyroid hormone levels may be associated with ADHD.9–15 Thyroid hormones are essential for brain development during intrauterine and early life: thyroxine (T4) and the biologically more active hormone triiodothyronine (T3) regulate the expression of genes that influence neural migration, differentiation, myelination, and synaptogenesis.16 Thyroid stimulating hormone (TSH) initiates thyroid hormone synthesis in the thyroid gland, and TSH release is influenced by circulating T3 and T4 in a negative feedback system. Thyroid peroxidase antibodies (TPOAb) inhibit the synthesis of thyroid hormones. Any perturbations of the thyroid hormone system (e.g. by thyroid diseases including thyroid autoimmunity, low iodine intake, or thyroid disrupting toxicants) could negatively affect thyroid hormone action in the developing brain, potentially leading to irreversible cognitive deficits.16–20
Thyroid function tests are typically conducted on serum using immunoassays, and thyroid dysfunction is classified according to population- or laboratory-specific reference ranges of TSH and free T4 (fT4) or free T3 (fT3; free refers to unbound). The most common form of thyroid dysfunction during pregnancy is hypothyroidism (elevated TSH and suppressed fT4).21,22 Overt and subclinical hypothyroidism (elevated TSH with normal fT4) occur in approximately 0.5% (overt) and 2.0% to 3.0% (subclinical) of pregnant women.22–24 Approximately 1.3% of pregnant women experience hypothyroxinemia (normal TSH levels and low fT4).25 Hyperthyroidism (suppressed TSH and elevated fT4 and/or fT3) affects 0.1%−0.4% of pregnancies.22,24,26 During the first trimester of pregnancy, 10%−20% of women who present as euthyroid based on TSH and fT4 levels are positive for thyroid hormone antibodies, including TPOAb and thyroglobulin antibodies.22 Factors associated with thyroid function include iodine intake, diet quality, age, depression and anxiety, alcohol use, smoking, and body mass index,27–34 among others.
During prenatal neurodevelopment, the fetus is unable to synthesize its own thyroid hormones until approximately mid-gestation. Until mid-gestation, the fetus must rely on maternal thyroid hormone production and transport across the placenta and fetal blood–brain barrier.35,36 The fetus continues being partly reliant on maternal thyroid hormones until it is self-sufficient at term. Thus, especially during the first 20 weeks of gestation, healthy maternal thyroid function is crucial for fetal brain development.
Neural myelination and synaptogenesis continue after birth,37 and thyroid hormone levels during early neonatal life are critical for healthy neurodevelopment. Congenital hypothyroidism, a rare thyroid hormone deficiency at birth,38 is a prime example of the importance of thyroid hormones in neurocognitive development: untreated congenital hypothyroidism leads to severe intellectual disability.39 Similarly, prenatal thyroid disease (e.g., hypothyroidism) and even subclinical maternal thyroid dysfunction has been linked to adverse neurocognitive outcomes (e.g. lowered intelligence).40,41
In this paper, we systematically reviewed studies investigating the relationship between maternal or neonatal thyroid hormones and ADHD and related symptoms in children.
METHODS
We searched Embase, Pubmed, Cinahl, PsycInfo, ERIC, Medline, Scopus, and Web of Science for English articles published or available ahead of print as of April 2018. We combined search terms for maternal or child thyroid hormone function (e.g. thyrotropin, maternal hypothyroxinemia, thyroid function, congenital hypothyroidism) with search terms for ADHD symptoms (e.g. inattention, hyperactivity, impulsivity; eAppendix 1). In addition to “free” search terms, we used corresponding “subject headings” for Medline and Embase. We searched reference lists of relevant review articles.
Search procedure
After removing duplicates, we screened titles and abstracts for eligibility and evaluated the full text of remaining articles. Two authors independently followed the search procedure and abstracted study details; discrepancies in exclusion decisions or abstracted details were discussed with a third author until we reached consensus.
Inclusion criteria
Eligible articles:
Included human participants.
Assessed thyroid hormone concentrations or clinical thyroid diagnoses during pregnancy or in infants up to 6 months of age.
Assessed ADHD or its symptoms, defined as a diagnosis of ADHD, prescription for ADHD medications, or measures that evaluated ADHD symptoms (e.g., inattention, hyperactivity, or impulsivity), before 19 years of age.
Related thyroid hormone measures to ADHD symptoms.
Had an observational design, were not evaluations of ADHD treatments or clinical case studies.
Were full-length articles reporting original research.
RESULTS
The original search yielded 925 records (730 after duplicates were removed). Of the 730 articles, we excluded 669 after title and abstract review. Full text review resulted in the exclusion of 38 articles, yielding 23 eligible studies (Figure). The updated search yielded an additional five articles (Figure), for a total of 28 papers.
Figure.
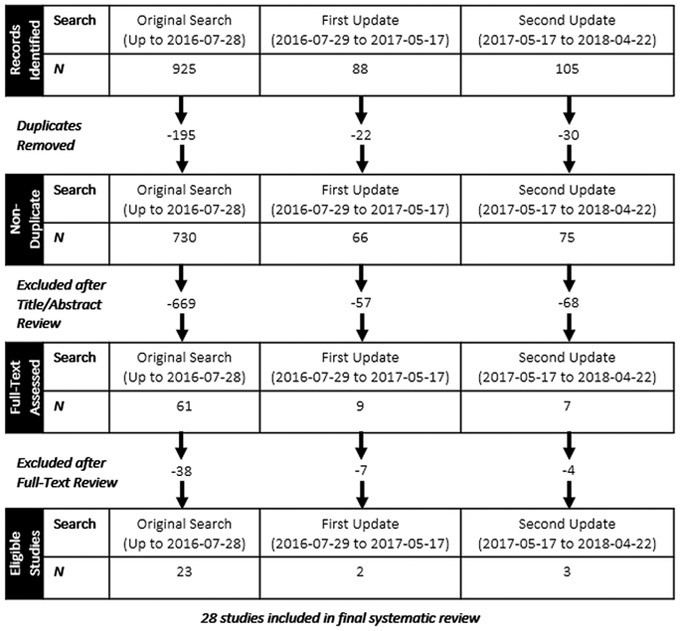
Summary of study selection for systematic review.
Clinical Maternal Thyroid Hormone Disorders
Maternal Hypothyroxinemia.
Four of the six studies of maternal hypothyroxinemia and ADHD used large population-based prospective birth cohorts (Table 1):9,42–44 Modesto et al. (2015) and Ghassabian et al. (2014) used the Dutch Generation R cohort (Ntotal=3783, Ntotal=3903, respectively);9,42 Päkkilä et al. (2014) used the Northern Finland Birth Cohort (Ntotal=5131);43 Oostenbroek et al. (2017) used the Amsterdam Born Children and their Development (Ntotal=2000).44 Andersen et al. (2018) nested a case–cohort study within the Danish National Birth Cohort (Ncases=1143, Ncontrols=7624 controls).45 Finally, Vermiglio et al. (2004) studied a small cohort in Italy (Ntotal=27).46
Table 1.
Studies of maternal thyroid hormones or dysfunction and offspring ADHD or related behaviors.
| First Author, Year Country |
Study Design (Sample Size) | Thyroid Function Measurement (Timing) | Outcome Measurement (Timing) | Adjustment Seta |
|---|---|---|---|---|
|
Andersen, 201845 Denmark |
Case-cohort (Ncases = 1143, Ncontrols = 7624) | TSH and fT4 measured using automated immunoassay (median of 9 weeks’ gestation, range 5-19 weeks) | 2+ filled prescriptions for ADHD medication based on National Registers (median age of 8.4 yrs) | 5, 8, 10, 12, 15, 18, 20, 45, 46 |
|
Andersen, 201448 Denmark |
Population-based prospective cohort (Ntotal = 857014) |
Maternal hyperthyroidism or hypothyroidism based on hospital diagnosis or prescription (diagnosed before or after birth) | ICD-10 diagnosis of ADHD, dispensed prescription of ADHD medication (3–18 yrs) | 2, 8, 9, 10, 11, 12, 26, 45, 46 |
|
Chevrier, 201151 USA |
Prospective birth cohort (Ntotal = 287) | TSH and TT4 measured using immunochemiluminometric assay; fT4 measured by direct equilibrium dialysis then radioimmunoassay (26.9 ±3.4 weeks gestation) | McCarthy memory tests, Child Behavior Checklist completed by mothers, Conner’s Kiddie Continuous Performance Test (5 yrs) | 1, 3, 8, 10, 19, 21, 23, 26, 35, 36, 37, 38, 39, 40, 41, 47 |
|
Endendijk, 201752 The Netherlands |
Prospective birth cohort (Ntotal = 442) | Serum TSH, fT4, and TPOAb measured by immunoassay (12, 24, and 36 weeks’ gestation) | Child Behavior Checklist completed by mothers and fathers (23-60 mos) | 1, 5, 8, 12, 13, 14, 15, 18, 22, 27, 33, 32, 42, 43, 44, 45 |
|
Ghassabian, 201442 The Netherlands |
Population-based prospective cohort (Ntotal = 3903) | Maternal hypothyroxinemia based on immunoassay TSH (<18 weeks gestation) | Child Behavior Checklist filled out by parents (6 yrs) | 2, 5, 8, 13, 42 |
|
Ghassabian, 201212 The Netherlands |
Population-based prospective cohort (Ntotal = 3139) | Maternal TPOAb based on immunoassay (7.9 – 17.9 weeks gestation) | Child Behavior Checklist filled out by parents (3 yrs) | 2, 8, 14, 15, 16, 33 |
|
Ghassabian, 201113 The Netherlands |
Population-based prospective cohort (Ntotal = 3736) | Maternal TSH based on immunoassay (13.3±1.7 weeks gestation) | Child Behavior Checklist filled out by parents (1.5, 3 yrs) | 1, 2, 5, 8, 13, 14, 15, 16, 17, 18, 19, 33, 42 |
|
Haddow, 199911 USA |
Prospective cohort (Ntotal = 186) | Maternal hypothyroidism based on TSH and fT4 immunoassay (second trimester gestation) | Conners’ Continuous Performance Test, WISC-III freedom from distractibility (7-9 yrs) | None |
|
Instanes 201610 Norway |
Population-based case-control (Ncases = 47 944, Ncontrols = 2 274 713) | Maternal hyperthyroidism or hypothyroidism based on medical registry diagnoses (Before or during pregnancy) | ADHD medication prescription (3+ yrs) | 1, 5, 8, 11, 12, 13, 46 |
|
Komendova, 201850 Czech Republic |
Cohort selected based on maternal thyroid hormone status (Ntotal = 192) | Maternal thyroid dysfunction based on serum TSH and TPOAb immunoassay (first trimester) | WISC-III freedom from distractibility (6-9 yrs). | None |
|
Modesto, 20159 The Netherlands |
Population-based prospective cohort (Ntotal = 3873) | Maternal hypothyroxinemia or subclinical hypothyroidism based on TSH and fT4 immunoassay (13.6±1.9 weeks gestation) | Conners’ Parent Rating Scale–Revised Short Form (8 yrs) | 2, 5, 8, 11, 12, 14, 15, 16, 18, 20, 26, 42, 45 |
|
Oostenbroek, 201744 The Netherlands |
Population-based prospective cohort (Ntotal = 2000) | Maternal hypothyroxinemia based on TSH and fT4 immunoassay (Median: 12.9 weeks gestation) | Strengths and Difficulties Questionnaire (5.1±0.2 years) | 5, 14, 15, 18, 20, 32, 33, 34, 45 |
|
Päkkilä, 201549 Finland |
Population-based prospective cohort (Ntotal = 4357 to 4370 depending on analysis) | Maternal thyroid dysfunction based on TSH, fT4, and TPOAb immunoassay (10.7±2.8 weeks gestation) | The Strengths and Weaknesses of ADHD Symptoms and Normal Behavior filled out by parents (16 yrs) | 1, 2, 3, 4, 8, 15, 21, 45 |
|
Päkkilä, 201443 Finland |
Population-based prospective cohort (Ntotal = 5131) | Maternal hypothyroxinemia based on TSH and fT4 immunoassay, and TPOAb levels using immunoassay (10.7±2.8 weeks gestation) |
Rutter B2 Scale –Finnish version filled out by parents (8 yrs) | 2, 4, 5, 8, 15, 21, 45 |
|
Samadi, 201547 Canada |
Cohort selected based on maternal thyroid hormone status (Ntotal = 44) | Maternal hypothyroidism based on medical records of diagnoses (during pregnancy) | Behavioral Rating Inventory of Executive Function filled out by parents (9-14 yrs) | None |
|
Vermiglio, 200446 Italy |
Cohort selected based on geographic region (Ntotal = 27) | Maternal hypothyroxinemia based on TSH and fT4 immunoassay (5-10, 11-14, 18-20 weeks gestation) | Clinical observation and diagnosis for ADHD, questionnaires based on DSM-IV-TR items (8-10 yrs) | 4 |
Key for adjustment set: 1 = gestational age at birth; 2 = child sex; 3 = SES indices; 4 = child intellectual disability; 5 = parental education; 6 = school term; 7 = evaluator psychologist; 8 = maternal age; 9 = maternal origin; 10 = maternal residence; 11 = maternal marital status; 12 = maternal parity; 13 = birth weight; 14 = ethnicity; 15 = maternal smoking; 16 = time of thyroid sampling during pregnancy; 17 = mode of birth delivery; 18 = maternal psychopathology; 19 = child apgar score; 20 = maternal weight/BMI; 21 = number of children in family; 22 = maternal breastfeeding; 23 = comorbid diagnoses; 24 = concomitant medication treatments; 25 = maternal bipolar subtype; 26 = income; 27 = alcohol use; 28 = protein intake; 29 = prenatal corticosteroids; 30 = bilirubin; 31 = sepsis; 32 = congenital anomalies; 33 = maternal use of thyroid medications; 34 = maternal hypertension; 35 = country of birth; 36 = diet quality index; 37 = home density and/or family structure; 38 = season of assessment; 39 = delivery complications; 40 = maternal vocabulary; 41 = child hospitalization; 42 = child age at testing; 43 = maternal diabetes I; 44 = assisted reproductive technology (e.g. hormone stimulation); 45 = multiple births; 46 = year of birth/birthday; 47 = maternal language.
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder, DSM = Diagnostic and Statistical Manual of Mental Disorders, fT4 = Free thyroxine, ICD = International Classification of Diseases, mos = months, TPOAb = Thyroid peroxidase antibodies, TSH = Thyroid stimulating hormone, WISC = Wechsler Intelligence Scale for Children, yrs = years.
The adjustment set includes variables that were statistically controlled for in analyses included in this review, or were assessed as potential covariates for analyses included in this review, as well as exclusion or restriction factors that were relevant for this review.
All six papers defined maternal hypothyroxinemia as TSH levels within given reference ranges and/or low fT4 measured from maternal blood sampled before 20 weeks’ gestation (eAppendix 2). All six studies used immunoassay to measure fT4.
The five larger studies adjusted for covariates including child sex, maternal age, and maternal smoking, among others (Table 1). Vermiglio et al. (2004) screened for intellectual disability but did not adjust for any covariates.
ADHD related behaviors in the four population-based prospective birth cohorts were assessed using screening instruments filled out by parents or teachers when children were 5–10 years old (Tables 1, 2).9,42–44 Andersen et al. (2018) measured their outcome as at least two ADHD medication prescription fills (based on national registers) in children with a median age of 8.4 years.45 Vermiglio et al. (2004) clinically evaluated ADHD using DSM-IV guidelines when children were 8–10 years old.46
Table 2.
Estimated associations between maternal thyroid disorders and offspring ADHD or related behaviors.
| Estimated associations between maternal thyroid disorders and ADHD or related behaviors | ||
|---|---|---|
| Exposure | Outcome (Time) | Estimate |
| Hypothyroxinemia | ||
| Andersen 201845 | ADHD prescription (8.4 yrs median) | HRoverall = 1.46 (1.04, 2.06) |
| HRboys = 1.16 (0.76, 1.77) | ||
| HRgirls = 2.28 (1.21, 4.29) | ||
| Oostenbroek, 201744 | Teacher SDQ hyperactivity/inattention (5 yrs) | ORft4<10th %tile = 1.47 (0.99, 2.20) |
| ORft4<5th %tile = 1.70 (1.01, 2.86) | ||
| Parent SDQ hyperactivity/inattention (5 yrs) | ORft4<10th %tile = 0.85 (0.50, 1.46) | |
| ORft4<5th %tile = 0.78 (0.36, 1.66) | ||
| Modesto 20159 | CPRS ADHD symptoms (8 yrs) | β = 0.07 (0.003, 0.14) |
| Vermiglio 200446 | DSM-IV ADHD diagnosis (8-10 yrs) | χ2 = 2.34, p = .001 |
| Ghassabian 201442 | CBCL ADHD symptoms (6 yrs) | β = 0.12 (−0.04, 0.27), p = 0.14 |
| Päkkilä 201443 | RB2 inattention & hyperactivity (8 yrs) | ORboys = 0.61 (0.18, 2.03) |
| RB2 inattention & hyperactivity (8 yrs) | No exposed girls with combined symptoms estimate | |
| Hypothyroidism | ||
| Andersen 201845 | ADHD prescription (8.4 yrs median) | HR = 1.05 (0.77, 1.43) |
| Instanes 201610 | ADHD prescription (3+ yrs) | OR = 1.2 (1, 1.4) |
| Samadi 201547 | BRIEF impaired working memory (9-14 yrs) | MD = 6.84, p = 0.067a |
| Haddow 199911 | CPT distractibility (7-9 yrs) | MD = −3, p = 0.08 |
| CPT impaired sustained attention (7-9 yrs) | OR = 3 (1, 5) | |
| Overt Hypothyroidism | ||
| Andersen 201845 | ADHD prescription (8.4 yrs median) | HR = 0.98 (0.44, 2.20) |
| Subclinical Hypothyroidism | ||
| Andersen 201845 | ADHD prescription (8.4 yrs median) | HR = 1.06 (0.76, 1.48) |
| Modesto 20159 | CPRS ADHD symptoms (8 yrs) | β = −0.01 (−0.07, 0.05) |
| Thyroid dysfunction | ||
| Komendova, 201850 | WISC-III freedom from distractibility (6-9 yrs) | MD = 0.38 (−4.76, 5.52) |
| Päkkilä 201549 | SWAN ADHD symptoms (16 yrs) | NS |
| Hyperthyroidism | ||
| Andersen 201845 | ADHD prescription (8.4 yrs median) | HR = 1.11 (0.79, 1.57) |
| Instanes 201610 | ADHD prescription (3+ years) | OR = 1.2 (0.9, 1.5) |
| Andersen 201448 | ICD-10 ADHD prescription/diagnosis (3-18 yrs) | NS based on figure 3 |
| Overt Hyperthyroidism | ||
| Andersen 201845 | ADHD prescription (8.4 yrs median) | HR = 0.93 (0.53, 1.62) |
| Subclinical Hyperthyroidism | ||
| Andersen 201845 | ADHD prescription (8.4 yrs median) | HR = 1.24 (0.81, 1.90) |
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder, DSM = Diagnostic and Statistical Manual of Mental Disorders, BRIEF = Behavioral Rating Inventory of Executive Function, CBCL = Child Behavior Checklist, CPRS = Conner’s Parent Rating Scale – Revised, CPT = Conners Continuous Performance Test, mos = months, HR = Hazard ratio, ICD = International Classification of Diseases, MD = Mean Difference, NR = Not Reported, NS = Not significant, OR = Odds ratio, RB2 = Rutter B2 Scale – Finnish Version, SDQ = Strengths and Difficulties Questionnaire, SWAN = Strengths and Weaknesses of ADHD Symptoms and Normal Behavior, WISC = Wechsler Intelligence Scale for Children, yrs = years, %tile = Percentile.
The mean difference was computed by subtracting the score in the unexposed group (48.06) from the score in the group exposed to maternal hypothyroidism (54.90).
Modesto et al. (2015), Ghassabian et al. (2014), and Andersen et al. (2018) reported positive, though mostly imprecise, associations between maternal hypothyroxinemia and ADHD outcomes (Table 2).9,42,45 Andersen et al. (2018) found that this association was stronger in girls than boys.45 Modesto et al. (2015) found no interaction between child sex and maternal hypothyroxinemia.9 Oostenbroek et al. (2017) reported positive associations between maternal hypothyroxinemia and teacher-rated inattention/hyperactivity, but reported inverse associations between maternal hypothyroxinemia and parent-rated inattention/hyperactivity (Table 2).44 Päkkilä et al. (2014) reported mixed effects (Table 2).43
Vermiglio et al. (2004) found that, in the region studied in which iodine was moderately sufficient, there were no cases of ADHD. In the moderately iodine-deficient region, the authors reported a higher prevalence of ADHD among offspring of mothers with low fT4 (hypothyroxinemia) compared to offspring of mothers with normal fT4 (euthyroid).46
Given the similarity in exposure and outcome assessment, we conducted a meta-analysis of these estimates. Due to insufficient information, we only meta-analyzed four of six studies, yielding an aggregate odds ratio estimate of 1.54 with 95% confidence intervals (1.02, 2.33). See eAppendix 3 for details.
Maternal hypothyroidism.
Four studies reported estimates of association between maternal hypothyroidism and ADHD outcomes (Table 1). Instanes et al. (2016) conducted a nested population-based case–control study of ADHD in Norway using registry data (Ncases=47 944, Ncontrols=2 274 713).10 Samadi et al. (2015) conducted a cohort study in Canada investigating brain development of children born to mothers with and without hypothyroidism (Ntotal=44).47 Haddow et al. (1999) studied a cohort of mothers in the U.S. selected based on their hypothyroid status (Ntotal=186).11 The study by Andersen et al. (2018) was described above.45
Two studies (Instanes et al. 2016, Samadi et al. 2015) used registered clinical diagnoses of maternal hypothyroidism (e.g. from nationwide and representative patient registries),10,47 whereas Haddow et al. (1999) used second trimester serum TSH and T4 measures to classify hypothyroidism and mild hypothyroidism.11
Instanes et al. (2016) linked the Medical Birth Registry of Norway and the Norwegian Prescription Database to identify children 3 years and older who were prescribed and dispensed ADHD medication.10 Samadi et al. (2015) measured ADHD related symptoms in children aged 9–14 years using two parent-completed screening instruments.47 Haddow et al. (1999) evaluated attention, vigilance, language, and other cognitive abilities at age 7–9 years using a neuropsychological battery.11
Instanes et al. (2016), Samadi et al. (2015), and Haddow et al. (1999) reported positive, though somewhat imprecise, associations between maternal hypothyroidism and ADHD medication prescription,10 impaired working memory,47 distractibility, and impaired sustained attention11 (Table 2). However, neither Samadi et al. (2015) nor Haddow et al. (1999) adjusted for covariates. Andersen et al. (2018) found a near-null association between maternal overt and overall hypothyroidism and ADHD medication prescription (Table 2).45
Maternal subclinical hypothyroidism.
Both Andersen et al. (2018) and Modesto et al. (2015) reported a near-null association between maternal subclinical hypothyroidism and ADHD prescription and symptoms around 8 years (Table 2; both studies described earlier).9,45
Maternal hyperthyroidism.
Three studies reported estimates of association between maternal hyperthyroidism and ADHD, two of which were described earlier (Andersen et al. 2018; Instanes et al., 2016). The third study, Andersen et al. (2014), used a population-based prospective cohort in Denmark and included mothers who were diagnosed with hyperthyroidism before or after the birth of their child (Ntotal=857014).48 Andersen et al. (2014) identified ADHD in children up to 18 years old based on diagnoses and dispensed ADHD medication prescriptions in national registers.
Andersen et al. (2018) and Instanes et al. (2016) both reported small positive and relatively imprecise estimates of association between maternal hyperthyroidism and ADHD, though Andersen et al.’s (2018) effect appears to be driven by subclinical—rather than overt—hyperthyroidism (Table 2).10,45 Andersen et al. (2014) did not report statistical results for maternal thyroid disorders diagnosed during pregnancy; however, their figure 3 indicates no association between maternal hyperthyroidism diagnosed before birth and ADHD.48 Andersen et al. (2014) additionally reported positive associations between maternal hypo- and hyperthyroidism diagnosed after birth and ADHD; however, because temporality cannot be clearly established for these associations, we excluded them from our review. The studies adjusted for maternal age, smoking, parity, and marital status, among other variables (Table 1).
Maternal thyroid dysfunction.
Päkkilä et al. (2015) reported that “maternal thyroid dysfunction” was not associated with parent-rated ADHD symptoms at 16 years (Ntotal=4,357 - 4,370; Table 2).49 It is unclear how thyroid dysfunction was classified. Komendová et al. (2018) found a near-null association between maternal thyroid disorder in the first trimester (high TSH and/or high TPOAb) and distractibility in 6–9 year olds (Ntotal=192).50 Komendová et al. (2018) additionally included a qualitative assessment of attention problems; however, because the methods are unclear, we have excluded these results from our review.
Maternal Thyroid Hormone Concentrations
Free Thyroxin (fT4).
Three studies investigated maternal fT4 and ADHD symptoms, two of which were described earlier (Andersen et al. 2018; Oostenbroek et al. 2017).44,45 The third study (Chevrier et al. 2011) used a birth cohort of mostly Latino children born in California (Ntotal=287). Chevrier et al. (2011) measured fT4 using direct equilibrium dialysis followed by radioimmunoassay from maternal serum sampled in the second half of gestation.51 When children were 5 years old, Chevrier et al. (2011) assessed attention and ADHD symptoms using a behavioral test and a parent-completed checklist.51
Oostenbroek et al. (2017) reported an inverse and relatively precise estimate of association between maternal fT4 levels and teacher-rated inattention/hyperactivity in 5-year-old children but a positive (though near-null) association between maternal fT4 levels and parent-rated inattention/hyperactivity (Table 3). Similarly, Chevrier et al. (2011) reported mixed results: higher fT4 was imprecisely associated with worse attention on a behavioral measure, but better attention on a parent-completed checklist measure, though both estimates were imprecise (Table 3). Andersen et al. (2018) reported a null effect (Table 3).
Table 3.
Estimated associations between maternal thyroid hormone biomarkers and offspring ADHD or related behaviors.
| Estimated associations between maternal thyroid hormone biomarkers and ADHD or related behaviors | ||
|---|---|---|
| Exposure | Outcome (Time) | Estimate |
| Higher fT4 | ||
| Oostenbroek 201744 | Teacher SDQ hyperactivity/inattention (5 yrs) | OR = 0.95 (0.85, 1.05) |
| Parent SDQ hyperactivity/inattention (5 yrs) | OR = 1.01 (0.90, 1.13) | |
| Chevrier 201151 | ↓ CBCL attention problems (5 yrs) | β = −0.10 (−2.03, 1.82) |
| ↑ KCPT attention problems (5 yrs) | β = 7.52 (−4.86, 19.91) | |
| Andersen 201845 | ADHD prescription (8.4 yrs median) | HR = 1.00 (0.63, 1.59) |
| TPOAb positive | ||
| Ghassabian 201212 | CBCL attention deficit/hyperactivity (3 yrs) | OR = 1.77 (1.15, 2.72) |
| Higher TSH | ||
| Endendijk, 201752 | CBCL attention problems (23-60 mos) | rboys(df=224) = 0.21, p<.01 |
| rgirls NR, not significant | ||
| Oostenbroek 201744 | Teacher SDQ hyperactivity/inattention (5 yrs) | OR = 1.02 (0.96, 1.09) |
| Parent SDQ hyperactivity/inattention (5 yrs) | OR = 1.01 (0.92, 1.11) | |
| Päkkilä 201443 | RB2 inattention & hyperactivity (5 years) | ORboys = 1.17 (1.00, 1.36) |
| ORgirls = 1.39 (1.07, 1.80) | ||
| Chevrier 201151 | ↑ CBCL attention (5 yrs) | β = −0.65 (−1.26, −0.04) |
| ↑ KCPT attention (5 yrs) | β = −0.75 (−4.61, 3.12) | |
| Ghassabian 201113 | CBCL attention deficit/hyperactivity (1.5-3 yrs) | βSD of TSH = 0.08 (0.01, 0.15) |
| Higher TT4 | ||
| Chevrier 201151 | CBCL attention problems (5 yrs) | β = 0.00 (−0.28, 0.27) |
| ↑ KCPT attention problems (5 yrs) | β = 0.09 (−1.70, 1.87) | |
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder, CBCL = Child Behavior Checklist, mos = months, HR = Hazard ratio, KCPT = Conners Kiddie Continuous Performance Test, OR = Odds ratio, RB2 = Rutter B2 Scale – Finnish Version, SDQ = Strengths and Difficulties Questionnaire, TPOAb = Thyroid peroxidase antibodies, TSH = Thyroid stimulating hormone, TT4 = Total thyroxin, yrs = years
Thyroid Peroxidase Antibodies (TPOAb).
Using the Generation R cohort (described previously), Ghassabian et al. (2012) found that elevated maternal TPOAb measured in early gestation was associated with parent-reported ADHD symptoms in 3-year-old children (Table 3).12
Thyroid Stimulating Hormone (TSH).
All five studies that reported an association between maternal TSH and ADHD used large prospective birth cohorts (Table 1), three of which were previously described (Chevrier et al. 2011, Oostenbroek et al. 2017, Päkkilä et al. 2014). Endendijk et al. (2017) recruited women in the Netherlands during their first antenatal appointment (Ntotal=442).52 Endendijk et al. (2017) measured TSH using immunoassay on serum samples collected at 12, 24, and 36 weeks’ gestation, and parents completed the Child Behavior Checklist when children were 23–60 months old. Ghassabian et al. (2011) assessed ADHD symptoms in children aged 1.5 and 3 years in the Generation R cohort using the parent-completed attention-deficit/hyperactivity subscale of the Child Behavior Checklist (Ntotal=3736).13 Ghassabian et al. (2011), Oostenbroek et al. (2017), and Päkkilä et al. (2014) measured TSH before 20 weeks’ gestation,13,43,44 whereas Chevrier et al. (2011) measured TSH in the second half of gestation.51
Ghassabian et al. (2011) found that higher maternal TSH was associated with ADHD symptoms at 1.5 and 3 years (pooling across ages; Table 3).13 Oostenbroek et al. (2017) reported near-null and precise estimates of association between maternal TSH and inattention/hyperactivity in 5-year-old children (Table 3).44 Päkkilä et al. (2014) found that increased maternal TSH was more strongly associated with inattention and hyperactivity in girls at 8 years than in boys (Table 3).43 By contrast, Endendijk found that first-trimester higher maternal TSH was associated with attention problems in boys but not girls (Table 3).52 Endendijk et al. (2017) also found that a thyroid hormone trajectory analysis including TSH and fT4 did not predict child attention problems better than the single-trimester analysis.52 Chevrier et al. (2017) found that higher TSH was associated with better attention and fewer ADHD symptoms at 5 years, though estimates were somewhat imprecise (Table 3).51 Four of the studies controlled for maternal smoking,13,43,44,52 and all four controlled for other covariates (Table 1).
Total Thyroxine (TT4).
Chevrier et al. (2011), described earlier, reported mixed estimates of association between maternal TT4 in the second half of gestation and ADHD symptoms at age 5 (Table 3).51
Early-Treated Congenital Hypothyroidism
All seven studies that reported on ADHD symptoms among children with early-treated congenital hypothyroidism included between 82 and 146 children (Table 4). Three studies were from Rovet et al., who used newborn congenital hypothyroidism screening programs in Canada.53–55 Three were from Kooistra et al, who used a Dutch longitudinal cohort study of the development of those with early-treated congenital hypothyroidism.56–58 Finally, one was from Tinelli et al. (2003), who studied a cohort in Italy.14
Table 4.
Studies of ADHD related behaviors among children with early-treated congenital hypothyroidism.
| First Author, Year Country |
Study Design (Sample Size) | Thyroid Function Measurement (Timing) | Outcome Measurement (Timing) | Adjustment Set a |
|---|---|---|---|---|
|
Kooistra, 200456 The Netherlands |
Cohorts selected based on congenital hypothyroidism status (Ntotal = 82) | Congenital hypothyroidism (birth) | Memory search task based on Sternberg response bias task (7.0–8.8 yrs) | 4 |
|
Kooistra, 200157 The Netherlands |
Cohorts selected based on congenital hypothyroidism status (Ntotal = 97) | Congenital hypothyroidism (birth) | Groningen Behavior Checklist School and Family Situation (7.5, 9.5 yrs) | None |
|
Kooistra, 199658 The Netherlands |
Cohorts selected based on congenital hypothyroidism status (Ntotal = 83) | Congenital hypothyroidism (birth) | Computer-paced continuous performance task, self-paced performance task (7.0-8.75 yrs) | 4 |
|
Rovet, 2001a (Attention…)53
Canada |
Retrospective cohort selected based on congenital hypothyroidism status (Ntotal = 98) | Congenital hypothyroidism (birth) | Child Behavior Checklist (filled out by parents), Battery of Behavioral Attention Tests (12.6-15.2 yrs) |
1 |
|
Rovet, 2001b (Dissociating…)54 Canada |
Retrospective cohort selected based on congenital hypothyroidism status (Ntotal = 144) | Congenital hypothyroidism, TSH, T4 (birth) | Two versions of Connors’ Continuous Performance Task (7-12 yrs) | 1, 2, 3, 4 |
|
Rovet, 199955 Canada |
Retrospective cohort selected based on congenital hypothyroidism status (Ntotal = 118) | Congenital hypothyroidism, T4 (birth) | Psychometric instruments including, but not limited to, Griffiths Test, Bayley Test, McCarthy Test, and the Wechsler Intelligence Scale for Children III (13 yrs) | None |
|
Tinelli, 200314 Italy |
Cohorts selected based on congenital hypothyroidism status (Ntotal = 146) | Congenital hypothyroidism (birth) | Child Behavior Checklist filled out by parents (10 and 13 years) | None |
Key for adjustment set: 1 = child age/bone age at testing; 2 = child sex; 3 = SES; 4 = child IQ.
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder, T4 = thyroxine, TSH = thyroid stimulating hormone, yrs = years.
The adjustment set includes variables that were statistically controlled for in analyses included in this review, or were assessed as potential covariates for analyses included in this review, as well as exclusion or restriction factors that were relevant for this review.
All seven studies of ADHD symptoms among children with early-treated congenital hypothyroidism had similar designs (Table 4): a convenience cohort of participants with early-treated congenital hypothyroidism was compared to a cohort of participants without congenital hypothyroidism, often matched on demographic variables such as gender and age. Outcomes were measured between 7 and 15 years using mostly continuous performance tasks or screening measures. Newborn screening for congenital hypothyroidism is similar in Canada, the Netherlands, and Italy: all used dried blood spots collected within the first week of life.59–62
Rovet et al. (2001a) and Rovet et al. (1999) reported positive associations between early-treated congenital hypothyroidism and ADHD symptoms (Table 5).53,55 Tinelli et al. (2003) reported positive associations between early-treated congenital hypothyroidism and inattention at age 13 but not at 10 years.14 Kooistra et al. (1996) found a positive association between the disorder and impaired attention on a self-paced sustained attention task but not on a computer-paced task.58 Finally, Kooistra et al. (2001, 2004) and Rovet et al. (2001b) reported null or near-null estimates of association (Table 5).54,56,57 Among those with early-treated congenital hypothyroidism, higher TSH and lower T4 at diagnosis was associated with impulsivity, inattention, and impaired memory (Table 5).54,55 The precision of these estimates is difficult to compare because most studies reported F and t statistics with p values (and no confidence intervals or indications of variability).
Table 5.
Estimated association between early-treated congenital hypothyroidism and ADHD or related behaviors.
| Estimated associations between early-treated congenital hypothyroidism and ADHD or related behaviors | ||
|---|---|---|
| Exposure | Outcome (Time) | Estimate |
| Congenital hypothyroidism | ||
| Kooistra 200456 | MST Impulsivity (7.0-8.8 yrs) | F = 0.66, p = 0.42 |
| Tinelli 200314 | CBCL impaired attention (10 yrs) | t = 0.007, p > .05 |
| CBCL impaired attention (13 yrs) | t = 2.665, p < .01 | |
| Kooistra 200157 | GBCS Impaired task orientation (7.5, 9.5 yrs) | NS |
| Rovet 2001a53 | Impaired attention across tasks (12.6-15.2 yrs) | F = 4.22, p < .001 |
| CBCL attention problems (12.6–15.2 yrs) | p < .001 | |
| Rovet 2001b54 | CPT impaired sustained attention (7-12 yrs) | NS |
| Rovet 199955 | PI impaired attention (vigilance), memory (13 yrs) | Significant, stats NR |
| Kooistra 199658 | ↓ sCPT self-paced sustained attention (7-8.75 yrs) | F = 5.70, p = .02 |
| ↓ cCPT computer-paced sustained attention (7-8.75 yrs) | F = .02, p = .9 | |
| Higher TSH at diagnosis | ||
| Rovet 2001b54 | CPT impulsivity (7-12 yrs) | r = 0.295 |
| CPT inattention (7-12 yrs) | r = 0.519, p < .01 | |
| Lower T4 at diagnosis | ||
| Rovet 2001b54 | CPT impulsivity (7-12 yrs) | r = .577, p<.001 |
| Rovet 199955 | PI impaired memory and attention (13 yrs) | Significant, stats NR |
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder, CBCL = Child Behavior Checklist, CPT = Conners Continuous Performance Task, cCPT = Computer-Paced Continuous Performance Task, GBCS = Groningen Behavior Checklist School/Family Situation, mos = months, MST = Memory Search Task, NR = Not reported, NS = Not significant, PI = A battery of behavioral Psychometric Instruments, sCPT = Self-Paced Continuous Performance Task, TSH = Thyroid stimulating hormone, T4 = Thyroxin, yrs = years.
Newborn Thyroid Hormone Concentrations
Three of the five studies of neonatal thyroid hormones and ADHD symptoms were cohort studies (Table 6): Trumpff et al. (2016) studied a Belgian cohort (Ntotal=310),63 and there were two studies of Canadian cohorts with less than 100 participants each (Ishaik et al. 2000; Simic et al. 2009).15,64 Two of the five studies had matched case–control designs:65,66 ADHD cases (identified through neurodevelopmental diagnostic clinics in Washington, D.C) were matched on the day of newborn screening and birth hospital. The first matched case–control study (Ncases=52, Ncontrols=71 to 130 depending on analysis)66 served as a pilot study for the second (Ncases=53, Ncontrols=231)65, and it is not clear whether participants in the pilot were included in the full-scale study.
Table 6.
Studies of neonatal thyroid hormone levels and later ADHD or related behaviors.
| First Author, Year Country |
Study Design (Sample Size) | Thyroid Function Measurement (Timing) | Outcome Measurement (Timing) | Adjustment Set a |
|---|---|---|---|---|
|
Trumpff, 201663 Belgium |
Retrospective cohort (Ntotal = 310) | TSH from dried bloodspots (3-5 days after birth) | French version of the Child Behavior Checklist for ages 1.5 - 5 years (4-5 yrs) | 1, 2, 6, 12, 13, 14, 15, 16 |
|
Ishaik, 200064 Canada |
Cohort (Ntotal = 36) | fT4, T4, TSH (2 weeks of life, and at 40 weeks post conceptual age) | Bayley Scales of Infant Development-II: Mental Development Index, including selective attention, sustained attention, and attention shift (3 mos corrected age) | 1 |
|
Simic, 200915 Canada |
Prospective cohort, case-control (Ntotal = 97) | fT4, TT3 (blood samples at 2 and 4 weeks of life, and 40 weeks postconceptual age) | Bayley Scales of Infant Development-II: Mental Development Index & Psychomotor development index, including attention (3 mos corrected age) | 1, 2, 3, 4, 7, 8, 9, 10, 11, 12, 14, 17, 18, 19, 20, 21 |
|
Soldin, 200365 USA |
Matched case-control (Ncases = 53, Ncontrols = 231) | T4 from dried bloodspots (2-3 days after birth) | DSM-IV ADHD diagnosis (5.5-12 yrs) | 1, 2, 5, 6, 22, 23 |
|
Soldin, 200266 USA |
Matched case-control (Ncases = 52, Ncontrols = 71 to 130 depending on analysis) | T4 from dried bloodspots (2-3 days after birth) | DSM-IV ADHD diagnosis (5.5-12 yrs) | 1, 2, 5, 6, 22, 23 |
Key for adjustment set: 1 = gestational age; 2 = child sex; 3 = SES; 4 = birth weight; 5 = child ethnicity; 6 = time of thyroid sampling; 7 = maternal alcohol use; 8: prenatal corticosteroids; 9: bilirubin; 10: neonatal illness (e.g., sepsis); 11 = congenital anomalies; 12 = infant congenital hypothyroidism; 13 = small for gestational age/low birth weight; 14 = co-adjustment for other thyroid function biomarkers; 15 = offspring neurological disease; 16 = multiple births; 17 = maternal history of thyroid disease; 18 = maternal exposure to organic solvents; 19 = other maternal medication use (e.g., anticonvulsants); 20 = maternal substance abuse (other than alcohol or tobacco); 21 = other child conditions (e.g. phenylketonuria, Down Syndrome); 22 = child age; 23 = birth hospital.
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder, DSM = Diagnostic and Statistical Manual of Mental Disorders, fT4 = Free thyroxine, mos = months, T4 = Thyroxine, TSH = Thyroid stimulating hormone, TT3 = Total triiodothyronine, yrs = years.
The adjustment set includes variables that were statistically controlled for in analyses included in this review, or were assessed as potential covariates for analyses included in this review, as well as exclusion or restriction factors that were relevant for this review.
Three of the five studies measured thyroid hormone concentrations using immunoassay on dried bloodspots collected after birth for congenital hypothyroidism screening programs.63,65,66 The other two studies used blood samples at 2 and 4 weeks of life, and at 40 weeks post-conceptual age.15,64
The most common covariates were child age and sex.63,65,66 Trumpff et al. (2016) only included children whose bloodspots were sampled 3–5 days after birth,63 and Soldin et al. (2002, 2003) collected bloodspots 2–3 days after birth.65,66
Both studies that reported on neonatal fT4 found that low fT4 was associated with impaired attention at 3 months,15,64 though effect sizes were modest (Table 7).15 One study found that higher total T3 (TT3) was associated with impaired selective attention in infancy (Table 7).15 One study of neonatal TSH did not find an association of TSH levels and ADHD-related outcomes at 4–5 years (Table 7).63 There was not sufficient information to compare the precision of the estimates.
Table 7.
Estimated association between neonatal thyroid hormone biomarkers and ADHD or related behaviors.
| Estimated associations between neonatal thyroid hormone biomarkers and ADHD or related behaviors | ||
|---|---|---|
| Exposure | Outcome (Time) | Estimate |
| Lower fT4 | ||
| Simic 200915 | BSID impaired selective attention (3 mos) | β = 0.022, SE = 0.024, NS |
| BSID impaired sustained attention (3 mos) | β = 0.020, SE = 0.10, p =.053 | |
| BSID impaired total attention (3 mos) | β = 0.098, SE = 0.034, p = .005 | |
| Ishaik 200064 | BSID impaired total attention (3 mos) | p < .01 |
| BSID impaired sustained attention (3 mos) | p < .05 | |
| Higher TT3 | ||
| Simic 200915 | BSID impaired selective attention (3 mos) | β = 0.320, SE = 0.185, p = .09a |
| Lower T4 | ||
| Soldin 200365 | DSM-IV ADHD diagnosis (5.5-12 yrs) | OR = 0.99, p = 0.81 |
| Soldin 200266 | DSM-IV ADHD diagnosis (5.5-12 yrs) | OR = 1.08 (0.95, 1.24) |
| TSH | ||
| Trumpff 201663 | CBCL ADHD symptoms (4-5 yrs) | NS |
Abbreviations: ADHD = Attention Deficit Hyperactivity Disorder, BSID = Bayley Scales of Infant Development-II, CBCL = Child Behavior Checklist, DSM = Diagnostic and Statistical Manual of Mental Diorders, fT4 = Free thyroxine, mos = months, NS = Not significant, OR = Odds ratio, β refers to a standardized regression coefficient, TSH = Thyroid stimulating hormone, TT3 = Total triiodothyronine, T4 = Thyroxine, yrs = years.
The direction of this effect was flipped for the purposes of interpretation and comparison.
DISCUSSION
There is growing interest in the role of maternal thyroid dysfunction in neurocognitive development,67–69 with accumulating evidence suggesting plausible associations. We sought to focus specifically on the role of maternal and neonatal thyroid function and incidence of ADHD and related behaviors, examining the literature from an epidemiologic perspective. Our search identified 28 articles: 16 studies of maternal thyroid hormones or thyroid dysfunction, seven studies of early-treated congenital hypothyroidism, and five studies of neonatal thyroid hormones. In this section, we summarize key findings, compare studies, and raise questions for future research.
Outcome Measures
Outcome assessment varied considerably across studies. Approximately half of the studies did not explicitly aim to assess ADHD, and instead measured attention, hyperactivity, and impulsivity. Because ADHD is a heterogeneous disorder,70 a single instrument may misclassify ADHD status, particularly when symptoms are mild.71,72 Most reviewed studies used a single outcome measurement, most commonly the Child Behavior Checklist (CBCL) or versions of Conners’ Continuous Performance Test (CPT). While the CBCL has good validity in predicting ADHD diagnosis73–75, the CPT has poor discriminant validity.71,76 Four of the reviewed studies used a clinical ADHD diagnosis as the outcome (ICD-1048 and DSM-IV46,65,66). Clinical classifications of ADHD are to some extent arbitrary, yet dimensional instruments designed to tap pathologies contributing to ADHD (as suggested by the Research Domain Criteria77) are also limited by our minimal understanding of ADHD’s causes. We reviewed both clinical and continuous measures of ADHD symptoms because converging evidence from both types of measures—which are affected by distinct limitations—would be more convincing than evidence from one outcome measure alone.
Maternal Thyroid Hormones and Offspring ADHD: Confounders
Maternal smoking is strongly associated with ADHD78,79 and with maternal thyroid function.31 Although this relationship is likely non-causal,80 maternal smoking is nonetheless on a key confounding path between maternal thyroid function and ADHD. Half of the reviewed studies of maternal thyroid hormones and ADHD adjusted for maternal smoking. Other maternal factors associated with both thyroid hormone dysfunction and offspring ADHD or related symptoms include: low iodine intake, older age, depression, anxiety, alcohol use, and maternal education (each of which were adjustment factors in at least one reviewed paper).27,32,33,81–85 The extent to which these variables contribute to confounding is unclear because their underlying biologic mechanisms are not well characterized. Furthermore, it is possible that genetic risk factors for ADHD, which may in turn affect maternal behaviors during pregnancy, confounds the association between maternal thyroid hormones and offspring ADHD. To our knowledge, there have been no sibling studies of the association between maternal thyroid hormones and offspring ADHD; such a study design would help disentangle the role of genetic risk factors.
Maternal Thyroid Hormone Disorders
Evidence for an association between maternal hypothyroxinemia and ADHD symptoms was mixed (effect estimates had variable magnitude and direction, and some were imprecise),9,42–44,46 and limitations of the literature prohibit clear conclusions. First, adjustment sets varied, with one study including no statistical adjustment for confounding. Second, one study had eight exposed women.46 Finally, all six studies used immunoassay for fT4 measurement, which may have resulted in misclassification of maternal hypothyroxinemia.
Measurement of fT4 during pregnancy is complicated by pregnancy-related increases in thyroxine binding globulin concentrations, increased total thyroid hormone levels, lower levels of albumin in blood, and circulating human chorionic gonadotropin (hCG).86–88 The gold-standard method of measuring free thyroid hormones (which uses equilibrium dialysis as the first step, and which was used in one of the reviewed studies51) can most accurately quantify fT4 levels during pregnancy,80 but is costly.87,89 All reviewed studies of maternal hypothyroxinemia, and all but one51 study of maternal fT4, used immunoassays to assess fT4 rather than the gold-standard equilibrium dialysis approach.
There was some evidence for an association between maternal hypothyroidism and ADHD. Three of five reviewed studies of maternal hypothyroidism reported positive associations between maternal hypothyroidism and offspring ADHD symptoms (inattention, distractibility,11 and impaired working memory10,47) and ADHD prescriptions.10 However, these studies did not explicitly differentiate overt from subclinical hypothyroidism and only one adjusted for potential confounders. The two studies that did distinguish clinical from subclinical hypothyroidism reported near-null associations.9,45
There was mixed evidence for an association between maternal hyperthyroidism and ADHD outcomes. Two of three studies did not separate overt from subclinical hyperthyroidism. One of these two studies reported a positive association between maternal hyperthyroidism diagnosed before birth and ADHD prescriptions,10 whereas the other found no association.48 The third study found that maternal subclinical hyperthyroidism was more strongly associated with ADHD prescription than overt hyperthyroidism.45
Maternal Thyroid Hormone Biomarkers
There is minimal evidence for (or against) an association between maternal fT4 and ADHD: two of three studies reported mixed effects, and the third reported a null association. Two of these studies used immunoassay to measure fT4, which may introduce measurement error during pregnancy.
There was mixed evidence for an association between maternal TSH and ADHD. One study found that higher maternal TSH was more strongly associated with attention problems in girls than in boys,43 whereas another study found a stronger association in boys than in girls.52 Of the three other studies, one reported a near-null effect,44 another found that higher maternal TSH was associated with more ADHD symptoms,13 and the third found that higher maternal TSH was associated with better attention.51
The one study that reported estimates for maternal TPOAb found that higher levels were associated with ADHD symptoms.12 It is unclear whether the observed association was a result of the disruptive effects of TPOAb on maternal thyroid hormone synthesis, or through some other (possibly more direct) mechanism.
Early-Treated Congenital Hypothyroidism and Later ADHD Outcomes
Four of the seven studies examining early-treated congenital hypothyroidism and ADHD symptoms (inattention14,53,55,58, impaired memory55) reported positive associations. The more severe the congenital hypothyroidism (indicated by elevated TSH and suppressed fT4 concentrations at diagnosis), the worse the impairment observed.54,55 Important research avenues remain. First, all seven studies included children aged 7 to 15 years; it is unclear whether the observed association would hold during the preschool years. Second, none of the studies used clinical ADHD assessments; however, dimensional and diagnostic measures of ADHD provide different insights into the disorder, so both are valuable. Finally, the reviewed studies each included fewer than 150 participants, and participants appeared to be a convenience sample of clinical cases, undercutting generalizability.
Neonatal Thyroid Hormones and ADHD Outcomes
There is insufficient evidence to draw conclusions about the association between neonatal thyroid hormones and ADHD. First, no more than two studies reported on a given biomarker. Second, two studies assessed outcomes during infancy,15,64 which, given current evidence, is too early to provide information about later ADHD (though neither study intended to assess ADHD per se). Third, effect sizes for most associations were small. Fourth, three of the five studies had fewer than 100 participants. Finally, the three studies that used dried bloodspots from newborn screening programs did not sufficiently control for sample time after birth. Newborn TSH levels rise within 30 minutes of birth, leading to increased T3 and T4 levels, which fall to adult levels 3–5 days after birth.90 Although one of the reviewed studies restricted the sample time frame to 3–5 days after birth,63 no reviewed study further adjusted for sample time.
Timing of Maternal Thyroid Hormone Deficiency
Because establishing temporality is crucial for drawing causal conclusions, we only reviewed associations for which thyroid hormone assessment clearly preceded outcome assessment. A recent review of human and animal literature found that maternal thyroid hormone dysfunction earlier in pregnancy has larger estimated effects on neurodevelopment (including intelligence, verbal development, and general cognition, among other outcomes),19,39 consistent with the fetus’s reliance on the mother during the first half of gestation. With respect to the estimates included in our review, 10 of 16 studies of maternal thyroid hormones and ADHD exclusively assessed maternal thyroid hormones during the first 20 weeks of gestation;9,12,13,42–46,49,50 three reported unclear timing (e.g., ‘before or during pregnancy’),10,47,48 two assessed thyroid hormones later in pregnancy,11,51 and one study (Endendijk et al., 2017) assessed the association between thyroid hormones in each trimester and ADHD outcomes.52 Endendijk et al. (2017) did find a stronger association between first trimester thyroid hormones and ADHD symptoms,52 consistent with past research and biological hypotheses. The effect of timing of maternal thyroid hormone dysfunction on ADHD remains a question for future studies.
Developmental Timing of ADHD Outcomes
Because most reviewed studies assessed ADHD at a single time point, our review provides minimal information about how thyroid–ADHD associations change with age. Relevant to this point is that a meta-analysis of longitudinal studies of ADHD among those 4 years and older found that the prevalence of ADHD decreased with age.91
Two of the reviewed studies assessed preschool ADHD (which found that maternal TPOAb and TSH were associated with ADHD symptoms12,13). Consistent with clinical recommendations to diagnose ADHD between 4 and 18 years,72,92 most of the reviewed studies assessed ADHD symptoms in children older than 4. Although current diagnoses of preschool ADHD have only moderate stability93,94 and evidence for the validity of preschool-aged ADHD diagnosis is minimal, more research of preschool ADHD is warranted to investigate the extent to which preschool ADHD and later ADHD have overlapping etiologies.
Two reviewed studies assessed attention in infancy.15,64 Although none of these authors claimed to study ADHD, their results nonetheless raise the question of whether infant attention is predictive of, or relevant to, childhood ADHD. Atkinson et al. (2012) describes three types of attention, each with different neural underpinnings;95 infant attention likely taps into the selective attention neurocognitive system, whereas childhood ADHD symptoms likely relate to the attentional control neurocognitive system. The extent to which early perceptual attentional deficits are indicative of, or related to, later cognitive attentional and executive function deficits is an area of active investigation.96–99
Dose–Response
Even mild disruptions in maternal thyroid function may influence fetal neurodevelopment.19 Most reviewed studies modelled thyroid hormone levels dichotomously or linearly. Recent evidence, however, suggests a U-shaped relationship between thyroid hormone concentrations and adverse neurodevelopmental effects;102 modeling thyroid hormones linearly or dichotomously obscures this dose–response. Only two reviewed studies explicitly assessed functional form, and neither found support for non-linear associations between maternal thyroid hormone concentrations and ADHD symptoms.45,51 Nonetheless, future studies of the association of thyroid hormones and ADHD should assess non-linearity before applying dichotomous or linear models.
The Importance of Iodine: A Potential Effect Measure Modifier
Iodine deficiency is the most preventable cause of neurocognitive deficits worldwide.103 During pregnancy, iodine intake requirements increase by approximately 50%,102 causing pregnant women to be at a higher risk of iodine deficiency.102 Under iodine insufficiency, the thyroid gland synthesizes relatively more T3 and less T4, and the expression of deiodinases in the nervous system is altered.103 As a result, iodine may be an effect measure modifier of the association between early thyroid hormone levels and ADHD. The importance of considering iodine deficiency is underscored by the fact that only three of eight countries represented in the systematic review (Canada, the Netherlands, and the Czech Republic) are considered to have adequate levels of iodine (eAppendix 4).104–108
Despite the importance of iodine for thyroid hormone function and brain development, only one reviewed study considered iodine’s role in thyroid hormone function. Vermiglio et al. (2004) found that ADHD was more common in the moderately iodine deficient region compared to the more iodine sufficient region.46 However, this study measured neither maternal iodine levels nor intake. Two other studies did assess iodine, but did not evaluate the interaction of iodine and thyroid hormones.42,63 Future studies of thyroid hormones and ADHD outcomes should consider iodine as a potential effect measure modifier.
Conclusions and Recommendations
This systematic review provides moderate evidence for an association between maternal thyroid function and childhood ADHD symptoms, some evidence for an association between early-treated congenital hypothyroidism and later ADHD symptoms, and minimal evidence for an association between neonatal thyroid hormones and later ADHD symptoms. Most reported effect estimates were small and imprecise in magnitude. The current literature suffers from methodologic limitations that prevent strong conclusions. Future studies should consider measurement issues for thyroid function and ADHD, as well as strive for population-based designs to improve generalizability. In addition, careful consideration of potential confounders and effect measure modifiers and investigating non-linear dose responses would strengthen conclusions.
Supplementary Material
Acknowledgments
Financial Support: This research was funded in part by NIEHS R01ES021777 and the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Conflicts of Interest: None declared.
Availability of data and code for replication: Because this is a systematic review of published articles, all data used to draw conclusions are presented in the manuscript tables (as well as in the original articles themselves). These data consist of effect estimates, confidence intervals, p values, and sample sizes. The meta-analysis described in the supplemental material was conducted using the metafor package (Wolfgang Viechtbauer, 2017) for R. Samantha SM Drover would be happy share the R program used to run the meta-analysis upon request (sdrover@live.unc.edu).
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). (2013). [DOI] [PubMed] [Google Scholar]
- 2.Polanczyk GV, Willcutt EG, Salum GA, Kieling C & Rohde LA ADHD Prevalence Estimates Across Three Decades: An Updated Systematic Review and Meta-Regression Analysis. Int. J. Epidemiol 43, 434–442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC et al. The Prevalence and Correlates of Adult ADHD in the United States: Results from National Comorbidity Survey Replication. Am. J. Psychiatry 163, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher J & Wolfe B Long-Term Consequences of Childhood ADHD on Criminal Activities. J Ment Health Policy Econ 12, 119–138 (2012). [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SS, Humphreys KL, Flory K, Liu R & Glass K Prospective Association of Childhood Attention-Deficit/Hyperactivity Disorder (ADHD) And Substance Use and Abuse/Dependence: A Meta-Analytic Review. Clin. Psychol. Rev 31, 328–341 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thapar A, Cooper M, Eyre O & Langley K Practitioner Review: What Have We Learnt About the Causes of ADHD? J. Child Psychol. Psychiatry 54, 3–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lichtenstein P, Carlstrom E, Rastam M, Gillberg C & Anckarsater H The Genetics of Autism Spectrum Disorders and Related Neuropsychiatric Disorders in Childhood. Am J Psychiatry 167, 1357–63 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Sciberras E, Mulraney M, Silva D & Coghill D Prenatal Risk Factors and The Etiology of ADHD — Review of Existing Evidence. Curr Psychiatry Rep 19, (2017). [DOI] [PubMed] [Google Scholar]
- 9.Modesto T et al. Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Pediatr 169, 838 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Instanes JT et al. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers with Inflammatory and Immune System Diseases. Biol. Psychiatry (2016). [DOI] [PubMed] [Google Scholar]
- 11.Haddow JE et al. Maternal Thyroid Deficiency During Pregnancy and Subsequent Neuropsychological Development of The Child. N. Engl. J. Med 341, 549–555 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Ghassabian A et al. Maternal Thyroid Autoimmunity During Pregnancy and The Risk of Attention Deficit/Hyperactivity Problems in Children: The Generation R Study. Thyroid 22, 178–186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghassabian A et al. Maternal Thyroid Function During Pregnancy and Parent-Report Problem Behavior of the Offspring up to Age Three Years. The Generation R Study. Pediatr. Res 69, 454–459 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Tinelli F et al. Behavioural Disorders in Adolescents With Early-Treated Congenital Hypothyroidism. Funct. Neurol 18, 161–164 (2003). [PubMed] [Google Scholar]
- 15.Simic N, Asztalos EV & Rovet J Impact of Neonatal Thyroid Hormone Insufficiency and Medical Morbidity on Infant Neurodevelopment and Attention Following Preterm Birth. Thyroid Off. J. Am. Thyroid Assoc 19, 395–401 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM & Ahmed RG Thyroid Hormones States and Brain Development Interactions. Int. J. Dev. Neurosci 26, 147–209 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Berbel P & Bernal J Hypothyroxinemia: A Subclinical Condition Affecting Neurodevelopment. Expert Rev. Endocrinol. Metab 5, 563–575 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Horn S & Heuer H Thyroid Hormone Action During Brain Development: More Questions Than Answers. Mol. Cell. Endocrinol 315, 19–26 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Moog NK et al. Influence of Maternal Thyroid Hormones During Gestation on Fetal Brain Development. Neuroscience 342, 68–100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y et al. Abnormalities of Maternal Thyroid Function During Pregnancy Affect Neuropsychological Development of their Children At 25–30 Months. Clin. Endocrinol. (Oxf.) 72, 825–829 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Teng W, Shan Z, Patil-Sisodia K & Cooper DS Hypothyroidism in Pregnancy. Lancet Diabetes Endocrinol 1, 228–237 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Stagnaro-Green A et al. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum. Thyroid Off. J. Am. Thyroid Assoc 21, 1081–125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stagnaro-Green A & Pearce E Thyroid Disorders in Pregnancy. Curr Opin Obstet Gynecol 17, 123–127 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Chang DL & Pearce EN Screening for Maternal Thyroid Dysfunction in Pregnancy: A Review of The Clinical Evidence and Current Guidelines. J Thyroid Res 2013, 851326 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey BM et al. Perinatal Significance of Isolated Maternal Hypothyroxinemia Identified in the First Half of Pregnancy. Obstet. Gynecol 109, 1129–1135 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Glinoer D Thyroid Hyperfunction During Pregnancy. Thyroid. 8, 859–865 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Obregon M-J, Del Rey FE & De Escobar GM The Effects of Iodine Deficiency on Thyroid Hormone Deiodination. Thyroid 15, 917–929 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Reinehr T, Isa A, De Sousa G, Dieffenbach R & Andler W Thyroid Hormones and their Relation to Weight Status. Horm. Res. Basel 70, 51–57 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Marzullo P et al. Investigations of Thyroid Hormones and Antibodies in Obesity: Leptin Levels Are Associated with Thyroid Autoimmunity Independent of Bioanthropometric, Hormonal, and Weight-Related Determinants. J. Clin. Endocrinol. Metab 95, 3965–3972 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Biondi B Thyroid and Obesity: An Intriguing Relationship. J. Clin. Endocrinol. Metab 95, 3614–3617 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Wiersinga WM Smoking and Thyroid. Clin. Endocrinol. (Oxf.) 79, 145–151 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Hoogendoorn EH et al. Thyroid Function and Prevalence of Anti-Thyroperoxidase Antibodies in a Population with Borderline Sufficient Iodine Intake: Influences of Age and Sex. Clin. Chem 52, 104–111 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Ittermann T, Völzke H, Baumeister SE, Appel K & Grabe HJ Diagnosed Thyroid Disorders Are Associated with Depression and Anxiety. Soc. Psychiatry Psychiatr. Epidemiol 50, 1417–1425 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Oguz A et al. Frequency of Isolated Maternal Hypothyroxinemia in Women with Gestational Diabetes Mellitus in a Moderately Iodine-Deficient Area. Gynecol. Endocrinol 31, 792–795 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Loubière LS et al. Expression and Function of Thyroid Hormone Transporters in the Microvillous Plasma Membrane of Human Term Synctiotrophoblast. Endocrinology 153, 6126–6135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel J, Landers K, Li H, Mortimer RH & Richard K Thyroid Hormones and Fetal Neurological Development. J. Endocrinol 209, 1–8 (2011). [DOI] [PubMed] [Google Scholar]
- 37.De Graaf-Peters VB & Hadders-Algra M Ontogeny of the Human Central Nervous System: What is Happening When? Early Hum. Dev 82, 257–266 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Rastogi MV & Lafranchi SH Congenital Hypothyroidism. Orphanet J. Rare Dis 5, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Préau L, Fini JB, Morvan-Dubois G & Demeneix B Thyroid Hormone Signaling During Early Neurogenesis and its Significance as a Vulnerable Window for Endocrine Disruption. Biochim. Biophys. Acta - Gene Regul. Mech 1849, 112–121 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Klein RZ et al. Relation of Severity of Maternal Hypothyroidism to Cognitive Development of Offspring. J. Med. Screen 8, 18–20 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Maraka S et al. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid 26, 580–590 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghassabian A, Henrichs J & Tiemeier H Impact of Mild Thyroid Hormone Deficiency in Pregnancy on Cognitive Function in Children: Lessons from the Generation R Study. Best Pract. Res. Clin. Endocrinol. Metab 28, 221–232 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Päkkilä F et al. The Impact of Gestational Thyroid Hormone Concentrations on ADHD Symptoms of The Child. J. Clin. Endocrinol. Metab 99, 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oostenbroek MHW et al. Maternal Hypothyroxinaemia in Early Pregnancy and Problem Behavior in 5-Year-Old Offspring. Psychoneuroendocrinology 81, 29–35 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Andersen SL, Andersen S, Vestergaard P & Olsen J Maternal Thyroid Function in Early Pregnancy and Child Neurodevelopmental Disorders: A Danish Nationwide Case-Cohort Study. Thyroid 28, 537–546 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Vermiglio F et al. Attention Deficit and Hyperactivity Disorders in The Offspring of Mothers Exposed to Mild-Moderate Iodine Deficiency: A Possible Novel Iodine Deficiency Disorder in Developed Countries. J. Clin. Endocrinol. Metab 89, 6054–6060 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Samadi A, Skocic J & Rovet JF Children Born to Women Treated for Hypothyroidism During Pregnancy Show Abnormal Corpus Callosum Development. Thyroid 25, 494–502 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Andersen SL, Laurberg P, Wu CS & Olsen J Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorder in Children Born to Mothers with Thyroid Dysfunction: A Danish Nationwide Cohort Study. BJOG Int. J. Obstet. Gynaecol 121, 1365–1374 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Päkkilä F et al. Maternal and Child’s Thyroid Function and Child’s Intellect and Scholastic Performance. Thyroid 25, 1363–1374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komendová I & Špitálníková S Intellectual Performance of Children of Mothers with an Untreated Thyroid Disorder in the First Pregnancy Trimester. Endokrynol. Pol (2018). Doi: 10.5603/EP.A2018.0025 [DOI] [PubMed] [Google Scholar]
- 51.Chevrier J et al. Maternal Thyroid Function During the Second Half of Pregnancy and Child Neurodevelopment at 6, 12, 24, and 60 Months of Age. J. Thyroid Res 2011, 1–13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Endendijk JJ, Wijnen HAA, Pop VJM & Van Baar AL Maternal Thyroid Hormone Trajectories During Pregnancy and Child Behavioral Problems. Horm. Behav 94, 84–92 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Rovet JF & Hepworth S Attention Problems in Adolescents with Congenital Hypothyroidism: A Multicomponential Analysis. J. Int. Neuropsychol. Soc 7, 734–744 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Rovet JF & Hepworth SF Dissociating Attention Deficits in Children with ADHD And Congenital Hypothyroidism Using Multiple Cpts. J. Child Adolesc. Psychiatry 42, 1049–1056 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Rovet JF Congenital Hypothyroidism: Long-Term Outcome. Thyroid 9, 741–748 (1999). [DOI] [PubMed] [Google Scholar]
- 56.Kooistra L, Vulsma T & Van Der Meere J An Investigation of Impulsivity in Children with Early-Treated Congenital Hypothyroidism. Dev. Neuropsychol 26, 595–610 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Kooistra L, Stemerdink N, Van Der Meere J, Vulsma T & Kalverboer AF Behavioural Correlates of Early-Treated Congenital Hypothyroidism. Acta Paediatr 90, 1141–1146 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Kooistra L, Meere J. J. Van Der, Vulsma T & Kalverboer AF Sustained Attention Problems in Children with Early Treated Congenital Hypothyroidism. Acta Paediatr 85, 425–429 (1996). [DOI] [PubMed] [Google Scholar]
- 59.Olivieri A, Study T & Hypothyroidism C The Italian National Register Of Infants With Congenital Hypothyroidism: Twenty Years Of Surveillance And Study Of Congenital Hypothyroidism. 5, 1–5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verkerk PH, Buitendijkl SE & Pauline S Congenital Hypothyroidism Screening and The Cutoff for Thyrotropin Measurement: Recommendations from The Netherlands. 83, (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Therrell BL & Adams J Newborn Screening in North America American Academy of Pediatrics American College of Medical Genetics. 447–465 (2007). Doi: 10.1007/S10545-007-0690-Z [DOI] [PubMed] [Google Scholar]
- 62.Newborn Screening Manual: A Guide for Newborn Care Providers. Newborn Screening Ontario. (2015). [Google Scholar]
- 63.Trumpff C et al. No Association Between Elevated Thyroid-Stimulating Hormone at Birth and Parent-Reported Problem Behavior at Preschool Age. Front. Endocrinol 7, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishaik G et al. Hypothyroxinemia of Prematurity and Infant Neurodevelopment: A Pilot Study. J. Dev. Behav. Pediatr. JDBP 21, 172–9 (2000). [PubMed] [Google Scholar]
- 65.Soldin OP, Lai S, Lamm SH & Mosee S Lack of a Relation Between Human Neonatal Thyroxine and Pediatric Neurobehavioral Disorders. Thyroid 13, 193–198 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soldin OP et al. Newborn Thyroxine Levels and Childhood ADHD. Clin. Biochem. 35, 131–136 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson W et al. Maternal Thyroid Hormone Insufficiency During Pregnancy and Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-Analysis. Clin. Endocrinol. (Oxf.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fetene DM, Betts KS & Alati R Maternal Thyroid Dysfunction During Pregnancy and Behavioural and Psychiatric Disorders of Children: A Systematic Review. Eur. J. Endocrinol 177, R261–R273 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Miranda A & Sousa N Maternal Hormonal Milieu Influence on Fetal Brain Development. Brain Behav 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wåhlstedt C, Thorell LB & Bohlin G Heterogeneity in ADHD: Neuropsychological Pathways, Comorbidity and Symptom Domains. 551–564 (2009). Doi: 10.1007/S10802-008-9286-9 [DOI] [PubMed] [Google Scholar]
- 71.Halpherin JM, Sharma V, Greenbatt E & Schwartz ST Assessment of The Continuous Performance Test: Reliability and Validity in a Nonreferred Sample. J. Consult. Clin. Psychol 3, 603–608 (1991). [Google Scholar]
- 72.Wolraich M et al. ADHD: Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 128, 1007–1022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aebi M Accuracy of The DSM -Oriented Attention Problem Scale of the Child Behavior Checklist in Diagnosing Attention-Deficit Hyperactivity Disorder. (2010). [DOI] [PubMed] [Google Scholar]
- 74.Chen WJ, Faraone SV, Biederman J & Tsuang MT Diagnostic Accuracy of the Child Behavior Checklist Scales for Attention-Deficit Hyperactivity Disorder: A Receiver-Operating Characteristic Analysis. J. Consult. Clin. Psychol 62, 1017–25 (1994). [DOI] [PubMed] [Google Scholar]
- 75.Ebesutani C et al. Concurrent Validity of the Child Behavior Checklist DSM-Oriented Scales: Correspondence with DSM Diagnoses And Comparison to Syndrome Scales. J. Psychopathol. Behav. Assess 32, 373–384 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mcgee RA, Clark SE & Symons DK Does the Conners’ Continuous Performance Test Aid in ADHD Diagnosis? J. Abnorm. Child Psychol 28, 415–424 (2000). [DOI] [PubMed] [Google Scholar]
- 77.Lilienfeld SO The Research Domain Criteria (Rdoc): An Analysis of Methodological and Conceptual Challenges. Behav. Res. Ther 62, 129–139 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Motlagh MG et al. Severe Psychosocial Stress and Heavy Cigarette Smoking During Pregnancy: An Examination of the Pre- and Perinatal Risk Factors Associated with ADHD and Tourette Syndrome. Eur. Child Adolesc. Psychiatry 19, 755–764 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Larsson H, Chang Z, D’Onofrio BM & Lichtenstein P The Heritability of Clinically Diagnosed Attention Deficit Hyperactivity Disorder Across the Lifespan. Psychol. Med 1–7 (2013). Doi: 10.1017/S0033291713002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gustavson K et al. Smoking in Pregnancy and Child ADHD. Pediatrics 139, E20162509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sagiv SK, Epstein JN, Bellinger DC & Korrick SA Pre- and Postnatal Risk Factors for ADHD in a Nonclinical Pediatric Population. J. Atten. Disord 17, 47–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sauver JLS et al. Early Life Risk Factors for Attention-Deficit/Hyperactivity Disorder: A Population-Based Cohort Study. Mayo Clin. Proc 79, 1124–1131 (2004). [PubMed] [Google Scholar]
- 83.Linnet KM et al. Maternal Lifestyle Factors in Pregnancy Risk of Attention Deficit Hyperactivity Disorder and Associated Behaviors: Review of the Current Evidence. Am. J. Psychiatry 160, 1028–1040 (2003). [DOI] [PubMed] [Google Scholar]
- 84.Motlagh MG et al. Severe Psychosocial Stress and Heavy Cigarette Smoking During Pregnancy: An Examination of The Pre- and Perinatal Risk Factors Associated with ADHD and Tourette Syndrome. Eur. Child Adolesc. Psychiatry 19, 755–764 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mick E, Biederman J, Faraone SV, Sayer J & Kleinman S Case-Control Study of Attention-Deficit Hyperactivity Disorder and Maternal Smoking, Alcohol Use, and Drug Use During Pregnancy. J. Am. Acad. Child Adolesc. Psychiatry 41, 378–385 (2002). [DOI] [PubMed] [Google Scholar]
- 86.Lee RH et al. Free T4 Immunoassays Are Flawed During Pregnancy. Am. J. Obstet. Gynecol 200, 260.E1–260.E6 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Kahric-Janicic N et al. Tandem Mass Spectrometry Improves the Accuracy of Free Thyroxine Measurements During Pregnancy. Thyroid Off. J. Am. Thyroid Assoc 17, 303–311 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Villanger GD et al. Effects of Sample Handling and Analytical Procedures on Thyroid Hormone Concentrations in Pregnant Women’s Plasma. Epidemiology 28, 365–369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thienpont LM, Van Uytfanghe K, Poppe K & Velkeniers B Determination of Free Thyroid Hormones. Best Pract. Res. Clin. Endocrinol. Metab 27, 689–700 (2013). [DOI] [PubMed] [Google Scholar]
- 90.Groot LJD & Jameson JL Endocrinology Adult and Pediatric: The Thyroid Gland E-Book. (Elsevier Health Sciences, 2013). [Google Scholar]
- 91.Faraone SV, Biederman J & Mick E The Age-Dependent Decline of Attention Deficit Hyperactivity Disorder: A Meta-Analysis of Follow-Up Studies. Psychol. Med 36, 159 (2005). [DOI] [PubMed] [Google Scholar]
- 92.Subcommittee on Attention-Deficit/Hyperactivity Disorder. ADHD: Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 128, 1007–1022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lahey BB, Pelham WE, Loney J, Lee SS & Willcutt E Instability of the DSM-IV Subtypes of ADHD from Preschool through Elementary School. Arch. Gen. Psychiatry 62, 896–902 (2005). [DOI] [PubMed] [Google Scholar]
- 94.Tandon M, Si X & Luby J Preschool Onset Attention-Deficit/Hyperactivity Disorder: Course and Predictors of Stability Over 24 Months. J. Child Adolesc. Psychopharmacol 21, 321–330 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Atkinson J & Braddick O Visual Attention in the First Years: Typical Development and Developmental Disorders. Dev. Med. Child Neurol 54, 589–595 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Miller M, Iosif A-M, Young GS, Hill MM & Ozonoff S Early Detection of ADHD: Insights from Infant Siblings of Children with Autism. J. Clin. Child Adolesc. Psychol 1–8 (2016). Doi: 10.1080/15374416.2016.1220314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papageorgiou KA et al. Individual Differences in Infant Fixation Duration Relate to Attention and Behavioral Control in Childhood. Psychol. Sci 25, 1371–1379 (2014). [DOI] [PubMed] [Google Scholar]
- 98.Skogan AH et al. Inhibition and Working Memory in Young Preschool Children with Symptoms of ADHD and/or Oppositional-Defiant Disorder. Child Neuropsychol 20, 607–624 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Skogan AH et al. Parent Ratings of Executive Function in Young Preschool Children with Symptoms of Attention-Deficit/-Hyperactivity Disorder. Behav. Brain Funct 11, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Korevaar TIM et al. Association of Maternal Thyroid Function During Early Pregnancy with Offspring IQ And Brain Morphology in Childhood: A Population-Based Prospective Cohort Study. Lancet Diabetes Endocrinol 4, 35–43 (2016). [DOI] [PubMed] [Google Scholar]
- 101.WHO. WHO, ICCIDD, UNICEF: Assessment of The Iodine Deficiency Disorders and Monitoring their Elimination. (2007). [Google Scholar]
- 102.Moleti M, Trimarchi F & Vermiglio F Thyroid Physiology in Pregnancy. Endocr. Pract 20, 589–596 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Verloop H, Dekkers OM, Peeters RP, Schoones JW & Smit JWA Genetic Variation in Deiodinases: A Systematic Review of Potential Clinical Effects in Humans. Eur. J. Endocrinol 171, 123–135 (2014). [DOI] [PubMed] [Google Scholar]
- 104.Pearce EN, Andersson M & Zimmermann MB Global Iodine Nutrition: Where Do We Stand In 2013? Thyroid 23, 523–528 (2013). [DOI] [PubMed] [Google Scholar]
- 105.Lazarus JH Iodine Status in Europe in 2014. Eur. Thyroid J 3, 3–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Medici M et al. Women with High Early Pregnancy Urinary Iodine Levels Have an Increased Risk of Hyperthyroid Newborns: The Population-Based Generation R Study. Clin. Endocrinol. (Oxf.) 80, 598–606 (2014). [DOI] [PubMed] [Google Scholar]
- 107.Zimmermann MB & Andersson M Prevalence of Iodine Deficiency in Europe in 2010. Ann. Endocrinol 72, 164–166 (2011). [DOI] [PubMed] [Google Scholar]
- 108.De Benoist B, World Health Organization & Nutrition for Health and Development. Iodine Status Worldwide: WHO Global Database on Iodine Deficiency. (Dept. Of Nutrition for Health and Development, World Health Organization, 2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


