Abstract
T cells play a key role in adaptive immune responses, and shifts among T cell classes occur in normal pregnancy. There is evidence for the role of TH17 cells and dysregulation of the TH17:Treg cell balance in morbidities and autoimmune diseases during pregnancy. Because TH17 responses may play a role in depression and anxiety outside of pregnancy, we hypothesize that TH17 responses and the balance of TH17:Treg activity may also contribute to the development of depression and anxiety during pregnancy. To explore this hypothesis, this review has three main aims: 1) to evaluate systematically the role of TH17 cells and cytokines during pregnancy; 2) to compare changes in the ratio of TH17:Treg cells during pregnancy morbidities with the changes that occur in depression and anxiety outside of pregnancy; and 3) to provide a basis for further research on TH17 cells in perinatal mood and anxiety disorders, with an eye toward the development of novel therapeutics. We also review the limited literature concerning perinatal mood and anxiety disorders, and hypothesize about the potential role of TH17 cells in these illnesses. Understanding the pathophysiology of perinatal mood and anxiety disorders will aid development of novel therapeutics that address immunological mechanisms, in addition to the serotonin system, which are targetable molecules in treating depression and anxiety during pregnancy.
Keywords: pregnancy, perinatal, anxiety, depression, TH17, Treg
1.1. Introduction
Early research into immune functioning during pregnancy focused on pregnancy as a time of immune suppression, with maternal immune responses hypothesized to be down-regulated to avoid rejection of the fetus (Chen et al., 2012). The improvement of some autoimmune diseases, such as multiple sclerosis, in pregnancy was thought to be evidence of immune suppression, but more nuanced assessment of immune responses during pregnancy led to the conclusion that, rather than blanket suppression, there were shifts among different classes of immune cells, including T cells, during normal pregnancy (Larocca et al., 2008). Initial research pointed to a shift away from helper T cell type 1 (TH1; pro-inflammatory) and toward helper T cell type 2 (TH2; anti-inflammatory) cells. This simplistic paradigm has also proved insufficient, as inflammation appears to change throughout pregnancy, and as our knowledge of other immune responses and cells has increased, there has been a paradigm shift away from simplistic assumptions about immunological changes during pregnancy and toward greater analysis and consideration of the kinetics of immune cells and responses during pregnancy (Kraus et al., 2012). One such cell population is TH17 cells, which can be both pro-inflammatory and anti-inflammatory in diverse tissue sites, including within the placenta and reproductive tract during pregnancy.
TH17 cells promote inflammation, help to amplify neutrophilic responses, promote B-cell class switching, activate barrier epithelial cells, and produce antimicrobial peptides (Murphy, 2017). Naïve T cells are prompted to differentiate into TH17 cells in response to IL-6 and transforming growth factor (TGF)-β, secreted primarily by innate immune cells. TH17 cells develop in the presence of IL-23 and mature TH17 cells produce IL-17(A-F) and IL-22, with IL-17 stimulating innate immune cells to enhance the production of IL-6. When TGF-β is present in the absence of IL-6, Treg cells will develop instead of TH17. Certain damage-associated molecular pattern molecules (DAMPs), including especially the alarmin high-mobility group box 1 (HMGB1), also stimulate TH17 responses (Li et al., 2014).
TH17 cells generally induce inflammation and are involved in the pathogenesis of some autoimmune conditions, including multiple sclerosis; in contrast, Treg cells induce tolerance by inhibiting the production and proliferation of cytokines, natural killer (NK) cells, dendritic cells (DCs), and immunoglobulins (Saito et al., 2010). The balance between these two types of CD4+ T cells – one promoting inflammation, the other controlling adaptive immune responses – is tightly regulated by the presence or absence of IL-6, and an enhanced TH17 response (thus throwing the TH17/Treg ratio out of balance) is increasingly thought to be associated with inflammatory morbidities of pregnancy, as well as with symptoms of depression and anxiety outside of pregnancy (Beurel and Lowell, 2017; Saito et al., 2010).
IL-6 is a pleiotropic cytokine produced by many cell types (Tanaka and Kishimoto, 2014) and is a marker of both acute and chronic inflammation. IL-6 has been implicated in a number of pregnancy morbidities that affect outcomes for both mother and fetus, including preeclampsia and preterm birth (Wei et al., 2010), as well as in depression and anxiety outside of pregnancy (Dowlati et al., 2010). The role of TH17 cells in both pregnancy morbidities and mood and anxiety symptoms raises the question of whether the actions of TH17 cells may be a mechanism through which IL-6 is exerting its deleterious effects – and whether we should consider the role of TH17 cells and cytokines in the pathophysiology of perinatal mood and anxiety disorders.
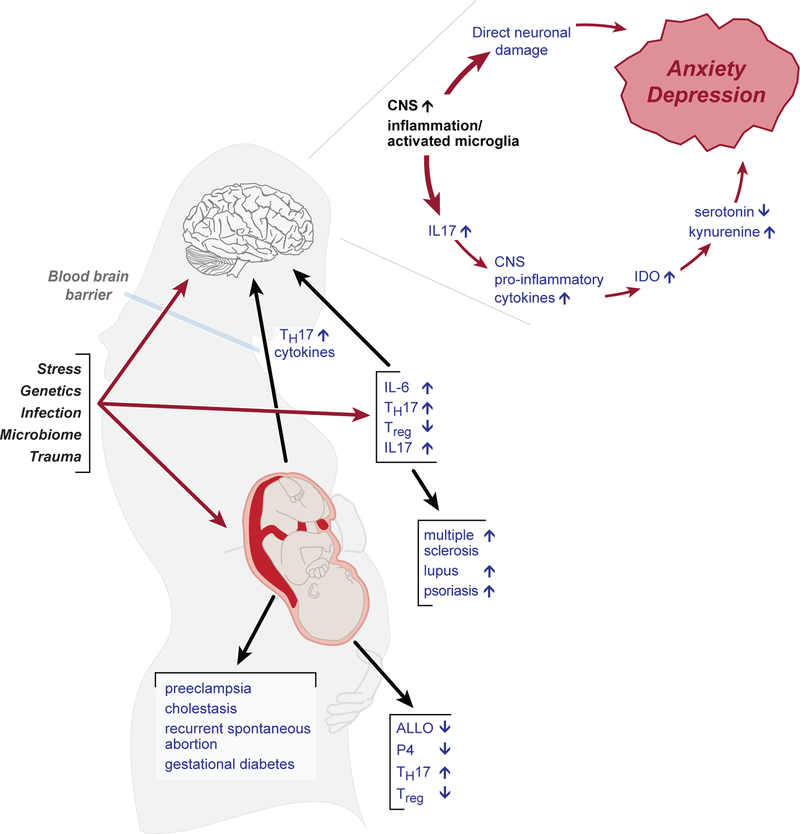
TH17 cells were first identified in 2005, and have since been linked to many inflammatory diseases, including rheumatic diseases (e.g., psoriasis, rheumatoid arthritis, systemic sclerosis, systemic lupus erythmatosus, and undifferentiated spondyloarthropathy), non-rheumatic autoimmune diseases (e.g., multiple sclerosis, autoimmune myocarditis, and endometriosis), pulmonary diseases (e.g., allergies and asthma), atopic dermatitis, inflammatory bowel disease, and periodontal disease (Tesmer et al., 2008). TH17 cells and cytokines also play a role in immune responses to cancer, infection, and transplantation, though the exact nature of this role is still unclear (Knochelmann et al., 2018; Tahmasebinia and Pourgholaminejad, 2017). There is substantial evidence of the involvement of TH17 cells in numerous pregnancy morbidities (e.g., preeclampsia, recurrent spontaneous abortion, cholestasis of pregnancy, and others) and in symptoms of mental illness, especially depression. The presence of increased TH17 cells and their cytokines in the central nervous system leads to inflammation and microglial activation, which can in turn lead to mood and anxiety symptoms in at least two ways – by direct neuronal damage and by induction of indoleamine 2,3, dioxygenase, the enzyme that shunts the metabolism of tryptophan away from serotonin (thus depleting the brain’s supply) and toward the neurotoxic kynurenic acid (see Figure 1) (Anderson et al., 2012).
Fig. 1.
Illustration of our hypothesized model, showing that environmental or genetic triggers may act directly to affect TH17/Treg balance in the brain, the periphery, and the placenta. In the placenta, this imbalance is connected to pregnancy morbidities (such as preeclampsia) and to endocrine dysregulation; in the periphery, to autoimmune disease; and in the brain (crossing in from the periphery across the blood-brain barrier), to depression and anxiety.
Because antenatal depression and anxiety are frequently co-morbid with other pregnancy morbidities that have now been closely associated with TH17 cells (Bansil et al., 2010; Osborne and Monk, 2013), we hypothesize that immunological changes in TH17 cells and cytokines during pregnancy may play a role in perinatal mood and anxiety disorders. The goals of this review are three-fold: 1) to evaluate systematically the role of TH17 cells and cytokines during pregnancy; 2) to compare changes in the ratio of TH17:Treg cells during pregnancy morbidities with the changes that occur in depression and anxiety outside of pregnancy; and 3) to provide a basis for further research on TH17 cells in perinatal mood and anxiety disorders, with an eye toward the development of novel therapeutics. Human literature on all of these topics is as yet limited, and we have elected throughout to make reference to animal literature as well – though animal models are not always reflective of human pathology.
2.1. Healthy Pregnancy
In order to understand the role of enhanced TH17 activity and dysregulation of the TH17:Treg balance during perinatal morbidities, it is important first to understand how these cell types behave throughout healthy pregnancy. Early in pregnancy, to help the blastocyst break through and damage uterine tissue in order to implant (and then repair it), a strong pro-inflammatory environment is required. During the second trimester, fetal growth is prioritized and an anti-inflammatory environment may prevail; whereas in late pregnancy, inflammation is again necessary to induce parturition (Mor et al., 2011). Over the course of pregnancy, paternal allo-antigens challenge the immune system and promote dominance of Tregs over TH17 in order to prevent fetal rejection (Figueiredo and Schumacher, 2016; Saito et al., 2011). But it does not, therefore, follow that TH17 cells are necessarily reduced; some studies show higher levels of circulating cells in pregnant women than in their non-pregnant counterparts in early pregnancy (Wu et al., 2014), while others show that levels of circulating TH17 cells do not actually change across the course of pregnancy (Nakashima et al., 2010), despite the fact that the uterine environment as a whole likely changes from pro to anti-inflammatory and back again. At the maternal-fetal interface, animal studies indicate that adequate frequencies of Tregs are essential for immunological tolerance at implantation (Saito et al., 2010), but this dominance may be a relative increase only (Mjosberg et al., 2009), as TH17 cells are higher in the decidua than they are in the periphery (Saito et al., 2010; Wu et al., 2014) and are perhaps necessary for immunological defense against infection at the maternal-fetal interface, with frequencies that may be increased in pregnancy compared to the non-pregnant state. It may be that the type of TH17 cells at the maternal-fetal interface is different from that found in the periphery, with more gamma-delta T (γδT) cells of a type that produces higher amounts of IL-17. These data suggest a pregnancy-specific recruitment or expansion of IL-17-producing cells at the maternal-fetal interface, with even higher levels in allogeneic as opposed to syngeneic pregnancies (Pinget et al., 2016). TH17 products (e.g., IL-17) in the human placenta (both term and those from spontaneous abortions) play a role in angiogenesis and immune regulation, which is not exhibited in mouse placentae (Pongcharoen et al., 2007). Thus, IL-17 may play a key role in placental development, and current mouse models may not be useful in describing this role.
Whether and how TH17 cells at the maternal-fetal interface are active in pregnancy (and at what times in pregnancy) depends not only on the presence of these cells and their regulatory counterparts, but also on the actions of hormones and signaling pathways that change across pregnancy. Animal studies illustrate that progesterone (P4) inhibits the expression of IL-17 in a dose-dependent fashion (Maeda et al., 2013). Progesterone also reduces the expression of IL-6 throughout normal pregnancy, with a sharp burst in expression at parturition (Telleria et al., 1998). Reduced IL-6 will in turn reduce the progression of naïve T cells to TH17 cells, and numerous studies have shown that optimal IL-6 regulation, with levels neither up or down, may be crucial to the avoidance of pregnancy morbidities and to carrying successful pregnancies to term (Markert et al., 2011). Leptin and ghrelin are also important. T cells express receptors for both, with pro-inflammatory leptin increased in early pregnancy and anti-inflammatory ghrelin increased in later pregnancy. The combined effect of the two is to enhance the presence of Tregs in early pregnancy and promote the presence of IL-17A in later pregnancy (Orlova and Shirshev, 2014).
Co-stimulatory pathways that are activated within TH17 and Treg cells may also play fundamental roles in the outcome of pregnancy. One such pathway is that of PD-1-PDL-1. PDL-1 is an immune regulatory molecule expressed on a variety of immune cells, and especially on macrophages. It is induced by IFN-γ and by TH1 cells. PDL-1 blockade has been associated with an increase in embryo resorption, a decrease in Treg cells, and an increase in TH17 cells, with resulting deleterious effects on fetomaternal tolerance; moreover, the increase in TH17 arises from cells newly differentiated from naïve T cells (rather than those converted from Treg). In one study, neutralizing IL-17 reversed the effect on fetal survival (D’Addio et al., 2011).
While our understanding of the role of TH17 and Tregs in healthy pregnancy is still evolving, the balance between defense and tolerance is crucial to sustaining a healthy pregnancy, and that balance changes across pregnancy, with Tregs higher in early pregnancy to promote fetal tolerance and lower in late gestation to promote the initiation of parturition by pro-inflammatory forces (Figueiredo and Schumacher, 2016). When this balance goes awry, pregnancy morbidities ensue – and it will be the argument of this paper that those morbidities likely include perinatal anxiety and depression.
2.2. Pregnancy Morbidities
2.2.1. Recurrent spontaneous abortion (RSA)
Recurrent spontaneous abortion (RSA) is a relatively common, multifactorial problem affecting 1–2% of reproductive women; in women with untreated antenatal depression, that rate is increased, leading to speculation about common etiologies (Field et al., 2006). Over half of women with RSA have an autoimmune condition, with antiphospholipid syndrome being the most common. Studies into other causes have focused on NK cells and TH1 cells, and, more recently, on TH17 and Treg cells. Findings have included higher proportions of TH17 cells and the cytokines they produce, including IL-17 and IL-23, in both peripheral blood and decidual tissue; gene polymorphisms; and increases in TH17 and decreases in Tregs (either peripheral or decidual) during early pregnancy in subjects with RSA compared to normal pregnancy. In addition, fetal loss has been induced by exogenously administered IL-17 in healthy mouse models, and improved pregnancy outcomes have been achieved in RSA subjects treated with intravenous immunoglobulin to address a TH17:Treg imbalance. These findings and others have been thoroughly reviewed elsewhere (Fu et al., 2014; Lee et al., 2012; Liu et al., 2017). Together, these findings make a strong argument for a role for enhanced TH17 activity in early pregnancy in women with RSA, and the literature supporting a decrease in fetal loss when IL-17 is blocked makes a strong case for this dysregulation as at least in part a causative mechanism. This is plausible, as Treg cells are known to regulate the maternal response to the fetus in normal pregnancy, and a dearth of them could therefore lead to an overactive immune response (from TH17 cells as well as other immune actors) that would result in pregnancy loss. Other literature points to upregulated activity of the HMGB1 gene in women with recurrent pregnancy loss (though the timing during pregnancy is unspecified), further indirect evidence of enhanced TH17 activity as this alarmin increases TH17 activity (Jin et al., 2015).
2.2.2. Preeclampsia.
Preeclampsia, a condition characterized by high blood pressure and proteinuria, usually after the 20th week of pregnancy, affects up to 7% of pregnancies worldwide and is a substantial cause of morbidity; some (but not all) literature shows an elevated risk in women with antenatal depression (Osborne and Monk, 2013), which may indicate shared pathophysiology. The exact cause in unknown, though research has implicated pre-existing maternal pathology (e.g., hypertension) and abnormal placentation, along with immunologic factors (Jido and Yakasai, 2013). Possible mechanisms include rejection of the fetus by an overactive immune response causing inflammation that affects placentation, angiogenesis, and endothelial dysfunction; the fact that women who have not been exposed to paternal immune priming (i.e., donor embryos) and those with a weaker exposure (e.g., first-time mothers, those with new partners, those with a longer break between children) have higher rates of preeclampsia lends credence to this theory. Numerous studies have found increased TH17 cells or an increase in the TH17/Treg ratio in preeclamptic women compared to normotensive and/or non-pregnant subjects, when measured either in peripheral blood, decidual tissue, or umbilical cord blood, while others have found correlations between IL-17 and increased blood pressure or intrauterine growth restriction; a substantial literature also supports a role for elevated IL-6 (a key player in the differentiation of TH17 cells) in preeclampsia. These and other findings have been extensively reviewed elsewhere (Cornelius and Lamarca, 2014; Fu et al., 2014; Lau et al., 2013; Saito, 2010). Other studies have found elevated levels of HMGB1, a marker for increased TH17 activity, at times in conjunction with increased levels of IL-17; most of these studies examined either late pregnancy or the postpartum period, but some also confirmed results in those with early onset (first trimester) preeclampsia (Brien et al., 2018; Chen et al., 2016; Li et al., 2018; Shao et al., 2016; Xu et al., 2018; Zhu et al., 2015).
2.2.3. Other pregnancy morbidities.
Although the amount of research on RSA and preeclampsia far outweighs that available for other pregnancy morbidities, there is some limited evidence for involvement of a TH17 bias in preterm birth, chorioamnionitis, cholestasis of pregnancy, and gestational diabetes, and these findings have not been reviewed elsewhere. For preterm birth a considerable amount of research links increased local IL-6 in the cervix (Wei et al., 2010), but evidence of TH17 involvement on a systemic basis is limited and conflicting. TH17 cells have been found to be decreased, and Tregs increased, in the mouse spleen just prior to preterm birth (Arenas-Hernandez et al., 2016), and IL-17 has been found to be lower in serum just before delivery (Hee et al., 2011) and higher in amniotic fluid at delivery (Ito et al., 2010) when subjects with preterm birth (at gestational week 24 and above) were compared to those with normal term pregnancies. HMGB1 has also been found to be elevated at birth in cases of preterm birth (Buhimschi et al., 2009; Romero et al., 2011). For chorioamnionitis, levels of both IL-17 (Ito et al., 2010) and IL-6 (Yoon et al., 1995) have been found to be elevated at birth in amniotic fluid, and several studies point to a role for elevated HMGB1 (Qiu et al., 2017; Romero et al., 2012). In intrahepatic cholestasis of pregnancy (ICP), a common condition in which the normal flow of bile is affected by pregnancy hormones, resulting in a buildup of bile salts, intense itching for the mother, and higher rates of fetal distress, preterm birth, and stillbirth, IL-17 has been shown to be elevated in the third trimester in both serum and placenta of women with ICP compared to normal pregnant controls (Kirbas et al., 2016; Kong et al., 2017). In gestational diabetes, there has been little research to date on TH17 cells or the cytokines they produce directly, but some evidence concerning IL-6 – that higher serum IL-6 in early pregnancy predicted the later development of gestational diabetes in a group of women at high risk (Abell et al., 2017), and that women with early onset gestational diabetes had higher serum levels of IL-6, and those with late-onset gestational diabetes higher levels of sIL-6R, when compared with normal pregnant women (Kuzmicki et al., 2014). One study found that serum HMGB1 was related to hyperglycemia and also independently to diagnoses of gestational diabetes in the third trimester (Giacobbe et al., 2016). Taken together, the literature on these other pregnancy morbidities is too limited to make any kind of judgment about the role of TH17 cells, even in areas such as gestational diabetes, preterm birth, and chorioamnionitis that are known to have an inflammatory component and have also been associated with antenatal depression (Osborne and Monk, 2013) – but there are enough positive signals (some increases in IL-17, some in IL-6 or HMGB1) to make further study in each of these warranted.
2.3. Asthma and Autoimmune Disease in Pregnancy.
Both asthma and autoimmune disease have increased prevalence in individuals with depression and anxiety (Andersson et al., 2015; Gao et al., 2015), though they have been explored little in antenatal depression and anxiety (and one recent review found that asthma was not associated with perinatal mental illness (Brown et al., 2018). The behavior of these diseases in pregnancy, however, indicates that this is an area ripe for future research. Certain autoimmune diseases (e.g., rheumatoid arthritis [RA] and multiple sclerosis [MS]) go into relative remission in pregnancy but flare in the postpartum, while others (e.g., systemic lupus erythematosus [SLE]) can flare during pregnancy; asthma and atopic diseases are variable (Fischer-Betz and Specker, 2017; Grosso et al., 2018; Voskuhl and Momtazee, 2017). A limited literature points to the role of TH17 in these patterns of inflammatory disease remissions and flares during pregnancy. Subjects with multiple sclerosis have been found to have no difference from healthy controls in the proportion of TH17 or in the pattern of change in Treg cells across pregnancy and postpartum (Neuteboom et al., 2010). Those with inflammatory bowel disease have a marked reduction in IL-17A from pre-conception to the second trimester (Seow et al., 2015). Subjects with SLE, on the other hand, have been found to have increased secretion of IL-17, IL-6 and TNF-α at various pregnancy timepoints (as well as outside of pregnancy) when compared to healthy controls (Su et al., 2001; Torricelli et al., 2011). Women with autoimmune antithyroid antibodies have been shown to have increased IL-17 (Turhan Iyidir et al., 2015) and IL-6 (Oztas et al., 2015) in early pregnancy when compared to healthy controls. Finally, several groups have looked at asthma in pregnancy, with one finding that pregnant asthmatics (>18 weeks gestation) have an enhanced IL-17 response compared to healthy pregnant controls (Vanders et al., 2013) and another finding that all asthmatic women (pregnant and not) had elevated prevalence of TH17 cells and pregnant asthmatic (weeks 20–35) women had blunting of Tregs when compared to healthy nonpregnant women; this resulted in an increased TH17/Treg ratio for asthmatic pregnant women when compared to healthy pregnant women (Toldi et al., 2011). Taken together, these data on autoimmune disease may indicate that TH17 activity is increased (and/or Treg activity decreased) when autoimmune or atopic disease flares in pregnancy, opening up a theoretically plausible reason for the comorbidity of these diseases with antenatal depression and anxiety. While there is currently no literature on HMGB1 and asthma/autoimmune disease in pregnancy, there are consistent findings of elevations of HMGB1 in these conditions outside of pregnancy (as reviewed in (Imbalzano et al., 2017; Zhu et al., 2018)), suggesting an area ripe for new research.
An increase in TH17 cells and cytokines and/or an imbalance in the TH17/Treg ratio is thus an integral characteristic of numerous pregnancy morbidities, with the greatest amount of evidence concerning recurrent spontaneous abortion and preeclampsia. Given the comorbidity of these conditions with antenatal depression and anxiety, it is a logical next step to examine how these cells behave in pregnant women with depression and anxiety, who are now thought by some to represent an inflammatory subtype of depression (Osborne and Monk, 2013). Because the evidence in the perinatal literature is sparse, we must begin by looking at psychiatric illness beyond pregnancy and extrapolating from these examples.
2.4. Depression and Anxiety Outside of Pregnancy
2.4.1. Depression.
The animal and human literature linking depression to elevation of pro-inflammatory markers is substantial (Miller and Raison, 2016). Inflammation has been noted to increase depressive symptoms, both when inflammatory agents are given directly (e.g., in the treatment of Hepatitis C virus infection with interferons (Machado et al., 2017)) and when inflammation is noted in people who are depressed. Numerous studies exist of elevated proinflammatory cytokines in depression, and the cytokines most frequently implicated include IL-6, IL-1β, and TNF-α (Dowlati et al., 2010). The fact that each of these cytokines is involved in the differentiation of TH17 cells has led researchers to investigate the involvement of TH17 in depression. There are several reviews of the role of TH17 and/or their cytokines in depression (Beurel and Lowell, 2017; Slyepchenko et al., 2016; Waisman et al., 2015), the most comprehensive of which posit theoretical mechanisms for the involvement of TH17 cells in depression. Although it is not yet known how closely measures of TH17 function in the periphery are mirrored in the central nervous system (CNS), it is clear that peripheral immune cells can access the CNS through lymphatic vessels, leaky areas of the blood-brain barrier, and possibly the choroid plexus; they are also extremely plastic and can differentiate from other T cell populations once in place (Baruch et al., 2016). TH17 cells and/or IL-17A are present in the CNS in other related disorders, including multiple sclerosis, autism spectrum disorders, epilepsy, and dementia (Tahmasebinia and Pourgholaminejad, 2017). Once present in the brain, there are numerous mechanisms by which TH17 cells and IL-17A may instigate depression, including activating astrocytes and microglia and stimulating the production of other pro-inflammatory cytokines (Beurel and Lowell, 2017). Those pro-inflammatory cytokines may in turn affect serotonin production by enhancing the actions of indoleamine 2,3 dioxygenase (IDO), the enzyme that preferentially shunts the metabolism of tryptophan away from serotonin and down the kynurenine pathway (Roomruangwong et al., 2018).
Transfer of TH17 cells to male mice results in characteristic of depression, including learned helplessness. Those mice that were deficient in RORγT (i.e., the transcription factor that regulates TH17 cell differentiation), as well as wild-type mice given an inhibitor of RORγT along with the TH17 cells, had reduced levels of learned helplessness when compared with mice that were administered undifferentiated CD4+ T cells or vehicle (Beurel et al., 2013). In a mouse model of psoriatic inflammation, increased levels of IL-17A correlated with increased depressive-like symptoms, which were reduced when an IL-17A inhibitor was administered; administering IL-17A alone to wild-type mice led to the same depressive-like symptoms (Nadeem et al., 2017). (In contrast, examinations of the spleen have turned up contradictory findings, including decreased TH17 splenic lymphocytes and increased splenic Treg cells (and TGF-β) in a mouse model of chronic unpredictable mild stress-induced depression (Hong et al., 2013), possibly indicating that results in the periphery ought not to be compared to those in lymphoid tissue). In human literature, subjects with major depressive disorder have been found to have a significant increase in circulating TH17 cells and a significant decrease in Treg cells (and thus an increased ratio) when measured by flow cytometry; increased levels of RORγT; and higher levels of circulating IL-17 when compared to healthy controls (Chen et al., 2011); and increases in both IL-17 and the stimulating cytokine TGF-β compared to age-matched controls (Davami et al., 2016). Mouse models have indicated that elevated HMGB1 is associated with increased depressive-like symptoms as well (Franklin et al., 2018; Wang et al., 2018; Wu et al., 2015).
Not all studies have found a positive association between TH17 and depression, however. One study found a decrease in the number of TH17 cells in depressed subjects compared to age and gender-matched healthy controls, but also found a decrease in the number of Treg cells from depressed subjects when the sample was restricted to those over 28 years of age (Grosse et al., 2016). Others have found no differences in plasma levels of IL-17A between a group with late-life depression and a group of healthy matched controls (Saraykar et al., 2017), or a decreased level of IL-17A in patients with recurrent depressive disorder (Rybka, 2013). This seemingly contradictory literature, in both animals and humans, may perhaps be explained by differences between acute depressive symptoms and those of chronic depression, which some have posited represent two different states of immune regulation, with activation at the time of acute symptoms and suppression in the face of chronic symptoms (Hong et al., 2013).
There also are many examples of studies highlighting the role of IL-6 in depression and depressive symptoms, in both human and animal literature. The vast majority of these studies, as reviewed in (Hodes et al., 2016), find a positive correlation between elevated IL-6 and symptoms of depression, or between lack of IL-6 and resistance to depressive-like symptoms. Elevated IL-6 in depression may also be directly related to level of traumatic experiences (Bob et al., 2010). Gimeno and colleagues measured IL-6 and cognitive symptoms of depression in a group of British civil servants at baseline and approximately 12 years later, and found that inflammatory markers at baseline predicted depressive symptoms at follow-up, but not vice versa (Gimeno et al., 2009). A few other studies find no relationship or opposite findings (i.e., decreased IL-6 in depressed populations (Carpenter et al., 2004; Podlipný et al., 2010). The Kern group found that decreased IL-6 predicted future depression, but that increased IL-6 was associated with concurrent depression in a population of older women (Kern et al., 2013, 2014).
Finally, the normalization of IL-6 in depressed subjects after treatment, as well as resistance to the antidepressant effects of medication in the presence of centrally administered IL-6, lends further support to the notion that IL-6 and the cells and cytokines induced by it may play a role in symptoms of depression (Sukoff Rizzo et al., 2012b). Mechanisms used to reduce both IL-6 and depressive symptoms include exercise (Lavebratt et al., 2017), anti-depressants (Hiles et al., 2012), anti-inflammatory agents (Abbasi et al., 2012), and electroconvulsive therapy (ECT) (Järventausta et al., 2017). In addition, IL-6 has been found to be a marker of antidepressant response to ketamine, raising the possibility that at least one mechanism to explain ketamine’s efficacy may lie in its ability to reduce inflammation (Yang et al., 2015).
If IL-6 and consequent dysregulation of the TH17/Treg ratio plays a role in antenatal depression, then any evidence on sex differences in IL-6 in depression would be informative. Transgenic mice with an overproduction of IL-6 in the CNS exhibited an increase in depression-like behaviors (Sukoff Rizzo et al., 2012a); the increase was stronger in male mice, whereas female mice had a stronger increase in anxiety-like behaviors. Tsuboi and colleagues found a positive correlation between IL-6 and depressive symptoms in women alone (Tsuboi et al., 2014). And Jha and colleagues recently found that increased IL-17 correlated with increased anhedonia in men only, with no relationship found for women (Jha et al., 2018)
In summary, there is a small number of studies directly examining the relationship between depression and TH17 cells and the cytokines they produce. While this literature is yet sparse, most studies do show an increase in TH17 and/or the TH17/Treg ratio in depression – though the one study that accounted for sex differences found that effect to be driven by men only. The literature linking IL-6 to depression, on the other hand, is vast, and almost uniformly shows a positive correlation between levels of IL-6 and depression outside the perinatal period, as well as a normalization with antidepressant treatment; studies that account for sex differences are too few to draw any conclusions.
2.4.2. Anxiety.
There is a vast literature concerning the role of the hypothalamic pituitary adrenal (HPA) axis and the stress response system in anxiety, but surprisingly few attempts to investigate immune functioning in anxious individuals. In the perinatal period, it is vital that we begin to expand our investigations of the biological causes of anxiety, as anxious symptoms may be at least as common as depressive symptoms in the perinatal period, and anxiety disorders are frequently comorbid with depression (Goodman et al., 2014). The literature linking anxiety outside of pregnancy to a TH17 cell imbalance so far is limited. When compared to cells of healthy controls, in vitro cell culture from individuals with generalized anxiety disorder shows an increased proportion of TH17 cells (Vieira et al., 2010), and a dominant TH17 phenotype in cells from individuals with GAD but not controls was enhanced by the administration of dopamine (Ferreira et al., 2011) and of Substance P (Barros et al., 2011). Several groups have reported on the role of IL-6, with one group finding that greater anxious attachment was significantly associated with higher levels of IL-6 following following coronary artery bypass surgery (Kidd et al., 2014) and another group finding that IL-6 expression increased with increasing anxiety scores on the Hospital Anxiety and Depression Scale, and that IL-6 expression in anxious individuals was also strongly correlated with DNA methylation of important epigenetic regulatory enzymes DNMT1 and EZH2 (Murphy et al., 2015). O’Donovan and colleagues, in the only study to address sex differences, found that IL-6 was elevated in individuals with clinically significant anxiety regardless of sex, age, or depressive symptoms (O’Donovan et al., 2010). Studies in anxiety thus are extremely limited, but those that exist support a possible role in anxiety for TH17 cells and IL-6.
2.5. Perinatal Depression and Anxiety
The literature cited above may convince the reader of the theoretical plausibility of the involvement of TH17 in perinatal depression and anxiety, but is there any direct evidence? Yes, but it is limited, confined mostly to evidence about IL-6 rather than about TH17 directly, and conflicting. The only study to look directly at a marker of TH17 activity (IL-17C), in the context of a study on 92 inflammatory markers at gestational weeks 35–39 in women with antenatal depressive symptoms (as defined by EPDS ≥ 13), women on SSRIs, and healthy controls, found that IL-17C was significantly lower in the women with antenatal depressive symptoms and the women on SSRIs when compared to the healthy controls, and found no relationship between IL-6 and depressive symptoms. This finding stands in contrast to the literature (undifferentiated by sex) for depression outside of pregnancy, and may indicate that this relationship differs by sex as well as by stage of pregnancy (Edvinsson et al., 2017). All other studies have considered IL-6 only. Walsh and colleagues found that interaction with early life adversity explained lower IL-6 levels in depressed pregnant adolescents at the second trimester (Walsh et al., 2016). One group found that elevated IL-6 at delivery predicted the later development of PPD (Liu et al., 2016), while another group found that it did not (Skalkidou et al., 2009). Increases in IL-6 at single-time measurements in pregnant (various time points) or early postpartum women with self-reported depressive symptoms compared to healthy controls (Azar and Mercer, 2013; Boufidou et al., 2009; Cassidy-Bushrow et al., 2012; Christian et al., 2009; Haeri et al., 2013; Maes et al., 2000) are also consistently reported. Osborne and colleagues recently examined IL-6 (and other pro-inflammatory cytokines) across pregnancy and up to 6 months postpartum, comparing women with higher depressive symptoms (as measured by the Beck Depression Inventory) to those with lower symptoms, and found significant differences in the slope of change across time as well as significant increases in IL-6 levels at the third trimester for more depressed women (Osborne et al., 2018a). Another recent study examined women with major depressive disorder (defined by DSM criteria) in the third trimester of pregnancy, and found elevated levels of IL-6 for the depressed women compared to healthy controls (Osborne et al., 2018b), while another found that IL-6 did not correlate with concurrent depressive symptoms in the third trimester of pregnancy but was a significant predictor of postpartum scores on the Edinburgh Postnatal Depression Scale (EPDS) (Simpson et al., 2016). Corwin and colleagues found no relationship between IL-6 and depressive symptoms when measured in the third trimester or early postpartum, but found that cortisol AUC increased with increasing levels of IL-6 for women with depressive symptoms but not for those without at 14 days postpartum (Corwin et al., 2015). Other studies have found no relationship at gestational week 24 (Karlsson et al., 2017) or at week 18 or 32 (Blackmore et al., 2011). The majority of the literature thus finds a correlation between increased depressive symptoms and increased IL-6, but not all studies support this.
Very few studies have tackled anxiety in the peripartum, with none looking directly at IL-17 or other markers of TH17 activity. Maes and colleagues found that higher IL-6 was correlated with higher STAI scores at 1 day postpartum (Maes et al., 2000). In a published poster abstract only, Fishe and colleagues found higher IL-6 levels in those with pregnancy-specific anxiety in the second trimester (Fishe et al., 2013). Osborne and colleagues found elevated levels of IL-6 among subjects with higher anxiety during late pregnancy and early postpartum (Osborne et al., 2018a). A study of infertile couples trying to conceive found that anxiety was correlated with stress, and stress with increased cervicovaginal IL-6 and decreased serum TGF-β1,and with a higher likelihood of IVF failure (Haimovici et al., 2018). As with depression, the results are not uniform, however. No relationship was found between IL-6 and either general or pregnancy-specific anxiety at gestational week 24 (though relationships were found with other cytokines not related to TH17) (Karlsson et al., 2017). IL-6 did not differ between African American women with high vs. low anxiety when measured on average at gestational week 20 (Catov et al., 2015), nor was it related to anxiety in a study of diverse women at high psychosocial risk when measured at gestational weeks 18 and 32 (Blackmore et al., 2011). These mixed findings, and the lack of any studies directly measuring IL-17 or other direct markers, make it impossible to draw any consistent conclusions about TH17 cells and anxious pregnancy.
3.1. Discussion.
There is substantial evidence of a role for TH17 dysregulation in numerous pregnancy morbidities, as well as more limited but growing evidence of a role for TH17 dysregulation in depression and anxiety outside of pregnancy and inconsistent evidence of IL-6 dysregulation (which may in turn lead to TH17 dysregulation) for depression and anxiety in pregnancy. The TH17 cells and cytokines produced in both the decidua and periphery in pregnancy morbidities, such as preeclampsia and recurrent spontaneous abortion, can easily cross into the CNS, promote disruption of the blood brain barrier (Kebir et al., 2007), and are found in higher numbers in the cerebrospinal fluid of individuals with other neuroinflammatory conditions such as multiple sclerosis (Matusevicius et al., 1999). While some of these data come from animal literature – which makes them inherently limited in informing our understanding of human physiology – there is a growing amount of data from human studies as well.
Could TH17 dysregulation be the missing link that connects pregnancy morbidities to antenatal depression and anxiety? Substantial (though not uniformly positive) literature links preeclampsia, preterm birth, and gestational diabetes to antenatal depression (as reviewed in (Osborne and Monk, 2013)), as well as to immune dysregulation – but the evidence is scattered and inconclusive on how or whether immune dysregulation is directly related to antenatal depression and anxiety. Perhaps this is because we are not asking the right questions. Most studies thus far are cross-sectional and are limited to examinations of a single or a few cytokines in the peripheral blood. If any consideration is given to T cell dysregulation, it is on the lines of the old TH1/TH2 paradigm. If the immune dysregulation that characterizes antenatal depression and anxiety is associated with shifts in immune cell populations, we are not using the right tools or the right lens to examine this question. Future studies should examine broader groups of cytokines, across time; immune cell phenotypes, both peripherally and centrally; and what happens to those immune cells when stimulated by a stressor. Moreover, studies must distinguish between the role of immune cells measured directly and that of cytokines, many of which are notoriously pleiotropic and may indicate the presence of more than one type of immune response. (IL-17, for example, the quintessential TH17 cytokine, is also associated with innate lymphoid cells – so studies that look only at IL-17 and not directly at cells may in fact be measuring innate lymphoid rather than T-cell pathology.) Only when we use the appropriate tools will we be able to answer our questions about what role the immune system may play in the mood and anxiety symptoms that plague up to 39% of pregnant women (as reviewed in (Goodman et al., 2014)) and affect their health and that of the generations to come. Symptoms of depression and anxiety have been linked to adverse pregnancy outcomes (such as preterm birth and growth restriction) as well as adverse outcomes in children, including increased rates of internalizing disorders, attention disorders, and conduct disorders (Capron et al., 2015; Field, 2011; O’Donnell et al., 2017).
Developing this knowledge will not only help us to understand mechanism; it may also help us to develop novel therapeutics. The target in nearly all antidepressant drugs remains serotonin – but the success of some anti-inflammatory treatments as adjunctive antidepressants (Faridhosseini et al., 2014); the recent though short-lived successes of ketamine (IL-6 has been found to be elevated in ketamine responders) (Yang et al., 2015); and the FDA breakthrough status given to a synthetic version of allopregnanolone (a progesterone metabolite, which may also act through inhibiting pro-inflammatory cytokines including IL-17 and IL-6)(Kanes et al., 2017), the first drug to be proposed to the FDA for postpartum depression specifically, indicate that drugs that affect immune dysregulation may be an exciting new target for antidepressant (and anti-anxiety) action in the perinatal period.
Highlights.
Immune dysregulation in pregnancy is known to be related to pregnancy morbidities, and recent literature supports a role for shifts among T cell classes
There is also evidence for the role of TH17 cells and dysregulation of the TH17:Treg cell balance in depression and anxiety outside of pregnancy
This paper combines the evidence about pregnancy morbidities and that concerning depression and anxiety to hypothesize about the role of TH17:Treg imbalance in antenatal depression and anxiety
Understanding the relationship between immune dysregulation and perinatal mood and anxiety disorders can help develop novel targets for treatment
Acknowledgments
The authors wish to acknowledge Cate Kiefe, who created the illustration used as Figure 1; Bridget Sundel and Aaron Lieberman, who assisted with organization of references; and the members of the Sabra Klein laboratory, who provided feedback on the illustration and manuscript structure. Funding: This work was supported by the National Institute of Mental Health, 1K23 MH110607-01A1.
Role of the Funding Source: Dr. Osborne’s work on this project has been supported by the National Institute of Mental Health, 1K23 MH110607-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None
References
- Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S, 2012. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: Randomized double-blind placebo-controlled study. Journal of Affective Disorders 141, 308–314. [DOI] [PubMed] [Google Scholar]
- Abell SK, Shorakae S, Harrison CL, Hiam D, Moreno-Asso A, Stepto NK, De Courten B, Teede HJ, 2017. High molecular weight adiponectin, omentin-1 and interleukin-6 in early pregnancy are associated with later development of gestational diabetes. Endocrine Reviews 38. [Google Scholar]
- Anderson G, Maes M, Berk M, 2012. Inflammation-related disorders in the tryptophan catabolite pathway in depression and somatization. Advances in protein chemistry and structural biology 88, 27–48. [DOI] [PubMed] [Google Scholar]
- Andersson NW, Gustafsson LN, Okkels N, Taha F, Cole SW, Munk-Jorgensen P, Goodwin RD, 2015. Depression and the risk of autoimmune disease: a nationally representative, prospective longitudinal study. Psychol Med 45, 3559–3569. [DOI] [PubMed] [Google Scholar]
- Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez-Lopez N, 2016. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cellular & molecular immunology 13, 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar R, Mercer D, 2013. Mild depressive symptoms are associated with elevated C-reactive protein and proinflammatory cytokine levels during early to midgestation: a prospective pilot study. Journal of women’s health (2002) 22, 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansil P, Kuklina EV, Meikle SF, Posner SF, Kourtis AP, Ellington SR, Jamieson DJ, 2010. Maternal and fetal outcomes among women with depression. Journal of women’s health (2002) 19, 329–334. [DOI] [PubMed] [Google Scholar]
- Barros PO, Ferreira TB, Vieira MM, Almeida CR, Araujo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Andrade AF, Bento CA, 2011. Substance P enhances Th17 phenotype in individuals with generalized anxiety disorder: an event resistant to glucocorticoid inhibition. J Clin Immunol 31, 51–59. [DOI] [PubMed] [Google Scholar]
- Baruch K, Deczkowska A, Rosenzweig N, Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, Kertser A, David E, Amit I, Schwartz M, 2016. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nature medicine 22, 135–137. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS, 2013. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biological Psychiatry 73, 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Lowell JA, 2017. Th17 cells in depression. Brain, Behavior, and Immunity [DOI] [PMC free article] [PubMed]
- Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG, 2011. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosom Med 73, 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bob P, Raboch J, Maes M, Susta M, Pavlat J, Jasova D, Vevera J, Uhrova J, Benakova H, Zima T, 2010. Depression, traumatic stress and interleukin-6. Journal of Affective Disorders 120, 231–234. [DOI] [PubMed] [Google Scholar]
- Boufidou F, Lambrinoudaki I, Argeitis J, Zervas IM, Pliatsika P, Leonardou AA, Petropoulos G, Hasiakos D, Papadias K, Nikolaou C, 2009. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord 115, 287–292. [DOI] [PubMed] [Google Scholar]
- Brien ME, Boufaied I, Soglio DD, Rey E, Leduc L, Girard S, 2018. Distinct inflammatory profile in preeclampsia and postpartum preeclampsia reveal unique mechanisms. Biol Reprod [DOI] [PubMed]
- Brown HK, Qazilbash A, Rahim N, Dennis CL, Vigod SN, 2018. Chronic Medical Conditions and Perinatal Mental Illness: A Systematic Review and Meta-Analysis. American journal of epidemiology [DOI] [PubMed]
- Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G, Bhandari V, Ehrenkranz RA, Weiner CP, Madri JA, Buhimschi IA, 2009. Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. The American journal of pathology 175, 958–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron LE, Glover V, Pearson RM, Evans J, O’Connor TG, Stein A, Murphy SE, Ramchandani PG, 2015. Associations of maternal and paternal antenatal mood with offspring anxiety disorder at age 18 years. J Affect Disord 187, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Heninger GR, Malison RT, Tyrka AR, Price LH, 2004. Cerebrospinal fluid interleukin (IL)-6 in unipolar major depression. Journal of Affective Disorders 79, 285–289. [DOI] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN, 2012. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J Reprod Immunol 94, 202–209. [DOI] [PubMed] [Google Scholar]
- Catov JM, Flint M, Lee M, Roberts JM, Abatemarco DJ, 2015. The relationship between race, inflammation and psychosocial factors among pregnant women. Maternal and child health journal 19, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yin YX, Wei J, Tong M, Shen F, Zhao M, Chamley L, 2016. Increased expression of high mobility group box 1 (HMGB1) in the cytoplasm of placental syncytiotrophoblast from preeclamptic placentae. Cytokine 85, 30–36. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Liu YL, Sytwu HK, 2012. Immunologic regulation in pregnancy: from mechanism to therapeutic strategy for immunomodulation. Clinical & developmental immunology 2012, 258391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, Sun Y, 2011. Emerging tendency towards autoimmune process in major depressive patients: A novel insight from Th17 cells. Psychiatry Research 188, 224–230. [DOI] [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, Iams JD, 2009. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun 23, 750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius DC, Lamarca B, 2014. TH17- and IL-17- mediated autoantibodies and placental oxidative stress play a role in the pathophysiology of pre-eclampsia. Minerva Ginecologica 66, 243–249. [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Pajer K, Paul S, Lowe N, Weber M, McCarthy DO, 2015. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav Immun 49, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addio F, Riella LV, Mfarrej BG, Chabtini L, Adams LT, Yeung M, Yagita H, Azuma M, Sayegh MH, Guleria I, 2011. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. Journal of immunology (Baltimore, Md. : 1950) 187, 4530–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davami MH, Baharlou R, Ahmadi Vasmehjani A, Ghanizadeh A, Keshtkar M, Dezhkam I, Atashzar MR, 2016. Elevated IL-17 and TGF-beta Serum Levels: A Positive Correlation between T-helper 17 Cell-Related Pro-Inflammatory Responses with Major Depressive Disorder. Basic Clin Neurosci 7, 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Edvinsson A, Brann E, Hellgren C, Freyhult E, White R, Kamali-Moghaddam M, Olivier J, Bergquist J, Bostrom AE, Schioth HB, Skalkidou A, Cunningham JL, Sundstrom-Poromaa I, 2017. Lower inflammatory markers in women with antenatal depression brings the M1/M2 balance into focus from a new direction. Psychoneuroendocrinology 80, 15–25. [DOI] [PubMed] [Google Scholar]
- Faridhosseini F, Sadeghi R, Farid L, Pourgholami M, 2014. Celecoxib: a new augmentation strategy for depressive mood episodes. A systematic review and meta-analysis of randomized placebo-controlled trials. Hum Psychopharmacol 29, 216–223. [DOI] [PubMed] [Google Scholar]
- Ferreira TB, Kasahara TM, Barros PO, Vieira MMM, Bittencourt VCB, Hygino J, Andrade RM, Linhares UC, Andrade AF, Bento CA, 2011. Dopamine up-regulates Th17 phenotype from individuals with generalized anxiety disorder. Journal of Neuroimmunology 238, 58–66. [DOI] [PubMed] [Google Scholar]
- Field T, 2011. Prenatal depression effects on early development: a review. Infant behavior & development 34, 1–14. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, 2006. Prenatal depression effects on the fetus and newborn: a review. Infant behavior & development 29, 445–455. [DOI] [PubMed] [Google Scholar]
- Figueiredo AS, Schumacher A, 2016. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 148, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Betz R, Specker C, 2017. Pregnancy in systemic lupus erythematosus and antiphospholipid syndrome. Best practice & research. Clinical rheumatology 31, 397–414. [DOI] [PubMed] [Google Scholar]
- Fishe KJ, Tell D, Mathews HL, Witek Janusek L, 2013. Pregnancy specific anxiety and distress is related to increased IL-6 during the second trimester of pregnancy. Brain, Behavior, and Immunity 32, e39–e40. [Google Scholar]
- Franklin TC, Xu C, Duman RS, 2018. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav Immun 72, 2–13. [DOI] [PubMed] [Google Scholar]
- Fu B, Tian Z, Wei H, 2014. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cellular and Molecular Immunology 11, 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YH, Zhao HS, Zhang FR, Gao Y, Shen P, Chen RC, Zhang GJ, 2015. The Relationship between Depression and Asthma: A Meta-Analysis of Prospective Studies. PLoS One 10, e0132424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe A, Granese R, Grasso R, Salpietro V, Corrado F, Giorgianni G, Foti G, Amadore D, Triolo O, Giunta L, Di Benedetto A, 2016. Association between maternal serum high mobility group box 1 levels and pregnancy complicated by gestational diabetes mellitus. Nutrition, metabolism, and cardiovascular diseases : NMCD 26, 414–418. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GDO, Rumley A, Marmot MG, Ferrie JE, 2009. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-Year follow-up of the Whitehall II study. Psychological Medicine 39, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JH, Chenausky KL, Freeman MP, 2014. Anxiety disorders during pregnancy: a systematic review. The Journal of clinical psychiatry 75, e1153–1184. [DOI] [PubMed] [Google Scholar]
- Grosse L, Hoogenboezem T, Ambree O, Bellingrath S, Jorgens S, de Wit HJ, Wijkhuijs AM, Arolt V, Drexhage HA, 2016. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav Immun 54, 38–44. [DOI] [PubMed] [Google Scholar]
- Grosso A, Locatelli F, Gini E, Albicini F, Tirelli C, Cerveri I, Corsico AG, 2018. The course of asthma during pregnancy in a recent, multicase-control study on respiratory health. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeri S, Baker AM, Ruano R, 2013. Do pregnant women with depression have a pro-inflammatory profile? The journal of obstetrics and gynaecology research 39, 948–952. [DOI] [PubMed] [Google Scholar]
- Haimovici F, Anderson JL, Bates GW, Racowsky C, Ginsburg ES, Simovici D, Fichorova RN, 2018. Stress, anxiety, and depression of both partners in infertile couples are associated with cytokine levels and adverse IVF outcome. Am J Reprod Immunol 79, e12832. [DOI] [PubMed] [Google Scholar]
- Hee L, Kirkegaard I, Vogel I, Thorsen P, Skogstrand K, Hougaard DM, Uldbjerg N, Sandager P, 2011. Low serum interleukin-17 is associated with preterm delivery. Acta Obstet Gynecol Scand 90, 92–96. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J, 2012. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychological medicine 42, 2015–2026. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Ménard C, Russo SJ, 2016. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiology of Stress 4, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Zheng J, Ding ZY, Chen JH, Yu L, Niu Y, Hua YQ, Wang LL, 2013. Imbalance between Th17 and treg cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice. NeuroImmunoModulation 20, 39–50. [DOI] [PubMed] [Google Scholar]
- Imbalzano E, Quartuccio S, Di Salvo E, Crea T, Casciaro M, Gangemi S, 2017. Association between HMGB1 and asthma: a literature review. Clinical and molecular allergy : CMA 15, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, Yoneda S, Shiozaki A, Sumi S, Tsuneyama K, Nikaido T, Saito S, 2010. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol 84, 75–85. [DOI] [PubMed] [Google Scholar]
- Järventausta K, Sorri A, Kampman O, Björkqvist M, Tuohimaa K, Hämäläinen M, Moilanen E, Leinonen E, Peltola J, Lehtimäki K, 2017. Changes in interleukin-6 levels during electroconvulsive therapy may reflect the therapeutic response in major depression. Acta Psychiatrica Scandinavica 135, 87–92. [DOI] [PubMed] [Google Scholar]
- Jha MK, Miller AH, Minhajuddin A, Trivedi MH, 2018. Association of T and non-T cell cytokines with anhedonia: Role of gender differences. Psychoneuroendocrinology 95, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jido TA, Yakasai IA, 2013. Preeclampsia: a review of the evidence. Annals of African medicine 12, 75–85. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu J, Yang Q, Cai Y, He W, Liu C, 2015. High mobility group box 1 protein polymorphism affects susceptibility to recurrent pregnancy loss by up-regulating gene expression in chorionic villi. J Assist Reprod Genet 32, 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, Hoffmann E, Rubinow D, Jonas J, Paul S, Meltzer-Brody S, 2017. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet (London, England) 390, 480–489. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Nousiainen N, Scheinin NM, Maksimow M, Salmi M, Lehto SM, Tolvanen M, Lukkarinen H, Karlsson H, 2017. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy-the FinnBrain Birth Cohort Study. Archives of women’s mental health 20, 39–48. [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A, 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nature medicine 13, 1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Skoog I, Börjesson-Hanson A, Blennow K, Zetterberg H, Östling S, Kern J, Gudmundsson P, Marlow T, Rosengren L, Waern M, 2013. Lower CSF interleukin-6 predicts future depression in a population-based sample of older women followed for 17 years. Brain, Behavior, and Immunity 32, 153–158. [DOI] [PubMed] [Google Scholar]
- Kern S, Skoog I, Börjesson-Hanson A, Blennow K, Zetterberg H, Östling S, Kern J, Gudmundsson P, Marlow T, Rosengren L, Waern M, 2014. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain, Behavior, and Immunity 41, 55–58. [DOI] [PubMed] [Google Scholar]
- Kidd T, Poole L, Leigh E, Ronaldson A, Jahangiri M, Steptoe A, 2014. Attachment anxiety predicts IL-6 and length of hospital stay in coronary artery bypass graft surgery (CABG) patients. Journal of Psychosomatic Research 77, 155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirbas A, Biberoglu E, Ersoy AO, Dikmen AU, Koca C, Erdinc S, Uygur D, Caglar T, Biberoglu K, 2016. The role of interleukin-17 in intrahepatic cholestasis of pregnancy. Journal of Maternal-Fetal and Neonatal Medicine 29, 977–981. [DOI] [PubMed] [Google Scholar]
- Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, Paulos CM, 2018. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cellular & molecular immunology 15, 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Kong Y, Zhang F, Wang T, Zhu X, 2017. Expression and significance of dendritic cells and Th17/Treg in serum and placental tissues of patients with intrahepatic cholestasis of pregnancy. Journal of Maternal-Fetal and Neonatal Medicine, 1–6. [DOI] [PubMed]
- Kraus TA, Engel SM, Sperling RS, Kellerman L, Lo Y, Wallenstein S, Escribese MM, Garrido JL, Singh T, Loubeau M, Moran TM, 2012. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol 32, 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmicki M, Telejko B, Lipinska D, Pliszka J, Wilk J, Wawrusiewicz-Kurylonek N, Zielinska A, Sobota A, Kretowski A, Gorska M, Szamatowicz J, 2014. The IL-6/IL-6R/sgp130 system and Th17 associated cytokines in patients with gestational diabetes. Endokrynologia Polska 65, 169–175. [DOI] [PubMed] [Google Scholar]
- Larocca L, Ramhorst R, Roca V, Calafat M, Aisemberg J, Franchi A, Perez Leiros C, 2008. Neuroimmune-endocrine interactions during early pregnancy in an autoimmune context: focus on macrophage activation. Neuroimmunomodulation 15, 84–90. [DOI] [PubMed] [Google Scholar]
- Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW, 2013. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: A systematic review and meta-analysis. American Journal of Reproductive Immunology 70, 412–427. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Herring MP, Liu JJ, Wei YB, Bossoli D, Hallgren M, Forsell Y, 2017. Interleukin-6 and depressive symptom severity in response to physical exercise. Psychiatry Research 252, 270–276. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J, 2012. Th17 and Regulatory T cells in Women with Recurrent Pregnancy Loss. American Journal of Reproductive Immunology 67, 311–318. [DOI] [PubMed] [Google Scholar]
- Li J, Huang L, Wang S, Zhang Z, 2018. Increased serum levels of high mobility group protein B1 and calprotectin in pre-eclampsia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 142, 37–41. [DOI] [PubMed] [Google Scholar]
- Li J, Wang FP, She WM, Yang CQ, Li L, Tu CT, Wang JY, Jiang W, 2014. Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. Journal of viral hepatitis 21, 129–140. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang Y, Gao Y, Zhang Z, 2016. Elevated levels of Hs-CRP and IL-6 after delivery are associated with depression during the 6 months post partum. Psychiatry Research 243, 43–48. [DOI] [PubMed] [Google Scholar]
- Liu ZZ, Sun GQ, Hu XH, Kwak-Kim J, Liao AH, 2017. The transdifferentiation of regulatory T and Th17 cells in autoimmune/inflammatory diseases and its potential implications in pregnancy complications. American Journal of Reproductive Immunology 78. [DOI] [PubMed] [Google Scholar]
- Machado MO, Oriolo G, Bortolato B, Kohler CA, Maes M, Solmi M, Grande I, Martin-Santos R, Vieta E, Carvalho AF, 2017. Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: A critical systematic review. J Affect Disord 209, 235–245. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Ohtsuka H, Tomioka M, Oikawa M, 2013. Effect of progesterone on Th1/Th2/Th17 and Regulatory T cell-related genes in peripheral blood mononuclear cells during pregnancy in cows. Veterinary Research Communications 37, 43–49. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Ombelet W, Stevens K, Kenis G, De Jongh R, Cox J, Bosmans E, 2000. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology 25, 121–137. [DOI] [PubMed] [Google Scholar]
- Markert UR, Morales-Prieto DM, Fitzgerald JS, 2011. Understanding the link between the IL-6 cytokine family and pregnancy: Implications for future therapeutics. Expert Review of Clinical Immunology 7, 603–609. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H, 1999. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 5, 101–104. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjosberg J, Berg G, Ernerudh J, Jenmalm MC, Matthiesen LS, 2009. Enrichment of Foxp3+ Tregs and reduction of TH17 cells in human early pregnancy decidua indicate immunosuppressive T cell dominance. American Journal of Reproductive Immunology 61, 421. [Google Scholar]
- Mor G, Cardenas I, Abrahams V, Guller S, 2011. Inflammation and pregnancy: the role of the immune system at the implantation site. Annals of the New York Academy of Sciences 1221, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.a.W., 2017. Janeway’s Immunobiology Garland Science, New York NY. [Google Scholar]
- Murphy TM, O’Donovan A, Mullins N, O’Farrelly C, McCann A, Malone K, 2015. Anxiety is associated with higher levels of global DNA methylation and altered expression of epigenetic and interleukin-6 genes. Psychiatric Genetics 25, 71–78. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Ahmad SF, Al-Harbi NO, Fardan AS, El-Sherbeeny AM, Ibrahim KE, Attia SM, 2017. IL-17A causes depression-like symptoms via NFkappaB and p38MAPK signaling pathways in mice: Implications for psoriasis associated depression. Cytokine 97, 14–24. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S, 2010. Circulating and decidual Th17 cell levels in healthy pregnancy. American Journal of Reproductive Immunology 63, 104–109. [DOI] [PubMed] [Google Scholar]
- Neuteboom RF, Verbraak E, Wierenga-Wolf AF, van Meurs M, Steegers EA, de Groot CJ, Laman JD, Hintzen RQ, 2010. Pregnancy-induced fluctuations in functional T-cell subsets in multiple sclerosis patients. Multiple sclerosis (Houndmills, Basingstoke, England) 16, 1073–1078. [DOI] [PubMed] [Google Scholar]
- O’Donnell KJ, Glover V, Lahti J, Lahti M, Edgar RD, Raikkonen K, O’Connor TG, 2017. Maternal prenatal anxiety and child COMT genotype predict working memory and symptoms of ADHD. PLoS One 12, e0177506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, Malone KM, 2010. Clinical anxiety, cortisol and interleukin-6: Evidence for specificity in emotion-biology relationships. Brain, Behavior, and Immunity 24, 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova EG, Shirshev SV, 2014. Role of leptin and ghrelin in induction of differentiation of IL-17-producing and T-regulatory cells. Bull Exp Biol Med 156, 819–822. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Monk C, 2013. Perinatal depression--the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology 38, 1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Yenokyan G, Fei K, Kraus T, Moran T, Monk C, Sperling R, 2018a. Innate immune activation and depressive and anxious symptoms across the peripartum: An exploratory study. Psychoneuroendocrinology 99, 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne S, Biaggi A, Chua TE, Du Preez A, Hazelgrove K, Nikkheslat N, Previti G, Zunszain PA, Conroy S, Pariante CM, 2018b. Antenatal depression programs cortisol stress reactivity in offspring through increased maternal inflammation and cortisol in pregnancy: The Psychiatry Research and Motherhood - Depression (PRAM-D) Study. Psychoneuroendocrinology [DOI] [PMC free article] [PubMed]
- Oztas E, Erkenekli K, Ozler S, Aktas A, Buyukkagnici U, Uygur D, Danisman N, 2015. First trimester interleukin-6 levels help to predict adverse pregnancy outcomes in both thyroid autoantibody positive and negative patients. Journal of Obstetrics and Gynaecology Research 41, 1700–1707. [DOI] [PubMed] [Google Scholar]
- Pinget GV, Corpuz TM, Stolp J, Lousberg EL, Diener KR, Robertson SA, Sprent J, Webster KE, 2016. The majority of murine gammadelta T cells at the maternal-fetal interface in pregnancy produce IL-17. Immunol Cell Biol 94, 623–630. [DOI] [PubMed] [Google Scholar]
- Podlipný J, Hess Z, Vrzalová J, Rosolová H, Beran J, Petrlová B, 2010. Lower serum levels of interleukin-6 in a population sample with symptoms of depression than in a population sample without symptoms of depression. Physiological Research 59, 121–126. [DOI] [PubMed] [Google Scholar]
- Pongcharoen S, Somran J, Sritippayawan S, Niumsup P, Chanchan P, Butkhamchot P, Tatiwat P, Kunngurn S, Searle RF, 2007. Interleukin-17 expression in the human placenta. Placenta 28, 59–63. [DOI] [PubMed] [Google Scholar]
- Qiu XY, Sun L, Han XL, Chang Y, Cheng L, Yin LR, 2017. Alarmin high mobility group box-1 in maternal serum as a potential biomarker of chorioamnionitis-associated preterm birth. Gynecol Endocrinol 33, 128–131. [DOI] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS, 2011. Damage -associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 24, 1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS, 2012. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 25, 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomruangwong C, Anderson G, Berk M, Stoyanov D, Carvalho AF, Maes M, 2018. A neuro-immune, neuro-oxidative and neuro-nitrosative model of prenatal and postpartum depression. Prog Neuropsychopharmacol Biol Psychiatry 81, 262–274. [DOI] [PubMed] [Google Scholar]
- Rybka J, 2013. HMGB1 and IL-17 are the mediators linking redox signalling and inflammation in depression. European Neuropsychopharmacology 23, S414. [Google Scholar]
- Saito S, 2010. Th17 cells and regulatory T cells: New light on pathophysiology of preeclampsia. Immunology and Cell Biology 88, 615–617. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Ito M, Shima T, 2011. Clinical implication of recent advances in our understanding of IL-17 and reproductive immunology. Expert Rev Clin Immunol 7, 649–657. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Shima T, Ito M, 2010. Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. American Journal of Reproductive Immunology 63, 601–610. [DOI] [PubMed] [Google Scholar]
- Saraykar S, Cao B, Barroso LS, Pereira KS, Bertola L, Nicolau M, Ferreira JD, Dias NS, Vieira EL, Teixeira AL, Silva APM, Diniz BS, 2017. Plasma IL-17A levels in patients with late-life depression. Revista brasileira de psiquiatria (Sao Paulo, Brazil :1999), 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seow CH, Leung Y, Hirota C, Afshar EE, Kaplan G, Bindra GK, Beck PL, Panaccione R, Ghosh S, Ueno A, 2015. Downregulation of IL-17 related cytokines in the second trimester of pregnancy in women with IBD supports pregnancy driven immunomodulatory effects involving the Th17 pathway. Gastroenterology 148, S454–S455. [Google Scholar]
- Shao J, Zhao M, Tong M, Wei J, Wise MR, Stone P, Chamley L, Chen Q, 2016. Increased levels of HMGB1 in trophoblastic debris may contribute to preeclampsia. Reproduction 152, 775–784. [DOI] [PubMed] [Google Scholar]
- Simpson W, Steiner M, Coote M, Frey BN, 2016. Relationship between inflammatory biomarkers and depressive symptoms during late pregnancy and the early postpartum period: a longitudinal study. Revista brasileira de psiquiatria (Sao Paulo, Brazil : 1999) 38, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalkidou A, Sylvén SM, Papadopoulos FC, Olovsson M, Larsson A, Sundström-Poromaa I, 2009. Risk of postpartum depression in association with serum leptin and interleukin-6 levels at delivery: A nested case-control study within the UPPSAT cohort. Psychoneuroendocrinology 34, 1329–1337. [DOI] [PubMed] [Google Scholar]
- Slyepchenko A, Maes M, Kohler CA, Anderson G, Quevedo J, Alves GS, Berk M, Fernandes BS, Carvalho AF, 2016. T helper 17 cells may drive neuroprogression in major depressive disorder: Proposal of an integrative model. Neurosci Biobehav Rev 64, 83–100. [DOI] [PubMed] [Google Scholar]
- Su Y, Hong S, Zhang Y, 2001. The changes of serum levels of interleukin-6 and interleukin-8 in pregnant women with systemic lupus erythematosus. Zhonghua fu chan ke za zhi 36, 660–662. [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Hughes ZA, Neal S, Roos JM, Rosenzweig-Lipson S, Moss SJ, Brandon N, 2012a. Depressive-like phenotype in transgenic mice with CNS over expression of IL-6. Neuropsychopharmacology 38, S89. [Google Scholar]
- Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, Brandon NJ, 2012b. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Translational psychiatry 2, e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebinia F, Pourgholaminejad A, 2017. The role of Th17 cells in auto-inflammatory neurological disorders. Prog Neuropsychopharmacol Biol Psychiatry 79, 408–416. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kishimoto T, 2014. The biology and medical implications of interleukin-6. Cancer immunology research 2, 288–294. [DOI] [PubMed] [Google Scholar]
- Telleria CM, Ou J, Sugino N, Ferguson S, Gibori G, 1998. The expression of interleukin-6 in the pregnant rat corpus luteum and its regulation by progesterone and glucocorticoid. Endocrinology 139, 3597–3605. [DOI] [PubMed] [Google Scholar]
- Tesmer LA, Lundy SK, Sarkar S, Fox DA, 2008. Th17 cells in human disease. Immunological reviews 223, 87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldi G, Molvarec A, Stenczer B, Muller V, Eszes N, Bohacs A, Bikov A, Rigo J Jr., Vasarhelyi B, Losonczy G, Tamasi L, 2011. Peripheral T(h)1/T(h)2/T(h)17/regulatory T-cell balance in asthmatic pregnancy. Int Immunol 23, 669–677. [DOI] [PubMed] [Google Scholar]
- Torricelli M, Bellisai F, Novembri R, Galeazzi LR, Iuliano A, Voltolini C, Spreafico A, Galeazzi M, Petraglia F, 2011. High levels of maternal serum IL-17 and activin A in pregnant women affected by systemic lupus erythematosus. American Journal of Reproductive Immunology 66, 84–89. [DOI] [PubMed] [Google Scholar]
- Tsuboi H, Sakakibara H, Tatsumi A, Yamakawa-Kobayashi K, Kaneko H, Mataunaga M, Shimoi K, 2014. The serum levels of n-3 fatty acids and IL-6 were independently associated with depressive symptoms in female population. Brain, Behavior, and Immunity 40, e29. [Google Scholar]
- Turhan Iyidir O, Konca Degertekin C, Sonmez C, Atak Yucel A, Erdem M, Akturk M, Ayvaz G, 2015. The effect of thyroid autoimmunity on T-cell responses in early pregnancy. J Reprod Immunol 110, 61–66. [DOI] [PubMed] [Google Scholar]
- Vanders RL, Gibson PG, Wark PA, Murphy VE, 2013. Alterations in inflammatory, antiviral and regulatory cytokine responses in peripheral blood mononuclear cells from pregnant women with asthma. Respirology 18, 827–833. [DOI] [PubMed] [Google Scholar]
- Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araujo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Linhares UC, Andrade AF, Bento CA, 2010. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol 229, 212–218. [DOI] [PubMed] [Google Scholar]
- Voskuhl R, Momtazee C, 2017. Pregnancy: Effect on Multiple Sclerosis, Treatment Considerations, and Breastfeeding. Neurotherapeutics 14, 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman A, Hauptmann J, Regen T, 2015. The role of IL-17 in CNS diseases. Acta neuropathologica 129, 625–637. [DOI] [PubMed] [Google Scholar]
- Walsh K, Basu A, Werner E, Lee S, Feng T, Osborne LM, Rainford A, Gilchrist M, Monk C, 2016. Associations among Child Abuse, Depression, and Interleukin-6 in Pregnant Adolescents: Paradoxical Findings. Psychosomatic Medicine 78, 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Lian YJ, Su WJ, Peng W, Dong X, Liu LL, Gong H, Zhang T, Jiang CL, Wang YX, 2018. HMGB1 mediates depressive behavior induced by chronic stress through activating the kynurenine pathway. Brain Behav Immun 72, 51–60. [DOI] [PubMed] [Google Scholar]
- Wei SQ, Fraser W, Luo ZC, 2010. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 116, 393–401. [DOI] [PubMed] [Google Scholar]
- Wu HX, Jin LP, Xu B, Liang SS, Li DJ, 2014. Decidual stromal cells recruit Th17 cells into decidua to promote proliferation and invasion of human trophoblast cells by secreting IL-17. Cellular & molecular immunology 11, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TY, Liu L, Zhang W, Zhang Y, Liu YZ, Shen XL, Gong H, Yang YY, Bi XY, Jiang CL, Wang YX, 2015. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J Psychiatr Res 64, 99–106. [DOI] [PubMed] [Google Scholar]
- Xu Q, Du F, Zhang Y, Teng Y, Tao M, Chen AF, Jiang R, 2018. Preeclampsia serum induces human glomerular vascular endothelial cell hyperpermeability via the HMGB1-Caveolin-1 pathway. J Reprod Immunol 129, 1–8. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Wang N, Yang C, Shi JY, Yu HY, Hashimoto K, 2015. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biological Psychiatry 77, e19–e20. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC, 1995. Amniotic fluid interleukin-6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. American Journal of Obstetrics and Gynecology 172, 960–970. [DOI] [PubMed] [Google Scholar]
- Zhu B, Zhu Q, Li N, Wu T, Liu S, Liu S, 2018. Association of serum/plasma high mobility group box 1 with autoimmune diseases: A systematic review and meta-analysis. Medicine 97, e11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhang Z, Zhang L, Shi Y, Qi J, Chang A, Gao J, Feng Y, Yang X, 2015HMGB1-RAGE signaling pathway in severe preeclampsia. Placenta 36, 1148–1152. [DOI] [PubMed] [Google Scholar]