Abstract
Introduction:
Traumatic brain injury (TBI) is a major worldwide neurological disorder of epidemic proportions. To date, there are still no FDA-approved therapies to treat any forms of TBI. Encouragingly, there are emerging data showing that biofluid-based TBI biomarker tests have the potential to diagnose the presence of TBI of different severities including concussion, and to predict outcome.
Area covered:
The authors provide an update on the current knowledge of TBI biomarkers, including protein biomarkers for neuronal cell body injury (UCH-L1, NSE), astroglial injury (GFAP, S100B), neuronal cell death (αII-spectrin breakdown products), axonal injury (NF proteins), white matter injury (MBP), post-injury neurodegeneration (total Tau and phospho-Tau), post-injury autoimmune response (brain antigen-targeting autoantibodies), and other emerging non-protein biomarkers. The authors discuss biomarker evidence in TBI diagnosis, outcome prognosis and possible identification of post-TBI neurodegernative diseases (e.g. chronic traumatic encephalopathy and Alzheimer’s disease), and as theranostic tools in pre-clinical and clinical settings.
Expert Commentary:
A spectrum of biomarkers is now at or near the stage of formal clinical validation of their diagnostic and prognostic utilities in the management of TBI of varied severities including concussions. TBI biomarkers could serve as a theranostic tool in facilitating drug development and treatment monitoring.
Keywords: biomarkers, traumatic brain injury, diagnostics, theranostics, neurotrauma
1. INTRODUCTION:
Traumatic brain injury (TBI), often referred to as a silent epidemic [1], is defined as a neurotrauma caused by a mechanical force that is applied to the head. In the United States, there are approximately 1.7–2.0 million incidents of TBI annually [2] (Table 1). In addition, CDC reported that approximately 5.3 million Americans live with the effects of TBI. About half of the Americans who experience TBI each year incur at least some short-term disability [2–7]. The Armed Forces Health Surveillance Center has reported that 375,230 US service members sustained TBI between 2000 and 2016 [3] (Table 1). TBI is a heterogeneous neurological disorder – it ranges from penetrating injury, focal contusion, different forms of hematoma (subdural, epidural) to diffuse injury to single or repetitive concussion /mild TBI. Currently, TBI can also be classified by Glasgow Coma Scale (GCS) score: severe (GCS 3–8), moderate (GCS 9–12) and mild (GCS 13–15) as well as cranial computer tomography (CT) abnormality (CT positive vs. CT negative) [6,7]. Mild TBI (mTBI, CT negative) accounts for over 85% of all cases of TBI. In the U.S., TBI accounts for 1.3% of all emergency department visits [8]. The direct medical costs for treatment of TBI in the U.S. have been estimated to be $4 billion annually. In addition to concussions that occur in the general populace in everyday activities, professional as well as recreational collegiate and scholastic athletes and high school athletes who participate in contact sports (e.g. American football, wrestling, hockey, boxing, soccer, lacrosse, rugby) are particularly vulnerable to experiencing concussions [9]. Between 1.6–3.8 million sports or recreational activities-related concussions occur annually in the United States and approximately half of these involve children and adolescents [10]. On average, 25% of athletes will report more than one concussion in their lifetime [11,12].
Table 1.
Spectrum of TBI in the USA.
| TBI Spectrum | Incidents | Point of Care | Glasgow Coma Scale |
|---|---|---|---|
| Severe TBI | ~ 170,000/per yr. US | Neuro-intensive care unit | 3–8 |
| Moderate TBI | ~ 85,000 / per yr. US | Hospital in-patient | 9–12 |
| Mild TBI | ~ 1.4 million/per yr. US | Emergency Dept.;Ambulance; | 13–15 |
| Military TBI(including blast brain injury) | ~375,000 cases (2000–2017) | In Theater, CSH | 3–15 |
| Sports-related Concussions | 1.6– 3.8 million/per year (CDC) | Sport-field, Athletic facility | (13–15) |
2. The need for TBI biomarkers
Significant scientific advances in the last decade have increased our understanding of the complex and heterogeneous pathophysiological processes associated with TBI. During the same period, numerous experimental drugs have been shown to be neuroprotective in animal models of brain injury. Unfortunately, none of these strategies have proven to be efficacious in TBI clinical trials [13–15]. The failure of clinical therapy trials has been attributed to the lack of therapeutic intervention-tracking CNS biomarkers complicated by the heterogeneity of TBI and poor translatability of preclinical TBI models. For example, it is now recognized that the pathophysiology of TBI is not only acute event, but is also a progressive and delayed neurodegenerative process made up of multiple, parallel, interacting, and interdependent cascades of biological reactions at the tissue, cellular, and subcellular levels. Due to the extended length of axonal fiber tracks, axons are particularly vulnerable to physical trauma to the brain. Thus, axonal injury is a common occurrence in both focal as well as diffuse brain trauma and can be found in TBI of all severities [16,17]. But in addition, neuronal body, dendrites, and synapses are also subjected to TBI-induced damage [18,19]. Similarly, not only are neurons at risk for injury, but also astroglia cells and the myelin-forming oligodendrocytes. For these reasons, a comprehensive understanding of these pathobiological processes at every cellular and subcellular level in greater detail is critical to bridging the knowledge gap that will allow new therapy development. Many agree that there is an unmet medical need for a rapid, simple biofluid-based diagnostic testing for the management of TBI patients, whether it is for monitoring severe TBI patients in the intensive care unit, or triaging mild and moderate TBI patients in the emergency room. Due to the multi-component pathobiology in brain injury, it would be ideal to have a panel of neuroinjury biomarkers that closely match with the various pathological processes we described above. There are emerging data from many recent studies from multiple research teams showing that biofluid-based TBI biomarker tests have the potential to assess the extent of TBI severity and determine a patient prognosis even at times when correlation with other neurological measures (neuroimaging) may not always be informative such as for mild TBI.
3. TBI biomarker attributes
In order for a biofluid-based TBI protein biomarker to be clinically useful, ideally it should have as many of the following attributes as possible (Table 2): (1) The protein biomarker levels should be readily measured in accessible biofluid such as cerebrospinal fluid (CSF), serum, plasma and/or whole blood in TBI patients. [For severe TBI (i.e. those TBI patients in neurointensive care unit), the biofluid type where the biomarker can be detected should be biofluids such as cerebrospinal fluid, serum, plasma and/or whole blood. For moderate and mild TBI (e.g. those patients managed in Emergency Departments or admitted to non ICU settings), the biofluid type should be serum, plasma and/or whole blood for rapid accessibility and convenience. (2) The biomarker levels must be elevated in various forms and/or severities of human TBI in the acute phase (3 h to 24 h post-injury), when compared to normal control counterparts, (3) the biomarker must have low background or basal biofluid levels in general non-injured healthy control population. (4) The biomarker detected in biofluid after TBI should be derived from or originated from the injured brain as the major source. (5) The biomarker levels in the above stated biofluids should be readily determined and quantified using sandwich ELISA or similar immunoassays with at least two assay formats or platforms. (6) There should be one or more available assay platform for such biomarker with test-retest reliability and reproducibility assay that meet assay analytical performance requirements acceptable to FDA. (7) The biomarker should be translational in nature with demonstrated evidence that there are similar to biofluid profiles in at least two different animal models of TBI (e.g. rodent control cortical impact (CCI), fluid percussion injury (FPI), close head injury (CHI), penetrating ballistic brain injury (PBBI) or blast overpressure-wave brain injury (OBI)). (8) The biomarker should be sensitive to severity of TBI as defined by GCS, CT abnormality. (9) The biomarker should allow for repeated detections in one of the above-mentioned biofluids within a 48 h window following brain injury. (10) The biomarker should have initial acute levels (within first 48 h postinjury) that correlate with currently available and commonly accepted TBI patient outcome indices (such as Glasgow outcome scale (GOS) or GOS-extended (GOS-E)), and (11) the post-TBI biofluid levels of the biomarker are responsive to therapeutic treatments. Based on cumulative evidence on a number of existing neuroinjury biomarkers and the discovery of additional biomarkers, we derived a TBI biofluid-based biomarker panel (Table 3).
Table 2.
Attributes of an ideal biofluid-based biomarker for TBI.
| 1 | The TBI biomarker levels can be readily measured in accessible biofluid. The biofluids will likely include whole blood, serum and/or plasma for moderate and mild TBI patients with the inclusion of CSF in cases of severe TBI patients. |
| 2 | The biomarkers are elevated in native and/or modified various forms which directly correlates to the degree of severity of human TBI in the acute phase (3 h to 24 h post-injury) |
| 3 | The biomarker has low background or basal biofluid levels in >95% of general non-injured healthy control population. |
| 4 | The biomarker detected in biofluid after TBI is the direct result of brain trauma. |
| 5 | The biomarker levels in the above-stated biofluids can be readily determined and quantified using at least two independent assay formats or platforms. |
| 6 | There are one or more available assays for such biomarker with test-retest reliability and reproducibility that meet analytical performance requirements acceptable to FDA. |
| 7 | The biomarker is translationally relevant relative to the model systems with demonstrated evidence that there are parallel biofluid-based biomarker profile patterns in at least two different animal models of TBI (e.g. rodent control cortical impact, fluid percussion injury, close head injury, penetrating brain injury or blast overpressure-wave brain injury) |
| 8 | The TBI protein biomarker should be qualitatively and quantitatively related to severity of TBI as defined by GCS, cranial CT and/or MRI abnormality. |
| 9 | The TBI protein biomarker should allow for repeated detections in one of the above mentioned biofluids within a 24–48 h window following brain injury. |
| 10 | The TBI protein biomarker should have initial acute levels (within first 24 h post-injury) that correlate with currently available and commonly accepted TBI patient outcome indices (such as Glasgow outcome scale-extended (GOS-E). |
| 11 | The post-TBI biofluid levels of the TBI biomarker is responsive to therapeutic treatments (i.e. Therapeutic treatment following TBI might reduce biomarker loads). |
Table 3.
TBI protein biomarker candidates that could have in vitro diagnostic, prognostic and therapeutic development /monitoring utilities.
| Origin/ Pathobiological mechanisms |
TBI Biomarker Candidates |
Evidence for Animal TBI detection |
Evidence for Human severe/moderate TBI detection |
Evidence for mild TBI detection (including concussion) |
|---|---|---|---|---|
| Neuronal cell body injury |
UCH-L1 | Liu et al. 2010 | Brophy et al. 2011 | Diaz-Arrastia 2014 Papa 2010, Papa 2012a Tate et al. 2013; Puvenna et al. 2014 |
| NSE | Agoston et al. 2012 Svetlov et a. 2012 Liu et al., 2015 |
Bohmer et al., 2011; Berger et al., 2012; |
Topolovec-Vranic et al., 2011; Buonora et al., 2015 |
|
| Astrogliosis, astroglial injury |
GFAP | Zoltewicz et al. 2013; Agoston et al.2012; Yan et al., 2015 Svetlov et al. 2012 |
Mondello et al., 2010 Vos et al. 2010 |
Papa et al. 2012, Okonkwo et al. 2013 Tate et al. 2013 |
| S100B | Agoston et al. 2012; Liu et al., 2015 |
Vos et al. 2010 | Cervellin:2012 Kiechle et al. 2014 |
|
| Axonal injury; Brain cell Necrosis- Apoptosis |
SBDPs (SBDP150, SBDP145, SBDP120,SNTF) |
SBDP150,145,1120: Pike et al, 2001 |
SBDP150, 145, 120: Mondello et al., 2010; SBDP150, 145, 120: Papa et al.., 2014 SNTF: Siman et al., 2009 |
SNTF: Siman et al., 2013, 2015 |
| Axonal injury |
NF-L, NF-M, NF-H (pNF-H) |
Anderson et al., 2008; Yan et al., 2015 Rostmi et al., 2012 |
Blyth et al., 2011 Zurek et al. 2011 |
NF-L: Gaston et al., 2014s; Shahim et al., 2016 NF-H: Oliver et al., 2015 |
| Axonal injury, CTE |
Tau, ,P-Tau | Yang et al., 2015 | Rubenstein et al., 2015 | Shahim et al., 2014 Olivera et al., 2016 Rubenstein et al., 2017 |
| Demyelination | MBP | Ringger et al. 2004 Liu et al. 2015 |
Berger et al., 2012; Zhang, et al. 2014 |
Berger et al., 2012 |
| Postsynaptic injury |
Neurogranin | - | Yang et al., 2015 | Yang et al., 2015 |
| Autoimmunity |
AutoAb[GFAP] Auto[S100b] |
- | Zhang et al., 2014; Wang et al. 2015 |
Wang et al. 2015 Marchi et al., 2013 |
Footnote: The above list of markers is not exhaustive but reflects the current state-of the art. Other possible markers include neurofilament proteins MAP2 (microtubule associated protein 2A, 2B), H-FABP (heart-type fatty acid binding protein) and BDNF (brain derived neurotrophic factor) and TDP-43 (TAR DNA-binding protein 43). But further studies are required for further clinical utility verification and biomarker characterization.
4. Current biomarker candidates
Mirroring the different pathophysiologic processes occurring in TBI, a panel of TBI biofluid-based protein biomarkers has now been identified. The processes covered by these biomarkers thus far include axonal injury, dendritic injury, neuronal cell body injury, demyelination, synaptic injury and astroglia injury and microglia responses (Figure 1) [20–22] [23].
Figure 1. Graphic representation of major TBI protein biomarkers linked to different pathophysiologic processes in TBI.
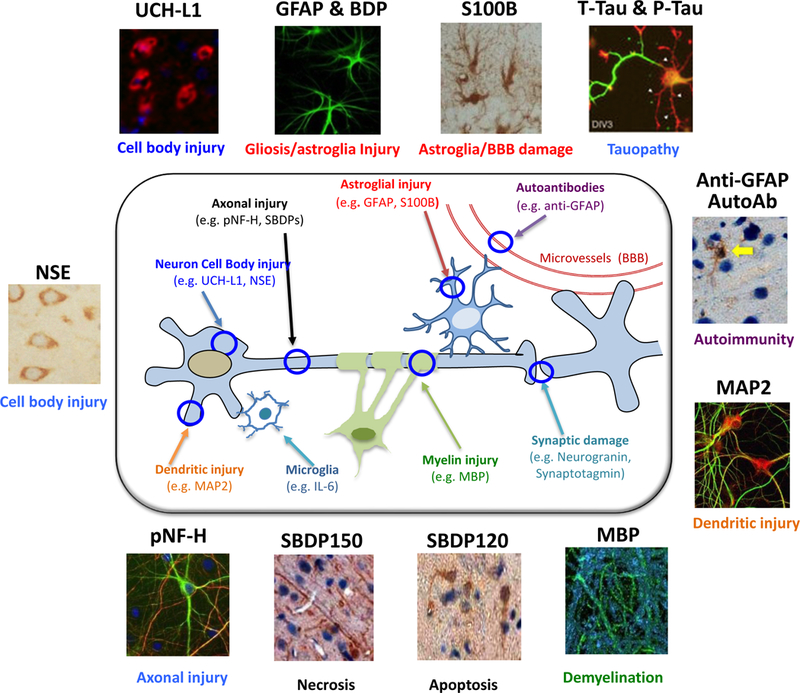
These processes thus far include axonal injury, dendritic injury, neuronal cell body injury, demyelination, synaptic injury and astroglia injury and microglia responses. Cellular and subcellular localization of representative TBI biomarkers are also shown with immunocytochemical staining images (based on mouse brain data).
4.1. Neuronal cell body injury markers:
4.1.1. Neuron specific enolase (NSE):
NSE is also known as gamma-enolase or enolase 2 (encoded by ENO2 gene) (Figure 1). NSE exists as a homodimer in mature neurons and neuroendocrine cells. NSE elevations in blood compartment has been documented in severe TBI [24–26]. NSE elevation in mTBI has also been documented [27–29]. NSE elevations were also reported in animal model of overpressure blast wave-induced brain injury in rodents [24–30] (Table 3). One major drawback of using NSE as specific marker for TBI is that it is also abundantly expressed in red blood cells, which prompted researchers to use a hemolysis correction when measuring NSE in blood [31].
4.1.2. Ubiquitin C-terminal hydrolase-L1 (UCH-L1):
UCH-L1 is a protein that mainly resides in the neuronal cell body cytoplasm. It was one of the few TBI biomarker candidates identified based on recent proteomic studies [32,33] (Figure 1). We reason that UCH-L1 is a functional biomarker and serves as a barometer of neuronal cell body injury (Table 3). UCH-L1 was first found to be released into CSF and serum among severe TBI patients [34–36]. The use of CSF UCH-L1 levels also appears to improve clinical outcome predictors of mortality following non-penetrating TBI. A new study also found UCH-L1 has utilities in long-term prognosis of severe TBI [37]. In addition, two independent studies demonstrated that UCH-L1 was released into serum/plasma in mTBI subjects [38]. It has been suggested that UCH-L1 together with GFAP form the foundation of a biomarker panel representing the two dominant cell types in the brain [39]. Interestingly, serum levels of both UCH-L1 and GFAP also appear elevated in professional breacher trainees who were exposed to repeated explosive discharges [40]. Lastly, Puvenna et al. (2014) also found significant UCH-L1 elevations in serum among athletes after concussions [41].
4.2. Astroglial biomarkers
4.2.1. S100B protein:
S100B is an astroglial 11 kDa calcium-binding protein. It is perhaps the most investigated brain injury biomarker to date [42,43] (Figure 1). Preclinical animal TBI model data is present [30,44] (Table 3). S100B has been studied in TBI of various severities [45–47]. However, since it has been noted that S100B can also be released from adipose tissue and cardiac/skeletal muscles, its levels are also elevated in orthopedic trauma without head injury [48]. Despite these confounders, S100B is actually a sensitive biomarker for predicting CT abnormality and post-concussive syndrome development among mTBI patients [49–51].
4.2.2. Glial fibrillary acidic protein (GFAP):
astroglial GFAP is emerging as the most robust TBI biomarker (Figure 1). GFAP biomarker levels are elevated within 3 to 34 h in CSF and serum/plasma following severe TBI [46,52–55] and in serum and plasma samples after moderate to mTBI [56]. GFAP as a biomarker, in the form of either the GFAP intact protein (50 kDa) or as breakdown products (GFAP-BDPs; 44–38 kDa) are predominantly released from injured brain tissue into biofluid such as cerebrospinal fluid and serum/plasma shortly following TBI [46,52,57]. In parallel with human TBI studies, GFAP elevations in CSF have been identified in several rat models of severe TBI (CCI, PBBI, OBI) [58,59] and as well as in serum /plasma samples in mTBI models [30,60–62]. There is additional evidence that the post-TBI elevation of GFAP is severity–dependent [59]. Lastly, GFAP levels are also linked to CT pathological alterations and patient outcomes [56,63,64] (Table 3).
4.3. αII-spectrin breakdown products/fragments as cell death markers:
More recently, C-terminal BDPs of axonal protein αII-spectrin (SBDP150 and SBDP145) produced by calpain during necrosis, and SBDP120 produced by caspase-3 during apoptosis) have been identified as potential cell death biomarkers in both animal models of TBI and human CSF samples [65–70] [71] (Figure 1). In parallel to SBDP150, the N-terminal spectrin fragment (SNTF) (~140 kDa) was also produced [65,66,72,73]. SNTF was shown to be elevated in circulation after concussion. One potential confound is that the αII-spectrin protein, although enriched in the CNS, is also expressed in other organs as well as peripheral blood mononuclear cells (PBMC). Thus, SBDP or SNTF elevations in blood alone may not be conclusively interpreted as the presence of brain trauma or concussion (Table 3).
4.4. Delayed axonal injury and demyelination markers:
4.4.1. Neurofilament proteins (NF):
NF belong to the so-called “class IV” intermediate filaments (10 nm diameter). They are found exclusively in neurons. NF exist as bundles known as neurofibrils and are a major cytoskeleton component that functions primarily to provide structural support for the axon and to regulate axon diameter. NF are composed of three polypeptide subunits of different molecular weight: neurofilament-light protein (NF-L; 68K) neurofilament-medium protein (NF-M, 150K) and neurofilament–heavy protein (NF-H; 200K) (Figure 1). NF-H is subjected to phosphorylation. Phospho-NF-H (pNF-H) is enriched in axons – making it a good immunohistochemical biomarker for staining fiber track. All three NF subunits are vulnerable to proteases such as calpain and cathepsin-B/D [74,75]. Following proteolysis, they can be dissociated from the cytoskeleton into cytosol or possibly extracellular fluid, especially if cell membrane integrity is compromised. pNF-H was found released in blood following experimental TBI [76]. Li et al. (2015) also showed that serum pNF-H elevations were correlated to mechanical impact responses elevated in serum following a weight drop closed head injury model [77]. Martínez-Morillo and colleagues showed that (NF-M) protein concentrations were increased in CSF and serum samples from patients with severe TBI [78]. Neselius et al. (2013) found increased CSF levels of pNF-H following bouts among amateur boxers [79], while pNF-H also appears to be a predictor of mortality after brain injury in children [80]. In addition, serum NF-L also appears elevated in American football players over the course of a season [81] and in TBI subjects [82],[79]. Importantly, NF protein release into biofluid is a delayed process with respect to the initial insult (days following insults) (Table 3). Thus, it could reflect on-going axonal degeneration and might be linked to cognitive decline and development in chronic TBI subjects. It is worth-noting that another axonally-located microtubule associated protein Tau is also an emerging TBI biomarker – it will be covered further in a later section.
4.4.2. Myelin basic protein (MBP):
MBP is an oligodendrocyte protein and a key structural component of the multi-layered myelin sheath covering nerve fibers (Figure 1). The myelin sheath found in the nervous system serves as an insulator to increase the velocity of axonal impulse conduction [3]. MBP maintains the correct structure of myelin, interacting with the lipids in the myelin membrane [83]. In myelinated fiber tracks of the white matter, MBP degradation by proteases, such as calpain, results in degradation of axons and the myelin sheath (demyelination) [84,85]. Thus, under these conditions, MBP or its fragmented forms might be released into biofluid after TBI. For example, MBP are shown to be released into CSF after rat CCI [44], while MBP is also found to be elevated in serum after severe TBI in children [20,44] and mTBI in adults [20] (Table 3).
4.5. Subacute, chronic TBI biomarkers:
Evidence show that biofluid (CSF, blood) levels of most acute TBI markers will return to baseline levels within a matter of days following TBI, especially for those who suffered from mild brain injury (Table 3). Yet subacute and chronic effects of TBI can persist for months following the initial injury event. These effects might include CNS and systemic sequelae such as cognitive impairment (memory and executive dysfunction), neurological symptoms (headache, sleep disturbance and pain), neuro-endocrine dysfunction and mental health impairment (depression, anxiety, apathy and suicidality) and, as yet, unclear processes leading to the development of neurodegenerative diseases such as chronic traumatic encephalopathy (CTE) and Alzheimer’s disease (AD) [86–90]. Thus, it might be appropriate to consider a continuum of TBI biomarkers that might be released at different time points following the initial brain injury event (Figure 2). These biomarkers could represent different but parallel pathways active at various time points after the initial injury. Some of the emerging subacute and chronic TBI biomarkers might include neurodegeneration markers such as total Tau (T-Tau) protein and phosphorylated Tau (P-Tau) and amyloid beta peptides (Aβ1–40, Aβ1–42), autoantibody response to neuroproteins [91,92] and inflammatory markers. Proinflammatory cytokines such as IL-1β, IL-6 IL-8 and TNF-α are released into brain tissue or biofluids (CSF, blood) in human and rat likely by reactive microglia or glia after TBI [93–99]. However this last group of markers is not specific to TBI.
Figure 2. A continuum of protein biomarkers in tracking different phases of TBI.
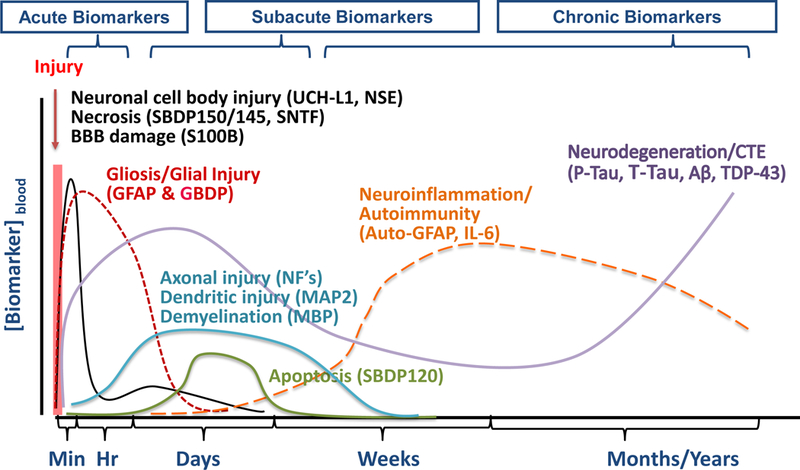
Acute neuronal cell body injury markers UCH-L1 (ubiquitin C-terminal hydrolase-L1), NSE (neuronal specific enolase), necrosis markers SBDP150/145 (αII-spectrin breakdown product 150 kDa & 145 kDa), SNTF (αII-spectrin N-terminal fragment), S100B (glial calcium-binding protein S100B), GFAP & BDP (glial fibrillary acidic protein & breakdown product), delayed axonal injury NF-H, M, L (neurofilament-heavy, medium & light), demyelination marker MBP (myelin basic protein), apoptosis marker SBDP120 (αII-spectrin breakdown product 120 kDa), autoimmunity markers auto[GFAP] (autoantibodies to GFAP), neurodegeneration markers Tau (tau protein), P-Tau (phosphorylated tau), Aβ (amyloid β-peptides) and TDP-43.
4.5.1. Postinjury Neurodegeneration/Tauopathy biomarkers:
Increasing evidence has suggested that TBI may also be a risk factor for the development of age-associated neurodegenerative disorders including AD, Parkinson’s Disease (PD), Amyotrophic Lateral Sclerosis and Multiple Sclerosis [100–103]. The increased relative risk is in the modest range (1.5 – 3-fold). The exact mechanism of this has not been elucidated. It is possible the TBI (either single or multiple impacts of varying severities) can cause initiation of a protein aggregation-related seeding event that, over time, leads to neurodegeneration-linked protein aggregate accumulation. Moderate to severe TBI has been shown in autopsy studies to result in increased amyloid deposition in the brain. As we are unable to perform brain biopsies on participants, Aβ1–40 and Aβ1–42 are proxies for altered amyloid metabolism and easily followed over time. Tau is a neuronal protein, which helps stabilize microtubules in the axon. Since axonal injury is a key feature of TBI, tauopathy and amyloidopathy could be chronic manifestations of TBI. Moreover, Smith and colleagues (1999) have shown deposition of P-Tau following TBI [104]. CTE is another emerging neurodegenerative condition believed to be induced by the repeated concussions. CTE is characterized clinically by changes in personality, increased in anxiety or aggression and other related psychiatric symptoms [105]. Neuropathologically, CTE is characterized by the presence of Tau and TAR DNA binding protein-43 kDa (TDP-43) deposits in the cerebral cortex [106]. Such Tau deposits are mostly readily detected by P-Tau specific antibodies [105,107]. Tau is phosphorylated at many sites potentially by kinases such as casein kinase II, tau tubulin kinases, GSK3β, and cdk5 [108] [109,110]. Tau hyperphosphorylation has also been reproduced in animal models of TBI [111].
Elevated levels of P-Tau are seen in the brain in CTE years following mTBI or repeated concussions as a significant tauopathic neurodegenerative disease likely occurring as a result of repeated concussions [105,107] (Figure 1, 2). Using the high sensitivity SIMOA platform (Quanterix), Tau can be observed in the acute to subacute/chronic stage following mTBI (hockey players and military veterans) [112,113]. In parallel, we recently developed an ultrasensitive rolling cycle amplification (RCA) based ELISA format platform for both T-Tau and P-Tau (T231 and S202) assays and found elevations of serum P-Tau and T-Tau from severe human TBI and in rodent repetitive mTBI in both acute and subacute period [109,110]. In human cohort of mostly moderate-severe TBI (n=21 subjects), we detected plasma T-Tau and P-Tau elevations not only in the acute phase (< 24 h), but also in the chronic phase (average 6 mo. post-injury) [114]. The availability of such ultrasensitive tests of T-Tau and P-Tau holds promise on tracking the possible development of post-TBI CTE and AD (Table 3). TDP-43 and its breakdown product were also found elevated in human CSF 24–48 h following severe TBI [115].
4.5.2. Autoantibodies as autoimmune response biomarkers:
Reports have documented brain-directed autoimmunity in neurological and neurodegenerative diseases such as AD, stroke, epilepsy, spinal cord injury and paraneoplastic syndromes [116–121]. In human TBI and stroke, however, autoimmunity has only been examined in a limited manner, and current studies focused on autoantibodies against preselected antigens such as MBP, S100B, and glutamate receptors [91,122–125]. Recently, Marchi et al. demonstrated that anti-glial protein S100B antibodies are elevated in football players with repeated concussions [126]. In parallel, we have gathered evidence that unexpectedly shows an immunodominant autoantibody response to GFAP and its BDPs in a subset of subacute and chronic TBI patients (day 5 to 6 mo. post injury) [52,127] (Figure 1, 2). Our hypothesis is that TBI-induced release of GFAP-BDPs is in substantive quantity through the compromised brain-blood-barrier into circulation and becomes accessible to and recognized by the immune systems as a non-self protein, triggering autoantibody response in these vulnerable individuals. As autoantibody specifically targeting a major brain protein (GFAP) might trigger a persistent autoimmune attack of the CNS, it might negatively affect TBI patient long-term outcome (Table 3). Also, Tanriverdi et al. showed the presence of anti-pituitary antibodies in patient serum 3 years after TBI [128,129]. This could be linked to hypopituitarism observed in the chronic phase of TBI [130]. Growth hormone deficiency was also found by Tanriverdi et al. (2013) in amateur boxers and kick boxers [131]. Additional neuroantigen targets capable of triggering TBI autoimmunity response are likely to be identified and verified in the future [132].
5. Other emerging biomarkers:
5.1. New protein biomarker candidates:
There are other TBI biomarker candidates with biofluid evidence from animal models of TBI from severe to mTBI in human. These include dendritic protein microtubule-associated protein-2 (MAP2) [133], [134], brain derived nerve growth factor (BDNF) [135], and postsynaptic protein neurogranin [136] (Table 3). Further evaluation of the utility and TBI specificity of these biomarkers are needed. Post-TBI vascular injury is also an emerging pathological mechanism under investigation [137,138]. Thus, it has been suggested that it might be possible to use vascular dysfunction/markers to track such events. They include fibrinogen, D-dimer and von Willebrand factor [139], [140], [141].
5.2. MicroRNA (miRNA)
miRNA are a class of small (19–28 nt) endogenous RNA molecules that regulate gene expression at post transcriptional level. Circulating miRNAs have been shown to be associated with several human diseases and disorders. A number of candidate miRNAs have in fact been reported to be elevated in biofluid (CSF, serum or plasma) in several rodent models of TBI with different severities. These include miR-Let-7i in acute CSF and serum using a rat overpressure blast brain injury model [142], miRNA (MiR-376a, MiR-214, MiR-199a-3p) candidates in acute serum samples using a mild close head injury model {Sharma:2014ig}. For human data, recently, Redell, et al. were the first to identify elevations of three miRNA (MiR-16, MiR-92a, MiR-765) in acute plasma samples after severe TBI [143]. Bhomia and our group also identified a total of ten candidate miRNAs (miR-151–5p, miR-195, miR-20a, miR328, miR-362–3p, miR30d, miR-451, miR-486, miR-505 and miR-92a) using acute CSF, and/or serum samples from human TBI that ranging from mild, moderate to severe TBI [144]. These miRNAs in fact can distinguish mTBI from healthy volunteers and orthopedic injury control samples. Another recent study showed that two miRNAs (miR-142–3p and miR-423–3p) can identify mild TBI patients who are likely to post-concussive syndrome [145]. Pietrao et al. also showed that miR-425–5p and miR-502 were elevated in mTBI in the early time points, while the latter also predicted the 6-month outcome [146].
5.3. Circulating nucleic acids
Circulating nucleic acids have been considered as diagnostic tools in trauma patients [147], [148]. Most researchers focused on DNA rather than mRNA, as unlike miRNA, DNA are highly labile and subjected to degradation by RNase found in biofluids. Total plasma cell-free DNA levels were found to be an independent mortality predictor for severe TBI subjects in several studies [148] [149] [150] [151]. However, since total plasma DNA is not brain-specific, it is unlikely DNA-based test will become a specific TBI diagnostic tool.
5.4. Exosome / Microvesicles
Another emerging field is the study of circulating microvesicles and exosomes (MV/E) in CSF and/or blood after TBI [152]. Exosomes and microvesicles are lipid-bilayer encapsulated particles (10–100 nm in diameter) that are released from cells (healthy or injured) into biofluids including extracellular fluid, CSF and blood. Often they contain distinct protein or miRNA content when released under a disease or disorder state. For example, plasma of TBI patients has a distinct set of proteins, as revealed by mass spectrometry-based method [153]. In addition, Manek et al. showed that MV/E released into CSF in TBI patients contains elevated levels of several protein biomarkers (SBDPs, synaptophysin, UCH-L1 and GFAP) [154]. Nekludov et al. (2017) also found that microvesicles isolated from TBI subject plasma (n=15) contains brain-derived GFAP and aquaporin-4 [155]. Circulating exosomes embedded with tau might be a better diagnostic tool for chronic TBI subjects at risk of developing chronic traumatic encephalopathy (CTE) [156].
6. Utilities of TBI biomarkers
As brain protein biomarkers detected in biofluid originated from the brain, they offer an organic measure of the pathophysiological processes at various time points following TBI. Since they can be readily measured in accessible biofluids, they can be referred to as a “liquid brain biopsy”. Such TBI biomarker tests can be used in acute, subacute and chronic patient care and management (Table 4).
Table 4.
Potential Utilities of TBI biomarkers.
| As diagnostic tools | As patient stratification tools in therapeutic clinical trials |
As disease prognosticator |
As monitor for chronic TBI |
|
|---|---|---|---|---|
| Setting | Acute (up to first 24 h post- injury |
Acute (within hours post- injury) |
Acute to subacute (hours to days) |
Weeks, months, years |
| Clinical utilities | • To reduce usage of CT • To triage patients for hospitalization |
• To screen and enroll mild TBI subjects who are likely to have poor outcome or develop post-concussive syndrome • To screen and enroll subjects who are likely to respond to a certain therapy |
• To inform patient and/or family of likelihood of mid- and long-term prognosis |
• Monitor development of CTE, AD or PD • Monitor autoimmune response |
| Examples | GFAP, UCH-L1, S100b or β, SBDP150/145/SNTF, T-Tau/P-Tau, pNF-H, NF-L |
GFAP, pNF-H | GFAP, P-Tau, S100B, NF-L |
T-Tau, P-Tau, Aβ peptides, AutoAb[GFAP] |
6.1. TBI diagnostics:
6.1.1. Neurointensive Care:
Severe TBI patients (about 10–15% of all TBI, GCS 3–8) are often managed in neurointensive care units (neuro-ICU) in major hospitals. Monitoring of patient’s brain and systemic status (including intracranial pressure) is critical in enhancing patient’s survival rate and long-term outcome. Here, ventriculostomy procedure is often performed as a means of cranial decompression. Thus, the availability of both blood samples and CSF samples can be obtained for possible “real time” biomarker monitoring. For example, the biomarker load (e.g. biomarker levels over a period time, or area under the curve) for UCH-L1 in both CSF and serum can distinguish injury severity and mortality [35]. Thus, we envision repeated monitoring of TBI biomarker levels over time in a neuro-ICU setting might provide useful and actionable information in the management of patients with severe TBI.
6.1.2. Emergency Department:
In the civilian setting the majority of TBI cases are mild-moderate TBI (about 80–85%, GCS 13–15 for mild, GCS 9–12 for moderate). Most of these patients would arrive at Emergency Departments for treatment and care. Currently, the cranial CT is the primary diagnostic tool for assessing injury severity. However, while CT can detect blood hemorrhage, it is not particularly sensitive in detecting DAI (Diffuse Axonal Injury) or other more subtle forms of brain injuries. In addition, repeated CT scanning can present high radiation exposure to at risk population such as children. Thus we envision a rapid POC device being developing as a screening prior to the use of CT. It is possible that repeated measurement of biomarkers over time could potentially be used to access evolving lesion, worsening of injury or the course of brain recovery.
6.1.3. Sports-related concussions/ mTBI:
On the opposite end of the TBI spectrum, a common form of everyday mild is sometimes referred to concussion. Sports-related single or repeated concussions are especially common among professional, recreational and collegiate athletes. In this setting, it is very important to access (a) if a concussion indeed occurred, and (ii) the degree of injury. If a biomarker is present in circulation within 30 min post-impact, its detection might be useful in making decision for return to play (RTP) in real time. Alternatively, with repeated testing of the same biomarkers within the days following concussion, in conjunction with other measures such as sports concussion assessment tools (SCAT3), they are useful in RTP decision. Another important feature of sports-related mTBI, the athletes who take part in such impact-prone sports activity often experience not only a single concussion, but also repeated concussion over time, both in recreational or professional sport setting. There is another potential life-threatening but rare condition – “second impact syndrome” – it occurs if an athlete who has not fully recovered from a recent concussion suffers another concussion, resulting in a greatly increase risk of severe disability or mortality [157] [158] [159].
6.1.4. Military mTBI:
Mild TBI also commonly occurs in military operations – including in the theater of operations as well as during training. Similar to sports concussion, triaging decisions rely on having accurate information on the severity of the impact in individual subjects. It is possible to use a POC diagnostic test to monitor biomarker levels over time. Biomarker testing might also be useful in assisting the decision of return to duty when blood levels of a biomarker return to below a preset cutoff value.
6.2. TBI prognostics:
TBI biomarkers can also be used as a prognostic tool in the ED setting, in neurointensive care unit for the more severely injured patients or even in an out-of-hospital setting. For mTBI cases, similar biomarkers might predict the possible development of persistent post-concussive syndrome (PCS). Several protein biomarkers have shown some promise in this emerging area.
6.2.1. S100B
One of the most powerful uses of biomarkers is to utilize acute phase levels of such biomarkers to inform on the outcome of patients. For example, serum levels of S100B within 12–36 h from TBI in neurointensive care units correlate with patient outcomes. [160]. These studies have been confirmed by a German research group showing a significant correlation of S100B concentrations in serum and Glasgow Outcome Score (GOS) score at 6 months. In addition, they also find that serum levels of S100B > 0.7 ng/mL correlate with 100% mortality [161]. Di Battista et al. (2015) reported that unfavorable neurological outcomes are associated with elevation in serum levels of S100B and GFAP [162]. Combined admission concentrations of these three markers are able to discriminate favorable versus unfavorable outcome and survival versus death. S100B can also detect brain death development or mortality after severe TBI [163], [164]. In another study, GFAP and S100B were found to be strong predictors of unfavorable outcome among severe TBI patients [46]. Another study also confirmed that acute S100B, GFAP, and UCH-L1 in severe TBI correlated significantly with the injury severity and clinical outcome [165]. One review analyzed how S100B may be utilized in TBI patients and pointed out S100B appears to be an important and useful predictor of functional outcome in moderate-to-severe TBI [166].
6.2.2. GFAP:
Acute plasma GFAP levels also modestly correlate with patient with poor outcomes (GOSE ≤4) (AUC, 0.74) or with good outcome (GOSE ≥ 7) (AUC, 0.65) [38]. Similar results were obtained in early studies that demonstrated the relationship of increased serum-GFAP in patients with severe TBI and clinical outcomes [53] [167]. Serum GFAP levels were also significantly higher in patients who died or had an unfavorable outcome and have predicted neurological outcome at 6 months [168]. These findings were confirmed by Czeiter and colleagues (2012) when evaluating the correlation between serum values of GFAP and the 6-month mortality [169]. Another study confirmed that serum concentration of GFAP and UCH-L1 proteins also strongly predicted poor outcomes and performed better than S100B [170]. In addition, as far as TBI in children, serum GFAP measured on day 1 of injury correlated with functional outcomes at 6 months as determined with Pediatric Cerebral Performance Category scores [171]. Taken together, GFAP and S100B levels in serum may enhance prognostication when combined with clinical variables [46].
6.2.3. UCH-L1:
Similarly, serum UCH-L1 can distinguish mild-moderate TBI subjects that require neurosurgical intervention from those that do not [172]. Both serum UCH-L1 and GFAP concentrations on the second day predicate the recovery and unfavorable outcome by distinguishing patients with GOS score 1–3 from patients with GOS score 4–5 [173]. Besides, in a pediatric TBI serum biomarker study, UCH-L1 was correlated with GOS, which was used to predict outcome [82].
6.2.4. NF proteins:
A study in Sweden involving 182 TBI patients reported that serum NF-L independently predicted TBI outcomes assessed at 6 to 12 months after injury using GOS [82]. Another study by Shahim et al. (2016) reported that initial serum levels of NF-L correlated with clinical outcome as assessed by the GOS scale at 12 months follow-up [174]. Similarly, the usefulness of NF-H (in addition to S100B and GFAP) as predictive biomarker of outcome in children with TBI has been also reported [175].
6.2.5. Tau protein:
Tau protein in serum is found to predict outcomes after severe TBI with a significantly higher concentration in the poor outcome group than that in the good outcome group. Both GCS score and Tau protein levels are independent prognostic factors for poor outcome which is determined by abnormal pupil light reflex and basal cistern compression on CT [176]. An early study showed that serum “cleaved Tau protein” levels (c-tau) in CSF is a predictor of clinical outcomes in severe TBI subjects [177]. Another recent study also revealed that serum “c-tau” levels in good outcome group (74.26 pg/ml) is lower than the poor outcome group (127.32 pg/mL) at 6 months follow up [178]. But the exact protein characterization of c-Tau remains elusive.
6.2.6. Other markers:
There are new biomarkers showing predictive value in TBI outcome. Early levels (within 24 h of injury) of MAP-2 in ventricular CSF MAP-2 provide enhanced prognostic capabilities for mortality at 6 months [72]. A TRACK-TBI pilot study revealed that day-of-injury serum BDNF provides 6-month prognostic information regarding recovery from TBI [160]. They found that BDNF has a higher prognostic value among mTBI subjects than moderate/severe TBI subjects. In addition, plasma Aβ42 levels at day 30 after the TBI correlate with clinical outcome assessed at 6 months after injury [179].
6.2.7. Opposing results:
However, others have reported opposite results, possibly due to the current lack of standardization of biomarker assays. Some studies found that serum S100B concentrations neither correlate with clinical outcome using GOS, GOS-E nor with imaging studies [49] [174]. In another study, while serum S100β levels increased in patients with minor to moderate TBI, S100B serum levels did not predict one-month neuropsychological outcomes [180]. Similarly, in multivariate analysis, GFAP was not predictive for outcome determined by GOS-E and return to work (RTW) time line. Lastly, for c-tau, an earlier study shows that its serum level is a poor predictor after mTBI [181].
7. TBI biomarkers as drug development Tools
7.1. Preclinical animal experimental therapeutic studies supporting the use of TBI biomarkers as therapeutic development tools:
As pointed out above, TBI biomarkers such as GFAP levels in CSF have been found to be elevated in several rodent models of severe TBI (CCI, PBBI, OBI) [58,59] and as well as in serum /plasma samples in mTBI models [30,60–62]. There is additional evidence that the post-TBI elevation of GFAP is severity–dependent [59]. In terms of using biomarkers to track therapeutic intervention in animal models of TBI, the best example is perhaps the Operation Brain Trauma Therapy (OBTT) study, which is a DoD–sponsored multi-center consortium study [182]. This study employed three rat models: CCI, PBBI and FPI to screen for more than 10 drugs with existing promising preclinical data in TBI already [182,183]. A composite scoring system was used to assess drug effects involving histopathology score, neurobehavioral /functional outcome and blood-based biomarkers (GFAP and UCH-L1 levels). Interestingly, it was shown that 4 h and 24 h GFAP levels significantly correlated with lesion volume as well as functional outcome [184]. Most importantly, one experimental drug, Simvastatin, was found to suppress the levels of serum GFAP at 24 h in FPI and PBBI models [185]. Another experimental drug, nicotinamide, also attenuated serum GFAP levels at 24 h in PBBI and CCI models [186].
7.2. Human clinical trials involving the use of biomarkers:
Large-scale therapeutic clinical trials are also beginning to incorporate blood-based biomarkers as a secondary endpoint (Table 4). The following are two examples: (1) INTREPID-2566 study; (ClinicalTrials.gov Identifier: NCT00805818), The purpose of this randomized, double-blind, placebo-controlled, dose-escalation study of NNZ-2566 is to assess the effect of an experimental drug, NNZ-2566 which is being developed as a treatment to decrease neuronal damage/death to the brain following moderate to severe TBI [187]. Among the secondary outcomes is examination of the modification of the acute physiological processes in TBI by evaluating electroencephalographic (EEG) determinants in patients with moderate to severe TBI (defined as GCS 4–12) and biomarker levels (GFAP, UCH-L1). (2) Blood Biomarkers of Injury and Outcome in Traumatic Brain Injury (BIO-ProTECT) (ClinicalTrials.gov Identifier: NCT01730443) and the parent ProTECT III study (ClinicalTrials.gov Identifier: NCT00822900) [188], [189]. The Bio-ProTECT study examines serum biomarkers of structural brain injury in subjects with severe TBI (S100B, GFAP, UCH-L1, SBDP150) with and without progesterone treatment.
The use of blood-based biomarkers for mTBI is particularly appealing. Currently most of the FDA-approval seeking therapeutic clinical trials target the severe to moderate TBI. Yet, the majority (>80%) or all TBI patients are in the mild category. One of the key factors is that over 80–85% of all mTBI subjects will recover spontaneously. Therefore, large numbers of patients would need to be recruited to adequately power a clinical trial designed to show therapeutic benefit in subjects with long-term neurological deficits. In addition, therapeutic effects might be difficult to detect in mTBI as the majority of mTBI subjects who get better without treatment. This makes achieving clinical efficacy much more challenging. As indicated above, acute biomarkers have the potential to inform on those subjects who are more likely to have poor outcome and experience persistent post-concussive syndrome (PCS). Thus it follows that in a therapeutic trial setting, one could administrate such a blood test in the acute phase to potential subjects and then only enroll subjects who are more likely to have poor outcome or develop PCS, based on the biomarker test results. This approach might enhance the probability of demonstrating therapeutic efficacy (Table 4).
8. Expert Commentary
Collective evidence shows that TBI protein biomarkers are a promising diagnostic and prognostic tool, which may ultimately improve patient treatment and management. Some biomarkers might facilitate the development of guidelines for management of mTBI or concussion including decisions regarding returning to work, duty or sports / athletic events while providing opportunities for other short and long term interventions for patients suffering the sequelae of TBI [14,66,88,190]. TBI biomarkers could also provide major opportunities for the conduct of clinical research including confirmation of injury mechanism(s) and drug target identification. A temporal profile of changes in biomarkers would guide timing of treatment. In addition, biomarkers can be used in clinical trials of new therapeutic interventions as an early outcome predictor thus potentially reducing the risks and costs of clinical trials. Potential gender and developmentally related differences in biomarker profiles [191–194], as well as sensitivity to therapeutic interventions can be further examined in future studies. With the accelerated development of biofluid-based protein biomarker assays, there is a strong future promise for significant advances in TBI management and treatment.
9. Five-year view:
The full validation of the diagnostic and prognostic utilities of a TBI biomarker has been a slow process. This is in part due to the lack of clinically compatible platforms that can run such TBI biomarker assays (with the exception of S100B being available on the Roche Elecsys platform), and the lack of formal regulatory agency approval (e.g. FDA). Within the next five years – we anticipate that at least one major diagnostic company (e.g. Abbott i-STAT platform) will overcome these hurdles and make newer markers (e.g. UCH-L1 and GFAP) available for clinical uses. Another trend we are seeing is that increasing number of large and smaller diagnostic companies are entering the race by adding new POC or automated/semi-automated clinical lab-based assay platforms to TBI -based diagnostics (e.g., including BioMerieux, Phillips, Sysmex, BioDirection and Banyan Biomarkers). We also anticipate that the clinical utilities of additional new markers will be independently validated in this period.
Key issues:
Assay platforms: Challenges remain in developing robust TBI assay - devices that can be used in POC environment with a rapid turnaround time and simple to use are on the horizon but the usefulness will need to be verified.
Assay standardization: Standardized assays format and qualified protein antigen standard and reference materials for the top TBI protein biomarkers candidates should be established. Assays may also undergo further analytical validation in accordance with good manufacturing practice (GMP) and good laboratory practice (GLP) guidance and Clinical & Laboratory Standards Institute (CLSI) guidelines.
Potential Confounds: The effects of age (e.g. age range from newborn to 80-year-old) on baseline (normal control) and post- TBI CSF, serum, plasma and whole blood values for the top biomarker candidates should be established. The ability of the candidate biomarkers to distinguish mTBI from non-TBI trauma patients (e.g. orthopedic injuries), and post-traumatic stress syndrome (PTSD) need to be firmly demonstrated.
Clinical usefulness validation of new biomarkers: As more researchers report on the discovery of “new” TBI biomarkers, many of them use a very small sample size, and they often show simple group comparison as evidence (e.g. showing statistically significant difference in the mean or median values between TBI group and control group, or between CT normal vs. CT abnormal groups, or outcome prediction). However, this exercise does not indicate any real-life practical clinical utilities of a given marker as a biomarker. For that, a larger study cohort is needed, and positive predictive (rate of true positive) and negative predictive values (rate of true negative) and a receiver operating characteristic curve (ROC) should be used in order to evaluate the clinical usefulness any new biomarker candidate.
Better classification of TBI: TBI is heterogeneous neurological disorder – it ranges from penetrating injury, focal contusion, and different forms of hematoma to diffuse injury to repetitive concussion. GCS is originally developed to assess functional responses after TBI (i.e. eye, muscle and verbal responses), but it informs very little about the size, location and type of brain injury. Thus, It is possible that a biomarker does not coorelate well with GCS. In fact, one could argue that in the future, a more complete classification of TBI might include the use of one or more TBI biofluid-biomarkers.
Neurodegeneration monitoring: A direct link between elevated and sustained blood levels of P-Tau, T-Tau or related markers and risk of development of post-traumatic CTE and/or AD needs to be further studied.
Acknowledgments
Funding
This study is supported in part by U.S. DOD TED Seed Project TED1506 (KK Wang), NIH R21NS085455–01 (KK Wang), VA I01 RX001859–02 Merit Award (KK Wang), and Florida State/McKnight Brain Institute BSCIRT Fund (#110587) (KK Wang), NIH RC2 NS069409 (GT Manley), NIH 1U01 NS086090–01 (GT Manley), DOD Grant W81XWH-14–2-0176 (GT Manley), DOD Grant W81XWH-13–1-04 (GT Manley), DOD-Army grant W81XWH-14–2-0166 (R Rubenstein). European Commission Framework Program 7, FP7-HEALTH-2013-INNOVATION-1 grant # 602150–2; (KK Wang; overall PI Andrew Maas, David Menon).
Footnotes
Declaration of Interest
KK Wang is a stockholder of Banyan Biomarkers, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
REFERENCES
Reference annotations
* Of interest
** Of considerable interest
- 1.Hoffman SW, Harrison C. The interaction between psychological health and traumatic brain injury: a neuroscience perspective. The Clinical Neuropsychologist. 23(8), 1400–1415 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease, Control, Prevention. CDC grand rounds: reducing severe traumatic brain injury in the United States. MMWR Morb. Mortal. Wkly. Rep. 62(27), 549–552 (2013). [PMC free article] [PubMed] [Google Scholar]
- 3.DOD-DVBIC. DVBIC Worldwide-TBI 2000–2013 Report. DOD Report. 1–5 (2014). [Google Scholar]
- 4.Elovic E, Doppalapudi HS, Miller M. Endocrine abnormalities and fatigue after traumatic brain injury. J Head Trauma Rehab. 21(5), 426–427 (2006). [Google Scholar]
- 5.Englander J, Bushnik T, Oggins J, Katznelson L. Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain Inj. 24(12), 1379–1388 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. The Lancet Neurology. 7(8), 728–741 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Kibby MY, Long CJ. Minor head injury: attempts at clarifying the confusion. Brain Inj. 10(3), 159–186 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Pearson WS, Sugerman DE, McGuire LC, Coronado VG. Emergency department visits for traumatic brain injury in older adults in the United States: 2006–08. West J Emerg Med. 13(3), 289–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012, e55–71 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. PsycEXTRA Dataset. [Google Scholar]
- 11.Pellman EJ, Viano DC, Casson IR, et al. Concussion in professional football: repeat injuries--part 4. Neurosurgery. 55(4), 860–73– discussion 873–6 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Slobounov S, Slobounov E, Sebastianelli W, Cao C, Newell K. Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery. 61(2), 338–44– discussion 344 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Dambinova SA, Bettermann K, Glynn T, et al. Diagnostic potential of the NMDA receptor peptide assay for acute ischemic stroke. PLoS ONE. 7(7), e42362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papa L, Akinyi L, Liu MC, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 38(1), 138–144 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saatman KE, Duhaime A-C, Bullock R, Maas AIR, Valadka A, Manley GT. Classification of Traumatic Brain Injury for Targeted Therapies. J. Neurotrauma. 25(7), 719–738 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. The Journal of Head Trauma Rehabilitation. 18(4), 307–316 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Xu JJ, Rasmussen I-AI, Lagopoulos JJ, Håberg AA. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J. Neurotrauma. 24(5), 753–765 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Merlo L, Cimino F, Angileri FF, et al. Alteration in synaptic junction proteins following traumatic brain injury. J. Neurotrauma. 31(16), 1375–1385 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Winston CN, Noël A, Neustadtl A, et al. Dendritic Spine Loss and Chronic White Matter Inflammation in a Mouse Model of Highly Repetitive Head Trauma. Am. J. Pathol. 186(3), 552–567 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Moghieb A, Kevin K Wang W. Acute, Subacute and chronic Biomarkers for CNS injury In: Biomarkers of Brain Injury and Neurological Disorders. Wang KKW, Zhang Z, Kobeissy FH (Eds.). CRC Press, 1–25 (2014). [Google Scholar]
- 21.Wang KK, Moghieb A, Yang Z, Zhang Z. Systems biomarkers as acute diagnostics and chronic monitoring tools for traumatic brain injury. SPIE Defense, Security, and Sensing. 87230O–87230O–15 (2013). [Google Scholar]
- 22.Mondello S, Muller U, Jeromin A, Streeter J, Hayes RL, Wang KKW. Blood-based diagnostics of traumatic brain injuries. Expert Rev. Mol. Diagn. 11(1), 65–78 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nature Reviews Neurology. 12(10), 563–574 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Bohmer AE, Oses JP, Schmidt AP, et al. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery. 68(6), 1624–30– discussion 1630–1 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Stein DM, Kufera JA, Lindell A, et al. Association of CSF Biomarkers and Secondary Insults Following Severe Traumatic Brain Injury. Neurocrit Care. 14(2), 200–207 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Berger RP, Pierce MC, Wisniewski SR, et al. Neuron-specific enolase and S100B in cerebrospinal fluid after severe traumatic brain injury in infants and children. PEDIATRICS. 109(2), E31 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Liu M, Zhang C, Liu W, et al. A novel rat model of blast-induced traumatic brain injury simulating different damage degree: implications for morphological, neurological, and biomarker changes. Front Cell Neurosci. 9, 168 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topolovec-Vranic J, Pollmann-Mudryj M-A, Ouchterlony D, et al. The value of serum biomarkers in prediction models of outcome after mild traumatic brain injury. J Trauma [Internet]. 71(5 Suppl 1), S478–86 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Buonora JE, Yarnell AM, Lazarus RC, et al. Multivariate Analysis of Traumatic Brain Injury: Development of an Assessment Score. Front Neurol. 6(Suppl 1), 503 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agoston DV, Elsayed M. Serum-based protein biomarkers in blast-induced traumatic brain injury spectrum disorder. Front Neurol. 3, 107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verfaillie CJ, Delanghe JR. Hemolysis correction factor in the measurement of serum neuron-specific enolase. Clin. Chem. Lab. Med. 48(6) (2010). [DOI] [PubMed] [Google Scholar]
- 32.Kobeissy FH, Ottens AK, Zhang Z, et al. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Molecular \& Cellular Proteomics [Internet]. 5(10), 1887–1898 (2006).* Original paper describing the identification of UCH-L1 as a potential TBI biomarker
- 33.Liu MC, Akinyi L, Scharf D, et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 31(4), 722–732 (2010).* First paper using ELISA assay to show UCH-L1 elevations in biofluid in rat model of TBI.
- 34.Mondello S, Linnet A, Büki A, et al. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery [Internet]. 70(3), 666–675 (2012).* Key paper describing UCH-L1 as a potential severe TBI biomarker
- 35.Brophy GM, Mondello S, Papa L, et al. Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J. Neurotrauma. 28(6), 861–870 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papa L, Oli MW, Akinyi L, et al. UCH-L1 is a novel biomarker for severe traumatic brain injury in human. Critical Care Med. 38(1), 138–144 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z-Y, Zhang L-X, Dong X-Q, et al. Comparison of the performances of copeptin and multiple biomarkers in long-term prognosis of severe traumatic brain injury. Peptides. 60, 13–17 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Diaz-Arrastia R, Wang KKW, Papa L, et al. Acute Biomarkers of Traumatic Brain Injury: Relationship between Plasma Levels of Ubiquitin C-Terminal Hydrolase-L1 and Glial Fibrillary Acidic Protein | Abstract. J. Neurotrauma. 31(1), 19–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch RD, Ayaz SI, Lewis LM, et al. Ability of Serum Glial Fibrillary Acidic Protein, Ubiquitin C-Terminal Hydrolase-L1, and S100B To Differentiate Normal and Abnormal Head Computed Tomography Findings in Patients with Suspected Mild or Moderate Traumatic Brain Injury. J. Neurotrauma. 33(2), 203–214 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tate CM, Wang KKW, Eonta S, et al. Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: a breacher pilot study. J. Neurotrauma. 30(19), 1620–1630 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Puvenna V, Brennan C, Shaw G, et al. Significance of Ubiquitin Carboxy-Terminal Hydrolase L1 Elevations in Athletes after Sub-Concussive Head Hits. PLoS ONE. 9(5), e96296 (2014).* UCH-L1 as a possible biomarker for sports-related concussions.
- 42.Schulte S, Podlog LW, Hamson-Utley JJ, Strathmann FG, Strüder HK. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train. 49(6), 830–850 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filippidis AS, Papadopoulos DC, Kapsalaki EZ, Fountas KN. Role of the S100B serum biomarker in the treatment of children suffering from mild traumatic brain injury. Neurosurg Focus. 29(5), E2 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Ringger NC, O’steen BE, Brabham JG, et al. A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J. Neurotrauma. 21(10), 1443–1456 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Kiechle K, Bazarian JJ, Merchant-Borna K, et al. Subject-Specific Increases in Serum S-100B Distinguish Sports-Related Concussion from Sports-Related Exertion. PLoS ONE. 9(1), e84977 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vos PE, Jacobs B, Andriessen TM, et al. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology [Internet]. 75(20), 1786–1793 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Cervellin G, Benatti M, Carbucicchio A, et al. Serum levels of protein S100B predict intracranial lesions in mild head injury. Clin. Biochem. 45(6), 408–411 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Papa L, Silvestri S, Brophy GM, et al. GFAP out-performs S100β in detecting traumatic intracranial lesions on computed tomography in trauma patients with mild traumatic brain injury and those with extracranial lesions. J. Neurotrauma. 31(22), 1815–1822 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metting Z, Wilczak N, Rodiger LA, Schaaf JM, van der Naalt J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology. 78(18), 1428–1433 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Barbosa RR, Jawa R, Watters JM, et al. Evaluation and management of mild traumatic brain injury. Journal of Trauma and Acute Care Surgery. 73, S307–S314 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Zongo D, Ribéreau-Gayon R, Masson F, et al. S100-B protein as a screening tool for the early assessment of minor head injury. Ann Emerg Med. 59(3), 209–218 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Zoltewicz JS, Mondello S, et al. Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS ONE. 9(3), e92698 (2014).** First paper describing anti-GFAP autoantibody as a dominant post-TBI autoimmune response.
- 53.Nylén K, Öst M, Csajbok LZ, et al. Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J. Neurol. Sci. 240(1–2), 85–91 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Mondello S, Jeromin A, Buki A, et al. Glial neuronal ratio: a novel index for differentiating injury type in patients with severe traumatic brain injury. J. Neurotrauma. 29(6), 1096–1104 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vos PE, Lamers KJ, Hendriks JC, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 62(8), 1303–1310 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Okonkwo DO, Yue JK, Puccio AM, et al. GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J. Neurotrauma. 30(17), 1490–1497 (2013).** First paper describing GFAP / GFAP-BDP as diagnostic and prognostic biomarker for full spectrum of TBI severity.
- 57.Yang Z, Wang KKW. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends in neurosciences. 38(6), 364–374 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glushakova OY, Jeromin A, Martinez J, et al. Cerebrospinal fluid protein biomarker panel for assessment of neurotoxicity induced by kainic acid in rats. Toxicological Sciences. 130(1), 158–167 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Zoltewicz JS, Mondello S, Yang B, et al. Biomarkers track damage after graded injury severity in a rat model of penetrating brain injury. J. Neurotrauma. 30(13), 1161–1169 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Svetlov SI, Prima V, Kirk DR, et al. Neuro-Glial and Systemic Mechanisms of Pathological Responses to Primary Blast Overpressure (OP) Compared to Severe “Composite”Blast in Rat Models. NATO Research and Technology Organisation; RTO-MP-HFM. 207, 37–1 – 37–10 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed F, Gyorgy A, Kamnaksh A, et al. Time-dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis. 33(24), 3705–3711 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Liu M-D, Luo P, Wang Z-J, Fei Z. Changes of serum Tau, GFAP, TNF-α and malonaldehyde after blast-related traumatic brain injury. Chin J Traumatol. 17(6), 317–322 (2014). [PubMed] [Google Scholar]
- 63.Kou Z, Gattu R, Kobeissy F, et al. Combining biochemical and imaging markers to improve diagnosis and characterization of mild traumatic brain injury in the acute setting: results from a pilot study. PLoS ONE. 8(11), e80296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schiff LL, Hadker NN, Weiser SS, Rausch CC. A literature review of the feasibility of glial fibrillary acidic protein as a biomarker for stroke and traumatic brain injury. Mol Diagn Ther. 16(2), 79–92 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Pike BR, Flint J, Dutta S, Johnson E, Wang KK, Hayes RL. Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J. Neurochem. 78(6), 1297–1306 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Mondello S, Robicsek SA, Gabrielli A, et al. alphaII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma. 27(7), 1203–1213 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vartanian MG, Cordon JJ, Kupina NC, et al. Phenytoin pretreatment prevents hypoxic-ischemic brain damage in neonatal rats. Developmental brain research [Internet]. 95(2), 169–175 (1996). [DOI] [PubMed] [Google Scholar]
- 68.Berger RP, Houle J-F, Hayes RL, Wang KK, Mondello S, Bell MJ. Translating biomarkers research to clinical care: applications and issues for rehabilomics. PM & R. 3(6 Suppl 1), S31–8 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Pike BR, Flint J, Dave JR, et al. Accumulation of calpain and caspase-3 proteolytic fragments of brain-derived alphaII-spectrin in cerebral spinal fluid after middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 24(1), 98–106 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Yokobori S, Zhang Z, Moghieb A, et al. Acute Diagnostic Biomarkers for Spinal Cord Injury: Review of the Literature and Preliminary Research Report. World Neurosurgery. 83(5), 867–878 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Massaro AN, Jeromin A, Kadom N, et al. Serum Biomarkers of MRI Brain Injury in Neonatal Hypoxic Ischemic Encephalopathy Treated With Whole-Body Hypothermia. Pediatr Crit Care Med. 14(3), 310–317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papa L, Robertson CS, Wang KKW, et al. Biomarkers improve clinical outcome predictors of mortality following non-penetrating severe traumatic brain injury. Neurocrit Care. 22(1), 52–64 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Siman R Evidence that the blood biomarker SNTF predicts brain imaging changes and persistent cognitive dysfunction in mild TBI patients. 1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Posmantur RM, Zhao X, Kampfl A, Clifton GL, Hayes RL. Immunoblot analyses of the relative contributions of cysteine and aspartic proteases to neurofilament breakdown products following experimental brain injury in rats. Neurochemical research [Internet]. 23(10), 1265–1276 (1998). [DOI] [PubMed] [Google Scholar]
- 75.Posmantur R, Hayes RL, Dixon CE, Taft WC. Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury. J. Neurotrauma. 11(5), 533–545 (1994). [DOI] [PubMed] [Google Scholar]
- 76.Anderson KJ, Scheff SW, Miller KM, et al. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. J. Neurotrauma [Internet]. 25(9), 1079–1085 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Zhang L, Kallakuri S, Cohen A, Cavanaugh JM. Correlation of mechanical impact responses and biomarker levels: A new model for biomarker evaluation in TBI. J. Neurol. Sci. 359(1–2), 280–286 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Martínez-Morillo E, Childs C, García BP, et al. Neurofilament medium polypeptide (NFM) protein concentration is increased in CSF and serum samples from patients with brain injury. Clinical Chemical Laboratory Medicine. 53(10), 1575–1584 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Neselius S, Zetterberg H, Blennow K, Marcusson J, Brisby H. Increased CSF levels of phosphorylated neurofilament heavy protein following bout in amateur boxers. PLoS ONE. 8(11), e81249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Žurek J, Bartlová L, Fedora M. Hyperphosphorylated neurofilament NF-H as a predictor of mortality after brain injury in children. Brain Inj. 25(2), 221–226 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Oliver JM, Jones MT, Kirk KM, et al. Serum Neurofilament Light in American Football Athletes over the Course of a Season. J. Neurotrauma. 33(19), 1784–1789 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Nimer Al F, Thelin E, Nyström H, et al. Comparative Assessment of the Prognostic Value of Biomarkers in Traumatic Brain Injury Reveals an Independent Role for Serum Levels of Neurofilament Light. PLoS ONE. 10(7), e0132177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deber CM, Reynolds SJ. Central nervous system myelin: structure, function, and pathology. Clin. Biochem. 24(2), 113–134 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ottens AK, Golden EC, Bustamante L, Hayes RL, Denslow ND, Wang KKW. Proteolysis of multiple myelin basic protein isoforms after neurotrauma: characterization by mass spectrometry. J. Neurochem. 104(5), 1404–1414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu MC, Akle V, Zheng W, et al. Extensive degradation of myelin basic protein isoforms by calpain following traumatic brain injury. J. Neurochem. 98(3), 700–712 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Front Neurol. 3, 11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ponsford JL, Ziino C, Parcell DL, et al. Fatigue and sleep disturbance following traumatic brain injury--their nature, causes, and potential treatments. The Journal of Head Trauma Rehabilitation. 27(3), 224–233 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Wagner AK, Zitelli KT. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology. 20(1), 39–48 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Clin Pract Neurol. – (2013). [DOI] [PubMed] [Google Scholar]
- 90.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 30(1), 179–88– xi (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sorokina EGE, Semenova ZBZ, Granstrem OKO, et al. [S100B protein and autoantibodies to S100B protein in diagnostics of brain damage in craniocerebral trauma in children]. Zh Nevrol Psikhiatr Im S S Korsakova. 110(8), 30–35 (2010). [PubMed] [Google Scholar]
- 92.Mecocci P, Parnetti L, Romano G, et al. Serum anti-GFAP and anti-S100 autoantibodies in brain aging, Alzheimer’s disease and vascular dementia. J Neuroimmunol [Internet]. 57(1–2), 165–170 (1995). [DOI] [PubMed] [Google Scholar]
- 93.Gordon WA, Zafonte R, Cicerone K, et al. Traumatic brain injury rehabilitation: state of the science. Am J Phys Med Rehabil. 85(4), 343–382 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Ziebell JM, Morganti-Kossmann MC. Involvement of Pro- and Anti-Inflammatory Cytokines and Chemokines in the Pathophysiology of Traumatic Brain Injury. Neurotherapeutics [Internet]. 7(1), 22–30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26(8), 1191–1201 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Maier B, Laurer H-L, Rose S, Buurman WA, Marzi I. Physiological levels of pro- and anti-inflammatory mediators in cerebrospinal fluid and plasma: a normative study. J. Neurotrauma. 22(7), 822–835 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Chiaretti A, Genovese O, Aloe L, et al. Interleukin 1? and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv Syst. 21(3), 185–193 (2004). [DOI] [PubMed] [Google Scholar]
- 98.Folkersma H, Brevé JJP, Tilders FJH, Cherian L, Robertson CS, Vandertop WP. Cerebral microdialysis of interleukin (IL)-1beta and IL-6: extraction efficiency and production in the acute phase after severe traumatic brain injury in rats. Acta Neurochir. 150(12), 1277–1284 (2008). [DOI] [PubMed] [Google Scholar]
- 99.Bonneh-Barkay D, Zagadailov P, Zou H, et al. YKL-40 expression in traumatic brain injury: an initial analysis. J. Neurotrauma. 27(7), 1215–1223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang J-H, Lin H-C. Increased risk of multiple sclerosis after traumatic brain injury: a nationwide population-based study. J. Neurotrauma. 29(1), 90–95 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J. Neurol. Neurosurg. Psychiatr. 84(2), 177–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci Biobehav Rev. 36(5), 1376–1381 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Lee P-C, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology. 79(20), 2061–2066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith DH, Chen XH, Nonaka M, et al. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. 58(9), 982–992 (1999). [DOI] [PubMed] [Google Scholar]
- 105.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. 68(7), 709–735 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 136(Pt 1), 43–64 (2013).** A key review paper describing post-TBI chronic traumatic encephalopathy.
- 107.Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League Player: Case report and emerging medicolegal practice questions. Journal of Forensic Nursing. 6(1), 40–46 (2010). [DOI] [PubMed] [Google Scholar]
- 108.Duan Y, Dong S, Gu F, Hu Y, Zhao Z. Advances in the Pathogenesis of Alzheimer’s Disease: Focusing on Tau-Mediated Neurodegeneration. Transl Neurodegener. 1(1), 24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Z, Wang P, Morgan D, et al. Temporal MRI characterization, neurobiochemical and neurobehavioral changes in a mouse repetitive concussive head injury model. Sci. Rep. 5(1), 11178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rubenstein R, Chang B, Davies P, Wagner AK, Robertson CS, Wang KKW. A novel, ultrasensitive assay for tau: potential for assessing traumatic brain injury in tissues and biofluids. J. Neurotrauma. 32(5), 342–352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic Traumatic Encephalopathy in Blast-Exposed Military Veterans and a Blast Neurotrauma Mouse Model. Science Translational Medicine [Internet]. 4(134), 134ra60–134ra60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shahim P, Tegner Y, Wilson DH, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol. 71(6), 684–692 (2014).* First paper describing the detection of Tau elevations in blood in the acute phase following concussions, with the use of high sensitivity assay platform (SIMOA).
- 113.Olivera A, Lejbman N, Jeromin A, et al. Peripheral Total Tau in Military Personnel Who Sustain Traumatic Brain Injuries During Deployment. JAMA Neurol. 72(10), 1109–1116 (2015). [DOI] [PubMed] [Google Scholar]
- 114.Rubenstein R, Chang B, Yue JK, et al. Comparing Plasma Phospho Tau, Total Tau, and Phospho Tau-Total Tau Ratio as Acute and Chronic Traumatic Brain Injury Biomarkers. JAMA Neurol. 74(9), 1063–1072 (2017).* First paper to show P-Tau (and Tau) elevations in blood not only in the acute phase but also chronic phase of TBI, using a high sensitivity platform (SOFIA).
- 115.Yang Z, Lin F, Robertson CS, Wang KKW. Dual vulnerability of TDP-43 to calpain and caspase-3 proteolysis after neurotoxic conditions and traumatic brain injury. Journal of Cerebral Blood Flow & Metabolism. 34(9), 1444–1452 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dambinova SAS, Khounteev GAG, Izykenova GAG, Zavolokov IGI, Ilyukhina AYA, Skoromets AAA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clinical Chemistry. 49(10), 1752–1762 (2003). [DOI] [PubMed] [Google Scholar]
- 117.Colasanti TT, Barbati CC, Rosano GG, Malorni WW, Ortona EE. Autoantibodies in patients with Alzheimer’s disease: pathogenetic role and potential use as biomarkers of disease progression. Autoimmunity Reviews. 9(12), 807–811 (2010). [DOI] [PubMed] [Google Scholar]
- 118.D’Andrea MR. Add Alzheimer’s disease to the list of autoimmune diseases. Medical Hypotheses. 64(3), 458–463 (2005). [DOI] [PubMed] [Google Scholar]
- 119.Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 99(4), 1073–1087 (2006). [DOI] [PubMed] [Google Scholar]
- 120.Popovich PGP, Stokes BTB, Whitacre CCC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J. Neurosci. Res. 45(4), 349–363 (1996). [DOI] [PubMed] [Google Scholar]
- 121.Lancaster E, Dalmau J. Neuronal autoantigens—pathogenesis, associated disorders and antibody testing. Nature Reviews Neurology. 8(7), 380–390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ponomarenko NA, Durova OM, Vorobiev II, et al. Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunology Letters. 103(1), 45–50 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Hedegaard CJ, Chen N, Sellebjerg F, et al. Autoantibodies to myelin basic protein (MBP) in healthy individuals and in patients with multiple sclerosis: a role in regulating cytokine responses to MBP. Immunology [Internet]. 128(1pt2), e451–e461 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cox AL, Coles AJ, Nortje J, et al. An investigation of auto-reactivity after head injury. Journal of Neuroimmunology. 174(1–2), 180–186 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Goryunova AVA, Bazarnaya NAN, Sorokina EGE, et al. Glutamate receptor autoantibody concentrations in children with chronic post-traumatic headache. Neurosci Behav Physiol. 37(8), 761–764 (2007). [DOI] [PubMed] [Google Scholar]
- 126.Marchi N, Bazarian JJ, Puvenna V, et al. Consequences of Repeated Blood-Brain Barrier Disruption in Football Players. PLoS ONE. 8(3), e56805 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang KKW, Yang Z, Yue JK, et al. Plasma Anti-Glial Fibrillary Acidic Protein Autoantibody Levels during the Acute and Chronic Phases of Traumatic Brain Injury: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury Pilot Study. J. Neurotrauma. 33(13), 1270–1277 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tanriverdi F, De Bellis A, Bizzarro A, et al. Antipituitary antibodies after traumatic brain injury: is head trauma-induced pituitary dysfunction associated with autoimmunity? European Journal of Endocrinology. 159(1), 7–13 (2008). [DOI] [PubMed] [Google Scholar]
- 129.Tanriverdi F, De Bellis A, Battaglia M, et al. Investigation of antihypothalamus and antipituitary antibodies in amateur boxers: is chronic repetitive head trauma-induced pituitary dysfunction associated with autoimmunity? European Journal of Endocrinology. 162(5), 861–867 (2010). [DOI] [PubMed] [Google Scholar]
- 130.Aimaretti G, Ambrosio MR, Di Somma C, et al. Hypopituitarism induced by traumatic brain injury in the transition phase. J. Endocrinol. Invest. 28(11), 984–989 (2005). [DOI] [PubMed] [Google Scholar]
- 131.Tanriverdi F, De Bellis A, Ulutabanca H, et al. A Five Year Prospective Investigation of Anterior Pituitary Function after Traumatic Brain Injury: Is Hypopituitarism Long-Term after Head Trauma Associated with Autoimmunity? J. Neurotrauma. 30(16), 1426–1433 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Yang Z, Zhu T, Weissman AS, et al. Autoimmunity and Traumatic Brain Injury. 1–8 (2017). [Google Scholar]
- 133.Mondello S, Gabrielli A, Catani S, et al. Increased levels of serum MAP-2 at 6-months correlate with improved outcome in survivors of severe traumatic brain injury. Brain Injury. 26(13–14), 1629–1635 (2012). [DOI] [PubMed] [Google Scholar]
- 134.Papa L, Robicsek SA, Brophy GM, et al. Temporal Profile of Microtubule-Associated Protein 2: A Novel Indicator of Diffuse Brain Injury Severity and Early Mortality after Brain Trauma. J. Neurotrauma. neu.20174994–9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Korley FK, Diaz-Arrastia R, Wu AHB, et al. Circulating Brain-Derived Neurotrophic Factor Has Diagnostic and Prognostic Value in Traumatic Brain Injury. J. Neurotrauma. 33(2), 215–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang J, Korley FK, Dai M, Everett AD. Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin. Biochem. 48(13–14), 843–848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kenney K, Amyot F, Haber M, et al. Cerebral Vascular Injury in Traumatic Brain Injury. Exp. Neurol. 275(Part 3), 353–366 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Tomkins O, Feintuch A, Benifla M, Cohen A, Friedman A, Shelef I. Blood-Brain Barrier Breakdown Following Traumatic Brain Injury: A Possible Role in Posttraumatic Epilepsy. Cardiovascular Psychiatry and Neurology. 765923, 1–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muradashvili N, Lominadze D. Role of fibrinogen in cerebrovascular dysfunction after traumatic brain injury. Brain Inj. 27(13–14), 1508–1515 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Oliveira CO, Reimer AG, Da Rocha AB, et al. Plasma von Willebrand factor levels correlate with clinical outcome of severe traumatic brain injury. J. Neurotrauma. 24(8), 1331–1338 (2007). [DOI] [PubMed] [Google Scholar]
- 141.Berger RP, Fromkin J, Rubin P, Snyder J, Richichi R, Kochanek P. Serum D-dimer concentrations are increased after pediatric traumatic brain injury. J. Pediatr. 166(2), 383–388 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Balakathiresan N, Bhomia M, Chandran R, Chavko M, McCarron RM, Maheshwari RK. MicroRNA Let-7i Is a Promising Serum Biomarker for Blast-Induced Traumatic Brain Injury. J. Neurotrauma. 29(7), 1379–1387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Redell JB, Moore AN, Ward NH, Hergenroeder GW, Dash PK. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma. 27(12), 2147–2156 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bhomia M, Balakathiresan NS, Wang KK, Papa L, Maheshwari RK. A Panel of Serum MiRNA Biomarkers for the Diagnosis of Severe to Mild Traumatic Brain Injury in Humans. Sci. Rep. 6(1), 28148–12 (2016).* A comprehensive study identifying a panel of CSF- and blood-based miRNA’s as candidate biomarkers for mild to severe TBI in human.
- 145.Mitra B, Rau TF, Surendran N, et al. Plasma micro-RNA biomarkers for diagnosis and prognosis after traumatic brain injury: A pilot study. Journal of Clinical Neuroscience. 38, 37–42 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Di Pietro V, Ragusa M, Davies D, et al. MicroRNAs as Novel Biomarkers for the Diagnosis and Prognosis of Mild and Severe Traumatic Brain Injury. J. Neurotrauma. 34(11), 1948–1956 (2017). [DOI] [PubMed] [Google Scholar]
- 147.Ferrari M, Cremonesi L, Galbiati S. 10. Circulating Nucleic Acids as Diagnostic Tool. EJIFCC. 19(1), 68–74 (2008). [PMC free article] [PubMed] [Google Scholar]
- 148.Rodrigues Filho EM, Simon D, Ikuta N, et al. Elevated cell-free plasma DNA level as an independent predictor of mortality in patients with severe traumatic brain injury. J. Neurotrauma. 31(19), 1639–1646 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Campello Yurgel V, Ikuta N, Brondani da Rocha A, et al. Role of Plasma DNA as a Predictive Marker of Fatal Outcome following Severe Head Injury in Males. J. Neurotrauma. 24(7), 1172–1181 (2007). [DOI] [PubMed] [Google Scholar]
- 150.Macher H, Egea-Guerrero JJ, Revuelto-Rey J, et al. Role of early cell-free DNA levels decrease as a predictive marker of fatal outcome after severe traumatic brain injury. Clinica Chimica Acta. 414(C), 12–17 (2012). [DOI] [PubMed] [Google Scholar]
- 151.Rodrigues Filho EM, Ikuta N, Simon D, Regner AP. Prognostic value of circulating DNA levels in critically ill and trauma patients. Revista Brasileira de Terapia Intensiva. 26(3), 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Agoston DV, Shutes-David A, Peskind ER. Biofluid biomarkers of traumatic brain injury. Brain Injury. 31(9), 1195–1203 (2017). [DOI] [PubMed] [Google Scholar]
- 153.Moyron RB, Wall NR. Differential protein expression in exosomal samples taken from trauma patients. Proteomics Clin Appl. 11(9–10), 1700095 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Manek R, Moghieb A, Yang Z, et al. Protein Biomarkers and Neuroproteomics Characterization of Microvesicles/Exosomes from Human Cerebrospinal Fluid Following Traumatic Brain Injury. Mol Neurobiol. 24(2), 133–31 (2017). [Google Scholar]
- 155.Nekludov M, Bellander BM, Gryth D, Wallen H, Mobarrez F. Brain-Derived Microparticles in Patients with Severe Isolated TBI. Brain Injury. 31(13–14), 1856–1862 (2017). [DOI] [PubMed] [Google Scholar]
- 156.Stern RA, Tripodis Y, Baugh CM, et al. Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J Alzheimers Dis. 51(4), 1099–1109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McCrory P, Davis G, Makdissi M. Second impact syndrome or cerebral swelling after sporting head injury. Curr Sports Med Rep. 11(1), 21–23 (2012). [DOI] [PubMed] [Google Scholar]
- 158.Cantu RC. Second Impact Syndrome: Concussion and Second Injury Brain Complications. Yearbook of Sports Medicine. 2011, 26–28 (2011). [Google Scholar]
- 159.Stovitz SD, Weseman JD, Hooks MC, Schmidt RJ, Koffel JB, Patricios JS. What Definition Is Used to Describe Second Impact Syndrome in Sports? A Systematic and Critical Review. Curr Sports Med Rep. 16(1), 50–55 (2017). [DOI] [PubMed] [Google Scholar]
- 160.Thelin EP, Nelson DW, Bellander B-M. Secondary Peaks of S100B in Serum Relate to Subsequent Radiological Pathology in Traumatic Brain Injury. Neurocrit Care. 20(2), 217–229 (2013). [DOI] [PubMed] [Google Scholar]
- 161.Kellermann I, Kleindienst A, Hore N, Buchfelder M, Brandner S. Early CSF and Serum S100B Concentrations for Outcome Prediction in Traumatic Brain Injury and Subarachnoid Hemorrhage. Clin Neurol Neurosurg. 145, 79–83 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Di Battista AP, Buonora JE, Rhind SG, et al. Blood Biomarkers in Moderate-To-Severe Traumatic Brain Injury: Potential Utility of a Multi-Marker Approach in Characterizing Outcome. Front Neurol. 6(8), 45–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Egea-Guerrero JJ, Murillo-Cabezas F, Gordillo-Escobar E, et al. S100B protein may detect brain death development after severe traumatic brain injury. J. Neurotrauma. 30(20), 1762–1769 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rainey T, Lesko M, Sacho R, Lecky F, Childs C. Predicting outcome after severe traumatic brain injury using the serum S100B biomarker: results using a single (24h) time-point. Resuscitation. 80(3), 341–345 (2009). [DOI] [PubMed] [Google Scholar]
- 165.Lee JY, Lee CY, Kim HR, Lee C-H, Kim HW, Kim JH. A Role of Serum-Based Neuronal and Glial Markers as Potential Predictors for Distinguishing Severity and Related Outcomes in Traumatic Brain Injury. J Korean Neurosurg Soc. 58(2), 93–100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Thelin EP, Nelson DW, Bellander B-M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir. 159(2), 209–225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pelinka LE, Kroepfl A, Leixnering M, Buchinger W, Raabe A, Redl H. GFAP Versus S100B in Serum after Traumatic Brain Injury: Relationship to Brain Damage and Outcome. J. Neurotrauma. 21(11), 1553–1561 (2004). [DOI] [PubMed] [Google Scholar]
- 168.Lei J, Gao G, Feng J, et al. Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: a prospective cohort study. Critical care (London, England). 19(1), 362–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Czeiter E, Mondello S, Kovacs N, et al. Brain injury biomarkers may improve the predictive power of the IMPACT outcome calculator. J. Neurotrauma. 29(9), 1770–1778 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mondello S, Kobeissy F, Vestri A, Hayes RL, Kochanek PM, Berger RP. Serum Concentrations of Ubiquitin C-Terminal Hydrolase-L1 and Glial Fibrillary Acidic Protein after Pediatric Traumatic Brain Injury. Sci. Rep. 6, 28203 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fraser DD, Close TE, Rose KL, et al. Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum*. Pediatr Crit Care Med. 12(3), 319–324 (2011). [DOI] [PubMed] [Google Scholar]
- 172.Papa L, Lewis LM, Silvestri S, et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg. 72(5), 1335–1344 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Takala RSK, Posti JP, Runtti H, et al. Glial Fibrillary Acidic Protein and Ubiquitin C-Terminal Hydrolase-L1 as Outcome Predictors in Traumatic Brain Injury. World Neurosurgery. 87, 8–20 (2016). [DOI] [PubMed] [Google Scholar]
- 174.Shahim P, Gren M, Liman V, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci. Rep. 6, 36791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Žurek J, Fedora M. The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir. 154(1), 93–103– discussion 103 (2011). [DOI] [PubMed] [Google Scholar]
- 176.Liliang P-C, Liang C-L, Lu K, et al. Relationship between injury severity and serum tau protein levels in traumatic brain injured rats. Resuscitation. 81(9), 1205–1208 (2010). [DOI] [PubMed] [Google Scholar]
- 177.Zemlan FP, Jauch EC, Mulchahey JJ, et al. C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain research. 947(1), 131–139 (2002). [DOI] [PubMed] [Google Scholar]
- 178.Pandey S, Singh K, Sharma V, et al. A prospective pilot study on serum cleaved tau protein as a neurological marker in severe traumatic brain injury. Br J Neurosurg. 73, 1–8 (2017). [DOI] [PubMed] [Google Scholar]
- 179.Bogoslovsky T, Wilson D, Chen Y, et al. Increases of Plasma Levels of Glial Fibrillary Acidic Protein, Tau, and Amyloid β up to 90 Days after Traumatic Brain Injury. J. Neurotrauma. 34(1), 66–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Abbasi M, Sajjadi M, Fathi M, Maghsoudi M. Serum S100B Protein as an Outcome Prediction Tool in Emergency Department Patients with Traumatic Brain Injury. Turk J Emerg Med. 14(4), 147–152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bazarian JJ, Zemlan FP, Mookerjee S, Stigbrand T. Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 20(7), 759–765 (2006). [DOI] [PubMed] [Google Scholar]
- 182.Kochanek PM, Bramlett HM, Dixon CE, et al. Operation Brain Trauma Therapy: Approach to Modeling, Therapy Evaluation, Drug Selection, and Biomarker Assessments, for a Multi-Center Pre-Clinical Drug Screening Consortium for Acute Therapies in Severe Traumatic Brain Injury. J. Neurotrauma. neu.20154113 (2015). [DOI] [PubMed] [Google Scholar]
- 183.Kochanek PM, Bramlett H, Dietrich WD, et al. A novel multicenter preclinical drug screening and biomarker consortium for experimental traumatic brain injury: operation brain trauma therapy. J Trauma. 71(1 Suppl), S15–S24 (2011). [DOI] [PubMed] [Google Scholar]
- 184.Mondello S, Shear DA, Bramlett HM, et al. Insight into Preclinical Models of Traumatic Brain Injury Using Circulating Brain Damage Biomarkers: Operation Brain Trauma Therapy. J. Neurotrauma. neu.20154132 (2015). [DOI] [PubMed] [Google Scholar]
- 185.Mountney A, Bramlett HM, Dixon CE, et al. Simvastatin Treatment in Traumatic Brain Injury: Operation Brain Trauma Therapy. J. Neurotrauma [Internet]. neu.20154130 (2015). [DOI] [PubMed] [Google Scholar]
- 186.Shear DA, Bramlett HM, Dixon CE, et al. Nicotinamide Treatment in Traumatic Brain Injury: Operation Brain Trauma Therapy. J. Neurotrauma. neu.20154115 (2015). [DOI] [PubMed] [Google Scholar]
- 187.Wei HH, Lu XC, Shear DA, et al. NNZ-2566 treatment inhibits neuroinflammation and pro-inflammatory cytokine expression induced by experimental penetrating ballistic-like brain injury in rats. Journal of Neuroinflammation. 6, 19 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wright DW, Kellermann AL, Hertzberg VS, et al. ProTECT: A Randomized Clinical Trial of Progesterone for Acute Traumatic Brain Injury. Ann Emerg Med. 49(4), 391–402.e2 (2007). [DOI] [PubMed] [Google Scholar]
- 189.Wright DW, Yeatts SD, Silbergleit R, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 371(26), 2457–2466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kobeissy FH, Guingab-Cagmat JD, Razafsha M, et al. Leveraging biomarker platforms and systems biology for rehabilomics and biologics effectiveness research. PM & R. 3(6, Supplement), S139–S147 (2011). [DOI] [PubMed] [Google Scholar]
- 191.Zhang Z, Mondello S, Kobeissy F, et al. Protein biomarkers for traumatic and ischemic brain injury: from bench to bedside. Translational Stroke Research [Internet]. 3, 1–32 (2011). [DOI] [PubMed] [Google Scholar]
- 192.Varma S, Janesko KL, Wisniewski SR, et al. F2-isoprostane and neuron-specific enolase in cerebrospinal fluid after severe traumatic brain injury in infants and children. J. Neurotrauma. 20(8), 781–786 (2003). [DOI] [PubMed] [Google Scholar]
- 193.Berger RP, Kochanek PM, Pierce MC. Biochemical markers of brain injury: could they be used as diagnostic adjuncts in cases of inflicted traumatic brain injury? Child Abuse & Neglect. 28(7), 739–754 (2004). [DOI] [PubMed] [Google Scholar]
- 194.Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J. Neurotrauma. 21(2), 125–136 (2004). [DOI] [PubMed] [Google Scholar]


