Abstract
Background:
Sex differences in the incidence and manifestation of cardiovascular disease (CVD) suggest the involvement of sex hormones in disease pathogenesis. Coronary artery calcium (CAC) and its progression, measured by non-contrast cardiac computed tomography, are markers of subclinical atherosclerosis and predict CVD, even among low-risk women. We hypothesized that sex hormone levels were associated with CAC progression among women in the Multi-Ethnic Study of Atherosclerosis.
Methods:
We studied 2,759 post-menopausal women (age 65±9 years), free of baseline CVD, with baseline serum sex hormones and CAC measured at Exam 1 (2000–2002). Of this sample, 2,427 had ≥1 follow-up CAC measurement through Exam 5 (2010–2012). Using mixed effects linear regression methods, we tested change in log[CAC+1] score by log[sex hormone] levels (continuous, comparing the 90th versus 10th percentiles). Models adjusted for demographics, lifestyle factors, cardiovascular risk factors, hormone therapy, and years since menopause.
Results:
At baseline, we found no associations between sex hormones and prevalent CAC. Over a median of 4.7 years, in fully-adjusted models, women with higher free testosterone levels had a greater relative CAC progression ratio (95% CI) [1.26 (1.011.56)], whereas higher sex hormone binding globulin (SHBG) was associated with lower progression risk [0.80 (0.64–0.99). No associations were seen for total testosterone, estradiol, or dehydroepiandrosterone.
Conclusion:
A more androgenic hormone profile of higher free testosterone and lower SHBG is associated with a greater CAC progression up to 10-years in post-menopausal women. Sex hormone levels may help identify women at increased risk for CVD who may benefit from additional risk-reducing strategies.
Keywords: Testosterone, sex hormones, coronary artery calcium, menopause, women, risk factors, cardiac computed tomography
Introduction:
Although cardiovascular disease (CVD) mortality rates are declining, CVD remains the leading cause of death among older women.1 Sex differences exist in the risk factors, presentation, and outcomes of CVD2, suggesting the influence of sex hormones. The coronary artery calcium (CAC) score measured by non-contrast cardiac computed tomography (CT) is a marker of subclinical atherosclerosis and is prognostic of risk for future coronary heart disease (CHD) events, independently of traditional risk factors.3–6 CAC is prognostic of CVD risk, even among women at low predicted risk.7, 8
At menopause there is a drastic change in the endogenous hormonal milieu, with a decrease in estrogen resulting in a relative androgen excess, which can influence a woman’s cardiovascular risk. Post-menopausal women with higher androgen levels have an increased burden of CVD risk factors,9 and have a more adverse cardiovascular phenotype, including increased concentric left ventricular remodeling,10 greater aortic stiffness11 and increased NT-proBNP levels12. In a prior analysis from the Multi-Ethnic Study of Atherosclerosis (MESA), post-menopausal women with a higher Testosterone (T): Estradiol (E2) ratio also had a greater risk for incident CVD, CHD, and heart failure events.13
Progression of subclinical atherosclerosis may be one mechanism linking higher androgen levels with CVD risk in women after menopause. Prior studies have shown some relationships between sex hormone levels and the presence of subclinical atherosclerosis, but findings were mixed.14–16 However, these prior studies were limited by cross-sectional design or a single assessment of CAC. Further work is still needed to delineate biological mechanisms that underlie atherosclerosis progression in women,17 and prospective long-term analyses are needed to further establish relationships between sex hormone levels and CAC progression. Identification of women at increased CVD risk is necessary for the appropriate targeting of risk-reducing strategies.
Thus, our study aimed to examine the associations of endogenous sex hormone levels with long-term (10-year) progression of subclinical atherosclerosis as measured by CAC, among post-menopausal women in a multi-ethnic community-based cohort. We hypothesized that a more androgenic hormone profile [i.e. higher free T and lower sex hormone binding globulin (SHBG)] would be associated with greater CAC progression.
Methods:
Study population
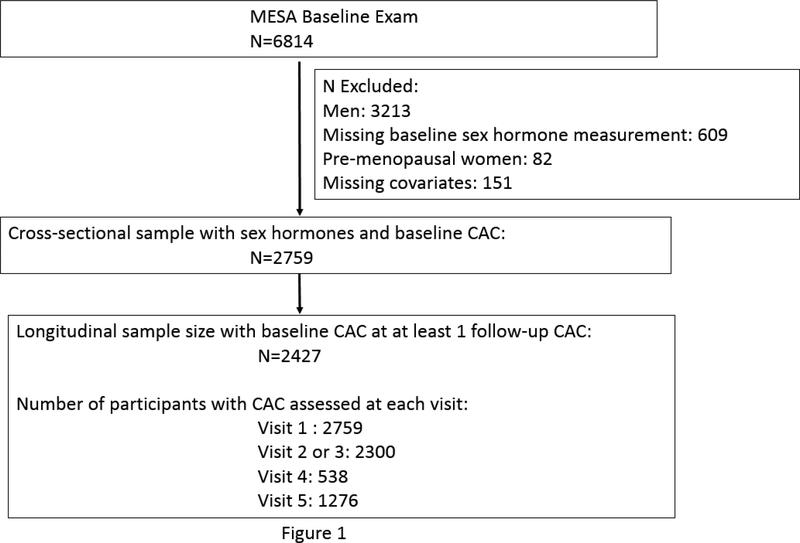
MESA is an ongoing prospective cohort study conducted at six centers across the U.S with 6,814 women and men enrolled, aged between 45–84 years and free of clinical CVD at baseline.18 The baseline exam (Exam 1) was conducted between 2000–2002 with subsequent follow-up exams held during, 2002–2004 (Exam 2), 2004–2005 (Exam 3), 2005–2007 (Exam 4) and 2010–2012 (Exam 5).
Our study population (Figure 1) consisted of all post-menopausal women enrolled in MESA who had endogenous sex hormone levels and CAC measured at the baseline exam (N=2,759). Menopausal status was determined through an algorithm developed using answers to a series of self-reported questions including, age, age at menopause, history of surgical menopause and age at surgical menopause as compared to age. Details and an explanatory algorithm have been previously reported,12 and presented again in Supplemental Figure 1.
Figure 1:
Participant inclusion/exclusion in study
Of this sample, 2,427 women had at least one additional measurement of CAC at the follow-up MESA exams. We restricted our sample to post-menopausal women as both sex hormone levels and cardiovascular risk differ between pre- and postmenopausal women, and there were relatively few pre-menopausal women in MESA, which limited analyses in this subset. Furthermore, we were interested in studying the progression of CAC in the post-menopausal state to examine women’s CVD risk after menopause to expand upon our prior work.10–13
MESA study protocols were approved by the IRB’s of all collaborating institutions and the MESA Coordinating Center. Participation was voluntary and informed written consent was obtained at each study examination.
Sex hormones
Endogenous sex hormone levels were measured at baseline from fasting serum samples at the University of Massachusetts Medical Center in Worcester, MA. The assays and kits used to measure the hormone levels were an ultrasensitive radioimmunoassay kit for E2 (Diagnostic System Laboratories, Webster, TX), radioimmunoassay kits for total T and dehydroepiandrosterone (DHEA) and a chemiluminescence enzyme immunometric assay using Immulite kits for SHBG (Diagnostic Products Corporation, Los Angeles, CA).10–13 Free T was estimated (as a percent of total T) using the Sodergard equation.19 The intra-assay coefficients of variation for total T, SHBG, DHEA, and E2 were 12.3%, 9.0%, 11.2%, and 10.5%, respectively.
Coronary artery calcium score:
CAC was measured using either electron-beam CT scanner or a multi-detector CT system. Each participant was scanned twice at baseline and results were averaged, as has been previously described.20 CAC was quantified using the Agatson scoring method.21 Scores were adjusted using a standard radiologic phantom used at the time of the scanning.22 All scans were read at the Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles Medical Center by trained readers blinded to participant characteristics. Intraobserver and interobserver agreement was high (κ= 0.93 and κ=0.90, respectively) for CT image analysis.20
CAC was measured in all post-menopausal women in our study sample at Exam 1 (N=2,759). Subsequently, participants were selected to get a repeat CT scan at either Exams 2 or 3, with the time point of the repeat scan randomly assigned (N=2,300). An additional random subset were selected for a third scan at Exam 4 (n=538). Finally, a subset who were participants of the MESA AIR ancillary study underwent repeat scanning at Exam 5, approximately 10-years after baseline scan (n=1,276). Thus, participants could have had up to four cardiac CT scans (since Exam 2 or 3 scan was mutually exclusive) over a 10-year period; 2,437 women had at least 1 follow-up CAC measurement and were included in these longitudinal analyses (Figure 1).
Other covariates:
Covariates were obtained from standardized questionnaires, physical exam, and laboratory measures at each study visit as previously reported.18 For this analysis, we used covariates ascertained at the baseline exam. Age, race/ethnicity, education level, smoking status, physical activity (intentional moderate + vigorous exercise in METS*min/week), and age at menopause were self-reported. A medication inventory determined current medication use including use of hormone therapy (HT). Diabetes was assessed by self-reported physician diagnosis, a fasting glucose level of ≥126 mg/dL, or hypoglycemic medication use. Height and weight, measured using standardized procedures, were used to calculate body mass index (BMI). Resting blood pressure was measured using a Dinamap automated sphygmomanometer in the seated position with the average of the 2nd and 3rd readings used.
Statistical methods:
Differences in baseline characteristics by presence or absence of baseline CAC were assessed by t-tests or χ2 tests. Sex hormones were positively skewed and thus log transformed for analysis. Our statistical models evaluated each sex hormone as a continuous measure and compared the 90th versus 10th percentiles for contrast. CAC scores were also skewed and had a high prevalence of zero scores, so we used the natural log of (CAC+1) as a continuous measure as our primary outcome measure to evaluate for progression.
Participants could contribute data to up to 4 time-points (Exams 1 to 5). We assessed the longitudinal change in CAC scores associated with each of the sex hormone levels separately by using multivariable-adjusted linear mixed effects models allowing for random variations in baseline CAC scores and longitudinal slope for CAC score progression across participants. The mixed effects model for longitudinal data leverages all available CAC information from all participants, including those without follow-up measurements, to jointly model the amount of CAC at baseline and CAC progression over time and has been described previously.23 As log-units of sex hormones and CAC are difficult to interpret, the beta-coefficients from our linear models were exponentiated and results were presented as CAC progression ratios and their 95% confidence intervals (CI). Progression ratios >1 suggest the hormone is positively associated with CAC progression; ratios <1 suggest reduced risk of progression.
In secondary analyses, we confirmed the association of each sex hormone with presence of prevalent CAC (Agatston score >0) at the baseline exam using modified Poisson regression and presented as prevalence ratio (PR) and 95% CIs. The cross-sectional relationship of sex hormones and CAC had already been previously reported in MESA.14 We now newly examined the association of sex hormones with incident CAC (among participants without baseline CAC) using survival analysis with interval censoring and presented as hazard ratios (HR) and 95% CI.
In all analyses, we used progressively adjusted models. In the minimally adjusted model (Model 1), we adjusted for the variables of age, race/ethnicity and study site. In our primary analytical model (Model 2), we additionally adjusted for education, smoking, physical activity, BMI, years since menopause, and current HT use. In the final model (Model 3), we additionally adjusted for CVD risk factors that may be in the causal pathway between sex hormone levels and CAC progression. This latter model included systolic blood pressure, use of anti-hypertensive medication, total cholesterol, HDL cholesterol, use of lipid lowering medication, prevalent diabetes mellitus (Yes/No), high-sensitivity C-reactive protein (CRP) levels, and estimated glomerular filtration rate (eGFR) by the CKD-Epi equation24.
We performed several sensitivity analyses for exploratory purposes. First, we repeated analyses after excluding women on current HT (n=1,879). Additionally, we repeated analyses stratified by years since menopause (≤10 vs >10 years) and by age categories (≤65 vs >65 years). Stata version 14.2 was used for all analyses.
Data availability statement
The MESA cohort participates in the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository (BioLINCC). The MESA data are available upon request, including data from exams 1 to 5, used in this analysis. Requests for data can be made through the following website: https://biolincc.nhlbi.nih.gov/studies/mesa/
Results:
Baseline characteristics
The mean (SD) age of study population was 65 (9), and 32% were current HT users. Baseline characteristics are shown in Table 1, stratified by the presence (46%) or absence of baseline CAC (54%). Women with baseline CAC were older, more likely to be White, had lower levels of physical activity, were more likely to be smokers, had a greater prevalence of hypertension, dyslipidemia and diabetes mellitus, and had lower eGFR, DHEA and E2 levels than woman without CAC.
Table 1:
| Characteristic | Baseline CAC absent (N=1503) | Baseline CAC present (N=1256) | p-value |
|---|---|---|---|
| Demographic factors | |||
| Age (years) | 61.8 (8.2) | 68.8 (8.4) | <0.001 |
| Race/Ethnicity | |||
| White, Caucasian | 34.5 | 42.8 | <0.001 |
| Chinese American | 11.8 | 12.9 | |
| Black, African-American | 29.3 | 24.5 | |
| Hispanic | 24.4 | 19.8 | |
| Education | |||
| < high school | 21.0 | 23.2 | <0.001 |
| high school | 19.8 | 24.6 | |
| < college | 28.3 | 28.0 | |
| ≥ college | 31.0 | 24.3 | |
| Lifestyle Factors | |||
| Physical activity (METS*min/wk) | 3840 (1890, 6990) | 3124 (1496, 5843) | <0.001 |
| Smoking status | |||
| Never | 62.7 | 54.3 | <0.001 |
| Former | 27.0 | 34.3 | |
| Current | 10.4 | 11.4 | |
| Cardiovascular Risk factors | |||
| Body Mass Index (kg/m2) | 28.6 (6.0) | 28.6 (6.1) | 1 |
| Systolic Blood Pressure (mm Hg) | 125.6 (22.7) | 134.0 (23.9) | <0.001 |
| Diastolic Blood pressure (mm Hg) | 69.1 (10.4) | 69.1 (10.1) | 0.92 |
| Anti-hypertensive medication | 34.1 | 50.9 | <0.001 |
| Hypertension | 42.0 | 61.4 | <0.001 |
| Total cholesterol (mg/dl) | 200.6 (35.4) | 203.0 (36.2) | 0.08 |
| HDL cholesterol (mg/dl) | 57.6 (15.4) | 55.5 (15.1) | <0.001 |
| Cholesterol lowering meds | 13.5 | 25.5 | <0.001 |
| Fasting glucose (mg/dl) | 95 (89, 104) | 97 (90, 108) | <0.001 |
| Diabetes mellitus | |||
| Normal | 79.0 | 70.8 | <0.001 |
| Impaired fasting glucose | 11.0 | 13.9 | |
| Diabetes mellitus | 10.0 | 15.3 | |
| Glomerular Filtration Rate | 78.6 (15.0) | 71.4 (16.0) | <0.001 |
| Current HT | 35.1 | 28.0 | <0.001 |
| Sex Hormones | |||
| Total T (nmol/L) | 0.9 (0.6, 1.3) | 0.9 (0.6, 1.4) | 0.65 |
| Free T (%) | 1.3 (0.9, 1.7) | 1.3 (0.9, 1.7) | 0.07 |
| Estradiol (nmol/L) | 0.08 (0.05, 0.18) | 0.07 (0.04, 0.13) | <0.001 |
| DHEA (nmol/L) | 10.9 (7.6, 15.1) | 9.5 (6.3, 14.0) | <0.001 |
| SHBG (nmol/L) | 58.4 (39.8, 95) | 60.4 (42.4, 92.2) | 0.92 |
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; HDL, high density lipoprotein; T, testosterone; DHEA, dehydroepiandrosterone; SHBG, sex hormone binding globulin; CAC, coronary artery calcium
Data are mean (S.D), for normally distributed variables, median (25th, 75th percentiles) for skewed variables, or (%) of subjects for categorical variables. P values were obtained using t-test or chi-square test.
Prevalent and incident CAC
In cross-sectional analysis, there were no significant associations of sex hormones with prevalent CAC at baseline (Supplemental Table 1). This corroborated findings from an earlier study in the same population.14
Participants were followed for up to 10-years, with a median follow-up of 4.7 years. Among participants without CAC at baseline (N=1,366) (Table 2), women with higher levels of free T had a greater risk of developing incident CAC>0 [HR 1.41 (95% CI 1.03, 1.92)] and women with higher levels of SHBG had lower risk [0.71 (0.52, 0.96)], after adjusting for demographics, lifestyle factors, menopausal duration and use of HT (Model 2). However, this association was not statistically significant after adjusting for cardiovascular risk factors (Model 3). There were no statistically significant associations between total T, E2, or DHEA and incident CAC in any model.
Table 2:
Association of baseline sex hormone levels with incident CAC among women with CAC=0 at baseline: The Multi-Ethnic Study of Atherosclerosis (2000 through 2012) *,†
| Sex Hormones | Incident CAC score > 0 |
||
|---|---|---|---|
| Model 1‡ aHR (95% CI) | Model 2ǁ aHR (95% CI) | Model 3§ aHR (95% CI) | |
| Total T | 1.03 (0.82, 1.28) | 0.94 (0.75, 1.18) | 0.94 (0.75, 1.18) |
| (N) | 1366 | 1366 | 1360 |
| E2 | 0.91 (0.71, 1.17) | 0.79 (0.56, 1.10) | 0.74 (0.52, 1.05) |
| (N) | 1366 | 1366 | 1360 |
| DHEA | 1.02 (0.81, 1.30) | 0.99 (0.78, 1.26) | 0.91 (0.71, 1.17) |
| (N) | 1366 | 1366 | 1360 |
| Free T | 1.50 (1.16, 1.96) | 1.41 (1.03, 1.92) | 1.01 (0.73, 1.42) |
| (N) | 1365 | 1365 | 1359 |
| SHBG | 0.66 (0.51, 0.86) | 0.71 (0.52, 0.96) | 0.98 (0.71, 1.37) |
| (N) | 1365 | 1365 | 1359 |
Results reflect the associations [in adjusted Hazard ratios (95% CI)] of baseline (log transformed) sex hormone levels with incident CAC score >0 over a 10-year period from baseline (Exam 1, 2000–2002) to follow-up (Exam 5, 2010–2012) among postmenopausal women with absent baseline CAC. Each sex hormone level were assessed in the model as a continuous measure, and the 90th vs the 10th percentiles were compared for contrast. Results were obtained from survival analyses with interval censoring. Hazard Ratios >1 suggest hormone is positively associated with CAC incidence; Hazard Ratios <1 suggest reduced risk of incident CAC. Bolded results are statistically significant (p <0.05)
Abbreviations: T, testosterone; E2, estradiol; DHEA, dehydroepiandrosterone; SHBG, sex hormone binding globulin; CAC, coronary artery calcium
Model 1 adjusts for age, race/ ethnicity, site
Model 2 adjusts for variables in Model 1 along with education, physical activity, smoking status, BMI, years since menopause, current hormone therapy
Model 3 adjusts for variables in Model 2 plus systolic blood pressure, use of antihypertensive medication, total cholesterol, HDL cholesterol, use of lipid lowering medication, diabetes mellitus status, CRP levels, estimated glomerular filtration rate
10-year CAC Progression
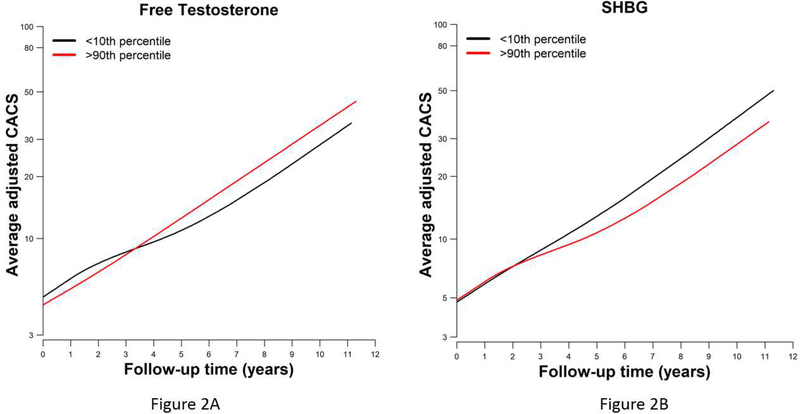
Among all participants (including those with or without CAC at baseline), the associations of sex hormones (comparing the 90th vs 10th percentile of their continuous distribution) with the progression of log(CAC+1) were estimated using linear mixed effect models that accounted for baseline CAC. Results are presented as CAC progression ratios, which are the exponentiated coefficients from the linear mixed effects models (Table 3). Women with higher levels of free T and lower levels of SHBG had a greater progression of CAC. The CAC progression ratio (95% CI) associated with higher free T and higher SHBG were 1.28 (1.03, 1.59) and 0.78 (0.63, 0.97), respectively, after adjusting for demographic and lifestyle factors including menopausal duration and use of HT (Model 2). CAC progression was generally linear over follow-up as shown in Figure 2A (for free T) and Figure 2B (for SHBG). These associations remained statistically significant after additional adjustment for cardiovascular risk factors (Model 3). There were no associations between total T, E2, or DHEA levels and CAC progression.
Table 3:
The associations of baseline sex hormones (log transformed) and change of coronary artery calcium scores from MESA Exam 1 (2000–2002) to Exam 5 (2010–2012) : The Multi-Ethnic Study of Atherosclerosis *,†
| Sex Hormones | 10-year progression of CAC (N=2427) |
||
|---|---|---|---|
| Model 1‡ | Model 2ǁ | Model 3§ | |
| Total T | 1.03 (0.84, 1.26) | 1.03 (0.84, 1.26) | 1.03 (0.85, 1.26) |
| E2 | 0.96 (0.78, 1.19) | 0.96 (0.78, 1.19) | 0.96 (0.78, 1.19) |
| DHEA | 0.92 (0.76, 1.13) | 0.93 (0.76, 1.13) | 0.92 (0.75, 1.12) |
| Free T | 1.28 (1.03, 1.59) | 1.28 (1.03, 1.59) | 1.26 (1.01, 1.56) |
| SHBG | 0.78 (0.63, 0.97) | 0.78 (0.63, 0.97) | 0.80 (0.64, 0.99) |
Results reflect the change of log [CAC+1] score during up to 10 years of follow-up by log [sex hormone] levels. Using mixed effect models that accounted for baseline CAC, each sex hormone level was assessed as a continuous measure, and the 90th vs the 10th percentiles were compared for contrast. Results are presented as CAC progression ratios (95% confidence interval). Progression Ratios >1 suggest the hormone is positively associated with CAC progression; Progression Ratios <1 suggest reduced risk of progression. Bolded results are statistically significant (p <0.05).
Abbreviations: T, testosterone; E2, estradiol; DHEA, dehydroepiandrosterone; SHBG, sex hormone binding globulin; CAC, coronary artery calcium
Model 1 adjusts for age, race/ ethnicity, site
Model 2 adjusts for variables in Model 1 along with education, physical activity, smoking status, BMI, years since menopause, current hormone therapy
Model 3 adjusts for variables in Model 2 plus systolic blood pressure, use of anti-hypertensive medication, total cholesterol, HDL cholesterol, use of lipid lowering medication, diabetes mellitus status, CRP levels, estimated glomerular filtration rate
Figure 2:
Association of log transformed sex hormones (comparing the 90th percentile to the 10th percentile) with progression of CAC scores over a 10-year follow-up for Free T (Figure 2A) and SHBG (Figure 2B). Models are adjusted for age, race/ ethnicity, site, education, physical activity, smoking status, BMI, years since menopause, current hormone therapy
In a sensitivity analysis excluding women on current HT (Supplemental Table 2), we saw trends consistent with those in our main model, with higher levels of free T and SHBG associated with greater [1.71 (1.19, 2.47)] and lesser [0.59 (0.42, 0.83)] progression of CAC with a larger effect size.
We also conducted a stratified analysis by years since menopause (≤10 vs >10 years) for incident CAC (Supplemental Table 3). In models adjusted for demographic factors (Model 1), free T was associated with a greater risk, and SHBG with a lower risk, of incident CAC, similar to our primary model. After adjusting for lifestyle and cardiovascular risk factors though, these associations were not statistically significant but trended in the same direction. The analyses of sex hormones and progression of CAC score (Supplemental Table 4), stratified by ≤10 vs >10 years post-menopause, did not show any statistically significant associations, though the associations trended in the same directions as seen in the whole cohort of women combined. While there was some statistically significant interactions by categories of menopausal years (noted in Supplemental Table 4), the results were qualitatively in the same direction of association for both categories.
Finally, we also performed an age-stratified analysis (≤65 years and >65 years). In women ≤65 years, the associations of free T and SHBG with incident CAC (Supplemental Table 5) and progression of CAC (Supplemental Table 6) were similar to our primary analysis; however these results were not statistically significant in women aged >65 years. There were some statistically significant interaction by these age categories for CAC progression (Supplemental Table 6), but again these results should be considered exploratory.
Discussion:
In a well-characterized cohort of post-menopausal women, free of CVD at baseline, we found that a more androgenic hormone profile (i.e., higher levels of free T and lower levels of SHBG) was associated with greater CAC progression up to 10years, even after adjustment for traditional CVD risk factors. These associations remained significant after excluding women on current HT with a greater magnitude of association, and these associations also appeared stronger for women ≤65 years of age.
CAC is a marker of total coronary atherosclerotic burden and considered in guidelines as a sufficient risk marker that could guide risk assessment for consideration of pharmacological therapy such as statins and aspirin.5, 25, 26 Women, compared to similarly aged-men, have lower CAC scores on average, but the presence of CAC is prognostic of future CVD risk even among women at low predicted risk.7, 8 Given sex differences in both the prevalence of CAC and CVD risk, the role of sex hormones in atherosclerosis progression warrants further study.
Sex hormones may influence CAC progression directly through traditional risk factors. Elevated androgens in older women are associated with elevated blood pressure, insulin resistance, and metabolic syndrome.9, 27, 28 On the other hand, estrogen replacement is associated with favorable lipid changes including decreases in LDL-cholesterol and increases in HDL-cholesterol levels.29 We found an independent association of an androgenic sex hormone profile with 10-year CAC progression that remained significant even after adjusting for CVD risk factors that may be in the causal pathway including lipids, blood pressure, and diabetes.
Beyond its relationship to traditional risk factors, estrogen is also thought to exhibit a direct inhibitory effect on the coronary calcification process through its effects on vascular smooth muscle cells, bone macrophages and matrix proteins, all components of the vessel wall.30 In this study, we did not see any associations between E2 and CAC progression, although E2 levels are very low in women not on HT after the menopausal transition. Our findings support work from previous cross-sectional studies in MESA where E2 levels were not associated with baseline CAC or abdominal aortic calcium in post-menopausal women.14, 15
However, studies in other populations have shown conflicting results of the relationship of estrogen with CAC and with coronary atherosclerosis progression. One retrospective analysis found that women with higher endogenous E2 levels have lower CAC scores,31 and in another observational study, women who used HT had less CAC progression.32 These findings were consistent with an ancillary study of the Women’s Health Initiative that found that women receiving estrogen-only HT had lower CAC scores when assessed on a single cardiac CT scan obtained ~7 years after randomization, as compared to those receiving placebo.33 However this benefit was not found with combined estrogen/progestin therapy or confirmed in other studies.34 For example, the Early versus Late Intervention Trial with Estradiol (ELITE)35 and the Estrogen Replacement and Atherosclerosis (ERA) trial36 showed no effect of HT (estrogen/progesterone and placebo) on coronary atherosclerosis progression as assessed by cardiac CT angiography and by quantitative coronary angiography, respectively.35, 36 Within our study, in a sensitivity analysis excluding women on current HT, we found a greater magnitude of association between an androgenic hormone profile and CAC progression, perhaps due to the loss of a potentially protective effect of estrogen in HT. However, it is important to note that randomized clinical trials have failed to show a beneficial effect of HT on CHD risk in post-menopausal women in a primary prevention setting.37, 38
The role of T in vascular calcification is less well elucidated. A previous study in mice found that exogenous T administration resulted in increased vascular calcification in both sexes by potentially modulating androgen (upregulation) and estrogen (downregulation) receptor expression in the vessel walls.39 SHBG is a glycoprotein that binds to both E2 and T but with greater affinity to the latter; it determines the circulating levels of the free hormones (i.e. greater SHBG means lower levels of free T for a given total T concentration).40 SHBG has effects on cardiovascular risk factors indirectly through its binding of sex hormones and directly through its receptors via other pathways.41
Few prior studies have evaluated the association of androgens and SHBG with CAC. Previous cross-sectional study in MESA and prospective analyses in the Rotterdam and CARDIA studies found no associations between free T or total T and SHBG with CAC in post-menopausal and/or young to middle-aged women.14, 42, 43 SHBG was found to be associated with presence and extent of abdominal aortic calcium in women in MESA; however this association was not independent of cardiovascular risk factors.15 A prior study in peri-menopausal women found that higher levels of free T and SHBG were associated with calcified coronary plaque, but this association was no longer seen after adjustment for traditional CVD risk factors.44
In this present study, we found that higher levels of free T and lower levels of SHBG were associated with greater CAC progression over 10-years, an association that persisted after adjusting for cardiovascular risk factors. The association was greater in magnitude after excluding women on HT. Our study adds to a growing body of literature that suggests excess androgens after menopause are associated with increased risk of traditional cardiometabolic risk factors,27 and with a variety of subclinical CVD measurements including greater left ventricular concentric remodeling10 and aortic stiffness11. Moreover, a more androgenic sex hormone profile is independently associated increased risk of incident CVD, CHD, and heart failure clinical events in post-menopausal women.13 Our findings suggest that CAC progression may be an intermediate step linking excess androgens with CVD risk in post-menopausal women.
Our study should be considered in the context of several limitations. We restricted our study to post-menopausal women, so we were unable to examine the association of sex hormones with CAC progression around the menopausal transition specifically. We were also unable to incorporate factors related to participants’ reproductive history due to missingness/unavailability of data. Sex hormones were measured only at a single time-point (baseline) so we were unable to evaluate the association between change in sex hormones levels and CAC progression. In our stratified analysis, we had sub-groups with greatly reduced sample sizes and while some results were not statistically significant, they were generally in the same direction as our main results. Cross-sectional analysis is subject to selection bias and longitudinal analysis to survival bias. We also performed multiple testing, resulting in the possibility that some of our results were obtained by chance. However, our results were consistent with our a priori hypothesis and prior literature.10–13 Exploring these associations prospectively in a younger cohort with multiple measurements of sex hormones and CAC could provide additional insight into the role of sex hormones in subclinical atherosclerosis pathogenesis.
Our study had many strengths. We had a well-characterized cohort of postmenopausal women free of CVD at baseline. We adjusted for relevant demographics, lifestyle and cardiovascular risk factors. We had multiple CT measurements of CAC over a period of 10-years, resulting in up to four follow-up CAC measurements for some participants. We used the mixed effects models to analyze CAC progression which allowed us to use all available data of participants including those with missing follow-up to jointly model the CAC at baseline and progression over time.
Conclusion:
To summarize, our study concentrated on exploring a better understanding of the association of sex hormones in the atherosclerotic process in post-menopausal women, with a focus on CAC progression. We found that an androgenic hormone milieu in postmenopausal women predisposes to greater CAC progression over 10-years
Supplementary Material
Acknowledgements:
The authors thank the MESA participants, staff, and other investigators for their valuable contributions to the MESA study. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
Dr. Subramanya was funded by grant 16SFRN27870000 from the American Heart Association (AHA) Go Red for Women Strategically Focused Research Network (SFRN). Drs. Zhao, Guallar, Michos, Ouyang, Vaidya, Post and Ying also receive some funding from the AHA SFRN through grants 16SFRN27870000 and 16SFRN28680004 from AHA Go Red for Women SFRN. Dr. Michos and Dr. Zhao received additional funding through the Blumenthal Scholars Award in Preventive Cardiology at Johns Hopkins School of Medicine. This research was funded by R01 HL074406 and R01 HL074338 from the National Heart, Lung, and Blood Institute (NHLBI). The MESA study was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS.
Footnotes
Disclosures
Dr. Budoff has received grant support from General Electric (GE) healthcare. No other authors report any conflicts of interest related to this work.
Clinical trial registration:
MESA is not a clinical trial. However the cohort is registered at: https://clinicaltrials.gov/ct2/show/NCT00005487
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Sidney S, Quesenberry CP, Jr., Jaffe MG, et al. Recent Trends in Cardiovascular Mortality in the United States and Public Health Goals. JAMA Cardiol. 2016;1:594–599. [DOI] [PubMed] [Google Scholar]
- 2.Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J. 2010;18:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;61:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). Journal of the American College of Cardiology. 2015;66:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michos ED, Blaha MJ, Blumenthal RS. Use of the Coronary Artery Calcium Score in Discussion of Initiation of Statin Therapy in Primary Prevention. Mayo Clinic proceedings. 2017;92:1831–1841. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multiethnic study of atherosclerosis (MESA). European heart journal. 2018;39:24012408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA). Arch Intern Med. 2007;167:2437–2442. [DOI] [PubMed] [Google Scholar]
- 8.Kelkar AA, Schultz WM, Khosa F, et al. Long-Term Prognosis After Coronary Artery Calcium Scoring Among Low-Intermediate Risk Women and Men. Circ Cardiovasc Imaging. 2016;9:e003742. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 2005;111:1242–1249. [DOI] [PubMed] [Google Scholar]
- 10.Subramanya V, Zhao D, Ouyang P, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas. 2018;108:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanya V, Ambale-Venkatesh B, Ohyama Y, et al. Relation of Sex Hormone Levels with Prevalent and 10-year Change in Aortic Distensibility Assessed by MRI: The Multi-Ethnic Study of Atherosclerosis. American journal of hypertension. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying W, Zhao D, Ouyang P, et al. Sex Hormones and Change in N-Terminal ProB-Type Natriuretic Peptide Levels: The Multi-Ethnic Study of Atherosclerosis. The Journal of clinical endocrinology and metabolism. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao D, Guallar E, Ouyang P, et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. Journal of the American College of Cardiology. 2018;71:2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouyang P, Vaidya D, Dobs A, et al. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;204:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michos ED, Vaidya D, Gapstur SM, et al. Sex hormones, sex hormone binding globulin, and abdominal aortic calcification in women and men in the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2008;200:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, 2nd, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:25622568. [DOI] [PubMed] [Google Scholar]
- 17.Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126:604–611. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 19.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. Journal of steroid biochemistry. 1982;16:801–810. [DOI] [PubMed] [Google Scholar]
- 20.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 22.Nelson JC, Kronmal RA, Carr JJ, et al. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005;235:403–414. [DOI] [PubMed] [Google Scholar]
- 23.Gassett AJ, Sheppard L, McClelland RL, et al. Risk Factors for Long-Term Coronary Artery Calcium Progression in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e001726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 25.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 26.Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017. [DOI] [PubMed] [Google Scholar]
- 27.Patel SM, Ratcliffe SJ, Reilly MP, et al. Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2009;94:4776–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–1295. [DOI] [PubMed] [Google Scholar]
- 29.Guetta V, Cannon RO, 3rd. Cardiovascular effects of estrogen and lipid-lowering therapies in postmenopausal women. Circulation. 1996;93:1928–1937. [DOI] [PubMed] [Google Scholar]
- 30.Christian RC, Harrington S, Edwards WD, Oberg AL, Fitzpatrick LA. Estrogen status correlates with the calcium content of coronary atherosclerotic plaques in women. J Clin Endocrinol Metab. 2002;87:1062–1067. [DOI] [PubMed] [Google Scholar]
- 31.Jeon GH, Kim SH, Yun SC, Chae HD, Kim CH, Kang BM. Association between serum estradiol level and coronary artery calcification in postmenopausal women. Menopause. 2010;17:902–907. [DOI] [PubMed] [Google Scholar]
- 32.Budoff MJ, Chen GP, Hunter CJ, et al. Effects of hormone replacement on progression of coronary calcium as measured by electron beam tomography. J Womens Health (Larchmt). 2005;14:410–417. [DOI] [PubMed] [Google Scholar]
- 33.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronaryartery calcification. N Engl J Med. 2007;356:2591–2602. [DOI] [PubMed] [Google Scholar]
- 34.Becker A, Leber A, von Ziegler F, Becker C, Knez A. Comparison of progression of coronary calcium in postmenopausal women on versus not on estrogen/progestin therapy. Am J Cardiol. 2007;99:374–378. [DOI] [PubMed] [Google Scholar]
- 35.Hodis HN, Mack WJ, Henderson VW, et al. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med. 2016;374:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrington DM, Reboussin DM, Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522–529. [DOI] [PubMed] [Google Scholar]
- 37.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- 38.Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal Hormone Therapy and Long-term All-Cause and Cause-Specific Mortality: The Women’s Health Initiative Randomized Trials. JAMA. 2017;318:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McRobb L, Handelsman D, Heather A. Androgen-induced progression of arterial calcification in apolipoprotein E-null mice is uncoupled from plaque growth and lipid levels. Endocrinology. 2009;150:841–848. [DOI] [PubMed] [Google Scholar]
- 40.Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroidbinding globulin in human plasma. The Journal of Clinical Endocrinology & Metabolism. 1981;53:58–68. [DOI] [PubMed] [Google Scholar]
- 41.Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Molecular and cellular endocrinology. 2010;316:79–85. [DOI] [PubMed] [Google Scholar]
- 42.Meun C, Franco OH, Dhana K, et al. High androgens in postmenopausal women and the risk for atherosclerosis and cardiovascular disease: the Rotterdam Study. The Journal of Clinical Endocrinology & Metabolism. 2018. [DOI] [PubMed] [Google Scholar]
- 43.Calderon-Margalit R, Schwartz S, Wellons M, et al. Prospective association of serum androgens and sex hormone-binding globulin with subclinical cardiovascular disease in young adult women: the “Coronary Artery Risk Development in Young Adults” women’s study. The Journal of Clinical Endocrinology & Metabolism. 2010;95:4424–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munir JA, Wu H, Bauer K, et al. The perimenopausal atherosclerosis transition: relationships between calcified and noncalcified coronary, aortic, and carotid atherosclerosis and risk factors and hormone levels. Menopause. 2012;19:10–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MESA cohort participates in the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository (BioLINCC). The MESA data are available upon request, including data from exams 1 to 5, used in this analysis. Requests for data can be made through the following website: https://biolincc.nhlbi.nih.gov/studies/mesa/