Abstract
A better understanding of the oviposition behaviour of malaria vectors might facilitate development of new vector control tools. However, factors that guide aquatic habitat selection of gravid females is poorly understood.
This study explored the relative attractiveness of similar artificial ponds (0.8 m2) aged at varying length prior to opening in such a way that wild Anopheles arabiensis could choose between ponds that were freshly set up, 4 or 17 days old to lay eggs. Physicochemical parameters, bacterial profile and volatile organic compounds emitted from ponds were investigated over 3 experimental rounds.
Fresh ponds contained on average twice as many An. arabiensis instar larvae (mean: 50, 95% confidence interval (CI): 29–85) as the ponds that had aged 4 days (mean: 24, 95% CI: 14–42) and 17 days (mean: 20, 95% CI: 12–34). Fresh ponds were associated with significantly higher turbidity combined with higher water temperature, higher nitrite levels and lower pH and chlorophyll level than the older ponds. Round by round analyses suggested that bacteria communities differed between age groups and 4-heptanone, 2-ethylhexanal and an isomer of octenal, were exclusively detected from the fresh ponds.
These characteristics may be useful for developing attract and kill strategies for malaria vector control.
Keywords: Malaria, oviposition, denaturing gradient gel electrophoresis, volatile compounds, physicochemical parameter
Introduction
Knowledge of the cues utilized by gravid female mosquitoes to select suitable habitats for their offspring may facilitate development of more effective or novel mosquito control tools. Oviposition site seeking mosquitoes may respond to cues of visual, tactile and olfactory nature (McCrae, 1984; Bentley & Day, 1989; Millar et al., 1992; Eneh et al., 2016). An. gambiae s.l., one of the principal vectors of malaria in Africa, is often described to prefer temporary, shallow, sunlit pools or puddles for oviposition (Mereta et al., 2013), however, a great range of habitats has been described for different eco-epidemiological settings (Fillinger et al., 2004; Fillinger et al., 2009). The age of the standing water providing potential oviposition sites has been associated with differential attractiveness for malaria vectors in a previous study (Munga et al., 2013), where older substrates had a lower abundance of An. gambiae s.l. larvae. Moreover Anopheles larvae have been found to be less prominently present in permanent larval habitats compared to temporary habitats (Gimnig et al., 2001; Mereta et al., 2013).
The cues associated with habitats more frequently colonized are not fully understood. Physicochemical, microbial, and volatile chemical cues as well as presence of competitors and predators may be involved in oviposition site selection and all of these may vary over time. While some studies have found a significant association between physicochemical parameters of potential oviposition sites and the density of An. gambiae s.l. larvae, others have not and the result from these studies are often ambiguous (Robert et al., 1998; Minakawa et al., 1999; Gimnig et al., 2001; Gimnig et al., 2002; Ye-Ebiyo et al., 2003; Fillinger et al., 2004; Mutero et al., 2004; Minakawa et al., 2005; S. Munga et al., 2005; Edillo et al., 2006; Kaufman et al., 2006; Awolola et al., 2007; Muturi et al., 2007; Mwangangi, Mbogo, et al., 2007; Mwangangi, Muturi, et al., 2007; Muturi et al., 2008; Fillinger et al., 2009; Antonio-Nkondjio et al., 2011; Kenea et al., 2011; Ndenga et al., 2011; Kweka et al., 2012; Mereta et al., 2013; Munga et al., 2013; Dida et al., 2015). For instance, the pH of the water has been suggested to be associated with abundance of An. gambiae s.l. larvae in some studies (Gimnig et al., 2001; Awolola et al., 2007; Mereta et al., 2013) whereas it was not associated with larval density in others (Edillo et al., 2006; Muturi et al., 2007; Mwangangi, Muturi, et al., 2007; Antonio-Nkondjio et al., 2011; Kenea et al., 2011; Ndenga et al., 2011; Chirebvu & Chimbari, 2015; Dida et al., 2015).
Previous studies have suggested that An. gambiae s.s. utilize volatile microbial metabolites to select between oviposition substrates in cage bioassays (Sumba et al., 2004; Lindh, Kannaste, et al., 2008). Furthermore, recent studies have shown that they can use water vapour and chemical cues to locate and select oviposition sites in semi-field settings and in the field (Okal et al., 2013; Lindh et al., 2015; Okal et al., 2015). The first identified oviposition attractant for An. gambiae s.l., cedrol, has been suggested to be of microbial origin (Lindh et al., 2015; Eneh et al., 2016).
Earlier studies also indicated that the presence of Anopheles larvae may influence the oviposition behaviour of conspecifics (Munga et al., 2006; Sumba et al., 2008), possibly by influencing the volatile signals emitted from the sites through dead larvae or their waste products. In addition, larval density and presence of predators have been observed to affect An. gambiae s.s. oviposition choices in a cage experiment (Sumba et al., 2008; Warburg et al., 2011).
To date, no studies involving wild malaria mosquitoes have investigated bacterial, chemical and physicochemical profiles of pond water preceding larval colonization and thereby excluding larval based cues. Here we study the colonization of similar artificial aquatic habitats in close vicinity (less than 5 meters apart) by wild Anopheles. The habitats were set-up with the same material and oviposition substrates but staggered in time and allowed to age for different duration under natural condition in the field covered by a fine net. The habitats were then opened for colonization at the same time. This study aimed to investigate if gravid Anopheles females make a choice between similar habitats in close range and what cues might mediate the de-novo oviposition site selection.
Materials and Methods
Experiment location
The experiment was conducted in two open fields located approximately one km apart at the International Centre of Insect Physiology and Ecology -Thomas Odhiambo Campus (icipe-TOC), Mbita, western Kenya, on the shores of Lake Victoria (0°26′06.19″ South; 34°12′53.13″ East). The climate of Mbita is tropical with annual temperature between 18–28 °C and an average annual rainfall of 1769 mm (data from 2010–2013 obtained from icipe’s weather station). The experiment was set-up in the rainy season of 2013 between the months of April and May. The malaria vector species composition reported for the study area was dominated by Anopheles arabiensis; other species included Anopheles gambiae s.s., Anopheles funestus s.s., Anopheles rivulorum and Anopheles coustani (Kawada et al., 2011; Herrera-Varela et al., 2014; Lindh et al., 2015).
Experimental set-up
Ponds were made from a plastic basin of 55 cm upper diameter that was placed in a hole in the ground, so the top of the basin was in level with the ground. The ponds received 2 kg of dried soil mixed with 30 L of non-chlorinated tap water pumped from Lake Victoria. The water surface area of each pond was 0.8 m2. The soil (silty clay loam (Herrera-Varela et al., 2014)) was collected from a site that was a natural Anopheles oviposition site. The site was dry and did not contain any viable Anopheles eggs or larvae at the time. The ponds were fitted with overflow points at two sides, protected with a mesh of 0.6 mm by 0.6 mm to prevent loss of larva with rain. Daily rainfall data for the duration of experiments was collected from icipe’s meteorological station.
Ponds were set up 4 m apart in 5 rows containing 3 ponds each. The location of the ponds at icipe-TOC was at least 70 m away from the next natural aquatic habitat to avoid direct competition between the artificial set up and natural sites. Five ponds of each treatment (age 1 (opened for colonization when 0 day old), age 2 (opened for colonization when 4 day old) and age 3 (opened for colonization when 17 day old)) were set-up at random positions in such a way that all treatments were present in each row (Figure 1). During ageing, colonization by mosquitoes and other organisms was prevented by covering the ponds with pieces of nets with a mesh size of 0.6 × 0.6 mm. The water level at the start of the experiment was marked on each pond and if water evaporated below this level, it was topped up.
Figure 1:
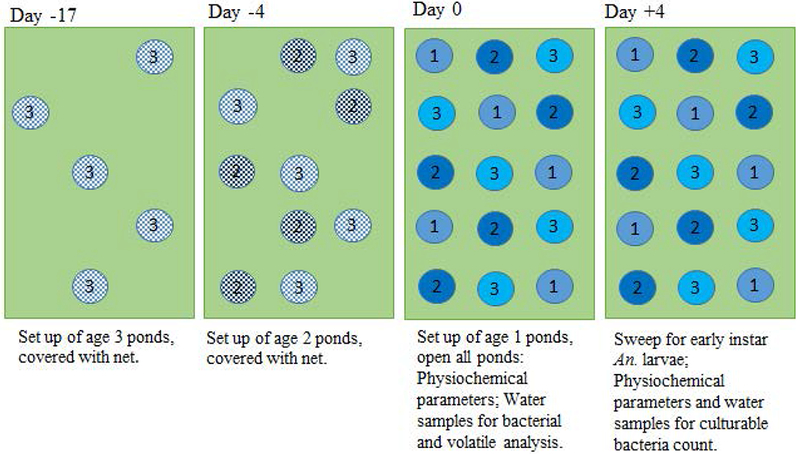
Experimental set up: distribution of open field ponds shown for a round.
A sweep net (length= 40 cm, width = 5 cm, height = 10 cm) was used to count the number of Anopheles early instar larvae on the fourth day after opening the ponds. Based on experience with this set up and published work (Yaro et al., 2006) it was assumed that at this time 90% of all eggs laid in the first 24 hours of opening had hatched. The sweep net was passed over the surface of the water ten times to exhaustively collect all mosquito larvae present. The content of the net was emptied into white plastic bowls providing a contrast for larva counting. Anopheles larvae were differentiated from culicine larvae based on morphology and the number of Anopheles larvae in each pond was recorded. Anopheles larvae were brought to the laboratory and allowed to grow to late instars before PCR-based species analysis was implemented. All Anopheles collected from the artificial ponds were Anopheles arabiensis, the pre-dominant species in the study area (Herrera-Varela et al., 2014). Water samples were taken for bacterial and chemical profiling on the day when ponds were opened for colonization (pre-colonization). Physicochemical profiling of pond water was performed on site and water samples taken for viable count of bacteria on the day the ponds were opened and on the day larval surveys were performed (4 days later, Figure 1). Larval count, physicochemical measurements and water collections for bacterial and chemical analyses were carried out between 9.00 and 11.00 a.m. on the day of sampling.
The experiment was replicated three times.
Physicochemical parameters
A hand-held multimeter (Multi 340i, WTW, Germany) was used to measure pH, dissolved oxygen (parts per million; ppm) and conductivity (µS/cm). Turbidity (Nephelometric Turbidity Unit; NTU) was measured with a turbidity meter (TURB 355IR, WTW Germany). Aquamerck® test kits (Aquamerck® No.111151, Germany) were used to measure nitrite (mg/l), nitrate (mg/l), phosphate (mg/l), carbonate hardness (mmol/l) and total hardness (mg/l). Water chlorophyll (µg Chl/l) content was measured with an AquaPen AP100 (Photon Systems Instruments, Czech Republic). Biochemical oxygen demand (BOD) was based on measuring dissolved oxygen (Multi 340i, WTW, Germany) in 50 ml water samples before and after storing in brown bottles at 25 °C for 5 days. BOD (ppm) was calculated as the difference between oxygen level at the start and after 5 days. All variables were measured according to the instructions by the manufacturer.
Bacterial viable count
Bacterial viable counts were performed for three randomly selected ponds of each age group Bacterial culture plates were prepared by dissolving 18.2 g Reasoner’s 2 agar (R2A, Sigma-Aldrich Sweden AB, Stockholm Sweden) medium in 1 L of deionized water. The resulting mixture was sterilized in an autoclave and poured into sterile Petri dishes (diameter 9 cm) with approximately 15 ml per dish. Water samples from the ponds were diluted a hundred fold with sterile saline solution (0.7% sodium chloride) and 100 µl of the dilution was plated by spreading the content equally over a plate. Three replicate plates were used for each pond. The plates were incubated at 30 °C for 24 hours. Thereafter, the number of colonies was counted and the colony forming units per ml (CFU/ml) were calculated.
Extraction of bacterial DNA from water samples
Water samples (50 ml) were taken from each of the ponds in Falcon tubes and centrifuged at 4000 rpm for 30 minutes. The supernatant was carefully decanted. The pellet was re-suspended in the remaining water and transferred to 1.5 ml Eppendorf tubes and then centrifuged at 14000 rpm for 1 minute. Subsequent steps were performed according to the handbook for QIAGEN blood and tissue kit (QIAGEN Ltd., North Manchester M15 6SH, UK). Extracted DNA was stored at minus 80 °C.
PCR amplification of 16S rDNA and purification of PCR products
The extracted DNA samples were used as template to amplify the V3 region of the 16s rDNA gene with bacteria universal primers (Eurofin AB, Stockholm, Sweden): forward primer 968F (5’-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GAA CGC GAA GAA CCT TAC-3’) with GC clamp and reverse primer 1601R (5’-CGG TGT GTA CAA GAC CC-3’). PCR were carried out using PuReTaq Ready-To-Go™ PCR Beads (GE Healthcare illustra™, Fisher Scientific, Stockholm, Sweden). To each PCR tube, 22 µL of nuclease free water (Life Technologies Europe BV, Stockholm, Sweden), 10 pmol forward and reverse primer and 1 µL of DNA template were added. The initial denaturation of the double stranded DNA was at 94 °C for 5 minutes followed by a touch down temperature programme of denaturation at 94 °C for 30 seconds and annealing at 58–48 °C for 30 seconds (1 °C decrease in temperature per cycle) and extension at 72 °C for 30 seconds. This was followed by 20 cycles at 94 °C, 50 °C and 72 °C for 30 seconds each. Final extension was 72 °C for 5 minutes. Each template was amplified in two replicate 25 µl PCR reactions. These were pooled, purified and concentrated to 10 µl using the Qiagen MinElute PCR purification kit following the protocol from the manufacturer (QIAGEN AB, Sollentuna, Sweden). Mixing of replicate PCR reactions have been reported to be effective in reducing possible bias in 16S rDNA gene amplification of environmental samples that have a complex mixture of templates (Polz & Cavanaugh, 1998). The DNA concentrations of the eluted samples were determined using nanodrop 1000 (Thermo Scientific, Mytogen Limited, Sweden). Agarose gel (1.5%) electrophoresis was carried out to confirm amplicon size by comparing band to the GelPilot 100 bp Ladder (QIAGEN AB, Sollentuna, Sweden) after staining with gelRed (VWR International AB, Spånga, Sweden).
Denaturing gradient gel electrophoresis (DGGE) analysis
Denaturing gradient gel electrophoresis (DGGE) was used to examine the bacterial profile of the water samples. The purified and concentrated PCR products derived from water sample from ponds were analysed using Dcode™ Universal Mutation Detection System (Bio-Rad Laboratories AB, Solna, Sweden). HyperLadder™ 25 bp DNA marker (Nordic Biosite AB, Stockholm, Sweden) was utilized as standard and 10 µl was loaded in the well in the middle of the gel. The two outermost wells were left empty. Separation of DNA fragments were carried out on polyacrylamide gel (6% acrylamide/bis-acrylamide, 37.5:1.0 wt/wt). The denaturing gradient ranged from 30% to 60% where 100% denaturant was 7 M urea and 40% (wt/v) deionized formamide. Each well was loaded with 400 ng of PCR product. Electrophoresis was performed in a buffer of 1 × TAE (Tris/acetate, pH 8; 0.5 M -ethylenediaminetetraacetic acid (EDTA)) at an initial voltage of 200 V for 10 minutes and subsequently 70 V for 16.5 h using an isothermal temperature of 60 °C. After the run, the gels were stained with 3x gelRed in 0.1 M NaCl in deionized water for 1.5 h with gentle shaking at 200 rpm. Stained gels were visualized and photographed using ChemiDoc XRS (Bio-Rad Laboratories AB, Stockholm, Sweden) with a UV filter fitted.
Sampling of volatiles
Tenax TA traps were made by packing 25 mg of Tenax TA of mesh size 60/80 (Supelco, Sigma-Aldrich Sweden AB, Stockholm, Sweden) into a GERSTEL-Twister Desorption glass liners (GERSTEL, Muelheim an der Ruhr, Germany) stopped with glass wool on both sides (Supelco, Sigma-Aldrich Sweden AB, Stockholm, Sweden). The traps were washed 10 times with 2 ml of methyl-tert butyl ether (MTBE, Supelco, Sigma-Aldrich Sweden AB, Stockholm Sweden) and openings covered with polytetrafluorethylene (PTFE) before being placed in a 50 ºC oven for at least 6 h before use. All glassware utilized for volatile collections were washed with an odourless detergent (Teepol, general purpose detergent, Teepol Industries, Nairobi, Kenya) and then rinsed in water and acetone before being placed in an oven at 200 °C for at least two hours before use. Volatile compounds were collected for 20 h from the headspace above 300 ml water samples in 500 ml Erlenmeyer flasks (E-flasks). The E-flasks were fitted with gas wash bottle heads (QuickFit joined ware, Staffordshire, United Kingdom). Water from the same source used to set-up the ponds and empty E-flasks were included as controls to screen for background volatiles. Due to a limited number of headspace trapping instruments 12 of 15 pond water samples in each round were randomly selected for sampling. Charcoal filtered air was pumped into the E-flasks at 0.1 l/min and drawn out at the same speed through the outlet which had a Tenax trap fitted. Polytetrafluoroethylene (PTFE) tubing and PTFE tape were used to make the connections airtight. After collections, the traps were sealed with Teflon tape and stored at −70 °C prior to analysis with GC-MS.
Gas chromatography – mass spectrometry (GC-MS) analysis
The Tenax traps were analysed for volatile compounds with an Agilent 7890A gas chromatograph connected to an Agilent 5975C inert MSD with Triple Axis detector mass spectrometer (Agilent, Santa Clara CA, United States). The GC system was fitted with GERSTEL Multi-Purpose Sampler (MPS: Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany). The GC capillary column (30 m, 250 µm internal diameter and 0.25 µm film thickness) was Agilent’s HP-5MS (5% phenyl and 95% dimethyl polysiloxane). Prior to analysis one microliter heptyl actetate (3.16 ng / µl) was added to the Tenax trap in a GERSTEL thermal desorption unit (TDU) followed by thermal desorption of the trap in splitless mode at initial temperature 40 °C and then increased by 120 °C / min to 270 °C, this end temperature was held for 5 min. The desorbed volatiles were focused on a tenax liner in a GERSTEL CIS inlet at 10 °C. The CIS inlet, which operated in splitless mode, was then heated at a rate of 12 °C/s to 280 °C during which the volatiles were transferred to the column. The GC oven temperature was initially held at 40 °C for 1 min and then increased by 4 °C / min to 280 °C which was held for 3 min. The carrier gas was helium at a pressure of 34 psi. The MS was at full scan and identified mass ranges from 30–400 m/z with electron ionization at 70 eV and ion source temperature at 230 °C.
Data analysis
The association between the number of early instar Anopheles larvae collected four days after opening the ponds and the three age treatments was analysed using a generalized linear model fitted with a negative binomial distribution with a log link. The different age groups were included in the model as main effect. Round and the interaction between the rounds and treatments were included in the initial model but since they had no significant association they were excluded from the final model. The mean number of early instar larvae and their 95% confidence intervals were estimated from this model. Analyses were done with IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
Partial least square – discriminant analysis (PLS-DA) was used to explore the physicochemical data using SIMCA statistical software version 13.0.3. Statistical comparison of physicochemical parameters was performed with non-parametric Kruskal-Wallis test.
GC-MS data were analysed with Agilent’s enhanced Chemstation software version E.02.01.1177. Chromatograms and mass spectra of samples were compared to that of the empty bottle and tap water controls. Sample peaks that had a different retention time and/or mass spectrum compared to the controls were assumed to be volatiles that were emitted from the ponds and therefore integrated. This was performed manually. When volatiles were present in control and sample but with a peak-area at least thrice as large in the sample compare to the controls they were also included. Volatiles with the same retention time and mass spectrum were given a unique volatile identification (volatile ID) number. Volatile ID numbers were assigned in increasing order of retention time. Mass spectra were matched to the spectra present in National Institute of Standards and Technology library 2008.
The DGGE gel images were analysed using the Image Lab gel analysis software (Bio-Rad Laboratories AB, Stockholm, Sweden). The bands and lanes were first automatically detected and then manually adjusted. Band detection was set to custom sensitivity followed by a manual addition of bands which were visually detected. The relative front (Rf) represents the band position of each band in relation to the highest and lowest band in each lane with a value of 0–1. The migration distance of each band was estimated by a regression method relative to the migration of the bands in the standard (HyperLadder™ 25 bp DNA marker). DGGE bands with same migration distance were assigned the same band identification number (Spp ID).
The volatile and bacteria profiles were explored using principal component analysis (PCA) to visualize the chemical or bacterial relationship between ponds of the different age groups using Canoco multivariate statistical software version 5.02. The presence/absence of the volatiles or bacteria were included in the analysis and the data were centred and standardized by volatiles/bacteria prior to analysis.
Results
Pond colonisation
In all rounds, the majority of the ponds of all age groups were colonized (40 of 45) by early instar Anopheles arabiensis larvae four days after they had been opened. However, the possibility of finding an early instar larva in ponds that were freshly set up and open for colonization on day 0 was 2 times higher (50, 95% CI 29–85; p=0.017) than in ponds that were 4 and 17 days older. The mean number of early instar Anopheles larvae in the 0.8 m2 fresh ponds was 50 (95% CI 29–85) compared to 24 (95% CI 14–42) in “4 days ponds” and 20 (95% CI 12–34) in “17 day ponds”). There was no position effect on larval distribution (Figure 2).
Figure 2:
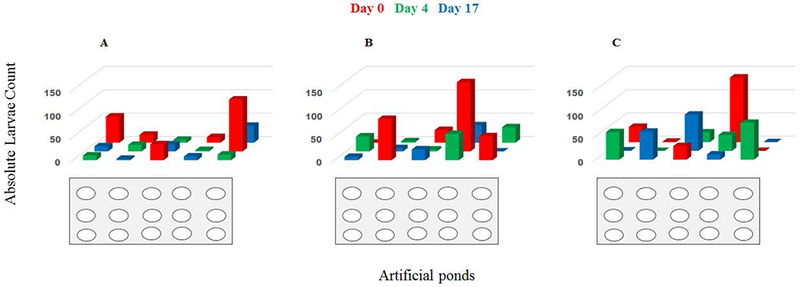
Investigating position effect on larval distribution. A, B and C show round 1, 2, and 3 respectively of larval distribution in all 15 replicates per treatment. The red bar represent fresh ponds left open for colonisation after set up, whilst green bars represent ponds that had aged for 4 days and blue bars represent ponds that aged for 17 before opening for colonization.
Physicochemical profiles at pre-colonization
Ponds were aged under natural climate conditions including rainfall. Especially during the first round of experiments, rainfall was abundant with 9 rain days of a total of 407 mm rain affecting the oldest age group (Figure 3). Round 2 and 3 of the experiments experienced less rain, however, the oldest age groups were affected by heavy rains beginning of May. Little rain was experienced when ponds were opened. At the day when the ponds were opened, the ponds differently exposed to environmental conditions differed in water turbidity, water temperature, chlorophyll content, nitrite content and water pH (Table 1). Using partial least square – discriminant analysis, fresh ponds (0 day old: age 1) grouped differently from the other age groups (Figure 4a) and were associated with a higher turbidity, water temperature and nitrite and a lower pH and chlorophyll content (Figure 4b) at the time when measurements were taken.
Figure 3:
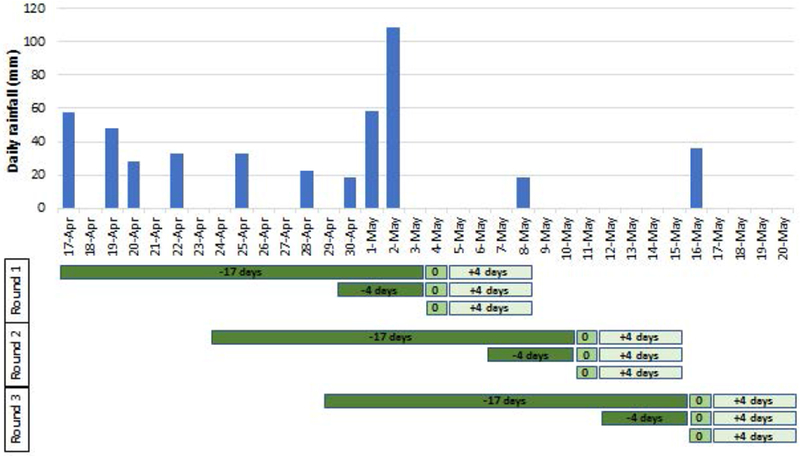
Daily rainfall during the 3 rounds of experiments. The green bars indicate the duration of aging (for 17 and 4 days) prior to opening the ponds on day 0. Larvae were sampled 4 days later.
Table 1:
Comparison of physicochemical water parameters for 0, 4 and 17 day old ponds at pre-colonization.
| Day 0 | Day 4 | Day 17 | |||
|---|---|---|---|---|---|
| Colonization stage | Physicochemical Parameters | mean (± 95% CI) |
mean (± 95% CI) |
mean (± 95% CI) |
P-value |
| Pre-colonization | pH | 7.7 (±0.2) | 8.5 (±0.2) | 8.7 (±0.2) | <0.001 |
| Temperature (°C) | 28.1 (±0.4) | 24.9 (±0.7) | 25.1 (±0.7) | <0.001 | |
| Dissolved oxygen (ppm) | 5.7 (±0.8) | 5.7 (±0.5) | 5.6 (±0.7) | 0.764 | |
| Conductivity (µS/cm) | 115 (±16) | 152 (±40) | 112 (±6) | 0.275 | |
| Chlorophyll | 183 (±20) | 697 (±175) | 831 (±416) | <0.001 | |
| Turbidity (NTU) | 191 (±86) | 77 (±21) | 98 (±21) | 0.028 | |
| Phosphate (mg/l) | 0.9 (±0.4) | 0.7 (±0.3) | 0.8 (±0.2) | 0.821 | |
| BOD (ppm) | 2.6 (±0.8) | 3.1 (±0.6) | 3.1 (±0.7) | 0.547 | |
| Nitrate (mg/l) | 7.3 (±2.5) | 8.0 (±2.3) | 7.3 (±2.5) | 0.889 | |
| Nitrite (mg/l) | 0.02 (±0.01) | 0.01 (±0.01) | 0 | <0.001 | |
| Carbonate hardness (mmol/l) | 1.1 (±0.2) | 1.6 (±0.4) | 1.2 (±0.1) | 0.061 | |
| Total hardness (mg/l) | 32 (±7) | 27 (±7) | 22 (±4) | 0.090 | |
Figure 4:
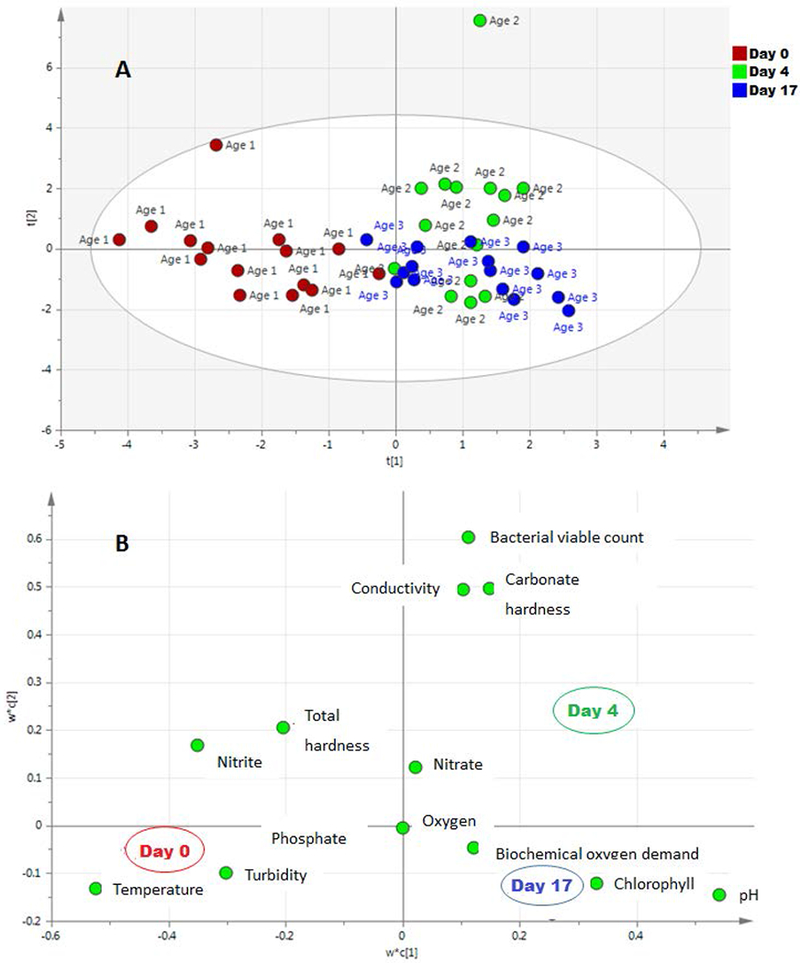
Partial least square – discriminant analysis (PLS-DA) score plot (A) of fresh pond 0 day old; age 1 (red dots), 4 day old; age 2 (green dots) and 17 day old; age 3 ponds (blue dots) and loading plot (B) based on physicochemical and bacterial viable count data at pre-colonization. Physicochemical data (green dots) included were: Conductivity (μS/cm); Carbonate hardness (mmol/l); Total hardness (mg/l); Phosphate (mg/l); Turbidity (NTU); Nitrite (mg/l); Nitrate (mg/l); Temperature (°C) ; pH; Oxygen dissolved (ppm); Biochemical oxygen demand (ppm) and Chlorophyll (μg Chl/l).
Chemical profiles at pre-colonization
In total 384 volatile compounds were detected from 36 water samples; 149 of these were detected in more than five samples and were included in the multivariate PCA analysis (Figure 5). Three of these volatile compounds (Table 2) were only detected in the fresh ponds. They were observed in more than 50% of the water samples taken from fresh ponds.
Figure 5:
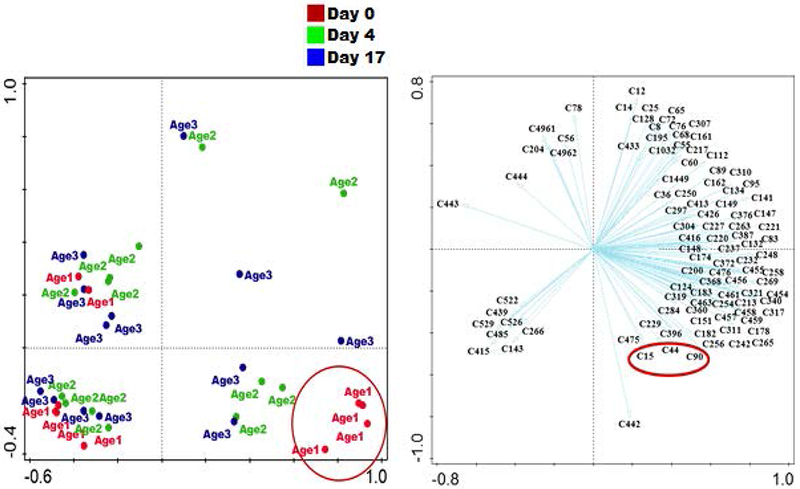
Principal component analysis (PCA) of volatile compounds detected in pond water of different age group. Axis 1 and 2 explains 33% of the total variation in data. Compounds C15 = 4-heptanone, C44 = 2-ethylhexanal and C90= an octenal isomer associated with age 1 ponds (in red circle) were detected in up to 80% of the water samples from age group 1. Ages 1, 2 and 3 represented 0, 4 and 17 day old ponds respectively.
Table 2:
Volatile compounds detected only in the fresh ponds at pre-colonization.
| Volatile ID | Number of replicates with compound (n=10) | Mass spectra ions (ion abundance) | Compound namea |
|---|---|---|---|
| C15 | 8 | 43 (100), 71 (96), 41(29), 114 (21), 39 (14) | 4-Heptanone |
| C44 | 7 | 57 (100), 72 (94), 43(82), 41 (57), 55 (38) | 2-Ethylhexanal |
| C90 | 6 | 70 (100), 41 (96), 39(81), 82 (79), 55 (75) | Octenal isomer |
Tentatively identified based on mass spectra and comparison to the NIST08 library.
Bacterial profile of ponds at pre-colonization
The average number of colony forming units (CFU) derived from fresh pond water samples (0 day old) were 50% lower than from 4 day old ponds and similar to 17 day old ponds. However, the number of CFU almost doubled from pre-colonization to post-colonization for fresh ponds (5.3 *104 ± 95% CI 2.2 *104 and 8.8 *104 ± 95% CI 5.1 *104 pre- and post-colonization, respectively) while a reduction by half were observed for the other two age groups (Age 2: 1.06 *105 ± 95% CI 5.9 *104 and 5.0 *104 ± 95% CI 3.1 *104; Age 3: 4.3*104 ± 95% CI 3.8 *104 and 3.5 *104 ± 95% CI 1.2 *104 pre- and post-colonization, respectively).
In the culture independent analysis of the microbial profile of pre-colonization water, 46 different bands (Spps) were detected (Figure 6a) using denaturing gradient gel electrophoresis (DGGE). No band was unique to any of the three age groups, furthermore, the profile of bands detected for each age group varied between rounds. Due to this round variation in band profile, principal component analysis (PCA) of DGGE data did not show any consistent age dependent grouping of the ponds (Figure 6b). However, round by round DGGE profile analysis showed that the fresh ponds grouped separately from the other age groups in round 1 and 3 (Figure 6c).
Figure 6:
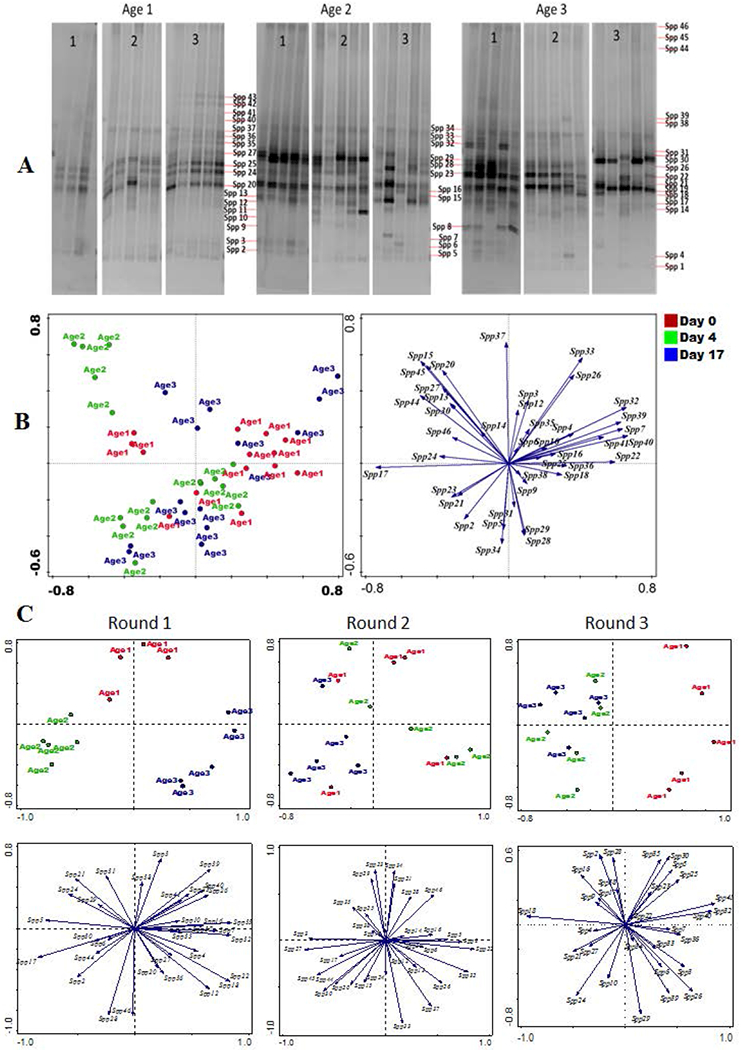
A: Denaturing gradient gel electrophoresis (DGGE) of samples from pre-colonization pond water. The numbers 1, 2 and 3 represented different rounds while Ages 1, 2 and 3 represented 0, 4 and 17 day old pond water samples respectively. (B): Principal component analysis (PCA) of presence/absence of DGGE bacteria bands (Spp) in the three age groups at pre-colonization in all rounds combined. Axis 1 and 2 together represent 23% of the total variation in the data. (C): PCA of presence/absence of DGGE bacteria bands in the three age groups at pre-colonization round by round.
Discussion
This study explored the relative attractiveness of similar aquatic habitats of different persistence to wild gravid Anopheles arabiensis females. Freshly prepared artificial ponds contained twice as many early instar Anopheles arabiensis larvae than ponds that were exposed to natural climate conditions for 4 and 17 days before opening them for colonisation. Hence, it is concluded that gravid females made a choice between these habitats that coexisted in close vicinity. This choice might have been based on attraction or stimulation by cues of the fresh ponds or on avoidance of cues associated with the older ponds. In contrast to previous field studies, which investigated water qualities of already colonized water, it was possible here to observe cues emanating from pond water directly preceding oviposition and excluding any confounding from conspecific larvae. Previous studies have shown that the presence of mosquito larvae may influence the oviposition behaviour of gravid Anopheles mosquitoes (McCrae, 1984; Munga et al., 2006; Sumba et al., 2008).
The artificial pond system has been used effectively at icipe-TOC for a large range of experiments over the past two decades (Fillinger et al., 2003; Herrera-Varela et al., 2014) and larval densities encountered per square meter during the experiments were well within the range published for previous experimental studies as well as within the range of natural aquatic Anopheles habitats (Fillinger et al., 2009; Ndenga et al., 2011).
It was shown that the fresh ponds differed from the older ponds in the physicochemical characteristics, bacteria and volatile chemical profile of the water before colonization potentially explaining the habitat selection by gravid females. The results presented here complement those of Munga and colleagues (Munga et al., 2013) who found significantly more larvae of Anopheles gambiae s.l. (based on daily larval sampling) in habitats in the western Kenya highlands where water was replenished every 10 days as compared to habitats that persisted for 30 days. Whilst this confirms that gravid females make a choice, it cannot be concluded from their study that habitat age alone was responsible for this, or whether it might have been associated with other cues manipulated during the study, like the removal of shading vegetation.
Similarly, the here presented results do not suggest that water age is the actual driving factor for oviposition selection but changes in habitat quality that have taken place over time, as demonstrated in the presented study in which all ponds were set up in exactly the same way yet at different time points. The older ponds were exposed to environmental conditions for extended time. Ponds were open to rainfall and specifically the ponds allowed to age for 17 days before opening, received substantial rain during the study periods which is assumed to have contributed to the changes in habitat characteristics.
The fresh ponds were associated with a higher temperature, higher turbidity, and lower pH than the older ponds with lower early instar Anopheles densities. These three factors are correlated. Higher turbidity increases near-surface water temperature (Paaijmans et al., 2008) due to the heat absorbing property of suspended water particles from solar radiation. (Herrera-Varela, 2015). Additionally, older ponds had a significantly higher chlorophyll content which again in turn might explain the higher pH. Notably, water temperature and pH change over a daily cycle based on climatic and biotic conditions hence a single measurement taken in the morning, as done here, does not allow strong inferences between these factors and oviposition which takes place at dusk. However, increased Anopheles larval abundance has previously been associated with these factors (Fillinger et al., 2009; Mwangangi et al., 2010; Mala & Irungu, 2011; Mereta et al., 2013;Herrera-Varela, 2015; Gimnig et al., 2001; Awolola et al., 2007; Chirebvu & Chimbari, 2015). Specifically, findings from field surveys in the same study area in western Kenya showed that highly turbid water of around 200 NTU and above was consistently colonized by early instars (Manuela Herrera-Varela et al., 2014). Turbidity could affect egg-laying of Anopheles mosquitoes in multiple ways. It is likely that turbid surfaces reflect polarized light especially during dusk when malaria vectors seek oviposition sites and make these habitats more visible in the landscape (Bernáth et al., 2004; Polarized Light and Polarization Vision in Animal Sciences, 2014). The fact that turbidity increases surface-water temperatures might support a faster larval development (Bayoh & Lindsay, 2003; Kirby & Lindsay, 2009) which might be beneficial in transient habitats. High turbidity might also lower the risk of being observed by predators.
The fresh ponds also had a slightly higher nitrite level which is in agreement with two previous studies (Kweka et al., 2012; Ndenga et al., 2012). Nitrites occur in water as an intermediate product in the biological breakdown of organic nitrogen, being produced either through the oxidation of ammonia or the reduction of nitrate by microorganisms. The decomposition of organic matter introduced with the soil when the ponds were set up is likely responsible for this.
Microbial activity over time might have affected the habitat quality and the volatile chemicals released from the ponds. Mosquito larvae feed on detritus and microorganism including bacteria (Briegel, 2003) and bacterial associated cues have been shown to influence oviposition site selection of Aedes mosquitoes (Ponnusamy et al., 2008; Albeny-Simoes et al., 2014). However the evidence for bacteria derived cues in Anopheles oviposition site selection is contradictive; while two studies have shown a positive oviposition response to bacteria containing oviposition media (Sumba et al., 2004; Lindh, Kannaste, et al., 2008) another study show the opposite effect (Huang et al., 2006). In the present study, there was no round independent association between bacterial profile and age of the oviposition substrates due to a high variability in bacteria profile. The differences in profile between rounds were bigger than between age groups. Interestingly however, round by round analysis revealed that the bacteria community in the fresh ponds differed strongly from older ponds in two out of the three rounds an observation that warrants further investigations into the role of bacteria in oviposition site selection by malaria vector mosquitoes. It has been previously shown that specific volatile compounds can be produced by more than one bacterium species (Lindh, Kaennaste, et al., 2008) hence different bacteria communities might still release similar volatile profiles. As with all techniques the methods utilized here to screen for bacteria have limitations and it is possible that a more general association between Anopheles larval abundance and bacteria could have been observed if other techniques like large scale sequencing had been utilized.
Three volatile compounds: 4-heptanone, 2-ethylhexanal and one isomer of octenal were selectively detected in the higher colonised fresh ponds and not in the older ponds. These volatile compounds should be further evaluated in bioassays as putative oviposition attractants for An. gambiae. The ketone (4-heptanone) which was detected in 80% of the fresh ponds in this study, has previously been included in a patented chemical blend for attracting host-seeking mosquitoes (Bernier et al., 2000) and for capturing and killing bed bugs (Frutos et al., 2015).
By analysing the water characteristics in ponds prior to colonization, differences were observed that may explain what brought the first generation of malaria vectors to a new, oviposition naïve site and this knowledge might be used to inform the development of new vector control tools. However, a number of study limitations need to be mentioned to highlight that the here presented results need to be interpreted in the local context in which data was collected and further studies are needed to confirm for example whether the observed changes in water characteristic are common when habitats age or were specific to the here used set up. The major aim of this study was to directly correlate the physicochemical parameters, bacteria community and chemical volatiles released. Due to the technically involving chemical analyses only a relatively small number of samples and replicates were feasible to analyse in this study. High heterogeneity in the outcome (larval density) as well as in the explanatory variables required a more descriptive approach to data analysis and makes results more indicative than conclusive. More replication will be required for this work going forward. Two rounds of the experiments strongly suggested an association with bacteria, not only the population composition but also abundance, which was previously found (Herrera-Varela et al., 2014) and should be more systematically investigated. More standardisation reducing variability in the system might help to further discover attractive or repellent volatiles for oviposition. Three volatiles were associated with fresh ponds exclusively and should be tested as oviposition attractants.
Acknowledgements:
We thank Benard Oyembe, Elisha Obudho and David Alila of icipe-TOC, Mbita for maintaining colonies of mosquitoes used for experiments and Elizabeth Masinde, Paul Ouma, Rose Atieno, Gregory Masinde, and Joel Odero for technical assistance.
Funding: This project was funded through a National Institute of Health (NIH) grant no. R01AI082537 and the Swedish Research Council (grant no SWE-2010–129). The funders had no role in study design, data collection or interpretation, or the decision to submit the work for publication.
Declarations
Conflict of Interest: The authors declare that they have no competing interests.
References
- Albeny-Simoes D, Murrell EG, Elliot SL, Andrade MR, Lima E, Juliano SA, et al. (2014) Attracted to the enemy: Aedes aegypti prefers oviposition sites with predator-killed conspecifics. Oecologia, 175, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio-Nkondjio C, Fossog BT, Ndo C, Djantio BM, Togouet SZ, Awono-Ambene P, et al. (2011) Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaounde (Cameroon): influence of urban agriculture and pollution. Malaria Journal, 10, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola TS, Oduola AO, Obansa JB, Chukwurar NJ & Unyimadu JP (2007) Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, southwestern Nigeria. Journal of Vector Borne Diseases, 44, 241–244. [PubMed] [Google Scholar]
- Bayoh MN & Lindsay SW (2003) Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae). Bulletin of Entomological Research, 93, 375–381. [DOI] [PubMed] [Google Scholar]
- Bentley MD & Day JF (1989) Chemical ecology and behavioral aspects of mosquito oviposition. Annual Review of Entomology, 34, 401–421. [DOI] [PubMed] [Google Scholar]
- Bernier UR, Kline DL, Barnard DR, Booth MM & Yost RA (2000) Chemical compositions that attract mosquitoes, pp. 75 pp. The United States of America, as Represented by the Secretary of Agriculture, USA; University of Florida; . [Google Scholar]
- Bernáth B, Gál J & Horváth G (2004) Why is it worth flying at dusk for aquatic insects? Polarotactic water detection is easiest at low solar elevations. Journal of Experimental Biology, 207, 755. [DOI] [PubMed] [Google Scholar]
- Briegel H (2003) Physiological bases of mosquito ecology. Journal of Vector Ecology, 28, 1–11. [PubMed] [Google Scholar]
- Chirebvu E & Chimbari MJ (2015) Characteristics of Anopheles arabiensis larval habitats in Tubu village, Botswana. Journal of Vector Ecology, 40, 129–138. [DOI] [PubMed] [Google Scholar]
- Dida GO, Gelder FB, Anyona DN, Abuom PO, Onyuka JO, Adoka SO, et al. (2015) Presence and distribution of mosquito larvae predators and factors influencing their abundance along the Mara River, Kenya and Tanzania. Springerplus, 4, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edillo FE, Tripét F, Touré YT, Lanzaro GC, Dolo G & Taylor CE (2006) Water quality and immatures of the M and S forms of Anopheles gambiae s.s. and An. arabiensis in a Malian village. Malaria Journal, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eneh LK, Okal MN, Borg-Karlson AK, Fillinger U & Lindh JM (2016) Gravid Anopheles gambiae sensu stricto avoid ovipositing in Bermuda grass hay infusion and it’s volatiles in two choice egg-count bioassays. Malaria Journal, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eneh LK, Saijo H, Borg-Karlson AK, Lindh JM & Rajarao GK (2016) Cedrol, a malaria mosquito oviposition attractant is produced by fungi isolated from rhizomes of the grass Cyperus rotundus. Malaria Journal, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U, Knols BG & Becker N (2003) Efficacy and efficiency of new Bacillus thuringiensis var israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Tropical Medicine and International Health, 8, 37–47. [DOI] [PubMed] [Google Scholar]
- Fillinger U, Sombroek H, Majambere S, van LE, Takken W & Lindsay SW (2009) Identifying the most productive breeding sites for malaria mosquitoes in The Gambia. Malaria Journal, 8, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U, Sombroek H, Majambere S, Van Loon E, Takken W & Lindsay SW (2009) Identifying the most productive breeding sites for malaria mosquitoes in the Gambia. Malaria Journal, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U, Sonye G, Killeen GF, Knols BGJ & Becker N (2004) The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: operational observations from a rural town in western Kenya. Tropical Medicine and International Health, 9, 1274–1289. [DOI] [PubMed] [Google Scholar]
- Frutos U, Elkashef S & Brown MA (2015) Compositions and methods for capturing, killing or repelling bed bugs, pp. 83pp. Olfactor Laboratories, Inc., USA: . [Google Scholar]
- Gimnig JE, Ombok M, Kamau L & Hawley WA (2001) Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. Journal of Medical Entomology, 38, 282–288. [DOI] [PubMed] [Google Scholar]
- Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM & Walker ED (2002) Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. Journal of Medical Entomology, 39, 162–172. [DOI] [PubMed] [Google Scholar]
- Herrera-Varela M (2015) Larval habitat discrimination by the African malaria vector Anopheles gambiae sensu lato: Observations from standardized experiments and field studies.
- Herrera-Varela M, Lindh J, Lindsay SW & Fillinger U (2014) Habitat discrimination by gravid Anopheles gambiae sensu lato--a push-pull system. Malaria Journal, 13, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Miller JR, Chen SC, Vulule JM & Walker ED (2006) Anopheles gambiae (Diptera: Culicidae) oviposition in response to agarose media and cultured bacterial volatiles. Journal of Medical Entomology, 43, 498–504. [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Wanja E, Maknojia S, Bayoh MN, Vulule JM & Walker ED (2006) Importance of algal biomass to growth and development of Anopheles gambiae larvae. Journal Medical Entomology, 43, 669–676. [DOI] [PubMed] [Google Scholar]
- Kawada H, Dida GO, Ohashi K, Komagata O, Kasai S, Tomita T, et al. (2011) Multimodal Pyrethroid Resistance in Malaria Vectors, Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus s.s. in Western Kenya. PLOS ONE, 6, e22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenea O, Balkew M & Gebre-Michael T (2011) Environmental factors associated with larval habitats of anopheline mosquitoes (Diptera: Culicidae) in irrigation and major drainage areas in the middle course of the Rift Valley, central Ethiopia. Journal of Vector Borne Diseases, 48, 85–92. [PubMed] [Google Scholar]
- Kirby MJ & Lindsay SW (2009) Effect of temperature and inter-specific competition on the development and survival of Anopheles gambiae sensu stricto and An. arabiensis larvae. Acta Tropica, 109, 118–123. [DOI] [PubMed] [Google Scholar]
- Kweka EJ, Zhou G, Munga S, Lee MC, Atieli HE, Nyindo M, et al. (2012) Anopheline Larval Habitats Seasonality and Species Distribution: A Prerequisite for Effective Targeted Larval Habitats Control Programmes. PLoS ONE, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh JM, Kaennaste A, Knols BGJ, Faye I & Borg-Karlson AK (2008) Oviposition responses of Anopheles gambiae s.s. (Diptera: Culicidae) and identification of volatiles from bacteria-containing solutions. Journal of Medical Entomology, 45, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Lindh JM, Okal MN, Herrera-Varela M, Borg-Karlson A-K, Torto B, Lindsay SW, et al. (2015) Discovery of an oviposition attractant for gravid malaria vectors of the Anopheles gambiae species complex. Malaria Journal, 14, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mala AO & Irungu LW (2011) Factors influencing differential larval habitat productivity of Anopheles gambiae complex mosquitoes in a western Kenyan village. Journal of Vector Borne Diseases, 48, 52–57. [PubMed] [Google Scholar]
- McCrae AW (1984) Oviposition by African malaria vector mosquitoes. II. Effects of site tone, water type and conspecific immatures on target selection by freshwater Anopheles gambiae Giles, sensu lato. Annals of Tropical Medicine and Parasitology, 78, 307–318. [PubMed] [Google Scholar]
- Mereta ST, Yewhalaw D, Boets P, Ahmed A, Duchateau L, Speybroeck N, et al. (2013) Physico-chemical and biological characterization of anopheline mosquito larval habitats (Diptera: Culicidae): implications for malaria control. Parasites Vectors, 6, 320/321–320/316, 316 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JG, Chaney JD & Mulla MS (1992) Identification of oviposition attractants for Culex quinquefasciatus from fermented Bermuda grass infusions. Journal of the American Mosquito Control Association, 8, 11–17. [PubMed] [Google Scholar]
- Minakawa N, Munga S, Atieli F, Mushinzimana E, Zhou G, Githeko AK, et al. (2005) Spatial distribution of anopheline larval habitats in Western Kenyan highlands: Effects of land cover types and topography. American Journal of Tropical Medicine and Hygiene, 73, 157–165. [PubMed] [Google Scholar]
- Minakawa N, Mutero CM, Githure JI, Beier JC & Yan G (1999) Spatial distribution and habitat characterization of anopheline mosquito larvae in Western Kenya. American Journal of Tropical Medicine and Hygiene, 61, 1010–1016. [DOI] [PubMed] [Google Scholar]
- Munga S, Minakawa N, Zhou G, Barrack OOJ, Githeko AK & Yan G (2005) Oviposition site preference and egg hatchability of Anopheles gambiae: Effects of land cover types. Journal of Medical Entomology, 42, 993–997. [DOI] [PubMed] [Google Scholar]
- Munga S, Minakawa N, Zhou G, Mushinzimana E, Barrack OOJ, Githeko AK, et al. (2006) Association between land cover and habitat productivity of malaria vectors in western Kenyan highlands. American Journal of Tropical Medicine and Hygiene, 74, 69–75. [PubMed] [Google Scholar]
- Munga S, Vulule J & Kweka EJ (2013) Response of Anopheles gambiae s.l. (Diptera: Culicidae) to larval habitat age in western Kenya highlands. Parasites Vectors, 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutero CM, Ng’ang’a PN, Wekoyela P, Githure J & Konradsen F (2004) Ammonium sulphate fertiliser increases larval populations of Anopheles arabiensis and culicine mosquitoes in rice fields. Acta Tropica, 89, 187–192. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Mwangangi J, Shililu J, Jacob BG, Mbogo C, Githure J, et al. (2008) Environmental factors associated with the distribution of Anopheles arabiensis and Culex quinquefasciatus in a rice agro-ecosystem in Mwea, Kenya. Journal of Vector Ecology, 33, 56–63. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Mwangangi J, Shililu J, Muriu S, Jacob B, Kabiru E, et al. (2007) Mosquito species succession and physicochemical factors affecting their abundance in rice fields in Mwea, Kenya. Journal of Vector Ecology, 44, 336–344. [DOI] [PubMed] [Google Scholar]
- Mwangangi JM, Mbogo CM, Muturi EJ, Nzovu JG, Githure JI, Yan G, et al. (2007) Spatial distribution and habitat characterisation of Anopheles larvae along the Kenyan coast. Journal of Vector Borne Diseases, 44, 44–51. [PMC free article] [PubMed] [Google Scholar]
- Mwangangi JM, Muturi EJ, Shililu JI, Muriu S, Jacob B, Kabiru EW, et al. (2007) Environmental covariates of Anopheles arabiensis in a rice agroecosystem in Mwea, Central Kenya. Journal of the American Mosquito Control Association, 23, 371–377. [DOI] [PubMed] [Google Scholar]
- Mwangangi JM, Shililu J, Muturi EJ, Muriu S, Jacob B, Kabiru EW, et al. (2010) Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malaria Journal, 9, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndenga BA, Simbauni JA, Mbugi JP & Githeko AK (2012) Physical, chemical and biological characteristics in habitats of high and low presence of anopheline larvae in Western Kenya highlands. PLoS One, 7, e47975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndenga BA, Simbauni JA, Mbugi JP, Githeko AK & Fillinger U (2011) Productivity of malaria vectors from different habitat types in the western kenya highlands. PLoS ONE, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okal MN, Francis B, Herrera-Varela M, Fillinger U & Lindsay SW (2013) Water vapour is a pre-oviposition attractant for the malaria vector Anopheles gambiae sensu stricto. Malaria Journal, 12, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okal MN, Herrera-Varela M, Fillinger U, Ouma P, Torto B, Lindsay SW, et al. (2015) Analysing chemical attraction of gravid Anopheles gambiae sensu stricto with modified BG-Sentinel traps. Parasites Vectors, 8, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Takken W, Githeko AK & Jacobs AFG (2008) The effect of water turbidity on the near-surface water temperature of larval habitats of the malaria mosquito Anopheles gambiae. International Journal of Biometeorology, 52, 747–753. [DOI] [PubMed] [Google Scholar]
- Polarized Light and Polarization Vision in Animal Sciences. (2014). Springer. [Google Scholar]
- Polz MF & Cavanaugh CM (1998) Bias in template-to-product ratios in multitemplate PCR. Applied and Environmental Microbiology, 64, 3724–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy L, Ning X, Stav G, Wesson DM, Schal Cand Appersson CS (2008) Diversity of Bacterial Communities in Container Habitats of Mosquitoes. Microbial Ecology, 56, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Awono-Ambene HP & Thioulouse J (1998) Ecology of larval mosquitoes, with special reference to Anopheles arabiensis (Diptera: Culcidae) in market-garden wells in urban Dakar, Senegal. Journal of Medical Entomology, 35, 948–955. [DOI] [PubMed] [Google Scholar]
- Sumba LA, Guda TO, Deng AL, Hassanali A, Beier JC & Knols BGJ (2004) Mediation of oviposition site selection in the African malaria mosquito Anopheles gambiae (Diptera: Culicidae) by semiochemicals of microbial origin. International Journal of Tropical Insect Science, 24, 260–265. [Google Scholar]
- Sumba LA, Ogbunugafor CB, Deng AL & Hassanali A (2008) Regulation of oviposition in Anopheles gambiae s.s.: role of inter- and intra-specific signals. Journal of Chemical Ecology, 34, 1430–1436. [DOI] [PubMed] [Google Scholar]
- Warburg A, Faiman R, Shtern A, Silberbush A, Markman S, Cohen JE, et al. (2011) Oviposition habitat selection by Anopheles gambiae in response to chemical cues by Notonecta maculata. Journal of Vector Ecology, 36, 421–425. [DOI] [PubMed] [Google Scholar]
- Yaro AS, Dao A, Adamou A, Crawford JE, Ribeiro JM, Gwadz R, et al. (2006) The distribution of hatching time in Anopheles gambiae. Malaria Journal, 5, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye-Ebiyo Y, Pollack RJ, Kiszewski A & Spielman A (2003) Enhancement of development of larval Anopheles arabiensis by proximity to flowering maize (Zea mays) in turbid water and when crowded. American Journal of Tropical Medicine and Hygiene, 68, 748–752. [PubMed] [Google Scholar]


