Abstract
The framework of the uncontrolled manifold (UCM) hypothesis was used to explore variables related to stability of task performance in the two hands of young healthy individuals. Fourteen young adults performed four-finger accurate constant force production tasks interrupted by a voluntary quick force pulse production and by an externally imposed displacement of all fingers. Three groups of variables were used to quantify stability of steady force production: (1) indices of the inter-trial variance were computed within the UCM and orthogonal to the UCM; (2) indices of motor equivalence were computed between steady-state intervals separated by the force pulse and by the inverse piano action; and (3) referent coordinate and apparent stiffness were computed using the data during the ascending phase of the finger-lifting episode. In another task, the subjects performed accurate constant force production with visual feedback removal after the 8th s and the drop in the total force after the removal was computed. There were differences between the right and left hand in some outcome variables such as variance within the UCM, and the timing of anticipatory synergy adjustments prior to the force pulse, consistent with the dynamic dominance hypothesis. There were significant correlations between the two hands for indices that were unrelated to accuracy of performance: variance within the UCM, index of motor equivalence, referent coordinate, apparent stiffness, and the drop of total force after visual feedback removal. We interpret these findings within the concept of stability-optimality trade-off. In particular, we conclude that individual subjects select particular, person-specific solutions within the spectrum allowed by the explicit task constraints, and this choice is consistent between the two hands. We conclude with a hypothesis that selecting specific solutions within the stability-optimality trade-off may represent an individual’s personal preference consistent between the two hands.
Keywords: hand, synergy, variance, motor equivalence, referent coordinate
INTRODUCTION
Recent studies of multi-finger force production have revealed differences between the dominant and non-dominant hands in indices of finger coordination. In particular, the non-dominant hand showed higher indices of performance stability during steady-state four-finger force production tasks (Park et al. 2012, Jo et al. 2015), whereas the dominant hand showed smaller loss of stability during quick force pulse production (Zhang et al. 2006). These studies used the framework of the uncontrolled manifold (UCM) hypothesis (Scholz and Schöner 1999) to quantify indices of inter-trial variance in the abundant space of finger forces (Latash 2012) in directions that do not affect performance (within the UCM for total force, VUCM) and in directions that affect performance (orthogonal to the UCM, VORT). These observations are consistent with the dynamic dominance hypothesis (Sainburg 2002), which suggests that the hemisphere contralateral to the dominant arm specializes in ensuring accurate movement dynamics through predictive mechanisms, whereas the non-dominant hemisphere specializes in impeding unanticipated perturbations and accurately controlling steady-state tasks (Wang and Sainburg, 2007).
Most studies of motor control and coordination focus on common features among participants that are viewed as representing the general population. Note that indices of both performance and stability during multi-finger actions show large variability across healthy young adults (Scholz et al. 2002). In this study, we explored both differences between the hands, common within a group of young healthy persons, and correlations between the hands for indices of performance and stability. In other words, we asked the question: Are there individual traits that are reflected in correlated indices of performance and/or stability between the two hands?
We used several methods to estimate the stability of force production. In addition to the mentioned analysis of inter-trial variance (VUCM vs. VORT) in spaces of elemental performance variables produced by individual fingers (finger forces), we used analysis of motor equivalence (motion magnitude within UCM and ORT) and analysis of performance-stabilizing synergies in the space of hypothetical control variables defined within the theory of motor control with spatial referent coordinates for the involved effectors (Latash 2010; Feldman 2015; see below for details). Analysis of motor equivalence compares the effects of quick actions or external perturbations on the deviations of the abundant system of four fingers along the UCM and along its orthogonal space (ORT), motor equivalent and non-motor equivalent deviations (ME and nME), respectively (Mattos et al. 2011, 2015). In our experiments, we quantified ME and nME deviations caused by a self-paced quick force pulse and by a brief positional finger perturbation produced by the “inverse piano” device (Martin et al. 2011).
Recently, the idea of task-specific stability of actions has been coupled with the idea of the neural control of movements using spatial referent coordinates (RCs; Latash 2010, 2016; Feldman 2015). Within this framework, we performed analysis of performance stability assuming that the neural control of action can be expressed adequately as time changes in referent coordinates for the agonist and antagonist muscle groups participating in the hand force production (cf. Feldman 2015; Reschechtko et al. 2017). Since every human effector, including the hand, is controlled by two groups of muscles – agonists and antagonists – even one-effector natural tasks are abundant (cf. Latash 2012) at the level of control. For example, hand force (F) production in isometric conditions may be viewed as a consequence of a change in RCs for the agonist and antagonist muscle groups. The superposition of these two control variables can be characterized as RC for the hand and its apparent stiffness, k: F = k(AC ‒ RC), where AC is the actual coordinate of the hand. If there is a force-stabilizing synergy at the level of two elemental variables, RC and k, one can expect high inter-trial variability of the {RC; k} data points along the hyperbolic UCM for this task compared to variability orthogonal to this UCM. Such {RC; k}-synergies have been documented in recent studies and quantified using the coefficient of determination, R2, in the hyperbolic regression analysis and an index, RSD (Ambike et al. 2016a,b; Reschechtko and Latash 2017, de Freitas et al. 2018), computed with the method of using randomized sets of elemental variables, RC and k, to create surrogate data sets (cf. Müller and Sternad 2003).
The indices computed in the current study can be divided into two groups. VORT and nME reflected the accuracy of hand force production. Given that most tasks in our study were performed under continuous visual feedback for total hand force, we expected no significant differences between the two hands and no between-hand correlations for VORT and nME, as well as for indices directly reflecting the accuracy of total force production (such as root mean squared error, RMSE). In contrast, other indices were not constrained by the available visual feedback. Based on the dynamic dominance hypothesis and earlier studies (Park et al. 2012; Jo et al. 2015), we expected higher VUCM and ME in the non-dominant hand and larger indices related to preparation of the force pulse (anticipatory synergy adjustments, ASAs, Olafsdottir et al. 2005) in the dominant hand (Hypotheses 1A and 1B). We also expected across-subject correlations between the non-feedback (implicit) indices such as VUCM, ME, RC, and k recorded in the two hands (Hypothesis 2), potentially reflecting individual preferences by the subjects.
In addition, we explored characteristics of unintentional force drift that is seen in constant force production tasks after the visual feedback on force has been turned off (Vaillancourt and Russell 2002; Ambike et al. 2015). Hand force drift reflects the loss of force stability as confirmed in several recent studies (Parsa et al. 2016; Reschechtko et. al. 2017). Because of the absence of visual feedback, force drift magnitude represents another index not constrained by the availability of visual feedback and we expected it to correlate between the two hands (Hypothesis 3).
Individuals may use different motor strategies, for example as reflected in kinematic and/or kinetic performance characteristics, to achieve the same task goal as shown in a study of ball catching (Cesqui et al, 2012). The way a person implements a solution or group of solutions could be part of his/her personal preference, a coordination trait that can distinguish this person from others. Effects of personal traits on motor coordination have been studied typically in populations with neurological or psychological abnormalities such as autism, dyspraxia, developmental coordination disorder, and others (Harris et al. 2008; Lingham et al. 2010; Cheng et al. 2017; Curioni et al. 2017). Personality traits such as risk taking, novelty seeking, reward dependence, general arousal, and fear of falling have been linked to cerebellar activity (Picerni et al. 2013; Petrosini et al. 2015) and are known to correlate with indices of performance (Miller and Saygin 2013; Zaback et al. 2015). Our experiments differ from earlier studies in addressing variables that are not directly related to the performance of the explicit task and receive no visual feedback.
METHODS
Participants
Fourteen healthy, self-reported right-handed adults (eight males and six females) between 20 and 38 years old (26.9 ± 4.5 years; mean ± standard deviation), with an average body mass of 66.6 (±10.3) kg and body height of 1.72 (±0.09) m, participated in the study. All participants had neither history of neurological disease nor discomfort or injury in the upper extremities. Before participation in the study, all subjects gave written informed consent according to the protocol approved by the Penn State Hershey Institutional Review Board.
Apparatus
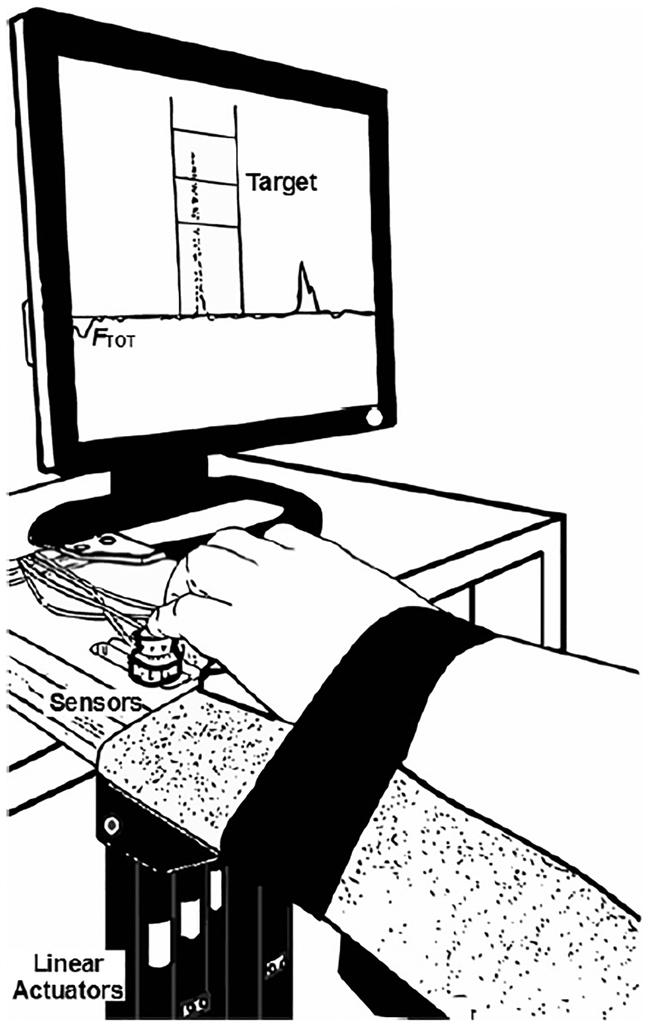
The “inverse piano” (IP) device (Martin et al. 2011), which allows forces exerted by the tip of the fingers to be recorded while the fingers are lifted and lowered, was used in this study. This device has four force sensors (Honeywell, Model 31, 25 LBS, Columbus, OH, USA) that were used to measure the downward pressing forces produced by the fingertips. The sensors were mounted on linear actuators (PS01–23×80; Linmot, Spruitenbach, Switzerland) placed within the slots of a steel frame (140 × 90 mm), 3-cm apart from each other in the medial-lateral direction. The actuators were controlled by a four-channel servo drive (Linmot E400-AT), which allowed each sensor to be lifted and lowered along its vertical axis. The sensor position could be adjusted in the anterior-posterior direction to match individual finger anatomy. The top surface of each sensor was covered with sandpaper (100-grit) to increase friction. A Velcro strap was placed slightly above the wrist joint of the participant to restrain the forearm on a board and avoid movement of the forearm during the tasks. Figure 1 shows a schematic representation of the experimental setup.
Figure 1:
An illustration of the experimental setup with finger position on the force sensors and linear actuators used to generate the “inverse piano” perturbations.
Subjects sat with their forearm in the sagittal plane, parallel to the ground, and placed each finger of a hand on a corresponding force sensor of the IP device (Figure 1A). A 19” monitor, positioned approximately 0.6 m from the subject’s face, provided visual feedback on the total downward force (FTOT) produced by the fingers. Sensor positions were adjusted to allow for each finger to be curved slightly when the fingertips were placed on the sensor, making the hand to look like a dome. A customized LabVIEW routine (2014 version, National Instruments, Austin, TX, USA) was used to present visual feedback, control the inverse piano, and acquire the force data. The force data were sampled at 200 Hz with a 16-bit resolution (NI PCI-6225, National Instruments) using a desktop computer and stored for offline data analyses.
Procedures
The participants performed three unimanual four-finger pressing tasks. Both hands were tested in a counterbalanced way, with half of the participants starting with the right hand and the other half starting with the left hand. The tasks were the maximal voluntary contraction (MVC), the accurate constant FTOT production, and the accurate FTOT production with visual feedback removal. In the MVC task, participants were asked to press with the four fingertips as hard as they could to reach maximal FTOT within 3–4 s until a command “relax” was given by the experimenter; FTOT was displayed on the monitor. Two MVC trials were performed with a 30-s interval in-between. The trial with the highest FTOT value was used to set the target force levels for the subsequent tasks.
Immediately after the MVC task, participants performed the accurate constant FTOT production task. In this task, the participants were asked to accurately produce a constant FTOT level set at 10% of FTOT MVC by pressing naturally with all four fingers to match a red horizontal target line shown on the monitor. The actual FTOT was shown on the monitor as a black, left-to-right running line. Two vertical lines were shown on the screen corresponding to 6 s and 10 s from the trial initiation. Three horizontal lines were shown in-between the vertical lines showing a 25%±5% of the FTOT MVC target window. The instruction was to press on the sensors with all four fingers and match FTOT with the initial target line (10% MVC) as accurately as possible. Once the line representing FTOT crossed the first vertical line, the subjects were instructed to produce a rapid force pulse into the target at a self-selected time within the next 4 s. The instruction emphasized speed of the force pulse over accuracy.
After producing the force pulse, the participants were asked to “go back to the horizontal red target line” as fast as they could and continue matching this line with the FTOT cursor until the end of the trial. The participants knew that at some point during the rest of the trial their fingers would be lifted by 1 cm over 0.5 s (2 cm/s) and immediately lowered over the same distance and time. The external perturbation was applied at the 14th s from the beginning of the trial. The subjects were asked not to try to predict when the perturbation would happen and not react to the perturbations (i.e., not to correct the force changes caused by the finger motion). This instruction was effective: Some participants in our pilot study were deviating from the force target during the practice trials in anticipation of the force-lifting episode, and after we told them to stop predicting the moment of the perturbation, they showed no visible change in performance prior to the finger lifting. As soon as the perturbation ended, the subjects continued matching the target line. There were two reasons to use the finger-lifting perturbation. First, we wanted to quantify indices of motor equivalence (ME and nME) over episodes with self-initiated “perturbation” (the force pulse) and external perturbation (finger lifting). Second, the finger-lifting perturbation was necessary to estimate outcome variables, RC and k, used in the {RC, k}-synergy analysis (see below).
The subjects performed six practice trials (recorded but not used in the data analysis) followed by 24 more trials. The 30 trials were performed in three blocks of 10 trials. Each trial lasted 20 s and 15 s of rest were given between trials. A more extended rest (≈ 1 min) was given between blocks of trials to prevent fatigue. Participants were encouraged to ask for extra rest as needed; fatigue was never reported.
The final task involved accurate force production with visual feedback removal. The participants performed four 30-s trials. In the first trial, they were asked to match a horizontal target line set at 25% of FTOT MVC as accurately as possible. This first trial had visual feedback at all times. In the next three trials, the participants knew that the visual feedback on FTOT would disappear 8 s after the trial initiation; they were instructed to continue producing the same level of FTOT until the end of the trial. The participants rested for 30 s after each trial and extra rest was provided when requested by the participants.
Data Analysis
Customized LabVIEW (2017 version) routines were used to analyze the data. The force signals were filtered with a 4th−order, zero-lag, Butterworth low-pass filter at 5 Hz.
Trial alignment
Analysis of performance in the accurate FTOT production task was performed separately for the two parts involving the force pulse and the finger lifting by the inverse piano device. For the force pulse, the trials were aligned by the time of force pulse initiation (t0), which was defined as the time when the rate of FTOT (dFTOT/dt) reached 5% of its peak value in that specific trial. The episodes with finger lifting by the inverse piano were aligned by the time of finger-lifting initiation, which was the same across all trials and subjects (t = 14 s).
Task performance
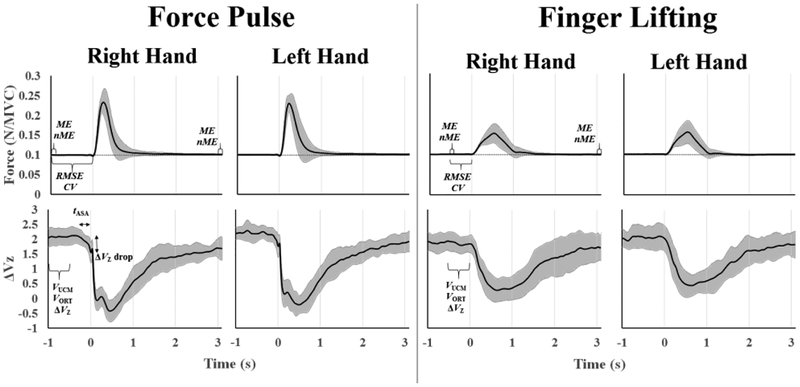
We calculated the coefficient of variation (CV) of FTOT and the root mean square error (RMSE) of FTOT with respect to the prescribed force target. Both CV and RMSE were calculated within the time interval {-1:0} s before the force pulse initiation and within the time interval {−0.5:0} s before the finger lifting by the IP. These time intervals are illustrated in Figure 2, which shows averaged across-subjects time series of total force and the synergy index (ΔVZ, see below). These time intervals were selected to include the time intervals in which the outcome variables in the UCM-based analysis of variance were computed for the two episodes (see below).
Figure 2:
Across-subjects averaged FTOT time-series (± 1 SD, in gray) normalized by the MVC are shown in the top panels, whereas across-subjects averaged ΔVZ time-series (± 1 SD, in gray) are depicted in the bottom panels. The right panels are from the force-pulse phase and the left panels are from the finger-lifting phase. The data for the right and left hands are presented. The time intervals for the calculation of the main outcome variables are illustrated in some plots.
UCM-based analysis of variance
The inter-trial variances within the UCM (VUCM) and within the space orthogonal to the UCM (VORT) were computed within the framework of the UCM hypothesis (Scholz and Schöner 1999). VUCM was computed as the variance that did not affect FTOT. The two variance components were computed for each time-sample using the null-space of the Jacobian matrix, J, as an approximation of the UCM in the space of finger forces. Additionally, an index of synergy was calculated: ΔV = (VUCM/3 - VORT/1)/(VTOT/4), where VTOT is the total variance and each variance index is normalized by the dimensionality of the corresponding space (Scholz et al. 2002). Because ΔV is not normally distributed, it was log-transformed taking into account its computational boundaries using a modified Fisher z-transformation, resulting in ΔVZ = 0.5×ln[(ΔV + 4)/(1.33 − ΔV)] (Park et al. 2010). Values of VUCM, VORT, and ΔVZ were averaged within each of the two steady-state time intervals. Prior to the force pulse, these variables were averaged from −1 s to −0.5 s from the pulse initiation. Prior to the finger lifiting, VUCM, VORT, and ΔVZ, were calculated between - 0.5 and 0 s from the finger lifting initiation (Figure 2). The duration of the time intervals for computing these outcome variables was the same in both episodes (i.e., 0.5 s). The difference in the timing of the two episodes was due to the phenomenon of ASA expected prior to the force-pulse but not prior to the finger-lifting episode. To avoid possible effects of ASAs on the computed steady-state values of VUCM, VORT, and ΔVZ, we shifted the time interval for the computation of these outcome variables by 0.5 s. This selection represented a compromise of getting this time interval close to the force-change episode and avoiding effects of ASAs.
The ASAs prior to the force pulse initiation were quantified using two indices, the time of ASA initiation (tASA) and the ΔVZ drop over the ASA, quantified as the difference between the average ΔVZ at steady-state and the ΔVZ at t0. The tASA was defined as the time when ΔVZ dropped below its average steady-state value by more than one standard deviation and stayed below that level until t0. A drop in ΔVZ starting prior to t0 was reflected in negative values of tASA.
Motor equivalence (ME) analysis
This analysis quantified the magnitude of motion in the finger force space in-between two time intervals in a single trial, within the UCM (motor equivalent, ME) and orthogonal to the UCM (non-motor equivalent, nME). For each trial, ME and nME indices were quantified for pairs of time intervals separated by the force pulse and by the finger lifting episode. For the analysis with the force pulse in-between the time intervals, the forces produced by each finger in a window of 100-ms before {−1:−0.9} s and after {3:3.1} s the pulse initiation (Figure 2), were averaged to calculate ME and nME. Similarly, for the analysis with the finger lifting in-between the time intervals, the ME and nME were calculated between the two 100-ms time intervals: {−0.5:−0.4} s before and after {3:3.1} s the finger lifting initiation (Figure 2). For each pair of measurements, the vectors of motion in the force space (ΔF) were projected onto the corresponding UCM and ORT spaces. The magnitudes of these two projections normalized by the square root of the space dimensionality were taken as ME and nME, respectively (for details see Mattos et al. 2015).
We observed in this data set that the within-trial variation of the contribution of each finger after the subject reached a stable FTOT was negligible. Therefore, the 100-ms interval has been considered sufficient to record a stable contribution of each finger to FTOT. The location of the intervals {-1:-0.9} and {-0.5:-0.4} before the force pulse and finger lifting, respectively, were selected based on the selected intervals for the UCM-based analysis of variance. We selected the first 100 ms of each of those time intervals. We also assumed (based on visual analysis of performance) that 3 s after the beginning of a perturbation was enough for the subjects to return to the level of performance seen prior to the perturbation.
For each subject, the ME and the nME values were averaged across trials and used for further statistical analysis.
{RC, k}-synergy analysis
This analysis quantified the stability of FTOT within the space of hypothetical control variables of the hand, RC (referent coordinate) and k (apparent stiffness) (Ambike et al. 2016a,b; Reschechtko and Latash 2017, de Freitas et al. 2018). {RC; k} pairs for each trial were calculated using the ascending phase of the finger lifting by the inverse piano. We excluded the first 50 and last 250 ms for each of the 500 ms ascending phases to remove data that did not include reflex-mediated changes in FTOT and to minimize chances of the hand response being affected by voluntary corrections. For each trial, a linear regression was run between the hand force and vertical coordinate. Only the trials with the correlation coefficient r ≥ 0.9 were accepted. For each accepted trial, k was computed as the slope and RC was computed as the X−axis intercept of the force-coordinate regression line.
Since the UCM in the two-dimensional {RC; k} space corresponding to a fixed FTOT value was hyperbolic, we could not use variance analysis. Hence, we employed the method of creating a surrogate data set using data randomization (Müller and Sternad 2003). First, the mean and standard deviation of the actual FTOT values across trials for each subject were calculated (FACT and SDACT). Further, a random permutation of RC and k values obtained from different trials was used to generate surrogate {RC; k} pairs. During this procedure, 1000 {RC; k} pairs were formed per subject. These data were used to compute values of FTOT and its standard deviation (FSUR and SDSUR). Finally, an index was calculated: RSD = SDSUR/SDACT. High RSD values indicate that the stability of FTOT is ensured by the co-variation of RC and k across trials. In addition, hyperbolic regressions were fitted to each participant’s {RC; k} data set and the coefficient of determination (R2) was computed. For further statistical analysis, R2 values were Fisher z-transformed.
Analysis of FTOT drift in trials with visual feedback removal
The FTOT drift after the visual feedback removal was quantified using two characteristics: The change in FTOT (ΔFTOT) and the coefficient (b) of the exponential equation fitting the FTOT time series after the visual feedback removal [FTOT(t)=aebt+c, where a, b, and c are constants, and t is time]. ΔFTOT was measured, in percent, as the drop in FTOT from the FTOT magnitude averaged over 1 s before the moment of the visual feedback removal and the averaged FTOT within the time interval {19; 20} s, after the visual feedback removal. The across-trials (n=3) averaged ΔFTOT, b, and Fisher z-transformed R2 (coefficient of determination of the exponential equation fitting) were used for statistical analyses.
Statistical Analyses
Statistical analyses were run in the IBM SPSS statistics package (version 25). Most of the data are presented in the text, Tables, and Figures as means ± standard errors. The normality of the data distributions was confirmed for all dependent variables with the Shapiro-Wilks test.
For the accurate FTOT production task, a two-way (Hand: left and right; Episode: force pulse and finger lifting) repeated measures (RM) multivariate analysis of variance (MANOVA) was performed for the two task performance variables (CV and RMSE). Two three-way (Hand, Episode, and Component: UCM and ORT) RM analyses of variance (ANOVA) were run on the indices of inter-trial variance (VUCM and VORT) and of motor equivalence (ME and nME). A two-way RM ANOVA was performed to test the effects of Hand and Episode on ΔVz. Differences between the left and right hands in tASA and ΔVZ drop were tested with paired Student’s t−tests. Two one-way RM MANOVAs were used to test the effect of Hand on RC, k, RSD, and z-transformed R2 for the hyperbolic fitting of RC and k.
For the accurate FTOT production with visual feedback removal task, a one-way RM MANOVA was performed to test the effect of hand on ΔFTOT, b, and z-transformed R2 for the exponential fitting of FTOT after feedback removal.
Hypotheses-2 and -3 were tested using Pearson’s correlation tests between the values of outcome variables in the right and left hands across subjects. The outcome variables (CV, RMSE, VUCM, VORT, ME, and nME) measured in the two steady-state episodes, before the force pulse and before the finger lifting by the inverse piano, were averaged between the two episodes, in order to reduce the number of correlation tests performed to avoid excessive corrections for multiple comparisons. Overall, alpha value was set at 0.05, but for the correlation analyses, it was corrected by the number of tests performed directly related to our two hypotheses using the Dunn-Sidak corrections. Therefore, the alpha value set for significant correlations was 0.0051 and the correspondent was r ≥ 0.7.
RESULTS
The right (dominant) hand was stronger across subjects compared to the left hand. The averaged MVC was 102.3 ± 8.89 N for the right hand and 94.4 ± 9.06 N for the left hand (t13 = 2.89; p < 0.05). There was also a significant strong correlation between the MVC magnitudes in the right and left hands across subjects (r12 = 0.95; p < 0.001).
Task performance
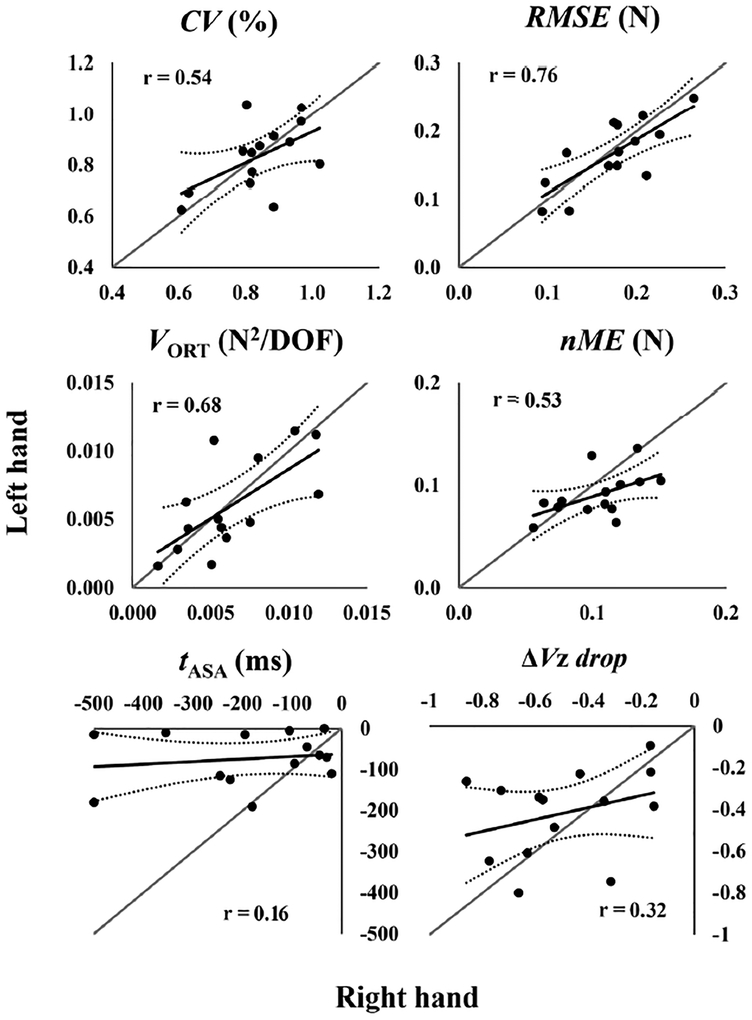
During accurate FTOT production trials with visual feedback, participants were able to keep a stable FTOT magnitude with CV ~1% and no significant difference between the two hands. There was also no significant difference in RMSE between the hands. These data are presented in Table 1. CV was larger before the force pulse than before finger lifting (effect of episode, F1,13 = 57.9, p<0.001), whereas there was no significant difference between the two episodes for RMSE. The correlation between the CV values (averaged across the two episodes) for the right and left hands was not significant after the alpha value correction (r12= 0.54). In contrast, individuals with higher RMSE for the right hand showed higher RMSE for the left hand (r12 = 0.76, p = 0.0016; for RMSE averaged across the two episodes, Figure 3).
Table 1:
Average magnitudes for the main outcome variables.
| Outcome Variable | Hand | Force pulse | Finger lifting | Relation to Performance Accuracy |
|---|---|---|---|---|
| CV (%) | Right | 0.95 (0.028) | 0.72 (0.045) | Explicit |
| Left | 0.98 (0.038) | 0.69 (0.045) | ||
| RMSE (N) | Right | 0.168 (0.014) | 0.178 (0.014) | Explicit |
| Left | 0.162 (0.014) | 0.171 (0.015) | ||
| VUCM (N2/DOF) | Right | 0.115 (0.017) | 0.114 (0.021) | Implicit |
| Left | 0.162 (0.034) | 0.135 (0.025) | ||
| VORT (N2/DOF) | Right | 5.23 (0.7) × 10−3 | 7.43 (1.06) × 10−3 | Explicit |
| Left | 5.11 (1.06) × 10−3 | 6.02 (0.88) × 10−3 | ||
| ΔVZ | Right | 2.07 (0.075) | 1.88 (0.068) | Implicit |
| Left | 2.21 (0.064) | 2.12 (0.085) | ||
| ME (N) | Right | 0.328 (0.031) | 0.327 (0.035) | Implicit |
| Left | 0.397 (0.058) | 0.319 (0.037) | ||
| nME (N) | Right | 0.111 (0.009) | 0.097 (0.009) | Explicit |
| Left | 0.088 (0.008) | 0.093 (0.007) | ||
| tASA (ms) | Right | −185.7 (44.1) | ------------ | __________ |
| Left | −72.6 (16.5) | ------------ | ||
| ΔVZ drop | Right | −0.495 (0.063) | ------------ | __________ |
| Left | −0.417 (0.056) | ------------ | ||
| RC (cm) | Right | ------------ | −1.4 (0.063) | Implicit |
| Left | ------------ | −1.34 (0.091) | ||
| k (N/cm) | Right | ------------ | 7.54 (0.61) | Implicit |
| Left | ------------ | 7.28 (0.72) | ||
| RSD | Right | ------------ | 14.74 (1.38) | Implicit |
| Left | ------------ | 13.08 (1.26) | ||
| Fisher-z R2 | Right | ------------ | 1.78 (0.081) | Implicit |
| Left | ------------ | 1.74 (0.064) |
Across-subject means (standard errors) are shown for the right and left hands. CV – coefficient of variation; RMSE – root mean square error; VUCM and VORT – across-trials variance components within the UCM and ORT spaces; ΔVZ – synergy index; ME and nME – motor equivalent and non-motor equivalent deviations; tASA and ΔVZ drop – the timing and magnitude of anticipatory synergy adjustments; RC – referent coordinate; k – apparent stiffness; RSD – index of synergy in the {RC; k} space; Fisher-z R2 – coefficient of determination in the hyperbolic regression. The last column shows whether an outcome variable was (Explicit) or was not (Implicit) directly related to accuracy of performance.
Figure 3:
Across-subjects scatter plots for variables related to accuracy of task performance (CV, RMSE, VORT, and nME) and to the anticipatory synergy adjustment (tASA and ΔVZ drop). Each CV, RMSE, VORT, and nME data point corresponds to the average value between the force-pulse and finger-lifting episodes. The tASA and ΔVZ drop were only measured before the force-pulse. The Y-axis shows the data for the left hand whereas the X-axis shows the data for the right hand. Identity lines, trend lines, and 95% confidence bands also are depicted.
UCM-based analysis of variance
The analysis of inter-trial variance during steady-states revealed, independent of hand, much higher VUCM compared to VORT (the difference was at least 10-fold, see Table 1). This result was confirmed by the significant effect of Component in the RM ANOVA (F1,13 = 31, p<0.001). As a result, the index of synergy, ΔVZ was consistently high (Table 1). The ΔVZ was higher before the pulse initiation than before the finger lifting, confirmed by a significant effect of Episode on ΔVZ (F1,13 = 8.22, p = 0.013). The difference in ΔVZ was due mainly to higher VUCM and lower VORT in the steady-state before the force pulse initiation than before the finger lifting (Episode × Component interaction close to significance, F1,13 = 4.37, p = 0.057).
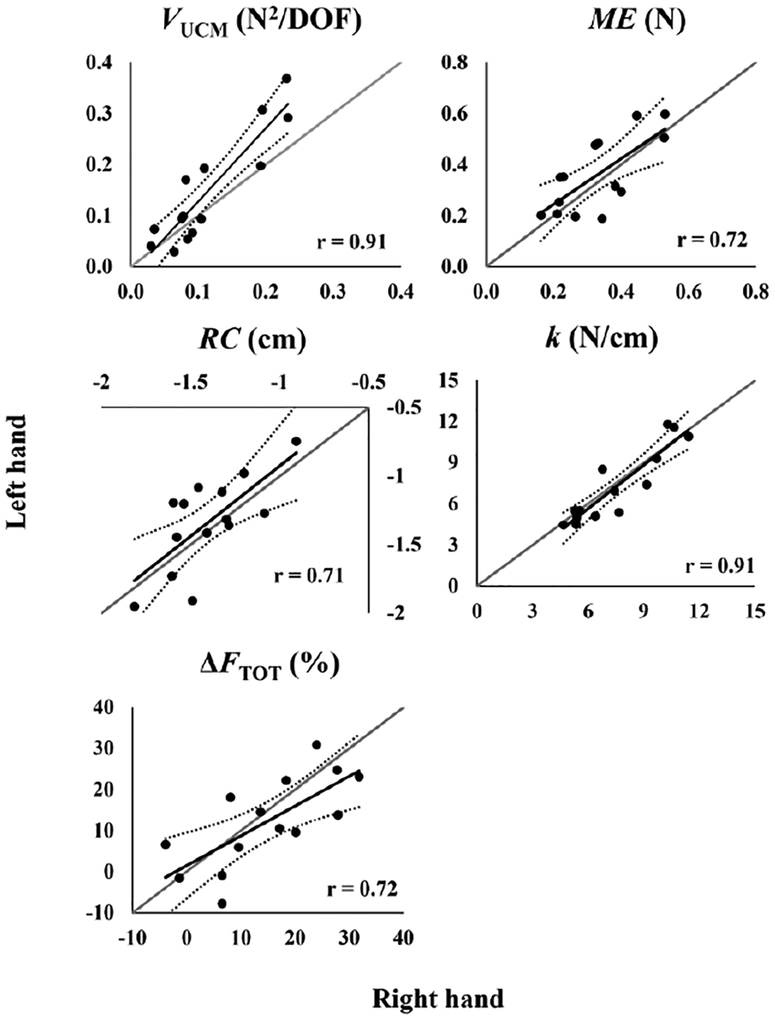
Whereas no significant difference between hands was revealed for VORT, VUCM was higher for the left hand when compared to the right hand (Hand × Component interaction: F1,13 = 5.46, p = 0.036). Consequently, ΔVZ tended to be higher for the left hand when compared to the right one (F1,13 = 4.1, p = 0.064). There was a strong positive correlation between the VUCM indices for the right and left hands (r12= 0.91, p < 0.001, Figure 4), while for VORT, the correlation between the two hands did not reach significance after alpha value correction (r12 = 0.68, Figure 3).
Figure 4:
Across-subjects scatter plots for variables not directly related to accuracy of task performance (VUCM, ME, RC, and k – the top four plots). The bottom plot shows the magnitude of force drop (ΔFTOT) in the task with visual feedback removal. The Y-axis shows the data for the left hand whereas the X-axis shows the data for the right hand. VUCM and ME values were averaged between the force-pulse and finger-lifting episodes. Identity lines, trend lines, and 95% confidence bands also are depicted.
Prior to the self-initiated force pulse, the subjects showed a drop in the ΔVZ index, which started significantly earlier in the right hand (about 200 ms before the pulse initiation time t0) compared to the left hand (about 100 ms before t0; effect of hand: t13 = 2.51, p = 0.026). No significant hand difference was observed for the ΔVZ drop magnitude. In addition, no correlations between hands were observed for tASA and ΔVZ drop (Figure 4, bottom panels).
Motor Equivalence Analysis
The changes in the finger forces between steady states separated by the force pulse and separated by the finger lifting episode were confined primarily to the UCM for FTOT. As a result, ME was about three times larger than nME (effects of Component: F1, 13 = 70.7, p<0.001, Table 1). No significant differences between the hands were found for ME and nME. Also, only ME for the left hand was higher in the steady-state before the pulse initiation than before the fingers lifting resulting in a three-way interaction (Hand × Episode × Component, F1,13 = 11.13, p < 0.01). While there was no significant between-hand correlation for nME (Figure 3), subjects with high ME in the right hand showed high ME in the left hand (r12 = 0.72, p = 0.004, Figure 4).
{RC, k} Synergy Analysis
For all participants, RC and k values from individual trials were aligned closely with a hyperbolic line corresponding to the prescribed FTOT value. The coefficient of determination (R2) for the hyperbolic regressions ranged from 0.83 to 0.97 for the right hand and from 0.85 to 0.97 for the left hand. No significant difference between the hands was found. There were also no significant differences between the left and right hands in the RC and k values, or in the index RSD. The RSD values were much higher than 1, indicating the presence of a strong across-trial covariation between RC and k stabilizing FTOT. There were significant positive between-hand correlations for RC and k (Figure 4).
Force drifts after visual feedback removal
Overall, the subjects showed consistent FTOT drifts toward smaller magnitudes after the removal of visual feedback, but no significant difference between the hands was observed for ΔFTOT. ΔFTOT was 14.7 ± 3% and 12.1 ± 3% for the right and left hands, respectively. Exponential fitting of the force drift curves showed similar exponent coefficients (b) for the two hands: ‒8.7±1.6 × 10−3 for the right hand and −6±1.6 × 10−3 for the left hand. There were moderate correlations between the right and left hands in ΔFTOT, b, and z-transformed R2, but only the correlation for ΔFTOT reached statistical significance after corrections for multiple comparisons (Figure 4).
DISCUSSION
Our main finding is the significant across-subjects correlation between indices related to stability of force production in the right and left hands. It is important to note that indices that had no direct relation to performance in the explicit task and received no visual feedback – implicit outcome variables – showed such correlations; these include VUCM and ME, indices quantified within the framework of the UCM hypothesis (Scholz and Schöner 1999). The same framework was used to quantify variables that were related to performance accuracy (explicit outcome variables), such as VORT and nME; these variables showed no significant correlations between the two hands. Overall, these observations confirm our specific hypotheses formulated in the Introduction.
We also observed differences between the right and left hands in some of the outcome variables. These differences confirm earlier observations (e.g., Zhang et al. 2006; Park et al. 2012; Jo et al. 2015) and are in line with the dynamic dominance hypothesis (Sainburg 2002) that postulates specialization of the two hemispheres for the control of fast (dominant) and steady-state (non-dominant) actions. For the first time, we observed significant hand differences in the index of feed-forward preparation to a quick action, tASA.
Hand dominance and stability of action
When a right-handed person uses a hammer to put a nail into a board, the right hand moves the hammer and the left hand holds the nail. If this person is asked to switch hands, he/she will feel equally uncomfortable moving the hammer with the left hand and holding the nail with the right hand. Indeed, if this task is performed by two right-handed persons, the one holding the nail would prefer to do this with the left hand. This is an illustration of the basic idea that humans do not have a “good hand” and a “bad hand” but rather two good hands that specialize in different aspects of everyday tasks. This idea has been formalized as the dynamic dominance hypothesis reflecting hemispheric specialization of the neural control of movements (Sainburg 2002).
Earlier studies on multi-finger force production reported results compatible with the dynamic dominance hypothesis. In particular, during quick force pulse production, there typically is a drop in the synergy index reflecting the relative amounts of VUCM and VORT in total inter-trial variance (Latash et al. 2002; Olafsdottir et al. 2005; Shim et al. 2005). This drop was shown to be smaller in the right hand compared to the left hand of right-handed subjects (Zhang et al. 2006). In the current study, for the first time we observed significant differences between the two hands in the duration of anticipatory synergy adjustments (ASAs). ASAs were longer in the right hand demonstrating that this hand has an advantage not only in its ability to avoid excessive destabilization of action during the force pulse (documented by Zhang et al. 2006), but also in the ability to prepare for the pulse. Note that ASAs are functionally important: they allow the controller to reduce the degree of stabilization of a variable in preparation to a quick action. ASA reduction is typical of some neurological disorders (reviewed in Latash and Huang 2015), in particular those associated with difficulties with action initiation such as Parkinson’s disease and cortical stroke.
Other studies documented the advantage of the non-dominant hand in ensuring high stability of action in steady-state tasks (Park et al. 2012; Jo et al. 2015). Our findings corroborate those results by showing higher VUCM magnitudes in the left hand. Overall, our data are consistent with the dynamic dominance hypothesis, although some findings did not reach significance (e.g., ΔVZ). It is possible that the protocol of the study combining accurate force production, quick force pulse production, and non-interference during the inverse piano episode was challenging for the subjects and led to excessive variability.
Relevance of variables along “irrelevant directions”
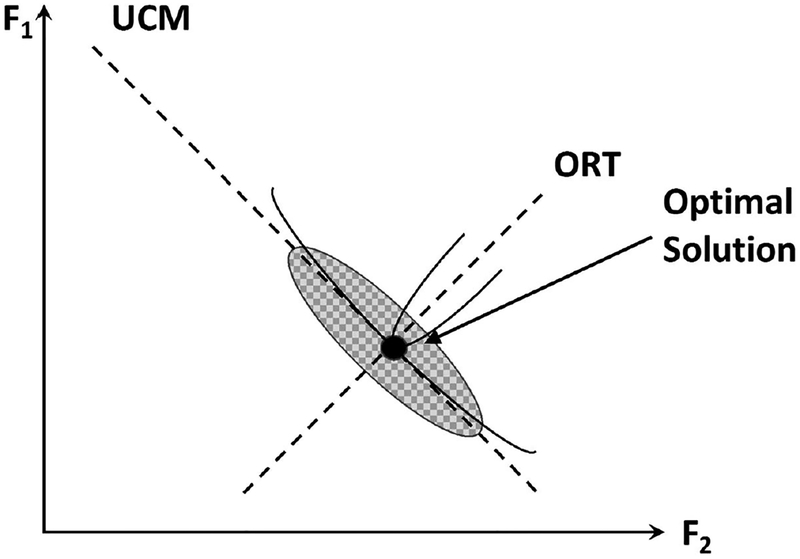
Studies of task-specific action stability have been based on the UCM hypothesis (Schöner 1995; Scholz and Schöner 1999), which analyzes inter-trial variance within two spaces, the UCM (where the salient performance variable does not change) and within ORT (where it does change). Both spaces are parts of the abundant space of elemental variables used in the analysis. In our study, we explored the stability of total force, FTOT, production using two sets of elemental variables, forces produced by the individual fingers and hypothetical control variables formulated within the theory of control with referent coordinates (see Table 1; Latash 2010; Feldman 2015). By definition, distributions and motion within the UCM do not affect the salient performance variable (FTOT). However, not all possible parts of the UCM are used during natural performance suggesting that this space is not truly “uncontrolled” but that the elements are given larger freedom to travel along that space compared to the ORT. In other words, ORT shows high stability, whereas UCM shows low (but not zero!) stability. As a result, inter-trial variance within UCM is higher than within ORT but still limited (see VUCM and VORT in Table 1). This idea is illustrated in Figure 5, which uses the task of total force production with two fingers only. It shows a typical ellipsoid of data points elongated along the UCM (dashed line with negative slope) and demonstrates schematically the potential fields aligned about the center of the data point distribution (solid lines). The steep potential well along ORT corresponds to higher stability, whereas the shallow potential well along UCM illustrates lower stability.
Figure 5:
A typical ellipsoid of data points elongated along the UCM (dashed line with negative slope) in a task of two-finger accurate total force production. The center of the ellipsoid is assumed to reflect an optimal solution. The drawing shows schematically the potential fields centered about the center of the data point distribution (solid lines). The steep potential well along the orthogonal to the UCM direction (ORT) corresponds to higher stability, while the shallow potential well along the UCM illustrates lower stability.
Stability properties along the UCM may look irrelevant to performance, but in fact, the lower stability within the UCM is an important feature reflecting performance stability. If a perturbation acts on a multi-element system, its effects are going to be mainly channeled into the UCM if stability within the UCM is lower than within ORT (reflected in high VUCM values in our study, VUCM ≫ VORT, see Table 1). If, for the same VORT values, VUCM < VORT, stability within the UCM becomes higher than within ORT, and the same perturbation is expected to produce much larger motion within ORT, i.e. stability of performance becomes lower. The same logic works if there is no perturbation but spontaneous changes in magnitudes of the elements, e.g., due to the signal-dependent noise (Harris and Wolpert 1998).
Consider the following example. Imagine that you walk along the hallway with a cup of coffee in your hand. Not spilling the coffee requires that the cup orientation is close to vertical at all times. Note that the cup orientation is perturbed continuously by the arm and whole-body movement, and by the not perfectly predictable forces when your feet hit the ground. This makes the dynamic stability of the cup orientation in space paramount for the task. Note that, sometimes, unexpected perturbations happen, e.g., someone or something makes contact with your elbow. If the body configuration is unstable in certain directions (along the UCM for the vertical cup orientation), the perturbation primarily will produce motor equivalent motion along the UCM, and the cup contents may not be spilled. Therefore, having low stability along the UCM is functionally important and may be reflected in such indices as VUCM and ME.
In fact, most of our everyday actions are performed in conditions of unexpected changes in both intrinsic body states and external forces. It makes stability arguably the most crucial feature of functional actions. Note that patients with various neurological disorders show significant impairments of action stability (Latash and Huang 2015). For example, this is true for patients with Parkinson’s disease at very early stages of the disorder when they are tested on optimal medication (Park et al. 2012) and even for healthy persons who are at high risk for basal ganglia-related disorders, such as professional welders (Lewis et al. 2016). Studies in patients with multiple sclerosis have shown a significant reduction in the synergy index (ΔV), which was due to a nearly two-fold drop in the seemingly irrelevant component of inter-trial variance (VUCM), whereas the component that affected performance (VORT), showed no difference between the patients and control subjects (Jo et al. 2017).
Trade-off between stability and optimality
Two aspects of the problem of motor redundancy (Bernstein 1967) have been explored in recent years. The first one is how to select a solution from an infinite set afforded by the larger number of elements contributing to typical tasks compared to the number of constraints associated with the tasks. One of the commonly invoked ideas is that of optimality. According to this approach, the controller selects a solution that minimizes (less commonly, maximizes) a specific cost function (reviewed in Prilutsky and Zatsiorsky 2002). Note that we imply in this context finding an optimal solution for a steady-state task, not optimal feedback control schemes that have gained prominence lately (Todorov and Jordan 2002; Todorov 2004; Diedrichsen et al. 2010). For example, with respect to the aforementioned task of constant force production with two fingers (Figure 5), a cost function can define a specific sharing pattern of FTOT between the fingers. The second approach is based on the principle of abundance (Gelfand and Latash 1998; Latash 2012, 2017). It assumes that no unique solution is selected but that elements are organized to ensure high stability of their combined outcome in some directions (along ORT in Figure 5) compared to other directions (UCM in Figure 5).
The two approaches seem incompatible: A single solution vs. a family of solutions. One of the approaches that reconciles the idea of optimization with the experimentally observed clouds of data points is optimal feedback control (Todorov and Jordan 2002, Diedrichsen et al. 2010). This approach assumes that the cost function depends on the actual feedback and, as a result, optimal solutions vary across trials depending on the actual trajectories of the system. The other approach suggests that an optimization criterion is used to define coordinates of the centers of data clouds (shown by the black dot in Figure 5), while stability properties are reflected in the shapes and sizes of the data clouds (Park et al. 2010). According to this idea, high VUCM is a sign of high selective stability (along ORT) and, at the same time, a sign of large violations of the optimality criterion (see VUCM ≫ VORT in Table 1). In contrast, a very small VUCM, smaller than VORT, suggests using a stereotypical set of solutions that may always be very close to optimal but does not ensure the stability of the salient performance variable if an external perturbation happens. We will refer to this as a stability-optimality (STOP) trade-off: One cannot be optimal and stable at the same time.
Personal preferences as reflected in the stability-optimality (STOP) trade-off
It is common knowledge that people differ from each other with respect to their abilities, strengths, and weaknesses. In particular, some people are clumsy, whereas others are better coordinated. This is true for the general population and becomes striking when one considers patients with an impaired motor function that may have implications for treatment strategies (Latash and Anson 1996). Recognition of this fact has led to studies of the effects of personality traits on the indices of motor performance correlated with neurophysiological findings (Picerni et al. 2013; Petrosini et al. 2015; Zaback et al. 2015; Cheng et al. 2017).
Whereas many earlier studies have emphasized differences between the dominant and non-dominant hands (reviewed in Sainburg 2002, 2005), similarities between the two hands that distinguish among individuals have received less attention. Some of these similarities are obvious. For instance, stronger people are likely to show higher strength in both hands (confirmed by the MVC data in our study). Between-hand correlations have been reported and analyzed recently in studies of kinematic variability correlated with the variability of ƒMRI signals in the posterior parietal cortex (Haar et al. 2017a,b).
In our study, we demonstrated for the first time significant correlations between the hands primarily for outcome variables that had no effect on task performance, such as VUCM and ME, whereas no similar correlations were found for variables related to the accuracy of performance, such as VORT and nME. The only variable reflecting accuracy of task performance that showed a significant correlation between the hands was RMSE. This could be related to the fact that both hands of stronger participants performed at higher absolute force magnitudes (the tasks were matched by a percentage of MVC) and, hence, were expected to show higher absolute force variability due to the signal-dependent noise (Newell and Carlton 1993; Harris and Wolpert 1998).
Our findings show that subjects differed in the VUCM and ME magnitudes and these differences were consistent across hands. Note that large VUCM (and ME) reflects more stable performance that also shows larger deviations from the center of data distributions, presumably defined by an optimization principle. In contrast, a small VUCM value suggests more optimal and less stable set of solutions. So, the differences among the subjects, correlated between the two hands, could be viewed as reflections of different choices within the STOP trade-off: Some persons preferred to facilitate stable but less optimal (more sloppy) performance, while others consistently preferred to facilitate optimal performance that could be less stable. Given that the conditions of performance were consistent and predictable, the latter strategy may look more reasonable. On the other hand, in everyday life, external conditions are frequently unpredictable favoring the former strategy.
Along similar lines, the exact values of the RC and k variables by themselves did not matter as long as they were confined to the hyperbolic UCM compatible with the required FTOT. Subjects showed correlated magnitudes of RC and k across the two hands. These findings lead to two conclusions: Subjects differed in the average across trials values of RC and k and these differences were consistent between the two hands.
Note that some outcome variables could correlate between the two hands because of factors common for the hands (e.g., the aforementioned higher MVC magnitudes), which might be unrelated to issues of motor coordination. In particular, our task was set in percent to the MVC force values. As a result, stronger subjects performed tasks that required larger finger forces. Given the dependence of force variability on force magnitude (reviewed in Newell and Carlton 1993; Sosnoff and Newell 2006), higher finger force variance could be expected in both hands of stronger subjects. This could lead to correlated across-subjects magnitudes of the variance components within both UCM and ORT. Note, however, that we observed strong correlations for only one component, namely VUCM (and ME) while the correlations for the other component, VORT (and nME), were weaker or non-significant. In addition, we observed a strong between-hands correlation for the force drift index, ΔF, expressed in percent of MVC force. Overall, the mentioned factor could affect some of our results but we see its effects as modest and not defining the general pattern of the main findings.
Taken together, our findings may reflect the individualized choice by each subject in reaching explicit motor goals set by the task. The abundance of the four-finger set in FTOT producing tasks affords different strategies corresponding to different coordinates along the stability-optimality axis. Searching for neurophysiological mechanisms that encode specific solutions preferred by individual subjects (individual traits) would be extremely interesting and might lead to a better understanding of the origins of the STOP trade-off.
An earlier study (Cesqui et al. 2012) explored the kinematics of ball catching and reported inter-individual variability in several kinematic parameters, such as wrist trajectory, wrist velocity profile, timing and spatial distribution of the impact point, upper limb posture, trunk motion, and submovement decomposition. Individual idiosyncratic behaviors were consistent across different ball flight time conditions and across two experimental sessions carried out at one-year intervals. These observations are consistent with our general idea that individuals differ in their preferred solutions for tasks involving abundant sets of effectors.
Concluding comments
We view our results as the first step toward formulating and quantifying personal coordination profiles. As with any first study of its kind, our experiment has a number of drawbacks. The most important ones are limiting the study to a single task and a single subject group. This limitation is relatively easy to overcome in future studies. Our protocol may have been too challenging, leading to excessive variability. On the other hand, it is possible that the relatively large across-subject variability was essential in bringing about quantifiable differences in the implicit outcome variables, such as VUCM, ME, RC, and k that reflect stability of the hand action.
ACKNOWLEDGMENTS
The study was in part supported by NIH grants NS082151 and NS095873.
REFERENCES
- Ambike S, Zatsiorsky VM, Latash ML (2015) Processes underlying unintentional finger force changes in the absence of visual feedback. Exp Brain Res 233: 711–721. [DOI] [PubMed] [Google Scholar]
- Ambike S, Mattos D, Zatsiorsky VM, Latash ML (2016a) Synergies in the space of control variables within the equilibrium-point hypothesis. Neurosci 315: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambike S, Mattos D, Zatsiorsky VM, Latash ML (2016b) Unsteady steady-states: Central causes of unintentional force drift. Exp Brain Res 234: 3597–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesqui B, d’Avella A, Portone A, Lacquaniti F (2012). Catching a ball at the right time and place: individual factors matter. PLoS one, 7(2), e31770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein NA (1967) The Co-ordination and Regulation of Movements. Pergamon Press, Oxford [Google Scholar]
- Cheng M, Kato M, Tseng CH (2017) Gender and autistic traits modulate implicit motor synchrony. PLoS One. 12(9):e0184083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curioni A, Minio-Paluello I, Sacheli LM, Candidi M, Aglioti SM (2017) Autistic traits affect interpersonal motor coordination by modulating strategic use of role-based behavior. Mol Autism. 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Freitas PB, Freitas SMSF, Lewis MM, Huang X, Latash ML (2018) Stability of steady hand force production explored across spaces and methods of analysis. Exp Brain Res 236: 1545–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R, Ivry RB (2010) The coordination of movement: optimal feedback control and beyond. Trends Cogn Sci 14: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AG (2015) Referent control of action and perception: Challenging conventional theories in behavioral science. 244 p. Springer, NY. [Google Scholar]
- Gelfand IM, Latash ML (1998) On the problem of adequate language in movement science. Motor Control 2: 306–313. [DOI] [PubMed] [Google Scholar]
- Haar S, Dinstein I, Shelef I, Donchin O (2017a) Effector-invariant movement encoding in the human motor system. J Neurosci 37: 9054–9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haar S, Donchin O, Dinstein I (2017b) Individual movement variability magnitudes are explained by cortical neural variability. J Neurosci 37: 9076–9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Best CS, Moffat VJ, Spencer MD, Philip RC, Power MJ, Johnstone EC (2008) Autistic traits and cognitive performance in young people with mild intellectual impairment. J Autism Dev Disord. 38: 1241–1249. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM (1998) Signal-dependent noise determines motor planning. Nature 394: 780–784. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Park J, Lewis MM, Huang X, Latash ML (2015) Prehension synergies and hand function in early-stage Parkinson’s disease. Exp Brain Res 233: 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Lucassen E, Huang X, Latash ML (2017) Changes in multi-digit synergies and their feed-forward adjustments in multiple sclerosis. J Mot Behav 49: 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML (2010) Motor synergies and the equilibrium-point hypothesis. Motor Control 14: 294–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML (2012) The bliss (not the problem) of motor abundance (not redundancy). Exp Brain Res 217: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML (2016) Towards physics of neural processes and behavior. Neurosci Biobehav Rev 69: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML (2017) Biological movement and laws of physics. Motor Control 21: 327–344. [DOI] [PubMed] [Google Scholar]
- Latash ML, Anson JG (1996) What are normal movements in atypical populations? Behav Brain Sci 19: 55–106. [Google Scholar]
- Latash ML, Huang X (2015) Neural control of movement stability: Lessons from studies of neurological patients. Neurosci 301: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Scholz JF, Danion F, Schöner G (2002) Finger coordination during discrete and oscillatory force production tasks. Exp Brain Res 146: 412–432. [DOI] [PubMed] [Google Scholar]
- Lewis MM, Lee E-Y, Jo HJ, Park J, Latash ML, Huang X (2016) Synergy as a new and sensitive marker of basal ganglia dysfunction: A study of asymptomatic welders. Neurotoxicology 56: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingam R, Golding J, Jongmans MJ, Hunt LP, Ellis M, Emond A (2010) The association between developmental coordination disorder and other developmental traits. Pediatrics 126(5):e1109–18. [DOI] [PubMed] [Google Scholar]
- Martin JR, Budgeon MK, Zatsiorsky VM, Latash ML (2011) Stabilization of the total force in multi-finger pressing tasks studied with the ‘inverse piano’ technique. Hum Move Sci 30: 446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos D, Latash ML, Park E, Kuhl J, Scholz JP (2011) Unpredictable elbow joint perturbation during reaching results in multijoint motor equivalence. J Neurophysiol 106: 1424–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattos D, Schöner G, Zatsiorsky VM, Latash ML (2015) Motor equivalence during accurate multi-finger force production. Exp Brain Res 233: 487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Saygin AP (2013) Individual differences in the perception of biological motion: links to social cognition and motor imagery. Cognition 128: 140–148. [DOI] [PubMed] [Google Scholar]
- Müller H, Sternad D (2003) A randomization method for the calculation of covariation in multiple nonlinear relations: illustrated with the example of goal-directed movements. Biol Cybern 89: 22–33. [DOI] [PubMed] [Google Scholar]
- Newell KM, Carlton LG (1993) Force variability in isometric responses. J Exp Psychol: Human Percept Perform 14: 37–44. [PubMed] [Google Scholar]
- Olafsdottir H, Yoshida N, Zatsiorsky VM, Latash ML (2005) Anticipatory covariation of finger forces during self-paced and reaction time force production. Neurosci Lett 381: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zatsiorsky VM, Latash ML (2010) Optimality vs. variability: An example of multi-finger redundant tasks. Exp Brain Res 207: 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wu Y-H, Lewis MM, Huang X, Latash ML (2012) Changes in multi-finger interaction and coordination in Parkinson’s disease. J Neurophysiol 108: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa B, O’Shea DJ, Zatsiorsky VM, Latash ML (2016) On the nature of unintentional action: A study of force/moment drifts during multi-finger tasks. J Neurophysiol 116: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosini L, Cutuli D, Picerni E, Laricchiuta D (2015) Cerebellum and personality traits. Cerebellum 14:43–6. [DOI] [PubMed] [Google Scholar]
- Picerni E, Petrosini L, Piras F, Laricchiuta D, Cutuli D, Chiapponi C, Fagioli S, Girardi P, Caltagirone C, Spalletta G (2013) New evidence for the cerebellar involvement in personality traits. Front Behav Neurosci. 7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prilutsky BI, Zatsiorsky VM (2002) Optimization-based models of muscle coordination. Exer Sport Sci Rev 30: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschechtko S, Latash ML (2017) Stability of hand force production: I. Hand level control variables and multi-finger synergies. J Neurophysiol 118: 3152–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschechtko S, Latash ML (2018) Stability of hand force production: II. Ascending and descending synergies. J Neurophysiol doi: 10.1152/jn.00045.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschechtko S, Zatsiorsky VM, Latash ML (2017) The synergic control of multi-finger force production: Stability of explicit and implicit task components. Exp Brain Res 235: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL (2002) Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142: 241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL (2005) Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev 33:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JP, Danion F, Latash ML, Schöner G (2002) Understanding finger coordination through analysis of the structure of force variability. Biol Cybern 86: 29–39. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schöner G (1999). The uncontrolled manifold concept: Identifying control variables for a functional task. Exp Brain Res 126, 289–306. [DOI] [PubMed] [Google Scholar]
- Schöner G (1995) Recent developments and problems in human movement science and their conceptual implications. Ecol Psychol 8: 291–314 [Google Scholar]
- Shim JK, Olafsdottir H, Zatsiorsky VM, Latash ML (2005) The emergence and disappearance of multi-digit synergies during force production tasks. Exp Brain Res 164: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnoff JJ, Newell KM (2006) The generalization of perceptual-motor intra-individual variability in young and old adults. J Gerontol B Psychol Sci Soc Sci 6: P304–P310. [DOI] [PubMed] [Google Scholar]
- Todorov E (2004). Optimality principles in sensorimotor control. Nat Neurosci 7: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Jordan MI (2002) Optimal feedback control as a theory of motor coordination. Nat Neurosci 5: 1226–1235. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Russell DM. Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res 145:275–285, 2002. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL (2007) The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp Brain Res 178: 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaback M, Cleworth TW, Carpenter MG, Adkin AL (2015) Personality traits and individual differences predict threat-induced changes in postural control. Hum Mov Sci 40:393–409. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sainburg RL, Zatsiorsky VM, Latash ML (2006) Hand dominance and multi-finger synergies. Neurosci Lett 409: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]