Abstract
StartReact is the ability of the startle reflex to involuntarily release a planned movement in the presence of a loud acoustic stimulus resulting in muscle activity patterns and kinematics that are tightly regulated and scaled with the intended action. Previous studies demonstrated startReact’s robustness during simple single-joint reaching tasks and found no difference between startReact and voluntary movements for movement kinematics and muscle activation patterns. However, startReact has not been evaluated during multi-joint reaching movements with multiple degrees of freedom. It is unclear if startReact would evoke accurate and precise multi-joint reaching movements in an unrestricted workspace. Furthermore, if tested more rigorously, multi-joint startReact movement kinematics and muscle activation patterns might not be truly equivalent despite showing no difference through traditional ANOVAs.
A previous study found multi-joint startReact was possible during unrestricted elbow and shoulder movement when reaching to a forward target. Therefore, we hypothesized that startReact would evoke similar multi-joint reaching movements for movement accuracy and muscle activation patterns when compared to voluntary movements in a multi-directional workspace. Expanding upon the previous study, our study uses a larger workspace and fully evaluates movement kinematics and muscle activations patterns. Results confirmed our hypothesis and found startReact movements were readily evoked in all directions. StartReact responses presented stereotypically earlier muscle activation, but the relative timing of agonist/antagonist firing pairs between startReact and voluntary movements remained similar. Results demonstrate that startReact is robustly present and equivalent in multi-joint reaching tasks and has potential clinical use for evaluating healthy and impaired movement.
Keywords: startReact, startle, multi-joint, two-dimensional, reaching, stroke
Introduction
The ability of the startle reflex to involuntarily release a planned movement in humans has received much attention since its discovery (Valls-Solé et al. 1999). While the classic startle response results in brief, synchronous activity in the body, startReact responses are more sophisticated and release muscle activity patterns and kinematics that are tightly regulated and scaled with the intended action (Valls-Solé et al. 1999; Carlsen et al. 2003, 2004a; Rothwell 2006). Because of these characteristics, the startReact phenomenon has been utilized to investigate movements extensively in both healthy and impaired populations (Carlsen et al. 2003, 2004a, b, 2008, 2011, 2012, Maslovat et al. 2009, 2011; Honeycutt and Perreault 2012; MacKinnon et al. 2013; Fisher et al. 2013; Nonnekes et al. 2014, 2015; Tresch et al. 2014; Honeycutt et al. 2017, 2015; Baker and Perez 2017).
One of the most provocative uses of startReact has been in clinical populations. startReact enhances movement in individuals following a stroke (Honeycutt and Perreault 2012; Honeycutt et al. 2015), spinal cord injury (Baker and Perez 2017), or hereditary spastic paraplegia (Fisher et al. 2013; Nonnekes et al. 2014). Additionally, it has been used to distinguish freezing of gait in the Parkinson’s population (Nonnekes et al. 2015). These studies highlight the unique potential of startReact as a clinical tool across multiple populations for enhancing movement and inaccessible muscle activation. However, most reports to date have examined single joint tasks (e.g. wrist extension) where all other joints are immobilized; thus startReact has not been evaluated during functional reaching movements requiring control of more than one joint (Carlsen et al. 2004a; Maslovat et al. 2009, 2011, 2012a, b; Honeycutt and Perreault 2012). Though previous work has found unrestricted startReact to be possible for elbow extension (Castellote and Valls-Sole 2015), the analysis focused primarily on muscle activation patterns without full evaluation of movement kinematics. Further analysis of both muscle activation patterns and movement kinematics during more functionally relevant and unrestricted movement is needed before startReact can be used more rigorously as an effective clinical tool.
While previous studies have demonstrated the robustness of startReact during these simple single-joint tasks, it is unclear if startReact would evoke accurate and precise multi-joint reaching movements in an unrestricted environment. In prior studies, muscle activity patterns and kinematics are not different; however, startReact movements are often reported to be less precise (increased variability of endpoint target) than voluntary movements (Carlsen et al. 2004a, b). Results show that while startReact movements are not different in position accuracy when compared to voluntary movements, they are more variable even during simple, single-joint tasks. The variability in movement kinematics for single joint tasks could be compounded during unrestricted, multi-joint movements leading to deficits in accuracy along with precision. Finally, previous work has used traditional statistical models (ttest, ANOVA) to test for differences not equivalence. These traditional testing methods have declared movement kinematics and muscle activation patterns between startReact and voluntary movements to be “not different”; however, the lack of difference between startReact and voluntary movements opens the question: could movements be similar if tested for equivalence using appropriate statistical testing? The objective of this study was to evaluate the ability of startReact to evoke unrestricted, multi-joint movements during two-dimensional reaching tasks that are equivalent to voluntary movements in all metrics except onset latency. Based on previous work showing that startReact was present during multi-joint reaching in a single direction (Wright et al. 2015), we hypothesized that startReact would evoke similar multi-joint reaching movements when compared to voluntary movements. We expand upon Wright, et al., by more extensively quantifying multi-joint reaching characteristics including movement accuracy and muscle activation patterns within a larger workspace. Furthermore, we use statistical tests of equivalence. Confirmation of our hypothesis would indicate that startReact is accessible during functionally relevant reaching movements and make the use of startReact as a clinical tool a more realistic implementation.
Methods and Materials
Subjects
Data was collected from 4 male and 6 female subjects (age: 20.57 ± 0.87). Subjects were required to have no prior head or neck injuries, no neurological disease or injury, and to be right-handed. Arizona State University’s Institutional Review Board (IRB) approved and oversaw all protocols (STUDY00002440). Risks and procedures of the study were provided to the subject and written consent was obtained prior to the experiment.
Equipment
Ag/Cl surface electrodes [MVAP Medical Supplies, Newbury Park, CA] were used to record activity from the brachioradialis (BR), biceps (BIC), triceps lateral head (TRI), pectoralis (PEC), anterior deltoid (AD), and posterior deltoid (PD) muscles. Electrodes were also placed on the left (LSCM) and right (RSCM) sternocleidomastoid muscles. EMG signals were amplified by the Bortec AMT-8 system [Bortec Biomedical, Calgary, Alberta, Canada]. This system has a bandwidth of 10–1000Hz, an input impedance of 10GΩ, and a common mode rejection ratio of 113dB at 60Hz. Electromyography (EMG) data were recorded at gain of 1500 and frequency of 3000Hz by a 32-channel, 16-bit data acquisition system [NI USB-6363, National Instrumentation, Austin, TX].
For this study, the InMotion2 Interactive Therapy System (Interactive Motion Technologies, Inc, Watertown, MA 02472 USA) was used to record time, position, and acceleration data for the reaching tasks performed by the subject at a sampling frequency of 1000 Hz. The InMotion2 system is a commercial version of the MIT-Manus and is designed for use in a clinical environment. A custom-made arm-rest was used to reduce arm fatigue and ensure correct elbow alignment (Fig. 1a).
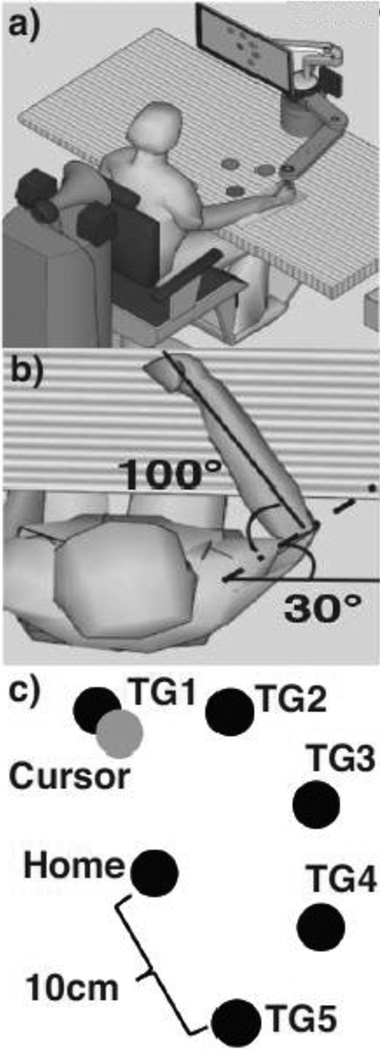
Fig. 1.
Experimental Setup a) Subjects used the InMotion2 system’s robot arm to move from the Home and Target positions. Auditory cues and acoustic stimuli were delivered through speakers behind the subject. b) Subject’s arm placement in Home position c) Computer screen visual feedback for the subject. The cursor, all five Targets and Home position were displayed for each trial
Target and Arm Placement
A computer screen displayed six circles. The “Home” target was located centrally on the screen and placed to match the subjects’ resting arm position (shoulder flexion at 30°, shoulder abduction at 85°, and elbow extension angle at 100° – Fig. 1b). Five surrounding Targets –abbreviated as TG1, TG2, TG3, TG4, and TG5 – surrounded Home (Fig. 1c). Using a standard xy-coordinate plane (where x-axis is medio-lateral, and y-axis is posterior-anterior), TG1 was positioned at 110° and TG5 was positioned 180° away from it to match where arm stability is greatest (Hu et al. 2012). TG2, TG3, and TG4 were spaced equally at 45° between TG1 and TG5 to define a functional workspace. Each of the five Targets were positioned 10cm from Home.
Protocol
Subjects were seated in a chair placed in front of the InMotion2 system. Two straps were placed over the subject’s upper body and fastened to limit torso movement. (Fig. 1c). Subjects were instructed to wait in a relaxed position for a sequence of two, low-intensity auditory cues (60dB). The first auditory cue (“Get Ready”) signaled subjects to prepare to reach to the selected Target, which was highlighted as a red circle. The second auditory cue (“Go”) instructed subjects to initiate the reaching movement. The “Get Ready” and “Go” cues were separated randomly by 1.5–2.5s to prevent task anticipation. The speaker was positioned directly behind the subject (Fig 1a).
Subjects were trained to perform the movement until it was clear they understood and were performing the reaching task at a consistent pace, which typically occurred after 20 trials. Following the completion of the training period for each Target, subjects performed three blocks of 75 trials. Each block contained five sub-blocks – one subblock for each Target. The sub-blocks were randomized using a Matlab script so Target order was different within each block. Each sub-block was composed of 15 trials of the same Target. Within each sub-block, five trials of the 15 trials were randomly selected to be Startle trials. During Startle trials, the “Go” cue was substituted with a startling, high intensity acoustic stimulus (113dB) emitted by a loudspeaker (TS-333S Siren Speaker, Chowsons International Inc., 8 Ohms, 30 Watts) positioned directly behind the subject’s head (15 cm). Startle protocols, specifically block order randomization and auditory cue sequence, follow procedures used in previous reports (Honeycutt and Perreault 2012; Honeycutt et al. 2013).
Data Analysis
EMG Analysis
All EMG data were processed in Matlab (R2014b; Mathworks). Raw EMG data were rectified and filtered using a 10 point moving average. A conservative custom Matlab code was used to detect onset latency, where the processed EMG signal remained above three times the standard deviation of the previous 500 ms for at least 5 ms. Each identified onset latency was visually evaluated to ensure accuracy by a reviewer trial type, target, and subject.
Activity in the SCMs is accepted as an indicator of startle (Carlsen et al. 2011). This study used a cut-off of 120ms (Carlsen et al. 2011) and classified activity in either the LSCM or RSCM within 120ms of the 113 dB acoustic stimulus “Go” cue as an Startle+ trial. Trials with no SCM activity or SCM activity after 120ms of the 113 dB acoustic stimulus cue were categorized as Startle-trials.
Movement Kinematics
The two-dimensional coordinates of the InMotion2 robot arm’s end point were used to calculate kinematics, including average movement velocity, time to peak velocity, maximum linear deviation, movement start time, total movement time, and movement direction at 100ms. Kinematic values were processed using a custom Matlab script. Movement start time was recorded as the time when the robot arm leaves the Home target (radius = 1cm) and movement end time was recorded when the robot arm first enters the Target radius (radius= 1cm; distance from Home target=10cm). Average velocity was calculated using the trajectory displacement divided by the total movement time. Maximum linear deviation was defined for hand-path curvature, calculated as linear deviation = (Sainburg et al. 1993; Bagesteiro and Sainburg 2002; Schaefer et al. 2009). Major axis was the straight-line path from Home to each Target and the minor axis was the largest perpendicular distance between major axis and the subject’s pathway. Movement direction at 100ms was defined using the xy coordinate plane of the workspace to calculate the angle of the vector between the Home target (0,0) and the selected Target (x,y). The angle, measured in degrees and converted to radians, was found using the formula arctan(dy/dx).
Trials where subjects moved prior to the “Go” cue or did not fully reach the Target were excluded from statistical analysis (3.7% of trials excluded). Additionally, subjects that did not have at least one Startle+ and Startle-trial condition were excluded for that particular Target.
Statistical Analysis
We hypothesized that startReact would evoke multi-joint reaching movements similar to voluntary movements as measured by muscle activation patterns, movement kinematics, and movement accuracy, but not in onset latencies. Comparisons between voluntary (low intensity GO with no SCM activity), Startle- (high intensity GO with SCM activity after 120 ms), and Startle+ (high intensity with SCM activity before 120 ms) were conducted. Comparisons between Startle+ and voluntary movements allow us to demonstrate that startReact is similar to voluntarily initiated movements, while comparisons between Startle+ and Startle-allow us to differentiate between intensity-dependent and startle-dependent effects on muscle onset latency and movement start time. Faster onset latencies occur in the presence of a startle due to the startle-dependent effect (Valls-Solé et al. 1999) and when the intensity of the “Go” cue is increased (intensity-dependent effect) (Kohfeld 1969). All statistics were computed using R and used a Type I error of 5%, or a 95% confidence interval.
First, onset latency and movement start time were tested for statistical differences. We fit a linear mixed effects model (lmerTest package in R) with target, muscle, and trial condition (Voluntary, Startle+, or Startle-) as independent factors, and the subject as the random factor. By setting subject as a random factor, we account for within subject correlations and avoid violating the assumptions of independence. Muscle onset latency and movement start time latency were used as dependent factors. We used a Satterthwaite approximation to account for the unequal variances in the sample data. Post-hoc analyses were conducted using the emmeans package in R to obtain pairwise comparisons for each muscle between the trial conditions (voluntary vs Startle+, Startle-vs Startle+).
In order to test the kinematic equivalence between voluntary, Startle+, and Startle-trials, we computed similarity metrics between trials using methods previously described in the literature (Avella et al. 2003; Avella and Bizzi 2005). For this computation, each trial was represented by a vector of the kinematic measures consisting of total movement time, linear deviation, time to peak velocity, average velocity, movement direction at 100ms. Then, we calculated the cosine of the angle between the vectors representing the individual trials as a measure of similarity by computing the normalized scalar dot product between the two trials (Avella et al. 2003; Avella and Bizzi 2005). We computed similarities for trials within voluntary, Startle+, and Startle-conditions.
We used two one-sided Welch’s t-tests (TOST) of the kinematic similarity measures for testing the kinematic equivalence of the voluntary versus Startle+ and Startle-versus Startle+ trials for each Target. We used Welch’s t-test due to the unbalanced design with unequal variances. We used the TOSTER package in R to run these similarity tests. As is convention for TOST, we utilized a 90% confidence interval (5% for each side of the t-tests). Given the repeated measures design, we used the 80th percentile from a one-sided chi-squared distribution (i.e. with γ = 0.2) as the estimate of measurement precision (Limentani et al. 2005). Matching this, we used the 80th percentile of distribution (i.e. 1.3 * smallest standard deviation) as the equivalence bounds for testing equivalence of the two distributions – voluntary vs Startle+ and Startle-vs Startle+ conditions.
The same methodology used for testing statistical equivalence for kinematic measures was applied to determine if muscle activation patterns between voluntary, Startle+, and Startle-trials were statistically equivalent. The differences between onset muscle latencies were calculated between flexor-extensor pairs (BIC and TRI, AD and PD) at each Target, and were used to create a vector for each trial. Delta values between flexor-extensor pairs were used as a quantitative metric for TOST analysis to measure the qualitative muscle onset latency observed. Once again, the bounds for the equivalence testing were set to 1.3 times the smallest standard deviation of the two distributions.
The TOST test described above is able to handle the hierarchical nature of our experiment. However it is more susceptible to outliers (being based on normal distributions). We therefore also utilized a Bayesian paired t-test to test for equivalence of the voluntary vs Startle+ and Startle-vs Startle-conditions for the kinematic measures and muscle activation data collected. To lessen the impact of the outliers, we used a Cauchy distribution as the prior. We averaged the similarity measure for each of the subjects under each of the conditions. We then used the Bayesian paired t-test in JASP software to conduct the analysis JASP Team (2018). JASP (Version 0.9) [Computer software].
A Brown-Forsythe Test was used to calculate variability between Startle+ and Startle-trials for linear deviation, movement direction at 100ms, and muscle activation patterns. Variability was analyzed by testing the difference in the standard deviations of the two distributions resulting from the similarity measures for linear deviation, movement direction at 100ms, and flexor-extensor pairs across all Targets.
Last, we tested the probability of eliciting a Startle+ response across all Targets. A linear mixed effects model using lmerTest in RStudio was used with Target direction as the independent factor. The percentage of Startle+ trials (calculated as ) was the dependent factor. Subjects were treated as the random factor.
Results
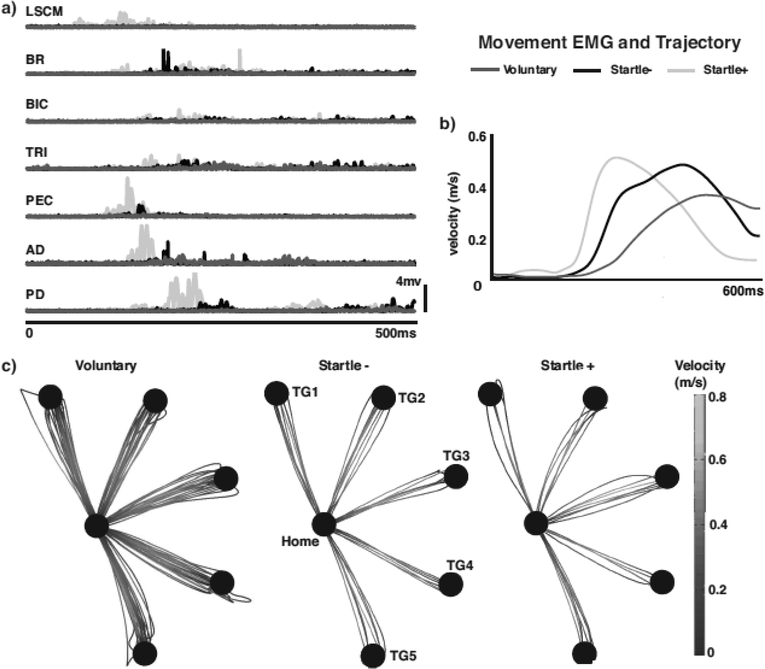
StartReact was readily evoked in all five directions generating similar muscle activation patterns and movement kinematics between Startle+ and voluntary trials and Startle+ and Startle-trials (Fig. 2). Startle+ trials presented stereotypically earlier muscle activation compared to both voluntary and Startle-trials as shown by a representative subject’s EMG activity (Fig. 2). The relative timing of agonist/antagonist firing pairs also remained similar between Startle+ and voluntary trials and Startle+ and Startle-trials. Movement kinematics, trajectory, and velocity profiles also appear similar between voluntary and Startle+ and Startle+ and Startle-trials (Fig. 2b, c).
Fig. 2.
a) EMG for Startle+ (light gray), Startle- (black), and voluntary (dark gray) trials for representative subject. b) Velocity trace comparison between Startle+ trial (light gray), Startle-trial (black), and voluntary (dark gray) trial. c) All movement trajectories for Startle- (left) and Startle+ (right) trials to each of the five Targets for a representative subject. Grayscale shows velocity during reaching movement
Group results confirmed earlier muscle activation (and movement start times) in Startle+ trials across all Targets and muscles (Fig. 3). ANOVA results for muscle onset latency found a difference for both trial condition (F2,10076= 2474.39, p<0.0001) and muscle type (F5,10071=143.93, p<0.001). The interaction between trial condition and muscle type also showed significance (F10,10070 = 2.37, p<0.00863). Post-hoc analysis for muscle onset latency found faster muscle onset latencies for Startle+ trials for all muscles (Table 1) when compared to both voluntary and Startle-trials (p<0.0001). Correspondingly, movement start time was also faster in Startle+ trials (Table 1) based on ANOVA results for trial condition (F2,1796= 412.28, p<0.0001). No difference was found in movement start time for Target (F4,1794.5=1.90, p=0.11), and the interaction between movement start time and Target was also not significant (F8,1792.8=0.82, p=59). Post-hoc analysis for both voluntary vs. Startle+ and Startle-vs. Startle+ at each Target showed Startle+ movement start times occurred faster (p<0.05).
Table 1.
Delta values for each muscle (Voluntary/Startle+ and Startle-/Startle+ comparisons) and movement start time averaged across all of the Targets.
| Onset Latency Omnibus Results | ||||
|---|---|---|---|---|
| Voluntary vs Startle+ | Startle-vs Startle+ | |||
| Delta (ms) | p-value | Delta (ms) | p-value | |
| Muscle Onset | ||||
| AD | 95.56 | <0.001 | 39.33 | <0.001 |
| BIC | 97.99 | <0.001 | 41.70 | <0.001 |
| BR | 88.85 | <0.001 | 34.84 | <0.001 |
| PD | 95.52 | <0.001 | 43.29 | <0.001 |
| PEC | 105.61 | <0.001 | 50.86 | <0.001 |
| TRI | 84.57 | <0.001 | 33.64 | <0.001 |
| Movement Start Time | ||||
| TG1 | 83.83 | <0.001 | 34.70 | 0.0016 |
| TG2 | 93.07 | <0.001 | 39.66 | 0.0001 |
| TG3 | 88.00 | <0.001 | 31.51 | 0.0028 |
| TG4 | 99.53 | <0.001 | 58.30 | <0.0001 |
| TG5 | 89.21 | <0.001 | 32.99 | 0.0016 |
Despite the faster initiation, Startle+ trials were equivalent in both kinematic measures (average velocity, linear deviation, total movement time, time to peak velocity, and movement direction at 100ms) and muscle activation patterns (flexor-extensor pairs) when compared to Startle-trials and voluntary trials. For each target, the confidence intervals for kinematic measures between Startle+ and Startle- and voluntary and Startle+ trials were well within the bounds and therefore statistically equivalent. The muscle activation patterns as measured by the time between the onset of the flexor-extensor pairs were also found to be equivalent. Tables 2 and 3 shows the results of both TOST analyses.
Table 2.
Results of TOST for flexor-extensor muscle activation patterns.
| Muscle Activation TOST Test Results γ = 0.2 and 1.3*SD (both 80th percentile) | ||||
|---|---|---|---|---|
| Target | Mean and 90% CI (5% for each of the 2 sides of the TOST) | Lower and upper equivalence bounds | Test statistics and p-val | |
| Muscle Activation Patterns (voluntary vs Startle+) | TG1 | 0.072 [0.023, 0.121] | [−0.469, 0.469] | t(201.564) = −13.416; p < 0.0001 |
| TG2 | 0.066 [−0.008, 0.141] | [−0.807, 0.807] | t(327.367) = −16.386; p < 0.0001 | |
| TG3 | 0.071 [0.016, 0.126] | [−0.578, 0.578] | t(271.874) = −15.141; p < 0.0001 | |
| TG4 | 0.148 [0.091, 0.205] | [−0.437, 0.437] | t(313.793) = −8.385; p < 0.0001 | |
| TG5 | −0.027 [−0.051, −0.003] | [−0.29, 0.29] | t(375.068) = 16.468; p < 0.0001 | |
| Muscle Activation Patterns (Startle -vs Startle+) | TG1 | 0.148 [0.088, 0.207] | [−0.317, 0.317] | t(279.419) = −4.701; p < 0.0001 |
| TG2 | 0.09 [−0.024, 0.203] | [−0.822, 0.822] | t(313.513) = −10.646; p < 0.0001 | |
| TG3 | −0.044 [−0.124, 0.036] | [−0.639, 0.639] | t(502.938) = 12.225; p < 0.0001 | |
| TG4 | 0.085 [0.016, 0.154] | [−0.515, 0.515] | t(518.769) = −10.306; p < 0.0001 | |
| TG5 | −0.162 [−0.224, −0.1] | [−0.263, 0.263] | t(285.365) = 2.701; p = 0.004 | |
Table 3.
Results of TOST for kinematic metrics.
| Kinematic Metrics TOST Test Results γ = 0.2 and 1.3*SD (both 80th percentile) | ||||
|---|---|---|---|---|
| Target | Mean and 90% CI (5% for each of the 2 sides of the TOST) | Lower and upper equivalence bounds | Test statistics (all p<0.001) | |
| Kinematic metrics (voluntary vs Startle+) | TG1 | 0.049 [0.014, 0.084] | [−0.266, 0.266] | t(185.184) = −10.379; p < 0.0001 |
| TG2 | −0.045 [−0.062, −0.027] | [−0.218, 0.218] | t(380.477) = 16.265; p < 0.0001 | |
| TG3 | 0.019 [−0.006, 0.046] | [−0.274, 0.274] | t(261.375) = −15.782; p < 0.0001 | |
| TG4 | −0.012 [−0.036, 0.013] | [−0.316, 0.316] | t(350.141) = 20.485; p < 0.0001 | |
| TG5 | −0.053 [−0.067, −0.038] | [−0.168, 0.168] | t(374.170) = 13.175; p < 0.0001 | |
| Kinematic metrics (Startle -vs Startle+) | TG1 | −0.016 [−0.061, 0.03] | [−0.314, 0.314] | t(340.480) = 10.856; p < 0.0001 |
| TG2 | −0.014 [−0.043, 0.014] | [−0.218, 0.218] | t(403.123) = 11.889; p < 0.0001 | |
| TG3 | 0.052 [0.022, 0.083] | [−0.212, 0.212] | t(398.749) = −8.623; p < 0.0001 | |
| TG4 | 0.006 [−0.028, 0.04] | [−0.318, 0.318] | t(560.946) = −15.093; p < 0.0001 | |
| TG5 | −0.121 [−0.152, −0.09] | [−0.168, 0.168] | t(368.164) = 2.469; p = 0.007 | |
Results of the Bayesian paired samples t-test also showed moderate evidence that both the voluntary versus Startle+ trials (BF01 = 4.826) and Startle-versus Startle+ trials (BF01 = 2.293) are equivalent in kinematic measures (Table 4) across all targets. These Bayes factors provide moderate evidence for the null hypothesis: kinematic measures (the same five metrics used for TOST analysis - average velocity, linear deviation, total movement time, time to peak velocity, and movement direction at 100ms) are equivalent. We further separated this analysis by each target (Table 4). Targets 2 and 4, show a moderate evidence for the alternate hypothesis that there is a difference between Voluntary and Startle+ measures. The Bayes Factor of 0.938 for Startle-vs Startle+ for Target 5 indicates an ambivalent result. Despite these individual differences, the overall analysis across all targets indicates evidence in favor of the kinematic equivalence of the Voluntary vs Startle+ and Startle-vs Startle+ trials.
Table 4.
Results of Bayesian paired samples t-test for kinematic metrics.
| Results of the Bayesian paired samples t-Test of kinematic similarity showing evidence for equivalence between the comparisons across all targets | |||
|---|---|---|---|
| Comparison of kinematic similarities | Target | BF01 | error % |
| TG1 | 2.391 | 6.615e -4 | |
| TG2 | 0.366 | 1.115e -4 | |
| TG3 | 2.965 | 0.035 | |
| Voluntary vs Startle+ | TG4 | 0.359 | 0.001 |
| TG5 | 3.018 | 0.006 | |
| Across all targets | 4.826 | 0.054 | |
| TG1 | 2.475 | 5.612e -4 | |
| TG2 | 2.747 | 0.008 | |
| TG3 | 1.933 | 2.676e -4 | |
| Startle-vs Startle+ | |||
| TG4 | 1.5 | 0.004 | |
| TG5 | 0.938 | 0.003 | |
| Across all targets | 2.293 | 0.013 | |
Results of the Bayesian paired samples t-test also showed moderate evidence that both the voluntary versus Startle+ trials (BF01 = 3.267) and Startle-versus Startle+ trials (BF01 = 5.226) are equivalent in muscle activation patterns (Table 5) across all targets. These Bayes factors provide moderate evidence for the null hypothesis – muscle activation patterns are equivalent.
Table 5.
Results of the Bayesian paired samples t-test for muscle activation patterns.
| Results of the Bayesian paired samples t-Test for muscle activations patterns showing evidence for equivalence between the comparisons across all targets | |||
|---|---|---|---|
| Comparison of Muscle Activations | Target | BF01 | error % |
| TG1 | 2.178 | 7275e-4 | |
| TG2 | 3.197 | 0.007 | |
| TG3 | 1.05 | 0.004 | |
| Voluntary vs Startle+ | TG4 | 1.699 | 0.02 |
| TG5 | 2.811 | 0.008 | |
| Across All targets | 3.267 | 9.028e-6 | |
| TG1 | 0.076 | 2.359e-4 | |
| TG2 | 3.046 | 0.006 | |
| TG3 | 2.96 | 0.035 | |
| Startle-vs Startle+ | |||
| TG4 | 2.787 | 1.434e-5 | |
| TG5 | 1.979 | 3.926e-4 | |
| Across All targets | 5.226 | 9.060e-6 | |
Similar to previous reports for a single joint, Startle+ trials showed increased variability in linear deviation and muscle activation patterns when compared to voluntary and Startle-trials but only for a subset of directions. Variability tests found a difference between voluntary and Startle+ trials for both linear deviation (F1,428=12.96, p=0.0003) and muscle activation patterns (F1,1413=22.7, p<0.0001). For Startle+ versus Startle-, variability tests did not show a difference for muscle activation (F1,2196=2.11, p=0.146) but did for linear deviation (F1,601 =8.346, p=0.004). However, there were no statistically significant differences in the variability of movement direction at 100 ms. This was the case for both Voluntary vs Startle+ trials (F1,493 = 0.493, p = 0.483) and for Startle-vs Startle+ trials (F1,623 = 0.185, p = 0.667).
The ability of evoking startReact was found to be equally probable across the five directions in the workspace. The linear mixed effects model found that the probability of eliciting startReact in each direction was not different across Targets (F4, 32=0.3176, p=0.864) at approximately 50% at each (TG1=50.05%, TG2=52.7%, TG3=47.1%, TG4= 51.5%, TG5=50.7%).
Discussion
Summary
We found that startReact is not only readily accessible during unrestricted, multi-joint reaching movements, but that startReact evokes equivalent movement kinematics and muscle activation patterns compared to voluntary movements. Previous studies have shown that startReact is robust across multiple individual joints, but startReact has also been shown to be more variable in kinematics such as mean peak displacement and peak angular velocity (Carlsen et al. 2004a). Many previous studies restricted all joints not specifically related to the task (Carlsen et al. 2004a; Maslovat et al. 2011; Honeycutt and Perreault 2012, 2013) raising the question of whether startReact would be accurate during unrestricted multi-joint movements that more closely resemble functional reaching movements in everyday environments. Further, previous work to date (including our own) has primarily utilized statistical models that test for differences, not similarity, leaving in doubt if startReact movements were truly similar or simply “not different.” Our results show that multi-joint startReact movements are indeed equivalent to voluntarily-initiated movement demonstrating that startReact evokes a sophisticated motor plan with tuned direction, force, and muscle activation patterns that can be involuntarily initiated. Though previous studies have demonstrated that startReact is more than a simplistic reflex (Valls-Solé et al. 1999; Carlsen et al. 2003, 2004a; Rothwell 2006), our results showcase that startReact can elicit spatially and temporarily tuned movement (Maslovat et al. 2011) in multiple directions and further strengthens startReact as a sophisticated tool that can be utilized to study and evaluate complex motor plans.
Movement Variability
Though we hypothesized that multi-jointed reaching movements would be similar between Startle+ and voluntary movements, there was some reason to consider an alternative result. Many previous studies restricted all joints not specifically related to the task (Carlsen et al. 2004a, b; Maslovat et al. 2011; Honeycutt and Perreault 2012, 2013) raising the question of whether startReact would be accurate during unrestricted multi-joint movements that more closely resemble functional reaching in everyday environments. Natural arm biomechanics, which demonstrate higher stability in certain directions (Hu et al., 2012), might influence variability in movement trajectory accuracy during the reaching task. Moreover, previous studies have also shown that startReact movements are more variable (Carlsen et al. 2004a) particularly within the first 100ms (Maslovat et al. 2011). Carlsen, et al (2004a) noted that reaching tasks under a startle condition appeared to have variability in standard deviation for mean peak displacement when compared to control trials. Similarly, results from Maslovat, et al (2011) also show qualitatively larger movement displacement between startle condition trials and non-startle trials; however neither study conducted statistical analysis to precisely quantify variability in peak displacement.
To more precisely quantify and understand the variability in movement trajectory in our results, we tested the differences in standard deviation for muscle activation patterns and linear deviation across all Targets. Though linear deviation does provide a slightly different measure of movement accuracy than total displacement, our methods capture how far a movement trajectory deviated from an expected Home to Target pathway. Interestingly, while we did find an increase in variability for linear deviation and muscle activation between Startle+ versus voluntary trials and Startle+ versus Startle-trials, our TOST equivalence measures for muscle activation patterns and movement kinematics demonstrate on average 2% or 10% differences (Tables 2 and 3) indicating that the added variability does not dramatically impact the movement as a whole. Furthermore, variability in movement direction at 100ms showed no difference between both voluntary versus Startle+ and Startle+ versus Startle-trials which aligns with previous results showing that initial movement direction is not affected by startReact (Marinovic et al. 2017). In conclusion, while we more precisely quantified and observed an increase in variability, an unrestricted workspace does not appear to have a large impact on the quality or accuracy of the movement. Our results highlight the robust nature of startReact to generate movement patterns that are tightly regulated and scaled to the intended action even when multiple joints are activated.
Clinical ramifications
One of the most provocative uses of startReact has been in clinical populations. StartReact enhances movement in individuals following a stroke (Honeycutt and Perreault 2012; Honeycutt et al. 2015), spinal cord injury (Baker and Perez 2017), or hereditary spastic paraplegia (Fisher et al. 2013; Nonnekes et al. 2014). In the Parkinson’s population, startReact can help identify freezing of gait (Nonnekes et al. 2015). These studies highlight the unique potential of startReact as a clinical tool across multiple populations for enhancing movement and inaccessible muscle activation. While most of these studies show a simple decrease in onset latency, which may or may not be clinically significant, more recent work demonstrates that startReact also enhances muscle activation patterns (Honeycutt and Perreault 2012; Marinovic et al. 2016). StartReact at the elbow in stroke survivors are not statistically different from those of unimpaired individuals both in muscle coordination patterns and in onset timing. In other words, when a stroke survivor’s movement plan is released by startReact, we find that their movements are indistinguishable from those of unimpaired individuals (Honeycutt and Perreault 2012). This is particularly striking given the differences in voluntary movements of these two groups, and has been replicated (Marinovic et al. 2016) which highlights its robustness. Still, Honeycutt and Perrault (2012) evaluated restricted single-joint elbow movements and Marinovic, et al (2016) immobilized the arm to prevent active movement in the wrist and elbow as there was no precedent for more functionally relevant reaching tasks that allowed movement with multiple degrees of freedom. Our findings that startReact is fully accessible during unrestricted, multi-joint reaching allow a more serious discussion about the utilization of startReact to facilitate functional movement patterns in stroke survivors. Because startReact can enhance the movements in the stroke survivor population for simple, single-jointed tasks, our results indicate that startReact can evoke more complex movements. This opens the door to begin evaluating startReact’s potential to enhance and facilitate more complex multi-joint movements of stroke survivors. Although additional research should be conducted to further understand its robustness as a clinical diagnostic tool, startReact might offer a viable option for initiation of movement in severely paralyzed patients with little or no ability to move. Indeed, our preliminary work in this population shows just this: startReact can initiate muscle activation in severe patients with little or no volitional activation (Rahimi and Honeycutt 2017).
Acknowledgements
This study was made possible through funding from the National Institutes of Health (R00 HD073240), ASU’s Fulton Undergraduate Research Initiative (FURI), and Barrett, The Honors College. Many thanks to members of the Human Mobility Lab for the guidance, assistance, and support throughout this research study.
References
- Avella A, Bizzi E (2005) Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci United States Am 102:3076–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avella AD, Saltiel P, Bizzi E (2003) Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci 6:300. [DOI] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL (2002) Handedness: Dominant Arm Advantages in Control of Limb Dynamics. J Neurophysiol 88:2408–2421. doi: 10.1152/jn.00901.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Perez MA (2017) Reticulospinal Contributions to Gross Hand Function after Human Spinal Cord Injury. 37:9778–9784. doi: 10.1523/JNEUROSCI.3368-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, et al. (2008) Differential Effects of Startle on Reaction Time for Finger and Arm Movements. J Neurophysiol 101:306–314. doi: 10.1152/jn.00878.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, et al. (2003) Startle response is dishabituated during a reaction time task. Exp Brain Res 152:510–518. doi: 10.1007/s00221-003-1575-5 [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, et al. (2004a) Prepared Movements Are Elicited Early by Startle. J Mot Behav 36:253–264 [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, et al. (2004b) Can prepared responses be stored subcortically? Exp Brain Res 159:301–309. doi: 10.1007/s00221-004-1924-z [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Franks IM (2012) Preparation for voluntary movement in healthy and clinical populations: Evidence from startle. Clin Neurophysiol 123:21–33. doi: 10.1016/j.clinph.2011.04.028 [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, et al. (2011) Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev 35:366–376. doi: 10.1016/j.neubiorev.2010.04.009 [DOI] [PubMed] [Google Scholar]
- Castellote JM, Valls-Sole J (2015) The StartReact effect in tasks requiring end-point accuracy. Clin Neurophysiol 126:1879–1885. doi: 10.1016/j.clinph.2015.01.028 [DOI] [PubMed] [Google Scholar]
- Fisher KM, Chinnery PF, Baker SN, Baker MR (2013) Enhanced reticulospinal output in patients with (REEP1) hereditary spastic paraplegia type 31. J Neurol 260:3182–3184. doi: 10.1007/s00415-013-7178-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt C, Ravichandran V, Perrault E (2017) The influence of startReact on long-latency reflexive muscle activation during the transition from posture to movement. bioRxiv 180554:1–27 [Google Scholar]
- Honeycutt CF, Kharouta M, Perreault EJ (2013) Evidence for reticulospinal contributions to coordinated finger movements in humans. J Neurophysiol 110:1476–1483. doi: 10.1152/jn.00866.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ (2012) Planning of Ballistic Movement following Stroke: Insights from the Startle Reflex. PLoS One 7:. doi: 10.1371/journal.pone.0043097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ (2013) Deficits in startle-evoked arm movements increase with impairment following stroke. Clin Neurophysiol doi 10.1016/j.clinph.2013.12.102. doi: 10.1016/j.immuni.2010.12.017.Two-stage [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt CF, Tresch UA, Perreault EJ (2015) Startling acoustic stimuli can evoke fast hand extension movements in stroke survivors. Clin Neurophysiol 126:160–164. doi: 10.1016/j.clinph.2014.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Murray WM, Perreault EJ (2012) Modeling the biomechanical constraints on the feedforward control of endpoint stiffness. J Neurophysiol 108:2083–2091. doi: 10.1109/IEMBS.2010.5626027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohfeld DL (1969) EFFECTS OF THE INTENSITY OF AUDITORY AND VISUAL READY SIGNALS ON SIMPLE REACTION TIME. J Exp Psychol 82:88–95 [DOI] [PubMed] [Google Scholar]
- Limentani GB, Ringo MC, Ye F, et al. (2005) Beyond the t-Test: Statistical Equivalence Testing. Anal Chem 77:1–6 [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Allen DP, Shiratori T, Rogers MW (2013) Early and Unintentional Release of Planned Motor Actions during Motor Cortical Preparation. PLoS One 8:. doi: 10.1371/journal.pone.0063417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinovic W, Brauer SG, Hayward KS, et al. (2016) Electric and acoustic stimulation during movement preparation can facilitate movement execution in healthy participants and stroke survivors. Neurosci Lett 618:134–138. doi: 10.1016/j.neulet.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Marinovic W, Tresilian J, Chapple JL, et al. (2017) UNEXPECTED ACOUSTIC STIMULATION DURING ACTION PREPARATION REVEALS GRADUAL RE-SPECIFICATION OF MOVEMENT DIRECTION. Neuroscience 348:23–32. doi: 10.1016/j.neuroscience.2017.02.016 [DOI] [PubMed] [Google Scholar]
- Maslovat D, Carlsen AN, Chua R, Franks IM (2009) Response preparation changes during practice of an asynchronous bimanual movement. Exp Brain Res 195:383–392. doi: 10.1007/s00221-009-1801-x [DOI] [PubMed] [Google Scholar]
- Maslovat D, Carlsen AN, Franks IM (2012a) Subcortical motor circuit excitability during simple and choice reaction time. Behav Neurosci 126:499–503. doi: 10.1037/a0028285 [DOI] [PubMed] [Google Scholar]
- Maslovat D, Hodges NJ, Chua R, Franks IM (2011) Motor preparation of spatially and temporally defined movements: evidence from startle. J Neurophysiol 125:226–240. doi: 10.1037/a0022567 [DOI] [PubMed] [Google Scholar]
- Maslovat D, Kennedy PM, Forgaard CJ, et al. (2012b) The effects of prepulse inhibition timing on the startle reflex and reaction time. Neurosci Lett 513:243–247. doi: 10.1016/j.neulet.2012.02.052 [DOI] [PubMed] [Google Scholar]
- Nonnekes J, DeKam D, Nijhuis LBO, et al. (2015) StartReact effects support different pathophysiological mechanisms underlying freezing of gait and postural instability in Parkinson’s disease. PLoS One 10:1–17. doi: 10.1371/journal.pone.0122064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnekes J, Nijhuis LBO, Niet M De, et al. (2014) StartReact Restores Reaction Time in HSP : Evidence for Subcortical Release of a Motor Program. 34:275–281. doi: 10.1523/JNEUROSCI.2948-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi M, Honeycutt CF (2017) Does startle enhance unrestricted, 2D reaching movement in stroke survivors ? Soc Neurosci 1 [Google Scholar]
- Rothwell JC (2006) Chapter 18 The startle reflex, voluntary movement, and the reticulospinal tract Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Poizner H, Ghez C (1993) Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol 70:2136–2147. doi: 10.1152/jn.1993.70.5.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Haaland KYH, Sainburg RL (2009) Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. 47:2953–2966. doi: 10.1016/j.neuropsychologia.2009.06.025.Hemispheric [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch UA, Perreault EJ, Honeycutt CF (2014) Startle evoked movement is delayed in older adults: Implications for brainstem processing in the elderly. Physiol Rep 2:1–11. doi: 10.14814/phy2.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Rothwell JC, Goulart F, et al. (1999) Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516:931–938. doi: 10.1111/j.1469-7793.1999.0931u.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ZA, Carlsen AN, Mackinnon CD (2015) Degraded Expression of Learned Feedforward Control in Movements Released by Startle. 233:2291–2300. doi: 10.1007/s00221-015-4298-5.Degraded [DOI] [PMC free article] [PubMed] [Google Scholar]