Abstract
Background
Post-operative atrial fibrillation (POAF) is a common arrhythmia following cardiac surgery and is associated with increased healthcare costs, complications, and mortality. The etiology of POAF is incompletely understood and its prediction remains suboptimal. Using data from published studies, we performed a systemic review and meta-analysis to identify preoperative clinical risk factors associated with patients at increased risk for POAF.
Methods
A systematic search of PubMed, MEDLINE, and EMBASE databases was performed.
Results
Twenty-four studies that reported univariate analysis results regarding POAF risk factors, published from 2001 to May 2017, were included in this meta-analysis with a total number of 36,834 subjects. Eighteen studies were carried out in the USA and Europe and 16 studies were prospective cohort studies. The standardized mean difference (SMD) between POAF and Non-POAF groups was significantly different [reported as (SMD: 95% CI)] for age (0.55: 0.47 to 0.63), left atrial diameter (0.45: 0.15 to 0.75), and left ventricular ejection fraction (0.30: 0.14 to 0.47). The pooled odds ratios [(reported as (OR: 95% CI)) demonstrated that heart failure (1.56: 1.31 to 1.96), chronic obstructive pulmonary disease (1.36: 1.13 to 1.64), hypertension (1.29: 1.12 to 1.48), and myocardial infarction (1.18: 1.05 to 1.34) were significant predictors of POAF incidence, while diabetes was marginally significant (1.06: 1.00 to 1.13).
Conclusion
The present analysis suggested that older age and history of heart failure were significant risk factors for POAF consistently whether the included studies were prospective or retrospective data sets.
Keywords: Atrial Fibrillation, Cardiac Surgery, Odd Ratio, Standardized Mean Difference
1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia following cardiac surgery and post-operative atrial fibrillation (POAF) is associated with prolonged hospitalization, increased healthcare costs, complications, and long-term mortality [1–25]. Kosmidou et al.[26] reported POAF after coronary artery bypass surgery (CABG) was associated with a 4-fold increase of 3-year stroke risk and 3-fold increase of all-cause mortality. Therefore, understanding the causes of POAF and reducing the risk of POAF will lead to improved care of cardiac surgery patients. On the other hand, the etiology of POAF is incompletely understood and its prediction remains suboptimal. Several risk factors [27,28], biomarkers [29,30], and genetic variants [31,32] have been investigated and linked to POAF but have not proven to be particularly sensitive in predicting which patients will develop POAF. Risk indexes that attempt to predict an individual’s risk for developing POAF have been developed [29,33–36], but these too suffer from a lack of specificity predicting which patients will develop POAF.
Electrical cardioversion or pharmacological interventions to restore sinus rhythm have been reported to yield improvements in symptoms and quality of life [37,38]. However, these interventions demonstrated the possibility of the inherent adverse side effects related to the interventions outweighing the benefits of AF suppression on postoperative outcomes [39]. Identifying specific patients at high risk for developing POAF may help to define a population that is more likely to benefit from anti-arrhythmic drugs or other preventive strategies. While there are many studies that individually evaluate the clinical risk factors for POAF, there is disagreement as to which clinical factors are associated with the greatest risk for POAF. Therefore, we conducted a systematic review and meta-analysis to identify the preoperative risk factors that are associated with POAF occurrence.
2. Methods
2.1. Search Strategy
A systematic electronic search was performed on the PubMed, MEDLINE, and EMBASE databases. We combined the terms: “atrial fibrillation”, “post-operative atrial fibrillation”, “cardiac surgical procedure”, and “cardiac surgery” as either keywords or MeSH terms (See Fig 1). The search included the time period between 2001 and May 2017 was conducted without language restrictions. To achieve maximum sensitivity of the search strategy and identify all studies, the reference lists of all retrieved articles were manually screened for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria.
Figure 1:
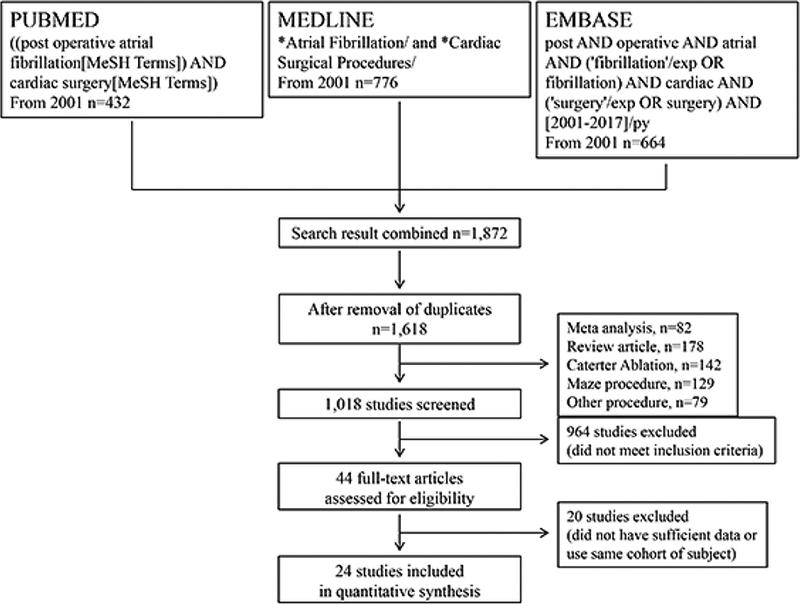
Flowchart of the literature search strategy.
2.2. Selection criteria
The major inclusion criteria were: (i) patients in the study underwent cardiac surgery; (ii) non-interventional study; (iii) the main outcomes included the incidence of new onset of AF; (iv) data available regarding clinical patient characteristics; (v) the data included information about patients with POAF and without POAF. The major reasons for exclusion of studies were: (i) patients with prior History of AF (ii) overlapping data sets; (iii) patients aged <18years old; (iv) data published in the form of abstracts without peer-reviewed publication of manuscripts.
2.3. Data extraction
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each retrieved article (D.D. and K.Y.). Discrepancies between the two reviewers were resolved by discussion and consensus with a third reviewer (N.H.). These points include: (i) clear identification of study population, (ii) clear definition of outcome and outcome assessment, (iii) independent assessment of outcome parameters, (iv) selective loss during follow-up, and (v) important confounders or prognostic factors or both identified.
2.4. Statistical analysis
The odds ratios (ORs), standard errors (SEs), and the 95% confidence intervals (CIs) from the univariable logistic regression model in each study were collected during the data extraction phase. Pooled ORs for each risk factor were calculated using the inversed variance (IV) random effect (RE) method [40]. The standardized mean difference (SMD) was used to compare the continuous variables (demographic features and clinical risk variables) between patients with and without POAF. To calculate the SMD, Hedges’ adjusted g formula was used, which includes an adjustment for small sample bias. The calculation of the SMD is given by:
where m1i is the mean for those with POAF, m2i is the mean for those without POAF, si is the pooled standard deviation (SD), and Ni = n1i+ni2 is the total number of observations where n1i and n2i are the total number of subjects with and without POAF, respectively [41]. The SE of SMD is calculated as:
All statistical tests were two-sided, and results with p-value lower than 0.05 were considered as statistically significant. Data analyses were carried out using Review Manager (Copenhagen, Denmark) [41], or RevMan, version 5.3, SAS (SAS Institute Inc., Cary, NC, USA) version 9.4 and Stata (Stata Corp., College Station, TX, USA) version 14.
2.5. Assessment of heterogeneity
To check for heterogeneity, for each analysis we first calculated the pooled OR and SMD estimate using a random effects model. The I2 statistic tests the null hypothesis that each study is evaluating the same effect. Large I2 values indicate high heterogeneities.
2.6. Assessment of publication bias
Each of the pooled analysis outcomes was quantitatively assessed for publication bias using the Egger’s test.
3. Results
Twenty-four studies reporting POAF incidence were included in this meta-analysis with a total of 36,834 subjects (Table 1). Sixteen out of the 24 studies (66.7%) used prospective study design. Most studies (18 out of 24, or 75.0%) were conducted in the United States or Europe. The number of patients ranges from 57 (Dogan et al. [11]) to 18,517 (El-Chami et al. [12]). Incidence of POAF in included studies was 28.0% (95% CI: 23.5 to 32.6). Males dominated in most studies (20 out of 24, or 83.3%). On pump CABG were involved in 14 studies, while off pump CABG were used in 10 studies.
Table 1:
Characteristics of Included Studies
| Author (year) | Country | Type of Study | Number of Subjects | Number of POAF | Incidence (%) of POAF (95% CI) | Number (%) of Male | Type of Surgery |
|---|---|---|---|---|---|---|---|
| Abdel-Massih (2012) | Lebanon | Prospective | 113 | 18 | 15.9 (15.8, 16.0) | 94 (83.2) | On pump CABG |
| Anderson (2017) | US | Prospective | 324 | 77 | 23.8 (23.7, 23.9) | 233 (71.9) | CABG or valve surgery |
| Antoniades (2009) | UK | Prospective | 144 | 43 | 29.9 (29.8, 30.0) | 120 (83.3) | Off pump CABG |
| Başaran (2016) | Turkey | Prospective | 90 | 23 | 25.6 (25.4, 25.8) | 71 (78.9) | On or Off pump CABG |
| Bolesta (2015) | US | Retrospective | 244 | 74 | 30.3 (30.2, 30.4) | 103 (42.2) | Valve surgery |
| Bouchot(2015) | France | Prospective | 100 | 34 | 34.0 (33.8, 34.2) | 92 (92.0) | On or Off pump CABG |
| Budeus (2006) | Germany | Prospective | 101 | 37 | 36.6 (36.4, 36.8) | 79 (78.2) | On pump CABG |
| Çetin (2012) | Turkey | Prospective | 272 | 62 | 22.8 (22.7, 22.9) | 224 (82.4) | On or Off pump CABG |
| Choi (2009) | Korea | Prospective | 315 | 66 | 21.0 (20.9, 21.1) | 225 (71.4) | Off pump CABG |
| Di Gioia (2017) | Italy | Retrospective | 134 | 41 | 30.6 (30.4, 30.8) | 98 (73.1) | Valve surgery |
| Dogan (2007) | Turkey | Prospective | 57 | 10 | 17.5 (17.3, 17.7) | 37 (64.9) | On pump CABG |
| EL-Chami (2012) | US | Retrospective | 18,517 | 3,486 | 18.8 (18.8, 18.8) | 13,276 (71.7) | On or Off pump CABG |
| Gang (2004) | UK | Prospective | 151 | 40 | 26.5 (26.4, 26.6) | 126 (83.4) | On or Off pump CABG |
| Gode (2016) | Turkey | Prospective | 90 | 15 | 16.7 (16.5, 16.9) | 70 (77.8) | On pump CABG |
| Guo (2017) | China | Prospective | 109 | 19 | 17.4 (17.3, 17.5) | 38 (34.9) | CABG and myectomy |
| Hernández-Romero (2017) | Spain | Prospective | 100 | 29 | 29.0 (28.8, 29.2) | 77 (77.0) | CABG or valve surgery |
| Ngai (2016) | US | Retrospective | 562 | 215 | 38.3 (38.3, 38.3) | 229 (40.7) | On pump CABG and/or Valve |
| O’Neal (2013) | US | Retrospective | 13,165 | 2,907 | 22.1 (22.1, 22.1) | 9,281 (70.5) | On or Off pump CABG |
| Osranek (2006) | US | Prospective | 203 | 84 | 41.4 (41.3, 41.5) | 133 (65.5) | CABG and/or valve surgery |
| Park (2014) | Korea | Prospective | 113 | 39 | 34.5 (34.3, 34.7) | 81 (71.7) | On or Off pump CABG |
| Saskin (2017) | Turkey | Retrospective | 662 | 153 | 23.1 (23.1, 23.1) | 541 (81.7) | On or Off pump CABG |
| Shariff (2010) | US | Retrospective | 757 | 144 | 19.0 (19.0, 19.0) | 558 (73.7) | On pump CABG |
| Silva (2010) | Brazil | Prospective | 448 | 145 | 32.4 (32.4, 32.4) | 287 (64.1) | CABG or valve surgery |
| Takahashi (2014) | Japan | Retrospective | 63 | 41 | 65.1 (64.7, 65.5) | 23 (36.5) | Valve surgery |
| Total | 36,834 | 7,802 | 28.0 (23.5, 32.6) | 26,096 (70.8) |
POAF=post-operative atrial fibrillation, CABG=coronary artery bypass graft, CI=confidence interval
Table 2 summarizes the results of our meta-analysis. Among the four continuous variables being examined, age, left atrial diameter (LAd), and left ventricular ejection fraction (EF) were found to be statistically significantly different between patients with and without POAF.
Table 2:
Summary of Meta-analysis Results
| Variables | I2 | Number of studies | Number of subjects | Std. Mean Difference [IV, Random, 95% CI] |
|---|---|---|---|---|
| Age [3,4,6–15,17,19,22,24] | 25% | 16 | 21,665 | 0.55 [0.47, 0.63] * |
| LA diameter [1,3,7,8,11,14,15,24] | 71% | 8 | 949 | 0.45 [0.15, 0.75] * |
| Ejection Fraction [6–12,14,15,19,24] | 69% | 11 | 19,961 | −0.30 [−0.47, −0.14]* |
| BMI [7,8,10–12,15,24] | 0% | 7 | 19,253 | 0.01 [−0.03, 0.04] |
| (b) Pooled ORs | ||||
| Variables | I2 | Number of studies | Number of subjects | Odds Ratio [M-H, Random, 95% CI] |
| History of HF [5,12,13,18−20,23] | 62% | 7 | 32,841 | 1.56 [1.31, 1.86]* |
| COPD [2,5–9,12–14,18,19,22,24] | 40% | 13 | 34,302 | 1.36 [1.13, 1.64]* |
| Hypertension [2–15,18,19,21,22,24] | 39% | 19 | 35,498 | 1.29 [1.12, 1.48]* |
| History of MI [2,5–7,9,11–13,18,19] | 39% | 10 | 33,177 | 1.18 [1.05, 1.34]* |
| Diabetes [2–15,18–24] | 3% | 21 | 36,059 | 1.06 [1.00, 1.13]** |
| β-blocker use [2–10,13–15,18–22,24] | 38% | 18 | 17,037 | 1.13 [0.95, 1.36] |
| Male gender [2–15,17–22,24] | 32% | 21 | 36,173 | 0.98 [0.87, 1.11] |
| Dyslipidemia [3,6,8,10,13,15,19,21,23,24] | 49% | 10 | 2,286 | 1.03 [0.75, 1.41] |
significant difference at the 0.05 test level
marginally significant difference at the 0.05 test level
IV=inverse variance, M-H=Mantel-Haenszel, BMI=body mass index, LA=left atrial, COPD=chronic obstructive pulmonary disease, MI=myocardial infarction, HF=heart failure, SMDs=standardized mean differences, ORs=odds ratios
Age data was analyzed from sixteen studies and 21,665 patients. The SMD was calculated to be 0.55 (95% CI: 0.47 to 0.63), with POAF patients having a greater mean in age of 5.45 (95% CI: 4.29 to 6.61) years compared to patients that did not have POAF. There was a moderate heterogeneity (I2 = 25%) in this analysis. See Fig. 2A.
Figure 2:
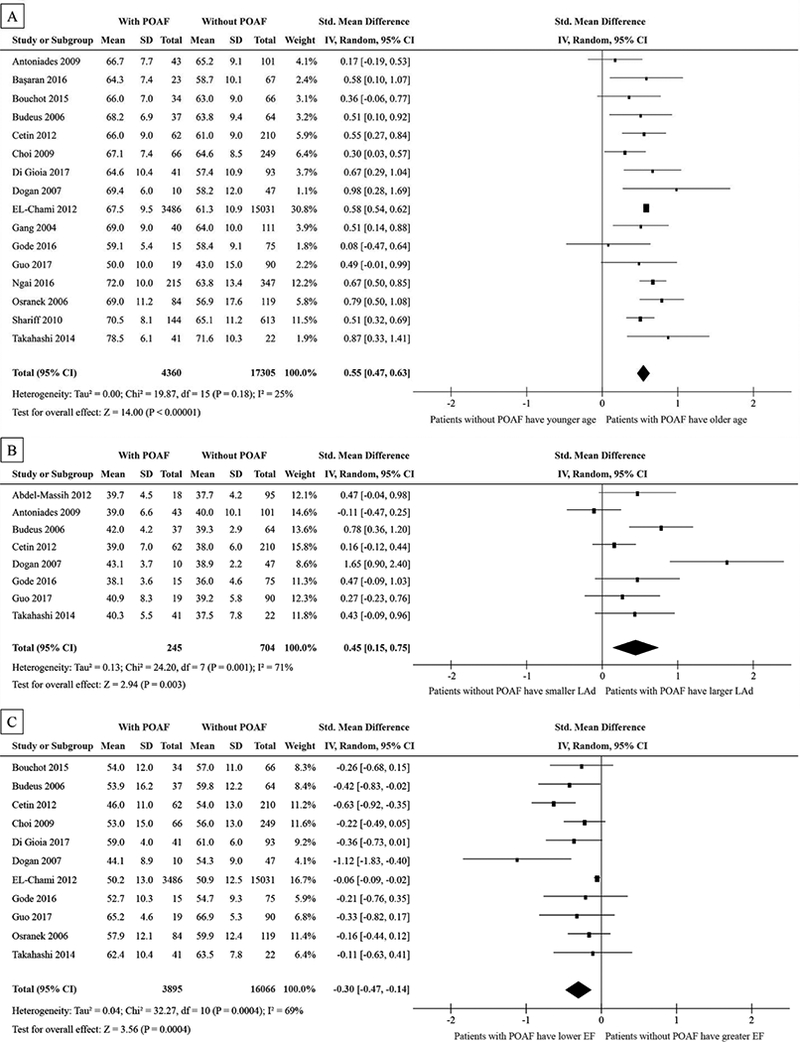
Forest plots showing std. mean difference of age in years (A), LAd in mm (B), and left ventricular EF (C) in percentage between patients with POAF without POAF.
POAF=post-operative atrial fibrillation, SD=standard deviation, IV=inverse variance, CI=confidence interval, M-H=Mantel-Haenszel, LAd=left atrial diameter, EF=ejection fraction, HF=heart failure, BMI=body mass index, COPD=chronic obstructive pulmonary disease, MI=myocardial infarction,
LAd data was analyzed from eight studies and 949 patients. The estimated SMD was 0.45 (95% CI: 0.15 to 0. 75), with POAF patients having a larger mean in LAd of 2.01 (95% CI: 1.03 to 2.99) mm compared to patients without POAF. There was a large heterogeneity (I2 = 71%) in this analysis (Fig. 2B).
EF data was analyzed in eleven studies and 19,961 patients. The estimated SMD was 0.30 (95% CI: 0.14 to 0.47), with POAF patients having a lower mean in EF by 3.01% (95% CI: 1.43 to 4.58) lower than patients that did not have POAF. There was a moderate heterogeneity (I2 = 57%) in this analysis. See Fig. 2C.
Among the dichotomous clinical factors, History of heart failure (HF), chronic obstructive pulmonary disease (COPD), hypertension, History of myocardial infarction (MI) and diabetes were found to be the significant risk factors for POAF.
History of HF was analyzed in seven studies and 32,841 patients with a total number of 6,729 POAF events. The pooled ORs was 1.56 and (95% CI: 1.31 to 1.86), in favor of the subjects without a History of HF. Patients with a HF history are expected to have a 56% higher odds of POAF than those without HF. There was a relatively large heterogeneity (I2 = 62%) in this analysis. See Fig. 3A.
Figure 3.
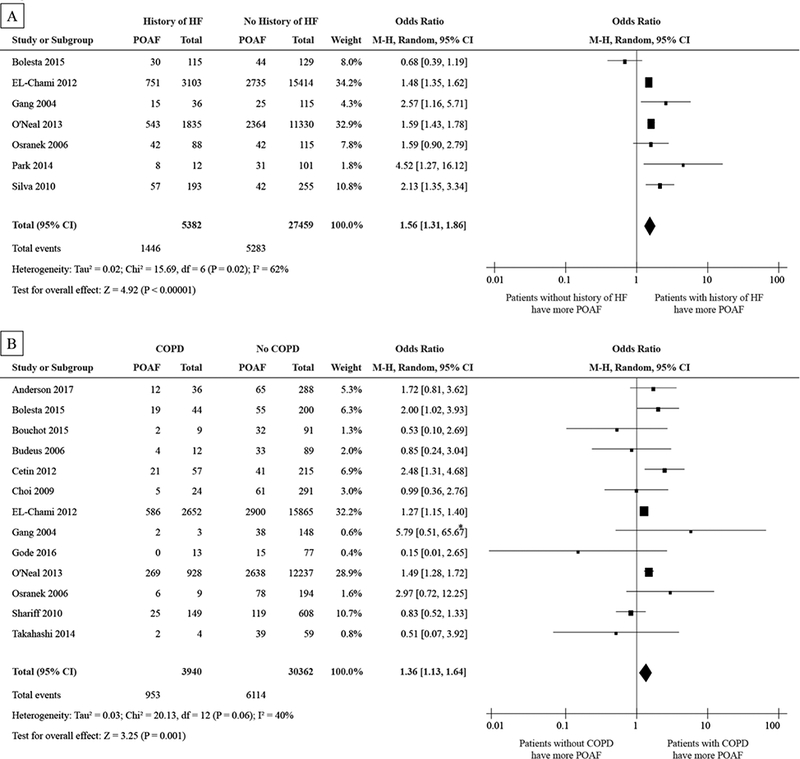
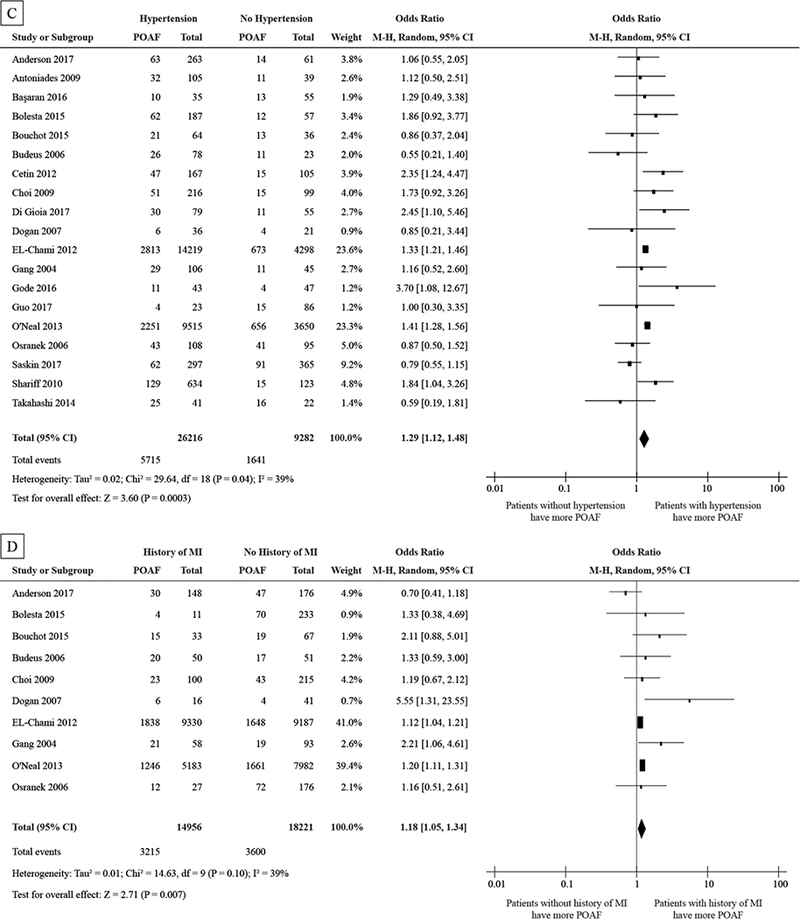
Forest plot showing the pooled POAF odds ratios for HF (A), COPD (B), hypertension (C), and MI (D).
Abbreviations as in Figure 2
*Large OR because of the small N for patients with COPD
Prevalence of COPD was evaluated from thirteen studies and 34,302 patients with a total number of 7,067 POAF events. The pooled OR was 1.36 and (95% CI: 1.13 to 1.64), in favor of the subjects without COPD. On average, patients with COPD have a 36% higher odds of POAF than those without COPD. There was a moderate heterogeneity (I2 = 40%) in this analysis. See Fig. 3B.
Hypertension data was analyzed from nineteen studies and 35,498 with a total number of 7,356 POAF events. The pooled OR was 1.29 and (95% CI: 1.12 to 1.49), in favor of the subjects without POAF. On average, patients with hypertension are expected to have a 29% higher odds of POAF when compared with their counterparts without hypertension. There was moderate heterogeneity (I2 = 39%) in this analysis. See Fig. 3C.
History of MI was analyzed in ten studies and 33,177 patients, with a total number of 6,815 POAF events. The pooled OR was 1.18 and (95% CI: 1.05 to 1.34), in favor of the subjects without the History of MI. Patients with the History of MI are expected to have an 18% higher odds of POAF than those without History of MI. There was a moderate heterogeneity (I2 = 39%) in this analysis. See Fig. 3D.
Twenty-one studies and 36,059 patients were included in the analysis of diabetes, with a total number of 7494 POAF events. There is a marginally significant association between diabetes and POAF, with a pooled ORs of 1.06 and (95% CI: 1.00 to 1.13), in favor of the subjects without the diabetes. Patients with diabetes are expected to have a 6% higher odds of POAF than those without diabetes. There was a very small heterogeneity (I2 = 3%) in this analysis. See Fig. 4.
Figure 4:
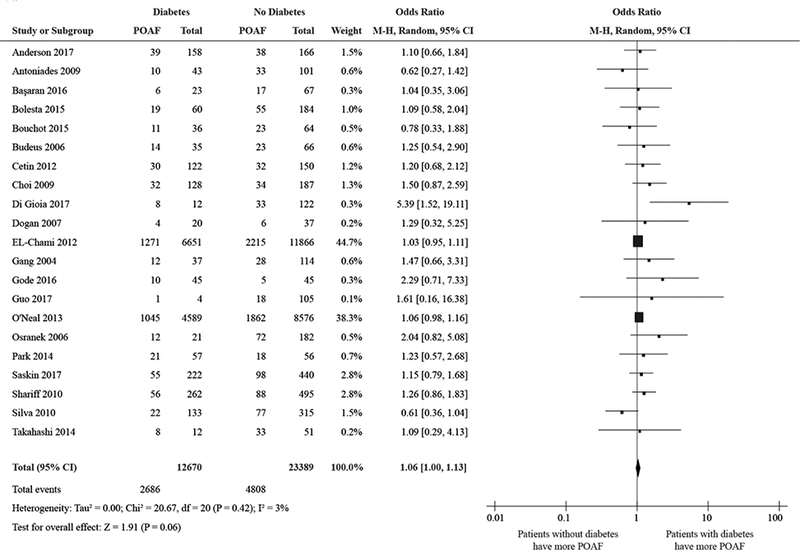
Forest plot showing the pooled odds ratio for diabetes.
Abbreviations as in Figure 2
Body mass index (BMI) data, β-blocker use, patient gender, and dyslipidemia were also analyzed with respect to POAF incidence. These factors did not reach statistical significance in our meta-analysis. See Fig. 5A, 5B, 5C, and 5D.
Figure 5:
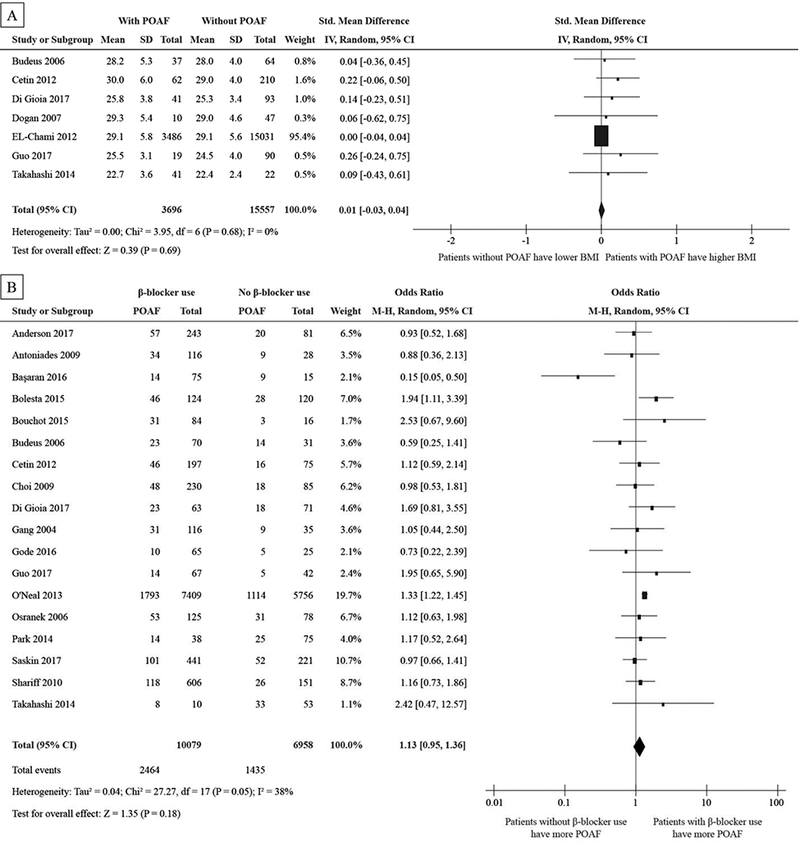
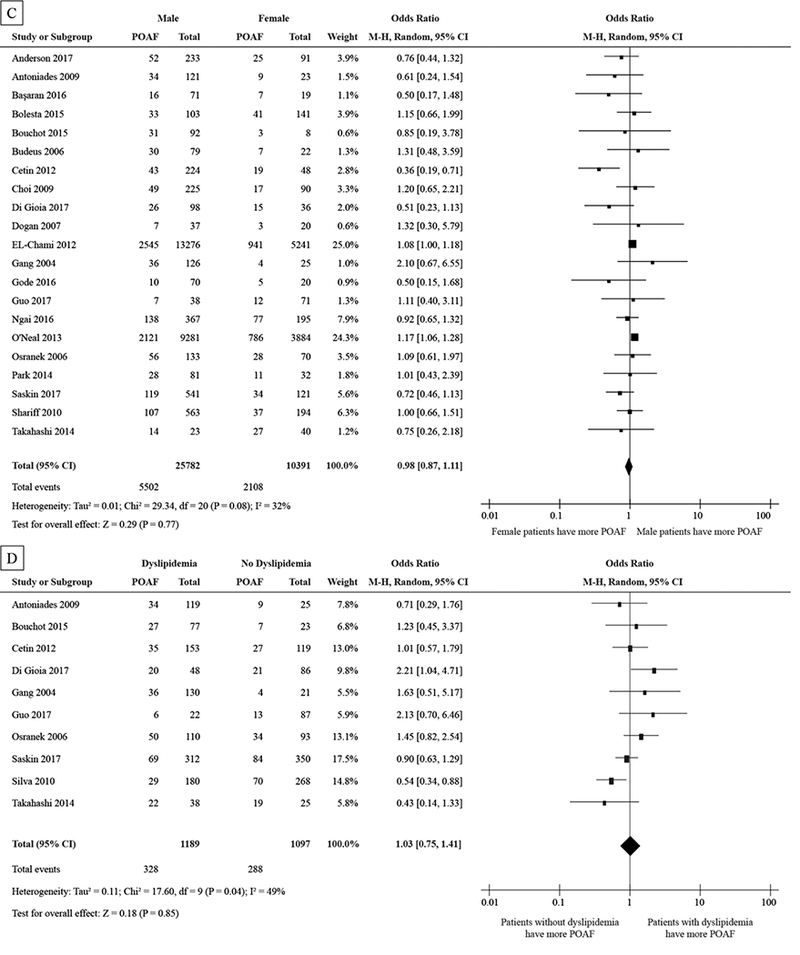
Forest plot showing std. mean difference of BMI (A) in kg/m2 and the pooled odds ratio for β-blocker use (B), gender (C), and dyslipidemia (D).
Abbreviations as in Figure 2
Because of the types of included studies, we divided all studies into prospective and retrospective design groups (Table 3). In this sub-analysis, Age and History of HF were consistently significant predictors for POAF incidence despite of study design. On the other hand, LAd and EF were not significant predictors in retrospective design group. Moreover, in prospective design group, COPD, HT, and History of MI also did not predict the incidence of POAF significantly.
Table 3:
Comparison between analysis in prospective and retrospective studies
| Prospective Study | Retrospective Study | |||||||
|---|---|---|---|---|---|---|---|---|
| I2 | Number of studies | Number of subjects | Std. Mean Difference [IV, Random, 95% CI] | I2 | Number of studies | Number of subjects | Std. Mean Difference [IV, Random, 95% CI] | |
| Age | 27 | 11 | 1,632 | 0.47 [0.34, 0.61]* | 0 | 5 | 20,033 | 0.58 [0.55, 0.62]* |
| LA diameter | 75 | 7 | 886 | 0.46 [0.12, 0.80]* | 0 | 1 | 63 | 0.43 [−0.09, 0.96] |
| Ejection Fraction | 38 | 8 | 1,247 | −0.37 [−0.54, −0.19]* | 25 | 3 | 18,714 | −0.11 [−0.26, 0.05] |
| BMI | 0 | 4 | 539 | 0.17 [−0.03, 0.37] | 0 | 3 | 18,714 | 0.00 [−0.03, 0.04] |
| (b) Pooled ORs | ||||||||
| Prospective Study | Retrospective Study | |||||||
| I2 | Number of studies | Number of subjects | Odds Ratio [M-H, Random, 95% CI] | I2 | Number of studies | Number of subjects | Odds Ratio [M-H, Random, 95% CI] | |
| History of HF | 0 | 4 | 915 | 2.09 [1.53, 2.86]* | 78 | 3 | 31,926 | 1.42 [1.18, 1.72]* |
| COPD | 30 | 8 | 1,556 | 1.51 [0.91, 2.48] | 56 | 5 | 32,746 | 1.31 [1.09, 1.58]* |
| Hypertension | 20 | 12 | 1,956 | 1.21 [0.93, 1.58] | 60 | 7 | 33,542 | 1.33 [1.13, 1.56]* |
| History of MI | 53 | 7 | 1,251 | 1.41 [0.93, 2.15] | 0 | 3 | 31,926 | 1.16 [1.10, 1.22]* |
| Diabetes | 0 | 14 | 2,517 | 1.10 [0.90, 1.35] | 24 | 7 | 33,542 | 1.07 [0.98, 1.17] |
| β-blocker use | 27 | 12 | 2,012 | 0.95 [0.72, 1.26] | 12 | 6 | 15,025 | 1.31 [1.14, 1.51] |
| Male gender | 17 | 13 | 2,069 | 0.85 [0.65, 1.10] | 33 | 8 | 34,104 | 1.06 [0.95, 1.18] |
| Dyslipidemia | 45 | 7 | 1,427 | 1.03 [0.70, 1.51] | 70 | 3 | 859 | 1.02 [0.48, 2.15] |
significant difference at the 0.05 test level
IV=inverse variance, M-H=Mantel-Haenszel, BMI=body mass index, LA=left atrial, COPD=chronic obstructive pulmonary disease, MI=myocardial infarction, HF=heart failure
In order to evaluate the sensitivity of our analysis to surgery type, we also conducted a sub-group analysis by analyzing the CABG studies and valve surgery studies separately (Table 4). The sub-analysis of CABG showed that large LA diameter was not a significant predictors of POAF but that the other clinical risk factors were similar to original analysis. The sub-group analysis of the valve surgery studies showed that older age and βblocker use were significant predictors of POAF. The difference in the sub-group analysis are likely due to the smaller sample size of the groups, particularly in the valve surgery studies.
Table 4:
Comparison between analysis in CABG and Valve Surgery
| CABG | Valve Surgery | |||||||
|---|---|---|---|---|---|---|---|---|
| I2 | Number of studies | Number of subjects | Std. Mean Difference [IV, Random, 95% CI] | I2 | Number of studies | Number of subjects | Std. Mean Difference [IV, Random, 95% CI] | |
| Age | 33 | 11 | 20,594 | 0.49 [0.39, 0.59]* | 0 | 2 | 197 | 0.73 [0.42, 1.04]* |
| LA diameter | 92 | 6 | 777 | 0.39 [−0.25, 1.02] | 0 | 1 | 63 | 0.43 [−0.09, 0.96] |
| Ejection Fraction | 79 | 7 | 19,452 | −0.35 [−0.60, −0.11]* | 0 | 2 | 197 | −0.28 [−0.58, 0.02] |
| BMI | 0 | 4 | 18,947 | 0.00 [−0.03, 0.04] | 0 | 2 | 197 | 0.12 [−0.18, 0.42] |
| (b) Pooled ORs | ||||||||
| CABG | Valve Surgery | |||||||
| I2 | Number of studies | Number of subjects | Odds Ratio [M-H, Random, 95% CI] | I2 | Number of studies | Number of subjects | Odds Ratio [M-H, Random, 95% CI] | |
| History of HF | 45 | 4 | 31,946 | 1.57 [1.38, 1.79]* | 0 | 1 | 244 | 0.68 [0.39, 1.19] |
| COPD | 50 | 9 | 33,468 | 1.29 [1.05, 1.59]* | 36 | 2 | 307 | 1.44 [0.46, 4.52] |
| Hypertension | 44 | 13 | 34,421 | 1.31 [1.13, 1.52]* | 53 | 3 | 441 | 1.54 [0.74, 3.18] |
| History of MI | 45 | 7 | 32,406 | 1.21 [1.07, 1.37]* | 0 | 1 | 244 | 1.33 [0.38, 4.69] |
| Diabetes | 0 | 14 | 34,534 | 1.06 [1.00, 1.12]* | 61 | 3 | 441 | 1.72 [0.65, 4.54] |
| β-blocker use | 50 | 12 | 15,960 | 1.01 [0.79, 1.29] | 0 | 3 | 441 | 1.88 [1.22, 2.89]* |
| Male gender | 43 | 14 | 34,534 | 1.00 [0.86, 1.15] | 29 | 3 | 441 | 0.82 [0.49, 1.39] |
| Dyslipidemia | 0 | 5 | 1,329 | 0.96 [0.73, 1.25] | 82 | 2 | 197 | 1.03 [0.21, 5.09] |
significant difference at the 0.05 test level
IV=inverse variance, M-H=Mantel-Haenszel, BMI=body mass index, LA=left atrial, COPD=chronic obstructive pulmonary disease, MI=myocardial infarction, HF=heart failure
4. Discussion
The primary findings of this study are as follows: 1) Older age (as a continuous measurement), increased LAd, and lower EF were associated with increased POAF incidence. 2) Binary risk factors of a History of HF, COPD, hypertension, MI, and diabetes were associated with increased POAF incidence. 3) Findings about age and History of HF were consistent whether the included studies were prospective or retrospective data sets.
The value of this meta-analysis is that while individual studies may not show a significant relationship between clinical variables and POAF incidence, pooling results from many studies can show relationships that are not consistent in individual studies. Some of the clinical factors in this analysis were predictably significant. For example, 12 of the 16 studies analyzed for age demonstrated that POAF patients were significantly older than those without POAF, and the other 4 studies trended towards older age in POAF patients. However, LA diameter was significantly larger in only 2 of 8 studies, with 1 of the 8 studies showing a lower average LA diameter. The meta-analysis found larger LA diameter to be significantly associated with POAF incidence.
Advanced age is considered an independent predictor for POAF after cardiac surgery as well as AF incidence outside of the cardiac surgery population [42]. Patients with advanced age experience remodeling in the atrial tissue including changes in ion channel function and distribution [43], systemic inflammation [3,6,9,21], impulse propagation [7,8,10,13], fibrosis distribution [15,16], and structural substrate [4,17,19,24], which lead to increased POAF inducibility and burden with increased age. Since age related remodeling is chronic and slow, older patients undergoing cardiac surgery likely have developed the substrate for increased POAF inducibility and burden. With the increased inflammation and sympathetic tone experienced by cardiac surgery patients, underlying age related structural remodeling may facilitate the new onset of POAF.
LAd may be increased due to patient characteristics such as age, gender, or body size. Pathophysiological conditions such as mitral valve regurgitation, arteriovenous fistula, and left to right shunt that lead to pressure and/or volume overload may also lead to increased LAd. Increased LAd has been associated with increased AF incidence in the general population [42], and in this meta-analysis, we have found that patients with POAF had larger LAd than the patients without POAF. Increased atrial dimension may lead to increased AF burden.
COPD is associated with an increased incidence of new onset of POAF as well as non-surgical AF. According to Mangnas et al. [44], patients with severe COPD experienced POAF more frequently than patients with mild to moderate COPD. However, Fuster et al. [45] also reported severity of COPD was correlated with pulmonary infections and length of hospital stay while the incidence of POAF after CABG was not significantly associated with significant COPD. The specific mechanisms by which POAF and COPD are associated are currently not known.
Hypertension is a leading cause of cardiovascular disease and is the most prevalent co-morbidity in patients with AF [46]. Hypertension may induce atrial cardiomyopathy, increased LAd, and atrial stretch, which induce structural and electrophysiological changes conducive to AF onset and burden. Gap junction remodeling associated with hypertension may lead to conduction abnormalities and ectopic firing, contributing to both the substrate and triggers required for AF onset and maintenance [47].
Patients with a History of MI are at increased risk for non-surgical AF [37,42]. Patients with coronary artery disease and History of MI may be at risk for compromised atrial perfusion, which has been proposed as a mechanism for atrial fibrosis deposition and electrophysiological remodeling [48]. Coronary artery disease may also lead to heterogeneous atrial blood flow and variations in dispersion of refractoriness. Since many of the cardiac surgery patients have a History of MI, this risk factor is of particular importance to POAF incidence. Particularly CABG patients may have the substrate and heterogeneous arterial blood flow that places them at higher risk for POAF.
Patients with diabetes are 50% more likely to have nonsurgical AF [42]. Diabetes is associated with increased atrial fibrosis, which has been shown to increase AF burden [49]. Autonomic dysfunction, particularly with an imbalance towards increased sympathetic tone, is prevalent in diabetic patients and may play a key role in the triggering or initiating AF [50]. In our meta-analysis, diabetes was only marginally significantly associated with POAF incidence, and patients with diabetes only have a 6% increase in risk of POAF incidence as compared to patients without POAF.
Other factors, including β-blocker use, gender, and dyslipidemia were not significantly different in patients with POAF from those that did not experience POAF. While BMI has been shown to be a clinical risk factor for non-surgical AF [51,52], our meta-analysis did not show increased BMI to be associated with increased POAF incidence. Another study determined that increased BMI led to increased AF risk, but when BMI was adjusted for LAd, BMI was no longer associated with AF risk [53]. BMI may be a secondary marker for atrial dilatation and stretch, but alone it may not be a direct measure of substrate remodeling in the atria that would lead to increased POAF for surgical patients.
Preoperative β-blocker use has been recommended before CABG as first-line therapy in guidelines because preoperative β-blocker use has been shown to reduce the incidence of POAF [54]. However, in this meta-analysis and subanalysis, β-blocker was not associated with POAF incidence. One reason was that this meta-analysis was not about assessing the effect of preoperative β-blocker use in prospective randomized controlled trial. β-blockers may be administered more often in patients with higher age, lower EF, and hypertension, negating the benefits of β-blocker administration. Another reason was that the dose and type of β-blocker were not consistent in this analysis. DoNicolantonie et al. [55] concluded that, compared with metoprolol, carvedilol significantly reduced the incidence of POAF in patients undergoing CABG.
5. Study Limitations
Our study contains several limitations. First, the type of studies in this meta-analysis is a mixture of prospective and retrospective studies. The unadjusted odds ratios from the univariable analysis in retrospective studies were more likely to be biased due to confounding. To correct for this possible confounding, we performed a sensitivity analysis that includes only the prospective studies in all analyses. The results of all these analyses were similar to those incorporating all studies.
Second, studies in our meta-analysis include different type of surgeries. Among these studies, the most common surgery type is valve surgery and CABG. In addition, studies we considered in the meta-analysis often included a mixture of several surgery types. Types of surgeries being used in each study can be found in Table 1. Our sensitivity analysis showed that the meta-analysis results was sensitive to the surgery type. Hence, caution should be used to generalize our study finding to one specific surgery type.
Third, our meta-analysis used studies from different geographic populations that can be quite different in genotypes. There is also a large variability in terms of sample sizes. Two studies had large sample sizes of more than 13,000 patients, while four studies had a sample size less than 100.
Fourth, the criteria for defining the POAF and monitoring methods varied across studies. Some studies defined POAF as any incidence of AF during the follow-up period while others defined it as AF lasting more than 1 hour. Also, patients in most included studies were continuously monitored until discharge but in some studies, heart rhythm was continuously recorded for the first 48 to 72 hours and monitored every 4 hours. Moreover, follow-up time varied substantially, with some studies following patients only during their ICU stay following surgery, with others utilized monitoring for up to 1 month following cardiac surgery.
Fifth, some meta-analyses of pooled OR and SMD has a relatively large I2 value (> 60%). For example, the SMD of LAd and EF as well as the pooled OR of History of HF. These indicate a relatively large heterogeneity in the studies used in these meta-analyses. Caution should be used when trying to generalize these results.
6. Conclusion
Advanced age, increased LA diameter, reduced LVEF, and a History of HF, COPD, hypertension, and a History of MI are all associated with increased risk of POAF. Patient characteristics including advanced age and a History of HF were at increased risk for developing POAF following cardiac surgery, whether the data come from prospective or retrospective studies. Body mass index, β-block use, gender, and dyslipidemia were not associated with POAF incidence.
Acknowledgments
Dr. Ravi Ranjan is partially supported by the National Heart, Lung, and Blood Institute (NHLBI) grants K23HL115084 and R56HL128674.
Partial support for Dr. Nan Hu was provided by the University of Utah Study Design and Biostatistics Center (SDBC), with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067–02 (formerly 8UL1TR000105 and UL1RR025764)
Research reported in this publication was supported by the NHLBI to Dr. Derek J. Dosdall under award number R01HL128752.
Footnotes
This paper has been presented at the American College of Cariology’s 67th Annual Scientific Session & Expo in Orlando, taking place from March 10th to 12th.
Conflict of interest statement
None of the listed grant support represent a conflict of interest. None of the authors have a financial interest related to the work presented in this manuscript.
References
- 1.Abdel-Massih TE, Sarkis A, Sleilaty G et al. Myocardial extraction of intracellular magnesium and atrial fibrillation after coronary surgery. Int J Cardiol 2012; 160: 114–118 [DOI] [PubMed] [Google Scholar]
- 2.Anderson EJ, Efird JT, Kiser AC et al. Plasma Catecholamine Levels on the Morning of Surgery Predict Post-Operative Atrial Fibrillation. JACC: Clinical Electrophysiology 2017, DOI: 10.1016/j.jacep.2017.01.014: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniades C, Van-Assche T, Shirodaria C et al. Preoperative sCD40L levels predict risk of atrial fibrillation after off-pump coronary artery bypass graft surgery. Circulation 2009; 120: S170–176 [DOI] [PubMed] [Google Scholar]
- 4.Basaran O, Tigen K, Gozubuyuk G et al. Predictive role of left atrial and ventricular mechanical function in postoperative atrial fibrillation: a two-dimensional speckle-tracking echocardiography study. Turk Kardiyol Dern Ars 2016; 44: 45–52 [DOI] [PubMed] [Google Scholar]
- 5.Bolesta S, Kong F. Effect of Statins on the Incidence of Postoperative Atrial Fibrillation after Cardiac Valve Surgery. Pharmacotherapy 2015; 35: 998–1006 [DOI] [PubMed] [Google Scholar]
- 6.Bouchot O, Guenancia C, Kahli A et al. Low Circulating Levels of Growth Differentiation Factor-15 Before Coronary Artery Bypass Surgery May Predict Postoperative Atrial Fibrillation. J Cardiothorac Vasc Anesth 2015; 29: 1131–1139 [DOI] [PubMed] [Google Scholar]
- 7.Budeus M, Hennersdorf M, Rohlen S et al. Prediction of atrial fibrillation after coronary artery bypass grafting: the role of chemoreflex-sensitivity and P wave signal averaged ECG. Int J Cardiol 2006; 106: 67–74 [DOI] [PubMed] [Google Scholar]
- 8.Cetin M, Kocaman SA, Erdogan T et al. Fragmented QRS may predict postoperative atrial fibrillation in patients undergoing isolated coronary artery bypass graft surgery. Anadolu Kardiyol Derg 2012; 12: 576–583 [DOI] [PubMed] [Google Scholar]
- 9.Choi YS, Shim JK, Hong SW, Kim DH, Kim JC, Kwak YL. Risk factors of atrial fibrillation following off-pump coronary artery bypass graft surgery: predictive value of C-reactive protein and transfusion requirement. Eur J Cardiothorac Surg 2009; 36: 838–843 [DOI] [PubMed] [Google Scholar]
- 10.Di Gioia G, Mega S, Nenna A et al. Should pre-operative left atrial volume receive more consideration in patients with degenerative mitral valve disease undergoing mitral valve surgery? Int J Cardiol 2017; 227: 106–113 [DOI] [PubMed] [Google Scholar]
- 11.Dogan SM, Buyukates M, Kandemir O et al. Predictors of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis 2007; 18: 327–331 [DOI] [PubMed] [Google Scholar]
- 12.El-Chami MF, Kilgo PD, Elfstrom KM et al. Prediction of new onset atrial fibrillation after cardiac revascularization surgery. Am J Cardiol 2012; 110: 649–654 [DOI] [PubMed] [Google Scholar]
- 13.Gang Y, Hnatkova K, Mandal K, Ghuran A, Malik M. Preoperative electrocardiographic risk assessment of atrial fibrillation after coronary artery bypass grafting. J Cardiovasc Electrophysiol 2004; 15: 1379–1386 [DOI] [PubMed] [Google Scholar]
- 14.Gode S, Aksu T, Demirel A et al. Effect of vitamin D deficiency on the development of postoperative atrial fibrillation in coronary artery bypass patients. J Cardiovasc Thorac Res 2016; 8: 140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y, Wu X, Zheng X, Lu J, Wang S, Huang X. Usefulness of Preoperative Transforming Growth Factor-Beta to Predict New Onset Atrial Fibrillation After Surgical Ventricular Septal Myectomy in Patients With Obstructive Hypertrophic Cardiomyopathy. Am J Cardiol 2017; 120: 118–123 [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Romero D, Vilchez JA, Lahoz A et al. Galectin-3 as a marker of interstitial atrial remodelling involved in atrial fibrillation. Sci Rep 2017; 7: 40378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngai J, Leonard J, Echevarria G, Neuburger P, Applebaum R. Left Atrial Appendage Velocity as a Predictor of Atrial Fibrillation After Cardiac Surgery. J Cardiothorac Vasc Anesth 2016; 30: 413–417 [DOI] [PubMed] [Google Scholar]
- 18.O’Neal WT, Efird JT, Davies SW et al. Impact of race and postoperative atrial fibrillation on long-term survival after coronary artery bypass grafting. J Card Surg 2013; 28: 484–491 [DOI] [PubMed] [Google Scholar]
- 19.Osranek M, Fatema K, Qaddoura F et al. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: a prospective study. J Am Coll Cardiol 2006; 48: 779–786 [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, On YK, Kim JS, Jeong DS, Kim WS, Lee YT. Heart rate turbulence for predicting new-onset atrial fibrillation in patients undergoing coronary artery bypass grafting. Int J Cardiol 2014; 174: 579–585 [DOI] [PubMed] [Google Scholar]
- 21.Saskin H, Serhan Ozcan K, Yilmaz S. High preoperative monocyte count/high-density lipoprotein ratio is associated with postoperative atrial fibrillation and mortality in coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2017; 24: 395–401 [DOI] [PubMed] [Google Scholar]
- 22.Shariff N, Zelenkofske S, Eid S, Weiss MJ, Mohammed MQ. Demographic determinants and effect of pre-operative angiotensin converting enzyme inhibitors and angiotensin receptor blockers on the occurrence of atrial fibrillation after CABG surgery. BMC Cardiovasc Disord 2010; 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva RG, Lima GG, Guerra N, Bigolin AV, Petersen LC. Risk index proposal to predict atrial fibrillation after cardiac surgery. Rev Bras Cir Cardiovasc 2010; 25: 183–189 [DOI] [PubMed] [Google Scholar]
- 24.Takahashi S, Fujiwara M, Watadani K et al. Preoperative Tissue Doppler Imaging-Derived Atrial Conduction Time Can Predict Postoperative Atrial Fibrillation in Patients Undergoing Aortic Valve Replacement for Aortic Valve Stenosis. Circulation Journal 2014; 78: 2173–2181 [DOI] [PubMed] [Google Scholar]
- 25.Ha AC, Mazer CD, Verma S, Yanagawa B, Verma A. Management of postoperative atrial fibrillation after cardiac surgery. Curr Opin Cardiol 2016; 31: 183–190 [DOI] [PubMed] [Google Scholar]
- 26.Kosmidou I, Chen S, Kappetein AP et al. New-Onset Atrial Fibrillation After PCI or CABG for Left Main Disease: The EXCEL Trial. J Am Coll Cardiol 2018; 71: 739–748 [DOI] [PubMed] [Google Scholar]
- 27.Djaiani G, Phillips-Bute B, Podgoreanu M et al. The Association of Patent Foramen Ovale and Atrial Fibrillation After Coronary Artery Bypass Graft Surgery. Anesthesia & Analgesia 2004, DOI: 10.1213/01.ane.0000099721.67426.de: 585–589 [DOI] [PubMed] [Google Scholar]
- 28.Tran DT, Perry JJ, Dupuis JY, Elmestekawy E, Wells GA. Predicting New-Onset Postoperative Atrial Fibrillation in Cardiac Surgery Patients. J Cardiothorac Vasc Anesth 2015; 29: 1117–1126 [DOI] [PubMed] [Google Scholar]
- 29.Mathew JP, Fontes ML, Tudor IC et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291: 1720–1729 [DOI] [PubMed] [Google Scholar]
- 30.Wazni OM, Martin DO, Marrouche NF et al. Plasma B-type natriuretic peptide levels predict postoperative atrial fibrillation in patients undergoing cardiac surgery. Circulation 2004; 110: 124–127 [DOI] [PubMed] [Google Scholar]
- 31.Body SC, Collard CD, Shernan SK et al. Variation in the 4q25 chromosomal locus predicts atrial fibrillation after coronary artery bypass graft surgery. Circ Cardiovasc Genet 2009; 2: 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolek MJ, Muehlschlegel JD, Bush WS et al. Genetic and clinical risk prediction model for postoperative atrial fibrillation. Circ Arrhythm Electrophysiol 2015; 8: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexandre J, Saloux E, Chequel M et al. Preoperative plasma aldosterone and the risk of atrial fibrillation after coronary artery bypass surgery: a prospective cohort study. J Hypertens 2016; 34: 2449–2457 [DOI] [PubMed] [Google Scholar]
- 34.Kashani RG, Sareh S, Genovese B et al. Predicting postoperative atrial fibrillation using CHA2DS2-VASc scores. J Surg Res 2015; 198: 267–272 [DOI] [PubMed] [Google Scholar]
- 35.Lancaster TS, Schill MR, Greenberg JW et al. Potassium and Magnesium Supplementation Do Not Protect Against Atrial Fibrillation After Cardiac Operation: A Time-Matched Analysis. Ann Thorac Surg 2016; 102: 1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sareh S, Toppen W, Mukdad L et al. CHADS2 score predicts atrial fibrillation following cardiac surgery. J Surg Res 2014; 190: 407–412 [DOI] [PubMed] [Google Scholar]
- 37.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014; 130: e199–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagens VE, Ranchor AV, Van Sonderen E et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol 2004; 43: 241–247 [DOI] [PubMed] [Google Scholar]
- 39.Crystal E, Connolly SJ, Sleik K, Ginger TJ, Yusuf S. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: a meta-analysis. Circulation 2002; 106: 75–80 [DOI] [PubMed] [Google Scholar]
- 40.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188 [DOI] [PubMed] [Google Scholar]
- 41.Deeks JJ, Higgins J. Statistical algorithms in Review Manager 5 2010, DOI: [Google Scholar]
- 42.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994; 271: 840–844 [PubMed] [Google Scholar]
- 43.Dun W, Boyden PA. Aged atria: electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol 2009; 25: 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manganas H, Lacasse Y, Bourgeois S, Perron J, Dagenais F, Maltais F. Postoperative outcome after coronary artery bypass grafting in chronic obstructive pulmonary disease. Can Respir J 2007; 14: 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuster RG, Argudo JA, Albarova OG et al. Prognostic value of chronic obstructive pulmonary disease in coronary artery bypass grafting. Eur J Cardiothorac Surg 2006; 29: 202–209 [DOI] [PubMed] [Google Scholar]
- 46.Lip GYH, Coca A, Kahan T et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace 2017; 19: 891–911 [DOI] [PubMed] [Google Scholar]
- 47.Fialova M, Dlugosova K, Okruhlicova L, Kristek F, Manoach M, Tribulova N. Adaptation of the heart to hypertension is associated with maladaptive gap junction connexin-43 remodeling. Physiol Res 2008; 57: 7–11 [DOI] [PubMed] [Google Scholar]
- 48.Pacchia CF, Dosdall DJ, Ranjan R, DiBella E. Alterations in atrial perfusion during atrial fibrillation. Exp Physiol 2014; 99: 1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato T, Yamashita T, Sekiguchi A et al. What are arrhythmogenic substrates in diabetic rat atria? J Cardiovasc Electrophysiol 2006; 17: 890–894 [DOI] [PubMed] [Google Scholar]
- 50.Pop-Busui R Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010; 33: 434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gami AS, Hodge DO, Herges RM et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol 2007; 49: 565–571 [DOI] [PubMed] [Google Scholar]
- 52.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med 2005; 118: 489–495 [DOI] [PubMed] [Google Scholar]
- 53.Wang TJ, Parise H, Levy D et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004; 292: 2471–2477 [DOI] [PubMed] [Google Scholar]
- 54.Hillis LD, Smith PK, Anderson JL et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 124: e652–735 [DOI] [PubMed] [Google Scholar]
- 55.DiNicolantonio JJ, Beavers CJ, Menezes AR et al. Meta-analysis comparing carvedilol versus metoprolol for the prevention of postoperative atrial fibrillation following coronary artery bypass grafting. Am J Cardiol 2014; 113: 565–569 [DOI] [PubMed] [Google Scholar]


