Abstract
Anxiety and sensory symptoms are highly prevalent and meaningful in the daily lives of individuals with autism spectrum disorder (ASD). Despite the importance of carefully measuring, researching, and treating these symptoms, current methods in ASD seldom include self-report. This study investigated the consistency of adolescent and parent reports of anxiety and auditory sensitivity in individuals with ASD, and examined their validity via comparisons with sympathetic arousal at baseline and in response to an auditory challenge. Fifty adolescent-parent dyads (n=26 ASD, n=24 typically developing; 12–16 years old; IQ>80) completed parallel versions of both anxiety and auditory hypersensitivity scales, which were compared to heart rate collected at rest and during an aversive noise task. Adolescents with ASD exhibited greater anxiety and auditory hypersensitivity than their peers, based on both self and parent report. Across groups, self-report was higher than parent report. In individuals with ASD, a significant relationship was found between self-reported anxiety and autonomic arousal at rest, and between self-reported auditory sensitivity and autonomic reactivity during the noise task. These relationships were not significant for parent-report. These findings extend past work by demonstrating greater self-reported (than parent-reported) anxiety and sensory symptoms. Furthermore, the presence of significant correlations between self-reported symptoms and sympathetic arousal supports the validity of self-report in adolescents with ASD with average or above average cognitive abilities. This indicates that adolescents with ASD have a unique perspective on their internal experience, which can complement parent reports and provide a more comprehensive assessment of symptoms in research and clinical settings.
Keywords: Autism, Anxiety, Sensory Sensitivity, Assessment, Autonomic Arousal
Individuals with autism spectrum disorder (ASD) experience difficulties in social communication, as well as restricted and repetitive interests and behaviors (APA, 2013). In addition to these core symptoms, anxiety and atypical sensory processing symptoms are highly prevalent and meaningful in the everyday lives of individuals with ASD. Notably, while anxiety disorders are considered relatively common in the general population (prevalence rate = 28.8%; Kessler et al., 2005), prevalence estimates within ASD range as high as 84%, with many more experiencing subclinical levels of anxiety (White, Oswald, Ollendick, & Scahill, 2009).
Similarly, sensory processing differences occur at a much higher rate in individuals with ASD compared to the general population, with up to 95% of individuals with ASD demonstrating meaningful sensory processing challenges (Baker, Lane, Angley, & Young, 2008; Robertson & Baron-Cohen, 2017; Tomchek & Dunn, 2007), compared to approximately 16% in the general population (Ben-Sasson, Carter, & Briggs-Gowan, 2009). Developmental trends in ASD suggest that sensory symptoms are most prevalent and severe in young children (Kern et al., 2006) and in individuals with higher general symptom severity (Ben-Sasson et al., 2009).
While research across neural and behavioral approaches demonstrates both hyper- and hypo-responsiveness to stimuli across sensory domains in ASD (for a review, see Schauder & Bennetto, 2016), atypical responses to auditory stimuli often stand out as one of the most common and challenging sensory concerns (Klintwall et al., 2011; O’Connor, 2012). Furthermore, auditory hypersensitivity (e.g., exhibiting a larger response than would be expected given the level of stimuli) is a common and challenging pattern of auditory processing differences in ASD (Baranek, Boyd, Poe, David, & Watson, 2007; Mazurek et al., 2013). Several past studies have also identified potential bidirectional links between sensory and anxiety symptoms in ASD (for a review, see Green & Ben-Sasson, 2010), which impacts theoretical perspectives on ASD symptomatology as well as treatment development. Thus, because anxiety and auditory sensitivity symptoms are both prevalent and meaningful in the day-to-day experience of many individuals with ASD, it is crucial to better understand their presence and impact through valid and appropriate assessment measures, to inform proper diagnosis and treatment.
In addition to the conceptual and potentially mechanistic links between anxiety and sensory symptoms in ASD, a shared feature of these symptoms that makes measurement difficult is their primarily internalizing nature. For example, an individual may show some outward signs of anxiety by pacing, breathing heavier, shaking, or even talking about their feelings, but it is likely that they may also be experiencing racing thoughts, an increased heartbeat, difficulty concentrating, or feelings of unease and uncertainty. While a close observer, such as a parent, may be able to report on the individual’s outward signs of anxiety, they may miss or underestimate the frequency and full extent of that anxiety because of their necessarily limited perspective. Similarly, an observer’s understanding of a person’s hypersensitivity to sensory input is often based on behavioral signs. For example, observers may recognize hypersensitivity to auditory input if a child is covering his/her ears or running away from loud noises, but they may miss internal reactions to sounds, such as an increased heartbeat, and worrying about and choosing to avoid certain situations. Because of this, in neurotypical populations, measurement of anxiety and sensory symptoms typically includes self-report for both adults (for a review, see Julian, 2011) and children (for a review, see Silverman & Ollendick, 2005).
Parent-child agreement in neurotypical populations
In general, correlations between self-report and parent-report of psychiatric symptoms tend to be low to moderate in neurotypical, child and adolescent populations (Achenbach, McConaughy, & Howell, 1987; for a review see Klein, 1991; Miller, Martinez, Shumka, & Baker, 2014), with some studies finding that parents report higher levels of symptoms than their children and others finding the opposite pattern (Cosi, Canals, Hernández-Martinez, & Vigil-Colet, 2010; K. Hodges, Gordon, & Lennon, 1990; for a review, see Silverman & Ollendick, 2005). A recent large-scale study by Rappaport, Pagliaccio, Pine, Klein, and Jarcho (2017) investigated child-parent agreement on anxiety symptoms in children (8–17 yrs) and confirmed past findings that non-clinical, healthy children report higher levels of symptoms than their parents, while anxious children typically report the same level of anxiety or less anxiety than their parents, indicating potential reporting differences in clinical and non-clinical samples (e.g., Cosi et al., 2010; Su, Wang, Fan, Su, & Gao, 2008; Wren, Bridge, & Birmaher, 2004). However, as might be expected, child-parent agreement is consistently higher for observable behaviors (e.g., crying, covering ears) than internal mental and physiological experiences (e.g., racing thoughts, increased heart beat Comer & Kendall, 2004; Klein, 1991; Lapouse & Monk, 1958; Sourander, Helstelä, & Helenius, 1999). While differential access to internal states seems to contribute to discordance between child and parent reports, other possibilities have been raised, which suggests that both child and parent reports are limited in their own ways. For example, while parents can only report on what they are told or what they observe, children may be influenced by wanting to appear more or less impaired than they are (Dadds, Perrin, & Yule, 1998), as well as by developmental limitations, such as their ability to understand complex questions (Schwab-Stone, Fallon, Briggs, & Crowther, 1994). Interestingly, however, past research that investigated moderators of child-parent discrepancies, including the children’s age, gender, and social desirability, along with parent’s psychopathology, found that none of these significantly affected overall disagreement (Grills & Ollendick, 2003). Taken together, this research suggests that the discordance between children and their parents is largely due to each informant having unique information on the construct being assessed. Thus, because of the ample evidence indicating child-parent reporting discrepancies and the unique contributions of each reporter on the child’s experience, standard practice in many pediatric settings is to collect diagnostic information from multiple informants (De Los Reyes et al., 2015; Grills & Ollendick, 2002; Wren et al., 2004).
Current assessment practices with adolescents with ASD
While the literature overwhelmingly supports multi-informant report when assessing children and adolescents from the general population, clinicians and researchers have been hesitant to consider any self-reported symptoms in ASD (Mazefsky, Kao, & Oswald, 2011; White et al., 2009). This hesitancy is primarily attributed to presumed challenges in identifying and communicating internal states and emotions in ASD, yet the research in this area is notably mixed and limited. For instance, individuals with ASD are often believed to have lower interoceptive abilities (i.e., the ability to detect internal regulatory states, such as respiration and heart rate) compared to neurotypical individuals. However, the research on group differences in interoceptive accuracy in individuals with ASD is mixed (for a review, see DuBois, Ameis, Lai, Casanova, & Desarkar, 2016), with some studies suggesting decreased accuracy in ASD (e.g., Garfinkel et al., 2016; Quattrocki & Friston, 2014) and others finding comparable or enhanced interoceptive abilities in ASD (e.g., Schauder, Mash, Bryant, & Cascio, 2015).
The studies that have investigated parent-child reports of anxiety symptoms in individuals with ASD with average or above average cognitive abilities have found varying levels of agreement between parents and their children, with the majority of studies finding moderate concordance between informants (Blakeley-Smith, Reaven, Ridge, & Hepburn, 2012; Kerns et al., 2015; Lohr et al., 2017; Ozsivadjian, Hibberd, & Hollocks, 2014). This variability may depend on child and adolescent factors including verbal IQ and abstract reasoning abilities (Blakeley-Smith et al., 2012). However, despite the moderate levels of reporter concordance found in previous studies, it is still common practice to rely solely on parent report of anxiety symptoms in adolescents with ASD. Few studies have included self-report of sensory symptoms in children and adolescents with ASD (De la Marche, Steyaert, & Noens, 2012; Jones et al., 2009), and no existing research has directly compared child and parent report of sensory symptoms in ASD, making it difficult to determine the relative contributions of each informant in understanding these symptoms. While it is likely that, similar to anxiety assessment, sensory symptoms will be best captured via multi-informant reports that provide complementary information, more empirical data is needed to inform future ASD research and clinical practice.
Furthermore, the similarities in the degree of parent-child reporting discrepancies between ASD and neurotypical populations suggests that children and adolescents with ASD may be reporting on different symptom components, which are less available to their parents. Therefore, the absence of self-report measures in the ASD literature is problematic, given that this misses the individual’s perspective on his or her symptoms, and relies on measuring internally experienced symptoms based purely on observable behaviors and spontaneous sharing of emotions and internal states.
Examining the relative validity of parent and adolescent reports
While there are several extant studies in ASD and non-ASD populations documenting discrepancies in multi-informant reporting of symptoms, past research has lacked objective comparison measures to address the relative utility and validity of child versus parent report. One potential method for objectively measuring internalizing conditions is to use biologically-based indices of symptoms. In past research, measures of sympathetic arousal have frequently been related to anxiety and sensory dysfunction in ASD and in non-ASD clinical populations (Green & Ben-Sasson, 2010; for a review, see W. F. Hodges, 2015; White et al., 2009). Specifically, cardiac indices of autonomic dysregulation at rest (e.g., resting heart rate, heart rate variability) have been consistently identified in individuals with higher levels of anxiety; this is true for both individuals with ASD (Kushki et al., 2013; Panju, Brian, Dupuis, Anagnostou, & Kushki, 2015; White et al., 2014) and without ASD (for a review, see Friedman, 2007; Rogeness, Cepeda, Macedo, Fisher, & Harris, 1990). Reactivity to aversive auditory stimuli has also been consistently demonstrated using cardiac indices in individuals with (Rogers & Ozonoff, 2005; Schoen, Miller, Brett-Green, & Nielsen, 2009) and without ASD (for a review, see Babisch, 2006). Many of these studies also included self-reported measures of anxiety and sensory symptoms, and found a significant relationship between these two forms of measurement. However, to date no study includes all three assessment methods (sympathetic arousal, child report, and parent report). Filling this gap in the literature is critical because concurrence of effects provides crucial information for the convergent validity of child and parent report and has the potential to inform future research and clinical assessment of these highly prevalent and clinically meaningful symptoms.
Present study
The current study sought to investigate adolescent-parent agreement on measures of anxiety and auditory hypersensitivity in adolescents with ASD who have average or above-average IQ, and directly compare these questionnaire reports to levels of sympathetic arousal, an objective, biologically-based measure. Specifically, anxiety assessed via questionnaire was compared to arousal levels at baseline, while state-level auditory hypersensitivity assessed via questionnaire was compared to autonomic reactivity to an in-laboratory noise task. To do this, we selected measures for anxiety and sensory symptomatology that included mirrored child (self) and parent reports. To index sympathetic arousal, we measured heart rate based on its past use as a measure and correlate of both anxiety and sensory reactivity in ASD and non-ASD populations (e.g., Friedman, 2007; Kushki et al., 2013), as well as its research and clinical feasibility.
Based on the research reviewed above, we hypothesized that adolescents with ASD and their parents would endorse greater levels of anxiety and auditory hypersensitivity than typically developing adolescents and their parents. Further, we hypothesized that there would be a significant difference in the self- and parent-reported total anxiety and auditory hypersensitivity scores across both groups, with adolescents reporting greater symptoms than their parents. We also hypothesized that self-report of anxiety symptoms, but not parent report, would demonstrate a significant relationship with heart rate at rest and that self-report of auditory hypersensitivity (reactivity), but not parent report, would demonstrate a significant relationship with heart rate reactivity during an aversive noise task. These final hypotheses address the posited reporting discrepancy and were based on the fact that heart rate is an internally-experienced symptom of anxiety and sensory dysregulation, which is likely not outwardly observed or understood by an outside informant.
Directly examining adolescent-parent discrepancies in the reporting of anxiety and sensory symptoms via the use of an objective comparison measure is a novel contribution to this literature and will inform our theoretical understanding of these conditions in individuals with ASD with average or above average cognitive abilities, as well as our use of assessment tools for measuring these internalizing symptoms in research and clinical settings.
Methods
Participants
Twenty-six adolescents with ASD (24 males) and 24 typically developing adolescents (22 males) completed this study (see Table 1 for demographic information). Adolescents were recruited to be between 12–17 years old and with verbal IQ >80, based on the content and verbal demands of the questionnaires and the stabilization of the autonomic nervous system by early adolescence (for a review, see Benevides & Lane, 2015).
TABLE 1.
Enrolled Participant Characteristics
| ASD | TD | |||||
|---|---|---|---|---|---|---|
| M(SD) | Range | M(SD) | Range | |||
| n | 26 | 24 | ||||
| Age (years) | 14.2 (1.4) | 12.0 – 16.7 | 14.8 (1.3) | 12.4 – 16.9 | ||
| Verbal IQ | 113.9 (16.0) | 87 – 138 | 117.7 (14.0) | 87 – 135 | ||
| Performance IQ | 103.6 (11.9) | 78 – 122 | 109.2 (13.4) | 83 – 136 | ||
| Full Scale IQ | 109.9 (12.9) | 84 – 133 | 114.8 (13.4) | 84 – 139 | ||
| SRS Total T-Score | 79.6 (10.6) | 61 – 95 | 40.9 (4.1) | 34 – 49 | ||
| Gender (M:F) | 24:2 | 22:2 |
Note: IQ was measured using the Wechsler Intelligence Scale for Children, 4th Ed. or the Wechsler Adult Intelligence Scale, 4th Ed; values presented are standard scores. SRS=Social Responsiveness Scale.
IQ was measured using an abbreviated version of the Wechsler Intelligence Scale for Children, 4th Ed. (WISC-IV; Wechsler, 2003) or the Wechsler Adult Intelligence Scale, 4th Ed. (WAIS-IV; Wechsler, 2008). Participants were matched by group on Verbal Comprehension Index scores, F(1,46)=0.77, p=.39, , mean age, F(1,48)=2.78, p=.10, , and gender composition, χ2=.007, p=.93. Groups were also not different on race and ethnicity (90% White, 10% Black; χ2=2.28, p=.13), Perceptional Reasoning Index score, F(1,46)=2.38, p=.13, , or Full Scale IQ, F(1,46)=1.66, p=.21, .
Individuals in both groups were recruited from a database of individuals who had participated in past studies or expressed an interest in research. Individuals with ASD originally learned about these research opportunities from advertisements in the general community, through clinic referrals, and through related community organizations/support groups. Control participants were recruited using advertisements in the community (e.g., flyers, post in an e-newsletter about child/adolescent activities in the area), which includes urban and rural settings.
Diagnoses were confirmed in the ASD group using the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 2008), the Autism Diagnostic Interview-Revised (ADI-R; Rutter, Le Couteur, & Lord, 2003), and by clinician judgment. A diagnosis of ASD was ruled out in the TD group using the ADOS and the Social Communication Questionnaire (Rutter, Bailey, Lord, & Berument, 2003). Based on parent report, TD participants did not have any behavioral, learning, or psychiatric diagnoses, nor did they have any first- or second-degree relatives with a diagnosis of ASD. Additional eligibility criteria for all participants included the absence of any history of seizures, use of a pacemaker, medically diagnosed hypertension, a history of cardiac irregularities, and any genetic, neurological, visual, or auditory abnormalities. All participants’ hearing was screened at the time of their visit using audiometry (Maico Diagnostics; Eden Prairie, MN) and each participant had normal clinical thresholds (≤ 20 dB SPL for frequencies .5–4 kHz; and ≤ 25 dB SPL for 8 kHz).
The current study was a project within a larger study examining the impact of aversive noise on autonomic and cognitive functioning in individuals with ASD. The current research focused on child and parent reports of anxiety and auditory sensitivity symptoms, along with indices of autonomic arousal at baseline and in response to a noise challenge. We report all analyses conducted to test a priori hypotheses. All procedures were approved by the University of Rochester’s research subjects review board. Informed consent was obtained from all parents and assent from all children included in the study. Children were paid $15/hour for their participation in the overall study.
Measures
Anxiety Questionnaire.
To compare multiple perspectives on participants’ levels of anxiety, the child- and parent-report versions of the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al., 1997) was included in the current study. The SCARED is commonly used in clinical and research settings and has demonstrated similar psychometric properties in samples of children with ASD compared to those reported for non-ASD samples (Stern, Gadgil, Blakeley-Smith, Reaven, & Hepburn, 2014), supporting its use in the current study. In the current study, the SCARED was programmed in an electronic database and participants and their parents completed their respective versions at home on a computer prior to the laboratory task visit. This was done to eliminate any potential differences in reporting that could arise from completing the questionnaires with an experimenter watching and/or before completing an anxiety-provoking laboratory task. Each version of the questionnaire has 41 items, which are rated on a 0–2 scale (0= Not True or Hardly Ever True; 2= Very True or Often True) and then summed to create a total score. The total score on the SCARED has a suggested clinical cutoff of 25. Both child (self-report) and parent-report versions demonstrated excellent internal consistency in the current sample (αchild=.91; αparent=.95).
Auditory Sensory Sensitivity Questionnaire.
To specifically compare multiple perspectives on participants’ auditory sensory symptoms, the self- and parent-report versions of the Brain Body Center (BBC) Sensory Scales for Children (Kolacz, Raspa, Heilman, & Porges, 2018) were included in the current study. These scales were selected because they have matching self and parent versions, and because they were constructed based on evidence from clinical settings and neuroscience, and consider difficulties in self-regulation as a foundation for the development of sensory vulnerabilities. The BBC Sensory Scales were also programmed in an electronic database and participants and their parents completed them at home on a computer prior to their laboratory visit. The Auditory Threat Hypersensitivity subscale was specifically used for the analyses in the current study because of the study’s focus on auditory sensitivity. This subscale has 9 items, which were rated on a 0–4 scale (0= Not sure/Not applicable; 4=Almost Always) and then summed to create a subscale total score. Clinical cutoffs are not currently available for this measure. The Auditory Threat Hypersensitivity subscale demonstrated good to excellent internal consistency in the current sample (αchild =.79; αparent =.93).
Autism Symptomatology.
The Social Responsiveness Scale (Constantino & Gruber, 2002) is a 65-item parent-report questionnaire designed to assess autism spectrum symptomatology in daily life across a wide range of severity. Parents were asked to rate their child’s behavior over the last 6 months. The SRS items are rated on a 1–4 scale (1=Not True; 4=Almost Always True) and then summed to create a total score. A gender-normed Total T-score was used in analyses.
Noise Task.
The noise task involved participants completing simple memory tasks on the computer while intermittent-gated broadband noise presented at 75dB was played in the background. This task lasted for approximately 15 minutes. The noise was created using Praat software (Boersma, 2002) by randomly mixing short periods of broadband noise and silence. Each moment of noise and silence ranged from 0.3–1.5 seconds. The noise was presented using noise-cancelling Sennheiser HDA200 headphones. The noise level was calibrated using the fast scale of a Quest Model 1900 sound level meter with a ½ inch B&K microphone.
Heart Rate.
Electrocardiogram (ECG) signals were collected at 1000 Hz using Biopac MP150 hardware (Biopac Systems Inc., Goleta, CA) and Acqknowledge software (AcqKnowledge software, Biopac Systems, Santa Barbara, CA, USA). A trained experimenter attached electrodes in a Lead III configuration upon arrival at the lab. After acclimating, participants sat quietly for a 5-minute baseline recording. Heart rate was also measured during a noise task (described above), which represented a sensory challenge in the current study. ECG signals were continuously monitored on a computer in an adjacent room; the experimenter working with the participant was immediately notified if signals looked atypical, at which point adjustments were made to improve signal collection. Following data collection, ECG signals were visually inspected for artifacts and were processed using Mindware software (HRV v3.0.21; Mindware Technologies, Gahanna, OH) by trained personnel. Consistent with standard practices cardiovascular data were ensembled in one-minute segments (for a similar approach, see Jamieson, Nock, & Mendes, 2012). All R-points in the ECG signal (indicating left ventricle contraction) were detected by Mindware HRV software and were also visually examined by trained coders to correct for noise artifacts and inaccurate placements when necessary.
Analytic Strategy
Statistical analyses were performed using SPSS version 24 (IBM, Armonk, NY). Heart rate was successfully collected during baseline and the noise condition for all participants. Two children (1 ASD, 1 TD) and one parent (ASD) did not complete the SCARED. Two children (1 ASD, 1 TD) and one parent (TD) did not complete the BBC Sensory Scales. All participants contributed usable heart rate data at baseline and during the auditory challenge. Pearson correlations were initially used to examine the correlations between the parent- and child-report versions on both the SCARED and the BBC Auditory Hypersensitivity scores, separately by group. We also performed correlations between anxiety, sensory, and overall autism symptoms. Group and reporter differences on measures of anxiety and auditory sensory symptomatology were examined in separate analyses via 2 (group) × 2 (reporter) mixed model ANOVAs. In the ASD-group only, Pearson correlations were used to examine relationships between baseline heart rate and anxiety (measured by the SCARED child and parent reports), and heart rate reactivity to the noise challenge and auditory sensory symptomatology (measured by the BBC Auditory Hypersensitivity child and parent reports). Heart rate reactivity was calculated as the difference in heart rate at rest (averaged across baseline) and during the noise task (averaged across task).
Results
Preliminary analyses.
The distribution of the self and parent reports on the SCARED and the BBC Auditory Hypersensitivity Scale were evaluated for normality and homogeneity of variance. Parent-report on the SCARED was not normally distributed in the TD group, (Shapiro-Wilk p<.001) and the parent report for both groups was not normally distributed on the BBC (Shapiro-Wilk p’s <.05). Levene’s test also indicated unequal variances across groups for adolescent and parent report on both questionnaires (p’s<.05). Although ANOVA has been consistently found to be robust to distributional differences within and across groups (e.g., Blanca, Alarcón, Arnau, Bono, & Bendayan, 2017; Schmider, Ziegler, Danay, Beyer, & Bühner, 2010), we re-ran analyses presented below using Wilcoxon Signed Rank test for the within-group reporter analysis and the Mann-Whitney U test for the between group analysis.
Correlations between child- and parent-report of symptoms
Pearson correlations were used to examine the relationship between self-report and parent-report versions of each questionnaire. Self- and parent-reported scores on the SCARED were marginally correlated in the ASD group, r(24)=.34, p=.10, but were not correlated in the TD group, r(23)=.01, p=.96. Child- and parent-reported scores on the BBC Auditory Hypersensitivity subscale were significantly correlated in the ASD group, r(24)=.49, p=.01, but not the TD group r(23)= .02, p=.92.
Correlations between anxiety and sensory symptomatology
Based on conceptual links between anxiety and sensory symptoms in ASD, Pearson correlations were used to examine the relationship between these symptoms in the current study. Self-reported anxiety and auditory hypersensitivity symptoms were marginally correlated in both the ASD, r(22)=.35, p=.09, and TD, r(20)=.37, p=.09 groups. However, parent-reports of these symptoms were not significantly correlated in either the ASD, r(22)=.27, p=.19, or TD, r(21)=.11, p=.60 groups. Notably, these correlations were all non-significant (p’s>.10) when controlling for the SRS Total T-score. This suggests that a significant portion of the shared variance between anxiety and auditory sensory symptomatology is accounted for by general ASD symptom severity, and that anxiety and sensory symptoms represent distinct constructs in this sample.
Group and reporter differences in anxiety and sensory symptomatology
Anxiety.
To examine group differences on measures of anxiety across self- and parent report a 2 (group: ASD vs. TD) × 2 (reporter: parent vs. child) mixed model ANOVA was performed. As predicted, a main effect of group emerged, with the ASD group showing significantly higher anxiety than the TD group, F(1,46)=42.38, p<.001, . Results also revealed a main effect of reporter, F(1,46)=12.03, p=.001, η2= 0.21, with adolescents reporting higher levels of anxiety than their parents’ ratings of them. See Table 2 for means and standard deviations of the SCARED across groups and reporters. There was not a significant group x reporter interaction, F(1,46)=0.74, p=0.40, , indicating that both groups showed a similar discrepancy between the child and parent reports (see Figure 1). To better understand the null finding for this interaction, we conducted post-hoc Bayes factor analyses (Dienes, 2014), which allowed us to more conclusively determine the likelihood of the null effect in the population (as opposed to a lack of power in our sample to detect statistically significant differences). Bayes factor values less than 0.33 are indicative of evidence in favor of the null hypothesis and values less than 0.10 are strongly indicative. Values greater than 3.0 are indicative of evidence in favor of the alternative hypothesis and values greater than 10.0 are strongly indicative. Values between 0.33 and 3.0 are considered inconclusive based on the data (Lee & Wagenmakers, 2014). Because it was possible that our effects could be in either direction (i.e., either adolescents or parents endorsing greater symptoms), we used a mean of p(population value|theory) of 0 and a 2-tailed distribution. The standard deviation of p(population value|theory) is defined as the maximum plausible effect and this was set to the difference score between the highest and lowest individual values from our sample (Dienes, 2008). The Bayes factor for this interaction effect was 0.11, which confirms that our data supports the null hypothesis whereby the reporter effect did not vary by group.
TABLE 2:
Means, standard deviations, and ranges of questionnaire and autonomic data by research group
| ASD | TD | |||||
|---|---|---|---|---|---|---|
| M(SD) | Range | M(SD) | Range | |||
| n | 26 | 24 | ||||
| Anxiety (SCARED) | ||||||
| Child Report | 22.8 (10.8) | 3 – 44 | 11.04 (6.6) | 0 – 23 | ||
| Parent Report | 18.8 (10.4) | 3 – 35 | 4.6 (5.0) | 0 – 18 | ||
| Auditory Hypersensitivity (BBC Sensory Scales) | ||||||
| Child Report | 18.7 (5.6) | 8 – 35 | 12.2 (2.6) | 7 – 17 | ||
| Parent Report | 16.0 (5.5) | 10 – 27 | 8.7 (2.8) | 4 – 14 | ||
| Heart Rate | ||||||
| Baseline | 83.8 (11.0) | 64.0 – 106.5 | 80.1 (10.9) | 59.5 – 102.4 | ||
| Noise Task minus Baseline | 5.4 (5.0) | −5.0 – 15.0 | 3.2 (4.5) | −7.5 – 13.4 | ||
Note: SCARED = Screen for Child Anxiety Related Emotional Disorders; BBC= Brain Body Center. Possible SCARED range is 0–82 (clinical cutoff at 25). Possible BBC-Auditory Hypersensitivity range is 0–36. Missing data was as follows: Two SCARED-child reports (1ASD, 1 TD), one SCARED-parent report (ASD), two BBC-child reports (1 ASD, 1 TD), and one BBC-parent report (TD). All participants provided usable heart rate data.
Fig. 1.
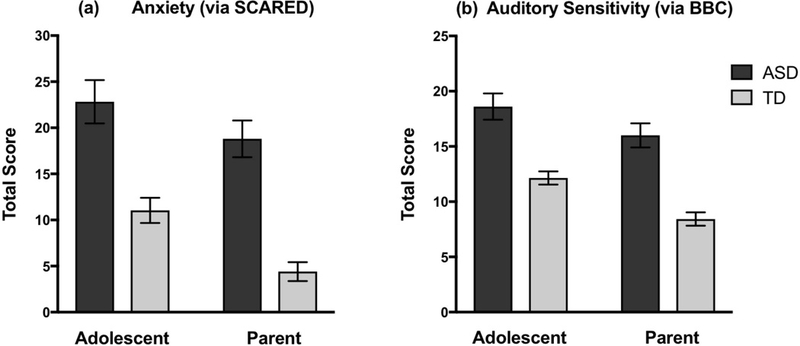
Group and reporter differences in anxiety and auditory sensory symptomatology Note: SCARED = Screen for Child Anxiety Related Emotional Disorders; BBC= Brain Body Center. Error bars represent ± standard error of the mean.
Auditory Sensory Symptomatology.
The BBC Auditory Hypersensitivity subscale was also examined via a 2 (group) x 2 (reporter) mixed model ANOVA. As expected, there was a main effect of group, F(1,46)=42.75, p<.001, , such that individuals with ASD had significantly higher sensory symptomatology than TD individuals. There was also a significant main effect of reporter, F(1,46)=18.58, p<.001, , with adolescents reporting significantly greater sensory symptoms than their parents reported for them. See Table 2 for means and standard deviations of the BBC Auditory Hypersensitivity subscale across groups and reporters. There was not a significant group x reporter interaction, F(1,46)=0.65, p=0.42, , indicating that the reporter difference between children and their parents was similar in both groups. To determine the likelihood of this null finding, we once again conducted post-hoc Bayes factor analyses (Dienes, 2008, 2014). The Bayes factor for this interaction effect was 0.07, which confirms that our data is highly indicative of the null hypothesis, whereby the reporter effect did not vary by group.
We also examined the potential role of age in the group and reporter effects discussed above for the SCARED and the BBC Auditory Hypersensitivity Scale. To do this we performed a median split on age (14.6 years) and re-ran all of the above analyses in the younger and older groups of participants. These results were consistent across the two age groups and with the results in the full sample.
The above analyses of group and reporter effects on anxiety and sensory symptomatology were repeated with non-parametric statistics; all findings were consistent, in direction and significance, with those from the parametric analyses.
Relationship between questionnaire measures and sympathetic arousal
Basal Heart Rate and Anxiety.
Next, we examined the relationship between questionnaire measures (SCARED and BBC Auditory Hypersensitivity subscale) and sympathetic arousal to investigate the relative validity of the child and parent reports of symptoms. These correlations were only performed in the ASD group due to minimal levels of self- and parent-reported symptomatology in the TD group (see Table 2). As a reminder, this lack of variance in symptoms in the TD group was expected given that this sample was specifically recruited to have no current or past psychopathology. Within the ASD group, baseline mean heart rate was positively associated with self-report of anxiety, r(24)=0.47, p=.02 (see Figure 2), but not parent report of anxiety symptoms, r(24)= −0.18, p=.42. The difference between these correlations was significant when converted using Steiger’s Z (test for differences between dependent correlations; Steiger, 1980), Z= 2.80, p=.005.
Fig. 2.
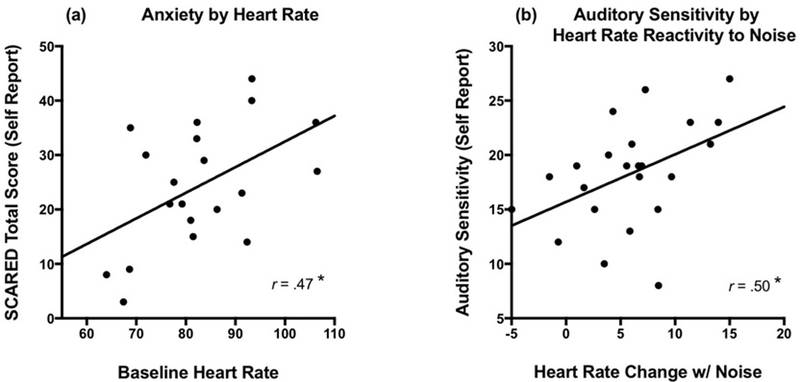
Self-report of symptoms compared to sympathetic arousal in ASD.
Note: SCARED = Screen for Child Anxiety Related Emotional Disorders. Auditory sensitivity was measured using the Brain Body Center Sensory Scales- Auditory Hypersensitivity subscale.
*p<.05
Heart Rate Reactivity and Auditory Hypersensitivity.
To relate day-to-day auditory sensory symptomatology to in vivo reactivity to noise stimuli, the change in mean heart rate from baseline to a noise challenge was examined in relation to self and parent report of auditory sensitivity symptoms. In the ASD group, self-report of everyday auditory hypersensitivity was significantly related to their change in heart rate from baseline during this noise challenge, r(24)=0.50, p=.01 (see Figure 3). Parent report of auditory sensitivity symptoms was not related to the children’s heart rate during the noise challenge, r(25)=0.17, p=.40, although this relationship was only marginally different than the correlation between self-report and heart rate, Z= 1.67, p=.09.
Again, we examined the potential role of age in the relationships between the questionnaire measures and heart rate by performing partial correlations accounting for age. These results were consistent with the results reported above, indicating significant relationships between self-reported symptoms and heart rate in the ASD group (p’s <.02) above and beyond the influence of age. Parent report of symptoms were again not related to heart rate (p>.49) when accounting for age. Additionally, based on known sex differences in the prevalence of adolescent anxiety (Merikangas et al., 2010), all analyses were re-done in a subsample of only male participants. All effects from these analyses were consistent – in direction and level of significance – with those in the full sample reported above.
Discussion
The current study investigated the contributions of self- versus parent-report of anxiety and auditory sensory symptoms in adolescents with and without ASD. These questionnaire reports were then directly compared to levels of sympathetic arousal indexed by resting heart rate (baseline) and heart rate reactivity to an auditory challenge in those with ASD. Results supported previous findings that indicated greater anxiety and auditory sensory symptoms in individuals with ASD compared to TD controls (for reviews, see Rogers & Ozonoff, 2005; White et al., 2009), and that the adolescents in both groups reported greater symptoms than their parent. Critically, this research fills an important gap in the literature by indicating that self-reports (but not parent reports) of anxiety and auditory sensory symptoms from adolescents with ASD with average or above average cognitive abilities were associated with sympathetic arousal. Additionally, follow-up analyses considering age and gender demonstrated the same pattern of results.
The current study makes a valuable contribution to the emerging body of research investigating discrepancies between adolescent self-report and parent report in the level of overall anxiety symptoms in ASD. Of the previous studies investigating self and parent reports of anxiety in ASD, several also used the SCARED (Blakeley-Smith et al., 2012; Lohr et al., 2017; Stern et al., 2014; van Steensel, Deutschman, & Bögels, 2013). These studies confirmed that both the child- and parent-report versions of the SCARED demonstrated similar psychometric properties to those seen in typically developing populations and those with anxiety disorders (Stern et al., 2014; van Steensel et al., 2013). Additionally, van Steensel et al. (2013) concluded that children and adolescents with ASD were able to reliably report on their anxiety symptoms, with 80% of their sample with ASD and comorbid anxiety disorder (based on diagnostic classification using the Anxiety Disorder Interview Schedule) reporting clinically significant levels of anxiety. Of the studies that directly examined the child-parent agreement of symptoms on the SCARED in an ASD group, correlations were generally considered moderate and ranged from r=.39 (van Steensel et al., 2013) to r=.52 (Blakeley-Smith et al., 2012). These correlations are comparable or somewhat larger than those observed in the current study (r=.34), which may be attributable to past studies having a larger proportion of individuals with comorbid anxiety disorder diagnoses, as this has been found to increase child-parent agreement (Nauta et al., 2004; van Steensel et al., 2013).
With regard to the direction of the informant discrepancy, Stern et al. (2014) found that parents generally reported higher levels of anxiety than their children on the SCARED, the opposite pattern of what was observed in the current study. This different pattern may be due to the greater clinical severity of the participants in the Stern et al. (2014) study compared to those in the current sample (Rappaport et al., 2017). It should also be noted that, consistent with the results of the current study, the majority of the neurotypical literature examining child-parent agreement on internalizing symptoms/disorders tends to find greater endorsement of symptoms by child report than parent report (e.g., Cosi et al., 2010; Rappaport et al., 2017), which has contributed to the standard practice of including self-report in neurotypical child and adolescent assessment. This reporting pattern (greater child than parent report of symptoms) is also seen in the results of the current study, with similar reporting patterns observed between the ASD and TD groups. Together, these findings underscore the importance of considering the valuable perspective of children with ASD when measuring their anxiety.
There are currently no existing empirical studies that have directly examined the child-parent agreement on sensory symptoms in individuals with ASD, which makes the current study a notable and novel contribution to the sensory processing literature in ASD. Although some studies have included self-report of sensory experiences in individuals with ASD (e.g., Crane, Goddard, & Pring, 2009; Minshew & Hobson, 2008; Tavassoli, Miller, Schoen, Nielsen, & Baron-Cohen, 2014), these reports were from older adolescents and adults, and were not directly compared to parent report of symptoms or physiological measures of functioning. Adolescent-parent comparisons were possible in the current study because of the use of the BBC Auditory Threat Hypersensitivity subscale, which has parallel self-report and parent-report versions. It is notable that the reporting pattern observed with the Auditory Hypersensitivity scale was very similar to that observed with the anxiety scale. This likely reflects the many similarities inherent in measuring anxiety and sensory symptoms, including that they are both primarily experienced internally and are not fully or readily communicated to outside observers. However, given the degree of variation in past research investigating multi-informant reporter discrepancies in other symptom domains, it will be important to further examine multi-informant reporting of auditory sensory symptoms in future studies.
A major finding of the current study is that self-report of both anxiety and auditory symptoms was significantly correlated with sympathetic arousal in the ASD group, which supports the relative utility and validity of self-report in adolescents with ASD. Importantly, this significant association between adolescent-report of symptoms and arousal was present across measures of both anxiety and auditory symptoms, and across basal arousal and in response to an aversive stimulus. This suggests that adolescents’ insight into their internal experience crosses symptom domains, and that this insight relates to both their basal arousal and their autonomic reactivity to environmental stimuli. While past studies have examined reporter discrepancies in questionnaires by evaluating their convergent validity with other questionnaires (e.g., Lohr et al., 2017) or semi-structured interviews (e.g., van Steensel et al., 2013), these comparisons are confounded by the many similarities between questionnaires and diagnostic interviews. For instance, research on anxiety measurement in neurotypical populations indicates that, similar to questionnaires, interviews often exhibit informant discrepancies that suggest complementary, rather than overlapping contributions from each informant (Klein, 1991). With regard to individuals with ASD, any concerns about their ability to detect and share emotions or internal states would impact questionnaires and interviews alike. Thus, using physiological measures of arousal offers a more distinct and appropriate way to examine the convergent validity of child and parent report. In fact, researchers have specifically recommended a similar approach to that taken here (Silverman & Ollendick, 2005).
Interestingly, we found that self-, but not parent, report of anxiety symptoms correlated with baseline autonomic arousal and that child, but not parent, report of auditory sensory symptoms correlated with autonomic reactivity in response to an aversive auditory task. This suggests that individuals with ASD are able to self-report on components of their anxiety and auditory sensory sensitivities that are related to internal experiences (such as regulatory states or physical sensations), while their parents, understandably, may not have insight into these areas. This demonstrates an added benefit of including child report when assessing individuals with ASD, and affirms that they are able to speak to aspects of their experience that others cannot. Importantly, having greater insight into these internal components, such as internal regulatory states, thoughts, and feelings, is crucial as these are the components of anxiety and sensory processing differences that are highly implicated in underlying theory (Schaaf et al., 2010; Spielberger, 2013) and as targets of treatment (Leahy, 2004; Schwartz & Andrasik, 2017). Moreover, this may be particularly important when trying to understand anxiety symptomatology in individuals with ASD, given that conceptualizing and differentially diagnosing anxiety and ASD can be especially challenging. Part of this difficulty is due to shared clinical presentations across diagnoses, as well as emerging theory that suggests that individuals with ASD may experience an “atypical” anxiety that is not yet fully captured by existing diagnostic manuals or tools (Kerns et al., 2014).
Clinical Implications
The importance of using adolescent self-report in research and clinical settings was supported by the current study’s results indicating (1) that adolescents reported higher levels of anxiety and auditory sensory symptoms than their parents, and (2) that adolescents’ self-reports of symptoms significantly correlated with autonomic measures of anxiety and sensory dysregulation in ASD. While parent report is much more commonly used in assessments of children with ASD, the reliance on the parent’s observation misses the child’s distinct perspective on internally experienced symptoms, which may capture a broader range or greater severity of difficulties. To demonstrate the importance of this distinct perspective, the SCARED can also be analyzed using clinical cutoff scores for the total scale and subscale scores (Birmaher et al., 1997). In the current study, approximately 44% of the ASD sample was above the clinical cut-off for the total score (> 25) based on the children’s self-report, while only 36% were above this cutoff based on parent report (χ2=1.93, p=.09). Given that most clinical referrals for further evaluations and treatment are made based on cutoff scores, this discrepancy would mean that solely collecting parent-reported information on symptoms would miss approximately 8% of the ASD population who may be experiencing clinical levels of anxiety.
Another important clinical implication of the adolescent-parent reporting discrepancy is that it suggests that outside observers may be consistently missing or misinterpreting behaviors that are related to underlying conditions, such as anxiety or sensory difficulties. For example, an adolescent may appear noncompliant or disruptive in a certain context when they may actually be anxious. However, because those around them cannot observe their internal dysregulation or thoughts that precipitated their behaviors, the adolescent’s reaction may be seen as exaggerated or inappropriate. Thus, including the adolescent’s perspective in research can hone our understanding of symptoms and may change impressions of individuals with ASD who may be less likely to spontaneously share information about their internal experiences. Because of this, future work should examine methods and strategies to refine self-report versions of questionnaires and interviews for individuals with ASD and should encourage self-report whenever possible.
Limitations and Future Directions
There are several limitations of the current study which should be considered when interpreting these findings. First, groups were matched on age and results were consistent after accounting for age within the current sample. However, cognitive and biological factors that often—but not always—track with age may better account for changes in emotional development that occur across adolescence. For example, extant research suggests that language level and pubertal status are two important factors to consider (Dahl & Gunnar, 2009). Participants in the current study all had verbal abilities in the average or above average ranges and groups were also matched on verbal IQ, so it is unlikely that general language level influenced emotional development in the current study. However, we did not include a measure of pubertal maturation in this study and there is very limited research examining pubertal maturation in individuals in ASD (Knickmeyer, Wheelwright, Hoekstra, & Baron-Cohen, 2006). Future research should aim to examine pubertal timing in individuals with ASD, as well as the role of pubertal timing in self-report of symptomatology in ASD.
While participants’ age did not impact results within the fairly narrow age range of the current study (12–16 yrs), it will be important to consider age when investigating younger children’s ability to report on internal experiences and emotions. Future research should seek to explore the relationship between multi-informant reports of anxiety and sensory symptomatology and sympathetic arousal in younger children. For example, school-age children (~5–10 yrs) have sometimes been found to be less reliable than older children and adolescents in reporting on anxiety (for a review, see Klein, 1991). As stated above, one potential barrier to assessing young children is the role of language in perceiving and communicating emotions. Behavioral and neurophysiological research within the construction theory of emotion has consistently demonstrated the importance of language in understanding one’s own emotional experiences (for a review, see Barrett, Lindquist, & Gendron, 2007). For example, research in this area suggests that children with language impairment or delays show deficits on emotion perception tasks (Spackman, Fujiki, & Brinton, 2006).
Many factors, including language development, that may present potential challenges to extending this work to younger children may also apply to working with individuals who are minimally verbal or who have lower cognitive abilities. Investigating these relationships in these individuals may require adapting questionnaire measures to accommodate difficulties with verbal comprehension (e.g., using visual aids, inquiring about simpler emotions/physical sensations). However, it has proven important to include self-report in neurotypical populations (Grills & Ollendick, 2002), and the current study’s results support the unique insight gained by using self-report from adolescents with ASD who have average or above average cognitive abilities, suggesting that it is just as important to include the perspectives of individuals with below average cognitive abilities, even if that requires measurement accommodations. Based on the current study’s results, it is also possible that measures of autonomic functioning could be useful in better understanding the internal experience of very young individuals and/or individuals with lower cognitive/language abilities, however more research is needed to establish the utility of autonomic measures as indices of emotional experiences in these populations. For example, if self-report in these populations is not feasible, future research could examine the concurrence of effects between autonomic measures and emotion-driven behaviors, as well as the potential to utilize wearable technology to track arousal and behaviors in real-time (Sano et al., 2018).
While this study supports the use of self-report when assessing anxiety and auditory sensitivity symptoms, future work should expand to other clinical conditions, including other affective disorders (e.g., depression, specific forms of anxiety) and sensory dysfunction in other domains (e.g., visual, tactile). Given that there has been little research specifically examining self-report of sensory symptoms in ASD, it will also be important to refine existing sensory questionnaires and continue to develop new measures that assess a range of sensory experiences for individuals with ASD. Additionally, the use of self-report may help with understanding symptoms that are particularly difficult to assess in individuals with ASD because of similarities in observable components, such as the repetitive behaviors observed in both ASD and obsessive-compulsive disorder. The role of the autonomic nervous system in both anxiety and sensory dysregulation is well supported, making it an appropriate correlate to questionnaire reports of these domains. Future work in other symptom areas will need to be critical in selecting appropriate correlates based on their underlying biological mechanisms.
Based on exclusion criteria specifying that individuals in the control group could not have psychiatric diagnoses, our sample’s typically developing group exhibited few clinically meaningful symptoms of either anxiety or auditory sensory difficulties as measured on either self- or parent-report questionnaires. Because of this, we did not explore the relationship between this group’s self and parent reports and autonomic arousal. While it is unlikely that these questionnaire-autonomic relationships are exclusive to adolescents with ASD, the absence of a comparison group that was more representative of a community or clinical sample makes this difficult to confirm. Investigating the relationship between these different informant reports and arousal in populations of non-ASD children with anxiety and sensory processing differences in the future will be an important next step to expand this work beyond individuals with ASD. Based on the internal nature of autonomic reactivity for all individuals, it is likely that these patterns would extend to non-ASD, clinical populations. However, it is also possible that children with ASD are less likely to spontaneously share information about their internal states to their caregivers than other children, making the differences between the child report and arousal correlation and the parent report and arousal correlation larger in this population. If this is true, researchers may find more similar relationships between child and parent report and arousal in non-ASD populations.
Finally, moderators of the child-parent discrepancy and the questionnaire and arousal correlations were not formally examined in the current study. While this was primarily based on the relatively small sample size and racial/ethnic homogeneity of the current study, future research should also include other measures that may impact this relationship. For example, past research in non-ASD populations found that parent psychopathology acted as a significant moderator of parent ratings of their child’s anxiety (Krain & Kendall, 2000). Importantly, because parents of children with ASD have been found to have higher rates of psychopathology than parents of typically developing children (likely due to both genetic factors and the added stress of raising a child with a disability; Hodge, Hoffman, & Sweeney, 2011) this potential moderator would most likely differentially impact the informant discrepancies seen between groups.
In conclusion, the current study made several important and novel contributions to the anxiety and sensory literature in ASD, as well as the general assessment literature in this population. These contributions support the use of self-report in adolescents with ASD, based on results that suggest that adolescent reports differ from parent reports and that adolescent reports are associated with objective measures of their internal regulatory states. Appreciating the unique contributions of self-report in ASD is critical as we continue to refine our understanding of the symptoms of ASD and how these symptoms intersect with a variety of highly prevalent, but poorly understood comorbid conditions. Additionally, appropriate and valid assessment is crucial to proper diagnosis and treatment of individuals with ASD, and including the individual with ASD in these processes is respectful of their experience and will likely increase both treatment engagement and effectiveness.
Acknowledgements:
This project was supported by grant funding from the Organization for Autism Research (PI: Keith) and the National Institute on Deafness and Other Communication Disorders, grant numbers R01 DC009439 (PI: Bennetto) and R21 DC011094 (PI: Bennetto). We would like to extend our sincere thanks to all of the families that participated in this research. We also thank the research assistants that assisted in data collection and processing, including Meredith Watson, Kelsey Lisbon, Emily Richardson, and Allison Havens.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Informed consent: Informed consent was obtained from all parents and assent from all children included in the study.
Ethical approval: This study was approved by the University of Rochester’s research subjects review board. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Achenbach TM, McConaughy SH, & Howell CT (1987). Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychological Bulletin, 101(2), 213. [PubMed] [Google Scholar]
- APA. (2013). Diagnostic and statistical manual of mental disorders (DSM-5) (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Babisch W (2006). Transportation noise and cardiovascular risk: Updated review and synthesis of epidemiological studies indicate that the evidence has increased. Noise and Health, 8(30), 1. [DOI] [PubMed] [Google Scholar]
- Baker AE, Lane A, Angley MT, & Young RL (2008). The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism and Developmental Disorders, 38(5), 867–875. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Boyd BA, Poe MD, David FJ, & Watson LR (2007). Hyperresponsive sensory patterns in young children with autism, developmental delay, and typical development. American Journal on Mental Retardation, 112(4), 233–245. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Lindquist KA, & Gendron M (2007). Language as context for the perception of emotion. Trends in cognitive sciences, 11(8), 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Carter A, & Briggs-Gowan M (2009). Sensory over-responsivity in elementary school: prevalence and social-emotional correlates. Journal of Abnormal Child Psychology, 37(5), 705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, & Gal E (2009). A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Benevides TW, & Lane SJ (2015). A review of cardiac autonomic measures: Considerations for examination of physiological response in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(2), 560–575. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The screen for child anxiety related emotional disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry, 36(4), 545–553. [DOI] [PubMed] [Google Scholar]
- Blakeley-Smith A, Reaven J, Ridge K, & Hepburn S (2012). Parent–child agreement of anxiety symptoms in youth with autism spectrum disorders. Research in Autism Spectrum Disorders, 6(2), 707–716. [Google Scholar]
- Blanca MJ, Alarcón R, Arnau J, Bono R, & Bendayan R (2017). Non-normal data: Is ANOVA still a valid option? Psicothema, 29(4). [DOI] [PubMed] [Google Scholar]
- Boersma P (2002). Praat, a system for doing phonetics by computer. Glot international, 5(9/10), 341–345. [Google Scholar]
- Comer JS, & Kendall PC (2004). A symptom-level examination of parent–child agreement in the diagnosis of anxious youths. Journal of the American Academy of Child & Adolescent Psychiatry, 43(7), 878–886. [DOI] [PubMed] [Google Scholar]
- Constantino J, & Gruber C (2002). The Social Responsiveness Scale Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Cosi S, Canals J, Hernández-Martinez C, & Vigil-Colet A (2010). Parent–child agreement in SCARED and its relationship to anxiety symptoms. Journal of Anxiety Disorders, 24(1), 129–133. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, & Pring L (2009). Sensory processing in adults with autism spectrum disorders. Autism, 13(3), 215–228. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Perrin S, & Yule W (1998). Social desirability and self-reported anxiety in children: An analysis of the RCMAS Lie Scale. Journal of Abnormal Child Psychology, 26(4), 311–317. [DOI] [PubMed] [Google Scholar]
- Dahl RE, & Gunnar MR (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Development and psychopathology, 21(1), 1–6. [DOI] [PubMed] [Google Scholar]
- De la Marche W, Steyaert J, & Noens I (2012). Atypical sensory processing in adolescents with an autism spectrum disorder and their non-affected siblings. Research in Autism Spectrum Disorders, 6(2), 639–645. [Google Scholar]
- De Los Reyes A, Augenstein TM, Wang M, Thomas SA, Drabick DA, Burgers DE, & Rabinowitz J (2015). The validity of the multi-informant approach to assessing child and adolescent mental health. Psychological Bulletin, 141(4), 858–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienes Z (2008). Understanding psychology as a science: An introduction to scientific and statistical inference: Palgrave Macmillan. [Google Scholar]
- Dienes Z (2014). Using Bayes to get the most out of non-significant results. Frontiers in psychology, 5, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois D, Ameis SH, Lai M-C, Casanova MF, & Desarkar P (2016). Interoception in autism spectrum disorder: A review. International Journal of Developmental Neuroscience, 52, 104–111. [DOI] [PubMed] [Google Scholar]
- Friedman BH (2007). An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Tiley C, O’Keeffe S, Harrison NA, Seth AK, & Critchley HD (2016). Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biological Psychology, 114, 117–126. [DOI] [PubMed] [Google Scholar]
- Green SA, & Ben-Sasson A (2010). Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: Is there a causal relationship? Journal of Autism and Developmental Disorders, 40(12), 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grills AE, & Ollendick TH (2002). Issues in parent-child agreement: The case of structured diagnostic interviews. Clinical Child and Family Psychology Review, 5(1), 57–83. [DOI] [PubMed] [Google Scholar]
- Grills AE, & Ollendick TH (2003). Multiple informant agreement and the anxiety disorders interview schedule for parents and children. Journal of the American Academy of Child & Adolescent Psychiatry, 42(1), 30–40. [DOI] [PubMed] [Google Scholar]
- Hodge D, Hoffman CD, & Sweeney DP (2011). Increased psychopathology in parents of children with autism: Genetic liability or burden of caregiving? Journal of Developmental and Physical Disabilities, 23(3), 227–239. [Google Scholar]
- Hodges K, Gordon Y, & Lennon MP (1990). Parent-child agreement on symptoms assessed via a clinical research interview for children: The Child Assessment Schedule (CAS). Journal of Child Psychology and Psychiatry, 31(3), 427–436. [DOI] [PubMed] [Google Scholar]
- Hodges WF (2015). The psychophysiology of anxiety. In Zuckerman M & Spielberger CD (Eds.), Emotions and Anxiety: New Concepts, Methods, and Applications (pp. 175–194). New York, NY: Psychology Press. [Google Scholar]
- Jamieson JP, Nock MK, & Mendes WB (2012). Mind over matter: Reappraising arousal improves cardiovascular and cognitive responses to stress. Journal of Experimental Psychology: General, 141(3), 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CR, Happé F, Baird G, Simonoff E, Marsden AJ, Tregay J, … Charman T. (2009). Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia, 47(13), 2850–2858. [DOI] [PubMed] [Google Scholar]
- Julian LJ (2011). Measures of anxiety. Arthritis Care & Research, 63(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, … Schroeder JL. (2006). The pattern of sensory processing abnormalities in autism. Autism, 10(5), 480–494. [DOI] [PubMed] [Google Scholar]
- Kerns CM, Kendall PC, Berry L, Souders MC, Franklin ME, Schultz RT, … Herrington J. (2014). Traditional and atypical presentations of anxiety in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(11), 2851–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Maddox BB, Kendall PC, Rump K, Berry L, Schultz RT, … Miller J (2015). Brief measures of anxiety in non-treatment-seeking youth with autism spectrum disorder. Autism, 19(8), 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Klein RG (1991). Parent-child agreement in clinical assessment of anxiety and other psychopathology: A review. Journal of Anxiety Disorders, 5(2), 187–198. [Google Scholar]
- Klintwall L, Holm A, Eriksson M, Carlsson LH, Olsson MB, Hedvall Å, … Fernell E (2011). Sensory abnormalities in autism: A brief report. Research in Developmental Disabilities, 32(2), 795–800. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Wheelwright S, Hoekstra R, & Baron-Cohen S (2006). Age of menarche in females with autism spectrum conditions. Developmental Medicine & Child Neurology, 48(12), 1007–1008. [DOI] [PubMed] [Google Scholar]
- Kolacz J, Raspa M, Heilman KJ, & Porges SW (2018). Evaluating Sensory Processing in Fragile X Syndrome: Psychometric Analysis of the Brain Body Center Sensory Scales (BBCSS). Journal of Autism and Developmental Disorders, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krain AL, & Kendall PC (2000). The role of parental emotional distress in parent report of child anxiety. Journal of Clinical Child Psychology, 29(3), 328–335. [DOI] [PubMed] [Google Scholar]
- Kushki A, Drumm E, Mobarak MP, Tanel N, Dupuis A, Chau T, & Anagnostou E (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS One, 8(4), e59730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouse R, & Monk MA (1958). An epidemiologic study of behavior characteristics in children. American Journal of Public Health and the Nations Health, 48(9), 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy RL (2004). Cognitive-behavioral therapy. In Heimberg RG, Turk CL, & Mennin DS (Ed.), Generalized Anxiety Disorder: Advances in Research and Practice (pp. 265–292). New York: Guilford. [Google Scholar]
- Lee MD, & Wagenmakers E-J (2014). Bayesian cognitive modeling: A practical course New York, New York: Cambridge University Press. [Google Scholar]
- Lohr WD, Daniels K, Wiemken T, Williams PG, Kelley RR, Kuravackel G, & Sears L (2017). The Screen for Child Anxiety-Related Emotional Disorders is sensitive but not specific in identifying anxiety in children with high-functioning autism spectrum disorder: A pilot comparison to the Achenbach System of Empirically Based Assessment Scales. Frontiers in Psychiatry, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, & Risi S (2008). Autism diagnostic observation schedule: ADOS Los Angeles, CA: Western Psychological Services [Google Scholar]
- Mazefsky C, Kao J, & Oswald D (2011). Preliminary evidence suggesting caution in the use of psychiatric self-report measures with adolescents with high-functioning autism spectrum disorders. Research in Autism Spectrum Disorders, 5(1), 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, … Lowery LA (2013). Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. Journal of Abnormal Child Psychology, 41(1), 165–176. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J. p., Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LD, Martinez YJ, Shumka E, & Baker H (2014). Multiple informant agreement of child, parent, and teacher ratings of child anxiety within community samples. The Canadian Journal of Psychiatry, 59(1), 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, & Hobson JA (2008). Sensory sensitivities and performance on sensory perceptual tasks in high-functioning individuals with autism. Journal of Autism and Developmental Disorders, 38(8), 1485–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta MH, Scholing A, Rapee RM, Abbott M, Spence SH, & Waters A (2004). A parent-report measure of children’s anxiety: Psychometric properties and comparison with child-report in a clinic and normal sample. Behaviour Research and Therapy, 42(7), 813–839. [DOI] [PubMed] [Google Scholar]
- O’Connor K (2012). Auditory processing in autism spectrum disorder: A review. Neuroscience & Biobehavioral Reviews, 36(2), 836–854. [DOI] [PubMed] [Google Scholar]
- Ozsivadjian A, Hibberd C, & Hollocks MJ (2014). Brief report: the use of self-report measures in young people with autism spectrum disorder to access symptoms of anxiety, depression and negative thoughts. Journal of Autism and Developmental Disorders, 44(4), 969–974. [DOI] [PubMed] [Google Scholar]
- Panju S, Brian J, Dupuis A, Anagnostou E, & Kushki A (2015). Atypical sympathetic arousal in children with autism spectrum disorder and its association with anxiety symptomatology. Molecular Autism, 6(1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocki E, & Friston K (2014). Autism, oxytocin and interoception. Neuroscience & Biobehavioral Reviews, 47, 410–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport B, Pagliaccio D, Pine D, Klein D, & Jarcho J (2017). Discriminant validity, diagnostic utility, and parent-child agreement on the Screen for Child Anxiety Related Emotional Disorders (SCARED) in treatment-and non-treatment-seeking youth. Journal of Anxiety Disorders [DOI] [PMC free article] [PubMed]
- Robertson CE, & Baron-Cohen S (2017). Sensory perception in autism. Nature Reviews Neuroscience [DOI] [PubMed]
- Rogeness GA, Cepeda C, Macedo CA, Fisher C, & Harris WR (1990). Differences in heart rate and blood pressure in children with conduct disorder, major depression, and separation anxiety. Psychiatry Research, 33(2), 199–206. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, & Ozonoff S (2005). Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry, 46(12), 1255–1268. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C, & Berument S (2003). The social communication questionnaire Los Angeles, California: Western Psychological Services. [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2003). Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services, 29, 30. [Google Scholar]
- Sano A, Taylor S, McHill AW, Phillips AJK, Barger LK, Klerman E, & Picard R (2018). Identifying objective physiological markers and modifiable behaviors for self-reported stress and mental health status using wearable sensor. Journal of Medical Internet Research [DOI] [PMC free article] [PubMed]
- Schaaf RC, Benevides T, Blanche EI, Brett-Green BA, Burke JP, Cohn ES, … May-Benson TA (2010). Parasympathetic functions in children with sensory processing disorder. Frontiers in Integrative Neuroscience, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder KB, & Bennetto L (2016). Towards an interdisciplinary understanding of sensory dysfunction in autism spectrum disorder: An integration of the neural and symptom literatures. Frontiers in Neuroscience, 10, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder KB, Mash LE, Bryant LK, & Cascio CJ (2015). Interoceptive ability and body awareness in autism spectrum disorder. Journal of Experimental Child Psychology, 131, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmider E, Ziegler M, Danay E, Beyer L, & Bühner M (2010). Is it really robust? Methodology
- Schoen SA, Miller LJ, Brett-Green BA, & Nielsen DM (2009). Physiological and behavioral differences in sensory processing: A comparison of children with autism spectrum disorder and sensory modulation disorder. Frontiers in Integrative Neuroscience, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab-Stone M, Fallon T, Briggs M, & Crowther B (1994). Reliability of diagnostic reporting for children aged 6–11 years: A test-retest study of the Diagnostic Interview Schedule for Children-Revised. American Journal of Psychiatry, 151(7), 1048–1054. [DOI] [PubMed] [Google Scholar]
- Schwartz MS, & Andrasik F (2017). Biofeedback: A practitioner’s guide New York, New York: Guilford Publications. [Google Scholar]
- Silverman WK, & Ollendick TH (2005). Evidence-based assessment of anxiety and its disorders in children and adolescents. Journal of Clinical Child and Adolescent Psychology, 34(3), 380–411. [DOI] [PubMed] [Google Scholar]
- Sourander A, Helstelä L, & Helenius H (1999). Parent-adolescent agreement on emotional and behavioral problems. Social Psychiatry and Psychiatric Epidemiology, 34(12), 657–663. [DOI] [PubMed] [Google Scholar]
- Spackman MP, Fujiki M, & Brinton B (2006). Understanding emotions in context: The effects of language impairment on children’s ability to infer emotional reactions. International Journal of Language & Communication Disorders, 41(2), 173–188. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (2013). Anxiety: Current trends in theory and research (Vol. 2). New York, New York: Elsevier. [Google Scholar]
- Steiger JH (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin, 87(2), 245. [Google Scholar]
- Stern JA, Gadgil MS, Blakeley-Smith A, Reaven JA, & Hepburn SL (2014). Psychometric properties of the SCARED in youth with autism spectrum disorder. Research in Autism Spectrum Disorders, 8(9), 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Wang K, Fan F, Su Y, & Gao X (2008). Reliability and validity of the screen for child anxiety related emotional disorders (SCARED) in Chinese children. Journal of Anxiety Disorders, 22(4), 612–621. [DOI] [PubMed] [Google Scholar]
- Tavassoli T, Miller LJ, Schoen SA, Nielsen DM, & Baron-Cohen S (2014). Sensory over-responsivity in adults with autism spectrum conditions. Autism, 18(4), 428–432. [DOI] [PubMed] [Google Scholar]
- Tomchek SD, & Dunn W (2007). Sensory processing in children with and without autism: A comparative study using the short sensory profile. American Journal of Occupational Therapy, 61(2), 190–200. [DOI] [PubMed] [Google Scholar]
- van Steensel FJ, Deutschman AA, & Bögels SM (2013). Examining the Screen for Child Anxiety-Related Emotional Disorder-71 as an assessment tool for anxiety in children with high-functioning autism spectrum disorders. Autism, 17(6), 681–692. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children, 4th ed. San Antonio, TX: Pearson Clinical [Google Scholar]
- Wechsler D (2008). Wechsler Adult Intelligence Scale, 4th ed. San Antonio, TX: Pearson Clinical. [Google Scholar]
- White SW, Mazefsky CA, Dichter GS, Chiu PH, Richey JA, & Ollendick TH (2014). Social-cognitive, physiological, and neural mechanisms underlying emotion regulation impairments: Understanding anxiety in autism spectrum disorder. International Journal of Developmental Neuroscience, 39, 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, & Scahill L (2009). Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review, 29(3), 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren FJ, Bridge JA, & Birmaher B (2004). Screening for childhood anxiety symptoms in primary care: integrating child and parent reports. Journal of the American Academy of Child & Adolescent Psychiatry, 43(11), 1364–1371. [DOI] [PubMed] [Google Scholar]


