Abstract
Although substance use and abuse may impact brain and behavior, it is still unclear why some people become addicted while others do not. Neuroscientific theories explain addiction as a series of between- and within-system neuroadaptations that lead to an increasingly dysregulating cycle, affecting reward, motivation, and executive control systems. In contrast, psychoanalysis understands addiction through a relational perspective wherein there is an underlying failure in affect regulation, a capacity shaped early developmentally. Considering recent findings suggesting the neurobiological overlap of addiction and attachment, it may be possible to integrate both perspectives into a developmental model through the lens of attachment. The goal of the present review is to evaluate the value of neurobiological and psychodynamic perspectives to inform our understanding of addiction, particularly substance-use disorders.
Keywords: Attachment, Addiction, Neurobiology, Psychoanalysis, Developmental Psychopathology
Introduction
Addiction is a psychiatric disorder characterized by a pathological and compulsive pattern of drug-seeking and drug-taking behaviors that occupy an extraordinary amount of an individual’s time and efforts, leading to significant functional impairments to meet the responsibilities of work, school, or home (APA, 2013). Data from the 2013 National Survey on Drug Use and Health suggested that 24.6 million Americans aged 12 years or older had consumed a psychoactive drug a month prior to the survey (NIDA, 2015). Persistent use of psychoactive drugs may lead to long-term changes in the brain, leading to the multiple symptoms and features of addictions, including craving, withdrawal, and tolerance (Robbins, Everitt, & Nutt, 2008; Volkow, Wang, Fowler, Tomasi, & Telang, 2011). The disruptive pattern of drug-seeking behaviors persists despite the negative consequences of addiction, with many individuals struggling to reduce or abstain substance use (Johnson, 2013; Robinson & Berridge, 2008).
Importantly, the initiation of substance use does not necessitate a pathway that leads to abuse and dependence. Therefore, additional factors that may increase susceptibility to addiction warrant consideration. When examining such factors, it may be beneficial to adopt a multidisciplinary perspective, appraising the potential value of integrating the breadth of literature that exists on addiction within individual disciplines. In beginning to address this notion, the goal of the present review is to evaluate whether the consideration of neurobiological and psychodynamic perspectives provides insight to our understanding of addiction, particularly substance-use disorders (SUDs). First, addiction will be discussed from a neurobiological perspective, based on recent neuroscientific findings and with a critical consideration of two central neurobiological theories of addiction –namely the “Opponent-process” (Solomon & Corbit, 1974) and the “Incentive-sensitization” (Robinson & Berridge, 1993) theories. Second, addiction will be explored through a psychodynamic lens to understand some subjective and relational aspects of the disorder. Finally, the value in synthesizing neuroscience and psychodynamic perspectives to our understanding of addiction will be considered, particularly in relation to attachment bonds.
Neurobiology of addiction
From a neurobiological perspective, addictions are understood as a series of within- and between-system neuroadaptations, which may lead to structural and functional brain changes that impact reward processing, executive functioning, and emotion regulation (Koob & Le Moal, 2008; Potenza, 2008; Volkow et al., 2011). In the case of SUDs, the neurochemical properties of substances may exert differential effects on neurotransmitter systems. For example, alcohol and benzodiazepines act upon the γ-aminobutyric acid (GABA) and glutamate systems (Davies, 2003; Gonzales & Jaworski, 1997), heroin and codeine impact the opiate system (Tanda, Pontieri, & DiChiara, 1997), hallucinogens such as lysergic acid diethylamide (LSD), phencyclidine (PCP), and ketamine, influence glutamatergic neurons (Muschamp, Regina, Hull, Winter, & Rabin, 2004), and stimulants such as cocaine, and amphetamines, act on dopaminergic and noradrenergic neurons (Nestler, 2005; Potenza, 2008).
Despite the varying neurotransmitter targets of psychoactive substances, it has been hypothesized that long-term substance use and abuse alters the mesolimbic and nigrostriatal dopaminergic systems, particularly the ventral tegmental area (VTA) and the nucleus accumbens (NAcc) (Everitt, 2006; Rutherford, Potenza, & Mayes, 2013; Volkow et al., 2011). Stimulating drugs have a direct effect on dopaminergic neurotransmission from the VTA to the NAcc (Nestler, 2005; Volkow et al., 2011). For instance, cocaine and methamphetamine block dopamine reuptake, which leads to increased dopaminergic activity from the VTA to the NAcc (Niehaus, Murali, & Kauer, 2010). Nicotine receptors also activate dopaminergic neurons in the VTA (Nestler, 2005). Other substances, including opioids, marijuana, alcohol, and benzodiazepines, work indirectly with this reward neural system, by interacting with the opioid system or with GABAergic interneurons that inhibit the dopaminergic neurotransmission from the VTA to the NAcc (Nestler, 2005; Volkow et al., 2011). Despite data implicating dopamine and related pathways in SUDs, there have also been data questioning how central dopamine is to addictions, with arguably the strongest data in humans being for stimulant addictions (Nutt et al., 2015; Potenza, 2013).
The VTA and NAcc have been conceptualized as an instinctual SEEKING system (Panksepp, 1998), which may underscore shifting or coordinating different motivational behaviors (Johnson, 2013; Robbins & Everitt, 1996). Altered dopaminergic neurotransmission in this SEEKING system may affect other brain regions, including the prefrontal cortex (PFC), hippocampus, anterior cingulate cortex, amygdala, and insula (Koob & LeMoal, 2008; Robbins et al., 2008; Rutherford et al., 2013). For instance, reduced activity in the PFC, particularly in the ventromedial (vmPFC) and orbitofrontal (OFC) areas (Elliott, Dolan, & Frith, 2011), has been associated with compromised executive control and decision-making in SUDs. Further, studies have shown that individuals with SUDs also evidence a reduced activation in the vmPFC and OFC, compared to comparison subjects without SUDs (Bechara, 2003). A similar pattern of reduced vmPFC activation has been found in individuals with impulse control problems such as impulsive aggression and gambling disorders (New et al., 2002; Potenza et al., 2003a; Potenza et al., 2003b; Tanabe et al., 2007). Executive functioning has been consistently associated with the development of self-regulation skills (Barkley, 2001; Baumeister, Smeichel, & Vohs, 2007; Hoffman, Schmeichel, & Baddeley, 2012). Therefore, the aforementioned between-system neuroadaptations that lead to impairments of executive functioning skills (e.g., cognitive control, attention, inhibition, reasoning) might explain the difficulties of addicted individuals in regulating difficult affective states, as will be discussed in following sections when considering the theoretical basis of the neurobiology of addiction.
Neurobiological theories of addiction
There are multiple theories of the neurobiology of addiction, and two prominent theories include the Opponent Process theory and the Incentive-Sensitization theory. Other non-mutually exclusive theories, such as those relating to self-medication (Khantzian, 1985), reward deficiency (Blum et al., 1996), allostatis (Koob & LeMoal, 2001), neurodevelopment (Chambers, Taylor, & Potenza, 2003; Somerville, Jones, & Casey, 2010), novelty/impulsivity and habit/compulsivity (Everitt & Robbins, 2005), and genetics (Ducci & Goldman, 2012), among others (Buisman-Pijlman et al., 2014; Sussman & Sussman, 2011) have also been proposed. To focus this review, we will direct attention to the Opponent Process theory and the Incentive-Sensitization theories.
The Opponent-Process theory (Solomon & Corbit, 1974) suggests that once a pleasurable state (a-process) is initiated in the brain, a series of opposing mechanisms (b-process) down-regulate or reduce the intensity of that hedonic or aroused state to bring the body back to homeostasis. In the case of SUDs, the a-process may be the initial rewarding state generated by the substance (e.g., euphoria, relaxation, disinhibition). In contrast, the b-process reflects the counter-adaptive changes that restore the body to its original state, which would include negative affective states or withdrawal symptoms (e.g., shakiness, sweating, irritability, thirst). Given the long-term chemical, structural, and functional effects of substance use on the brain, over time the b-process does not fully return the body to homeostasis (Koob & LeMoal, 2001). The remaining discomfort or distressing affective states are associated with memories of the original rewarding experience from the a-process, thus motivating the individual to crave or keep procuring the substance (Koob & LeMoal, 2008). This leads to an increasingly dysregulating cycle of positive (i.e., pleasure) and negative (i.e., withdrawal) reinforcement processes that eventually lead to the generalization of all negative emotions as needing to be modulated by substance use, perpetuating the addictive cycle (Koob & LeMoal, 2001, 2008). The opponent-process theory provides a model to understand the within- and between-system neuroadaptations that lead to the downward spiral of symptoms that characterize addictions; however, although not excluded, the model does not speak to the individual variation in vulnerability to addiction wherein some, but not all, substance users transition to substance abuse and dependence.
The Incentive-Sensitization theory (Robinson & Berridge, 1993) proposes that the persistent use of psychoactive substances leads to a process of brain sensitization toward substance-related cues or incentives. This sensitization manifests behaviorally through increased attentional bias towards these substance-related cues or incentives, which can be measured experimentally in research settings (Lubman, Peters, Mogg, & Bradley, 2000; Townshend & Duka, 2001). One example could be the dot-probe task, used to assess reaction times to certain stimuli, where pairs of images (one of them the desired cue) are shown side by side on a screen for 500 ms and then replaced by a probe (asterisk) replacing one of the photographs; the participant must then press a key as quickly as possible to indicate where the probe was located. Using this task, Ehrman and colleagues (2002) evidenced that current nicotine smokers displayed a significantly greater attentional bias (i.e., faster reaction times) toward cigarette cues than non-smokers. Concurrently, impaired executive functions that may reflect existing deficits and/or consequences of substance use itself (Buisman-Pijlman et al., 2014; Volkow et al., 2011) alongside increased sensitization to substance-related cues or incentives, are thought to underlie the core symptoms of addiction, namely tolerance, withdrawal, and a long-lasting incentive motivation for the substance even after cessation (Potenza, 2008).
Importantly, incentive sensitization compels persistence in substance use irrespective of whether the individual dislikes the substance and its negative consequences, if they are attempting to abstain, or even in the absence of withdrawal symptoms (Robinson & Berridge, 2004). Also, Robinson and Berridge (2008) argue that the insidious brain changes that sensitize the brain to drug-related cues can lead to relapse even long after the disappearance of withdrawal symptoms. Furthermore, incentive sensitization can be both explicit and implicit. This means that it can take the form of a conscious “craving” when triggered by explicit drug-related cues, such as images or conversations; or of unconscious “wanting” when triggered by implicit or context-specific cues, such as internal states, scents, environments, or interactions in which the drug used to be consumed (Anagnostaras, Schallert, & Robinson, 2002; Nocjar & Panksepp, 2002). Therefore, this model considers addiction as self-sustaining, wherein the individual becomes addicted to procuring the substance of abuse rather than to the psychophysiological effects of using that substance (Robinson & Berridge, 1993, 2001, 2004, 2008), and might explain why not everyone that consumes a psychoactive substance becomes addicted to them.
Taken together, neurobiological approaches have provided critical insight into the mechanisms that may underscore the transition from substance use to abuse and dependence. This neuroscience perspective offers the opportunity to understand more regarding the physical and chemical mechanisms behind addictive processes. However, these theories may not fully capture aspects of conceptualizing the subjective and relational factors in the pathway from substance use to abuse and dependence, which may play a critical role to increasing addiction vulnerability – particularly across development. This may limit the value of neurobiological approaches to addiction when considered in isolation of these subjective and relational factors.
Psychodynamic theories of addiction
A significant strength of psychodynamic theory has been the focus on interpersonal and intrapersonal factors, beginning as early as during parent-child interactions and the emergence of unconscious motivations, factors that may manifest in behavior across development. Overall, modern psychodynamic theories (based primarily on case studies) suggest that there are three interrelated factors that lead to addiction: (1) underdeveloped ego-functions and defense mechanisms; (2) a failure in symbolization of the soothing qualities of internal objects; and, (3) a deviant positioning towards pleasure in the form of jouissance (tr. enjoyment).
First, from an ego-psychology perspective, addiction is more likely in individuals with underdeveloped executive functioning or ego-functions, primarily reality testing, stimulus barrier, judgement, impulse control, and the synthetic-integrative function (Bellak, Hurvich, & Gedeman, 1973). This vulnerability, coupled with a stressful or demanding environment, hinders the proper development of the superego and limits the ego’s ability to develop more mature defense mechanisms for self-regulation (e.g., repression, displacement, sublimation, or humor; Freud, A., 1937). The ego is therefore limited to more primitive defensive strategies, including denial, idealization, and projective identification –defense mechanisms that are commonly reported in patients with addiction (Freud, A., 1937; Kernberg, 1975). Therefore, when such a fragile core-ego is faced with the hedonic demands of the Id, it may give in to the demands, for example, by procuring and consuming psychoactive drugs, or engaging compulsively in gambling, sex, or binge-eating (Freud, S, 1915; Fonagy & Target, 2008).
Second, an object-relations perspective proposes that to understand addiction vulnerability, a focus on the relational and representational aspects of development is needed, wherein, over time, the mind develops in relation to others, primarily with early caregivers. Through experience, these interactions become embedded in the child’s internal world as mental representations or internal objects (Beres & Joseph, 1970; Kohut, 1979; Stern, 1983). These imaginary representations are imbued with real (i.e., conscious) and fantasized (i.e., unconscious) qualities of significant others and relationships. Throughout development, these internal objects and their imaginary interaction with the external world model and guide future social interactions by means of associative learning (Johnson, 2013). Therefore, addictions may be viewed as the consequence of poor object relations. The failure of primary caregivers at providing proper care and affection is thought to be experienced by the infant as a “nameless dread” (Bion, 1962), or as having lost the object’s love (Freud, S., 1917; Klein, 1940). This highly distressing internal state is thought to thwart the infant’s ability to integrate the good (i.e., nurturing, soothing) and bad (i.e., distressing, frustrating) qualities of the internal object. Individuals with addiction may reach out for an “external regulator” (i.e., drug) to emulate the soothing qualities of the good object and “wall-off” the distressing bad object (Kernberg, Diamond, Yeomans, Clarkin, & Levy, 2008; Krystal, 1978).
Eventually, most individuals with an addiction reach a more mature and realistic psychological state, in which defenses may be more stable and the individual less threatened by the internal and external worlds (see depressive position in Klein, 1946). However, it has been argued that this developmental achievement is not stable enough given the absence of “containment” (Bion, 1962) or psychological support from early caregivers. Therefore, individuals with addiction are more prone to retreat to more primitive coping strategies and psychological states when negative emotions emerge (e.g., withdrawing, or returning to past relationships, behaviors, or fears; Kernberg, 1975). Therefore, addiction may be understood as a failure in the ability to evoke the soothing qualities of the good internal object (i.e., symbolization; Bion, 1962; Klein, 1930; Segal, 1998), or as an attempt to “control” these object qualities through the use of drugs to modulate feelings of distress (Waska, 2006).
Third, the Lacanian psychoanalytic movement explains addiction from the perspective of jouissance: a paradoxical –enjoyable yet intolerable– form of pleasure (Bazan & Detandt, 2013; Loose, 2002). From this perspective, the way in which an individual subjectively positions themself in relation to the social contract (i.e., The Other), the notion of otherness in society, and most importantly, the prohibition (i.e., castration) from overindulging with jouissance, will result in the different personality structures and psychopathologies (Bailly, 2009; Fink, 1999; Lacan, 1964). For instance, the psychotic structure “forecloses” or rules out castration and The Other. Thus the psychotic individual is subjectively ‘entrapped’ in their own logic and is incapable of using the symbolic meaning of symptoms to communicate and reduce distress (Fink, 1999; Loose, 2002). Therefore, whenever these individuals experience intense physical or psychic symptoms, such as hallucinations, racing thoughts, anxiety, or fear (i.e., jouissance), they may become overwhelmed and seek out substances to protect themselves from the experience (Loose, 2002). In that sense, one could argue that psychotic-type addictions use substances as pacifiers against extreme jouissance irruptions that threaten to ‘destroy’ the psyche.
On the contrary, when an individual recognizes castration, and thus the notion of rules, impossibility, and otherness in society, two alternatives become available to deal with the resulting frustration. The first alternative is to repress the castration, as is the case of the neurotic structure. Such an approach would defy the rules of jouissance (e.g., by only using drugs in social gatherings) but with a resulting quota of guilt or shame for challenging The Other (i.e., social contract). The second alternative is to disavow or “pretend” as if castration never took place, resulting in the perverse structure where the individual bends the rules of jouissance to their own benefit despite The Other; for example, by knowing exactly when or how to use drugs to avoid testing positive in a drug test from work. In both cases, addiction would be understood as an act of rebellion against castration, by self-administering an extra quota of jouissance (i.e., plus de jouir) with substance use (Bazan & Detandt, 2013; Lacan, 1969; Loose, 2002). Therefore, addiction in neurosis and perversion may be understood as overindulgence in the hedonic properties of drug-taking behaviors in an attempt to avoid acknowledging, and effectively dealing with, frustration. Some forms of frustration may be social norms and boundaries, social rejection, loneliness, or loss (Bazan & Detandt, 2013; Loose, 2002).
Taken together, these theories consider addictions as originating during the earliest stages of human interaction, whereby constitutional vulnerabilities –paired with demanding environments– lead to a more overarching failure in the individual to recognize, understand, contain, and regulate difficult affective states. The psychodynamic approach may provide particular insight to what protects, predisposes, and maintains substance abuse from a longitudinal and intrapsychic point of view; however, the individualized nature of its methodology make it difficult to test its hypothesis on the wider population. Therefore, attempts at grounding psychodynamic theories on replicable methodologies and objective data are important.
Integrating perspectives: The role of attachment
Addiction can be understood from multiple perspectives and here we have focused on addiction through the lens of neurobiology and psychoanalysis. The neurobiological perspective provides a considerably structured and empirically-based approach, acknowledging that substance use leads to a series of neurochemical reactions in the brain that have structural and functional neuroadaptations. The opponent-process approach (Solomon & Corbit, 1974) suggests that the shift from substance use to substance abuse is generated by the transition from positive to negative reinforcement processes motivating continued substance use. From the perspective of incentive sensitization (Robinson & Berridge, 1993), the shift reflects an associative learning process mediated by a neurobiological sensitization to substance-related cues. Taken together, and according to incentive-learning principles (Bouton & Nelson, 1998), it is possible that before drug-related cues become meaningful enough to ‘incentivize’ drug use, they first need to be paired with the consequences of drug-use via repetition and reinforcement. Notwithstanding the specific mechanism, it seems that an important factor leading to substance dependence may be the (internal and external) context in which the individual and its addiction are embedded.
Psychodynamic theories also emphasize the role of context in the development of addictions. Parallel to neurobiological accounts, addictive processes are intrasystemic Id conflicts (Johnson, 2013) related to (a) underdeveloped ego functions and defense mechanisms (Freud, A. 1937), (b) failures in symbolization (Kernberg et al., 2008), or (c) a pathological relationship to the pleasure principle (Bazan & Detandt, 2013). However, adverse caregiving experiences in early life may in particular foster the aforementioned deficits, as they lead to conflicting mental representations of self and others (Fonagy & Target, 2008). Such disorganized mental representations may thwart the individual’s ability to make sense of their own mental and physical experience, and consequently motivate substance use and abuse to escape discomfort (Kernberg, Diamond, Yeomans, Clarkin, & Levy, 2008).
Despite the contrast of neuroscience and psychoanalysis with respect to their differential methodology and focus on objectivity versus subjectivity, these disciplines may share some commonalities with respect to our understanding of addiction. First, addiction may be viewed as impairments of executive functioning that thwart effective self-regulation in the face of internal or external stressors –albeit by seemingly different mechanisms: compromised neural activity (Volkow et al., 2011) or underdeveloped ego-functions and defense mechanisms (Freud, A. 1937). Second, the pathological motivation to persist in substance-using behaviors is associated with aberrant reward-processing –again with seemingly different underlying mechanisms: compromised neural activities relating to reward-processing (Panksepp, 1998) or the pleasure principle overriding the reality principle (Loose, 2002). Third, both neurobiological and psychodynamic approaches indicate the importance of internal subjective processes –either in the form of associative learning (Robinson & Berridge, 1993) or mental representations of good and bad objects (Waska, 2006).
Given the aforementioned commonalities amongst neurobiological and psychodynamic accounts of addiction, can we synergistically bring these approaches together to further understand the nature of addiction to optimize intervention and prevention efforts? We propose that adopting an attachment-based framework to identify mechanisms that promote, or protect against, the development of addiction could be valuable –reflecting the proposal that addiction may be better understood as a developmental disorder, in which genetic, epigenetic, and neurobiological factors interact with adverse caregiving experiences during key developmental stages, increasing the risk of future SUDs (McCrory & Mayes, 2015; Puetz & McCrory, 2015). Consistent with this notion, high rates of comorbidity between SUDs, trauma histories, and psychiatric disorders have been reported (Espinosa, Beckwith, Howard, Tyler, & Swanson, 2001; Milby, Sims, Khuder, Schumacher, & Huggins, 1996; Suchman & Luthar, 2000). A growing body of evidence also suggests an intergenerational transmission of attachment patterns and poor developmental outcomes of children of addicted parents, including substance abuse (Salo & Flykt, 2013; Slade, Grienenberger, Bernbach, Levy, & Locker, 2005; Fonagy, & Target, 2005; Lyden & Suchman, 2013; Stacks et al., 2014). Finally, individuals who have experienced early adversity may have greater difficulties with affect regulation and engaging in rewarding relationships, which may render them vulnerable to turn to drug use as a means of coping (Crittenden, 2015; Fonagy & Target, 2008). Taken together, an attachment perspective affords the opportunity to adopt a developmental stance in the understanding of addiction and the contribution of neurobiology and psychoanalysis in this endeavor.
Attachment Theory: Mental representations, reflective functioning, and addiction
Central to this attachment-based integration of neuroscience and psychoanalysis are the mental representations of attachment or the internal working models of expectations and attributions about the mother, the child, and the dyadic relationship (Bowlby, 1988). These representations guide behaviors, attitudes, and expectations, and emerge during the first mother-infant interactions (Huth-Bocks, Muzik, Beeghly, Earls, & Stacks, 2014; Suchman, McMahon, Zhang, Mayes, & Luthar, 2006). Furthermore, these representations are constantly revised and expanded to adapt to increasingly complex relationships, environments, and –most importantly– danger, thus having a direct impact on self-regulatory, motivational, and executive skills (Crittenden, 2015; Fonagy, Gergely, Jurist, & Target, 2004; Isosävi et al., 2016; Lyden & Suchman, 2013; Wiseman, Hashmonay, & Harel, 2006).
Considering their role in organizing behaviors and expectations in relation to danger and adversity, attachment representations may be a central element in understanding addictions across generations. Studies have emphasized how extreme childhood experiences, including trauma, abuse, and adversity, can be barriers to coherent and secure attachment representations (Speranza, Nicolais, Vergano, & Dazzi, 2017). For instance, using the Adult Attachment Interview (AAI; George, Kaplan, & Main, 1985), Speranza and colleagues (2017) identified that adults with extreme early childhood adversity were more likely to present a collapse in reasoning and discourse, alongside extreme behavioral reactions, with odd or lacking descriptions of primary attachment relationships. This complex pattern is common in clinical samples and is typically defined either as “unresolved/disorganized” (U/D) or as an unspecific “cannot classify” (CC) attachment category, with the development of new coding systems and extension of existing coding systems to provide more insight into this complex behavioral pattern observed in the AAI (Crittenden, 2015; Goldwyn & Hugh-Jones, 2011; Koren-Karie et al., 2003; Lyons-Ruth, Yellin, Melnick, & Atwood, 2005). Notably, Speranza and colleagues (2017) redefined this apparent breakdown of reasoning and discourse observed in the AAI into a more flexible and clinically meaningful “low-coherence CC” category, which is characterized by emptiness, inconsistency, and fragmentation. Consequently, absent or traumatically ruptured attachments are expected to impact the development of personal identity and affect regulation (Berner, Carlos, & Whipple, 2010; Fonagy et al., 2004; Speranza et al., 2017).
This ‘cannot classify’ category is important to the current discussion given the high index of early childhood adversity, rejection, neglect, and low support in their upbringing, that has been reported in substance-abusing populations (Kaltenbach, 2013; Suchman et al., 2012). In fact, mothers with SUDs –especially those with comorbid psychiatric problems– are more likely to have their own histories of abuse and neglect (Isosävi et al., 2016; Freeman, Collier, & Parillo, 2002; Medrano, Hatch, Zule, & Desmond, 2002; Suchman et al., 2012). Likewise, numerous studies have found that unresolved and insecure attachment representations are more common in mothers with SUDs than in those without SUDs (Espinosa, Beckwith, Howard, Tyler, & Swanson, 2001; Isosävi et al., 2016; Medrano et al., 2002; Sokolowsky, Hans, Bernstein, & Cox, 2007; Suchman et a., 2012). What is more, those with dismissive and intrusive internal representations are also more likely to lose custody of their children (Suchman et al., 2006). This might explain why children born to mothers with SUDs are more likely to be insecurely attached or present disorganized attachment patterns, possibly reflecting –as with their mothers– compromised internal representations and unintegrated states of mind (Crittenden, 2015; Lyons-Ruth et al., 2005; O’Connor, Kogan, & Findlay, 2006; Rodning, Beckwith, & Howard, 2008; Speranza et al., 2017; Swanson, Beckwith, & Howard, 2010; Zeanah & Boris, 2000). Thus, an attachment-based perspective begins to illuminate mechanisms that may underscore intergenerational transmission of risk for addiction vulnerability.
Early attachment experiences can also affect a mother’s capacity to interpret her behavior and that of her child in terms of mental states and intentions (Fonagy & Target, 1997; Fonagy, Steele, Steele, Moran, & Higgitt, 1991; Huth-Bocks et al., 2014). This capacity, also known as parental reflective functioning, may be essential for mothers to make sense –and adapt to– the child’s preverbal demands (Fonagy & Target, 1997; Slade, 2005). Parental reflective functioning is correlated with maternal sensitivity –even more so than attachment representations– and has been associated with, and may potentially develop from, executive functioning skills and attachment interactions (Alvarez-Monjaras, McMahon, & Suchman, 2017; Demers, Bernier, Tarabulsy, & Provost, 2010; Farrow & Blissett, 2014; Huth Bocks et al., 2014; Rutherford et al., 2018; Stacks et al., 2014). However, reflective functioning is limited in mothers with addiction, who tend to find it difficult to take their infant’s perspective, interpret and respond to their affective needs, respect their interests, and modulate their emotional responses (Hans, Bernstein, & Henson, 1999; Suchman et al., 2008, 2010, 2011, 2012). Notably, lower levels of parental reflective function have also been associated with poorer executive functioning in mothers with substance use disorders (Håkansson et al., 2018). Given that prenatal exposure to drugs may make infants more irritable and difficult to soothe (Eiden, Schuetze, & Coles, 2011; Siqveland, Haabrekke, Wentzel-Larsen, & Moe, 2014; Siqveland & Moe, 2014), the already underdeveloped reflective functioning capacities and potentially compromised executive functions may significantly disadvantage mothers with addiction during complex caregiving interactions. Consequently, children of substance-abusing mothers may also be more vulnerable for developing maladaptive attachment representations and poor executive functioning and self-regulatory skills, increasing their own vulnerability for addiction in the future. Therefore, it is important to acknowledge that direct effects of attachment on addiction may be mediated by multiple mechanisms, including executive functioning and parental reflective functioning.
Considering their close links with executive functioning and emotion regulation, both attachment representations and parental reflective functioning have been considered essential mechanisms of change in improving maternal caregiving behaviors and thus reducing the risk for addictive processes in the offspring (Alvarez-Monjaras et al., 2017; Isosävi et al., 2016; Rutherford, Wallace, Laurent & Mayes, 2015; Rutherford, Booth, Crowley, & Mayes, 2016; Suchman et al., 2012). As such, holistic treatment alternatives targeting these factors in both the child and the mother have been recommended (Neger & Prinz, 2015; Suchman, Mayes, Conti, Slade & Rounsaville, 2004). Attachment- and mentalization-based interventions have gained popularity as effective treatments for patients with SUDs (e.g., Dawe, Harnett, Staiger, & Dadds, 2000; Söderström & Skarderud, 2009; Suchman, DeCoste, Castiglioni, Legow, & Mayes, 2008). These interventions are effective in improving emotional bonds, maternal reflective functioning skills, and mother-infant interactions so as to reduce the likelihood of addiction and maladaptive attachment styles in future generations (Pajulo & Kalland, 2013; Suchman, et al., 2008, 2011, 2013).
Attachment and Neurobiology
An attachment perspective also allows the integration of neurobiological processes. Converging research suggests that addiction and attachment have overlapping neural pathways. Specifically, addiction and attachment may engage the mesocorticolimbic and nigrostriatal dopaminergic systems as well as the oxytocinergic system (Buisman-Pijlman et al., 2014; Johns, Lubin, Walker, Meter, & Mason, 1997; Strathearn, 2011). While the dopaminergic system has been implicated in motivation and reward processing (Robbins & Everitt, 1992), the oxytocinergic system plays a central role in mood and self-regulation and social behaviors important to attachment (Buisman-Pijlman et al., 2014; Feldman, Weller, & Zagoory-Sharon, & Levine, 2007; Strathearn, 2011; Tops et al., 2014). Data suggest that early stress and traumatic attachment experiences may hinder the development of the endogenous oxytocinergic system, increasing vulnerability to future addictive behaviors (Ammerman, Kolko, Kirisci, Blackson, & Dawes, 1999; Bremner & Narayan, 2008; Chaplin & Sinha, 2013; Sinha, 2001; Tops et al., 2014). Extending this attachment and addiction model further, it has been hypothesized that when the addicted adult transitions to their parenting role, the co-optation of reward neural circuits by addiction decreases the salience and pleasure in caregiving (Rutherford & Mayes, 2017; Rutherford, Potenza, & Mayes, 2013; Rutherford, Williams, Moy, Mayes, & Johns, 2011). This resonates with the aforementioned object relations theories, where early mother-infant interactions become embedded into the child’s psyche as mental representations of soothing, caregiving, and interacting that influence behavior and attribution biases across the lifetime.
Echoing psychodynamic object relations theories, secure attachment bonds have been suggested to protect an individual from developing an addiction (Crittenden, 2015). For instance, sensitive parenting has been found to promote the development of the executive functioning skills and self-regulation (Berner et al., 2010). It has been argued that growing up in a nurturing environment could promote a more effective distress regulation system and a greater ability to refrain from overindulging in recreational drugs (Fonagy et al., 2004). Importantly, not all insecurely attached children develop an addiction later in life (Schindler & Bröning, 2015). Therefore, while distorted or negative attachment representations, along with a shortage of salient soothing and positive attachment experiences, may function as important vulnerability factors for the derogation or substitution of social rewards with the intensely rewarding effects of drugs (Rutherford & Mayes, 2017; Tops et al., 2014), other factors are important to consider, which might include the potential genetic basis of addiction.
Twin, adoption, and molecular genetic studies have established that substance abuse has a significant heritable component, with gene-environment interactions having an effect on the risk of developing substance abuse problems during adolescence (Enoch, 2012; Goldman, Oroszi, & Ducci, 2005; Young-Wolff, Enoch, & Prescott, 2011). Gene-environment interactions, or the effect of certain environments over the phenotypic expression or suppression of genomic traits, have been found between early adversity and substance abuse, particularly for the gene PER1 (involved in stress-related alcohol consumption in rats; Dong, 2011), the long allele of the SLC6A4 gene of the serotonin transporter (involved in addictions and psychiatric disorders; Graff-Guerrero et al., 2005), and GABRA2 haplotype (associated with resilience to developing an addiction in individuals exposed to childhood maltreatment; Enoch, 2012). Overall, findings from genetic studies suggest that even though genes modulate addiction susceptibility, it may be the interaction between genes and environmental circumstances that is responsible for the individual differences predisposing to drug use, abuse, and dependence (for a more detailed account see Goldman, Oroszi, & Ducci, 2005). In other words, the mere genetic predisposition for substance abuse and dependence (e.g., SLC6A6 long allele) may not be not enough to develop an addiction as it may also require the presence of environmental risk factors (e.g., childhood maltreatment or other adverse childhood experiences) to manifest. As psychiatric disorders like addictions are thought to involve multiple small contributions from many genes (as well as interactions with multiple environmental factors), considerable additional research is needed to determine the proportion of variance that specific allelic variants contribute in specific environmental contexts.
Epigenetic mechanisms are important to consider, given that environmental factors may shape the way in which genes are expressed in the genome, without modifying the DNA sequence. For instance, DNA methylation is an epigenetic mechanism associated with decreased gene expression that may lead to long-term changes in the brain (Cecil, Walton, & Viding, 2015). Repeated administration of drugs in rodents has been found to modify DNA methylation patterns in the “reward” regions of the brain (e.g., striatum and NAcc) and to influence synaptic plasticity and memory consolidation, thus contributing to the physiology of addictive processes (Colvis et al., 2005; Renthal et al., 2005; Wong, Mill, & Fernandez, 2011). Cecil, Walton, and Viding (2015) also have found that, across multiple studies, the proopiomelanocortin gene POMC (associated with stress response in rodents; Wu et al., 2014), the opioid receptor μ1 gene (OPRM1; implicated in alcohol dependence in humans; Ray et al., 2014) and the serotonin receptor 3A (HTR3A; involved in emotion processing; Gatt, 2010) have been consistently hypermethylated in drug-induction (i.e., administration) animal studies. Nevertheless, there are still insufficient data to assess how varying substances of abuse may affect DNA methylation, and how this may manifest across the lifespan. However, research is beginning to address this critical gap in the literature. For instance, in their sample of 244 youth from the Avon Longitudinal Study of Parents and Children, Cecil and colleagues (2016) found that prenatal maternal tobacco-smoking was associated with epigenetic variations in the genes PACSIN1 (involved in synaptic neurotransmission in regions implicated in addiction; Liu et al., 2012), NEUROD4 (involved in neuronal differentiation; Lee, 1997), and NTRK2 (involved in nerve growth and associated with treatment-resistant depression; Li, 2013) in infants at birth that were later associated with increased substance abuse (i.e., tobacco, cannabis, and alcohol) during adolescence. These findings provide initial insight into how substance use may exert effects on the developing brain via epigenetic changes. Nevertheless, at this stage it is still unclear how these epigenetic changes contribute to addiction. In particular, future studies employing larger samples are needed to examine these processes, and studies such as the Adolescent Brain Cognitive Development Study (ABCD; https://abcdstudy.org) offer promise for providing important insight.
A developmental model of addiction
Taken together, it is possible to conceptualize a developmental model of addictions (Figure 1) using an attachment lens wherein: (1) an individual’s genetic makeup provides the configuration for neurobiological (and potentially behavioral) phenotypic expressions of vulnerability to substance use and abuse (Goldman, Oroszi & Ducci, 2005); (2) specifically, structural and functional differences in specific brain regions or systems may delimit the parameters for reward processing (related to jouissance), executive functioning (potentially including defense mechanisms), and emotion regulation (possibly modelled by object relations), all being potential factors involved in attachment, self-regulation, and addictive processes; (3) environmental (i.e., intrauterine, perinatal, and postnatal) circumstances may trigger epigenetic changes that either protect against or facilitate addictive processes; and, (4) factors such as early adversity and attachment trauma may set the foundation for a latent vulnerability (McCrory & Mayes, 2015) for addiction in adolescence or adulthood. Considering the epigenetic effect of the environment over phenotypic expression and the potential intergenerational transmission of addiction (Rutherford & Mayes, 2017), it might be essential for addiction treatment to help clients develop more adaptive patterns of interaction and self-regulation even if it is later in life. It is possible that, by modeling sufficiently rewarding social interactions (e.g., through attention, praising, and emotional containment; all in the safety of a therapeutic relationship), an individual’s neural functioning may become gradually (a) more attuned towards implicit social cues and interactions, making drug-related cues and hedonic responses less reinforcing (Tops et al., 2014) and (b) more effective (self-) reflective functioning capacity to identify, interpret, and regulate drug-seeking urges or negative affect without the need for psychoactive agents.
Figure 1.
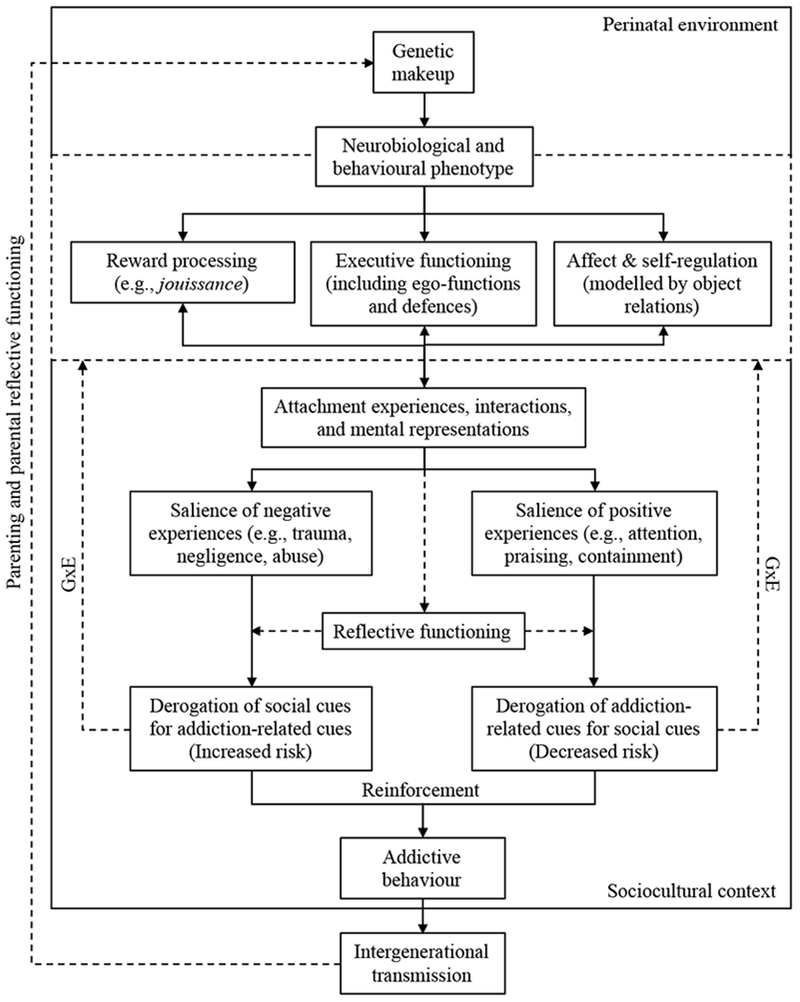
Developmental model of addiction. The genetic makeup of an individual defines the neurobiological and behavioural phenotypical variations of the structure and function of brain areas involved in reward processing, executive functioning, affect- and self-regulation. Phenotypes are both related to peri- and postnatal environments, and influence attachment experiences, representations and interactions. These, in turn, further influence the phenotypes and guide addictive behaviour based on the salience of social and addiction-related cues (i.e., cues related to alcohol, drugs, gambling or other addiction-related foci) and the developing reflective functioning capacity. Addictive behaviour is then learned and maintained via reinforcement and gene-environment interactions with a sociocultural context. Ultimately, vulnerability to said mechanisms is potentially transmitted to the next generation via parent-infant interactions, parental reflective functioning, as well as parental genetic and maternal perinatal contributions.
Taken together, while multiple theories of addiction exist, many are not mutually exclusive. As we describe above, viewing addictive disorders from an attachment perspective may help promote an improved understanding of these conditions that often carry negative individual and familial impacts. That being said, attachment likely does not capture all elements of addictive disorders. Further research directly investigating specific aspects of attachment and how they may mitigate against the development of addictive disorders and perhaps promote recovery from these conditions is needed.
Conclusion
This review has considered the value of synthesizing neurobiological and psychodynamic perspectives to better understand addictions, identifying potential pathways to the initiation of substance use, as well as mechanisms that may maintain substance use and abuse. The neurobiological approach allows biological mechanisms to be identified that may contribute to substance abuse and dependence; the psychodynamic approach provides an alternate framework for understanding relational and representational aspects of addiction within a developmental perspective. Attachment theory may present a unique opportunity to bring together these lines of enquiry, enabling an integrative developmental model of addiction with early experiences laying the foundation for psychological as well as neurobiological trajectories to substance use, abuse, and dependence. Fostering secure attachment bonds through sensitive parenting during childhood or through psychological interventions later in life may represent a unique opportunity to promote healthy socio-emotional and motivational growth across the lifespan.
Acknowledgments
This work was supported by the Anna Freud Centre (UK), the John Leopold Weil and Geraldine Rickard Weil Memorial Charitable Foundation (USA), the National Council for Science and Technology (CONACYT, Mexico) under scholarship 600356, a National Center for Responsible Gaming Center of Excellence grant, the Connecticut Council on Problem Gambling, the Connecticut Department of Mental Health and Addiction Services, and the National Institute of Health grants R01 DA039136, R01DA035058, and R01 DA026437. The views presented are those of the authors and may not reflect those of the funding agencies who did not have input into the manuscript.
Disclosure Statement
None of the authors have any relevant financial disclosures. Dr. Potenza has consulted for Ironwood, Lundbeck, Shire, INSYS, Rivermend Health, Opiant/Lightlake Therapeutics, and Jazz Pharmaceuticals; has received research support (to Yale) from Pfizer, Mohegan Sun Casino and the National Center for Responsible Gaming; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse-control disorders or other health topics; has consulted for or advised gambling and legal entities on issues related to impulse-control/addictive disorders; provides clinical care in a problem gambling services program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
References
- Alvarez-Monjaras M, McMahon T, & Suchman N (2017). Does Maternal Reflective Functioning Mediate Associations between Representations of Caregiving With Maternal Sensitivity in a High-Risk Sample?. Attachment and Human Development. DOI: 10.1037/pap0000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Ammerman R, Kolko D, Kirisci L, Blackson T, & Dawes M (1999). Child abuse potential in parents with histories of substance use disorder. Child Abuse and Neglect , 23(12), 1225–1238. [DOI] [PubMed] [Google Scholar]
- Bailly L (2009). Lacan: A beginners guide. Oxford, England: Oneworld Publications. [Google Scholar]
- Bazan A, & Detandt S (2013). On the physiology of jouissance: interpreting the mesolimbic dopaminergic reward functions from a psychoanalytic perspective. Frontiers in Human Neuroscience, 7(709), 1–13. DOI: 10.3389/fnhum.2013.00709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A (2003) Risky business: emotion, decision-making, and addiction. Journal of Gambling Studies, 19, 23–51. DOI: 10.1023/a:102123113233 [DOI] [PubMed] [Google Scholar]
- Bellak L, Hurvich M, & Gedeman H (1973) Ego functions in schizophrenics, neurotics and normal: A systematic study of conceptual, diagnostic, and therapeutic aspects. New York, NY: John Wiley. [Google Scholar]
- Beres D, & Joseph E (1970). The concept of mental representation in psychoanalysis. The International Journal of Psychoanalysis, 51, 1–8. [PubMed] [Google Scholar]
- Berner A, Carlson E, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of Young children’s executive functioning. Child Development, 81(1), 326–339. [DOI] [PubMed] [Google Scholar]
- Bion W (1962). Learning from experience. London, England: Karnak. [Google Scholar]
- Blum K, Sheridan P, Wood R, Braverman E, Chen T, Cull J, & Cummings D (1996). The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine, 89, 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton M, & Nelson JB (1998). The role of context in classical conditioning: Some implications for cognitive behavior therapy. Learning and behavior therapy, 59–84. [Google Scholar]
- Bowlby J (1988). A secure base: clinical applications of attachment theory. London, England: Routledge. [Google Scholar]
- Bremner J, & Narayan M (1998). The effects of stress on memory and the hippocampus throughout the life cycle: Implications for childhood development and aging. Development and Psychopathology, 10(04), 871–885. [DOI] [PubMed] [Google Scholar]
- Buisman-Pijlman F, Sumracki N, Gordon J, Hull P, Carter S, & Tops M (2014). Individual differences underlying susceptibility to addiction: Role of the endogenous oxytocin system. Pharmacology, Biochemistry and Behavior, 119, 22–38. DOI: 10.1016/j.pbb.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Cecil C, Walton E, Smith R, Viding E, McCrory E, Relton C, Suderman M, Pingault J-B, McArdle E, Gaunt T, Mill J, & Barker B (2016). DNA methylation and substance-use risk: a prospective, genome-wide study spanning gestation to adolescence. Translational Psychiatry, 6, e976 DOI: 10.1038/tp.2016.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil C, Walton E, & Viding E (2015). DNA Methylation, Substance Use and Addiction: A Systematic Review of Recent Animal and Human Research from a Developmental Perspective. Current Addiction Reports, 2(4), 331–346. DOI: 10.1007/s40429-015-0072-9 [DOI] [Google Scholar]
- Chambers R, Taylor J, & Potenza M (2003). Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry, 160, 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin T, & Sinha R (2013). Stress and parental addiction In Suchman N, Pajulo M, & Mayes L (Eds.), Parenting and substance abuse: Developmental approaches to intervention. New York, NY: Oxford University Press. [Google Scholar]
- Colvis C, Pollock J, Goodman R, Impey S, Dunn J, Mandel G, Champagne F, Mayford M, Korzus E, Kumar A, Renthal W, Theobald D, & Nestler E (2005). Epigenetic Mechanisms and Gene Networks in the Nervous System. The Journal of Neuroscience, 25(45), 10397–10389. DOI: 10.1523/jneurosci.4119-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden P (2015). Danger, development and adaptation. Hampshire, England: Waterside Press. [Google Scholar]
- Davies M (2003). The role of GABA-A receptors in mediating the effects of alcohol in the central nervous system. Journal of Psychiatry and Neuroscience, 28(4), 263–274. [PMC free article] [PubMed] [Google Scholar]
- Dawe S, Harnett P, Straiger P, & Dadds M (2000). Parent training skills and methadone maintenance: Clinical opportunities and challenges. Drug and Alcohol Dependence, 60, 1–11. [DOI] [PubMed] [Google Scholar]
- Demers I, Bernier A, Tarabulsy G, & Provost M (2010). Mind-mindedness in adult and adolescent mothers: Relations to maternal sensitivity and infant attachment. International Journal of Behavioral Development, 34(6), 529–537. doi: 10.1177/0165025410365802 [DOI] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Desrivieres S, Clarke T, Lourdusamy A, Smolka M, Cichon S, Blomeyer D, Treutlein J, Perreau-Lenz S, Witt S, Leonardi-Essman F, Wodarz N, Zill P, Soyka M, Albrecht U, Rietschel M, Lathrop M, Bakalkin G, Spanagel R, & Schumann G (2011). Effects of the Circadian Rhythm Gene Period 1 (Per1) on Psychosocial Stress-Induced Alcohol Drinking. American Journal of Psychiatry, 168 (10), 1090–1098. [DOI] [PubMed] [Google Scholar]
- Ducci F, & Goldman D (2012). Genetic basis of addictive disorders. Psychiatric Clinics of North America, 35(2), 495–519. DOI: 10.1016/j.psc.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman R, Robbins S, Bromwell M, Lankford M, Monterosso J, & O’Brien C (2002). Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug and alcohol dependence, 67(2), 185–191. [DOI] [PubMed] [Google Scholar]
- Eiden R, Schuetze P, & Coles C (2011). Maternal cocaine use and mother-infant interactions: Direct and moderated associations. Neurotoxicology and Teratology, 33, 120–128. DOI: 10.1016/j.ntt.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan R, & Frith C (2000). Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex 10, 308–317. DOI: 10.1093/cercor/10.3.30 [DOI] [PubMed] [Google Scholar]
- Espinosa M, Beckwith L, Howard J, Tyler R, & Swanson K (2001). Maternal psychopathology and attachment in toddlers of heavy cocaine-using mothers. Infant Mental Health Journal, 22(3), 316–333. [Google Scholar]
- Everitt B (2006). From the dark side to the bright side of drug addiction. Science. 314 59–60. DOI: 10.1126/science.1129565 [DOI] [Google Scholar]
- Everitt B, & Robbins T (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience, 8, 1481–1489. DOI: 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Farrow C, & Blisset J (2014). Maternal mind-mindedness during infancy, general parenting sensitivity and observed child feeding behavior: A longitudinal study. Attachment and Human Development, 16(3), 230–241. DOI: 10.1080/14616734.2014.898158 [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, & Levine A (2007). Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science, 18(11), 965–970. [DOI] [PubMed] [Google Scholar]
- Fink B (1999). A clinical introduction to Lacanian psychoanalysis. Cambridge, Mass: Harvard University Press. [Google Scholar]
- Fonagy P, & Bateman A (2008). Mentalization-based treatment of borderline personality disorder In Jurist E, Slade A, & Bergner S (Eds.), Mind to mind: Infant research, neuroscience, and psychoanalysis. New York, NY: Other Press. [Google Scholar]
- Fonagy P Gergely G, Jurist E, & Target M (2004). Affect regulation, mentalization and the development of the self. London, England: Karnac. [Google Scholar]
- Fonagy P, Steele M, Steele H, Moran G, & Higgitt A (1991). The capacity for understanding mental states: The reflective self in parent and child and its significance for security of attachment. Infant Mental Health Journal, 12(3), 201–218. [Google Scholar]
- Fonagy P, & Target M (1997). Attachment and reflective function: Their role in selforganization. Development and Psychopathology, 9, 679–700. [DOI] [PubMed] [Google Scholar]
- Fonagy P, & Target M (2005). Bridging the transmission gap: An end to an important mystery of attachment research?. Attachment and Human Development, 7(3), 333–343. DOI: 10.1080/14616730500269278 [DOI] [PubMed] [Google Scholar]
- Fonagy P, & Target M (2008). Attachment, trauma and psychoanalysis In Jurist E, Slade A & Bergner S (Eds.), Mind to mind: Infant research, neuroscience, and psychoanalysis. New York, NY: Other Press. [Google Scholar]
- Freeman R, Collier K, & Parillo K (2002). Early life sexual abuse as a risk factor for crack cocaine use in a sample of community-recruited women at high risk for illicit drug use. The American Journal of Drug and Alcohol Abuse, 28(1), 109–131. [DOI] [PubMed] [Google Scholar]
- Freud A (1937). The mechanisms of defence In Freud A (Ed.). The ego and the mechanisms of defence. London, England: Hogarth Press. [Google Scholar]
- Freud S (1915). Instincts and their vicissitudes In Strachey J (Ed. & Trans.), The standard edition of the complete psychological works of Sigmund Freud (Vol. 14). London, England: The Hogarth Press & The Institute of Psychoanalysis. [Google Scholar]
- Freud S (1917). Mourning and melancholia In Strachey J (Ed. & Trans.), The standard edition of the complete psychological works of Sigmund Freud (Vol. 14). London, England: The Hogarth Press & The Institute of Psychoanalysis. [Google Scholar]
- Gatt J, Williams L, Schofield P, Dobson-Stone C, Paul R, Grieve S, Clark R, Gordon E, & Nemeroff E (2010). Impact of the HTR3A gene with early life trauma on emotional brain networks and depressed mood. Depression and Anxiety, 27, 752–759. DOI: 10.1002/da.20726 [DOI] [PubMed] [Google Scholar]
- George C, Kaplan N, & Main M (1985). Adult Attachment Interview (Unpublished Manuscript). Department of Psychology. University of California, Berkeley. [Google Scholar]
- Goldwyn R, & Hugh-Jones S (2011). Using the Adult Attachment Interview to understand reactive attachment disorder: Findings from a 10-case adolescent sample. Attachment & Human Development, 13, 169–191. DOI: 10.1080/14616734.2011.554006 [DOI] [PubMed] [Google Scholar]
- Gonzales R, & Jaworski J (1997). Alcohol and glutamate. Alcohol Health and Research World, 21(2), 120–127. [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, De la Fuente-Sandoval C, Cameran B, Apiquián R, Fresán A, Aguilar A, Méndez-Núñez J, Escalona-Huerta C, Drucker-Colín R, & Nicolini H (2005) Frontal and limbic metabolic differences in subjects selected according to genetic variation of the SLC6A4 gene polymorphism. NeuroImage, 25, 1197–1204. DOI: 10.1016/j.neuroimage.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Håkansson U, Söderström K, Watten R, Skårderud F, & Øie M (2018). Parental reflective functioning and executive functioning in mothers with substance use disorder. Attachment & Human Development, 20(2), 181–207, DOI: 10.1080/14616734.2017.1398764 [DOI] [PubMed] [Google Scholar]
- Hans S, Bernstein V, & Henson L (1999). The role of psychopathology in the parenting of drug-dependent women. Development and Psychopathology, 11, 957–977. [DOI] [PubMed] [Google Scholar]
- Huth-Bocks A, Muzik M, Beeghly M, Earls L, & Stacks A (2014). Secure base scripts are associated with maternal parenting behavior across contexts and reflective functioning among trauma-exposed mothers. Attachment and Human Development, 16(6), 535–556. DOI: 10.1080/14616734.2014.967787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isosävi S, Flykt M, Belt R, Posa T, Kuittinen S, Puura K, & Punamäki R (2016). Attachment representations among substance-abusing women in transition to motherhood: implications for prenatal emotions and mother–infant interaction. Attachment & Human Development, 18(4), 391–417, DOI: 10.1080/14616734.2016.1151904 [DOI] [PubMed] [Google Scholar]
- Johns J, Lubin D, Walker C, Meter K, & Mason G (1997). Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague-Dawley rats. Neuropeptides, 31(5), 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B (2013). Addiction and will. Frontiers in Human Neuroscience, 7(545), 1–11. DOI: 10.3389/fnhum.2013.00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach K (2013), Biopsychosocial characteistics of parenting women with substance use disorders In Suchman N, Pajulo M, & Mayes L (Eds.), Parenting and substance abuse: Developmental approaches to intervention. New York, NY: Oxford University Press. [Google Scholar]
- Kernberg O (1975). Borderline conditions and pathological narcissism. New York, NY: Aronson. [Google Scholar]
- Kernberg O, Diamond D, Yeomans F, Clarkin J, & Levy K (2008). Mentalization and attachment in borderline patients in transference focused psychotherapy In Jurist E, Slade A & Bergner S (Eds.), Mind to mind: Infant research, neuroscience, and psychoanalysis. New York, NY: Other Press. [Google Scholar]
- Khantzian E (1985). The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. American Journal of Psychiatry, 142(11), 1259–1264. [DOI] [PubMed] [Google Scholar]
- Klein M (1930). The importance of symbol-formation in the development of the ego. The International Journal of Psychoanalysis, 11, 24–39. [Google Scholar]
- Klein M (1940). Mourning and its relation to manic-depressive states. International Journal of Psychoanalysis, 21, 125–153. [Google Scholar]
- Klein M (1946). Notes on some schizoid mechanisms. The International Journal of Psychoanalysis, 27, 99–110. [PubMed] [Google Scholar]
- Kohut H (1979). Four basic concepts in self psychology In Ornstein P (Ed.). The Search for the Self: Selected Writings of Heinz Kohut: 1950-1978. Madison, WI: International Universities Press. [Google Scholar]
- Koob G, & LeMoal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97–129. [DOI] [PubMed] [Google Scholar]
- Koob G, & LeMoal M (2008). Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3113–3123. DOI: 10.1098/rstb.2008.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren-Karie N, Sagi-Schwartz A, & Joels T (2003). Absence of Attachment Representations (AAR) in the adult years: The emergence of a new AAI classification in catastrophically traumatized Holocaust child survivors. Attachment & Human Development, 5, 381–397. DOI: 10.1080/14616730310001633456 [DOI] [PubMed] [Google Scholar]
- Krystal H (1978). Self representation and the capacity for self care. Annual of Psychoanalysis, 6, 209–246. [Google Scholar]
- Lacan J (1964). Book XI: The four fundamental concepts of psychoanalysis. New York, NY: W.W. Norton & Company. [Google Scholar]
- Lacan J (1969). D’un Autre à l’autre In Miller J (Ed.). Le séminaire de Jacques Lacan: Livre XVI. Paris, France: Seuil. [Google Scholar]
- Lee J (1997). Basic helix-loop-helix genes in neural development. Current Opinion in Neurobiology, 7, 13–20. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Wang Z, Chen J, Fan J, Guan Y, Chang C, Yuan C, Hong W, Wang Y, Wu Z, Huang J, Hu Y, Cao L, Yi Z, Cui D, Yun S, & Fang Y (2013). The role of BDNF, NTRK2 gene and their interaction in development of treatment-resistant depression: Data from multicenter, prospective, longitudinal clinic practice. Journal of Psychiatric Research, 47(1), 8–14. DOI: 10.1016/j.jpsychires.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lv K, Li Z, Yu A, Chen J, & Teng J (2012). PACSIN1, a Tau-interacting protein, regulates axonal elongation and branching by facilitating microtubule instability. Journal of Biological Chemistry, 287(47), 39911–39924. DOI: 10.1074/jbc.M112.403451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose R (2002). A Lacanian approach to clinical diagnosis and addiction In Glynos J & Stavrakakis Y (Eds.) Lacan and Science. London: Karnac. [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, & Deakin JFW (2000). Attentional bias for drug cues in opiate dependence. Psychological medicine, 30(1), 169–175. [DOI] [PubMed] [Google Scholar]
- Lyden H, & Suchman N (2013). Transmission of parenting models at the level of representation: Implications for mother-child dyads, affected by maternal substance abuse In Suchman N, Pajulo M, & Mayes L (Eds.), Parenting and substance abuse: Developmental approaches to intervention. New York, NY: Oxford University Press. [Google Scholar]
- Lyons-Ruth K, Yellin C, Melnick S, & Atwood G (2005). Expanding the concept of unresolved mental states: Hostile/helpless states of mind on the Adult Attachment Interview are associated with disrupted mother–infant communication and infant disorganization. Development and Psychopathology, 17, 1–23. DOI: 10.1017/S0954579405050017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, & Mayes L (2015). Understanding Addiction as a Developmental Disorder: An Argument for a Developmentally Informed Multilevel Approach. Current Addictions Report, 2, 326–330. DOI: 10.1007/s40429-015-0079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano M, Hatch J, Zule W, & Desmond D (2002). Psychological distress in childhood trauma survivors who abuse drugs. The American Journal of Drug and Alcohol Abuse, 28(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Milby J, Sims M, Khuder S, Schumacher J, & Huggins N (1996). Psychiatric comorbidity: Prevalence in methadone maintenance treatment. American Journal of Alcohol and Drug Abuse, 22(1), 95–107. [DOI] [PubMed] [Google Scholar]
- Muschamp J, Regina M, Hull E, Winter J, & Rabin R (2004). Lysergic acid diethylamide and [–]-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Research, 1023, 134–140. DOI: 10.1016/j.brainres.2004.07.044 [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (2015, June). National Trends [Online Article]. Retrieved from https://www.drugabuse.gov/publications/drugfacts/nationwide-trends.
- Neger E, & Prinz R (2015). Interventions to address parenting and parental substance abuse: Conceptual and methodological considerations. Clinical Psychology Review, 39, 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E (2005). Is there a common molecular pathway for addiction?. Nature Neuroscience, 8(11), 1445–1449. [DOI] [PubMed] [Google Scholar]
- Nestler E (2014). Epigenetic mechanisms of drug addiction. Neuropharmacology, 76, 259–68. DOI: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New A, Hazlett E, Buchsbaum M, Goodman M, Reynolds D, Mitropoulou V, Sprung L, Shaw R, Koenigsberg H, Platholi J, Silverman J, & Siever L (2002) Blunted prefrontal cortical 18 fluorodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Archives of General Psychiatry, 59(7), 621–629. DOI: 10.1001/archpsyc.59.7.621 [DOI] [PubMed] [Google Scholar]
- Niehaus J, Murali M, & Kauer J (2010). Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. European Journal of Neuroscience, 32(1), 108–117. DOI: 10.1111/j.1460-9568.2010.07256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, & Panksepp J (2002). Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behavioral Brain Research, 128(2), 189–203. [DOI] [PubMed] [Google Scholar]
- Nutt D, Lingford-Hughes A, Erritzoe D, & Stokes P (2015). The dopamine theory of addiction: 40 years of highs and lows. National Review of Neuroscience, 16(5), 305–12. DOI: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- O’Connor M, Kogan N, & Findlay R (2006). Prenatal alcohol exposure and attachment behavior in children. Alcoholism: Clinical and Experimental Research, 26(10), 1592–1602. DOI: 10.1111/j.1530-0277.2002.tb02460.x [DOI] [PubMed] [Google Scholar]
- Pajulo M, & Kalland M (2013). Mentalizing-based intervention with mother-baby dyads In Suchman N, Pajulo M, & Mayes L (Eds.), Parenting and substance abuse: Developmental approaches to intervention. New York, NY: Oxford University Press. [Google Scholar]
- Panksepp J (1998). SEEKING systems and anticipatory states in the nervous system In Panksepp J (Ed.). Affective neuroscience: the foundations of human and animal emotions. Oxford, England: Oxford University Press. [Google Scholar]
- Parrott A (2002). Recreational ecstasy/MDMA, the serotonin syndrome, and serotonin neurotoxicity. Pharmacology, Biochemistry and Behavior, 71, 837–844. [DOI] [PubMed] [Google Scholar]
- Potenza M (2008). The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3181–3189. doi: 10.1098/rstb.2008.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M (2013). How central is dopamine to pathological gambling or gambling disorder? Frontiers in Behavioral Neuroscience, 7, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M, Leung H, Blumberg H, Peterson B, Fulbright R, Lacadie C, Skudlarski P, & Gore J (2003a). An fMRI Stroop study of ventromedial prefrontal cortical function in pathological gamblers. American Journal of Psychiatry, 160, 1990–1994. [DOI] [PubMed] [Google Scholar]
- Potenza M, Steinberg M, Skudlarski P, Fulbright R, Lacadie C, Wilber M, Rounsaville B, Gore J, & Wexler B (2003b). Gambling urges in pathological gamblers: An fMRI study. Archives of General Psychiatry, 60, 828–836. [DOI] [PubMed] [Google Scholar]
- Puetz V, & McCrory E (2015). Exploring the Relationship Between Childhood Maltreatment and Addiction: A Review of the Neurocognitive Evidence. Current Addictions Report, 2, 318–325. DOI: 10.1007/s40429-015-0073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L, Bujarski S, MacKillop J, Courtney K, Monti P, & Miotto K (2014). Subjective Response to Alcohol among Alcohol Dependent Individuals: Effects of the Mu-Opioid Receptor (OPRM1) Gene and Alcoholism Severity. Alcohol: Clinical and Experimental Research, 47(1), E116–E124. DOI: 10.1111%2Fj.1530-0277.2012.01916.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T, & Everitt B (1992). Functions of dopamine in the dorsal and ventral striatum. Seminars in Neuroscience, 4, 119–127. [Google Scholar]
- Robbins T, & Everitt B (1996). Neurobehavioral mechanisms of reward and motivation. Current Opinion in Neurobiology, 6, 228–236. [DOI] [PubMed] [Google Scholar]
- Robbins T, Everitt B, & Nutt D (2008). Introduction. The neurobiology of drug addiction: new vistas. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3109–3111. DOI: 10.1098/rstb.2008.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T, & Berridge K (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Review, 18, 247–291. DOI: 10.1016/01650173(93)90013-P [DOI] [PubMed] [Google Scholar]
- Robinson T, & Berridge K (2001). Incentive-sensitization and addiction. Addiction, 93, 103–114. DOI: 10.1080/09652140020016996 [DOI] [PubMed] [Google Scholar]
- Robinson T, & Berridge K (2004). Incentive-sensitization and drug ‘wanting’. Psychopharmacology, 171, 352–353. DOI: 10.1007/s00213-003-1602-z [DOI] [Google Scholar]
- Robinson T, & Berridge K (2008). The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 363(1507), 3137–3146. DOI: 10.1098/rstb.2008.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodning C, Beckwith L, & Howard J (2008). Characteristics of attachment organization and play organization in prenatally drug-exposed toddlers. Development and Psychology, 1(4), 277–289. DOI 10.1017/S095457940000047X [DOI] [Google Scholar]
- Rutherford HJV, Booth CR, Crowley MJ & Mayes LC (2016). Investigating the relationship between working memory and emotion regulation in mothers. Journal of Cognitive Psychology, 28(1), 52–59. [Google Scholar]
- Rutherford HJV, Byrne SP, Crowley MJ, Bornstein J, Bridgett DJ, & Mayes LC (2018). Executive functioning predicts reflective functioning in mothers. Journal of Child and Family Studies, 27(3), 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJ, & Mayes LJ (2017). Parenting and addiction: Neurobiological insights. Current Opinion in Psychology, 15, 55–60. DOI: 10.1016/j.copsyc.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJ, Potenza MN, & Mayes LC (2013). The neurobiology of addiction and attachment In Suchman N, Pajulo M, & Mayes L (Eds.), Parenting and substance abuse: Developmental approaches to intervention. New York, NY: Oxford University Press. [Google Scholar]
- Rutherford HJV, Wallace NS, Laurent HK, & Mayes LC (2015). Emotion regulation in parenthood. Developmental Review, 36, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford H, Williams S, Moy S, Mayes L, & Johns J (2011). Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Frontiers in psychiatry, 2, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo S, & Flykt M (2013). The impact of parental addiction on child development In Suchman N, Pajulo M, & Mayes L (Eds.), Parenting and substance abuse: Developmental approaches to intervention. New York, NY: Oxford University Press. [Google Scholar]
- Schindler A, & Bröning S (2015). A Review on Attachment and Adolescent Substance Abuse: Empirical Evidence and Implications for Prevention and Treatment. Substance Abuse, 36, 304–313. DOI: 10.1080/08897077.2014.983586 [DOI] [PubMed] [Google Scholar]
- Segal H (1998). ‘The importance of symbol-formation in the development of the ego’: in context. Journal of child psychotherapy, 24, 349–357. [Google Scholar]
- Sinha R (2001). How does stress increase the risk of drug abuse and relapse? Psychopharmacology, 158, 343–359. DOI: 10.1007/s002130100917 [DOI] [PubMed] [Google Scholar]
- Siqveland T, Haabrekke K, Wentzel-Larsen T, & Moe V (2014). Patterns of mother-infant interaction from 3 to 12 months among dyads with substance abuse and psychiatric problems. Infant Behavior and Development, 37, 772–786. DOI: 10.1016/j.infbeh.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Siqveland T, & Moe V (2014). Longitudinal development of mother-infant interaction during the first year of life among mothers with substance abuse and psychiatric problems and their infants. Child Psychiatry and Human Development, 45, 408–421. [DOI] [PubMed] [Google Scholar]
- Slade A, Grienenberger J, Bernbach E, Levy D, & Locker A (2005). Maternal reflective functioning, attachment and the transmission gap: A preliminary study. Attachment and Human Development, 7(3), 283–298. [DOI] [PubMed] [Google Scholar]
- Slade A (2005). Parental reflective functioning: An introduction. Attachment and Human Development. 7(3), 269–281. DOI: 10.1080/14616730500245906 [DOI] [PubMed] [Google Scholar]
- Söderström K, & Skarderud F (2009). Minding the baby: Mentalization-based treatment in families with parental substance use disorder: Theoretical framework. Nordic Psychology, 61(3), 47–65. DOI: 10.1027/1901-2276.61.3.47 [DOI] [Google Scholar]
- Sokolowsky M, Hans S, Bernstein V, & Cox S (2007). Mothers’ representations of their infants and parenting behavior: Associations with personal and social-contextual variables in a high-risk sample. Infant Mental Health Journal, 28(3), 344–365. DOI: 10.1002/imhj.20140 [DOI] [PubMed] [Google Scholar]
- Solomon R, & Corbit J (1974). An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review, 81(2), 119–145 [DOI] [PubMed] [Google Scholar]
- Somerville L, Jones R, & Casey B (2010). A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–145. DOI: 10.1016/j.bandc.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza A, Nicolais G, Vergano C, & Dazzi N (2017). Emerging criteria for the low-coherence cannot classify category. Attachment & Human Development, 19(6), 613–634. DOI: 10.1080/14616734.2017.1355396 [DOI] [PubMed] [Google Scholar]
- Stacks A, Muzik M, Wong K, Beeghly M, Huth-Bocks A, Irwin J, Rosenblum K (2014). Maternal reflective functioning among mothers with childhood maltreatment histories: Links to sensitive parenting and infant attachment security. Attachment and Human Development, 16(5), 515–533.DOI: 10.1080/14616734.2014.935452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D (1983). The early development of schemas of self, other, and “self with other” In Lichtenberg J & Kaplan S (Eds.), Reflections of Self Psychology. Hillsdale, NJ: Analytic Press. [Google Scholar]
- Strathearn L (2011). Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. Journal of Neuroendocrinology, 23, 1054–1065. DOI: 10.1111/j.1365-2826.2011.02228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman N, DeCoste C, Leigh D, & Borelli J (2010). Reflective functioning in mothers with drug use disorders: Implications for dyadic interactions with infants and toddlers. Attachment and Human Development, 12(6), 567, 586 DOI: 10.1080/14616734.2010.501988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman N, DeCoste C, McMahon T, Rounsaville B, & Mayes L (2011). The mothers and toddlers program, an attachment-based parenting intervention for substance-using women: Results at 6-week follow-up in a randomized clinical pilot. Infant Mental Health Journal, 32(4), 427–449. DOI: 10.1002/imhj.20303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman N, DeCoste C, Rosenberger P, & McMahon T (2012). Attachment-based intervention for substance-using mothers: A preliminary test of the proposed mechanisms of change. Infant Mental Health Journal, 33(4), 360–371. DOI: 10.1002/imhj.21311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman N DeCoste C, Ordway M, & Bers S (2013). Mothering from the inside-out: A mentalization-based individual therapy for mothers with substance-use disorders In Suchman N, Pajulo M, & Mayes L (Eds.), Parenting and substance abuse: Developmental approaches to intervention. New York, NY: Oxford University Press. [Google Scholar]
- Suchman N, DeCoste C, Castiglioni N, Legow N, & Mayes L (2008). The Mothers and Toddlers Program: Preliminary findings from an attachment-based parenting intervention for substance abusing mothers. Psychoanalytic Psychology, 25, 499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman N, & Luthar S (2000). Maternal addiction, child maladjustment, and socio-demographic risks: Implications for parenting behaviors. Addiction, 95(9), 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman N, Mayes L, Conti J, Slade A, & Rounsaville B (2004). Rethinking parenting interventions for drug-dependent mothers: From behavior management to fostering emotional bonds. Journal of Substance Abuse Treatment, 27, 179–185. DOI: 10.1016/j.jsat.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Suchman N, McMahon T, Zhang H, Mayes L, & Luthar S (2006). Substance-abusing mothers and disruption in child custody: An attachment perspective. Journal of Substance Abuse Treatment, 30, 197–204. DOI: 10.1016/j.jsat.2005.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, & Sussman A (2011). Considering the definition of addiction. International Journal of Environmental Research and Public Health, 8(10), 4025–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K, Beckwith L, & Howard J (2010). Intrusive caregiving and quality of attachment in prenatally drug- exposed toddlers and their primary caregivers. Attachment and Human Development, 2(2), 130–148. DOI: 10.1080/14616730050085527 [DOI] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, & Banich M (2007). Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Human Brain Mapping, 28, 1276–1286. DOI: 10.1002/hbm.20344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri F, & DiChiara G (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science, 276, 2048–2050. [DOI] [PubMed] [Google Scholar]
- Tops M, Koole S, IJzerman H, & Buisman-Pijlman F (2014). Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacology, Biochemistry and Behavior, 119, 39–48. DOI: 10.1016/j.pbb.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Torrado M, Ouakinin S, & Bacelar-Nicolau L (2013). Alexythimia, emotional awareness, and perceived dysfunctional parental behaviors in heroin dependents. International Journal of Mental Health and Addictions, 11, 703–718. DOI: 10.1007/s11469-013-9448-z [DOI] [Google Scholar]
- Townshend J, & Duka T (2001). Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology, 157(1), 67–74. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Tomasi D, & Telang F (2011). Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences, 108(37), 15037–15042. DOI: 10.1073/pnas.1010654108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waska R (2006). Addictions and the quest to control the object. American Journal of Psychoanalysis, 66(1), 43–63. DOI: 10.1007/s11231-005-9002-2 [DOI] [PubMed] [Google Scholar]
- Wiseman H, Hashmonay R, & Harel J (2006). Interplay of parent-child representations from a psychoanalytic perspective: An analysis of two mother-father-child triads In Mayseless O (Ed.), Parenting representations; theory, research and clinical implications. New York, NY: Cambridge University Press. [Google Scholar]
- Wong C, Mill J, & Fernandes C (2011). Drugs and addiction: an introduction to epigenetics. Addiction, 106(3), 480–9. DOI: 10.1111/j.1360-0443.2010.03321.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Patchev A, Daniel G, Almeida O, & Spengler D (2014). Early-Life Stress Reduces DNA Methylation of the Pomc Gene in Male Mice. Endocrinology, 155(5), 1751–1752. DOI: 10.1210/en.2013-1868 [DOI] [PubMed] [Google Scholar]


