Abstract
Sepsis is the overwhelming systemic immune response to infection, which can result in multiple organ dysfunction and septic shock. Myocardial dysfunction during sepsis is associated with advanced disease and significantly increased in-hospital mortality. Our group has shown that energetic failure and excess reactive oxygen species (ROS) generation constitute major components of myocardial dysfunction in sepsis. Because ROS production is central to cellular metabolic health, we tested if the synthetic anti-oxidant lignan secoisolariciresinol diglucoside (SDG; LGM2605) would alleviate septic cardiac dysfunction and investigated the underlying mechanism. Using the cecal ligation and puncture (CLP) mouse model of peritonitis-induced sepsis, we observed impairment of cardiac function beginning at 4 hours post-CLP surgery. Treatment of mice with LGM2605 (100mg/kg body weight, i.p.) 6 hours post-CLP surgery reduced cardiac ROS accumulation and restored cardiac function. Assessment of mitochondrial respiration (Seahorse XF) in primary cardiomyocytes obtained from adult C57BL/6 mice that had undergone CLP and treatment with LGM2605 showed restored basal and maximal respiration, as well as preserved oxygen consumption rate (OCR) associated with spare capacity. Further analyses aiming to identify the cellular mechanisms that may account for improved cardiac function showed that LGM2605 restored mitochondria abundance, increased mitochondrial calcium uptake and preserved mitochondrial membrane potential. In addition to protecting against cardiac dysfunction, daily treatment with LGM2605 and antibiotic ertapenem (70 mg/kg) protected against CLP-associated mortality and reversed hypothermia when compared against mice receiving ertapenem and saline. Therefore, treatment of septic mice with LGM2605 emerges as a novel pharmacological approach that reduces cardiac ROS accumulation, protects cardiac mitochondrial function, alleviates cardiac dysfunction, and improves survival.
1. INTRODUCTION
Sepsis is a life-threatening condition that is caused by the uncontrolled inflammatory response of the host to infection resulting in substantial hospital-associated morbidity and mortality. If not identified early and treated properly, sepsis can progress to septic shock which is a lethal condition characterized by hypotension, decreased tissue perfusion, and multiple organ failure[1]. Current treatment guidelines are limited to source control, administration of broad-spectrum antibiotics, and supportive therapy. For this reason, targeted therapies aimed at protecting against sepsis-associated organ dysfunction will benefit the clinical management of patients with sepsis.
Cardiovascular dysfunction is a major complication of sepsis associated with advanced disease and poor prognosis[2–4]. Septic cardiac dysfunction is characterized by impaired contractility, diastolic dysfunction, and reduced cardiac index and ejection fraction (EF)[2, 5]. This outcome has been correlated with various pathophysiological events, such as increased inflammation, oxidative stress, impaired β-adrenergic signaling, energetic deficiency, and mitochondrial dysfunction[6–9]. Currently, there is no therapy for sepsis-induced cardiomyopathy. Previous work by our lab has established cardiac energetic changes associated with sepsis as a feasible target for intervention to improve cardiac function during sepsis[9–12]. We have further shown that inhibition of reactive oxygen species (ROS) formation via inhibition of the extra-mitochondrial protein NOX2 protected against septic cardiomyopathy[12]. Based upon these results, we investigated how sepsis affects mitochondria-related parameters, and assessed the impact of an anti-oxidant therapy which has never been applied to sepsis, on cardiac function.
In the present study, we evaluated the therapeutic potential of the mammalian lignan precursor secoisolariciresinol diglucoside (SDG). SDG is an ingredient of flaxseed, a non-toxic whole grain that consists of high concentrations of omega-3 fatty acids and lignans. Both flaxseed and SDG are potent antioxidants with anti-inflammatory and anti-fibrotic properties[13–15]. Previous studies have identified beneficial effects of SDG in treating a variety of conditions including hypercholesterolemia, diabetes, postmenopausal symptoms, cardiovascular disease, metabolic syndrome, bone disease, ARDS, ischemia-reperfusion injury, radiation-induced pneumonopathy, and hyperoxia[13–18]. In this study, we show for the first time that chemically synthesized SDG, LGM2605, is cardioprotective and protective against mortality in a mouse model of peritonitis-induced sepsis. In addition, we show major beneficial effects of LGM2605 in increasing mitochondrial abundance, mitochondrial calcium uptake, and mitochondrial respiration, which are significantly compromised during septic cardiac dysfunction[11]. Thus, treatment with LGM2605 emerges as a potential therapeutic intervention for the alleviation of septic cardiac dysfunction.
2. METHODS
2.1. Animal care, cecal ligation and puncture procedure, surface temperature measurements, and echocardiography –
Animal protocols were approved by the Temple University Institutional Animal Care and Use Committee and were carried out in accordance with the NIH guidelines for the care and use of laboratory animals. Wild type (WT) 7 to 12-week old C57BL/6 mice were purchased from Jackson labs. Male and female mice were used for experiments assessing the effect of CLP surgery on cardiac function. As only male mice exhibited signs of cardiac dysfunction, subsequent studies were restricted to male animals. Cecal ligation and puncture (CLP) was performed as previously described[12]. Mice were anesthetized with 3.5% inhaled isoflurane. Under aseptic conditions, a 1–2 cm midline laparotomy was performed and exposure of the cecum with adjoining intestine. The cecum was tightly ligated at its base below the ileo-cecal valve at a distance of 1cm and was punctured twice with a 19-gauge needle. The length of the ligated cecum was defined as the distance from the distal end of cecum to ligation point, which affects the degree of disease severity. Fecal material was extruded from the punctured cecum, and it was returned to the peritoneal cavity. The peritoneum and the skin were closed with three sutures. The mice were resuscitated by injecting subcutaneously 1ml of pre-warmed 0.9% saline solution to induce the hyperdynamic phase of sepsis and for post-operative analgesia the mice received subcutaneously buprenorphine (0.05mg/kg body weight). Mice received a single intraperitoneal injection of LGM2605 (100mg/kg i.p.) that was administrated either 2h prior to CLP or 6h post CLP.
Two-dimensional echocardiography was performed on anesthetized mice (1.5% inhaled isoflurane) using a VisualSonics Vevo 2100 machine 12h post-CLP. Echocardiographic images were recorded in a digital format. A single observer blinded to the respective treatments of mice analyzed short-axis M-mode images by LV trace. To measure myocardial strain and strain rate, speckle-tracking probe was applied to the long-axis B-mode images using VevoStrain software. For this analysis, B-mode images of 300 frames at greater than 200 frames/second were used. Strain calculates the change in length relative to initial length was calculated for the anterior, posterior, and apical regions of the myocardium. Global longitudinal strain (GLS) and strain rate (GLS rate) was calculated by the software using these values. Reverse GLS rate, as a measurement of early diastolic filling, was calculated using the reverse peak function as previously described[19].
Xiphoid surface temperature was assessed using the Etekcity Lasergrip infrared thermometer held approximately 3 inches from xiphoid process as previously described[20]. The number of mice used for each experiment are mentioned in the figure legends.
2.2. RNA purification and gene expression analysis-
Total RNA was purified from heart tissue using the TRIzol reagent according to the instructions of the manufacturer (Invitrogen). DNase-treated RNA was used for cDNA synthesis using the ProtoScript II First Strand cDNA Synthesis Kit (New England Biolabs). Quantitative real-time PCR was performed with the SYBR Select Master Mix (Applied Biosystems). Incorporation of the SYBR green dye into the PCR products was monitored with the Applied Biosystems StepOnePlus Real-Time PCR System. Samples were normalized against mouse 36B4. The sequences of the primers used for realtime PCR are shown in Supplemental Table 1.
2.3. Protein purification and analysis-
Isolated heart tissue was homogenized in radioimmune precipitation assay buffer containing protease and phosphatase inhibitors (Pierce Protease and Phosphatase Inhibitor Mini Tablets, Thermo Scientific). 30–50 μg of total protein extract was applied to SDS-PAGE and transferred onto PVDF membranes. Antibodies used for this study include anti-IκBα (sc-1643, Santa Cruz), anti-phosphoIκBα at Ser-32 (sc-7977, Santa Cruz), anti-LC3B (Sigma L7543), anti-MCU (Cell Signaling D2Z3B), anti-MICU1[21], and anti-ATP5A (Santa Cruz sc-136178).
2.4. Inflammatory cytokines measurement—
Circulating levels of IL-1α, IL-1β, IL-6, IL-10 and TNFα were quantified simultaneously from frozen plasma samples using the Milliplex MAP Mouse Cytokine kit (MCYTOMAG-70K-05) following the kit specifications. Samples were read using the Luminex MAGPIX multiplexing unit.
2.5. Adult mouse cardiomyocytes isolation –
Adult mouse cardiomyocytes (ACMs) were isolated from ventricles of C57BL/6 mice 12 hours post-sham surgery, CLP surgery or combined performance of CLP and treatment with LGM2605 at 6 hours post-surgery. Hearts from heparinized mice (90 USP; ip) were cannulated through the aorta. Hearts were perfused with perfusion buffer (120.4 mM NaCl, 14.7 mM KCL, 0.6 mM NaH2PO4, 0.6 mM KH2PO4, 1.2 mM MgSO4, 10 mM HEPES, 4.6 mM NaHCO3, 30 mM taurine, 10 mM BDM, 5.5 mM glucose; pH 7.4) for 3 min followed by digestion with perfusion buffer containing 19,250 units Collagenase type II (Worthington), 5–6 mg trypsin and 0.02 mM CaCl2 for 7 min. Ventricles were gently teared into small pieces, perfusion buffer containing 5 mg/ml BSA and 0.125 mM CaCl2 was added and filtered with 100 μm nylon. The filtrate was pelleted by gravity for 5 min, centrifuged for 30 sec at 700 rpm and the pellet resuspended in perfusion buffer containing 5 mg/ml BSA and 0.225 mM CaCl2. The cells were pelleted by gravity for 10 min, centrifuged for 30 sec at 700 rpm and the pellet resuspended in perfusion buffer containing 5 mg/ml BSA and 0.525 mM CaCl2. Following isolation, cells were counted via hemocytometer and assessed for viability via Tryptan blue staining and observation of cell morphology. Only cell preparations with greater than 90% viable cells were used for subsequent experiments.
2.6. Cell culture –
A human ventricular cardiomyocyte-derived cell line, designated AC16[22], was used for in vitro experimentation. Cells were maintained in Dulbecco’s modified Eagle’s medium-nutrient mixture F-12 (DMEM-F-12; Invitrogen, Carlsbad, CA).
2.7. Measurement of mitochondrial superoxide, mitochondrial membrane potential and mitochondrial number –
AC16 cells were cultured in sterile cell culture dishware at approximately 80% confluence. After overnight incubation, cells were treated with LPS (1 μg/ml), LPS and LGM2605 (50 μM). LPS and LGM2605 were diluted in serum free media and the control group was incubated as well with serum free media. At 12 h after the treatment the AC16 cells were loaded with mitochondrial superoxide indicator MitoSOX Red (5 μΜ, M36008, Molecular Probes) and incubated for 30 min in the dark. Excess MitoSOX Red was removed following three washes with PBS solution. MitoSOX red fluorescence was imaged at a fluorescent microscope using the Cy3 filter (510 nm excitation wavelength). Fluorescence intensity subtracted for background intensity was quantified for single cells using ImageJ software.
To assess changes in the mitochondrial number, LPS-stimulated AC16 cells and adult cardiomyocytes isolated 12 hours post-CLP were stained with 200 nM Mitotracker Red (M22425, Molecular Probes), and incubated for 30 min in the dark. Excess Mitotracker Red was removed with three washed of PBS solution. Mitotracker Red fluorescence was recorded at 581 nm (excitation) and quantified for single cells using ImageJ software. To quantify mitochondrial copy number, heart tissue collected 12 hours after surgery was homogenized and DNA isolated by chloroform extraction. Primers directed against the mitochondrial COXII gene (FWD-GCCGACTAAATCAAGCAACA; REV-CAATGGGCATAAAGCTATGG) and nuclear β-Globin (FWD-GAAGCGATTCTAGGGAGCAG; REV-GGAGCAGCGATTCTGAGTAGA) using 100 ng of template DNA.
To measure mitochondrial membrane potential, AC16 cells were stained with TMRM (62.5 nM, T668, Molecular Probes) for 30 min in the dark. TMRM stain was washed in warm PBS three times and imaged at 200x magnification using the Cy3 filter (510 nm excitation wavelength).
2.8. DHE staining of cardiac tissue-
Live myocardium was isolated from mice 12 hours after surgery and sectioned into 10 sections using a clean razor blade. Tissue was stained with 20 μM dihydroethidium (DHE) for 30 min at room temperature and imaged on a Zeiss Axio Observer Z1 fluorescent microscope at 490 ± 10 nm excitation and 632 ± 30 nm emission. Oxidized DHE fluoresces red and intercalates DNA. Individual nuclei were measured within each visual field using Zeiss Zen Blue software, and visual fields were averaged to measure mean fluorescence intensity for each mouse.
2.9. Radioligand binding assay –
Plasma membranes from excised mouse hearts were prepared, and saturation radio-ligand binding was performed as described previously[23], using 125I-CYP (iodocyanopindolol; PerkinElmer, Waltham, MA) for β-AR density measurement. Data were analyzed by nonlinear regression analysis using GraphPad Prism (GraphPad Software, La Jolla, CA).
2.10. Seahorse analysis –
Isolated primary ACMs were counted with Hematocytometer. Dead cells were detected with Trypan Blue Dye staining. Cells were platted (3000 cells per well) in XF96 Seahorse® plates pre-coated with laminin with 20 μg/ml laminin (Invitrogen, 23017). In order to assess oxygen consumption rates (OCR) for fatty acid oxidation (FAO) recordings, cells were incubated in FA-limited medium (DMEM containing 10mM Glucose, 1.025mM CaCl2, 0.5mM carnitine, pH=7.4) and assayed with FAO medium as per manufacturer’s protocol. Before starting the assay, 1mM palmitate conjugated with BSA was added in each well. Drugs used for maximal response during FAO were: Oligomycin (3μM) (Sigma, O4875), which blocks complex V, FCCP (2μM) (Sigma, C2920) that leads to the collapse of the proton gradient, and rotenone/antimycin A (0.5μM) (Sigma, A8674)/ (Sigma, R8875) where rotenone blocks complex I and antimycin A blocks complex III. The pre-hydrated with XF assay calibrant, XF cartridges were filled with the drugs and the cartridge was calibrated for 30 min in Seahorse Analyzer. All experiments were performed at 37°C. Calculations were made as described in the Seahorse manual and XF Seahorse Mito Stress Test kit user guide, and OCR measurements were normalized to the number of viable rod-shaped cardiomyocytes plated. Briefly, basal respiration was calculated with subtraction of non-mitochondrial respiration rate from the last measurement prior to first injection. Maximal respiration was calculated by subtraction of the non-mitochondrial respiration measurement from maximum measurement after FCCP injection. ATP synthesis-related OCR was obtained indirectly by measuring ATP-linked respiration in the presence of complex V inhibitor (oligomycin). The decrease of oxygen consumption rate representing the portion of basal respiration that was used to drive ATP synthesis was calculated with subtraction of the minimum measurement after oligomycin injection from the last measurement prior to oligomycin injection. Spare Respiratory Capacity was equal to (maximum respiration)- (basal respiration).
2.11. In vivo cardiac adrenergic sensitivity measurements –
Hemodynamics measurements were performed, as published[24]. Mice were anesthetized with 2% Tribromoethanol (Avertin). The right carotid artery was cannulated with a 1.4 French micro-manometer (Millar Instruments, Houston, TX) and was advanced into LV cavity, measuring LV pressure, LV end-diastolic pressure (LVEDP) and heart rate (HR). These parameters as well as maximal values of the instantaneous first derivative of LV pressure (+dP/dtmax, as a measure of cardiac contractility) and minimum values of the instantaneous first derivative of LV pressure (−dP/dtmin, as a measure of cardiac relaxation) were recorded at baseline and after administration, through the jugular vein, of increasing doses of the β-adrenergic receptor (βAR) agonist, isoproterenol (0.1 ng, 0.5 ng, 1 ng, 5 ng, 10 ng). Data was recorded and analyzed on a PowerLab System (AD Instruments).
2.12. Calcium uptake assay in permeabilized adult cardiomyocytes –
Cardiomyocytes were isolated from mice 12 hours after CLP or sham surgery and 6 hours after LGM2605 or saline injection. Calcium uptake was performed as previously described [25] and detailed below. Before permeabilization, cardiomyocytes were washed in a Ca2+-free buffer (120 mM NaCl, 5 mM KCl, 1 mM KH2PO4, 0.2 mM MgCl2, 0.1 mM EGTA and 20 mM HEPES–NaOH, at pH 7.4) and stored on ice for at least 10 min. Cardiomyocytes were pelleted by centrifugation and transferred to an intracellular-like medium (permeabilization buffer: 120 mM KCl, 10 mM NaCl, 1 mM KH2PO4, 20 mM HEPES–Tris, at pH 7.2, protease inhibitors (EDTA-free complete tablets, Roche Applied Science), 2 μM thapsigargin and digitonin (80 μg ml−1). The cell suspension supplemented with succinate (2 mM) was placed in a fluorimeter and permeabilized by gentle stirring. Fura2FF (0.5 μM) was added at 0 s, and JC-1 (800 nM) at 20 s. Fluorescence signal was monitored in a temperature-controlled (37 °C) multiwavelength-excitation dual-wavelength-emission spectrofluorometer (Delta RAM, Photon Technology International) using 490 nm excitation/535 nm emission for the JC-1 monomer, 570 nm excitation/595 nm emission for the J-aggregate of JC-1 and 340 nm/380 nm for Fura2FF. At 400 s, a single 10 μM Ca2+ pulse was added, and changes in cytosolic [Ca2+] was monitored. Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) was added at 750 s to collapse the mitochondrial membrane potential and measure calcium expelled from the mitochondria.
2.13. Survival Analysis-
Mice were assessed every 12 h for survival until 72 h post-CLP. Mice were randomized to receive either daily administration of ertapenem (70 mg/kg) and saline or ertapenem (70 mg/kg) and LGM2605 (100 mg/kg). Mice were dosed beginning 6 h after CLP and subsequently every 24 h for three days. Following 72 h, animals were euthanized in accordance with Temple University guidelines for appropriate animal care.
2.14. Statistical analysis-
Results are presented as mean ± SEM. The unpaired t-test was used for comparisons of two means; a 2-tailed value of P<0.05 was considered statistically significant. Statistical analysis was performed using Prism v5, GraphPad Software. For groups of 2 or more, ANOVA was used with Bonferroni post-hoc test or Kruskall-Wallis non-parametric test when a normal distribution could not be assumed. Survival curves were compared using Mantel-Cox (logrank) test.
3. RESULTS
3.1. LGM2605 treats cardiac dysfunction in a mouse model of cecal ligation and puncture (CLP)- induced sepsis –
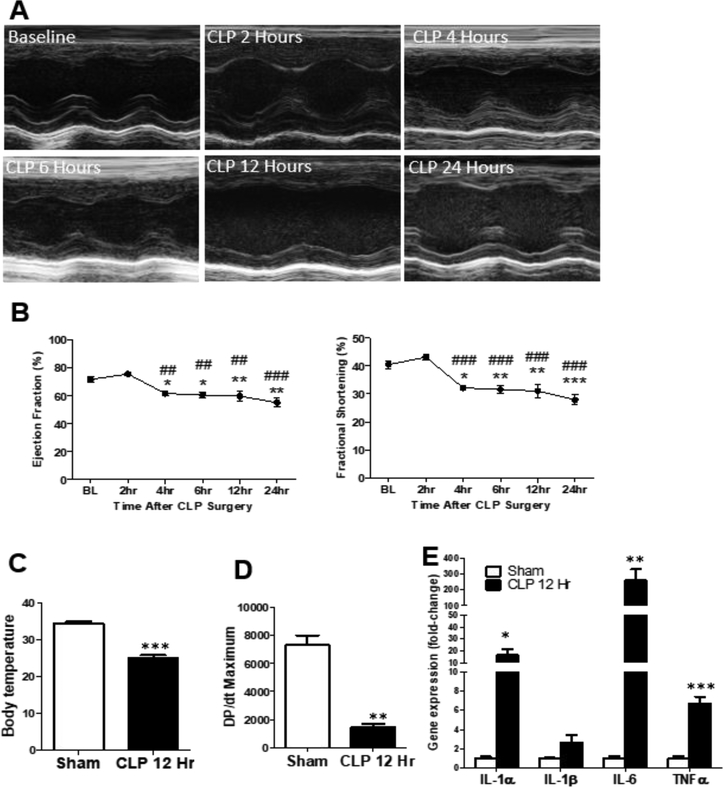
We induced mid-to-low grade sepsis (ligation site: 1cm) in male C57BL/6 mice using CLP and assessed cardiac function with 2D-echo up to 12 h post-surgery. Cardiac function significantly declined 6 h post-CLP which was sustained up to 24 h post-CLP (Fig. 1A-B). Septic mice showed a significant decrease in body temperature (Fig. 1C), as well as in contractility represented by dP/dtmax (Fig. 1D) and increased expression of cardiac inflammatory genes (Fig. 1E) 12 h post-CLP. As opposed to male mice, female C57BL/6 mice that underwent CLP surgery were protected against septic cardiac dysfunction and did not develop reduced EF or FS 12 h post-CLP (Suppl. Fig. 1). We therefore focused the remainder of our investigation to male mice.
Figure 1: Establishment of septic cardiac dysfunction using the cecal ligation and puncture model (CLP) –
(A) Representative M-mode echocardiograms after CLP surgery. (B) Graph of ejection fraction (Ef) and fractional shortening (FS); n=5 mice, One-way ANOVA analysis *p:<0.05 vs baseline (BL), **p:<0.01 vs BL, ##p<0.01 vs 2h, ###p<0.001 vs 2 h by ANOVA with Bonferroni post-test. (C) Body temperature of mice 12 h after sham or CLP surgery. n=3–4 mice. (D) Graph of DP/dt maximum of mice 12 h post-sham and CLP surgery. n=3 mice. (E) Gene expression of inflammatory cytokines in ventricular tissue of mice 12hours post-sham and CLP surgery, n = 4–5 mice per group. *p < 0.05, **p < 0.01, ***p < 0.001 vs Sham by t-test.
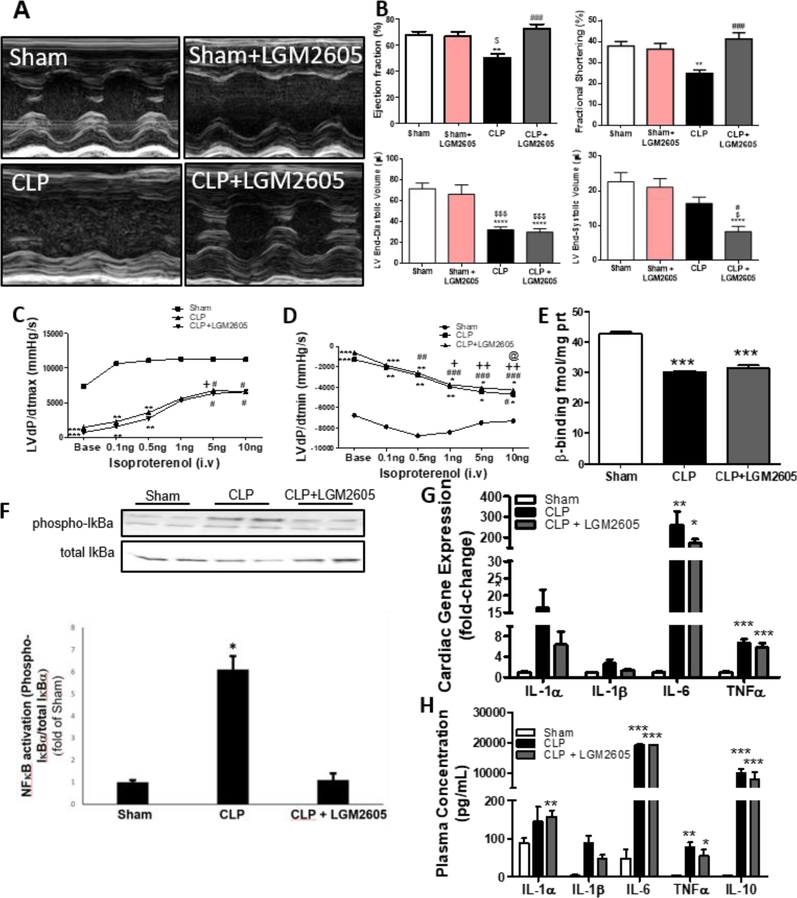
To assess the effect of LGM2605 in septic cardiac dysfunction, we first administered LGM2605 via i.p. injection (100 mg/kg body weight) 2 h prior to CLP in one group of mice and 6 h post-CLP in another group of mice. Both pre-CLP (Suppl. Fig. A,B and Suppl. Table 2) and post-CLP (Fig. 2A-B and Suppl. Table 2) treatments prevented CLP-mediated cardiac systolic dysfunction. As opposed to septic mice that did not receive LGM2605, which showed a 17.61% reduction in ejection fraction, and 13.07% reduction in fractional shortening, the mice that were treated with LGM2605 had normal EF/FS. This occurred despite reduced end-diastolic volume following CLP that was not restored by LGM2605 at the tested dose and mode of administration. Using speckle-tracking echocardiography (Suppl. Fig. 2C), we assessed if the treatment with LGM2605 would affect GLS and GLS rate, as alternative measurements of systolic function, and reverse GLS rate, an indicator of early diastolic filling. In accordance with our previous observation, GLS and GLS rate were impaired by CLP and restored to normal by LGM2605 (Suppl. Fig. 2D-E). However, treatment with LGM2605 did not restore reverse GLS rate (Suppl. Fig. 2F). Thus, LGM2605 restores cardiac systolic function but not diastolic function in sepsis, and the beneficial effect of LGM2605 occurred when LGM2605 was administered either preventively or after the onset of cardiac dysfunction.
Figure 2. LGM2605 corrects septic cardiac dysfunction in C57BL/6 mice following CLP surgery without reducing inflammatory cytokines.
(A-B) Representative M-mode echocardiograms (A) and ejection fraction (EF), fractional shortening (FS), end diastolic volume, and end-systolic volume of C57BL/6 mice treated with LGM2605 6hrs post-CLP and monitored for 12hrs after the surgery (B). Sham: n=9, Sham+6hrs SDG: n=4, CLP: n=12, CLP+6hrs SDG: n=12, **P < 0.01 vs Sham, ###P < 0.001 vs CLP, $P < 0.05 vs Sham + LGM2605 by ANOVA with Bonferroni post-test. (C) LVdP/dtmax and (D) LVdP/dtmin in response to increasing doses of isoproterenol in mice that underwent sham surgery, CLP and combined CLP and LGM2605 treatment (6 h post-CLP), at 12 hrs timepoint. n=3 mice per group, **P < 0.01, ***P < 0.001 versus sham at corresponding timepoints, #P < 0.05 versus baseline, ##P < 0.01 versus baseline, ###P < 0.001 versus baseline, +P < 0.05 and ++ P < 0.01 versus 0.1ng isoproterenol, @ P<0.05 versus 0.5ng isoproterenol, by ANOVA with Bonferroni posttest. (E) Density of p adrenergic receptors using radio ligand binding assay, n=4–5 mice per group. ***P < 0.001 by ANOVA with Bonferroni post-test. (F) Immunoblotting and densitometric analysis of phosphorylated and total IkBa from ventricular tissue of mice 12 hours post-surgery. (G) Cardiac mRNA expression and (H) plasma levels of cytokines 12-hours post-surgery, n = 4–5 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001 by ANOVA with Bonferroni post-test.
We tested if improvement in cardiac function by LGM2605 was associated with improved β-AR sensitivity. Hemodynamic measurements showed that septic mice had lower basal myocardial LV dP/dtmax (Fig. 2C) and LV dP/dtmin (Fig. 2D) with or without LGM2605 administration, compared to sham surgery. Responsiveness to isoproterenol was less robust in both septic groups regardless of LGM2605 treatment (Fig. 2C,D). From this approach, we also observed that septic mice had lower heart rate and systolic blood pressure that was not restored by LGM2605 (Suppl. Fig. 2G,H). As basal levels of LV dP/dtmax and LV dP/dtmin were lower in mice with CLP, we performed radioligand binding assay to assess the density of β-AR in hearts obtained from septic mice (12 h post-CLP) that were treated with LGM2605 (6 h post-CLP). This analysis showed a significant decrease of cardiac β-AR density in mice that underwent CLP, which was not reversed by treatment with LGM2605 (Fig. 2E).
3.2. LGM2605 influences cardiac NF-κB activation but not cardiac expression and plasma inflammatory cytokines levels –
To assess if the cardioprotective effect of LGM2605 relies on anti-inflammatory properties, we tested the expression of inflammatory markers in the hearts of septic C57BL/6 mice. Mice with CLP had increased phosphorylation of IκBα, suggesting activation of NF-κΒ, which was prevented by LGM2605 (Fig. 2F). Analysis of inflammatory markers in septic mice showed increased inflammatory cytokine mRNA levels at 6 h post-CLP which was not reduced by LGM2605 administered at the time of surgery (Suppl. Fig 3A), and 12 h post-CLP which was not affected by LGM2605 administered 6 h post-surgery (Fig. 2G). Accordingly, LGM2605 administration 6 h post-surgery did not reduce circulating levels of proinflammatory cytokines IL-1α, IL-1β, IL-6, and TNFα, or the anti-inflammatory cytokine IL-10, which was also elevated during sepsis (Fig. 2H). Thus, although inhibition of NF-κB signaling seems to be alleviated by LGM2605 treatment, LGM2605-mediated cardiac function improvement does not lower production of inflammatory cytokines.
3.3. LGM2605 does not affect glucose and fatty-acid metabolism related gene expression –
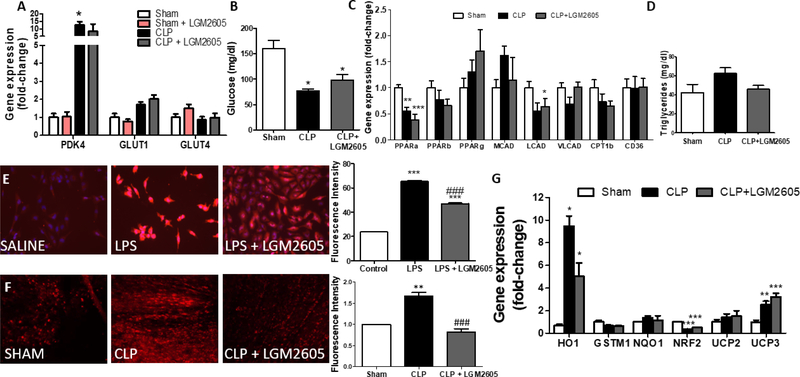
We measured cardiac mRNA levels of glucose uptake and catabolism markers in mice that underwent CLP with and without LGM2605 6 h post-surgery. GLUT1 and GLUT4 cardiac mRNA levels did not change significantly in septic heart tissue, but we observed a significant increase in the mRNA levels of cardiac PDK4, which inhibits pyruvate utilization by inactivating pyruvate dehydrogenase (Fig. 3A). This increase was not alleviated by administration of LGM2605 (Fig. 3A). We measured plasma glucose levels in septic mice 6 h with administration of LGM2605 2 h pre-CLP (Suppl. Fig. 3B) and 12 h post-CLP with LGM2605 administration 6 hours after surgery (Fig.3B). We observed that hypoglycemia in septic mice was not alleviated by the administration of LGM2605.
Figure 3: LGM2605 alleviates mitochondrial oxidative stress without altering fatty acid and glucose metabolism-related gene expression program –
(A) Expression of glucose metabolism-related genes in ventricular tissue 12 h post-CLP or control sham surgery, n=4 mice per group. (B) Plasma glucose levels 12 h post-CLP or control sham surgery; n=4 mice per group. (C) Expression of lipid metabolism-related genes in ventricular tissue 12 h post-CLP or control sham surgery; n=4–5 mice per group. (D) Plasma triglyceride content 12 hours post-CLP or control sham surgery; n=4 mice per group. (E) Representative fluorescence microscopy images and quantification of Mitosox Red staining in AC16 cells treated with LPS or LPS+LGM2605 for 12 hours, n=250. (F) Representative confocal microscopy images and quantification of DHE staining intensity in ventricular tissue of mice 12 h post-CLP or control sham surgery; n=3 mice per group. (G) Antioxidant-related gene expression in ventricular tissue 12 h post-CLP or control sham surgery; n=4–5 mice per group. *P<0.05, ** P<0.01, ***P < 0.001 vs Control/Sham ###p<0.001 vs LPS/CLP; ANOVA with Bonferroni post-test.
We then assessed cardiac expression of genes associated with fatty-acid metabolism, which constitutes approximately 70% of the cardiac ATP production[26], that are known to be affected during sepsis. Mice with CLP had lower expression levels of PPARα and LCAD, while PPARβ/δ, PPARγ, MCAD, VLCAD, CPT1β and CD36 mRNA levels were not altered (Fig. 3C). LGM2605 6 h post-surgery did not reverse the CLP-mediated changes in the PPARα and LCAD mRNA levels (Fig. 3C). Plasma triglyceride levels showed a trend of increase, which did not occur in septic mice treated with LGM2605 (Fig. 3D). Collectively, these data suggest that the beneficial effect of LGM2605 in septic cardiac dysfunction is not associated with alterations in fatty-acid metabolism-related gene expression.
3.4. LGM2605 alleviates oxidative stress without altering antioxidant-related gene expression –
We measured mitochondrial superoxide generation using Mitosox Red staining in AC16 cells treated with E. coli lipopolysaccharides (LPS). Treatment of AC16 cells with LPS for 12 h increased mitochondrial superoxide levels, which was suppressed by LGM2605 (Fig. 3E). Accordingly, dihydroethidium (DHE) staining of ventricular tissue isolated from septic mice 12 h post-CLP showed increased staining intensity which was alleviated significantly by LGM2605 administration 6 h after surgery (Fig. 3F). The beneficial effect of LGM2605 was not accompanied by prevention of the CLP-mediated changes in cardiac expression of antioxidant genes, including nuclear respiratory factor 2 (NRF2), heme oxygenase 1 (HO1), glutathione Stransferase Mu 1 (GSTM1), NAD(P)H:quinone oxidoreductase 1 (NQO1), and uncoupling proteins 2 and 3 (UCP2, UCP3) (Fig. 3G). Taken together, these results show that the beneficial effect of LGM2605 is accompanied by direct alleviation of ROS accumulation, while gene expression program of antioxidant systems is not altered.
3.5. LGM2605 increases mitochondrial abundance without affecting mitochondrial biogenesis-related gene expression or autophagy markers –
To assess whether the beneficial effect of LGM2605 involves changes in mitochondrial number that is known to be affected in septic cardiac dysfunction[10], we treated AC16 cells with LPS and LGM2605 for 12 h and stained them with Mitotracker Red. LPS treatment decreased mitochondrial number, which was prevented by treatment with LGM2605 (Fig. 4A-B). In accordance with these results, Mitotracker Staining analysis performed in adult cardiomyocytes isolated from septic mice 12 h post-CLP suggests that LGM2605 administration 6 h post-surgery prevents CLP-associated reduction in mitochondrial abundance (Fig. 4C-D). We also observed a robust decrease in mitochondrial DNA copy number 12 h after CLP surgery which is reversed by LGM2605 6 h post-surgery(Fig. 4E). Based upon these data, we conclude that LGM2605 restores mitochondrial abundance in sepsis.
Figure 4: LGM2605 increases mitochondrial abundance and restores oxygen consumption during sepsis –
(A) Representative fluorescent images and (B) quantification of mitotracker signal in AC16 cells treated with LPS, LPS+LGM2605 or vehicle for 12 hours, n=250 cells per group. (C) Representative fluorescent images and (D) quantification of mitochondria detection by Mitotracker Red in adult cardiomyocytes isolated from mice 12 h post-CLP or control sham surgery, n=150 cells. (E) Mitochondrial copy number from cardiac tissue collected 12 hours post-CLP or control sham surgery. n=6–9 per group. (F-J) Oxygen consumption rate using Seahorse XF Mito Stress analysis in isolated adult cardiomyocytes 12 h after CLP surgery or CLP followed by treatment with LGM2605 at 6 h post-CLP measured. Basal respiration (G), respiration related to ATP production (H), maximal respiration (I), and spare capacity (J). *P < 0.05, ***P < 0.001 vs Ctrl, #P<0.05 vs CLP, ###P<0.001; ANOVA with Bonferroni post-test n = 3–6 mice per group. (K) Graph of mitochondrial biogenesis- related gene expression from ventricular tissue of mice 12 h alter surgery, n = 4–5 mice per group. (L) Fusion and fission gene expression-related markers in ventricular tissue of mice 12hrs after surgery, n=4 mice per group. (M,N) Representative LC3B and densitometric quantification of LC3BII/LC3BI ratio, n=8–9 mice per group. (O) Quantitative RT-PCR analysis of autophagy related genes. *p<0.05, **p<0.01, ***p<0.001 vs Sham/Saline, #p<0.05, ###p<0.001 vs LPS/CLP; ANOVA +
To determine if restoration in mitochondrial abundance is associated with increased oxygen consumption rate, we isolated cardiomyocytes from mice 12 h after CLP treated with or without LGM2605 6 hours after surgery and performed Seahorse XF mitostress analysis (Fig. 4F). In coordination with reduced mitochondrial abundance, we observed suppression of basal respiration (Fig. 4G), respiration associated with ATP production (Fig.4H), maximal respiration (Fig.4I), and spare capacity (Fig.4J). Suppression of basal respiration, maximal respiration, and spare capacity was reversed by LGM2605 treatment. To investigate the mechanism that may mediate LGM2605-driven restoration of mitochondria abundance, we measured the expression of various markers of mitochondrial biogenesis, fusion/fission, and mitophagy. Cardiac gene expression of mitochondrial biogenesis markers including PGC1α and PGC1β were reduced 12 h post-CLP, whereas mtTFA was not significantly changed. None of these changes were affected by LGM2605 administered 6 h post-surgery (Fig. 4K).
We then examined whether the effect of LGM2605 was associated with changes in the expression of mitochondrial fusion and fission markers. Heart tissue of mice that underwent CLP had increased mRNA levels of MFN1, MFN2, and DRP1, and a trend towards reduced mRNA levels of Fis1 (Fig 4L). LGM2605 treatment restored the CLP-mediated changes in MFN1, MFN2, DRP1, and FIS1 expression (Fig.4L), suggesting that LGM2605 may affect the dynamics between mitochondrial fission and fusion by suppressing cardiac expression of fusion markers that seem to be induced in mice with CLP.
To assess activation of autophagy pathways that mediate mitochondrial flux in sepsis, we measured conversion of LC3BI to LC3BII, a known marker of autophagosome formation. We found increased LC3BII/LC3BI ratio (Fig. 4M, 4N) demonstrating activation of autophagy pathways in cardiac tissue isolated from septic mice. We further found an induction of Map1lc3b and BNIP3 mRNA levels (Fig. 4O). Neither marker was alleviated by LGM2605 treatment 6 h post-surgery suggesting that LGM2605 does not prevent activation of autophagy pathways.
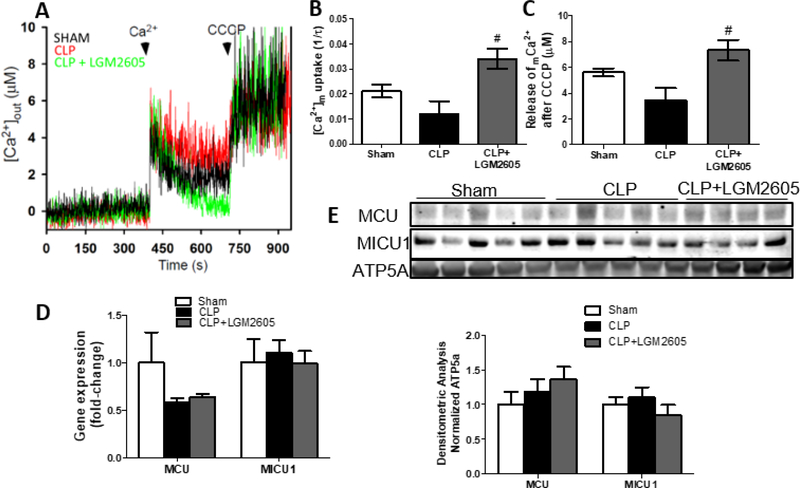
3.6. Increased cardiac mitochondrial abundance in LGM2605-treated mice is associated with increased mitochondrial calcium uptake –
Mitochondrial calcium uptake was assessed in digitonin-permeabilized adult cardiomyocytes treated with thapsigargin that inhibits SERCA-mice 12 h after CLP surgery showed reduced Ca2+ uptake and lower release of Ca2+ following CCCP administration (Fig. 5B, 5C). We found that administration of LGM2605 6 h post-surgery to septic mice increased mitochondrial Ca2+ uptake rate and release of Ca2+ following CCCP administration beyond that observed for sham operated mice (Fig. 5B, 5C). To determine if changes in Ca2+ uptake were associated with changes in mitochondrial Ca2+ uptake-associated genes, we measured expression of mitochondrial calcium uniporter (MCU) and mitochondrial calcium uptake protein 1 (MICU1). On the mRNA and protein level, neither MCU nor MICU1 were altered 12 h post-CLP, and LGM2605 did not affect expression of these proteins. (Fig. 5D, 5E).
Figure 5: LGM2605 increases mitochondrial calcium uptake in isolated primary cardiomyocytes from septic mice –
(A-C) Mitochondrial calcium uptake after a single bolus of calcium in permeabilized adult cardiomyocytes isolated from mice at 12 h post-CLP or control sham surgery; n=3 mice per group. #P<0.05 vs CLP; Kruskall-Wallis test with Dunn’s multiple comparisons. (D) MCU, MICU1 gene expression from ventricular tissue of mice 12 h after sham surgery, CLP with or without treatment with LGM2605 at 6 h post-CLP, n=4–5 mice. (E) Immunoblots and densitometric analysis of cardiac MCU, MICU1 normalized with ATP5A 12 h after
3.7. LGM2605 prevents mitochondrial membrane depolarization –
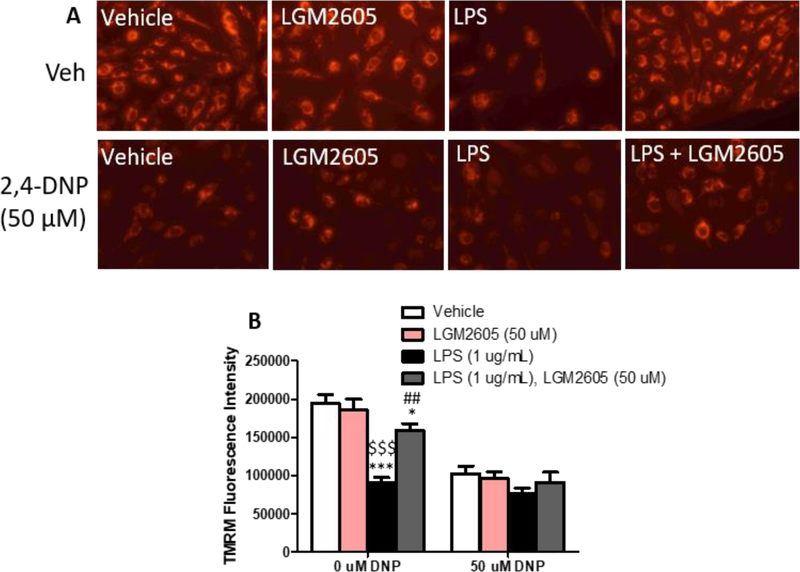
Because the mitochondrial membrane potential is the major driving force for mitochondrial calcium uptake and mitochondrial respiration, we tested if LGM2605 alters mitochondrial depolarization using TMRM staining, which is sequestered by active mitochondria dependent on the mitochondrial membrane potential. LPS treatment significantly reduced TMRM staining intensity compared to vehicle and LGM2605-treated controls, suggesting that LPS reduces the mitochondrial membrane potential (Fig. 6A, 6B). Treatment with LGM2605 significantly restored TMRM staining toward baseline (Fig. 6A, 6B).
Figure 6: LGM2605 preserves mitochondrial membrane potential in LPS stimulated AC16 cardiomyocytes –
(A) Representative images and (B) fluorescence intensity quantification from AC16 cells treated for 12 hours with LPS and LGM2605 with and without uncoupling agent 2,4-DNP (50 mM). *p<0.05, ***P<0.001 vs Vehicle; ##P<0.01 vs LPS, $ $ $P<0.001 vs LGM2605; ANOVA + Bonferroni post-hoc analysis.
As a control experiment, we tested if LGM2605 would affect membrane depolarization that is driven by the uncoupling agent 2,4-dinitrophenol (2,4-DNP), which depolarizes the mitochondrial membrane independent of ROS generation. First, to select the right dose of 2,4-DNP that would not incur significant toxicity for AC16 cells, we applied a series of treatments with increasing concentrations of 2,4-DNP (Suppl. Fig 4). Based on this analysis, we selected to treat cells with 50 μΜ 2,4-DNP. Combined treatment of AC16 cells with LGM2605 and 2,4-DNP showed that LGM2605 lost its beneficial effect in restoring TMRM staining in cells that received 50 μM 2,4-DNP either with or without LPS (Fig. 6B).
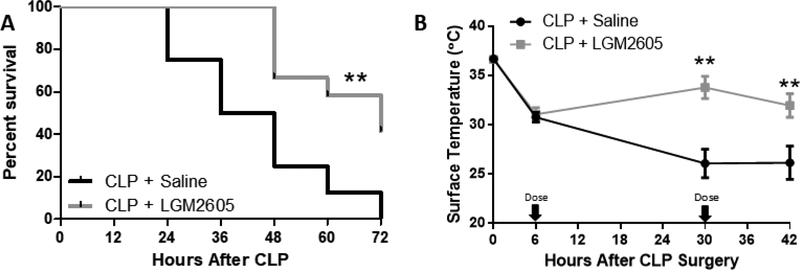
3.8. LGM2605 prevents CLP-associated mortality and reverses hypothermia –
To assess if LGM2605 improves survival after CLP surgery, we performed survival analysis in mice that were treated once daily with Ertapenem (70 mg/kg) and either saline or LGM2605 (100 mg/kg) only treated with ertapenem and saline (Fig. 7A). We also observed that LGM2605 reverses CLP-associated hypothermia, which has previously been identified as a negative prognostic indicator in septic patients and murine models[20, 27]. Septic animals that received LGM2605 were more active than the control septic mice that received saline only (Suppl. Videos). Therefore, systemic LGM2605 administration not only reverses septic cardiac dysfunction, but also improves survival and markers of animal well-being.
Figure 7: LGM2605 prevents mortality and improves body temperature following CLP surgery –
(A) Survival curve and (B) xiphoidal surface temperature (measured by infrared thermometer) of C57BL/6 mice that underwent CLP surgery and received ertapenem (70mg/kg) and either saline or LGM2605 (100 mg/kg) every 24 hours beginning 6 hours post-surgery. n=8–12 mice **p<0.01 by Mantel-Cox test. **p<0.01 vs CLP + Saline; t-test.
4. DISCUSSION
Sepsis is the most common cause of death among critically ill patients in intensive care units (ICU)[28]; particularly when it is accompanied by acute organ dysfunction. Myocardial damage has been described in patients with bacteremia, as shown by higher serum troponin levels[29]. Nevertheless, cardiovascular impairments have been associated with significantly higher sepsis-related mortality[30]. Despite years of research, the pathophysiology of sepsisinduced myocardial dysfunction has not yet been defined, and the responsible cellular mechanisms still remain unclear[3]. No effective treatments or specific medications are used in clinical practice to reverse sepsis-induced cardiomyopathy[31]. The pathophysiology of septic cardiac dysfunction has been attributed to increased oxidative stress, elevated inflammation, impaired β-adrenergic signaling, activation of apoptosis, suppression of metabolic pathways, and reduced ATP synthesis in the cardiomyocytes[11, 12, 32, 33].
Previous evidence from our group has shown that a major component of myocardial dysfunction in sepsis is energetic failure, the correction of which improves cardiac function despite increased levels of inflammatory cytokines[9–12]. Mitochondria constitute major cellular organelles involved in the energetic machinery of the heart and other organs. Thus, mitochondrial dysfunction can be detrimental for cardiac function in sepsis and other diseases[34–36]. ROS such as superoxide and peroxide compromise mitochondrial integrity and function during sepsis. Previous work from our team has shown that the inhibition of NOX2, which is an extramitochondrial protein involved in the generation of superoxide, alleviated oxidative stress and preserved cardiac function in a murine model of sepsis, suggesting a crucial role of ROS stress in aberrant cardiac function associated with sepsis[12].
In the present study, we focused on the effects of antioxidant therapy in mitochondrial function during sepsis. The antioxidant LGM2605 successfully restored normal cardiac function in septic mice when administered either after or prior to the induction of sepsis despite sustained elevation of inflammatory cytokines. The cardioprotective effect of LGM2605 was associated with restoration in mitochondrial abundance, and a reduction in LPS and CLPmediated increase in ROS in vitro and in vivo respectively. Mitochondria are central to the detrimental effects of oxidative stress on cellular function in cardiomyocytes. Increased ROS generation is associated with mitochondrial membrane depolarization, reduced mitochondrial respiration, and initiation of apoptotic pathways[37]. In our study, we observed restoration in cardiomyocyte mitochondrial respiration and preservation of mitochondrial membrane potential with LGM2605 antioxidant therapy, suggesting that LGM2605 protects mitochondrial function.
Various other studies have proposed that antioxidant therapy alleviates organ dysfunction in sepsis. Antioxidants acting on various components of the redox signaling pathway including redox defense systems[38], ROS scavenging systems[39], mitochondrial ROS generation[40], and extra-mitochondrial ROS generation[12] have been shown to improve outcome in preclinical animal models and in patients. LGM2605, a novel compound with ROS scavenging and anti-inflammatory properties, is currently under development for the treatment of radiation-induced pneumopathy and lung injury[13–17]. This compound has the benefit of being a non-toxic compound with favorable bioavailability and pharmacokinetics, and a potent ability to reduce multiple ROS species with a lower EC50 compared with ascorbic acid which also improves outcome in septic patients[17, 41]. Further, the ROS scavenging capabilities of LGM2605 do not rely on endogenous antioxidant defense systems which are depleted during sepsis [38, 42]. Previous studies using SDG to prevent diabetes have suggested that single daily oral administration of 22mg/kg is sufficient to confer its anti-oxidant effects with minimal side effects[43]. Once daily administration of 100 mg/kg LGM2605 I.P. improved survival in our septic animals compared to standard treatment alone thereby suggesting that LGM2605 may constitute a supplemental therapy for severe sepsis and organ dysfunction.
Surprisingly, we observed that LGM2605 did not restore dP/dt in our in situ measurements despite increasing EF and FS. These measurements of cardiac contractility must be interpreted in alignment with the decrease in preload and afterload that occurs during sepsis, as well as the lack of reversal of β-AR density with LGM2605 treatment. Reduced dP/dt may reflect the lower load that the heart must overcome during the ejection phase [44–46]. We observed that LGM2605 treatment did not improve diastolic volume, blood pressure, and heart rate, but it did improve GLS and GLS rate. GLS and GLS rate are calculated using speckle-tracking echocardiography and represent myocardial wall deformation along the longitudinal axis. GLS has been proposed as an early and sensitive marker of septic cardiac dysfunction and independent predictor of mortality[47]. LGM2605 administration restored GLS and GLS rate to normal levels as it did for EF and FS. This indicates greater myocardial wall movement, which may account for the improved efficiency of the heart to pump higher blood volume particularly in a situation of reduced peripheral resistance that happens in sepsis. Furthermore, the diastolic dysfunction that is manifested by combined reduction in end-diastolic volume, impaired reverse GLS rate and lower dP/dtmin is not resolved by LGM2605 treatment. Thus, LGM2605 restores systolic but not diastolic function in septic mice.
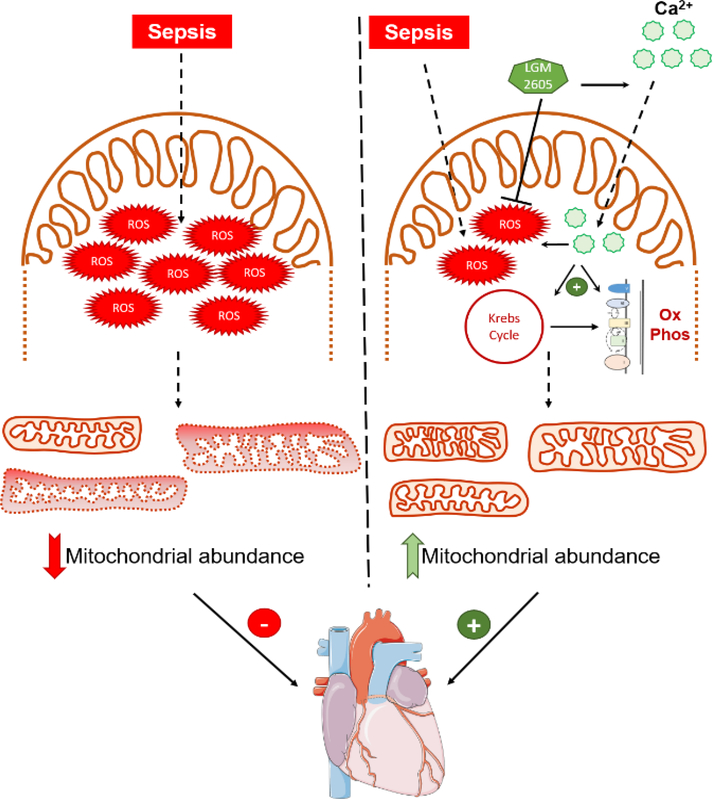
Alterations in mitochondrial abundance, regulation of fission/fusion pathways, and energetic failure have been described in cardiac disease[48]. Our present study indicates the role of reduced mitochondrial abundance resulting from oxidative stress in aggravating cardiac function in sepsis. LGM2605 treatment was accompanied by increased mitochondrial calcium uptake and mitochondrial respiration. Calcium import in mitochondria takes place primarily through the mitochondria calcium uniporter (MCU) and is driven by the mitochondrial membrane potential. As MCU protein levels are not significantly altered in septic mice treated with LGM2605, the improvement in mitochondrial calcium uptake may be driven by restoration of mitochondrial potential that the beneficial treatment incurs and the increase in mitochondrial abundance. Mitochondrial calcium serves as a regulator of enzymes associated with fatty acid and pyruvate oxidation, the Krebs cycle and oxidative phosphorylation and stimulates enzymatic activity associated with cellular respiration[49]. On the other hand, inhibition of increased Ca2+ uptake has been proposed as a therapeutic intervention during cardiac stress[50, 51], and unregulated Ca2+ uptake by mitochondria increases ROS production[52, 53]. In our study, increased Ca2+ uptake was associated with lower ROS accumulation. This effect may be attributed to either increased mitochondrial abundance or the anti-oxidant effect of LGM2605, which seems to act as a dual mitochondrial Ca2+ uptake inducer and ROS scavenger (Fig.8).
Figure 8: Proposed mechanism of action for alleviation of cardiac dysfunction by LGM2605 –
Graphical model of the proposed mechanism by which LGM2605 alleviates oxidative stress, increases mitochondrial respiration, and restores cardiac systolic function. Figure was produced using Servier Medical Art (http://www.servier.com/ ).
Increased mitochondrial calcium uptake has also been proposed as an essential process underlying the energetic adaptations to adrenergic signaling in the heart[50]. A prior study, using a different sepsis animal model, reported reduced mitochondrial calcium uptake in isolated non-permeabilized rat cardiomyocytes. They suggested that reduced calcium uptake may underlie lower responsiveness to adrenergic challenge in septic rats[54]. In our study, LGM2605 increased calcium uptake in permeabilized cardiomyocytes but did not improve β-AR responsiveness. Therefore, we propose that increased mitochondrial abundance and calcium uptake restore mitochondrial respiration, but they do not suffice to reverse lack of β-AR responsiveness in sepsis.
Based on our previous studies that identified energetic failure as a major cause of septic cardiomyopathy[9–12], our present study focuses on the role of the oxidative stress-mediated impairment of mitochondrial parameters in aggravating cardiac function in sepsis. We observed that sepsis activated the expression of mitofusin genes, which was suppressed by LGM2605. This result may suggest that LGM2605 alleviates cardiac stress thereby reducing stressinduced mitochondrial hyperfusion, that has been previously described as a pre-apoptotic cellular response to stress conditions[55]. In agreement with our observations, others have shown that oxidative stress promotes mitochondrial hyperfusion in myocytes[56]. Surprisingly, LGM2605 did not affect gene expression associated with mitochondrial biogenesis or activation of autophagy pathways, which mediate mitophagy. Importantly, our gene expression analyses show trends of restored expression of autophagy-related markers by LGM2605. Further, the beneficial effect of LGM2605 on mitochondrial membrane potential suggests that LGM2605 may affect mitophagic flux by preventing mitochondrial damage. Nevertheless, mitochondrial membrane depolarization is an important signal that targets mitochondria to mitophagy pathways[57], which could explain the elevation in mitochondrial abundance despite sustained activation of autophagy pathways. This response may constitute early secondary signals that ameliorate mitophagy due to lower oxidative stress, preserved mitochondrial membrane potential, increased mitochondrial respiration, and reduced mitochondrial damage.
We found that the beneficial effects of LGM2605 exceeded restoration of cardiac function. LGM2605 also improved survival and reversed hypothermia, suggesting that our treatment alleviates hypodynamic sepsis. These findings imply that systemic administration of LGM2605 may be protective for other organs beyond the heart, alleviate ischemia- and inflammatory-related organ injury and eventually prevent multiple organ failure that accounts for mortality in sepsis [58]. Despite the beneficial effects that LGM2605 may incur in other organs, improvement of cardiac systolic function per se is an important event that can prevent shock and reverse its complications. Cardiac output is directly involved in the regulation of arterial pressure[59]. In addition, improvement in cardiac function can improve the ability to provide fluids without causing fluid overload following antibiotic and fluid treatment[60].
This study was designed to be consistent with current preclinical guidelines for sepsis research including documentation of organ dysfunction, comparison to standard of care, and clinically relevant dosing schedules[61]. Future studies may apply this protocol to assess how antioxidant therapy with LGM2605 affects function of other organs that are damaged during sepsis, and to validate the beneficial effect of LGM2605 in large animal studies.
Limitations:
While we observed that CLP results in a progressive deterioration in EF and FS in male C57BL/6 mice, we did not see the same phenotype in female mice at the timepoints we investigated. Previous studies have shown that females are protected against sepsis-induced organ dysfunction[62]. For this reason, we focused our study on male animals. It is also unknown what biological factors mediate the beneficial effect of LGM2605 in sepsis, as well as if this treatment may be beneficial in other organ systems that may affect cardiac function.
In our study, we found that LGM2605 increased mitochondrial abundance which was associated with improved cardiomyocyte respiration and mitochondrial calcium uptake. While increases in these measurements may reflect an increase in mitochondrial abundance to some extent, assessment of individual mitochondrial function remains to be tested. Importantly, we observed in vitro that mitochondrial membrane potential is reduced by LPS stimulation, which seems to be a ROS driven phenomenon since LGM2605 restored TMRM staining. As maintenance of membrane potential is important for both mitochondrial respiration and calcium uptake, the combined reduction in mitochondrial abundance and quality likely contribute to the deficits we observed in our study.
5. CONCLUSIONS
We demonstrate for the first time that CLP deteriorates cardiac systolic function that is dependent on oxidative stress. We also demonstrate that oxidative stress underlies metabolic dysfunction, which underlies CLP-induced septic cardiomyopathy. Treatment with LGM2605 is a novel pharmacological approach that reduces cardiac ROS accumulation, protects cardiac mitochondrial function, reverses cardiac dysfunction, and improves survival.
Supplementary Material
HIGHLIGHTS.
Sepsis results in progressive cardiac dysfunction associated with oxidative stress.
ROS formation underlies mitochondrial dysfunction in septic cardiomyopathy.
Antioxidant LGM2605 scavenges ROS and alleviates mitochondrial dysfunction
LGM2605 treats septic cardiac dysfunction and improves survival.
ACKNOWLEDGEMENTS
We would like to thank Brett R. Brown for technical assistance. D.K and C.K. were MSc students of the “Molecular Basis of Human Diseases” graduate program of the Medical School, University of Crete, Greece. All persons named in the “Acknowledgments” section have provided the corresponding author with permission to be named in the manuscript.
SOURCES OF FUNDING
This study was supported by the National Heart Lung and Blood Institute of the NIH “Pathway to Independence” K99/R00 award HL112853 (K.D.), HL130218 (K.D.), the W.W. Smith Charitable Trust (K.D), and P01HL091799 (W.J.K.). The work was also supported in part by 1P42ES023720–01 (M.C.S.). S.S. is supported by an NIH K99/R00 grant (1 K99 HL13826801). M.H. was supported by an American Heart Association pre-doctoral fellowship (18PRE34060115). I.D.K. was supported by the American Heart Association and the Kahn Family Post-Doctoral Fellowship in Cardiovascular Research (18POST34060150). D.T. was supported by American Heart Association post-doctoral fellowships (17POST33660251).
Footnotes
DISCLOSURES
Melpo Christofidou-Solomidou (MCS) reports grants from the NIH and NASA during the conduct of the study. In addition, MCS has patents No. PCT/US2015/033501, PCT/US2016/049780, PCT/US17/35960, PCT/US2014/041636, No. PCT/US15/22501 pending and has a founders equity position in LignaMed, LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama. 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ren J, Wu S. A burning issue: do sepsis and systemic inflammatory response syndrome (SIRS) directly contribute to cardiac dysfunction? Frontiers in Bioscience. 2006;11:8. [DOI] [PubMed] [Google Scholar]
- [3].Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. [DOI] [PubMed] [Google Scholar]
- [4].Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15:392–7. [DOI] [PubMed] [Google Scholar]
- [5].Hunter JD, Doddi M. Sepsis and the heart. Br J Anaesth. 2010;104:3–11. [DOI] [PubMed] [Google Scholar]
- [6].Turdi S, Han X, Huff AF, Roe ND, Hu N, Gao F, et al. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced myocardial contractile dysfunction: role of autophagy. Free Radic Biol Med. 2012;53:1327–38. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [7].Kumar A Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. Journal of Experimental Medicine. 1996;183:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Montmollin E, Aboab J, Mansart A, Annane D. Bench-to-bedside review: Beta-adrenergic modulation in sepsis. Crit Care. 2009;13:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Drosatos K, Drosatos-Tampakaki Z, Khan R, Homma S, Schulze PC, Zannis VI, et al. Inhibition of c-Jun-N-terminal kinase increases cardiac peroxisome proliferator-activated receptor alpha expression and fatty acid oxidation and prevents lipopolysaccharide-induced heart dysfunction. The Journal of biological chemistry. 2011;286:36331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Drosatos K, Khan RS, Trent CM, Jiang H, Son NH, Blaner WS, et al. Peroxisome proliferator-activated receptor-gamma activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circulation Heart failure. 2013;6:550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Current heart failure reports. 2015;12:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Joseph LC, Kokkinaki D, Valenti MC, Kim GJ, Barca E, Tomar D, et al. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Christofidou-Solomidou M, Pietrofesa R, Arguiri E, McAlexander MA, Witwer KW. Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: a novel mechanism of tissue radioprotection by flaxseed. Cancer Biol Ther. 2014;15:930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pietrofesa RA, Solomides CC, Christofidou-Solomidou M. Flaxseed Mitigates Acute Oxidative Lung Damage in a Mouse Model of Repeated Radiation and Hyperoxia Exposure Associated with Space Exploration. J Pulm Respir Med. 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pietrofesa RA, Velalopoulou A, Arguiri E, Menges CW, Testa JR, Hwang WT, et al. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis. 2016;37:177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mishra OP, Pietrofesa R, Christofidou-Solomidou M. Novel synthetic (S,S) and (R,R)-secoisolariciresinol diglucosides (SDGs) protect naked plasmid and genomic DNA From gamma radiation damage. Radiat Res. 2014;182:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mishra OP, Simmons N, Tyagi S, Pietrofesa R, Shuvaev VV, Valiulin RA, et al. Synthesis and antioxidant evaluation of (S,S)- and (R,R)-secoisolariciresinol diglucosides (SDGs). Bioorg Med Chem Lett. 2013;23:5325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Velalopoulou A, Tyagi S, Pietrofesa RA, Arguiri E, Christofidou-Solomidou M. The Flaxseed-Derived Lignan Phenolic Secoisolariciresinol Diglucoside (SDG) Protects Non-Malignant Lung Cells from Radiation Damage. Int J Mol Sci. 2015;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schnelle M, Catibog N, Zhang M, Nabeebaccus AA, Anderson G, Richards DA, et al. Echocardiographic evaluation of diastolic function in mouse models of heart disease. J Mol Cell Cardiol. 2018;114:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Laitano O, Van Steenbergen D, Mattingly AJ, Garcia CK, Robinson GP, Murray KO, et al. Xiphoid Surface Temperature Predicts Mortality in a Murine Model of Septic Shock. Shock. 2018;50:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hoffman NE, Chandramoorthy HC, Shamugapriya S, Zhang X, Rajan S, Mallilankaraman K, et al. MICU1 Motifs Define Mitochondrial Calcium Uniporter Binding and Activity. Cell Reports. 2013;5:1576–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Davidson MM, Nesti C, Palenzuela L, Walker WF, Hernandez E, Protas L, et al. Novel cell lines derived from adult human ventricular cardiomyocytes. J Mol Cell Cardiol. 2005;39:133–47. [DOI] [PubMed] [Google Scholar]
- [23].Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–23. [DOI] [PubMed] [Google Scholar]
- [24].Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, et al. A Novel and Efficient Model of Coronary Artery Ligation and Myocardial Infarction in the Mouse. Circ Res. 2010;107:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nature cell biology. 2012;14:1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lopaschuk GD, Ussher JR, Folmes CDI, Jaswal JS, Stanley WC. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol Rev. 2010;90:52. [DOI] [PubMed] [Google Scholar]
- [27].Kushimoto S, Gando S, Saitoh D, Mayumi T, Ogura H, Fujishima S, et al. The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Critical Care. 2013;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, R. PM. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:8. [DOI] [PubMed] [Google Scholar]
- [29].Kalla C, Raveh D, Algur N, Rudensky B, Yinnon AM, Balkin J. Incidence and significance of a positive troponin test in bacteremic patients without acute coronary syndrome. Am J Med. 2008;121:909–15. [DOI] [PubMed] [Google Scholar]
- [30].Parrillo JE, Parker MM, Natanson C, Suffredial AF, Danner RL, Cunnion RE, et al. Septic Shock in Humans. Annals of Internal Medicine. 1990;113:16. [DOI] [PubMed] [Google Scholar]
- [31].Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cimolai MC, Alvarez S, Bode C, Bugger H. Mitochondrial Mechanisms in Septic Cardiomyopathy. Int J Mol Sci. 2015;16:17763–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Neviere R, Fauvel H, Chopin C, Formstecher P, Marchetti P. Caspase Inhibition Prevents Cardiac Dysfunction and Heart Apoptosis in a Rat Model of Sepsis. Am J Respir Crit Care Med. 2001;163:8. [DOI] [PubMed] [Google Scholar]
- [34].Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. The Lancet. 2002;360:219–23. [DOI] [PubMed] [Google Scholar]
- [35].Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovascular research. 2004;64:279–88. [DOI] [PubMed] [Google Scholar]
- [36].Levy RJ. Mitochondrial dysfunction, bioenergetic impairment, and metabolic downregulation in sepsis. Shock. 2007;28:24–8. [DOI] [PubMed] [Google Scholar]
- [37].Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol Rev. 2014;94:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Biolo G, Antonione R, Marcell DC. Glutathione metabolism in sepsis. Critical care medicine. 2007;35:5. [DOI] [PubMed] [Google Scholar]
- [39].Marik PE, Khangoora V, River R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock. Chest. 2017;151:9. [DOI] [PubMed] [Google Scholar]
- [40].Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Setchell KD, Brown NM, Zimmer-Nechemias L, Wolfe B, Jha P, Heubi JE. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food & Function. 2014;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Malmezat T, Breuillé D, Capitan P, Mrand PP, Obled C. Glutathione Turnover Is Increased during the Acute Phase of Sepsis in Rats. Nutrient Metabolism. 2000;130:8. [DOI] [PubMed] [Google Scholar]
- [43].Prasad K Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: Effect of secoisolariciresinol diglucoside (SDG). Molecular and Cellular Biochemistry. 2000;209:7. [DOI] [PubMed] [Google Scholar]
- [44].Konishi T, Nakamura Y, Kato I, Kawai C. Dependence of peak dP/dt and mean ejection rate on load and effect of inotropic agents on the relationship between peak dP/dt and left ventricular developed pressure - assessed in the isolated working rat heart and cardiac muscles International Journal of Cardiology. 1992;35:9. [DOI] [PubMed] [Google Scholar]
- [45].Blaudszun G, Licker MJ, Morel DR. Preload adjusted left ventricular dP/dtmax: a sensitive, continuous, load independent contractility index. Experimental Physiology. 2013;98:11. [DOI] [PubMed] [Google Scholar]
- [46].Hamlin RL, del Rio C. dP/dt(max)-a measure of ‘baroinometry’. J Pharmacol Toxicol Methods. 2012;66:3. [DOI] [PubMed] [Google Scholar]
- [47].Chang WT, Lee WH, Lee WT, Chen PS, Su YR, Liu PY, et al. Left ventricular global longitudinal strain is independently associated with mortality in septic shock patients. Intensive Care Medicine. 2015;41:9. [DOI] [PubMed] [Google Scholar]
- [48].Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW, 2nd, Kitsis RN, et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association. Circ Res. 2016;118:1960–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Williams GS, Boyman L, Lederer WJ. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol. 2015;78:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, et al. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell reports. 2015;12:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, et al. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell reports. 2015;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:8. [DOI] [PubMed] [Google Scholar]
- [53].Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Annals of the New York Academy of Sciences. 2010;1201:6. [DOI] [PubMed] [Google Scholar]
- [54].Pinto BB, Dyson A, Umbrello M, Carre JE, Ritter C, Clatworthy I, et al. Improved Survival in a Long-Term Rat Model of Sepsis Is Associated With Reduced Mitochondrial Calcium Uptake Despite Increased Energetic Demand. Crit Care Med. 2017;45:e840–e8. [DOI] [PubMed] [Google Scholar]
- [55].Shutt TE, McBride HM. Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim Biophys Acta. 2013;1833:417–24. [DOI] [PubMed] [Google Scholar]
- [56].Redpath CJ, Bou Khalil M, Drozdzal G, Radisic M, McBride HM. Mitochondrial hyperfusion during oxidative stress is coupled to a dysregulation in calcium handling within a C2C12 cell model. PLoS One. 2013;8:e69165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Twig G, Shirihai OS. The Interplay Between Mitochondrial Dynamics and Mitophagy. Antioxidants & Redox Signaling. 2011;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wen H Sepsis Induced by Cecal Ligation and Puncture. Methods Mol Biol. 2014;1031:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guyton AC. The Relationship of Cardiac Output and Arterial Pressure Control. Circulation. 1981;64:10. [DOI] [PubMed] [Google Scholar]
- [60].Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid Overload in Patients with Severe Sepsis and Septic Shock Treated with Early-Goal Directed Therapy is Associated with Increased Acute Need for Fluid-Related Medical Interventions and Hospital Death. Shock. 2015;43:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Osuchowski MF, Ayala A, Bahrami S, Bauer M, Boros M, Cavaillon JM, et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (Mqtipss): An International Expert Consensus Initiative for Improvement of Animal Modeling in Sepsis. Shock. 2018;50:377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen J, Chiazza F, Collino M, Patel NS, Coldewey SM, Thiemermann C. Gender dimorphism of the cardiac dysfunction in murine sepsis: signalling mechanisms and age-dependency. PloS one. 2014;9:e100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.