Abstract
Background:
Although some metals are endocrine-disrupting compounds and known or probable carcinogens, the distribution of exposures to many metals, particularly among school-aged children, is not well characterized.
Objectives:
To examine whether a community-level stressor, neighborhood deprivation, was associated with urinary concentrations of thirteen metals, and whether any observed relationships varied by race/ethnicity.
Methods:
We obtained neighborhood characteristics from the 2005–2009 American Community Survey. Demographic information and urine samples from 401 healthy young girls in Northern California were obtained during a clinical visit. Urine samples were analyzed for metals using inductively-coupled plasma-mass spectrometry and levels were corrected for creatinine. We ran analysis of variance and generalized linear regression models to estimate associations of urinary metal concentrations with neighborhood deprivation and race/ethnicity and stratified multivariable models to evaluate possible interactions among predictors on metals concentrations.
Results:
Urinary concentrations of three metals (barium, lead, antimony) varied significantly across neighborhood deprivation quartiles, and four (barium, lead, antimony, tin) varied across race/ethnicity groups. In adjusted models, both race/ethnicity (F3,224=3.89, p=0.01) and neighborhood deprivation (F3,224=3.82, p=0.01) were associated with antimony concentrations, but neither were associated with lead, barium, or tin, concentrations. Examining neighborhood deprivation within race/ethnicity groups, barium levels (pinteraction<0.01) decreased with neighborhood deprivation among Hispanic girls (ptrend<0.001) and lead levels (pinteraction=0.07) increased with neighborhood deprivation among Asian girls (ptrend=0.03).
Conclusions:
Children are especially vulnerable to environmental exposure to metals. Our results indicate that community- and individual-level stressors may influence vulnerability to some metals and contribute to environmental health disparities.
Keywords: environmental health disparities, metals, vulnerability, lead, children
1. Introduction
Metals are ubiquitous chemical elements that arise from both natural and anthropogenic sources and can have adverse health effects at high levels of exposure (Agency for Toxic Substances & Disease Registry (ATSDR) 2008; Occupational Safety & Hazard Administration (OSHA) 2009). Some metals have been classified as endocrine-disrupting compounds (EDCs) (Iavicoli et al. 2009), and others have been identified as known, probable, or possible carcinogens (International Agency for Research on Cancer (IARC) 2013). Children may be particularly vulnerable to these environmental toxicants due to their smaller bodies and immature metabolic pathways (Landrigan and Miodovnik 2011).
General population studies suggest a range of health effects from metals exposure, including positive associations between urinary barium and indices of obesity (Padilla et al. 2010), cadmium and all-cause and cardiovascular mortality (Tellez-Plaza et al. 2012), and antimony, cadmium, cobalt, and tungsten exposure with cerebrovascular disease (Agarwal et al. 2011). In addition to their classification as human carcinogens, cadmium and arsenic are also associated with kidney damage (Järup 2003) and respiratory, dermal, reproductive, and neurological effects (Jomova et al. 2011). The limited epidemiological evaluations of tin exposure suggest a link with occupational lung disease (Chonan et al. 2007). In children specifically, metals exposure is most frequently associated with neurodevelopmental toxicity. Several studies report associations between antimony, cadmium, chromium, arsenic, lead, manganese, mercury, and/or nickel and autism spectrum disorder (Palmer et al. 2009; Roberts et al. 2013; Windham et al. 2006). Exposure to lead has also consistently been linked to neurocognitive deficits such as attention deficit disorder (Kim et al. 2013) and lowered IQ (Lanphear et al. 2005), as well as behavioral problems such as aggression and delinquency (Olympio et al. 2009). Similarly adverse effects from manganese exposure include impairments in motor skills, intelligence, and olfactory function (Grandjean and Landrigan 2014; Roels et al. 2012).
Knowledge of the severe effects of lead and other metals on children’s health has resulted in regulations to reduce exposure, including the removal of lead from gasoline and paint, and remediation of indoor sources, such as lead-based paint (Mauss 1994; Needleman 2004). Nevertheless, a primary source of childhood exposure to lead is ingestion of household lead-based paint chips or dust (ATSDR 2007b; Levin et al. 2008). Household paint also contains other potentially toxic metals, such as cobalt and manganese (Fjelsted and Christensen 2007; Mielke et al. 2001). Living in dilapidated housing has been linked to lead exposure (Hood 2005), and the age of housing stock is the primary determinant of differential exposure (Needleman 2004; Oyana and Margai 2007). In addition to deteriorating paint, traffic and industrial activities have also been linked to the presence of lead and other trace metals in urban environments (Akkus and Ozdenerol 2014; Wong et al. 2006).
Blood lead levels in the U.S. population have decreased dramatically over the past 40 years, but remain significantly higher among Black children and those living in poverty (Environmental Protection Agency (EPA) 2013). Levels of other metals such as antimony, thallium, cadmium, and mercury have also been found to be elevated in racial/ethnic minority populations (Belova et al. 2013; Said and Hernandez 2015). For most heavy metals, exposures to which may be concomitant due to common sources, the published data suggest a lack of knowledge around factors driving individual susceptibility, such as age, sex, and the timing and duration of exposure (Karagas et al. 2012). Consideration not only of physical environmental hazards, but also of individual and social stressors, is critical to an enhanced understanding of the etiology of disparities in vulnerability and susceptibility to environmental exposures (Gee and Payne-Sturges 2004; Morello-Frosch et al. 2011). Despite calls for increased attention to the social determinants of environmental health (Wright 2009), very few quantitative studies (e.g., Hicken et al. 2012) have explored how socio-environmental processes contribute to the differential distribution of exposures associated with health disparities (Burger and Gochfeld 2011; Schwartz et al. 2011).
2. Theory
Whereas traditional exposure pathway models focus on the source and routes of exposure, dose, and biological characteristics of the exposed population, the multilevel stress-exposure-disease model proposes that individual-level and community-level stressors contribute to differential distributions of exposures (i.e., vulnerability) and differential health impacts of exposures (i.e., susceptibility) (Gee and Payne-Sturges 2004; Gochfeld and Burger 2011; Payne-Sturges and Gee 2006). This model is consistent with cumulative risk approaches that recognize the potential for social stressors to contribute to the adverse effects of environmental toxicants (Environmental Protection Agency (EPA) 2003; Sexton and Linder 2010). Using the stress-exposure-disease model as a framework, the current study examines whether urinary biomarkers of exposure to thirteen metals vary across levels of a community-level stressor, neighborhood deprivation, and whether exposure vulnerability is similar for girls from various racial/ethnic groups when accounting for family income. Higher levels of stress, assessed with both self-report and physiological measures, have been documented among racial/ethnic populations (Borders et al. 2015; Byrd 2012; Dowd et al. 2014; Juster et al. 2010; Merkin et al. 2009; Morello-Frosch et al. 2011; Peek et al. 2010). Thus, in this study, race/ethnicity serves as a proxy for stressors associated with being a member of a minority group. We do not address exposure-disease associations; rather, we focus on predictors of differential vulnerability to better understand the relationships between stressors and metals exposures. We hypothesized that urinary metals concentrations would be highest among girls living in areas with the highest levels of neighborhood deprivation, and that these associations would be most pronounced among Black and Hispanic girls.
3. Materials and Methods
3.1. Participants and Procedures
The Cohort Study of Young Girls’ Nutrition, Environment, and Transitions (CYGNET) is a longitudinal study aimed at exploring the influence of environmental factors on pubertal development, in order to further understand the etiology of breast cancer within the Breast Cancer and the Environment Research Program (Hiatt et al. 2009). One of three similar studies, recruitment and enrollment details are described elsewhere (Biro et al. 2013). In brief, a Kaiser Permanente Northern California (KPNC) database was used to identify all current female members between the ages of 6 to 7 years in 2005–2006, who lived in Marin or San Francisco counties or select East Bay communities in Contra Costa and Alameda Counties, CA at the time of recruitment and at the time of birth. Girls who had been diagnosed with precocious puberty or other endocrinological conditions known to influence pubertal timing were excluded. Families of these girls received printed materials describing the study and inviting their participation; those who planned to move in the near future were ineligible. A total of 444 girls and their caregivers enrolled in the CYGNET study. The study received approval from Institutional Review Boards at the University of California San Francisco, the University of California at Berkeley, Kaiser Permanente Northern California, and the Centers for Disease Control and Prevention (CDC).
3.2. Data Collection
Data and biospecimens were collected at study visits that occurred at one of three Kaiser Permanente clinic sites in Oakland, San Francisco, or San Rafael. At the first study visit, which occurred between June 2005 and August 2006, data were collected by interview with the primary caregiver, and through anthropometry and pubertal maturation assessment of the child participant as previously described (Biro et al. 2013). The primary caregiver (more than 90% were the child’s mother) provided demographic information and an updated home address; the latter was used to derive neighborhood deprivation measures.
Urine was collected and stored using materials that were determined to be metal-free by the CDC. A urine sample was collected at the time of study visit and stored temporarily in a refrigerator. At the end of the clinic visit, specimens were transported on ice to the KPNC Division of Research laboratory and aliquoted for long-term storage at −80° C. Single spot urine samples such as these allow for assessment of multiple toxicological elements (CDC, 2012). The National Health and Nutrition Examination Survey (NHANES) measures lead and cadmium (bioaccumulative inorganic materials) in both blood and urine, whereas the other 11 metals explored in this study (non-bioaccumulative inorganic materials) are measured in urine only (CDC 2012; Needham and Sexton 2000; Needham et al. 2005).
A total of 422 girls provided urine samples during the baseline visit. Of these girls, we conducted analyses among the 401 with complete data on neighborhood deprivation, race/ethnicity, and family income.
3.3. Neighborhood and Family Socioeconomic Characteristics
Neighborhood was defined as the census tract (based on 2000 Census boundaries) of the participant’s residence at baseline (between 2005 and 2006). Census tracts contain about 4,000 residents and are designed to be homogenous with respect to socio-demographic characteristics and living conditions (US Census Bureau 2012). We used data from the 2005–2009 American Community Survey to calculate neighborhood deprivation scores based on an index comprised of eight sociodemographic census tract characteristics (Messer et al. 2006). These characteristics included percentages of: males in management and professional occupations; crowded housing (i.e., housing units with more than one occupant per room); households in poverty; female-headed households with dependents; households on public assistance; households earning less than $30,000 annually; adults not earning a high school diploma; and unemployed. Higher scores indicate higher levels of neighborhood deprivation.
Annual household income for each girl’s family was assessed using a series of questions that asked whether household income was greater than or less than increments of $25,000. For this analysis, we categorized this information as: <$50,000; $50,000-$100,000, and $100,000 or more.
3.4. Race/ethnicity
The primary caregiver provided information regarding the child’s race and ethnicity from two questions: “What race do you consider [child’s name] to be?” and “Do you consider [child’s name] to be Hispanic/Latina?” We used a hierarchical approach to assign a single, mutually exclusive racial/ethnic group for analytic purposes. Any girl who was reported to be Black was coded as such, regardless of other racial or ethnic selections. Non-Black girls for whom Hispanic ethnicity was answered “Yes” were coded as Hispanic. Any girl who was reported by her caregiver to be Asian, but not Black or Hispanic, was coded as Asian. Lastly, girls for whom White was the only racial or ethnic group selected on the questionnaire were coded as such. No study participants fell into other commonly-used race/ethnicity categories (e.g., American Indian).
3.5. Urinary metal and cotinine concentrations
The National Center for Environmental Health (NCEH) at the CDC analyzed the urine samples for 15 metals and cotinine. Urinary metal concentrations were determined using inductively coupled plasma-mass spectrometry, as described previously (Jarrett et al. 2007; Jarrett et al. 2008). After hydrolysis with β-glucuronidase, urine samples were analyzed for total cotinine by liquid chromatography-tandem mass spectrometry. Urinary creatinine concentrations were determined using the Roche/Hitachi Modular P Chemistry Analyzer. This method is described in Roche’s Creatinine plus Product Application # 11775685216V18. Urine metal and cotinine concentrations are adjusted by using creatinine concentrations to correct for variable urine excretion rates at the time of spot urine specimen collection.
We analyzed creatinine-corrected urinary metal concentrations (μg/g creatinine) of antimony, barium, beryllium, cadmium, cesium, cobalt, lead, manganese, molybdenum, platinum, strontium, thallium, tin, tungsten, and uranium. Very few girls had detectable levels of beryllium (n=14) and platinum (n=22), so we did not explore these metals further. Because they are largely undetectable, urinary concentrations of these two metals have not been reported by the NHANES after 2009–2010 (CDC, 2015). In addition to the internal CDC quality control procedures, we incorporated approximately 10% masked quality control specimens (n=43) from a single urine pool. The coefficients of variation (CVs) were <10% for seven of the metals, between 10–25% for four, and 37% for the remaining metal (manganese). Where concentrations were below the LOD, we replaced the undetectable values with the LOD divided by the square root of 2 (Needham et al. 2005) for all metals except cadmium. Due to the high percentage of girls in the sample with urinary cadmium concentrations below the limit of detection (54%), we assessed urinary cadmium concentrations as a continuous outcome among the 183 girls with detectable cadmium levels and alternatively as a dichotomous variable (below LOD, above LOD) and a categorical variable (below LOD, lower than the median, higher than the median) in the full sample. Results were the same regardless of how cadmium was assessed, so we present findings for the continuous measure of urinary cadmium concentrations to be consistent with how other metals were analyzed.
3.6. Statistical Analysis
Following Messer and colleagues (2006), a principal components analysis was conducted on the eight items included in the neighborhood deprivation index to confirm a single factor structure and obtain weights to calculate factor scores. All weights were positive, with the exception of the weight for percentage of males in professional occupations. Neighborhood deprivation scores ranged from −3.28 to 6.90. Using quartile cutpoints, we created a categorical neighborhood deprivation variable for analysis. Because the original deprivation score variable was not normally distributed, we derived a continuous variable from the median value within each quartile for tests of linear trend.
We used univariate and bivariate methods to examine the distribution of, and associations with, analytic variables. Because all urinary metal concentrations were non-normally distributed, we applied natural log transformations for multivariable analyses and then transformed back to the original scale for interpretation. Because of the high precision of the laboratory procedures, we eliminated only four very extreme outliers in analyses of the respective metals: the highest values for cadmium and manganese and the lowest values for cesium and cobalt. Several invidiual-level potential confounding factors were evaluated for inclusion in multivariable analyses, including number of people in the household, primary language (English vs. non-English), number of smokers in the home, and creatinine-adjusted cotinine. Most were not associated with urinary metal concentrations or were highly correlated with family income.
We used analysis of variance to obtain unadjusted estimates of the associations between urinary metal concentrations and quartiles of neighborhood deprivation as well as categorical indicators for demographic covariates, and used the Tukey procedure to compare geometric means of urinary metal concentrations across categories of these variables. For urinary metal concentrations that differed across quartiles of neighborhood deprivation or race/ethnicity (barium, lead, antimony, tin), we ran multivariate regression models that included neighborhood deprivation, race/ethnicity, and family income, a factor that has been associated with increased and decreased exposures to metals and metallic compounds (Tyrrell et al. 2013). White girls with annual household incomes greater than $100,000 and living in the least deprived neighborhoods (Q1) served as the reference group in these models. We also ran sensitivity analyses that further controlled for natural log transformed creatinine-adjusted cotinine to determine whether observed associations between neighborhood deprivation and urinary metal concentrations could be explained by exposure to secondhand smoke. Significance of main effects was evaluated with full versus restricted F-tests that included and excluded indicator variables for both quartiles of neighborhood deprivation and race/ethnicity. To determine whether the effect of neighborhood deprivation on urinary metal concentrations varied across racial/ethnic groups, we stratified models by race/ethnicity. We then assessed the statistical significance of interactions using full versus restricted F-tests, comparing models inclusive of interaction terms to models without these terms.
For both main and interaction effects, we calculated the percent change in geometric mean (GM) from the referent group. The two-sided alpha criterion for statistical significance was set at 0.10 for interaction effects and 0.05 for main effects and linear trends. Alpha was increased to 0.10 to attain more statistical power to detect the interactions of interest (Selvin 2004).We accounted for clustering of girls within neighborhoods in all regressions by using robust variance estimation procedures. All analyses were conducted using Stata® v. 13 (College Station, TX).
4. Results
The mean age of participants at baseline was 7.34 years (SD=0.42). Nearly one quarter of the girls were Hispanic (23.7%), 20.7% were Black, 12.2% were Asian, and 43.4% were White (Table 1). Compared to the general population in the Bay Area, our sample of girls ages 6 to 8 had greater representation of Hispanics and Blacks and fewer Asians (U.S. Census Bureau 2015). Approximately two-thirds of the CYGNET girls had family incomes greater than $75,000, which was above the 2005–2007 median household income for the Bay Area ($72,059) (U.S. Census Bureau 2015). The distribution of girls of various racial/ethnic backgrounds and family income levels was not uniform across quartiles of neighborhood deprivation (Table 1). Furthermore, patterns of neighborhood deprivation varied greatly by county; girls living in Alameda county were the most likely to live in the most deprived neighborhoods and girls living in Marin county were the least likely. Girls living in the most deprived neighborhoods (Q4) had significantly higher urinary cotinine levels than girls living in less deprived neighborhoods.
Table 1.
Sample Characteristics, by Neighborhood Deprivation Quartilea
| Total | Q1 | Q2 | Q3 | Q4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [-3.28, -1.96] | [-1.95, -1.08] | [-1.07, 0.35] | [0.36, 6.9] | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (in years) | 7.34 | 0.42 | 7.28 | 0.43 | 7.29 | 0.41 | 7.40 | 0.40 | 7.37 | 0.43 |
| Cotinine (μg/g)b | 0.34 | 3.49 | 0.23 | 2.77 | 0.33 | 3.43 | 0.32 | 3.21 | 0.56 | 4.05 |
| N | % | n | % | n | % | n | % | n | % | |
| Race/Ethnicity | ||||||||||
| Black | 83 | 20.70 | 7 | 8.43 | 13 | 15.66 | 27 | 32.53 | 36 | 43.37 |
| Hispanic | 95 | 23.69 | 12 | 12.63 | 14 | 14.74 | 21 | 22.11 | 48 | 50.53 |
| Asian | 49 | 12.22 | 16 | 32.65 | 14 | 28.57 | 12 | 24.49 | 7 | 14.29 |
| White | 174 | 43.39 | 65 | 37.36 | 58 | 33.33 | 41 | 23.56 | 10 | 5.75 |
| Family Income | ||||||||||
| <$50,000 | 82 | 20.45 | 4 | 4.88 | 12 | 14.63 | 22 | 26.83 | 44 | 53.66 |
| $50,000-$100,000 | 148 | 36.91 | 26 | 17.57 | 39 | 26.35 | 40 | 27.03 | 43 | 29.05 |
| >$100,000 | 171 | 42.64 | 70 | 40.94 | 48 | 28.07 | 39 | 22.81 | 14 | 8.19 |
| County | ||||||||||
| San Francisco | 84 | 20.95 | 15 | 17.86 | 22 | 26.19 | 33 | 39.29 | 14 | 16.67 |
| Marin | 94 | 23.44 | 40 | 42.55 | 38 | 40.43 | 12 | 12.77 | 4 | 4.26 |
| Alameda | 218 | 54.36 | 42 | 19.27 | 39 | 17.89 | 55 | 25.23 | 82 | 37.61 |
| Unknown | 5 | 1.25 | --- | --- | --- | --- | --- | --- | --- | --- |
| Total | 401 | 100.00 | 100 | 24.94 | 99 | 24.69 | 101 | 25.19 | 101 | 25.19 |
Q1=lowest deprivation, Q4=highest deprivation
Geometric mean and SD
Compared with national estimates from the 2005–2006 NHANES for 6 to 11 year-old children in the U.S., the geometric means for barium, cadmium, cesium, tungsten, and uranium for girls in our CYGNET sample were higher (Table 2). Geometric means for lead, cobalt, molybdenum, thallium, and antimony were lower for the CYGNET girls than in NHANES (CDC 2013a). National averages for urinary concentrations of manganese, tin, or strontium were not reported in 2005–2006; however, geometric means for girls in the CYGNET sample were higher for manganese, and lower for tin and strontium, than the geometric means for 6 to 11 year-old children in the U.S. in 2011–2012 (CDC 2015). The largest differences between the two samples were observed for manganese, tungsten, and uranium. Correlations between all 13 metals assessed in the CYGNET sample were varied (Supplemental Table 1).
Table 2.
Geometric Means (GM) and Selected Percentiles of Urinary Metal Concentrations (μg metal/g creatinine)
| NHANES | CYGNET Sample |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metal | GM (95% CI) | GM (95% CI) | Selected percentiles |
Min | Max | LODc | % belowLODc,d | |||
| 50th | 75th | 90th | 95th | |||||||
| Antimonya | 0.09 (0.08, 0.10) | 0.08 (0.07, 0.08) | 0.08 | 0.12 | 0.18 | 0.24 | 0.03 | 1.26 | 0.041 | 17.71 |
| Bariuma | 2.14 (1.85, 2.48) | 2.21 (2.04, 2.41) | 2.32 | 4.03 | 6.75 | 8.68 | 0.16 | 20.80 | 0.100 | 0.00 |
| Cadmiuma,d | 0.08 (0.07, 0.09) | 0.10 (0.09, 0.11) | 0.09 | 0.13 | 0.19 | 0.26 | 0.06 | 0.69 | 0.056 | 54.36 |
| Cesiuma | 5.85 (5.41, 6.34) | 6.28 (5.93, 6.66) | 7.17 | 9.43 | 11.80 | 13.10 | 0.56 | 29.00 | 0.048 | 0.00 |
| Cobalta | 0.52 (0.49, 0.55) | 0.49 (0.46, 0.52) | 0.52 | 0.75 | 1.01 | 1.29 | 0.05 | 3.23 | 0.048 | 0.25 |
| Leada | 0.63 (0.56, 0.70) | 0.62 (0.57, 0.67) | 0.68 | 1.08 | 1.57 | 1.90 | 0.06 | 4.05 | 0.080 | 1.75 |
| Manganeseb | 0.20 (0.18, 0.23) | 0.37 (0.34, 0.40) | 0.33 | 0.57 | 1.20 | 2.08 | 0.06 | 8.53 | 0.080 | 4.74 |
| Molybdenuma | 81.00 (71.90, 91.30) | 74.93 (69.15, 81.2) | 85.20 | 128.00 | 184.00 | 234.00 | 3.45 | 571.00 | 0.990 | 0.00 |
| Strontiumb | 118.00 (104.00, 133.00) | 100.61 (92.87, 109) | 113.35 | 186.07 | 255.29 | 287.66 | 3.92 | 456.57 | 2.500 | 0.00 |
| Thalliuma | 0.22 (0.20, 0.23) | 0.20 (0.19, 0.21) | 0.22 | 0.32 | 0.41 | 0.46 | 0.01 | 0.69 | 0.020 | 0.25 |
| Tinb | 1.67 (1.46, 1.90) | 1.36 (1.23, 1.50) | 1.21 | 2.47 | 5.32 | 7.72 | 0.16 | 119.93 | 0.220 | 0.75 |
| Tungstena | 0.18 (0.15, 0.20) | 0.35 (0.32, 0.38) | 0.42 | 0.65 | 0.98 | 1.16 | 0.02 | 4.17 | 0.026 | 1.50 |
| Uraniuma | 0.01 (0.01–0.01) | 0.02 (0.02, 0.02) | 0.02 | 0.04 | 0.06 | 0.08 | 0.00 | 0.35 | 0.003 | 3.74 |
2005–2006 NHANES estimates for 6–11 year old boys and girls (CDC 2013a)
2011–2012 NHANES estimates for 6–11 year old boys and girls (CDC 2015)
LOD=Limit of detection
Undetectable values replaced with LOD/sqrt(2) for all metals except cadmium. For cadmium, we only examined the subset with values>LOD (n=183).
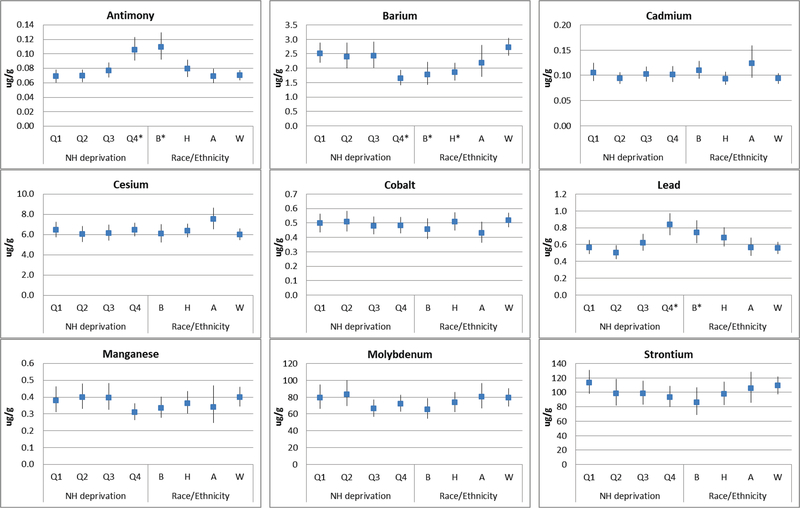
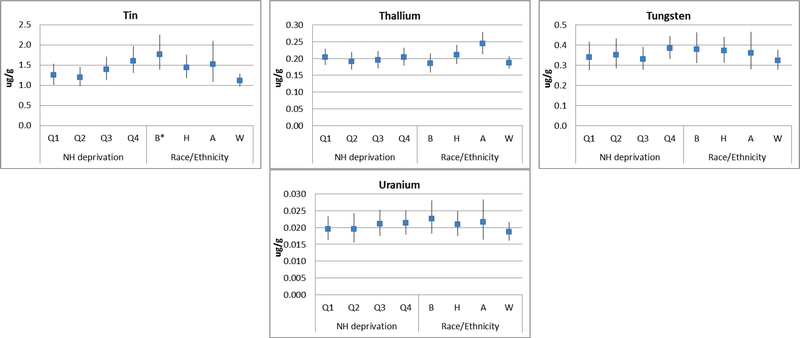
Significant differences in urinary metal concentrations were observed across neighborhood deprivation quartile and race/ethnicity for only four metals: in the hypothesized directions for lead, antimony, and tin, but in the opposite direction for barium (Figure 1, Supplemental Table 2). Girls living in neighborhoods with the highest levels of neighborhood deprivation (Q4) had the highest concentrations of lead and antimony and lowest concentrations of barium compared to girls living in neighborhoods with lower levels of deprivation. Urinary concentrations of antimony, lead, and tin were significantly higher among Black girls compared to White girls. Black and Hispanic girls had significantly lower urinary barium concentrations as compared to their White counterparts.
Figure 1.
Distributions of Creatinine-Corrected Urinary Metal Concentrations across Neighborhood Deprivation Quartilesa and Race/Ethnicityb
aQ1=lowest deprivation, Q4=highest deprivation
bB=Black, H=Hispanic, A=Asian, W=White
*Significantly different (p<0.05) than the category referent (NH deprivation=Q1, Race/Ethnicity=W)
In multivariable analyses, we observed statistically-significant main effects of neighborhood deprivation (F3,224=3,82, p=0.01) and race/ethnicity (F3,224=3.89, p=0.01) on urinary concentration for only one metal: antimony (Table 3). Black girls and those living in the most deprived neighborhoods (Q4) had significantly-higher urinary antimony concentrations than White girls (p=0.01) and those living in the least deprived neighborhoods (Q1) (p=0.01), respectively, when controlling for family income, with a significant linear trend across quartiles of deprivation (ptrend<0.01). No statistically-significant main effects of race/ethnicity or neighborhood deprivation were found for urinary lead concentration in comparison to referent groups, but we observed a positive linear trend across quartiles of neighborhood deprivation (ptrend=0.05) (Table 3). Although the main effect of race/ethnicity on barium concentration was only marginally significant (F3, 224=2.22, p=0.09), barium concentrations were lower among Black and Hispanic girls as compared to White girls (p=0.05 for both groups) (Table 3). Similarly, despite significant differences in urinary tin concentrations between Black and White girls (p=0.04), an overall association between race/ethnicity and urinary tin concentrations was not observed (F3, 224=2.17, p=0.09). Nor was neighborhood deprivation associated with urinary tin concentrations (Table 3). In sensitivity analyses that additionally controlled for creatinine-adjusted cotinine, the differences in tin and barium concentrations between Black and White girls remained suggestive although they ceased to be statistically significant (p=0.06 and p=0.07, respectively) as did the positive linear trend for lead (ptrend=0.07).
Table 3.
Linear Regression Models Estimating Main Effectsa of Neighborhood Deprivation Quartile and Race/Ethnicity on Urinary Antimony, Barium, Lead, and Tin Concentrations (μg metal/g creatinine), Adjusted for Family Income
| Adj. GMb (95% CI) | % change in GM | β | F | p-value | Adj. GMb(95% CI) | % change in GM | β | F | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Antimony |
Barium |
|||||||||
| Quartile | 3.82 | 0.01 | 1.42 | 0.24 | ||||||
| Q1 | 0.07 (0.06, 0.08) | --- | Ref. | --- | 2.80 (2.40, 3.26) | --- | Ref. | --- | ||
| Q2 | 0.07 (0.06, 0.07) | −3.55 | −0.04 | 0.67 | 2.79 (2.23, 3.47) | −0.31 | 0.00 | 0.98 | ||
| Q3 | 0.07 (0.06, 0.08) | 1.51 | 0.01 | 0.87 | 3.02 (2.37, 3.85) | 8.15 | 0.08 | 0.54 | ||
| Q4 | 0.09 (0.07, 0.11) | 32.43 | 0.28 | 0.01 | 2.33 (1.80, 3.00) | −16.81 | −0.18 | 0.15 | ||
| Trend-test | <0.01 | 0.10 | ||||||||
| Race/Ethnicity | 3.89 | 0.01 | 2.22 | 0.09 | ||||||
| White | 0.07 (0.06, 0.08) | --- | Ref. | --- | 2.80 (2.40, 3.26) | --- | Ref. | --- | ||
| Black | 0.09 (0.07, 0.11) | 28.32 | 0.25 | 0.01 | 2.06 (1.50, 2.82) | −26.44 | −0.31 | 0.05 | ||
| Hispanic | 0.06 (0.05, 0.08) | −10.39 | −0.11 | 0.24 | 2.22 (1.73, 2.85) | −20.55 | −0.23 | 0.05 | ||
| Asian | 0.06 (0.05, 0.08) | −4.58 | −0.05 | 0.60 | 2.27 (1.74, 2.98) | −18.65 | −0.21 | 0.12 | ||
| Lead |
Tin |
|||||||||
| Quartile | 2.00 | 0.11 | 0.18 | 0.91 | ||||||
| Q1 | 0.54 (0.45, 0.65) | --- | Ref. | --- | 1.13 (0.94, 1.37) | --- | Ref. | --- | ||
| Q2 | 0.46 (0.35, 0.60) | −14.94 | −0.16 | 0.25 | 1.03 (0.81, 1.31) | −9.22 | −0.10 | 0.48 | ||
| Q3 | 0.54 (0.43, 0.70) | 0.72 | 0.01 | 0.96 | 1.10 (0.75, 1.60) | −3.04 | −0.03 | 0.86 | ||
| Q4 | 0.69 (0.48, 0.98) | 26.88 | 0.24 | 0.16 | 1.12 (0.79, 1.60) | −1.03 | −0.01 | 0.95 | ||
| Trend-test | 0.05 | 0.87 | ||||||||
| Race/Ethnicity | 0.39 | 0.76 | 2.17 | 0.09 | ||||||
| White | 0.54 (0.45, 0.65) | --- | Ref. | --- | 1.13 (0.94, 1.37) | --- | Ref. | --- | ||
| Black | 0.58 (0.43, 0.78) | 7.71 | 0.07 | 0.58 | 1.59 (1.16, 2.18) | 40.17 | 0.34 | 0.04 | ||
| Hispanic | 0.51 (0.39, 0.68) | −5.16 | −0.05 | 0.68 | 1.26 (0.93, 1.69) | 10.88 | 0.10 | 0.51 | ||
| Asian | 0.53 (0.41, 0.69) | −1.22 | −0.01 | 0.92 | 1.53 (1.08, 2.17) | 34.98 | 0.30 | 0.08 | ||
Main effects were assessed in three different ways: a global F-test for overall differences across neighborhood deprivation or race/ethnicity; t-tests comparing each quartile and race/ethnicity to the referent category (Q1 and White); and a test of linear trend across neighborhood deprivation quartiles.
Adjusted for family income
Formal tests of interaction indicated differences across race/ethnicity groups for associations between neighborhood deprivation and urinary barium concentrations (F9,224=3.04, pinteraction<0.01) and lead concentrations (F9,224=1.82, pinteraction=0.07) (Table 4). Stratified analyses indicated that barium decreased across quartiles of neighborhood deprivation among Hispanic girls (ptrend<0.001). In contrast, a significant linear trend indicated that lead levels increased across quartiles of neighborhood deprivation among Asian girls (ptrend=0.03). Differences in lead (F3,68=5.38, p<0.01) and barium (F3,68=4.97, p<0.01) concentrations across neighborhood deprivation were observed among Black girls, although not in a linear pattern (Table 4). A formal test of interaction did not support significant differences in antimony concentrations by neighborhood deprivation among different racial/ethnic groups (F9,224=1.32, pinteraction=0.23), but these analyses suggested that the significant linear association between neighborhood deprivation quartile medians and urinary antimony concentration (Table 3) was driven mostly by White girls (Table 4; ptrend=0.04). While differences in urinary tin concentration across neighborhood deprivation quartiles were observed among Black girls (F3,68=3.58, p=0.02), there was not a statistically significant trend (ptrend=0.32) or interaction with race/ethnicity (F9,224=1.55, pinteraction=0.13) (Table 4). These results held in sensitivity analyses that additionally controlled for creatinine-adjusted cotinine.
Table 4.
Linear Regression of Creatinine-Corrected Urinary Antimony, Barium, Lead and Tin Concentrations (μg metal/g creatinine) on Neighborhood Deprivation, Stratified by Race/Ethnicity and Adjusted for Family Income
| Antimony |
Barium |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adj. GMa (95% CI) | % change in GM | β | F | p-value | Adj. GMa (95% CI) | % change in GM | β | F | p-value | |
| Interaction | 1.32 | 0.23 | 3.04 | <0.01 | ||||||
| Black | 2.86 | 0.04 | 4.97 | <0.01 | ||||||
| Q1 | 0.11 (0.08, 0.16) | --- | Ref. | --- | 3.68 (2.61, 5.20) | --- | Ref. | --- | ||
| Q2 | 0.07 (0.05, 0.12) | −35.04 | −0.43 | 0.08 | 1.25 (0.54, 2.93)† | −65.99 | −1.08 | 0.02 | ||
| Q3 | 0.07 (0.04, 0.11) | −39.72 | −0.51 | 0.03 | 1.43 (0.79, 2.59) | −61.17 | −0.95 | <0.01 | ||
| Q4 | 0.11 (0.07, 0.16) | −5.75 | −0.06 | 0.77 | 1.76 (1.06, 2.91) | −52.29 | −0.74 | <0.01 | ||
| Trend | 0.13 | 0.97 | ||||||||
| Hispanic | 2.60 | 0.06 | 8.45 | <0.001 | ||||||
| Q1 | 0.07 (0.04, 0.12) | --- | Ref. | --- | 3.22 (2.21, 4.71) | --- | Ref. | --- | ||
| Q2 | 0.05 (0.03, 0.07) | −27.92 | −0.33 | 0.21 | 2.31 (1.27, 4.22)† | −28.22 | −0.33 | 0.28 | ||
| Q3 | 0.07 (0.05, 0.11) | 2.40 | 0.02 | 0.93 | 2.02 (1.17, 3.47) | −37.43 | −0.47 | 0.08 | ||
| Q4 | 0.08 (0.05, 0.12) | 15.32 | 0.14 | 0.55 | 1.23 (0.78, 1.94) | −61.82 | −0.96 | <0.001 | ||
| Trend | 0.08 | <0.001 | ||||||||
| Asian | 0.23 | 0.87 | 2.05 | 0.12 | ||||||
| Q1 | 0.07 (0.05, 0.09) | --- | Ref. | --- | 1.81 (1.13, 2.90) | --- | Ref. | --- | ||
| Q2 | 0.06 (0.04, 0.08) | −12.76 | −0.14 | 0.50 | 2.37 (1.20, 4.70)† | 31.11 | 0.27 | 0.47 | ||
| Q3 | 0.07 (0.05, 0.09) | −0.32 | 0.00 | 0.99 | 3.53 (2.40, 5.21) | 95.41 | 0.67 | 0.02 | ||
| Q4 | 0.07 (0.05, 0.09) | −1.79 | −0.02 | 0.94 | 2.07 (0.71, 6.04)† | 14.49 | 0.14 | 0.80 | ||
| Trend | 0.97 | 0.44 | ||||||||
| White | 1.67 | 0.18 | 0.88 | 0.46 | ||||||
| Q1 | 0.06 (0.05, 0.08) | --- | Ref. | --- | 2.59 (2.15, 3.10) | --- | Ref. | --- | ||
| Q2 | 0.07 (0.06, 0.08) | 10.86 | 0.10 | 0.34 | 2.96 (2.32, 3.77) | 14.38 | 0.13 | 0.33 | ||
| Q3 | 0.07 (0.06, 0.09) | 11.37 | 0.11 | 0.42 | 3.22 (2.45, 4.23) | 24.45 | 0.22 | 0.18 | ||
| Q4 | 0.10 (0.07, 0.16) | 62.35 | 0.48 | 0.03 | 2.58 (1.88, 3.54) | −0.38 | 0.00 | 0.98 | ||
| Trend | 0.04 | 0.40 | ||||||||
| Antimony |
Tin |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adj. GMa (95% CI) | % change in GM | β | F | p-value | Adj. GMa (95% CI) | % change in GM | β | F | p-value | |
| Interaction | 1.82 | 0.07 | 0.13 | |||||||
| Black | 5.38 | <0.01 | 0.13 | 0.02 | ||||||
| Q1 | 0.94 (0.70, 1.28) | --- | Ref. | --- | 2.52 (1.49, 4.26) | --- | Ref. | --- | ||
| Q2 | 0.38 (0.23, 0.62) | −59.82 | −0.91 | <0.01 | 0.92 (0.45, 1.86)† | −63.68 | −1.01 | 0.02 | ||
| Q3 | 0.50 (0.31, 0.81) | −46.94 | −0.63 | 0.01 | 1.03 (0.66, 1.59) | −59.22 | −0.90 | 0.01 | ||
| Q4 | 0.69 (0.44, 1.09) | −26.86 | −0.31 | 0.15 | 1.68 (1.03, 2.72) | −33.50 | −0.41 | 0.20 | ||
| Trend | 0.25 | 0.32 | ||||||||
| Hispanic | 0.78 | 0.51 | 0.70 | 0.56 | ||||||
| Q1 | 0.45 (0.26, 0.75) | --- | Ref. | --- | 1.10 (0.68, 1.79) | --- | Ref. | --- | ||
| Q2 | 0.40 (0.21, 0.76)† | −9.11 | −0.10 | 0.79 | 1.42 (0.62, 3.28)† | 28.77 | 0.25 | 0.56 | ||
| Q3 | 0.34 (0.19, 0.62)† | −23.70 | −0.27 | 0.45 | 1.32 (0.67, 2.59)† | 19.43 | 0.18 | 0.61 | ||
| Q4 | 0.49 (0.30, 0.80) | 10.14 | 0.10 | 0.73 | 0.98 (0.46, 2.07)† | −11.47 | −0.12 | 0.74 | ||
| Trend | 0.17 | 0.26 | ||||||||
| Asian | 2.03 | 0.12 | 0.32 | 0.81 | ||||||
| Q1 | 0.48 (0.31, 0.75) | --- | Ref. | --- | 1.62 (0.81, 3.22)† | --- | Ref. | --- | ||
| Q2 | 0.51 (0.32, 0.82) | 6.67 | 0.06 | 0.82 | 1.14 (0.63, 2.09) | −29.26 | −0.35 | 0.44 | ||
| Q3 | 0.69 (0.53, 0.88) | 42.98 | 0.36 | 0.13 | 1.63 (0.72, 3.69)† | 0.53 | 0.01 | 0.99 | ||
| Q4 | 0.98 (0.54, 1.79) | 104.11 | 0.71 | 0.05 | 1.36 (0.83, 2.22) | −15.86 | −0.17 | 0.68 | ||
| Trend | 0.03 | 0.81 | ||||||||
| White | 0.38 | 0.77 | 0.09 | 0.97 | ||||||
| Q1 | 0.54 (0.45, 0.65) | --- | Ref. | --- | 1.09 (0.87, 1.36) | --- | Ref. | --- | ||
| Q2 | 0.47 (0.35, 0.64) | −12.44 | −0.13 | 0.43 | 1.13 (0.87, 1.48) | 3.72 | 0.04 | 0.82 | ||
| Q3 | 0.58 (0.41, 0.82) | 7.03 | 0.07 | 0.71 | 1.18 (0.75, 1.84) | 7.72 | 0.07 | 0.75 | ||
| Q4 | 0.58 (0.20, 1.72)† | 7.80 | 0.08 | 0.89 | 1.30 (0.61, 2.76)† | 19.17 | 0.18 | 0.65 | ||
| Trend | 0.77 | 0.61 | ||||||||
Estimate may be unreliable (relative SE>30%)
Adjusted for family income
5. Discussion
In these analyses, we sought to identify upstream predictors of metals exposure among girls in Northern California based on objective exposure markers and indicators of individual (race/ethnicity) and community-level (neighborhood deprivation) stressors. Our hypothesis that urinary metals concentrations would be highest among girls living in areas with the highest levels of neighborhood deprivation, and that these associations would be most pronounced among Black and Hispanic girls, was only partially supported. We found associations with several metals: increased neighborhood deprivation scores associated with higher concentrations of lead and antimony, but lower concentrations of barium. We observed differences in urinary metal concentrations among race/ethnicity groups, with higher levels of lead, antimony and tin in Blacks and Hispanics compared to Whites, and the opposite direction for barium.
The stress-exposure-disease framework that guided the current study explicitly links social conditions with environmental health and posits that variation in community- and individual-level stressors may account for differences in susceptibility to health risks (Gee and Payne-Sturges 2004). Published findings for lead levels support this paradigm. A recent study found a positive relationship between blood lead and blood pressure among Black adults, but not White adults (Hicken et al. 2012). In our study, which focused on potentially damaging metal exposures rather than a specific health outcome, we found increasing levels of lead with increasing neighborhood deprivation only among Asian girls. While this increase in vulnerability was hypothesized, the fact that the association was limited to Asian girls was unexpected. One possible explanation, differential exposure to secondhand smoke, is unlikely as this association persisted when controlling for cotinine in a sensitivity analysis. Asian girls and their families may encounter additional stressors that would promote vulnerability to lead. For example, approximately 60% of the Asian population in the Bay Area of California during the time period that this study was conducted was foreign-born (U.S. Census Bureau, 2015). Immigration-related factors such as limited English language abilities may prevent some families from knowing and minimizing their lead exposure risk. However, we might expect the same linguistic stressor to affect Hispanic girls, and no such relationship between neighborhood deprivation and urinary lead concentration was observed in this group. In addition, in this study population, language was more of an issue among the Hispanic than the Asian participants. Additional research is needed to understand why Asian girls living in the most deprived neighborhoods are more vulnerable to lead exposure than their White, Black, and Hispanic counterparts.
Urinary lead concentrations were highest for Black girls living in the most deprived neighborhoods but did not differ significantly from Black girls living in the least deprived neighborhoods. One potential explanation for this finding could be heightened exposure to lead as a result of housing renovations in the most advantaged areas. Alternatively, the patterns observed for urinary lead concentrations among Black girls may suggest that individual-level stressors offset the benefits of living in less deprived neighborhoods, as has also been observed in behavioral studies of alcohol and drug consumption (Molina et al. 2012; Tanner-Smith 2012). These findings highlight the need to consider both individual and community stressors in environmental health disparities research (DeFur et al. 2007; Gee and Payne-Sturges 2004; Morello-Frosch et al. 2011).
In addition to lead, we observed social patterning of barium, antimony, and tin exposures. Contrary to our hypotheses, but consistent with recent NHANES findings (Belova et al. 2013), Hispanic and Black girls living in the least deprived neighborhoods had the highest urinary barium concentrations, although a monotonic trend was observed only among Hispanics. This pattern, not observed for Asian or White girls, suggests a possible unique exposure to barium for Hispanic and Black girls living in more privileged neighborhoods. Dietary preferences may offer one explanation for this unexpected pattern. The ATSDR (2007a) identifies Brazil nuts, seaweed, and fish as being high in barium. Such foods may be preferentially accessible to or consumed by Hispanic and Black girls living in neighborhoods with less deprivation. Deficits in iron intake may also explain these results, as studies with animals (Reeves and Chaney 2008) and humans (Nawrot et al. 2010) have documented increased absorption of cadmium when levels of calcium, iron, or zinc intake are low. The determinants, mechanisms, and toxicity of barium are understudied (Kravchenko et al. 2014), but it is possible that deficiencies in micronutrients could result in higher urinary concentrations of barium as well. Other studies have similarly documented higher mercury concentrations in more advantaged populations due to differential dietary patterns (Belova et al. 2013; Tyrrell et al. 2013).
In both unadjusted and adjusted models, Black girls and girls living in neighborhoods with the highest levels of deprivation had the highest urinary concentrations of antimony. These findings align with those of Belova and colleagues (2013), who documented disparities in biomarker levels of antimony among non-Hispanic Blacks. Although no differences across neighborhood deprivation were observed, Black girls also had the highest urinary concentrations of tin. The elevated urinary concentrations of antimony and tin among Black girls were reduced to marginal statistical significance, but were still suggestive when cotinine was included in the models. This may be because antimony and tin are not the metals of greatest concern in cigarette smoke (Bernhard et al. 2005; Chiba and Masironi 1992). Antimony is a soft metal that is used not only in household paint, but also mixed with other, harder metals to form alloys used in industrial products such as storage batteries, solder, sheet and pipe metal, and ammunition. Tin is another soft metal that is commonly found in household objects such as cans, toothpaste, soaps, and plastics. Exposures to antimony and tin occur through ingestion, inhalation, and dermal contact, although the primary source of exposure for both is ingestion (ATSDR 1992; 2005). Patterns for these two metals were the most consistent with hypotheses of higher burden of exposure among girls living in the most disadvantaged neighborhoods or experiencing the most individual-level stressors. Antimony trioxide has been labeled as a possible carcinogen by the IARC (2013), and there is some evidence from animal studies to suggest that triphenyltin hydroxide, an organotin compound, may cause pituitary or liver cancer (ATSDR 2005). Additional research should be directed toward enhancing our understanding of the vulnerabilities of young Black girls and girls living in neighborhoods with high levels of deprivation as they relate to these exposures.
Three metals recognized as potential EDCs (cadmium, lead and manganese), were explored in the current analysis (Iavicoli et al. 2009). Exposure to EDCs during childhood may influence the development of the reproductive organs by mimicking reproductive hormones or by functioning as agonists and antagonists for hormone receptors (Iavicoli et al. 2009). Disruptions or changes during this critical window of susceptibility can alter biological systems and physiological functions that have immediate and long-term effects on health (Biro and Deardorff 2013; Diamanti-Kandarakis and Gore 2012; Selevan et al. 2000). Evidence for the endocrine-disrupting potential of cadmium and lead has been documented in in vitro, in vivo, and epidemiological studies. Evidence that manganese functions as an EDC is weaker, but results from in vivo and in vitro animal studies suggest that it may influence the production and secretion of hormones (Iavicoli et al. 2009).
In addition to being a potential EDC (Iavicoli et al. 2009), manganese is an essential element (ATSDR 2012) that has been linked to neurodevelopmental and behavioral disorders in children (Rodriguez-Barranco et al. 2013). Girls in the 2005–2006 CYGNET sample had urinary concentrations of manganese that were nearly two times higher than those in U.S. children ages 6 to 11 in 2011–2012 (CDC 2015). The CVs were highest for manganese, so the high concentrations observed in the CYGNET sample may reflect technical challenges measuring this compound in the lab. Furthermore, we do not know if the difference in timing of specimen collection may account for some of the large difference in urinary manganese concentrations between the CYGNET and national samples if manganese exposures decreased overall between 2006 and 2011–12. Although low levels of manganese are present in ambient air, levels are higher near industrial sites (EPA 2007). Heightened exposure to manganese from the Chevron oil refinery in nearby Richmond, CA (City of Richmond March 2014), is one possible explanation for the high urinary concentrations observed in the CYGNET sample, but a recent study concluded that airborne concentrations of metals generated by petroleum refineries, including manganese, are quite low (Lewis et al. 2012) and a local air monitoring program did not detect manganese in the air in three neighborhoods surrounding the Chevron oil refinery in 2015 (Argos Scientific April 2015). This issue may warrant further investigation as earlier studies have documented high levels of air pollutants in the San Francisco Bay Area (Morello-Frosch et al. 2000). Urinary lead concentrations in the NHANES and CYGNET samples were nearly equivalent (CDC 2013a). Although not classified as potential EDCs, tungsten and uranium levels were also elevated in the CYGNET sample. We also examined several other metals that are largely understudied in pediatric populations, including barium, cadmium, cesium, cobalt, molybdenum, antimony, thallium, tin, and strontium. There is no evidence to support these metals as EDCs, but researchers have called for in vitro and in vivo studies to assess the endocrine-disrupting potential of a broader array of metals (Iavicoli et al. 2009).
Although the partial application of the stress-exposure-disease model to explore patterns of urinary metal concentrations by neighborhood sociodemographic characteristics and across racial and ethnic groups was a strength of the current study, we note several limitations. First, despite being racially and ethnically diverse, study participants were comprised of girls with Kaiser Permanente health insurance and medical care. The generalizability of these results to boys and more economically-diverse populations is unknown. We note that collinearity between neighborhood deprivation and family income variables was not a concern in our analyses because the two were only moderately correlated (ρ=−0.45) and thus likely captured different features of socioeconomic status.
Second, we had limited precision to evaluate relationships at the extremes of our data (e.g., for those girls with the lowest household incomes living in neighborhoods with the lowest levels of deprivation), as we had very few girls in these groups. The distribution of deprivation scores was one reason we used a variable parameterized from the median of each quartile to evaluate trend, rather than the continuous score variable. In the tables, we also indicate which estimates may be unreliable due to small cell sizes. Replacement of undetectable metal concentrations with the metal-specific limit of detection divided by the square root of 2 allowed us to use the full sample for 12 of the 13 metals of interest (cadmium excepted), but may have also impacted the precision and reliability of our estimates (particularly for antimony, as 18% of the girls in our sample had undetectable concentrations).
Third, urinary biomarkers of exposure may not yield the same results as blood biomarkers of exposure. This limitation may be most relevant for lead and cadmium, bioaccumulative inorganic chemicals that are best assessed in blood. However, most of the other metals explored in this study (with the exception of tin, strontium, and uranium) have been classified as non-bioaccumulative inorganic chemicals that are non-persistent and thus readily assessed in the urine (Needham et al. 2005). Urinary concentrations also may not reflect accurately the long-term exposure to these compounds, but our approach is consistent with that used by the NCEH to monitor metal exposures for national surveillance purposes (CDC, 2012).
The aggregate measure of neighborhood deprivation is an indirect measure of one’s immediate geographic residence and may not capture important domains of the social environment (Bernard et al. 2007) that may contribute to exposure vulnerability (Gee and Payne-Sturges 2004). While useful for our evaluation of differential exposures to chemicals by geographic residence, the neighborhood deprivation index itself does not suggest clear intervention points, and causality cannot be assumed.
Lastly, we did not assess routes of exposure to metals and metallic compounds. The four metals (barium, lead, antimony, tin) for which we observed differences in urinary concentrations across quartiles of neighborhood deprivation or race/ethnicity share a common exposure source, household paint (Fjelsted and Christensen 2007), but concentrations are not uniform and household paint is not the only source of exposure to these metals and metallic compounds. For several of the metals examined (antimony, cesium, cobalt, manganese, molybdenum, thallium, uranium), ingestion through food sources is the primary route of exposure (ATSDR 1992a; 1992b; 2004; 2013; Biomonitoring California 2010; CDC 2013b). Studies employing methods capable of assessing multiple exposures to environmental, psychosocial, and biological stressors may provide further insight into our findings.
6. Conclusion
This study of young, healthy girls contributes to our knowledge of the distribution of exposure to metals and metallic compounds across racial and ethnic groups as well as levels of neighborhood deprivation. Increased neighborhood deprivation was associated with higher urinary concentration levels of lead, antimony and tin, and lower levels of barium, with findings more striking for some race/ethnicity groups than others. Children are especially vulnerable to environmental exposure to metals and our results indicate that community- and individual-level stressors may influence vulnerability to some metals. Additional research should further explore not only distributions of these exposures (vulnerability) but also associated health effects (susceptibility) in various racial and ethnic groups.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge Samuel Blanchard of the College of Natural Resources in the Department of Environmental Sciences, Policy, and Management at the University of California Berkeley for providing the 2005–2009 American Community Survey neighborhood deprivation scores and Dr. Barbara Laraia for her input regarding the calculation of the deprivation score. We are grateful to Dr. Kathleen Caldwell of the Division of Laboratory Sciences at the National Center for Environmental Health (CDC) for her expert consultation regarding the details of the measurement of urinary metal concentrations. We would also like to thank Dr. Ivo Iavocoli and Dr. Ellen Silbergeld for their feedback on the findings for barium.
This publication was made possible by the Breast Cancer and the Environment Research Program (BCERP) award numbers U01ES012801, U01ES019435 and U01ES019457 from the National Institute of Environmental Health Sciences (NIEHS) and the National Cancer Institute (NCI), and UL1RR024131 from the National Center for Research Resources (NCRR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NCI, NCCR or National Institutes of Health. Support was also provided by the California Department of Public Health and the Avon Foundation.
References
- Agarwal S; Zaman T; Tuzcu EM; Kapadia SR Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Angiology 62:422–9; 2011. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances & Disease Registry (ATSDR), 1992a. Toxicological profile for antimony. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=332&tid=58
- ATSDR, 1992b. Toxicological profile for thallium. http://www.atsdr.cdc.gov/toxprofiles/tp54.pdf [PubMed]
- ATSDR, 2004. Toxicological profile for cesium. http://www.atsdr.cdc.gov/toxprofiles/tp157.pdf [PubMed]
- ATSDR, 2005. Toxicological profile for tin and tin compounds. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=543&tid=98 [PubMed]
- ATSDR, 2007a. Public health statement for barium. http://www.atsdr.cdc.gov/PHS/PHS.asp?id=325&tid=57
- ATSDR, 2007b. Toxicological profile for lead. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=96&tid=22
- ATSDR, 2008. Toxic substances portal: Metals/Elements. http://www.atsdr.cdc.gov/substances/toxchemicallisting.asp?sysid=33
- ATSDR, 2012. Toxicological profile for manganese. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=102&tid=23 [PubMed]
- ATSDR, 2013. Toxicological profile for uranium. http://www.atsdr.cdc.gov/toxprofiles/tp150.pdf [PubMed]
- Akkus C and Ozdenerol E Exploring childhood lead exposure through GIS: a review of the recent literature. Int J Environ Res Public Health 11:6314–34; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos Scientific, April 2015. Event sampling report: Chevron Richmond community air monitoring program. http://fenceline.org/richmond/report.php?download=event/RCAMP_Event_Report_EV_25%20(April%202015).pdf
- Belova A; Greco SL; Riederer AM; Olsho LE; Corrales MA A method to screen U.S. environmental biomonitoring data for race/ethnicity and income-related disparity. Environ Health 12:114,069X-12–114; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P; Charafeddine R; Frohlich KL; Daniel M; Kestins Y; Potvin L Health inequalities and place: A theoretical conception of neighbourhood. Soc Sci Med 65:1839–1852; 2007. [DOI] [PubMed] [Google Scholar]
- Bernhard D; Rossmann A; Wick G Metals in cigarette smoke. IUBMB Life 57:805–9; 2005. [DOI] [PubMed] [Google Scholar]
- Biomonitoring California, 2010. Manganese fact sheet. http://www.biomonitoring.ca.gov/downloads/manganese-fact-sheet
- Biro FM; Greenspan LC; Galvez MP; Pinney SM; Teitelbaum S; Windham GC et al. Onset of breast development in a longitudinal cohort. Pediatr 132:1019–1027; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro FM and Deardorff J Identifying opportunities for cancer prevention during preadolescence and adolescence: Puberty as a window of susceptibility. J Adolesc Health 52:S15–20; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borders AE; Wolfe K; Qadir S; Kim KY; Holl J; Grobman W Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J Perinatol 35:580–4; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J and Gochfeld M Conceptual environmental justice model for evaluating chemical pathways of exposure in low-income, minority, Native American, and other unique exposure populations. Am J Public Health 101:S64–S73; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DR Race/Ethnicity and self-reported levels of discrimination and psychological distress, California, 2005. Prev Chronic Dis 9:E156; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2012. Laboratory procedure manual: Multi-element in urine, NHANES 2011–2012. http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/UHM_G_met_heavy_metals.pdf
- CDC, 2015. Fourth report on human exposures to environmental chemicals: Updated tables, February 2015. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- CDC, 2013a. Fourth report on human exposures to environmental chemicals: Updated tables. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]
- CDC, 2013b. Biomonitoring summaries. http://www.cdc.gov/biomonitoring/biomonitoring_summaries.html
- Chiba M and Masironi R Toxic and trace elements in tobacco and tobacco smoke. Bull World Health Organ 70:269–75; 1992. [PMC free article] [PubMed] [Google Scholar]
- Chonan T; Taguchi O; Omae K Interstitial pulmonary disorders in indium-processing workers. Eur Respir J 29:317–24; 2007. [DOI] [PubMed] [Google Scholar]
- City of Richmond, March 2014. Chevron refinery modernization project: Environmental Impact Report (Volume 1: Draft EIR). http://chevronmodernization.com/wp-content/uploads/2014/03/Volume-1_DEIR.pdf
- DeFur PL; Evans GW; Cohen Hubal E.A.; Kyle AD; Morello-Frosch RA; Williams DR,. Vulnerability as a function of individual and group resources in cumulative risk assessment. Environ Health Perspect 115:817–24; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E and Gore AC 2012. Endocrine disruptors and puberty. New York, NY: Humana Press. [Google Scholar]
- Dowd JB; Palermo T; Chyu L; Adam E; McDade TW Race/ethnic and socioeconomic differences in stress and immune function in The National Longitudinal Study of Adolescent Health. Soc Sci Med 115:49–55; 2014. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA), 2013. America’s children and the environment (3rd ed.). EPA [Google Scholar]
- EPA, 2003. Framework for cumulative risk assessment. http://www2.epa.gov/sites/production/files/2014-11/documents/frmwrk_cum_risk_assmnt.pdf
- EPA, 2007. Toxicity and Exposure Assessment for Children’s Health (TEACH) chemical summary: Manganese. http://epa.gov/teach/chem_summ/manganese_summary.pdf
- Fjelsted L and Christensen T Household hazardous waste: Composition of paint waste. Waste Manag Res 25:502–9; 2007. [DOI] [PubMed] [Google Scholar]
- Gee GC and Payne-Sturges DC Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ Health Perspect 112:1645–1653; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochfeld M and Burger J Disproportionate exposures in environmental justice and other populations: The importance of outliers. Am J Public Health 101:S53–S63; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P and Landrigan PJ Neurobehavioural effects of developmental toxicity. Lancet Neurol 13:330–8; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchette CL The political ecology of lead poisoning in eastern North Carolina. Health Place 14:209–16; 2008. [DOI] [PubMed] [Google Scholar]
- Hiatt RA; Haslam SZ; Osuch J The Breast Cancer and the Environment Research Centers: Transdisciplinary research on the role of the environment in breast cancer etiology. Environ Health Perspect 117:1814–22; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicken MT; Gee GC; Morenoff J; Connell CM; Snow RC; Hu H A novel look at racial health disparities: The interaction between social disadvantage and environmental health. Am J Public Health 102:2344–2351; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood E Dwelling disparities: How poor housing leads to poor health. Environ Health Perspect 113:A310–7; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavicoli I; Fontana L; Bergamaschi A The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev 12:206–23; 2009. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic risks to humans: List of classifications. 2014; 2013. [PMC free article] [PubMed] [Google Scholar]
- Jarrett JM; Jones RL; Caldwell KL; Verdon CP Total urine arsenic measurements using inductively coupled plasma mass spectrometry with a dynamic reaction cell. Atomic Spectroscopy 28:113–122; 2007. [Google Scholar]
- Jarrett JM; Xiao G; Caldwell KL; Henahan D; Shakirova G; Jones RL Eliminating molybdenum oxide interference in urine cadmium biomonitoring using ICP-DRC-MS. J Anal At Spectrom 23:962–967,doi:10.1039/B801927D; 2008. [Google Scholar]
- Järup L Hazards of heavy metal contamination. Br Med Bull 68:167–182; 2003. [DOI] [PubMed] [Google Scholar]
- Jomova K; Jenisova Z; Feszterova M; Baros S; Liska J; Hudecova D et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107; 2011. [DOI] [PubMed] [Google Scholar]
- Juster RP; McEwen BS; Lupien SJ Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 35:2–16; 2010. [DOI] [PubMed] [Google Scholar]
- Karagas MR; Choi AL; Oken E; Horvat M; Schoeny R; Kamai E et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect 120:799–806; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S; Arora M; Fernandez C; Landero J; Caruso J; Chen A Lead, mercury, and cadmium exposure and attention deficit hyperactivity disorder in children. Environ Res 126:105–10; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko J; Darrah TH; Miller RK; Lyerly HK; Vengosh A A review of the health impacts of barium from natural and anthropogenic exposure. Environ Geochem Health 36:797–814; 2014. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ and Miodovnik A Children’s health and the environment: An overview. Mt Sinai J Med 78:1–10; 2011. [DOI] [PubMed] [Google Scholar]
- Lanphear BP; Hornung R; Khoury J; Yolton K; Baghurst P; Bellinger DC et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113:894–9; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R; Brown MJ; Kashtock ME; Jacobs DE; Whelan EA; Rodman J et al. Lead exposures in U.S. Children, 2008: implications for prevention. Environ Health Perspect 116:1285–93; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RC; Gaffney SH; Le MH; Unice KM; Paustenbach DJ Airborne concentrations of metals and total dust during solid catalyst loading and unloading operations at a petroleum refinery. Int J Hyg Environ Health 215:514–21; 2012. [DOI] [PubMed] [Google Scholar]
- Mauss EA Childhood lead poisoning prevention: The tortuous trail from human health impact assessment to effective environmental policy. Environ Impact Assess Rev 14:403–23; 1994. [Google Scholar]
- Merkin SS; Basurto-Davila R; Karlamangla A; Bird CE; Lurie N; Escarce J et al. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Ann Epidemiol 19:194–201; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer L; Laraia B; Kaufman J; Eyster J; Holzman C; Culhane J et al. The development of a standardized neighborhood deprivation index. J Urban Health 83:1041–62; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke HW; Powell ET; Shah A; Gonzales CR; Mielke PW Multiple metal contamination from house paints: consequences of power sanding and paint scraping in New Orleans. Environ Health Perspect 109:973–8; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina KM; Alegria M; Chen CN Neighborhood context and substance use disorders: A comparative analysis of racial and ethnic groups in the United States. Drug Alcohol Depend 125S:S35–43; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello-Frosch R; Zuk M; Jerrett M; Shamasunder B; Kyle AD Understanding the cumulative impacts of inequalities in environmental health: Implications for policy. Health Aff 30:879–87; 2011. [DOI] [PubMed] [Google Scholar]
- Morello-Frosch RA; Woodruff TJ; Axelrad DA; Caldwell JC Air toxics and health risks in California: the public health implications of outdoor concentrations. Risk Anal 20:273–91; 2000. [DOI] [PubMed] [Google Scholar]
- Nawrot TS; Staessen JA; Roels HA; Munter E; Cuypers A; Richart T et al. Cadmium exposure in the population: From health risks to strategies of prevention. Biometals 23; 2010. [DOI] [PubMed] [Google Scholar]
- Needham LL, Sexton K Assessing children’s exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J Expo Anal Environ Epidemiol 10; 2000. [DOI] [PubMed] [Google Scholar]
- Needham LL; Ozkaynak H; Whyatt RM; Barr DB; Wang RY; Naeher L et al. Exposure assessment in the National Children’s Study: introduction. Environ Health Perspect 113:1076–82; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman H Lead poisoning. Annu Rev Med 55:209–22; 2004. [DOI] [PubMed] [Google Scholar]
- Occupational Safety & Hazard Administration (OSHA). Toxic metals. 2013; 2009. [Google Scholar]
- Olympio KP; Goncalves C; Gunther WM; Bechara EJ Neurotoxicity and aggressiveness triggered by low-level lead in children: a review. Rev Panam Salud Publica 26:266–75; 2009. [DOI] [PubMed] [Google Scholar]
- Oyana TJ and Margai FM Geographic analysis of health risks of pediatric lead exposure: A golden opportunity to promote healthy neighborhoods. Arch Environ Occup Health 62:93–104; 2007. [DOI] [PubMed] [Google Scholar]
- Padilla MA; Elobeid M; Ruden DM; Allison DB An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99–02. Int J Environ Res Public Health 7:3332–47; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RF; Blanchard S; Wood R Proximity to point sources of environmental mercury release as a predictor of autism prevalence. Health Place 15:18–24; 2009. [DOI] [PubMed] [Google Scholar]
- Payne-Sturges D and Gee GC National environmental health measures for minority and low-income populations: Tracking social disparities in environmental health. Environ Res 102:154–71; 2006. [DOI] [PubMed] [Google Scholar]
- Peek MK; Cutchin MP; Salinas JJ; Sheffield KM; Eschbach K; Stowe RP et al. Allostatic load among non-Hispanic Whites, non-Hispanic Blacks, and people of Mexican origin: effects of ethnicity, nativity, and acculturation. Am J Public Health 100:940–6; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PG and Chaney RL Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci Total Environ 398:13–19; 2008. [DOI] [PubMed] [Google Scholar]
- Roberts AL; Lyall K; Hart JE; Laden F; Just AC; Bobb JF et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect 121:978–84; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barranco M; Lacasana M; Aguilar-Garduno C; Alguacil J; Gil F; Gonzalez-Alzaga B et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 454–455:562–77; 2013. [DOI] [PubMed] [Google Scholar]
- Roels HA; Bowler RM; Kim Y; Claus Henn B; Mergler D; Hoet P et al. Manganese exposure and cognitive deficits: A growing concern for manganese neurotoxicity. Neurotoxicology 33:872–880; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said S and Hernandez GT Environmental exposures, socioeconomics, disparities, and the kidneys. Adv Chronic Kidney Dis 22:39–45; 2015. [DOI] [PubMed] [Google Scholar]
- Schwartz J; Bellinger D; Glass T Expanding the scope of environmental risk assessment to better include differential vulnerability and susceptibility. Am J Public Health 101:S88–S93; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG; Kimmel CA; Mendola P Identifying critical windows of exposure for children’s health. Environ Health Perspect 108:451–5; 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin S 2004. Statistical analysis of epidemiologic data. 4th ed. New York, NY: Oxford University Press. [Google Scholar]
- Sexton K and Linder SH The role of cumulative risk assessment in decisions about environmental justice. Int J Environ Res Public Health 7:4037–49; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner-Smith EE Pubertal development and adolescent girls’ substance use: Race, ethnicity, and neighborhood contexts of vulnerability. J Early Adolesc 32:621–49; 2012. [Google Scholar]
- Tellez-Plaza M; Navas-Acien A; Menke A; Crainiceanu CM; Pastor-Barriuso R; Guallar E Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect 120:1017–22; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell J; Melzer D; Henley W; Galloway TS; Osborne NJ Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001–2010. Environ Int 59:328–35; 2013. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau, American FactFinder. 2005–2007 American Community Survey 3-Year Estimates, San Francisco-Oakland-Fremont, CA metro area. ACS demographic and housing estimates: 2005–2007 (Table DP05); Median income in the past 12 months (In 2007 inflation-adjusted dollars) (Table S1903); Selected population profile in the United States: Asian alone or in combination with one or more other races (Table S0201). http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml
- US Census Bureau, 2012. Geographic terms and concepts – census tract. http://www.census.gov/geo/reference/gtc/gtc_ct.html
- Windham GC; Zhang L; Gunier R; Croen LA; Grether JK Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ Health Perspect 114:1438–44; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CS; Li X; Thornton I Urban environmental geochemistry of trace metals. Environ Pollut 142:1–16; 2006. [DOI] [PubMed] [Google Scholar]
- Wright RJ Moving towards making social toxins mainstream in children’s environmental health. Curr Opin Pediatr 21:222–9; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.