Abstract
For patients with advanced cancers there has been a concerted effort to transition from a generic treatment paradigm to one based on tumor-specific biologic, and patient-specific clinical characteristics. This approach, known as precision therapy has been made possible owing to widespread availability and a reduction in the cost of cutting-edge technologies that are used to study the genomic, proteomic, and metabolic attributes of individual tumors. This review traces the evolution of precision therapy for lung cancer from the identification of molecular subsets of the disease to the development and approval of tyrosine kinase, as well as immune checkpoint inhibitors for lung cancer therapy. Challenges of the precision therapy era including the emergence of acquired resistance, identification of untargetable mutations, and the effect on clinical trial design are discussed. We conclude by highlighting newer applications for the concept of precision therapy.
Keywords: precision medicine, non–small cell lung cancer, ALCHEMIST, MATCH trial, exceptional responders initiative, immune checkpoint inhibitors
INTRODUCTION
With an estimated 224,000 cases diagnosed in 2014, lung cancer continues to account for a large share of the cancer burden in the United States. Additionally, the estimated 159,000 deaths related to this disease account for 27% of all cancer deaths, and the overall 5-year survival for patients with lung cancer remains distressingly low (17%).1 Non–small cell lung cancer (NSCLC) is the most common type of lung cancer, and adenocarcinoma is the most common histologic subtype of this disease.2 Unfortunately most of patients with lung cancer are diagnosed with locally advanced or metastatic disease which is unamenable to surgical resection or definite radiation therapy; the 5-year survival of these patients is 4%.1
Advanced NSCLC has traditionally been treated with platinum-doublet chemotherapy with the addition of the vascular endothelial growth factor inhibitor, bevacizumab when clinically indicated. Response rates (RRs) of 12%−37% have been observed in the frontline setting with median overall survival (OS) ranging from 10–14 months.3 The identification of molecular subsets of NSCLC in the genomic era, and the development of drugs targeting specific oncogenic mutations have resulted in significant alterations in the management of advanced lung cancer. These changes provide a vivid illustration of the evolution of the therapeutic paradigm from a “one-size-fits-all” approach to that of “precision therapy” with the biology of the disease forming the bedrock of treatment choice. In this article we focus on the identification of molecular subsets of NSCLC and describe the effect of these discoveries on clinical management of advanced NSCLC, as well as its effects on clinical trial design.
CLASSIFICATION OF NSCLC: EFFECT OF THE GENOMIC ERA
The classification of NSCLC has traditionally been based on the histologic appearance of the tumor supplemented by information provided by immunohistochemistry.4 With the advent of genomic testing, it has been recognized that NSCLC is composed of multiple molecular subsets with distinct phenotypes, natural histories, and sensitivities to targeted therapies. The discovery of oncogenic drivers, which has been facilitated by large multi-institutional studies using cutting-edge genomic technologies forms the bedrock of precision therapy for lung cancer.5–8
The Cancer Genome Atlas Project
The Cancer Genome Atlas (TCGA) analysis of lung cancer was conducted to identify molecular alterations in NSCLC and uncover potential targets for biologic therapy. Tumor samples and matched normal controls from patients with previously untreated NSCLC (230 adenocarcinomas and 178 squamous cell carcinomas) were analyzed using multiple platforms including whole genome, messenger RNA, and microRNA sequencing, as well as DNA copy number, methylation, and proteomic analyses.5,7 These studies confirmed the presence of a large number of somatic mutations in NSCLC (mean mutation rate per megabase, adenocarcinoma:8.9, squamous cell carcinoma: 8.1) including statistically significant mutations in 18 genes in adenocarcinoma and 11 genes in squamous cell carcinomas. Adenocarcinomas without known oncogenic mutations (EGFR, KRAS, and BRAF) were found to harbor aberrations in NF1, RIT1, KEAP1, TP53, MET, and ERBB2. Biologic pathways with recurrent alterations included the RTK/RAS/RAF, PI3K/mTOR, p53, and oxidative stress response pathways, cell cycle regulators, and chromatin and RNA splicing factors. Squamous cell carcinomas nearly always harbored mutations in TP53. Other important findings in squamous cell carcinomas included newly discovered loss-of-function mutations in the human leukocyte antigen-A class 1 major histocompatibility gene, and recurrent alterations affecting the PI3K/AKT, CDKN2A/RB1, NFE2L2/KEAP1/CUL3, and SOX2/TP63/NOTCH1 pathways.
Lung Cancer Mutation Consortium Trial
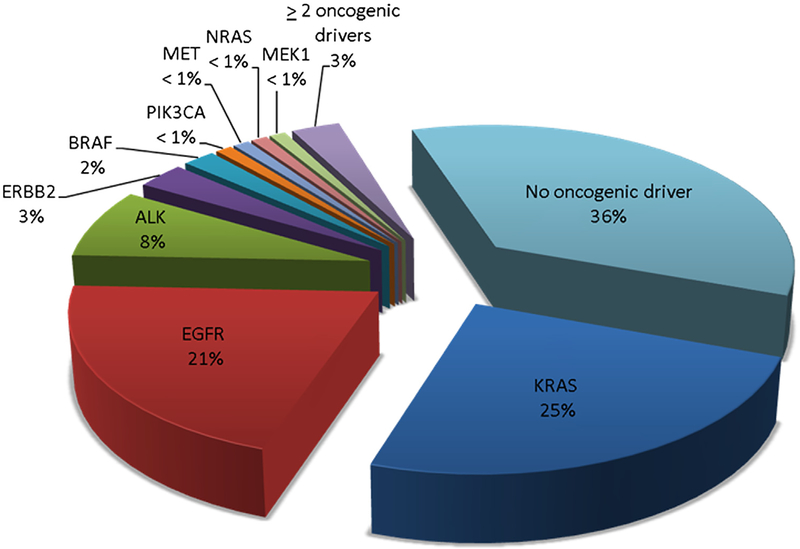
The Lung Cancer Mutation Consortium (LCMC) conducted a study designed to determine the frequency of 10 oncogenic drivers in lung adenocarcinoma, select treatment based on the identified target, and determine survival.8 Unlike the TCGA project, tumors analyzed in the LCMC study were derived from patients with advanced or recurrent adenocarcinoma of the lung. More than 50% of the patients had received prior chemotherapy, and analyzed tumor specimens were not exclusively obtained by surgical resection. Among 1102 eligible patients, analysis of at least 1 gene was possible in 1007 patients including 341 (34%) never-smokers. Tumor samples were screened for EGFR, KRAS, ERBB2, AKT1, BRAF, MEK1, NRAS, and PIK3CA mutations, as well as ALK rearrangements and MET amplification. Full genotyping was feasible in 733 (67%) cancers, and an oncogenic driver was detected in 466 (64%) cases (Fig. 1). Based on these results, 260 (26%) patients received appropriate targeted therapy, and had a median survival of 3.5 years compared with 2.4 years for 318 (32%) patients with an oncogenic driver who did not receive genotype-targeted therapy (P = 0.006). This study demonstrated the feasibility of conducting comprehensive molecular profiling in patients with advanced adenocarcinoma of the lung, and suggested that targeted therapy based on molecular profiling improved patient survival.
Figure 1.
Frequency of oncogenic drivers in adenocarcinoma of the lung detected by the Lung Cancer Mutation Consortium.8
PRECISION THERAPY BASED ON GENOMIC DATA
The identification of molecular subsets of lung cancer has resulted in the exponential growth of biologic agents designed to target specific oncogenic drivers. Scores of drugs are at various stages of development and a few small molecule tyrosine kinase inhibitors (TKIs) have been approved by the U.S. Food and Drug Administration (FDA) for treatment of NSCLC with EGFR mutations or ALK translocations.9
NSCLC With EGFR Mutations
In-frame deletions in exon 19 and the L858R substitution in exon 21 account for 90% of all mutations in the tyrosine kinase domain of the EGFR gene, and confer sensitivity to EGFR TKIs.10 Several randomized clinical trials have demonstrated the ability of EGFR TKIs to significantly improve RRs and progression-free survival (PFS) in NSCLC harboring EGFR sensitizing mutations (Table 1).11–17 In May 2013 the U.S. FDA approved the use of the competitive EGFR inhibitor, erlotinib for first-line treatment of metastatic NSCLC with EGFR exon 19 deletions or exon 21 substitution mutations based on the results of the randomized, phase III European Tarceva vs Chemotherapy (EURTAC) trial. In this study, 174 patients with previously untreated meta-static NSCLC harboring the aforementioned sensitizing mutations were randomized to receive frontline treatment with platinum-doublet chemotherapy or oral erlotinib at a dose of 150 mg daily. The primary end point of the study was investigator-assessed PFS. The results demonstrated a clear benefit of erlotinib with a median PFS of 9.7 months in the erlotinib arm vs 5.2 months in the chemotherapy arm (hazard ratio [HR] = 0.37; 95% CI: 0.25–0.54; P < 0.0001). Erlotinib was well tolerated; treatment-related serious adverse events (AEs) were observed in 6% of patients in the erlotinib arm vs 20% in the chemo-therapy arm.11
Table 1.
Selected Trials of EGFR Tyrosine Kinase Inhibitors in Patients With EGFR Activating and Acquired Resistance Mutations
| TrialRef (Clinical Trial ID) | Phase | Drug | Line of Treatment | Tumor Mutation Status | Patients Enrolled | Response Rate (%) | Median PFS (mo) | Hazard Ratio for PFS |
|---|---|---|---|---|---|---|---|---|
| EURTAC11 (NCT00446225) |
III | Erlotinib Platinum-doublet* |
Front line | EGFR del 19 or L858R | 174 | 58 15 |
9.7 5.2 |
0.37 |
| OPTIMAL12 (NCT00874419) |
III | Erlotinib Carboplatin + gemcitabine |
Front line | EGFR del 19 or L858R | 154 | 83 36 |
13.1 4.6 |
0.16 |
| NEJSG 00213
(UMIN000000376) |
III | Gefitinib Carboplatin + paclitaxel |
Front line | EGFR del 19, L85SR, L861Q, G719A, G719C, G719S |
200 | 74 31 |
10.8 5.4 |
0.30 |
| WJTOG340514
(UMIN000000539) |
III | Gefitinib Cisplatin + docetaxel |
Front line | EGFR del 19 or L858R | 177 | 62 32 |
9.2 6.3 |
0.49 |
| LUX-Lung 315
(NCT00949650) |
III | Afatinib Cisplatin + pemetrexed |
Front line | EGFR del 19, L858R or other activating mutations |
345 | 56 23 |
11.1 6.9 |
0.58 |
| LUX-Lung 616
(NCT01121393) |
III | Afatinib Cisplatin + gemcitabine |
Front line | EGFR del 19, ins 20, L858R, L861Q, G719A, G719C G719S, T790M, S7G8I |
364 | 67 23 |
11.0 5.6 |
0.28 |
| NCT00818441†,17 | II | Dacomitinib | Front line | EGFR del 19, L858R | 45 | 76 | 18.2 | |
| TiGERX‡,24 (NCT01526928) |
I | CO-1686 | Relapse after prior EGFR TKI therapy |
T790M present | 40 | 58 | Not reached | |
| AURA25
(NCT01802632) |
I | AZD9291 | Relapse after prior EGFR TKI therapy |
T790M present T790M absent |
107 50 |
64 22 |
Not reported Not reported |
del, deletion; ins, insertion; Ref, reference.
Cisplatin + docetaxel or gemcitabine, or carboplatin + docetaxel or gemcitabine.
Results from EGFR del 19 or L858R-positive patients only.
Results from T790M+ patients within the therapeutic dose range.
In July 2013, afatinib, a second-generation, non-competitive TKI targeting all members of the ErbB family of receptors was approved as frontline therapy for patients with NSCLC with EGFR-deletion 19 and L858R mutations. Approval was based on the results of a randomized, phase III trial (LUX-Lung 3) that showed an improvement in PFS with oral afatinib at a dose of 40 mg daily vs up to 6 cycles of cisplatin and pemetrexed in patients with advanced EGFR-mutated NSCLC.15 Among 345 patients randomized to treatment, the median PFS was 11.1 months in the afatinib arm and 6.9 months in the chemotherapy arm (HR = 0.58; 95% CI: 0.43–0.78; P = 0.001). AEs were manageable with the frequency of treatment-related grade ≥3 AEs comparable in both arms (49% in the afatinib arm and 48% in the chemotherapy arm). Afatinib demonstrated comparable benefit in a randomized, phase III trial in treatment-naïve Asian patients with EGFR-mutated NSCLC (LUX-Lung 6) with a median PFS of 11 months in the afatinib group vs 5.6 months in the chemotherapy group (HR = 0.28; 95% CI: 0.20–0.39; P < 0.0001).16 Although neither trial showed an improvement in OS for the entire study populations, an OS benefit was observed in patients with exon 19 deletions, suggesting innate differences between exon 19 deletions and L858R mutations in pulmonary adenocarcinomas.18
Despite these impressive results, a significant challenge associated with the use of small molecule TKIs is acquired resistance.19,20 Several mechanisms account for this phenomenon, the most common of which is the emergence of a secondary mutation (T790M) in exon 20, which is detected in approximately 50% of cases.21 Initial attempts to target the T790M mutation with second-generation EGFR TKIs yielded disappointing results.22,23 However, 2 new third-generation TKIs that target common EGFR mutations including T790M have shown promising results in early-phase trials (Table 1). In a dose finding study of CO-1686, 88 patients were treated including 55 (63%) with the T790M mutation. A partial response was achieved in 23 of 40 (58%) evaluable patients with a T790M mutation.24 A phase I study evaluated AZD9291 in 199 patients with EGFR-mutated NSCLC who had developed acquired resistance to EGFR TKIs. Among 107 patients with a T790M mutation, the overall RR was 64% vs 22% in 50 patients without a T790M mutation.25 Additional therapeutic strategies to overcome acquired resistance to EGFR TKIs are currently under evaluation.26
NSCLC With ALK Translocations
ALK translocations were identified as a driver event in NSCLC in 2007.27 ALK rearrangements occur in 3%−7% of NSCLCs, and the fusion product can be targeted by small molecule TKIs. There are 2 drugs approved for treatment of advanced NSCLC with ALK rearrangements, and others are being evaluated in clinical trials (Table 2).28–33
Table 2.
Selected Trials of ALK Inhibitors in Advanced NSCLC Harboring ALK Rearrangements
| TrialRef (Clinical Trial ID] | Phase | Drug | Line of Treatment | Patients Enrolled | Response Rate (%) | Median PFS (mo) | Hazard Ratio for PFS |
|---|---|---|---|---|---|---|---|
| PROFILE 101428
(NCT01154140) |
III | Crizotinib Pemetrexed + Platinum* |
Front line | 172 171 |
74 45 |
10.9 7.0 |
0.45 |
| PROFILE 100729
(NCT00932893) |
III | Crizotinib Pemetrexed or Docetaxel |
Second line | 173 174 |
65 20 |
7.7 3.0 |
0.49 |
| NCT01283516†,30 | I | Ceritinib | Relapse after prior chemotherapy or crizotinib |
114 | 58 | 7.0 | |
| NCT01449461‡,31 | I/II | AP26113 | Relapse after prior crizotinib |
16 | 75 | Not reported | |
| NCT0158802832 | I | Alectinib | Relapse after prior chemotherapy and crizotinib |
37 | 48 | Not reached | |
| JapicCTI 101264§,33 |
I/II | Alectinib | Relapse after prior chemotherapy but ALK inhibitor-naive |
46 | 94 | Not reported |
Ref, reference.
Cisplatin or Carboplatin.
Data for patients with NSCLC treated with ceritinib doses ≥400 mg/d.
Data from ALK-positive NSCLC patients previously treated with crizotinib.
Data from the phase II portion of the study.
In November 2013 the U.S. FDA approved the use of crizotinib for treatment of metastatic NSCLC with ALK rearrangements. This approval was based on the results of a randomized, phase III trial in which 347 patients with locally advanced or metastatic NSCLC with ALK rearrangements who had received 1 prior line of platinum-containing chemotherapy were randomized to receive oral crizotinib at a dose of 250 mg twice daily or chemotherapy (intravenous pemetrexed 500 mg/m2 or docetaxel 75 mg/m2 once every 3 weeks). The primary end point was PFS. Patients receiving crizotinib had a median PFS of 7.7 vs 3.0 months for patients in the chemotherapy arm (HR = 0.49; 95% CI: 0.37–0.64; P < 0.001). Crizotinib was well tolerated, and AEs were usually mild. The incidence of treatment-related grade 3 or 4 AEs and other serious AEs were similar in the crizotinib and chemotherapy arms (33% vs 32% and 12% vs 14%, respectively).29
Ceritinib is an adenosine triphosphate-competitive, TKI targeting ALK with 20 times the potency of crizotinib, which has shown preclinical activity against crizotinib-sensitive, as well as crizotinib-resistant tumors.34 Ceritinib was evaluated at doses of 50–750 mg once daily in a phase I study in patients with tumors harboring ALK rearrangements.30 A total of 130 patients were enrolled including 122 with advanced, previously treated NSCLC of whom 83 (68%) had received crizotinib previously. Ceritinib doses of 400 mg or more per day were administered in 114 patients with NSCLC, and resulted in an overall RR of 58% (95% CI: 48%−67%) and median PFS of 7 months (95% CI: 5.6–9.5 months). Among 80 patients with NSCLC previously treated with crizotinib and receiving at least 400 mg/d of ceritinib, the RR was 56% (95% CI: 45%−67%) and median PFS was 6.9 months (95% CI: 5.3–8.8 months). In April 2014, ceritinib received FDA approval for treatment of patients with metastatic NSCLC with disease progression or intolerance to crizotinib.
Similar to EGFR-targeted therapies, a consequence of prolonged treatment with ALK TKIs is the development of acquired resistance. Multiple mechanisms have been implicated in this phenomenon including development of secondary resistance mutations, amplification of the ALK fusion gene, and activation of bypass pathways including the KIT and EGFR signaling cascades.35,36 As mentioned above, ceritinib is the first drug approved for treatment of crizotinib-resistant NSCLC with ALK rearrangements. Other drugs that are under evaluation for treatment of ALK resistance include alectinib, AP26113, and heat shock protein 90 (Hsp90) inhibitors including AUY922 and ganetespib.37 In phase I studies, AP26113 and alectinib have shown promising activity with RRs of 75% and 48% in patients with NSCLC who have progressed on crizotinib.31,32 Development of acquired resistance remains a recurring problem that limits the benefits of these drugs as illustrated by the occurrence of novel ALK mutations in a patient treated with alectinib.38 Additional approaches need to be explored to circumvent the problem of acquired resistance including the identification of newer compounds for targeted therapy, evaluation of sequential therapy using existing drugs, and development of patient-derived models of acquired resistance to identify drug combinations that can overcome resistance.39
Other Targets for Precision Therapy in NSCLC
In addition to EGFR and ALK, efforts such as TCGA and LCMC have identified several genomic aberrations that have the potential to serve as targets for precision therapy in NSCLC. Examples include BRAF and HER2 mutations and ROS1 gene rearrangements.
The LCMC data showed that the prevalence of BRAF mutations in advanced lung adenocarcinoma is approximately 2%, and these mutations occur more often in current or former smokers.40 The BRAF inhibitor, dabrafenib has demonstrated activity in a phase II study in patients with advanced NSCLC harboring a BRAF V600E mutation after failure of chemotherapy, with an overall RR of 32%, median duration of response of 11.8 months, and an acceptable safety profile.41 In January 2014 Dabrafenib received “Breakthrough Therapy” designation by the FDA for treatment of patients with metastatic, BRAF V600E mutation-positive NSCLC who had received at least 1 prior line of platinum-containing chemotherapy.
HER2 mutations and amplifications are found in approximately 2%−6% of NSCLCs. Various attempts at targeted therapy for these mutations/amplifications are presently under evaluation.8,9,42–44
ROS1 and RET gene rearrangements have each been identified in 1%−2% of patients with NSCLC. These gene rearrangements result in fusion products with constitutive activation of the respective tyrosine kinases which act as oncogenic drivers.45 Both ROS1 and RET tyrosine kinases have been shown to be targetable with small molecule TKIs. Owing to structural similarities between the ROS1 and ALK tyrosine kinases, crizotinib is a potent inhibitor of the ROS1 kinase. In a retrospective European study of 32 patients with advanced adenocarcinoma of the lung with ROS1 rearrangements who received crizotinib for off-label use, the overall RR was 80% and median PFS was 9.1 months.46 These results validate the observations from an expansion cohort of a phase I study of crizotinib in which 50 patients with advanced NSCLC with ROS1 rearrangements were treated with the standard crizotinib dose of 250 mg orally twice daily resulting in a RR of 72% (95% CI: 58%−84%) and median PFS of 19.2 months (95% CI: 14.4 months to not reached).47 Early reports also document the activity of the RET inhibitors, cabozantinib and vandetanib in patients with NSCLC harboring RET rearrangements.48,49 Several clinical trials are evaluating other TKIs in patients with ROS1- and RET-positive NSCLC.9,45
NEWER CHALLENGES IN THE PRECISION THERAPY ERA
The ability to perform genomic analyses of lung cancers and make treatment decisions based on the identification of oncogenic drivers has resulted in remarkable benefits for patients with advanced disease in need of effective options for systemic therapy. However, these developments have brought into focus a unique set of challenges uncovered by the precision medicine approach.
Undruggable Targets
Although several drugs are being developed to inhibit a variety of oncogenic drivers, some of the most common genetic changes associated with lung cancer have proven to be notoriously difficult to target, and are essentially “undruggable” at present. Of particular relevance in this regard are mutations in RAS and p53. KRAS mutations occur in 20%−30% of NSCLCs.8 The oncogenic properties of RAS are influenced by high-affinity binding to guanosine triphosphate leading to activation, attachment to the inner aspect of the cell membrane by the process of prenylation, and subsequent activation of downstream signaling pathways including BRAF and PIK3CA, which themselves are also mutated in NSCLC.50 Farnesyltransferase inhibitors which block prenylation, as well as inhibitors of downstream effectors, such as MEK and PI3K/AKT/mTOR, have been evaluated without much success.51,52 Nevertheless, renewed efforts to target RAS are focusing on direct RAS inhibitors, novel downstream targets like TBK1, CDK4, and GATA-2, newer methods to target RAS localization, and identification of targets that have synthetic lethal interactions with RAS.50,53
Mutations in the tumor suppressor p53 are seen in 46% of lung adenocarcinomas.7 Although restoring p53 activity has proved to be challenging thus far, there have been renewed efforts in this direction in recent years.54
Tumor Heterogeneity
The success of precision medicine is predicated on the accurate identification of genomic aberrations in a tumor. The existence of pretreatment tumor heterogeneity, and clonal evolution following initiation of treatment influence the clinical outcome of precision therapy. Clonal heterogeneity provides an explanation for de novo resistance to a targeted drug, as well as the development of acquired resistance. Efforts are underway to study tumor heterogeneity to better understand the genomic landscape of a tumor both at a certain point in time, as well as longitudinally by acquiring tumor tissue at the time of disease progression. TRAcking non–small cell lung Cancer Evolution through therapy (Rx) (TRACERx) is a prospective trial designed to study intratumor heterogeneity by using an array of techniques including next-generation sequencing, immunohistochemistry, fluorescence in situ hybridization, fluorescence-activated cell sorting, and analysis of circulating tumor cells, as well as circulating tumor DNA. The aims of this trial are to follow tumors from diagnosis to relapse, study the effect of therapy on the evolutionary trajectory of lung cancer, and determine the effects of tumor heterogeneity and clonal dominance on clinical outcome.55
Development of Resistance
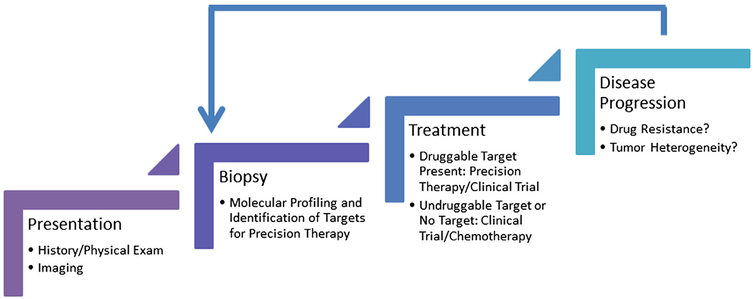
Presently acquired resistance is an inevitable consequence of precision therapy as illustrated above in reference to the use of EGFR and ALK TKIs. With increasing availability of sophisticated genomic techniques and the development of trials such as TRACERx, significant efforts are underway to delineate the mechanisms of resistance to targeted therapy. As development of resistance is a dynamic process, it is imperative to evaluate a tumor in real time for identification of new genomic abnormalities that mediate acquired resistance. This necessitates a new strategy to evaluate tumors with repeat biopsies at the time of disease progression (Fig. 2). A challenging dilemma is how to deal with radio-graphic progression when a patient is receiving a targeted drug. It is not uncommon to observe progression of lesions in distinct organ sites, whereas the disease remains stable elsewhere. Additionally, abrupt withdrawal of a targeted agent can precipitate a growth flare owing to the presence of clonal populations of tumor cells that remain sensitive to the targeted drug.56 Studies have shown that under these circumstances, local therapy for sites of progression and continued use of the targeted agent results in additional clinical benefit.57 Even in the setting of widespread slow growing disease that meets Response Evaluation Criteria In Solid Tumors (RECIST) guidelines for progression, continuation of the targeted drug can provide sufficient disease control for a considerable period of time.58
Figure 2.
The precision therapy paradigm. Successful application of the concept of precision medicine relies on the ability to identify constantly evolving molecular aberrations that occur in response to treatment and as a result of the passage of time. The initial step in this process is to obtain sufficient tissue at the time of diagnosis for molecular profiling and identification of targets for precision therapy. The initial choice of systemic therapy should be based on the presence of a druggable target. Upon disease progression, attempts should be made to acquire tumor tissue from the sites of progression to perform molecular analyses and determine the cause of treatment failure.
IMMUNOTHERAPY
Despite the use of cutting-edge technology, a driver event cannot be identified in approximately one-third of patients with NSCLC. In addition, aforementioned issues such as the presence of undruggable targets and the emergence of acquired resistance limits the efficacy of precision therapy for NSCLC based on TKIs alone. The use of immuno-therapy to surmount these challenges has gained widespread recognition in recent years. Several immunotherapeutic interventions have been evaluated with mixed results. Vaccines designed to target proteins such as mucin-1 (MUC-1) and melanoma-associated antigen E-3 (MAGE-3), allogenic whole-tumor vaccines like belagenpumatucel-L and the immunostimulatory compound, talactoferrin alpha failed to demonstrate an improvement in survival in most clinical trials.59
Cellular immune responses to normal self-antigens, microbial agents, and cancer cells are regulated by diverse interactions of stimulatory, as well as inhibitory ligands and receptors on lymphocytes, professional antigen presenting cells, as well as cancer cells.60–62 CTLA-4 is a receptor on T cells, which interacts with B7.1 and B7.2 stimulatory ligands on antigen presenting cells to dampen response of CD28 engagement with these ligands during T-cell activation. CTLA-4 activation results in down modulation of CD4 T cells and enhanced immunosuppressive function of regulatory T cells. Program death-1 (PD-1) is a receptor, which is typically expressed on activated T cells. Engagement of PD-1 with its ligand (PD-L1) on normal cells inhibits destruction of normal tissues during inflammatory responses to infectious agents. Approximately, 50% of lung cancers express PD-1 or PD-L1.63 Engagement of PD-1 with PD-L1 on cancer cells results in T-cell exhaustion and anergy.
Although the monoclonal antibodies, ipilimumab, and tremelimumab, which inhibit CTLA-4 activation, have been shown to be effective against melanoma, these agents by themselves have limited activity against lung cancer.61,62 A randomized phase II trial has been performed to evaluate carboplatin and paclitaxel with placebo or ipilimumab (concurrent or phased) in 204 chemotherapy-naïve patients with stage III/IV NSCLC. Steroids (to limit ipilimumab-mediated autoimmune responses) were administered in all arms.64 The primary end point of the study was immune-related PFS (ir-PFS). Although ir-PFS was improved in the phased treatment vs placebo group (5.7 vs 4.6 months; HR = 0.72; P = 0.05), there was no improvement in OS. In an unplanned analysis, PFS and OS appeared to be improved in squamous cell carcinomas.
Carboplatin and paclitaxel with placebo or ipilimumab (concurrent or phased) has also been evaluated in 130 chemotherapy-naïve patients with extensive disease–small cell lung cancer (SCLC) in a randomized phase II trial.65 Steroids were administered in all arms. The primary end points were ir-PFS and clinical PFS. Although ir-PFS was improved in the phased vs placebo group (6.4 vs 5.3 months, HR = 0.64; P = 0.03), there was no improvement in clinical PFS or OS. These studies prompted 2 large randomized phase III trials (NCT01285609 and NCT01450761) evaluating ipilimumab vs placebo with paclitaxel and carboplatin in squamous cell lung cancers, and ipilimumab vs placebo with etoposide and platinum therapy in extensive disease–SCLC, respectively.
A phase I trial has evaluated the safety and clinical activity of an anti-PD-1 antibody (nivolumab) in cancer patients.66 Clinical responses were observed in 14 of 76 (18%) patients with NSCLC. In another phase I trial, an anti-PD-L1 antibody (BMS-936559) was evaluated in patients with advanced cancers, and objective responses were observed in 5 of 49 (10%) patients with NSCLC.67 Treatment-related toxicities included fatigue, rash, transaminitis, and pneumonitis (only in the PD-1 trial). Responses in both studies were durable. Subsequent efforts have con-firmed results of these seminal studies.61,62 RRs in patients with NSCLC treated with nivolumab or pembrolizumab (another humanized monoclonal anti-PD-1 antibody) are approximately 18% with median durations of response of 74 and 31 weeks, respectively. Rare and fatal pneumonitis has been associated with both of these agents. MPDL3280A is a human monoclonal antibody to PD-L1. RRs in patients with lung cancer following treatment with this antibody are in the range of 23%. No pneumonitis has been observed with this antibody. In general RRs for anti-PD-1 and anti-PD-L1 antibodies appear to coincide with levels of intratumoral PD-L1 expression. Additionally RRs for these antibodies appear to be higher in smokers or former smokers compared with never-smokers, possibly owing to the mutational load in tobacco-associated cancers.
Preliminary results from a phase I study evaluating a combination of ipilimumab and nivolumab in 49 patients with chemo-naïve NSCLC revealed a RR of 16%.68 Responses were seen in squamous cell and nonsquamous cell cancers, as well as PD-L1 positive and PD-L1 negative tumors. Grades 3 and 4 AEs were seen in nearly 50% of patients. Totally 3 patients died from treatment-related toxicities (respiratory failure, pulmonary hemorrhage, and toxic epidermal necrolysis).
The emergence of immune checkpoint inhibitor therapy represents a major paradigm shift in lung cancer therapy. These impressive results have prompted the initiation of numerous trials evaluating immune checkpoint inhibitors alone or in combination with other agents (Table 3).61,62,69–75
Table 3.
Selected Trials of Immune Checkpoint Inhibitors in Patients With Advanced NSCLC
| DrugRef | Target | Patient Characteristics | Patients Enrolled | Response Rate (%) | Progression-Free Survival (wk) | Overall Survival (wk) | Clinical Trial ID | |
|---|---|---|---|---|---|---|---|---|
| Line of Treatment | Histology | |||||||
| Nivolumab69 | PD-1 | Front line | Sq Non-Sq |
9 11 |
22 36 |
15 47 |
68 NR |
NCT01454102 |
| Nivolumab + Chemo70
N 10 mg/kg + Gem/Cis N 10 mg/kg + Pem/Cis N 10 mg/kg + Pac/Car N 5 mg/kg + Pac/Car |
PD-1 | Front line | Sq Non-Sq Sq + non-Sq Sq + non-Sq |
12 15 15 14 |
33 47 47 50 |
25 30 21 31 |
51 83 65 NR |
NCT01454102 |
| Nivolumab + Ipilimumab68
N 1 mg/kg + Ipi 3 mg/kg N 1 mg/kg + Ipi 3 mg/kg N 3 mg/kg + Ipi 1 mg/kg N 3 mg/kg + Ipi 1 mg/kg |
PD-1, CTLA-4 | Front line | Sq Non-Sq Sq Non-Sq |
9 15 9 16 |
11 13 33 13 |
9 33 21 10 |
44 NR NR NR |
NCT01454102 |
| Nivolumab + Erlotinib71 | PD-1, EGFR | Chemo-naïve; EGFR TKI-naïve, post-EGFR-TKI |
Non-Sq | 21 | 19 | 29 | NR | NCT01454102 |
| Nivolumab72 | PD-1 | Relapsed | Sq + non-Sq | 129 | 17 | 10 | 43 | NCT00730639 |
| Pembrolizumab73,74 | PD-1 | Front line Relapsed |
Sq + non-Sq | 45 217 |
26*,† 20 |
27*
11* |
Not reported Not reported |
NCT01295827 |
| MEDI473675 | PD-L1 | Front line + relapsed | Sq + non-Sq | 155 | 16† | Not reported | Not reported | NCT01693562 |
Carbo, carboplatin; Cis, cisplatin; EGFR, epidermal growth factor receptor; Gem, gemcitabine; Ipi, ipilimumab; N, nivolumab; Non-Sq, nonsquamous; NR, not reached; Pac, paclitaxel; Pem, pemetrexed; Ref, reference; Sq, squamous; TKI, tyrosine kinase inhibitor.
Central review using RECIST version 1.1.
In response-evaluable patients.
EFFECT OF THE PRECISION THERAPY ERA ON CLINICAL TRIAL DESIGN
Traditional clinical trial designs with enrollment predicated on histology and end points based on the phase of the clinical trial are not well suited for evaluating targeted therapies in patients with tumors harboring specific genomic abnormalities. Early-phase proof-of-concept studies with novel designs to match a targeted treatment to its corresponding molecular abnormality provides a more efficient means of evaluating precision therapy.76 These studies can be histology-independent, and based on specific molecular abnormalities (also known as basket trials) or histology-based, and designed to evaluate multiple molecular abnormalities (umbrella trials). An alternative approach is to consider single patient (n-of-1) trials in which an individual patient receives precision therapy for a specific molecular abnormality.77
The U.S. National Cancer Institute (NCI) has developed a series of precision medicine initiatives that illustrate the use of these novel clinical trial designs. These include the NCI Molecular Analysis for Therapy Choice (MATCH) trial, Adjuvant Lung Cancer Enrichment. Marker Identification and. Sequencing Trials (ALCHEMIST), Lung Cancer Master Protocol (Lung-MAP), and the Exceptional Responders Initiative.78
NCI MATCH is an example of a prospective basket trial that will enroll patients with relapsed or refractory, advanced or metastatic solid tumors or lymphomas that harbor actionable mutations.78 A mandatory pretreatment biopsy will be performed, and a targeted next-generation sequencing assay will be used to analyze approximately 200 genes. Patients with actionable mutations will receive appropriate targeted therapy and be followed for response and PFS. At progression patients will be eligible for rebiopsy and further targeted therapy if additional mutations are detected.
The ALCHEMIST trial and Lung-MAP are examples of umbrella trials. The ALCHEMIST trial is designed to evaluate the benefit of adding erlotinib or crizotinib to standard adjuvant therapy vs adjuvant therapy alone, in resectable, early-stage lung cancer harboring EGFR mutations or ALK trans-locations respectively.78 Lung-MAP will enroll patients with advanced squamous cell carcinoma of the lung who have progressed after frontline therapy and evaluate targeted therapies based on the results of an NGS panel of approximately 250 genes.78,79 Patients with tumors harboring actionable mutations will be assigned to a substudy that uses a phase II/III trial design. A phase II interim analysis will determine if the assigned intervention is beneficial based on a predefined improvement in PFS. Co-primary end points for the phase III component will be assessment of PFS and OS. Selection of drugs for the various substudies that comprise Lung-MAP will be a fluid process that is based on the latest findings from ongoing precision medicine studies. Initially Lung-MAP will consist of 5 substudies with 10 treatment arms. Among these substudies 4 will enroll patients based on the presence of a relevant biomarker and will compare appropriate precision therapy with chemotherapy or a combination of precision therapy and chemotherapy with chemotherapy alone. If the patient’s tumor does not harbor a biomarker that can be paired with a study drug, the patients will be assigned to a “nonmatch” arm, and will be randomly assigned to receive immunotherapy vs standard chemotherapy.
The Exceptional Responders pilot study aims to use genome sequencing to understand the molecular basis of an exceptional response of an individual patient to a targeted agent to which most patients do not usually demonstrate significant or durable response. Eligible patients should have demonstrated a complete response or partial response lasting for at least 6 months to a drug whose overall response is less than 10%.78,80 The exceptional responders initiative illustrates the concept underlying n-of-1 studies.
FUTURE AVENUES
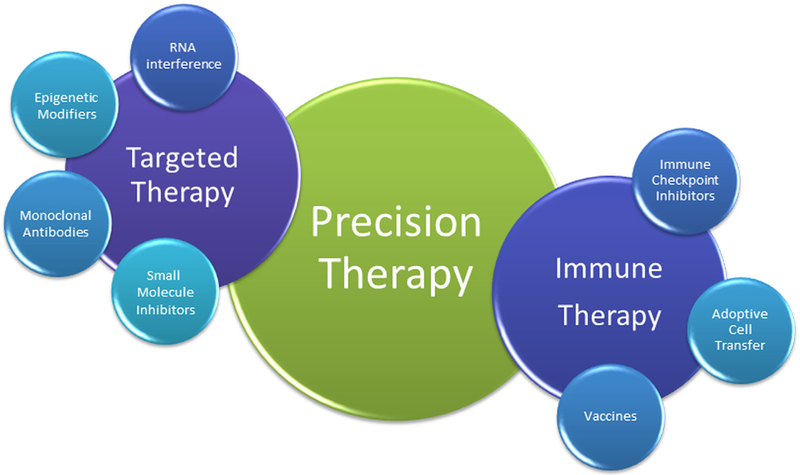
Despite several challenges that need to be overcome, precision medicine has yielded impressive dividends for patients with advanced cancers, including NSCLC. In the future, it is conceivable that the benefits of precision medicine will extend beyond the discovery of genomic aberrations and the development of TKIs to target these abnormalities (Fig. 3). Novel targets that are being explored for precision therapy include chemokines and their receptors and aberrant glycosylation associated with cancer.81,82 Immunotherapy provides a vivid example of the concept of precision medicine. Several clinical trials exploring different immunotherapeutic interventions to treat a variety of tumors have been successfully completed in recent years.83 A personalized approach to immunotherapy could involve recognition of tumor heterogeneity, identification of tumor-specific antigens, detection of differences in antitumor immunity in individual patients, and development of adoptive T-cell therapies.84 Precision therapies could potentially involve RNA interference after identification of microRNA-based signatures of individual tumors.85 Finally, the possibility of coupling information about tumors gained from various molecular methods with nanomedicine to improve diagnostic tools and drug delivery is under evaluation.86 Integration of these approaches to generate robust therapies to target individual tumors could be considered the holy grail of precision medicine.
Figure 3.
The components of precision therapy. Abnormalities in the genome, epigenome, and transcriptome can be targeted using several interventions including small molecule inhibitors, monoclonal antibodies, epigenetic strategies, and small interfering RNAs. Immune dysregulation can be targeted using vaccines against tumor-specific antigens, immune checkpoint inhibitors to block cell surface inhibitory molecules, and adoptive cell transfer technologies.
References
- 1.National Cancer Institute: SEER cancer statistics factsheets: Lung and bronchus cancer. Bethesda, MD: http://seer.cancer.gov/statfacts/html/lungb.html. Accessed 12 February, 2015. [Google Scholar]
- 2.Travis WD, Brambilla E, Noguchi M, et al. : International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6:244–285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenthal GM, Karuri SW, Zhang H, et al. : Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: A US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol 10.1200/JCO.2014.59.0489, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Riely GJ: New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J Clin Oncol 31:992–1001, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network: Comprehensive genomic characterization of squamous cell lung cancers. Nature 489: 519–525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical Lung Cancer Genome Project, Network Genomic Medicine: A genomics-based classification of human lung tumors. Sci Transl Med 5: 209ra153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network: Comprehensive molecular profiling of lung adeno-carcinoma. Nature 511:543–550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kris MG, Johnson BE, Berry LD, et al. : Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. J Am Med Assoc 311:1998–2006, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgensztern D, Campo MJ, Dahlberg SE, et al. : Molecularly targeted therapies in non-small-cell lung cancer annual update 2014. J Thorac Oncol 10:S1–S63, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma SV, Bell DW, Settleman J, et al. : Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7:169–181, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Rosell R, Carcereny E, Gervais R, et al. : Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Wu YL, Chen G, et al. : Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Maemondo M, Inoue A, Kobayashi K, et al. : Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Mitsudomi T, Morita S, Yatabe Y, et al. : Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 11:121–128, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Sequist LV, Yang JC, Yamamoto N, et al. : Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adeno-carcinoma with EGFR mutations. J Clin Oncol 31:3327–3334, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Wu YL, Zhou C, Hu CP, et al. : Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol 15: 213–222, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Janne PA, Ou SH, Kim DW, et al. : Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: A multicentre, open-label, phase 2 trial. Lancet Oncol 15: 1433–1441, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Yang JC, Wu YL, Schuler M, et al. : Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16:141–151, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Gainor JF, Shaw AT: Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol 31: 3987–3996, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camidge DR, Pao W, Sequist LV: Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol 11: 473–481, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Sequist LV, Waltman BA, Dias-Santagata D, et al. : Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3:75ra26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller VA, Hirsh V, Cadranel J, et al. : Afatinib versus placebo for patients with advanced, metastatic non–small cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol 13: 528–538, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Reckamp KL, Giaccone G, Camidge DR, et al. : A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer 120:1145–1154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sequist LV, Soria J-C, Gadgeel SM, et al. : First-in-human evaluation of CO-1686, an irreversible, highly selective tyrosine kinase inhibitor of mutations of EGFR (activating and T790M). J Clin Oncol 32, 2014 [abstr 8010] [Google Scholar]
- 25.Janne PA, Ramalingam SS, Yang JC-H, et al. : Clinical activity of the mutant-selective EGFR inhibitor AZD9291 in patients (pts) with EGFR inhibitor-resistant non-small cell lung cancer (NSCLC). J Clin Oncol 32, 2014 [abstr 8009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu HA, Riely GJ, Lovly CM: Therapeutic strategies utilized in the setting of acquired resistance to EGFR tyrosine kinase inhibitors. Clin Cancer Res 20:5898–5907, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soda M, Choi YL, Enomoto M, et al. : Identi-fication of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448: 561–566, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Solomon BJ, Mok T, Kim DW, et al. : First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371: 2167–2177, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Shaw AT, Kim DW, Nakagawa K, et al. : Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368: 2385–2394, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Shaw AT, Kim DW, Mehra R, et al. : Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 370:1189–1197, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camidge DR, Bazhenova L, Salgia R, et al. : Updated results of a first-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies. J Thorac Oncol 8:S296, 2013. (suppl 2) [Google Scholar]
- 32.Gadgeel S, Ou SH, Chiappori AA, et al. : A phase 1 dose escalation study of a new ALK inhibitor, CH5424802/RO5424802, in ALK+non-small cell lung cancer (NSCLC) patientsþwho have failed crizotinib (AF-002JG/NP28761, NCT01588028). J Thorac Oncol 8: S199, 2013. (suppl 2) [Google Scholar]
- 33.Seto T, Kiura K, Nishio M, et al. : CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): A single-arm, open-label, phase 1–2 study. Lancet Oncol 14:590–598, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Friboulet L, Li N, Katayama R, et al. : The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 4:662–673, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katayama R, Shaw AT, Khan TM, et al. : Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 4: 120ra17, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doebele RC, Pilling AB, Aisner DL, et al. : Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 18: 1472–1482, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez CA, Velez M, Raez LE, et al. : Overcoming the resistance to crizotinib in patients with non-small cell lung cancer harboring EML4/ALK translocation. Lung Cancer 84: 110–115, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Katayama R, Friboulet L, Koike S, et al. : Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res 20:5686–5696, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crystal AS, Shaw AT, Sequist LV, et al. : Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 346:1480–1486, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villaruz LC, Socinski MA, Abberbock S, et al. : Clinicopathologic features and outcomes of patients with lung adenocarcinomas harboring BRAF mutations in the Lung Cancer Mutation Consortium. Cancer 121:448–456, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Planchard D, Kim TM, Mazieres J, et al. : Dabrafenib in patients with BRAF V600E-mutant advanced non-small cell lung cancer (NSCLC): A multicenter, open-label, phase II trial (BRF113928). Ann Oncol 25, 2014. (suppl 4) [abstr LBA38_PR] [Google Scholar]
- 42.Arcila ME, Chaft JE, Nafa K, et al. : Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 18:4910–4918, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazieres J, Peters S, Lepage B, et al. : Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 31:1997–2003, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Mar N, Vredenburgh JJ, Wasser JS: Targeting HER2 in the treatment of non-small cell lung cancer. Lung Cancer: 10.1016/j.lungcan.2014.12.018, 2015. [DOI] [PubMed] [Google Scholar]
- 45.Gainor JF, Shaw AT: Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 18:865–875, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazieres J, Zalcman G, Crino L, et al. : Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1 Cohort. J Clin Oncol 10.1200/JCO.2014.58.3302 [DOI] [PubMed] [Google Scholar]
- 47.Shaw AT, Ou SH, Bang YJ, et al. : Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371:1963–1971, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drilon A, Wang L, Hasanovic A, et al. : Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov 3:630–635, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautschi O, Zander T, Keller FA, et al. : A patient with lung adenocarcinoma and RET fusion treated with vandetanib. J Thorac Oncol 8: e43–e44, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Cox AD, Fesik SW, Kimmelman AC, et al. : Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov 13:828–851, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riely GJ, Johnson ML, Medina C, et al. : A phase II trial of Salirasib in patients with lung adenocarcinomas with KRAS mutations. J Thorac Oncol 6:1435–1437, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Carter CA, Rajan A, Szabo E, et al. : Two parallel randomized phase II studies of selumetinib (S) and erlotinib (E) in advanced non-small cell lung cancer selected by KRAS mutations. J Clin Oncol 31, 2013. [abstr 8026] [Google Scholar]
- 53.Vasan N, Boyer JL, Herbst RS, et al. : Renaissance: Emerging targeted therapies for KRAS-mutated non-small cell lung cancer. Clin Cancer Res 20:3921–3930, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khoo KH, Verma CS, Lane DP: Drugging the p53 pathway: Understanding the route to clinical efficacy. Nat Rev Drug Discov 13: 217–236, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Jamal-Hanjani M, Hackshaw A, Ngai Y, et al. : Tracking genomic cancer evolution for precision medicine: The lung TRACERx study. PLoS Biol 12:e1001906, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaft JE, Oxnard GR, Sima CS, et al. : Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin Cancer Res 17:6298–6303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu HA, Sima CS, Huang J, et al. : Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 8:346–351, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishie K, Kawaguchi T, Tamiya A, et al. : Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: A retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol 7: 1722–1727, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Seetharamu N: The state of the art in non-small cell lung cancer immunotherapy. Semin Thorac Cardiovasc Surg 26:26–35, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Brahmer JR, Pardoll DM: Immune checkpoint inhibitors: Making immunotherapy a reality for the treatment of lung cancer. Cancer Immunol Res 1:85–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anagnostou VK, Brahmer JR: Cancer immuno-therapy: A future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res 21:976–984, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Helissey C, Champiat S, Soria JC: Immune checkpoint inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol 27:108–117, 2015 [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Wang L, Li Y, et al. : Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 7:567–573, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch TJ, Bondarenko I, Luft A, et al. : Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 30:2046–2054, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Reck M, Bondarenko I, Luft A, et al. : Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 24:75–83, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brahmer JR, Tykodi SS, Chow LQ, et al. : Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antonia SJ, Gettinger SN, Chow LQM, et al. : Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol 32, 2014. [abstr 8023] [Google Scholar]
- 69.Gettinger SN, Shepherd FA, Antonia SJ, et al. : First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol 32, 2014. [abstr 8024] [Google Scholar]
- 70.Antonia SJ, Brahmer JR, Gettinger SN, et al. : Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with platinum-based doublet chemotherapy (PT-DC) in advanced non-small cell lung cancer (NSCLC). J Clin Oncol 32, 2014. [abstr 8113] [Google Scholar]
- 71.Rizvi NA, Chow LQM, Borghaei H, et al. : Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. J Clin Oncol 32, 2014. [abstr 8022] [Google Scholar]
- 72.Brahmer JR, Horn L, Gandhi L, et al. : Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): Survival and clinical activity by subgroup analysis. J Clin Oncol 32, 2014. [abstr 8112] [Google Scholar]
- 73.Rizvi NA, Garon EB, Patnaik A, et al. : Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC). J Clin oncol 32, 2014. [abstr 8007] [Google Scholar]
- 74.Garon EB, Leighl NB, Rizvi NA, et al. : Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 32, 2014. [abstr 8020] [Google Scholar]
- 75.Brahmer JR, Rizvi NA, Lutzky J, et al. : Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 32, 2014. [abstr 8021] [Google Scholar]
- 76.Sleijfer S, Bogaerts J, Siu LL: Designing transformative clinical trials in the cancer genome era. J Clin Oncol 31:1834–1841, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Doroshow JH: Selecting systemic cancer therapy one patient at a time: Is there a role for molecular profiling of individual patients with advanced solid tumors? J Clin Oncol 28: 4869–4871, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Abrams J, Conley B, Mooney M, et al. : National Cancer Institute’s precision medicine initiatives for the new National Clinical Trials Network. Am Soc Clin Oncol Educ Book: 71–76, 2014 [DOI] [PubMed] [Google Scholar]
- 79.Herbst RS, Gandara DR, Hirsch FR, et al. : Lung Master Protocol (Lung-MAP)-A Biomarker-Driven Protocol for accelerating development of therapies for squamous cell lung cancer: SWOG S1400. Clin Cancer Res. 10.1158/1078-0432.CCR-13-3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takebe N, McShane L, Conley B: Biomarkers: Exceptional responders-discovering predictive biomarkers. Nat Rev Clin Oncol. 10.1038/nrclinonc.2015.19, 2015. [DOI] [PubMed] [Google Scholar]
- 81.Weitzenfeld P, Ben-Baruch A: The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett 352:36–53, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Padler-Karavani V: Aiming at the sweet side of cancer: Aberrant glycosylation as possible target for personalized-medicine. Cancer Lett 352: 102–112, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Rosenberg SA: Decade in review-cancer immunotherapy: Entering the mainstream of cancer treatment. Nat Rev Clin Oncol 11:630–632, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wayteck L, Breckpot K, Demeester J, et al. : A personalized view on cancer immunotherapy. Cancer Lett 352:113–125, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Ballarin-Gonzalez B, Ebbesen MF, Howard KA: Polycation-based nanoparticles for RNAi-mediated cancer treatment. Cancer Lett 352:66–80, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Rosenblum D, Peer D: Omics-based nanomedicine: The future of personalized oncology. Cancer Lett 352:126–136, 2014 [DOI] [PubMed] [Google Scholar]