Abstract
Background
Avian Haemosporida are vector-borne parasites that commonly infect Passeriformes. Molecular analyses revealed a high number of different lineages and lineage specific traits like prevalence and host-specificity, but knowledge of parasite prevalence and lineage diversity in wild birds in Central Germany is still lacking.
Results
Blood samples from a total of 238 adult and 122 nestling songbirds belonging to six species were investigated for infections with avian haemosporidian genera and lineages (Haemoproteus spp., Plasmodium spp., Leucocytozoon spp.) and Trypanosoma avium using PCR, targeting the parasite mitochondrial cytochrome b gene and 18S ribosomal RNA. In total, the prevalence in adult birds was 31.3% infected with Haemoproteus, 12.5% with Plasmodium and 71.0% with Leucocytozoon (nestlings excluded). None of the tested birds was infected with Trypanosoma avium. Only in two nestling birds, aged 12–17 days, a Leucocytozoon spp. infection was proven. Among 225 successfully sequenced samples, we found four Haemoproteus, three Plasmodium and 19 Leucocytozoon lineages, including two new Leucocytozoon lineages. Furthermore, we report two new host-lineage associations.
Conclusions
As first study investigating avian haemosporidian parasites in Central Germany, we provide new information on genetic diversity of Haemosporida infecting Passeriformes. We show that even with a small sample size new lineages as well as previously unknown linkages between certain lineages and host species can be detected. This may help to elucidate the diversity of lineages as well as lineage-host-connections of avian Haemosporida.
Keywords: Haemosporida, Passeriformes, Lineage diversity, Avian pathogens, Lineage networks
Introduction
Avian blood parasites (Hematozoa) infect both domestic and wild birds, and therefore they have been objects of intensive scientific research over a long period (Valkiūnas, 1996; Valkiūnas, 2005; Bensch et al., 2013). Among the most common blood parasite orders are the Haemosporida. These include among others the avian malaria-like genera Haemoproteus and Leucocytozoon as well as the pathogen of avian malaria Plasmodium. These avian Haemosporida belonging to the phylum Apicomplexa share a similar but complex life cycle (Schmid et al., 2017), including asexual stages of reproduction in a bird host and sexual stages within a vector (Valkiūnas, 2005; Santiago-Alarcon, Palinauskas & Schaefer, 2012). Another common blood parasite is Trypanosoma avium, flagellate protozoans living in the bloodstream of birds (Hamilton, Gibson & Stevens, 2007). Hematophagous dipterans are the vectors of these parasites. Leucocytozoon parasites are vectored by blood-sucking dipterans of two families: black flies (Simuliidae) and biting midges (Ceratopogonidae) (Freund et al., 2016; Lotta et al., 2016). Ceratopogonid midges are also vectors for Haemoproteus spp. (Desser & Bennett, 1993). The main vectors for Plasmodium spp. are several species of mosquitos (Culicidae) (Medeiros, Hamer & Ricklefs, 2013). The transmission for T. avium remains still unclear with various blood-sucking insects mentioned as possible vectors (Votypka et al., 2002). Haemosporida are generally considered as pathogens with a low pathogenicity and harmless in bird populations (Wiersch et al., 2007; Ciloglu et al., 2016), but several studies demonstrated different costs on life-history traits associated with Haemosporida infections: Haemosporidian parasites can affect the body condition (Valkiūnas et al., 2006), reproductive success (e.g., Hunter, Rohner & Currie, 1997; Merino et al., 2000; Marzal et al., 2005; Tomás et al., 2007a; Knowles, Palinauskas & Sheldon, 2010) and the survival (e.g., Dawson & Bortolotti, 2000; Møller & Nielsen, 2007; Donovan et al., 2008; Bueno et al., 2010).
Haemosporida are abundant in many avian families and occur worldwide except in Antarctica (Valkiūnas, 2005; Bensch, Hellgren & Perez-Tris, 2009; Clark, Clegg & Lima, 2014; Vanstreels et al., 2014). However, there are interspecific differences in the parasite prevalence (e.g., Bennett, Bishop & Peirce, 1993; Bennett, Peirce & Ashford, 1993; Valkiūnas, 2005) and in some orders, e.g., Passeriformes, the parasites are more abundant (Valkiūnas, 2005). On the basis of recent molecular studies, the diversity of Haemosporida species and lineages may be assessed more precisely. The species diversity seems to be as high as the diversity of avian species (Bensch et al., 2004) or even higher (Schmid et al., 2017) and thousands of lineages may exist (Szollosi et al., 2011). Lineages can differ from each other in only one single nucleotide (e.g., one substitution) of the mitochondrial cytochrome b gene (Bensch et al., 2004; Hellgren, Waldenström & Bensch, 2004; Bensch, Hellgren & Perez-Tris, 2009; Chagas et al., 2017). For the order Passeriformes alone, 912 Haemoproteus, 852 Plasmodium and 600 Leucocytozoon lineages are deposited in the avian haemosporidian parasite database MalAvi (Bensch, Hellgren & Perez-Tris, 2009; MalAvi, 2018). In addition, molecular surveys vastly improved our understanding about the host specificity of avian haemosporidian infections (Ciloglu et al., 2016). The Haemosporida occupy niches that vary from extreme host generalization to extreme host specialization (Okanga et al., 2014). Generally, Plasmodium spp. is known to be more host-generalized (e.g., Waldenström et al., 2002; Križanauskiene et al., 2006; Dimitrov, Zehtindjiev & Bensch, 2010; Mata et al., 2015), whereas species of Leucocytozoon as well as Haemoproteus are considered to be more host-specific (e.g., Waldenström et al., 2002; Beadell et al., 2004; Forrester & Greiner, 2008; Dimitrov, Zehtindjiev & Bensch, 2010; Jenkins & Owens, 2011). Trypanosoma avium seems to possess a relatively low host-specificity (Bennett, Earle & Squires-Parsons, 1994; Sehgal, Jones & Smith, 2001).
Furthermore, prevalence varies not merely interspecific but also with the age of the birds. A common pattern observed in host-parasite assemblages is a higher abundance of parasites in juvenile compared to adult birds (Sol, Jovani & Torres, 2003). However, for blue tits nesting in nest boxes, blood parasites are far less prevalent in nestlings than in adult birds (Cosgrove et al., 2006; Martinez-de la Puente et al., 2013). Possibly because box-nesting species may be shield from vector exposure due to their enclosed surroundings (Dunn et al., 2017).
The aims of the present study were (i) to assess the prevalence of the Haemosporida: Plasmodium, Haemoproteus and Leucocytozoon as well as T. avium in six species of wild Passeriformes in Central Germany, (ii) to identify and compare the lineage diversity among the birds and to record interspecific-shared lineages by means of mitochondrial cytochrome b sequencing as well as (iii) to compare the prevalence in adult and nestling birds.
Material & Methods
Origin and preparation of the samples
Bird capture and sampling were carried out under license (Animal welfare officer of the University of Giessen, no. 662_GP and 828_GP, and the Regierungspräsidium Giessen, no. 109-2012 and 77-2016) in accordance with the German legislation. Blood samples from 360 Passeriformes of four families and six species (blue tit Cyanistes caeruleus, Paridae; great tit Parus major, Paridae; coal tit Periparus ater, Paridae; eurasian tree sparrow Passer montanus, Passeridae; european pied flycatcher Ficedula hypoleuca, Muscicapidae and eurasian nuthatch Sitta europaea, Sittidae) were collected during April to June in the years 2015, 2017 and 2018 (Table 1).
Table 1. Numbers of sampled adult and nestling songbirds.
Sample sizes per sex and parasite genus are given (P, Plasmodium spp.; H, Haemoproteus spp.; L, Leucocytozoon spp.; T, Trypanosoma avium).
| Species | Year of sampling | Number of specimens (n) | |||||
|---|---|---|---|---|---|---|---|
| Adult (male/female) | Nestling | ||||||
| P | H | L | T | L | T | ||
| Blue tit (C. caeruleus) | 2017 | 58 (30/28) | 58 (30/28) | 58 (30/28) | 58 (30/28) | 65a | 65a |
| Great tit (P. major) | 2015/2018 | 32 (14/18) | 32 (14/18) | 142 (49/93) | 68 (18/50) | 57b | 57b |
| Coal tits (P. ater) | 2017 | 4 (2/2) | 4 (2/2) | 4 (2/2) | 4 (2/2) | 0 | 0 |
| Eurasian tree sparrow (P. montanus) | 2015/2017 | 10 (1/9) | 10 (1/9) | 10 (1/9) | 10 (1/9) | 0 | 0 |
| European pied flycatcher (F. hypoleuca) | 2015/2017 | 14 (2/12) | 14 (2/12) | 14 (2/12) | 14 (2/12) | 0 | 0 |
| Eurasian nuthatch (S. europaea) | 2015/2017 | 10 (3/7) | 10 (3/7) | 10 (3/7) | 10 (3/7) | 0 | 0 |
Notes.
Age: 16 days.
Age: 12–17 days.
Sample sites were located in and closely around the city Giessen (50°35′2.584″N 8°40′42.251″E, Hesse, Central Germany). All birds were captured at their nest boxes by hand and blood-sampled by brachial venipuncture. The blood was stored on Whatman FTA classic cards (Whatman®, UK). For DNA isolation a 3 × 3 mm piece of each sample was cut out of the FTA card with a sterile scalpel blade. Subsequently the DNA was extracted according to the ammonium-acetate protocol by Martinez et al. (2009) and purified with NZYspintech-columns (NZYTech, Lda.-Genes & Enzymes, Portugal) or Zymo-Spin™ IIC columns (Zymo Research, USA). The presence and concentration of DNA were confirmed and determined with NanoDrop2000c UV-Vis Spectrophotometer (NanoDrop Technologies, USA). If the DNA concentration was higher than 80 ng/µl, samples were diluted to 30 ng/µl.
Parasite screening
A partial amplification of the mitochondrial cytochrome b gene of the different Haemosporida was accomplished by polymerase chain reaction (PCR) with the respective primers (Table 2).
Table 2. Primer pairs and their PCR conditions used for blood parasite screening.
| Primer pair | Fragment size (bp) | Initial denaturation | Denaturation annealing extension | Cycles | Final extension | Target gene |
|---|---|---|---|---|---|---|
| HaemF | 480 | 3 min/94 °C | 30 s/94 °C | 35 | 10 min/72 °C | Cytochrome b |
| HaemR2 | 30 s/55 °C | |||||
| 45 s/72 °C | ||||||
| HaemFL | 600 | 3 min/94 °C | 30 s/94 °C | 35 | 10 min/72 °C | Cytochrome b |
| HaemNR3 | 30 s/51 °C | |||||
| 45 s/72 °C | ||||||
| TryF | 122 | 10 min/95 °C | 15 s/95 °C | 40 | 10 min/72 °C | 18S rRNA |
| TryR | 30 s/56 °C | |||||
| 60 s/72 °C |
For the detection of Haemoproteus/Plasmodium species the primer pair HaemF (5′-ATGGTGCTTTCGATATATGCATG 3′) and HaemR2 (5′-GCATTATCTGGATGTGATAA TGGT-3′) (Bensch et al., 2000) was used. For Leucocytozoon detection we applied primer pair HaemFL (5′- ATGGTGTTTTAGATACTTACATT-3′) and HaemNR3 (5′-ATAGAAAGATAAGAAATACCATTC-3′) (Hellgren, Waldenström & Bensch, 2004). To test for T. avium infections the accomplished primer pair was TryF (5′-ATGCACTAGGCACCGTCG-3′) and TryR (5′-GGAGAGGGAGCCTGAGAAATA-3′) (Martinez-de la Puente et al., 2013) targeting the 18S ribosomal RNA (Table 2).
Nestlings were only checked for possible Leucocytozoon spp. and T. avium infections. The PCR reactions with HaemF and HaemR2 consisted of 20 µl reaction volumes containing 2 µl DNA template (14.2–80 ng/µl), 1.2 µl of each primer (10 µM), 10 µl of InnuMix PCR Master Mix (2×, Analytik Jena AG, Germany) and 5.6 µl nuclease-free water. The PCR reaction for the detection of Leucocytozoon spp. contained 2.5 µl DNA template (8.0–80 ng/µl), 0.6 µl of each primer (20 µM), 10.6 µl InnuMix PCR Master Mix and 5.7 µl nuclease-free water. For T. avium evidence the PCR reaction volume was 20 µl consisting of 2.5 µl DNA template, 0.6 µl of each primer (20 µM), 10.6 µl DreamTaq PCR Master Mix (Thermo Fisher Scientific, Germany) and 5.7 µl nuclease-free water. The PCR reaction parameters using thermal cyclers peqSTAR 96Q (Peqlab, Germany) and Tone (Biometra, Germany) are given in Table 2. A positive as well as a negative control were included in each run to ensure PCR was working properly. PCR amplicons were visualized using QIAxcel Advanced (Qiagen, Switzerland) high-resolution capillary gel electrophoresis. Samples rendering a clear peak during gel electrophoresis were bi-directional Sanger sequenced by Microsynth-Seqlab (Sequence Laboratories Goettingen GmbH, Germany).
Phylogenetic and statistical analyses
The forward and reverse sequences were assembled and trimmed in CLC Main Workbench 7.6.4 (CLC Bio, Qiagen, Denmark). PCR and sequencing were repeated, if not all nucleotides of a sequence could be determined unambiguously. Sequences were excluded from network construction when repetitions did not improve sequence quality.
The consensus sequences were assigned to a parasite lineage by BLAST (BLASTN 2.3.0 +, Zhang et al., 2000) using the database MalAvi (Bensch, Hellgren & Perez-Tris, 2009). Constructions of lineage networks for each Haemosporida genus, using the median-joining network method, were performed with PopART 1.7 (Bandelt, Forster & Röhl, 1999; Leigh & Bryant, 2015) after aligning the sequences in BioEdit v7.2.5 (Hall, 1999). For these alignments, we used 123 Leucocytozoon (478 bp), 39 Haemoproteus (463 bp) and 14 Plasmodium consensus sequences (440 bp).
Statistical evaluation of comparing prevalences and number of interspecific shared lineages per genus was performed with R ( R Core Team, 2016) using the R Package R commander. To compare the equality of proportions of parameters mentioned above the frequency distribution test Pearson‘s Chi2-test was applied. A significance level of p < 0.05 was used.
Results
Blood parasite prevalence
We detected three Haemosporida parasite genera in the local bird population in and around Giessen. The overall prevalence was 31.3% for Haemoproteus, 12.5% for Plasmodium and 71.0% for Leucocytozoon (nestlings excluded) (Table 3). Only blue tits and great tits were infected with all three genera. Coal tits were infected with Plasmodium and Leucocytozoon and tree sparrows with Plasmodium and Haemoproteus. Nuthatches showed infections with Haemoproteus and Leucocytozoon. For pied flycatchers we only found evidence of Haemoproteus infections (Table 3).
Table 3. Haemosporida and Trypanosoma avium prevalence in six songbird species.
| Species (No. of samples) | Haemoproteus spp. | Plasmodium spp. | Leucocytozoon spp. | Trypanosoma avium | ||||
|---|---|---|---|---|---|---|---|---|
| positive/ negative | Prevalence (%) | positive/ negative | Prevalence (%) | positive/ negative | Prevalence (%) | positive/ negative | Prevalence (%) | |
| C. caeruleus | ||||||||
| Adult (58) | 21/37 | 36.2 | 6/52 | 10.3 | 55/3 | 94.8 | 0/58 | 0.0 |
| Nestling (65) | 0/65 | 0.0 | 0/65 | 0.0 | ||||
| P. major | ||||||||
| Adult (142)a | 13/19 | 40.6 | 7/25 | 21.9 | 110/32 | 77.5 | 0/68 | 0.0 |
| Nestling (57) | 2/55 | 3.5 | 0/57 | 0.0 | ||||
| P. ater | ||||||||
| Adult (4) | 0/4 | 0.0 | 1/3 | 25.0 | 1/3 | 25.0 | 0/4 | 0.0 |
| P. montanus | ||||||||
| Adult (10) | 1/9 | 10.0 | 2/8 | 20.0 | 0/10 | 0.0 | 0/10 | 0.0 |
| F. hypoleuca | ||||||||
| Adult (14) | 3/11 | 21.4 | 0/14 | 0.0 | 0/14 | 0.0 | 0/14 | 0.0 |
| S. europaea | ||||||||
| Adult (10) | 2/8 | 20.0 | 0/10 | 0.0 | 3/7 | 30.0 | 0/10 | 0.0 |
| Total | 40/88 | 31.3 | 16/112 | 12.5 | 171/189 | 47.5 | 0/286 | 0.0 |
| Nestlings excluded | 169/69 | 71.0 | 0/164 | 0.0 | ||||
Notes.
For Haemoproteus spp. and Plasmodium spp. the sample size was 32 and for Trypanosoma avium 68 adult great tits.
Prevalence rates of Leucocytozoon spp. were highest in blue tits (94.8%). The highest prevalence of Plasmodium infections occurred in coal tits (25.0%). Haemoproteus was almost equally common in blue tits (36.2%) and great tits (40.6%) (Table 3). The prevalence for the three haemosporidian genera in adult birds was different between the genera (Pearson‘s Chi2-test: χ2 = 129.1, df = 2, p < 0.001). Comparing the prevalence of Haemoproteus and Plasmodium, Haemoproteus spp. was significantly more prevalent (Pearson‘s Chi2-test: χ2 = 13.2, df = 1, p < 0.001), and Leucocytozoon was significantly more prevalent than the other two genera (Pearson‘s Chi2-test: Haemoproteus/Leucocytozoon: χ2 = 53.7, df = 1, p < 0.001; Plasmodium/Leucocytozoon: χ2 = 114.0, df = 1, p < 0.001). For none of the tested birds a T. avium infection was detected. In blue tit nestlings a haemosporidian infection could not be detected by PCR. In great tit nestlings we proved an infection with Leucocytozoon, although the prevalence with 3.5% tits infected (2 out of 57 nestlings) was low.
Lineage diversity
In total, 26 haemosporidian lineages could be identified in the MalAvi database. We found four Haemoproteus spp., three Plasmodium spp. and 19 Leucocytozoon spp. lineages (Table 4). Great and blue tits showed similar high lineage diversities (n = 15 lineages: 2 Haemoproteus, 1 Plasmodium and 12 Leucocytozoon lineages; n = 17: 1 Haemoproteus, 3 Plasmodium and 13 Leucocytozoon lineages, respectively). We identified two new Leucocytozoon lineages (CYACAE02, GenBank accession number MH758695, and CYACAE03, MH758696), infecting blue tits which differ each in 0.21% (1 nucleotide) from their closest MalAvi match. For new lineages PCR and sequencing were performed twice to verify the results.
Table 4. Parasite lineages found in the six songbird species with their closest MalAvi match, the respective accession number and query cover in %.
| Parasite | Lineage (MalAvi) | Accession number (GenBank) | n | Match (%) | Host species (Number of individuals) | Lineage prevalence (%)a | Reference accession number |
|---|---|---|---|---|---|---|---|
| Haemoproteusb | PARUS1 | JQ778282 | 34 | 100 | C. caeruleus (21) P. major (11) S. europaea (2) | 15.1 | Glaizot et al. (2012) |
| Haemoproteus | PADOM03 | KJ488647 | 1 | 100 | P. montanus (1) | 0.4 | Drovetski et al. (2014) |
| Haemoproteus | PHSIB1 | KJ396634 | 1 | 100 | P. major (1) | 0.4 | Scordato & Kardish (2014) |
| H. pallidus | PFC1 | JX026899 | 3 | 100 | F. hypoleuca (3) | 1.3 | Valkiūnas et al. (2013) |
| Plasmodium | GRW11 | AY831748 | 3 | 100 |
C. caeruleus (2) P. montanus (1) |
1.3 | Perez-Tris & Bensch (2005) |
| Plasmodium | SGS1 | AB542064 | 11 | 99–100 |
C. caeruleus (3) P. major (7) P. montanus (1) |
4.9 | Ejiri et al. (2011) |
| P. circumflexum | TURDUS1 | KP000842 | 2 | 100 |
C. caeruleus (1) P. ater (1) |
0.9 | Ciloglu et al. (2016) |
| Leucocytozoonc | PARUS4 | KJ488615 | 11 | 99–100 |
P. major (10) C. caeruleus (1) |
4.9 | Mata et al. (2015) |
| Leucocytozoon | PARUS7 | KJ488817 | 3 | 97–100 | P. major (1) S. europaea (2) | 1.3 | Mata et al. (2015) |
| Leucocytozoon | PARUS11 | HM234019 | 5 | 99–100 | C. caeruleus (5) | 2.2 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS12 | HM234020 | 3 | 100 | C. caeruleus (3) | 1.3 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS13 | HM234021 | 2 | 100 | C. caeruleus (2) | 0.8 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS14 | HM234022 | 15 | 100 | C. caeruleus (15) | 6.7 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS15 | HM234023 | 1 | 100 | C. caeruleus (1) | 0.4 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS16 | HM234024 | 14 | 100 | P.major (14) | 6.2 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS18 | HM234026 | 6 | 99–100 |
C. caeruleus (2) P. major (4) |
2.7 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS19 | HM234027 | 67 | 99–100 |
C. caeruleus (13) P. major (53) P. ater (1) |
29.8 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS20 | KJ488629 | 4 | 99–100 |
P. major (3) S. europaea (1) |
1.8 | Mata et al. (2015) |
| Leucocytozoon | PARUS22 | HM234031 | 2 | 100 |
P. major (1) C. caeruleus (1) |
0.9 | Jenkins & Owens (2011) |
| Leucocytozoon | PARUS33 | JX867108 | 2 | 100 | P. major (2) | 0.9 | Van Rooyen et al. (2013b) |
| Leucocytozoon | PARUS34 | JX855049 | 2 | 100 | P. major (2) | 0.9 | Van Rooyen et al. (2013b) |
| Leucocytozoon | PARUS72 | KJ488759 | 3 | 99 | P. major (3) | 1.3 | Mata et al. (2015) |
| Leucocytozoon | PARUS74 | KJ488766 | 9 | 100 |
P. major (6) C. caeruleus (3) |
4.0 | Mata et al. (2015) |
| Leucocytozoon | PARUS84 | KJ488911 | 6 | 100 | C. caeruleus (6) | 2.7 | Mata et al. (2015) |
| Leucocytozoon | CYACAE02 | MH758695 | 1 | 100 | C. caeruleus (1) | 0.4 | New lineage |
| Leucocytozoon | CYACAE03 | MH758696 | 3 | 98–100 |
P. major (1) C. caeruleus (2) |
1.3 | New lineage |
Notes.
Percentage of each lineage among all infected birds (n = 225).
One sample could be determined as Haemoproteus spp., but could not be assigned to one certain lineage by BLAST against the MalAvi database due to an insufficient sequence quality.
10 samples could be determined as Leucocytozoon spp., but could not be assigned to one certain lineage by BLAST against the MalAvi database due to an insufficient sequence length and quality.
The lineage diversity of the other four species was less pronounced probably due to smaller sample sizes. For pied flycatchers we detected one Haemoproteus lineage (PFC1). For tree sparrows we found three lineages (1 Haemoproteus: PADOM03 and 2 Plasmodium: GRW11 and SGS1), for coal tits one lineage each for Plasmodium (TURDUS1) and Leucocytozoon (PARUS19) and for nuthatches one Haemoproteus (PARUS1) and two Leucocytozoon lineages (PARUS7 and PARUS20).
Several sequences (n = 11) could not be assigned to a single reference sequence due to an insufficient sequence length or quality. In these cases, the sequences were determined to the closest related reference lineages (Table S1).
Host specificity
Maximally three out of six Passeriformes species were found infected with the same lineage (Table 4). We recorded the highest number of lineages occurring in several host species for Plasmodium. According to the BLAST results, all identified Plasmodium lineages occurred in more than one species (Table 4). In contrast, smaller percentages of lineages infecting more than one host species, were found for Leucocytozoon (36.8% of the lineages infected two host species and only 5.3% (one lineage: PARUS19) infected three host species) and Haemoproteus with 25% (only one lineage (PARUS1) infected more than one host species) (Table 4). The difference in the percentage of interspecific shared lineages was not significant between the three Haemosporida genera (Pearson’s Chi2-test: χ2 = 6.82, df = 4, p = 0.15).
Lineage networks
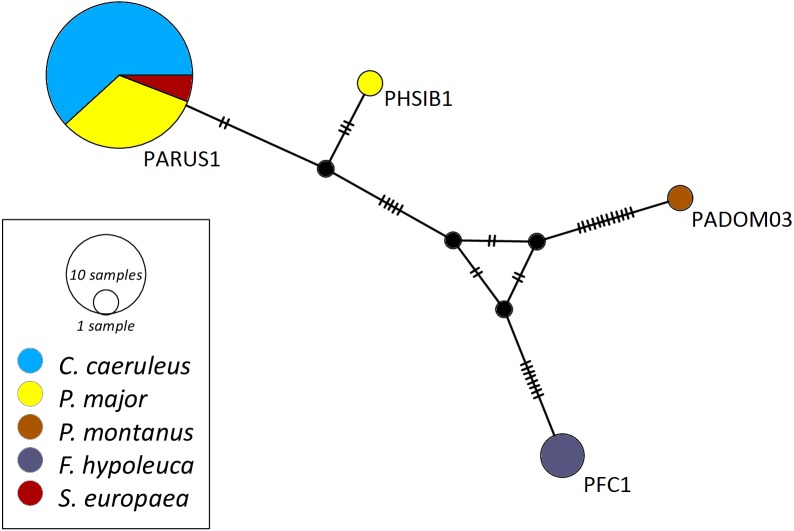
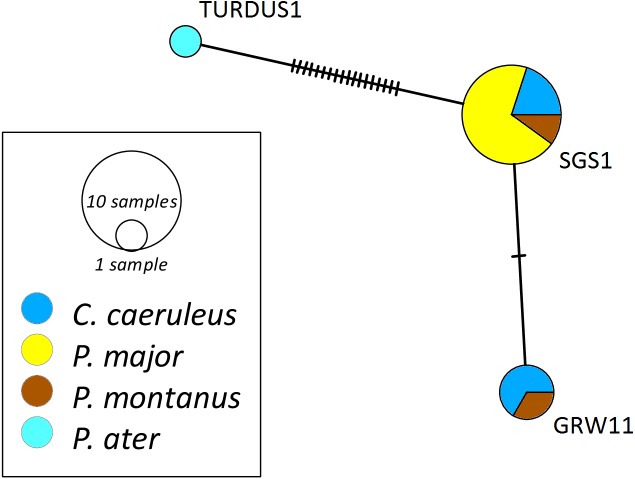
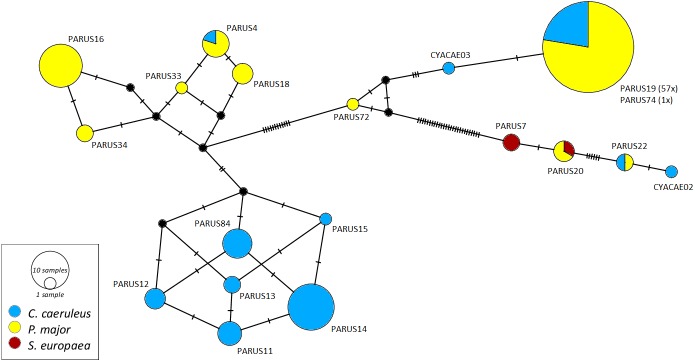
The networks for each haemosporidian genus revealed the occurrence of four lineages for Haemoproteus (Fig. 1), three for Plasmodium (Fig. 2) and 18 for Leucocytozoon (Fig. 3). In the Leucocytozoon network sequences from the samples NK17_P01 (C. caeruleus) and NK17_Y40 (C. caeruleus) occurred each separately from all the other sequences and had a BLAST of maximum 99% match with lineages deposit in MalAvi, indicating a new lineage (named CYACAE02 and CYACAE03). The genetic divergence to the closest MalAvi match of CYACAE02 (sample NK17_P01) is 0.21% (1 nucleotide difference to PARUS22, HM234031). The difference of CYACAE03 (sample NK17_Y40) to its closest related match PARUS19 (99% match in 477 bp) is 0.21%. The genetic divergence for the Leucocytozoon lineages in the network is 0.21–7.95%. All found lineages of the dataset are separated at least by one mutation (equivalent to one hatch mark in the network). With the exception of PARUS19 and PARUS74 (Fig. 3). The mutation differentiating these lineages is located in the cytochrome b gene before the fragment we used for network construction.
Figure 1. Median-joining network of mitochondrial cytochrome b gene lineages of Haemoproteus spp. (n = 39, 463 bp fragment).
Circles represent lineages, and the circle sizes are proportional to the lineage frequencies in the population. One hatch mark represents one mutation. Sampled host species are represented by different colors. Lineage names are noted at the associated circles.
Figure 2. Median-joining network of mitochondrial cytochrome b gene lineages of Plasmodium spp. (n = 14, 440 bp fragment).
Circles represent lineages, and the circle sizes are proportional to the lineage frequencies in the population. One hatch mark represents one mutation. Sampled host species are represented by different colors. Lineage names are noted at the associated circles.
Figure 3. Median-joining network of mitochondrial cytochrome b gene lineages of Leucocytozoon spp. (n = 123, 478 bp fragment).
Circles represent lineages, and the circle sizes are proportional to the lineage frequencies in the population. One hatch mark represents one mutation. Sampled host species are represented by different colors. Lineage names are noted at the associated circles.
The Haemoproteus network (Fig. 1) shows that the lineage PFC1 (H. pallidus), occurring in all three positively tested pied flycatchers, and the lineage PADOM03, found in one tree sparrow, are clearly separated from the other Haemoproteus spp. lineages. Haemoproteus lineages have a genetic divergence of 1.08–5.18%. The Plasmodium network (Fig. 2) illustrates the three lineages (SGS1, GRW11 and TURDUS1) found in this study. The results of this network lead to the assumption that TURDUS1 exclusively infects coal tits. However, it must be noted that also a blue tit was infected with TURDUS1, but the sample sequence was too short to be used for the alignment. Plasmodium lineages in the network range from 0.23–4.32% in their genetic divergence.
Discussion
Prevalence in nestling birds
Contrary to a high percentage of adult blue and great tits infected with at least one haemosporidian parasite we did not detect a single haemosporidian or T. avium infection in nestling blue tits and a very low infection rate of Leucocytozoon spp. in great tit nestlings. Generally, nestlings should be highly susceptible to vector-borne infection diseases due to their confinement to the nest, nakedness and immunological naivety during nestling period (Baker, 1975). Possibly, more nestlings might have been infected in our study, but our methodology did not accomplish detection if the disease was still at the prepatent stage. Infections cannot be detected immediately after transmission because of the prepatency period (i.e., the period between initial infection and the release of gametocytes into the peripheral blood). We can distinguish between parasites with shorter prepatency periods like Leucocytozoon (5 to 6 days) (Desser & Bennett, 1993) or T. avium (24 to 48 h) (Bennett, 1970), and with longer prepatency periods like Haemoproteus or Plasmodium. The period for Haemoproteus spp. varies from 11 to 21 days, for Plasmodium it can last from few days up to more than one month until an infection is detectable in the peripheral blood, depending on host and parasite species (Valkiūnas, 2005; Cosgrove et al., 2006). Alternatively, it could simply mean that the nestlings had not been infected yet.
To test if an infection was transmitted already, it would have been necessary to remove nestlings from their nests, raising them in vector-free cages and checking regularly for subsequent development of patent infections. Valkiūnas (2005) applied this approach in chaffinch (Fringilla coelebs) nestlings. In his study, only two out of 67 chicks (3%), removed from the nest at 6 to 12 days of age, subsequently developed infections. Contrary, the infection rate of 25 to 50 day old wild fledglings was 36.2%, suggesting that most infections occurred after the nestlings had left the nest (Valkiūnas, 2005). The very low rate of infections in nestling tits in our study might also result from a lack of vector activity during the nestling period as in northern temperate climes dipteran vector populations reach their peak not until late summer (Beaudoin et al., 1971).
Prevalence in adult birds
Within Germany, only a few studies deal with the prevalence and distribution of infections with avian haemosporidian parasites (microscopic examination: Haberkorn, 1984; Krone et al., 2001; PCR-based methods: Wiersch et al., 2007; Jenkins & Owens, 2011; Santiago-Alarcon et al., 2016) (see Table 5 for an overview of prevalences in great and blue tits sampled in Germany). Wiersch et al. (2007) sampled birds in the northern part of Germany. Infection prevalences in great tits were 30.4% for Haemoproteus and 46.4% for Plasmodium. In line with the present study, coal tits had no infections with Haemoproteus spp. and a similar infection rate with Plasmodium spp. (18.7%). The infection rate for pied flycatchers with Haemoproteus spp. (0.9%) was less than in the present study (21.4%). In contrast to our findings, Wiersch et al. (2007) reported Plasmodium infections in pied flycatchers (5.9%). Santiago-Alarcon et al. (2016) found no haemosporidian infection in eurasian nuthatches in Germany (20.0% Haemoproteus spp. and 30.0% Leucocytozoon spp. in the present study) and Haberkorn (1984) found no haemosporidian infections in coal tits (25.0% Plasmodium spp. and 25.0% Leucocytozoon spp. in this study). As far as we know, no other data are available for european tree sparrow infection rates in Germany.
Table 5. Overview of publications dealing with Haemosporida and Trypanosoma avium prevalences in great and blue tits sampled in Germany.
Research method, sample sizes, study region and prevalences of the different blood parasites are given (P, Plasmodium spp.; H, Haemoproteus spp.; L, Leucocytozoon spp.; T, Trypanosoma avium). NT, Not tested in the listed study.
| Reference | Study region | Method | Species (sample size) | Prevalence P | Prevalence H | Prevalence L | Prevalence T |
|---|---|---|---|---|---|---|---|
| Haberkorn (1984) | Western and northern Germany | Microscopic examination | P. major (82) | 2.4% | 8.5% | 1.2% | 0.0% |
| C. caeruleus (66) | 0.0% | 7.6% | 1.5% | 0.0% | |||
| Wiersch et al. (2007) | Northern Germany | PCR-based | P. major (56) | 46.4% | 30.4% | NT | NT |
| Jenkins & Owens (2011) | Southern Germany | PCR-based | P. major (33) | NT | NT | 33.3% | NT |
| C. caeruleus (24) | NT | NT | 41.6% | NT | |||
| Santiago-Alarcon et al. (2016) | Southwestern Germany | PCR-based | P. major (43) | 46.5%a | NT | ||
| C. caeruleus (6) | 0.0%a | NT | |||||
| Present study | Central Germany | PCR-based | P. major (142)b | 21.9% | 40.6% | 94.8% | 0.0% |
| C. caeruleus (58) | 10.3% | 36.2% | 77.5% | 0.0% |
Notes.
Only overall prevalence for the three Haemosprida was given in the publication (20 from 43 great tits were infected); no prevalences for the single genera were mentioned.
For Haemoproteus spp. and Plasmodium spp. the sample size was 32 and for Trypanosoma avium 68 adult great tits.
In contrast to our results, low infection rates in blood smears from great tits were reported by Haberkorn (1984) (Haemoproteus spp.: 7.3%, Leucocytozoon spp.: 1.2%). These variations in prevalences might be based on methodological differences.
Jenkins & Owens (2011) recorded 33% Leucocytozoon prevalence in South Germany for great tits and 50% for blue tits, which is much lower than the prevalences reported here for the two species (77.3% and 94.8%, respectively). However, some studies report even higher prevalences especially for the avian malaria pathogen Plasmodium (e.g., 91% for blue tits from Switzerland, Glaizot et al., 2012; 100% in a blue tits population in England, Szollosi et al., 2011) and for T. avium (e.g., 49% of infected blue tits in Spain, Fargallo & Merino, 2004; 40% in female blue tits in Spain, Tomás et al., 2007b).
However, it should be considered that sensitive PCR-based diagnostics are able to detect sporozoites of Leucocytozoon in the peripheral blood (Valkiūnas et al., 2009). Sporozoites are transmitted to the bird during the blood meal of the vector fly. As it is unclear whether all of these sporozoites result in an actual infection of the host, the evidence of haemosporidian lineages by PCR based method does not necessarily allow the conclusion that the parasites complete their entire life cycle in the host (Valkiūnas et al., 2009).
The high prevalence of Leucocytozoon spp. in blue and great tits in this study compared to the parasite genera Plasmodium and Haemoproteus may be associated with the vector abundance and behavior. Dipteran vectors of some Haemosporida genera may be more strictly ornithophilic than Culicidae vectors of Plasmodium spp., which feed on a broader range of vertebrates, reducing their potential for transmitting diseases to birds (Savage et al., 2009). The reasons for the high Leucocytozoon prevalence in our study area Hesse compared to other parts of Germany are speculative. One reason might be that Hesse is the most richly forested of all German states (42% of the state area are forests) (Forest report Hesse, 2015). Moreover, these forest sites are mostly near-natural and with a lot of woodland-running-waters, that are mostly (70%) in a good ecological condition (Forest report Hesse, 2015). As Simuliidae, the vectors of all Leucocytozoon species (except L. caulleryi, that is vectored by a biting midge; Lotta et al., 2016), need running waters for reproduction (Lacey & Merritt, 2003) the conditions for their reproduction in Hesse are good according to the habitat parameters mentioned above. However, we did not record any habitat parameters during our study and similarly no nationwide data throughout Germany on black fly distribution is available at the moment. Therefore, it is not possible to test regional prevalence of Leucocytozoon depending on the distribution and density of black flies so far.
Unfortunately, many studies do not consider all three avian haemosporidian genera and especially Leucocytozoon is underrepresented in the literature (Van Rooyen et al., 2013a). Hence, a detailed comparison of prevalences in different avian hosts is difficult to assess. However, comparison of our results with studies from Germany and Europe show that the prevalences of avian haemosporidian infections differ among local bird populations (e.g., Haberkorn, 1984; Wiersch et al., 2007; Santiago-Alarcon et al., 2016). The factors causing these local differences are still poorly understood. Szollosi et al. (2011) showed that the distribution and prevalence of avian malarial parasite species are influenced by multiple factors, such as host and dipteran vector density, habitat characteristics or climatic conditions (see also Wood et al., 2007; Merino et al., 2008). Moreover, prevalence seems to be a lineage-specific trait (Szollosi et al., 2011). That shows the importance to investigate not only prevalence for the parasite genera, but rather identify the different parasite lineages infecting regional bird populations.
Lineage diversity and host specificity
By using molecular phylogeny, we detected different lineages for each parasite genus. Few lineages differed by only one nucleotide, resulting in low genetic divergences. Other authors (e.g., Bensch et al., 2000; Chagas et al., 2017) also found low sequence divergences in Haemosporida. In this study, the two new Leucocytozoon lineages (CYACAE02 and CYACAE03) differ in one nucleotide each from their closest matching lineage, indicating they may have diverged only recently (Bensch et al., 2000).
The lineage PFC1 (H. pallidus), infecting only pied flycatchers in this study, is clearly separated in the network. It is possible that the infection with this lineage was transmitted outside Germany, as pied flycatchers, wintering in Africa, are the only long-distance migratory bird species in our study and infection with the PFC1 lineage might be vectored from dipteran vectors in Africa. However, the distribution of vectors on wintering and breeding grounds, especially for Haemoproteus, is poorly understood (Dubiec et al., 2017) and transmission of PFC1 lineage possibly also occurs in Europe (Jones, 2017).
Generally, host specificity of haemosporidian lineages differs among the genera with host specialists being predominant among Haemoproteus and Leucocytozoon lineages but being absent among Plasmodium lineages (Mata et al., 2015). This general pattern of host specificity, with Haemoproteus being the most host specialized and Plasmodium being more host generalized, is supported by several studies (e.g., Ricklefs & Fallon, 2002; Waldenström et al., 2002; Križanauskiene et al., 2006; Dimitrov, Zehtindjiev & Bensch, 2010; Jenkins & Owens, 2011; Drovetski et al., 2014; Okanga et al., 2014; Mata et al., 2015; Ciloglu et al., 2016). Less studies regarding host specificity of Leucocytozoon lineages exist, therefore it is necessary to do further investigations to confirm Leucocytozoon lineages to be, as assumed, host-specific mostly at avian order level and in some cases even on species level (Forrester & Greiner, 2008; Ciloglu et al., 2016). In the present study, we found no significant difference in the host specificity of the three haemosporidian genera. But due to small sample sizes and closely related host species (all within the order Passeriformes) general patterns should be proven with a higher number of samples and an increased range of species.
Our data complements existing knowledge about host specificity and distribution of some individual lineages as we obtained first records for lineages infecting a specific host species. The two Leucocytozoon lineages PARUS20 (isolated from sample NK17_C05, MH758693) and PARUS7 (isolated from samples NK17_C04, MH758692 and NK17_C14, MH758694) were not detected in eurasian nuthatches (S. europaea) prior to this study (Bensch, Hellgren & Perez-Tris, 2009; MalAvi, 2018). This is also the first record of the PARUS7 lineage in the family Sittidae. Host specificity seems not to be determined by parasite genera but by the single lineages comparable with the lineage-specific prevalences. For example, several studies (e.g., Ricklefs & Fallon, 2002; Beadell et al., 2009; Loiseau et al., 2012) suggest that few lineages of avian malaria pathogen Plasmodium exhibit extreme generalization, whereas other lineages seem to be constrained to certain host families or even host species. Host shifts are often associated with a change in pathogen virulence (Toft & Krater, 1990). Therefore, invading a new host may increase or decrease parasite virulence (Bull, 1994). This might be the case for the two Leucocytozoon lineages (PARUS20 and PARUS7) infecting nuthatches.
Conclusion
In summary, we found avian malaria and avian malaria-like pathogens of three genera (Plasmodium, Haemoproteus and Leucocytozoon) infecting common Passeriformes in Central Germany. The findings presented here provide knowledge about the distribution and prevalence of avian haemosporidian parasites in a geographic region, which has not yet been subject to studies investigating this kind of parasites. On the basis of a relatively small sample size we found numerous lineages and detected several first records of lineage infections as well as two new Leucocytozoon lineages. Comparison with studies from other parts of Germany pointed out regional differences in Haemosporida prevalence, in particular for Leucocytozoon. Understanding these patterns resulting in regional differences could be important in future to understand the epidemiology of blood parasites in wild bird populations.
Supplemental Information
Acknowledgments
We would like to thank Wendy Gibson (School of Biological Sciences, University of Bristol) and Rasa Bernotienė (Nature Research Center, Vilnius) for trypanosome infected bird samples used as positive controls. We are grateful to academic editor Erika Braga and three anonymous reviewers for helpful comments on the manuscript. We thank all helpers of the nest box checks in 2015 (Jennifer Schwarz, David Ensslin and Carsten Hoth) and 2017/18 (Anna Bentele, Fabian Gausepohl, Benjamin Grünwald, Daniel Höhn and Michael Reis) and Tobias Warmann for labwork assistance.
Funding Statement
There was no external funding for this project.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yvonne R. Schumm conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Christine Wecker, Carina Marek and Anna Bentele performed the experiments, analyzed the data, approved the final draft.
Mareike Wassmuth performed the experiments, approved the final draft.
Hermann Willems performed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Gerald Reiner conceived and designed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Petra Quillfeldt conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All sampling was performed in accordance to animal welfare standards, supervised by the Animal welfare officer of the University of Giessen and the Regierungspräsidium Giessen. The German equivalent to an IACUC number is the Intern number of the University: 662_GP and 828_GP.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Bird capture and sampling were carried out under a license from Regierungspräsidium Giessen: GI 15/8-Nr.109/2012 and GI 15/8-Nr.77/2016.
Data Availability
The following information was supplied regarding data availability:
Newly generated sequences are available in GenBank: MH758692, MH758693, MH758694, MH758695 and MH758696. The Haemoproteus and Plasmodium sequences are available in Table 4. The hosts and sites table is available at the MalAvi database: http://mbio-serv2.mbioekol.lu.se/Malavi/.
References
- Baker (1975).Baker JR. Epizootiology of some haematozoic protozoa of English birds. Journal of Natural History. 1975;9:601–609. doi: 10.1080/00222937500770491. [DOI] [Google Scholar]
- Bandelt, Forster & Röhl (1999).Bandelt H, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- Beadell et al. (2009).Beadell JS, Covas R, Gebhard C, Ishtiaq F, Melo M, Schmidt BK, Perkins S, Graves GR, Fleischer R. Host associations and evolutionary relationships of avian blood parasites from West Africa. International Journal for Parasitology. 2009;39:257–266. doi: 10.1016/j.ijpara.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadell et al. (2004).Beadell JS, Gering E, Austin J, Dumbacher JP, Peirce MA, Pratt TK, Atkinson CT, Fleischer RC. Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Molecular Ecology. 2004;13:3829–3844. doi: 10.1111/j.1365-294X.2004.02363.x. [DOI] [PubMed] [Google Scholar]
- Beaudoin et al. (1971).Beaudoin RL, Applegate JE, David DE, Mclean RG. A model for the ecology of avian malaria. Journal of Wildlife Diseases. 1971;7:5–13. doi: 10.7589/0090-3558-7.1.5. [DOI] [PubMed] [Google Scholar]
- Bennett (1970).Bennett GF. Trypanosoma avium Danilewsky in the avian host. Canadian Journal of Zoology. 1970;48:803–807. doi: 10.1139/z70-140. [DOI] [Google Scholar]
- Bennett, Bishop & Peirce (1993).Bennett GF, Bishop MA, Peirce MA. Checklist of the avian species of Plasmodium Marchiafava and Celli, 1885 (Apicomplexa) and their distribution by avian family and Wallacean life zones. Systematic Parasitology. 1993;26:171–179. doi: 10.1007/BF00009724. [DOI] [Google Scholar]
- Bennett, Earle & Squires-Parsons (1994).Bennett GF, Earle RA, Squires-Parsons D. Trypanosomes of some sub-Saharan birds. Onderstepoort Journal of Veterinary Research. 1994;61:263–271. [PubMed] [Google Scholar]
- Bennett, Peirce & Ashford (1993).Bennett GF, Peirce MA, Ashford RW. Avian haematozoa: mortality and pathogenicity. Journal of Natural History. 1993;27:993–1001. doi: 10.1080/00222939300770621. [DOI] [Google Scholar]
- Bensch et al. (2013).Bensch S, Hellgren O, Križanauskiené A, Palinauskas V, Valkiūnas G, Outlaw D, Ricklefs RE. How can we determine the molecular clock of malaria parasites? Trends in Parasitology. 2013;29:363–369. doi: 10.1016/j.pt.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Bensch, Hellgren & Perez-Tris (2009).Bensch S, Hellgren O, Perez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Bensch et al. (2004).Bensch S, Perez-Tris J, Waldstrom J, Hellgren O. Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution. 2004;58:1617–1621. doi: 10.1111/j.0014-3820.2004.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Bensch et al. (2000).Bensch S, Stjernman M, Hasselquist H, Ostman O, Hansson B, Westerdahl H, Torres Pinheiro R. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proceedings of the Royal Society of London Series B: Biological Sciences. 2000;267:1583–1589. doi: 10.1098/rspb.2000.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno et al. (2010).Bueno MG, Lopez RP, de Menezes RM, Costa-Nascimento MdeJ, Lima GF, Araujo RA, Guida FJ, Kirchgatter K. Identification of Plasmodium relictum causing mortality in penguins (Spheniscus magellanicus) from Sao Paulo Zoo, Brazil. Veterinary Parasitology. 2010;173:123–127. doi: 10.1016/j.vetpar.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Bull (1994).Bull JJ. Perspective-virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Chagas et al. (2017).Chagas CRF, Valkiūnas G, Guimarães LDO, Monteiro EF, Guida FJV, Simões RF, Rodrigues PT, Luna EJDA, Kirchgatter K. Diversity and distribution of avian malaria and related haemosporidian parasites in captive birds from a Brazilian megalopolis. Malaria Journal. 2017;16:83. doi: 10.1186/s12936-017-1729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciloglu et al. (2016).Ciloglu A, Yildirim A, Duzlu O, Onder Z, Dogan Z, Inci A. Investigation of avian haemosporidian parasites from raptor birds in Turkey, with molecular characterisation and microscopic confirmation. Folia Parasitologica. 2016;63:023. doi: 10.14411/fp.2016.023. [DOI] [PubMed] [Google Scholar]
- Clark, Clegg & Lima (2014).Clark NJ, Clegg SM, Lima MR. A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): new insights from molecular data. International Journal for Parasitology. 2014;44:329–338. doi: 10.1016/j.ijpara.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Cosgrove et al. (2006).Cosgrove CL, Knowles SCL, Day KP, Sheldon BC. No evidence for avian malaria infection during the nestling phase in a passerine bird. The Journal of Parasitology. 2006;92:1302–1304. doi: 10.1645/GE-878R.1. [DOI] [PubMed] [Google Scholar]
- Dawson & Bortolotti (2000).Dawson RD, Bortolotti GR. Effects of hematozoan parasites on condition and return rates of American Kestrels. The Auk: Ornithological Advances. 2000;117:373–380. doi: 10.1642/0004-8038(2000)117[0373:EOHPOC]2.0.CO;2. [DOI] [Google Scholar]
- Desser & Bennett (1993).Desser SS, Bennett GF. The genera Leucocytozoon, Haemoproteus and Hepatocystis. In: Kreier JP, editor. Parasitic Protozoa. Academic Press; San Diego: 1993. pp. 273–307. [Google Scholar]
- Dimitrov, Zehtindjiev & Bensch (2010).Dimitrov D, Zehtindjiev P, Bensch S. Genetic diversity of avian blood parasites in SE Europe: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Bulgaria. Acta Parasitologica. 2010;55:201–209. doi: 10.2478/s11686-010-0029-z. [DOI] [Google Scholar]
- Donovan et al. (2008).Donovan TA, Schrenzel M, Tucker TA, Pessier AP, Stalls IH. Hepatic hemorrhage, hemocoelom, and sudden death due to Haemoproteus infection in passerine birds: eleven cases. Journal of Veterinary Diagnostic Investigation. 2008;20:304–313. doi: 10.1177/104063870802000307. [DOI] [PubMed] [Google Scholar]
- Drovetski et al. (2014).Drovetski SV, Aghayan SA, Mata VA, Lopes RJ, Mode NA, Harvey JA, Voelker G. Does the niche breadth or trade-off hypothesis explain the abundance-occupancy relationship in avian Haemosporidia? Molecular Ecology. 2014;23:3322–3329. doi: 10.1111/mec.12744. [DOI] [PubMed] [Google Scholar]
- Dubiec et al. (2017).Dubiec A, Podmokła E, Harnist I, Mazgajski TD. Haemoparasites of the pied flycatcher: inter-population variation in the prevalence and community composition. Parasitology. 2017;145:912–919. doi: 10.1017/S0031182017001913. [DOI] [PubMed] [Google Scholar]
- Dunn et al. (2017).Dunn JC, Stockdale JE, Bradford EL, McCubbin A, Morris AJ, Grice PV, Goodman SJ, Hamer KC. High rates of infection by blood parasites during the nestling phase in UK Columbids with notes on ecological associations. Parasitology. 2017;144:622–628. doi: 10.1017/S0031182016002274. [DOI] [PubMed] [Google Scholar]
- Ejiri et al. (2011).Ejiri H, Sato Y, Kim KS, Hara T, Tsuda Y, Imura T, Murata K, Yukawa M. Entomological study on transmission of avian malaria parasites in a zoological garden in Japan: bloodmeal identification and detection of avian malaria parasite DNA from blood-fed mosquitoes. Journal of Medical Entomology. 2011;48:600–607. doi: 10.1603/ME10197. [DOI] [PubMed] [Google Scholar]
- Fargallo & Merino (2004).Fargallo JA, Merino S. Clutch size and haemoparasite species richness in adult and nestling blue tits. Écoscience. 2004;11:168–174. doi: 10.1080/11956860.2004.11682821. [DOI] [Google Scholar]
- Forest report Hesse (2015).Forest report Hesse Regionaler Waldbericht Hessen 2015. 2015. Regionale PEFC-Arbeitsgruppe Hessen e.V. (eds.). https://pefc.de/media/filer_public/c2/2d/c22d6ce7-3bcb-4580-aee1-c465b85dd094/hessen_waldbericht_2015.pdf .
- Forrester & Greiner (2008).Forrester DJ, Greiner EC. Leucocytozoonosis. In: Atkinson CT, Thomas NJ, Hunter DB, editors. Parasitic diseases of wild birds. Blackwell Publishing; Ames: 2008. pp. 54–107. [Google Scholar]
- Freund et al. (2016).Freund D, Wheeler SS, Townsend AK, Boyce WM, Ernest HB, Cicero C, Sehgal RNM. Genetic sequence data reveals widespread sharing of Leucocytozoon lineages in corvids. Parasitology Research. 2016;115:3557–3565. doi: 10.1007/s00436-016-5121-3. [DOI] [PubMed] [Google Scholar]
- Glaizot et al. (2012).Glaizot O, Fumagalli L, Iritano K, Lalubin F, Van Rooyen J, Christe P. High prevalence and lineage diversity of Avian Malaria in wild populations of great tits (Parus major) and mosquitoes (Culex pipiens) PLOS ONE. 2012;7(4):e34964. doi: 10.1371/journal.pone.0034964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkorn (1984).Haberkorn A. Observations on malaria in European perching birds (Passeriformes) Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene Series A. 1984;256:288–295. [PubMed] [Google Scholar]
- Hall (1999).Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium. 1999;41:95–98. [Google Scholar]
- Hamilton, Gibson & Stevens (2007).Hamilton PB, Gibson WC, Stevens JR. Patterns of co-evolution between trypanosomes and their hosts deduced from ribosomal RNA and protein-coding gene phylogenies. Molecular Phylogenetics and Evolution. 2007;44:15–25. doi: 10.1016/j.ympev.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Hellgren, Waldenström & Bensch (2004).Hellgren O, Waldenström J, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. Journal of Parasitology. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- Hunter, Rohner & Currie (1997).Hunter DB, Rohner C, Currie DC. Mortality in fledgling great horned owls from black fly hematophaga and leucocytozoonosis. Journal of Wildlife Diseases. 1997;33:486–491. doi: 10.7589/0090-3558-33.3.486. [DOI] [PubMed] [Google Scholar]
- Jenkins & Owens (2011).Jenkins T, Owens IP. Biogeography of avian blood parasites (Leucocytozoon spp.) in two resident hosts across Europe: phylogeographic structuring or the abundance-occupancy relationship? Molecular Ecology. 2011;20:3910–3920. doi: 10.1111/j.1365-294X.2011.05221.x. [DOI] [PubMed] [Google Scholar]
- Jones (2017).Jones W. Parasitism and speciation in a changing world. Uppsala University, Swedenhttps://uu.diva-portal.org/smash/get/diva2:1088708/FULLTEXT01.pdf Introductory Research Essay (104) 2017
- Knowles, Palinauskas & Sheldon (2010).Knowles SCL, Palinauskas V, Sheldon BC. Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. Journal of Evolutionary Biology. 2010;23:557–569. doi: 10.1111/j.1420-9101.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- Križanauskiene et al. (2006).Križanauskiené A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiūnas G. Variation in host specificity between species of avian hemosporidian parasites: evidence from parasite morphology and cytochrome B gene sequences. Journal of Parasitology. 2006;92:1319–1324. doi: 10.1645/GE-873R.1. [DOI] [PubMed] [Google Scholar]
- Krone et al. (2001).Krone O, Priemer J, Streich J, Soemmer J, Langgemach T, Lessow O. Haemosporida of birds of prey and owls from Germany. Acta Protozoologica. 2001;40:281–289. [Google Scholar]
- Lacey & Merritt (2003).Lacey LA, Merritt RW. The safety of bacterial microbial agents used for black fly and mosquito control in aquatic environments. In: Hokkanen HMT, Hajek AE, editors. Enviromental impacts of microbial insecticides. Springer; Dordrecht: 2003. pp. 151–168. (Progress in biological control, volumne 1). [Google Scholar]
- Leigh & Bryant (2015).Leigh JW, Bryant D. PopART: full-feature software for haplotype network construction. Methods in Ecology and Evolution. 2015;6:1110–1116. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- Loiseau et al. (2012).Loiseau C, Harrigan RJ, Robert A, Bowie RCK, Thomassen HA, Smith TB, Sehgal RNM. Host and habitat specialization of avian malaria in Africa. Molecular Ecology. 2012;21:431–441. doi: 10.1111/j.1365-294X.2011.05341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta et al. (2016).Lotta AL, Pacheco MA, Escalante AA, Gonzalez AD, Mantilla JS, Moncada LI, Adler PH, Matta NE. Leucocytozoon diversity and possible vectors in the neotropical highlands of Colombia. Protist. 2016;167:185–204. doi: 10.1016/j.protis.2016.02.002. [DOI] [PubMed] [Google Scholar]
- MalAvi (2018).MalAvi 2018. [09 August 2018]. A database for avian haemosporidian parasites. Table: host and sites. http://mbio-serv2.mbioekol.lu.se/Malavi/
- Martinez et al. (2009).Martinez J, Martinez-de La Puente J, Herrero J, Del Cerro S, Lobato E, Aguilar JR, Vasquez RA, Merino S. A restriction site to differentiate Plasmodium and Haemoproteus infections in birds: on the inefficiency of general primers for detection of mixed infections. Parasitology. 2009;136:713–722. doi: 10.1017/S0031182009006118. [DOI] [PubMed] [Google Scholar]
- Martinez-de la Puente et al. (2013).Martinez-de la Puente J, Martinez J, Rivero-de Aguilar J, DelCerro S, Merino S. Vector abundance determines Trypanosoma prevalence in nestling blue tits. Parasitology. 2013;140:1009–1015. doi: 10.1017/S0031182013000371. [DOI] [PubMed] [Google Scholar]
- Marzal et al. (2005).Marzal A, De Lope F, Navarro C, Moller AP. Malarial parasites decrease reproductive success: an experimental study in a passerine bird. Oecologia. 2005;142:541–545. doi: 10.1007/s00442-004-1757-2. [DOI] [PubMed] [Google Scholar]
- Mata et al. (2015).Mata VA, Da Silva LP, Lopes RJ, Drovetski SV. The Strait of Gibraltar poses an effective barrier to host-specialised but not to host-generalised lineages of avian Haemosporidia. International Journal for Parasitology. 2015;45:711–719. doi: 10.1016/j.ijpara.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Medeiros, Hamer & Ricklefs (2013).Medeiros MCI, Hamer GL, Ricklefs RE. Host compatibility rather than vector–host-encounter rate determines the host range of avian Plasmodium parasites. Proceedings of the Royal Society B. 2013;280 doi: 10.1098/rspb.2012.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino et al. (2000).Merino S, Moreno J, Sanz JJ, Arriero E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus) Proceedings of the Royal Society B. 2000;267:2507–2510. doi: 10.1098/rspb.2000.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino et al. (2008).Merino S, Moreno J, Vásquez RA, Martínez J, Sánchez-Monsálvez I, Estades CF, Ippi S, Sabat P, Rozzi R, McGehee S. Haematozoa in forest birds from southern Chile: latitudinal gradients in prevalence and parasite lineage richness. Austral Ecology. 2008;33:329–340. doi: 10.1111/j.1442-9993.2008.01820.x. [DOI] [Google Scholar]
- Møller & Nielsen (2007).Møller AP, Nielsen JT. Malaria and risk of predation: a comparative study of birds. Ecology. 2007;88:871–881. doi: 10.1890/06-0747. [DOI] [PubMed] [Google Scholar]
- Okanga et al. (2014).Okanga SM, Cumming GS, Hockey PAR, Nupen L, Peters JL. Host speciation and co-speciation in avian hemosporidia in the Western Cape, South Africa. PLOS ONE. 2014;9(2):e86382. doi: 10.1371/journal.pone.0086382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Tris & Bensch (2005).Perez-Tris J, Bensch S. Dispersal increases local transmission of avian malarial parasites. Ecology Letters. 2005;8:838–845. doi: 10.1111/j.1461-0248.2005.00788.x. [DOI] [Google Scholar]
- R Core Team (2016).R Core Team . Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- Ricklefs & Fallon (2002).Ricklefs RE, Fallon SM. Diversification and host switching in avian malaria parasites. Proceedings of the Royal Society B. 2002;269:885–892. doi: 10.1098/rspb.2001.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Alarcon et al. (2016).Santiago-Alarcon D, MacGregor-Fors I, Kühnert K, Segelbacher G, Schaefer HM. Avian haemosporidian parasites in an urban forest and their relationship to bird size and abundance. Urban Ecosystems. 2016;19:331–346. doi: 10.1007/s11252-015-0494-0. [DOI] [Google Scholar]
- Santiago-Alarcon, Palinauskas & Schaefer (2012).Santiago-Alarcon D, Palinauskas V, Schaefer HM. Diptera vectors of avian haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biological Reviews of the Cambridge Philosophical Society. 2012;87:928–964. doi: 10.1111/j.1469-185X.2012.00234.x. [DOI] [PubMed] [Google Scholar]
- Savage et al. (2009).Savage AF, Robert V, Goodman SM, Raharimanga V, Raherilalao MJ, Andrianarimisa A, Ariey F, Greiner EC. Blood parasites in birds from Madagascar. Journal of Wildlife Diseases. 2009;45:907–920. doi: 10.7589/0090-3558-45.4.907. [DOI] [PubMed] [Google Scholar]
- Schmid et al. (2017).Schmid S, Fachet K, Dinkel A, Mackenstedt U, Woog F. Carrion crows (Corvus corone) of southwest Germany: important hosts for haemosporidian parasites. Malaria Journal. 2017;16:369. doi: 10.1186/s12936-017-2023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordato & Kardish (2014).Scordato ESC, Kardish MR. Prevalence and beta diversity in avian malaria communities: host species is a better predictor than geography. Journal of Animal Ecology. 2014;83:1387–1397. doi: 10.1111/1365-2656.12246. [DOI] [PubMed] [Google Scholar]
- Sehgal, Jones & Smith (2001).Sehgal RNM, Jones HI, Smith TB. Host specificity and incidence of Trypanosoma in some African rainforest birds: a molecular approach. Molecular Ecology. 2001;10:2319–2327. doi: 10.1046/j.1365-294X.2001.01339.x. [DOI] [PubMed] [Google Scholar]
- Sol, Jovani & Torres (2003).Sol D, Jovani R, Torres J. Parasite mediated mortality and host immune response explain age-related differences in blood parasitism in birds. Oecologia. 2003;135:542–547. doi: 10.1007/s00442-003-1223-6. [DOI] [PubMed] [Google Scholar]
- Szöllősi et al. (2011).Szöllősi E, Cichoń M, Eens M, Hasselquist D, Kempenaers B, Merino S, Nilsson JÅ, Rosivall B, Rytkönen S, Török J, Wood MJ, Garamszegi LZ. Determinants of distribution and prevalence of avian malaria in blue tit populations across Europe: separating host and parasite effects. Journal of Evolutionary Biology. 2011;24:2014–2024. doi: 10.1111/j.1420-9101.2011.02339.x. [DOI] [PubMed] [Google Scholar]
- Toft & Krater (1990).Toft CA, Krater AJ. Parasite-host coevolution. Trends in Ecology & Evolution. 1990;5:326–329. doi: 10.1016/0169-5347(90)90179-H. [DOI] [PubMed] [Google Scholar]
- Tomás et al. (2007b).Tomás G, Merino S, Moreno J, Morales M. Consequences of nest reuse for parasite burden and female health and condition in blue tits, Cyanistes caeruleus. Animal Behaviour. 2007b;73:805–814. doi: 10.1016/j.anbehav.2006.06.016. [DOI] [Google Scholar]
- Tomás et al. (2007a).Tomás G, Merino S, Moreno J, Morales J, Martìnez-de la Puente J. Impact of blood parasites on immunoglobulin level and parental effort: a medication field experiment on a wild passerine. Functional Ecology. 2007a;21:125–133. [Google Scholar]
- Valkiūnas (1996).Valkiūnas GA. Ecological implications of Hematozoa in birds. Bulletin for the Scandinavian Society for Parasitology. 1996;6:103–113. [Google Scholar]
- Valkiūnas (2005).Valkiūnas G. Avian malaria parasites and other Haemosporidia. CRC Press; Boca Raton: 2005. [Google Scholar]
- Valkiūnas et al. (2009).Valkiūnas G, Iezhova TA, Loisseau C, Sehgal RNM. Nested cythochrome b polymerase chain reaction diagnostics detect sporozoites of haemosporidian parasites in peripheral blood of naturally infected birds. Journal of Parasitology. 2009;95:1512–1515. doi: 10.1645/GE-2105.1. [DOI] [PubMed] [Google Scholar]
- Valkiūnas et al. (2013).Valkiūnas G, Palinauskas V, Križanauskiene A, Bernotiene R, Kazlauskiene R, Iezhova TA. Further observations on in vitro hybridization of hemosporidian parasites: patterns of ookinete development in Haemoproteus spp. Journal of Parasitology. 2013;99:124–136. doi: 10.1645/GE-3226.1. [DOI] [PubMed] [Google Scholar]
- Valkiūnas et al. (2006).Valkiūnas G, Zickus T, Shapoval AP, Iezhova TA. Effect of Haemoproteus belopolskyi (Haemosporida: Haemoproteidae) on body mass of the blackcap Sylvia atricapilla. Journal of Parasitology. 2006;92:1123–1125. doi: 10.1645/GE-3564-RN.1. [DOI] [PubMed] [Google Scholar]
- Van Rooyen et al. (2013a).Van Rooyen J, Lalubin F, Glaizot O, Christe P. Altitudinal variation in haemosporidian parasite distribution in great tit populations. Parasites & Vectors. 2013a;6:139. doi: 10.1186/1756-3305-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooyen et al. (2013b).Van Rooyen J, Lalubin F, Glaizot O, Christe P. Avian haemosporidian persistence and co-infection in great tits at the individual level. Malaria Journal. 2013b;12:40. doi: 10.1186/1475-2875-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstreels et al. (2014).Vanstreels RET, Miranda FR, Ruoppolo V, Almeida Reis AO de, Costa ES, Lira Pessôa AR de, Torres JPM, Cunha LSTda, Cruz Piuco R da, Valiati VH, González-Acuña D, Labruna MB, Petry MV, Epiphanio S, Catão-Dias JL. Investigation of blood parasites of pygoscelid penguins at the King George and Elephant Islands, South Shetlands Archipelago, Antarctica. Polar Biology. 2014;37:135–139. doi: 10.1007/s00300-013-1401-x. [DOI] [Google Scholar]
- Votypka et al. (2002).Votypka J, Obornik M, Volf P, Svobodova M. Trypanosoma avium of raptors (Falconiformes): phylogeny and identification of vectors. Parasitology. 2002;125:253–263. doi: 10.1017/s0031182002002093. [DOI] [PubMed] [Google Scholar]
- Waldenström et al. (2002).Waldenström J, Bensch S, Kiboi S, Hasselquist D, Ottosson U. Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Molecular Ecology. 2002;11:1545–1554. doi: 10.1046/j.1365-294X.2002.01523.x. [DOI] [PubMed] [Google Scholar]
- Wiersch et al. (2007).Wiersch SC, Lubjuhn T, Maier WA, Kampen H. Haemosporidian infection in passerine birds from Lower Saxony. Journal of Ornithology. 2007;148:17–24. doi: 10.1007/s10336-006-0094-0. [DOI] [Google Scholar]
- Wood et al. (2007).Wood MJ, Cosgrove CL, Wilkin TA, Knowles SC, Day KP, Sheldon BC. Within-population variation in prevalence and lineage distribution of avian malaria in blue tits, Cyanistes caeruleus. Journal of Animal Ecology. 2007;16:3263–3273. doi: 10.1111/j.1365-294X.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2000).Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology. 2000;7:203–214. doi: 10.1089/10665270050081478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- MalAvi 2018. [09 August 2018]. A database for avian haemosporidian parasites. Table: host and sites. http://mbio-serv2.mbioekol.lu.se/Malavi/
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Newly generated sequences are available in GenBank: MH758692, MH758693, MH758694, MH758695 and MH758696. The Haemoproteus and Plasmodium sequences are available in Table 4. The hosts and sites table is available at the MalAvi database: http://mbio-serv2.mbioekol.lu.se/Malavi/.