Abstract
In recent years, microbial degradation and bioremediation approaches of polychlorinated biphenyls (PCBs) have been studied extensively considering their toxicity, carcinogenicity and persistency potential in the environment. In this direction, different catabolic enzymes have been identified and reported for biodegradation of different PCB congeners along with optimization of biological processes. A genome analysis of PCB-degrading bacteria has led in an improved understanding of their metabolic potential and adaptation to stressful conditions. However, many stones in this area are left unturned. For example, role and diversity of uncultivable microbes in PCBs degradation is still not fully understood. Improved knowledge and understanding on this front will open up new avenues for improved bioremediation technologies which will bring economic, environmental and societal benefits. This article highlights on recent advances in bioremediation of PCBs in soil. It is demonstrated that bioremediation is the most effective and innovative technology which includes biostimulation, bioaugmentation, phytoremediation, rhizoremediation etc. and acts as a model solution for pollution abatement. More recently, transgenic plants and genetically modified microorganisms have proved to be revolutionary in the bioremediation of PCBs. Additionally, other important and aspects such as pretreatment using chemical/physical agents for enhanced biodegradation are also addressed. Efforts have been made to identify challenges, research gaps and necessary approaches which in future can be harnessed for successful use of bioremediation under field conditions. Emphases have been given on the quality/efficiency of bioremediation technology and its related cost which determines its ultimate acceptability.
Keywords: Polychlorinated biphenyls (PCBs), Dechlorination, Bioremediation, Pre-treatment, Plant-microbe interaction, Genetically modified organisms (GMOs), Phytoremediation
Introduction
Increased industrialization in developed/ developing countries has resulted in many types of pollutants contaminating the environment over recent decades. Anthropogenic activities have introduced numerous xenobiotic hydrophobic organic compounds into the natural environment. Many of these contaminants have multiple toxic and mutagenic effects on human health as well as environment. Polychlorinated biphenyls are among one such group of notorious contaminant. PCBs are member of chlorinated organic chemicals which may theoretically contain 209 different congeners. These congeners are formed with different number and position of chlorine atoms on the biphenyl ring. (Bedard et al. 2003; Piper 2005; Tu et al. 2011; Passatore et al. 2014). However, only 60–90 congeners have actually been reported in commercial chemical PCB formulations (Wiegel and Wu, 2000; Field and Sierra-Alvarez, 2008). Highly chlorinated congeners are more stable and tend to have lower solubility in aqueous solution and also have higher octanol water partition coefficients (KOW) than low molecular weight PCBs (Hawker and Connell 1988). The high KOW is partly responsible for their persistence and enables them to strongly absorb in the soil (Wiegel and Wu 2000; Passatore et al. 2014). The semi-volatility is the reason of their spread throughout the environment according to the “grasshopper effect” which bioaccumulate, volatilize in warmer conditions and deposits in colder climates (Gomes et al. 2013). They are persistently showing their presence in the list of 10 top most toxic priority pollutants of the United States Agency for Toxic Substances and Disease Registry (ATSDR) (Agency for Toxic Substances and Disease Registry 2011; Meggo and Schnoor 2013). Considering the environmental and ecological impacts of PCBs, an international chemical treaty i.e. Stockholm Convention has listed them among the priority persistent organic pollutants (POPs) which also documents that the PCBs must be eliminated from the environment by the year 2025 (Egorova et al. 2013). Commercially, PCBs were sold under several trade names at various parts of the globe, e.g. Aroclor (Monsanto, USA), Santotherm (Mitsubishi, Japan), Clophen (Bayer, Germany), Phenoclor and Pyralene (Prodolec, France) etc. on the basis of percent of chlorine (by weight).
It is reported that between 1920 and early 1980s approximately 1.5 million tons of PCBs were manufactured throughout the globe and as a result of which a significant amount of PCBs has been released in the environment (Piper 2005; Sharma et al. 2014). Due to their extensive use in past and lack of appropriate disposal technologies, PCBs and their congeners have attained ubiquitous distribution from the Arctic to the Antarctic. Although, the extent of global PCB contamination is still to be studied, Holoubek (2000) made an attempt to examine sites contaminated with PCBs throughout the world and provided detailed information with respect to its production, import/export, fate, contaminated sites and management approaches. However, inventorization of sites contaminated with PCBs has to be carried out by each signatory country to Stockholm Convention which is to be documented under their respective national implementation plans (NIP) POPs.
According to a report of the USEPA (2011), 350 sites are contaminated with PCB in the United States of America, while 148 sites in Canada are contaminated with PCBs as per Federal Contaminated Sites Inventory (TBS-SCT, 2011). Meijer et al. (2003) estimated global soil total PCB burden of 21,000 tons in an inventory on atmospheric deposition in background surface soil. The threshold concentration for contaminated soil varies between 10 and 50 mg kg−1 in some countries, while in some countries it may be as low as 0.5 mg kg−1 (CCME 1999; EPA 2009; UKEPA 2004; USEPA 2012).
PCBs enter the environment during their production; due to accidental spills and leaks; and during transportation, use, disposal etc. PCBs own “dioxin-like toxicity” which makes it probable carcinogen due to this reason (Baars et al. 2004). Some PCB congeners posses dioxin-like activity with associated toxicity. PCBs have recently been categorized in carcinogen class I by the International Agency for Research on Cancer (IARC) (Lauby-Secretain et al. 2013). Initially toxicity testing was restricted to animals only which with time was extrapolated to humans also. Three decades ago, Safe (1984) has reported development of hepatic tumors in rats due to exposure to PCBs. Reduced reproduction and mass mortality of sea birds is reported as a result of bioaccumulation of PCBs (O’Riordan, 1995; Borja et al. 2005). Several studies have reported a variety of exposure-related effects of PCBs in humans, including endocrine disruption, non-specific reproductive effects, dermal abnormalities including chloroacne and abnormal neurobehavioral effects in children (ASTDR 2000; Ross 2004). Serious health effects among approximately 14,000 people have been reported in Yusho, Japan after ingestion of PCB contaminated rice oil and the effects of which can still be observed (Gomes et al. 2013). PCBs also indirectly affect humans by entering the food chain through transfer by phytoplanktons to invertebrates, fish and mammals. Owing to these hazards to both humans and environment as a result of PCBs bioconcentration, it is necessary to deal with this toxic contaminant in an eco friendly and cost effective manner.
Remediation measures for PCBs
Due to their long persistence and deleterious environmental and health impacts, it is important to secure and decontaminate the polluted sites. This is highlighted in many in many national and international studies. To address this challenging problem, UNIDO has prepared a toolkit on investigation and management of sites contaminated with POPs (UNIDO Contaminated site toolkit 2010). Several methods have been reviewed and suggested for effective remediation/destruction of PCBs which include; landfilling, landfarming, incineration, thermal desorption, chemical dehalogenation, plasma arc, catalytic hydrogenation, ultrasonic technology, advanced oxidation processes etc (UNIDO 2000; Gomes et al. 2013). The selection of appropriate technology for remediation depends upon the socio economical & climatic conditions of a particular place and availability of particular technology plus concentration, volume and matrix of the PCB contamination along with any co-contaminant. Half life of PCBs varies with respect to different environmental conditions. Further, Weber (2007) has suggested a list of criteria for evaluation of PCBs/POPs destruction technologies which include a) applicability (target contaminants); b) overall cost reliability and maintenance safety; c) residuals produced (by-products: PCDD/PCDF, other POPs, other toxic compounds); d) minimum achievable concentration; e) public acceptability; f) development status; g) environmental impacts; h) performance dependency on site characteristics; i) clean-up time required; j) decontaminated soil quality and k) site data needed.
Several technologies listed earlier have their own advantages and disadvantages with respect to time required, efficiency & effectiveness, amount of waste/unintentional byproduct generated etc. Therefore, development of cost-effective and environmentally sound technology becomes urgent need of the time. In this context, bioremediation has incredible potential to satisfy the requirement and holds faith for protection of environmental and its management (Juwarkar et al. 2014). Bioremediation is an increasingly popular alternative to conventional methods for treating pollutants with the possibility to degrade contaminants, since it uses natural microbial activity mediated by different consortia of microbial strains. Many studies on bioremediation have been reported and the scientific literature has revealed the progressive emergence of various advances in bioremediation techniques (Vidali 2001; Juwarkar et al. 2010; Aken et al. 2010; Nanekar and Juwarkar, 2015).
The bioremediation process is divided in two techniques as ex situ and in situ. In situ techniques are preferred compared to ex situ techniques as they cut down the cost of excavation and also restrict the contaminant transfer by carrying out the remediation process at the site of contamination. In situ techniques comprise of both bioremediation (including microbial and fungal) and phytoremediation techniques which when clubbed can emerge as an effective weapon for eradication or breakdown of organic contaminants like PCBs. Remediation using fungal strains in some cases proved as a highly effective remediation approach (Kuba´tova´ et al. 2001; Juwarkar et al. 2014).
The super-hydrophobicity of PCBs makes its biodegradation a very difficult task. The rate and efficiency of biodegradation of PCBs can be increased using chemical reduction/oxidation process which modifies molecular structures that are resistant to biodegradation (Dercova et al. 1999; Baciocchi et al. 2005; Prządo et al. 2007). Chemical reduction/oxidation is a process in which hazardous contaminant is chemically converted into a non toxic or less hazardous compound resulting in more stable, less mobile and inert products (Li 2006). Chemical oxidation is an effective and innovative technology for degradation of a range of contaminants in which chemical oxidants are delivered to contaminated media either to destroy the contaminants or to convert them into easily biodegradable compounds (Goi et al. 2006). This process combined with biodegradation forms an emerging technique having a chemo-biological approach towards breakdown of PCBs and is briefed in this article.
Bioremediation: strategies and outline
Bioremediation is defined as breakdown of contaminant by biological mechanisms that include organisms in order to clean a contaminated site. Use of microorganisms capable of utilizing organic contaminant as a carbon source is the essence of bioremediation. Some microorganisms survive in the contaminated site by degrading the contaminant using an enzyme or cofactor during the oxidation or reduction of carbon containing complex organic compounds. Indeed, many congeners can be degraded by multiple pathways (Bedard et al. 2003; Laore et al. 2014). Additionally, rate of degradation strongly depends on nature of microbial population and their metabolic specificity which actually depends on number and position of chlorine atoms along with presence of electron donors (Weigel and Wu 2000). This intrinsic property of catabolism possessed by microorganisms favours their use in the bioremediation process. Ideally (Chávez et al. 2006) microbes can be used for bioremediation of PCBs contaminated sites if it posses properties such as; (a) PCBs tolerance, (b) surfactant(s) producing which increases bioavailability of PCBs, (c) chemotactic towards PCBs, (d) possess and expresses various dehalogenating enzymes responsible for dechlorination of PCBs (e) degrades PCBs without or with minimal generation of toxic intermediates, and (f) able to survive till the completion of cleanup process.
Further, it has been comprehensively explained by Wiegel and Wu (2000) that various environmental factors including temperature and pH affect the growth and variety of metabolic processes of different microorganisms and hence affect their ability of dechlorination of PCBs. Accordingly, a better understanding of whether and to what extent environmental factors are affecting is important and this knowledge will help in predicting potential for PCB degradation in soil and will support in developing PCB bioremediation plans. Isolation of these kinds of organisms and their augmentation at contaminated site ensures that the contaminant is degraded completely or transformed into a non-toxic compound.
Bioremediation technology can be effective in both aerobic and anaerobic environment which has been one of the reasons for its wide acceptance. Bioremediation may be either aerobic or anaerobic (Wiegel and Wu 2000; Juwarkar et al. 2014). Owing the problem associated with either of this method to treat highly complex compounds, sometime sequential anaerobic–aerobic bioremediation processes are also adopted to remediate contaminated sites (Master et al. 2002).
Anaerobic bioremediation
Anaerobic bioremediation is facilitated by anaerobic organisms which break chemical compounds in the soil to release energy required for their metabolic processes. Anaerobic bacteria respire by means of electron acceptors like sulfates and nitrates in uncontaminated soils. However, in case of PCB contaminated soils, they switch to dehalorespiration (May et al. 2008; Payne et al. 2011). Dehalorespiration is a process in which bacteria attack chlorine substituents in para and meta position, replacing them with hydrogen. The numbers of these bacteria are small or negligible in soil which explains the reduced natural anaerobic degradation of PCBs. The process of dehalorespiration transforms higher chlorinated congeners to less chlorinated congeners thus decreasing their toxicity and further making them available for aerobic degradation (Lehtinin 2010).
Biostimulation of anaerobic organisms
Biostimulation simply refers to a stimulation of the indigenous flora by providing optimum survival conditions. In case of soil, anaerobic organisms, creating anaerobic conditions can be stimulation. Several researchers in nineties have performed dechlorination of Aroclor congeners under anaerobic conditions and have found promising results and concluded that anaerobic bioremediation can act as the only way to breakdown highly chlorinated PCBs (Quensen III et al. 1990; Tiedje et al. 1993; Alexander 1999). For activation of these organisms, anaerobic conditions can be created by flooding of soil with water.
Microbial dechlorination of Aroclor 1260 was stimulated with the use of other halogenated aromatic compounds (De Weerd and Bedard 1999). Although, dechlorination of PCBs can be stimulated by using some non specific inducers such as fatty acids, alcohols, glucose, hydrogen and zero valent metals; but the final products formed are the same (Rysavy et al. 2005). Moreover, significant increase in microbial activity was found after the addition of defined minimal medium comprising of suitable nutrients and trace elements. The addition of FeSO4 to the Aroclor 1242 contaminated soils showed promising results in stimulation of dechlorination process for PCBs as it stimulated the growth of sulphate reducing microorganisms that were responsible for PCB dechlorination (Borja et al. 2005; Anyasi and Atagana 2013). It was also reported that the direct addition of Fe0 to contaminated sediments might significantly reduce the lag period before dechlorination (Rysavy et al. 2005). Concentration of sodium bicarbonate was also found to affect the dechlorination of 2,3,4,5-CB in sediments (Yan et al. 2006).
Bioaugmentation of anaerobic organisms
The term bioaugmentation stands for the addition/supplementation of microbial strains capable of degrading the pollutant at respective contaminated site. The success of the process generally depends on efficient and reliable microbes which are to be augmented with knowledge of their survival and metabolic activities. The availability of robust information on augmented population can provide insight for an effective management of the contaminant (Chi et al. 2013).
Laboratory as well as in situ reductive dechlorination of PCBs using anaerobic microorganisms has been demonstrated extensively. Bioaugmentation of granular anaerobic methanogenic microbial consortium along with a suitable carbon source was successfully demonstrated (Nollet et al. 2005). In this line, anaerobic dechlorination of PCBs has also been reported for various contaminated sites (Pakdeesusuk et al. 2005). Macedo et al. (2007) observed transformation of highly chlorinated PCB congeners as an effect of adaptation of microbial communities in contaminated soil. A group of microorganisms within the dechlorinating Chloroflexi that appear to be common in PCB contaminated sites may catalyse reductive dechlorination activity (Watts et al. 2005).
Moreover, several studies on dechlorination of Aroclors (1260 and 1254) contaminated soils demonstrated positive results, by priming the indigenous microorganisms in sediments with PCBs (Rysavy et al. 2005). Yan et al. (2006) found that chemistry and origin of contaminated soil considerably affected the activity of bioaugmented PCB degrading cultures. Certain macro and microelements are crucial in the process of dechlorination of PCBs in soil (Zeeb et al. 2006). Recently, it was showed that paddy field soils have the potential for anaerobic microbial degradation of a wide range of PCB congeners (Baba et al. 2007; Chen et al. 2014). However, it was also found from the biological data estimation (e.g., biomarkers of contamination, structure of the microbial community) that the results were comparatively similar in both biostimulated soils as well as in soils without addition of Dhc (Dehalococcoide) consortium indicating specialized PCB dechlorinators were not adopted to the harsh conditions (high PCB concentrations) existing in the contaminated soil (Matturro et al. 2016).
Aerobic bioremediation
Aerobic bioremediation makes use of microorganisms that uses atmospheric oxygen to perform breakdown of contaminant. The main approach towards encouraging aerobic degradation of PCBs has been via addition of oxygen, co-substrates, inducers, surfactants and sometimes bioaugmentation of PCB-degrading bacteria (Abraham et al. 2002; Ohtsubo et al. 2004; Field and Sierra-Alvarez 2008). Biphenyl, which is vital primary substrate that supports PCB co-metabolism, has been successfully employed to stimulate degradation of PCB contaminated soil aerobically (Field and Sierra-Alvarez, 2008). During aerobic degradation oxidative destruction of PCBs takes place involving several genes and their associated enzymes (Piper 2005; Field and Sierra-Alvarez 2008; Hashmi et al. 2016). These genes are mainly Bph gene clusters viz BphA, BphB, BphC, BphD, BphE, BphF, BphG which give rise to enzymes like BphB (dehydrogenase), BphC (ring-cleavage dioxygenase), BphD (hydrolase), BphE (hydratase), BphF (aldolase) and BphG (acetaldehyde dehydrogenase). These enzymes are major enzymes required in PCB degradation pathway as documented by Ohtsubo and coworkers (Ohtsubo et al. 2004). Figure 1 represents one of the most accepted and uncomplicated schematic representation of pathways of aerobic degradation of PCBs as described by Bedard (2003) and Pieper (2005). According to the process stated by Lehtinin (2010), bacteria first transform PCBs to chlorobenzoic acid (CBA) using biphenyl as a carbon and energy source. Furthermore, CBA degrading bacteria transform CBA to less toxic end products and aerobic degradation is known to breakdown lower chlorinated congeners.
Figure 1.
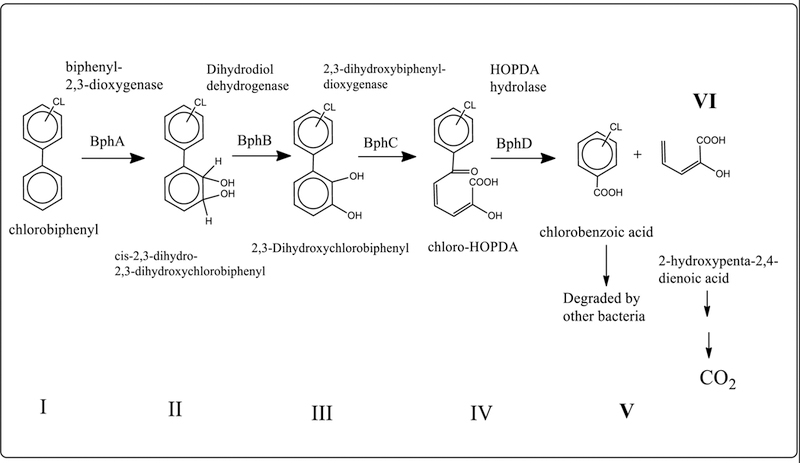
Pathway of aerobic PCB degradation by biphenyl oxidizing bacteria (Novakovaet al., 2002; Bedard, D.L., 2003; Pieper, D.H., 2005). (I) biphenyl, (II) 2; 3-dihydroxy-4-phenylhexa-4; 6-diene, (III) 2; 3-dihydroxybiphenyl, (IV) 2-hydroxy-6-oxo-6-phenylhea-2; 4-dienoic acid, (V) chlorobenzoic acid, (VI) 2-hydroxypenta-2; 4-dienoic acid. (bphA) biphenyl 2; 3-dioxygenase, (bphB) dihydrodiol dehydrogenase, (bphC) 2;3dihydroxybiphenyl 1; 2-dioxygenase, (bphD) 2-hydroxy-6-oxo-6-phenylhexa-2; 4-dienoate hydrolase.
Biostimulation of aerobic organisms
For biostimulation of PCB degradation, pH in neutral range, optimum salt concentrations, an adequate availability of macro- and microelements along with addition of mineral sources of nitrogen, phosphorus, and potassium distinctly accelerate PCB biodegradation in some soils and sediments (Fava et al. 2003). It majorly covers several remedial technologies which enhance biodegradation of the contaminant by supplementing soils with growth substrates/co-substrates. The most common biostimulation agents include bulking agents, nutrient supplementation, halogenated priming compounds (halo-priming), and surfactants (Passatore et al. 2014; Nanekar et al. 2015). The rate of PCB dechlorination can be increased by priming with halogenated compound. During this process, a halogenated aromatic substrate is used to stimulate PCB degrading indigenous organisms (Bedard et al. 1998; Passatore et al. 2014). This process is based on the assumption that high concentration of dehalogenation substrate will enhance the growth of dehalogenating microorganisms selectively as they use the compound as electron acceptor. This enhanced population of dehalogenators will further dechlorinate PCBs in the contaminated site. In a study by Haggblom and co-workers (2003), a 74% decrease in sediment associated PCBs was observed in a year by addition of 26-BB (bromobiphenyl) as a priming agent which activated PCB dechlorinating indigenous bacteria.
Biphenyls, chlorobiphenyls and some more easily degradable brominated analogues of PCBs along with CBAs (chlorobenzoic acids/chlorobenzoate) have been postulated to be inducers of aerobic biodegradation of PCBs in soils. (Piper 2005). Ferrer et al. (2003) have documented the use of maltotriose fatty acid monoesters drastically increases the bioavailability which in turn accelerate the biodegradation of higher PCBs. Addition of nutrients such as ammonia-nitrogen and phosphate, biphenyl and oxygen enhances PCB biodegradation (Wiegel and Wu, 2000). Several studies conducted in soil microcosms spiked with known mixture of PCBs have revealed that they can be significantly biodegraded, particularly when they are amended with biphenyl and oxygen and inoculated with PCB degrading bacteria. Reports shows that studies conducted on PCBs contaminated soils have confirmed this finding (Field and Sierra-Alvarez, 2008). Bacteria of species Rhodococci, such as strain RHA1, are recognized to be effective PCB degraders which have good survival in soil (Leigh et al. 2006). A study demonstrates isolation of three aerobic bacterial strains from Nigerian polluted soils, which could grow on all mono-CBs and on a wide range of di-CBs (68 to 100% removal) (Adebusoye et al. 2007). When dealing with hydrophobic contaminants like PCBs, certain surfactants can be augmented to enhance the process of degradation. Several bacterial and fungal species produce metabolic products which mimic surfactants when they are grown on hydrophobic compounds. These are called biosurfactants which cell wall associated and secreted externally. The excreted ones can be used for emulsification and hence enhanced remediation of PCBs (Zhang et al. 2012). Biosurfactants increase the surface area of hydrophobic compounds and increase their bioavailability owing to their amphiphilic structure. Moreover, these compounds are biodegradable and non hazardous (Pacwa et al. 2011). In some studies, it was reported that biosurfactants can increase the bioavailability of non-aqueous and soil bound phases of PCBs through desorption and solubilization (Robinson et al. 1996; Fiebig et al. 1997; Cho et al. 2004; Viisimaa et al. 2013). It could be presumed that biosurfactants can not only enhance the bioavailability of PCBs but also their availability to chemical oxidants as both processes are predetermined by similar functionalities (Viisimaa et al. 2013). Biosurfactants also reduced the lag time before dechlorination. Amendment of biosurfactant can be accomplished through in situ enrichment of biosurfactant producing microorganisms or direct application of biosurfactants (Cho et al. 2004; Field and Sierra Alvarez 2008).
Bioaugmentation of aerobic organisms
Aerobic organisms actively degrade mono- and dichlorbiphenyls (Egorova et al. 2013). However, many aerobic bacterial strains like Pseudomonas, Burkholderia, Rhodococcus display degradative activity to lower and highly chlorinated biphenyls (Piper 2005; Egorova et al. 2011; De et al. 2006; Hatamian-Zarmi et al. 2009; Petrić et al. 2011). Fava and Bertin (1999) have reported that exogeneous PCB and CBA degrading bacteria can be used in slurry phase in presence of biphenyl and oxygen for effective bioremediation of PCB contaminated soil. The following bacteria showed encouraging results: a mixture of gfp-transformed strains in soil microcosm (Pseudomonas sp. Cam-1-gfp1 and Sag-50G-gfp1); a mixture of strain Pseudomonas testosteroni B-356 along with a surfactant-producing, hydrocarbon-degrading strain in soil microcosm (Ahn et al. 2001); a strain of Janibacter sp. in liquid medium and soil (Sierra et al. 2003); Pseudomonas fluorescens HK 44 bearing a naphthalene degradation plasmid and the bioluminescence gene lux in field condition (Ang et al. 2005); biphenyl-degrading strains of Arthrobacter sp. B1B and H850 in presence with carvone, salicylic acid, and surfactant sorbitol trioleate in soil (Singer et al. 2000). Among the most methodically studied among these are Burkholderia xenovorans LB400 and Rhodococcus jostii RHA1 (Pieper 2005; Furukawa and Fujihara 2008). In studies carried out by various researchers PCB-degrader strains Rhodococcus ruber P25 and Microbacterium sp. B51 degraded broad range of PCB congeners. Strains P25 and B51 were found to degrade chlorinated biphenyls efficiently from mono to hexachlorobiphenyls which includes planar congeners too. It was recognized that these strains are able to utilize a variety of chlorobiphenyls as medium for growth without requiring supplementary carbon source and accumulated non/less toxic byproducts in the environment (Rybkina et al. 2003; Egorova et al. 2011; Plotnikova et al. 2012). In recent past, R. ruber P25 and Microbacterium sp. B51 demonstrated high degradation capability to all type of congeners present in Sovol (Egorova et al. 2013).
Effectiveness of sequential anaerobic and aerobic treatment for PCB degradation
Anaerobic microbes use reductive dechlorination to reduce the number of chlorine atoms whereas aerobic oxidation is brought about by addition of oxygen to biphenyl ring. Replacement of a chlorine substituent by a hydrogen and the departure of chlorine as chloride ion is termed as reductive dechlorination. Furthermore, decrease in chlorine number results in decreased anaerobic reductive dechlorination rates while the same results in increased aerobic oxidation rates (Anid et al. 1993). Owing to high redox potentials of highly chlorinated congeners, they are less susceptible to aerobic degradation. Low chlorinated congeners are reduced to greater extent and hence more vulnerable oxidation. These processes (dechlorination and oxidation) are dependent on substitution position of chlorine and not only on its number. Therefore, sequential anaerobic-aerobic biodegradation has been proposed as an efficient strategy for treatment of PCBs contaminated soils and sludges which have been tested successfully in sediment microcosms (Klasson et al. 1994; Rodrigues et al. 2006). A study conducted by Master et al. (2002) revealed that the higher chlorinated congeners were converted into lesser chlorinated congeners predominantly tetrachlorobiphenyls which were subsequently degraded by Burkholderia LB400 in a sequential anaerobic–aerobic treatment of Aroclor 1260. Decrease in average chlorine content by 20–30% has also been observed in Canadian arctic soils contaminated with Aroclor 1260 upon inoculation of anaerobic river sediments (Kuipers et al. 2003). Later on, several two-step anaerobic-aerobic bioremediation experiments were carried out for Aroclor 1242 contaminated sediment (Rodrigues et al. 2006). A biological tilled soil reactor which functioned under sequential anaerobic-aerobic conditions has achieved 75% reduction of total PCBs concentration in sludge from Ralston street lagoon which was heavily contaminated by Aroclor 1248 (Tharakan et al. 2006). More recently, in a two stage organic composting, 25% reduction of PCBs was observed during a 98 day’s experiment (70 days anaerobic and 28 days aerobic) suggesting benefits of sequential approach for remediation. The study also suggested that further research on two stage composting to get better results for tackling of higher chlorinated biphenyls (Long et al. 2015).
Scope of Metagenomics approach
Although degradation of PCB by isolated microbes are well characterized as discussed above, knowledge on role of uncultivable microbes in PCB bioremediation is very limited. Considering >99% environmental microbes are uncultivable (Singh 2010), the diversity of majority of the degrading but uncultivable microbes and their physiological capabilities are still to be discovered. Recent advancements in technologies provide unprecedented opportunities to generate in-depth knowledge and harness them for better, effective, economic bioremediation technologies (Ray et al. 2012). For example, metagenomics not only provides enormous opportunity to characterize the PCB degrading uncultivable bacteria but can be also be exploited to provide novel gene enzyme systems which can increase the efficiency of transgene based bioremediation technologies. However, environmental microbes are extremely diverse and metagenomics approach to find new gene/ enzyme system for bioremediation may be too costly and time consuming to be practically and economically feasible in some cases. However, because several microbes can utilize PCB as a carbon source, stable-isotope probing (SIP) combined with metagenomes can overcome this problem to some extent (Singh 2009). In the approach, soil is first incubated with 13C PCBs, the cellular components including genetic materials (DNA, RNA) of microbes which will utilize PCB as a source of energy get enriched 13C. Heavy DNA (13C DNA) of PCB degrading microbes can be separated from other environmental microbes by ultracentrifugation before metagenomic analysis. Such an approach has already produced a number of genes for bioremediations (Sul et al. 2009).
Integrated chemo-biological approach
One of the most effective approaches for enhanced degradation of PCBs is the application of pretreatment step prior to the biological degradation (Aronstein et al. 1995, Dervova et al. 1999). For this pretreatment, advanced oxidation process is among the most commonly used method which are based on generation of hydroxyl radicals to initiate oxidation of organic compounds ( Aronstein et al. 1995; Dervova et al. 1999; Manzano et al. 2003; Przado et al. 2007). These hydroxyl radicals can be generated using hydrogen peroxide in the presence of catalysts i.e. ferrous ion and is commonly referred as Fenton’s type reaction. Fenton’s type reaction is effective owing to its non specificity towards the aromatic rings during the chemical oxidation process. Generally, partial chemical oxidation increases the water solubility of organics that in turns increases bioavailability and facilitates biological action for enhanced degradation (Dercova et al. 1999). By combination of these two processes, one can expect more efficient degradation of PCBs at a low cost and lesser time when compared with the classical bioremediation technologies. Dercova et al. (1999) has suggested that partial chemical oxidation is responsible for increased water solubility of the organic compounds which leads to increase in biosusceptibility and thus facilitates microbial action which in turns enhances the biodegradation rate. Scanty work has been carried out on chemical pretreatment of PCBs using Fenton’s type reaction (Viisimaa et al. 2013) however; researchers have documented the process for pretreatment of different organic compounds such as, PAHs, phenols, PCPs, chlorinated organic pesticides etc. for their subsequent biodegradation. Yang (1994) reported that the pretreatment of 4,4′-dichlorobiphenyl (DCB) and 2,2′,4,4′,6,6′-hexachlorobiphenyl (HCB) using Fenton’s reagent in which the biodegradation rate constant for pretreated sample was 5 times faster than the untreated sample. Two possible means of enhanced biodegradation of PCBs post Fentos’s pretreatment were suggested by Aronstein et al. (1995) i.e. a) utilization of partially oxidized compounds in the system and b) direct microbial attack on transformed compound. Subsequently, various researchers have reported the effectiveness of Fenton pretreatment for degradation of PCBs (Dercova et al. 1999; Manzano et al. 2003; Przado et al. 2007). Studies have also been carried out using UV radiations, ozone as well as photo-Fenton process for pretreatment of PCBs (Quiroga et al. 2009; Javorska et al. 2009; Dasary et al. 2010; Liu et al. 2011). Liu et al. (2012) demonstrated the combined effect of pretreatment of PCB contaminated soils using biosurfactant washing and UV-irradiation on subsequent increase in biodegradation rate. More recently, it was also reported that joint application of microorganisms, biosurfactant, and oxidizing chemicals in moderate quantity to PCB contaminated soil led to increase in soil respiration along with dehydrogenase activity as compared to that obtained by microbial consortium alone, demonstrating stimulation of microflora integrating these processes (Viisimaa et al. 2013).
Another approach for pretreatment has been use of activated carbon (AC) which is used as an adsorbent for many volatile/ hydrophobic /organic contaminants because of its high specific surface area and microporous structure (Payne et al. 2011; Kjellerup et al. 2013). It has an inherent property to attract PCB degraders to form biofilm and also to keep PCB adsorbed to the surface. Hence use of dechlorinating bacterial biofilm coated activated carbon at contaminated site ensures close proximity of PCB and its degraders. Use of biofilm adsorbed AC also provides high density of PCB degraders on its surface increasing the direct interaction between PCB and bacteria, required for electron transfer and subsequent PCB degradation. The adsorption also protects the dechlorinating bacteria from being washed off and scavenging from indigenous organisms which helps in their long-term presence at contaminated site (Edward and Kjellurup 2013).
Further, nanoscale zero-valent metals have great potential for in situ PCB remediation. Zero valent iron (ZVI) oxidizes to the environmentally friendly Fe(III) and can be applied through direct subsurface injection (Gardner et al 2004). Researchers are now focusing on iron-reducing cultures that may dechlorinate PCBs co-metabolically. Wiegel and Wu (2000) have studied PCB dechlorination to occur under iron (III) reducing conditions. Use of nanoscale ZVI reduces the oxidation reduction potential of contaminated sediments and stimulates anaerobic organisms. Such conditions are favourable for sulfate reducers and methanogens. Thus, indigenous/augmented cultures were stimulated by the use of zero valent metals to enhance the reductive dechlorination (Mikszewski. 2004). A very recent research conducted by Le et al. (2015) revealed that bimetallic nanoparticles Pd/nFe used for pretreatment of Aroclor 1242 resulted in dechlorination of tri-, tetra-, penta-, and hexachlorinated biphenyls upto 99%, 92%, 84%, and 28% respectively. The resulted biphenyls were later subjected to rapid biodegradation by Burkholderia xenovorans LB400 in which benzoic acid was formed as an intermediate.
White Rot Fungi as an attractive candidate for bioremediation (mycoremediation)
Breakdown of PCB’s is majorly restricted by their hydrophobic nature making them less bioavailable for microbial breakdown. White rot fungi are a group of basidiomycetes are considered the most efficient organisms in mineralizing lignin in nature. Lignin degradation (ligninolysis) is brought about by a group of extracellular lignin modifying enzymes (LME) which comprise of lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase. The non specificity of these enzymes provides white rot fungi with the unique ability to degrade a wide range of environmental pollutants such as dioxins, PCBs, petroleum hydrocarbons, munitions wastes (such as trinitrotoluene), industrial dyes, herbicides and pesticides. Alike ligninolysis, degradation of a number of pollutants by these organisms is activated by limitation for nutrients, such as N and C and is also temporally correlated to lignin mineralization. Furthermore, they utilize other available sources of energy in the environment, such as sugars and polysaccharides and not the pollutants and in turn needlessly breakdown various pollutant chemicals, which are usually present in minute amounts (Urrea and Reddy 2012).
These organisms have become a positive option for remediation due to following features/ characteristics (Baldrian 2008; Pinedo-Rilla et al. 2009);
-
-
The availability of organisms for bioremediation studies due to their wide distribution in the nature
-
-
The flexibility to degrade a range of chlorinated organic pollutants either individually or in consortium
-
-
Inherent biodegradation enzymes helps in acclimatization in the polluted environment
-
-
Extracellular enzymes peroxidases and laccases break down the pollutants via oxidation and avoids internalization of substrates
-
-
Their growth via hyphal extension helps in attaining better contact to few contaminants which accumulates in small pores in soil
Phanerochaete chrysosporium decreased PCB concentration of Aroclors 1242, 1254, and 1260 (Yadav et al. 1995; Borazjani et al. 2005; Gomes et al. 2013). Additionally, congeners of lower chlorine numbers were shown to be degraded more extensively (Borazjani et al. 2005; Gomes et al. 2013). Beaudette et al. (1998) evaluated biodegradation of six selected PCB congeners using 12 white rot fungi. However, only a few PCB degradation studies were performed in soil systems. Lower concentration of surfactants increased fungal mineralization of PCB congeners (Beaudette et al. 2000). The ability of fungi to degrade low PCB concentrations has been demonstrated for several strains (Kamei et al. 2006).
Phytoremediation of PCBs
Microbial remediation faces some difficulties due to the presence of wide range of congeners and their low bioavailability and also due to their positional selectivity in attacking the chlorine substituent. In addition there is complexity in the interaction of contaminated sites with microbes and individual congeners (Borja et al. 2005). Metabolites of PCB degradation can also affect the viability of the organisms involved in degradation due to their high toxicity effects (e.g. dihydrodiols and dihydroxybiphenyls) (Beatriz et al. 2004). Though microbial degradation is effective till some extent, the cost constraints increase when we aim maximum degradation of PCBs (owing to the cost of augmented biosurfactants, bacterial consortiums and other co-subsrates). Therefore, it needs to be clubbed with some other remediation technique like phytoremediation for better and harmless degradation outcomes. Use of plants will help to overcome these issues and help in maximum degradation of PCBs. Details are discussed further in this article.
Phytoremediation is an emerging technology that uses living green plants and associated bacteria or fungi for in situ treatment of contaminated soil, sludges, sediments, and groundwater through removal, degradation, or containment of the contaminant (Aken et al. 2010). Although, phytoremediation is a natural process; investigation of its efficiency and progress in its application as a modern and innovative treatment technology at waste sites is not very old (Newman and Reynolds 2004; Liu and Schnoor 2008; Aken et al. 2010; Abhilash et al. 2012). Very recently Arslan et al (2015) has provided critical view of factors that affect absorption and translocation of POPs in plants along with the limitations that plant have to deal with during the POPs remediation. Phytoremediation of PCBs may take place by one of several ways: pollutants can be taken up inside the plant tissues (phytoextraction/phytoaccumulation); enzymatic transformations of PCBs can occur within the plant (phytotransformation); or volatilize into the atmosphere through leaves (phytovolatilization), secondary metabolites released by plants also enhance microbial activity, improving the degradation of PCBs in the root zone (rhizoremediation); it can be adsorbed to the roots (rhizofiltration); or contained to the soil material (phytostabilization) (Aken et al. 2010). Out of the above processes, phytoextraction and rhizoremediation are found to be most effective way of PCB degradation and are discussed further in this article. Figure 2 represents different processes and phases for phytoremediation of PCBs from contaminated soil.
Figure 2.
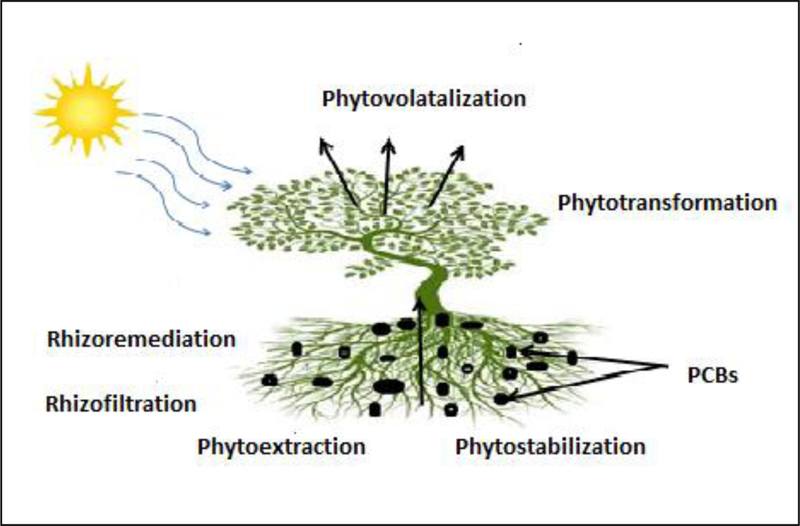
Phytoremediation of Polychlorinated Biphenyls (PCBs) may involve several processes viz; pollutants from contaminated soil, sediment and groundwater can be taken up by the plant tissues (phytoextraction) or adsorbed to the roots (rhizofiltration); pollutants inside plant tissues can be transformed by plant enzymes (phytotransformation) or can be volatilized into the atmosphere (phytovolatilization); pollutants in soil can be degraded by microbes in the root zone (rhizoremediation).
Phytoextraction of PCBs
Phytoextraction involves two major processes i.e. phytoaccumulation and translocation. Metabolism of the contaminant starts from absorption of PCBs to roots followed by active translocation to the shoots. Once accumulation of the contaminant is complete, transformation of the contaminant to less toxic metabolites takes place which are then phytoevaporated/evapotranspirated through plant leaves (Prasad 2011). PCBs diffuse into the free spaces in the endodermis of the root and then must bypass the Casparian Strip, where they can be translocated up into the shoots via the vascular tissues (Zeeb et al. 2006). Several studies highlighted the potential of phytoextraction for PCBs from contaminated soils (Teng et al. 2010; Fiko et al. 2011; Wang et al. 2011).
Many researchers have studied the uptake and translocation of PCBs in different plant species such as corn (Zea mays L.), cabbages (Brassica oleracea var. capotata L.), pumpkin (Cucurbita pepo ss pepo cv. Howden), carrots (Daucus carota L.), squash (C. pepo ssp ovifera), zucchini (C. pepo ssp pepo), beets (Beta vulgaris), turnips (Barssica rapa L.) and beans (Phaseolus vulgaris) (Iwata and Gunther 1976; Fries and Marrow 1981; Webber et al. 1994; White et al. 2006; Low et al. 2010). For the same reason, they are taken up by plant tissues in negligible amounts. However, there are some plant species belonging to Cucurbitaceae family which are known to accumulate PCBs in roots as well as shoots. It is reported that pumpkins are efficient in taking up and translocating PCBs from soil (Aslund et al. 2008). Also, it has been documented that C. pepo ssp pepo plants maximizes phytoextraction of PCBs when the plant shoot has reached its maximum biomass (Low et al. 2010). Also, Hulstler and his co workers (1994) have studied the uptake of PCBs in zucchini and found that zucchini fruits could accumulate two orders of magnitude more polychlorinated dibenzo-p-dioxins and dibenzofurans than other fruits and vegetables in the same contaminated site. Findings of some researchers (Mattina et al. 2007; Aslund et al. 2007 and 2008; White et al. 2006) support the hypothesis that plant species C. pepo spp pepo are efficient in phytoextraction of PCBs containing POPs by uptake and translocation to shoots via roots. Currently the research focuses on the uptake mechanisms of C. pepo for POPs containing PCBs. The higher uptake of PCBs in this plant can be an induced phytoextraction process wherein the compounds secreted by plant roots into the soil facilitate the uptake of PCBs (Dakora and Philips 2002). List of selected plants species studied for phytoextraction of PCBs along with the major findings are given in Table 1.
Table: 1.
List of selected plants species studied for phytoextraction of PCBs
| Plant(s) | Contaminant(s) | Accumulation in plant |
Major Findings | Reference |
|---|---|---|---|---|
| Cucurbita pepo ssp. pepo cv. Howden | Aroclor 1248 | Roots and shoots | PCBs accumulated in roots and shoots during the growth period with stable concentration. | Low et al., 2010 |
| Cucurbita pepo ssp. pepo cv. Howden | Aroclor 1254–1260 | Roots and shoots | Greater accumulation of PCBs in shoots as compared to roots owing to higher uptake and translocation. | Aslund et al., 2008 |
| Medicago sativa L | PCB contaminated soil | Roots and shoots | Inoculation with rhizhobium and fungal species increased the accumulation of PCBs in shoots along with higher yield of alfalfa. | Teng et al., 2010 |
| Cucurbita pepo ssp. Pepo Bidens cernua Chenopodium album Daucus carota Plantago major Rumex crispus | PCB contaminated soil | Roots and shoots | Equal distribution of PCBs in roots and shoots was observed. Root exudates were found to affect uptake and transport of contaminants within specific plant species. | Ficko et al., 2011 |
| Zea mays | Mix of PCB congeners (15, 28, 47) | Roots and shoots | Higher translocation factor of PCBs resulted in higher PCB concentration in shoots as compared to roots. | Wang et al., 2011 |
| Nicotiana tabacum Solanum nigrum | Mix of PCB congeners (total PCB concentration 110 mg kg−1). | Roots, leaves and berries | Both plant species differed in the amount of accumulation and distribution of PCBs within the plant species. Uptake of PCBs was observed in roots, leaves and berries. | Kurzawova et al., 2012 |
| Amaranthus retroflexus Ambrosia artemisifolia Brassica nigra | Mixture of Aroclors 1254/1260 | Roots and shoots | All the studied plant species showed significant accumulation of PCBs in their root and shoot tissues. However, it was also observed that changes in | Ficko et al., 2010 |
| Cirsium vulgare Daucus carota Echinochloa crusgalli Lythrum salicaria Polygonum persicaria Setaria viris Solidago Canadensis Soncus asper Symphyotrichum ericoides Symphyotrichum novae-angliae Vicia cracca Chrysanthemum leucanthemum | Aroclor 1248 | environmental conditions such as precipitation and temperature possibly affected plant growth, and therefore potentially the plant tissue concentrations. | ||
| Zea mays L | e-waste contaminated soil | Roots and shoots | In this comparative study on effect of chelant and plant growth promoting bacteria (PGPB) on PCB uptake, P GPB resulted in higher accumulation of PCBs in shoot owing to higher plant growth and biomass. | Luo et al. 2015 |
Rhizoremediation of PCBs
Rhizoremediation is a promising phytoremediation strategy that banks on the ability of plant roots to facilitate growth and activity of pollutant degrading bacteria present in its rhizosphere. Researchers have reported significant reduction of PCBs in planted soils as compared to unplanted controls (Chaudhry et al. 2005; Gerhardt et al. 2009). PCBs get sorbed to soil particles strongly owing to their hydrophobic nature. Bittsánszky and his co-workers (2011) have compiled a review on different species of plants belonging to Cucurbitaceae family having the potential to accumulate PCBs in plant root and shoots. Tall fescue can accumulate considerable amount of PCB in root and are hence good candidates for phytoaccumulation (Pinsker 2011) Although, PCBs are accumulated within the plant, reports on their transformation to non toxic components is not available. Hence, the fear of introduction of accumulated PCBs in the soil matrix upon death of plant persists. In the view of this problem, breakdown of the contaminant is an apt solution for its remediation. This is brought about by rhizospheric bacteria and the process is called rhizodegradation. Leigh et al. (2006) documented that the indigenous PCBs degrading microorganisms are associated with the plants growing in the contaminated soils. Research also suggest the significance of the plant and rhizospheric microbial interactions (Macková et al. 2006) concerning their ability to degrade PCBs. Arslan et al (2015) have comprehensively reviewed and compiled information on plant-rhizobacteria partnership for remediation of POPs including PCBs.
Fate of PCBs in rhizosphere:
Degradation of organic pollutants can still be a problem even with sufficient microbial biomass and availability of the contaminant. This can be due to uninduced degradation pathway genes or insufficient energy for performing the degradation process. The part of gene inducers and surfactants in making the compound available for both rhizospheric bacteria and plant has been discussed above. Energy supply to the degrading cells is also of equal importance while studying the degradation of organic contaminants. It is quite possible that the energy produced by the cells after degradation of the contaminant is not enough for the survival of the organisms. Plants are capable of ameliorating this deficit of energy (McCutcheon and Schnoor 2004). Aerobic degradation of higher chlorinated compounds in rhizospheric soil is either very slow or negligible. Plants secrete certain compounds in root exudates that are similar to biphenyl and act as co metabolites for stimulation of PCBs degrading microorganisms in rhizosphere (Singer et al. 2000; McCutcheon and Schnoor 2004). Plant roots also provide certain compounds that drive metabolism of PCBs as secondary substrates (McCutcheon and Schnoor 2004).
It has been reported that few compounds present in root exudates released by plant may stimulate microbial degradation of PCBs (Mackova et al. 2006). Brassica nigra directly contributed to enhanced removal of PCBs in Aroclor 1242 contaminated soil (Singer et al. 2003). Carex aquatalis and Spartina pectinata are predicted to be among the most efficient and effective plants for phytoremediation of PCBs (Smith et al. 2007). Efforts were undertaken to expand the degradation capacity of rhizosphere competent bacteria. Strain F113 has been found to be an excellent coloniser in several plant rhizospheres which helped in co-metabolism of PCBs better than strain LB400 (Villacieros et al. 2005). Zeeb et al. (2006) recently studied the phytoremediation of a soil with slight contamination of Aroclor 1260. However, no considerable PCB removal was found in highly contaminated soil, but the plants performed well in lower contamination. Table 2 presents the PCB-metabolizing bacteria isolated from various rhizospheres of plant species. Further studies are needed to characterize both cultivable and uncultivable microbes which can mineralize PCB in rhizosphere. Emerging technologies such as metagenomics, transcriptomics and proteomics not only provide huge opportunity to do so but also allow an understanding about their physiological capabilities. Such information is key for successful exploitation of microbial capability for industrial processes including bioremediation. For example, these technologies can be employed to know whether intrinsic microflora have degrading capability of PCB (by examining degrading genes/ protein) and what nutritional amendment is needed for biostimulation.
Table: 2.
Identification of PCB-metabolizing bacteria isolated from various rhizospheres of plant species by different biochemical and 16S rRNA/rDNA gene sequence analysis.
| Bacteria | Plant(s) | Contaminant(s) | Probable Effect | Reference |
|---|---|---|---|---|
| Arthrobacter sp., Rhodococcus sp., Staphylococcus sp., Pseudomonas sp. | Austrian pine (Pinus nigra L.) | PCB contaminated soil | Biphenyl Degradation | Leigh et al., 2007 |
| Burkholderia Cepacia | Barley (Hordeum vulgare) | 2,4-Dichlorobiphenyl | Biotransformation of PCBs | Petroutsos et al., 2008 |
| Pseudomonas plecoglossicida, Ochrobactrum anthropi, Achromobacter xylosoxidans, Pseudomonas sp., P. stutzeri, P. fluorescens, P. putida, P. fluorescens, P. pseudoalcaligenes | Nightshade (Solanum nigrum), Horseradish (Armoracia rusticana), Thistle (Silybum marianum), Alfalfa (Medicago sativa), Tobacco (Nicotiana tabacum) | PCB mixture Delor 103 | Phyto/rhizoremediation of PCBs | Mackova et al., 2009 |
| Hydrogenophaga palleronii (AF019073), Achromobacter xylosoxidans subsp. xylosoxidans (AJ491839), Methylobacillus flagellatus (CP000284) | Horseradish (Armoracia rusticana) | PCBs | Metabolism and catabolism of biphenyl in the rhizosphere | Uhlik et al., 2009 |
| Rhizobium meliloti | Alfalfa (Medicago sativa L.) | PCBs | Enhanced removal of PCBs | Xu et al., 2010 |
However, phytoremediation has a limitation that this technology is quite slower and is climate dependent. To overcome this limitation, genetically modified organisms and transgenic plant species for speeding up the remediation process can be implemented. The rhizopheric degradation can be enhanced by inducing degradation genes in organisms as well as plants to reduce the time required. Also transgenic plants which are able to survive in given climatic conditions can be designed. The use of both in remediation process is discussed below.
Genetically Modified Organisms (GMOs) and transgenic plants for Phytoremediation of PCBs
In the face of the complex detoxification pathway present in plants, their slow generation time compared with that of microorganisms means that plants have had less time to evolve efficient methods for detoxifying these synthetic compounds (Aken et al. 2010). Although bacteria isolated from contaminated soil can rapidly detoxify PCBs in laboratory cultures, the fact that these PCBs persist in the environment suggests that bacteria do not possess enough biomass or metabolic activity to decontaminate these areas significantly (Liste and Alexander 2000; Zuang et al. 2007). Rhizosphere provides a natural environment for in situ bioremediation of the contaminant in soil. Inserting genes encoding biphenyl pathway into the host bacteria which already exists in the rhizosphere ensures rapid degradation. Also, exudates from plants can act as co substrates that biostimulate degradation activity of microbes (Rein, 2006). Plant roots in turn help to reduce leaching of contaminants, aerates the soil and release exudates that foster selective microorganisms (Amos and Younger 2003). This kind of rhizoremediation technology which conjugates use of GMOs and Plants is a promising bioremediation technology.
The microbial and mammalian catabolic genes possess the metabolic enzymes for complete mineralization of organic molecules. These genes can therefore be used to harmonize the metabolic abilities of the plants. Transgenic plants have been developed for the phytoremediation of PCBs. Recently, the use of transgenic plants for PCBs phytoremediation has been evaluated by several researchers (Cherian and Oliveira 2005; Eapen et al. 2007; Doty 2008; Aken et al. 2008, 2010). In an innovative study, Francova et al. (2003), developed transgenic tobacco plants (Nicotiana tabacum) by inserting gene responsible for 2,3- dihydroxybiphenyl ring cleavage, bphC, from the PCB degrader Comamonas testosteroni. Similarly bph genes from B. xenovorans LB 400 were cloned into tobacco plants, one of the most efficient PCB degrading bacteria. bphAE, bphF, and bphG which are essential components of the bph operon needed for dioxygenation of the biphenyl ring were independently cloned and expressed in transgenic plants. It was observed that purified enzymes extracted from plants were capable of oxidizing 4-chlorobiphenyl into 2,3-dihydro-2,3-dihydroxy-4′-chlorobiphenyl. Sylvestre et al. (2009) reported that transgenic plants can also generate three components of the biphenyl dioxygenase and the 2, 3-dihydroxybiphenyl dioxygenase which catalyze vital steps in bacterial PCB degradation taking this into account this type of microbe assisted phytoremediation wherein transgenic plants initiate PCB metabolism and exude nutrients for rhizospheric degradation can be implemented. Table 3 presents a list of transgenic plants and bacteria engineered for phytoremediation of PCBs. Recently, Novakova et al. (2009) successfully cloned 2,3-dihydroxybiphenyl-1,2-dioxygenase, bphC gene obtained from Pseudomonas testosteroni B-356 into one of the highly suitable Tobacco (Nicotiana tabacum) plant during which the growing transgenic plants in presence of 2,3-dihydroxybiphenyl shows higher resistance to the toxic compounds in comparison to wild type plants. The growing transgenic plants in presence of 2,3-dihydroxybiphenyl shows higher resistance to the toxic compounds in comparison to wild type plants. In an effort to improve rhizoremediation performances, several researchers have cloned key catabolic genes of known PCB degraders into specific rhizosphere bacteria. Villacieros et al. (2005) isolated bph operon from B. xenovorans strain LB400 and introduced into strain F113 under the influence of strong promoter, nodbox 4, from Sinorhizobium meliloti. The modified strain, F113::1180, expressed a high level of biphenyl dioxygenase which was capable of metabolizing biphenyl and various PCB congeners at a much higher rate than strain F113pcb (Rein et al. 2007). Mesocosm experiments with PCB-contaminated soil demonstrated a good survival capability of F113 strains in willow plant rhizosphere, signifying that alliance of transgenic rhizosphere bacteria with plants represents a promising approach for the management of PCB-contaminated soils.
Table: 3.
Transgenic plants for the phytoremediation of PCBs.
| Gene | Source | Target | Contaminant(s) | Effect | Reference |
|---|---|---|---|---|---|
| Biphenyl dioxygenase, bph, located on transposon, TnPCB | Pseudomonas sp. strain LB400 | Sugar beet seeds (cv. Rex) | PCBs | Rhizodegradation of PCBs by Pseudomonas | Brazil et al., 1995 |
| Biphenyl dioxygenase, bphC | Comamonas testosteroni B-356 | Tobacco (Nicotiana tabacum) | PCBs | Phytodegradation of PCBs | Francova et al., 2003 |
| Oxygenolytic ortho-dechlorination (ohb) gene | Pseudomonas aeruginosa strain 142 | Sinorhizobium meliloti colonizing Alfalfa (Medicago sativa) | 2’,3,4-Trichloro-biphenyl | Enhanced orth-dechlorination of PCBs | Toure et al., 2003 |
| Biphenyl dioxygenase, bph Green fluorescent protein, gfp | Burkholderia xenovorans LB400 | Alfalfa roots (Medicago sativa, var. Resis, DLF Trifolium) | 3-Chloro-biphenyl | Rhizosphere degradation of PCBs | Boldt et al., 2004 |
| PCB-degrading genes, ortho-halobenzoate 1,2-dioxygenase (ohb) genes | Pseudomonas aeruginosa strain 142 | Sinorhizobium meliloti colonizing Alfalfa (Medicago sativa) | 2’,3,4-Trichloro-biphenyl | Enhanced bioremediation of PCB | Chen et al., 2005 |
| Biphenyl dioxygenases, bphA, bphE, bphF, bphG | Burkholderia xenovorans LB400 | Nicotiana tabacum, Nicotiana benthamiana | 4-Chloro-biphenyl | Oxygenation of 4-chloro-biphenyl | Mohammadi et al., 2007 |
| Biphenyl dioxygenase, bph | Burkholderia xenovorans LB400 | Pseudomonas fluorescens F113 colonizing willow (Salix sp.) rhizosphere | Individual PCBs congeners | Degradation of PCB congeners | Rein et al., 2007 |
| Biphenyl dioxygenase, bph | Burkholderia xenovorans LB400 | Pseudomonas fluorescens colonizing rhizosphere of willow (Salix sp.) | PCBs-contaminated soil | Rhizoremediation of PCBs through genetically modified organisms | de Carcer et al., 2007 |
| 2,3-dihydroxybiphenyl-1,2-dioxygenase, bphC | Pseudomonas testosteroni B-356 | Tobacco (Nicotiana tabacum) | 2,3-Dihydroxy-biphenyl | Increase the efficiency of the biodegradation of PCBs | Novakova et al., 2009 |
| 2,3-dihydroxybiphenyl-1,2-dioxygenase, bphC | Pandoraea pnomenusa | Tobacco (Nicotiana tabacum) | PCBs (Delor 103) | Increased efficiency degradation of total PCBs | Viktorová et al., 2014 |
| 2,3-dihydroxybiphenyl-1,2-dioxygenase, bphC | Agrobacterium tumefaciens | Alfalfa (Medicago sativa) | PCBs and 2,4-dichlorophenol (2,4-DCP) | Expression of bphC enhanced tolerance of plants and remediation of PCBs and 2,4-DCP | Wang et al., 2015 |
Basidiomycetes like white rot fungus produces unique extracellular oxidative enzymes like lignin peroxidase (Lip), manganese dependent peroxidase (Mnp) and laccase (Lac) which are found to be important in degradation of PCBs. in the study conducted by Sonoki and co workers (2007), genes responsible for production of Lip, Mnp and Lac enzymes produced by Phaenerochete chrysoporium have been introduced into the DNA of A thaliana to make a transgenic species that efficiently degrades PCBs. More research needs to be done on plant microbe interactions both GMO, and inbuilt rhizhospheric bacteria and plant. The in-depth knowledge of the fate of the contaminant inside the plant is of utmost importance to avoid undesired effects in field application. Exploiting the knowledge on molecular communication between plants and microbes will be helpful in achieving better results in the elimination of contaminants will be fascinating area of research.
These studies might disclose the microbe–plant interactions and can be used to study the induction of catabolic pathways in polluted soils undergoing rhizoremediation. These emerging techniques will also allow to monitor or selection of catabolic genes to improve remediation strategies (Kiely et al. 2006). The development of metagenomic analysis will perhaps reveal new degradative genes that will be worth introducing into strains with other interesting qualities like superior root colonization capability. To find the perfect combination of plant and augmented organism for degradation, the signals that plant and microbes exchange when they identify each other will have to be understood along with dissection of molecular basis of the specific interactions between certain plant genotypes with specific bacteria (Segura et al, 2009).
However, impact of transgenic plants on environment is still being debated and we strongly recommend the use of native plant species of the contaminated site as primary option for phytoremediation owing to their adaptability to the conditions.
Estimation of global cost for remediation
Most of the above mentioned technologies are going through their developmental stages. Additional information regarding field data and pilot scale experiments is required to evaluate the effectiveness and efficiency of these technologies. As discussed above, owing to the complexity of PCBs, a single technology does not seem conveniently applicable to both ex situ and in situ remediation of PCB contaminated soil. Every case is different and various factors need to be considered while calculating the cost constraint (Gomes et al. 2013). Investigations on application of bio and phytoremediation are growing rapidly all over the globe because of to its advantages over conventional physico-chemical treatments. The ever growing demand of developing countries has pushed international community to harness biological remediation technologies, wherever applicable and as well as to assess the estimated cost for remediation of contaminated sites. There is a vastly growing market for environmentally sound management of hazardous waste and remediation of contaminated site which was estimated around US$ 1 trillion (Masons Water Yearbook 2000–2001).
The cost including the risk associated with a particular technology is of high priority. The financial aspects includes costs of capital and operation, installation and management, energy consumption, chemicals, manpower, monitoring, pre-treatment, post-treatment etc. On the other hand, risk assessment should include loading flexibility, emergency management, transient control etc (Rahuman et al. 2000; Li and Mohamed 2007). Literature shows that the worldwide market for the contaminated site remediation is possibly in the range between US$30 and 35 billion (Singh et al. 2009). On the other hand, it is calculated that approximately US$1.5 billion is the global requirement of bioremediation technologies per annum (Passatore et al. 2014). The market for bioremediation of contaminated sites is ready to explore in various countries such as Western European countries, Canada, Australia, Japan, US etc. Whereas, developing countries such as Asian, Latin American and Eastern European countries correspond to the budding market for contaminated site bioremediation. However, it is very complicated to assess the cost of this promising market for remediation because of lack of an established comprehensive catalogues for contaminated sites in several countries (Singh et al. 2009). Nonetheless, Li and Mohamed (2007) has comprehensively assembled a few recognized bioremediation technologies under the assignment of United Nations Industrial Developmental Organization (UNIDO) viz. DARAMEND®, XenoremTM estimating approximately US$55 to $360/m3 for remediation of sites contaminated with chlorinated POPs. USEPA has also calculated costs for phytoremediation of halogenated POPs (specifically chlorinated) including PCBs which estimates in the range of $150 and $630/m3. These estimates may vary according to different essential factors including site characteristics, availability of expertise, national law and legislation etc. Table 4 represents comparison of various technical aspects/requirements between bio/phytoremediation and other physicochemical technologies (Rahuman et al. 2000; Li and Mohamed 2007; Gomes et al. 2013). Inspite of all these factors, the worldwide market for bioremediation of contaminated sites is experiencing a qualitative transformation and evidently, it will attain market maturity and stability (Passatore et al. 2014).
Table: 4.
Comparison between different technical aspects/requirements for bio/phytoremediation and other physicochemical technologies
| Factors (dependency/requirement) | Bio/phytoremediation | Other physico-chemical technologies e.g. incineration, pyrolysis, solvent extraction, etc. |
|---|---|---|
| Soil Temperature | High | Average to high |
| Soil Moisture | Average to high | Average to high |
| Particle Size | High | Average to high |
| Permeability/clay content | High | Low |
| Space Requirement | Depends whether In situ or Ex situ | Average to high |
| Pre-treatment | Low to average | Average |
| Power | Low | High |
| Water | Average | Low to average |
| Monitoring | Low | High |
| Skilled labour | Average | High |
| Transportation | Low | High |
| Excavation | Low | High |
| Post treatment | Low | Low to average |
| Impact on Environment | Low | Average (sometimes high) |
| Hazardous by products | Low | Average to high |
| Field testing | Limited to average | Average to high |
| Developmental stage | Initial to practical | Practical |
| Societal acceptance | Average to high | Low to average |
| Efficiency | Average to high | High |
| Cost | Low to average | High |
Referred from Rahuman et al. 2000; Li and Mohamed 2007; Gomes et al 2013
Conclusions and Future Perspectives
Owing to the physic-chemical properties of PCBs such as persistence, low bioavailability, and toxicity, sustainable remediation measures are warranted from time to time. Rapid progress has been made in developing effective, economical and socially viable bioremediation processes. It is expected that in the future, bioremediation using microbes, plants (including transgenic plants) and plant-microbe interactions will be used widely to significantly reduce PCBs from the environment. As pre-treatment for enhanced biodegradation of PCBs, an integrated approach for chemo-biological treatment seems to be an effective tool for remediation. However, to exploit these possibilities on large scale, several scientific, regulatory and social aspects are needed to be addressed as follows;
-
a)
Up scaling laboratory finding to field scale needs further understanding of microbial and plant’s physiological requirements. In this case, examples of petroleum industries that have successfully implemented bioremediation technology on field scale can be followed for remediation of PCBs contaminated sites.
-
b)
Emerging consensus is that phytoremediation in combination with microbial degradation can proved to be an effective technology to remediate PCB contamination. On this front, further understanding of rhizospheric microbial interactions is fundamental for effective remediation. Novel knowledge and investigations on mode of interactions and communication between different biotic factors in rhizospheric zone will provide further tools for effective remediation technology.
-
c)
Combining phytoremediation with other economic and environmental benefits is needed. For example use of biofuel plants for bioremediation will provide both remediation of the site as well as biomass for energy generation. This in turn will make technology economically attractive and environmental friendly. Therefore, there is a need to study such plants with additional benefits.
-
d)
Characterization of uncultivable PCB degrading microbes and evaluating their physiological capabilities and nutritional requirements using emerging technologies of omics will provide a strong platform for exploitation of intrinsic microflora for bioremediation.
-
e)
Discovery of novel and more efficient gene/ enzymes using metagnomics and their expression in plants and microbes will benefit bioremediation efficacy. Here, improvement in bioinformatics’ support for metagenomic works is needed which is considered to be the main bottleneck for this technology.
-
f)
The adoption of an integrated approach for enhanced degradation of PCBs needs to be investigated with minimal requirement of chemical dechlorinators which may lead to complete mineralization of PCBs. Here, it is important to carry out further advanced investigations on optimum utilization of readily available strong oxidizing agents along with employment of several physic-chemical agents such as H2O2, nanoscale zero valent metals, activated carbon, ozone, UV radiations etc.
-
g)
Social and regulatory acceptance of transgenic technology need to be settled as soon as possible. Until then non-transgenic technologies such as designer plants can be exploited for multi-purpose bioremediation.
-
h)
Use of GMOs has difficulties in field applications even after their known and reported benefits. There are legal restrictions on release of recombinant organisms in the field in many countries which should be studied (along with its scientific concerns) before release of GMOs into the environment (Bloom and de Serres 1995). An important obstacle for field application of transgenic plants for bioremediation is linked with the true or apparent risk of horizontal gene transfer to wild or cultivated plants. Therefore, need for more risk-benefit analysis and risk mitigation plan becomes significant to guarantee that transgenic biotechnologies would result in wide recognition and application of bioremediation (Aken et al. 2010).
Additionally, it is necessary to achieve modifications in the existing legislation, overcome regulatory obstructions and educate public to improve their views on GM plants and microbes. Current knowledge implies that bioremediation is an effective technology but requires time and needs to be tailored to achieve desired results for decontamination of PCBs contaminated sites.
Acknowledgments
The authors are thankful to the Director, CSIR-NEERI, Nagpur for the kind support and encouragement. JKS is thankful to Council of Scientific and Industrial Research, New Delhi, India for the award of Senior Research Fellowship.
References
- Abhilash PC, Singh HB, Powell JR and Singh BK (2012) Plant-microbe interactions: novel applications for exploitation in multi-purpose remediation technologies. Trends Biotechnol 30: 416–420. [DOI] [PubMed] [Google Scholar]
- Abraham WR, Nogales B, Golyshin PN, Pieper DH, Timmis KN (2002) Polychlorinated biphenyl-degrading microbial communities in soils and sediments. Curr Opin Microbiol 5: 246–253. [DOI] [PubMed] [Google Scholar]
- Adebusoye SA, Picardal FW, Ilori MO, Amund OO, Fuqua C, Grindle N (2007) Growth on dichlorobiphenyls with chlorine substitution on each ring by bacteria isolated from contaminated African soils. Appl Microbiol Biot 74: 484–492. [DOI] [PubMed] [Google Scholar]
- Ahn YB, Beaudette LA, Lee H, Trevors JT (2001) Survival of a gfp-Labeled Polychlorinated Biphenyl Degrading Psychrotolerant Pseudomonas spp. in 4 and 22 Degrees C Soil Microcosms. Microb Ecol 42: 614–623. [DOI] [PubMed] [Google Scholar]
- Aken BV (2008) Transgenic plants for phytoremediation: Helping nature to clean up environmental pollution. Trends Biotechnol 26: 225–227. [DOI] [PubMed] [Google Scholar]
- Aken BV, Correa PA, Schnoor JL (2010) Phytoremediation of Polychloinated Biphenyls: New trends and promises. Environ Sci Technol 44: 2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M (1999) Biodegradation and Bioremediation, 2nd edition. California, USA: Acedemic Press. [Google Scholar]
- Amos PW, Younger PL (2003) Substrate characterization for a sub-surface reactive barrier to treat colliery spoil leachate. Water Res 37: 108–120. [DOI] [PubMed] [Google Scholar]
- Ang EL, Zhao HM, Obbard JP (2005) Recent advances in the bioremediation of persistent organic pollutants via biomolecular engineering. Enzyme Microb Tech 37: 487–496. [Google Scholar]
- Anid PJ, Ravest-Webster BP, Vogel TM (1993) Effect of hydrogen peroxide on the biodegradation of PCBs in anaerobically dechlorinated river sediments. Biodegradation 4(4): 241–248 [DOI] [PubMed] [Google Scholar]
- Arslan M, Imran A, Khan QM, Afzal M (2015) Plant–bacteria partnerships for the remediation of persistent organic pollutants. Environ Sci Pollut Res 1–15. [DOI] [PubMed] [Google Scholar]
- Aslund MLW, Rutter A, Reimer KJ, Zeeb BA (2008) The effects of repeated planting, planting density, and specific transfer pathways on PCB uptake by Cucurbita pepo grown in field conditions. Sci Total Environ 405(1): 14–25. [DOI] [PubMed] [Google Scholar]
- Aslund MLW, Zeeb BA, Rutter A, Reimer JK (2007) In situ phytoextraction of polychlorinated biphenyl (PCB) contaminated soil. Sci Total Environ 374(1): 1–12. [DOI] [PubMed] [Google Scholar]
- ASTDR (2000) Toxicological profile for polychlorinated biphenyls (PCBs) [PubMed] [Google Scholar]
- Baars AJ, Bakkera MI, Baumanna RA, Boonb PE, Freijera JI, Hoogenboomb LAP, Hoogerbruggea R, Van Klaverenb JD, Liema AKD, Traagb WA, Vriesc JD (2004). Dioxins, Dioxin-Like PCBs and Non-Dioxin-Like PCBs in foodstuffs: Occurrence and dietary intake in the netherlands. Toxicol Lett 151(1):51–61. [DOI] [PubMed] [Google Scholar]
- Baba D, Yasuta T, Yoshid N, Kimura Y, Miyake K, Inoue Y, Toyota K, Katayama A (2007) Anaerobic biodegradation of polychlorinated biphenyls by a microbial consortium originated from uncontaminated paddy soil. World J Microb Biot 23: 1627–1636. [Google Scholar]
- Baciocchi R, Ciotti C, Gavasci R, Lombardi F (2005) Site remediation of an industrial waste dump: fenton treatment of PCB contaminated soil Solid Waste Association. [Google Scholar]
- Baldrian P (2008) Wood-inhabiting ligninolytic basidiomycetes in soils: ecology and constraints for applicability in bioremediation. Fungal Ecol 1:4–12 [Google Scholar]
- Beaudette LA, Davies S, Fedorak PM, Ward OP, Pickard MA (1998) Comparison of Gas Chromatography and Mineralization Experiments for Measuring Loss of Selected Polychlorinated Biphenyl Congeners in Cultures of White Rot Fungi. Appl Environ Microb 64: 2020–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudette LA, Ward OP, Pickard MA, Fedorak PM (2000) Low surfactant concentration increases fungal mineralization of a polychlorinated biphenyl congener but has no effect on overall metabolism. Lett Appl Microbiol 30: 155–160. [DOI] [PubMed] [Google Scholar]
- Bedard DL (2003) Polychlorinated Biphenyls in Aquatic Sediments: Environmental Fate and Outlook for Biological Treatment. In: Dehalogenation: Microbial Processes and Environmental Applications, (Haggblom MM and Bossert I, eds.), Kluwer Press, pp. 443–465. [Google Scholar]
- Bedard DL, Van Dort H, Deweerd KA (1998) Brominated biphenyls prime extensive microbial reductive dehalogenation of Aroclor 1260 in Housatonic River sediment. Appl. Environ. Microbiol 64: 1786–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittsánszky A, Gullner G, Gyulai G, Komives T (2011) A case study: uptake and accumulation of persistent organic pollutants in Cucurbitaceae species. In Organic xenobiotics and plants, Springer Netherlands; 77–85. [Google Scholar]
- Bloom AD, de Serres F (1995) Ecotoxicity and Human Health: A Biological Approach to Environmental Remediation CRC Press. [Google Scholar]
- Boldt TS, Sorensen J, Karlson U, Molin S, Ramos C (2004) Combined use of different GFP reporters for monitoring single cell activities of a genetically modified PCB degrader in the rhizosphere of alfalfa. FEMS Microbiol Ecol 48: 139–148. [DOI] [PubMed] [Google Scholar]
- Borazjani H, Wiltcher D, Diehl S (2005) Bioremediation of polychlorinated biphenyl and petroleum contaminated soil. In: Lyon WG, Hong JJ, Reddy RK, editors. Proceedings of the International Conference on Environmental Science and Technology New Orleans, USA: American Science Press: 502–7. [Google Scholar]
- Borja J, Taleon DM, Auresenia J, Gallardo S (2005) Polychlorinated Biphenyls and their biodegradation. Process Biochem 40: 1999–2013. [Google Scholar]
- Brazil GM, Kenefick L, Callanan M, Haro A, Delorenzo V, Dowling DN, Ogara F (1995) Construction of a rhizosphere Pseudomonad with potential to degrade polychlorinated biphenyls and detection of bph gene expression in the rhizosphere. Appl Environ Microb 61: 1946–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCME (1999). Canadian soil quality guidelines for the protection of environmental and human health. Total PCBs. Canadian environmental quality guidelines Canadian Council of Ministers of Environment. [Google Scholar]
- Chaudhry Q, Blom-Zandstra M, Cupta S, Joner EJ (2005) Utilizing the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ Sci Pollut R 12(1): 34–48. [DOI] [PubMed] [Google Scholar]
- Chávez FP, Gordillo F, Jerez CA (2006) Adaptive responses and cellular behaviour of biphenyl-degrading bacteria toward polychlorinated biphenyls. Biotechnol adv 24(3): 309–320. [DOI] [PubMed] [Google Scholar]
- Chen Y, Adam A, Toure O, Dutta SK (2005) Molecular evidence of genetic modification of Sinorhizobium meliloti: enhanced PCB bioremediation. J Ind Microbiol Biot 32(11–12): 561–566. [DOI] [PubMed] [Google Scholar]
- Cherian S, Oliveira MM (2005) Transgenic plants in phytoremediation: Recent advances and new possibilities. Environ Sci Technol 39: 9377–9390. [DOI] [PubMed] [Google Scholar]
- Chi XQ, Zhang JJ, Zhao S, Zhou NY (2013). Bioaugmentation with a consortium of bacterial nitrophenol-degraders for remediation of soil contaminated with three nitrophenol isomers. Environ Pollut 172, 33–41. [DOI] [PubMed] [Google Scholar]
- Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant and soil 245(1): 35–47. [Google Scholar]
- Dasary SSR, Saloni J, Fletcher A, Anjaneyulu Y, Yu H (2010). Photodegradation of selected PCBs in presence of nano Ti-O2 as catalyst and H2O2 as ana oxidant. Int J Environ Res Public Health 7:3987–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carcer DA, Martin M, Mackova M, Macek T, Karlson U, Rivilla R (2007) The introduction of genetically modified microorganisms designed for rhizoremediation induces changes on native bacteria in the rhizosphere but not in the surrounding soil. ISME Journal 1: 215–223. [DOI] [PubMed] [Google Scholar]
- De J, Ramaiah N, Sarkar A (2006) Aerobic degradation of highly chlorinated polychlorobiphenyls by a marine bacterium, Pseudomonas CH07. World J Microbiol Biotechnol 22: 1321–1327. [Google Scholar]
- Doty SL (2008) Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol 179, 318–333. [DOI] [PubMed] [Google Scholar]
- Eapen S, Singh S, D’Souza SF (2007) Advances in development of transgenic plants for remediation of xenobiotic pollutants. Biotechnol Adv 25: 442–451. [DOI] [PubMed] [Google Scholar]
- Egorova DO, Demakov VA, Plotnikova EG (2013) Bioaugmentation of a polychlorobiphenyl contaminated soil with two aerobic bacterial strains. J Hazard Mater 261: 378–386. [DOI] [PubMed] [Google Scholar]
- Egorova DO, Demakov VA, Plotnikova EG (2011) Destruction of mixture of tri-hexa-chlorinated biphenyls by Rhodococcus genus strains. Appl Bioche Micro 47: 599–606 [PubMed] [Google Scholar]
- EPA (2009). Industrial waste resource guidelines. Environment protection (industrial waste resource) regulations 2009 Environmental Protection Agency; Victoria: (Publication IWRG643.1. September). [Google Scholar]
- Fava F, Bertin L (1999) Use of exogenous specialised bacteria in the biological detoxification of a dump site-polychlorobiphenyl-contaminated soil in slurry phase conditions. Biotechnol Bioeng 64: 240–249. [DOI] [PubMed] [Google Scholar]
- Fava F, Bertin L, Fedi S, Zannon D (2003) Methyl-Beta-Cyclodextrin-enhanced solubilization and aerobic biodegradation of polychlorinated biphenyls in two aged-contaminated soils. Biotechnol Bioeng 81: 381–390. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Golyshin P, Timmis KN (2003) Novel maltotriose esters enhance biodegradation of Aroclor 1242 by Burkholderia cepacia LB400. World J Microb Biot 19: 637–643. [Google Scholar]
- Ficko S, Rutter A, Zeeb B (2011) Effect of pumpkin root exudates on ex situ polychlorinated biphenyl (PCB) phytoextraction by pumpkin and weed species. Environ Sci Pollut Res 18 :1536–43. [DOI] [PubMed] [Google Scholar]
- Ficko SA, Rutter A, Zeeb BA (2010) Potential for phytoextraction of PCBs from contaminated soils using weeds. Sci Total Environ 408:3469–76. [DOI] [PubMed] [Google Scholar]
- Field JA, Sierra-Alvarez R. (2008). Microbial transformation and degradation of polychlorinated biphenyls. Environ Pollut 155: 1–12 [DOI] [PubMed] [Google Scholar]
- Francova K, Sura M, Macek T, Szekeres M, Bancos S, Demnerova K, Sylvestre M, Mackova M (2003) Preparation of plants containing bacterial enzyme for degradation of polychlorinated biphenyls. Fresen Environ bull 12: 309–313. [Google Scholar]
- Fries GF, Marrow GS (1981) Chlorobiphenyl movement from soil to soybean plants. J Agr Food Chem 29(4): 757–759. [DOI] [PubMed] [Google Scholar]
- Gardner K, Aulisio D, Spear JM (2004) In-Situ Dechlorination of Polychlorinated Biphenyls in Sediments Using Zero-Valent Iron. PowerPoint presentation from the RTDF Sediments meeting of February 18–19, 2004 [Google Scholar]
- Gerhardt KI, Huang XD, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminant Potential and challenges. Plant Sci 176: 20–30. [Google Scholar]
- Goi A, Kulik N, Trapido M (2006) Combined chemical and biological treatment of oil contaminated soil. Chemosphere 63(10): 1754–1763. [DOI] [PubMed] [Google Scholar]
- Gomes HI, Dias-Ferreira C, Ribeiro AB (2013) Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci Total Environ 445–446: 237–260. [DOI] [PubMed] [Google Scholar]
- Häggblom MM, Haggblom MH, Bossert ID (2003) Dehalogenation Springer Science & Business Media. [Google Scholar]
- Hashmi MZ, Qin Z, Yao X, Ahmed Z, Xiaomei S, Shen C, Tang X (2016). PCBs attenuation and abundance of Dehalococcoides spp., bphC, CheA, and flic genes in typical polychlorinated biphenyl-polluted soil under floody and dry soil conditions. Environ Sci Pollut Res 23(4): 3907–3913. [DOI] [PubMed] [Google Scholar]
- Hatamian-Zarmi A, Shojaosadati SA, Vasheghani-Farahani E, Hosseinkhani S, Emamzadeh A (2009) Extensive biodegradation of higly chlorinated biphenyl and Aroclor 1242 by Pseudomonas aeruginosa TMU56 isolated from contaminated soils. Int Biodeter Biodegr 63: 788–794. [Google Scholar]
- Hawker DW, Connell DW (1988) Octanol-water partition coefficients of polychlorinated biphenyl congeners. Environ Sci Technol 22: 382–387. [DOI] [PubMed] [Google Scholar]
- Holoubek I (2000) Polychlorinated Biphenyls (PCBs) - World-Wide Contaminated Sites. TOCOEN Report No. 173 [Google Scholar]
- Hulster A, Muller J, Marschner H (1994) Soil-plant transfer of polychlorinated dibenzo-p-dioxins and dibenzofurans to vegetables of the cucumber family (Cucurbitaceae) Environ Sci Technol 28: 1110–1115. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Gunther FA (1976) Translocation of the polychlorinated biphenyl Aroclor 1254 from soil into carrots under field conditions. Arch Environ Con Tox 4(1): 44–59. [DOI] [PubMed] [Google Scholar]
- Javorská H, Tlustoš P, Komárek M, Leštan D, Kaliszová R, Száková J (2009). Effect of ozonation on polychlorinated biphenyl degradation and on soil physico-chemical properties. J Hazard Mater 161(2): 1202–1207. [DOI] [PubMed] [Google Scholar]
- Juwarkar AA, Misra RR, Sharma JK (2014) Recent trends in bioremediation. In Geomicrobiology and Biogeochemistry (pp. 81–100). Springer Berlin Heidelberg. [Google Scholar]
- Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Biotechnol 9:215–288 [Google Scholar]
- Kamei I, Kogura R, Kondo R (2006) Metabolism of 4,4′-Dichlorobiphenyl by White-Rot Fungi Phanerochaete chrysosporium and Phanerochaete sp. MZ142 Appl Microbiol Biot 72: 566–575. [DOI] [PubMed] [Google Scholar]
- Kiely PD, Haynes JM, Higgins CH, Franks A, Mark GL, Morrissey JP, O’Gara F (2006) Exploiting new systems‐ based strategies to elucidate plant‐ bacterial interactions in the rhizosphere. Microb Ecol 51: 257–266 [DOI] [PubMed] [Google Scholar]
- Kjellerup BV, Naff C, Edwards SJ, Ghosh U, Baker JE, Sowers KR (2013) Effects of activated carbon on reductive dechlorination of PCBs by halorespiring bacteria indigenous to sediments. Water Res 52: 1–10. [DOI] [PubMed] [Google Scholar]
- Klasson KT, Reeves ME, Evans BS, Dudley CA (1994) Sequential anaerobic-aerobic degradation of indigenous PCBs in a contaminated soil matrix (No. CONF-941245−−2) Oak Ridge National Lab., TN (United States). [Google Scholar]
- Kubatova A, Erbanova P, Eichlerova I, Homolka L, Nerud F, Šašek V (2001) PCB congener selective biodegradation by the white rot fungus Pleurotus ostreatus in contaminated soil. Chemosphere 43(2): 207–215 [DOI] [PubMed] [Google Scholar]
- Kuipers B, Cullen WR, Mohn WW (2003) Reductive dechlorination of weathered aroclor 1260 during anaerobic biotreatment of Arctic soils. Can J Microbiol 49: 9–14. [DOI] [PubMed] [Google Scholar]
- Kurzawova V, Stursa P, Uhlik O, Norkova K, Strohalm M, Lipov J, et al. (2012) Plant/microorganism interactions in bioremediation of polychlorinated biphenyl-contaminated soil. New Biotechnol 30 :15–22. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L,... & Straif K (2013) Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol 14(4): 287. [DOI] [PubMed] [Google Scholar]
- Le TT, Nguyen KH, Jeon JR, Francis AJ, Chang YS (2015) Nano/bio treatment of polychlorinated biphenyls with evaluation of comparative toxicity. J Hazard Mater 287: 335–341. [DOI] [PubMed] [Google Scholar]
- Leigh MB, Pellizari VH, Uhlik O, Sutka R, Rodrigues J, Ostrom NE, Zhou J, Tiedje JM (2007) Biphenyl-utilizing bacteria and their functional genes in a pine root zone contaminated with polychlorinated biphenyls (PCBs). ISME J 1: 134–148. [DOI] [PubMed] [Google Scholar]
- Leigh MB, Prouzova P, Mackova M, Macek T, Nagle DP, Fletcher JS (2006) Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Appl Environ Microb 72: 2331–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Eng P, Mohamed EISA (2007) Remediation Treatment Technologies: Reference Guide for Developing Countries Facing Persistent Organic Pollutants [Google Scholar]
- Li LKY (2006), Chemical Reduction/Oxidation Advanced Physicochemical Treatment Processes, in: Wang LK, Hung Y-T, Shammas NK (Eds.), Humana Press, 483–519. [Google Scholar]
- Liste HH, Alexander M (2000) Accumulation of phenanthrene and pyrene in rhizosphere soil. Chemosphere 40(1): 11–14. [DOI] [PubMed] [Google Scholar]
- Liu JY, Schnoor JL (2008) Uptake and translocation of lesser-chlorinated polychlorinated biphenyls (PCBs) in whole hybrid poplar plants after hydroponic exposure. Chemosphere 73(10): 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ma M, Shi Z, Deng Y, Zeng X, Hong Y (2012) A study on remediation of PCBs-contaminated soil by a combination of biosurfactant washing, UV-irradiation and biodegradation. Adv Sci Lett 10(1): 344–348. [Google Scholar]
- Liu YS, Ma MY, Shi Z (2011) Application of rhamnolipid biosurfactant for removing polychlorinated biphenyls from contaminated soil. Adv Mat Res 233: 608–613. [Google Scholar]
- Long YY, Fang Y, Zhang C, Du Y, Shentu J, Shen DS (2015) Degradation of Polychlorinated Biphenyls by Sequential Anaerobic–Aerobic Composting. Water Air Soil Poll 226(3): 1–12. [Google Scholar]
- Low JE, Whitfield Åslund ML, Rutter A, Zeeb BA (2010) Effect of Plant Age on PCB Accumulation by Cucurbita pepo ssp. Pepo. J Environ Qual 39(1): 245–250. [DOI] [PubMed] [Google Scholar]
- Luo C, Wang S, Wang Y, Yang R, Zhang G, Shen Z (2015) Effects of EDDS and plant-growth-promoting bacteria on plant uptake of trace metals and PCBs from e-waste–contaminated soil. J Hazard Mater 286: 379–385. [DOI] [PubMed] [Google Scholar]
- Macedo AJ, Neu TR, Kuhlicke U, Abraham WR (2007) Adaptation of microbial communities in soil contaminated with polychlorinated biphenyls, leading to the transformation of more highly chlorinated congeners in biofilm communities. Biofilms 3: 37–46. [Google Scholar]
- Mackova M, Barriault D et al. (2006) Phytoremediation of polychlorinated biphenyls in “Phytoremediation Rhizoremediation Springer Netherlands; 143–167. [Google Scholar]
- Mackova M, Prouzova P, Stursa P, Ryslava E, Uhlik O, Beranova K, Rezek J, Kurzawova V, Demnerova K, Macek T (2009) Phyto/rhizoremediation studies using long-term PCB-contaminated soil. Environ Sci Pollut R 16: 817–829. [DOI] [PubMed] [Google Scholar]
- Master ER, Lai VV, Kuipers B, Cullen WR, Mohn W (2002) Sequential anaerobic–aerobic treatment of soil contaminated with weathered Aroclor 1260. Environ Sci Technol 36: 100–103. [DOI] [PubMed] [Google Scholar]
- Mattina MI, Berger WA, Eitzer BD (2007) Factors affecting the phytoaccumulation of weathered, soil-borne organic contaminants: analyses at the ex planta and in planta sides of the plant root. Plant Soil 291: 143–154 [Google Scholar]
- Matturro B, Ubaldi C, Grenni P, Caracciolo AB, Rossetti S (2016) Polychlorinated biphenyl (PCB) anaerobic degradation in marine sediments: microcosm study and role of autochthonous microbial communities. Environ Sci Pollut Res 23(13): 12613–12623. [DOI] [PubMed] [Google Scholar]
- May HD, Miller GS, Kjellerup BV, Sowers KR. (2008) Dehalorespiration with polychlorinated biphenyls by an anaerobic ultramicrobacterium. Appl Environ Microbioly 74(7): 2089–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon SC, Schnoor JL (2004) Phytoremediation: transformation and control of contaminants (Vol. 121). John Wiley & Sons. [Google Scholar]
- Meggo RE, Schnoor JL (2013) Cleaning polychlorinated biphenyl (PCB) contaminated garden soil by phytoremediation. Environ Sci 1:33–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer SN, Ockenden WA, Sweetman A, Breivik K, Grimalt JO, Jones KC (2003). Global distribution and budget of PCBs and HCB in background surface soils: implications for sources and environmental processes. Environ Sci Technol 37:667–72. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Chalavi V, Novakova-Sura M, Laliberté JF, Sylvestre M (2007): Expression of bacterial biphenyl-chlorobiphenyl dioxygenase genes in tobacco plants. Biotechnol Bioeng, 97(3): 496–505. [DOI] [PubMed] [Google Scholar]
- Nanekar S, Dhote M, Kashyap S, Singh SK, Juwarkar AA (2015) Microbe assisted phytoremediation of oil sludge and role of amendments: a mesocosm study. Int J Environ Sci Technol, 12(1): 193–202. [Google Scholar]
- Nanekar SV, Juwarkar AA (2015). Environmental Biotechnology: A Quest for Sustainable Solutions. In Plant Biology and Biotechnology (pp. 663–671). Springer India. [Google Scholar]
- Newman LA, Reynolds CM (2004) Phytodegradation of organic compounds. Curr Opin Biotech 15: 225–230. [DOI] [PubMed] [Google Scholar]
- Nollet H, Van de Putte I, Raski L, Verstraete W (2005) Carbon/electron source dependence of polychlorinated biphenyl dechlorination pathways for anaerobic granules. Chemosphere 58: 299–310. [DOI] [PubMed] [Google Scholar]
- Novakova H, Vosahlíkova M, Pazlarova J, Mackova A, Burkhard J, Demnerova K (2002) PCB metabolism by Pseudomonas sp. P2. Int Biodeter Biodegr 50:47–54. [Google Scholar]
- Novakova M, Mackova M, Chrastilova Z, Viktorova J, Szekeres M, Demnerova K, Macek T, (2009) Cloning the bacterial bphC gene into Nicotiana tabacum to improve the efficiency of PCB phytoremediation. Biotechnol Bioeng 102: 29–37. [DOI] [PubMed] [Google Scholar]
- O’Riordan T (1995). Environmental sciences for environmental management New York: John Wiley and Sons Inc. [Google Scholar]
- Ohtsubo Y, Kudo T, Tsuda M, Nagata Y (2004). Strategies for bioremediation of polychlorinated biphenyls. Appl Microbiol Biot 65: 250–258 [DOI] [PubMed] [Google Scholar]
- Pakdeesusuk U, Lee CM, Coates JT, Freedman DL (2005) Assessment of natural attenuation via in situ reductive dechlorination of polychlorinated biphenyls (PCBs) in sediments of the Twelve Mile Creek Arm of Lake Hartwell, SC. Environ Sci Technol 39: 945–952. [DOI] [PubMed] [Google Scholar]
- Passatore L, Rossetti S, Juwarkar AA, Massacci A (2014) Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): state of knowledge and research perspectives. J Hazard Mater 278: 189–202. [DOI] [PubMed] [Google Scholar]
- Payne RB, May HD, Sowers KR (2011) Enhanced reductive dechlorination of polychlorinated biphenyl impacted sediment by bioaugmentation with a dehalorespiring bacterium. Environ. Sci. Technol 45: 8772–8779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petric I, Hrsak D, Fingler S, Udikovic-Koli´ c N, Bru D, Martin-Laurent F (2011) Insight in the PCB-degrading functional community in long-term contaminated soil under bioremediation. J Soils Sediments 11: 290–300. [Google Scholar]
- Petroutsos D, Katapodis P, Samiotaki M, Panayotou G, Kekos D (2008) Detoxification of 2, 4-dichlorophenol by the marine microalga Tetraselmis marina. Phytochemistry 69: 707–714. [DOI] [PubMed] [Google Scholar]
- Pieper DH (2005) Aerobic degradation of Polychlorinated Biphenyls. Appl Microbiol Biot 67: 170–191. [DOI] [PubMed] [Google Scholar]
- Pinedo-Rilla C, Aleu J, Collado IG (2009) Pollutants biodegradation by fungi. Curr Org Chem 13:1194–1214 [Google Scholar]
- Pinsker NI (2011) Phytoremediation of PCB Contaminated Soil: Effectiveness and Regulatory Policy (Doctoral dissertation, Virginia Commonwealth University; Richmond, Virginia: ). [Google Scholar]
- Plotnikova EG, Solyanikova IP, Egorova DO, Shumkova ES, Golovleva LA (2012) Degradation of 4-chlorobiphenyl and 4-chlorobenzoic acid by the strain Rhodococcus ruber P25. Microbiology 81: 143–153 [PubMed] [Google Scholar]
- Prasad MNV (2011) A state-of-the-art report on bioremediation, its applications to contaminated sites in India Ministry of Environment and Forests, New Delhi: URL: http://moef.nic.in/downloads/public-information/BioremediationBook.pdf [Google Scholar]
- Prządo D, Kafarski P, Steininger M (2007) Studies on degradation of polychlorinated biphenyls by means of Fenton’s reagent. Pol J Environ Stud 16: 881–887. [Google Scholar]
- Qin H, Brookes PC, Xu J (2014) Cucurbita spp. and Cucumis sativus enhance the dissipation of polychlorinated biphenyl congeners by stimulating soil microbial community development. Environ Pollut 184: 306–312. [DOI] [PubMed] [Google Scholar]
- Quensen JF III, Boyd SA, Tiedje JM (1990) Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl Environ Microb 56(8), 2360–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahuman M, Pistone L, Trifirò F, Miertus S (2000) Destruction Technologies for Polychlorinated Biphenyls. Proceedings of Expert Group Meetings on POPs and Pesticides Contamination: Remediation Technologies (April 2000) and on Clean Technologies for the Reduction and Elimination of POPs (May 2000) United Nations Industrial Development Organization (ICS-UNIDO). [Google Scholar]
- Ray S, Karpouzas D and Singh BK (2012) Emerging technologies in bioremediation: constraints and opportunities. Biodegradation, 23: 917–926 [DOI] [PubMed] [Google Scholar]
- Rein A (2006) Remediation of PCB-contaminated soils-Risk analysis of biological in situ processes (Doctoral dissertation, Universität Tübingen; ). [Google Scholar]
- Rein A, Fernqvist MM, Mayer P, Trapp S, Bittens M, Karlson UG (2007) Degradation of PCB congeners by bacterial strains. Appl Microbiol Biot 77: 469–481. [DOI] [PubMed] [Google Scholar]
- Rodrigues JLM, Kachel CA, Aiello MR, Quensen JF III, Maltseva OV, Tsoi TV, Tiedje JM (2006) Degradation of Aroclor 1242 Dechlorination Products in Sediments by Burkholderia xenovorans LB400(Ohb) and Rhodococcus sp. Strain RHA1(Fcb). Appl Environ Microb 72: 2476–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G (2004) The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotox Environ Safe 59: 275–291. [DOI] [PubMed] [Google Scholar]
- Rybkina DO, Plotnikova EG, Dorofeeva LV, Mironenko YL, Demakov VA (2003) A new aerobic gram-positive bacterium with a unique ability to degrade ortho- and para-chlorinated biphenyls. Microbiology 72: 672–677 [PubMed] [Google Scholar]
- Rysavy JP, Yan T, Novak PJ (2005) Enrichment of anaerobic Polychlorinated Biphenyl dechlorinators from sediment with Iron as a Hydrogen source. Water Res 39: 569–57. [DOI] [PubMed] [Google Scholar]
- Safe S (1984) Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): Biochemistry, toxicology, mechanism of action. CRC Cr Rev Toxicol 13:310–395. [DOI] [PubMed] [Google Scholar]
- Segura A, Rodríguez-Conde S, Ramos C, Ramos JL (2009) Bacterial responses and interactions with plants during rhizoremediation. Microb Biotech 2(4): 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma JK, Gautam RK, Misra RR, Kashyap SM, Singh SK, Juwarkar AA (2014) Degradation of di-through hepta-chlorobiphenyls in clophen oil using microorganisms isolated from long term PCBs contaminated soil. Indian J Microbiol 54(3): 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra I, Valera JL, Marina ML, Laborda F (2003) Study of the biodegradation process of polychlorinated biphenyls in liquid medium and soil by a new isolated aerobic bacterium (Janibacter sp.). Chemosphere 53: 609–618. [DOI] [PubMed] [Google Scholar]
- Singer AC, Gilbert ES, Luepromchai E, Crowley DE (2000) Bioremediation of polychlorinated biphenyl-contaminated soil using carvone and surfactant-grown bacteria. Appl Microbiol Biot 54: 838–843. [DOI] [PubMed] [Google Scholar]
- Singer AC, Smith D, Jury WA, Hathuc K, Crowley DE (2003) Impact of the plant rhizosphere and augmentation on remediation of polychlorinated biphenyls contaminated soil. Environ Toxicol Chem 22: 1998–2004. [DOI] [PubMed] [Google Scholar]
- Singh A, Kuhad RC, Ward OP (2009) Biological remediation of soil: an overview of global market and available technologies. Advances in applied bioremediation 1–19, Springer Berlin; Heidelberg. [Google Scholar]
- Singh BK (2009) Organophosphorus-degrading bacteria: ecology and industrial applications. Nat Rev Microbiol 7: 156–164. [DOI] [PubMed] [Google Scholar]
- Singh BK (2010) Exploring microbial diversity for biotechnology: the way forward. Trends Microbiol 28: 111–116. [DOI] [PubMed] [Google Scholar]
- Smith KE, Schwab AP, Banks MK (2007) Phytoremediation of Polychlorinated Biphenyl (PCB)-contaminated sediment: A greenhouse feasibility study. J Environ Qual 36: 239–44. [DOI] [PubMed] [Google Scholar]
- Sonoki S, Fujihiro S, Hisamatsu S (2007). Genetic engineering of plants for phytoremediation of polychlorinated biphenyls. In Phytoremediation, Humana Press; 3–13 [Google Scholar]
- Sul WJ, Park J, Tsoi TV, Zysstra GJ, Tiedje JM (2009) DNA- stable isotope probing integrated with metagenomics for retrieval of biphenyl dioxygnase genes from polychlorinated biphenyl contaminated river sediments. Appl Environ Microb 75: 5501–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre M, Macek T, Mackova M (2009) Transgenic plants to improve rhizoremediation of polychlorinated biphenyls (PCBs). Cur Opin Biotech 20(2): 242–247. [DOI] [PubMed] [Google Scholar]
- Teng Y, Luo Y, Sun X, Tu C, Xu L, Liu W et al. (2010) Influence of arbuscular Mycorrhiza and Rhizobium on phytoremediation by alfalfa of an agricultural soil contaminated with weathered PCBs: a field study. Int J Phytoremediat 12:516–33. [DOI] [PubMed] [Google Scholar]
- Tharakan J, Tomlinson D, Addagada A, Shafagati A (2006) Biotransformation of PCBs in contaminated sludge: Potential for novel biological technologies. Eng Lif Sci 6: 43–50. [Google Scholar]
- Tiedje JM, Quensen JF III, Chee-Sanford J, Schimel JP, Cole JA Boyd SA (1993) Microbial reductive dechlorination of PCBs. Biodegradation 4: 231–240. [DOI] [PubMed] [Google Scholar]
- Toure O, Chen YQ, Dutta SK (2003) Sinorhizobium meliloti electrotransporant containing ortho-dechlorination gene shows enhanced PCB dechlorination. Fresen Environ Bull 12(3): 320–322. [Google Scholar]
- Tu C, Teng Y, Luo Y, Li X, Sun X, Li Z, Liu W, Christie P (2011) Potential for biodegradation of polychlorinated biphenyls (PCBs) by Sinorhizobium meliloti. J Hazard Mater 186: 1438–1444. [DOI] [PubMed] [Google Scholar]
- Uhlik O, Jecna K, Mackova M, Vlcek C, Hroudova M, Demnerova K, Paces V, Macek T (2009) Biphenyl-metabolizing bacteria in the rhizosphere of Horseradish and bulk soil contaminated by polychlorinated biphenyls as revealed by stable isotope probing. Appl Environ Microb 75: 6471–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKEPA (2004). Framework for the classification of contaminated soils as hazardous waste United Kingdom Environmental Protection Agency. [Google Scholar]
- UNIDO (2010) persistent organic pollutants: contaminated site investigation and management toolkit [Google Scholar]
- USEPA (2004) Emerging Technologies for the in Situe Remediation of PCB-contaminated Soils and Sediments: Bioremediation and Nanoscale Zero-valent Iron [Google Scholar]
- USEPA (2011) Search Superfund Site Information http://cumulis.epa.gov/supercpad/cursites/srchsites.cfm. [Google Scholar]
- USEPA (2012) PCB regulations at 40 CFR Part 761 United States Environmental Protection Agency. Toxic Substances Control Act. [Google Scholar]
- Vidali M (2001) Bioremediation. An overview. Pure Appl Chem 73(7): 1163–1172. [Google Scholar]
- Viisimaa M, Karpenko O, Novikov V, Trapido M, Goi A (2013) Influence of biosurfactant on combined chemical–biological treatment of PCB-contaminated soil. Chem Eng J 220: 352–359. [Google Scholar]
- Viktorová J, Novakova M, Trbolova L, Vrchotova B, Lovecka P, Mackova M, Macek T (2014) Characterization of Transgenic Tobacco Plants Containing Bacterial bphc Gene and Study of Their Phytoremediation Ability. Int J Phytoremediat 16(9): 937–946. [DOI] [PubMed] [Google Scholar]
- Villacieros M, Whelan C, Mackova M, Molgaard J, Sanchez-Contreras M, Lloret J, de Carcer A, Oruezabal RI, Bolanos L, Macek T, Karlson U, Dowling DN, Martin M, Rivilla R (2005) Polychlorinated biphenyl rhizoremediation by Pseudomonas fluorescens F113 derivatives, using a Sinorhizobium meliloti nod system to drive bph gene expression. Appl Environ Microb 71: 2687–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang S, Huang H, Zhao M, Lv J (2011) Uptake, translocation and metabolism of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in maize (Zea mays L.). Chemosphere 85 :379–85. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ren H, Pan H, Liu J, Zhang L. (2015) Enhanced tolerance and remediation to mixed contaminates of PCBs and 2, 4-DCP by transgenic alfalfa plants expressing the 2, 3-dihydroxybiphenyl-1, 2-dioxygenase. J Hazard Mater 286: 269–275. [DOI] [PubMed] [Google Scholar]
- Watts JEM, Fagervold SK, May HD, Sowers KR (2005) A PCR-based specific assay reveals a population of bacteria within the Chloroflexi associated with the reductive dehalogenation of polychlorinated biphenyls. Microbiology 151: 2039–2046. [DOI] [PubMed] [Google Scholar]
- Webber MD, Pietz RI, Granato TC, Svoboda ML (1994) Plant uptake of PCBs and other organic contaminants from sludge-treated coal refuse. J Environ Qual 23(5): 1019–1026. [DOI] [PubMed] [Google Scholar]
- Weber R (2007) Relevance of PCDD/PCDF formation for the evaluation of POPs destruction technologies–review on current status and assessment gaps. Chemosphere 67(9): S109–S117. [DOI] [PubMed] [Google Scholar]
- White J, Parrish Z, Isleyen M, Gent M, Iannucci-Berger W, Eitzer B, Kelsey J, Mattina M (2006) Influence of citric acid amendments on the availability of weathered PCBs to plant and earthworm species. Int J Phytorem 8: 63–79. [DOI] [PubMed] [Google Scholar]
- WHO (2003) Polychlorinated biphenyls: Human health aspects [Google Scholar]
- Wiegel J, Wu Q (2000) Microbial Reductive Dehalogenation of Polychlorinated Biphenyls. FEMS Microbiol Ecol 32: 1–15. [DOI] [PubMed] [Google Scholar]
- Xu L, Teng Y, Li ZG, Norton JM, Luo YM (2010) Enhanced removal of polychlorinated biphenyls from alfalfa rhizosphere soil in a field study: the impact of a rhizobial inoculum. Sci Total Environ 408(5): 1007–1013. [DOI] [PubMed] [Google Scholar]
- Yadav JS, Quensen JF, Tiedje JM, Reddy CA (1995) Degradation of polychlorinated biphenyl mixtures (Aroclors 1242, 1254, and 1260) by the white rot fungus Phanerochaete chrysosporium as evidenced by congener-specific analysis. Appl Environ Microbiol 61(7): 2560–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, LaPara TM, Novak PJ (2006) The reductive dechlorination of 2,3,4,5-tetrachlorobiphenyl in three different sediment cultures: evidence for the involvement of phylogenetically similar Dehalococcoides-like bacterial populations. FEMS Microbiol Ecol 55: 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S (1994) Enhancement of PCB congener biodegradation by pre-oxidation with Fenton’s reagent. Water Sci Technol 30(7): 105–113. [Google Scholar]
- Yearbook MW (2000). Yearbook 2000–2001 [Google Scholar]
- Zeeb BA, Amphlett JS, Rutter A, Reimer KJ (2006) Potential for Phytoremediation of Polychlorinated Biphenyl-(PCB)-contaminated soil. Int J Phytoremediat 8: 199–221. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zhu Z, Jing L, Cai Q, Li Z (2012) Pilot-scale demonstration of biosurfactant-enhanced in-situ bioremediation of a contaminated site in newfoundland and labrador [Google Scholar]


