Abstract
Background
Sunitinib, used widely in metastatic renal cell carcinoma (mRCC), can result in hypertension, left ventricular (LV) dysfunction, and heart failure. However, the relationships between vascular function and cardiac dysfunction with sunitinib are poorly understood.
Methods and Results
In a multi-center prospective study of 84 mRCC patients, echocardiography, arterial tonometry, and b-type natriuretic peptide (BNP) measures were performed at baseline, 3.5, 15, and 33 weeks following sunitinib initiation, correlating with sunitinib cycles 1, 3, and 6. Mean change in vascular function parameters and 95% confidence intervals were calculated. Linear regression models were used to estimate associations between vascular function and LV ejection fraction (LVEF), longitudinal strain, diastolic function (E/e’), and BNP. Following 3.5 weeks of sunitinib, mean systolic blood pressure (BP) increased by 9.5 mmHg (95% CI 2.0, 17.1, p=0.02) and diastolic BP by 7.2 mmHg (95% CI 4.3, 10.0, p<0.001) across all participants. Sunitinib resulted in increases in large artery stiffness (carotid-femoral pulse wave velocity) and resistive load (total peripheral resistance (TPR), arterial elastance (EA)) (all p<0.05) as well as changes in pulsatile load (total arterial compliance, wave reflection). There were no statistically significant associations between vascular function and systolic dysfunction (LVEF, longitudinal strain). However, baseline TPR, EA, and aortic impedance were associated with worsening diastolic function and filling pressures over time.
Conclusions
In patients with mRCC, sunitinib resulted in early, significant increases in BP, arterial stiffness, resistive and pulsatile load within 3.5 weeks of treatment. Baseline vascular function parameters were associated with worsening diastolic, but not systolic function.
Keywords: Hypertension, Tonometry, Sunitinib, Cardio-Oncology, Cardiotoxicity
Introduction
Several vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors (TKI) have led to increases in progression-free and overall survival in the metastatic renal cell cancer (mRCC) population (1–7). Sunitinib, an oral VEGF receptor directed therapeutic agent with activity against all 3 VEGF receptors, platelet-derived growth factor receptor (PDGF) α and β, stem cell factor receptor (cKIT), and fms-like tyrosine kinase receptor 3 (FLT3) (8), is used as first line therapy for mRCC (9).
Unfortunately, these therapies are associated with significant cardiovascular toxicities as they also interfere with fundamental cardiovascular signaling pathways. VEGF-inhibitor TKIs have been associated with hypertension (HTN), heart failure (HF), and left ventricular (LV) dysfunction (10–14). HTN associated with VEGF-inhibitor therapy is the most common cardiovascular side effect, with an estimated incidence of 19 to 47% (15). LV dysfunction can also occur, and data from our own group, acquired through careful prospective cardiac monitoring, suggests that overall, 9.7% experience declines in LV ejection fraction (LVEF) of ≥10% to <50% (16). LVEF declines tended to occur early, primarily within the first cycle (3.5 weeks) of exposure to sunitinib therapy.
Despite the significance of this problem, detailed assessments and a comprehensive understanding of the effects of VEGF inhibitors on vascular function parameters are lacking. While arterial blood pressure (BP) is often used clinically as a surrogate for LV afterload, LV afterload is actually a complex time-varying process determined by arterial stiffness, steady-state resistive and pulsatile load (Figures 1 and 2) (17). The mechanical load imposed by the systemic circulation to the LV is a critical part of normal cardiovascular function, but also plays an important role in the pathophysiology of various cardiac conditions, including HTN, HF, and LV remodeling. While it is speculated that the development of HTN leads to LV dysfunction with sunitinib, the relationship between the development of sunitinib related HTN and HF has not been precisely defined (10, 12, 15, 18–19). In this multi-center, longitudinal prospective cohort study, we performed detailed non-invasive characterization of hemodynamic and arterial function among patients with mRCC receiving sunitinib, and determined the relationships between the vasculature and LV function, using repeated measures derived from arterial tonometry and transthoracic echocardiography. A priori, we hypothesized that baseline and early changes in vascular function would be associated with LV dysfunction.
Figure 1. Example of Measurements of Central Pressure and Flow Data.
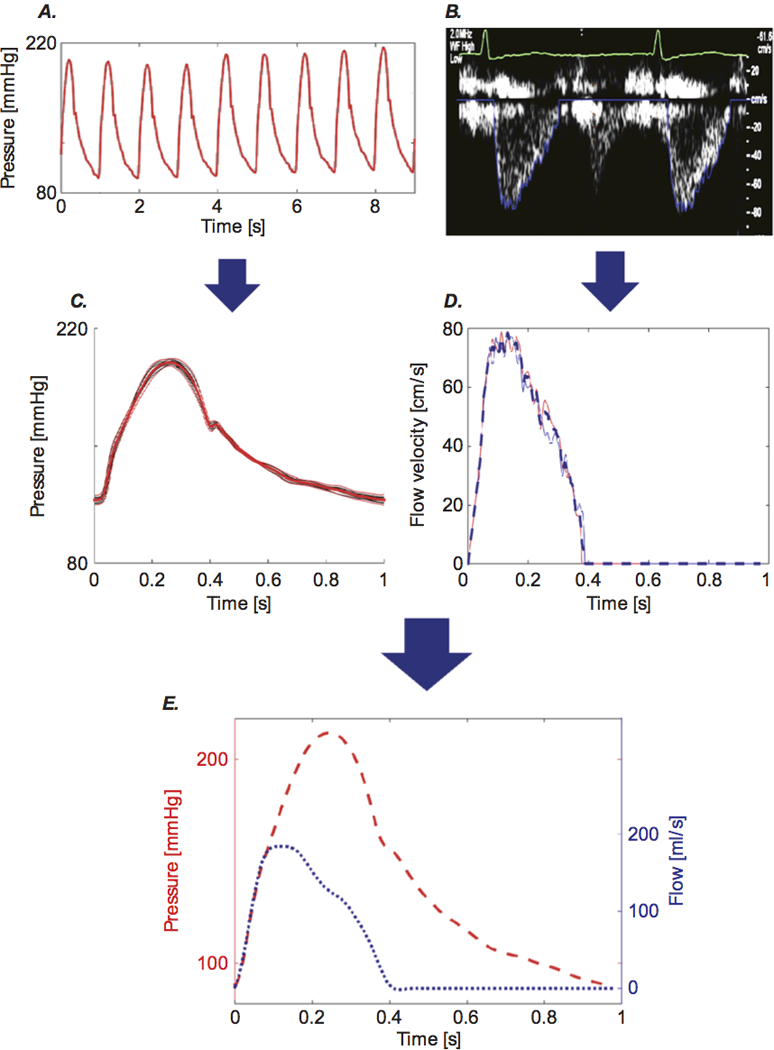
Carotid tonometry recordings of pressure (A) and Doppler recordings of aortic inflow (B) are processed to obtain signal-averaged waveforms (C and D), which are subsequently aligned to obtain a pressure-flow pair (E). Reproduced with permission, from Chirinos JA and Sweitzer N, Cardiac Failure Review. 2017 (17).
Figure 2. Assessment of Arterial Load and Ventricular-Arterial Interactions with Pressure-Flow Relations.
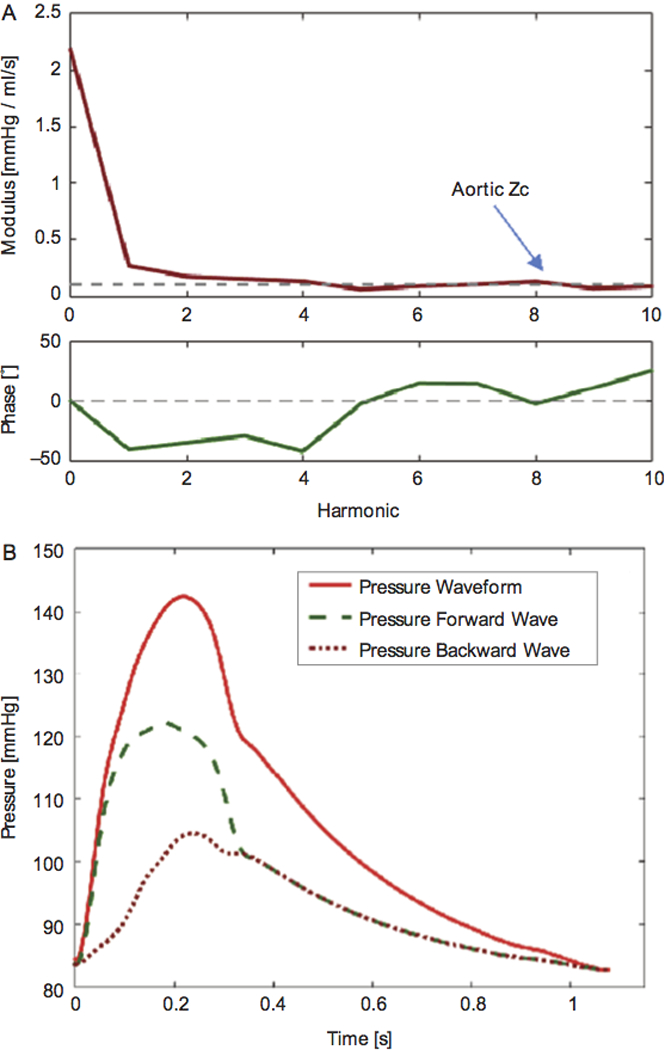
(A) Modulus (top) and phase (bottom) of aortic input impedance. The dashed line in the modulus plot represents the pulsatile load measure aortic characteristic impedance (Zc). (B) Wave separation analysis showing forward (green dashed line) and backward (red dotted line) waves. Reproduced with permission, from Chirinos JA and Sweitzer N, Cardiac Failure Review. 2017 (17).
Methods
Study Design
This was a multicenter prospective cohort study performed at the University of Pennsylvania, Vanderbilt University Medical Center, University of Wisconsin, University Hospitals Case Medical Center, and University of Utah Hospital (16). Eligible participants were accrued between December 2011 and December 2015 and included patients with mRCC who planned to initiate sunitinib therapy. Sunitinib starting dose, schedule, and dose adjustments were determined at the discretion of the treating medical oncologist. All participants provided written informed consent, and the study protocol was approved by the Institutional Review Board at each participating site.
Prior to the initiation of sunitinib, enrolled participants underwent a detailed review of their medical history, including prior cardiac events, cardiovascular risk factors, and current medications, with all findings verified by the oncology provider. All participants underwent a baseline transthoracic echocardiogram, BP assessment, and arterial tonometry.
Study follow-up was designed to detect both the peak and late incidences of LV dysfunction as a result of sunitinib exposure (20). Accordingly, echocardiograms and arterial tonometry were performed at 3.5 weeks (+/− 1 week), 15 weeks (+/− 2 weeks), and 33 weeks (+/− 2 weeks) after starting sunitinib. These follow-up visits coincided with cycles 1, 3 and 6 of sunitinib therapy, respectively, and were timed to occur while the participant was actively taking sunitinib. The decision to initiate anti-hypertensive or other cardiovascular medications was at the discretion of the treating medical provider. In the event of sunitinib discontinuation due to disease progression or intolerable toxicity, follow-up cardiac assessments were obtained per protocol.
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Transthoracic Echocardiography
Transthoracic echocardiograms were performed at the participating sites according to a standardized protocol. Two-dimensional images were acquired using the Philips IE33 platform (Andover, MA). Echocardiography quantitation was independently performed in the imaging core laboratory at the University of Pennsylvania by trained sonographers blinded to patient characteristics and tonometry data. Quantification of end-systolic and end-diastolic LV volumes, LVEF, and diastolic function indices were performed using TomTec Image Arena (TomTec Imaging Systems, Unterschleissheim, Germany), as previously described (16, 21–22) (Supplemental Methods). The E/e’ ratio was used as an estimate of diastolic function and LV filling pressures. LV outflow tract (LVOT) velocities were measured with pulsed wave Doppler in the apical 5-chamber view. LVOT cross-sectional area was computed from its diameter (d) measured in the parasternal long axis view (area=π(d/2)2). LV volume outflow (equal to aortic inflow) was computed as velocity times the cross-sectional area. Intraobserver coefficient of variation (CV) for LVEF was 4.4% respectively. The intraobserver CVs for mitral inflow and tissue Doppler velocities were 2.3–5.4%.
Peak longitudinal systolic strain measurements were performed on digitally archived images on the TomTec Cardiac Performance Analyses (TomTec Imaging Systems, Unterschleissheim, Germany) as previously described (16, 23) (Supplemental Methods). Intraobserver CV for longitudinal strain was 10.9%.
Arterial Tonometry and Assessment of Arterial Function
Arterial applanation tonometry was performed using a commercially available applanation tonometry system (SphygmoCor Vx System, AtCor Medical, Sydney, Australia) equipped with a high-fidelity Millar tonometer (SPT 301, Millar instruments, Houston Tx) at the same time as the echocardiograms. Sites were required to submit qualification studies prior to patient enrollment, and quality control metrics were assessed real time with each study. Resting peripheral BP was also recorded using a validated oscillometric device (Omron HEM-705CP, Omron Corp, Kyoto, Japan). Arterial tonometry was performed at the radial, carotid, and femoral arteries. Radial tonometry waveforms were calibrated using oscillometric brachial systolic (SBP) and diastolic blood pressure (DBP). Mean arterial pressure (MAP) was computed from the radial pressure waveform. Carotid tonometry waveforms were calibrated using MAP and DBP. Carotid-femoral PWV was measured with sequential carotid and femoral arterial tonometry, to compute the time delay between the two sites, using the QRS complex as a fiducial point. The distance between the suprasternal notch and the carotid recording point was subtracted from the distance between the sternal notch and the femoral recording point (femoral pulse to the umbilicus and then to the midline point of the suprasternal notch), and carotid-femoral PWV was calculated as distance/change in time.
Vascular Hemodynamics
We used custom-designed software in MATLAB (R2014b, MathWorks, Natick, MA) to characterize arterial load, as previously described (24–28). A trained operator assessed the reproducibility of beat-to-beat tonometry signals, the signal averaged waveform, and evaluated all Doppler waveforms visually. Doppler waveforms were then digitized using semi-automated algorithms. A cardiologist experienced in pressure-flow analyses (JAC) provided a final quality control (Supplemental Methods). Central pressure measurements were ensemble-averaged and time-aligned with LVOT flow such that the upstroke of pressure and flow occurred simultaneously, peak flow was coincident with the first systolic peak or inflection point in the pressure waveform, and flow ceased at the dicrotic notch. Zc was quantified in the frequency domain as the average modulus at higher frequencies. Total peripheral resistance (TPR) was quantified as the ratio of mean pressure to mean flow. Total arterial compliance (TAC) was determined using the pulse-pressure method (24). Linear wave separation was performed to obtain the amplitude of the forward (Pf) and backward (Pb) pressure waves. Reflection magnitude was defined as the ratio of Pb to Pf (Pb/Pf) (25–27). Reflected wave transit time was estimated via tube-load modeling (27). Figures 1 and 2 illustrate the vascular function parameters derived from arterial tonometry and echocardiography (17). Effective arterial elastance (EA), a commonly used index of arterial load, was computed as the ratio of end-systolic pressure/stroke volume. EA depends on the interplay between systemic vascular resistance and heart rate, with negligible influence of pulsatile load (28). After identification of the 1st (P1) and 2nd (P2) systolic peaks in the central pressure waveform, augmentation index was computed as the augmented pressure (P2-P1) expressed as a percentage of central pulse pressure. Augmentation index is a composite measure dependent on several factors, including the magnitude and timing of wave reflections, and the LV ejection pattern.
Biomarker Analyses
Plasma samples were collected in EDTA tubes, processed at 3353 RPM for 20 minutes at room temperature, aliquoted, and stored at −80°C until the time of assay. BNP was quantitatively measured using the Singulex Single Molecule Counting laboratory assay (Alameda, CA) (29).
Statistical Analysis
Baseline patient characteristics were described using counts and percentages for categorical variables and medians and interquartile ranges (IQR) for continuous variables. Baseline vascular function parameters were also described using medians and IQRs. Longitudinal trends in vascular function measures were assessed using piecewise linear regression with confidence intervals (CI) estimated using robust (Huber-White) sandwich-based standard errors (30–32) to illustrate the mean trend in vascular function measures across visits.
For BP and all vascular function parameters, the mean changes from visit 1 to visit 2 and the 95% CI were computed. Hypothesis tests for change from visit 1 to visit 2 were conducted using one sample t-tests. These changes were also evaluated in a subgroup of participants who did not have any new anti-hypertensive therapy initiated between visits 1 and 2. The effect of adjustment for heart rate and mean arterial pressure (MAP) on these changes was also explored.
The relationship between baseline vascular function measures and change in LVEF at each visit relative to baseline was examined using linear regression models estimated via generalized estimating equations (GEE) with an independence working correlation structure to account for within-subject correlation (33). The independence working correlation was chosen to avoid the possibility of bias which may be induced when using more complex working correlation structures (34). Unadjusted models included visit number and individual vascular function variables. Adjusted models additionally included age and gender. To allow for comparison of the presented relationships, vascular function measures were scaled by their standard deviation. Therefore, reported parameter estimates represent estimated average difference in the change in LVEF relative to baseline associated with a one standard deviation increase in a given vascular function measure. Unadjusted and adjusted regression models were also estimated for longitudinal strain, E/e’, and log10BNP (outcome measure) using GEE, with a separate regression model estimated for each combination of outcome measure and vascular function measure. BNP was log transformed given its skewed distribution. The associations between changes in vascular function measures between visit 1 and visit 2 and changes in outcome measures (LVEF, longitudinal strain, E/e’, log10BNP) between visit 1 and visit 2 and, separately, between visit 1 and visit 3 were estimated using linear regression.
All statistical analyses were conducted using R 3.4.0 (R Foundation for Statistical Computing, Vienna Austria). Statistical significance was evaluated at a two-sided alpha of 0.05 level.
Results
Patient Population
This study consisted of 84 patients who were prospectively enrolled prior to initiation of sunitinib therapy and underwent baseline echocardiography and arterial tonometry. Baseline characteristics are summarized in Table 1. The median age was 62.5 years (IQR 55.8, 68.0). HTN (55%) and hyperlipidemia (52%) were highly prevalent at baseline, as was the use of anti-hypertensive therapy, although no patients were treated with nitrates. Baseline BP measurements and vascular parameters are shown in Table 2.
Table 1. Baseline Patient Characteristics (N=84).
| Variable | N (%) |
|---|---|
| Age | |
| Median (IQR) | 62.5 (55.8, 68.0) |
| Sex | |
| Male | 56 (67.0) |
| Prior Systemic RCC Therapy | |
| None | 73 (86.9) |
| IL-2 | 6 (7.1) |
| Other targeted agent | 5 (6.0) |
| Sunitinib Starting Dose | |
| 50 mg | 50 (59.5) |
| 37.5 mg | 4 (4.8) |
| 25 mg | 5 (6.0) |
| Other (Escalating Dose) | 25 (29.8) |
| Baseline Echo Parameters | |
| LVEF (%) Median (IQR) | 50.4 (45.1, 54.2) |
| GLS (%) Median (IQR) | -14.0 (−11.5, −15.5) |
| E/e’ Median (IQR) | 8.5 (6.9, 10.8) |
| Baseline Cardiac Biomarkers | |
| BNP (IQR), pg/ml | 31.7 (16.6, 64.8) |
| Baseline BMI | |
| Median (IQR), kg/m2 | 27.9 (24.2, 33.4) |
| Baseline Co-morbidities | |
| Hypertension | 46 (55.4) |
| Coronary artery disease | 8 (9.5) |
| Heart Failure | 3 (3.6) |
| Hyperlipidemia | 44 (52.4) |
| Diabetes Mellitus | 20 (23.8) |
| Tobacco Use | 7 (8.4) |
| Baseline Cardiac Medication Use | |
| Aspirin | 22 (26.2) |
| ACEI or ARB | 23 (27.4) |
| Beta Blocker | 22 (26.2) |
| Calcium Channel Blocker | 19 (22.6) |
| Diuretic | 14 (16.7) |
| Alpha Blocker | 3 (3.6) |
| Nitrate | 0 (0) |
| Statin | 27 (32.1) |
Abbreviations: IQR, interquartile range; RCC, renal cell carcinoma; IL-2, interleukin-2; LVEF, left ventricular ejection fraction; BNP, b-type natriuretic peptide; BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; Statin, HMG CoA reductase inhibitor.
Table 2. Baseline Levels and Mean Change in Vascular Function from Visit 1 to Visit 2.
| Vascular Function Parameter | Baseline Median (IQR) | Mean Change | 95% CI | p value |
|---|---|---|---|---|
| Blood Pressure | ||||
| Peripheral SBP (mmHg) | 130.5 (116.8, 141.0) |
9.5 | (2.0, 17.1) | 0.02 |
| Peripheral DBP (mmHg) | 74.5 (65.8, 81.2) |
7.2 | (4.3, 10.0) | <0.001 |
| Central SBP (mmHg) | 115.7 (104.4, 128.5) |
11.3 | (4.8, 17.9) | 0.001 |
| Peripheral PP (mmHg) | 56.5 (45.0, 67.0) |
2.4 | (−4. 6, 9.3) | 0.50 |
| Peripheral MAP (mmHg) | 91.5 (84.8, 100.0) |
7.9 | (4.3, 11.6) | <0.001 |
| Arterial Stiffness | ||||
| Carotid-Femoral PWV (m/s) | 9.5 (7.7, 11.0) |
0.7 | (0.4, 1.1) | <0.001 |
| Resistive Load | ||||
| Total peripheral resistance (TPR) (dyn·sec·cm−5) | 1369.8 (1101.9, 1938.6) |
640.0 | (346.7, 920.0) | <0.001 |
| Effective arterial elastance (EA) (mmHg/mL) | 1.3 (1.0, 1.7) |
0.6 | (0.3, 0.8) | <0.001 |
| Pulsatile Load | ||||
| Aortic characteristic impedance (Zc) (dyn·sec·cm−5) | 138.9 (94.3, 171.8) |
13.3 | (−40.0, 66.7) | 0.59 |
| Total arterial compliance (TAC) (mL/mmHg) | 1.2 (0.8, 1.6) |
-0.3 | (−0.5, −0.2) | <0.001 |
| Augmentation Pressure (mmHg) | 3.0 (−2.0, 8.0) |
3.9 | (1.1, 6.7) | 0.008 |
| Augmentation index (AI) (%) | 9.0 (−7.0, 17.5) |
6.9 | (2.4, 11.5) | 0.004 |
| Reflected wave transit time (msec) | 26.2 (14.2, 43.3) |
-7.4 | (−13.1, −1.7) | 0.01 |
| Reflection magnitude | 0.4 (0.3, 0.4) |
0.0 | (0.0, 0.1) | 0.14 |
| Forward wave amplitude (mmHg) | 42.2 (33.8, 50.0) |
1.6 | (−4.9, 8.1) | 0.63 |
| Backward wave amplitude (mmHg) | 15.9 (11.4, 19.8) |
1.8 | (−0.6, 4.2) | 0.15 |
Abbreviations: IQR, interquartile range; PWV, pulse wave velocity; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure.
The median follow-up time was 30.9 weeks (IQR 6.3, 35.0 weeks). Of the 84 participants enrolled at baseline, 92% had follow up echocardiography and tonometry at 3.5 weeks (visit 1/cycle 1), 69% at 15 weeks (visit 3/cycle 3), and 51% at 33 weeks (visit 4/cycle 6). The decrease in participants over time was primarily secondary to progressive oncologic disease.
Changes in Blood Pressure with Sunitinib Over Time
Overall, and as expected, significant increases in BP occurred early (Table 2, Figure 3). Between baseline and 3.5 weeks, the mean increase in systolic BP was 9.5 mmHg (95% CI 2.0, 17.1). Diastolic BP demonstrated an adjusted mean increase of 7.2 mmHg (95% CI 4.3, 10.0). In exploratory analyses, we evaluated BP changes in the subgroup of participants (n=41) who did not initiate new anti-hypertensive medications between baseline and visit 2. Here, the mean changes in BP were similar to those who had initiated anti-hypertensive therapy during this time frame (SBP ~ 9.3–9.7mmHg; DBP 5.3–8.7mmHg) (Supplementary Table 1).
Figure 3. Longitudinal Trajectories in Vascular Function and Echo Parameters Across All Visits.
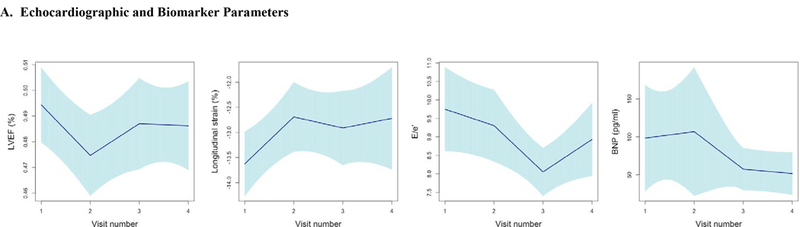
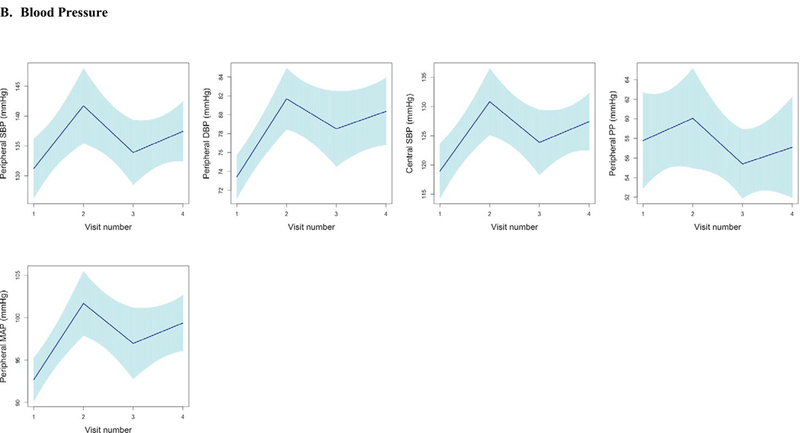
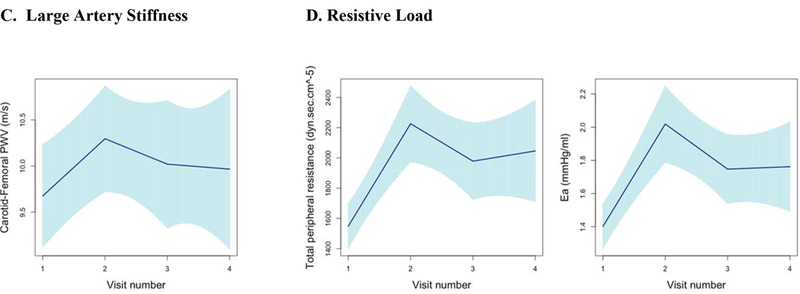
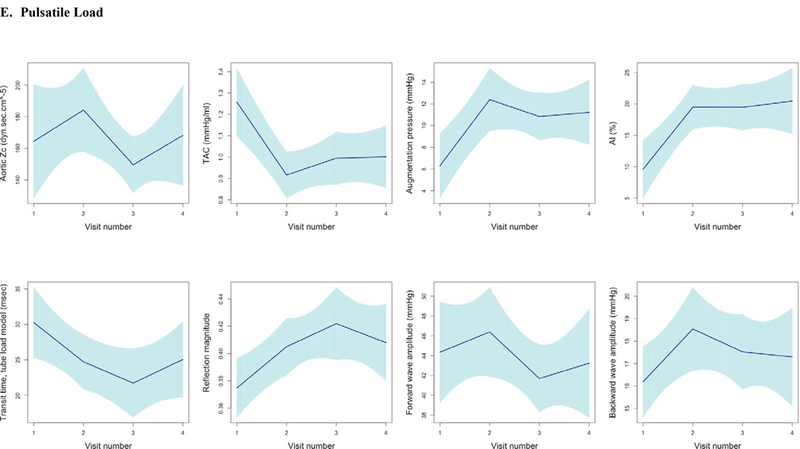
Longitudinal trends in vascular function, echocardiographic parameters, and cardiac biomarker b-type natriuretic peptide using piecewise linear regression with confidence intervals estimated using robust (Huber-White) sandwich-based standard errors (30–32) to illustrate the mean trend in vascular function measures across visits. Figures grouped according to echocardiographic parameters and b-type natriuretic peptide (A), Blood Pressure (B), Large Artery Stiffness (C), Resistive Load (D), Pulsatile Load (E). Abbreviations: LVEF, left ventricular ejection fraction; BNP, b-type natriuretic peptide; PWV, pulse wave velocity; SBP, systolic blood pressure; PP, pulse pressure; EA, Effective arterial elastance; TAC, Total arterial compliance; Zc, Characteristic impedance of the aortic root; AI, Augmentation index.
Changes in Vascular Function with Sunitinib Over Time
Vascular function parameters are shown across all visits in Figure 3, with the most pronounced changes noted between visit 1 and visit 2. The mean changes and 95% CIs in various vascular function indices between baseline and 3.5 weeks are further detailed in Table 2. Across all participants, carotid-femoral PWV increased significantly by 0.7 m/s (95% CI 0.4, 1.1). This finding was independent of adjustment for HR and MAP (adjusted mean change 0.7 m/s, 95% CI 0.3, 1.0). Resistive load indices also increased with sunitinib. Total peripheral resistance (TPR) demonstrated a mean increase of 640.0 dyn·sec·cm−5 (95% CI 346.7, 920.0) and effective arterial elastance, EA, increased by 0.6 mmHg/ml (95% CI 0.3, 0.8).
Pulsatile load, characterized by total arterial compliance (TAC) and measures of wave reflection also showed significant changes, providing additional evidence for adverse vascular function. There was a notable reduction in TAC with a mean change of −0.3 mL/mmHg (95% CI −0.5, −0.2). While early systolic pulsatile load, defined by aortic impedance (Zc), did not significantly change, measures of wave reflection did demonstrate significant changes. Augmentation index (AI) increased by 6.9% (95% CI 2.4, 11.5), and reflected wave transit time decreased by 7.4 msec (95% CI −13.1, −1.7).
We also explored the impact of changes in BP medications on changes in vascular function parameters. Participants who newly initiated anti-hypertensive medications, on average, had an attenuation of the changes in vascular function parameters observed between visits 1 and 2, as compared to the subgroup who did not initiate new medications. Although measures of peripheral BP were similar between the 2 groups, changes in resistive and pulsatile load qualitatively appeared to be more pronounced in those who did not start new anti-hypertensive medications, although there was no statistically significant interaction (Supplementary Table 1).
Relationship Between Baseline Vascular Function Measures and LV Systolic Function Over Time
TNext, the relationship between vascular function at baseline as it related to LV systolic function, specifically LVEF and longitudinal strain were assessed over time. As previously reported (16), we found an overall 9.7% risk of developing LV dysfunction (LVEF decline ≥10% to a value of <50%) in this study. The majority of patients of those who developed LV dysfunction did so within 3.5 weeks after initial sunitinib treatment. On average, there was a significant, but modest decrease in LVEF across the entire cohort, mean LVEF decline of 1.9% (95% CI 3.2, 0.5) at 3.5 weeks (cycle 1) when compared to baseline (p=0.007). There was also a slight worsening in longitudinal strain over the duration of follow up, of borderline significance (mean change of 0.30 (−0.05, 0.65) per visit; p=0.067) (Figure 3A).
There was no significant relationship between baseline vascular function, including markers of both resistive and pulsatile load, and change in LVEF over time in adjusted (Table 3) and unadjusted analyses (data not shown). Similarly, no significant association was observed between baseline measures and longitudinal strain over time (Table 4). Early changes in vascular parameters between baseline (visit 1) and 3.5 weeks (visit 2/cycle 2) after initiation of sunitinib were not associated with changes in LVEF or longitudinal strain over the same time interval or subsequent visit at 15 weeks (visit 3/cycle 6) (Supplementary Tables 2–5).
Table 3. Association Between Baseline Vascular Function and Longitudinal Change in LVEF Relative to Baseline.
| Vascular Function Parameter | Adjusted Beta | 95% CI | p value |
|---|---|---|---|
| Blood Pressure | |||
| Peripheral SBP (mmHg) | 0.2 | (−0. 8, 1.3) | 0.64 |
| Peripheral DBP (mmHg) | 0.2 | (−0. 8, 1.3) | 0.66 |
| Central SBP (mmHg) | 0.7 | (−0.4, 1.8) | 0.21 |
| Peripheral PP (mmHg) | 0.2 | (−0.8, 1.1) | 0.73 |
| Peripheral MAP (mmHg) | 0.3 | (−0.8, 1.4) | 0.60 |
| Arterial Stiffness | |||
| Carotid-Femoral PWV (m/s) | 0.7 | (−0.5, 1.9) | 0.23 |
| Resistive Load | |||
| Total peripheral resistance (TPR) (dyn·sec·cm−5) | 0.6 | (−0.5, 1.8) | 0.29 |
| Effective arterial elastance (EA) (mmHg/mL) | 0.3 | (−0.9, 1.6) | 0.60 |
| Pulsatile Load | |||
| Aortic characteristic impedance (Zc) (dyn·sec·cm−5) | 0.2 | (−0.6, 0.9) | 0.63 |
| Total arterial compliance (TAC)(mL/mmHg) | -1.0 | (−2.3, 0.3) | 0.12 |
| Augmentation Pressure (mmHg) | 0.2 | (−1.0, 1.33) | 0.78 |
| Augmentation index (AI) (%) | 0.1 | (−1.2, 1.5) | 0.87 |
| Reflected wave transit time (msec) | -0.8 | (−2.3, 0.6) | 0.27 |
| Reflection magnitude | 0.5 | (−0.8, 1.8) | 0.42 |
| Forward wave amplitude (mmHg) | 0.6 | (−0.3, 1.5) | 0.21 |
| Backward wave amplitude (mmHg) | 0.1 | (−0.3, 2.3) | 0.13 |
Vascular function variables scaled by their standard deviations, and regression coefficients are multiplied by 100 and adjusted for age, gender, and categorical visit number. For example, each standard deviation increase in SBP was associated with a nonsignificant 0.2% increase in LVEF (p=0.64). Repeated measures accounted for using GEE. Abbreviations: LVEF, left ventricular ejection fraction; PWV, pulse wave velocity; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure.
Table 4. Association Between Baseline Vascular Function and Change in Longitudinal Strain Relative to Baseline.
| Vascular Function Parameter | Adjusted Beta | 95% CI | p value |
|---|---|---|---|
| Blood Pressure | |||
| Peripheral SBP (mmHg) | -0.1 | (−1.6, 1.4) | 0.92 |
| Peripheral DBP (mmHg) | 0.5 | (−0.3, 1.3) | 0.24 |
| Central SBP (mmHg) | -0.2 | (−1.4, 1.1) | 0.79 |
| Peripheral PP (mmHg) | -0.8 | (−2.3, 0.7) | 0.28 |
| Peripheral MAP (mmHg) | 0.3 | (−0.7, 1.3) | 0.54 |
| Arterial Stiffness | |||
| Carotid-Femoral PWV (m/s) | -0.1 | (−0.8, 0.7) | 0.90 |
| Resistive Load | |||
| Total peripheral resistance (TPR) (dyn·sec·cm−5) | -0.1 | (−0.7, 0.6) | 0.88 |
| Effective arterial elastance (EA) (mmHg/mL) | 0.0 | (−0.6, 0.7) | 0.93 |
| Pulsatile Load | |||
| Aortic characteristic impedance (Zc) (dyn·sec·cm−5) | -0.1 | (−0.7, 0.5) | 0.75 |
| Total arterial compliance (TAC) (mL/mmHg) | 0.4 | (−0.4, 1.2) | 0.29 |
| Augmentation Pressure (mmHg) | 0.3 | (−0.4, 1.0) | 0.36 |
| Augmentation index (AI) (%) | 0.6 | (−0.2, 1.3) | 0.14 |
| Reflected wave transit time (msec) | -0.4 | (−1.2, 0.3) | 0.23 |
| Reflection magnitude | -0.3 | (−1.0, 0.4) | 0.38 |
| Forward wave amplitude (mmHg) | -0.6 | (−1.5, 0.3) | 0.19 |
| Backward wave amplitude (mmHg) | -0.7 | (−1.6, 0.1) | 0.09 |
Vascular function variables scaled by their standard deviations. Regression coefficients adjusted for age, gender, and categorical visit number. Repeated measures accounted for using GEE. Abbreviations: CI, confidence interval; PWV, pulse wave velocity; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure.
Relationship Between Baseline Vascular Function Measures and LV Diastolic Function and BNP Over Time
Next, we estimated the relationship between baseline vascular function and changes in a surrogate marker of diastolic function and LV filling pressures (E/e’) during sunitinib treatment (Table 5). Resistive load at baseline, as assessed by TPR and EA, was associated with increased E/e’ after the initiation of sunitinib. Each standard deviation change in baseline TPR was associated with a 0.8-unit increase in E/e’ from baseline to visit 2 (95% CI 0.2, 1.4, p=0.01). For each standard deviation increase in EA at baseline, there was a 0.9-unit increase in E/e’ from baseline to visit 2 (95% CI 0.3, 1.5, p 0.004). Similarly, aortic root impedence (Zc), a measure of the pulsatile load imposed by the aortic root (p<0.001), and augmentation pressure were associated with increased E/e’ over time (p=0.01). Significant associations were also observed between baseline vascular function (EA and Zc) and log10BNP (Table 5). However, early changes in vascular parameters between baseline (visit 1) and 3.5 weeks (visit 2/cycle 2) after initiation of sunitinib were not consistently associated with changes in diastolic function (E/e’ and log10BNP) over the same time interval or subsequent visit at 15 weeks (visit 3/cycle 6) (Supplementary Tables 6–9).
Table 5. Association Between Baseline Vascular Function and Longitudinal Changes in LV Diastolic Function (E/e’ and Log10 BNP) Relative to Baseline.
| Vascular Function Parameter | Adjusted Beta E/e’ | 95% CI | p value | Adjusted Beta Log10 BNP (pg/ml) | 95% CI | p value |
|---|---|---|---|---|---|---|
| Blood Pressure | ||||||
| Peripheral SBP (mmHg) | 0.3 | (−0.2, 0.9) | 0.26 | 0.1 | (−0.0, 0.1) | 0.15 |
| Peripheral DBP (mmHg) | -0.3 | (−0.8, 0.2) | 0.23 | -0.1 | (−0.1, 0.0) | 0.24 |
| Central SBP (mmHg) | 0.3 | (−0.4, 0.9) | 0.41 | 0.0 | (−0.1, 0.1) | 0.52 |
| Peripheral PP (mmHg) | 0.5 | (−0.01, 0.9) | 0.06 | 0.1 | (0.0, 0.2) | 0.02 |
| Peripheral MAP (mmHg) | 0.1 | (−0.5, 0.6) | 0.87 | 0.0 | (−0.1, 0.1) | 0.83 |
| Arterial Stiffness | ||||||
| Carotid-Femoral PWV (m/s) | 0.0 | (−0.7, 0.7) | 0.98 | 0.1 | (−0.0, 0.2) | 0.30 |
| Resistive Load | ||||||
| Total peripheral resistance (TPR) (dyn·sec·cm−5) | 0.8 | (0.2, 1.4) | 0.01 | 0.1 | (−0.0, 0.2) | 0.21 |
| Effective arterial elastance (EA) (mmHg/mL) | 0.9 | (0.3, 1.5) | 0.004 | 0.1 | (0.0, 0.2) | 0.01 |
| Pulsatile Load | ||||||
| Aortic characteristic impedance (Zc) (dyn·sec·cm−5) | 0.7 | (0.3, 1.0) | <0.001 | 0.1 | (0.0, 0.1) | 0.01 |
| Total arterial compliance (TAC)(mL/mmHg) | -0.3 | (−0.8, 0.3) | 0.35 | -0.1 | (−0.2, 0.0) | 0.11 |
| Augmentation Pressure (mmHg) | 1.0 | (0.3, 1.7) | 0.01 | 0.0 | (−0.1, 0.2) | 0.55 |
| Augmentation index (AI) (%) | 0.7 | (−0.01, 1.4) | 0.05 | -0.0 | (−0.1, 0.1) | 0.84 |
| Reflected wave transit time (msec) | 0.7 | (−0.02, 1.5) | 0.06 | -0.0 | (−0.1, 0.0) | 0.34 |
| Reflection magnitude | -0.2 | (−0.8, 0.5) | 0.61 | 0.0 | (−0.1, 0.1) | 0.55 |
| Forward wave amplitude (mmHg) | 0.4 | (−0.3, 1.1) | 0.26 | 0.1 | (−0.0, 0.1) | 0.06 |
| Backward wave amplitude (mmHg) | 0.4 | (−0.3, 1.1) | 0.30 | 0.1 | (−0.0, 0.2) | 0.06 |
Vascular function variables scaled by their standard deviations. Regression coefficients adjusted for age, gender, and categorical visit number. Repeated measures accounted for using GEE. Abbreviations: BNP, b-type natriuretic peptide; CI, confidence interval; PWV, pulse wave velocity; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure.
Discussion
VEGF-inhibitor therapies including sunitinib are associated with significant adverse cardiovascular effects, namely HTN, LV dysfunction, and HF (10–14). However, detailed relationships between vascular function parameters and HTN and LV dysfunction have not been specifically studied. To our knowledge this is the first prospective study performed using serial echocardiography including systolic and diastolic function assessment, longitudinal biomarkers, and repeated measures derived from arterial tonometry to comprehensively characterize cardiotoxicity with exposure to sunitinib in mRCC patients.
Our study demonstrated several important findings. First, vascular function in this mRCC cohort worsened early; increases in large artery stiffness with sunitinib were observed within the first 3.5 weeks of therapy. Second, we demonstrated worsening in both resistive and pulsatile load after initiation of sunitinib, similar to sorafenib (35). Our study extends these findings through a more detailed assessment of vascular function, beyond PWV and augmentation index alone. Third, we found no significant associations between vascular function measures and LV systolic function (LVEF or longitudinal strain), although vascular function was associated with diastolic function and filling pressures (E/e’ and BNP).
Total peripheral resistance and EA, markers of resistive load, showed significant increases with sunitinib. TPR is determined by the number and caliber of arterioles, in contrast to pulsatile load, which is more closely linked to the properties of conduit arteries. Decreased arteriole wall nitric oxide production, increased endothelin-1 production, and microvascular rarefaction have been proposed as possible mechanisms for VEGF-inhibitor related HTN (15, 18). Microvascular rarefaction is a process of endothelial cell apoptosis and remodeling of the capillary beds, eventually leading to diminished number of microvessels (36). Increased resistive load demonstrated in our study supports the hypothesis that microvascular rarefaction may have a role in the pathophysiology of sunitinib related HTN.
We found significant increases in carotid-femoral PWV, suggesting worsening large artery stiffness. While it has been speculated that HTN itself leads to increasing arterial stiffness, arterial stiffness may actually represent the cause rather than the consequence of isolated systolic HTN (37). Data from the Framingham Heart Study found that increased PWV preceded development of HTN by 7 years (37). Given the rapid changes in arterial stiffness and HTN we observed, increasing arterial stiffness is likely involved in the pathophysiology of HTN induced by sunitinib and other VEGF-inhibitors. Increasing arterial stiffness has been demonstrated to be an independent risk factor for coronary and cerebrovascular disease (9, 10) as well as diastolic and systolic LV dysfunction (38–39).
Measures of pulsatile load include the aortic root Zc, measures of wave reflection magnitude and timing, and the total compliance of the arterial tree (40–41). Aortic root Zc, the main determinant of early systolic pulsatile load, is dependent upon the elastic properties of the aorta and governs the pressure-flow relation in the absence of wave reflections (41–42). The load imposed by wave reflections depends upon complex physical properties (stiffness, taper and branching) of the arterial tree, PWV and the distance to reflection sites, and the reflected wave transit time from the heart to the periphery and back (40–41, 43). In our study, Zc was only minimally increased while augmentation index increased more significantly, likely representing the effects of premature wave reflections, as supported by reduction in reflected wave transit time. Augmentation of reflected pressure waves results in an increase in mid to late systolic load of the LV (41). This pattern of late systolic load has been shown to lead to myocardial hypertrophy (44–45); fibrosis (41); LV systolic and diastolic dysfunction (39, 46–48); and HF (49). The reduction in TAC observed in just a short period with sunitinib therapy is equivalent to observed changes over decades of aging of the arterial tree in the general population (25), although it may be largely functional.
We identified important relationships between baseline vascular function and worsening diastolic function and elevations in BNP over time with sunitinib treatment. We showed that increased pre-treatment TPR, EA, Zc, and augmentation pressure, representative of resistive and pulsatile load (both early and late systolic load) were each associated with worsening diastolic function. There was also a significant relationship between BNP and EA and Zc. This relationship between arterial stiffness and diastolic dysfunction and elevated filling pressures due to abnormal load and loading sequence is well recognized (38–39). However, our study is the first to demonstrate this with VEGF inhibitors. Moreover, these findings suggest that baseline assessment of vascular function indices can result in an improved understanding of cardiovascular risk.
Given the associations between baseline measures of abnormal resistive load and arterial compliance with worsening diastolic function over time, we hypothesize that ideal anti-hypertensive therapies in sunitinib-treated patients may be those with arterial vasodilating properties and the ability to improve conduit artery function, such as a combination of calcium channel blockers and inorganic nitrates. By improving muscular artery compliance, the phase of reflected waves could be shifted to reduce the late systolic load by wave reflections. As selective vasodilators of conduit arteries, inorganic nitrates may be ideal agents to mitigate these effects in the sunitinib-treated population (50). Dihydropyridine calcium channel blockers which affect resistive load may also be effective in this population, and clinical experience supports this. In contrast, pure beta blockers may be less preferred given prolongation in ejection time which may increase vulnerability to reflected waves. Of note, of the 23 patients prescribed a beta blocker, the majority (19/23) were treated with a pure beta blocker. The role of these agents, and of non-invasive hemodynamic testing to personalize therapy in this clinical setting, should be assessed in future trials.
Contrary to our hypothesis, measures of vascular function were not associated with LV systolic function. Our small sample size may have limited our ability to demonstrate a relationship between increased arterial stiffness and the subsequent development of systolic dysfunction, as assessed by LVEF and longitudinal strain. The population average changes in LVEF were also relatively modest, on the order of −1.9%, which likely also limited our ability to detect a significant association (16). Moreover, we were unable to investigate associations with the categorical outcome of LV dysfunction or changes over the long term given limitations in power. We focused on E/e’ as a surrogate of diastolic function and LV filling pressures, but acknowledge that a comprehensive assessment of diastolic function includes multiple additional parameters. An observed lack of association may also be related to the effect of medications. At baseline, 55% of patients had HTN and 52% of patients were treated with anti-hypertensive therapy. At the first follow up visit after sunitinib therapy (visit 2), 50% of patients were treated with anti-hypertensive therapies, and these agents attenuated the changes in vascular function (Supplementary Table 1).
In conclusion, we demonstrated that sunitinib therapy in mRCC patients is associated with increased large artery stiffness, resistive and pulsatile load within 3.5 weeks of treatment. While we did not find an association between changes in vascular function parameters and LV systolic dysfunction as originally hypothesized, we did demonstrate a significant association with worsening diastolic dysfunction (E/e’) and LV filling pressures (BNP) and change in vascular function over time. Our study offers insight into the complex time-varying process of vascular function in the setting of VEGF inhibitor therapy. Moreover, our findings emphasize the importance of phenotypic characterization of the changes in vascular function parameters with therapies that affect BP.
Supplementary Material
What is New?
This study is the first to comprehensively characterize the changes in vascular function in metastatic renal cell cancer patients receiving sunitinib therapy, and relate these to measures of left ventricular systolic and diastolic function.
Sunitinib resulted in significant early increases in blood pressure, large artery stiffness, resistive and pulsatile load within 3.5 weeks of therapy initiation.
Changes in indices of vascular function observed with sunitinib were of significant magnitude, equivalent functionally to those observed over decades of aging of the arterial tree. Current anti-hypertensive therapies may attenuate these changes.
What are the Clinical Implications?
Baseline measures of resistive and pulsatile load are associated with worse diastolic function and elevations in BNP over time with sunitinib.
Changes in vascular function occur within the first 3.5 weeks of sunitinib therapy, suggesting the critical importance of careful monitoring and treatment early, within the first cycle of therapy initiation.
Optimal anti-hypertensive therapies in the sunitinib treated population may be those with arterial vasodilating properties and those with the ability to improve conduit artery function and central pulsatile hemodynamics.
Acknowledgments
Source(s) of Funding
This study was funded in part by an Investigator Initiated Research Award to Bonnie Ky from Pfizer, Inc. BNP analyses was performed by Singulex, Inc. in a blinded fashion. Sponsors were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict(s) of Interest Disclosures: J.A.C. has received consulting honoraria from Bristol-Myers-Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft, Merck, Ironwood Pharmaceuticals, Sanifit Laboratories, and Vital Labs. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers-Squibb, Microsoft and CVRx Inc., and device loans from Atcor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. The other authors declare no potential conflicts of interest.
References
- 1.Motzer RJ, Hutson TE, Tomczak P, Michaelson D, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Dr PH., Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New Engl J Med. 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. J Clin Oncol. 2009;27:3584.19487381 [Google Scholar]
- 3.Patil S, Figlin RA, Hutson TE, Michaelson MD, Négrier S, Kim ST, Huang X, Motzer RJ. Prognostic factors for progression-free and overall survival with sunitinib targeted therapy and with cytokine as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2011;22:295. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarbá JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Castellano YC Lim HY D, Uemura H, Tarazi J Cella D, Chen C, Rhosbrook B, Kim S, Motzer RJ. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378:1931–9. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, Shah S, Bukowski RM. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Polska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, for the TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. New Engl J Med. 2007;356:125–34. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. [DOI] [PubMed] [Google Scholar]
- 9.NCCN Guidelines: Kidney Cancer. National Comprehensive Cancer Network;Version I; 2016. [Google Scholar]
- 10.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Wolfe K, Pravda E, Cassola F, Desai J, George S, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetria GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007; 370: 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–12. [DOI] [PubMed] [Google Scholar]
- 12.Di Lorenzo G, Autorino R, Bruni G, Cartenì G, Ricevuto E, Tudini M, Ficorella C , Romano C, Aieta M, Giordano A, Giuliano M, Gonnella A, De Nunzio C, Rizzo M, Montesarchio V, Ewer M, De Placido S. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol. 2009;20:1535–1542. [DOI] [PubMed] [Google Scholar]
- 13.Faruque LI, Lin M, Battistella M, Wiebe N, Reiman T, Hemmelgarn B, Thomas C, Tonelli M. Systematic review of the risk of adverse outcomes associated with vascular endothelial growth factor inhibitors for the treatment of cancer. PLoS One. 2014;9:e101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aslam S, Eisen T. Vascular endothelial growth factor receptor tyrosine kinase inhibitors in metastatic renal cell cancer: latest results and clinical implications. Ther Adv Med Oncol. 2013;5:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014; 3:e000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narayan V, Keefe S, Haas N, Wang L, Puzanov I, Putt M, Catino A, Fang J, Agarwal N, Hyman D, Smith AM, Finkelman BS, Narayan HK, Ewer S, ElAmm C, Lenihan D, Ky B. Prospective Evaluation of Sunitinib-Induced Cardiotoxicity in Patients with Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2017;23: 3601–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirinos JA, Sweitzer N. Ventricular–Arterial Coupling In Chronic Heart Failure. Cardiac Fail Rev. 2017;3:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.X de Jesus-Gonzalez N, Robinson E, Moslehi J, Humphreys BD. Management of antiangiogenic therapy-induced hypertension. Hypertension. 2012;60:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. [DOI] [PubMed] [Google Scholar]
- 20.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19:1613–8. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Victor M, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancillotto P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1,39.e14. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 23.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick T, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d’Hooge J, Aurigemma GP, Thomas JD, and Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015; 28:183–193. [DOI] [PubMed] [Google Scholar]
- 24.Stergiopulos N, Segers P, and esterhof N. Use of pulse pressure method for estimating total arterial compliance in vivo. Am J Physiol Heart Circ Physiol 1999;276:H424-H428. [DOI] [PubMed] [Google Scholar]
- 25.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007:49: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 26.Chirinos JA, Segers P, Raina A, Saif H, Swillens A, Gupta AK, Townsend R, Emmi AG Jr., Kirkpatrick JN, Keane MG, Ferrari VA, Wiegers SE, St John Sutton MG. Arterial pulsatile hemodynamic load induced by isometric exercise strongly predicts left ventricular mass in hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H320–H330. [DOI] [PubMed] [Google Scholar]
- 27.Phan TS, Li JK, Segers P, Reddy‐Koppula M, Akers SR, Kuna ST, Gislason T, Pack AI, Chirinos JA. Aging is Associated With an Earlier Arrival of Reflected Waves Without a Distal Shift in Reflection Sites. J Am Heart Assoc. 2016;5:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chirinos JA, Rietzschel ER, Shiva-Kumar P, De Buyer ML, Zamani P, Claessens T, Geraci S, Konda P, Bacquer DB, Akers SR, Gillebert TC, Segers. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension 2014; 64: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 29.Wu A, Estis J, Heseltine P, Bui K, Todd J, Kavsak P. High-sensitivity cardiac troponin I in a large community-based population at risk for cardiovascular disease. Clin Chem 2015;61, No. 10, Supplement. [Google Scholar]
- 30.Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. In: Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley: University of California Press; 1967:221–233. [Google Scholar]
- 31.White H A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. [Google Scholar]
- 32.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, England: Clarendon Press; 1994. [Google Scholar]
- 33.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988; 1049–60. [PubMed] [Google Scholar]
- 34.Pepe MS, Anderson GL. A cautionary note on inference for marginal regression models with longitudinal data and general correlated response data. Communications in statistics-simulation and computation. 1994;23:939–51. [Google Scholar]
- 35.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, O’Dwyer PJ. Mechanisms of Hypertension Associated With BAY 43–9006. J Clin Onc. 2006; 24:9; 1363–1369. [DOI] [PubMed] [Google Scholar]
- 36.Van der Veldt AA, de Boer MP, Boven E, Eringa EC, van den Eertwegh AJ, van Hinsbergh VW, Smulders YM, Serné EH. Reduction in skin microvascular density and changes in vessel morphology in patients treated with sunitinib. Anticancer Drugs. 2010;21:439–46. [DOI] [PubMed] [Google Scholar]
- 37.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic Stiffness, Blood Pressure Progression, and Incident Hypertension. J Am Med Assoc. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. [DOI] [PubMed] [Google Scholar]
- 39.Weber T, Wassertheurer S, O’Rourke MF, Haiden A, Zweiker R, Rammer M, Hametner B, Eber B. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:1874–1883. [DOI] [PubMed] [Google Scholar]
- 40.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 1: pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension. 2010;56:555–562. [DOI] [PubMed] [Google Scholar]
- 41.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 2015; 66: 698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Rourke MF, Pauca AL. Augmentation of the aortic and central arterial pressure waveform. Blood Press Monit. 2004; 9:179–185. [DOI] [PubMed] [Google Scholar]
- 43.O’Rourke MF. Wave travel and reflection in the arterial system. J Hypertens Suppl. 1999; 17:S45-S47. [PubMed] [Google Scholar]
- 44.Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. [DOI] [PubMed] [Google Scholar]
- 45.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O’Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto N, Onishi K, Tanabe M, Dohi K, Funabiki K, Kurita T, Yamanaka T, Nakajima K, Ito M, Nobori T, Nakano T. Nitroglycerin improves left ventricular relaxation by changing systolic loading sequence in patients with excessive arterial load. J Cardiovasc Pharmacol. 2005;45:211–216. [DOI] [PubMed] [Google Scholar]
- 47.Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. [DOI] [PubMed] [Google Scholar]
- 48.Shah SJ, Wasserstrom JA. Increased arterial wave reflection magnitude: a novel form of stage B heart failure? J Am Coll Cardiol. 2012;60:2178–2181. [DOI] [PubMed] [Google Scholar]
- 49.Chirinos JA, Kips JG, Jacobs DR Jr., Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Omar SA, Fok H, Tilgner KD, Nair, Hunt J, Jiang B, Taylor P, Chowienczyk P, Webb AJ. Paradoxical Normoxia-Dependent Selective Actions of Inorganic Nitrite in Human Muscular Conduit Arteries and Related Selective Actions on Central Blood Pressures. Circulation. 2015;131:381–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


