Abstract
Purpose:
To determine how PRK and MMC impact corneal nerves and their regeneration over time after surgery.
Methods:
Twenty-eight New Zealand rabbits had: corneal epithelial scrape with MMC 0.02% (n=3) and without MMC (n=3), −9.0 diopter PRK with MMC (n=6) and without MMC (n=16). Corneas were removed after euthanasia and corneal nerve morphology was evaluated using acetylcholinesterase immunohistochemistry and β-III tubulin staining after 1 day for all the groups, after 1 month for PRK with and without MMC, and 2, 3 and 6 months after PRK without MMC. Image-Pro was used to quantitate the area of nerve loss after the procedures and, consequently, regeneration of the nerves overtime. Opposite eyes were used as controls.
Result:
Epithelial scrape with MMC treatment did not show a statistically significant difference in nerve loss compared to epithelial scrape without MMC (p=0.4). PRK with MMC was significantly different from PRK without MMC at 1 day after surgery (p=0.0009) but not different at one month after surgery (p=0.9). In the PRK without MMC group, nerves regenerated at two months (p=<0.0001) but did not return to the normal preoperative level of innervation until three months after surgery (p=0.05). However, the morphology of the regenerating nerves was abnormal—with more tortuosity and aberrant innervation compared to the preoperative controls—even at six months after surgery.
Conclusion:
PRK damages the corneal nerves but they are partially regenerated by three months after surgery in rabbits. Nerve loss after PRK extended peripheral to the excimer laser ablated zone, indicating that there was retrograde degeneration of nerves after PRK. MMC had a small additive toxic effect on the corneal nerves when combined with PRK that was only significant prior to one month after surgery.
Introduction
The cornea is a densely innervated structure that is richly supplied by sensory and autonomic nerve fibers originated from the ophthalmic division of the trigeminal nerve.1 Many corneal nerve receptors are polymodal nociceptors,2 which are stimulated by different mechanical, thermal and chemical stimuli that serve perceptions such as touch, pain and temperature. Many of these fibers also modulate functions such as the blink reflex, tear production and the corneal wound healing response.1,3
Corneal nerves also have a critical role in maintaining a healthy ocular surface and traumatic or surgical wounds commonly alter nerve morphology and function.3 Surgery-related injury to corneal innervation results in transient or persistent clinical changes to corneal function and may cause post-operative complications such as dry eye syndrome, neurotrophic epitheliopathy, photophobia, hypoesthesia, and persistent epithelial defects.4–7 Mitomycin C (MMC) used with photorefractive keratectomy (PRK) also has the potential to alter nerve morphology and function but few studies have investigated this possibility.8 This study aimed to better characterize corneal nerve injury and regeneration after PRK performed with and without MMC in a rabbit model.
Methods
A). Animals and Surgeries
All animals were treated in accordance with the tenets of the ARVO Statement for theUse of Animals in Ophthalmic and Vision Research and the Animal Control Committee at the Cleveland Clinic Foundation approved these studies.
Twenty-eight 12–15 weeks old female New Zealand White female rabbits weighing 2 to 3 kg each were included in this study. One eye of each rabbit was selected to have epithelial scrape, with or without 0.02% MMC, or photorefractive keratectomy (PRK), with or without 0.02% MMC. The opposite eye was used as a control. Each group had three animals at each time point, except four rabbits were included in the six-month time point.
Epithelial scrape with 0.02% MMC for 30 seconds (n=3) and without 0.02% MMC (n=3) was studied one day after the procedure. PRK with MMC 0.02% for 30 seconds (n=3 at each time point) and PRK without MMC (n=3 at each time point) were analyzed one day and one month after the surgery. In addition, PRK without MMC was studied at two months (n=3), three months (n=3) and six months (n=4) after surgery.
General anesthesia for corneal scrape or PRK was performed by intramuscular injection of ketamine hydrochloride (30mg/kg) and xylazine hydrochloride (5mg/kg). Topical proparacaine hydrochloride 1% (Alcon, Fort Worth, TX) was also applied to each treated eye and the control eye prior surgery. Euthanasia was performed with an intravenous injection of 100 mg/kg Beuthanasia (MERCK, Kenilworth, NJ) while the animal was under general anesthesia.
1). Epithelial scrape technique
With the animal under general and local anesthesia, a sterile lid speculum was positioned within interpalpebral fissure and an 8 mm diameter area of epithelium and epithelial basement membrane concentric with the pupil was removed by scraping with a #64 blade (Beaver, Becton-Dickinson & Co, Franklin Lakes, NJ). Care was taken to insure all epithelial basement membrane was removed within the area of scrape.
2). PRK Technique
With the animal under general and local anesthesia, a sterile lid speculum was positioned within the interpalpebral fissure and an 8 mm diameter area of epithelium concentric with the pupil was removed by scraping with a #64 Beaver blade. A spherical PRK ablation of −9 diopters was performed with a 6 mm ablation zone centered over the entrance pupil using a VISX Star S4 IR excimer laser (Abbot Medical Optics, Irvine, CA).
3). Mitomycin C application
0.02% MMC (Intas Pharmaceutical LTD, Accord Health Care, Durham, NC) was applied after epithelial scrape or PRK using a round 8 mm diameter X 1mm thick surgical sponge that was wet with solution and placed on the central cornea for 30 seconds. The cornea was irrigated with 3 ml of balanced salt solution (BSS) (Alcon, Fort Worth, Texas) after removal of the sponge.
4). Post procedure antibiotics
One drop of ciprofloxacin hydrochloride 0.3% (Ciloxan, Alcon, Fort Worth, Texas) was applied after epithelial scrape, PRK or to control eyes four times a day until the corneal epithelium has healed in the treated eye (4–6 days).
B). Corneal nerve staining methods
Corneal nerves were stained using the Karnovsky-Roots acetylcholinesterase (AChE) technique.9–11 Briefly, after removing the corneo-scleral rim, the cornea was rinsed in D-PBS (Dulbecco’s Phosphate Buffered Saline, D8662, Sigma- Aldrich, St. Louis, MO, USA) and the tissue was fixed in a 4% paraformaldehyde in 50mM Na-K phosphate buffer (pH 7.2) and 8% sucrose solution for 30 minutes. The Descemet’s membrane-endothelial complex was removed before fixation, otherwise the membrane becomes strongly adherent to the posterior stroma and this membrane has non-specific AchE staining.10 The cornea was then rinsed overnight at 4°C in 0.1 M sodium phosphate buffer (pH 7.2). Preliminary experiments were performed to determine if nerves were affected by removing the Descemet’s membrane-endothelial complex prior to fixation and found no difference in nerve area per mm2 in corneas whether the Descemet’s membrane-endothelial complex was removed prior to (18.4 ± 0.9) or after (18.6 ± 0.5) paraformaldehyde fixation (n=3 in each group, p=0.4).
The following day, the corneas were pre-incubated for 7 hours at 4°C in 10 ml of 0.1M sodium phosphate buffer (pH 5.5) solution containing 65mM sodium acetate, 10mM sodium citrate, 4mM copper sulfate and 0.5mM potassium ferricyanide, and then reacted with acetylcholine iodide (1.0 mg/ml; Sigma-Aldrich) in incubation medium for 20–22 hours at 4°C. The acetylcholinesterase present in the corneal nerves reacted with acetylcholine iodide in the substrate to produce a brown staining of the nerves. Subsequently, the corneas were rinsed for 15 minutes in 0.1mg/ml sodium sulphate. Finally, the corneo-scleral rims were placed in dilute ammonium sulfide solution (Sigma-Aldrich, St. Louis, MO) prepared by placing two drops of 0.1 mg/ml sodium sulphate in 10 ml of distilled water for 30 minutes and then rinsing twice for five minutes in distilled water. Four radial corneal incisions were made to flatten the cornea and the sample was mounted with the epithelium facing up in 1x PBS onto a microscope slide (Superfrost plus #1255015, Thermo Fisher Scientific, Pittsburgh, PA, US) and a cover slip was placed over the tissue.
Nerve morphology was also subsequently examined using three-dimensional analysis with β-III tubulin staining.12 The corneas that had been fixed, as previously described, were cut using a 9 mm trephine (Beaver-Visitec, Waltham, MA). The corneal button was then washed with 0.1 M PBS containing 0.1% bovine serum albumin (PBS-BSA, #9048–46-8, Sigma-Aldrich, St. Louis, MO) three times during a 24-hour interval. The button was incubated with 10% normal goat serum (#S-100, Vector Laboratories, Burlingame, CA) with 0.3% Triton X-100 solution (#9002–93-1, Sigma-Aldrich, St. Louis, MO) in 0.1% BSA-PBS solution for 60 minutes at room temperature.
Each corneal button was subsequently incubated with shaking in 1:3000 monoclonal antibody specific for neuronal class β III-tubulin (Tuj1, MMS-435P, Covance Antibody Services Inc, Berkeley, CA) in 0.1 M PBS containing 1.5% normal goat serum with 0.1% Triton X-100 for 72 hours at 4°C. Then, the corneal buttons were washed with PBS-BSA for 24 hours and incubated with gentle shaking in the secondary antibody Alexa Fluor® 568 goat anti-mouse IgG (Cat #A11004, ThermoFisher Scientific, Rockford, IL) at a dilution of 1:1500 in 0.1 M PBS containing 1.5% normal goat serum with 0.1% Triton X-100 for 24 hours at 4°C. Finally, the corneal buttons were washed in PBS-BSA solution for 24 hours and mounted with Vectashield (Vector Laboratories Inc, Burlingame, Calif) onto microscope slides (Superfrost plus #1255015, Thermo Fisher Scientific, Pittsburgh, PA, US) with the epithelium facing up and covered with a coverslip.
C). Microscopy
Corneas that had acetylcholinesterase staining were imaged using a Retiga SRV Cooled CCD camera with liquid crystal tunable RGB filter (QImaging, Surrey, BC Canada) on a Leica MZ16FA stereomicroscope (Leica Microsystems, GMbH, Wetzlar, Germany) using Image-Pro Plus and Scope-Pro software (Media Cybernetics, Rockville, MD).
Confocal images of corneal buttons that were β-III tubulin stained were obtained using a Leica SP8 confocal microscope (Leica Microsystems, GMbH, Wetzlar, Germany). Confocal images were montaged using the Leica LAS-X capture and processing software.
D). Computerized quantification of nerve area
The nerves located in the intended area of laser ablation (6mm diameter, total area 28.2mm2) and in the surrounding cornea out to a 9.4 mm diameter circle were analyzed in control corneas using Image-Pro software (Media Cybernetics, Rockville, US). In order to always measure the same area in all the corneas, a 9.4 mm diameter circle with a total area of 69.3 mm2 was drawn on each image and saved for future analyses. Subsequently, in the area selected, a filter was applied to convert the stained nerve images to black and white (nerves were white) in order to increase the contrast and facilitate the measurements. Image-Pro software automatically measured the area of the nerves in mm2 inside the area of interest. Identical measurements were performed to calculate the nerve damage area after epithelial scrape or PRK at the different time points.
E). Statistical analysis
Data were analyzed using statistical software SAS® 9.4 (Cary, NC). Variations were expressed as standard deviation of the mean (SD). Statistical comparisons between the groups were performed using analysis of variance (ANOVA) and for comparisons between two groups a T-test assuming unequal variances (Satterthwaite). All statistical tests were conducted at an alpha level at 0.01 due to the large number of statistical comparisons. Normality of the data is assumed for statistical analyses.
Results
A). Acetylcholinesterase staining of corneal nerves in the central cornea
Corneas that had epithelial scraping had lower nerve density compared to control corneas at one day after scrape (Fig. 1, Table 1 and Table 2), indicating there was mild damage to the superficial nerves produced by the scrape. PRK had a major impact on corneal nerve density at one day after surgery (Fig. 1, Table 1 and Table 2). There was progressive recovery of corneal nerve density within the ablated zone from one to six months after PRK (Fig. 1, Fig. 2, Table 1 and Table 2) compared to control corneas. At three months and six months the difference in nerve density in the PRK ablated zone compared to controls was not statistically significant at a p-value <0.01, although there was a trend towards a persistent decrease in nerve density at these time points (p=0.05). There was no difference between scrape with and scrape without MMC at one day after injury (Fig. 1, Table 1 and Table 2), indicating MMC itself had no effect on corneal nerve density after a scrape injury. However, in the context of PRK injury, there was a small effect of MMC on corneal nerve density within the ablated zone at one day after surgery (p=0.0009, Fig. 1, Table 1 and Table 2), but this effect of MMC was no longer present at one month after PRK and, therefore, was not examined at later time points.
Fig. 1. Acetylcholinesterase histochemistry staining in corneas at one day after different injuries.
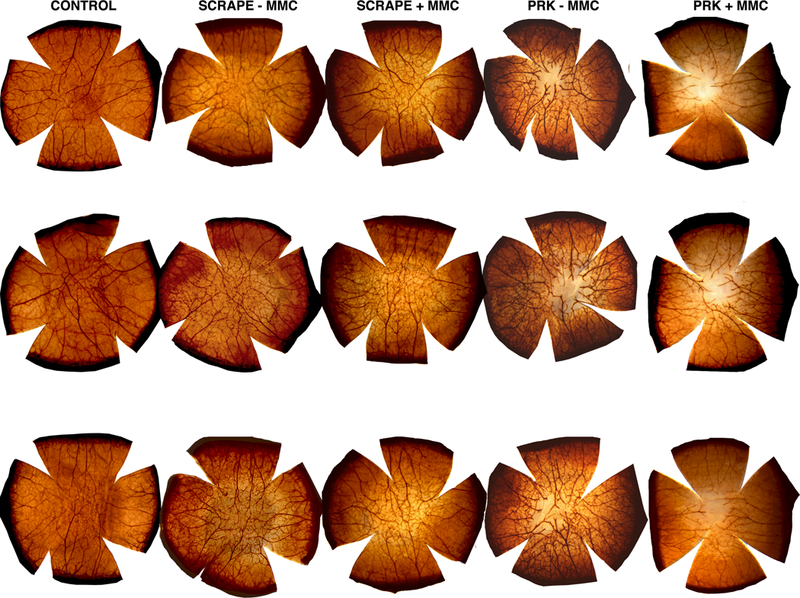
Scrape – MMC group had 8 mm epithelial scrape without the use of MMC. Scrape + MMC group had MMC 0.02% applied for 30 seconds after 8 mm epithelial scrape. PRK – MMC group had −9D PRK performed without the use of MMC. PRK + MMC had −9D PRK with MMC treatment. Three corneas were analyzed per group.
Table 1-. Nerve Area per mm2 over time, Mean ± SD values of the area of the nerve staining after different injuries over time and p-values compared to control group.
Scrape – and Scrape + indicates that an epithelial scrape was performed without and with the use of MMC, respectively. PRK – and PRK + indicates that −9D PRK was performed without and with the use of MMC, respectively.
| Groups | Control | Scrape - MMC 1d |
Scrape + MMC 1d |
PRK - MMC 1d |
PRK + MMC 1d |
PRK- MMC lm |
PRK + MMC lm |
PRK - MMC 2m |
PRK - MMC 3m |
PRK - MMC 6m |
|---|---|---|---|---|---|---|---|---|---|---|
| Nerve area per mm2 (Mean±SD) | 18.4 ± 0.9 | 16.9 ± 0.3 | 17.3 ± 0.4 | 11.2 ± 0.1 | 9.5 ± 0.6 | 14.1 ± 0.4 | 14.2 ± 0.3 | 15.3 ± 0.1 | 17.5 ± 0.4 | 17.6 ± 0.3 |
Table 2-. ANOVA p values per group / per time-point, ANOVA multiple comparisons between the groups over different time-points.
ANOVA was used to analyze the difference between the multiple groups and time points. 1d is the one-day time point, and 1m, 2m, 3m and 6m respectively mean one, two, three and six months, respectively. When using this table, the p-value for a comparison between two groups is found at the intersection of one group on the y-axis with the other group on the x-axis.
| Groups | Control | Scrapc - MMC 1d |
Scrapc + MMC 1d |
PRK - MMC 1d |
PRK + MMC 1d |
PRK - MMC lm |
PRK + MMC 1m |
PRK - MMC 2m |
PRK - MMC 3m |
PRK - MMC 6m |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.002 | 0.02 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.05 | 0.05 | |
| Scrape - MMC Id | 0.4 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.002 | 0.1 | 0.1 | ||
| Scrapc + MMC 1d | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0002 | 0.6 | 0.5 | |||
| PRK - MMC 1d | 0.0009 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
| PRK + MMC 1d | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| PRK - MMC 1m | 0.9 | 0.01 | <0.0001 | <0.0001 | ||||||
| PRK + MMC 1m | 0.01 | <0.0001 | <0.0001 | |||||||
| PRK - MMC 2m | <0.0001 | <0.0001 | ||||||||
| PRK - MMC 3m | 0.9 | |||||||||
| PRK - MMC 6m |
Fig. 2. Acetylcholinesterase histochemistry staining in corneas at different time points after −9D PRK with and without MMC.
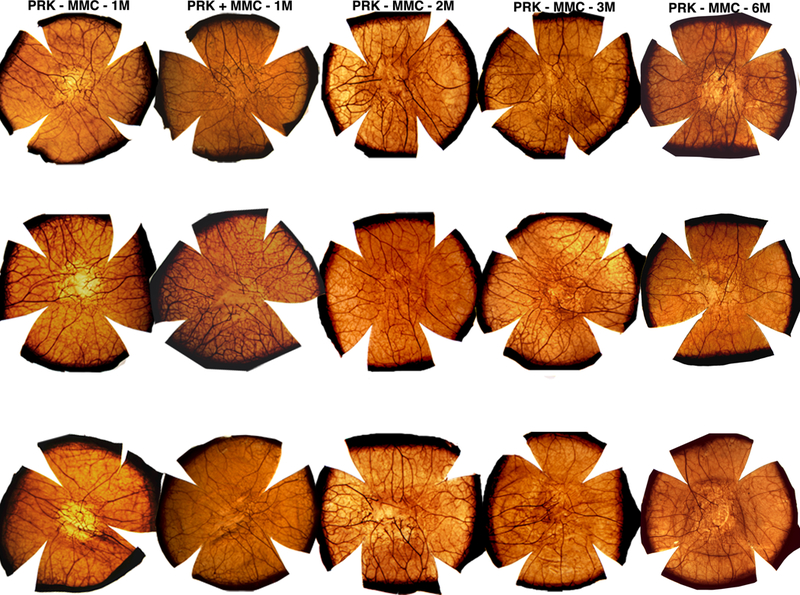
PRK + MMC-1m group had a −9.0 D PRK with 0.02% MMC for 30 seconds and analyzed after one month after surgery. PRK - MMC-1m, −2m, −3m and −6m corneas had −9.0D PRK without the use of MMC and were analyzed at one, two, three and six months, respectively, after surgery. Three corneas were analyzed per group, per time-point, except the sixth month time point had four corneas.
B). Corneal nerve morphology using β-III Tubulin staining after PRK without MMC
Extensive damage to the corneal nerves was noted at one day after PRK at the level of the sub-basal nerve plexus (Fig. 3), in the anterior stroma at a depth of approximately 125 μm (Fig. 4), and in the mid-stroma at a depth of approximately 250 μm (Fig. 5). It can be noted that nerve damage extended beyond the 6 mm excimer laser ablated zone at one day after PRK (Fig. 3B, Fig. 4B, Fig. 5B), especially in the superficial zone of the sub-basal nerve plexus (Fig. 3B).
Fig. 3. β-III Tubulin histochemistry staining the corneal sub-basal plexus overtime after −9D PRK without MMC.
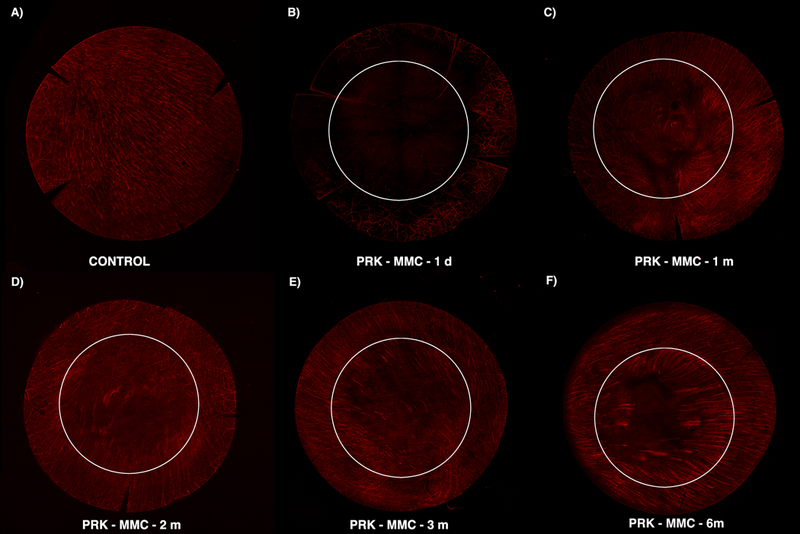
This composite shows the early damage peripheral to the ablated zone (B) and subsequent (C and D) regeneration following the margin of the wound in a perpendicular pattern (E and F). An oblique orientation of the regenerated fibers at the edge of the wound was observed. However, the original central ablated zone is not fully regenerated. The six mm white circle delineates the estimated excimer laser ablation zone. Note that nerve density is decreased beyond the ablated zone.
Fig. 4. β-III Tubulin histochemistry staining the corneal anterior stroma at a depth of approximately 125 μm overtime after −9D PRK without MMC.
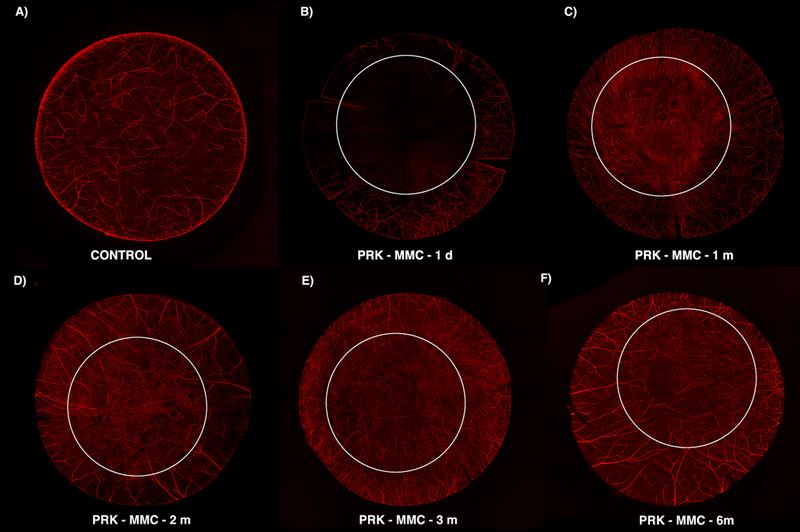
This composite shows that the anterior corneal stroma contributed substantially to the neuro-remodeling process. Abnormal morphology and a very tortuous pattern are observed one month after the initial injury (C). The small and tortuous neurites were replaced by longer fibers that repopulate the treated area but with dichotomous or trichotomous branching patterns (D). A high density of nerves, but with thinner fibers, is observed in the central cornea (E). A more organized pattern is noted at six months after surgery than three months after surgery (F). The six mm white circle delineates the estimated excimer laser ablation zone.
Fig. 5. β-III Tubulin histochemistry staining the corneal mid-stroma at a depth of approximately 250 μm overtime after −9D PRK without MMC.
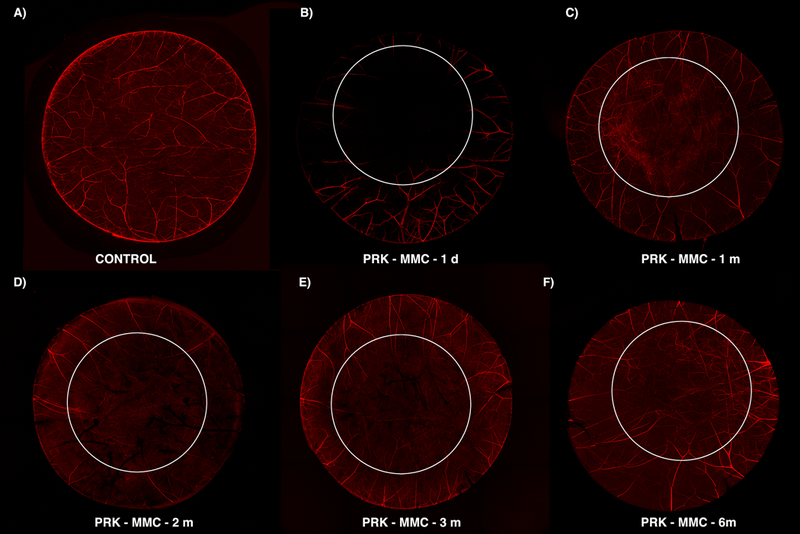
This composite shows that the nerve loss extends to deeper cornea layers in the mid-stroma. There is retrograde axonal death (B) and then neuron recovery (C). The first attempt of regeneration was also observed on the deeper layers (D). At 3 months after PRK, some long fibers are present at the ablated zone (E). This population was increased after 6 months (F), but not fully recovered compared to unwounded controls. The six mm white circle delineates the estimated excimer laser ablation zone.
At one month following PRK, signs of nerve regeneration were noted in sub-basal plexus (Fig. 3C) and anterior stroma (Fig. 4C). Collateral sprouts arose from the edge of the wound on sub-epithelial plexus and migrated perpendicularly to reach the sub-basal plexus and epithelium (Fig. 3). In addition, stromal nerve changes were also present in the deeper ablated zone, where there were limited larger fibers even at 6 months after surgery (Fig. 5). Small nerves that exhibited aberrant morphology with localized twisting, looping and multiple branches were also observed and tended to be concentrated at the margin of the excimer laser ablation (Fig. 5C).
Over time out to six months after PRK (Figs. 3, 4 and 5), the corneal nerves continue to regenerate, and the morphology became more normal, but even at six months after surgery has not returned fully to the control density at any of the three levels in the cornea (Figs. 3 and Fig. 5).
Nerve damage at all layers of the cornea compared to the control (Video 1) can be best noted in movies of β-III tubulin staining at one day (Video 2), one month (Video 3), two months (Video 4), three months (Video 5), and six months (Video 6) after PRK.
Discussion
Important observations have been made to provide a detailed understanding of the distribution of innervation in the cornea.13,14 The nerves penetrate the cornea from the limbus in the mid-stroma and terminate as free nerve endings in the epithelial layer of the cornea.1 The majority of the nerve fibers are located in the anterior third of the stroma, but the thick stromal nerve trunks are located beneath the anterior third of the stroma. The path of the stromal nerves makes a 90° turn in the superficial cornea and they divide into several branches that parallel the basal epithelium and give rise to the sub-basal plexus.1
The delicate nerve network within the epithelium and the sub-basal plexus are damaged during epithelial removal as a first step of PRK surgery. Subsequently, the excimer laser injures deeper stromal nerves during the ablation process and the amount of damage is related to the level of correction, and, therefore, the amount of stromal ablation, as well as whether the treatment is for myopia or hyperopia. The greater the intended correction, the deeper the excimer laser ablation extends into the cornea and the more nerves are damaged.
Several different methods can be used to study corneal nerve architecture—including probing tissue sections with dyes or immunohistochemistry for nerve components, as in the present study, or in vivo confocal microscopy (IVCM) or electron microscopy.1 Most studies of corneal nerves used IVCM. This approach has provided important insights into corneal nerve morphology and function, but it has disadvantages when studying the impact of PRK on corneal nerves. A major disadvantage is a decrease in the contrast of the images attributable to backscattered light that originates from “activated” keratocytes (in cell biology referred to as corneal fibroblasts) and altered extracellular matrix that occurs in the months post-PRK and is referred to as normal haze. These factors make the use of IVCM to evaluate corneal nerves more difficult, especially when examining small fine bundles.15,16
The acetylcholinesterase staining method with a stereomicroscope was used in the present study as the first method to analyze whole rabbit corneas. The stereomicroscope allows the examination of thicker specimens by providing a full thickness visualization of the sample. The acetylcholinesterase staining was used to quantify nerve damage after the different procedures because it allows the visualization of every type of fibers present in corneal tissue. Thus, the combination of the acetylcholinesterase staining method with the stereomicroscope analysis of the whole cornea provided high contrast images of the total nerve network for quantitative analysis.
The second nerve staining method used in this study was immunofluorescent detection of the tubulin beta III subunit of tubulin III, a protein that is primarily expressed in neurons and is considered to play a critical role in proper axon guidance and maintenance.17 The use of the in vitro confocal microscope for this study enabled imaging at different depths within the stroma to provide a more complete analysis of nerve damage after PRK at different levels in the cornea and, therefore, provided a better understanding of nerve regeneration after surgery.
As expected, the corneal nerves were damaged at one day in all groups, including after manual epithelium layer scrapping alone. Epithelial debridement damaged the free nerve endings in the epithelium and the complex network of the fibers in the sub-basal plexus. The excimer laser PRK ablation for high myopia used in this study caused nerve loss that extended peripheral to the excimer laser treated zone (Fig. 3B and4B). This indicates that the ablation of nerves caused a retrograde nerve degeneration beyond the 6 mm central ablated area.18–21 Thus, PRK doesn’t merely ablate the nerve endings, as has been suggested by clinicians, but rather the damage to the nerves extends well beyond the excimer laser ablation and, therefore, regeneration of the nerves requires months to approach the preoperative density in the center of the ablated zone.
Different from previous reports that used sub-epithelial plexus to study the corneal nerves,22–24 this confocal three-dimensional analysis of the different corneal depths throughout the whole corneal thickness showed extensive nerve damage at all corneal depths from the epithelium to the mid-stroma. However, the degree of nerve loss would be variable and likely less when PRK ablations were made to correct lower levels of myopia.25, 26
Neurotoxicity has been described with the use of mitomycin C (MMC) in other organs. For example, the topical application of MMC produced a substantial sensory-neural hearing loss—demonstrating toxicity to acoustic nerves.27 When used for advanced breast cancer treatment it also produced peripheral neurotoxicity.28 A decrease of the thickness of myelin sheath was observed after local use of MMC at concentrations over 0.7mg/ml for preventing post-laminectomy epidural scar formation, indicating an adverse effect on the peripheral nerves.29 Neurotoxicity effect of MMC was also published in studies in the eyes.30,31 The present study revealed only mild MMC nerve toxicity in the early postoperative period that did not persist at one month after PRK with 0.02% MMC and was not noted at all after simple epithelial debridement with MMC treatment. This finding agrees with a study of the long-term safety of MMC in the cornea.32 Other authors reported a dose-dependent neurotoxicity of MMC in the eye and other tissues.29–31 Thus, this study did not find any longterm effects of MMC treatment on corneal nerves when used in conjunction with PRK for the correction of high myopia.
After the nerve damage produced by PRK, there is gradual recovery and reorganization of the nerve fibers extending over a period of at least six months. In this study, the area of regenerated nerve fibers in the central cornea reached a comparable value with unoperated control eyes at three months after surgery, which agrees with other studies.22,33 However, other studies differ considerably in relation to the functional recovery time of the corneal nerves that vary between 5–8 months,24,34–36 one year,37 two years,38–40 three years,16 and five years23 depending on the methods used for nerve study.
A prior study in rabbits also made important contributions regarding nerve regeneration after corneal lesions.25 One important observation was that new fibers grew in a perpendicular arrangement to the edge of the wound,25 which was also observed in the present study at one month after PRK (Fig. 3C). These wound-oriented neurites were the primary nerves to repopulate the cornea and migrated from the sub-basal plexus into the epithelium, but they were also detected in the anterior stroma (Fig. 4C) albeit with abnormal morphology.
Nerve regeneration in the mid-stroma also started at one month after PRK (Fig 5C), but with a different pattern than was noted in unwounded control corneas. A very dense, multiple- branched and tortuous morphology was noted specifically at the margin of the ablation zone in rabbit corneas that had −9D PRK in the present study, was similar to findings previously reported at the literature as an early, and probably immature or aborted, attempt to regenerate into the ablated zone that was described as the first phase of regeneration.25,41
Rósza et al25,41described a subsequent event that consisted of degeneration of the neurites from the first phase and recovery of the nerve terminals. This process is known as the second phase of nerve regeneration and refers to the previously described vestiges of wound-oriented neurites being replaced by growing axon terminals, with higher density, and shorter in length.25,42 In the current study, this neuron behavior was observed at the third month and was not completed until the sixth month (Fig 4-E, F). The oblique arrangement of the nerve fibers noted at three months is a characteristic sign of this neuro-regeneration phase.
When acetylcholinesterase images at three months after −9D PRK are observed alone, there is no statistical difference in the area of the regenerated nerves compared to the unwounded control corneas in the central cornea (Fig. 2). However, in the β-III tubulin confocal images, where the nerves were analyzed not only by area but also by depth, it could be observed that the nerve regeneration of the PRK-treated area occurred mainly in the anterior stroma at this point (Fig 4). The second phase of nerve growth is considered the first sign of neurogenesis.25 This phase was initiated in the corneal stroma three months after −9D PRK in rabbits but a substantial intraepithelial nerve population did not reach the central cornea even after six months (Fig 3F). Also, a persistent abnormal architecture and orientation of the nerve fibers was still observed at six months after PRK. Thus, the neural remodeling is not completed until after sixth months following high-correction PRK and abnormalities could persist for months or years in some corneas. The slow neural recovery present at sub-epithelial and sub-basal plexus could be a consequence of the dense and complex network of the nerve fibers that compose these structures, which likely need a longer time to recover fully, although longer term studies would be needed to study this. What can be concluded from this study is that nerve defects persist for a minimum of six months following PRK surgery for high myopia and it is possible that subtle defects in nerve function persist indefinitely.
Supplementary Material
A 9 mm unwounded cornea with a normal distribution of corneal nerves from the very thin nerve terminals perpendicular to the surface at the epithelium layer and sub-basal plexus that is dense and multi-branched parallel to the surface. Larger trunks are seen in the mid-stroma.
A corneal button at one day after -9D PRK without MMC shows extensive nerve damage from the epithelium to the thick mid-stromal nerve trunks. Fibers peripheral to the treated zone are decreased after the excimer laser treatment, indicating retrograde neuronal damage beyond the ablated area.
A corneal button at one month after -9D PRK without MMC showing limited nerve regeneration. Nerve regeneration can be seen in the sub-basal plexus and the anterior stroma. Collateral nerve sprouts can be seen arising from the subepithelial edge of the wound that migrate to reach the sub-basal plexus and epithelium. Aberrant nerve morphology concentrated at the edge of the wound and in the central ablation zone can be noted.
A cornea at two months after -9D PRK without MMC. Further regeneration of the corneal nerves can be noted. A higher number of nerves are repopulating the central area, with a low branching pattern and atypical morphology are concentrated in the area of the sub-basal plexus.
A cornea at three months after the surgery shows further nerve regeneration within the treated zone.
A cornea at six months after the surgery shows a persistent decrease in nerves within the ablation zone at the sub-basal plexus. However, even in the mid-stroma the density of nerve trunks appears diminished compared to unwounded control corneas.
Acknowledgments
Supported in part by US Public Health Service grants RO1EY10056 (SEW) and P30-EY025585 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY. Dr. Lassance is supported by NEI training grant T32 EY007157. Dr. Medeiros is supported by CAPES training grant PDSE2016 Brasília, Brazil. Research reported in this publication was supported by the Office of the Director, National Institutes of Health under award number 1S10OD019972–01 for light and confocal microscopy imaging. The authors thank James F. Bena, MS, biostatistician in the Quantitative Health Sciences Department, Cleveland Clinic, for help with statistical analyses, and Paramananda Saikia, PhD, and Rodrigo Carlos de Oliveira, MD, Cleveland Clinic, for help with animal work during the last time-point and data analysis.
Footnotes
None of the authors or their family members have any financial interests in the topics of this manuscript.
References
- 1.Müller LJ, Marfurt CF, Kruse F, Tervo TM Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. [DOI] [PubMed] [Google Scholar]
- 2.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78(3):513–525. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohlhaas M, Draeger J, Bohm A, et al. Aesthesiometry of the cornea after refractive corneal surgery. Klin Monbl Augenheilkd. 1992;201(4):221–223. [DOI] [PubMed] [Google Scholar]
- 5.Wilson SE. Laser in situ keratomileusis-induced (presumed) neurotrophic epitheliopathy. Ophthalmology. 2001;108(6):1082–1087. [DOI] [PubMed] [Google Scholar]
- 6.Ambrósio R, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24(4):396–407. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SE, Medeiros CS, Santhiago MR. Pathophysiology of corneal scarring in persistent epithelial defects after PRK and other corneal injuries. J Refract Surg. 2018;34(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santhiago MR, Netto M V., Wilson SE Mitomycin C: Biological effects and use in refractive surgery. Cornea. 2012;31(3):311–321. [DOI] [PubMed] [Google Scholar]
- 9.Koelle GB, Friedenwald JS. The histochemical localization of cholinesterase in ocular tissues. Am J Ophthalmol. 1950;33(2):253–256. [DOI] [PubMed] [Google Scholar]
- 10.Tervo T Histochemical demonstration of cholinesterase activity in the cornea of the rat and the effect of various denervations on the corneal nerves. Histochemistry. 1976;47(2):133–143. [DOI] [PubMed] [Google Scholar]
- 11.Xia Y, Chai X, Zhou C, Ren Q. Corneal nerve morphology and sensitivity changes after ultraviolet A/riboflavin treatment. Exp Eye Res. 2011;93(4):541–547. [DOI] [PubMed] [Google Scholar]
- 12.He J, Bazan NG, Bazan HEP. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91(4):513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zander E, Weddell G. Observations on the innervation of the cornea. J Anat. 1951;85(1):68–99. [PMC free article] [PubMed] [Google Scholar]
- 14.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90(4):478–492. [DOI] [PubMed] [Google Scholar]
- 15.Tervo T, Moilanen J. In vivo confocal microscopy for evaluation of wound healing following corneal refractive surgery. Prog Retin Eye Res. 2003;22(3):339–358. [DOI] [PubMed] [Google Scholar]
- 16.Linna T, Tervo T. Real-time confocal microscopic observations on human corneal nerves and wound healing after excimer laser photorefractive keratectomy. Curr Eye Res. 1997;16(7):640–649. [DOI] [PubMed] [Google Scholar]
- 17.Moskowitz PF, Smith R, Pickett J, Frankfurter A, Oblinger MM. Expression of the class III beta-tubulin gene during axonal regeneration of rat dorsal root ganglion neurons. J Neurosci Res. 1993;34(1):129–134. [DOI] [PubMed] [Google Scholar]
- 18.Catapano J, Zhang J, Scholl D, Chiang C, Gordon T, Borschel GH. N-Acetylcysteine prevents retrograde motor neuron death after neonatal peripheral nerve injury. Plast Reconstr Surg. 2017;139(5):1105–1115. [DOI] [PubMed] [Google Scholar]
- 19.Al-Louzi O, Button J, Newsome SD, Calabresi PA, Saidha S. Retrograde trans-synaptic visual pathway degeneration in multiple sclerosis: a case series. Mult Scler. 2017; 23(7):1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handley SE, Panteli VS, Liasis A. Trans-synaptic retrograde degeneration following hemispherectomy in childhood. Neuroophthalmology. 2017;41(2):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchihara T An order in Lewy body disorders: Retrograde degeneration in hyperbranching axons as a fundamental structural template accounting for focal/multifocal Lewy body disease. Neuropathology. 2017;37(2):129–149. [DOI] [PubMed] [Google Scholar]
- 22.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: A 1-year confocal microscopic study. Ophthalmology. 2000;107(7):1235–1245. [DOI] [PubMed] [Google Scholar]
- 23.Moilanen JA, Vesaluoma MH, Müller LJ, Tervo TM. Long-term corneal morphology after PRK by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2003;44(3):1064–1069. [DOI] [PubMed] [Google Scholar]
- 24.Kauffmann T, Bodanowitz S, Hesse L, Kroll P. Corneal reinnervation after photorefractive keratectomy and laser in situ keratomileusis: an in vivo study with a confocal videomicroscope. Ger J Ophthalmol. 1996;5(6):508–512. [PubMed] [Google Scholar]
- 25.Rózsa AJ, Guss RB, Beuerman RW. Neural remodeling following experimental surgery of the rabbit cornea. Invest Ophthalmol Vis Sci. 1983;24(8):1033–1051. [PubMed] [Google Scholar]
- 26.De Felipe C, Belmonte C. c-Jun expression after axotomy of corneal trigeminal ganglion neurons is dependent on the site of injury. Eur J Neurosci. 1999;11(3):899–906. [DOI] [PubMed] [Google Scholar]
- 27.Moody MW, Lang H, Spiess AC, Smythe N, Lambert PR, Schmiedt RA. Topical application of mitomycin C to the middle ear is ototoxic in the gerbil. Otol Neurotol. 2006;27(8):1186–1192. [DOI] [PubMed] [Google Scholar]
- 28.Kornek GV, Haider K, Kwasny W, Lang F, Krauss G, Hejna M, Raderer M, Weinlaner G, Depisch D, Scheithauer W. Effective treatment of advanced breast cancer with vinorelbine, 5-fluorouracil and l-leucovorin plus human granulocyte colony-stimulating factor. Br J Cancer. 1998;78(5):673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sui T, Zhang J, Du S, Su C, Que J, Cao X. Potential risk of mitomycin C at high concentrations on peripheral nerve structure. Neural Regen Res. 2014;9(8):821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mietz H, Addicks K, Diestelhorst M, Krieglstein GK. Intraocular toxicity to ciliary nerves after extraocular application of mitomycin C in rabbits. Int Ophthalmol. 1995;19(2):89–93. [DOI] [PubMed] [Google Scholar]
- 31.Mietz H, Prager TC, Schweitzer C, Patrinely J, Valenzuela JR, Font RL. Effect of mitomycin C on the optic nerve in rabbits. Br J Ophthalmol. 1997;81(7):584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gambato C, Miotto S, Cortese M, Ghirlando A, Lazzarini D, Midena E. Mitomycin C- assisted photorefractive keratectomy in high myopia: a long-term safety study. Cornea. 2011;30(6):641–645. [DOI] [PubMed] [Google Scholar]
- 33.Tervo K, Latvala TM, Tervo TM. Recovery of corneal innervation following photorefractive keratoablation. Arch Ophthalmol. 1994;112(11):1466–1470. [DOI] [PubMed] [Google Scholar]
- 34.Heinz P, Bodanowitz S, Wiegand W, Kroll P. In vivo observation of corneal nerve regeneration after photorefractive keratectomy with a confocal videomicroscope. Ger J Ophthalmol. 1996;5(6):373–377. [PubMed] [Google Scholar]
- 35.Nejima R, Miyata K, Tanabe T, Okamoto F, Hiraoka T, Kiuchi T, Oshika T. Corneal barrier function, tear film stability, and corneal sensation after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2005;139(1):64–71. [DOI] [PubMed] [Google Scholar]
- 36.Alio JL, Javaloy J. Corneal inflammation following corneal photoablative refractive surgery with excimer laser. Surv Ophthalmol. 2013;58(1):11–25. [DOI] [PubMed] [Google Scholar]
- 37.Erie JC, Patel SV, Bourne WM. Aberrant corneal nerve regeneration after PRK. Cornea. 2003;22(7):684–686. [DOI] [PubMed] [Google Scholar]
- 38.Frueh BE, Cadez R, Bohnke M. In vivo confocal microscopy after photorefractive keratectomy in humans. A prospective, long-term study. Arch Ophthalmol. 1998;116(11):1425–1431. [DOI] [PubMed] [Google Scholar]
- 39.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140(6):1059–1064. [DOI] [PubMed] [Google Scholar]
- 40.Neira-Zalentein W, Moilanen JA, Tiusku IS, Holopainem JM, Tervo TM. Photorefractive keratectomy retreatment after LASIK. J Refract Surg. 2008;24(7):710–712. [DOI] [PubMed] [Google Scholar]
- 41.Rózsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14(2):105–120. [DOI] [PubMed] [Google Scholar]
- 42.Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69(1):196–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A 9 mm unwounded cornea with a normal distribution of corneal nerves from the very thin nerve terminals perpendicular to the surface at the epithelium layer and sub-basal plexus that is dense and multi-branched parallel to the surface. Larger trunks are seen in the mid-stroma.
A corneal button at one day after -9D PRK without MMC shows extensive nerve damage from the epithelium to the thick mid-stromal nerve trunks. Fibers peripheral to the treated zone are decreased after the excimer laser treatment, indicating retrograde neuronal damage beyond the ablated area.
A corneal button at one month after -9D PRK without MMC showing limited nerve regeneration. Nerve regeneration can be seen in the sub-basal plexus and the anterior stroma. Collateral nerve sprouts can be seen arising from the subepithelial edge of the wound that migrate to reach the sub-basal plexus and epithelium. Aberrant nerve morphology concentrated at the edge of the wound and in the central ablation zone can be noted.
A cornea at two months after -9D PRK without MMC. Further regeneration of the corneal nerves can be noted. A higher number of nerves are repopulating the central area, with a low branching pattern and atypical morphology are concentrated in the area of the sub-basal plexus.
A cornea at three months after the surgery shows further nerve regeneration within the treated zone.
A cornea at six months after the surgery shows a persistent decrease in nerves within the ablation zone at the sub-basal plexus. However, even in the mid-stroma the density of nerve trunks appears diminished compared to unwounded control corneas.


