Abstract
Introduction:
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin cancer that lacks durable responses to traditional chemotherapy.
Areas covered:
After MCC was shown to be an immunogenic tumor, small trials revealed high objective response rates to PD-1/PD-L1 checkpoint inhibitors. The JAVELIN Merkel 200 (NCT02155647) trial tested the use of avelumab, a human IgG1 monoclonal antibody against PD-L1, in metastatic MCC. Avelumab recently became the first approved drug for metastatic MCC.
Expert commentary:
By conducting broad phase I studies assessing the safety of avelumab and a small phase II study demonstrating efficacy in this rare orphan tumor type, avelumab gained accelerated approval for the treatment of metastatic MCC. Additional studies are needed to determine how the antibody-dependent cellular cytotoxicity (ADCC) competent Fc region of avelumab contributes to disease control.
Remaining questions:
Longer follow-up will determine the durability of checkpoint blockade in controlling metastatic MCC. Additional studies will assess the utility and safety of adjuvant checkpoint blockade in patients with excised MCC. How to increase response rates by combining PD-1/PD-L1 blockade with other treatment approaches needs to be explored. In addition, treatment options for MCC patients who fail or do not respond to avelumab need to be identified.
Keywords: anti-PD-1, anti-PD-L1, avelumab, Bavencio, check point inhibitor, immunotherapy, Merkel cell carcinoma, pembrolizumab
1. INTRODUCTION
1.1. Merkel cell Carcinoma
Merkel cell carcinoma (MCC) is a rare and aggressive skin cancer with a predilection for UV-damaged skin of older and immunocompromised patients [1, 2]. MCC is rare, occurring with an incidence of 0.2 to 0.4 cases per 100,000 people per year in Europe, 0.79 in the U.S., and 1.6 in Australia [3, 4, 5]. Although rare, MCC’s incidence has tripled over the past three decades [6] and it has a disease-associated mortality of 46%, three-times that of melanoma [7, 8]. Up to 80% of MCC patients eventually develop metastases [9]. On average, patients with stage IV disease treated with cytotoxic chemotherapy face an 18% 5 year survival [7], and a median progression-free survival of only 3 months [10, 11].
MCC is an immunogenic cancer arising more often and with a worse prognosis in immunocompromised patients [12]. One of the most important factors in illuminating the pathogenesis of MCC was the discovery of the Merkel cell polyomavirus (MCV). In most studies MCV is detected in 69-85% of MCC tumors, however in countries with high UV-exposure, such as Australia and New Zealand, MCC tumors are mostly MCV-negative [13, 14, 15, 16]. MCV DNA is clonally integrated into the tumor genome [13, 17], and viral T antigens are required for proliferation and survival of MCV-positive MCC tumor cells [17, 18, 19]. Antibodies against these T antigens are specifically detected in the blood of patients with MCC, and serology titers reflect tumor burden and predict recurrence [20]. MCV-associated MCC carry very few somatic mutations. In contrast, UV-induced, MCV-negative MCC are characterized by mutational loads approximately 100 times greater than MCV-positive MCC [21, 22, 23].
Early stage MCC is treated with surgical excision and radiation [24] . Until recently, effective treatments for metastatic MCC were lacking [25]. There are no FDA-approved chemotherapy protocols for managing MCC, and treatments are largely derived from those used for small cell lung cancer, a more common neuroendocrine tumor [11]. Commonly used chemotherapy regimens include platinum-containing agents combined with etoposide or cyclophosphamide, doxorubicin, and vincristine (CAV) [11]. MCC has high response rates to chemotherapy, but tumors recur within 4-15 months [25]. As a consequence, treatment guidelines recommended enrolment in a clinical trial for patients with metastatic disease [3, 24]. However, within the last year, the anti-PD-L1 immune checkpoint inhibitor avelumab (Bavencio®) was approved for the treatment of metastatic MCC in the United States, the European Union, Canada, Australia, Israel, Japan and Switzerland.
1.2. Overview of immunotherapy and checkpoint inhibitors
Over the last decade, the use of monoclonal antibodies against immune checkpoint receptors like cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death receptor (PD-1) has changed the practice of oncology. There is an expanding catalogue of immune checkpoint inhibitors available for the treatment of human malignancies (Table 1). Immune checkpoints are signaling pathways that restrict continued immune responses, thereby preventing host autoimmunity and excessive immune reactions against certain antigens [26]. Drugs that block immune checkpoint signaling can reactivate cellular immune responses to facilitate tumor clearance. When effective, checkpoint inhibitors can provide durable clinical benefit in close to one third of cancer patients [27, 28, 29, 30]. There is particular interest in using PD-1 blockers, alone or in combination with other agents [27, 31, 32, 33], to engage anti-tumor immune responses [33, 34].
Table 1.
Check-point inhibitors and their approval history as of February 14, 2018
| Action | Agent/trade name/company | Antibody type | Efficacy | Ref. | Approval |
|---|---|---|---|---|---|
| Anti-CTLA4 | Ipilimumab AKA: MDX-010 Trade name: Yervoy BMS | IgG1κ fully humanized | Metastatic malignant melanoma | [40] | FDA/EMA 2011 |
| Anti-PD-1 | Nivolumab AKA: Nivolumabum, ONO-4538, BMS-936558, MDX1106 Trade name: Optivo BMS | IgG4 fully humanized | Unresectable or metastatic Melanoma | [101] [102] |
FDA 2014 EMA 2015 |
| Metastatic NSCLC | FDA/EMA 2015 | ||||
| Metastatic renal cell Carcinoma | FDA/EMA 2015 | ||||
| Hodgkin lymphoma | FDA/EMA 2016 | ||||
| Head and neck cancer | FDA 2016 EMA 2017 |
||||
| Advanced or metastatic urothelial carcinoma | FDA 2017 EMA 2016 |
||||
| Hepatocellular carcinoma | FDA 2017 | ||||
| Colorectal cancer | FDA 2017 | ||||
| Adjuvant therapy in completely resected stage III/IV melanoma | [103] | FDA 2017 | |||
| Anti-PD-1 | Pembrolizumab AKA: Lambrolizumab, MK-3475 Trade name: Keytruda Merck/MSD | IgG4 fully humanized | Metastatic malignant melanoma | [104] [93] |
FDA 2014 EMA 2015 |
| Metastatic head and neck squamous cell carcinoma | FDA 2016 | ||||
| Metastatic non-small cell lung cancer | FDA 2016 EMA 2016 |
||||
| Classical Hodgkin Lymphoma | FDA 2017 EMA 2017 |
||||
| Locally advanced or metastatic urothelial carcinoma | FDA 2017 EMA 2017 |
||||
| Any solid tumor with a specific genetic feature (microsatellite instability-high or mismatch repair deficient) | FDA 2017 | ||||
| PD-L1 positive gastric/gastroesophageal junction cancer | FDA 2017 | ||||
| Anti-PD-L1 | Atezolizumab AKA: MPDL3280 Trade name: Tecentriq Roche Genentech | IgG1 fully humanized | Advanced Urothelial carcinoma | FDA 2016 | |
| Metastatic lung cancer | FDA 2016 EMA 2017 |
||||
| Advanced bladder cancer | FDA 2017 EMA 2017 |
||||
| Anti-PD-L1 | Avelumab AKA: MSB0010718C Trade name: Bavencio Merck KgaA and Pfizer | IgG1 fully humanized | Merkel cell carcinoma | [50] | FDA 2017 EMA 2017 |
| Urothelial carcinoma | FDA 2017 | ||||
| Anti-PD-L1 | Durvalumab Trade name: Imfinzi AstraZeneca | IgG1κ fully humanized | Advanced bladder cancer | FDA 2017 | |
| Advanced non-small lung cancer | EMA 2017 |
Ab: antibody; ADR: adverse drug reaction; EMA: European Medicines Agency; FDA: food and drug administration; IgG: immunoglobulin G; ORR: overall response rate; PFS: progression-free survival; PD-1: programmed cell death protein 1; PD-L1: programmed cell death protein 1 ligand
The PD-1 immune checkpoint pathway was described in 1992 as a mechanism for T cell death [35]. The PD-1 receptor is expressed on activated B, T, and NK cells, including activated tumor-infiltrating T lymphocytes (TIL) [36, 37]. Upon ligand binding, PD-1 signaling inactivates cytotoxic T cell (CTL), promotes-their apoptosis, and reduces production of pro-inflammatory cytokines [38, 39]. There are two PD-1 ligands, PD-L1 and PD-L2. These ligands can be expressed by hematopoietic cells (B, T, dendritic, and myeloid) and tumor cells [37, 39]. Upregulation of PD-L1 by tumor cells can inactivate cytotoxic effector cells, allowing tumors to evade the immune system [34, 38, 39]. Reversing this immune escape with checkpoint inhibitors allows anti-tumor immune responses to proceed (Figure 1). At the same time, use of immune checkpoint inhibitors can induce auto-immune related side effects [40, 41, 42].
Figure 1.
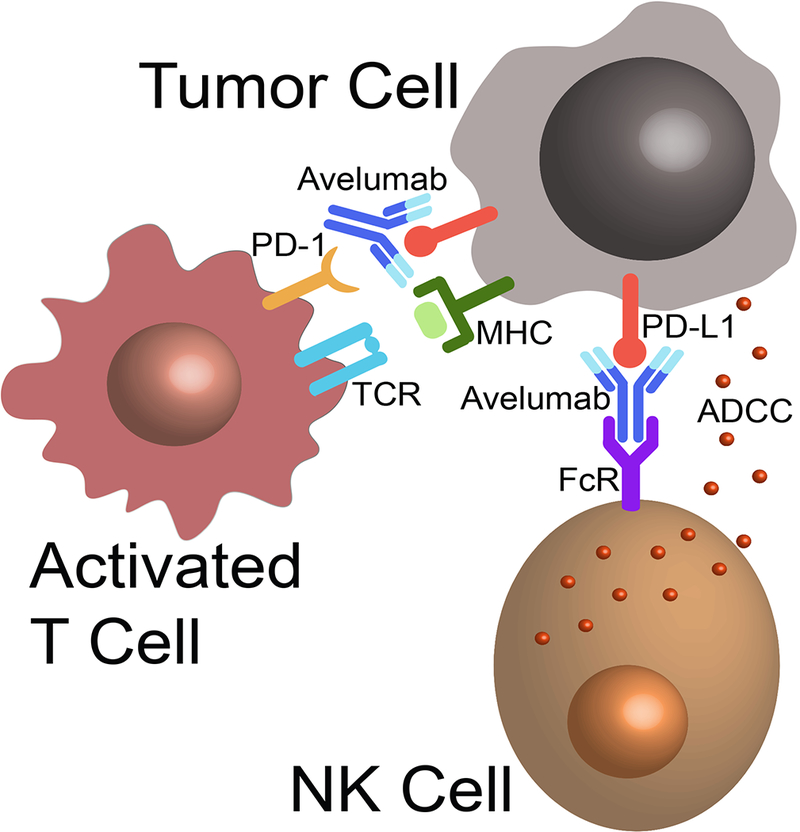
Proposed mechanisms of action for avelumab. By binding PD-L1 the monoclonal antibody blocks signaling to PD-1 on anti-tumor T cells, reversing their inhibition. When bound to tumor cells, the IgG1 Fc domain on avelumab can bind Fc receptors on NK cells to activate ADCC.
1.3. PD-L1 expression
Although data is inconsistent across malignancies, expression of PD-L1on tumor cells correlates with worse prognosis and predicts a higher likelihood of response to PD-1 blockade in some cancers [10, 31, 43, 44] . A reactive pattern of PD-L1 expression has been described in melanoma cells proximate to TIL, whereas melanomas with sparse TIL infiltration were less likely to express PD-L1 [45]. These results suggest that in response to immune recognition tumor cells upregulate PD-L1 in order to inactivate effector T cells [45]. Immunohistochemical tests for PD-L1 expression were recently approved by the FDA to stratify patients with advanced non-small-cell lung cancer as being more likely to respond to anti-PD-1 therapy [46, 47]. In contrast, other studies have shown that PD-L1 overexpression is not a robust predictor for response, and investigations of its utility as a biomarker are ongoing across multiple tumor types [10, 43, 48].
Approximately 50% of MCC express PD-1 on TIL and PD-L1 on tumor cells and adjacent immune cell infiltrates [21, 49, 50]. Moreover, circulating MCV-specific CD8-positive T cells from patients with MCC express higher levels of PD-1 than T cells specific to other common human viruses. In MCC tumors, higher PD-L1 expression correlated with TIL infiltration and a better prognosis [23, 50]. These expression patterns provided a rationale for investigating the therapeutic potential of PD-1/PD-L1 checkpoint inhibitors in MCC. In 2011 the first phase 1 clinical trial involving the PD-L1 antibody atezolizumab in advanced solid and hematologic malignancies, including MCC was initiated. At the time of writing 17 clinical trials involving MCC and checkpoint inhibitors have been initiated (Table 2).
Table 2.
Clinical trials including checkpoint inhibitors for treatment of Merkel Cell Carcinoma - as of September 26, 2017
| Study Sponsor Collaborator | Phase | Official Title | Status | Tumor | Interventions | ClinicalTrials.gov Identifier Date of first submitted |
|---|---|---|---|---|---|---|
| National Cancer Institute (NCI) | II | Phase II Open-Label Trial of Ipilimumab for Metastatic Merkel Cell Carcinoma | Study withdrawn prior to enrolment | MCC | Ipilimumab | NCT01913691 July 28, 2013 |
| JAVELIN Merkel 200 EMD Serono | II | A Phase II, Open-Label, Multicenter Trial to Investigate the Clinical Activity and Safety of Avelumab (MSB0010718C) in Subjects With Merkel Cell Carcinoma | Recruiting | MCC | Avelumab | NCT02155647 June 2, 2014 |
| ADMEC Prof. Schadendorf University Hospital Essen BMS | II | Prospective Randomized Trial of an Adjuvant Therapy of Completely Resected Merkel Cell Carcinoma (MCC) With Immune Checkpoint Blocking Antibodies (Nivolumab, Opdivo®; Ipilimumab (Yervoy®) Every 3 Weeks for 12 Weeks Versus Observation | Recruiting | MCC | Ipilimumab Nivolumab | NCT02196961 June 20, 2014 |
| National Cancer Institute (NCI) | II | A Phase II Study of MK-3475 in Patients With Advanced Merkel Cell Carcinoma (MCC) | Ongoing, but not recruiting | MCC | Pembrolizumab | NCT02267603 October 14, 2014 |
| Fred Hutchinson Cancer Research Center National Cancer Institute (NCI) EMD Serono | I / II | Study to Evaluate Cellular Adoptive Immunotherapy Using Polyclonal Autologous CD8+ Antigen-Specific T Cells for Metastatic Merkel Cell Carcinoma in Combination With MHC Class I Up-Regulation and the Anti-PD-L1 Antibody Avelumab | Recruiting | MCC | Avelumab, Laboratory Biomarker Analysis MCPyV Tag-specific polyclonal Autologous CD8-positive T-cells, Radiation Therapy Recombinant IFN Beta | NCT02584829 October 21, 2015 |
| Ludwig Institute for Cancer Research MedImmune LLC Cancer Research Institute, New York City | I / II | A Phase 1/2 Study of In Situ Vaccination With Tremelimumab and IV Durvalumab (MEDI4736) Plus the Toll-like Receptor Agonist PolyICLC in Subjects With Advanced, Measurable, Biopsy-accessible Cancers | Recruiting | MCC among other tumors | Durvalumab, Tremelimumab, Poly ICLC | NCT02643303 December 18, 2015 |
| National Cancer Institute (NCI) | II | A Phase II Study of T-VEC Followed by T-VEC + Nivolumab in Refractory T Cell and NK Cell Lymphomas, Cutaneous Squamous Cell Carcinoma, Merkel Cell Carcinoma, and Other Rare Skin Tumors | Not yet recruiting | MCC among other tumors | Nivolumab Talimogene Laherparepvec | NCT02978625 November 30, 2016 |
| AbbVie | I | A Multicenter, Phase 1, Open-Label, Dose-Escalation Study of ABBV-181, a Monoclonal Antibody, as Monotherapy and in Combination With Another Anti-Cancer Therapy in Subjects With Advanced Solid Tumors | Recrutiting | MCC among other tumors | ABBV-181 (anti-PD1) Rovalpituzumab Tesirine | NCT03000257 December 15, 2016 |
| H. Lee Moffitt Cancer Center and Research Institute BMS | II | A Phase 2, Randomized, Multi-institutional Study of Nivolumab and Ipilimumab Versus Nivolumab, Ipilimumab and Stereotactic Body Radiation Therapy for Metastatic Merkel Cell Carcinoma | Recruiting | MCC Skin Cancer | Nivolumab, ipilimumab, stereotactic body radiation therapy (SBRT) | NCT03071406 March 1, 2017 |
| AbbVie | I | A Multicenter, Phase 1, Open-Label, Dose-Escalation Study of the Safety, Tolerability and Pharmacokinetics of ABBV-368 as a Single Agent and Combination in Subjects With Locally Advanced or Metastatic Solid Tumors | Recruiting | MCC among other tumors | Nivolumab ABBV-368 (anti-c-Met) | NCT03071757 March 2, 2017 |
| Merck KGaA Pfizer | Temporary Authorization for Use (ATU) to Avelumab for Treatment of Adult Patients With Metastatic Merkel Cell Carcinoma (mMCC) with progress after at least one prior chemotherapy | Expanded access | MCC | Avelumab | NCT03089658 March 20, 2017 | |
| Incyte Biiosciences International Sàrl | I / II | A Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of INCAGN01876 in Combination With Immune Therapies in Subjects With Advanced or Metastatic Malignancies | Recruiting | MCC among other tumors | NCAGN01876 (anti-GITR) Nivolumab, Ipilimumab | NCT03126110 April 19, 2017 |
| NantCell, Inc. | IB / II | NANT Merkel Cell Carcinoma (MCC) Vaccine: Combination Immunotherapy in Subjects With MCC Who Have Progressed on or After Anti-programmed Death-ligand 1 (PD-L1) Therapy | Not yet recruiting | MCC | Avelumab Bevacizumab Capecitabine Cisplatin Cyclophosphamide 5-fluorouracil Leucovorin nab-Paclitaxel omega-3-acid ethyl esters Stereotactic Body Radiation Therapy ALT-803 ETBX-051 ETBX-061 GI-6301 haNK | NCT03167164 May 23, 2017 |
| Checkpoint Therapeutics, Inc. Novotech (Australia) Pty Limited | I | A Phase 1, Open-label, Multicenter, Dose-escalation Study of CK-301 Administered Intravenously as a Single Agent to Subjects With Advanced Cancers | Recruiting | MCC among other tumors | CK-301 (anti-PD-L1) | NCT03212404 July 6, 2017 |
| Incyte Biosciences International Sàrl | I / II | A Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of INCAGN01949 in Combination With Immune Therapies in Subjects With Advanced or Metastatic Malignancies | Not yet recruiting | MCC among other tumors | INCAGN01949 (OX40 agonist) Nivolumab Ipilimumab | NCT03241173 August 2, 2017 |
| Incyte Biosciences International Sàrl | I / II | A Phase 1/2 Safety and Efficacy Study of INCAGN01876 in Combination With Immune Therapies in Subjects With Advanced or Metastatic Malignancies | Recruiting | MCC among other tumors | INCAGN01876 (anti-GITR) Epacadostat Pembrolizumab | NCT03277352 August 14, 2017 |
| University of Washington National Cancer Institute | III | A Multicenter, Randomized, Double-Blinded, Placebo-Controlled, Phase 3 Trial of Adjuvant Avelumab (Anti-PDL-1 Antibody) in Merkel Cell Carcinoma Patients With Clinically Detected Lymph Node Metastases | Not yet recruiting | MCC | Avelumab | NCT03271372 August 31, 2017 |
IgG: immunoglobulin G; MCC: merkel cell carcinoma; PD-1: programmed cell death protein 1; PD-L1: programmed cell death protein 1 ligand
1.4. MCC as an immunogenic tumor
Many lines of evidence suggest that MCC is a highly immunogenic tumor [23, 49, 50]. MCV-positive MCC tumors express polyomavirus antigens that can serve as epitopes for immune detection. Among virus-negative MCC tumors there is an exceptionally high mutational burden [21, 22, 23] and the somatic mutation levels in tumors correlate with the production of novel peptide epitopes (neoantigens) and improved response to immunotherapy [51]. In addition to possessing candidate immune targets, expression of PD-L1 in the tumor microenvironment is evidence for immune evasion by some MCC tumors. The immunogenicity of MCC is also evidenced by the increased incidence rates observed in immune compromised populations [12, 52]. Moreover, spontaneous regression, even of metastatic MCC, has been repeatedly observed [53]. Spontaneous regression is often associated with a dense infiltrate of T cells [54, 55], suggesting an immune-mediated clearance of MCC. Furthermore, in 40% of stage IIIB patients, the primary skin tumor cannot be identified, presumably because of immunological elimination. Interestingly, these cases carry a distinctly better prognosis than stage IIIB MCC with known primary tumors [52, 56, 57]. The importance of an anti-tumor immune response in controlling MCC is also reflected in the observations that robust intratumoral CD8+ lymphocytes and a competent immune system are both independent predictors of improved survival [12, 58]. These results strongly suggest that MCC can be efficiently cleared by the immune system and supports the use of immunotherapy for this malignancy. At the same time, 8-10% of the MCC patients are immunosuppressed [12, 52], and may not respond to immunotherapy.
1.5. Immunotherapy in MCC
Immune check-point modulators are important therapies for a variety of malignancies. To date, ipilimumab (anti-CTLA-4), nivolumab (anti-PD-1), and pembrolizumab (anti-PD-1) have all been approved by the FDA and EMA as therapies against various malignancies. Avelumab, atezolizumab, and durvalumab (all anti-PD-L1) are FDA approved for the treatment of bladder cancer. Additionally, atezolizumab is also approved as therapy against metastatic lung cancer (Tables 1–3). Avelumab is the only drug that is approved for the treatment of MCC, however a number of checkpoint inhibitors have been demonstrated to be effective in MCC [30, 49, 50, 59, 60, 61, 62] .
Table 3.
Clinical trials of checkpoint inhibitors for Merkel cell carcinoma
| Drug | Pembrolizumab | Nivolumab | Avelumab | Avelumab |
|---|---|---|---|---|
| Checkpoint | Anti-PD-1 | Anti-PD-1 | Anti-PD-L1 | Anti-PD-L1 |
| Study | NCT02267603 | CheckMate 358, NCT02488759 | JAVELIN Merkel 200, NCT02155647 Part A | JAVELIN Merkel 200, NCT02155647 Part B |
| Study design | Multicenter, open-label, single arm, phase 2 study | Non-comparative, open-label, multiple cohort, phase 1/2 study | Multicenter, single-group, open-label, phase 2 | Multicenter, single-group, open-label, phase 2 |
| Inclusion criteria | Distant metastatic or recurrent locoregional MCC, chemotherapy-naïve | Advanced MCC, ≤ 2 prior therapies | Stage IV MCC, ≥ 1 prior therapy | Stage IV MCC, chemotherapy-naïve |
| No of patients | 26 (1 without evaluation) | 25 | 88 | 39 |
| Median follow up (FU) | 7.6 months | 6.0 months (preliminary analysis) | 16.4 months | 5.1 months |
| ORR | 14 (56 %) | 22 (68 %) | 31.1% (≥ 6 months FU) 33% (≥ 1 year FU) | 62.1% (≥ 3 months FU) 71.4% (≥ 6 months FU) |
| Complete Response | 4 (16 %) | 3 (14 %) | 9.1% (≥ 6 months FU) 11.4% (≥ 1 year FU) | 13.8% (≥ 3 months FU) 28.6% (≥ 6 months FU) |
| Partial response | 10 (40 %) | 12 (55 %) | 22.7% (≥ 6 months FU) 21.6% (≥ 1 year FU) | 48.2% (≥ 3 months FU) 42.9% (≥ 6 months FU) |
| Stable disease | 1 (4 %) | 4 (18 %) | 10.2% (≥ 6 months FU) 10.2% (≥ 1 year FU) | 10.3% (≥ 3 months FU) 7.1% (≥ 6 months FU) |
| Progressive disease | 9 (36 %) | 3 (14 %) | 36.4% (≥ 6 months FU) 36.4% (≥ 1 year FU) | 24.1% (≥ 3 months FU) 14.3% (≥ 6 months FU) |
| Ongoing responses | 14 (56 %) | 13 (52 %) | 72.4% (at data cutoff) | 77.8% (≥ 3 months FU) 14.3% (≥ 6 months FU) |
| Progression-free | 67 % (at 6 months) | 86 % (at 3 months) | 30 % (at 1 year) | 67% (at 3 months) |
| Treatment-related adverse events of any grade | 77 %, mainly fatigue and lab abnormalities | 17 (68 %) | 70 %, mainly fatigue (24 %) and infusion-related reactions (17 %) | 72 % |
| Grade 3 treatment-related adverse events | 4 (15 %) grade 3 | 5 (20 %) (grade 3 and 4) | 4 (5 %) lymphopenia, blood creatine phosphokinase increase, aminotransferase increase, blood cholesterol increase | 8 (20.5 %) elevated AST and ALT, cholangitis, paraneoplastic syndrome, gait disturbance, paraneoplastic encephalitis, polyneuropathy in malignant disease |
| Serious treatment-related adverse events | 2 (8 %), myocarditis, elevated alanine aminotransferase and aspartate aminotranferase | na | 5 (6 %), enterocolitis, infusion-related reaction, aminotransferases increased, chondrocalcinosis, synovitis, interstitial nephritis | none |
Ipilimumab
Ipilimumab (Yervoy®, MDX-010) is a fully humanized monoclonal antibody targeting CTLA-4. Clinical trials testing ipilimumab in MCC were eagerly expected. Unfortunately, a phase 2 trial investigating the effect of ipilimumab on patients with metastatic MCC (NCT01913691) was canceled due to withdrawal of sponsor support. Winkler et al. described the use of ipilimumab in five cases of metastatic MCC. One patient experienced progressive disease, two had stable disease (SD), and two patients had complete responses (CR). Progression free survival (PFS) was between 4.8 and 23.5 months [59].
Nivolumab
Nivolumab (Opdivo®, ONO-4538, BMS-936558, MDX1106) is a fully human monoclonal IgG4 antibody directed against PD-1, disrupting the binding of PD-1 to PD-L1. A recent case report described a stable and rapid partial disease remission (> 8 months) in a patient with MCV-positive MCC treated with 3mg/kg nivolumab administered every 2 weeks [60]. Nivolumab was further tested in a recent open-label, multiple cohort, non-comparative, phase 1/2 study (CheckMate 358, NCT02488759) to evaluate its efficacy in patients with virus-associated tumors, including MCC. Among 25 MCC patients, the overall response rate (ORR) was 68% with ongoing responses in 13 of 15 (87%) patients. Responses occurred in treatment-naïve patients (71%) and in patients with 1-2 prior systemic therapies (63%) independent of MCV-status [30] (Table 3).
Pembrolizumab
Pembrolizumab (Keytruda®, MK-3475, lambrolizumab) is a fully humanized monoclonal IgG4 antibody targeting PD-1. In a single case report, a patient with etoposide-refractory metastatic MCC achieved a partial response (PR) with pembrolizumab [62]. In a phase 1 clinical trial (KEYNOTE-001, NCT01295827) of pembrolizuamb 2 mg/kg every 3 weeks in advanced tumors, one patient with previously untreated advanced MCC achieved a durable complete remission (>56 weeks) [61]. This dose regimen was therefore selected for a subsequent phase 2 clinical trial [49]. In this trial (NCT02267603) 25 patients with chemotherapy-naïve stage IIIB and IV MCC received pembrolizumab and reached an ORR of 56%, including 4 complete remissions in MCV-positive or negative tumors. Clinical responses to pembrolizumab were not correlated to PD-L1 expression on tumor cells or presence of tumor infiltrating immune cells. PD-L1 expression was more prevalent in MCV-positive tumors than in MCV-negative tumors (71% vs. 25%, p=0.049). These findings in 25 patients strongly supports PD-1/PD-L1 blockade as a therapeutic option for advanced MCC [49] .
2. INTRODUCTION TO AVELUMAB
Avelumab (Bavencio®, MSB0010718C) is a fully human IgG1 anti-PD-L1 antibody, developed by EMD Serono Inc., the biopharmaceutical division of Merck KGaA, Darmstadt, Germany. On March 23, 2017 avelumab received accelerated FDA approval for treatment of patients aged ≥12 years with metastatic MCC, Avelumab was approved by Swissmedic on September 05 (only as second line treatment), followed by the EMA on September 18. To date, it has been approved in the US, Canada, Switzerland, EU, Australia, Israel and Japan. Avelumab is administrated 10 mg/kg intravenously over 60 minutes every 2 weeks. To prevent infusion reactions, patients should be treated with acetaminophen and an antihistamine prior to treatment for the first four infusions and if necessary thereafter [63]. Approval of avelumab was based on the results of a phase 2 clinical trial including 88 patients with advanced MCC [50]. Safety data was evaluated in 1738 patients who received avelumab (JAVELIN Solid Tumor) [41, 64]. Continued approval for MCC will depend on the results of ongoing confirmatory trials.
Merck KGaA is investigating avelumab’s potential in numerous additional clinical trials. The company has partnered with Pfizer for the clinical co-development of avelumab.
2.1. Avelumab chemistry
Avelumab is a fully human monoclonal IgG1 antibody (mAb) directed against PD-L1 that inhibits PD-L1/PD-1 interactions but does not alter PD-L2/PD-1 signaling [41, 65]. In addition to disinhibiting T-cells, avelumab was designed to have the structure of a natural human IgG1 antibody. Most human or humanized antibodies directed to PD-1 or PD-L1 are of the IgG4 isotype or are IgG1 that has been mutated to minimize induction of antibody-dependent cellular cytotoxicity (ADCC) activity. Due to its native Fc domain, avelumab preserves the ability to induce natural killer-mediated ADCC in vitro [66]. There were initial concerns that avelumab might deplete tumor-specific PD-L1 expressing effector cells via ADCC. In vitro stimulation assays demonstrated that avelumab enhanced antigen-specific immune activation, indicating that avelumab did not deplete the cells required for immune stimulation [67]. In addition, when co-cultured with purified autologous NK cells, avelumab did not induce lysis of peripheral blood mononuclear cells (PBMCs) [66]. In its phase 1A dose-escalation trial, avelumab did not show any significant effect on patients’ absolute lymphocyte count or on the number of circulating PD-L1 expressing immune cells [41, 64, 68], suggesting that avelumab does not measurably deplete any immune cell subsets. Although avelumab-mediated ADCC can cause direct killing of PD-L1-expressing tumor cells and immunosuppressive antigen-presenting cells, to date there is no in vivo evidence of an additive clinical effect from ADCC [41, 64].
Avelumab is the only therapeutic antibody which exploits immune checkpoint inhibition and ADCC-mediated killing of tumor cells simultaneously. However, compared to other checkpoint inhibitor antibodies, infusion reactions are more frequent, and this is possibly related to avelumab’s native IgG1 Fc-domain.
2.2. Competing compounds in clinical development
As of June 29, 2017 140 clinical studies investigating PD-L1 inhibitors are listed on ClinicalTrials.gov including BMS-936559 (anti-PD-L1, phase 1, BMS, NCT02576457), LY3300054 (anti-PD-L1, phase 1, Lilly, NCT02791334), MEDI4736 (anti-PD-L1, phase 2, Swiss Group for Clinical Cancer Research, NCT02572843), REGN2810 (anti-PD-L1, phase 1, Regeneron Pharmaceuticals, NCT02383212), KN035 (anti-PD-L1, phase 1, 3D Medicines (Sichuan) Co., Ltd., NCT02827968), FAZ053 (anti-PD-L1, phase 1, Novartis, NCT02936102), MSB0011359C (bifunctional fusion protein targeting PD-L1 and TGF-β, phase 1, EMD Serono, NCT02517398), and CA-170 (small molecule targeting PD-L1, PD-L2 and VISTA, phase 1, Curis Inc., NCT02812875).
Clinically available PD-L1 inhibitors include atezolizumab (Tecentriq®, Roche/Genentech, FDA-approval for lung cancer in April 2016 and bladder cancer in May 2016), avelumab (Bavencio®, Merck/Pfizer, FDA-approval for MCC in March 2017 and bladder cancer in May 2017, Swissmedic, and EMA-approval for MCC in September 2017), and durvalumab (Imfinzi®, Medimmune/AstraZeneca, FDA-approval for urothelial carcinoma in May 2017). Atezolizumab, a phage-derived human IgG1 monoclonal antibody, was engineered with a mutated Fc domain to prevent N-linked glycosylation and ADCC activity. Durvalumab is a human IgG1 monoclonal Ab with high affinity and specificity to PD-L1 and an Fc region modified to prevent ADCC.
2.3. Avelumab Safety and Side Effects:
Avelumab has demonstrated a manageable safety profile. Treatment related adverse events (TRAE) occurring under treatment with avelumab were similar to other agents targeting the PD-1/PD-L1 axis [69, 70, 71, 72]. Safety data was evaluated in a pool of 1738 patients from the JAVELIN Solid tumor (NCT01772004) and JAVELIN Merkel 200 (NCT02155647) trials who received 10mg/kg avelumab every 2 weeks for a median of 12 weeks [41, 64, 73]. The most common any grade TRAE included fatigue (18%), infusion related reactions (IRR) (17%), and nausea (9%). TRAE led to drug discontinuation in 107 patients (6%) and four patients (0.2%) died. The rate of IRR with avelumab is elevated relative to other monoclonal antibody immune checkpoint inhibitors (1-2%). IRR or related symptoms (e.g. chills, pyrexia, hypersensitivity) occurred in 439 patients (25%) receiving avelumab, usually at first infusion (79%) and within the first 4 doses in 99% of cases. Among patients with IRR, 14% had IRR recurrence in later cycles. IRR led to discontinuation of drug in 35 patients (2%). Autoimmune adverse events can occur in association with immunotherapy. Any grade immune-related adverse events (irAE) were seen in 247 patients (14%) treated with avelumab. These irAE were considered serious in 43 patients (2%) and led to discontinuation in 34 patients (2%). The most common any grade irAEs were thyroid disorder (6%) and rash (5%). Other irAE included colitis, pneumonitis hepatitis, adrenal insufficiency, and myositis, each occurring in <2% of patients. IrAE mostly manifested during treatment, but also occurred after discontinuation of treatment.
Anti-drug antibodies (ADA) arose after treatment initiation in 4.1% of 1558 evaluable patients. However, the emergence of ADA did not alter the pharmacokinetics (PK) of avelumab or the incidence of IRR [41, 64].
TRAE occurred in 70% of the 88 patients with MCC treated with avelumab in the JAVELIN Merkel 200 (part A) trial (66% grade 1-2, 5% grade 3). The most frequently TRAE of any grade were fatigue (24% of patients) and IRR (17% of patients). No grade 4 TRAE or treatment-related deaths occurred during the study, and only 6% of patients experienced serious TRAE (chondrocalcinosis, enterocolitis, increased aminotransferases, infusion-related reactions, intestinal nephritis or synovitis), leading to discontinuation of treatment in one patient [50]. IrAE were manageable and occurred in less than 13% of patients (grade 1 or 2: diarrhea, hyperthyroidism, hypothyroidism, nephritis, pneumonitis, rash, type 1 diabetes mellitus; grade 3: increased aminotransferase). A similar safety profile was reported for pembrolizumab when treating metastatic MCC [49].
At data cutoff on March 24, 2017, results from a separate cohort of 39 patients (112 patients planned) with chemotherapy-naïve MCC enrolled in the JAVELIN Merkel 200 (part B) trial revealed TRAE in 28 patients (72%) including grade 3-4 TRAE in 8 patients (21%). Six patient had to discontinue avelumab treatment. There were no treatment-related deaths [74].
As discussed above, the frequency of IRR with avelumab is elevated relative to other checkpoint inhibitors. IRR, such as influenza-like symptoms, are often associated with release of pro-inflammatory cytokines by direct activation of immune cells [75]. Cytokine release occurs during and shortly after therapeutic antibody infusion [76, 77]. It has been speculated that avelumab binding PD-L1 expressed on immune cells could triggering cytokine release. The retained native IgG1 Fc-region on avelumab may also be involved in provoking cytokine release during infusion. All IRR that occurred in patients enrolled in the phase 1 dose-escalation trial [41] were medically manageable and resolved quickly with antihistamines, meperidine, acetaminophen, and rarely, corticosteroids. Patients who had an IRR subsequently had their infusions restarted at 50% reduced rate, and only one of four patients had a recurrent event, suggesting that reducing the infusion rate helps to avoid recurrence [41]. Because of the frequency of IRR, it is recommended that all patients should be premedicated with an antihistamine and acetaminophen prior to their first four infusions of avelumab. Premedication should be continued as needed, thereafter. Ongoing phase 3 trials with avelumab should further elucidate the frequency, significance, and severity of IRR and how best to manage them.
2.4. Avelumab pharmacokinetics and metabolism
The pharmacokinetics (PK) of avelumab and PD-L1 receptor occupancy (RO) were determined in samples collected from the phase 1a multi-cohort, dose-escalation JAVELIN Solid Tumor trial (NCT01772004) [41]. In vitro data using blood samples from healthy donors indicated that 1 µg/ml avelumab was sufficient for >95 % RO. In the dose escalation study, 53 patients with advanced solid tumors received either 1, 3, 10, or 20 mg/kg of avelumab every two weeks. There was a linear increase of both Cmax and area under curve with dosage. Half-lives for the antibody were 66, 86, 92, and 115 hours corresponding to the four dose levels. The PK data were consistent with a two-compartment model with linear elimination of avelumab. As 10 mg/kg was sufficient for >95% RO, this dose was chosen for phase 2 and 3 trials. Steady state plasma levels of avelumab were achieved after 4-6 weeks (2-3 cycles) of treatment. In patients treated with 10 mg/kg avelumab, the mean volume of distribution at steady state was 4.72 liters, the terminal half-life was 6.1 days, and the total systemic clearance was 0.59 liters/day. The primary elimination mechanism for avelumab is proteolytic degradation. Patients with MCC demonstrated a decreased avelumab clearance over time, with a mean maximal reduction of approximately 42% (based on a post hoc analysis). Age, race, gender, tumor burden, PD-L1 expression status, and the presence of renal or hepatic impairment had no significant impact on avelumab clearance. Bodyweight and total systemic clearance of avelumab were positively correlated. The impact of severe liver dysfunction on avelumab PK is uncertain [41].
2.5. Avelumab clinical efficacy:
As of May 2017, avelumab has been investigated in phase 1 clinical trials for various cancers, including bladder, stomach, lung (NSCLC), head and neck, ovary, kidney, and mesothelioma. For MCC, a phase 2 trial is ongoing. Numerous phase 3 trials are investigating the use of avelumab in malignancies including NSCLC, bladder, ovarian and gastric cancer. Additionally, avelumab received orphan drug designation by the EMA for the treatment of gastric cancer in January 2017.
At the point of writing, Avelumab has been under investigations in 56 different clinical trials including the following malignancies: colorectal carcinoma, NSCLC, glioblastoma multiforme, nasopharyngeal cancer, MCC, small intestinal adenocarcinoma, endometrial cancer, gestational trophoblastic neoplasia (GTN), recurrent respiratory papillomatosis, acute myeloid leukemia, epithelial ovarian cancer, triple negative breast neoplasms, osteosarcoma, squamous cell carcinoma of head and neck, thymoma and thymic carcinoma, renal cell cancer, neuroendocrine carcinoma grade 3, relapsed and refractory T-cell lymphoma, metastatic leiomyosarcoma and metastatic liposarcoma, clear-cell renal cell carcinoma, gastric cancer, urothelial cancer, Hodgkins and Non-Hodgkins Lymphoma, prostate cancer, melanoma, diffuse large B-cell lymphoma, and pancreatic cancer.
3. AVELUMAB IN CLINICAL STUDIES
3.1. Phase I studies
The ongoing multinational, open-label, phase 1b, dose-expansion component of the JAVELIN Solid Tumor trial involves >4000 patients comprising 16 different cancers of various entities, including breast, pancreas, ovary, lung (NSCLC), urothelium as well as mesothelioma, all regardless of PD-L1 expression [64, 78]. All patients receive 10mg/kg avelumab intravenously every 2 weeks up to the point of unacceptable toxicity, disease progression, or other reason for withdrawal. Although safety is the primary endpoint of the phase 1b dose-expansion trial [64], avelumab demonstrated preliminary efficacy in various malignancies. For instance, 50% of patients of the non-treatment-naive advanced NSCLC cohort of JAVELIN Solid Tumor (184 patients, 8.8 months median follow-up) demonstrated disease control (one CR, 21 PR, and 67 SD) [64], and the confirmed ORR was 12%. PD-L1 expression or histopathological features of the tumor had no influence on the treatment responses. According to RECIST version 1.1, median PFS was 11.6 weeks and median overall survival (OS) was 8.4 months [64]. Based on the results of this trial, numerous phase 2 and 3 trials were introduced, including JAVELIN Merkel 200, which led to the FDA approval of avelumab for MCC [41].
In additional studies, interim efficacy results demonstrated different objective response rates (ORR) for the following JAVELIN Solid Tumor cohorts: advanced NSCLC (145 patients, first-line treatment, ORR 18.7%) [79], advanced gastric or gastro-esophageal junction cancer (151 patients, first-line maintenance or second-line therapy, ORR 9.7%) [80], metastatic urothelial carcinoma (249 patients, after platinum-based therapy or cisplatin-ineligible, ORR 17.6%) [81], advanced adrenocortical carcinoma (37 patients, progressed after platinum-based therapy, ORR 10.5%) [82], advanced thymic epithelial tumor (8 patients, second-line therapy, ORR 57%) [83], locally advanced or metastatic breast cancer (168 patients, refractory to or progressing after standard therapy, ORR 5.4%) [84], recurrent/refractory ovarian cancer (124 patients, refractory to or progressing after standard therapy, ORR 9.7%) [85], and advanced unresectable mesothelioma (53 patients, progressed after platinum-pemetrexed-containing regimen, ORR 9.4%) [86].
JAVELIN Solid Tumor JPN (NCT01943461) has been initiated as an open-label, phase 1b trial of avelumab in Japanese patients suffering from advanced solid tumors consisting of a dose-escalation cohort and as a dose-expansion cohort [87, 88]. So far, preliminary results of 20 patients with advanced gastric or gastroesophageal junction adenocarcinoma are available. The 20 patients were treated with 10mg/kg avelumab every two weeks in the dose expansion part of the study based on level of PD-L1 expression. [88]. Disease control rate (CR, PR and SD; median follow-up of 6 months) was 65% based on three patients with confirmed PR. There was a trend towards higher ORR and PFS rates in patients with PD-L1-positive tumors compared with PD-L1-negative patients [88].
3.2. Efficacy in MCC - Phase II studies
JAVELIN Merkel 200 (NCT02155647)
This multicenter, international, prospective, open-label, single-group, phase 2 trial enrolled 88 patients with chemotherapy-resistant (part A) metastatic MCC measurable by RECIST criteria v1.1 [3]. All patients had distant metastatic disease (M1, defined as metastases beyond regional lymph nodes) at the time of study enrolment. Patient selection was not based on MCV or PD-L1 expression status [50, 89]. Patients received avelumab 10 mg/kg by 1 hour intravenous infusion once every 2 weeks until unacceptable toxicity, confirmed disease progression, or occurrence of any other criterion for withdrawal. All patients received premedication with an H1-antihistamine, such as diphenhydramine, and acetaminophen 30-60 min before avelumab treatment. Tumors were evaluated by an independent review committee every 6 weeks. ORR was the primary efficacy endpoint, identified as the fraction of patients with CR or PR [50, 89]. At a median follow-up of 16.4 months (primary analysis; data cut-off date of 3 September 2016), using modified immune-related response criteria, 29 patients (33.0%) had an ORR (11% CR and 22% PR; 10% SD and 36% PD);18 patients (21%) were not assessable [89]. Among responses, 76% were noted at the first post-baseline assessment (6 weeks). The median duration of response had not been reached (range 2.8-23.3 months; 95% CI 18.0 months-not estimable). Responses were ongoing in 21 patients (72% of responders). Median PFS was 2.7 months, and median OS was 12.9 months[89]. Subgroup analyses suggested a higher probability of response in patients who received fewer prior lines of chemotherapy, with a lower baseline disease burden, and with PD-L1-positive tumors. Nonetheless, durable responses occurred irrespective of baseline factors, including MCV status [89]. Insofar as patient-reported outcomes, a recent sub-analysis of the first cohort of 88 MCC patients determined that non-progression of metastatic MCC during treatment with avelumab correlated with gains in health-related quality of life [90].
As of March 24, 2017, results from a separate cohort of 39 patients with chemotherapy-naïve MCC enrolled in the JAVELIN Merkel 200 (part B) trial revealed in patients with ≥ 3 months of follow up (n=29) an ORR of 62% (14% complete response, 48% partial response). Patients with ≥ 6 months of follow up (n=14) had an ORR of 71% (29% complete response, 43% partial response). PFS rate at 3 months was 67%. These results suggest that first-line treatment with avelumab also achieves durable responses in patients with advanced MCC [74, 91].
3.3. Phase III studies:
As of May 2017, avelumab is being tested in numerous phase 3 studies e.g. patients suffering from NSCLC, bladder, gastric, renal, head and neck cancer, and ovarian cancer. Efficacy analysis has yet to be performed. The JAVELIN program is ongoing, further trials are running in solid tumors, as well as in hematologic malignancies. Running definitive phase 3 trials with chemotherapy as the comparator would be problematic for ethical reasons in an orphan disease like MCC where conventional therapies do not provide durable responses.
3.4. Comparison of checkpoint inhibitor studies in MCC:
When considering the two published studies on treating MCC with checkpoint inhibitors, there are differences between the JAVELIN Merkel 200 trial (part A) using avelumab [89] and the first-line pembrolizumab study [49] (Table 3). The patients in the first-line pembrolizumab study had treatment-naïve stage IIIB and stage IV disease, whereas the patients in the avelumab study had chemotherapy-refractory stage IV MCC. The more advanced, treatment-resistant disease in the avelumab study may have contributed to the lower ORR (33% versus 56%). Consistent with this idea, preliminary data from the chemotherapy-naïve (part B) MCC cohort of the JAVELIN Merkel 200 trial showed a confirmed ORR of 62% in 29 patients with ≥ 3 months of follow up and an ORR of 71% in 14 patients with ≥ 6 months of follow up [74, 91]. In addition, preliminary analysis of the phase 2 study of nivolumab in 25 patients with MCC, patients with treatment-naïve disease had a better ORR (71%) than treatment-resistant disease (63%) [30]. Similar trends have been observed with other malignancies, suggesting that immunotherapy achieves better results in patients who have not received prior chemotherapy. This could be due to treatment-resistant tumors being more aggressive in addition to host immune system impairment by chemotherapy [92, 93].
All studies of checkpoint inhibitors in MCC have shown durable responses, albeit with relatively short median follow-up times. All studies have also reported responses in patients regardless of tumor PD-L1 expression and MCV-status. When considering virus status, it is noteworthy that the percentage of MCV-negative tumors among patients enrolled in these studies was slightly higher than would be expected from previous reports [94].
4. REGULATORY AFFAIRS
In late 2015, avelumab obtained orphan drug, fast track, and breakthrough therapy designations from the FDA for the treatment of MCC. On March 23, 2017, after priority review, the FDA granted accelerated approval to avelumab for the treatment of adults and pediatric patients ≥12 years with metastatic MCC, irrespective of prior treatments. Approval was based on data from part A of the JAVELIN Merkel 200 trial. The ongoing part B of this trial will confirm the clinical efficacy of avelumab in treatment-naïve advanced MCC, a required condition of accelerated approval.
According to Merck KGaA, the wholesale price for avelumab is $13,000 US dollar/month. Avelumab is co-commercialized by EMD Serono, the biopharmaceutical business of Merck KGaA in the US and Canada, and Pfizer.
In mid-2016 avelumab received EMA orphan drug designation for the treatment of MCC. In July of 2017 the Committee for Medicinal Products for Human Use (CHMP) of the EMA recommended approval of avelumab for the treatment of adults with metastatic MCC. On September 5, 2017 avelumab was approved by Swissmedic for MCC treatment. On September 18, 2017 avelumab was approved by the EMA for treatment of MCC. To date, it has been approved in the US, Canada, Switzerland, EU, Australia, Israel and Japan.
5. EXPERT COMMENTARY
Avelumab is the first approved therapy for MCC, and it is the first therapy to offer durable treatment responses to a meaningful portion of patients with metastatic disease. The immunogenicity of MCC and its notably high response rates to single agent checkpoint inhibitors draw interest to this rare malignancy. As immunotherapy is adopted as the primary treatment for metastatic MCC, more will be learned about the characteristics and durability of responses in MCC, and perhaps about the nature of immunotherapy in the treatment of solid tumors.
5.1. Adjuvant immunotherapy treatment of resected MCC
Patients with stage I-III MCC who are free of disease after surgical resection are being recruited to a clinical trial in Germany testing checkpoint inhibition as an adjuvant treatment to prevent disease recurrence (NCT02196961; CA184-205; ADMEC). Initially, patients were randomized to either receive ipilimumab 3 mg/kg every 3 weeks for 12 weeks or to an observation arm. However, the treatment arm was changed, and now patients receive nivolumab 480 mg every 4 weeks for up to one year.
A placebo-controlled clinical trial testing the efficacy of avelumab as an adjuvant treatment for patients with resected stage III MCC is currently being planned in the US. These studies will hopefully quantify the utility of adjuvant checkpoint blockade in preventing progression of resected MCC so that it can be weighed against the risks associated with treatment.
5.2. Seeking predictors of response to immunotherapy
To date, all the studies of checkpoint inhibition in MCC have been relatively small. This limits what can be learned from subgroup analyses to identify predictors of treatment response. As discussed above, chemotherapeutic-naïve patients are more likely respond more frequently than patients who have been treated with multiple lines of chemotherapy. However, with avelumab being used as a first-line therapy, going forward, it is likely that patients with MCC will primarily get chemotherapy after failing a checkpoint inhibitor.
Although no difference was detected, it is possible that avelumab might achieve differential therapeutic benefits in patients whose MCC is driven by different underlying mechanisms. Specifically, MCV-positive tumors that express viral antigens and have low somatic mutational burdens are likely to be immunogenic in a different way than MCV-negative tumors that have a high burden of UV-mutagenesis driven neoantigens [21, 22, 23]. Results demonstrating clinical activity in both, virus-related and UV-radiation-induced MCC tumors provide an impetus for investigating avelumab in other tumor types with similar causes. However, tumor markers that suggest impaired ability to present immune antigens such as low predicted neoantigen levels, lack of viral protein expression, or reduced major histocompatibility complex I (MHC I) expression levels may help identify less immunogenic tumors.
Theoretical treatment response differences between virus positive and virus negative MCC might be confounded by the fact that MCV-positive tumors tend to express PD-L1 more frequently than MCV-negative tumors [23, 49]. Similar to most tumor types, there has been a trend in MCC whereby tumors that stain positively for PD-L1 have slightly higher response rates, especially when using more stringent testing thresholds (>5% tumor cells staining positive versus >1%) [49, 50]. In September 2015, Merck and Pfizer started a cooperation with Dako (Agilent Technologies), for the development of a companion diagnostic test for use with avelumab [72]. This test uses immunostaining to analyze PD-L1 expression within the tumor as well as the tumor microenvironment, including tumor-associated immune cells. The investigational assay is being assessed as part of ongoing trials [72]. When considering the potential utility of PD-L1 staining as a predictor of response to anti PD-1/PD-L1 immunotherapy, it is important to define the staining protocol and thresholds being used. A recent comparison of four PD-L1 assays (28-8, 22C3, SP142, SP263) on lung carcinoma samples showed that, with the exception of the SP142 antibody in the Ventana automated staining system (which was less sensitive), the remaining assays yielded similar results [95]. Although PD-L1 appears to have some prognostic value, there will need to be a standardized assay and interpretation for tumor PD-L1 protein expression before it can be effectively tested as a biomarker to predict the probability of response to checkpoint inhibitors in MCC or other malignancies.
The fact that complete responses are also achieved in patients whose tumors were negative for PD-L1 staining suggests that high levels of PD-L1 are not needed on tumor cells to maintain immune quiescence once it is established. However, blocking the PD-L1 that is upregulated in response to inflammation is a potential mechanism to prevent the reestablishment of tumor immune evasion. It is possible that the presence of detectible PD-L1 within a tumor reflects an active anti-tumor immune response that is being inhibited - the so called “hot tumor”. In contrast, a tumor with no PD-L1 may have achieved immune quiescence and is now a “cold tumor”. There are efforts to identify combinations of biomarkers that will better distinguish hot from cold tumors, including PD-L1 expression and the presence of CD8 T cells along the tumor margins [32], or quantifying markers of activation and immune exhaustion on lymphocytes. The hope is that such combinations will be effective in predicting response to immunotherapy.
Patients with known immunosuppression were not included in the trials of avelumab, as functional cellular immunity is necessary for anti-cancer immunotherapy to be effective. Immune senescence, intercurrent disease, medications, and prior cancer therapies can weaken immunity in cancer patients. Developing assays to detect impaired immunity may help in determining patients’ chances of responding to checkpoint inhibitors. A panel of in vitro tests, including flow cytometric measurements of PBMC subpopulations, T cell responsiveness to antigenic stimuli, and tests for NK activity may provide biomarkers for the immunocompetence of the patients and possibly explain some of the treatment failures of immune checkpoint inhibition.
5.3. Understanding and overcoming resistance
In addition to the patients who fail to respond, some individuals develop resistance to immune checkpoint inhibitors. In tumors progressing after initial response to anti-PD-1 therapy, an upregulation of the immune checkpoint receptor TIM-3 has been observed on cytotoxic T cells, and addition of a TIM-3-blocking antibody reversed the treatment resistance [96]. Identifying and blocking alternative immune checkpoints in combination with PD-1/PD-L1 inhibition will likely improve response rates and prevent resistance. However, the safety risk of routinely using combination immunotherapy will need to be weighed against the improved benefits. The benefit and risk of combining PD-1 and CTLA-4 inhibition in MCC patients is presently being investigated in the phase I/II clinical trial CheckMate 358 which compares monotherapy with nivolumab to the combination of nivolumab and ipilimumab in metastatic MCC (NCT02488759). Patients with other virus-associated tumors, that is, Epstein-Barr virus and HPV-positive tumors, are also being included.
Genomic and transcriptomic investigations seek to define additional mechanisms of response or resistance. Tumors resistant to checkpoint inhibitors have been shown to exhibit some recurrent alterations, such as mutations in beta 2 microglobulin, loss of Phosphatase and Tensin homolog (PTEN), modifications on the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, upregulation of WNT signaling, and elevated expression of co-inhibitory molecules [65]. Efforts to identify and countermand the changes that confer tumor resistance are ongoing.
5.4. Approaches to improve response to immunotherapy
It has been reported that the combination of immunotherapy and radiotherapy (RT) for the treatment of melanoma can have synergistic effects [97, 98]. Melanoma cells destroyed by RT release tumor antigens, induce inflammation, and reverse immune escape mechanism such as the down-regulation of MHC I. These radiation-induced changes could enhance immune checkpoint therapy. Down-regulation of MHC I expression has also been observed in MCC, notably in MCV-positive MCC. Therapies such as interferon, etoposide, and RT have been proposed as interventions to re-establish MHC I expression in MCC. Thus, combining these therapies with checkpoint inhibitors is worthy of further study [99].
Because avelumab has the ability to induce ADCC, co-administration of effector NK cells may result in synergistic anti-tumor responses. Similarly, studies combining checkpoint blockade with other cell-based immunotherapies such as autologous TIL infusion or chimeric antigen receptor T cells are being explored. The combination of checkpoint inhibition with cytokine-based immunotherapy (e.g. IL-2, IFN, IL-12, or IL-15), tumor vaccines, or oncolytic viruses may also prove to be effective.
Finally, the patient’s microbiome may influence antitumor immunity. The introduction of commensal bacteria (Bifidobacterium) to mice caused spontaneous antitumor immunity as well as an improved response to checkpoint blockade [100]. Interindividual variations in the microbiota could potentially predict response to immunotherapy. Future studies may demonstrate a potential benefit of enhancing the microbiota prior to treatment with immunotherapy.
5.5. Conclusion
MCC is a rare and aggressive skin cancer that lacked highly effective therapies for advanced disease. Recent studies have demonstrated that metastatic MCC responds to immunotherapy with PD-1/PD-L1 checkpoint inhibitors with surprisingly high response rates and manageable safety profiles. The largest study of immunotherapy in MCC was the JAVELIN Merkel 200 trial that demonstrated the efficacy and safety of PD-L1 inhibition with the human monoclonal antibody avelumab. Based on this study, avelumab became the first FDA approved treatment for metastatic MCC. This change in the first-line management of MCC brings the possibility of durable disease management to affected patients, and will likely impact the natural history of MCC going forward.
6. FIVE-YEAR VIEW
Immune checkpoint inhibitors like avelumab can be life extending drugs for patients with metastatic MCC, however not every patient responds and their use comes with the risk of adverse events. Overall, the available data support the early use of immune checkpoint inhibitors over chemotherapy in MCC. With the exception of patients with immunosuppression or autoimmune disease, who were excluded from the studies, most patients benefited from first-line use of PD-1/PD-L1 blocking antibodies. Future studies will investigate primary and secondary therapeutic resistances in addition to examining drug combinations that may improve response rates and limit resistance. It will also be important to define the utility of adjuvant checkpoint blockade in patients with resected MCC. To ensure optimal MCC patient treatment, there remain questions in terms of patient selection, treatment strategies in immunosuppressed patients, and how to best combine checkpoint inhibitors with radiation therapy and other treatment modalities.
7. KEY ISSUES.
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin tumor with high relative mortality.
MCC is an immunogenic tumor that responds exceptionally well to PD-1/PD-L1 checkpoint inhibitors (objective response rates > 50%).
PD-L1 blockade with avelumab is the first and only approved treatment for patients with metastatic MCC.
Avelumab is unique in its ability to target PD-1/PD-L1 signaling and also induce ADCC.
Acknowledgments
Funding
This paper was funded by the Intramural Research Program, NCI, NIH ZIA BC 011450.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Merck Group provided a scientific accuracy review at the request of the journal editor.
8. INFORMATION RESOURCES
Avelumab (BAVENCIO®) full prescribing information: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761049s000lbl.pdf
Merkelcell.org is an authoritative source of information on Merkel cell carcinoma for patients, physicians, and researchers. https://www.merkelcell.org/
Key publications:
Kaufman et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016 Oct;17(10):1374-1385.
Nghiem et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016 Jun 30;374(26):2542-52.
9. REFERENCES
Papers of special note have been highlighted as:
* of interest to readers
** of considerable interest to readers
- 1.Schrama D, Becker JC. Merkel cell carcinoma--pathogenesis, clinical aspects and treatment. J Eur Acad Dermatol Venereol. 2011. October;25(10):1121–9. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaidou E, Mikrova A, Antoniou C, et al. Advances in Merkel cell carcinoma pathogenesis and management: a recently discovered virus, a new international consensus staging system and new diagnostic codes. Br J Dermatol. 2012. January;166(1):16–21. [DOI] [PubMed] [Google Scholar]
- 3.Lebbe C, Becker JC, Grob JJ, et al. Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015. November;51(16):2396–403. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald TL, Dennis S, Kachare SD, et al. Dramatic Increase in the Incidence and Mortality from Merkel Cell Carcinoma in the United States. Am Surg. 2015. August;81(8):802–6. [DOI] [PubMed] [Google Scholar]
- 5.Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014. August;150(8):864–72. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005. January 1;89(1):1–4. [DOI] [PubMed] [Google Scholar]
- 7.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010. November;63(5):751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S, Afanasiev O, Nghiem P. Immunobiology of Merkel cell carcinoma: implications for immunotherapy of a polyomavirus-associated cancer. Curr Oncol Rep. 2011. December;13(6):488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai P Merkel cell cancer: update on biology and treatment. Curr Opin Oncol. 2008. March;20(2):196–200. [DOI] [PubMed] [Google Scholar]
- 10.*.Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016. September;5(9):2294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai PT, Yu E, Winquist E, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000. June;18(12):2493–9. [DOI] [PubMed] [Google Scholar]
- 12.Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013. March;133(3):642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.*.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008. February 22;319(5866):1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008. July 1;68(13):5009–13. [DOI] [PubMed] [Google Scholar]
- 15.Becker JC, Houben R, Ugurel S, et al. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J Invest Dermatol. 2009. January;129(1):248–50. [DOI] [PubMed] [Google Scholar]
- 16.Garneski KM, Warcola AH, Feng Q, et al. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009. January;129(1):246–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shuda M, Kwun HJ, Feng H, et al. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011. September;121(9):3623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houben R, Shuda M, Weinkam R, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010. July;84(14):7064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houben R, Adam C, Baeurle A, et al. An intact retinoblastoma protein-binding site in Merkel cell polyomavirus large T antigen is required for promoting growth of Merkel cell carcinoma cells. Int J Cancer. 2012. February 15;130(4):847–56. [DOI] [PubMed] [Google Scholar]
- 20.Paulson KG, Carter JJ, Johnson LG, et al. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010. November 1;70(21):8388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.*.Wong SQ, Waldeck K, Vergara IA, et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015. December 15;75(24):5228–34. [DOI] [PubMed] [Google Scholar]
- 22.*.Harms PW, Vats P, Verhaegen ME, et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015. September 15;75(18):3720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.*.Goh G, Walradt T, Markarov V, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy]. Oncotarget. 2016. January 19;7(3):3403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassler NM, Merrill D, Bichakjian CK, et al. Merkel Cell Carcinoma Therapeutic Update. Curr Treat Options Oncol. 2016. July;17(7):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desch L, Kunstfeld R. Merkel cell carcinoma: chemotherapy and emerging new therapeutic options. J Skin Cancer. 2013;2013:327150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006. February 09;439(7077):682–7. [DOI] [PubMed] [Google Scholar]
- 27.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012. June 28;366(26):2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014. September 20;384(9948):1109–17. [DOI] [PubMed] [Google Scholar]
- 29.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012. June 28;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.*.Topalian SL, Bhatia S, Hollebecque A. Non-comparative, open-label, multiple cohort, phase 1/2 study to evaluate nivolumab (NIVO) in patients with virus associated tumors (CheckMate 358): Efficacy and safety in merkel cell carcinoma (MCC). AACR 2017; [Google Scholar]
- 31.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014. November 27;515(7528):563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014. November 27;515(7528):568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moshiri AS, Doumani R, Yelistratova L, et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J Invest Dermatol. 2017. April;137(4):819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulson KG, Lewis CW, Redman MW, et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: A prospective validation study. Cancer. 2017. April 15;123(8):1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992. November;11(11):3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009. August 20;114(8):1537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008. June;8(6):467–77. [DOI] [PubMed] [Google Scholar]
- 39.Messal N, Serriari NE, Pastor S, et al. PD-L2 is expressed on activated human T cells and regulates their function. Mol Immunol. 2011. September;48(15-16):2214–9. [DOI] [PubMed] [Google Scholar]
- 40.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017. September 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.*.Heery CR, O’Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017. May;18(5):587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa R, Carneiro BA, Agulnik M, et al. Toxicity profile of approved anti-PD-1 monoclonal antibodies in solid tumors: a systematic review and meta-analysis of randomized clinical trials. Oncotarget. 2017. January 31;8(5):8910–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritter C, Fan K, Paulson KG, et al. Reversal of epigenetic silencing of MHC class I chain-related protein A and B improves immune recognition of Merkel cell carcinoma. Sci Rep. 2016. February 23;6:21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014. October 01;20(19):5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012. March 28;4(127):127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agilent. PD-L1 IHC 28-8 pharmDX IFU Overview [Internet] http://www.agilent.com/en/products/pharmdx/pd-l1-ihc-28-8-overview, accessed on February 14,2018.
- 47.Phillips T, Simmons P, Inzunza HD, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015. September;23(8):541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012. March 22;12(4):252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.**.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016. June 30;374(26):2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.**.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016. October;17(10):1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015. April 03;348(6230):124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asgari MM, Sokil MM, Warton EM, et al. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014. July;150(7):716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh NM. Complete spontaneous regression of Merkel cell carcinoma (1986-2016): a 30 year perspective. J Cutan Pathol. 2016. December;43(12):1150–1154. [DOI] [PubMed] [Google Scholar]
- 54.Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991. April;127(4):550–3. [PubMed] [Google Scholar]
- 55.Takenaka H, Kishimoto S, Shibagaki R, et al. Merkel cell carcinoma with partial spontaneous regression: an immunohistochemical, ultrastructural, and TUNEL labeling study. Am J Dermatopathol. 1997. December;19(6):614–8. [DOI] [PubMed] [Google Scholar]
- 56.Foote M, Veness M, Zarate D, et al. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol. 2012. September;67(3):395–9. [DOI] [PubMed] [Google Scholar]
- 57.Moshiri AS, Nghiem P. Milestones in the staging, classification, and biology of Merkel cell carcinoma. J Natl Compr Canc Netw. 2014. September;12(9):1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paulson KG, Iyer JG, Tegeder AR, et al. Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol. 2011. April 20;29(12):1539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winkler JK, Dimitrakopoulou-Strauss A, Sachpekidis C, et al. Ipilimumab has efficacy in metastatic Merkel cell carcinoma: a case series of five patients. J Eur Acad Dermatol Venereol. 2017. September;31(9):3389–e391. [DOI] [PubMed] [Google Scholar]
- 60.Walocko FM, Scheier BY, Harms PW, et al. Metastatic Merkel cell carcinoma response to nivolumab. J Immunother Cancer. 2016;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patnaik A, Kang SP, Rasco D, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015. October 01;21(19):4286–93. [DOI] [PubMed] [Google Scholar]
- 62.Winkler JK, Bender C, Kratochwil C, et al. PD-1 blockade: a therapeutic option for treatment of metastatic Merkel cell carcinoma. Br J Dermatol. 2017. January;176(1):216–219. [DOI] [PubMed] [Google Scholar]
- 63.EMD Serono Inc and Pfizer Inc. Bavencio® (avelumab) injection: US prescribing information. 2017 [Internet] http://www.fda.gov, accessed on February 14,2018.
- 64.Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017. May;18(5):599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braun DA, Burke KP, Van Allen EM. Genomic Approaches to Understanding Response and Resistance to Immunotherapy. Clin Cancer Res. 2016. December 01;22(23):5642–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diaz LA, Jr., Le DT PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015. November 12;373(20):1979. [DOI] [PubMed] [Google Scholar]
- 67.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016. April 09;387(10027):1540–50. [DOI] [PubMed] [Google Scholar]
- 68.*.Donahue RN, Lepone LM, Grenga I, et al. Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody. J Immunother Cancer. 2017;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weber JS, Yang JC, Atkins MB, et al. Toxicities of Immunotherapy for the Practitioner. J Clin Oncol. 2015. June 20;33(18):2092–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016. May 07;387(10031):1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016. March;17(3):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016. August;13(8):473–86. [DOI] [PubMed] [Google Scholar]
- 73.Kelly K, Infante JR, Taylor MH, et al. Safety profile of avelumab in patients with advanced solid tumors: A JAVELIN pooled analysis of phase 1 and 2 data. J Clin Oncol. 2017;35(15_suppl):3059. [Google Scholar]
- 74.**.D´Angelo SP, Russell J, Hassel JC, et al. Avelumab treatment in chemotherapy-naive patients with distant metastatic Merkel cell carcinoma (mMCC). Ann Oncol. 2017;28(suppl_5):v428–v448. [Google Scholar]
- 75.Descotes J, Gouraud A. Clinical immunotoxicity of therapeutic proteins. Expert Opin Drug Metab Toxicol. 2008. December;4(12):1537–49. [DOI] [PubMed] [Google Scholar]
- 76.Freeman CL, Morschhauser F, Sehn L, et al. Cytokine release in patients with CLL treated with obinutuzumab and possible relationship with infusion-related reactions. Blood. 2015. December 10;126(24):2646–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas K, Eisele J, Rodriguez-Leal FA, et al. Acute effects of alemtuzumab infusion in patients with active relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2016. June;3(3):e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kelly K, Heery CR, Patel MR. Avelumab (MSB0010718C); anti-PD-L1) in patients with advanced cancer: safety data from 1300 patients enrolled in the phase 1b JAVELIN Solid Tumor trial. J Clin Oncol. 2016;34(Suppl)(abstract no.):3055. [Google Scholar]
- 79.Verschraegen C, Chen F, Spigel DR, et al. Avelumab (MSB0010718C); anti PE-L1) as a first-line treatment for patients with advanced NSCLC from the JAVELIN Solid Tumor phase 1b trial: safety, clinical activity, and PD-L1 expression. J Clin Oncol. 2016;34(Suppl)(abstract no.):9036. [Google Scholar]
- 80.Chung HC, Arkenau HT, Wyrwicz L, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: Analysis of safety and clinical activity. J Clin Oncol. 2016;34(15_suppl):4009. [Google Scholar]
- 81.Apolo AB, Ellerton J, Infante JR, et al. Avelumab treatment of metastatic urothelial carcinoma (mUC) in the phase 1b JAVELIN Solid Tumor study: updated analysis with ≥6 months of follow-up in all patients. Ann Oncol. 2017;28(supp_5):v295–v329. [Google Scholar]
- 82.Le Tourneau C, Hoimes CJ, Zarwan C, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced adrenocortical carcinoma from the JAVELIN solid tumor phase Ib trial: Safety and clinical activity. J Clin Oncol. 2016;34(15_suppl):4516. [Google Scholar]
- 83.Rajan A, Heery CR, Perry S, et al. Safety and clinical activity of anti-programmed death-ligand 1 (PD-L1) antibody (ab) avelumab (MSB0010718C) in advanced thymic epithelial tumors (TETs). J Clin Oncol. 2016;34(15_suppl):e20106. [Google Scholar]
- 84.Dirix LY, Takacs I, Nikolinakos P, et al. Abstract S1-04: Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase Ib JAVELIN solid tumor trial. Cancer Res. 2016;76(4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Disis ML, Patel MR, Pant S, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: Safety and clinical activity. J Clin Oncol. 2016;34(15_suppl):5533. [Google Scholar]
- 86.Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol. 2016;34(15_suppl):8503. [Google Scholar]
- 87.Shitara K, Yamada Y, Yoh K, et al. Phase I, open-label, multi-ascending dose trial of avelumab (MSB0010718C), an anti-PD-L1 monoclonal antibody, in Japanese patients with advanced solid tumors. J Clin Oncol. 2015;33(15_suppl):3023. [Google Scholar]
- 88.Nishina T, Shitara K, Iwasa S, et al. Safety, PD-L1 expression, and clinical activity of avelumab (MSB0010718C), an anti-PD-L1 antibody, in Japanese patients with advanced gastric or gastroesophageal junction cancer. J Clin Oncol. 2016;34(4_suppl):168. [Google Scholar]
- 89.**.Kaufman HL, Russell JS, Hamid O, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥ 1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018. January 19;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.**.Kaufman HL, Hunger M, Hennessy M, et al. Nonprogression with avelumab treatment associated with gains in quality of life in metastatic Merkel cell carcinoma. Future Oncol. 2017. December 8. [DOI] [PubMed] [Google Scholar]
- 91.MERCK. Press release September 20,2017 [Internet] https://www.merckgroup.com/en/news/bavencio-ec-approval-2017-09-20.html, accessed on February 14, 2018.
- 92.Feldmeyer L, Hudgens CW, Ray-Lyons G, et al. Density, Distribution, and Composition of Immune Infiltrates Correlate with Survival in Merkel Cell Carcinoma. Clin Cancer Res. 2016. November 15;22(22):5553–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Voog E, Biron P, Martin JP, et al. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer. 1999. June 15;85(12):2589–95. [DOI] [PubMed] [Google Scholar]
- 94.Liu W, MacDonald M, You J. Merkel cell polyomavirus infection and Merkel cell carcinoma. Curr Opin Virol. 2016. October;20:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017. February;12(2):208–222. [DOI] [PubMed] [Google Scholar]
- 96.Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016. February 17;7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: a review of clinical outcomes. Int J Radiat Oncol Biol Phys. 2014. April 1;88(5):986–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barker CA, Postow MA, Khan SA, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013. August;1(2):92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paulson KG, Tegeder A, Willmes C, et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol Res. 2014. November;2(11):1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015. November 27;350(6264):1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015. April;16(4):375–84. [DOI] [PubMed] [Google Scholar]
- 102.Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016. November;17(11):1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017. November 9;377(19):1824–1835. [DOI] [PubMed] [Google Scholar]
- 104.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015. June 25;372(26):2521–32. [DOI] [PubMed] [Google Scholar]


