Abstract
Alcohol abuse causes profound damage to both the developing brain and the adult brain. Prenatal exposure to alcohol results in a wide range of deficits known as fetal alcohol spectrum disorders (FASD). Alcohol abuse in adults is associated with brain shrinkage, memory and attention deficits, communication disorders and physical disabilities. Monocyte chemoattractant protein-1 (MCP-1/CCL2) is one of the key chemokines that regulate the recruitment and activation of monocytes and microglia. Both MCP-1 and its receptor C-C chemokine receptor type 2 (CCR2) expressed in the brain are involved in various neuroinflammatory disorders, such as multiple sclerosis (MS), Alzheimer’s disease (AD) and Parkinson’s disease (PD). However, the role of MCP1/CCR2 in alcohol-induced brain damage is unclear. Recent evidence indicates that alcohol exposure increased the activity of MCP-1/CCR2 in both mature and developing central nervous systems (CNS). MCP-1/CCR2 signaling in the brain was involved in alcohol drinking behavior. MCP-1/CCR2 inhibition alleviated alcohol neurotoxicity by reducing microglia activation/neuroinflammation in the developing brain and spinal cord. In this review, we discussed the role of MCP-1/CCR2 signaling in alcohol-induced neuroinflammation and brain damage. We also discussed the signaling cascades that are involved in the activation of MCP-1/CCR2 in response to alcohol exposure.
Keywords: Alcohol abuse, apoptosis, chemokines, development, glia, neurodegeneration
1. Introduction
Alcohol abuse is the third leading preventable cause of death in the United States. An estimated 88,000 people die from alcohol-related causes annually [1]. 16.6 million adults (around 7% of the population in the USA) aged 18 and older have an alcohol use disorder (AUD) [1]. The cost of excessive alcohol use in the United States reached $249 billion in 2010 [2], and is still a significant economic and medical burden to society. Alcohol contributes to more than 200 diseases and injury-related health conditions and the central nervous system (CNS) is particularly vulnerable to alcohol toxicity. Alcohol abuse causes severe damage to both developing and mature brains [4–6]. Maternal alcohol consumption during pregnancy may cause fetal alcohol spectrum disorders (FASD) which are characterized by a spectrum of physical, mental and behavioral disabilities with possible lifelong implications [2]. Fetal alcohol syndrome is the most severe form of FASD, and includes a group of pathological conditions, such as facial abnormalities, growth restrictions, learning/memory deficits, and defects of heart, kidney and bone [7]. Chronic alcohol exposure in adults results in neuronal degeneration and cognitive deficits [3]. However, the mechanisms underlying alcohol CNS neurotoxicity are unclear.
Multiple mechanisms have been proposed, including endoplasmic reticulum (ER) stress [4], oxidative stress [5, 6], interference of signaling by neurotrophic factors and disruption of microRNAs [7]. Recently, neuroinflammation has been proposed to play an important role in the pathogenesis of FASD and AUD [8–10]. Alcohol-induced neuronal death is accompanied by microglial activation and neuroinflammation in both developing and adult brains [8, 9, 11, 12]. We have recently shown that inhibition of microglial activation and neuroinflammation by minocycline or targeting monocyte chemoattractant protein 1 (MCP-1) signaling offered protection against alcohol-induced neuronal death in the developing brain and spinal cord [13–15], suggesting that of microglial activation and neuroinflammation is involved in alcohol-induced damage to in the developing CNS.
MCP-1, also known as chemokine (CC motif) ligand 2 (CCL2), is an important chemokine that regulates neuroinflammation [16–18]. MCP-1 and its receptor C-C chemokine receptor type 2 (CCR2) are mainly detected in microglia in mouse and human brains. [16]. Astrocytes are another source for MCP-1 [19], and there are reports showing that MCP-1 and CCR2 are detected in neurons [20, 21]. MCP-1/CCR2 signaling has been implicated in several neuroinflammatory disorders, such as Alzheimer’s disease [22], multiple sclerosis [23] and ischemic brain damage [24]. MCP-1/CCR2 signaling is also involved in some models of alcoholism [25–27]. The administration of alcohol increases the expression of MCP-1 in the rodent brain [28, 29] and genetic studies in animals indicate that elevated MCP-1 signaling is accompanied by increased alcohol consumption [30]. In human alcoholics, MCP-1 expression is augmented in the ventral tegmental area, substantia nigra, hippocampus and amygdala of the brain autopsy [27]. This review will discuss the involvement of MCP-1/CCR2 signaling activity in alcohol’s action in the CNS and potential underlying mechanisms.
2. MCP-1/CCR2 signaling
MCP-1, a member of the C-C chemokine family, is a potent chemotactic factor for monocytes. MCP-1 is the first discovered human CC chemokine. Located on chromosome 17 (chr.17, q11.2), human MCP-1 is composed of 76 amino acids and is 13 kDa in size [31]. The biological function of MCP-1 is mediated via its G protein-coupled receptor CCR2 [32]. CCR2 binds the five pro-inflammatory chemokines: MCP-1, CCL7, CCL8, CCL12 and CCL13 [33–35]. However, MCP-1 is the most potent among those chemokines for triggering signal transduction pathways mediated by CCR2 [36]. CCR2A and CCR2B are two alternatively spliced forms of CCR2 that differ in their carboxy-terminal tails [37]. CCR2B accounts for 90% of the CCR2 expressed on the cell-surface even though CCR2A and CCR2B can activate different signaling pathways and exert different actions [31]. The direct downstream target of CCR2 signaling is monocyte chemotactic protein-1-induced protein-1 (MCPIP1), a transcriptional activator that regulates the expression of IL-1β, MCP-1 and TNFα. Other targets of CCR2 include phosphatidylinositol-3-OH kinase (PI3K), mitogen activated protein kinases (MAPK) and protein kinase C [34, 38], indicating that a wide range of intracellular pathways may be involved in cellular responses elicited by MCP-1. MCP-1 has been demonstrated to recruit monocytes into foci of active inflammation [39] and is confirmed to be the main chemokine responsible for recruiting monocytes [40]. Besides recruiting and directing leukocyte movement, MCP-1 may also influence T-cell immunity. It has been reported that MCP-1 expression is associated with the development of polarized Th2 responses [41, 42] and that MCP-1 increases the secretion of IL-4 by T cells [43]. Gonzalo et al [44] also observed elevated expression of MCP-1 in Th2 immune-mediated diseases, such as asthma. In summary, in the peripheral immune system, MCP-1/CCR2 signaling plays a crucial role in guiding and directing immune cells in response to inflammatory challenges.
3. MCP-1/CCR2 in the CNS
In addition to its well-established role in the peripheral immune system, increasing evidence indicates that MCP-1/CCR2 plays an important role in the CNS [45–47]. MCP-1 and CCR2 are mainly expressed in microglia and astrocytes in the CNS [14, 48–52]. MCP-1/CCR2 signaling is involved in the activation of microglia. For example, Feng et al reported that expression of MCP-1 and CCR2 induced by photoreceptor apoptosis promotes the activation and migration of microglia and monocytes [53]. Another study demonstrated astrocyte-derived MCP-1 leads to increased migration of microglial cells, and inhibition of CCR2 attenuates MCP-1-mediated microglial activation [54]. Dubovy et al observed that microglial activation in periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) in rats following injury was mediated by CCR2, which may be activated by neuron or astrocyte-derived MCP-1 [55].
MCP-1 and CCR2 are also expressed in CNS neurons and cultured neuronal cell lines [20, 21, 46, 47, 56]. MCP-1 is constitutively expressed in the neurons of discrete brain regions in rats, such as the cerebral cortex, hippocampus, hypothalamus, substantia nigra, cerebellum and spinal cord [20, 56]. MCP-1/CCR2 signaling can regulate neuronal functions. For instance, Zhou et al observed that MCP-1 enhances neuronal excitability and synaptic transmission via presynaptic mechanisms in rat hippocampal slices [57]. Widera et al [58] reported that MCP-1 acts as a chemokine on neural stem cells to activate the migration capacity of rat-derived neural stem cells. Collectively, MCP-1/CCR2 signaling is involved in a variety of neurological activities in addition to its role in the immune system.
4. MCP-1/CCR2 in neurological disorders
An increased MCP-1 expression has been observed in the CNS astrocytes and microglia under pathological conditions. Elevated MCP-1 is an important mediator of the neuroinflammatory responses in brain trauma [59], ischemic brain injury [24, 60] and various neurodegenerative disorders, such as multiple sclerosis [23, 61] and Alzheimer’s disease [62, 63]. MCP-1 interacts with CCR2 to regulate neuroinflammatory processes in the CNS [63, 64]. We discuss several examples in which MCP-1/CCR2 is involved in CNS damage and neurological disorders.
4. 1. MCP-1/CCR2 in Alzheimer’s disease
Alzheimer’s disease (AD) is the most common neurodegenerative disorder characterized by the accumulation of β-amyloid peptide (Aβ). Elevated levels of MCP-1 have been observed in the brains of AD patients and in transgenic mouse models of the disease. Galimberti et al [22] reported that the transition from mild cognitive impairment to AD is associated with an elevated MCP-1 level in the cerebrospinal fluid (CSF). Several studies have also demonstrated that increased levels of β-amyloid induces MCP-1, which results in disrupting the blood–brain barrier (BBB) and facilitating recruitment of immune cells into the CNS [65]. However, the role of CCR2 in AD is controversial. Some studies using mouse models show that monocytes migrate from the bone marrow, infiltrate into the brain in a CCR2-dependent manner, and then differentiate into microglia or macrophages; others argue that CCR2 deficiency accelerates the onset of spatial and contextual memory deficits and aggravates amyloid pathology in a mouse model of AD [66]. Nevertheless, a majority of data support a role for the MCP-1/CCR2 system in the pathogenesis of Alzheimer’s disease. MCP-1 in peripheral blood may attract blood-derived monocytes to migrate into the brain [67], and a higher plasma MCP-1 level is associated with greater severity and faster cognitive decline.
4. 2. MCP-1/CCR2 in Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurological disorder in the elderly after AD [68]. Although the origin of this neuronal degeneration is unknown, substantial evidence has indicated the involvement of inflammatory processes in the pathology of PD [69, 70]. Several studies propose that PD is caused by programed cell death (apoptosis) due to increased levels of cytokines, such as TNFα and MCP-1 [71–76]. Elevated MCP-1 expression was observed in peripheral blood mononuclear cells (PBMCs) in PD patients compared with healthy control subjects [77]. Moderate MCP-1 over-induction led to increased neurotoxicity in MPTP mice (a mouse model of PD), likely due to the increased CCR2+ monocyte infiltration [78]. Liu et al reported that Nurr1 overexpression played neuroprotective and anti-inflammatory roles via down-regulating MCP-1 in both in vivo and in vitro PD models [79]. In another study of PD, Kempuraj et al observed that MPP+ activates mouse and human mast cells to release MCP-1 [80]. In addition, Lindqvist et al reported that MCP-1 levels in CSF were correlated with increased non-motor symptoms of PD, such as depression [81]. Furthermore, Nishimura et al found that MCP-1–2518A/G genotype affected the age-at-onset of PD patients [82], which suggested an association between the MCP-1 and CCR2 gene polymorphisms and PD risk.
4. 3. MCP-1/CCR2 in ischemic stroke
Accumulating evidence indicates that MCP-1 and CCR2 are involved in postischemic inflammation. An augmented MCP-1 expression has been observed in both the serum and CSF of patients after cerebral stroke [83, 84]. MCP-1−/− mice exhibit decreased activated microglia and phagocytic macrophage accumulation in the brain and smaller infarcts following permanent middle cerebral artery occlusion [85]. The expression of a nonfunctional MCP-1 gene (an N-terminal deletion mutant of human MCP-1) in rats significantly attenuated the infarct volume and macrophage infiltration [86]. CCR2 −/− mice have reduced blood–brain barrier permeability, decreased level of inflammatory cytokines and smaller infarct size in the affected ischemic hemisphere [87]. In summary, these data suggest that inhibition of MCP-1/CCR2 could improve the treatment of ischemic stroke.
4. 4. MCP-1/CCR2 in multiple sclerosis
Multiple sclerosis (MS) is a demyelinating autoimmune disease leading to severe and progressive neurological impairment. Activated microglia, infiltration of macrophages and lymphocytes, and reactive astrocytes are the major characteristics of MS [88, 89]. Increased expression of MCP-1 has been detected in patients with both acute and chronic MS [16]. It has been demonstrated that MCP-1 is expressed by astrocytes and macrophages within actively demyelinating MS plaques [61]. In experimental autoimmune encephalomyelitis (EAE), an animal model for MS, increased MCP-1 expression correlates with the severity of the disease [16]. Also in EAE, knocking out CCR2 inhibited mononuclear cell inflammatory infiltration and proinflammatory cytokine expression in the CNS of mice [90].
5. MCP-1/CCR2 in alcohol-induced neuropathology
In addition to the involvement in neurological disorders, recent studies indicates that MCP-1/CCR2 signaling also plays and important role in alcoholic neuropathology of both the adult CNS and the developmental CNS. These findings are discussed below.
5.1. MCP-1/CCR2 in alcohol’s action in the adult CNS
Heavy alcohol exposure causes neuroinflammation. For example, Increased MCP-1 expression and microglial activation have been observed in the brain of human alcoholics [27]. Greater amounts of TNFα were observed in the monocytes isolated from the blood of alcoholics [91]. Leclercq et al observed that lipopolysaccharides and peptidoglycans from the gut microbiota stimulate IL-8 and IL-1β in peripheral blood mononuclear cells that are correlated with alcohol craving [92]. Studies using animal models confirmed that alcohol increased the expression of multiple neuroimmune genes, such as cyclooxygenase 2 (COX2), NF-κB and cyclic AMP-responsive element binding protein (CREB) in the brain and that these alterations may persist over long periods even after alcohol withdrawal [93–95].
MCP-1 has been shown to regulate neuroinflammation and microglia activity [96]. As the first responder to environmental insults in the CNS, microglia are vital in neuroinflammation. Under resting conditions, microglia is in the ramified form, having long branching processes and a small cellular body [97]. In response to injury or pathogen invasion, quiescent ramified microglia proliferate and transform into reactive ameboid microglia, which have fewer and thicker processes with a larger cell body. The marker Iba-1 is upregulated in reactive microglia and is often used to visualize these cells [98]. It appears that alcohol could stimulate inflammatory pathways by activating microglia in the CNS and that MCP-1 plays an important role in these processes. For example, He et al [27] observed elevated MCP-1 levels and microglial markers, such as Iba-1 and Glucose transporter-5 (GluT5), in the ventral tegmental area (VTA), substantia nigra (SN), hippocampus and amygdala in the brains of human alcoholics. Alcohol exposure activated microglia, increased the expression of MCP-1 and other proinflammatory cytokines, such as TNF-α, IL-6 and IL-1β, and induced neuronal death in rats [8, 99]. Qin et al [28] revealed that pretreatment with alcohol potentiated LPS-induced increase in MCP-1 and microglial activation in the brain of adult mice. It has been proposed that MCP-1 lowers the “threshold sensitivity” for microglia as a “priming” stimulus and enhances the synthesis of proinflammatory cytokines in response to subsequent insult [100]. With a neuron/microglia co-culture system, Yang et al showed that MCP-1-induced neurotoxicity requires the presence of microglia and that exogenous MCP-1 was able to activate and stimulate microglia to produce cytokines [101]. An MCP-1 neutralizing antibody inhibited MCP-1-induced microglia activation and neuronal death in culture and in the thalamus [101].
Several studies have demonstrated that MCP-1/CCR2 signaling is involved in alcohol drinking behavior as well. Deletion of CCR2 and MCP-1 (in female mice) reduced alcohol preference and consumption in a two-bottle choice test, and alcohol administration produced a stronger conditioned taste aversion in CCR2−/− and MCP-1−/− mice [25]. The delivery of MCP-1 or TLR4 siRNA into the central nucleus of the amygdala (CeA) and ventral tegmental area (VTA) in alcohol preferring rats inhibited target gene expression and blunted binge drinking [96]. Although exactly how MCP-1 regulates drinking behavior is not clear, a potential explanation is that MCP-1 activates the dopamine system [102]. Taken together, MCP-1/CCR2 may participate in alcohol-induced brain damage and drinking behavior through the regulation of microglial activation and neurotransmission.
5. 2. MCP-1/CCR2 in alcohol neurotoxicity in the developing CNS
FASD is the leading cause of mental retardation in North America, ahead of Down syndrome and cerebral palsy [103–106]. FASD is estimated to affect as high as 5% of people in the United States and some Western European countries, and the economic burden of FASD in the US is significant [107]. The developing CNS is particularly susceptible to alcohol exposure, so even moderate maternal drinking could lead to cognitive and behavioral impairments [103, 108–111]. Despite attempts to raise awareness about the dangers of drinking during pregnancy, numbers of women drinking during pregnancy in the USA have not declined [30].
Microglial activation and neuroinflammation have been implicated in alcohol neurotoxicity in the developing CNS [8, 13–15, 112]. It has been proposed that microglia primed by ethanol exposure may lead to excess neuroinflammation and the subsequent neurotoxicity observed in AUD and FASD [8, 112]. Since MCP-1/CCR2 plays an important role in microglial activation and neuroinflammation, we investigated the involvement of MCP-1/CCR2 in alcohol neurotoxicity in the developing CNS. Using a third trimester equivalent mouse model of alcohol exposure, we compared the effects of alcohol on the developing spinal cord among wild type, MCP-1 deficient (MCP-1−/−) and CCR2 deficient (CCR2−/−) mice [14]. Alcohol caused apoptotic cell death in the dorsal horn of the spinal cord, which was accompanied by glial activation and macrophage infiltration. MCP-1 or CCR2 deficient mice were resistant to alcohol-induced apoptosis, inflammation, and glial activation. It appeared that deletion of CCR2 was more effective than MCP-1 in the protection against alcohol-induced damage to the spinal cord [14]. Therefore, MCP1/CCR2 signaling may mediate alcohol-induced microglial activation and neuronal death in the developing spinal cord.
The importance of MCP-1/CCR2 signaling in mediating alcohol-induced microglial activation and neuroinflammation is also demonstrated in the developing brain [15]. In the third trimester equivalent mouse model of alcohol exposure, alcohol caused widespread neuroapoptosis, microglial activation and neuroinflammation in the brain. MCP-1 synthesis inhibitor Bindarit and CCR2 antagonist RS504393 each significantly inhibited alcohol-induced microglial activation/neuroinflammation and neuroapoptosis in the brain of early postnatal mouse pups [15]. Further studies using MCP-1−/− or CCR2−/− mice also confirmed that the deficiency in MCP-1 or CCR2 made mice more resistant to alcohol-induced neurodegeneration. It appeared MCP-1 deficiency offered better protection against alcohol-induced damage to the developing brain. Moreover, alcohol and MCP-1 caused more neuronal death in the neuron/microglia co-culture system than the neuronal culture alone, suggesting that microglia are required for alcohol/MCP-1-induced neurotoxicity. Bindarit and RS504393 effectively protected neurons against alcohol/MCP1-induced neuronal death in the co-cultures, indicating that MCP-1/CCR2 signaling is involved in microglial contribution to ethanol neurotoxicity [15].
Minocycline is an antibiotic that inhibits microglial activation and alleviates neuroinflammation. Using the same third trimester equivalent mouse model of alcohol exposure, we showed that minocycline significantly inhibited alcohol-induced caspase-3 activation, and blocked alcohol-increased MCP-1/CCR2 expression as well as microglial activation in the developing brain [13]. Minocycline was also effective in protecting neurons against alcohol-induced neuronal death in neurons/microglia co-cultures. It is possible that minocycline-mediated neuroprotection operates through its inhibition of MCP-1/CCR2 signaling. Taken together, MCP-1/CCR2 is an important mediator for alcoholic neuropathogenesis.
6. Proposed mechanisms underlying the interaction between MCP-1/CCR2 and alcohol in microglial activation and neuroinflammation
The above evidence indicates that MCP-1/CCR2 signaling plays an important role in alcohol-induced neuroinflammation and microglial activation in both the adult and developing CNS. It is important to understand the mechanisms underlying MCP-1/CCR2induced microglia activation and neuroinflammation in the context of alcohol neurotoxicity.
GSK3β, TLR4, JNKs, and p38 MAPK are key regulators of microglia activation and neuroinflammation [113–115]. GSK3β is a multifunctional serine/threonine kinase and plays an important role in neurogenesis, neuronal differentiation, neuronal migration and survival in the developing CNS [116–119]. The activity of GSK3β is inhibited by the phosphorylation at Ser9 but stimulated by the phosphorylation at Tyr216 [120, 121]. GSK3β activation promotes microglia migration and the production of inflammatory molecules [115]. Over-expression of GSK3β makes neurons more sensitive to alcohol-induced neuronal death while inhibition of GSK3β ameliorates alcohol neurotoxicity in vitro and in vivo [122]. Lithium, a GSK3β inhibitor, protects neurons against alcohol-induced neurodegeneration and inhibition of neurite outgrowth [122, 123].
GSK3β was activated and involved in alcohol-induced microglial activation and up-regulation of proinflammatory cytokines in vitro and in vivo [15]. Blocking MCP-1/CCR2 signaling partially mitigated alcohol-mediated activation of GSK3β. TLR4 was also activated by alcohol in the developing brain and cultured microglial cells [15]. Inhibition of MCP-1/CCR2 signaling significantly blocked alcohol-induced increase of TLR4. These results suggest that MCP-1/CCR2 signaling is involved in alcohol-induced activation of GSK3β and TLR4. In addition, there is a cross-talk among MCP-1/CCR2 signaling, GSK3β, and TLR4 in response to alcohol exposure. Blocking TLR4 inhibited ethanol-induced activation of GSK3β and up-regulation of MCP-1 in cultured microglial cells. Furthermore, blocking GSK3β also attenuated ethanol-induced up-regulation of TLR4 and MCP-1 [15]. Similarly, alcohol-induced activation of GSK3β and JNKs were reduced in the developing spinal cord of MCP-1−/− mice and CCR2−/− mice compared to wild type mice [14]. Furthermore, minocycline inhibited alcohol-induced microglial activation and neuroinflammation in the developing brain; it also attenuated alcohol-mediated activation of MCP-1, GSK3β, and JNKs, supporting the notion that the interaction among MCP-1, GSK3β, and JNK contributes to alcohol-induced neuroinflammation [13]. Alcohol did not significantly affect p38 MAPK signaling in this mouse model of third trimester equivalent exposure [13].
According to these findings, we propose a model of alcohol-induced neuroinflammation and microglial activation (Fig. 1). In this model, alcohol up-regulates the expression of MCP-1 and activates CCR2 signaling. It is also possible that alcohol could directly activate CCR2; this possibility remains further investigation. Activated MCP1/CCR2 signaling regulates pro-inflammatory transcription factors, such as AP-1 and NF-κB through GSK3β or JNK. These transcription factors stimulate the expression of inflammatory cytokines and chemokines including MCP-1 itself, causing neuroinflammation and neuronal death. On the other hand, alcohol could activate TLR4 either directly or indirectly and turn on its down-stream effectors such as TRIF, MyD88 and JNK; at the same time, the active TLR4 may stimulate GSK3β, which further activates TLR4 and JNK. Consequently, the activation of TRIF, MyD88, JNK and GSK3β up-regulates transcription factors, resulting in an increase of proinflammatory cytokines (e.g. MCP-1, IL6, and TNF-α). The released MCP-1 binds its receptor CCR2 which further activates TLR4, GSK3β, and JNK; this positive feedback loop amplifies the neuroinflammation and neurotoxicity. In addition to its role in neuroinflammation and neurotoxicity, MCP-1 may regulate alcohol self-administration through unknown mechanisms.
Figure 1.
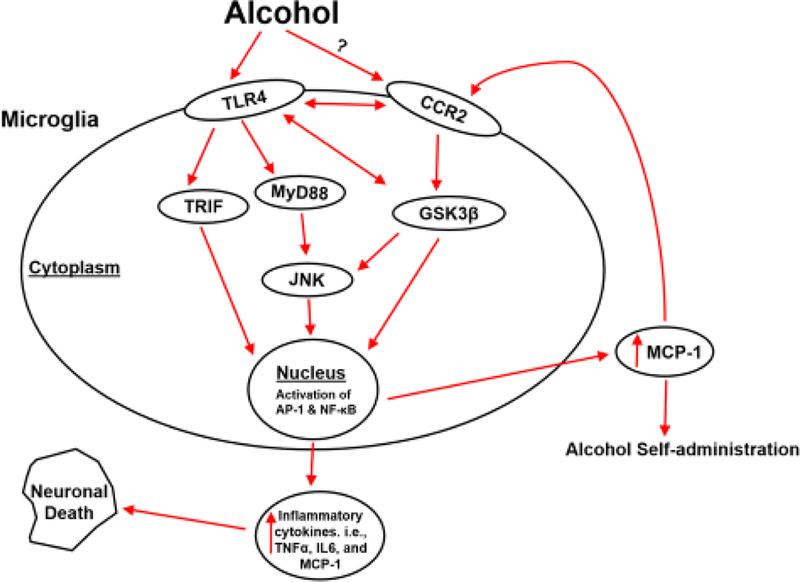
The cascade of MCP-1/CCR2 signaling in alcohol-induced neuroinflammation and neurotoxicity. Alcohol increases the expression of MCP-1 and activates CCR2 signaling. Activated MCP-1/CCR2 signaling regulates pro-inflammatory transcription factors, such as AP-1 and NF-κB through GSK3β or JNK. These transcription factors stimulate the expression of inflammatory cytokines and chemokines including MCP-1 itself, causing neuroinflammation and neuronal death. On the other hand, alcohol could directly or indirectly activate TLR4 and turn on its down-stream effectors such as TRIF, MyD88 and JNK; the active TLR4 meanwhile may stimulate GSK3β, which may further activate TLR4 and JNK. The activation of this cascade results in an increase of proinflammatory cytokines (e.g. MCP-1, IL6, and TNF-α). The released MCP-1 binds its receptor CCR2 which further activates TLR4, GSK3β, and JNK; this positive feedback loop amplifies the neuroinflammation and neurotoxicity. In addition to its role in neuroinflammation and neurotoxicity, MCP-1 is also reported to regulate alcohol self- administration.
7. Conclusions and future studies
Available evidence suggests that MCP-1/CCR2 interacting with GSK3β, TLR4, and JNKs may mediate alcohol’s action on neuroinflammation and neurotoxicity in the CNS, particularly the developing CNS. Therefore, suppression of MCP-1/CCR2 activity by selective inhibitors or genetic manipulation may ameliorate alcohol-induced damages to the CNS. These findings establish MCP-1/CCR2 signaling as a potential target for therapeutic efforts. Although there has been no definitive clinical proof-of-concept for antiMCP-1/CCR2 therapeutics, there has been great interest in targeting MCP-1/CCR2 signaling in treating some neurological disorders [124]. For this purpose, innovative technologies that facilitate effective and specific targeting of MCP-1/CCR2 signaling in neuroinflammation have been developed, such as dominant negative mutants of MCP-1, RNAi and potent small-molecule antagonists of CCR2 to block receptor activation [124–126]. Although the excessive MCP-1/CCR2 activation has detrimental effects on neurons, basal MCP-1/CCR2 signaling is required for normal immune response against disease [19]. It is therefore important to carefully design and evaluate anti-MCP-1/CCR2 therapy.
There are several interesting points for future study. First, although MCP-1 and CCR2 are mainly expressed by microglia, they are also expressed by astrocytes [19]. Mouse studies have demonstrated that MCP-1/CCR2 signaling could mediate astrocytosis in familial ALS [127] and that MCP-1 produced by spinal cord astrocytes contributes to central sensitization and neuropathic pain [52]. Astrocytes play critical roles in AUD by modulating neurotransmission [128]. It has also been demonstrated that astrocytes are involved in animal models of FASD [12, 129]. Therefore, it would be interesting to investigate the contribution of MCP-1/CCR2 to the activation of astrocytes in the context of alcohol neurotoxicity.
Second, several animal models of FASD have demonstrated that alcohol exposure could produce long lasting neurobehavioral deficits in adolescent and adult mice [130–139]. Since MCP-1−/− mice and CCR2−/− mice are more resistant to alcohol-induced neuronal death in the developing CNS, it would be important to determine whether deficiency of MCP-1/CCR2 protects mice against alcohol-induced behavioral deficits.
Third, it is well-established that the vulnerability of the developing brain to alcohol is temporal- and regional-dependent. Accordingly, is MCP-1/CCR2 developmentally regulated? Does the expression pattern of MCP-1/CCR2 contribute to the differential sensitivity to alcohol during the development? These questions warrant further investigation.
Lastly, in addition to microglial activation and neuroinflammation, MCP-1/CCR2 signaling may participate in other cellular stress processes, which contribute to alcohol neurotoxicity. It has been well-established that oxidative stress and endoplasmic reticulum (ER) stress play an important role in alcohol neurotoxicity [4, 140–142]. MCP1/CCR2 signaling may regulate ER stress. For example, the activation of MCP-1/CCR2 signaling may cause ER stress through the upregulation of MCPIP in cardiomyocytes and osteoclasts [143]. CCR2 inhibitor attenuates ER stress and reduces the expression of inflammatory cytokines in the liver of type 2 diabetic mice [144]. We recently showed that MCP-1−/− and CCR2−/−mice are more resistant to alcohol-induced ER stress in the developing spinal cord [14]. It appears that MCP-1/CCR2 signaling may also be involved in oxidative stress. Kim et al reported that MCP-1 deficiency attenuates oxidative stress and protects against ovariectomy-induced chronic inflammation in mice [145]. Therefore, it would be interesting to determine the interplay of MCP-1/CCR2 signaling, ER stress, and oxidative stress, as well as how the interplay contributes to alcohol neurotoxicity.
Acknowledgments
We thank Jacqueline Frank for reading this manuscript. This work was supported by grants from the National Institutes of Health (NIH) (AA017226 and AA015407). It is also supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development: BX001721).
Abbreviation
- AD
Alzheimer’s disease
- AUD
Alcohol use disorders
- BBB
Blood–brain barrier
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CCR2
Chemokine (C–C motif) receptor 2
- COX2
Cyclooxygenase 2
- ER
Endoplasmic reticulum
- EAE
Experimental autoimmune encephalomyelitis
- FASD
Fetal alcohol spectrum disorder
- GSK3β
Glycogen synthase kinase 3 beta
- Iba-1
Ionized calcium binding adaptor molecule 1
- IL
Interleukin
- MCP-1
Monocyte chemoattractant protein-1
- MS
Multiple sclerosis
- PD
Parkinson’s disease
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Gonzales K, et al. , Alcohol-attributable deaths and years of potential life lost−-11 States, 2006–2010. MMWR Morb Mortal Wkly Rep, 2014. 63(10): p. 213–216. [PMC free article] [PubMed] [Google Scholar]
- 2.Green PP, Vital signs: alcohol-exposed pregnancies—United States, 2011–2013. MMWR. Morbidity and mortality weekly report, 2016. 65. [DOI] [PubMed] [Google Scholar]
- 3.Collins MA and Neafsey EJ, Alcohol, Excitotoxicity and Adult Brain Damage: An Experimentally Unproven Chain-of-Events. Frontiers in molecular neuroscience, 2016. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ke Z, et al. , Ethanol induces endoplasmic reticulum stress in the developing brain. Alcohol Clin Exp Res, 2011. 35(9): p. 1574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodlett CR, Horn KH, and Zhou FC, Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood), 2005. 230(6): p. 394–406. [DOI] [PubMed] [Google Scholar]
- 6.Chen G and Luo J, Anthocyanins: are they beneficial in treating ethanol neurotoxicity? Neurotox Res, 2010. 17(1): p. 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyadjieva NI and Sarkar DK, Cyclic adenosine monophosphate and brain-derived neurotrophic factor decreased oxidative stress and apoptosis in developing hypothalamic neuronal cells: role of microglia. Alcohol Clin Exp Res, 2013. 37(8): p. 1370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chastain LG and Sarkar DK, Role of microglia in regulation of ethanol neurotoxic action. Int Rev Neurobiol, 2014. 118: p. 81–103. [DOI] [PubMed] [Google Scholar]
- 9.Kane CJ and Drew PD, Inflammatory responses to alcohol in the CNS: nuclear receptors as potential therapeutics for alcohol-induced neuropathologies. J Leukoc Biol, 2016. 100(5): p. 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crews FT, et al. , The role of neuroimmune signaling in alcoholism. Neuropharmacology, 2017. 122: p. 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito M, et al. , Ethanol-Induced Neurodegeneration and Glial Activation in the Developing Brain. Brain Sci, 2016. 6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm CJ and Guizzetti M, Fetal Alcohol Spectrum Disorders: An Overview from the Glia Perspective. Front Integr Neurosci, 2015. 9: p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, et al. , Minocycline protects developing brain against ethanol-induced damage. Neuropharmacology, 2018. 129: p. 84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Z, et al. , Ethanol-induced damage to the developing spinal cord: The involvement of CCR2 signaling. Biochim Biophys Acta, 2017. 1863(11): p. 2746–2761. [DOI] [PubMed] [Google Scholar]
- 15.Zhang K, et al. , Role of MCP-1 and CCR2 in ethanol-induced neuroinflammation and neurodegeneration in the developing brain. Journal of Neuroinflammation, 2018. 15(1): p. 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conductier G, et al. , The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol, 2010. 224(1–2): p. 93–100. [DOI] [PubMed] [Google Scholar]
- 17.Xu LL, et al. , Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol, 1996. 60(3): p. 365–71. [DOI] [PubMed] [Google Scholar]
- 18.Carr MW, et al. , Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A, 1994. 91(9): p. 3652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deshmane SL, et al. , Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of interferon & cytokine research, 2009. 29(6): p. 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banisadr G, et al. , Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: Evidence for its colocalization with neurotransmitters and neuropeptides. Journal of Comparative Neurology, 2005. 489(3): p. 275–292. [DOI] [PubMed] [Google Scholar]
- 21.Banisadr G, et al. , Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: Functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. Journal of Comparative Neurology, 2005. 492(2): p. 178–192. [DOI] [PubMed] [Google Scholar]
- 22.Galimberti D, et al. , Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiology of aging, 2006. 27(12): p. 1763–1768. [DOI] [PubMed] [Google Scholar]
- 23.Mahad DJ and Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). in Seminars in immunology. 2003. Elsevier. [DOI] [PubMed] [Google Scholar]
- 24.Minami M and Satoh M, Chemokines and their receptors in the brain: pathophysiological roles in ischemic brain injury. Life sciences, 2003. 74(2): p. 321–327. [DOI] [PubMed] [Google Scholar]
- 25.Blednov YA, et al. , Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behavioural brain research, 2005. 165(1): p. 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crews FT, et al. , Cytokines and alcohol. Alcohol Clin Exp Res, 2006. 30(4): p. 720–30. [DOI] [PubMed] [Google Scholar]
- 27.He J and Crews FT, Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol, 2008. 210(2): p. 349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin L, et al. , Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation, 2008. 5: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrlich D, Pirchl M, and Humpel C, Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience, 2012. 205: p. 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blednov YA, et al. , Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addiction biology, 2012. 17(1): p. 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Coillie E, Van Damme J, and Opdenakker G, The MCP/eotaxin subfamily of CC chemokines. Cytokine & growth factor reviews, 1999. 10(1): p. 61–86. [DOI] [PubMed] [Google Scholar]
- 32.Ransohoff RM, The chemokine system in neuroinflammation: an update. The Journal of infectious diseases, 2002. 186(Supplement_2): p. S152–S156. [DOI] [PubMed] [Google Scholar]
- 33.Gong J-H, et al. , An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. Journal of Experimental Medicine, 1997. 186(1): p. 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wain J, Kirby J, and Ali S, Leucocyte chemotaxis: Examination of mitogen-activated protein kinase and phosphoinositide 3-kinase activation by Monocyte Chemoattractant Proteins-1,−2,−3 and-4. Clinical & Experimental Immunology, 2002. 127(3): p. 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gouwy M, et al. , Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. Journal of leukocyte biology, 2004. 76(1): p. 185–194. [DOI] [PubMed] [Google Scholar]
- 36.Sozzani S, et al. , Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. The Journal of Immunology, 1994. 152(7): p. 3615–3622. [PubMed] [Google Scholar]
- 37.Charo IF, et al. , Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proceedings of the National Academy of Sciences, 1994. 91(7): p. 2752–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thelen M, Dancing to the tune of chemokines. Nature immunology, 2001. 2(2): p. 129134. [DOI] [PubMed] [Google Scholar]
- 39.Ajuebor MN, et al. , Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. Journal of leukocyte biology, 1998. 63(1): p. 108–116. [DOI] [PubMed] [Google Scholar]
- 40.Palframan RT, et al. , Inflammatory chemokine transport and presentation in HEV. Journal of Experimental Medicine, 2001. 194(9): p. 1361–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chensue SW, et al. , Monocyte chemotactic protein expression during schistosome egg granuloma formation. Sequence of production, localization, contribution, and regulation. The American journal of pathology, 1995. 146(1): p. 130. [PMC free article] [PubMed] [Google Scholar]
- 42.Handel TM and Domaille PJ, Heteronuclear (1H, 13C, 15N) NMR assignments and solution structure of the monocyte chemoattractant protein-1 (MCP-1) dimer. Biochemistry, 1996. 35(21): p. 6569–6584. [DOI] [PubMed] [Google Scholar]
- 43.Karpus WJ, et al. , Differential CC chemokine-induced enhancement of T helper cell cytokine production. The Journal of Immunology, 1997. 158(9): p. 4129–4136. [PubMed] [Google Scholar]
- 44.Gonzalo J-A, et al. , The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. Journal of Experimental Medicine, 1998. 188(1): p. 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerard C and Rollins BJ, Chemokines and disease. Nature immunology, 2001. 2(2): p. 108–116. [DOI] [PubMed] [Google Scholar]
- 46.De Haas A, et al. , Neuronal chemokines: versatile messengers in central nervous system cell interaction. Molecular neurobiology, 2007. 36(2): p. 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mélik-Parsadaniantz S and Rostène W, Chemokines and neuromodulation. Journal of neuroimmunology, 2008. 198(1): p. 62–68. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi M, et al. , Production and function of monocyte chemoattractant protein-1 and other β-chemokines in murine glial cells. Journal of neuroimmunology, 1995. 60(1): p. 143–150. [DOI] [PubMed] [Google Scholar]
- 49.Berman JW, et al. , Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. The Journal of Immunology, 1996. 156(8): p. 3017–3023. [PubMed] [Google Scholar]
- 50.Glabinski A and Ransohoff R, Chemokines and chemokine receptors in CNS pathology. Journal of neurovirology, 1999. 5(1): p. 3–12. [DOI] [PubMed] [Google Scholar]
- 51.Hanisch UK, Microglia as a source and target of cytokines. Glia, 2002. 40(2): p. 140155. [DOI] [PubMed] [Google Scholar]
- 52.Gao Y-J, et al. , JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. Journal of Neuroscience, 2009. 29(13): p. 4096–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng C, et al. , Expression of CCL2 and its receptor in activation and migration of microglia and monocytes induced by photoreceptor apoptosis. Molecular vision, 2017. 23: p. 765. [PMC free article] [PubMed] [Google Scholar]
- 54.He M, et al. , Astrocyte-derived CCL2 is associated with M1 activation and recruitment of cultured microglial cells. Cellular Physiology and Biochemistry, 2016. 38(3): p. 859–870. [DOI] [PubMed] [Google Scholar]
- 55.Dubový P, et al. , Activation of Astrocytes and Microglial Cells and CCL2/CCR2 Upregulation in the Dorsolateral and Ventrolateral Nuclei of Periaqueductal Gray and Rostral Ventromedial Medulla Following Different Types of Sciatic Nerve Injury. Frontiers in cellular neuroscience, 2018. 12: p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gosselin RD, et al. , Constitutive expression of CCR2 chemokine receptor and inhibition by MCP1/CCL2 of GABA-induced currents in spinal cord neurones. Journal of neurochemistry, 2005. 95(4): p. 1023–1034. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y, et al. , Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. Journal of neurochemistry, 2011. 116(3): p. 406414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Widera D, et al. , MCP-1 induces migration of adult neural stem cells. European journal of cell biology, 2004. 83(8): p. 381–387. [DOI] [PubMed] [Google Scholar]
- 59.Glabinski AR, et al. , Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. The Journal of Immunology, 1996. 156(11): p. 4363–4368. [PubMed] [Google Scholar]
- 60.Kim JS, et al. , Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. Journal of neuroimmunology, 1995. 56(2): p. 127–134. [DOI] [PubMed] [Google Scholar]
- 61.Simpson JE, et al. , Expression of monocyte chemoattractant protein-1 and other betachemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol, 1998. 84(2): p. 238–49. [DOI] [PubMed] [Google Scholar]
- 62.ISHIZUKA K, et al. , Identification of monocyte chemoattractant protein- 1 in senile plaques and reactive microglia of Alzheimer’s disease. Psychiatry and clinical neurosciences, 1997. 51(3): p. 135–138. [DOI] [PubMed] [Google Scholar]
- 63.Sokolova A, et al. , Monocyte Chemoattractant Protein-1 Plays a Dominant Role in the Chronic Inflammation Observed in Alzheimer’s Disease. Brain pathology, 2009. 19(3): p. 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banisadr G, et al. , Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. Journal of neurochemistry, 2002. 81(2): p. 257269. [DOI] [PubMed] [Google Scholar]
- 65.Roberts TK, et al. , CCL2 disrupts the adherens junction: implications for neuroinflammation. Laboratory investigation, 2012. 92(8): p. 1213–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naert G and Rivest S, CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Journal of Neuroscience, 2011. 31(16): p. 6208–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee W-J, et al. , Plasma MCP-1 and Cognitive Decline in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: A Two-year Follow-up Study. Scientific reports, 2018. 8(1): p. 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanner CM and Goldman SM, Epidemiology of Parkinson’s disease. Neurol Clin, 1996. 14(2): p. 317–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kannarkat GT, Boss JM, and Tansey MG, The role of innate and adaptive immunity in Parkinson’s disease. Journal of Parkinson’s disease, 2013. 3(4): p. 493–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amor S, et al. , Inflammation in neurodegenerative diseases–an update. Immunology, 2014. 142(2): p. 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagatsu T and Sawada M, Inflammatory process in Parkinson’s disease: role for cytokines. Current pharmaceutical design, 2005. 11(8): p. 999–1016. [DOI] [PubMed] [Google Scholar]
- 72.Sawada M, Imamura K, and Nagatsu T, Role of cytokines in inflammatory process in Parkinson’s disease, in Parkinson’s Disease and Related Disorders 2006, Springer; p. 373–381. [DOI] [PubMed] [Google Scholar]
- 73.McGeer P, et al. , Reactive microglia are positive for HLADR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology, 1988. 38(8): p. 1285–1285. [DOI] [PubMed] [Google Scholar]
- 74.McGeer PL and McGeer EG, Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism & related disorders, 2004. 10: p. S3–S7. [DOI] [PubMed] [Google Scholar]
- 75.Tansey MG, McCoy MK, and Frank-Cannon TC, Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Experimental neurology, 2007. 208(1): p. 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tansey MG, et al. , Neuroinflammation in Parkinson’s disease: is there sufficient evidence for mechanism-based interventional therapy. Front Biosci, 2008. 13(5): p. 709717. [DOI] [PubMed] [Google Scholar]
- 77.Reale M, et al. , Peripheral cytokines profile in Parkinson’s disease. Brain, behavior, and immunity, 2009. 23(1): p. 55–63. [DOI] [PubMed] [Google Scholar]
- 78.Parillaud VR, et al. , Analysis of monocyte infiltration in MPTP mice reveals that microglial CX3CR1 protects against neurotoxic over-induction of monocyte-attracting CCL2 by astrocytes. Journal of neuroinflammation, 2017. 14(1): p. 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W, Gao Y, and Chang N, Nurr1 overexpression exerts neuroprotective and anti-inflammatory roles via down-regulating CCL2 expression in both in vivo and in vitro Parkinson’s disease models. Biochemical and biophysical research communications, 2017. 482(4): p. 1312–1319. [DOI] [PubMed] [Google Scholar]
- 80.Kempuraj D, et al. , Mast Cells Release Chemokine CCL2 in Response to Parkinsonian Toxin 1-Methyl-4-Phenyl-Pyridinium (MPP(+)). Neurochem Res, 2016. 41(5): p. 1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindqvist D, et al. , Cerebrospinal fluid inflammatory markers in Parkinson’s disease– associations with depression, fatigue, and cognitive impairment. Brain, behavior, and immunity, 2013. 33: p. 183–189. [DOI] [PubMed] [Google Scholar]
- 82.Nishimura M, et al. , Influence of monocyte chemoattractant protein 1 gene polymorphism on age at onset of sporadic Parkinson’s disease. Movement disorders, 2003. 18(8): p. 953–955. [DOI] [PubMed] [Google Scholar]
- 83.Arakelyan A, et al. , Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators of Inflammation, 2005. 2005(3): p. 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Losy J and Zaremba J, Monocyte chemoattractant protein-1 is increased in the cerebrospinal fluid of patients with ischemic stroke. Stroke, 2001. 32(11): p. 2695–2696. [DOI] [PubMed] [Google Scholar]
- 85.Hughes PM, et al. , Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. Journal of Cerebral Blood Flow & Metabolism, 2002. 22(3): p. 308317. [DOI] [PubMed] [Google Scholar]
- 86.Kumai Y, et al. , Anti—Monocyte Chemoattractant Protein-1 Gene Therapy Protects against Focal Brain Ischemia in Hypertensive Rats. Journal of Cerebral Blood Flow & Metabolism, 2004. 24(12): p. 1359–1368. [DOI] [PubMed] [Google Scholar]
- 87.Dimitrijevic OB, et al. , Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke, 2007. 38(4): p. 1345–1353. [DOI] [PubMed] [Google Scholar]
- 88.Brosnan CF and Raine CS, Mechanisms of immune injury in multiple sclerosis. Brain Pathology, 1996. 6(3): p. 243–257. [DOI] [PubMed] [Google Scholar]
- 89.Lucchinetti CF, et al. , Distinct patterns of multiple sclerosis pathology indicates heterogeneity in pathogenesis. Brain pathology, 1996. 6(3): p. 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Izikson L, et al. , Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR2). Journal of Experimental Medicine, 2000. 192(7): p. 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McClain CJ, et al. , Recent advances in alcoholic liver disease IV. Dysregulated cytokine metabolism in alcoholic liver disease. American Journal of Physiology Gastrointestinal and Liver Physiology, 2004. 287(3): p. G497–G502. [DOI] [PubMed] [Google Scholar]
- 92.Leclercq S, et al. , Role of inflammatory pathways, blood mononuclear cells, and gutderived bacterial products in alcohol dependence. Biol Psychiatry, 2014. 76(9): p. 72533. [DOI] [PubMed] [Google Scholar]
- 93.Knapp DJ and Crews FT, Induction of Cyclooxygenase2 in Brain During Acute and Chronic Ethanol Treatment and Ethanol Withdrawal. Alcoholism: Clinical and Experimental Research, 1999. 23(4): p. 633–643. [PubMed] [Google Scholar]
- 94.Alfonso-Loeches S, et al. , Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. Journal of Neuroscience, 2010. 30(24): p. 82858295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zou J and Crews F, CREB and NF-κB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cellular and molecular neurobiology, 2006. 26(4–6): p. 383–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.June HL, et al. , CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology, 2015. 40(6): p. 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ladeby R, et al. , Microglial cell population dynamics in the injured adult central nervous system. Brain Research Reviews, 2005. 48(2): p. 196–206. [DOI] [PubMed] [Google Scholar]
- 98.Block ML, Zecca L, and Hong J-S, Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Reviews Neuroscience, 2007. 8(1): p. 57–69. [DOI] [PubMed] [Google Scholar]
- 99.Alfonso-Loeches S and Guerri C, Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Critical reviews in clinical laboratory sciences, 2011. 48(1): p. 19–47. [DOI] [PubMed] [Google Scholar]
- 100.Rankine E, et al. , Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. European Journal of Neuroscience, 2006. 24(1): p. 77–86. [DOI] [PubMed] [Google Scholar]
- 101.Yang G, et al. , Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol, 2011. 21(3): p. 279–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guyon A, et al. , Long term exposure to the chemokine CCL2 activates the nigrostriatal dopamine system: a novel mechanism for the control of dopamine release. Neuroscience, 2009. 162(4): p. 1072–80. [DOI] [PubMed] [Google Scholar]
- 103.Riley EP and McGee CL, Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood), 2005. 230(6): p. 357–65. [DOI] [PubMed] [Google Scholar]
- 104.May PA and Gossage JP, Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health, 2001. 25(3): p. 159–67. [PMC free article] [PubMed] [Google Scholar]
- 105.Stratton K, Howe C, and Battaglia FC, Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment 1996: National Academies Press. [Google Scholar]
- 106.Nash K, et al. , Understanding fetal alcohol spectrum disorders (FASDs): toward identification of a behavioral phenotype. The Scientific World Journal, 2008. 8: p. 873882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lupton C, Burd L, and Harwood R. Cost of fetal alcohol spectrum disorders. in American Journal of Medical Genetics Part C: Seminars in Medical Genetics 2004. Wiley Online Library. [DOI] [PubMed] [Google Scholar]
- 108.Mattson SN, Schoenfeld AM, and Riley EP, Teratogenic effects of alcohol on brain and behavior. Alcohol Research and Health, 2001. 25(3): p. 185–191. [PMC free article] [PubMed] [Google Scholar]
- 109.O’Malley KD and Nanson J, Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. The Canadian Journal of Psychiatry, 2002. 47(4): p. 349–354. [DOI] [PubMed] [Google Scholar]
- 110.O’Callaghan FV, et al. , Maternal alcohol consumption during pregnancy and physical outcomes up to 5 years of age: a longitudinal study. Early human development, 2003. 71(2): p. 137–148. [DOI] [PubMed] [Google Scholar]
- 111.O’Callaghan FV, et al. , Prenatal alcohol exposure and attention, learning and intellectual ability at 14 years: a prospective longitudinal study. Early human development, 2007. 83(2): p. 115–123. [DOI] [PubMed] [Google Scholar]
- 112.Zhang X, et al. , Prenatal alcohol exposure alters the course and severity of adjuvantinduced arthritis in female rats. Brain Behav Immun, 2012. 26(3): p. 439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Badshah H, et al. , Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. Journal of Neuroimmune Pharmacology, 2016. 11(1): p. 48–60. [DOI] [PubMed] [Google Scholar]
- 114.Wang M-J, et al. , Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades. Journal of Neuroinflammation, 2010. 7(1): p. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jope RS, Yuskaitis CJ, and Beurel E, Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochemical research, 2007. 32(4–5): p. 577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boku S, et al. , Glucocorticoids and lithium reciprocally regulate the proliferation of adult dentate gyrus-derived neural precursor cells through GSK-3β and β-catenin/TCF pathway. Neuropsychopharmacology, 2009. 34(3): p. 805–815. [DOI] [PubMed] [Google Scholar]
- 117.Maurer MH, et al. , Glycogen synthase kinase 3β (GSK3β) regulates differentiation and proliferation in neural stem cells from the rat subventricular zone. Journal of proteome research, 2007. 6(3): p. 1198–1208. [DOI] [PubMed] [Google Scholar]
- 118.Tong N, et al. , Activation of glycogen synthase kinase 3 beta (GSK-3β) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. European Journal of Neuroscience, 2001. 13(10): p. 1913–1922. [DOI] [PubMed] [Google Scholar]
- 119.Pap M and Cooper GM, Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. Journal of Biological Chemistry, 1998. 273(32): p. 19929–19932. [DOI] [PubMed] [Google Scholar]
- 120.Bhat RV, et al. , Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3β in cellular and animal models of neuronal degeneration. Proceedings of the National Academy of Sciences, 2000. 97(20): p. 11074–11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ji Z, et al. , Binge Alcohol Exposure Causes Neurobehavioral Deficits and GSK3beta Activation in the Hippocampus of Adolescent Rats. Sci Rep, 2018. 8(1): p. 3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Y, et al. , Overexpression of glycogen synthase kinase 3beta sensitizes neuronal cells to ethanol toxicity. J Neurosci Res, 2009. 87(12): p. 2793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Z, et al. , Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci, 2012. 32(34): p. 11706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Semple BD, Kossmann T, and Morganti-Kossmann MC, Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. Journal of Cerebral Blood Flow & Metabolism, 2010. 30(3): p. 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Begin-Lavallee V, et al. , Functional inhibition of chemokine receptor CCR2 by dicersubstrate-siRNA prevents pain development. Mol Pain, 2016. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stone MJ, et al. , Mechanisms of regulation of the chemokine-receptor network. International journal of molecular sciences, 2017. 18(2): p. 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kawaguchi-Niida M, et al. , MCP-1/CCR2 signaling-mediated astrocytosis is accelerated in a transgenic mouse model of SOD1-mutated familial ALS. Acta neuropathologica communications, 2013. 1(1): p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Adermark L and Bowers MS, Disentangling the role of astrocytes in alcohol use disorder. Alcoholism: Clinical and Experimental Research, 2016. 40(9): p. 1802–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guizzetti M, et al. , Glia and neurodevelopment: focus on fetal alcohol spectrum disorders. Frontiers in pediatrics, 2014. 2: p. 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schambra UB, et al. , Consequences of low or moderate prenatal ethanol exposures during gastrulation or neurulation for open field activity and emotionality in mice. Neurotoxicology and teratology, 2016. 57: p. 39–53. [DOI] [PubMed] [Google Scholar]
- 131.Schambra UB, et al. , Low and moderate prenatal ethanol exposures of mice during gastrulation or neurulation delays neurobehavioral development. Neurotoxicology and teratology, 2015. 51: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fish E, et al. , the enduring impact of neurulation stage alcohol exposure: A combined behavioral and structural neuroimaging study in adult male and female C57bl/6j mice. Behavioural Brain Research, 2018. 338: p. 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marquardt K and Brigman JL, The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: Insights from rodent models. Alcohol, 2016. 51: p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wilson D, et al. , Developmental ethanol exposure-induced sleep fragmentation predicts adult cognitive impairment. Neuroscience, 2016. 322: p. 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wagner JL, Zhou FC, and Goodlett CR, Effects of one-and three-day binge alcohol exposure in neonatal C57BL/6 mice on spatial learning and memory in adolescence and adulthood. Alcohol, 2014. 48(2): p. 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lewin M, et al. , Developmental ethanol induced sleep fragmentation, behavioral hyperactivity, cognitive impairment and parvalbumin cell loss are prevented by lithium co-treatment. Neuroscience, 2017. [DOI] [PMC free article] [PubMed]
- 137.Mantha K, Kleiber M, and Singh S, Neurodevelopmental timing of ethanol exposure may contribute to observed heterogeneity of behavioral deficits in a mouse model of fetal alcohol spectrum disorder (FASD). Journal of Behavioral and Brain Science, 2013. 3(01): p. 85. [Google Scholar]
- 138.Houlé K, Abdi M, and Clabough EB, Acute ethanol exposure during late mouse neurodevelopment results in long-term deficits in memory retrieval, but not in social responsiveness. Brain and behavior, 2017. 7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baculis BC, Diaz MR, and Valenzuela CF, Third trimester-equivalent ethanol exposure increases anxiety-like behavior and glutamatergic transmission in the basolateral amygdala. Pharmacology Biochemistry and Behavior, 2015. 137: p. 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ke Z, et al. , Cyanidin-3-glucoside ameliorates ethanol neurotoxicity in the developing brain. J Neurosci Res, 2011. 89(10): p. 1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ren Z, et al. , Binge ethanol exposure causes endoplasmic reticulum stress, oxidative stress and tissue injury in the pancreas. Oncotarget, 2016. 7(34): p. 54303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu D and Cederbaum AI, Alcohol, oxidative stress, and free radical damage. Alcohol Research and Health, 2003. 27: p. 277–284. [PMC free article] [PubMed] [Google Scholar]
- 143.Kolattukudy PE and Niu J, Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circulation research, 2012. 110(1): p. 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim H-M, et al. , CC chemokine receptor 2 inhibitor ameliorates hepatic steatosis by improving ER stress and inflammation in a type 2 diabetic mouse model. PloS one, 2015. 10(3): p. e0120711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim W-K, et al. , Monocyte chemoattractant protein-1 deficiency attenuates oxidative stress and protects against ovariectomy-induced chronic inflammation in mice. PloS one, 2013. 8(8): p. e72108. [DOI] [PMC free article] [PubMed] [Google Scholar]


