Abstract
Introduction:
Polypharmacy, defined as the use of 5 or more medications is associated with multiple adverse outcomes in older adults, including falls and slow gait velocity. However, the relationship between polypharmacy and cortical control of locomotion has not been reported. The purpose of this study was to examine the relationship between polypharmacy and activation patterns in the prefrontal cortex (PFC), a brain region involved in higher order control of locomotion during attention-demanding conditions.
Methods:
Using Functional Near Infrared Spectroscopy (fNIRS) to quantify PFC oxygenated hemoglobin (HbO2) levels, we performed a cross sectional analysis of 325 community dwelling adults age ≥65 years, and examined HbO2 levels during single tasks (Single-Task-Walk (STW), (talking, cognitive interference (Alpha)) and Dual-Task Walk (DTW)).
Results:
The prevalence of polypharmacy was 33% (n = 104) amongst the 325 participants (mean age 76.4± 6.7 years, 56% women). Among the 221 participants with no polypharmacy there was an increase in HbO2 levels from STW to DTW (estimate=−0.625; p=<0.001) and from Alpha to DTW (estimate=−0.079; p=0.031). Polypharmacy status, however, moderated the change in HbO2 levels comparing the two single tasks to the dual-task walking condition. Specifically, the presence of polypharmacy was associated with an attenuated increase in HbO2 levels from STW to DTW (estimate=0.149; p=0.027) and with a decline in HbO2 levels from Alpha to DTW (estimate=0.169; p=0.009) after adjustments for potential confounders including medical comorbidities and the use of high-risk medications
Conclusion:
The results of this study further support the need for clinicians to reduce polypharmacy in older adults, given its significant association with the PFC hemodynamic response during attention-demanding locomotion.
Keywords: (6) Polypharmacy, Elderly, Dual task, Prefrontal Cortex
Graphical Abstract
The effect of Polypharmacy on HbO2 levels during Tasks
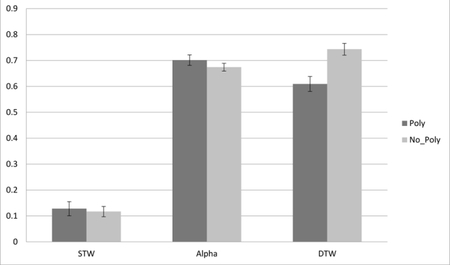
Changes in Hb02 levels (Y-axis-expressed in micromolar units), and tasks from Normal Walk(STW) and Alpha to Walk-While-Talk(DTW) as a function of polypharmacy status (Poly=Polypharmacy; No_Poly= No Polypharmacy).
1. INTRODUCTION
Polypharmacy, the use of more medications than clinically indicated1, has also been defined using numerical cutoffs related to adverse clinical outcomes2. For example, using 5 or more medications was associated with falls, frailty, hospitalization, and institutionalization in older adults2–5. While it is plausible that polypharmacy is a marker of multimorbidity and the consequences that follow, many studies have reported that the effects of polypharmacy persisted even after adjusting for comorbidities6. One explanation is that the presence of polypharmacy leads to medication interactions, adverse medication events and non-adherence; thereby predisposing older adults to adverse outcomes beyond what might be expected from their underlying medical conditions2. A long-term pharmacovigilance study reported that 7.3% of adverse drug reactions in patients with polypharmacy were probably preventable and related to known medication interactions7. The presence of polypharmacy has been linked to clinical outcomes such as cognitive impairment, mortality, incident falls, disability and frailty3. We have recently reported that polypharmacy was associated with slower gait velocity during single task condition8. There is, however, little data on the effects of polypharmacy on brain structure and function.
Medication induced cognitive impairment is a recognized entity that can manifest as delirium, and has been reported with the use of both psychotropic agents such as benzodiazepines and opioid analgesics, and non-psychotropic agents such as histamine receptor blockers, anticholinergic agents, and beta blockers9,10. There has been some discussion about the role of neurotoxic modifiers including medications, medical conditions, aging and stress in promoting both hippocampal neurodegeneration and gray matter volume atrophy11. It would follow that the multiplicative effects of medications coupled with age related changes in brain neurochemistry and capacity for handling of neurotransmitters might also play a role in changes in the brain’s structure and function11. While data exists about specific classes of medications and cognitive impairment, there is no known data on the mechanism of the association between polypharmacy and brain structure or function.
Dual-task-walk (DTW) requires the individual to allocate attention concomitantly to the walking and cognitive interference task (Alpha), and is more dependent on executive functions as evidenced by behavioral studies12,13. Poor dual-task walking performance is a risk factor of falls14 as well as disability, frailty and mortality15. Moreover, our previous work demonstrated reliable increases in prefrontal cortex (PFC) Oxygenated Hemoglobin (HbO2) levels in dual compared to single-task walking 16–18providing strong validation to the key functional role this brain region plays in higher order control of locomotion, notably under attention-demanding conditions.
Using functional-Near-Infrared Spectroscopy (fNIRS), the current study was designed to examine the effect of polypharmacy on the change in PFC HbO2 levels from single tasks (Single-Task-Walk – STW and cognitive interference-Alpha) to dual-task walking (DTW). We hypothesized that older adults with polypharmacy would demonstrate attenuated increases in PFC HbO2 levels from the single tasks to DTW compared to older adults without polypharmacy. This prediction was based on capacity limitations19–21, a model delineating brain behavior relations wherein the presence of neuropathology is implicated in attenuated brain responses in relation to cognitive tasks that increase in difficulty and complexity.
2. MATERIALS AND METHODS
2.1. Participants
A cross sectional study in 325 community dwelling adults age 65 years and older enrolled in the “Central Control of Mobility in Aging” (CCMA) study was performed. CCMA is a longitudinal study at Albert Einstein College of Medicine in the Bronx, New York. The study design has been previously reported13. The main aim is to determine cognitive and brain predictors of mobility in aging. Participants are initially screened by telephone with the AD8 Dementia Screening interview22 and the Memory Impairment Screen23 to exclude those with dementia. Participants who pass the screen are invited for further in-person testing in our research center. Inclusion criteria are aged 65 and older, English speaking, ambulatory, residence in the community, and plan to be in the area for the next three years. Exclusion criteria for the parent study include presence of dementia (self-reported, detected on the CCMA telephone cognitive screen, or diagnosed based on in-house data using established case conference diagnostic procedures24), inability to walk independently, history of severe neurological or psychiatric disorders, significant loss of vision or hearing, recent or planned surgical procedures that could affect mobility, or serious chronic or acute illnesses. Written informed consent was obtained in-person from all study participants. The Institutional review board approved the study protocol.
2.2. Medication history.
The study physician conducted structured neurological examinations, and review of medical history and medication usage. Medication history was further confirmed by review of medication bottles, interviewing family members when available, and any other available medical records. Prescription medications, Over the Counter (OTC) vitamin and mineral supplements and herbal agent use was documented. We have previously reported moderate to high medication adherence in the same cohort25. Polypharmacy definitions vary in previous studies both in terms of the medication count used3, as well as whether prescription and OTC medications and herbal agents were included in the definition26. In this study, polypharmacy was defined as the use of 5 or more medications including prescription medications, OTC vitamin and mineral supplements, and herbal agents based upon widely used operational definitions in the literature2–5, and previously applied to the CCMA cohort 8. High-risk medications were defined based upon the American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults27, and included medications such as anticholinergic agents, benzodiazepines and opioids.
2.3. Quantitative gait assessments
An electronic walkway (4×14 feet) which used ProtoKinetic Movement Analysis Software (PKMAS) was used for quantitative gait analysis (Zenometrics, LLC; Peekskill, NY). Stride velocity was measured under single task (STW) and dual-task (DTW) walking conditions. A cognitive (Alpha) task was also examined where participants were asked to recite alternate letters of the alphabet for 30 seconds while standing. For STW participants were asked to walk around the walkway for 3 consecutive loops. For DTW, they were instructed to walk while reciting alternate letters of the alphabet, and pay equal attention to both the walking and the cognitive task. This protocol has excellent reliability16, and been validated in other studies15,16.
2.4. fNIRS system
Functional Near Infrared Spectroscopy (fNIRS) is a portable system that can detect changes in the ratio of oxygenated and deoxygenated hemoglobin in the PFC during walking by using light-tissue interaction properties of light within the near infrared range28. It has been validated against traditional neuroimaging29 and is better able to handle motion artifact30. The fNI procedures have been described in detail in previous publications16,17,28. Briefly, changes in hemodynamic activity in the PFC were assessed using fNIRS Imager 1100 (fNIRS Devices, LLC, Potomac, MD). The system collects data at a sampling rate of 2Hz. The fNIRS sensor consists of 4 LED light sources and 10 photodetectors, which cover the forehead using 16 voxels, with a source-detector separation of 2.5 cm. The light sources on the sensor (Epitex Inc. type L4X730/4X805/4X850–40Q96-I) contain three built-in LEDs having peak wavelengths at 730, 805, and 850 nm, with an overall outer diameter of 9.2 ± 0.2 mm. The photodetectors (Bur Brown, type OPT101) are monolithic photodiodes with a single supply transimpedance amplifier. Given the sensitivity of the fNIRS recording device, the lighting in the test room was reduced such that the mean illumination of the forehead was approximately 150 lux. In this study, standard sensor placement and room lighting procedures were used. fNIRS was performed under four conditions; rest (baseline), STW, Alpha, and DTW. During baselines participants are asked to remain still and count from 1 to 10 in their head for 10 seconds. HbO2 levels were determined relative to the baseline condition for each of the tasks. We used a block study design. The three test conditions were presented in a counterbalanced order using a Latin-square design to estimate the effect of test order.
2.5. Clinical evaluations
Participants received detailed clinical, cognitive, and mobility assessments at their baseline in-house visit and at yearly follow-up visits. They are also interviewed about medical conditions, cognitive status, and had neurological examinations performed by the study clinician. As previously reported13, presence or absence of physician diagnosed chronic illnesses (depression, Parkinson’s disease, chronic obstructive lung disease, or severe arthritis) and vascular diseases (diabetes, heart failure, hypertension, angina, myocardial infarction, or stroke) is reported by the participants upon entry in the study to calculate a Global Health Score (GHS) ranging from 0–1024.
Medical history is further confirmed using available medical records and by interviewing family members. The Repeatable Battery for Assessment of Neuropsychological Status (RBANS) total score was used to evaluate global cognitive status31. Body Mass Index (BMI) was calculated using the participant’s weight and height.
2.6. Statistical Analysis
Baseline characteristics in those with and without polypharmacy were compared using descriptive statistics and compared using two-sample-test for continuous variables and Chi Square test for categorical variables. Model assumptions were examined and met.
Linear mixed effects models (LMEM) and Generalized Estimating Equations (GEE) Poisson models were used to assess the main and moderating effects of polypharmacy on change in stride velocity (STW, DTW), HbO2 levels (Alpha, STW, DTW), and rate of correct letter generation (Alpha, DTW). The presence or absence of Polypharmacy was the two-level between subject factor, and task condition (STW, DTW, Alpha) the repeated within subject factors. A random intercept was included to allow for varying entry points across individuals. The interaction term of polypharmacy x task was included in each model to determine if polypharmacy is associated with changes on the dependent variable between tasks. The covariates to be included in the models were chosen if they were significant at a P value of .05 or less in the univariate analyses (see Table 1) or based upon biological plausibility. Analyses adjusted for age, sex, educational level, falls within the last year, high-risk medication use, stride velocity and medical comorbidities including HTN, DM, MI, and stroke. Additional sensitivity analyses were executed to further examine the possible effects of chronic disease burden, high-risk medications and an alternative definition for polypharmacy. All analyses were performed on SPSS version 25, IBM.
Table 1:
Baseline Characteristics of participants with and without polypharmacy
| Baseline Characteristic | Total N=325 |
Polypharmacy N=104 |
No Polypharmacy N= 221 |
P-value |
|---|---|---|---|---|
| Age (years) mean, SD | 76.4±6.7 | 77.4 ± 6.9 | 76.0 ± 6.6 | 0.065 |
| Female, n (%) | 182 (56.0) | 55 (52.9) | 127 (57.9) | 0.473 |
| Educational level (years), mean, SD | 14.4 ± 3.0 | 14.1 ± 3.0 | 14.6 ± 3.0 | 0.161 |
| Global Health Score, mean, SD | 1.6±1.1 | 2.1 ± 1.0 | 1.4 ± 1.0 | <.001* |
| Medical Conditions | ||||
| Hypertension | 197 (60.6) | 85 (81.0) | 112 (50) | <.001* |
| Congestive Heart Failure, n (%) | 4(1.2) | 3 (2.9) | 1 (0.5) | 0.097 |
| Diabetes, n (%) | 56(17.2) | 29 (27.9) | 27 (12.2) | 0.001* |
| Myocardial Infarction, n (%) | 18(5.5) | 10 (9.6) | 8 (3.6) | 0.037* |
| Chronic Obstructive Pulmonary Disease, n (%) | 25(7.7) | 10 (9.6) | 15 (6.8) | 0.380 |
| Stroke, n (%) | 17(5.2) | 10 (9.6) | 7 (3.2) | 0.029* |
| Depression, n (%) | 33(10.2) | 14 (13.6) | 19 (8.6) | 0.172 |
| Osteoarthritis, n (%)a | 154(87.5) | 48 (88.9) | 106 (86.9) | 0.809 |
| Measures | ||||
| Systolic Blood pressure (mm Hg), SD | 130.1±13.2 | 128.9 ± 14.2 | 130.7 ± 12.7 | 0.273 |
| Diastolic Blood pressure (mm Hg), SD | 77.7±7.8 | 77.0 ± 8.5 | 78.0± 7.4 | 0.280 |
| Knee Extensor Strength (KG), mean, SD | 38.0±81.5 | 30.1 ± 13.0 | 41.3 ± 98.0 | 0.149 |
| Falls within the last year, n (%) | 51(15.7) | 25 (24.0) | 26 (11.8) | 0.005* |
| Body Mass Index (kg/m2), mean, SD | 29.2±6.5 | 30.3 ± 8.0 | 28.8 ± 5.7 | 0.066 |
| Grip strength, mean, SD | 23.9±8.9 | 23.7 ± 9.2 | 24.0 ± 8.8 | 0.828 |
| Total RBANSb score (0–100), SD | 91.4± 11.9 | 92.4 ±11.9 | 90.4 ± 11.9 | 0.319 |
| Stride Velocity STW(cm/sec) | 80.0±17.5 | 76.8±15.5 | 81.5±18.1 | 0.018* |
| Stride Velocity DTW(cm/sec) | 65.0±18.8 | 61.9±17.3 | 66.4±19.3 | 0.040* |
| Alpha: rate of correct letter generation | 16.8±6.0 | 16.4±6.0 | 17.0±6.0 | 0.331 |
| DTW: rate of correct letter generation | 22.7±9.8 | 22.3±9.5 | 23.0±9.9 | 0.555 |
| HbO2 Levels | ||||
| STWc | 0.110±1.2 | 0.128±1.1 | 0.117±1.16 | 0.745 |
| Alpha | 0.682±.86 | 0.701±.822 | 0.674±.889 | 0.280 |
| DTWd | 0.705±1.28 | 0.609±1.17 | 0.743±1.34 | <0.001* |
Based upon an n of 176 total, 54 in Polypharm and 106 in no polypharm
RBANS: Repeatable Battery for Assessment of Neuropsychological Status
Based upon an n of 176 total, 54 in the Polypharm and 106 in the No Polypharm group
STW=Normal Walk
DTW=Walk While Talk
3.0. Results
The prevalence of polypharmacy was 33% (n = 104) amongst the 325 participants examined at baseline in the CCMA sample between June 2011 and February 2014. he baseline characteristics of the 104 patients with polypharmacy and the 221 without polypharmacy are listed in Table 1. The mean age was 77 ± 6.9 years in the polypharmacy group and 76 ± 6.6 years in the No polypharmacy group. Participants who had polypharmacy were more likely to have hypertension, diabetes, history of a stroke, and a history of a myocardial infarction. The polypharmacy group was also more likely to have a fall within the last year (24% vs. 11%, p= 0.005), than those without polypharmacy. There was a statistically significant difference in stride velocity in STW (76.8 ±15.5 vs. 81.5 ±18.1, p=.018) and DTW (61.9 ±17.3 vs. 66.4 ±19.3, p=.040 between the polypharmacy and the no polypharmacy groups. Blood pressure, total RBANS score, rate of correct letter generation, educational level, knee extensor strength, the presence of osteoarthritis and depression were comparable between participants with polypharmacy and those without polypharmacy.
Table 2 shows the frequency of medications used above 5% in the cohort, compares medication use among the polypharmacy and No polypharmacy group, and also includes the frequency of medications classified as high-risk.27 Non-prescription medications included OTC medications, vitamin and mineral supplements and herbal agents were used by 53% of the participants. On average, participants in the cohort were on 1.1 ±1 .3 SD nonprescription medications (not shown). HMGCOa (3-hydroxy-3-methyl-glutaryl-coenzyme A) reductase inhibitors followed by beta blockers and angiotensin converting enzyme inhibitors were the more commonly used prescription medications among participants in the sample. The prevalence of high-risk medications was 15.7% in our sample; antidepressants (4.6%) and benzodiazepines/anxiolytics (4.0%) were used with the highest frequency among high-risk medication users. nticholinergics and antihistamines were used in only .6% and 1.8% of the participants in the sample respectively. As expected, those with polypharmacy compared to those without polypharmacy were on more medications from all listed classes.
Table 2:
Medication Use Frequency for participants with and without Polypharmacy
| Medication | All N=325 n (%) |
Polypharm N=104 n (%) |
No Polypharm N= 221 n (%) |
P value |
|---|---|---|---|---|
| Non-Prescription Drug | 162(53.5) | 82(78.8) | 80(36.2) | <0.001 |
| HMGCoA Inhibitorsa | 168(51.7) | 76(73.1) | 92(41.6) | <0.001 |
| Beta Blockers | 86(26.5) | 51(49.0) | 35(15.8) | <0.001 |
| Ace Inhibitors | 67(20.6) | 31(29.8) | 36(16.3) | 0.008 |
| Antiplatelet Agents | 59 (18.2) | 35(33.7) | 24(10.9) | <0.001 |
| Angiotensin Receptor Blockers | 60(18.5) | 29(27.9) | 31(14.0) | <0.005 |
| Vitamin and Mineral Combinations | 32(9.8) | 21(20.2) | 11(5.0) | <0.001 |
| Oral hypoglycemic Agents | 43 (13.2) | 24(23.1) | 19(8.6) | 0.001 |
| Thyroid Hormone Replacements | 38 (11.7) | 19 (18.3) | 19(8.6) | 0.016 |
| Calcium | 34(10.5) | 20(19.2) | 14(6.3) | <0.001 |
| Vitamin D | 27 (8.3) | 18(17.3) | 9(4.1) | <0.001 |
| Proton Pump Inhibitors | 20 (6.2) | 13(12.5) | 7(3.2) | <0.002 |
| Antihypertensive Combination | 21(6.5) | 9(8.7) | 12(5.4) | 0.333 |
| Ophthalmic Agents | 21 (6.5) | 11(10.6) | 10(4.5) | 0.052 |
| Anticoagulants | 23(7.1) | 16(15.4) | 7(3.2) | <0.001 |
| Agents for Gout | 18(5.5) | 14(13.5) | 4(1.8) | <0.001 |
| NSAID analgesic | 14(4.3) | 5(4.8) | 9(4.1) | 0.774 |
| Loop Diuretic | 14(4.3) | 9 (8.7) | 5(2.3) | <0.015 |
| Thiazide diuretic | 17(5.2) | 12(11.5) | 5(2.3) | 0.001 |
| High Risk Medications | 51(15.7) | 29(27.9) | 22(10) | <0.001 |
| Antidepressants | 15(4.6) | 8(7.7) | 7(3.2) | 0.089 |
| Alpha 1 antagonists | 5(1.5) | 4(3.8) | 1(0.5) | 0.038 |
| Benzodiazepines/anxiolytics | 13(4.0) | 9(8.7) | 4(1.8) | 0.006 |
| Antihistamines | 6(1.8) | 3(2.9) | 3(1.4) | 0.389 |
| Opioids | 3(0.9) | 3(2.9) | 0 (0) | 0.032 |
| Anticholinergics | 2(0.6) | 2(1.9) | 0(0) | 0.102 |
| Muscle relaxants | 1(0.3) | 1(1.0) | 0(0) | 0.320 |
HMGCoA: 3-hydroxy-3-methyl-glutaryl-coenzyme A
3.1. Effects of polypharmacy on task-related changes in HbO2 levels
Fully adjusted LMEM was used to determine the main effects of task (three-level repeated measure variable with DTW serving as the reference) and polypharmacy (two-level between-subject factor) as well as their interactions on HbO2 levels (Table 3). HbO2 levels from the 16 fNIRS optodes were allowed to vary in the context of the LMEM and served as the dependent measure.
Table 3.
Effects of polypharmacy on HB02 levels during tasks
| Variables | Estimate | SE | 95% CI | P value |
|---|---|---|---|---|
| Unadjusted model | ||||
| STW ×DTW(npa) | −0.626 | 0.039 | −0.701 to −0.550 | <0.001 |
| Alpha × DTW(np) | −0.080 | 0.037 | −0.153 to −0.007 | <0.001 |
| STW × DTW(pb) | −0.470 | 0.056 | −0.580 to −0.360 | <0.001 |
| Alpha × DTW(p) | 0.086 | 0.054 | −0.020 to 0.193 | 0.111 |
| Polypharm(DTW) | −0.144 | 0.048 | −0.238 to −0.049 | 0.031 |
| Task effectsc | ||||
| Polypharm × STW × DTW | 0.156 | 0.068 | 0.022 to 0.290 | 0.003 |
| Polypharm × Alpha × DTW | 0.167 | 0.066 | 0.0375 to 0.296 | 0.022 |
| Adjusted model | ||||
| STW ×DTW(np) | −0.625 | 0.038 | −0.699 to −0.551 | <0.001 |
| Alpha × DTW(np) | −0.079 | 0.079 | −0.150 to 0.007 | 0.031 |
| STW × DTW (p) | −0.476 | 0.055 | −0.585 to −0.368 | <0.001 |
| Alpha × DTW(p) | 0.090 | 0.054 | −0.015 to 0.195 | 0.094 |
| Polypharm(DTW) | −0.199 | 0.050 | −0.296 to 0.102 | <0.001 |
| Task effects | ||||
| Polypharm × STW × DTW | 0.149 | 0.067 | 0.017 to 0.280 | 0.027 |
| Polypharm × Alpha × DTW | 0.169 | 0.065 | 0.042 to 0.296 | 0.009 |
| Adjusted Covariates | ||||
| Age | −0.0004 | 0.002 | −0.005 to 0.004 | 0.809 |
| Gender | −0.286 | 0.028 | −0.329 to −0.221 | <0.001 |
| Education | −0.009 | 0.002 | −0.016 to 0.002 | 0.114 |
| Fall past year | 0.068 | 0.037 | −0.006 to 0.141 | 0.071 |
| Hypertension | −0.015 | 0.029 | −0.072 to 0.043 | 0.620 |
| Diabetes | 0.090 | 0.005 | 0.019 to 0.161 | 0.014 |
| Myocardial infarction | 0.025 | 0.060 | −0.092 to 0.142 | 0.677 |
| Stroke | 0.085 | 0.062 | −0.036 to 0.207 | 0.169 |
| Velocity | 0.001 | 0.001 | −0.003 to −66 E-005 | 0.049 |
| High Risk Medications | 0.062 | 0.039 | −0.014 to 0.039 | 0.108 |
np=no polypharmacy
p=polypharmacy
Task effects describes the interaction terms of polypharmacy × task( STW ×DTW) and polypharmacy × task (alpha × DTW) in both the unadjusted and adjusted models.
Among participants with no polypharmacy, there was a statistically significant increase in HbO2 levels from STW to DTW (estimate= −0.625; p=<0.001) and from Alpha to DTW (estimate= −0.079; p=0.031). Among participants with polypharmacy there was also a statistically significant increase in HbO2 levels from STW to DTW (estimate=−0.476; p=<0.001), while the change in HbO2 levels from Alpha to DTW was in the opposite direction but did not reach statistical significance (estimate=0.090; p=0.094). Polypharmacy status moderated the effect of task on changes in HbO2 levels (see Figure 1 for visual depiction of the group by task interactions). Specifically, compared to controls, the presence of polypharmacy was associated with an attenuated increase in HbO2 levels from STW to DTW (estimate=0.149; p=0.027) and with an opposite trajectory delineating a decline in HbO2 levels from Alpha to DTW (estimate=0.169; p=<0.009). Analyses adjusted for age, sex, educational level, falls within the last year, high-risk medication use, stride velocity and medical comorbidities including HTN, DM, MI, and stroke.
Figure 1. The effect of Polypharmacy (≥5) on Hb02 levels during Tasks.
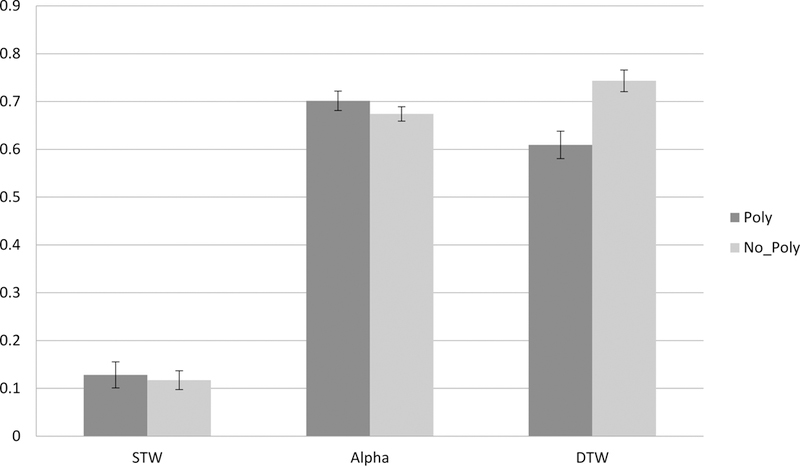
Changes in Hb02 levels (Y-axis-expressed in micromolar units), and tasks from Normal Walk(STW) and Alpha to Walk-While-Talk(DTW) as a function of polypharmacy status.
(Poly=Polypharmacy; No_Poly= No Polypharmacy).
Polypharmacy moderated the change in Hb02 levels during tasks. Error bars are based on the standard errors obtained from the linear mixed effects model.
3.2. Effects of polypharmacy on Stride velocity
Fully adjusted LMEM were used to examine the effects of polypharmacy on stride velocity with polypharmacy as the two-level between-subject factor, Walking Condition (STW, DTW) as the two-level repeated within-subject factor (DTW is the reference), and stride velocity as the outcome. There was a statistically significant change in stride velocity during DTW compared to STW among those without polypharmacy (estimate= 15.06; 95% CI= 13.54 to 16.59, p< 0.001). There was also a statistically significant change in stride velocity during DTW compared to STW among those with polypharmacy (estimate= 14.97; 95% CI= 12.73 to 17.21, p< 0.001). The presence of polypharmacy was associated with slower stride velocity (estimate = −4.527;95%CI=−8.817 to 0.237; p= 0.039). The two-way interaction of polypharmacy and task was not statistically significant (estimate= −0.090; 95% CI= −2.80 to 2.62, p= 0.948) adjusting for age, sex, educational level, falls within the last year, HTN, DM, MI, stroke, and high-risk drugs.
3.3. Effects of Polypharmacy on Rate of Correct Letter Generation
GEE was used to examine the rate of correct letter count generation. Among those without polypharmacy, there was no statistically significant difference in DTW compared to Alpha (estimate of log of rate ratio= −0.022; 95% CI= 0.096 to −0.051, p=0.554). Similarly, among those with polypharmacy, there was no statistically significant difference in DTW compared to Alpha (estimate of log of rate ratio= −0.027; 95% CI= −0.115 to 0.062, p=0.860). The presence of polypharmacy was not significantly associated with the rate of correct letter count generation (estimate of log of rate ratio = −0.092; 95%CI=−0.045 to 0.054; p= 0.120). The interaction between polypharmacy and correct letter generation during task was also not statistically significantly different (estimate of log or rate ratio= −0.027, 95% CI= −0.062 to 0.115, p =.554) after adjusting for age, sex, educational level, fall within the last year, HTN, DM, MI, stroke, high risk drugs and baseline gait velocity.
3.4. Sensitivity Analyses
3.4.1. The Effect of Chronic Disease on Study Outcomes
As reported earlier, the effect of polypharmacy on changes in HbO2 from single to dual-task conditions was significant when adjusting for medical comorbidities that were significantly different between the polypharmacy no polypharmacy group. An additional analysis that further adjusted for a total Global Health Score (GHS) revealed that the interaction between polypharmacy and STW vs DTW (estimate=0.149; 95% CI=0.0169 to 0.280, p=.027) and Polypharmacy and alpha vs. DTW (estimate=0.169; 95% CI=0.042 to 0.0297, p=.009) remained statistically significant.
3.4.2. The Effect of High-Risk Medication use on Study Outcome
We adjusted for high-risk medication use in all previously described models, but in order to further explore the possible impact of high-risk medications on the results, we performed a number of sensitivity analyses. 1) Since antidepressants were used with the greatest frequency (4.6%) among those on a high-risk medication, we adjusted for antidepressant use, and found that moderating effects of polypharmacy on task-related changes in HbO2 levels (polypharmacy x STW vs DTW, (estimate= 0.015; 95% CI= 0.017 to 0.280, p=0.027); and polypharmacy x alpha vs DTW, estimate= 0.169; 95% CI= 0.042 to 0.297, p=0.009) remained statistically significant. 2) We excluded participants who were on a high-risk medications in the primary analysis and found that there was still a statistically significant interaction between polypharmacy and task ((alpha vs. DTW); estimate=0.209; 95% CI=0.061 to 0.356, p=.006)) and the interaction between polypharmacy and task ((STW vs DTW); estimate=0.147; 95% CI=−0.005 to 0.299, p=0.058) was marginal. 3) To explore whether there was an interaction between high-risk medication use and task (STW vs. DTW and alpha vs. DTW), 2-way interactions (high risk x task) and (polypharmacy x task), and a 3-way interaction ( high risk x task x polypharmacy) were explored. In the 2-way interaction of polypharmacy x task results were statistically significant STW x DTW estimate=0.149; 95% CI=0.017 to 0.280, p=0.027 and alpha x DTW; estimate=0.169; 95% CI=0.0417 to 0.296, p=.009. In the 2-way interaction of high risk x task, the use of high risk medication moderated the effect of task on HbO2 levels for STW vs. DTW; estimate= 0.137; 95% CI= 0.014 to 0.260, p=0.029, but not for alpha vs. DTW; estimate= 0.033; 95% CI= −0.088 to 0.155, p = 0.593. Three-way interaction between polypharmacy x task x high risk medications to determine if the effect of polypharmacy on change in oxygenated levels differs by the presence of high risk medication use was not significant; STW vs. DTW; estimate= 0.169; 95% CI= −0.024 to 0.363, p= .086 and alpha vs. DTW; estimate= 0.048; 95% CI= −0.144 to 0.240, p=0.623.
3.4.3. Alternative Definition of Polypharmacy:
The Effect of Increased Medication Count An alternative definition of polypharmacy using 8 or more medication as cutoff was used to determine if an increase in the number of medications used would influence the results. Compared to controls, the presence of polypharmacy was associated with an attenuated increase in HbO2 levels from STW to DTW (estimate=0.245; 95% CI=0.028 to 0.462, p=0.027 and with an opposite trajectory delineating a decline in HbO2 levels from Alpha to DTW (estimate=0.335; 95% CI 0.124 to 0.545, p=<0.002). All sensitivity analyses adjusted for age, sex, educational level, falls within the last year, high-risk medication use, baseline velocity and medical comorbidities including HTN, DM, MI, and stroke.
4.0. Discussion
The current study was designed to determine the relationship between polypharmacy and cortical control of locomotion. We found that participants with polypharmacy had differential prefrontal cortex activation patters during tasks when compared with those without polypharmacy. Compared to controls, those with polypharmacy showed an attenuated increase in PFC HbO2 levels from STW to DTW, and an opposite trajectory delineating a decline in PFC HbO2 from Alpha to DTW. It appears that during the more cognitively demanding DTW task, participants with polypharmacy were unable to mount an increase in HbO2 levels, but instead had a significant decline in prefrontal cortex activation. This is consistent with the capacity limitations hypothesis19,20,21, which implicates neuropathology in attenuated brain responses in relation to cognitive tasks that increase in difficulty and complexity. Brain resources may have already been maximized during the single tasks such as Alpha; leading to a decrease in further activation in the face of increased demands.
While polypharmacy resulted in notable changes in brain function as suggested by differential brain activation patterns, its association with behavioral outcomes was variable and less marked. Specifically, polypharmacy was associated with both history of falls and slower gait velocity during single and dual-task walking in unadjusted analyses. Gait and cognitive performance, as assessed in the context of dual-task walking, however, were not associated with polypharmacy after adjusting for potential confounders. reasonable explanation is that changes in brain activity are more sensitive to the effect of polypharmacy and likely precede behavioral abnormalities during dual-task walking. These changes may become more evident as pathology progresses. It is possible that there is a threshold effect when multiple medications are used, and that at a cutoff of 5 or more, there is a saturation of brain synapses and receptors, which causes a slowing or a decrease in activation patterns in the prefrontal cortex ultimately leading to behavioral changes over time.
The results of this study indicated a significant interaction between polypharmacy and task after adjusting for potential confounders including the use of high-risk medication use. Given the relationship between high-risk medication use and cognition in aging, we performed extensive sensitivity analyses to further explore whether high-risk medications influenced the reported associations between polypharmacy and the study outcomes. It is noteworthy that the moderating effect of polypharmacy on changes in HbO2 levels across task conditions remained significant in all sensitivity analyses. Given the low frequency of high-risk medications including anticholinergic agents, in this cohort the additional analyses have limitations but clearly lend credibility and further bolster our primary findings.
The association of polypharmacy with task-related changes in HbO2 levels cannot be attributed to medical comorbidities and their neurobiological consequences. Adjustment for disease burden using the GHS and for specific medical comorbid conditions did not attenuate the association between polypharmacy and study outcomes. We also examined the relationship between prefrontal cortex activation patterns and polypharmacy defined as the use of 8 or more medications. We found that the estimates for change in oxygenation levels from single to DTW were similar when 8 or more medications ore 5 or more medications were used.
This study is the first to examine the relationship between polypharmacy and brain activity in aging. The PFC is an important region implicated in cortical control of mobility, and older adults with polypharmacy may have an alteration of the function of this brain region. In this study, imaging data identifies abnormalities that have not yet been noted during examination of behavior. Further strengths of this study include the reliability and novelty of our experimental procedures, clinical characterization of the participants and use of multivariate analysis to account for a range of possible confounders. While we adjusted for a number of possible confounders including several medical comorbidities and the use of high-risk medications, it was not possible to adjust for all possible unmeasured confounders. Furthermore, the cross-sectional nature of the study limits our ability to determine causation.
The current study did not include traditional imaging, which might have provided further insights into the underlying brain substrates that might influence the effects of polypharmacy on PFC substrates of locomotion, but fNIRS offers the advantage of assessing cortical activation during active walking. Though fNIRS is limited in terms of depth of penetration, its utility in measuring changes in PFC HbO2 levels in response to increased cognitive demands has been supported by the literature 16,32. Future studies using larger and more diverse samples and multi-modal neuroimaging methods that provides whole brain structural and functional information, could improve generalizability, complement the current findings and provide additional information regarding the neurobiological consequences of polypharmacy in aging. Furthermore, a future direction will be to examine the longitudinal effects of polypharmacy on gait and its underlying cortical control mechanisms.
5.0. Conclusion
Outcomes associated with polypharmacy include changes to brain function, as evidenced by differences in activation patterns in the prefrontal cortex in relation to tasks that increase in difficulty. The results of this study further support the need for clinicians to reduce the number of medications older adults are prescribed, even though measurable behavioral changes have not yet been recognized.
Acknowledgements:
This research was supported by the National Institutes on Aging grants (R01AG036921, R01AG044007), and by the NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number KL2TR001071.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest:
Dr. Izzetoglu has a very minor share in the company that manufactures the fNIRS device used in this study. All other authors have no conflicts of interest to report in relation to the current article.
References
- 1.CJ G Polypharmacy. In: Hirth VADM, ed. Case-Based Geriatrics: A Global Approach 1st ed. New York: McGraw Hill; 2011:431. [Google Scholar]
- 2.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert opinion on drug safety 2014;13:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–95. [DOI] [PubMed] [Google Scholar]
- 4.Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet 2007;370:185–91. [DOI] [PubMed] [Google Scholar]
- 5.Doan J, Zakrzewski-Jakubiak H, Roy J, Turgeon J, Tannenbaum C. Prevalence and Risk of Potential Cytochrome P450-Mediated Drug-Drug Interactions in Older Hospitalized Patients with Polypharmacy. Annals of Pharmacotherapy 2013;47:324–32. [DOI] [PubMed] [Google Scholar]
- 6.Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc 2014;62:2261–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnovale C, Gentili M, Fortino I, et al. The importance of monitoring adverse drug reactions in elderly patients: the results of a long-term pharmacovigilance programme. Expert Opin Drug Saf 2016;15:131–9. [DOI] [PubMed] [Google Scholar]
- 8.George C, Verghese J. Polypharmacy and Gait Performance in Community-dwelling Older Adults. J Am Geriatr Soc 2017. [DOI] [PMC free article] [PubMed]
- 9.Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging 1999;15:15–28 [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell L, Gnjidic D, Nahas R, Bell JS, Hilmer S. Anticholinergic burden:considerations for older adults. Journal of Pharmacy Practice and Research 2017;47:67–71. [Google Scholar]
- 11.Daulatzai MA. Neurotoxic saboteurs: straws that break the hippo’s (hippocampus) back drive cognitive impairment and Alzheimer’s Disease. Neurotox Res 2013;24:407–59. [DOI] [PubMed] [Google Scholar]
- 12.Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor Control 2012;16:64–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age (Dordr) 2014;36:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayers EI, Tow AC, Holtzer R, Verghese J. Walking while talking and falls in aging. Gerontology 2014;60:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verghese J, Holtzer R, Lipton RB, Wang C Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc 2012;60:1901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online frontocortical control of simple and attention-demanding locomotion in humans. Neuroimage 2015;112:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtzer R, Schoen C, Demetriou E, et al. Stress and gender effects on prefrontal cortex oxygenation levels assessed during single and dual-task walking conditions. Eur J Neurosci 2017;45:660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtzer R, Verghese J, Allali G, Izzetoglu M, Wang C, Mahoney JR. Neurological Gait Abnormalities Moderate the Functional Brain Signature of the Posture First Hypothesis. Brain Topogr 2016;29:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grady CL, McIntosh AR, Horwitz B, et al. Age-related reductions in human recognition memory due to impaired encoding. Science 1995;269:218–21. [DOI] [PubMed] [Google Scholar]
- 20.Madden DJ, Turkington TG, Coleman RE, Provenzale JM, DeGrado TR, Hoffman JM. Adult age differences in regional cerebral blood flow during visual world identification: evidence from H215O PET. Neuroimage 1996;3:127–42. [DOI] [PubMed] [Google Scholar]
- 21.Reuter-Lorenz PA, Jonides J, Smith EE, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci 2000;12:174–87. [DOI] [PubMed] [Google Scholar]
- 22.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology 2005;65:559–64. [DOI] [PubMed] [Google Scholar]
- 23.Lipton RB, Katz MJ, Kuslansky G, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc 2003;51:1382–90. [DOI] [PubMed] [Google Scholar]
- 24.Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB. Within-person across-neuropsychological test variability and incident dementia. JAMA 2008;300:823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George CJ, Verghese J. Gait Performance in Hypertensive Patients on Angiotensin-Converting Enzyme Inhibitors. J Am Med Dir Assoc 2016;17:737–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlesworth CJ, Smit E, Lee DS, Alramadhan F, Odden MC. Polypharmacy Among Adults Aged 65 Years and Older in the United States: 1988–2010. J Gerontol A Biol Sci Med Sci 2015;70:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Geriatrics Society Beers Criteria Update Expert. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012;60:616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol Biol Sci Med Sci 2011;66:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 2002;17:719–31. [PubMed] [Google Scholar]
- 30.Cooper RJ, Seib J, Gagnon L, et al. A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front Neurosci-Switz 2012;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol 2008;23:603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen M, Blumen HM, Izzetoglu M, Holtzer R. Spatial Coregistration of Functional Near-Infrared Spectroscopy to Brain MRI. J Neuroimaging 2017;27:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


