Abstract
Background:
Major depressive disorder (MDD) is one of the most prevalent and debilitating psychiatric disorders. Cognitive complaints are commonly reported in MDD and cognitive impairment is a criterion item for MDD diagnosis. As cognitive processes are increasingly understood as the consequences of distributed interactions between brain regions, a network-based approach may provide novel information about the neurobiological basis of cognitive deficits in MDD.
Methods:
51 Depressed (MDD, n = 23) and non-depressed (control, n = 28) adult participants completed neuropsychological testing and resting-state fMRI (rsfMRI). Cognitive domain scores (processing speed, working memory, episodic memory, and executive function) were calculated. Anatomical regions of interests were entered as seeds for functional connectivity analyses in: default mode (DMN), salience, and executive control (ECN) networks. Partial correlations controlling for age and sex were conducted for cognitive domain scores and functional connectivity in clusters with significant differences between groups.
Results:
Significant rsfMRI differences between groups were identified in multiple clusters in the DMN and ECN. Greater positive connectivity within the ECN and between ECN and DMN regions was associated with poorer episodic memory performance in the Non-Depressed group but better performance in the MDD group. Greater connectivity within the DMN was associated with better episodic and working memory performance in the Non-Depressed group but worse performance in the MDD group.
Conclusions:
These results provide evidence that cognitive performance in MDD may be associated with aberrant functional connectivity in cognitive networks and suggest patterns of alternate brain function that may support cognitive processes in MDD.
Keywords: Major Depression, Cognition, Functional Connectivity, Functional MRI
Although Major depressive disorder (MDD) is diagnosed through identification of behavioral symptoms and signs, deficits in cognitive performance are also common. Alterations in the connectivity and function of the networks likely affect emotional processes directly related to depressive symptoms but may also negatively affect cognitive function. Establishing the relationship between altered network connectivity and cognitive function in depressed individuals is necessary to fully understand to role of network connectivity in MDD and to guide the development of interventions aimed at improving cognition.
Resting-state functional connectivity alterations in MDD generally involve the default mode network (DMN), executive control network (ECN), and the salience network(Kaiser et al., 2015; Mulders et al., 2015). Patterns of altered connectivity in MDD include greater connectivity within the DMN (posterior and anterior hubs) with greater inclusion of the subgenual anterior cingulate cortex (sgACC) in depression (Greicius et al., 2007). In the ECN, the dorsolateral prefrontal cortex (dlPFC) typically shows decreased connectivity with other ECN regions (parietal and precuneus regions) in MDD (Kaiser et al., 2015; Mulders et al., 2015). There have been less consistent findings in the salience network (Mulders et al., 2015); however, its activity broadly reflects paralimbic emotional processing and emotional control(Hamilton et al., 2016; Uddin, 2015), which may be altered in MDD and contribute to depressive symptoms(Hamilton et al., 2016; Sliz and Hayley, 2012).
These patterns of altered functional connectivity in MDD suggest that intrinsic networks exhibit altered connectivity with brain regions involved in both emotional processes and broader cognitive function. The ECN is involved in emotional and behavioral regulation as well as coordinating cognitive processes. Although SN activity reflects emotional processing it is also thought to contribute to cognitive processing and coordinating activity of the DMN and ECN (Seeley et al., 2007). In MDD, connectivity within the DMN also includes brain areas that are important for self-referential emotional processing(Fox et al., 2005) , directing attention, and memory(Greicius et al., 2007). Because these brain networks are involved in both emotion regulation and cognitive processes including memory, attention, and executive function, alterations in networks that contribute to emotional dysregulation in MDD may also adversely affect cognitive performance. Optimal cognitive performance may rely on the functional capacity of all of these networks and the ability to effectively switch activity between networks as tasks demand.
Poorer cognitive performance on neuropsychological testing is common in MDD and recent meta-analyses support that depression is associated with poor executive function, memory, and attention performance compared to non-depressed individuals (Albert et al., 2017, 2018; Bora et al., 2013; Hasselbalch et al., 2011; Rock et al., 2014). Such differences may be related to altered function in both the ECN and DMN (Sheline et al., 2010, 2009). However, it is unclear whether network alterations in MDD are a mechanism underlying cognitive deficits or if they maintain cognitive performance in the context of other functional changes that occur in depression. While previous studies have examined functional connectivity and cognitive performance in MDD, few have examined the relationship between these measures concurrently in the same individuals. The aim of this study was to examine intrinsic functional network connectivity in antidepressant-free MDD and examine how network connectivity is associated with performance across multiple cognitive domains, including tasks of episodic memory, executive function, processing speed, and working memory. We hypothesized that depressed participants would have reduced connectivity in ECN and greater connectivity in DMN and SN compared to non-depressed participants and that these patterns of connectivity would be associated with worse cognitive performance in depressed participants.
METHODS
Participants
Participants between the ages of 20 and 50 years were enrolled at Duke University Medical Center. Participants were included in a previous, larger report examining cognitive performance in MDD.[REF] The current analyses include the sub-sample of participants who completed resting-state fMRI (Albert et al., 2018). Depressed participants had a Diagnostic and Statistical Manual of Mental Disorder (DSM-IV) diagnosis of recurrent MDD, as assessed by the Mini-International Neuropsychiatric Interview (MINI, version 5.0) (Sheehan et al., 1998) and interview with a study psychiatrist. Additional entry criteria included onset of first depressive episode before age 35 years and a Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) of 15 or greater. Entry criteria specified no antidepressant use in the last month (six weeks for fluoxetine); however, most participants reported no antidepressant use for at least three months or longer. Eligible control participants had no lifetime history of psychiatric disorders and no history of psychotropic medication use.
Exclusion criteria included other lifetime DSM-IV Axis I disorders including substance abuse or dependence. Participants were excluded for Axis II disorders determined by the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (Spitzer et al., 1992). Additional exclusion criteria included: history of psychosis, acute suicidality, use of illicit substances in the last month, ECT in the last 6 months, a family history of bipolar disorder, any unstable medical condition, or any history of neurological illness or head injury. Both the Duke University Medical Center Institutional Review Board and the Vanderbilt University Institutional Review Board approved this study. All study participants provided informed consent.
Clinical Assessment and Neuropsychological Testing
Participants provided demographic data through a structured interview. A study psychiatrist assessed depression severity with the MADRS and participants completed the Beck Depression Inventory (BDI). Participants then completed a battery of neuropsychological tests that covered cognitive domains relevant to depression. The battery was administered by a trained psychometric technician supervised by a clinical neuropsychologist.
As previously described (Taylor et al., 2017), we created rationally constructed composite domain variables from a broad test battery. To combine tasks, we created z-scores for each measure based on the performance of all participants and averaged the z-scores for all tests within each domain for each individual. Internal consistency for each domain was assessed using Cronbach’s coefficient alpha (CoA). This resulted in four composite neuropsychological measures: a) episodic memory (Logical Memory 1 and 2; Benton Visual Retention Test, number correct; Rey’s Verbal Learning Test, total I-V and total VII, CoA = 0.87); b) executive function (Controlled Oral Word Association [COWA] test (total score); Trail Making B time (reverse scored time to completion); semantic fluency (Animal Naming); Stroop Color-Word interference condition (number completed); CoA = 0.75); c) processing speed (Symbol-Digit Modality (number completed); Trail Making A (reverse scored time to completion); Stroop Color Naming condition (verbal, number completed); CoA = 0.70); and d) working memory (Digit Span forward (number of trials correctly completed); Digit Span backward (number of trials correctly completed); CoA = 0.75).
MRI Acquisition
Participants were scanned on a Siemens 3.0 Tesla Trio Tim scanner, with an 8 channel head coil. All participants received the following MR sequences:
T1-weighted 3D Magnetization-Prepared Rapid Gradient-Echo (MPRAGE) sequence, repetition time (TR) of 2300 ms, echo time (TE) of 3.46 ms, a flip angle of 9°, a 256 × 256 matrix, FOV 240 mm, 160 slices with a 1.2 mm slice thickness for voxel size of 0.9 × 0.9 × 1.2mm.
Echoplanar Blood Oxygenation Level Dependent (EpiBOLD) functional resting-state scan with transverse orientation, TR 2000 ms, TE 27 ms, 32 axial slices with voxel size 4.0 × 4.0 × 4.0mm.
Resting State Functional Connectivity
Images from the resting scan were preprocessed using the Conn toolbox (version 15.g) in SPM 12 including: realignment of the functional runs and correction for bulk-head motion (using artifact detection tools (ART) with thresholds for motion = 0.9mm and global signal z = 5, no participants were removed for excessive motion) , coregistration of functional and anatomical images for each participant, segmentation of the anatomical image, normalization of the anatomical and functional images to the standard MNI template, and spatial smoothing with a Gaussian filter (6 mm at full width half maximum). Denoising in Conn was conducted for white matter and cerebral spinal fluid signal, and realignment parameters. The resulting BOLD time series were band-pass filtered (0.01–0.1 Hz) to further reduce noise and increase sensitivity.
We created first-level whole brain seed-to-voxel individual subject functional connectivity maps for each seed (6mm sphere) correlated with cognitive domain scores for the executive control (bilateral dorsolateral prefrontal cortex (dlPFC) seeds(Seeley et al., 2007)), default mode (posterior cingulate cortex (PCC) seed(Vincent et al., 2008)), and salience networks (bilateral anterior insula seeds(Seeley et al., 2007)). In an exploratory analysis we also examined bilateral dorsal anterior cingulate cortex (dACC) seeds for the salience network (Seeley et al., 2007). Second-level ANCOVAs were completed for the difference between the MDD and Non-depressed groups in connectivity correlated with each cognitive domain score (fisher-transformed correlation coefficients). ANCOVAs were controlled for age, sex, and education and corrected for multiple comparisons with uncorrected p < 0.001, FDR = 0.05. Beta values for significant clusters were examined separately for each group to determine the strength and direction of correlation with cognitive domain score in the MDD and Non-Depressed groups.
Results
Fifty-three participants were enrolled and completed resting state fMRI. Two outliers (one from each diagnostic group) were removed for low cognitive performance scores (below 3X the interquartile range for scores) resulting in 51 participants’ data included in the current analysis (MDD n = 23, Non-Depressed n = 28). There were no significant differences between the MDD and Non-Depressed groups in age, sex, or education; however, the MDD group had significantly higher MADRS and BDI scores (Table 1). In contrast to findings from the larger sample(Albert et al., 2018), there were no significant group differences in this resting state MRI sub-sample for mean cognitive performance in any of the domains (Table 1).
Table 1.
| Control (n=28) Mean (SD) | MDD (n=23) Mean (SD) | t-test | |
|---|---|---|---|
| Age(years) | 31.86 (10.19) | 34.13 (9.67) | t(49) = −0.801, p = 0.421 |
| Sex (% female) | 67% | 57% | t(49) = 0.822, p = 0.415 |
| Education (years) | 15.53 (1.91) | 16.13 (1.87) | t(49) = −1.665, p= 0.269 |
| Beck Depression Inventory (BDI) | 1.84 (2.75) | 21.26 (11.04) | t(49) = −8.674, p <0.001 |
| Montgomery-Asberg Depression Rating Scale (MADRS) | 0.61 (0.99) | 24.45 (3.95) | t(49) = −30.817, p< 0.001 |
| Processing Speed Domain (z score) | 0.35 (0.67) | 0.11 (0.64) | t(49) = 1.287, p = 0.204 |
| Working Memory Domain (z score) | 0.01 (0.86) | −0.05 (0.81) | t(49) = 0.220, p = 0.826 |
| Episodic Memory Domain (z score) | 0.17 (0.63) | −0.07 (0.76) | t(49) = 1.273, p = 0.209 |
| Executive Function Domain (z score) | 0.15 (0.56) | 0.13 (0.65) | t(49) = 0.088, p = 0.930 |
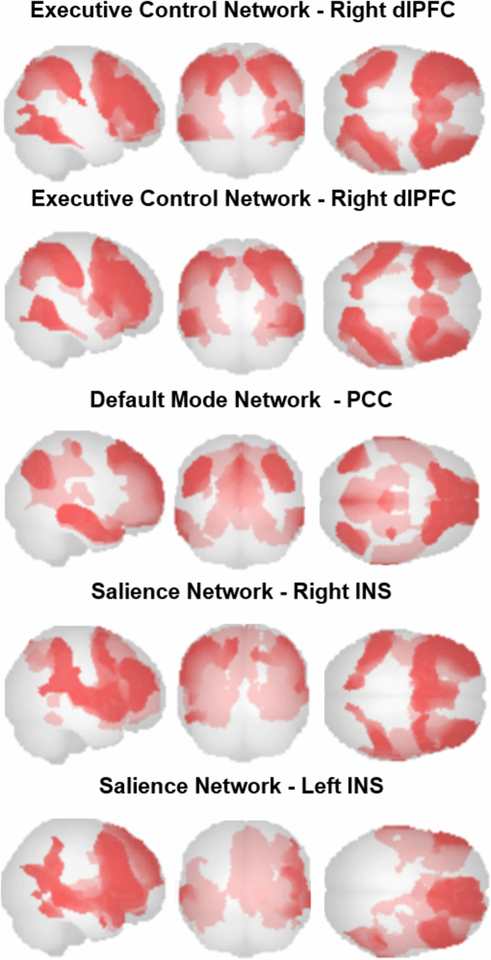
Resting-state functional connectivity analysis of the ECN using dlPFC seeds resulted in positive connectivity maps that included middle frontal, inferior parietal, and inferior temporal regions (Table 2, Figure 1). Analysis for the DMN using the PCC seed resulted in a positive connectivity map that included left precuenus, medial frontal, angular gyrus, middle occipital and precentral regions (Table 2, Figure 1). Analysis for the salience network using INS seeds resulted in positive connectivity maps that included frontal orbital, inferior temporal, caudate, and middle cingulum regions (Table 2, Figure 1). After correcting for multiple comparisons, there were no significant diagnostic group differences in functional connectivity in any of the network analyses.
Table 2.
| MNI x, y, z | Cluster Size (voxels) | Peak | |
|---|---|---|---|
| Executive Control Network | |||
| Right dIPFC | 46,38,18 | 25861 | right middle frontal |
| 42,−46,44 | 5741 | right inferior parietal | |
| −38,−50,46 | 5502 | left inferior parietal | |
| −54,−56,−18 | 2761 | left inferior temporal | |
| 60,−56,−18 | 1849 | right inferior temporal | |
| Left dIPFC | −42,34,20 | 28481 | left inferior frontal |
| −50,−40,46 | 6436 | left inferior parietal | |
| −56,−56,−14 | 2666 | left inferior temporal | |
| 56,−56,−12 | 1883 | right inferior temporal | |
| Default Mode Network | |||
| PCC | 00,−54,18 | 20270 | left precuneus |
| 00,62,20 | 15548 | left superior medial frontal | |
| −44,−70,34 | 2471 | left angular gyrus | |
| 50,−64,32 | 2221 | right angular gyrus | |
| 36,−24,16 | 188 | right middle occipital | |
| −34,−26,64 | 182 | left precentral | |
| Salience Network | |||
| Right INS | 36,26,−08 | 45273 | right frontal orbital |
| 56,−50,−22 | 348 | right inferior temporal | |
| Left INS | −32,26,−14 | 27474 | left frontal orbital |
| 32,28,−14 | 8272 | right frontal orbital | |
| 14,08,06 | 466 | right caudate | |
| −04,−02,32 | 306 | left middle cingulum | |
dlPFC: dorsolateral prefrontal cortex, PCC: posterior cingulate cortex
Figure 1:
Resting functional connectivity patterns in all participants, using the dorsolateral prefrontal cortex (dlPFC) seed for the executive control network, the posterior cingulate cortex (PCC) seed for the default mode network, and the, insula (INS) seed for the salience network.
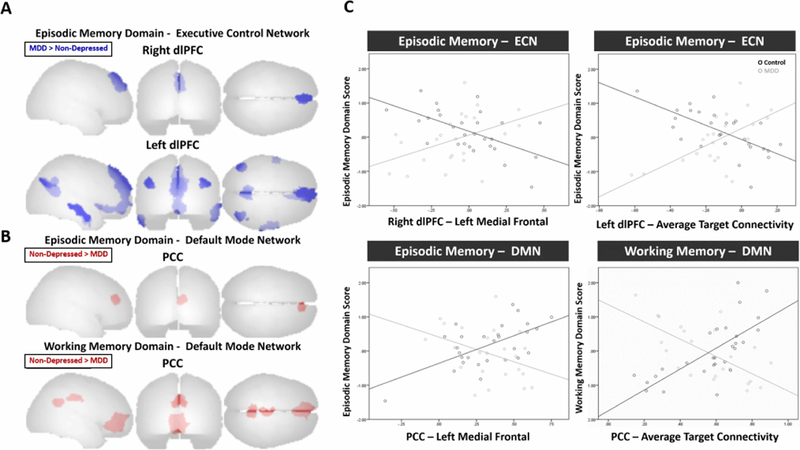
We next examined the relationship between resting functional connectivity and cognitive domain performance in each group, controlling for age, sex and education. We observed group differences in functional connectivity in the executive control and default mode networks related to episodic and working memory performance (Table 3, Figure 2). For episodic memory, greater resting functional connectivity between the right dlPFc and medial frontal regions was associated with poorer episodic memory performance only in the Non-Depressed group, while the inverse pattern was seen in the MDD group (Figure 2 - A, C). Similarly, the Non-Depressed group showed a negative relationship between episodic memory performance and resting functional connectivity between the left dlPFC and other regions in the executive control network and default mode network (medial frontal, precuneus, right middle occipital, left angular, left middle temporal, and right inferior temporal) (Figure 2 - A, C). In the Non-Depressed group, this was reversed, with a positive relationship between episodic memory performance and left dlPFC connectivity with these regions (except the left middle temporal gyrus) (Figure 2 - C).
Table 3.
| MNI x, y, z | Region | Control (n=28) | MDD (n=23) | |
|---|---|---|---|---|
|
Episodic Memory Domain Executive Control Network | ||||
| r = −0.580 , p = | r = 0.429, p = | |||
| Right dIPFC | 02, 54, 36 | Left Medial Frontal | 0.001 | 0.041 |
| r = −0.571, p = | r = 0.506, p = | |||
| Left dIPFC | −02, 64, 24 | Left Medial Frontal | 0.001 | 0.014 |
| r = −0.592, p = | r = 0.495, p = | |||
| 06, −56, 18 | Right Precuneus | 0.001 | 0.016 | |
| Left Middle | r = −0.656, p < | r = 0.236, p = | ||
| −58, −14, −14 | Temporal | 0.001 | 0.278 | |
| Right Middle | r = −0.550, p = | r = 0.584, p = | ||
| 46, −66, 28 | Occipital | 0.002 | 0.003 | |
| r = −0.483, p = | r = 0.557, p = | |||
| −38, −64, 28 | Left Angular | 0.009 | 0.006 | |
| Right Inferior | r = −0.474, p = | r = 0.583, p = | ||
| 62, −06, −30 | Temporal | 0.011 | 0.003 | |
|
Episodic Memory Domain Default Mode Network | ||||
| r = 0.607, p = | r = −0.385, p = | |||
| PCC | 02, 54, 36 | Left Medial Frontal | 0.001 | 0.070 |
|
Working Memory Domain Default Mode Network | ||||
| r = 0.638, p < | r = −0.659, p < | |||
| PCC | 08, 50, −02 | Right Medial Orbital | 0.001 | 0.001 |
| Left Posterior | r = 0.616, p < | r = −0.511, p = | ||
| 02, −48, 30 | Cingulum | 0.001 | 0.013 | |
| Left Middle | r = 0.607, p < | r = −0.616, p = | ||
| −04, −20, 38 | Cingulum | 0.001 | 0.002 | |
dlPFC: dorsolateral prefrontal cortex, PCC: posterior cingulate cortex
Figure 2:
A) Resting functional connectivity patterns associated with better cognitive performance (episodic memory) in participants with major depressive disorder (MDD) compared to non-depressed participants using the dorsolateral prefrontal cortex (dlPFC) seed for the executive control network. B) Resting functional connectivity patterns associated with better cognitive performance (episodic and working memory) in non-depressed participants compared to participants with major depressive disorder (MDD) using the posterior cingulate cortex (PCC) seed for the default mode network.
C) Functional connectivity for bilateral dlPFC and PCC and cognitive domain scores (episodic and working memory) in the MDD and Non-Depressed groups.
For the DMN PCC seed, the Non-Depressed group exhibited positive relationships between episodic memory performance and resting functional connectivity (Figure 2 - B). Specifically, the Non-Depressed group had a positive association between episodic memory and resting functional connectivity between the PCC and medial frontal regions (Figure 2 - C). These relationships were not present in the MDD group. The Non-Depressed group also showed positive relationships between working memory and connectivity of the PCC with medial orbital regions, and middle and posterior cingulum regions. The depressed group exhibited inverse relationships between working memory and PCC connectivity, wherein greater DMN functional connectivity was associated with poorer working memory performance in the MDD group (Figure 2 - C).
There were no significant differences in salience network resting functional connectivity related to cognitive domain performance between the two groups for either the INS or dACC seeds.
DISCUSSION
The primary findings of this study were that, when compared with non-depressed adults, depressed adults exhibited different associations between cognitive performance and functional connectivity in the executive control and default mode networks. In this sample, there were no significant differences in cognitive domain performance between MDD and Non-Depressed groups. However, greater positive connectivity within the ECN and between ECN and DMN regions was associated with poorer episodic memory performance in the Non-Depressed group but better performance in the MDD group. Greater connectivity within the DMN was associated with better episodic and working memory performance in the Non-Depressed group but worse performance in the MDD group.
We did not observe differences in network functional connectivity (not related to cognitive domain performance) between diagnostic groups. This is in contrast to previous findings of resting-state network connectivity alterations in MDD(Mulders et al., 2015) but may be due to methodological differences between the current and previous studies. Differences in seed placement (such as the choice of posterior PCC rather than anterior PCC (Sheline et al., 2010)) may alter functional connectivity findings or obscure findings that are only seen in MDD. Additionally, differences in methods of correction for multiple comparisons in the resting-state fMRI analysis may affect results. The current study included a relatively conservative uncorrected p value (<0.001) with FDR correction to p = 0.05 compared to previous studies that used uncorrected p values < 0.05 or p < 0.01 (for example see: Greicius et al. 2007 (Greicius et al., 2007), Zhou et al. 2010(Zhou et al., 2010) , Manoliu et al. 2014(Manoliu et al., 2014)). This may have limited the findings in the group comparisons.
The observed group differences in the relationships between connectivity patterns and cognitive performance may represent adaptive changes in MDD. Similar to the inverse associations observed here between diagnostic groups, greater positive connectivity between the ECN and DMN has been associated with worse memory performance in young and middle-aged adults, but associated with better memory performance in older adults (Fjell et al., 2015) and with increased cognitive load (such as greater number of items to be successfully remembered) (Liang et al., 2016). Greater positive connectivity between the ECN and DMN may be a marker of network inefficiency in healthy adults but serve as a compensatory mechanism in the context of changes in other brain networks related to aging or MDD(Sheline et al., 2010). In MDD, greater ECN connectivity may additionally be required to regulate the increased DMN activity seen in MDD and maintain cognitive function (Sheline et al., 2010). During response to emotional stimuli, individuals with MDD show less DMN activity than non-depressed but greater activity during rest (Hamilton et al., 2013). Additionally, greater dlPFC and dorsal anterior cingulate activity during a working memory task is associated with better performance in depressed individuals but not in non-depressed individuals (Harvey et al., 2005). This suggests that while DMN activity is increased in MDD, there may be a compensatory regulation of DMN activity during task-directed behavior. The current results support this as the negative association between greater DMN connectivity in cingulate regions and memory performance in the MDD group may reflect enhanced DMN activity (greater cognitive focus on internal emotional processes than task-related stimuli) that interferes with cognitive performance in depressed individuals (Leech and Sharp, 2014).
In MDD, greater regulation of DMN activity during tasks may be needed to maintain cognitive performance. We found that greater functional connectivity between DMN and ECN is associated with better memory performance, but only in MDD. Greater positive functional connectivity between the ECN and DMN at rest may reflect efforts to engage in stronger regulatory control by the ECN over DMN activity or concurrent ECN activity that controls cognitive processes or behavioral response down-stream of the DMN. In this study, ECN functional connectivity exhibited opposite patterns with memory performance between diagnostic groups, suggesting that the depressed individuals did not benefit cognitively from connectivity patterns that were closer to those of non-depressed controls. The absence of group differences in overall functional connectivity support that the altered relationships between cognitive performance and connectivity seen in MDD are not due to differential connectivity patterns. Other functional brain changes in MDD (such as increased DMN activity) may alter cognitive processing such that functional connectivity supporting cognitive performance in non-depressed individuals is not supportive in the context of these changes. In MDD, alternative connectivity patterns may develop to compensate for maladaptive or inefficient patterns and approaches that target restoring normal connectivity patterns may have negative cognitive effects.
ECN functional imaging results in MDD have been conflicting, generally finding decreased task-based activity and increased resting-state functional connectivity(Mulders et al., 2015). Sheline and colleagues suggest that the increased and variable functional connectivity at rest may limit the range for task-based activity fluctuations because of overall greater ECN activity (Sheline et al., 2010). Our results support that greater ECN functional connectivity is associated with better memory performance which may accord with greater ECN activity (Harvey et al., 2005). However, in a meta-analysis of functional imaging studies in depression, Diener and colleagues found that in depressed individuals greater resting state connectivity was associated with reduced ECN task-based activity (Diener et al., 2012). Future work that combines task-based activity and functional connectivity analyses would help clarify whether greater resting connectivity in the ECN is concurrent with reduced task-based activity changes and better characterize reciprocal activity between the ECN and DMN during cognitive tasks in depressed and non-depressed individuals.
This study includes a cross-sectional examination of currently depressed individuals which precludes addressing whether differences in functional brain connectivity related to cognitive performance precede the onset of depression or remain during remission. Thus, we cannot conclude whether altered functional connectivity is a trait vulnerability factor for depression or related specifically to depressive episodes and which may resolve with remission. Additionally, this study did not include assessments of emotional cognition, which may be particularly affected in depression (Joormann and Quinn, 2014) and may better distinguish between depressed and non-depressed individuals. The current examination of resting-state functional connectivity was designed to assess general activity patterns, however this approach may obscure functional connectivity changes that are specific for emotional cognition (Bressler and Menon, 2010). The lack of association between salience network connectivity and cognitive performance in this study may be due to the non-emotional nature of the tasks included in the cognitive domain measures. As the salience network is important for the detection of and attending to emotionally-valenced stimuli, functional connectivity within this network may be better examined during tasks that include emotional stimuli (Kaiser et al., 2015; Sliz and Hayley, 2012).
This study suggests that patterns of functional connectivity within and between the ECN and DMN are differentially associated with cognitive performance between individuals with and without MDD. These findings are in the absence of any statistically significant group difference in network connectivity. The inverse relationship between functional connectivity and cognitive performance between groups provides evidence that altered functional connectivity in MDD may support cognitive performance rather than mark cognitive dysfunction. The distinction between adaptive and maladaptive patterns should be considered when assessing treatment targets and strategies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert K, Gau V, Taylor WD, Newhouse PA, 2017. Attention bias in older women with remitted depression is associated with enhanced amygdala activity and functional connectivity. J. Affect. Disord 210 10.1016/j.jad.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert KM, Potter GG, Mcquoid DR, Taylor WD, 2018. Cognitive performance in antidepressant-free recurrent major depressive disorder. Depress. Anxiety 10.1002/da.22747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Yücel M, Pantelis C, 2013. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol. Med 43, 2017–2026. 10.1017/S0033291712002085 [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V, 2010. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H, 2012. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage 61, 677–685. 10.1016/j.neuroimage.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Fjell AM, Sneve MH, Grydeland H, Storsve AB, de Lange AMG, Amlien IK, Røgeberg OJ, Walhovd KB, 2015. Functional connectivity change across multiple cortical networks relates to episodic memory changes in aging. Neurobiol. Aging 36, 3255–3268. 10.1016/j.neurobiolaging.2015.08.020 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME, 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A 102, 9673–8. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF, 2007. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol. Psychiatry 62, 429–437. 10.1016/j.biopsych.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH, 2013. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiol. Dis 10.1016/j.nbd.2012.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Bagarinao E, Chang C, Mackey S, Sacchet MD, Gotlib IH, 2016. Effects of salience-network-node neurofeedback training on affective biases in major depressive disorder. Psychiatry Res. - Neuroimaging. 10.1016/j.pscychresns.2016.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, LeBastard G, Lehéricy S, Allilaire JF, Dubois B, 2005. Cognitive control and brain resources in major depression: An fMRI study using the n-back task. Neuroimage. 10.1016/j.neuroimage.2005.02.048 [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Kessing LV, 2011. Cognitive impairment in the remitted state of unipolar depressive disorder: A systematic review. J. Affect. Disord 10.1016/j.jad.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Joormann J, Quinn ME, 2014. Cognitive processes and emotion regulation in depression, in: Depression and Anxiety. pp. 308–315. 10.1002/da.22264 [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-Scale Network Dysfunction in Major Depressive Disorder. JAMA Psychiatry. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ, 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y, 2016. Topologically Reorganized Connectivity Architecture of Default-Mode, Executive-Control, and Salience Networks across Working Memory Task Loads. Cereb. Cortex 26, 1501–11. 10.1093/cercor/bhu316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthöffer D, Zimmer C, Förstl H, Bäuml J, Riedl V, Wohlschläger AM, Sorg C, 2014. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci 7, 930 10.3389/fnhum.2013.00930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I, 2015. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, Blackwell AD, 2014. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med 44, 2029–40. 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10, in: Journal of Clinical Psychiatry. pp. 22–33. 10.1016/S0924-9338(99)80239-9 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun M.a, Wang S, Coalson RS, Raichle ME, 2009. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A 106, 1942–7. 10.1073/pnas.0812686106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA, 2010. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci 107, 11020–11025. 10.1073/pnas.1000446107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliz D, Hayley S, 2012. Major Depressive Disorder and Alterations in Insular Cortical Activity: A Review of Current Functional Magnetic Imaging Research. Front. Hum. Neurosci 6 10.3389/fnhum.2012.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB, 1992. The Structured Clinical Interview for DSM-III-R (SCID) - History, rationale, and description. Arch. Gen. Psychiatry 49, 624–629. 10.1001/archpsyc.1992.01820080032005 [DOI] [PubMed] [Google Scholar]
- Taylor WD, Boyd B, Turner R, McQuoid DR, Ashley-Koch A, MacFall JR, Saleh A, Potter GG, 2017. APOE ε4 associated with preserved executive function performance and maintenance of temporal and cingulate brain volumes in younger adults. Brain Imaging Behav. 11, 194–204. 10.1007/s11682-016-9522-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL, 2008. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J. Neurophysiol 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, Li K, Jiang T, 2010. Increased neural resources recruitment in the intrinsic organization in major depression. J. Affect. Disord 121, 220–230. 10.1016/j.jad.2009.05.029 [DOI] [PubMed] [Google Scholar]