Abstract
Until recently, it was assumed that the only interaction between muscle and bone is mechanical, that the muscle acts as a pulley and the bone as a lever to move the organism. A relatively new concept is that muscle, especially contracted muscle, acts as a secretory organ, regulating metabolism. An even newer concept is that bone, especially the osteocytes in bone, act as endocrine cells targeting other organs such as kidney and more recently, muscle. These two new concepts logically led to the third concept: that muscle and bone communicate via soluble factors. Crosstalk occurs through muscle factors such as myostatin, irisin, and a muscle metabolite, β-aminoisobutyric acid, BAIBA, and through bone factors such as osteocalcin, transforming growth factor beta, TGFβ, Prostaglandin E2, PGE2 and Wnts. Some of these factors have positive and some negative effects on the opposing tissue. One feature both bone and muscle have in common is that their tissues are mechanically loaded and many of their secreted factors are regulated by load. This mechanical loading, also known as exercise, has beneficial effects on many systems leading to the hypothesis that muscle and bone factors can be responsible for the beneficial effects of exercise. Many of the characteristics of aging and diseases associated with aging such as sarcopenia and osteoporosis and neurological conditions such as Alzheimer’s disease and dementia, are delayed by exercise. This beneficial effect has been ascribed to increased blood flow increasing oxygen and nutrients, but could also be due to the secretome of the musculoskeletal system as outlined in this review.
Effects of aging on the Musculoskeletal system
Aging has overwhelming effects on bone and skeletal muscle mass. Reduced movement due to increased periods of rest and reduced physical activity most likely explains much of the reduced bone and muscle phenotype in older individuals. About the same time of age-related bone loss, osteoporosis occurs, so does age-related muscle loss, referred to as ‘sarcopenia’. Muscle loss, like bone loss, actually starts soon after age 30, but becomes a rapid, progressive, debilitating condition after age 60. It is projected that in 2050, 20% of the world’s population over 60 will suffer from sarcopenia and by 2150, this percentage will increase to 33% of the population[1]. Sarcopenia is associated with metabolic abnormalities, including changes in insulin sensitivity, increased fat and connective tissue infiltration in the skeletal muscle known as myosteatosis, reduced hormone levels and impaired oxidative defenses due to decreased mitochondrial activity. With aging, the number of muscle satellite cells decreases, resulting in lower capacity for muscle regeneration and neurodegeneration contributes to impaired contractile function and reduced muscle strength[1–3].
Aging-associated osteoporosis and sarcopenia
Sarcopenia and osteoporosis are often present in the same patients[4]. It is unclear whether one condition precedes the other or if the conditions are linked. The mechanical perspective implies that as muscle function declines, as with sarcopenia or cachexia, this would result in decreased loading of the skeleton leading to a decrease in bone mass. The mechanical perspective postulates that muscle weakness comes first before bone loss. However, patients exist with low bone mass before a diagnosis and sometimes low bone mass patients never receive a diagnosis of sarcopenia. Taken together, reduced regenerative capacity could be a shared mechanism for sarcopenia and osteoporosis. However, muscle atrophy alone cannot fully explain the totality of osteoporosis and, reciprocally, aging associated decreases in bone mass do not fully explain sarcopenia.
Aging-associated osteoporosis frequently coexists with sarcopenia or cachexia, creating a downward spiral between abnormal muscle and bone that contributes to the significant worsening of the quality of life and to shorter survival[5]. Sarcopenia is normally defined as age-associated decrease in muscle mass and function, whereas cachexia is defined as inflammatory mediated loss of muscle and fat. Both are wasting disorders. Despite significant mechanistic differences, sarcopenia is also frequently related to cachexia, a condition that severely impacts quality of life in patients affected with chronic diseases. Cachexia is weight loss generally due to disease. Frequently diseases associated with aging can result is muscle loss that may be cachexia or a combination with sarcopenia[6].
Sarcopenia becomes clinically evident when the ratio between appendicular skeletal muscle mass and height reaches two or more standard deviations below the value in young individuals of the same sex and ethnic background. It appears that about 50% of men and 40% of women over 80 years of age are affected by sarcopenia, with Hispanic men and women showing relatively higher rates. Lower birth weight is associated with reduced muscle mass and strength in adults[7]. Sarcopenia appears more evident in men than women which may be due to hormonal factors and genetic components.
The diagnosis and treatment of this sarcopenia can be complicated by disease-associated changes in body composition and by obesity. Fat mass may obscure body weight loss, which is normally a reflection of changes in muscle mass. This condition, known as ‘sarcopenic obesity’, has been described in conditions such as malignancy, rheumatoid arthritis, and aging. Sarcopenic obesity is primarily characterized by loss of lean body mass concurrent with preservation or even increase of fat mass[8].
Sarcopenia can follow a sedentary lifestyle, neuromuscular deficits, abnormal endocrine and hormonal function, disease, or trauma. Loss of muscle mass is due to an imbalance between protein synthesis and protein degradation, with degradation the predominant component. Insulin or IGF-1 deficits, malnutrition, reduced physical activity, bed rest, reduced sex hormones, and chronic disease have been linked to enhanced protein degradation leading to decreased muscle mass. The sex hormones estrogen and testosterone may directly affect muscle function and may indirectly affect muscle mass through their inhibitory effects on pro-inflammatory and pro-catabolic cytokines, such as IL-1 and IL-6.
Experiments using satellite cell transplantation or parabioses between young and old mice animals shows a rejuvenation of the satellite cell pools in aged mice demonstrating the positive effects of factors from young animals suggesting that aged animals no longer make these factors in sufficient quantities to protect muscle mass[9]. Though normally an essential process involved in the turnover of cellular components, enhanced or exagerated autophagy may also lead to increased muscle protein turnover responsible for sarcopenia.
Several approaches are being taken to date to reduce sarcopenia including resistance exercise, targeting hormonal changes, and nutritional supplementation[7]. As androgen signaling restores testosterone levels with improvement in muscle mass, strength and functional status, nonsteroidal selective androgen receptor modulators (SARMs), are being tested[10]. Nutritional supplementation has been controversial as some studies have reported beneficial effects of high protein intake, but others have reported unchanged synthesis rates. Creatine supplementation, especially in combination with exercise, has been shown to positively affect muscle size and function and supplementation with branched-chain amino acids (BCAAs) has been reported to increase the overall nitrogen balance, despite unchanged protein synthesis rates. Clearly more studies are needed to determine the consequences of nutritional supplementation on muscle mass in sarcopenia[11]. It would be important to conduct studies to determine if maintaining bone mass can also positively affect muscle mass.
Dogma: Only mechanical interactions occur between muscle and bone.
Until recently, it was assumed that the only interaction between muscle and bone was mechanical. Skeletal muscles attach to bone and contraction is responsible for movement of the bone and therefore locomotion by the organism. Muscle is attached to bone close to the axes of motion, generating small lever arms requiring large muscle forces to produce the motion-required torque (Figure 1). Forces generated by muscle are the source of mechanical loading that generates the strain in bone. Other support for the concept of a mechanical interaction between bone and muscle is that depletion of skeletal muscle mass also seems to trigger bone loss due to the unloading of bone. Mice paralyzed due to muscular dysgenesis in utero have bones but their shapes are abnormal. Individuals affected with muscular dystrophy also show reduced bone mass and enhanced bone frailty. It is well documented that muscle paralysis exacerbates bone loss and osteoporosis. Similarly, muscle atrophy due to spinal cord injury, motorneuron loss, immobilization, or absence of gravity as in space flight is also associated with precipitous bone loss.
Figure 1.
Not only mechanical, but also biochemical signaling is important for optimal functioning of the musculoskeletal system. Though the mechanical interaction between bone and muscle (the muscle acting as a pulley and the bone as a lever) has long been recognized, a new area of research focuses on molecular signaling between these two tissues. The combination of mechanical loading and positive biochemical signals are synergistic, providing more potent effects than either stimuli alone.
However, there are several lines of evidence that support the concept that muscle and bone interact beyond the mechanical. For example, muscle flaps appear to accelerate bone healing after injury[12], muscle and bone development are tightly linked, genes have been identified that affect both bone and muscle mass, and the twin diseases of osteoporosis and sarcopenia appear linked. New data suggests that bone and muscle are also linked through biochemical communication through the musculoskeletal secretome (Figure 1).
Bone and Muscle are linked through development and transdifferentiation.
During intrauterine development, bone and muscle cells share a common mesenchymal precursor and bone and muscle experience organogenesis through tightly orchestrated gene activation and inactivation so that bone and muscle develop synchronously[13]. In the adult animal, myoblasts maintain the capacity to transdifferentiate into osteogenic cells. For example, in fracture healing, satellite cells from muscle can transdifferentiate into chondrocytes and osteoblasts. In vitro, the cell line C2C12 retains the capacity to differentiate into either muscle or bone cells depending on culture conditions. Whether osteoblasts or osteocytes, thought of as terminally differentiated cells, can differentiate into muscle has not been shown[14]. Both tissues reach their peak tissue mass at the same time and both tissues start to lose mass at about the same age.
Pleiotropic genes for bone and muscle.
Evidence exists for genes that regulate both muscle and bone called ‘pleiotropic’genes[15]. Bivariate genome wide association studies (GWAS) have been used to identify pleiotropic candidate genes/SNPs/regions associated with traits in both bone and muscle, ie.; total-body lean mass and total-body less head bone mineral density[16]. Studies using over 10,000 children identified variants with pleiotropic effects in eight loci, seven of which are well known bone mineral density loci: WNT4, GALNT3, MEPE, CPED1/WNT16, TNFSF11, RIN3, and PPP6R3/LRP5. These variants gave a positive correlation between lean mass and bone mineral density, however, one did not. Variants in the TOM1L2/SREBF1 locus exert opposing effects on total body lean mass and bone mineral density suggesting a role in increasing muscle while decreasing bone.
Another potentially pleiotropic gene has been identified, METTL21C, a member of the methyltransferase superfamily. In vitro studies using cell lines, showed that Mettl21c signaling was linked to the NF-kB signaling pathway[17]. Down-regulation of this gene in C2C12 muscle cells reduced myogenic differentiation and myotube cell area in addition to reducing calcium release from the sarcoplasmic reticulum. Down regulation in MLO-Y4 osteocyte cells made the cells more susceptible to dexamethasone induced cell death. Another gene identified in these studies was MEF2C, a gene that encodes a transcription factor (myocyte enhancer factor 2C) shown to be involved in cardiac and skeletal muscle development[18]. Deletion of Mef2C in osteocytes results in mice with an increased bone density[19]. Therefore Mef2c plays critical functions in both bone and muscle. These studies show that shared genetic determinants and commonly shared signaling pathways are operational in both muscle and bone.
Several mutations not thought of as pleiotropic genes are now known to have effects on both tissues. Well known bone diseases caused by mutations in bone have such as Osteogenesis imperfecta (OI) also been shown to have muscle defects [20]. This bone disorder is caused by mutations in collagen and its processing enzymes leading to either a deficiency or mutated form of collagen. In a number of OI mouse models, therapeutics known to target bone such as anti-sclerostin antibody, anti-TGFβ antibody, ACVR2BFc, a soluble ACVR2B fusion protein and inhibitor of the receptor downstream signaling activin receptor type 2B (ACVR2B), and bisphosphonates in addition to showing an improvement in bone, also show an improvement in muscle[21]. The effects on muscle may be indirect through the bone, but direct effects of these therapeutics on muscle cannot be ruled out.
New Concept: Muscle as a secretory endocrine organ.
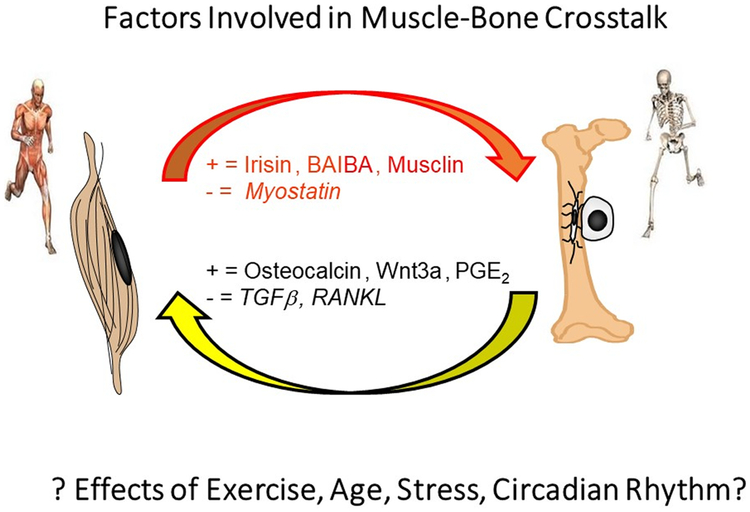
Pederson and colleagues in 2010 first described muscle as a secretory organ and called the muscle secreted factors ‘myokines’[22]. Muscle factors include myostatin, leukemia inhibitory factor (LIF), Insulin-like growth factor I (IGF-1), fibroblast growth factor 2 (FGF2), follistatin-like protein 1, brain-derived neutrophic factor (BDNF), irisin, a potent regulator of the conversion of white fat into brown fat, IL-8 that stimulates angiogenesis, and IL-15, a muscle factor that reduces adiposity[23]. In addition to secreted proteins, a metabolite, beta-aminoisobutyric acid, BAIBA, has been shown to be an osteocyte protective factor preventing bone loss due to immobilization[24]. Many of these factors have an effect, positive or negative, on bone (See Table 1) as described below.
Table 1.
Muscle to Bone Crosstalk
| Myostatin | Deletion results in animals with enlarged muscles and larger bones. Inhibitor of osteoblast differentiation and promoter of osteoclast activation. | Buerhing, 2013[73] |
| Irisin | May support or inhibit bone formation | Kim 2018 [36] |
| β-aminoisobutyric acid, BAIBA | Prevents osteocyte cell death, preserves bone and muscle | Kitase, 2018[24] |
| Brain-Derived Neurotrophic Factor, BDNF | Regulates VEGF secretion by osteoblasts | Zhang, 2017 [74] |
| Interleukin 6, Il-6 | Increases osteoclasts | Kurihara 1990[75] |
| IL-15 | Supports osteoblastic matrix formation | Loro, 2017[76] |
| Il-7 | promotes osteoclastogenesis | Weitzmann 2000[77] |
Myostatin is a negative regulator of bone as well as a negative regulator of muscle. It was the first myokine to be identified[25]. It is also known as growth and differentiation factor (GDF)-8, a member of the TGF-β superfamily and is highly expressed in pathologic conditions associated with muscle atrophy. As a negative regulator of bone, it was shown that myostatin directly regulates bone remodeling by stimulating the recruitment and differentiation of osteoclasts. Global deletion of myostatin resulted in the “Mighty Mouse” showing impressive muscle hypertrophy. Both animals and humans with deletion or low myostatin show dramatically enhanced muscle mass, reduced adiposity and increased insulin sensitivity, but surprisingly no improvement in muscle function or strength. A significant increase in bone mineral density in myostatin null mice supports the concept of direct biochemical communication[26]. Myostatin inhibitors such as ACVR2B/Fc, a soluble myostatin decoy receptor, has been shown to prevent both muscle and bone loss in models of muscular dystrophy[27], osteogenesis imperfecta[28], as well as cancer- and chemotherapy-induced cachexia[29]. Unfortunately, off target effects have been observed in humans which limits use in its current form[30].
Irisin is both a positive and negative regulator of bone mass depending on amount and hormonal milieu[31]. When the muscle transmembrane protein called Fibronectin type III domain-containing protein 5, FNDC5, is cleaved, the extracellular domain, called irisin, is released into the circulation[32]. It is mainly produced in response to exercise and appears to have autocrine effects on muscle and also has effects on fat metabolism[32]. Findings generated by using mass spectrometry demonstrate that human irisin is regulated by exercise[33]. Irisin appears to play a role as a pro-myogenic factor enhancing skeletal muscle size in experimental models of immobilization using denervation-induced atrophy. Irisin was also shown to modestly increase bone cortical mass in concentrations that have no effects on fat [34]. In humans, irisin was inversely correlated with the incidence of bone fractures in postmenopausal osteoporotic women, and with type 2 diabetes, cardiovascular disease and liver disease[35]. However, recently it has been shown that mice with global deletion of FNDC5 have little detectable phenotype but that with ovariectomy, no bone is lost[36]. This suggests an interaction of irisin with estrogen signaling and a potential negative effect of irisin on bone. The mechanisms behind the complex effects of irisin on bone remain to be identified.
Recently it has been shown that a muscle metabolite can preserve bone mass under conditions of unloading [24]. Beta-aminoisobutyric acid, BAIBA, (103.6Da) is secreted by contracting muscle though the actions of PGC1α. The metabolite has been shown to influence a number of metabolic processes such as activation of the β-oxidation pathway of hepatic fatty acid, the browning of white adipose tissue, improvement of insulin resistance and inflammation in skeletal muscle, reduction of hepatic ER stress and glucose/lipid metabolic disturbance in type 2 diabetes, and also reduction of renal fibrosis in mouse models of obstructed kidney. This muscle metabolite is also inversely correlated with cardiometabolic risks factors.[37, 38] Now to this list of activities has been added the function of an osteocyte protective factor against reactive oxygen species to prevent the loss of bone with hindlimb unloading. BAIBA was shown to be secreted by isolated young and old muscle, but the receptor, Mas-related G-protein receptor Type D, MRGPRD, though highly elevated in young osteocytes was significantly decreased with age, suggesting the aging defect with muscle-bone crosstalk in this model is in the osteocyte and not the muscle[24].
Newer Concept: Bone, specifically through osteocytes, functions as a secretory endocrine organ.
Osteoblasts are factories for the production of collagen and growth factors generating osteoid that mineralizes creating a ‘storehouse’ for factors in the body. The largest source of TGFβ in the body is in the bone along with other growth factors such as insulin-like growth factor (IGF-1) and the bone morphogenetic proteins (BMPs). These growth factors can be released from the bone matrix during repair by osteoclasts especially in response to bone injury[39]. However, it was the disregarded, overlooked osteocyte that became recognized as a true secretory bone cell. In 2006 it was first proposed osteocytes are endocrine cells with the first example of a secreted factor being fibroblast growth factor 23, (FGF23), highly elevated in osteocytes in patients with hypophosphatemic rickets[40]. Through the secretion of this factor, bone targets the kidney to regulate phosphate homeostasis. Since that time osteocytes have been described to produce other circulating factors such as receptor activator of nuclear factor-kappa B ligand (RANKL), sclerostin and small molecules such as PGE2[41]. Considering that the total cellular mass of osteocytes within the skeleton is approximately the same or greater mass as the brain, this is a relatively large source of factors[42]. The list of osteocyte factors continues to grow, and includes: Dickkopf-1 (DKK1), matrix extracellular phosphoglycoprotein (MEPE), osteoprotegerin (OPG), and small molecules adenosine triphosphate (ATP), and Nitric Oxide, in addition to PGE2. So similar to muscle, both proteins and small molecular weight molecules are secreted to regulate local and distant cells. Originally these factors were thought to mainly have effects on bone, but as discussed below, these ‘osteokines’ can also have effects on muscle (see Table 2).
Table 2.
Bone to Muscle Crosstalk
| Factor | Description | Reference |
|---|---|---|
| unknown | Primary osteocytes and MLO-Y4 cells induce C2C12 myoblasts to differentiate into myotubes | (Mo 2012[78]) |
| PGE2 | PGE2 mimics some of the effects of osteocyte secreted factors on myogenesis and muscle function. | (Mo 2012[61]) |
| Wnt3a, Wnt1 | Wnt3a accelerates C2C12 differentitation | Huang, 2017[52] |
| Osteocalcin | Osteocalcin has positive effects on muscle mass and function and is necessary for adaptation to exercise | Shen, 2015[79], Mera, 2016[80] |
| TGFβ | Excess TGFβ released from bone due to breast cancer metastasis is responsible for muscle weakness | Waning 2015[47] |
| TGFβ? | Pamidronate attenuates muscle loss after pediatric burn injury | Borsheim, 2017[81] |
| unknown | Osteocytes produce factors that decrease muscle mass and function with age | Gorski, 2016 [82] |
| RANKL | RANKL inhibits muscle mass and function | Boulanger, 2018; Dufresne, 2018[60, 83] |
Only bone produces osteocalcin, mainly by mature osteoblasts, but also by osteocytes. Osteocalcin has a very high affinity for hydroxyapatite, responsible for its storage in bone, but with decarboxylation due to low pH, can be released into the circulation. Osteocalcin binds to the Gprc6a receptor, affecting distant adipocytes and pancreatic β cells. Osteocalcin appears to have many functions in mice such as regulating glucose metabolism, energy metabolism, fertility, and ectopic calcification, and recently been shown to also have effects in muscle thereby affecting whole organism physiology[43]. The Gprc6a knockout mouse displays the phenotype of decreased muscle mass, while the Esp knockout mouse, a phosphatase that inhibits the function of osteocalcin, has increased muscle mass. Further evidence that osteocalcin is important for muscle mass and function is that supplementation with osteocalcin restores reduced exercise capacity in mice and increases muscle strength. Aerobic exercise increases circulating bioactive osteocalcin levels and induces osteocalcin signaling in muscle leading to myokine IL-6 production[44].
The largest source of TGFβ is in bone. This factor mainly appears to be produced by bone-forming osteoblasts, targeting the latent TGFβ to the matrix along with collagen. This inactive form must be activated to have biological effects. This is accomplished through low pH such as during osteoclast resorption, and through mechanical stretching.[45] Though long known for its interactions and effects on osteoblasts and osteoclasts, recently it has been shown that TGFβ has profound effects on osteocytes, specifically their capacity to remodel their perilacunar matrix. Lack of TGFβ signaling in osteocytes leads to bone fragility[46]. TGFβ plays a role in bone to muscle communication, as it is the active TGFβ released by breast cancer cells in bone that is responsible for muscle wasting[47].
The first factor to be described as acting as an endocrine factor produced by osteocytes was FGF23. In addition to FGF23, osteocytes regulate phosphate through several non-secreted molecules such as Phosphate Regulating Neutral Endopeptidase on Chromosome X, (Phex) and Dentin Matrix Protein 1, (Dmp1). Both Dmp1 and Phex down regulate FGF23 in osteocytes allowing reabsorption of phosphate by the kidney to maintain sufficient circulating phosphate to maintain normal bone mineral content. In the absence of either Dmp1 or Phex, FGF23 is systemically elevated in the osteocyte, leading to phosphate excretion by the kidney resulting in osteomalacia and rickets. Elevated FGF23 has negative effects on cardiac muscle[48], but appears to have no negative effects on skeletal muscle[49].
The Wnt/β-catenin pathway and mechanosensation/mechanotransduction are intimately connected in osteocytes[50, 51]. Components of this pathway are important regulators of bone mass and important in osteocyte transmission of mechanical loading signals to cells on the bone surface. The Wnt/β-catenin pathway is triggered by crosstalk with the prostaglandin pathway in response to loading. This leads to a decrease in negative regulators of bone formation such as sclerostin and Dkk1 and an increase in positive regulators of bone formation such as the Wnts. Wnt 1, highly expressed in osteocytes and Wnt3a, produced by osteocytes in response to shear stress, will support myogenesis and muscle function[52]. While the Wnts, (there are about nineteen described to date), are thought to mainly act locally, Wnt3a and others can be quantitated in serum. Wnt signaling seems to be involved in the control of the myogenic program and the differentiation of satellite cells by activating the expression of muscle regulatory factors during the early phases of embryogenesis. Wnts play a similar role in the specification of mesenchymal progenitors towards bone precursors during development or in response to loading[53, 54]
There are two major negative regulators of the Wnt/β-catenin signaling pathway, sclerostin and Dkk1 that are the targets of therapeutics[55]. Sclerostin is highly expressed in osteocytes and is a potent inhibitor of osteoblastic bone formation. Recently it has been shown that Dkk1 in bone is mainly secreted by osteoblasts but not osteocytes[56]. Deletion of Dkk1 in osteoblasts and osteocytes results in high bone mass in spite of high levels of circulating sclerostin. This suggests that circulating levels of sclerostin cannot override the effects of local Dkk1. It is not known if these inhibitors of β-catenin signaling have an effect on muscle, but as Wnts affect muscle by supporting myogenesis and muscle function, inhibitors of this pathway most likely will have effects.
Receptor activator of nuclear factor kappa-B ligand (RANKL), also known as tumor necrosis factor ligand superfamily member 11 (TNFSF11), was first identified as a product of immune cells, but has since been shown to be produced by osteocytes to activate osteoclasts [57, 58]. The receptor for RANKL, known as RANK is not only expressed by osteoclasts but also expressed in skeletal muscle [59]. In muscle, RANKL appears to regulate Ca2+ storage and SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase, activity. RANK expression may play a role in weakness in dystrophies as selective genetic deletion in dystrophin deficient mdx mice led to improvement in muscle force as did treating mdx mice with anti-RANKL antibody and truncated osteoprotegerin, soluble RANKL receptor, (OPG)-Fc [60]. These observations raise a number of questions such as what is the source of RANKL that affects muscle, is it the immune cells or osteocytes?
ProstaglandinE2, PGE2 is a potent stimulator of myogenic differentiation in primary myoblasts/myotubes. PGE2 production by osteocytes is more than 100 times greater than PGE2 secretion by muscle cells. PGE2, elevated in response to fluid flow shear stress and released from osteocytes through Connexin 43 hemichannels, was found to enhance myogenesis and ex vivo primary muscle function[61]. Targeted knockdown of connexin 43 in osteoblast/osteocytes not only decreases cortical bone thickness, but also causes a defective muscle phenotype in fast twitch extensor digitorum longus (EDL) muscle [62]. This suggests that a structural protein such as Connexin 43 can regulate both bone and muscle through the same mechanism, the release of prostaglandin and potentially through other small molecules through hemichannels.
Exercise has beneficial effects on many systems.
A healthy musculoskeletal system is essential for the health of the individual and maintaining a certain degree of activity and mobility over time has been shown to delay the effects of aging [63]. For example aerobic, endurance exercises (such as walking and jogging) are able to reduce coronary heart disease risk factors, while heavy resistance training is known to reduce muscle loss, reduce femoral neck fracture while maintaining balance necessary for proper gait and joint stability. Could the beneficial effects of exercise be mediated through secretion of factors by muscle and bone? Could muscle-bone crosstalk be playing a major role?[64, 65] Many of the characteristics and diseases associated with aging, such as dementia, Alzheimer’s disease, sarcopenia, and osteoporosis, are delayed by exercise. These beneficial effects of exercise may be through the secretion of muscle and bone factors. It is most likely not just one or a few factors, but factors working together to delay aging (Figure 2). Eventually though, even exercise, no longer has a beneficial effect. Aging and disease eventually overcome exercise. But, if we understood this cross-talk, we would be closer to preventing and treating the negative effects of aging.
Figure 2.
There are both positive and negative factors secreted by muscle and bone. Contraction of muscle and loading of bone appear to induce the release of positive factors. The muscle factor myostatin not only has negative effects on muscle mass but also on bone. The factors RANKL and TGFβ, released during bone resorption, have negative effects on muscle. At this time, the effects of exercise, age, stress, and circadian rhythm on this crosstalk is unknown providing a fertile area for future investigations.
Circadian Rhythm in Aging Muscle/Bone Communication
The circadian cycle is approximately 24 hrs based on light/dark to prepare the organism for sleeping and for waking activity such as feeding. This rhythm can be disrupted by jetlag, fasting, feeding, stress, and activity. Some hormones such as cortisol are influenced by the circadian clock, but can also depend on other factors such as stress and activity[66]. Scheduled exercise can entrain the circadian clocks in skeletal muscle.
Every cell in the body has a circadian rhythm and muscle and bone are no exception. The circadian rhythm in muscle has been shown to be important not only for muscle function but also for liver metabolism and regulation of glucose[67] as demonstrated when the clock gene Bmal1 was specifically deleted in muscle. Muscle specific rescue of Bmal1 null mice protected mice from decreased activity, body weight, and reduced longevity[68]. As muscle secretes a number of factors, including those that target bone, it is likely that bone tissue is also affected. Bmal1 deletion in osteoblasts resulted in a skeleton with low bone mass due to increased osteoclast numbers, whereas no effects were observed with Bmal1 deletion in osteocytes[69]. Deletion of Bmal1in osteoclasts resulted in mice with increased bone [70]. Neither study looked at effects on metabolism or muscle.
Bone formation and bone resorption demonstrate circadian rhythmicity. CTX, C-terminal cross-linked telopeptide of type 1 collagen, has been shown to have a circadian rhythm similar to osteocalcin with a low point in the afternoon and high point at night. No distinct rhythms were observed for PINP, sclerostin or Dkk1[71]. The circadian clock becomes less synchronous with age. Aged humans become more sensitive to sleep and circadian rhythm creating a condition call SCR frailty. This appears due to a lack of day/night contrast, reduced sensitivity to light, napping and a sedentary lifestyle[72]. As resistance exercise can enhance muscle chronicity in mice, it would be important to determine if this would also work in humans (Figure 2).
Summary
A close relationship exists between bone and muscle from embryogenesis, through growth and development, and into aging. Throughout life, bone and muscle integrate with each other and work physically and biochemically as one unit. Diseases associated with muscle usually have manifestations in the bone and vice versa. Likewise, aging results in the progressive and parallel loss of bone known as osteopenia and in skeletal muscle known as sarcopenia. The mechanical and biochemical interactions between muscle and bone may work together synergistically. Mechanical force might prime bone and muscle for regulation and release of specific factors to exert their effects on the opposing tissue. Understanding the mechanical and the cellular and molecular mechanisms responsible for biochemical communication between bone and muscle is important as a means to identify potential new therapies that may affect both bone and muscle concurrently in positive ways. Treatments targeting not only one but both tissues simultaneously could revolutionize the treatment of related bone and muscle diseases such as osteoporosis and sarcopenia and other consequences of aging.
Highlights:
The aging of the musculoskeletal system is strongly associated with health or morbidity.
Muscle and bone not only interact mechanically but through secreted biochemical signaling molecules.
The musculoskeletal system secretome most likely has effects on metabolism and function of other organs.
The musculoskeletal system is the mediator of the positive effects of exercise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Ali S, Garcia JM, Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review, Gerontology 60(4) (2014) 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L, Aging and Multimorbidity: New Tasks, Priorities, and Frontiers for Integrated Gerontological and Clinical Research, J Am Med Dir Assoc 16(8) (2015) 640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M, Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review, Front Physiol 3 (2012) 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reginster JY, Beaudart C, Buckinx F, Bruyere O, Osteoporosis and sarcopenia: two diseases or one?, Current opinion in clinical nutrition and metabolic care 19(1) (2016) 31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R, Fi A.T.i.g., Osteosarcopenia is more than sarcopenia and osteopenia alone, Aging clinical and experimental research 28(5) (2016) 895–9. [DOI] [PubMed] [Google Scholar]
- [6].Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC, Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”, Clin Nutr 29(2) (2010) 154–9. [DOI] [PubMed] [Google Scholar]
- [7].McCormick R, Vasilaki A, Age-related changes in skeletal muscle: changes to life-style as a therapy, Biogerontology (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Batsis JA, Villareal DT, Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies, Nat Rev Endocrinol 14(9) (2018) 513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA, Rejuvenation of aged progenitor cells by exposure to a young systemic environment, Nature 433(7027) (2005) 760–4. [DOI] [PubMed] [Google Scholar]
- [10].Shin MJ, Jeon YK, Kim IJ, Testosterone and Sarcopenia, World J Mens Health 36(3) (2018) 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Landi F, Sieber C, Fielding RA, Rolland Y, Guralnik J, Nutritional Intervention in Sarcopenia: Report from the International Conference on Frailty and Sarcopenia Research Task Force, J Frailty Aging 7(4) (2018) 247–252. [DOI] [PubMed] [Google Scholar]
- [12].Liu R, Schindeler A, Little DG, The potential role of muscle in bone repair, J Musculoskelet Neuronal Interact 10(1) (2010) 71–6. [PubMed] [Google Scholar]
- [13].Land C, Schoenau E, Fetal and postnatal bone development: reviewing the role of mechanical stimuli and nutrition, Best Practice & Research Clinical Endocrinology & Metabolism 22(1) (2008) 107–118. [DOI] [PubMed] [Google Scholar]
- [14].Rauch F, Schoenau E, The developing bone: slave or master of its cells and molecules?, Pediatr Res 50(3) (2001) 309–14. [DOI] [PubMed] [Google Scholar]
- [15].Karasik D, Kiel DP, Evidence for pleiotropic factors in genetics of the musculoskeletal system, Bone 46(5) (2010) 1226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Medina-Gomez C, Kemp JP, Dimou NL, Kreiner E, Chesi A, Zemel BS, Bonnelykke K, Boer CG, Ahluwalia TS, Bisgaard H, Evangelou E, Heppe DHM, Bonewald LF, Gorski JP, Ghanbari M, Demissie S, Duque G, Maurano MT, Kiel DP, Hsu YH, C.J.v.d.E. B, Ackert-Bicknell C, Reppe S, Gautvik KM, Raastad T, Karasik D, van de Peppel J, Jaddoe VWV, Uitterlinden AG, Tobias JH, Grant SFA, Bagos PG, Evans DM, Rivadeneira F, Bivariate genome-wide association meta-analysis of pediatric musculoskeletal traits reveals pleiotropic effects at the SREBF1/TOM1L2 locus, Nature communications 8(1) (2017) 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang J, Hsu YH, Mo C, Abreu E, Kiel DP, Bonewald LF, Brotto M, Karasik D, METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-kappaB signaling pathway, J Bone Miner Res 29(7) (2014) 1531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Edmondson DG, Lyons GE, Martin JF, Olson EN, Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis, Development 120(5) (1994) 1251–63. [DOI] [PubMed] [Google Scholar]
- [19].Kramer I, Baertschi S, Halleux C, Keller H, Kneissel M, Mef2c deletion in osteocytes results in increased bone mass, J Bone Miner Res 27(2) (2012) 360–73. [DOI] [PubMed] [Google Scholar]
- [20].Boot AM, de Coo RF, Pals G, de Muinck Keizer-Schrama SM, Muscle weakness as presenting symptom of osteogenesis imperfecta, Eur J Pediatr 165(6) (2006) 392–4. [DOI] [PubMed] [Google Scholar]
- [21].Phillips CL, Jeong Y, Osteogenesis Imperfecta: Muscle-Bone Interactions when Bi-directionally Compromised, Curr Osteoporos Rep (2018). [DOI] [PubMed] [Google Scholar]
- [22].Pedersen BK, Muscles and their myokines, J Exp Biol 214(Pt 2) (2011) 337–46. [DOI] [PubMed] [Google Scholar]
- [23].Hamrick MW, The skeletal muscle secretome: an emerging player in muscle-bone crosstalk, Bonekey Rep 1 (2012) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kitase Y, Vallejo J, Guthiel W, Vemula H, Jähn K, Yi J, Zhou J, Brotto M, Bonewald LF, Beta-aminoisobutyric acid, BAIBA, is a muscle-derived osteocyte survival factor., Cell Reports accepted (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McPherron AC, Lee SJ, Double muscling in cattle due to mutations in the myostatin gene, Proc. Natl. Acad. Sci. U. S. A 94(23) (1997) 12457–12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, Kang B, Isales CM, Fulzele S, Wenger KH, Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading, Bone 40(6) (2007) 1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Puolakkainen T, Ma H, Kainulainen H, Pasternack A, Rantalainen T, Ritvos O, Heikinheimo K, Hulmi JJ, Kiviranta R, Treatment with soluble activin type IIB-receptor improves bone mass and strength in a mouse model of Duchenne muscular dystrophy, BMC Musculoskelet Disord 18(1) (2017) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DiGirolamo DJ, Singhal V, Chang X, Lee SJ, Germain-Lee EL, Administration of soluble activin receptor 2B increases bone and muscle mass in a mouse model of osteogenesis imperfecta, Bone Res 3 (2015) 14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barreto R, Kitase Y, Matsumoto T, Pin F, Colston KC, Couch KE, O’Connell TM, Couch ME, Bonewald LF, Bonetto A, ACVR2B/Fc counteracts chemotherapy-induced loss of muscle and bone mass, Scientific reports 7(1) (2017) 14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Consitt LA, Clark BC, The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials, J Frailty Aging 7(1) (2018) 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Briganti SI, Gaspa G, Tabacco G, Naciu AM, Cesareo R, Manfrini S, Palermo A, Irisin as a regulator of bone and glucose metabolism: a narrative review, Minerva Endocrinol (2017). [DOI] [PubMed] [Google Scholar]
- [32].Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM, A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis, Nature 481(7382) (2012) 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM, Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry, Cell Metab 22(4) (2015) 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Colaianni G, Mongelli T, Colucci S, Cinti S, Grano M, Crosstalk Between Muscle and Bone Via the Muscle-Myokine Irisin, Curr Osteoporos Rep 14(4) (2016) 132–7. [DOI] [PubMed] [Google Scholar]
- [35].Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J, Mantzoros CS, Irisin in metabolic diseases, Endocrine 59(2) (2018) 260–274. [DOI] [PubMed] [Google Scholar]
- [36].Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman V, Novick SJ, Strutzenberg TS, Pascal BD, Le PT, Brooks DJ, Roche AM, Gerber KK, Mattheis L, Chen W, Tu H, Hynes RO, Bouxsen ML Griffin PR, Baron R, Rosen CJ, Bonewald LF, Spiegelman BM, Irisin Mediaes Effects on Bone and Fat via aV Integrin Receptors, Cell in press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kammoun HL, Febbraio MA, Come on BAIBA light my fire, Cell Metab 19(1) (2014) 1–2. [DOI] [PubMed] [Google Scholar]
- [38].Roberts LD, Bostrom P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, Chen MH, Ramachandran VS, Larson MG, Bouchard C, Rankinen T, Souza AL, Clish CB, Wang TJ, Estall JL, Soukas AA, Cowan CA, Spiegelman BM, Gerszten RE, beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors, Cell Metab 19(1) (2014) 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bonewald LF, Regulation and regulatory activities of transforming growth factor beta, Crit Rev Eukaryot Gene Expr 9(1) (1999) 33–44. [PubMed] [Google Scholar]
- [40].Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE, Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism, Nat. Genet 38(11) (2006) 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dallas SL, Prideaux M, Bonewald LF, The osteocyte: an endocrine cell … and more, Endocr Rev 34(5) (2013) 658–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Buenzli PR, Sims NA, Quantifying the osteocyte network in the human skeleton, Bone 75 (2015) 144–50. [DOI] [PubMed] [Google Scholar]
- [43].Karsenty G, Ferron M, The contribution of bone to whole-organism physiology, Nature 481(7381) (2012) 314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, Bacchetta J, Szulc P, Kitsis RN, de Cabo R, Friedman RA, Torsitano C, McGraw TE, Puchowicz M, Kurland I, Karsenty G, Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise, Cell Metab 25(1) (2017) 218. [DOI] [PubMed] [Google Scholar]
- [45].Dallas SL, Rosser JL, Mundy GR, Bonewald LF, Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix, J Biol Chem 277(24) (2002) 21352–21360. [DOI] [PubMed] [Google Scholar]
- [46].Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, Gludovatz B, Walsh F, Regan JN, Messina S, Evans DS, Lang TF, Zhang B, Ritchie RO, Mohammad KS, Alliston T, Osteocyte-Intrinsic TGF-beta Signaling Regulates Bone Quality through Perilacunar/Canalicular Remodeling, Cell Rep 21(9) (2017) 2585–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, Chiechi A, Wright LE, Umanskaya A, Niewolna M, Trivedi T, Charkhzarrin S, Khatiwada P, Wronska A, Haynes A, Benassi MS, Witzmann FA, Zhen G, Wang X, Cao X, Roodman GD, Marks AR, Guise TA, Excess TGF-beta mediates muscle weakness associated with bone metastases in mice, Nat Med 21(11) (2015) 1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B, Bonewald LF, Stubbs JR, Wacker MJ, FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy, Am J Physiol Endocrinol Metab 304(8) (2013) E863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Avin KG, Vallejo JA, Chen NX, Wang K, Touchberry CD, Brotto M, Dallas SL, Moe SM, Wacker MJ, Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility, Am J Physiol Endocrinol Metab 315(4) (2018) E594–E604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bonewald LF, Johnson ML, Osteocytes, mechanosensing and Wnt signaling, Bone 42(4) (2008) 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Baron R, Kneissel M, WNT signaling in bone homeostasis and disease: from human mutations to treatments, Nat. Med 19(2) (2013) 179–192. [DOI] [PubMed] [Google Scholar]
- [52].Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, Dallas SL, Johnson ML, Jahn K, Bonewald LF, Brotto M, Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/beta-catenin pathway, JBMR Plus 1(2) (2017) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Goldbeter A, Pourquie O, Modeling the segmentation clock as a network of coupled oscillations in the Notch, Wnt and FGF signaling pathways, J Theor Biol 252(3) (2008) 574–85. [DOI] [PubMed] [Google Scholar]
- [54].Fujimaki S, Hidaka R, Asashima M, Takemasa T, Kuwabara T, Wnt protein-mediated satellite cell conversion in adult and aged mice following voluntary wheel running, J Biol Chem 289(11) (2014) 7399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ke HZ, Richards WG, Li X, Ominsky MS, Sclerostin and Dickkopf-1 as Therapeutic Targets in Bone Diseases, Endocr. Rev 33(5) (2012) 747–783. [DOI] [PubMed] [Google Scholar]
- [56].Colditz J, Thiele S, Baschant U, Niehrs C, Bonewald LF, Hofbauer LC, Rauner M, Postnatal Skeletal Deletion of Dickkopf-1 Increases Bone Formation and Bone Volume in Male and Female Mice, Despite Increased Sclerostin Expression, J Bone Miner Res 33(9) (2018) 1698–1707. [DOI] [PubMed] [Google Scholar]
- [57].Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H, Evidence for osteocyte regulation of bone homeostasis through RANKL expression, Nat. Med 17(10) (2011) 1231–1234. [DOI] [PubMed] [Google Scholar]
- [58].Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA, Matrix-embedded cells control osteoclast formation, Nat. Med 17(10) (2011) 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dufresne SS, Dumont NA, Boulanger-Piette A, Fajardo VA, Gamu D, Kake-Guena SA, David RO, Bouchard P, Lavergne E, Penninger JM, Pape PC, Tupling AR, Frenette J, Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles, Am J Physiol Cell Physiol 310(8) (2016) C663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dufresne SS, Boulanger-Piette A, Bosse S, Argaw A, Hamoudi D, Marcadet L, Gamu D, Fajardo VA, Yagita H, Penninger JM, Russell Tupling A, Frenette J, Genetic deletion of muscle RANK or selective inhibition of RANKL is not as effective as full-length OPG-fc in mitigating muscular dystrophy, Acta Neuropathol Commun 6(1) (2018) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Mo C, Zhao R, Vallejo J, Igwe O, Bonewald L, Wetmore L, Brotto M, Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation, Cell Cycle 14(10) (2015) 1507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shen H, Grimston S, Civitelli R, Thomopoulos S, Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice, J. Bone Miner. Res 30(4) (2014) 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Castrogiovanni P, Trovato FM, Szychlinska MA, Nsir H, Imbesi R, Musumeci G, The importance of physical activity in osteoporosis. From the molecular pathways to the clinical evidence, Histol Histopathol 31(11) (2016) 1183–94. [DOI] [PubMed] [Google Scholar]
- [64].Brotto M, Bonewald L, Bone and muscle: Interactions beyond mechanical, Bone 80 (2015) 109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Maurel DB, Jahn K, Lara-Castillo N, Muscle-Bone Crosstalk: Emerging Opportunities for Novel Therapeutic Approaches to Treat Musculoskeletal Pathologies, Biomedicines 5(4) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Spencer RL, Chun LE, Hartsock MJ, Woodruff ER, Glucocorticoid hormones are both a major circadian signal and major stress signal: How this shared signal contributes to a dynamic relationship between the circadian and stress systems, Front Neuroendocrinol 49 (2018) 52–71. [DOI] [PubMed] [Google Scholar]
- [67].Riley LA, Esser KA, The Role of the Molecular Clock in Skeletal Muscle and What It Is Teaching Us About Muscle-Bone Crosstalk, Curr Osteoporos Rep 15(3) (2017) 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McDearmon EL, Patel KN, Ko CH, Walisser JA, Schook AC, Chong JL, Wilsbacher LD, Song EJ, Hong HK, Bradfield CA, Takahashi JS, Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice, Science 314(5803) (2006) 1304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Takarada T, Xu C, Ochi H, Nakazato R, Yamada D, Nakamura S, Kodama A, Shimba S, Mieda M, Fukasawa K, Ozaki K, Iezaki T, Fujikawa K, Yoneda Y, Numano R, Hida A, Tei H, Takeda S, Hinoi E, Bone Resorption Is Regulated by Circadian Clock in Osteoblasts, J Bone Miner Res 32(4) (2017) 872–881. [DOI] [PubMed] [Google Scholar]
- [70].Xu C, Ochi H, Fukuda T, Sato S, Sunamura S, Takarada T, Hinoi E, Okawa A, Takeda S, Circadian Clock Regulates Bone Resorption in Mice, J Bone Miner Res 31(7) (2016) 1344–55. [DOI] [PubMed] [Google Scholar]
- [71].van der Spoel E, Oei N, Cachucho R, Roelfsema F, Berbee JFP, Blauw GJ, Pijl H, Appelman-Dijkstra NM, van Heemst D, The 24-hour serum profiles of bone markers in healthy older men and women, Bone 120 (2018) 61–69. [DOI] [PubMed] [Google Scholar]
- [72].Martinez-Nicolas A, Madrid JA, Garcia FJ, Campos M, Moreno-Casbas MT, Almaida-Pagan PF, Lucas-Sanchez A, Rol MA, Circadian monitoring as an aging predictor, Scientific reports 8(1) (2018) 15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Buehring B, Binkley N, Myostatin--the holy grail for muscle, bone, and fat?, Curr Osteoporos Rep 11(4) (2013) 407–14. [DOI] [PubMed] [Google Scholar]
- [74].Zhang Z, Zhang Y, Zhou Z, Shi H, Qiu X, Xiong J, Chen Y, BDNF regulates the expression and secretion of VEGF from osteoblasts via the TrkB/ERK1/2 signaling pathway during fracture healing, Molecular medicine reports 15(3) (2017) 1362–1367. [DOI] [PubMed] [Google Scholar]
- [75].Kurihara N, Bertolini D, Suda T, Akiyama Y, Roodman GD, IL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 release, J. Immunol 144 (1990) 4226–4230. [PubMed] [Google Scholar]
- [76].Loro E, Ramaswamy G, Chandra A, Tseng WJ, Mishra MK, Shore EM, Khurana TS, IL15RA is required for osteoblast function and bone mineralization, Bone 103 (2017) 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R, Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines, Blood 96(5) (2000) 1873–8. [PubMed] [Google Scholar]
- [78].Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M, Prostaglandin E2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation, Recent patents on biotechnology 6(3) (2012) 223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shen H, Grimston S, Civitelli R, Thomopoulos S, Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice, J Bone Miner Res 30(4) (2015) 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, Bacchetta J, Szulc P, Kitsis RN, de Cabo R, Friedman RA, Torsitano C, McGraw TE, Puchowicz M, Kurland I, Karsenty G, Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise, Cell Metab 23(6) (2016) 1078–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Borsheim E, Herndon DN, Hawkins HK, Suman OE, Cotter M, Klein GL, Pamidronate attenuates muscle loss after pediatric burn injury, J. Bone Miner. Res 29(6) (2014) 1369–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gorski JP, Huffman NT, Vallejo J, Brotto L, Chittur SV, Breggia A, Stern A, Huang J, Mo C, Seidah NG, Bonewald L, Brotto M, Deletion of Mbtps1 (Pcsk8, S1p, Ski-1) Gene in Osteocytes Stimulates Soleus Muscle Regeneration and Increased Size and Contractile Force with Age, J Biol Chem 291(9) (2016) 4308–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Boulanger Piette A, Hamoudi D, Marcadet L, Morin F, Argaw A, Ward L, Frenette J, Targeting the Muscle-Bone Unit: Filling Two Needs with One Deed in the Treatment of Duchenne Muscular Dystrophy, Curr Osteoporos Rep 16(5) (2018) 541–553. [DOI] [PubMed] [Google Scholar]