Abstract
Purpose
The purpose of the study was to investigate the role of Prolactin-Induced Protein (PIP) as a predictive biomarker for Keratoconus (KC).
Participants
This study included one hundred and forty-seven patients with KC (105 male, 42 female), and sixty healthy controls (27 male, 33 female).
Methods
Tears, plasma and saliva samples were collected from all participants. In both KC and healthy groups all collected samples were divided into four age subgroups (15–24y), (25–34y), (35–44y) and (45y and up). Samples were analyzed using western blot (WB) and enzyme-linked immunosorbent assay (ELISA). Areas under the receiver operating characteristic curves (AUROCs) were used to evaluate diagnostic accuracy for distinguishing between KC and healthy eyes.
Main Outcome Measures
Difference in PIP protein levels between patients with KC and healthy controls.
Results
Results showed significant downregulation of PIP expression in all three biological fluids on KC patients when compared to healthy controls, independent of age, sex and severity. Since PIP is a hormonal-regulated protein, we also investigated the expression of major sex hormones. We detected significant upregulation in salivary and plasma Dehydroepiandrosterone sulfate (DHEA-S) levels and significant downregulation of estrone and estriol levels, in KC patients compared to healthy controls, independent of sex, age, and KC severity stage. ROC was used to determine the overall predictive accuracy of this protein in KC. Data showed an area under the curve (AUC) for PIP in tears of 0.937 (95%CI: 0.902–0.971), in plasma of 0.928 (95%CI: 0.890–0.968) and in saliva of 0.929 (95%CI: 0.890–0.968).
Conclusions
Conclusively, our results show that PIP levels are reduced in all three human biological fluids tested, and may independently or in combination with current imaging techniques aid in screening and diagnosis of KC. Our data revealed that PIP levels can potentially differentiate between disease and healthy cases, and PIP levels are stable in relation to KC severity, sex and age. Moreover, alterations in sex hormone levels in correlation with reduced PIP levels in KC provide an intriguing insight in the underlying KC pathophysiology and highlights the role of PIP as a KC biomarker.
Keywords: Keratoconus, Prolactin-Induced Protein, Biomarker, Saliva, Blood, Tears
Graphical Abstract
1. Introduction
Keratoconus (KC) is a multifactorial, progressive, degenerative disease of the cornea (Rabinowitz, 1998). Genetic, biochemical, and environmental factors play a potential role in pathogenesis, but the etiology remains obscure (Gordon-Shaag et al., 2015; Khaled et al., 2017). Corneal thinning, protrusion, and steepening are the main characteristics of the disease, which result in reduced visual acuity and reduced quality of life (Nowak and Gajecka, 2011).
KC frequently develops during puberty and commonly presents bilaterally, but with a high prevalence of inter-eye asymmetry (Khaled et al., 2017). This ectatic disorder is difficult to diagnose in early stages when the cornea appears healthy, even with modern scanning devices. However, in later stages, protrusion and thinning becomes apparent by slit lamp examination (Krachmer et al., 1984). KC management depends on the severity of the disease. In less severe cases, compensation of the reduced visual acuity with spectacles, contact lenses, or intracorneal ring segments may be sufficient. In more severe cases, treatments such as corneal collagen crosslinking (CXL) or corneal transplants may become necessary. CXL offers the opportunity to halt the progression of KC, making early diagnosis of this progressive disease critical (Spoerl et al., 1998; Wollensak et al., 2003).
A number of genes have been reported as casual candidates for KC, but genetic studies have been inconclusive (Abu-Amero et al., 2014; Gordon-Shaag et al., 2015). Various studies have reported a significant downregulation in Lysyl oxidase (LOX) gene and protein levels in cornea and tear samples form KC patients when compared to healthy control samples (Dudakova et al., 2012; Nishtala et al., 2016; Shetty et al., 2015). Further studies in larger cohorts are required to prove specificity and sensitivity of LOX as a potential biomarker of KC.
While other disease areas such as cancer and diabetes have taken the path of discovering and validating a biomarker, the KC scientific community has not (Nishtala et al., 2016). Such a discovery could lead to early detection of the disease, prediction of severity and rate of progression, and monitoring of response to treatment(s). Most importantly, it would accelerate the development of novel therapeutic modalities aimed towards personalized treatments.
Sex hormones are critical in KC pathogenesis based on multiple lines of evidence, including the long-standing observation of adolescent onset (McKay et al., 2016; Yin et al., 2017). It has been shown that corneal thickness changes occur during the menstrual cycle and that pregnancy may be a risk factor for KC progression (Ghahfarokhi et al., 2015; Naderan and Jahanrad, 2017), both of which highlight the role of sex hormones in KC. Yuksel et al. also observed hormonally driven acceleration of corneal thinning in three KC patients undergoing estrogen treatment for in vitro fertilization (Yuksel et al., 2016). Despite these connections, hormone receptors and their role within the cornea remain understudied (Gupta et al., 2005).
In 2014, our group identified significant downregulation of Prolactin-Induced Protein (PIP) expression in the human tear proteome from 36 KC patients and 17 healthy controls (Priyadarsini et al., 2014). Those findings were validated in vitro using corneal-derived cells grown onto our established 3D culture model. The current clinical study investigated levels of PIP in tears, plasma, and saliva. Our study is the first to include three biological fluids in KCrelated investigations, and underscores the need for a system-level understanding for biomarker research and personalized medicine, giving us the ability to establish/validate a novel protein biomarker in numerous biological fluids.
PIP is a 17-kDa glycoprotein originally identified as gross cystic disease fluid protein 15. It is a major component of human milk, breast cyst fluid, and saliva (Haagensen et al., 1990; Hassan et al., 2008b; Naderi and Meyer, 2012). The PIP gene is located on chromosome 7q32–36 and has four exons, but only one 900 bp mRNA transcript has been described (Murphy et al., 1987). PIP is a 146-amino acid polypeptide that is found in mammary glands, salivary glands, lacrimal glands, prostate, and other organs (Naderi, 2015). Determination of PIP’s crystal structure revealed an immunoglobulin fold composed of seven antiparallel beta-strands and seven loops (Hassan et al., 2008a). PIP has an aspartyl protease activity, which mediates its role as a secreted protein capable of extracellular matrix (ECM) degradation (Naderi and Meyer, 2012). Despite widespread expression, the exact function of PIP in healthy and disease state remains vague. Various studies have reported PIP as a multi-hormonally regulated gene in human breast cancer cell lines (Baniwal et al., 2012), and showed PIP to be overexpressed in situ in both primary and metastatic breast cancer labelling it as a breast cancer marker (Baniwal et al., 2013).
The aim of this study was to investigate PIP as a potential biomarker for KC. Our study is amongst the top three, ever reported, in terms of number of participants and the first to include and correlate three different biological fluids from those participants. Together, these aspects provide a sound scientific premise for our clinical studies.
Based on our findings, PIP is a robust biomarker, holding true independent of sex, age and KC severity. We therefore believe that PIP can serve as a novel biomarker for KC and enable clinicians to more effectively recognize disease in patients, particularly at early stages, and facilitate new treatment modalities to improve KC management.
2. Subjects and Methods
2.1. Ethics approval and consent to participate
The study adhered to the tenets of the Declaration of Helsinki. All participants signed a written informed consent before participation. The research protocol was approved by The Central Denmark Region Committees on Health Research Ethics (protocol number: 1–10-72–127-16), and by the Institutional Review Board (IRB)/Ethics committee at the University of Oklahoma Health Sciences Center-Dean McGee Eye Institute (IRB protocol #3450).
2.2. Participants and Inclusion/exclusion criteria
All patients registered with diagnosis of KC in The Danish National Patient Registry, residing in the Western Part of Denmark and a minimum of 15 years of age were invited to attend a clinical examination at The Department of Ophthalmology, Aarhus University Hospital, Denmark. This recruitment approach aimed to include as diverse a KC group as possible and specifically to include the older KC patients who usually are not compliant with regular patient follow-ups. All patients who responded to the invite underwent a thorough ophthalmologic examination including Pentacam HR, refraction and slit lamp examination to confirm KC diagnosis and exclude any other ophthalmic diseases. In addition, patients completed a study questionnaire about their medical history, use of medication, use of refraction, and family history of KC. Patients ethnicity was based on their country of origin. Healthy controls underwent a similar thorough ophthalmologic examination to ensure that they did not have KC or other eye diseases. Patients with a history of ocular trauma or ocular surgery including: corneal laser surgery, corneal transplantation, intra-corneal ring segments or CXL as well as ocular surgeries not related to keratoconus on either eye was excluded, except for uncomplicated strabismus operation and uncomplicated cataract operation more than 1 year previous. Persons with other eye diseases were likewise excluded, except for mild cataract. Patients with any present or previous cancer as well as serious systemic diseases were excluded. Systemic diseases not leading to exclusion included well-treated hypertension, well-treated hypercholesterolemia and well-treated asthma.
Tears, plasma and saliva samples were collected from all participants. All laboratory analyses of these human biological fluids were performed blindly, to minimize bias. KC severity groups, which were defined according to maximum corneal curvature (Kmax), and demographic characteristics of KC patients and healthy controls are presented in (Table 1).
Table 1:
Demographic characteristics of the KC group and the healthy control group
| Healthy control group | KC group | |
|---|---|---|
| Gender | ||
| Male | 27 (45%) | 105 (71.4%) |
| Female | 33 (55%) | 42 (28.6%) |
| Age Groups | ||
| 1: 15–24 years | 8 (13.3%) | 19 (12.9%) |
| 2: 25–34 years | 25 (41.7%) | 37 (25.2%) |
| 3: 35–44 years | 13 (21.7%) | 46 (31.3%) |
| 4: ≥ 45 years | 14 (23.3%) | 45 (30.6%) |
| Severity (kmax) | ||
| 1: <48D | - | 32 (21.8%) |
| 2: ≥48–53D | - | 44 (29.9%) |
| 3: ≥53–58 D | - | 42 (28.6%) |
| 4: ≥58D | - | 29 (19.7%) |
| Ethnicity | ||
| Danish | 58 (96.7%) | 135 (91.8%) |
| Europe Other | 0 (0.0%) | 4 (2.7%) |
| Middle East | 1 (1.7%) | 5 (3.4%) |
| Africa | 0 (0.0%) | 1 (0.7%) |
| South America | 0 (0.0%) | 1 (0.7%) |
| North America | 0 (0.0%) | 1 (0.7%) |
| Unknown | 1 (1.7%) | 0 (0.0%) |
Distribution of gender, age, severity and etnicity in the KC group along with distribution of gender, age and etnicity in the healthy control group. KC Severity groups were defined based on Kmax from Pentacam HR.
2.3. Tear sample collection
Hirschmann® microcapillary pipettes volume 6.66 μL (Hirchmann Laborgeräte, Germany) were used to collect tear fluid samples non-invasively from all participants. Tear fluid collection was performed from the outer third of the lower fornix and care was taken to avoid touching the conjunctiva or produce any reflex tearing (Stuchell et al., 1984). Tear fluid were expelled from the micropipette tubes into sterile microfuge tubes and stored at −80°C until further analysis.
2.4. Saliva sample collection
Patients were requested to rinse their mouth with water prior to sample collection. Saliva samples (~2 mL) were collected in 15 mL VWR® High-Performance Centrifuge Tubes (VWR®, United States) by passive drool (Navazesh, 1993). Prior to processing, saliva samples were aliquoted and stored at −80 °C until further use.
2.5. Plasma sample collection
Blood samples were collected from all participants in 10 ml EDTA-coated tubes (BD vacutainer®, United States). EDTA-coated tubes were gently inverted to secure mixture of whole blood and then centrifuged for 10 min at 1300g at 4°C to separate plasma (Tuck et al., 2013). Plasma was then transferred to sterile microfuge tubes and stored at −80°C until further analyses.
2.6. Western blot analysis
Tear, plasma, and saliva samples from both healthy and KC participants were processed for western blot analysis, as previously described (Chertov et al., 2004; Chevalier et al., 2007; Khurshid et al., 2017; Priyadarsini et al., 2014). Briefly, Bradford assay (Thermo scientific, IL, USA) was carried out to determine the protein concentration and purity of saliva and plasma samples, though not to tear samples due to small volume (~5ul). Samples were mixed with loading buffer and equal amounts of protein were loaded (15 μg per lane) on a 4–20% Tris-Glycine gel (Novex, Life technologies, Carlsbad, CA) and electrophoresed at 135–140V for 1.5 hours, and transferred on to a nitrocellulose membrane (Novex, Nitrocellulose membrane, Life Technologies, Carlsbad, CA), at 100V for 1 hour on ice. All samples were run in single. Thereafter, the membranes were blocked in milk (5% milk in TBST; Thermo scientific, IL, USA) as per the manufacturer’s antibody specification protocol; for 1 hour and incubated overnight at 4°C with primary antibody (Rabbit Monoclonal Anti-PIP, Abcam, Cambridge, MA) at 1:1000 dilution. The membranes were then washed and incubated with a donkey anti-rabbit Alexafluor 568 labeled secondary antibody (1:2000; Life Technologies, Eugene, OR). ChemiDoc It2 imaging system (UVP, Upland, CA) was used for band detection, and quantification by densitometry with grey scale conversion and background subtraction. Results were analyzed by normalizing to housekeeping GAPDH (Abcam, Cambridge, MA) and the fold expression was plotted.
2.7. Enzyme linked Immunosorbent Assay (ELISA)
2.7.1. Salivary ELISA
The following commercial immunoassay kits were used to detect hormone levels present in saliva samples: estrone enzyme immunoassay kit (Cat#1–3202, Salimetrics, State College, PA), estriol/HS estriol enzyme immunoassay kit (Cat#1–1802, Salimetrics, State College, PA), high sensitivity 17β-estradiol enzyme immunoassay kit (Cat#1–3702, Salimetrics, State College, PA), and DHEA-S enzyme immunoassay kit (Cat#1–1252, Salimetrics, State College, PA). Briefly, saliva samples were maintained on ice and centrifuged at 3000 rpm (4°C) for 15 minutes to pellet debris and mucins. All reagents were allowed to reach room temperature before use, and 100 μL of prepared standard, saliva sample, and enzyme conjugate were added to appropriate well (~ 2mg/mL protein extract) and incubated overnight at 4 °C with rocking. All standards and saliva samples were loaded in duplicate. Samples were then washed 4× with provided wash buffer and incubated with the 3,3’,5,5’-Tetramethylbenzidine (TMB) substrate solution at room temperature in the dark for 30 minutes, to allow the plate to develop. Finally, the reaction was stopped with 100 μL of stop solution added to each well and mixed briefly. Optical density was measured at 450 nm using a plate reader. A 4-parameter non-linear regression curve fit was used to calculate the concentrations of all samples based on the standard curve.
2.7.2. Plasma ELISA
The following commercial immunoassay kits were used to detect hormone levels present in plasma samples: estrone enzyme immunoassay kit (Cat# MBS494182, MyBioSource-San Diego, CA ), estriol enzyme immunoassay kit (Cat# MBS494179, MyBioSource -San Diego, CA), high sensitivity 17β -estradiol enzyme immunoassay kit (Cat# MBS494184, MyBioSource San Diego, CA), and DHEA-S enzyme immunoassay kit (Cat# MBS498882, MyBioSource -San Diego, CA). Briefly, plasma samples were maintained on ice, 100 μ L of prepared standard, plasma samples, and enzyme conjugate were added to the appropriate wells (~2mg/mL protein extract) and incubated overnight at 4 °C with rocking. All standard and plasma saliva samples were loaded in duplicate. Samples were then washed 4× with provided wash buffer and incubated with the TMB solution at room temperature in the dark for 30 minutes. 100 μL of stop solution was added to each well, mixed briefly, and measured in a plate reader at 450 nm. A curve-fitting statistical software was used to plot a 4-parameter logistic curve fit to the standards and then calculate results for the all the samples.
2.8. Statistical analysis
Using both, D’Agostino & Pearson normality test and Shapiro-Wilk statistical test we detected non-normally distributed data. Data are presented as mean +/− SEM using bar plots and scatter plots. Mann-Whitney U non-parametric test for comparing variables and determine significance was employed with GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). A value of P < 0.05 was considered significant. ROC curves were calculated to evaluate the ability of PIP to differentiate between patient with KC and normal controls. ROC curves were calculated with 95%CI and results reported as percent of specificity and sensitivity as well as AUROC.
3. Results
3.1. Protein Expression
3.1.1. Total PIP expression
In order to compare the amounts of PIP at the protein level in human tears, plasma and saliva samples from KC patients and healthy controls we performed western blot analysis. Densitometry analysis of examined samples showed significantly lower level of PIP protein in KC patients than in healthy controls across all three biological fluids.
Tears
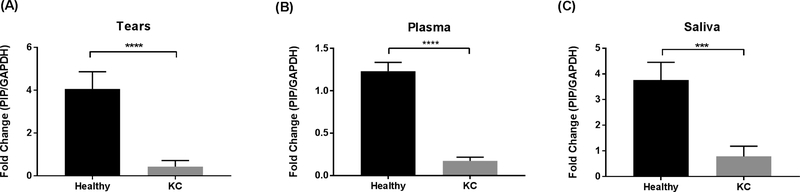
Despite the small volume for sampling, tears are an accessible source of biological material. The total protein concentration of human tears ranges from 6 to 11 mg/ml (Zhou and Beuerman, 2017) and are considered a rich source of biomarkers. Tears may hold an advantage over other fluids for finding relevant biomarkers in KC due to the close proximity to the diseased cornea. Protein levels of PIP were significantly downregulated in tear samples of KC patients compared to healthy controls (Fig 1A; p< 0.0001).
Fig. 1.
PIP Protein levels measured by western blot analysis from three biological fluids: A: Tears; B: Plasma; and C: Saliva, in patients with KC (n = 147) compared to healthy controls (n = 60). Estimates represent mean and error bars represent standard error of the mean. Mann-Whitney U test was used to test group difference, ***p < 0.001, ****p < 0.0001.
Plasma
Human plasma is widely used in clinical and biological studies, and is an easily accessible biological fluid (Yu et al., 2011). Protein levels of PIP were significantly downregulated in plasma samples of KC patients compared to healthy controls (Fig 1B; p< 0.001).
Saliva
The non-invasive collection and continuous availability of saliva make it an excellent source of biomarkers. The protein composition of saliva has been analyzed by several groups and is a medically relevant fluid (Lee and Wong, 2009). Saliva was first analyzed in KC patients by our group in 2014 (McKay et al., 2016). In our current study, protein levels of PIP were significantly downregulated in saliva samples of KC patients compared to healthy controls (Fig 1C; p< 0.001).
3.1.2. Correlation of PIP protein expression with clinical features in KC patients
In this study, the correlation of PIP protein expression with clinical features of KC patients demonstrated that protein levels of PIP were significantly downregulated when compared to healthy controls, as seen in (Fig 1). We also tested whether PIP expression showed correlation with age, sex and KC severity.
Age
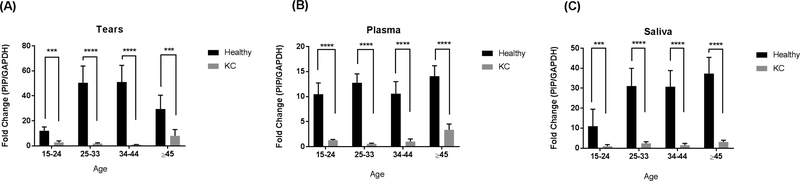
PIP expression was significantly downregulated in KC patients when compared to healthy controls across all age groups. Age did not affect PIP levels within healthy controls, or within KC patients. Excitingly, these significant differences were observed in all three biological fluids (Tears: Fig 2A, Plasma: Fig 2B, and Saliva: Fig 2C).
Fig. 2.
PIP Protein levels measured by western blot analysis from three biological fluids: A: Tears; B: Plasma; and C: Saliva, in patients with KC (n = 147) compared to healthy controls (n = 60). Four different age groups (15–14 yr, 25–33 yr, 34–44 yr, ≥45 yr) were analyzed and compared. Estimates represent mean and error bars represent standard error of the mean. Mann-Whitney U test was used to test group difference, ***p < 0.001, ****p < 0.0001.
Sex
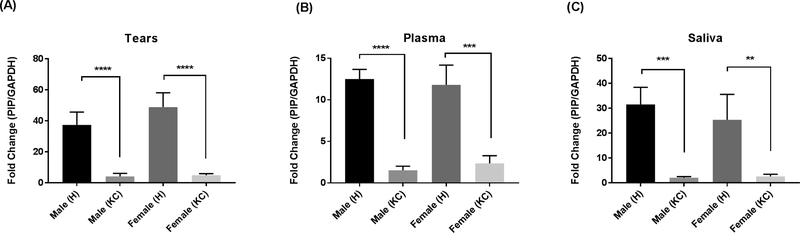
Both KC-Male and KC-Female populations showed significantly downregulated expression of PIP, across all three biological fluids (tears, plasma, and saliva). We found no differences between males and females, in healthy or KC population (Tears: Fig 3A, Plasma: Fig 3B, and Saliva: Fig 3C).
Fig. 3.
PIP Protein levels measured by western blot analysis from three biological fluids: A: Tears; B: Plasma; and C: Saliva, in patients with KC (n = 147) compared to healthy controls (n = 60). Male (H): healthy male: n = 27; Male (KC): male with KC: n = 105; Female (H): healthy female: n = 33; and Female (KC): female with KC: n = 42. Estimates represent mean and error bars represent standard error of the mean. Mann-Whitney U test was used to test group difference, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Severity
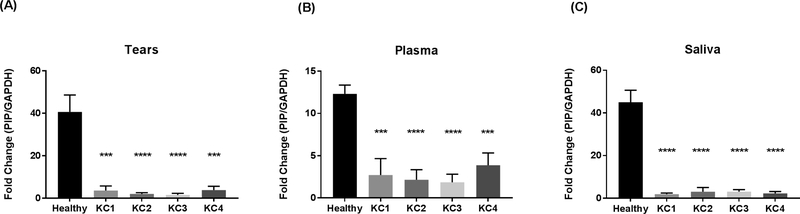
PIP protein expression was significantly downregulated across all three biological fluids in KC patients, in all four severity groups (KC1 through KC4), when compared to Healthy controls. No differences were found, in PIP expression, amongst the severity groups (Tears: Fig 4A, Plasma: Fig 4B, and Saliva: Fig 4C).
Fig. 4.
PIP Protein levels measured by western blot analysis from three biological fluids: A: tears, B: Plasma and C: Saliva in patients with KC (n = 147) compared to healthy controls (n = 60). Four KC severity stages were classified according to Kmax values (KC1, KC2, KC3, and KC4). Estimates represent mean and error bars represent standard error of the mean. Mann-Whitney U test was used to test group difference, ***p < 0.001, ****p < 0.0001.
3.2. Sex hormone levels and ELISA analysis
3.2.1. Total hormone expression
Several studies have demonstrated that PIP expression is regulated by hormones such as androgens, estrogens and prolactin (Baniwal et al., 2013; Naderi and Meyer, 2012). These hormones are essential for ocular surface homeostasis and structural organization, critical in maintaining a healthy cornea (Spoerl et al., 2007). In order to investigate the relationship between KC, PIP, and sex hormones, we quantified concentrations of estrone, estriol, 17β-estradiol and DHEA-S utilizing individual high sensitivity ELISAs.
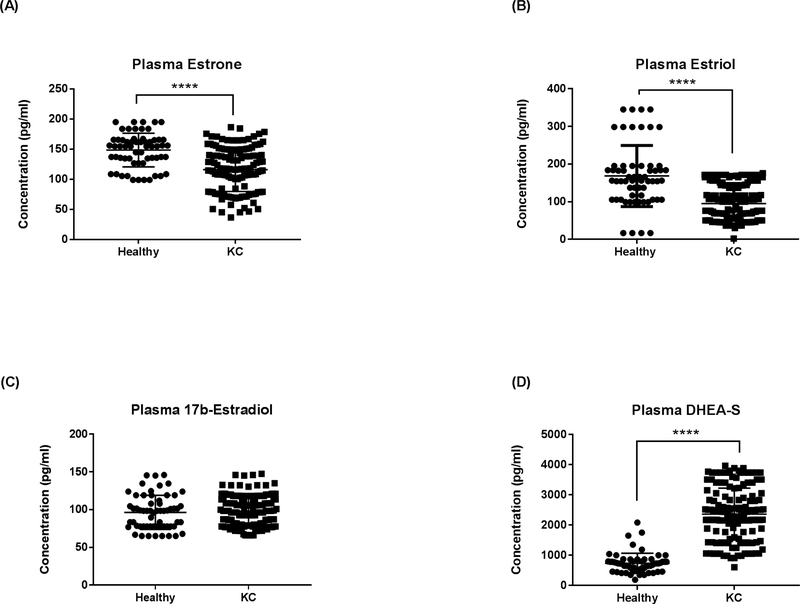
Plasma
Our results showed a significant downregulation in both estrone (Fig 5A; p<0.0001) and estriol (Fig 5B; p<0.0001) levels in KC plasma samples compared to healthy controls. No significant differences were seen in 17β -estradiol levels (Fig 5C; p>0.05). DHEA-S levels, on the other hand, were significantly higher in KC plasma samples compared to healthy controls (Fig 5D; p<0.0001).
Fig. 5.
ELISA analysis of total plasma sex hormone concentrations in KCs and healthy controls: (A) Estrone, (B) estriol, (C) 17β-estradiol, and (D) DHEA-S. Statistical significance was determined using Mann-Whitney U test with p ≤ 0.05 considered statistically significant. Estimates represent mean and error bars represent standard error of the mean. ****p < 0.0001.
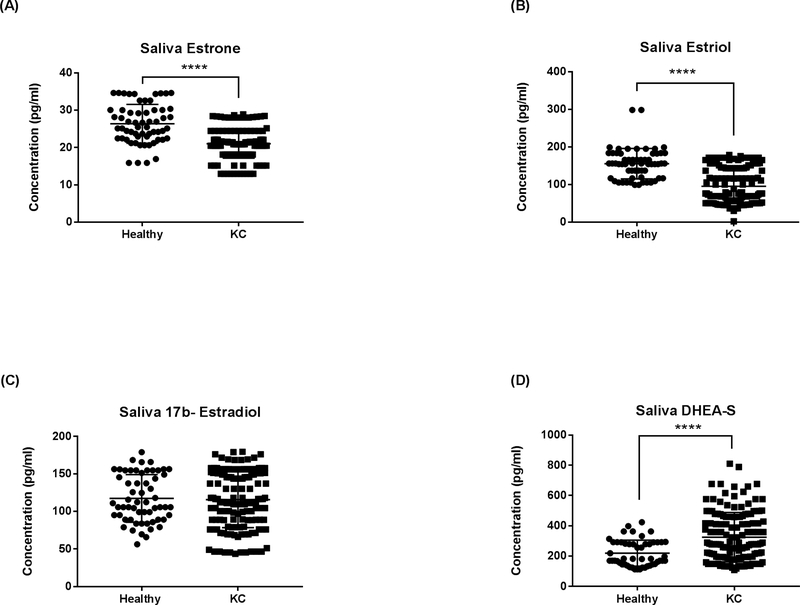
Saliva
Sex hormone levels were also determined in the saliva samples. Our results showed a significant downregulation in both salivary estrone (Fig 6A; p<0.0001) and estriol (Fig 6B; p<0.0001) levels in KC saliva compared to healthy controls. No significant differences were seen in 17β -estradiol levels (Fig 6C; p>0.05). In agreement with the plasma data (Fig 5D), salivary DHEA-S levels were significantly higher in KC patients when compared to healthy controls (Fig 6D; p=0.0001).
Fig. 6.
ELISA analysis of total Saliva sex hormone concentrations in KCs and healthy controls: (A) Estrone, (B) estriol, (C) 17β-estradiol, and (D) DHEA-S. Statistical significance was determined using Mann-Whitney U test with p ≤ 0.05 considered statistically significant. Estimates represent mean and error bars represent standard error of the mean, ****p < 0.0001.
3.2.2. Correlation of sex hormone levels with clinical features
3.2.2.1. Plasma
Age
Expression of Estrone and Estriol were significantly downregulated, in KC, under all age groups when compared to healthy controls. No differences were observed for 17b Estradiol. DHEA-S was significantly upregulated, in KC, under all age groups when compared to healthy controls. No significant differences were detected amongst the four different age groups in this study, whether in the healthy control group or in the KC group, as shown in Fig S1.
Sex
Expression of Estrone and Estriol were significantly downregulated in both KC-Male and KC-Female, when compared to healthy controls. No differences were observed for 17b Estradiol. DHEA-S was significantly upregulated in both KC-Male and KC-Female, when compared to healthy controls. No significant differences were observed amongst the genders in Healthy or KC populations as shown in Fig S2.
Severity
Expression of Estrone and Estriol were significantly downregulated, in all KC severity groups, when compared to healthy controls. No differences were observed for 17b Estradiol, whereas DHEA-S was significantly upregulated in all KC severity groups as shown in Fig S3.
3.2.2.2. Saliva
Age
Similar to plasma, expression of salivary Estrone and Estriol were significantly downregulated, in KC, under all age groups when compared to healthy controls. No differences were observed for 17b Estradiol. DHEA-S was also significantly upregulated, in KC, under all age groups when compared to healthy controls. No significant differences were detected amongst the four different age groups in this study, whether in the healthy control group or in the KC group, as shown in Fig S4.
Sex
Similar to plasma, expression of salivary Estrone and Estriol were significantly downregulated in both KC-Male and KC-Female, when compared to healthy controls. No differences were observed for 17b Estradiol. DHEA-S was significantly upregulated in both KC-Male and KC-Female, when compared to healthy controls. No significant differences were observed amongst the genders in Healthy or KC populations as shown in Fig S5.
Severity
Again similar to plasma, expression of salivary Estrone and Estriol were significantly downregulated, in all KC severity groups, when compared to healthy controls. No differences were observed for 17b Estradiol, whereas DHEA-S was significantly upregulated in all KC severity groups as shown in Fig S6.
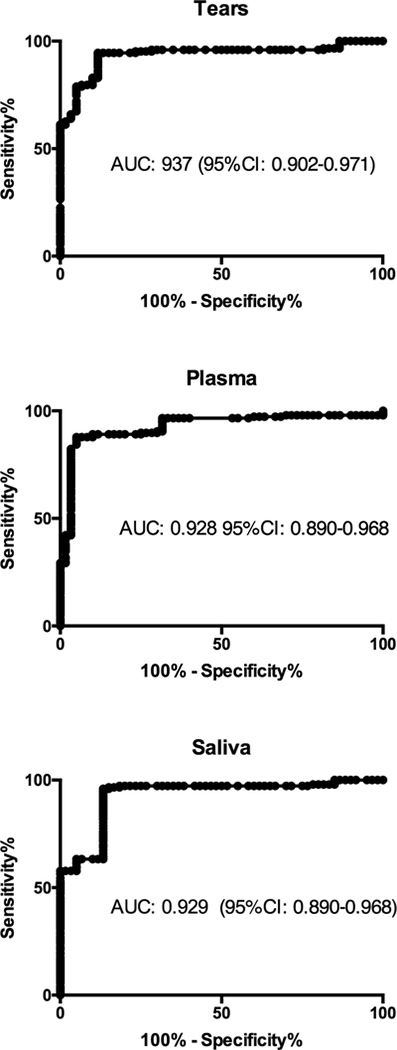
3.3. Receiver operating characteristic curves
ROC curves for determining the overall predictive accuracy of PIP in tears, plasma and saliva of KC patients as described by the AUC. Fig 7 shows AUC for PIP in tears of 0.937 (95%CI: 0.902–0.971), in plasma of 0.928 (95%CI: 0.890–0.968) and in saliva of 0.929 (95%CI: 0.890–0.968).
Fig 7:
Receiver operating characteristic (ROC) curves for KC patients compared to healthy controls for PIP levels in tears, plasma and saliva. Area under the curve (AUC) and 95%CI quantified the ability of PIP levels to discriminate between individuals with KC and healthy controls.
4. Discussion
With the constant development of diagnostic techniques, there is an unmet demand for noninvasive methods in medical screening and diagnosis. Given the lack of knowledge on KC etiology, those screening methods are vital to improve early diagnosis, survey therapeutic outcomes, and facilitate the development of novel drug candidates.
Today there is a strong clinical imperative to identify discerning molecular biomarkers of disease to improve diagnosis, prognosis, and treatment. Despite the advances in high-throughput genomics and proteomics, we are still searching for a distinct biomarker for KC. Consequently, the search for molecular biomarkers is more intense than ever amongst the scientific society. Here we present novel findings of a potential biomarker through the integration of a relationship among three biological fluids (tears, plasma, and saliva). This approach fulfills a clinical need for minimally invasive strategies for screening and diagnosis.
Human tear fluid has become the most popular body fluid to investigate ocular diseases, being a potential source of biomarkers (de Souza et al., 2006; Zhou and Beuerman, 2017; Zhou et al., 2012). Tear location can be informative in pathological conditions associated with the anterior segment of the eye, and also yields information related to retinal and systemic changes in the human body (von Thun und Hohenstein-Blaul et al., 2013). Various studies indicate that certain inflammatory molecules are elevated in tears from patients with KC. Lema and Duran (2005) found that levels of interleukin 6 (IL-6), Matrix metalloproteinases-9 (MMP-9) and tumor necrosis factor-alpha (TNF-α) are higher in KC in comparison to healthy controls (Lema and Duran, 2005). Pannebaker et al. (2010) showed that the level of Matrix metalloproteinases-1 (MMP-1), keratins, and mammaglobin B was increased in KC (Pannebaker et al., 2010). Various studies have also showed other molecules to be differentially expressed in KC tears such as zinc-a2glycoprotein, immunoglobulin kappa chain (IGKC), lactoferrin (Lema et al., 2009), α-enolase, and β-actin (Karamichos et al., 2015; Nishtala et al., 2016; Priyadarsini et al., 2014). PIP was initially discovered in KC by our group during proteomic screening of tear fluid (Priyadarsini et al., 2014).
Human plasma is another important biological fluid investigated as a possible source of biomarkers. Plasma has been shown to be higher in sensitivity, compared to serum, in biomarker detection (Yu et al., 2011). Analysis of the human plasma proteome for biomarkers has great potential for diagnosis and early detection of human disease (Sahab et al., 2007). For example, the detection of certain autoantibodies in patient blood is a reliable biomarker for autoimmune disease, and the detection of rheumatoid factors has been an important diagnostic marker for systemic lupus erythematosus, rheumatoid arthritis, lupus nephritis and ankylosing spondylitis (Shi et al., 2017). Plasma inflammatory cytokines and vascular growth factors were also investigated in different stages of Diabetic Retinopathy, age related macular degeneration, glaucoma and Dry Eye Syndrome (Nalini et al., 2017; Semba et al., 2013; Vehof et al., 2017). A 2012 study found increased superoxide dismutase (SOD) and reduced MMP-2 and zinc in KC patient blood (Ortak et al., 2012). However, this conflicts with a 2016 report which found no difference in SOD activity, although zinc was reduced in their cohort as well, corroborating a potential role for zinc deficiency (Kilic et al., 2016). Prolidase was found to be reduced in KC patient blood in a 2015 study which also found elevated oxidative stress markers (Goncu et al., 2015). In this study, the levels of PIP in plasma were downregulated as it was with tears. To challenge the biomarker even further, we measured its levels in human saliva.
Saliva contains many components also found in plasma and has several advantages over plasma in that saliva offers easy, cost-effective, and a noninvasive bodily fluid. This is highly applicable, especially in the case of oral cancers and Sjögren’s syndrome (Baldini et al., 2018). PIP protein levels were downregulated in human KC saliva, in agreement with our data from tears and plasma. To our knowledge, this is the first KC study and biomarker data that were similarly regulated in three bodily fluids.
Moreover, various studies showed that in both normal and diseased states, PIP expression is known to be regulated by prolactin and androgen hormones. Haagensen et al. (Haagensen et al., 1980) reported a significant upregulation of PIP levels in the plasma of pregnant women. The expression of PIP in human breast cancer cell lines was also shown to be up-regulated by prolactin, glucocorticosteroids and androgens, whereas, estrogens inhibited PIP expression (Carsol et al., 2002; Murphy et al., 1987). On the subcellular level, the signal transducer and activator of transcription 5 (Stat5) and Runx2 works through the androgen receptor (AR) to regulate PIP expression (Haagensen et al., 1980; Naderi, 2015). Nevertheless, further studies are required to understand the molecular mechanisms of PIP action in various tissues.
We previously utilized STRING database to determine the predicted protein-protein interactions of PIP using functional clustering (Sharif et al., 2018). Interestingly, progesterone receptor (PGR), estrogen receptor 1 (ESR1), and AR, were within the top ten highest protein-interaction scores (0.98, 0.96, 0.95, 0.95, and 0.8 respectively). These findings highlight the strong link that exist between PIP, hormones, and KC. These findings might explain the reduced PIP level and modulated sex hormone levels in KC patients detected in this study.
ROC curves highlight the strength of PIP as KC biomarker. Our data showed that KC patients and controls could be significantly differentiated based on PIP levels in all three fluids, with the maximal AUC in tear fluid of 0.937. KC patients are usually diagnosed based on clinical exam and tomographic measurements. Tomography such as the Pentacam have an AUC of around 0.972 when distinguishing KCs from healthy controls (Du et al., 2015). However, when discriminating subclinical KC and healthy controls, the AUC of the Pentacam is considerably lower with highest value of 0.887 (Shetty et al., 2017). As our study has shown that PIP is unaffected by KC severity, the AUC of PIP in discriminating between subclinical KC and healthy controls may possibly be higher than the Pentacam, which would make the PIP level clinically usable as a diagnostic test to supplement the Pentacam measurements in subclinical KC. Specifically this would be useful in patients before laser refractive surgery such as laser-assisted in situ keratomileusis (LASIK) and small incision lenticule extraction (SMILE). It is well known that a small percentage of patients who undergo laser refractive surgery develop ectasia of the cornea, even though their tomographic measurements are deemed normal before the operation. If patients who develop ectasia have low levels of PIP similar to keratoconus patients, then it would be extremely relevant to test the level of PIP in patients before they undergo laser-refractive surgery.
Overall, our study provides a number of conceptual innovations regarding the early diagnosis, and treatment of KC. We are the first to study PIP in the context of KC pathogenesis. Our previous studies together with the data shown here are consistent with the premise that PIP is a new KC biomarker. The fact that 147 KC patients were willing to donate all three fluids at the same time as part of our study highlights the demand driven by the KC patients themselves to discover a new KC biomarker.
Our collective efforts have been successful in providing a roadmap for future work in validating the specificity of PIP as a potential biomarker for KC. (Karamichos, 2018). Testing PIP on other corneal diseases in the near future will determine its specificity. The specificity of PIP in keratoconus is of key importance for its clinical usability. Future studies may consider testing PIP in corneal diseases such as ectasia post laser-refractive surgery, dry eyes disease including Sjögrens disease and in relation to clinical characteristics such as eye rubbing. Biomarkers with minimally invasive collection and reproducible metrics will aid in understanding the underlying causes of KC and clinically in diagnosing keratoconus in the early stages of the disease.
5. Conclusion
Our current work investigating PIP as a KC biomarker advances KC research, although further work to test PIP regulation in other diseases and validate its specificity as a biomarker for KC is still needed, Our data significantly enhances the understanding of KC pathogenesis and paves the way for early diagnosis, clinical trials, and new therapeutic targets for disease prevention. Validating PIP as a KCbiomarker has the potential to improve clinical approach to this disease..
Supplementary Material
Highlights.
Prolactin-Induced Protein significantly downregulated in Keratoconus patients.
Prolactin-Induced Protein significantly downregulated in tear fluid, plasma and saliva.
Prolactin-Induced Protein could serve as a potential biomarker for Keratoconus.
Acknowledgements:
The authors would like to thank all the participants for their time and contributions to this study.
Financial support
Authors would like to acknowledge the support of the National Institute of Health (NEI) Grant EY028888 (DK), unrestricted grant (DMEI) from Research to Prevent Blindness (New York, NY USA), and Synoptik Fonden, Fight for Sight, Denmark, Aarhus University and Einar Willumsens Mindelegat, The funding organizations had no role in study design or conduct of this research.
Abbreviations and Acronyms
- KC
Keratoconus
- PIP
Prolactin-Induced Protein
- WB
Western Blot
- ELISA
Enzyme-Labelled Immunosorbent Assay
- DHEA-S
Dehydroepiandrosterone sulfate
- CXL,
Collagen Cross-linking
- ECM
Extracellular Matrix
- TMB, 3,3’
5,5’-Tetramethylbenzidine
- LASIK
laser-assisted in situ keratomileusis
- IL-6
Interleukin 6
- MMP-9
Matrix metalloproteinases-9
- MMP-1
Matrix metalloproteinases-1
- TNF-α
Tumor Necrosis Factor-alpha
- IGKC
Immunoglobulin Kappa Chain
- SOD
Superoxide Dismutase
- ROC
Receiver Operating Characteristic Curve
- AUROC
Areas under the receiver operating characteristic curves
- PGR
progesterone receptor
- ESR1
estrogen receptor
- AR
androgen receptor
Footnotes
Declarations of interest: no conflicting relationship exists for any author.
Availability of data and material
All data generated and analyzed during this study are included in this published article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Amero KK, Al-Muammar AM, Kondkar AA, 2014. Genetics of Keratoconus: Where Do We Stand? Journal of Ophthalmology 2014, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini C, Ferro F, Elefante E, Bombardieri S, 2018. Biomarkers for Sjögren’s syndrome. Biomarkers in Medicine 12, 275–286. [DOI] [PubMed] [Google Scholar]
- Baniwal SK, Chimge N-O, Jordan VC, Tripathy D, Frenkel B, 2013. Prolactin-Induced Protein (PIP) Regulates Proliferation of Luminal A Type Breast Cancer Cells in an Estrogen-Independent Manner. PLoS ONE 8, e62361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniwal SK, Little GH, Chimge NO, Frenkel B, 2012. Runx2 controls a feed-forward loop between androgen and prolactin-induced protein (PIP) in stimulating T47D cell proliferation. Journal of cellular physiology 227, 2276–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsol JL, Gingras S, Simard J, 2002. Synergistic action of prolactin (PRL) and androgen on PRL-inducible protein gene expression in human breast cancer cells: a unique model for functional cooperation between signal transducer and activator of transcription-5 and androgen receptor. Mol Endocrinol 16, 1696–1710. [DOI] [PubMed] [Google Scholar]
- Chertov O, Biragyn A, Kwak LW, Simpson JT, Boronina T, Hoang VM, Prieto DA, Conrads TP, Veenstra TD, Fisher RJ, 2004. Organic solvent extraction of proteins and peptides from serum as an effective sample preparation for detection and identification of biomarkers by mass spectrometry. Proteomics 4, 1195–1203. [DOI] [PubMed] [Google Scholar]
- Chevalier F, Hirtz C, Chay S, Cuisinier F, Sommerer N, Rossignol M, de Périère DD, 2007. Proteomic Studies of Saliva: A Proposal for a Standardized Handling of Clinical Samples. Clinical Proteomics 3, 13–21. [Google Scholar]
- de Souza GA, Godoy LM, Mann M, 2006. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol 7, R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X-L, Chen M, Xie L-X, 2015. Correlation of basic indicators with stages of keratoconus assessed by Pentacam tomography. International Journal of Ophthalmology 8, 1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakova L, Liskova P, Trojek T, Palos M, Kalasova S, Jirsova K, 2012. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res 104, 74–81. [DOI] [PubMed] [Google Scholar]
- Ghahfarokhi NA, Vaseghi A, Ghahfarokhi NA, Ghoreishi M, Peyman A, Dehghani A, 2015. Evaluation of corneal thickness alterations during menstrual cycle in productive age women. Indian Journal of Ophthalmology 63, 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncu T, Akal A, Adibelli FM, Cakmak S, Sezen H, Yilmaz OF, 2015. Tear Film and Serum Prolidase Activity and Oxidative Stress in Patients With Keratoconus. Cornea 34, 1019–1023. [DOI] [PubMed] [Google Scholar]
- Gordon-Shaag A, Millodot M, Shneor E, Liu Y, 2015. The Genetic and Environmental Factors for Keratoconus. BioMed Research International 2015, 795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Johar K, Nagpal K, Vasavada A, 2005. Sex hormone receptors in the human eye. Survey of ophthalmology 50, 274–284. [DOI] [PubMed] [Google Scholar]
- Haagensen DE Jr., Dilley WG, Mazoujian G, Wells SA Jr., 1990. Review of GCDFP-15. An apocrine marker protein. Ann N Y Acad Sci 586, 161–173. [DOI] [PubMed] [Google Scholar]
- Haagensen DE Jr., Gall SA, Brazy JE, Giannola J, Wells SA Jr., 1980. Analysis of amniotic fluid, maternal plasma, and cord blood for a human breast gross cystic disease fluid protein. Am J Obstet Gynecol 138, 25–32. [DOI] [PubMed] [Google Scholar]
- Hassan MI, Bilgrami S, Kumar V, Singh N, Yadav S, Kaur P, Singh TP, 2008a. Crystal structure of the novel complex formed between zinc alpha2-glycoprotein (ZAG) and prolactin-inducible protein (PIP) from human seminal plasma. J Mol Biol 384, 663–672. [DOI] [PubMed] [Google Scholar]
- Hassan MI, Kumar V, Singh TP, Yadav S, 2008b. Purification and characterization of zinc alpha2glycoprotein-prolactin inducible protein complex from human seminal plasma. J Sep Sci 31, 2318–2324. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Zieske JD, Sejersen H, Sarker-Nag A, Asara JM, Hjortdal J, 2015. Tear metabolite changes in keratoconus. Experimental eye research 132, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled ML, Helwa I, Drewry M, Seremwe M, Estes A, Liu Y, 2017. Molecular and Histopathological Changes Associated with Keratoconus. BioMed Research International 2017, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid Z, Moin SF, Khan RS, Agwan MAS, Alwadaani AH, Zafar MS, 2017. Human salivary protein extraction from RNAPro·SAL™, Pure·SAL™, and passive drooling method. European Journal of Dentistry 11, 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic R, Bayraktar AC, Bayraktar S, Kurt A, Kavutcu M, 2016. Evaluation of Serum Superoxide Dismutase Activity, Malondialdehyde, and Zinc and Copper Levels in Patients With Keratoconus. Cornea 35, 1512–1515. [DOI] [PubMed] [Google Scholar]
- Krachmer JH, Feder RS, Belin MW, 1984. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol 28, 293–322. [DOI] [PubMed] [Google Scholar]
- Lee Y-H, Wong DT, 2009. Saliva: An emerging biofluid for early detection of diseases. American journal of dentistry 22, 241–248. [PMC free article] [PubMed] [Google Scholar]
- Lema I, Duran JA, 2005. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology 112, 654–659. [DOI] [PubMed] [Google Scholar]
- Lema I, Sobrino T, Durán JA, Brea D, Díez-Feijoo E, 2009. Subclinical keratoconus and inflammatory molecules from tears. British Journal of Ophthalmology 93, 820–824. [DOI] [PubMed] [Google Scholar]
- McKay TB, Hjortdal J, Sejersen H, Asara JM, Wu J, Karamichos D, 2016. Endocrine and Metabolic Pathways Linked to Keratoconus: Implications for the Role of Hormones in the Stromal Microenvironment. Scientific Reports 6, 25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LC, Tsuyuki D, Myal Y, Shiu RP, 1987. Isolation and sequencing of a cDNA clone for a prolactin-inducible protein (PIP). Regulation of PIP gene expression in the human breast cancer cell line, T-47D. J Biol Chem 262, 15236–15241. [PubMed] [Google Scholar]
- Naderan M, Jahanrad A, 2017. Topographic, tomographic and biomechanical corneal changes during pregnancy in patients with keratoconus: a cohort study. Acta Ophthalmol 95, e291–e296. [DOI] [PubMed] [Google Scholar]
- Naderi A, 2015. Prolactin-Induced Protein in Breast Cancer, in: Diakonova PM (Ed.), Recent Advances in Prolactin Research. Springer International Publishing, Cham, pp. 189–200. [Google Scholar]
- Naderi A, Meyer M, 2012. Prolactin-induced protein mediates cell invasion and regulates integrin signaling in estrogen receptor-negative breast cancer. Breast Cancer Res 14, R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalini M, Raghavulu BV, Annapurna A, Avinash P, Chandi V, Swathi N, Wasim, 2017. Correlation of various serum biomarkers with the severity of diabetic retinopathy. Diabetes Metab Syndr 11 Suppl 1, S451–s454. [DOI] [PubMed] [Google Scholar]
- Navazesh M, 1993. Methods for collecting saliva. Ann N Y Acad Sci 694, 72–77. [DOI] [PubMed] [Google Scholar]
- Nishtala K, Pahuja N, Shetty R, Nuijts RMMA, Ghosh A, 2016. Tear biomarkers for keratoconus. Eye and Vision 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DM, Gajecka M, 2011. The Genetics of Keratoconus. Middle East African Journal of Ophthalmology 18, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortak H, Sogut E, Tas U, Mesci C, Mendil D, 2012. The relation between keratoconus and plasma levels of MMP-2, zinc, and SOD. Cornea 31, 1048–1051. [DOI] [PubMed] [Google Scholar]
- Pannebaker C, Chandler HL, Nichols JJ, 2010. Tear proteomics in keratoconus. Molecular Vision 16, 1949–1957. [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini S, Hjortdal J, Sarker-Nag A, Sejersen H, Asara JM, Karamichos D, 2014. Gross cystic disease fluid protein-15/prolactin-inducible protein as a biomarker for keratoconus disease. PloS one 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz YS, 1998. Keratoconus. Surv Ophthalmol 42, 297–319. [DOI] [PubMed] [Google Scholar]
- Sahab ZJ, Semaan SM, Sang Q-XA, 2007. Methodology and Applications of Disease Biomarker Identification in Human Serum. Biomarker Insights 2, 21–43. [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Enghild JJ, Venkatraman V, Dyrlund TF, Van Eyk JE, 2013. The Human Eye Proteome Project: Perspectives on an emerging proteome. Proteomics 13, 2500–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R, Bak-Nielsen S, Hjortdal J, Karamichos D, 2018. Pathogenesis of Keratoconus: The intriguing therapeutic potential of Prolactin-inducible protein. Progress in retinal and eye research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty R, Sathyanarayanamoorthy A, Ramachandra RA, Arora V, Ghosh A, Srivatsa PR, Pahuja N, Nuijts RMMA, Sinha-Roy A, Mohan RR, Ghosh A, 2015. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Molecular Vision 21, 12–25. [PMC free article] [PubMed] [Google Scholar]
- Shetty R, Sharma A, Pahuja N, Chevour P, Padmajan N, Dhamodaran K, Jayadev C, M. M. A. Nuijts R, Ghosh A, Nallathambi J, 2017. Oxidative stress induces dysregulated autophagy in corneal epithelium of keratoconus patients. PloS one 12, e0184628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Zhang Z, Li Q, 2017. New Biomarkers in Autoimmune Disease. Journal of Immunology Research 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerl E, Huhle M, Seiler T, 1998. Induction of Cross-links in Corneal Tissue. Experimental Eye Research 66, 97–103. [DOI] [PubMed] [Google Scholar]
- Spoerl E, Zubaty V, Raiskup-Wolf F, Pillunat LE, 2007. Oestrogen-induced changes in biomechanics in the cornea as a possible reason for keratectasia. The British Journal of Ophthalmology 91, 1547–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchell RN, Feldman JJ, Farris RL, Mandel ID, 1984. The effect of collection technique on tear composition. Invest Ophthalmol Vis Sci 25, 374–377. [PubMed] [Google Scholar]
- Tuck M, Turgeon DK, Brenner DE, 2013. Chapter 5 - Serum and Plasma Collection: Preanalytical Variables and Standard Operating Procedures in Biomarker Research A2 - Issaq, Haleem J, in: Veenstra TD (Ed.), Proteomic and Metabolomic Approaches to Biomarker Discovery. Academic Press, Boston, pp. 77–85. [Google Scholar]
- Vehof J, Hysi PG, Hammond CJ, 2017. A Metabolome-Wide Study of Dry Eye Disease Reveals Serum Androgens as Biomarkers. Ophthalmology 124, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Thun und Hohenstein-Blaul N, Funke S, Grus FH, 2013. Tears as a source of biomarkers for ocular and systemic diseases. Experimental Eye Research 117, 126–137. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T, 2003. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. American journal of ophthalmology 135, 620–627. [DOI] [PubMed] [Google Scholar]
- Yin H, Luo C, Tian Y, Deng Y, 2017. Altered expression of sex hormone receptors in keratoconus corneas. Biomedical Research 28. [Google Scholar]
- Yu Z, Kastenmüller G, He Y, Belcredi P, Möller G, Prehn C, Mendes J, Wahl S, Roemisch-Margl W, Ceglarek U, Polonikov A, Dahmen N, Prokisch H, Xie L, Li Y, Wichmann HE, Peters A, Kronenberg F, Suhre K, Adamski J, Illig T, Wang-Sattler R, 2011. Differences between Human Plasma and Serum Metabolite Profiles. PLoS ONE 6, e21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel E, Yalinbas D, Aydin B, Bilgihan K, 2016. Keratoconus Progression Induced by In Vitro Fertilization Treatment. J Refract Surg 32, 60–63. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, 2017. The power of tears: how tear proteomics research could revolutionize the clinic. Expert Rev Proteomics 14, 189–191. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW, 2012. In-depth analysis of the human tear proteome. J Proteomics 75, 3877–3885. [DOI] [PubMed] [Google Scholar]
- Karamichos D,2018. Keratoconus: Challenges and Emerging Trends. J Mol Genet Med 2018, 12:3 DOI: 10.4172/1747-0862.1000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.