Abstract
Objective:
To determine the disease free survival (DFS) and recurrence after the treatment of patients with rectal cancer with open (OPEN) or laparoscopic (LAP) resection.
Background:
This randomized clinical trial (ACOSOG (Alliance) Z6051), performed between 2008–2013, compared LAP and OPEN resection of Stage II/III rectal cancer, within 12 cm of the anal verge (T1–3, N0–2, M0) in patients who received neoadjuvant chemoradiotherapy. The rectum and mesorectum were resected using open instruments for rectal dissection (included hybrid hand-assisted laparoscopic) or with laparoscopic instruments under pneumoperitoneum. The 2 year DFS and recurrence were secondary endpoints of Z6051.
Methods:
The DFS and recurrence were not powered and are being assessed for superiority. Recurrence was determined at 3, 6, 9, 12 and every 6 months thereafter using carcinoembryonic antigen, physical exam, computed tomography and colonoscopy. 486 patients were randomized to LAP (243) or OPEN (243), with 462 eligible for analysis (LAP=240 and OPEN=222). Median follow up is 47.9 months.
Results:
2 year DFS was LAP 79.5% (95%CI, 74.4–84.9) and OPEN 83.2% (95% CI, 78.3–88.3). Local and regional recurrence was 4.6% LAP and 4.5% OPEN. Distant recurrence was 14.6% LAP and 16.7% OPEN.
DFS was impacted by unsuccessful resection (HR 1.87, 95% CI, 1.21–2.91): (composite of incomplete specimen (HR 1.65, 95% CI, 0.85–3.18); positive circumferential resection margins (HR 2.31, 95% CI, 1.40–3.79); positive distal margin (HR 2.53, 95% CI, 1.30–3.77).
Conclusion:
Laparoscopic assisted resection of rectal cancer was not found to be significantly different to OPEN resection of rectal cancer based on the outcomes of DFS and recurrence.
Mini-Abstract:
Reporting of the secondary endpoint of the disease free survival of a multi-center non-inferiority randomized clinical trial (ACOSOG (Alliance) Z6051) performed between 2008–2013 comparing LAP and OPEN low anterior and abdominoperineal radical resection for Stage II/III rectal cancer. Disease free survival two years after resection was found to be similar for patients treated with laparoscopic or open techniques for proctectomy.
INTRODUCTION
Minimally invasive treatment of rectal cancer improves short-term outcomes for patients in the areas of pain, recovery, complications and quality of life. The mixed results from reports in 2015 on the immediate surrogate markers of oncologic outcomes after laparoscopic-assisted approaches to rectal resection for cancer have caused some of the surgical world to pause.1,2,3,4 The resulting controversy over the appropriateness of minimally invasive approaches to rectal cancer has been significant.5 It has forced the needed discussion and action to assure excellent surgical technique and develop ways to provide quality assurance for the surgical care of rectal cancer specifically.
The critical nature of surgical technique in the management of rectal cancer has been shown by Quirke and Heald and in the past.6 This stimulated surgeons to prove that the minimally invasive approach to rectal cancer was safe, feasible and appropriate on an oncologic basis.7 The American College of Surgeons Oncology Group (ACOSOG) Z6051 randomized controlled trial reported that the laparoscopic treatment of rectal cancer (LAP) did not meet criteria for non-inferiority because the composite score of completeness of total mesorectal excision (TME) specimen, negative circumferential radial margins (CRM) and negative distal margins (DM) was greater than 6% lower than the score for open (OPEN) resection of rectal cancer. The Laparoscopic Assisted Resection vs Open Resection on Pathologic Outcomes in Rectal Cancer (ALaCaRT) Study from Australia simultaneously confirmed this finding, using a protocol based on the ACOSOG Z6051 protocol with minor adjustments.1,2 In both studies, the never-before validated composite oncologic score reduced the number of necessary enrolled patients, provided a means for immediate evaluation of a new technique and a mechanism to avoid potential harm to our patients given the uncertainty of the ability to duplicate good outcomes. A metadata analysis of these and two other studies is planned based on similarity of design.
The secondary and clearly more relevant outcomes of the Alliance (ACOSOG) Z6051 RCT are the disease free survival (DFS) and local and regional recurrence (LR) rates at two years. The study was designed primarily to ascertain non-inferiority of LAP resection of Stage II-III rectal cancer compared to OPEN resection for the surgical quality composite endpoint.1 The follow-up period for the secondary outcomes is now complete and is reported herein.
METHODS
The details of this multicenter randomized non-inferiority study including eligibility criteria, interventions, sample size justification, randomization, hypothesis and primary results were reported previously in JAMA.1 Randomization was performed centrally. Through the use of a minimization algorithm, laparoscopic-assisted or open rectal resection was assigned to minimize imbalance with respect to the following stratification variables: surgeon, site of primary tumor (high, middle, or low rectum according to the sub-classification of the 12 cm of rectum into equal thirds), and planned operative procedure (low anterior resection with anastomosis or abdominoperineal resection with colostomy). No blinding of interventions was conducted. Patients received neoadjuvant therapy according to current recommendations for the participating institution, which usually included at least 25 fractions of 200 cGy of external beam irradiation over a 5-week period and a systemic radiation enhancer based on 5-fluorouracil (FU). Patients waited 6 to 12 weeks before undergoing operation. Adjuvant chemotherapy after operation was recommended based on pretreatment Stage II-III, following National Comprehensive Cancer Center Guidelines.8 Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
Patients were assessed after operation at day 3, 1–2 weeks, 4–6 weeks, 3, 6, 9, 12, 18, and 24 months, and every 6 months thereafter, for as long as possible, according to the practice of the institution. Reporting to the central data center through ACOSOG, (now part of the Alliance for Clinical Trials in Oncology (Alliance)), was required for five years. Patients underwent physical exam, review of symptoms suggestive of recurrence, yearly computed tomography (CT) of chest, abdomen and pelvis, carcinoembryonic antigen (CEA) at each visit after 3 months and colonoscopy at years 1, 3 and 5. Positron emission tomography (PET) or biopsy confirmation of recurrence was considered adequate by the Steering Committee.
Outcomes
This report of the Alliance (ACOSOG) Z6051 study addresses the secondary endpoint of 2-year DFS and LR rates. The trial was not powered in terms of the secondary endpoint and any lack of difference found between arms is not an indication of the two interventions not being different. DFS is determined at the first event of either recurrent disease (local, distant or regional) or death, if occurring without recurrence, and those without a recurrence or death were censored at their last assessment for follow-up. LR is assessed as either local or regional recurrence. Distant recurrence is metastasis to an organ or area outside the pelvis. Time-to-event is time from surgery to first event. Patients without recurrence were censored at their last recurrence-free assessment. Thirty-two patients had no assessment for recurrence conducted and are censored at date of death (n=9) or last date known alive (n=23, median follow-up 7.9 months, Interquartile Range (IQR) 3.0 – 37.9 months). A sensitivity analysis was conducted for recurrence without these patients to assess impact of inclusion. No difference was seen (results not shown).
Statistical Methods
Analysis was performed on a modified intent-to-treat basis according to the protocol where any patient who did not receive surgery (n=24) is removed from analysis.1 Patients in the LAP group who were converted to an open procedure were analyzed in the LAP group. Time-to-event of DFS and time to LR was analyzed by Kaplan-Meier plots and log-rank testing. Cox proportional hazard models were adjusted by sex, age, surgeon, ECOG performance status and location of tumor (low, middle or high) as determined in the original protocol. As these adjustments had little impact on conclusions, the results presented are from unadjusted analysis. The rates of local, distant and regional recurrence, as well as development of a new primary, are presented as frequencies and percentages.
Ancillary analysis was performed to identify factors of association with DFS and recurrence. These included the primary analysis of a composite score of surgery where all items had to meet criteria to be considered successful (composite endpoint of; quality of TME specimen (complete or nearly complete), CRM > 1 mm and DM > 1 mm)), as well as univariate analysis of each individual component of the composite score of successful operation which were modeled separately. A number of variables that are considered clinically relevant were also analyzed; location of primary tumor (low < 5 cm, middle 5–8 cm or high 9–12 cm above the anal verge) position in the rectum; surgical approach (abdominoperineal resection (APR) vs. low anterior resection (LAR) vs. low anterior resection and coloanal anastomosis (LAR + CAA)), rectal perforation, and tumor size (cm)). Multivariable Cox proportional hazards models were created to assess the association of clinically relevant variables with DFS and LR, while adjusting for the surgical technique (OPEN vs. LAP). Logistic regression was performed to assess the association of clinical factors (surgical approach and location of tumor) with rectal perforation.
Level of significance was set at 5% with 2-sided tests. Assumptions were verified for all results presented. Analysis was performed with SAS software v.9.4 (SAS Institute Inc., Cary, NC, USA) and verification of model assumptions and Cox proportional hazards models were performed with R v.3.2.3 (R Foundation for Statistical computing, Vienna, Austria) using the Survival Package. All analyses were based on the study database frozen on June 26, 2017. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. This phase III therapeutic trial was monitored by the ACOSOG (Alliance) Data and Safety Monitoring Committee.
RESULTS
Credentialed surgeons (49) at 35 Alliance/ACOSOG institutions participated between October 2008 and September 2013, to randomize 486 Stage II-III rectal cancer patients to LAP (n=243) or OPEN (n=243) arms. The baseline characteristics of these patients were balanced across arms and included only T1 to T3 and N0 to N2 (no T4) tumors. Of these, 462 are evaluable for multivariable analysis (LAP=240, OPEN=222). Median follow-up at the time of analysis was 47.9 months (IQR 31.2–59.4 months) for all patients and 49.5 months (IQR 36.1–60.1 months) for those still alive.
Adjuvant therapy compliance was similar between the two surgical groups; 196 (81.7%) LAP and 174 (78.4%) open. A majority of patients received either 5-FU or Oxaliplatin or some combination (Table 1).
Table 1:
Demographics and Comparison
| LAP N = 240 |
OPEN N = 222 |
|
|---|---|---|
| Follow-up, median (IQR), months | 47.7 (26.1 – 59.1) | 48.1 (33.9 – 59.8) |
| First Recurrence, No. (%) | ||
| None | 198 (82.5) | 179 (80.6) |
| Local | 4 (1.7) | 2 (0.9) |
| Regional | 6 (2.5) | 4 (1.8) |
| Distant | 32 (13.3) | 37 (16.7) |
| All Local Recurrences, No. (%) | 5 (2.1) | 4 (1.8) |
| All Regional Recurrences, No. (%) | 6 (2.5) | 6 (2.7) |
| All Distant Recurrences, No. (%) | 35 (14.6) | 37 (16.7) |
| Liver | 11 | 9 |
| Lung | 14 | 18 |
| Othera | 8 | 9 |
| Alive at last follow-up | 204 (85.0%) | 192 (86.5%) |
| Follow-up for those alive, median (IQR), months | 49.2 (36.0 – 60.1) | 49.6 (36.1 – 60.0) |
| Cause of death: Rectal cancer, No. (%) | 26 (10.8%) | 15 (6.8%) |
| Received Adjuvant Therapy, No. (%) | 196 (81.7%) | 174 (78.4%) |
| Received Adjuvant after Surgery, within 3 monthsb | 103 (42.9%) | 106 (47.8%) |
| Typec, No. (%): Fluorouracil (5FU) | 139 (70.9%) | 125 (71.8%) |
| Oxaliplatin | 122 (62.2%) | 103 (59.2%) |
| Leucovorin | 22 (11.2%) | 26 (14.9%) |
| Capecitabine | 10 (5.1%) | 11 (6.3%) |
| Bevacizumab | 7 (3.6%) | 4 (2.3%) |
| Irinotecan | 1 (0.5%) | 2 (1.2%) |
| Radiation | 5 (2.6%) | 1 (0.6%) |
| Adriamycin | 0 | 1 (0.5%) |
| Unknown | 4 (2.0%) | 1 (0.5%) |
– Other distant recurrent sites are: LAP: bone (n=2); lungs and liver (n=1); liver, lung and multiple myeloma (n=1); liver, lung and peritoneum (n=1); lymph nodes (n=2); spine (n=1). OPEN: bone (n=2); lungs and liver (n=2); lung, liver, retroperitoneal lymph nodes (n=1); liver and vagina (n=1); ovary (n=1); retro-peritoneum (n=1); breast (n=1).
– Date patient started adjuvant was not captured; instead it was reported at each follow-up point on whether it occurred since last visit. The above captures if the patients 3 month follow-up was completed on time and patient was reported to have started adjuvant since surgery. More patients could have started therapy during that time but the data cannot delineate that.
– Types of adjuvant therapy are not mutually exclusive categories. 246 patients (130 LAP, 116 OPEN) had two or more adjuvant therapies.
Disease Free Survival
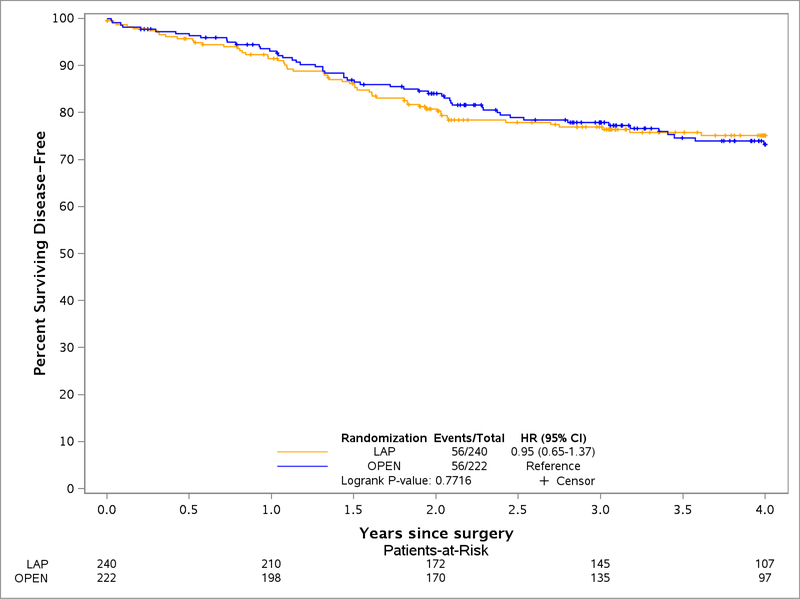
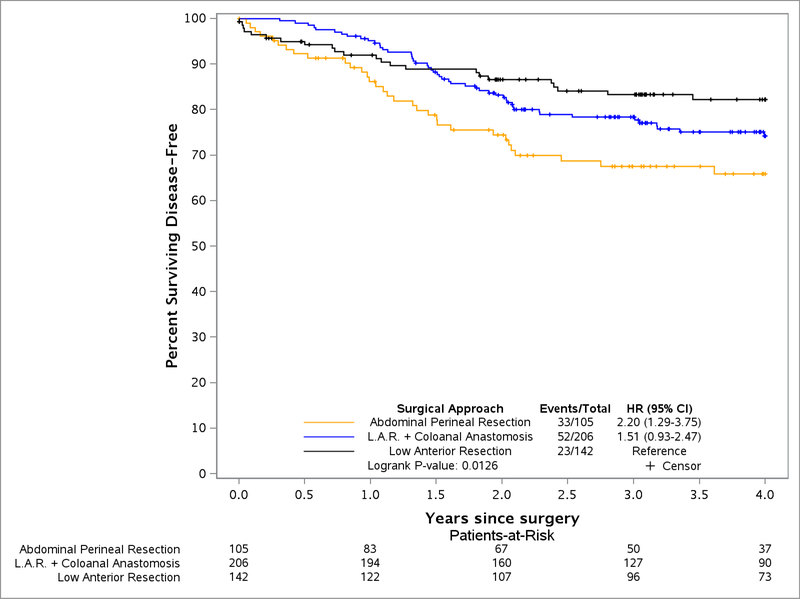
The 2 year DFS for LAP patients was 79.5% (95% CI, 74.4–84.9) and for OPEN was 83.2% (95% CI, 78.3–88.3), with no statistical difference found between LAP and OPEN groups (Figure 1). Similar rates of DFS were observed up to 4 years (LAP 75.2%, 95% CI, 69.6–81.1, OPEN 73.2%, 95% CI, 67.2–79.8). An unsuccessful composite score for surgery was associated with reduced DFS (1.87 HR, 95% CI, 1.21–2.91) (Table 2). When examining each component of a successful surgery separately, only the CRM significantly influenced DFS (HR 2.31, 95% CI 1.40–3.79). Additionally, DFS was significantly worse for patients with stage II/III rectal cancer who underwent APR (low rectal cancer) compared to LAR (HR 2.21, 95% CI, 1.30–3.77) whereas LAR+CAA and LAR were not significantly different (Figure 2). Rectal perforation had a lower Kaplan Meier estimate of DFS in patients at 2 years (70.7%, 95% CI, 59.6–83.9 vs. 82.7%, 95% CI, 79.1–86.6) and increased risk of any recurrence (HR of 1.65, 95% CI, 1.00–2.71).
Figure 1:
Disease Free Survival of Laparoscopic Resection compared to Open Resection of Stage II-III Rectal Cancer
Table 2:
Disease Free Survival Analysis
| DFS Events/N |
DFS KM 2 Year Estimate (95% CI) |
DFS a HR (95% CI) |
|
|---|---|---|---|
| Arm: OPEN | 56/222 | 83.2 (78.3, 88.3) | Ref |
| LAP | 56/240 | 79.5 (74.4, 84.9) | 0.95 (0.65, 1.37) |
| Composite Score: Successful | 86/389 | 82.9 (79.2, 86.8) | Ref |
| Unsuccessful resection | 26/73 | 69.1 (59, 81.1) | 1.87 (1.21, 2.91) |
| Distal Margin: Negative | 108/454 | 81.6 (78, 85.3) | Ref |
| Positive | 4/8 | 46.9 (21.5, 100) | 2.53 (0.93, 6.86) |
| Circumferential Margin: > 1mm | 93/416 | 83.2 (79.6, 87) | Ref |
| ≤ 1 mm | 19/46 | 61 (48.1, 77.5) | 2.31 (1.40, 3.79) |
| Completeness of TME: Complete | 83/356 | 82.7 (78.8, 86.8) | Ref |
| Nearly complete | 19/76 | 75.6 (66.4, 86.1) | 1.15 (0.70, 1.90) |
| Incomplete | 10/30 | 73.9 (58.9, 92.7) | 1.65 (0.85, 3.18) |
| Rectal Perforation: No | 93/404 | 82.7 (79.1, 86.6) | Ref |
| Yes | 19/58 | 70.7 (59.6, 83.9) | 1.65 (1.00, 2.71) |
| Surgical approach: LAR | 23/142 | 86.7 (81.2, 92.6) | Ref |
| LAR + Coloanal Anastomosis | 52/206 | 81.7 (76.5, 87.2) | 1.52 (0.93, 2.48) |
| Abdominal Perineal Resection | 33/105 | 72.4 (64, 81.9) | 2.21 (1.30, 3.77) |
| Location of tumor in rectum: | |||
| Other (Top, High) | 48/233 | 83 (78.2, 88.1) | Ref |
| Low | 64/229 | 79.4 (74.2, 85) | 1.38 (0.95, 2.0) |
| Tumor distance from anal verge (cm)b | ~ | ~ | 0.94 (0.89, 1.00) |
| Tumor size, largest dimension (cm)c | ~ | ~ | 1.03 (0.95, 1.13) |
Abbreviations: DFS, Disease Free Survival; KM, Kaplan Meier; TME, Total Mesorectal Excision; LAR, Lower Anterior Resection
– CoxPH model adjusted by arm reflecting Hazard Ratio and 95% Confidence Interval.
– Missing tumor distance for 3 patients (2 LAP).
– Missing tumor size for 15 patients (9 LAP).
Figure 2:
Disease Free Survival of Surgical Approach
Recurrence: Local, Regional and Distant
Overall, the use of LAP technique did not increase the risk of any recurrence compared to OPEN (Kaplan Meier Estimate at 2 years: LAP 80.6% (95% CI, 75.5–86.1); OPEN 83.9% (95% CI, 78.9–89.2)). (eTable 1 in the Supplement) LR was similar for the groups (LAP 2.1%; OPEN 1.8%; log-rank p=0.86). Distant metastasis was similar between groups (LAP 14.6%; OPEN 16.7%). (Table 1)
Unsuccessful surgery, based on the composite score and influenced mostly by positive CRM, significantly increased recurrence (p=0.006) (eTable 1 in the Supplement). Incomplete TME was not found to be a significant determinant of any recurrence. Rectal perforation resulted in a higher risk of any recurrence, though not significant (HR 1.59, 95% CI, 0.92–2.74). Patients undergoing APR had greater risk for any recurrence compared to LAR (p=0.007) whereas LAR+CAA was not significantly different. Tumor in the low rectum had a recurrence free survival rate of 80.5% (95% CI, 75.2–86.1) whereas those not in the lower rectum (middle and high) had a rate of 83.7% (95% CI, 78.9–88.9). The risk of recurrence decreased with each additional centimeter between the tumor and the anal verge in a consistent way (p=0.056). (eTable 1 in the Supplement)
Factors Influencing Perforation of the Rectal Specimen and Incomplete TME
In the previous report from the Z6051, it was noted that there was a higher rate of macroscopic rectal perforation than expected as reported by the pathologist (LAP-15.4%, OPEN-9.5%, p=0.054). Subset analysis reveals that APR has a significant relationship with the occurrence of rectal perforation in the entire study population compared to LAR and LAR+CAA.(36%, 4%, 6% respectively, p<0.0001).(eTable 2 in the Supplement) Patients with rectal perforation were found to have a higher incidence of low lying tumors than those without perforation (19% vs 6%, p<0.0001). As a result, the risk of rectal perforation is significantly influenced by the need for APR and not LAR or LAR+CAA (OR 10.8 95% CI, 3.9–29.9), p<0.0001). Laparoscopic approach did not significantly affect the risk of perforation (p=0.06) (eTable 3 in the Supplement).
DISCUSSION
Summary
Two year disease free survival and local recurrence rates were not found to be different between patients treated with laparoscopic and open proctectomy for Stage II-III rectal cancer enrolled and followed in the Alliance (ACOSOG) Z6051 randomized controlled trial. This is different than the finding of the earlier report for the short-term composite surrogate oncologic outcomes which suggested that laparoscopic methods could not be considered “non-inferior” to open techniques. These results are reassuring for patients undergoing proctectomy via a minimally invasive approach. It is now also apparent after multivariable analysis that the positive CRM is the most important factor in the composite score of an unsuccessful operation. APR was significantly related to rectal perforation, lower DFS and higher LR than LAR and LAR+CAA. Very importantly laparoscopic technique was not an independent predictor of rectal perforation. Low rectal tumors were more common in the setting of rectal perforation and the risk of LR increased incrementally by proximity of the tumor to the anal verge.
Interpretation
Even though the low rectal tumor position did not significantly negatively impact DFS or LR, there is a trend towards increased risk of poorer outcome. This fact is substantiated by a consistent increase in LR with diminishing distance of the tumor from the anal verge. Patients who underwent APR and LAR+CAA had higher rates of LR and lower DFS than LAR, with APR being significantly different. As a result, depending upon individual surgeon technical abilities, experience and judgment, it may be appropriate to take a more conservative approach to the use of minimally invasive procedures for low rectal cancer. The determining factor in the choice of operative therapy should thus be whether or not a clean resection without perforation can be accomplished.9 There is a report of a translevator approach to extralevator (wide) resection of advanced and locally invasive tumors using minimally invasive techniques but it is likely to be only in the hands of a select few experts.10 New alternatives such as robotic surgery, TaTME and extralevator perineal APR could provide lower recurrence and better outcomes by reducing positive CRM and rectal perforation during the operation.
Current data from Europe and the UK indicate that in a multidisciplinary setting selective use of the neoadjuvant and adjuvant therapies may be more appropriate than broad stroke application to all patients.11,12 Multimodality therapy could be limited to only those patients with biologically high risk tumors (poorly differentiated, extramural venous invasion, lymphovascular invasion, nodal involvement), threatened CRM and low rectal cancers planned for APR and LAR+CAA. Individuals with high and middle rectal cancer, where LAR is possible with low rates of rectal perforation and positive CRM, can be spared toxic and costly treatment.
Generalizability
The surgeons who participated in this trial are considered experts in minimally invasive colorectal surgery. The surgical outcomes are therefore applicable to surgeons with adequate training and ongoing experience with patients having rectal cancer. The risk of a positive CRM seems to predict the greatest risk of worse outcomes regardless of the use of minimally invasive techniques. Unfortunately we do not have data on preoperative CRM potential positivity since local staging was performed by endorectal ultrasound or MRI without specific mention of threatened CRM. If skill is adequate to achieve a clear CRM, any technique for proctectomy is appropriate, including laparoscopy, to provide a successful operation. In fact current data suggest that laparoscopic technique can provide wider margins and negative CRM in the pelvis.10
Any hesitancy or question of ability in that circumstance should result in either conversion to a technique which can obtain a negative CRM or obtaining assistance from a more expert individual.
Much has been written regarding the impact of poor surgical technique on local recurrence in patients with rectal cancer.13 This trial has built in technical credentialing of surgeons, audit for quality of the LAP procedure and audit of photographs of the TME specimen to guarantee the best surgical outcome. Some of these principles are being incorporated into the National Accreditation Program for Rectal Cancer treatment managed by The American College of Surgeons and the Commission on Cancer.14 The education of surgeons and the multidisciplinary treatment of rectal cancer have improved outcomes for patients in Europe and the UK.12 Expert surgeons are a critical feature of the improvement and our next task in the US is to create the infrastructure for all patients with rectal cancer to be treated only by expert surgeons, related specialists and oncologists focused on this disease.
Overall Evidence
The ALaCaRT study report of over 475 randomized Stage I-IV rectal cancer patients comparing LAP and OPEN resection of rectal cancer confirmed the lack of non-inferiority for LAP resection of rectal cancer.2 The protocol was based on the Z6051 protocol with an 8% clinical deficit in immediate composite surgical oncologic outcomes to declare inferiority as the endpoint. Incomplete TME and CRM positivity resulted in the unsuccessful operations that caused the laparoscopic arm of the study to fail to achieve non-inferiority. The COLOR II trial was an international trial enrolling over 1000 patients with Stage I-III rectal cancer, did not require neoadjuvant or adjuvant therapy and resulted in no difference in outcomes or survival between the LAP and open groups.4 This study used a different definition of the rectum (<15 cm from the anal verge) and allowed a 2 to 1 accrual for LAP to open cases. The LR rates for laparoscopy (4.4%) were superior to laparotomy (11.7%). The COREAN trial was designed from the Z6051 protocol and showed an improved composite operative success rate for LAP compared to open and has resulted in similar survival for both groups.3 The BMI for this group was <25 compared to <35 for the Z6051 and ALaCaRT groups. These 4 groups of patients will be tapped for a metadata analysis to look at immediate surgical oncologic outcomes and survival.
Limitations
The use of a composite score for a successful operation in the original publication from this study was designed to reduce the time needed to discover potential unfavorable outcomes of a new technique and allowed us to compare the end result of a surgical technique immediately. The immediate surgical specimen outcome has not translated into a large enough difference in clinical outcomes to be measured. While the intent was good, the composite score may have confused the issue. Both positive CRM and poor TME quality have been shown to be indicative of reduced survival and increased local recurrence but small numbers of events in this study may explain why TME quality did not impact DFS.
Also, high quality surgery may still yield positive CRM due to biologic behavior of the tumor and negatively influence LR and DFS. This can be wrongly attributed to the failure of the surgical technique. A study looking at the ability of laparoscopic techniques to achieve negative margins in the setting of threatened circumferential margins on rectal cancer protocol MRI would go a long way to answer this issue. Quality of life data were not available at the time of this report and will be reported separately.
Unfortunately, our study was not powered to find non-inferiority for DFS and LR. Therefore, the lack of difference found between arms cannot be used to conclude that no difference exists between laparoscopic resection and open operation, given some of the trends noted. Longer follow-up may still be informative since time to recurrence is longer after preoperative chemoradiation.
Conclusion
Laparoscopic assisted resection of rectal cancer was not found to be significantly different to open resection of rectal cancer based on the outcomes of disease-free survival and local/regional recurrence, the lack of statistical difference is not an indicator of no difference existing. Factors that negatively impact disease-free survival after resection of rectal cancer include operation (APR), low position of the tumor in the rectum, rectal perforation during the resection and unsuccessful operation based on circumferential radial margin positivity.
Supplementary Material
Key Point:
Disease free survival and local recurrence were found to be similar between patients treated by open or laparoscopic operation for Stage II-III rectal cancer within this population of patients.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180790, U10CA180833, U10CA18036, and U10CA180858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier NCT00726622
Eulogy
Daniel J. Sargent, PhD, one the founding members of this project, departed this life in September 2016. He was our mentor, leader, friend and an inspiration to all. His work will continue to guide us and set the bar for our efforts. We thank him for all of this and so much more and dedicate this work to his memory.
Study Group Members
Advocate Lutheran General Hospital, Park Ridge, IL (Leela Prasad)
Allegheny General Hospital, Pittsburgh, PA (James McCormick)
Baylor University Medical Center, Dallas, TX (James W. Fleshman)
Boone Hospital Center, Columbia, MO (Walter Peters)
Cleveland Clinic Foundation, Cleveland, OH (Luca Stocchi)
Cleveland Clinic-Weston, Weston, FL (Steven Wexner)
Columbia University Medical Center, New York, NY (Daniel Feingold)
Duke University Medical Center, Durham, NC (Linda Farkas)
Franciscan Saint Francis Health-Mooresville, Mooresville, IN (Dipen C. Maun)
Indiana University Hospital/Melvin and Bren Simon Cancer Center, Indianapolis, IN (Virgilio George)
Indiana University Health North Hospital, Carmel, IN (Virgilio George)
Integris Cancer Institute of Oklahoma, Oklahoma City, OK (Chris M. Davis)
John B Amos Cancer Center, Columbus, GA (William Taylor)
John F Kennedy Medical Center, Edison, NJ (Bertram Chinn)
John Muir Medical Center, Walnut Creek, CA (Samuel C. Oommen)
John Muir Medical Center-Concord Campus, Concord, CA (Samuel C. Oommen)
Kaiser Permanente Los Angeles Medical Center, Los Angeles, CA (Maher Abbas)
Lahey Hospital and Medical Center, Burlington, MA (Peter Marcello)
Lankenau Medical Center, Wynnewood, PA (John Marks)
MD Anderson Cancer Center, Houston, TX (George Chang)
Mayo Clinic, Rochester, MN (David Larson)
Mayo Clinic, Scottsdale, AZ (Tonia Young-Fadok)
Memorial Sloan Kettering Cancer Center, New York, NY (Martin Weiser)
NorthShore University HealthSystem-Evanston Hospital, Evanston, IL (Marc Singer)
Northwestern University, Chicago, IL (Amy Halverson)
Ochsner Medical Center Jefferson, New Orleans, LA (David Margolin)
Providence Portland Medical Center, Portland, OR (Mark Whiteford)
Rush University Medical Center, Chicago, IL (Marc Brand, Theodore Saclarides)
Saint Joseph’s Healthcare Charlton Campus, Hamilton, ON (Mehran Anvari)
Saint Paul’s Hospital, Vancouver, BC (P. Phang)
Sidney and Lois Eskenazi Hospital, Indianapolis, IN (Virgilio George)
Spectrum Health-Blodgett Campus, Grand Rapids, MI (Rebecca Hoedema)
State University of New York Upstate Medical University, Syracuse, NY (Jiri Bem)
Stony Brook University Medical Center, Stony Brook, NY (Roberto Bergamaschi)
Tampa General Hospital, Tampa, FL (Jorge Marcet)
University of Chicago, Chicago, IL (Alessandro Fichera, Mitchell Posner)
University of Iowa Hospitals and Clinics, Iowa City, IA (John Byrn)
University of Pittsburgh Cancer Institute (UPCI), Pittsburgh, PA (Herbert Zeh)
Vanderbilt University/Ingram Cancer Center, Nashville, TN (Alan Herline)
Washington University School of Medicine, Saint Louis, MO (Elisa Birnbaum, James Fleshman, Matthew Mutch, Paul Wise)
Western Pennsylvania Hospital, Pittsburgh, PA (James McCormick)
Contributor Information
Megan E. Branda, branda.megan@mayo.edu, Alliance Statistics and Data Center, Mayo Clinic, Rochester, MN.
Daniel J. Sargent, Formerly with Alliance Statistics and Data Center, Mayo Clinic, Rochester, MN.
Anne Marie Boller, aboller@northwestern.edu, Northwestern University, Keck School of Medicine, Chicago, IL.
Virgilio V. George, georgev@musc.edu, Medical University of South Carolina, Charleston, SC.
Maher A. Abbas, dubaicolorectal@gmail.com, Dubai Colorectal and Digestive Clinic, Dubai, United Arab Emirates.
Walter R. Peters, Jr, walter.peters@bswhealth.org, Baylor University Medical Center, Dallas, TX.
Dipen C. Maun, dipen.maun@gmail.com, Franciscan Health, Indianapolis, IN.
George J. Chang, gchang@mdanderson.org, The University of Texas M.D. Anderson Cancer Center, Houston, TX.
Alan Herline, aherline@augusta.edu, Augusta University, Augusta, GA.
Alessandro Fichera, Alessandro_fichera@med.unc.edu, University of North Carolina, Chapel Hill, NC.
Matthew G. Mutch, mutchm@wudosis.wustl.edu, Washington University School of Medicine, St. Louis, MO.
Steven D. Wexner, wexners@ccf.org, Cleveland Clinic-Weston, Fort Lauderdale, FL.
Mark H. Whiteford, mwhiteford@orclinic.com, The Oregon Clinic, Oregon Health and Sciences University, Portland, OR.
John Marks, marksj@mlhs.org, Lankenau Hospital, Main Line Health, Wynnewood, PA.
Elisa Birnbaum, elisa.birnbaum@ucdenver.edu, University of Colorado Denver, Denver, CO.
David A. Margolin, damargolin@ochsner.org, Ochsner Clinic Foundation, New Orleans, LA.
David W. Larson, larson.david2@mayo.edum, Mayo Clinic, Rochester, MN.
Peter W. Marcello, peter.w.marcello@lahey.org, Lahey Hospital & Medical Center, Burlington, MA.
Mitchell C. Posner, mposner@surgery.bsd.uchicago.edu, University of Chicago Medicine, Chicago, IL.
Thomas E. Read, thomas.read@lahey.org, Lahey Hospital & Medical Center, Burlington, MA.
John R.T. Monson, john.monson.md@flhosp.org, Florida Hospital Medical Group, Orlando, FL.
Sherry M. Wren, swren@stanford.edu, Stanford University School of Medicine, Palo Alto Veterans Health Care System, Palo Alto, CA.
Peter W. T. Pisters, ppisters@mdanderson.org, The University of Texas M.D. Anderson Cancer Center, Houston, TX.
Heidi Nelson, nelsonh@mayo.edu, Mayo Clinic, Rochester, MN.
REFERENCES
- 1.Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of Stage II or III rectal cancer on pathologic outcomes. The ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevenson ARL, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs Open resection on pathological outcomes in rectal cancer. The ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363. [DOI] [PubMed] [Google Scholar]
- 3.Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomized controlled trial. Lancet Oncol. 2010;11(7):637–645. [DOI] [PubMed] [Google Scholar]
- 4.van der Pas MH, Haglind E, Cuesta MA, et al. ; Colorectal Cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomized phase 3 trial. Lancet Oncol. 2013;14(3):210–218. [DOI] [PubMed] [Google Scholar]
- 5.Spinelli AA, D’Hoore Y, Pais WA, Bemelman DG, Jayne DG, Furst A. Critical appraisal of two randomized clinical trials on pathologic outcomes: laparoscopic vs. open resection for rectal cancer. Coloproctology. 2017;39(4):277. [Google Scholar]
- 6.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303–312. [DOI] [PubMed] [Google Scholar]
- 7.Baik SH, Gincherman M, Mutch MG, et al. Laparoscopic vs open resection for patients with rectal cancer: comparison of perioperative outcomes and long term survival. Dis Colon Rec. 2011;54(1):6–20. [DOI] [PubMed] [Google Scholar]
- 8.Benson AB, Bekaii-Saab T, Chan E, et al. Rectal cancer. Clinical practice guidelines in oncology. JNCCN. 2012;10(12):1528–1564. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Hevia M, Delgado S, Castells A, et al. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261(2):221–227. doi: 10.1097/sla.0000000000000865 [DOI] [PubMed] [Google Scholar]
- 10.Palter VN, MacLellan S, Ashamalla S. Laparoscopic translevator approach to abdominoperineal resection for rectal adenocarcinoma: feasibility and short-term oncologic outcomes. Surgical Endoscopy and Other Interventional Techniques. 2016;30(7):3001–3006. [DOI] [PubMed] [Google Scholar]
- 11.Low Rectal Cancer Study (MERCURY II), PI Gina Brown. ClinicalTrials.gov Identifier: NCT02005965, Sponsor: Royal Marsden NHS Foundation Trust, first posted December 9, 2013. [Google Scholar]
- 12.Nagtegaal ID, van de Velde CJ, van der Worp E, et al. Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group. Macroscopic evaluation of rectal cancer resection specimen. J Clin Oncol. 2002;20(7):1729–1734. [DOI] [PubMed] [Google Scholar]
- 13.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1(8496):1479–1482. [DOI] [PubMed] [Google Scholar]
- 14.Monson JRT, Dietz DW, Boughey JC, You YN. Improving rectal cancer outcomes through advocacy, education, and research: The OSTRiCh Consortium and the new NAPRC. Bulletin of the American College of Surgeons. November 1, 2016. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.