Abstract
Non‐small cell lung cancer (NSCLC) accounts for up to 85% of all lung cancers. Central nervous system metastases are a common complication of NSCLC and confer a poor prognosis and a dismal survival rate. Treatment is limited, has poor outcomes, and affects patient quality of life. Pembrolizumab is an anti‐PD‐1 antibody that has shown good results for the management of NSCLC. However, its penetration of the central nervous system has not been well studied, and patients with untreated brain metastases are excluded from most clinical trials. Herein, we report two cases of NSCLC with brain metastases in patients successfully treated with pembrolizumab, and discuss the efficacy and safety of pembrolizumab in these patients. Pembrolizumab has shown good control and the patients have had long progression‐free survival with a high quality of life. Neither patient has experienced serious or grade 3–4 treatment‐related adverse events. Pembrolizumab demonstrates activity in brain metastases in NSCLC patients with an acceptable safety profile. Thus, there may be a role for systemic immunotherapy in patients with untreated or progressive brain metastases.
Keywords: Central nervous system metastases, non‐small cell lung cancer (NSCLC), pembrolizumab
Introduction
Lung cancer is one of the leading causes of cancer‐related death worldwide, with an estimated 1.8 million new cases globally in 2012.1, 2 Non‐small cell lung cancer (NSCLC) accounts for up to 85% of all lung cancers.3 Central nervous system (CNS) metastases (including brain metastasis [BM] and leptomeningeal metastasis [LM]) are a common complication of NSCLC with poor prognosis and a dismal survival rate.4 Approximately 40% of patients develop CNS metastases in the course of their disease.5, 6 Treatment modalities are limited to a combined approach of surgical resection with radiotherapy, which also has poor outcomes, leads to various neurological deficits, and affects patient quality of life. New treatment modalities, such as immune checkpoint inhibitors (ICIs), have revolutionized the management of melanoma, renal cancer, and NSCLC.
PD‐1 is a receptor present on cytotoxic T cells that binds to its two natural ligands, PD‐L1 and PD‐L2. Its major role is to limit the activity of T cells in peripheral tissues during inflammatory responses to infection and to limit autoimmunity.7, 8, 9 Binding of PD‐L1 expressed in NSCLC cells to its receptor in T cells leads to an immunosuppressive tumor microenvironment and promotes tumor formation.10 Pembrolizumab is a humanized monoclonal immunoglobulin G4 antibody directed against human cell surface receptor PD‐1. Treatment with pembrolizumab in a first‐line setting achieves significantly longer progression‐free survival (PFS) and overall survival (OS) than conventional treatment with platinum doublets in patients with advanced NSCLC with high PD‐L1 protein expression.11
The CNS penetration of these effective drugs has not been well studied, and patients with untreated brain metastases are excluded from most clinical trials because of concerns about potential neurological sequelae. Clinical trials of patients with metastatic NSCLC rarely allow the enrollment of patients with untreated brain metastases, and local CNS therapy is typically required prior to trial enrollment.12, 13
Herein, we report two cases of NSCLC with brain metastases in patients successfully treated with pembrolizumab, and discuss the efficacy and safety of pembrolizumab in these patients.
Case 1
A 51‐year‐old man with a history of heavy smoking (smoking index 600) was admitted for lymphadenopathy in the left neck, mediastinum, and a tubercle in the right lung. Pathological examination of transbronchial needle aspiration biopsy specimens by endobronchial ultrasonography identified an adenocarcinoma, and the patient was clinically diagnosed with stage IV pulmonary adenocarcinoma (cTxN3M1c). The tumor harbored neither EGFR gene mutation nor ALK gene rearrangement. The patient was treated with four cycles of combination chemotherapy with cisplatin and etoposide from 21 September to 1 December 2012, together with 11 cycles of 400 mg nimotuzumab once a week, followed by 12 cycles of maintenance treatment with pemetrexed (from 16 January to 9 October 2013), and 38 cycles of autologous lymphocyte reinfusion (from 13 May to 12 June 2015). The patient underwent mediastinal lymphadenectomy and wedge resection of the lower lobe of the right lung by video‐assisted thoracic surgery because of reduction in the pulmonary focus on 25 November 2014. The postoperative pathology indicated large cell carcinoma. Immunohistochemical analysis showed: (CK)(+), CK7 (+), CK5/6(−), p63, CK20 (−), CD3, CD5, CD20 (diffused+), TTF‐1 (−), Napsin A (−), CgA (+/−), Syn (−), CD56 (−), CEA (−), EMA (−), Ki‐67(30%+), and AB‐PAS (−). Gene detection showed: FGFR3 (+), PDGFR (+), RET (+), ROS1 (−), ALK (−), c‐Met (−). After surgery the patient was administered three cycles of combination chemotherapy of docetaxel 130 mg and cisplatin 130 mg (from 1 January to 27 February 2015). However, subsequent metastasis to his CNS was detected in July 2015; brain magnetic resonance imaging (MRI) showed an abnormally enhanced nodule on the surface of the orbital frontal gyrus in the left frontal lobe and revealed multiple brain metastases. The patient experienced headaches, nausea, and vomiting in October and a subsequent brain MRI showed that the orbital gyrus of the left frontal lobe and right cerebellopontine angle was larger than in the previous MRI and the right frontal lobe occupied a new position. The patient then suffered an epileptic fit and received whole brain radiation therapy (WBRT) from 11 November to 12 December 2015. As a third‐line treatment, 18 cycles of pembrolizumab (100 mg/2w) was initiated from 21 January 2016 to 12 January 2017. In April 2015, chest and abdomen computed tomography (CT) and brain MRI revealed no enlargement of intrapulmonary lesions and obvious reduction of all brain lesions. The patient's headaches and other symptoms were improved. On the 15th month after initial pembrolizumab administration, the patient had joint swelling and pain, which improved after symptomatic treatment. At the time of submission of this report the patient continues on treatment and remains completely asymptomatic, 31 months after initiation of pembrolizumab (Fig 1).
Figure 1.
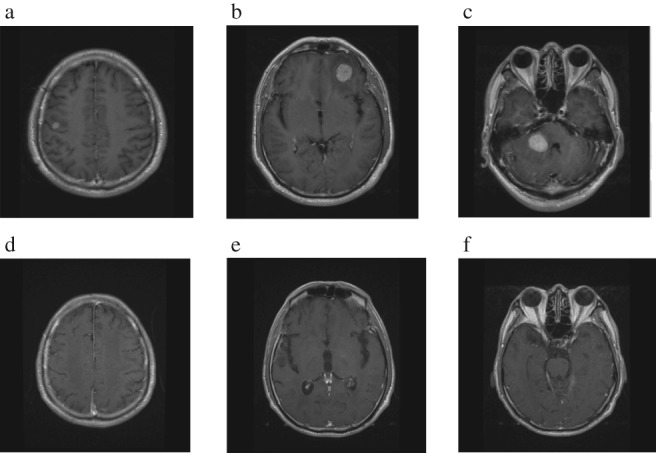
Brain magnetic resonance imaging before pembrolizumab treatment (a–c), after 18 cycles of pembrolizumab treatment, and 19 months after drug withdrawal (d–f).
Case 2
A 45‐year‐old man with a history of heavy smoking (smoking index 100) suffered an epileptic fit and brain MRI showed multiple abnormal spaces located in the bilateral occipital lobe, left thalamus, right cerebellum hemisphere, and pontine. Chest and abdomen CT showed a tubercle in the right lung. Pathological examination of the percutaneous lung biopsy specimens identified an adenocarcinoma, and the patient was clinically diagnosed with stage IV pulmonary adenocarcinoma (cT2N3M1c). The tumor harbored neither EGFR gene mutations nor ALK gene rearrangement. The patient was treated with two cycles of combination chemotherapy with cisplatin and pemetrexed from 1 December 2016 to January 2017. The patient was then administered WBRT from 24 January to 7 February 2017. As a second‐line treatment, pembrolizumab (120 mg/3w) was administered from 8 February to 1 December 2017. During this time, the patient underwent radiofrequency ablation of pulmonary lesions on 27 July 2017. In March 2018, brain MRI showed no obvious reduction of any brain lesions (Fig 2). Chest and abdomen CT performed in June 2018 showed no enlargement of the original lesion, and no new metastases. At the time of submission of this report the patient remains completely asymptomatic, 28 months after initiation of treatment with pembrolizumab.
Figure 2.
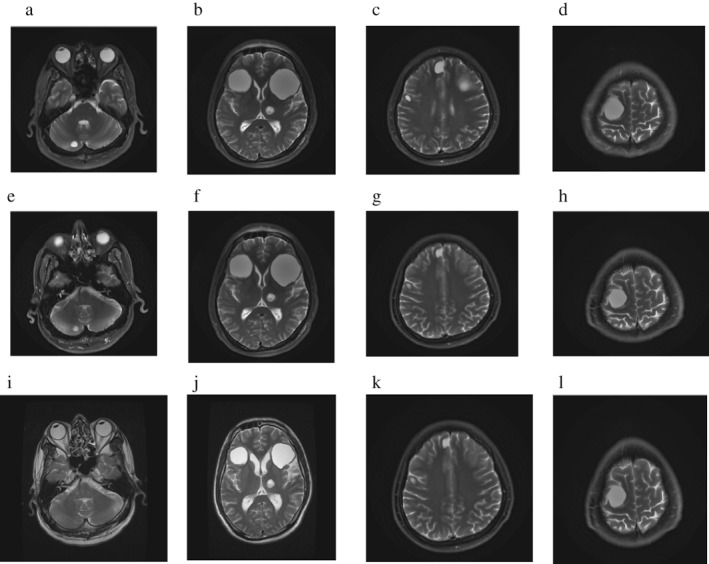
Brain magnetic resonance imaging (a–d) before and (e–h) after three cycles of pembrolizumab treatment, and (i–l), and three months after drug withdrawal.
Discussion
The cases reported herein show that pembrolizumab has activity in NSCLC brain metastases with acceptable treatment‐related adverse events (TRAEs). The majority of the TRAEs were grade 1–2. In case 1, the patient developed grade 3 autoimmune thyroiditis, which was controlled by timely treatment. After treatment with pembrolizumab, both patients have had long OS and PFS with high quality of life.
BM is one of the dreadful complications and a major cause of NSCLC morbidity and mortality. The prognosis after BM is extremely poor and life expectancy is severely curtailed. Currently, the treatment modalities for NSCLC are limited to combined surgical resection and radiotherapy, which also has poor outcomes, leads to various neurological deficits, and affects patient quality of life.
With advancements in diagnostic imaging techniques and new therapies for NSCLC, the detection rate of BM has increased. At present, the major treatment modalities are surgery, stereotactic radiosurgery, and WBRT. These therapies offer little survival benefit with risks such as neurological degeneration, infection, intracranial hemorrhage, seizures, postoperative strokes, and decline in cognition. EGFR and ALK tyrosine kinase inhibitors have shown significant results in BM in patients with sensitizing EGFR mutations or ALK rearrangements. However, patients with no sensitizing mutations have poor survival without targeted therapies.14 ICIs have revolutionized the management of melanoma, renal cancer, and NSCLC. Some clinical trials and case reports on immunotherapy have encouraged the use of these novel agents for the management of BM.
Anti‐PD‐1 and anti‐PD‐L1 antibodies are ICIs that have shown good results in the management of NSCLC. Two commercially available ICIs are pembrolizumab and nivolumab. Initial trials of these drugs excluded patients with BM. Clinical trials including patients with BM are rare, and the patients enrolled are few. Goldberg et al. conducted a single‐institution, two‐cohort phase II trial evaluating the activity and safety of pembrolizumab in patients with melanoma or NSCLC and untreated or progressive brain metastases. In their study, 34 NSCLC patients with untreated or progressive brain metastases were selected and 18 were treated with pembrolizumab. Six of the 18 patients treated with pembrolizumab had BM responses, including four complete responses (22%) and two partial responses (11%), for a BM response rate of 33% (95% confidence interval [CI] 14–59). Two additional patients had stable disease in the CNS. CNS responses were similarly durable for NSCLC, ranging from three to seven months, with five of the six continuing to respond at the time of data analysis.15, 16 Regarding safety, three patients (17%) experienced transient grade 3 cognitive dysfunction and grade 1–2 seizures. Similar clinical trials were conducted with nivolumab. Goldman et al. evaluated the efficacy and safety of nivolumab compared to docetaxel for the treatment of patients with advanced NSCLC and patients with pretreated stable CNS metastases. In patients with pretreated stable CNS metastases, the median OS was 7.61 months with nivolumab (n = 34) versus 7.33 months with docetaxel (n = 34; 30 vs. 27 months, 1.04, 95% CI 0.62–1.76; CheckMate 057). Any‐grade TRAEs occurred in 67% of patients and grade 3–4 TRAEs occurred in 7%, with no TR deaths.17 Studies conducted by Bidoli et al. and Dudnik et al. showed a similar result, indicating that nivolumab has intracranial activity in patients with NSCLC and untreated/progressing brain metastases with low‐grade toxicity in general.18, 19 In the latest clinical trials, Henon et al. evaluated 259 patients with NSCLC treated with ICIs. Among these, 48 (19%) had CNS involvement before immunotherapy. With a median follow up of 17 months (95% CI 15–21), 15 patients were evaluable for CNS outcome. The CNS overall response rate was 27% (4/15: 3 partial, 1 complete response) and the CNS disease control rate was 60% (9/15). The median OS was 8 (95% CI 6–11) months. No difference was observed in OS between the CNS+ versus CNS‐ population (P = 0.09). The overall response rate in the CNS+ was 18% versus CNS‐ at 20% (P = 1).20 We can draw the conclusion that immunotherapy can be used to treat CNS metastasis, and CNS involvement did not seem to be associated with a negative impact on outcomes in advanced NSCLC patients.
In addition, Vanda Téglási21, conducted a comprehensive analysis of immune cell infiltration, PD‐1 expression in immune cells, and PD‐L1 expression in tumor and immune cells and their clinical relevance in a large cohort of lung adenocarcinoma patients with brain metastases. It was the first study to show that mononuclear cell infiltration, especially in a peritumoral location, is associated with better survival in NSCLC patients with BM. Accordingly, reactivation of the immune response by ICIs will likely add further clinical benefit.
Prospective trials evaluating ICI efficacy in previously untreated and/or unstable BM are scarce. Based on a few published trials and case reports, anti‐PD‐1 and anti‐PD‐L1 antibodies as ICIs have shown promising results for the management of BM. However, deeper insight is required to understand the specific mechanism of action and how these drugs act in the BM microenvironment.
The efficacy of ICIs needs to be further explored in BM as their use is rapidly increasing for the management of extracranial tumors. Along with efficacy, further research is required to understand their side effect profile, long‐term effect on the immune system, and combination therapies. ICIs are a revolution for the management of cancers and have shown benefits for BM management. In the long term, if these drugs prolong survival and improve the quality of life of patients with BM, it will be a new era for BM management.15
Disclosure
No authors report any conflict of interest.
References
- 1. Bray F, Jemal A, Torre LA, Forman D et al Long‐term realism and cost‐effectiveness: primary prevention in combatting cancer and associated inequalities worldwide. J Natl Cancer Inst 2015; 107 (12): djv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 3. Ettinger DS, Akerley W, Borghaei H et al Non‐small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013; 11: 645–53. [DOI] [PubMed] [Google Scholar]
- 4. Yuankai SHI et al [China experts consensus on the diagnosis and treatment of brain metastases of lung cancer (2017 version)]. Zhongguo Fei Ai Za Zhi 2017; 20: 1–13 (In Chinese.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Yu J, Sun X, Meng X. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of central nerve system metastases from non‐small cell lung cancer. Cancer Lett 2014, 2014; 351: 6–12. [DOI] [PubMed] [Google Scholar]
- 6. Lombardi G, Di Stefano AL, Farina P et al Systemic treatments for brain metastases from breast cancer, non‐small cell lung cancer, melanoma and renal cell carcinoma: An overview of the literature. Cancer Treat Rev 2014; 40: 951–9. [DOI] [PubMed] [Google Scholar]
- 7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scarpace SL. Metastatic squamous cell non‐small‐cell lung cancer (NSCLC): Disrupting the drug treatment paradigm with immunotherapies. Drugs Context 2015; 4: 212289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hida T, Nishio M, Nogami N et al Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non‐small cell lung cancer. Cancer Sci 2017; 108: 1000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reck M, Rodríguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 12. Flanigan JC, Jilaveanu LB, Faries M et al Melanoma brain metastases: Is it time to reassess the bias? Curr Probl Cancer 2011; 35: 200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flanigan JC, Jilaveanu LB, Chiang VL, Kluger HM. Advances in therapy for melanoma brain metastases. Clin Dermatol 2013; 31: 264–81. [DOI] [PubMed] [Google Scholar]
- 14. Yoda S, Dagogo‐Jack I, Hata AN. Targeting oncogenic drivers in lung cancer: Recent progress, current challenges and future opportunities. Pharmacol Ther 2018; pii: S0163‐7258(18)30145‐1. [DOI] [PubMed] [Google Scholar]
- 15. Jindal V, Gupta S. Expected paradigm shift in brain metastases therapy—Immune checkpoint inhibitors. Mol Neurobiol 2018; 55: 7072–8. [DOI] [PubMed] [Google Scholar]
- 16. Goldberg SB, Gettinger SN, Mahajan A et al Pembrolizumab for patients with melanoma or non‐small‐cell lung cancer and untreated brain metastases: Early analysis of a non‐randomised, open‐label, phase 2 trial. Lancet Oncol 2016, 2016; 17: 976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldman JW, Crino L, Vokes EE et al Nivolumab (nivo) in patients (pts) with advanced (adv) NSCLC and central nervous system (CNS) metastases (mets): Track: Immunotherapy. J Thorac Oncol 2016; 11 (10 Suppl): S238–9.27676570 [Google Scholar]
- 18. Bidoli P, Chiari R, Catino A et al Efficacy and safety data from patients with advanced squamous NSCLC and brain metastases participating in the nivolumab Expanded Access Programme (EAP) in Italy. Ann Oncol 2016; 27 (6 Suppl): 1228. [Google Scholar]
- 19. Dudnik E, Yust‐Katz S, Nechushtan H et al Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer 2016; 98 (4 Suppl): 114–7. [DOI] [PubMed] [Google Scholar]
- 20. Henon C, Mezquita L, Auclin E et al P2.07‐005 impact of baseline leptomeningeal and brain metastases on immunotherapy outcomes in advanced non‐small cell Lung cancer (NSCLC) patients. J Thorac Oncol 2017; 12: S2417. [Google Scholar]
- 21. Livia R, Lilla R, Vand T et al Chemotherapy treatment is associated with altered PD‐L1 expression in lung cancer patients. J Cancer Res Clin Oncol 2018; 144: 1219–26. [DOI] [PubMed] [Google Scholar]


