Abstract
Background
Tumor infiltrating lymphocytes (TILs) are known to correlate with the prognosis of patients affected by a variety of cancer types. We evaluated TILs in patients who underwent surgery for lung squamous cell carcinoma (SCC).
Methods
Specimens obtained from patients during resection of lung SCC were examined for TIL density, lymphoid follicle formation, PD‐L1 expression, and the appearance of regulatory T cells (Tregs).
Results
We enrolled 72 patients who underwent surgery for SCC (TIL grades 0, 1, and 2: 29, 18, and 25, respectively). Lymphoid follicles were observed in 13 (18.1%) patients and 8 were positive for Tregs, which were always observed in association with lymphoid follicles (P < 0.001). Multivariate analysis revealed that lymphoid follicle formation, the appearance of Tregs, pathological stage, and pleural invasion were independent prognostic factors related to overall survival, whereas TIL density and PD‐L1 expression were not.
Conclusion
SCC patients with lymphoid follicle formation accompanied by Tregs show poor survival following lung resection surgery.
Keywords: Lung cancer, lymphoid follicle, squamous cell carcinoma, tumor‐infiltrating lymphocyte
Introduction
In the past decades, most of the advances made in the treatment of non‐small cell lung cancer (NSCLC) have focused on adenocarcinoma or non‐squamous cell carcinoma, while there has been no breakthrough in the treatment of squamous cell carcinoma (SCC). Recently, the use of immune checkpoint inhibitor (ICI) therapy has drastically changed treatment options for patients with NSCLC, particularly SCC. ICI administration blocks tumor immunoediting and induces tolerance between cancer cells and the immune system, thus affecting interaction between the PD‐1 pathway and its ligand (PD‐L1). Several reports have suggested that tumor‐infiltrating lymphocytes (TILs) are correlated with the prognosis of patients with various types of cancer, such as melanoma,1 colon,2 ovarian,3 breast,4 and pancreatic cancers.5 Furthermore, TILs play a complementary role for tumor node metastasis (TNM) classification, and TIL density or distribution is associated with lung cancer prognosis.6 A study found that the appearance of lymphoid follicles in tumor stroma is a possible prognostic factor;2 however, the study also noted that some studies reported favorable prognosis for patients with lymphoid follicles in tumor stroma, while others did not.2
In the present study, we focused on patients with resectable lung SCC and retrospectively analyzed the relationship between pathological factors, including TIL density, lymphoid follicles, PD‐1/PD‐L1 expression in tumor stroma, and patient prognosis in order to find prognostic markers to guide treatment.
Methods
Specimens obtained from consecutive lung SCC patients who underwent complete resection from January 2010 to December 2012 at our institution were analyzed. Informed consent for the use of materials was obtained from each patient and the Ethical Committee of Dokkyo Medical University Hospital approved this retrospective study (#R‐5‐8). Follow‐up examinations were completed for all patients by January 2018.
Resected specimens were fixed in 10% neutral buffered formalin at room temperature and then embedded in paraffin. Sections (2 μm thick) were obtained from a block including the largest cut surface of the tumor, stained with hematoxylin and eosin (H&E), and examined. Next, 4 μm thick sections were cut from the same blocks, deparaffinized in xylene, and dehydrated in graded alcohol solutions. A standard avidin‐biotin complex peroxidase technique was then used for immunohistochemical staining of primary antibodies against CD3, CD4, CD8, CD20, and CD25. Immunohistochemical analysis of PD‐L1 was performed by LSI Medience Corp. (Tokyo, Japan) using a commercially available antibody (22C3). A trained observer and a pathologist reviewed each slide in detail. When there was disagreement, other pathologists were introduced into the discussion.
The TILs were divided into three grades based on intensity and distribution: grade 0, low or focal intensity; grade 1, medium or multifocal intensity; and grade 2, high or diffuse intensity (Fig 1a–c). Lymphoid follicles were identified as CD20‐positive B cell accumulations with a germinal center (Fig 1d–f). The T cell:B cell ratio of TILs was determined based on a CD3:CD20 ratio. CD4 and CD25 double‐positive T cells in TILs were considered to be infiltration by regulatory T cells (Tregs) (Fig 1g–i).
Figure 1.
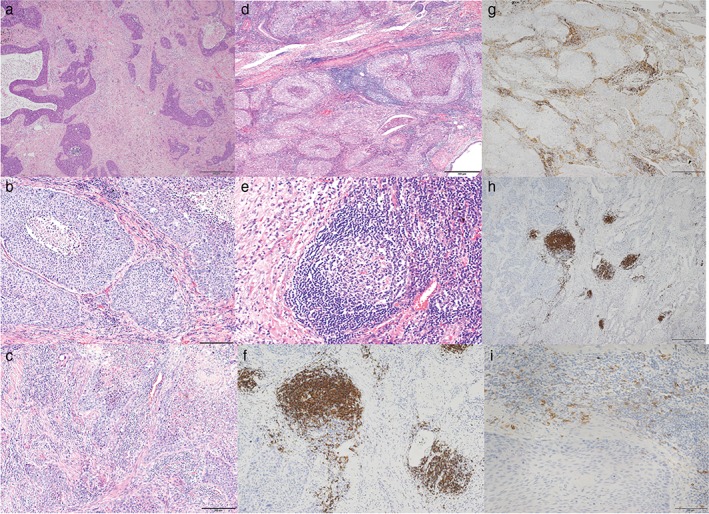
Representative images showing tumor‐infiltrating lymphocytes (TILs): (a) grade 0 (hematoxylin and eosin [H&E] stain, ×40), (b) grade 1 (HE stain, ×100), and (c) grade 2 (HE stain, ×100). Lymphoid follicles (d) in tumor stroma (HE stain, ×40), (e) with a germinal center (HE stain, ×200) and (f) composed of B cells (CD20 stain, ×100). (g) T cells (CD4 stain, ×40). (h) B cells (CD20 stain, ×40). (i) Regulatory T cells (CD25 stain, ×200).
Statistical analysis of the groups was performed using chi‐square or Fischer's exact tests to compare variables. Survival curves were obtained using the Kaplan–Meier method and comparisons within each group were performed using a log‐rank test. Risk factors for overall survival were evaluated via univariate and multivariate analyses using the Cox regression method. Statistical calculations were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). Significance was considered at P < 0.05.
Results
A total of 72 patients with SCC underwent lung resection during the study period. Patient characteristics are shown in Table 1. Nine out of 72 patients were administered neoadjuvant therapy before surgery, 5 were administered chemoradiotherapy, and 4 were administered chemotherapy. PD‐L1 expression was measured in all patients, with data from 63 available, which showed 0% in 19 and ≥ 1% in 44. Twenty‐four patients experienced postoperative complications: respiratory morbidity in 19 (pneumonia in 7, prolonged air leakage in 6, acute exacerbation of interstitial pneumonia in 4, and bronchopleural fistula in 2); cardiovascular complications in 4 (arrhythmia in 2 and heart failure in 2); and surgical site infection in 1. During the five‐year follow‐up period, 31 patients died: 18 as the result of lung cancer and 13 to other causes (pneumonia in 4, cardiovascular disease in 2, empyema in 2, interstitial pneumonia in 1, liver cirrhosis in 1, and unknown in 3).
Table 1.
Patient characteristics
| Characteristics | N = 72 |
|---|---|
| Gender | |
| Male | 67 |
| Female | 5 |
| Smoking index (packs/year) | |
| < 30 | 10 |
| ≥ 30 | 62 |
| Interstitial pneumonia | |
| Absent | 50 |
| Present | 22 |
| SCC | |
| ≤ 1.6 | 39 |
| > 1.6 | 32 |
| Unknown | 1 |
| Neoadjuvant therapy | |
| + | 9 |
| − | 63 |
| Type of resection | |
| Sublobar | 9 |
| Lobectomy or more | 63 |
| Pleural invasion | |
| 0 | 51 |
| 1 | 8 |
| 2 | 3 |
| 3 | 10 |
| Lymphatic invasion | |
| 0 | 60 |
| 1 | 12 |
| Vascular invasion | |
| 0 | 28 |
| 1 | 44 |
| Pathological stage | |
| 0 | 1 |
| IA | 20 |
| IB | 15 |
| IIA | 11 |
| IIB | 8 |
| IIIA | 17 |
| PD‐L1 expression | |
| 0% | 19 |
| 1–49% | 36 |
| ≥ 50% | 8 |
| Unknown | 9 |
| Postoperative complication | |
| + | 24 |
| − | 48 |
| Status | |
| Alive | 39 |
| Died | 31 |
| Unknown | 2 |
SCC, squamous cell carcinoma‐related antigen.
Among the 72 patients, the TIL grades were: 0 in 29, 1 in 18, and 2 in 25. Lymphoid follicles were observed in 13 (18.1%) of the 72 cases. Tregs were positive in eight cases and were exclusively observed in tumors of patients with lymphoid follicles (P < 0.001) (Table 2). The relationship between lymphoid follicles and PD‐L1 expression is shown in Table 2, although the correlation was not statistically significant (P = 0.16).
Table 2.
Relationship between lymphoid follicles and regulatory T cells
| Tregs | PD‐L1 | ||||||
|---|---|---|---|---|---|---|---|
| Absent | Present | P | 0% | ≥ 1% | P | ||
| Lymphoid follicle | Absent | 59 | 0 | 13 | 37 | ||
| Present | 5 | 8 | < 0.001 | 6 | 7 | 0.16 | |
Tregs, regulatory T cells.
Univariate analysis was performed using each factor (Table 3). The results revealed that pathological stage, pleural invasion, vascular invasion, and lymphoid follicles were associated with overall survival after lung resection, while neoadjuvant therapy, postoperative complications, TIL grade, and PD‐L1 were not correlated with survival. Multivariate analysis was conducted with a P of < 0.05 in univariate analysis, and revealed that pleural invasion and lymphoid follicles were independent prognostic factors related to overall survival in patients who underwent lung resection (Table 3). The five‐year survival rates of patients positive and negative for pleural invasion were 42.3% and 57.8%, respectively, representing a statistically significant difference (P = 0.008) (Fig 2a). Furthermore, the five‐year survival rates for patients positive and negative for lymphoid follicles were 19.2% and 60.5%, respectively (P = 0.002) (Fig 2b). The five‐year survival rates of patients positive and negative for Tregs were 18.8% and 58.3%, respectively (P = 0.003) (Fig 2c).
Table 3.
Univariate and multivariate analyses of overall survival
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | P | HR | 95% CI | P | ||
| Gender | ||||||||
| Male/female | 0.87 | 0.27 | 4.75 | 0.87 | ||||
| PS | ||||||||
| 0/≥ 1 | 2.26 | 0.79 | 6.49 | 0.13 | ||||
| BI | ||||||||
| < 600/≥ 600 | 5.59 | 0.76 | 41.0 | 0.091 | ||||
| IP | ||||||||
| −/+ | 1.90 | 0.92 | 3.94 | 0.082 | ||||
| SCC | ||||||||
| ≤ 1.6/> 1.6 | 1.65 | 0.81 | 3.35 | 0.17 | ||||
| pStage | ||||||||
| I/II + III | 2.54 | 1.19 | 5.41 | 0.012 | 1.51 | 0.66 | 3.47 | 0.33 |
| Neoadjuvant therapy | ||||||||
| −/+ | 0.63 | 0.19 | 2.07 | 0.45 | ||||
| Operation | ||||||||
| limited/lobectomy or more | 0.94 | 0.33 | 2.71 | 0.91 | ||||
| Postoperative complication | ||||||||
| −/+ | 1.48 | 0.72 | 3.02 | 0.29 | ||||
| Pleural invasion | ||||||||
| −/+ | 2.54 | 1.24 | 5.22 | 0.011 | 2.26 | 1.08 | 4.74 | 0.031 |
| Lymphatic invasion | ||||||||
| −/+ | 1.64 | 0.70 | 3.84 | 0.28 | ||||
| Vascular invasion | ||||||||
| −/+ | 2.43 | 1.08 | 5.46 | 0.031 | 1.60 | 0.68 | 3.79 | 0.28 |
| TIL grade | ||||||||
| 0/1–2 | 1.03 | 0.49 | 2.14 | 0.94 | ||||
| 0–1/2 | 0.97 | 0.45 | 2.06 | 0.93 | ||||
| T/B lymphocyte ratio | ||||||||
| < 1/≥ 1 | 0.94 | 0.46 | 2.17 | 0.99 | ||||
| PD‐L1 | ||||||||
| 0%/≥ 1% | 0.78 | 0.34 | 1.77 | 0.55 | ||||
| 0–49%/≥ 50% | 1.97 | 0.74 | 5.20 | 0.17 | ||||
| Lymphoid follicles | ||||||||
| −/+ | 3.33 | 1.50 | 7.43 | 0.003 | 2.61 | 1.11 | 6.01 | 0.026 |
BI, Brinkman index (smoking index); CI, confidence interval; HR, hazard ratio; IP, interstitial pneumonia; PS, performance status; pStage, pathological stage; SCC, squamous cell carcinoma‐related antigen; TIL, tumor‐infiltrating lymphocyte.
Figure 2.
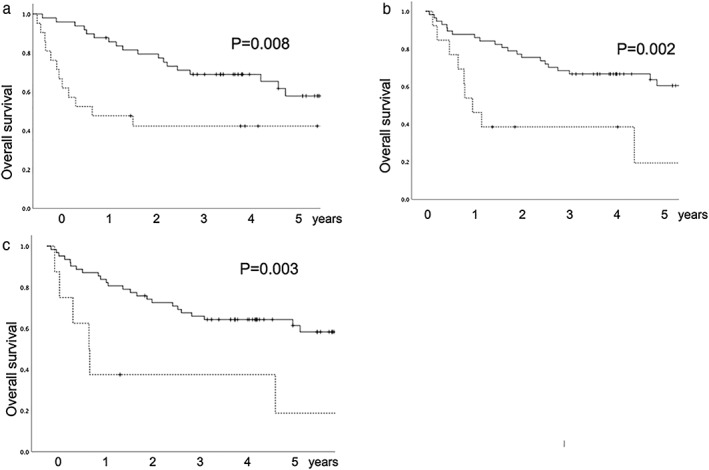
Overall survival curves. (a) Relationship between pleural invasion and overall survival. Solid line, negative for pleural invasion; dotted line, positive for pleural invasion. (b) Relationship between existence of lymphoid follicles and overall survival. Solid line, negative for lymphoid follicles; dotted line, positive for lymphoid follicles. (c) Relationship between the existence of regulatory T cells and overall survival. Solid line, negative for regulatory T cells; dotted line, positive for regulatory T cells.
Discussion
In the present study, we evaluated TIL density and lymphoid follicles in tumor stroma to determine their effectiveness as prognostic factors in patients with lung SCC who have undergone surgery. Our results indicate that lymphoid follicles, the appearance of Tregs, pathological stage, and pleural invasion are independent prognostic factors related to overall survival following resection of lung SCC, while TIL density and PD‐L1 expression are not associated with survival. In the study cohort, Tregs were exclusively observed in cases with the presence of lymphoid follicles.
Tumor‐infiltrating lymphocytes exist around cancer cells and play an important role in the mechanism of cancer immunity.7 Several studies have reported relationships between TIL subsets, such as CD3‐, CD8‐, and FOXP3‐positive cells, and prognosis.8, 9, 10, 11 Downregulation of the Fas/Fas‐ligand pathway leads to cancer growth as cancer cells escape from the immune checkpoint system as a result of the inactivation of CD4‐ and CD8‐positive lymphocytes, and subsequently avoid cytotoxic T cell attacks and apoptosis.12 We evaluated CD4 and CD8 density as parameters of TIL subsets in the present specimens, but did not find their density or distribution to be a prognostic factor, with the exception of the existence of Tregs. Hasegawa et al. reported the prognostic value of Tregs in NSCLC.13 We speculate that the existence of Tregs is more important to prognosis than TIL quantity, as shown in CD4‐ and CD8‐ positive cells.
Studies related to colon cancer have observed lymphoid follicle formation in advanced stage patients and superior prognosis in patients with lymphoid follicles compared to those without.14, 15, 16 The presence of lymphoid follicles has been termed a Crohn's‐like lymphoid reaction and a germinal center occurs with lymphocyte accumulation. The existence of these follicles has been proposed to be a prognostic factor independent of stage and TIL.17, 18, 19 In the present study, we considered that CD20‐positive B lymphocyte accumulation with a germinal center was evidence of lymphoid follicles and their existence in patients with lung SCC indicated poor prognosis compared to those without follicles. In the present study, all Treg positive patients had lymphoid follicles in their specimens. Because Treg existence is an indicator of poor prognosis in resected cancer,13 Tregs around lymphoid follicles may have affected the survival of our study cohort. Further case accumulation and analysis is required to explain the difference between our data and other references.
FoxP3 is a specific marker of CD4 and CD25 double‐positive Tregs, and Tregs play a role in the suppression and regulation of immune response, as well as the prevention of autoimmune disease.20 The existence of Tregs in TILs has been reported to be correlated with poor survival of patients with melanoma,21 kidney,22 breast,23 and ovarian cancers,24 while those with Hodgkin lymphoma showed superior survival.25 In colon cancer, the role of Tregs has not been consistently elucidated.26 For example, a study found that tumor‐infiltrating Tregs were associated with recurrence in pathologic stage I NSCLC patients,27 while another reported that lung cancer patients with a high density of tumor‐infiltrating Tregs had better prognosis compared to those without Tregs.28 In the present study, we defined CD4 and CD25 double‐positive lymphocytes as Tregs. These were only found around lymphoid follicles and all patients with Tregs had lymphoid follicle formation. We speculate that Tregs may suppress the immune response of lymphoid follicles, leading to poor prognosis for patients with such follicles.
Patients who undergo resection of advanced lung cancer often experience recurrence. Unfortunately, therapy options for recurrent disease are limited, especially for lung SCC. Recently, the utility of ICIs targeting PD‐1 or PD‐L1 has been reported, and PD‐L1 expression seems to be a biomarker of the effects of ICI. In the present series, the enrolled patients underwent surgery from 2010 to 2012, and were not administered ICI therapy during the postoperative course. We conducted our study to clarify whether PD‐L1 expression itself affected survival in patients that were not administered ICI therapy. Elevated PD‐L1 expression is associated with a poor outcome in patients with bladder and ovarian cancers,29, 30 while NSCLC patients with PD‐1 expression have also been shown to have a poor prognosis.31, 32 In our study, PD‐L1 expression itself was not a prognostic factor in patients with lung SCC following surgery and was not correlated with lymphoid follicle formation.
Limitations of this study include its retrospective study design, performance by a single institution, and the small number of cases. Additional case accumulation is needed to analyze the effects of TILs and lymphoid follicles on tumor‐immune interaction in cases of lung SCC.
In conclusion, lymphoid follicle formation, the appearance of Tregs, and pleural invasion were independent prognostic factors related to survival following resection of lung SCC, while TIL density and PD‐L1 expression were not.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank Masaru Kojima and Hajime Kuroda for their diagnostic assistance.
References
- 1. Ladányi A, Somlai B. T‐cell activation marker expression on tumor‐infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res 2004; 10: 521–30. [DOI] [PubMed] [Google Scholar]
- 2. Rozek LS, Schmit SL, Greenson JK, Tomsho LP, Rennert HS et al Tumor‐Infiltrating lymphocytes,crohn's‐like lymphoid reaction, survival from colorectal cancer. J Natl Cancer Inst 2016; 108: djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santoiemma PP, Powell DJ. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther 2015; 16: 6,807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loi S, Michiels S, Salgado R et al Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann Oncol 2014; 25: 1544–50. [DOI] [PubMed] [Google Scholar]
- 5. Fukunaga A, Miyamoto M, Cho Y et al CD‐8+ tumor‐infiltrating lymphocytes together with CD4+ tumor‐infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004; 28: e26–31. [DOI] [PubMed] [Google Scholar]
- 6. Geng Y, Shao Y, He W, Hu XY et al Prognostic role of tumor‐infiltrating lymphocytes in lung cancer: A meta analysis. Cell Physiol Biochem 2015; 37: 1560–71. [DOI] [PubMed] [Google Scholar]
- 7. Balch CM, Riley LB, Bae YJ et al Patterns of human tumor‐infiltrating lymphocytes in 120 human cancers. Arch Surg 1990; 125: 200–5. [DOI] [PubMed] [Google Scholar]
- 8. Kl AI‐S, Donnem T, Al‐Saad S, Persson M, Bremnes RM et al Prognostic effect of epithelial and stromal lymphocyte infiltration in non‐small cell lung cancer. Clin Cancer Res 2008; 14: 5220–7. [DOI] [PubMed] [Google Scholar]
- 9. Kawai O, Ishii G, Kubota K et al Predominant infiltration of macropharges and CD8+ Tcells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008; 113: 1387–95. [DOI] [PubMed] [Google Scholar]
- 10. Kayser G, Schulte‐Uentrop L, Sienel W et al Stromal CD4/CD25 positive T‐cells are a strong and independent prognostic factor in non‐small cell lung cancer patients, especially with adenocarcinomas. Lung Cancer 2012; 76: 445–51. [DOI] [PubMed] [Google Scholar]
- 11. Ruffini E, Asioli S, Filosso PL et al Clinical significance of tumor‐infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg 2009; 87: 365–72. [DOI] [PubMed] [Google Scholar]
- 12. Viard‐Leveugle I, Veyrenc S, French LE, Brambilla E. Frequent loss of Fas expression and function in human lung tumours with overexpression of FasL in small cell lung carcinoma. J Pathol 2003; 201: 268–77. [DOI] [PubMed] [Google Scholar]
- 13. Hasegawa T, Suzuki H, Yamamura T, Muto S, Okabe N et al Prognostic value of peripheral and local forkehead box P3+ regulatory T cells in patients with non‐small‐cell lung cancer. Mol Clin Oncol 2014; 2: 685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrison JC, Dean PJ, El‐Zeky F, Zwaag RV. Impact of the Crohn's‐like lymphoid reaction on staging of right‐sided colon cancer. Hum Pathol 1995; 26: 31–8. [DOI] [PubMed] [Google Scholar]
- 15. Graham DM, Appelman HD. Crohn's like lymphoid reaction and colorectal carcinoma: A potential histologic prognosticator. Mod Pathol 1990; 3: 332–5. [PubMed] [Google Scholar]
- 16. Murphy J, O'Sullivan GC, Lee G, Madden M, Shanahan F et al The inflammatory response within dukes’ B colorectal cancers: Implications for progression of micro metastases and patient survival. Am J Gastroenterol 2000; 95: 3607–14. [DOI] [PubMed] [Google Scholar]
- 17. Mahmoud SM, Paish EC, Powe DG, Macmillian RD, Grainge MJ et al Tumor‐infiltrating CD8+lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29: 1949–55. [DOI] [PubMed] [Google Scholar]
- 18. Vayrynen JP, Sajanti SA, Klintrup K, Makela J, Herzig KH et al Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer 2014; 134: 2126–35. [DOI] [PubMed] [Google Scholar]
- 19. Bento DC, Jones E, Junaid S et al High endothelial venules are rare in colorectal cancers but accumulate in extra‐tumoral areas with progression. OncoImmunology 2015; 4: e974374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains(CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol 1995; 155: 1151–64. [PubMed] [Google Scholar]
- 21. Gerber AL, Munst A, Schlapbach C, Shafighi M, Kiermeir D et al High expression of FOXP3 in primary melanoma is associated with tumour progression. Br J Dermatol 2014; 170: 103–9. [DOI] [PubMed] [Google Scholar]
- 22. Li JF, Chu YW, Wang GM, Zhu TY, Rong RM et al The prognostic value of peritumoral regulatory T cells and its correlation with intratumoral cyclooxygenase‐2 expression in clear cell renal cell carcinoma. BJU Int 2008; 103: 399–405. [DOI] [PubMed] [Google Scholar]
- 23. Kim MH, Koo JS, Lee S. FOXP3 expression is related to high Ki‐67 index and poor prognosis in lymph node positive breast cancer patients. Oncology 2013; 85: 128–36. [DOI] [PubMed] [Google Scholar]
- 24. Curiel TJ, Coukos G, Zou L et al Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10: 942–9. [DOI] [PubMed] [Google Scholar]
- 25. Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF et al Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 2005; 11: 1467–73. [DOI] [PubMed] [Google Scholar]
- 26. Salama P, Phillips M, Grieu F et al Tumor‐infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 2009; 27: 186–92. [DOI] [PubMed] [Google Scholar]
- 27. Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H et al Prognostic potential of FOXP3 expression in non‐small cell lung cancer cells combined with tumor‐infiltrating regulatory T cells. Lung Cancer 2011; 75: 95–101. [DOI] [PubMed] [Google Scholar]
- 28. Petersen RP, Campa MJ, Sperlazza J et al Tumor infiltrating FOXP3+ regulatory T‐cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer 2006; 107: 2866–72. [DOI] [PubMed] [Google Scholar]
- 29. Darb‐Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S et al Prognostic impact of programmed cell death‐1(PD‐1) and PD‐ligand 1 (PD‐L1) expression in cancer cells and tumor‐infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2015; 7: 1486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang Y, Zhang S, McCrudden C, Chan KW, Lin Y et al The prognostic significance of PD‐L1 in bladder cancer. Oncol Rep 2015; 33: 3075–84. [DOI] [PubMed] [Google Scholar]
- 31. Mazzaschi G, Madeddu D, Falco A et al Low PD‐1 expression in cytotoxic CD8+ tumor‐infiltrating lymphocytes confers an immune‐privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res 2018; 24: 407–19. [DOI] [PubMed] [Google Scholar]
- 32. Wu S, Shi X, Sun J et al The significance of programed cell death ligand 1 expression in resected lung adenocarcinoma. Oncotarget 2017; 8: 16421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


