Abstract
Background
The prognostic value of lymphovascular invasion (LVI) in esophageal cancer remains controversial. This study investigated the impact of LVI on prognosis in thoracic esophageal squamous cell carcinoma (ESCC).
Methods
A total of 1586 patients who underwent radical esophagectomy were selected for the study. Correlations between LVI and clinicopathological features were evaluated by χ2 test. Univariate analysis of the survival curve was conducted using the Kaplan–Meier method. Multivariate analysis was carried out by Cox regression. The Akaike information criterion (AIC) and the concordance index (c‐index) were employed to assess model prognostic accuracy of different pN staging systems.
Results
The presence of LVI was detected in 406 of 1586 (25.6%) patients. LVI frequency was significantly higher in patients with higher pN classifications (P < 0.001). LVI had independent significant prognostic value in ESCC (P < 0.001). In subgroup analyses, the presence of LVI significantly decreased overall survival in pN0, pN2, and pN3 stage patients. The AIC value of the pN staging system modified by LVI was lower than that of the current pN staging system, while the c‐index of the modified pN staging system was higher than that of the current pN staging system.
Conclusion
Our results suggest that LVI is an independent prognostic indicator in radically resected thoracic ESCC. LVI could potentially supplement the pN ESCC staging system. ESCC patients with LVI could be staged at more advanced pN classifications.
Keywords: Esophageal cancer, lymphovascular invasion, prognosis
Introduction
Esophageal cancer is the fifth most common cancer in China, accounting for more than 20 000 cancer‐related deaths annually. More than 90% of esophageal cancer cases have been classified as esophageal squamous cell carcinoma (ESCC) in China and esophageal adenocarcinoma is the predominant type in Western countries.1, 2 Despite advances made in chemoradiotherapy for ESCC, esophagectomy is still the most effective treatment measure at present.3, 4, 5 However, more than half of the patients who undergo radical esophagectomy develop locoregional or distant recurrence within three years.6, 7, 8
The current system predicating the prognosis of patients with ESCC is based on the
American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) Tumor Node Metastasis (TNM) classification comprising several clinicopathological features, such as depth of tumor invasion, tumor cell differentiation, regional lymph node metastasis, and distant metastasis.9 Lymphovascular invasion (LVI) provides the substantial basis for cancer cells metastasizing from the primary tumor to locoregional nodes or distant organs; therefore, LVI might have significant prognostic value in cancer patients.10 Several retrospective studies have reported that LVI is a poor prognostic factor for esophageal cancer, while others have reported that LVI is not a risk factor in esophageal cancer recurrence.11, 12, 13, 14, 15, 16 The aim of the current study was to analyze the correlation between the presence of LVI and clinicopathological characteristics in radically resected thoracic ESCC. The impact of LVI on prognosis was also investigated.
Methods
Patients
The Medical Ethics Committee of Fujian Cancer Hospital approved this study. We screened patients diagnosed with thoracic ESCC who underwent radical esophagectomy at Fujian Cancer Hospital from January 2001 to December 2010. The selection criteria included: (i) pathologically confirmed ESCC; (ii) transthoracic esophagectomy with three‐field lymphadenectomy had been performed; (iii) pathological T status of T1, T2, T3, or T4a; (iv) microscopically complete resection of the tumor (R0); and (v) aged ≥ 18 and ≤ 75 years. We did not include patients who died as a result of perioperative complications. Patients who received neoadjuvant chemotherapy or radiotherapy were also excluded. A total of 1586 patients were selected for the study.
Detailed clinicopathological data including patient age, gender, tumor location, depth of tumor invasion, tumor cell differentiation, lymph node status, and LVI, are summarized in Table 1. Pathological staging was reassessed based on the 7th AJCC staging system.9
Table 1.
Association between lymphovascular invasion and clinicopathologic variables
| Lymphovascular invasion | |||
|---|---|---|---|
| Variables | Yes (n = 406) | No (n = 1180) | P |
| Gender (%) | |||
| Male | 328 (27.4) | 868 (72.6) | 0.004 |
| Female | 78 (20.0) | 312 (80.0) | |
| Median age (years) | 55 | 56 | |
| Tumor location (%) | |||
| Upper thoracic | 58 (23.5) | 189 (76.5) | 0.150 |
| Middle thoracic | 320 (26.7) | 878 (73.3) | |
| Lower thoracic | 28 (19.9) | 113 (80.1) | |
| Pathological T stage (%) | |||
| T1 | 21 (17.2) | 101 (82.8) | 0.002 |
| T2 | 55 (21.2) | 204 (78.8) | |
| T3 | 245 (25.8) | 704 (74.2) | |
| T4a | 85 (33.2) | 171 (66.8) | |
| Pathological N stage (%) | |||
| N0 | 107 (17.1) | 520 (82.9) | < 0.001 |
| N1 | 117 (25.3) | 346 (74.7) | |
| N2 | 112 (32.6) | 232 (67.4) | |
| N3 | 70 (46.1) | 82 (53.9) | |
| Tumor cell differentiation (%) | |||
| Good | 40 (14.9) | 229 (85.1) | < 0.001 |
| Moderate | 274 (26.9) | 743 (73.1) | |
| Poor | 93 (31.0) | 207 (69.0) | |
Pathological review
A pathologist blinded to the pathological diagnoses and outcomes reviewed a minimum of two original histopathologic primary tumor slides. Any discrepancies between pathological diagnosis and diagnosis at the time of esophagectomy were resolved by simultaneous re‐examination of all primary tumor slides by two pathologists. LVI was defined as the presence of neoplastic structures inside the lumen of a vessel (Fig 1).17 The diameter of the lumen was generally < 300 μm, including venules, arterioles, and small lymph vessels. As they cannot be distinguished under a microscope on hematoxylin and eosin stained slides, all of these structures are referred to as LVI.
Figure 1.
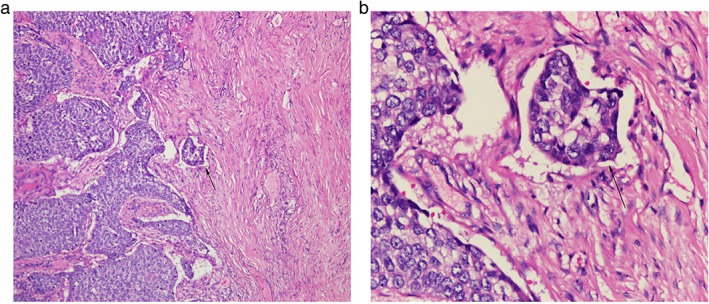
Hematoxylin and eosin staining for lymphovascular invasion. Arrows: (a) original magnification ×100, (b) ×400.
Follow up
After primary treatment, patients were followed up at the outpatient clinic every three months for the first two years, and every six months for the next three years thereafter. If a patient could not afford regular follow‐up visits, they were contacted via telephone and asked predetermined questions with special emphasis on their vital status. If the patient had died, family members were contacted to determine whether or not the patient's death was caused by esophageal cancer. All patients were followed up for at least five years or until death.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Correlations between LVI and clinicopathological features were evaluated by χ2 test. Multiple logistic regression analysis was performed to determine any association between clinicopathological features and the presence of LVI. Overall survival (OS) was defined from the date of esophagectomy to the date of death or final follow‐up. For univariate analysis, survival was derived using the Kaplan–Meier method, and the differences between curves were assessed by the log‐rank test. Factors with statistical significance (P < 0.05) in univariate analyses were included in multivariate analysis. Multivariate analysis was performed using the Cox proportional hazards regression model. We hypothesized that patients with LVI could be staged at more advanced pN classifications. The Akaike information criterion (AIC) and the concordance index (c‐index) were employed in the Cox proportional hazards regression model to assess the prognostic accuracy of the different pN staging systems. AIC was defined as previously described.
Lower AIC values indicate better goodness‐of‐fit. The c‐index was calculated using R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria), and larger c‐index values indicate better predicted precision of outcome.18
Results
Lymphovascular invasion (LVI) patterns and association with clinicopathological variables
The presence of LVI was detected in 406 out of 1586 (25.6%) ESCC patients. LVI was significantly associated with gender, depth of tumor invasion, pN classification, and tumor cell differentiation (Table 1). No correlation was observed between LVI and age or tumor location. Logistic analysis revealed that LVI frequency was significantly higher in patients with higher pN classifications (odds ratio 1.520, 95% confidence interval 1.353–1.708; P < 0.001).
Prognostic value of LVI in thoracic esophageal squamous cell carcinoma (ESCC) patients
Univariate analyses of clinicopathological factors and the presence of LVI were performed to explore the factors affecting the prognosis of thoracic ESCC patients who underwent radical esophagectomy. Among the factors analyzed, gender (P < 0.001), age (≥ 56/< 56, P = 0.007), pathological T stage (P < 0.001), pathological N stage (P < 0.001), and the presence of LVI (P < 0.001) were significant prognostic factors (Fig 2). Other factors, such as tumor location and tumor cell differentiation, did not significantly affect prognosis. We included the five factors detected by univariate analysis as significant in multivariate analysis to identify independent prognostic factors. As shown in Table 2, the presence of LVI had independent significant prognostic value in radically resected thoracic ESCC (P < 0.001).
Figure 2.
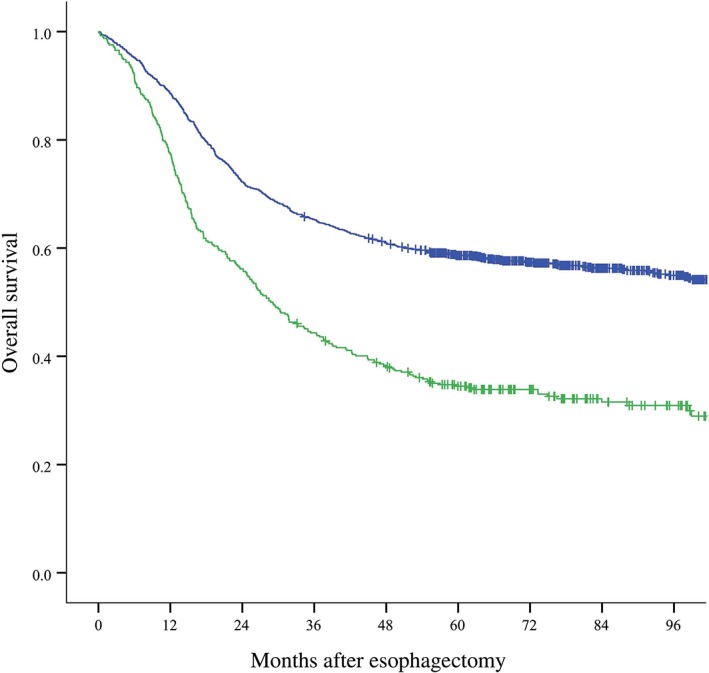
Association between the presence of lymphovascular invasion (LVI) and overall survival in thoracic ESCC patients. ( ) Tumor without LVI (n = 1180) and (
) Tumor without LVI (n = 1180) and ( ) Tumor with LVI (n = 406) (P < 0.001).
) Tumor with LVI (n = 406) (P < 0.001).
Table 2.
Multivariate Cox regression analysis
| Factor | HR (95% CI) | P |
|---|---|---|
| Current staging system | ||
| Gender (male/female) | 1.266 (1.063–1.508) | 0.008 |
| Age (≥ 56/< 56) | 1.282 (1.116–1.473) | < 0.001 |
| pT stage (T4a/T3/T2/T1) | 1.347 (1.214–1.494) | < 0.001 |
| pN stage (N3/N2/N1/N0) | 1.669 (1.558–1.788) | < 0.001 |
| Modified staging system | ||
| Gender (male/female) | 1.245 (1.045–1.483) | 0.014 |
| Age (≥ 56/< 56) | 1.307 (1.137–1.502) | < 0.001 |
| pT stage (T4a/T3/T2/T1) | 1.330 (1.200–1.457) | < 0.001 |
| pN stage (N4/N3/N2/N1/N0) | 1.615 (1.522–1.715) | < 0.001 |
CI, confidence interval; HR, hazard ratio.
We then performed univariate survival analyses for the presence of LVI stratified by pN stage. The results demonstrated that the presence of LVI significantly decreased OS in pathological N0 (P < 0.001), N2 (P = 0.004), and N3 (P = 0.002) stage patients, but not N1 (P = 0.131) stage patients (Fig 3). However, pN1 stage patients with LVI had poorer five‐year survival than patients without LVI (40.9% vs. 51.4%).
Figure 3.
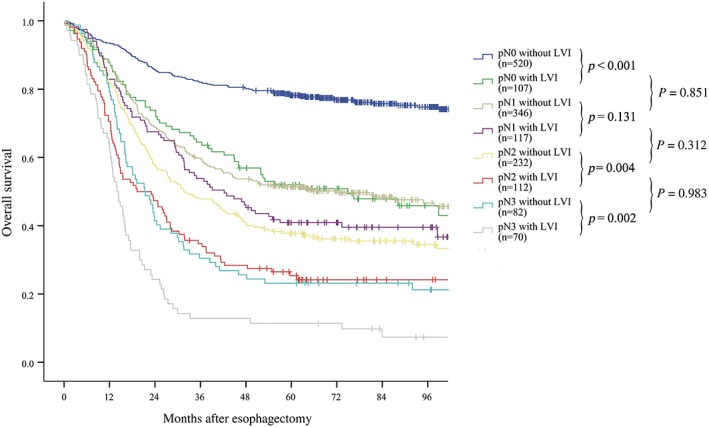
Univariate survival analyses of the presence of lymphovascular invasion (LVI) stratified by pN stage. The presence of LVI significantly decreased overall survival in pathological N0 (P < 0.001), N2 (P = 0.004), and N3 (P = 0.002) stage patients, but not in pathological N1 stage patients (P = 0.131). Patients at pN0 stage with LVI had a similar prognosis to pN1 patients without LVI (P = 0.851); pN1 patients with LVI had a similar prognosis to pN2 patients without LVI (P = 0.312); and pN2 patients with LVI had a similar prognosis to pN3 patients without LVI (P = 0.983).
LVI as a potential supplement to the pN classification of ESCC
Further univariate survival analyses were performed to compare the prognosis of patients with and without LVI at more advanced pN stages. The results showed that the prognosis in pN0 patients with LVI was similar to pN1 patients without LVI (P = 0.851) (Fig 3). The same results were also observed in patients with other pN classifications: pN1 patients with LVI had a prognosis similar to pN2 patients without LVI (P = 0.312); pN2 patients with LVI had a prognosis similar to pN3 patients without LVI (P = 0.983).
According to these results, we hypothesized that LVI is a potential supplement to the pN classification of ESCC: patients with LVI could be staged at more advanced pN classifications. That is, pN0 patients without LVI could be classified as pN0; pN0 patients with LVI and pN1 patients without LVI could be classified as pN1; pN1 patients with LVI and pN2 patients without LVI could be classified as pN2; pN2 patients with LVI and pN3 patients without LVI could be classified as pN3; and pN3 patients with LVI could be classified as pN4. Figure 4 shows the survival curves for the different classifications of the current and modified pN staging systems. Because both pN classifications were determined as prognostic factors in univariate analyses, two separate multivariate models were developed: one included age, gender, pT stage, and current pN stage; while the other included age, gender, pT stage, and modified pN stage. As shown in Table 2, using both models in accordance with the different staging systems, gender, age, pT stage, and pN stage were all independent prognostic factors for OS. The efficiency of the current and modified pN staging systems were quantified based on the likelihood ratio chi‐square, AIC, and c‐index. The results in Table 3 show that the AIC value in the modified model was lower and the c‐index value in the modified model was higher than that of the current model, indicating that the modified pN staging system is a better prognostic stratification.
Figure 4.
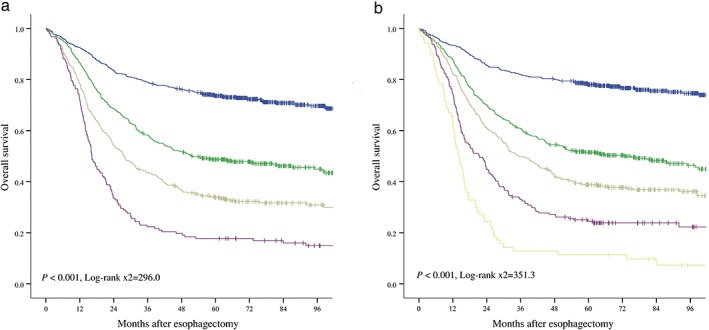
Overall survival analyses of the different classifications in the (a) current ( ) pN0 (n = 627), (
) pN0 (n = 627), ( ) pN1 (n = 463), (
) pN1 (n = 463), ( ) pN2 (n = 344), and (
) pN2 (n = 344), and ( ) pN3 (n = 152) and (b) modified pN staging systems (
) pN3 (n = 152) and (b) modified pN staging systems ( ) pN0 (n = 520), (
) pN0 (n = 520), ( ) pN1 (n = 453), (
) pN1 (n = 453), ( ) pN2 (n = 349), (
) pN2 (n = 349), ( ) pN3 (n = 194), and (
) pN3 (n = 194), and ( ) pN4 (n = 70). Both the current and modified pN classifications were prognostic factors in univariate analyses.
) pN4 (n = 70). Both the current and modified pN classifications were prognostic factors in univariate analyses.
Table 3.
Comparison between two multivariate Cox regression models in accordance with different pN staging systems
| Cox model | −2 log likelihood | AIC value | C‐index (95% CI) |
|---|---|---|---|
| Current model | 10892.25 | 10900.25 | 0.6870 (0.6683–0.7056) |
| Modified model | 10857.16 | 10865.16 | 0.6952 (0.6767–0.7138) |
Current model, multivariate Cox regression model in accordance with current pN staging system; modified model, multivariate Cox regression model in accordance with pN staging system modified by the presence of lymphovascular invasion. AIC, Akaike information criterion; C‐index, concordance index; CI, confidence interval.
Discussion
The current TNM staging system comprising depth of tumor invasion, tumor cell differentiation, regional lymph node metastasis, and distant metastasis is considered the most effective prognostic factor of esophageal cancer. Pathological studies have incorporated LVI into the TNM staging systems of multiple cancers;10, 19, 20 however the prognostic value of LVI in esophageal cancer remains controversial. Most retrospective studies indicated the presence of LVI as a poor prognostic factor for esophageal cancer.11, 12, 13, 14 Zhu et al. proposed a new prognostic model with the pN classification supplemented by LVI that might improve the ability to discriminate esophageal cancer patient outcomes.13 On the other hand, some studies suggested that the presence of LVI may not be a significant risk factor in esophageal cancer recurrence.15, 16
In this study, we retrospectively reviewed the presence of LVI in radically resected thoracic ESCC. Our results demonstrated that LVI was present in 406 out of 1586 (25.6%) ESCC patients and LVI frequency was significantly higher in patients with higher pN classifications, which is consistent with the results of previous reports.12, 13, 14 Survival analyses revealed that LVI was an independent prognostic indicator in radically resected thoracic ESCC. Khan et al. and Waraich et al. reported that LVI was not a prognostic indicator in esophageal cancer;15, 16 however, the histology of tumors in their studies included adenocarcinoma and squamous cell carcinoma, and adenocarcinoma was the predominant histology type.
Univariate survival analyses stratified by pN stage showed that patients with LVI had poorer survival than patients at the same pN stage without LVI, but a similar prognosis to patients with more advanced pN stage that in those without LVI. Therefore, we propose LVI as a potential supplement to the pN staging system of ESCC: patients with LVI could be promoted to a more advanced pN classification. Compared to the current pN staging system, the proposed modified system including the presence of LVI showed better prognostic stratification. To our knowledge, this is the first report to incorporate LVI into the ESCC pN staging system. Zhu et al. proposed the presence of LVI and lymph node metastasis as equal risk factors in ESCC; however, they did not stratify detailed pN stage.13
There are some limitations to our study. First, the presence of LVI was evaluated on hematoxylin and eosin stained slides without any distinction between lymphatic and vascular invasion. Wang et al. reported that the emergence of biomarkers for lymphatic endothelium (Podoplanin) and vascular endothelium (CD34) provides an opportunity to distinguish the existence of lymphatic and vascular invasion in ESCC.14 Second, we chose cancer‐related OS as the endpoint rather than disease‐free survival (DFS). Some authors consider DFS as a better endpoint because it can more significantly evaluate the association between the presence of LVI and locoregional or distant recurrence. However, we were unable to choose DFS as our research endpoint because of incomplete follow‐up data.
In conclusion, our findings suggest that the presence of LVI is significantly correlated with pN classification. LVI is considered an independent prognostic indicator in radically resected thoracic ESCC and could potentially supplement the pN staging system for thoracic ESCC. Patients with ESCC with LVI could be promoted to more advanced pN classifications.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This study was financed by the Fujian Provincial Health and Family Planning Research Talent Training Program (Grant number: 2018‐ZQN‐16, and 2018‐CXB‐3).
Peng Chen and Zhen Wang contributed equally to this study.
References
- 1. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Zeng H et al The incidence and mortality of major cancers in China, 2012. Chin J Cancer 2016; 35: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Hagen P, Hulshof MC, van Lanschot JJ et al Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. [DOI] [PubMed] [Google Scholar]
- 4. Ku GY, Ilson DH. Long‐term survival with salvage surgery for recurrent esophageal adenocarcinoma after chemoradiotherapy. J Clin Oncol 2015; 33: 3854–7. [DOI] [PubMed] [Google Scholar]
- 5. Yang H, Liu H, Chen Y et al Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A Phase III multicenter, randomized, open‐label clinical trial. J Clin Oncol 2018; 36: 2796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jang NY, Kim IA, Byun SS et al Patterns of failure and prognostic factors for locoregional recurrence after radical surgery in upper urinary tract transitional cell carcinoma: Implications for adjuvant radiotherapy. Urol Int 2013; 90: 202–6. [DOI] [PubMed] [Google Scholar]
- 7. Liu Q, Cai XW, Wu B et al Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: Implications for the clinical target volume design of postoperative radiotherapy. PLoS One 2014; 9: e97225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ninomiya I, Okamoto K, Tsukada T et al Recurrence patterns and risk factors following thoracoscopic esophagectomy with radical lymph node dissection for thoracic esophageal squamous cell carcinoma. Mol Clin Oncol 2016; 4: 278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rice TW, Gress DM, Patil DT et al Cancer of the esophagus and esophagogastric junction‐Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017; 67: 304–17. [DOI] [PubMed] [Google Scholar]
- 10. Fisseler‐Eckhoff A. New TNM classification of malignant lung tumors 2009 from a pathology perspective. Pathologe 2009; 30 (Suppl. 2): 193–9. [DOI] [PubMed] [Google Scholar]
- 11. Sugimachi K, Matsuura H, Kai H et al Prognostic factors of esophageal carcinoma: Univariate and multivariate analyses. J Surg Oncol 1986; 31: 108–12. [DOI] [PubMed] [Google Scholar]
- 12. Schoppmann SF, Jesch B, Zacherl J et al Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery 2013; 153: 526–34. [DOI] [PubMed] [Google Scholar]
- 13. Zhu CM, Ling YH, Xi SY et al Prognostic significance of the pN classification supplemented by vascular invasion for esophageal squamous cell carcinoma. PLoS One 2014; 9: e96129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang S, Chen X, Fan J et al Prognostic significance of lymphovascular invasion for thoracic esophageal squamous cell carcinoma. Ann Surg Oncol 2016; 23: 4101–9. [DOI] [PubMed] [Google Scholar]
- 15. Khan OA, Alexiou C, Soomro I et al Pathological determinants of survival in node‐negative oesophageal cancer. Br J Surg 2004; 91: 1586–91. [DOI] [PubMed] [Google Scholar]
- 16. Waraich N, Rashid F, Jan A et al Vascular invasion is not a risk factor in oesophageal cancer recurrence. Int J Surg 2011; 9: 237–40. [DOI] [PubMed] [Google Scholar]
- 17. Li H, Zhang Q, Xu L et al Factors predictive of prognosis after esophagectomy for squamous cell cancer. J Thorac Cardiovasc Surg 2009; 137: 55–9. [DOI] [PubMed] [Google Scholar]
- 18. Zheng Y, Wang Z, Wang F et al Proposed modifications of supraclavicular lymph node metastasis in the esophageal squamous cell carcinoma staging system for improved survival stratification. Oncotarget 2017; 8: 41563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Compton CC, Fielding LP, Burgart LJ et al Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000; 124: 979–94. [DOI] [PubMed] [Google Scholar]
- 20. Kojima M, Shimazaki H, Iwaya K et al Pathological diagnostic criterion of blood and lymphatic vessel invasion in colorectal cancer: A framework for developing an objective pathological diagnostic system using the Delphi method, from the Pathology Working Group of the Japanese Society for Cancer of the Colon and Rectum. J Clin Pathol 2013; 66: 551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


