Abstract
Indoor air pollution (IAP) or environmental tobacco smoke (ETS) exposure may influence nasopharyngeal carriage of bacterial species and development of lower respiratory tract infection (LRTI). The aim of this study was to longitudinally investigate the impact of antenatal or postnatal IAP/ETS exposure on nasopharyngeal bacteria in mothers and infants.
A South African cohort study followed mother–infant pairs from birth through the first year. Nasopharyngeal swabs were taken at birth, 6 and 12 months for bacterial culture. Multivariable and multivariate Poisson regression investigated associations between nasopharyngeal bacterial species and IAP/ETS. IAP exposures (particulate matter, carbon monoxide, nitrogen dioxide, volatile organic compounds) were measured at home visits. ETS exposure was measured through maternal and infant urine cotinine. Infants received the 13-valent pneumococcal and Haemophilus influenzae B conjugate vaccines.
There were 881 maternal and 2605 infant nasopharyngeal swabs. Antenatal ETS exposure was associated with Streptococcus pneumoniae carriage in mothers (adjusted risk ratio (aRR) 1.73 (95% CI 1.03–2.92)) while postnatal ETS exposure was associated with carriage in infants (aRR 1.14 (95% CI 1.00–1.30)) Postnatal particulate matter exposure was associated with the nasopharyngeal carriage of H. influenzae (aRR 1.68 (95% CI 1.10– 2.57)) or Moraxella catarrhalis (aRR 1.42 (95% CI 1.03–1.97)) in infants.
Early-life environmental exposures are associated with an increased prevalence of specific nasopharyngeal bacteria during infancy, which may predispose to LRTI.
Short abstract
Indoor air pollution and tobacco smoke exposure impacts on nasopharyngeal bacterial carriage in mothers and infants http://ow.ly/lonJ30n3REY
Introduction
Nasopharyngeal microbiota, comprised of a myriad of microorganisms, is rapidly acquired after birth and established in infancy [1, 2]. Nasopharyngeal carriage of potentially pathogenic bacteria in early life precedes the development of lower respiratory tract infections (LRTIs) including pneumonia and bronchiolitis [3]. Understanding the factors that impact on nasopharyngeal carriage may therefore be important in developing strengthened strategies to prevent LRTIs, a major cause of death in children in low and middle-income countries (LMICs).
Nasopharyngeal carriage may be influenced by several environmental factors including exposure to environmental tobacco smoke (ETS) or indoor air pollution (IAP) [4]. Rapid urbanisation particularly in LMIC, has resulted in an increased use of alternate fuel sources for household energy, with numerous by-products of combustion (including particulate matter (particulate matter <10 μm in diameter (PM10)), carbon monoxide (CO), nitrogen dioxide (NO2) and volatile organic compounds (VOCs)) contributing to IAP [5]. Antenatal and early-life exposure to ETS has many potentially detrimental effects on child health including an increased incidence and severity of LRTIs [6, 7].
While studies have assessed the effect of household air pollution on the lung microbiome [8], no studies have investigated the effects of IAP on nasopharyngeal carriage, particularly in infants. However, recently mouse models have shown black carbon (a component of particulate matter) induced structural, compositional and functional changes in bacterial biofilms and their responses to antibiotics as well as facilitated the micro-aspiration of Streptococcus spp. from the nasopharynx to the lung [9]. Further, ETS exposure increases the nasopharyngeal carriage of Streptococcus pneumoniae [10], alters innate immune mechanisms in the nasal mucosa and disrupts epithelial barriers [11].
This study longitudinally investigated the impact of antenatal and postnatal IAP and ETS exposure on nasopharyngeal carriage in mothers and infants enrolled in an African birth cohort study, from birth to 1 year of age.
Methods
Study population and procedures
This study was nested within the Drakenstein Child Health Study (DCHS), a birth cohort in a peri-urban area of South Africa [12]. Consenting pregnant women were enrolled at 20–28 weeks’ gestation at two public primary health clinics: Mbekweni (serving a predominantly black African population) and Newman (serving a predominantly mixed ancestry population) from March 2012 to July 2015.
All births occurred at a single, central hospital, Paarl hospital. Thereafter, mother–infant pairs were followed at 6, 10 and 14 weeks, 6, 9 and 12 months, for healthcare and immunisations including the 13-valent pneumococcal conjugate vaccine (PCV-13) given at 6, 14 weeks and 9 months and Haemophilus influenzae type b conjugate vaccine at 6, 10, 14 weeks according to the South African Expanded Programme on Immunisation schedule [13]. Study questionnaires and clinical data were collected at enrolment and follow-up visits. A validated socioeconomic score (SES) was used to categorise participants into quartiles as lowest, low-moderate, moderate-high or highest SES [5]. Clinical data collected at each follow-up visit included details on recent respiratory tract infections, including respiratory symptoms, otitis media, wheeze or LRTIs in the preceding month and any antibiotic use in the prior 6 months.
Assessing environmental exposures
Measuring IAP exposure
The participant's home environment was assessed and dwellings categorised based on having two or more defined household dimensions (type of home, building material, water supply, toilet facilities, kitchen type, ventilation in kitchen areas) [5]. IAP was measured at home visits conducted antenatally (within 4 weeks of enrolment) and postnatally (between 4–6 months of the infant's life) [5]. Home visits were conducted over 3 years with sampling occurring throughout the year covering all seasons and weather conditions. PM10 and CO were measured by separate monitors (AirChek 52; SKC, Eighty-Four, PA, USA for PM10 and Altair; Troy, MI, USA for CO) left in homes over 24 h. NO2 and VOCs, benzene and toluene, were measured using diffusion tubes (Radiello absorbent filters in polyethylene diffusive body; Sigma-Aldrich, St Louis, MO, USA) and (Markes thermal desorption tubes; Llantrisant, UK) left in homes for 2 weeks [5]. These monitors were internally calibrated for temperature and humidity as per the manufacturer information, whereas diffusion tubes were corrected for humidity during laboratory analysis [5, 14]. The South African National Ambient Air Quality Standards were used to define expected exposure levels for each pollutant based on an averaging period of 1 year for each measure [15, 16].
Measuring ETS exposure
Maternal, paternal and household tobacco smoking and exposure were assessed using questionnaires administered at enrolment, and antenatal and postnatal visits. These were validated using maternal and infant urine cotinine measures. Maternal cotinine was measured antenatally and at birth, and infant cotinine at birth and 6–10 week with the highest result used to assign smoking status and exposure. Cotinine levels were classified as <10 ng·mL−1 (nonsmoker), 10–499 ng·mL−1 (passive smoker/exposed), or ≥500 ng·mL−1 (active smoker) [7].
Assessing nasopharyngeal carriage of bacteria
Nasopharyngeal swabs were obtained from mothers (at the time of delivery) and infants at birth, 6 and 12 months by trained study staff according to World Health Organization recommendations [17]. The swabs were immediately stored in 1 mL of skimmed milk, tryptone, glucose and glycerol transport medium (STGG), transported on ice to the laboratory and frozen at −80°C for later batch processing. After thawing at room temperature (22°C), samples were vortexed for 15 s before plating out a 10 µL aliquot onto four different solid media (National Health Laboratory Services, Green Point Media Laboratory Cape Town, South Africa). Standard laboratory protocols were used for the phenotypic and biochemical identification of common bacterial species that asymptomatically colonise the upper respiratory tract. For S. pneumoniae culture, Columbia blood agar base with 2% agar, 5% horse blood and 4 mg·mL−1 gentamicin (CAG) was incubated at 37°C in 5% CO2 overnight. Presumptive S. pneumoniae isolates were identified by colony morphology, α-haemolysis, optochin disk susceptibility (Oxoid, Basingstoke, UK) and confirmed using lytA PCR [18]. For H. influenzae, bacitracin heated blood agar plates were incubated at 37°C with 5% carbon dioxide (CO2). Suspected H. influenzae colonies were inoculated onto Columbia agar and identified using Factors X, V and XV discs and by observing growth in the haemolytic zone of Staphylococcus aureus on blood agar plates. S. aureus isolates were identified by culturing on mannitol salt agar, and DNase testing whereas Moraxella catarrhalis isolates were identified by culturing on 2% blood agar and incubated overnight at 37°C. Isolates were presumptively identified by push test and confirmed by copB PCR [19]. Gram-negative bacteria were subcultured onto MacConkey agar and identified on Vitek 2® (bioMérieux, Marcy I'Etoile, France).
Ethics
The study was approved by the Human Research Ethics Committees of the Faculties of Health Sciences, University of Cape Town and Stellenbosch University, and by the Western Cape Provincial Health Research committee (HREC 149/2013). Mothers provided written informed consent at enrolment.
Statistical analysis
Study data were captured in a relational Microsoft Access® database or collected and managed using REDCap electronic data capture tools hosted at the University of Cape Town [20]. Statistical analyses were conducted in Stata version 14.2 for Windows (Stata Corp, College Station, TX, USA). Statistical tests were considered significant if the p-value was <0.05 or if p-value cut-offs were derived using the Benjamini–Hochberg procedure for assessing the association between IAP and the pathogens [21]. Categorical variables were summarised using frequency counts and percentages, n (%). Normally and non-normally distributed continuous variables were described using mean (sd) and median (interquartile range (IQR)) values, respectively. Mann–Whitney or Wilcoxon signed-rank tests, as appropriate, were used to compare medians as well as their spread. Cross tabulations with Fishers’ exact or Chi-squared tests were used to describe and compare the prevalence of pathogen carriage between the infants (at all time points) and their mothers or between different time points for infants.
Multivariable modified Poisson regression analyses with robust error variance [22] were performed to estimate adjusted risk ratios (aRRs) between each bacterial pathogen and IAP measures (individually (adjusted) or together (adjusted 2)). The association between antenatal exposures or maternal cotinine and maternal carriage was explored as was the association between postnatal exposure or infant cotinine and infant carriage. The multivariable Poisson regressions adjusted for demographic and clinical factors (weight-for-age z-score at birth, preterm, ethnicity, sex, HIV exposure, time on exclusive breastfeeding, average number of people per sleeping room, dwelling category, recent respiratory infection, day care attendance, vaccination, number of other children under 5 years in the household, antibiotic use) that have been associated with pathogen acquisition. Multivariable regressions were then further performed for each site [23, 24]. Further, we explored the possible confounding effects of bacterial co-carriage by including indicator variables for each pathogen.
Results
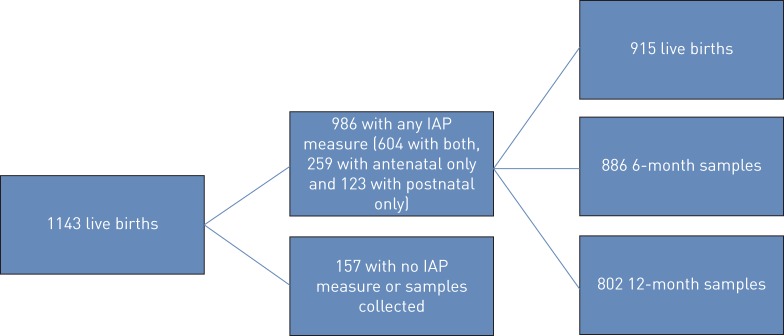
During the study period there were 1137 women enrolled with 1143 live births (four sets of twins and one set of triplets), of which 986 infants had at least one IAP home measurement and one nasopharyngeal swab collected and were included in this analysis (figure 1).
FIGURE 1.
Cohort description and samples collected. IAP: indoor air pollution.
Maternal characteristics
A total of 530 (54%) mothers were enrolled from Mbekweni (a predominantly black African population) and 452/982 (46%) from Newman (predominantly mixed ancestry). The median age of mothers enrolled was 25.8, IQR (22.0, 30.6) years. Significantly more Mbekweni mothers were HIV-infected; 193 (36%) compared with Newman, 13 (3%), p<0.001. However, with an effective prevention of mother-to-child transmission programme only two infants (<1%) were HIV-infected. Unemployment was high in both communities (74%) with more Mbekweni participants in the lowest SES (30%) compared with Newman (18%; table 1).
TABLE 1.
Maternal and infant characteristics
| Mbekweni (black African) | Newman (mixed ancestry) | Total | p-value | |
| Maternal characteristics | ||||
| Mothers | 530 (54%) | 452 (46%) | 982 (100%) | |
| Age at enrolment years | 26.9 (22.3, 31.6) | 24.6 (21.3, 29.1) | 25.8 (22.0, 30.6) | <0.001 |
| HIV-infected | 193 (36%) | 13 (3%) | 206 (21%) | <0.001 |
| Unemployed | 400 (75%) | 325 (72%) | 724 (74%) | 0.205 |
| SES quartile | ||||
| Lowest SES | 157 (30%) | 80 (18%) | 237 (24%) | <0.001 |
| Low–moderate SES | 150 (28%) | 109 (24%) | 259 (26%) | |
| Moderate–high SES | 126 (24%) | 127 (28%) | 253 (26%) | |
| Highest SES | 97 (18%) | 136 (30%) | 233 (24%) | |
| Household members | 4 (3, 6) | 5 (4, 7) | 4 (3, 6) | <0.001 |
| Married/cohabiting | 188 (35%) | 194 (43%) | 382 (39%) | 0.017 |
| Infant characteristics and birth outcomes | ||||
| Number of infants; sets of twins | 534 (54%); 4 | 452 (46%); 0 | 986 (100%); 4 | |
| Female | 280 (52%) | 204 (45%) | 484 (49%) | 0.022 |
| Gestation at delivery weeks | 39 (38, 40) | 39 (37, 40) | 39 (38, 40) | 0.032 |
| Birthweight g | 3180 (2810, 3460) | 2990 (2630, 3340) | 3080 (2720, 3415) | <0.001 |
| Weight-for-age z-score | −0.4 (−1.3, 0.2) | −0.7 (−1.4, −0.1) | −0.6 (−1.3, 0.0) | <0.001 |
| Birthweight <2500 g | 55 (10%) | 83 (18%) | 138 (14%) | <0.001 |
| Birth <37 weeks gestation | 16 (3%) | 19 (4%) | 35 (4%) | 0.314 |
| Feeding at 6 months | ||||
| Exclusive breastfeeding | 76 (14%) | 66 (15%) | 142 (14%) | <0.001 |
| Mixed | 89 (17%) | 170 (38%) | 259 (26%) | |
| Not breastfeeding | 369 (69%) | 216 (48%) | 585 (59%) | |
| Duration of exclusive breastfeeding months | 1.1 (0.5, 3.0) | 1.6 (0.9, 3.0) | 1.4 (0.7, 3.0) | 0.410 |
| Day care attendance | ||||
| 6 months of age | 17 (3%) | 28 (6%) | 45 (5%) | 0.024 |
| 12 months of age | 69 (13%) | 90 (20%) | 159 (16%) | 0.003 |
| Additional child under 5 years of age in household | 455 (85%) | 396 (88%) | 851 (86%) | 0.274 |
| Respiratory infection in prior month | ||||
| 6 months of age | 40 (7%) | 37 (8%) | 77 (8%) | 0.685 |
| 12 months of age | 29 (5%) | 22 (5%) | 51 (5%) | 0.691 |
| Antibiotic use in prior 6 months | ||||
| 6 months of age | 25 (5%) | 38 (8%) | 63 (6%) | 0.057 |
| 12 months of age | 14 (3%) | 13 (3%) | 27 (3%) | 0.957 |
Data are presented as median (interquartile range), unless otherwise stated. SES: socioeconomic score.
Infant characteristics
Of the 986 infants included, 484 (49%) were female; the median gestational age was 39 (IQR 38, 40) weeks. Most (585 (59%)) infants were not breastfed at 6 months and duration of exclusive breastfeeding was 1.4 (IQR 0.7, 3.0) months. Significantly more Newman infants attended day care at both 6 months (6%) and 12 months (20%) of age compared with Mbekweni infants; 6 months (3%) and 12 months (5%). In 851 (86%) of households there was at least one other child younger than 5 years. Overall, 77 (8%) children reported a recent respiratory infection at 6 months and 51 (5%) at 12 months of age. Antibiotic use was infrequent, (table 1), while vaccine coverage was high in infants (supplemental table 1).
Home environment and exposures
Almost 40% of homes were informal and one-third of homes had less than two of the defined household dimensions. Electricity access was high (95%); however, nearly one-third of Mbekweni homes used alternate fuels for cooking and heating. The median Mbekweni household size was 4 (IQR 3–6) people, smaller than Newman at 5 (IQR 4–7) people (table 2)
TABLE 2.
Home environment and exposures
| Mbekweni | Newman | Total | p-value | |
| Home environment | ||||
| Household dimensions# | ||||
| Has ≤2 dimensions | 185 (38%) | 121 (27%) | 306 (33%) | <0.001 |
| Has >2 dimensions | 302 (62%) | 320 (73%) | 622 (67%) | |
| Alternate fuel used (coal, wood, paraffin, gas) | ||||
| Cooking | 133 (31%) | 37 (10%) | 170 (21%) | <0.001 |
| Heating | 127 (29%) | 7 (2%) | 134 (16%) | <0.001 |
| Crowding | ||||
| Household size | 4 (3, 6) | 5 (4, 7) | 4 (3, 6) | <0.001 |
| Persons per sleeping room | 3 (2, 4) | 3 (2, 5) | 3 (2, 5) | 0.010 |
| Pollutants measured: antenatal | ||||
| PM10 g·m−3 (n=755) | 32.0 (12.3, 64.2) | 35.6 (12.8, 65.6) | 33.4 (12.4, 65.6) | 0.417 |
| Above threshold | 73 (19%) | 65 (18%) | 138 (18%) | 0.853 |
| NO2 µg·m−3 (n=747) | 7.3 (2.6, 14.6) | 7.1 (3.9, 11.3) | 7.1 (3.4, 12.7) | 0.494 |
| Above threshold | 16 (4%) | 3 (1%) | 19 (3%) | 0.005 |
| Benzene µg·m−3 (n=729) | 4.6 (1.5, 17.9) | 3.9 (1.8, 8.6) | 4.3 (1.8, 11.0) | 0.475 |
| Above threshold | 183 (47%) | 147 (43%) | 330 (45%) | 0.244 |
| Toluene µg·m−3 (n=729) | 16.1 (5.9, 43.0) | 17.4 (8.2, 46.5) | 16.9 (7.2, 44.6) | 0.282 |
| Above threshold | 36 (9%) | 30 (9%) | 66 (9%) | 0.803 |
| CO mg·m−3 (n=706) | 0.0 (0.0, 5.1) | 0.0 (0.0, 8.4) | 0.0 (0.0, 7.6) | 0.144 |
| Above threshold | 39 (10%) | 42 (14%) | 81 (11%) | 0.095 |
| Pollutants measured: postnatal | ||||
| PM10 g·m−3 (n=505) | 30.0 (14.7, 49.7) | 28.4 (10.5, 53.7) | 29.3 (12.6, 52.5) | 0.364 |
| Above threshold | 38 (16%) | 36 (14%) | 74 (15%) | 0.499 |
| NO2 µg·m−3 (n=532) | 6.3 (2.9, 14.6) | 5.3 (2.6, 11.3) | 5.8 (2.6, 12.67) | 0.119 |
| Above threshold | 6 (2%) | 1 (0%) | 7 (1%) | 0.041 |
| Benzene µg·m−3 (n=462) | 2.8 (0.8, 14.4) | 3.2 (1.5, 7.6) | 3.1 (1.1, 9.5) | 0.345 |
| Above threshold | 95 (39%) | 75 (35%) | 170 (37%) | 0.426 |
| Toluene µg·m−3 (n=462) | 15.1 (4.9, 50.0) | 15.9 (6.5, 51.7) | 15.5 (5.9, 50.0) | 0.342 |
| Above threshold | 24 (10%) | 23 (11%) | 47 (10%) | 0.728 |
| CO mg·m−3 (n=502) | 0.0 (0.0, 0.0) | 0.0 (0.0, 5.6) | 0.0 (0.0, 0.0) | 0.018 |
| Above threshold | 17 (7%) | 30 (12%) | 47 (9%) | 0.026 |
| Maternal antenatal tobacco smoking | ||||
| Number of mothers | 530 | 451 | 981 | |
| Maternal urine cotinine (n=954) | 512 | 442 | ||
| <10 ng·mL−1, non-smoker | 181 (35%) | 47 (11%) | 228 (24%) | <0.001 |
| 10–499 ng·mL−1, passive/exposed | 255 (50%) | 155 (35%) | 410 (43%) | |
| ≥500 ng·mL−1, active smoker | 76 (15%) | 240 (54%) | 316 (33%) | |
| Infant urine cotinine | ||||
| Urine cotinine at birth or 6–10 weeks (n=763) | 415 | 348 | ||
| <10 ng·mL−1 | 184 (44%) | 55 (16%) | 239 (31%) | <0.001 |
| 10–499 ng·mL−1 | 212 (51%) | 208 (60%) | 420 (55%) | |
| ≥500 ng·mL−1 | 19 (5%) | 85 (24%) | 104 (14%) |
Data are presented as median (interquartile range), unless otherwise stated. PM10: particulate matter <10 μm in diameter; NO2: nitrogen dioxide; CO: carbon monoxide. #: dimensions comprise type of home, building material, water supply, toilet facilities, kitchen type and ventilation in kitchen areas.
Of the pollutants measured antenatally, significantly more Mbekweni homes had NO2 levels above ambient standard compared with Newman (16 (4%) versus 3 (1%)); overall, 45% of homes had measured benzene levels above ambient standards. However, none of the median levels were above ambient standards. For pollutants measured postnatally, there were significant differences across sites in prevalence of above ambient standards for NO2 (p=0.041) and CO (p=0.026; table 2).
Using maternal cotinine measures, 316 (33%) of mothers were active smokers, significantly higher for Newman, 241 (55%) compared with Mbekweni, 76 (15%) and a further 423 (44%) were exposed to tobacco smoke. For infant ETS exposure, 524 (69%) had cotinine levels indicative of tobacco smoke exposure, of which 104 (14%) were that of a level of active smokers (table 2).
Nasopharyngeal bacterial carriage
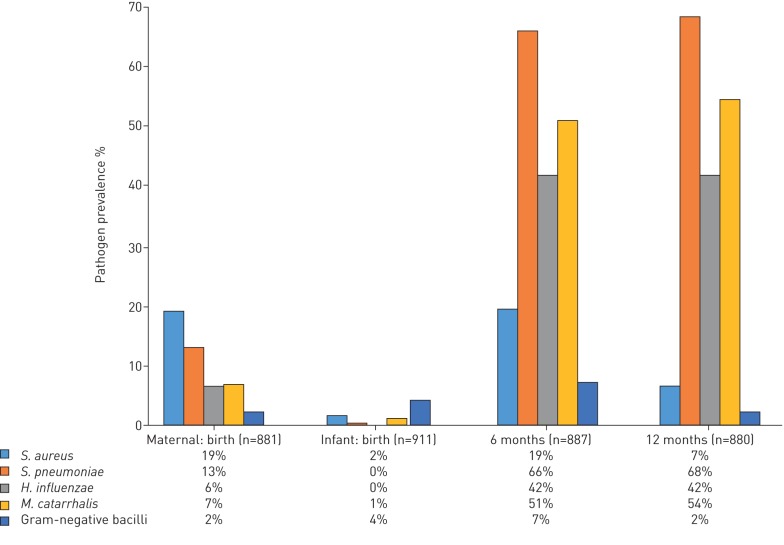
At delivery, 167/881 (19%; 95% CI 16–22) mothers carried S. aureus, 114/881 (13%; 95% CI 11–15) S. pneumoniae, 57/881 (6%; 95% CI 5–8) H. influenzae, 59/881 (7%; 95% CI 5–9) Moraxella catarrhalis and 18/881 (2%; 95% CI 1–3) other gram-negative bacilli (figure 2).
FIGURE 2.
Nasopharyngeal bacterial carriage at birth, 6 months and 1 year of life.
At birth, 15/911 (2%; 95% CI 1–4) infants had S. aureus while 40/911 (4%; 95% CI 3–6) had gram-negative bacilli on nasopharyngeal samples. By 6 months, 584/887 (66%; 95% CI 63–69) infants carried S. pneumoniae, which remained the predominant organism at 1 year of age, carried by 547/800 (68%; 95% CI 65–72). However, carriage of S. aureus 53/800 (7%; 95% CI 6–9) or gram-negative bacilli 18/800 (2%; 95% CI 1–4) decreased between 6 and 12 months (p<0.001). The median number of bacteria carried was two (IQR 1–3) and two (IQR 1–2) at 6 and 12 months respectively (figure 2).
Of the infants who carried H. influenzae at 6 months, 160 (50%) continued to carry this at 12 months. Likewise, 376 (74%) of infants who carried S. pneumoniae at 6 months also carried S. pneumoniae at 12 months (supplemental table 2).
Associations between environmental exposures and nasopharyngeal carriage
Antenatal exposures and maternal nasopharyngeal carriage
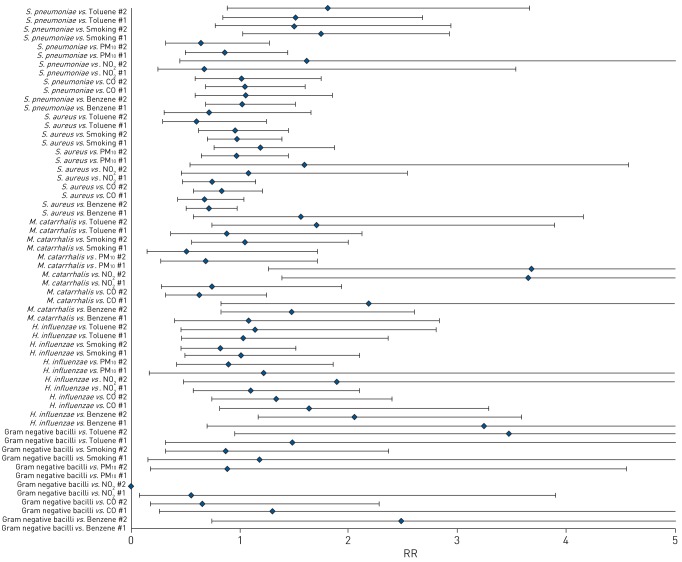
Antenatal exposure to NO2 above ambient standards was associated with increased maternal nasopharyngeal carriage of M. catarrhalis when adjusted for all clinical covariates (adjusted; aRR 3.65 (95% CI 1.39–9.58)) and when adjusted for clinical covariates as well as the other pollutants (adjusted 2; aRR 3.69 (95%CI 1.27–10.73)).
Benzene exposure was associated with maternal H. influenzae carriage when adjusted for clinical covariates (adjusted; aRR 2.06 (95% CI 1.18–3.59)) and tobacco smoke exposure almost doubled the risk of S. pneumoniae carriage in mothers (aRR 1.73 (95% CI 1.03–2.92)) (figure 3).
FIGURE 3.
Multivariate analysis of antenatal exposures and maternal nasopharyngeal bacterial carriage. PM10: particulate matter <10 µm in diameter; RR: risk ratio.
Site-specific multivariable analysis generally showed similar associations. Mbekweni showed gram-negative bacilli carriage being associated with toluene (aRR 5.92 (95% CI 1.16–30.08); supplemental table 3). For Newman, smoking also increased the risk of gram-negative bacilli carriage exponentially (supplemental table 4).
Postnatal exposures and infant nasopharyngeal carriage
PM10 was associated with an increased risk of H. influenzae at 6 months (adjusted 2; aRR 1.68 (95% CI 1.10–2.57)) and M. catarrhalis at 12 months (adjusted 2; aRR 1.42 (95% CI 1.03–1.97)), while NO2 increased the risk of gram-negative bacilli carriage at 12 months (adjusted; aRR 15.89 (95% CI 3.35–75.46); table 3). Further, when adjusting for co-carriage, clinical covariates and all the other pollutants (adjusted 2), PM10 was still associated with an increased risk of H. influenzae at 6 months, (aRR 1.60 (95% CI 1.04–2.46)) and M. catarrhalis at 12 months (aRR 1.39 (95% CI 1.02–1.90)), and NO2 with gram-negative bacilli at 12 months (adjusted; aRR 9.44 (95% CI 1.96–45.40)) while CO increased the risk of S. pneumoniae carriage at 6 months (aRR 1.33 (95% CI 1.03–1.72); supplemental table 7).
TABLE 3.
Multivariate analysis of postnatal exposures and infant nasopharyngeal bacterial carriage
| S. aureus | S. pneumoniae | H. influenzae | M. catarrhalis | Gram negative bacilli | ||||||
| Adj | Adj2 | Adj | Adj2 | Adj | Adj2 | Adj | Adj2 | Adj | Adj2 | |
| PM10 | ||||||||||
| 6 months | 0.98 (0.57–1.70) | 0.95 (0.42–2.14) | 0.96 (0.79–1.16) | 0.96 (0.69–1.34) | 1.09 (0.85–1.40) | 1.68 (1.10– 2.57) | 0.91 (0.70–1.18) | 0.88 (0.57–1.35) | 0.61 (0.19–1.95) | 0.13 (0.02–1.05) |
| 12 months | 0.56 (0.13–2.48) | 2.23 (0.40–12.35) | 1.02 (0.87–1.20) | 0.98 (0.72–1.35) | 1.15 (0.88–1.51) | 1.09 (0.69–1.73) | 1.12 (0.90–1.40) | 1.42 (1.03–1.97) | 0.92 (0.11–7.59) | |
| NO2 | ||||||||||
| 6 months | 0.90 (0.16–4.93) | 2.15×10−6 (1.39×10−7–3.32×10−5) | 1.32 (0.97–1.81) | 1.93 (0.98–3.80) | 0.58 (0.18–1.92) | 2.38×10−6 (2.62×10−7–2.16×10−5) | 0.96 (0.41–2.21) | 5.09×10−6 (6.23e×10−7–4.16×10−5) | 3.05 (0.48–19.48) | 1.77×10−5 (4.45×10−7–7.04×10−4) |
| 12 months | 2.32×10−6 (7.08×10−7–7.60×10−6) | 1.93×10−7 (2.74×10−9–1.35×10−5) | 1.07 (0.80–1.43) | 0.72 (0.27–1.92) | 0.58 (0.21–1.62) | 1.93×10−7 (2.74×10−9 – 1.35×10−5) | 15.89 (3.35–75.46) | |||
| Benzene | ||||||||||
| 6 months | 0.78 (0.52–1.17) | 0.78 (0.33–1.83) | 1.01 (0.87–1.17) | 0.88 (0.64–1.22) | 0.96 (0.77–1.19) | 1.68 (1.10–2.57) | 0.95 (0.48–1.20) | 1.00 (0.72–1.39) | 0.65 (0.30–1.42) | 0.38 (0.13–1.09) |
| 12 months | 0.81 (0.36–1.82) | 1.37 (0.20–9.59) | 1.01 (0.89–1.15) | 1.17 (0.90–1.51) | 0.83 (0.65–1.05) | 0.90 (0.55–1.46) | 0.89 (0.74–0.09) | 0.92 (0.66–1.28) | 2.06 (0.44–9.74) | |
| CO | ||||||||||
| 6 months | 1.17 (0.74–1.87) | 1.53 (0.78–2.98) | 1.03 (0.88–1.21) | 1.28 (0.99–1.66) | 1.03 (0.80–1.33) | 0.99 (0.63–1.54) | 1.04 (0.85–1.27) | 1.19 (0.88–1.60) | 1.15 (0.48–2.75) | 2.15 (0.90–5.12) |
| 12 months | 0.68 (0.22–2.12) | 0.56 (0.08–3.97) | 1.05 (0.91–1.22) | 1.13 (0.89–1.44) | 1.01 (0.76–1.34) | 0.91 (0.58–1.43) | 0.98 (0.79–1.22) | 1.01 (0.75–1.35) | 1.60 (0.38–6.72) | |
| Smoking | ||||||||||
| 6 months | 1.28 (0.87–1.88) | 0.90 (0.44–1.83) | 1.14 (1.00–1.30) | 1.12 (0.86–1.47) | 0.93 (0.75–1.15) | 0.92 (0.62–1.37) | 1.01 (0.84–1.22) | 1.15 (0.81–1.62) | 0.88 (0.46–1.70) | 0.93 (0.34–2.62) |
| 12 months | 0.79 (0.42–1.50) | 0.81 (0.18–3.58) | 1 (0.87–1.15) | 0.93 (0.74–1.18) | 1.21 (0.96–1.53) | 1.43 (0.95–2.17) | 1.03 (0.87–1.21) | 1.05 (0.77–1.41) | 6.77 (0.91–50.53) | |
| Toluene | ||||||||||
| 6 months | 0.55 (0.24–1.27) | 0.86 (0.23–3.17) | 0.95 (0.74–1.22) | 1.23 (0.82–1.83) | 0.88 (0.61–1.27) | 0.52 (0.25–1.09) | 0.99 (0.73–1.35) | 5.57×10−7 (3.22×10−7–9.64×10−7) | ||
| 12 months | 0.82 (0.22–3.04) | 1.00×10−7 (1.95×10−7–5.16×10−7) | 1.1 (0.90–1.34) | 1.00 (0.70–1.43) | 1.15 (0.83–1.59) | 1.14 (0.62–2.11) | 1.13 (0.85–1.51) | 5.84×10−7 (2.08×10−7–1.64×10−6) | ||
Data are presented as risk ratio (95% CI). Adj: adjusted for all the clinical-demographic variables; Adj2: adjusted for all the clinical-demographic variables, plus adjusting for other indoor air pollutants. Risk ratios in bold are statistically significant at p-values derived using Benjamini–Hochberg (p<0.05). Empty cells indicate that model convergence was not achieved. PM10: particulate matter <10 μm in diameter; NO2: nitrogen dioxide; CO: carbon monoxide.
In infants, ETS exposure was also associated with an increased risk of S. pneumoniae carriage at 6 months of age (aRR 1.14 (95% CI 1.00–1.30); adjusted; table 3) The association between smoke exposure and S. pneumoniae was also noted when adjusting for the other bacterial organisms co-carried (adjusted; aRR 1.16 (95% CI 1.02–1.32) at 6 months (supplemental table 7).
Site-specific analysis showed similar risks for Mbekweni with PM10 increasing the risk of H. influenzae (aRR 2.09 (95% CI 1.15–3.81)) carriage and NO2 gram-negative bacilli (aRR 3.88×108 (95% CI 1.32×108 to 1.14×109); supplemental table 5). However, for Newman, NO2 was associated with an increased risk of S. pneumoniae carriage at both 6 months (aRR 2.66 (95% CI 2.04–3.47)) and 12 months (aRR 1.75 (95% CI 1.26–2.42)). Also of note for Newman, was that the VOCs (benzene and toluene) were associated with an increased risk of H. influenzae carriage at 6 months (adjusted; aRR 1.45 (95% CI 1.06–2.00)) and S. pneumoniae carriage at 12 months (adjusted 2; aRR 1.31 (95% CI 1.06–1.60)) respectively (supplemental table 6).
Discussion
In this large peri-urban birth cohort study, we have shown an association between antenatal IAP exposure and maternal nasopharyngeal carriage of bacteria and the impact of tobacco smoke exposure on both maternal and infant bacterial carriage, particularly S. pneumoniae. We also report an association between exposure to other indoor air pollutants including PM10, NO2 and CO on infant bacterial nasopharyngeal carriage. Further, despite the socioeconomic and ethnic differences between the two communities studied, the environmental risks associated with carriage were similar.
While it is recognised that several factors including age, season, day care, number of siblings, acute respiratory illness, HIV infection, diet and vaccination may influence the acquisition of nasopharyngeal carriage in infants [4, 23]. the effect of IAP on nasopharyngeal carriage has not been well described particularly in LMICs [25]. In this study, significant associations between exposure to several IAP and maternal and infant nasopharyngeal carriage occurred even after adjusting for such clinical factors associated with carriage and for co-carriage. The associations noted between postnatal PM10 exposure and infant H. influenzae and M. catarrhalis carriage were consistent. It is well recognised that PM10 exposure is associated with LRTIs and childhood respiratory diseases [26–28]. Further, an in vitro study found that PM10 promoted bacterial invasion of epithelial airway cells by attenuating innate defence mechanisms, through the disruption of human defensins, a critical part of antimicrobial activity [29]. Black carbon, a component of PM10, has also been shown to impact bacterial colonisation as well as spread of bacteria from the nasopharynx to the lungs [9]. In this cohort, we have also previously shown an association between PM10 exposure and LRTIs in infancy [30]. However, the inclusion of nasopharyngeal swab sampling at only two time points (6 and 12 months) in this study, precluded a longitudinal analysis of the impact of bacterial carriage on LRTIs. Nevertheless, this finding highlights the impact of IAP exposure in several ways that may contribute to child respiratory illness. Although there were no consistent associations between postnatal IAP exposure and infant carriage at 6 and 12 months, this may reflect both the dynamic nature of bacterial carriage and the complex interplay of a number of factors that influence this and highlights the importance of IAP and ETS exposure in influencing nasopharyngeal bacterial carriage. Also of note was although there were differences between the communities studied, IAP exposure and the risks associated with bacterial carriage were similar. However, interestingly in the community of mixed ethnicity, postnatal VOC exposure from benzene and toluene significantly increased the risk of bacterial carriage of a number of organisms. VOCs are a by-product of combustion of alternate fuels such as paraffin and cigarette smoke [31] and may also reflect the much higher prevalence of maternal smoking and exposure in this population compared with the black African community.
In this cohort, maternal smoking and infant in utero and postnatal tobacco exposure was very high as indicated by urine cotinine in both mothers and infants. The high rates of maternal smoking especially among mothers of mixed ethnicity is concerning, as is the very high exposure to household smoke from both fathers and other household members [7]. ETS exposure was associated with an increased carriage of several bacteria in mothers and infants including S. pneumoniae, S. aureus and gram-negative bacilli. Nasopharyngeal carriage is recognised as a precursor to LRTIs and a source of person-to-person transmission among individuals [3, 32]. Mice models have shown cigarette smoke suppressed nasal inflammatory mediator expression, in particular neutrophil recruiting chemokines normally activated by S. pneumoniae carriage, thereby predisposing to invasive S. pneumoniae infection. Further, smoking cessation reversed this and prevented invasive pneumococcal disease [33]. Other animal studies have also shown an increased neutrophil response to H. influenzae in cigarette-exposed mice, with interleukin 1α produced by alveolar macrophages driving this process. This exaggerated response may underlie accelerated lung pathology seen when there is cigarette smoke and bacterial infection [34]. Tobacco smoke also has significant effects on the generation of adaptive immune responses to H. influenzae which may predispose to recurrent infections and exacerbations particularly in chronic lung diseases [35]. The host's ability to control bacterial colonisation of the upper airway is also impacted by cigarette smoke with increased inflammatory mediators and reduced blood granulocyte and monocyte phagocytosis activity [36]. These mechanisms may therefore increase the risk of developing LRTIs and warrant further investigation.
The association between S. pneumoniae nasopharyngeal carriage and the development of subsequent S. pneumoniae disease including LRTIs is well recognised [3, 32, 37]. Increased S. pneumoniae carriage in infants associated with both CO and ETS exposure suggests that reducing exposure to these pollutants could play a role in the prevention of childhood LRTIs. Further, tobacco smoke exposure almost doubled maternal nasopharyngeal pneumococcal carriage, providing a source for increased transmission between individuals. While we found no association between preceding or recurrent respiratory tract infection (either upper or LRTIs), on nasopharyngeal bacterial carriage, the association between nasopharyngeal pneumococcal carriage and the development of LRTIs (as an outcome) was not explored, as for this study infant nasopharyngeal carriage was assessed only at two time points (6 and 12 months) and the majority of LRTI cases occurred early, before 6 months [30]. Similarly, we were unable to assess the effect of 13-valent pneumococcal conjugate vaccine (PCV13) (administered at 6, 14 weeks and 9 months) on S. pneumoniae carriage due to the very high vaccination rates and the infrequent sampling period in this study. We have however, previously described the association between IAP and ETS exposure and LRTIs in this cohort [30], highlighting the role of IAP and ETS as potential risk factors for childhood respiratory illness.
The patterns of infant carriage seen in this cohort are consistent with those previously described, with S. aureus predominating in the first months, followed by S. pneumoniae which persisted until 12 months of age [23, 38]. The predominant maternal bacterium carried was S. aureus, however the prevalence was lower than other studies which have reported maternal S. aureus carriage of up to 50% [39–41]. The high rates of infant S. pneumoniae carriage are also consistent with those reported in low and low-middle-income settings [37, 42]. The study extends this knowledge by identifying factors associated with acquisition which have not previously been described in infants. A limitation of this study is reliance on bacterial culture to identify organisms, which may underestimate bacterial prevalence and the inability to subtype organisms. However, the four most common bacterial species identified are the major contributors to bacterial LRTI in infants. In addition to these species, we also identified a range of gram-negative enteric bacterial species in the nasopharynx, but at a considerably lower frequency, and with insufficient statistical power to draw conclusions. Gram-negative bacteria are important causes of LRTIs in early life, particularly in the neonates [43]. It is unclear whether nasopharyngeal carriage with gram-negative bacilli precedes invasive disease. Further work is required to understand the relationship between environmental exposures, colonisation with gram-negative bacteria and LRTIs in early life.
Although standard bacterial culture is likely to miss key components of the bacterial microbiota of the nasopharynx, one of the major advantages of culture over 16S-amplicon sequencing based microbiome studies, is the ability to identify bacteria at the species level [44]. This is highly relevant for nasopharyngeal bacteria, where there is substantial difference in potential for pathogenicity within a genus. Further, a range of viral infections are likely to impact carriage of bacteria in the nasopharynx [45], with viral infections playing a mediator role. IAP or ETS exposure may also impact on viral infections [46], which in turn may influence bacterial carriage. However, a limitation of this study is that comprehensive assessment of viral infections at a range of time points, including time points preceding our analysis of bacterial carriage was beyond the scope of this analysis. Further, we considered multiple hypotheses at once through multiple statistical tests. While we corrected for multiplicity using the Benjamini–Hochberg procedure, it is a conservative procedure with the consequence that some of the significant associations might still be due to chance. Strengths of this study were the large sample size, high cohort retention, longitudinal measurements of nasopharyngeal carriage, direct measurements of IAP both antenatally and postnatally, testing of maternal and infant samples and comprehensive analysis which considered numerous covariates.
The association between both IAP and ETS exposure on maternal and infant nasopharyngeal bacterial carriage has not been well described, particularly in LMICs and peri-urban communities who may rely on alternate fuel sources. This study highlights the need for effective, preventative measures to reduce such exposures. The population in this study represent a particularly vulnerable group of poor pregnant women and children in a LMIC setting, where there is a high burden of respiratory disease. Effective strategies to reduce smoking in pregnant women and to minimise household IAP exposure from both alternate fuel sources and ETS exposure are needed to improve child and maternal health particularly in LMICs.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables 00052-2018_supp_tables (373.4KB, pdf)
Acknowledgements
We thank the study and clinical staff at Paarl hospital and Mbekweni and Newman clinics and in particular the fieldworker teams. Thank you to SGS Environmental Services for supporting this project. We thank the participants and their families.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: A. Vanker reports receiving grants from the Bill & Melinda Gates Foundation (OPP1017641) and the Discovery Foundation, a South African Thoracic Society AstraZeneca Respiratory Fellowship, grants from the National Research Fund (South Africa), a CIDRI Clinical Fellowship, grants from the Medical Research Council (South Africa), during the conduct of the study.
Conflict of interest: P.M. Nduru reports receiving grants from the Bill and Melinda Gates Foundation during the conduct of the study.
Conflict of interest: W. Barnett reports receiving grants from the Bill and Melinda Gates Foundation (OPP1017641), the Discovery Foundation, the National Research Fund (South Africa) and the Medical Research Council (South Africa) during the conduct of the study.
Conflict of interest: F.S. Dube reports receiving grants from the Bill and Melinda Gates Foundation during the conduct of the study.
Conflict of interest: P.D. Sly has nothing to disclose.
Conflict of interest: R.P. Gie has nothing to disclose.
Conflict of interest: M.P. Nicol reports receiving grants from the Bill and Melinda Gates Foundation during the conduct of the study.
Conflict of interest: H.J. Zar reports receiving grants from the Bill and Melinda Gates Foundation, MRC South Africa and NRF South Africa during the conduct of the study.
Support statement: This study was funded by the Bill and Melinda Gates Foundation (OPP1017641), the Discovery Foundation, a South African Thoracic Society AstraZeneca Respiratory Fellowship, the National Research Foundation (South Africa), a CIDRI Clinical Fellowship, the SA Medical Research Council, the National Institute Of Allergy and Infectious Diseases of the National Institutes of Health (U01AI110466) and the National Human Genome Research Institute of the National Institutes of Health (54HG009824) under the H3Africa Initiative. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Zar HJ, Ferkol TW. The global burden of respiratory disease: impact on child health. Pediatr Pulmonol 2014; 49: 430–434. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet; 385: 430–440. [DOI] [PubMed] [Google Scholar]
- 3.Vissing NH, Chawes BLK, Bisgaard H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am J Respir Crit Care Med 2013; 188: 1246–1252. [DOI] [PubMed] [Google Scholar]
- 4.Harrison LM, Morris JA, Telford DR, et al. The nasopharyngeal bacterial flora in infancy: effects of age, gender, season, viral upper respiratory tract infection and sleeping position. FEMS Immunol Med Microbiol 1999; 25: 19–28. [DOI] [PubMed] [Google Scholar]
- 5.Vanker A, Barnett W, Nduru PM, et al. Home environment and indoor air pollution exposure in an African birth cohort study. Sci Total Environ 2015; 536: 362–367. [DOI] [PubMed] [Google Scholar]
- 6.Öberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011; 377: 139–146. [DOI] [PubMed] [Google Scholar]
- 7.Vanker A, Barnett W, Brittain K, et al. Antenatal and early life tobacco smoke exposure in an African birth cohort study. Int J Tuberc Lung Dis 2016; 20: 729–737. [DOI] [PubMed] [Google Scholar]
- 8.Rylance J, Kankwatira A, Nelson DE, et al. Household air pollution and the lung microbiome of healthy adults in Malawi: a cross-sectional study. BMC Microbiol 2016; 16: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussey SJK, Purves J, Allcock N, et al. Air pollution alters Staphylococcus aureus and Streptococcus pneumoniae biofilms, antibiotic tolerance and colonisation. Environ Microbiol 2017; 19: 1868–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg D, Givon-Lavi N, Broides A, et al. The contribution of smoking and exposure to tobacco smoke to Streptococcus pneumoniae and Haemophilus influenzae carriage in children and their mothers. Clin Infect Dis 2006; 42: 897–903. [DOI] [PubMed] [Google Scholar]
- 11.Jaspers I. Cigarette smoke effects on innate immune mechanisms in the nasal mucosa. Potential effects on the microbiome. Ann Am Thorac Soc 2014; 11: Suppl. 1, S38–S42. [DOI] [PubMed] [Google Scholar]
- 12.Zar HJ, Barnett W, Myer L, et al. Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax 2015; 70: 592–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zar HJ, Barnett W, Stadler A, et al. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med 2016; 4: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIOSH Manual of Analytical Methods (NMAM) - 4th edition, 3rd supplement. www.cdc.gov/niosh/docs/2003-154/ Date last accessed: October, 2014. Date last updated: September 20, 2018
- 15.Government Gazette Republic of South Africa. National Ambient Air Quality Standards. www.environment.gov.za/sites/default/files/legislations/nemaqa_airquality_g32816gon1210.pdf Date last accessed: August, 2014. Date last updated: December 24, 2009
- 16.Richardson M, Lukey P, Phahlane A. Department of Environmental Affairs, Republic of South Africa. State of Air Report 2005. Pretoria, South Africa; 2005.
- 17.Satzke C, Turner P, Virolainen-Julkunen A, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013; 32: 165–179. [DOI] [PubMed] [Google Scholar]
- 18.Dube FS, Kaba M, Whittaker E, et al. Detection of Streptococcus pneumoniae from different types of nasopharyngeal swabs in children. PLoS ONE 2013; 8: e68097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greiner O, Day PJ, Altwegg M, et al. Quantitative detection of Moraxella catarrhalis in nasopharyngeal secretions by real-time PCR. J Clin Microbiol 2003; 41: 1386–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald JH. Handbook of Biological Statistics. Baltimore, MD, Sparky House Publishing, 2014. [Google Scholar]
- 22.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–706. [DOI] [PubMed] [Google Scholar]
- 23.García-Rodríguez JÁ, Fresnadillo Martínez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother 2002; 50: Suppl. 3, 59–74. [DOI] [PubMed] [Google Scholar]
- 24.Bogaert D, Keijser B, Huse S, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS ONE 2011; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med 2014: 2: 823–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horne BD, Joy EA, Hofmann MG, et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am J Respir Crit Care Med 2018; 198: 759–766. [DOI] [PubMed] [Google Scholar]
- 27.Gurley ES, Homaira N, Salje H, et al. Indoor exposure to particulate matter and the incidence of acute lower respiratory infections among children: a birth cohort study in urban Bangladesh. Indoor Air 2013; 23: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grigg J. Particulate matter exposure in children: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009; 6: 564–569. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Liu J, Zhou J, et al. Urban particulate matter (PM) suppresses airway antibacterial defence. Respir Res 2018; 19: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanker A, Barnett W, Workman L, et al. Early-life exposure to indoor air pollution or tobacco smoke and lower respiratory tract illness and wheezing in African infants: a longitudinal birth cohort study. Lancet Planet Health 2017; 1: e328–e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants. Geneva, World Health Organization, 2010. [PubMed]
- 32.Simell B, Auranen K, Kayhty H, et al. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 2012; 11: 841–855. [DOI] [PubMed] [Google Scholar]
- 33.Shen P, Morissette MC, Vanderstocken G, et al. Cigarette smoke attenuates the nasal host response to Streptococcus pneumoniae and predisposes to invasive pneumococcal disease in mice. Infect Immun 2016; 84: 1536–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikota JK, Shen P, Morissette MC, et al. Cigarette smoke primes the pulmonary environment to IL-1alpha/CXCR-2-dependent non-typeable Haemophilus influenzae-exacerbated neutrophilia in mice. J Immunol 2014; 193: 3134–3145. [DOI] [PubMed] [Google Scholar]
- 35.Lugade AA, Bogner PN, Thatcher TH, et al. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol 2014; 192: 5226–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voss M, Wonnenberg B, Honecker A, et al. Cigarette smoke-promoted acquisition of bacterial pathogens in the upper respiratory tract leads to enhanced inflammation in mice. Respir Res 2015; 16: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adegbola RA, DeAntonio R, Hill PC, et al. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: a systematic review and meta-analysis. PLoS ONE 2014; 9: e103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogaert D, Van Belkum A, Sluijter M, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 2004; 363: 1871–1872. [DOI] [PubMed] [Google Scholar]
- 39.Shiri T, Nunes MC, Adrian PV, et al. Interrelationship of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus colonization within and between pneumococcal-vaccine naïve mother-child dyads. BMC Infect Dis 2013; 13: 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jimenez-Truque N, Tedeschi S, Saye EJ, et al. Relationship between maternal and neonatal Staphylococcus aureus colonization. Pediatrics 2012; 129: e1252–e1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lebon A, Moll HA, Tavakol M, et al. Correlation of bacterial colonization status between mother and child: the Generation R Study. J Clin Microbiol 2010; 48: 960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usuf E, Bottomley C, Adegbola RA, et al. Pneumococcal carriage in sub-Saharan Africa-a systematic review. PLoS ONE 2014; 9: e85001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toltzis P. Colonization with antibiotic-resistant gram-negative bacilli in the neonatal intensive care unit. Minerva Pediatr 2003; 55: 385–393. [PubMed] [Google Scholar]
- 44.Biesbroek G, Tsivtsivadze E, Sanders EA, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med 2014; 190: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 45.Shu M T, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015; 17: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol 2007; 19: 1135–1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables 00052-2018_supp_tables (373.4KB, pdf)